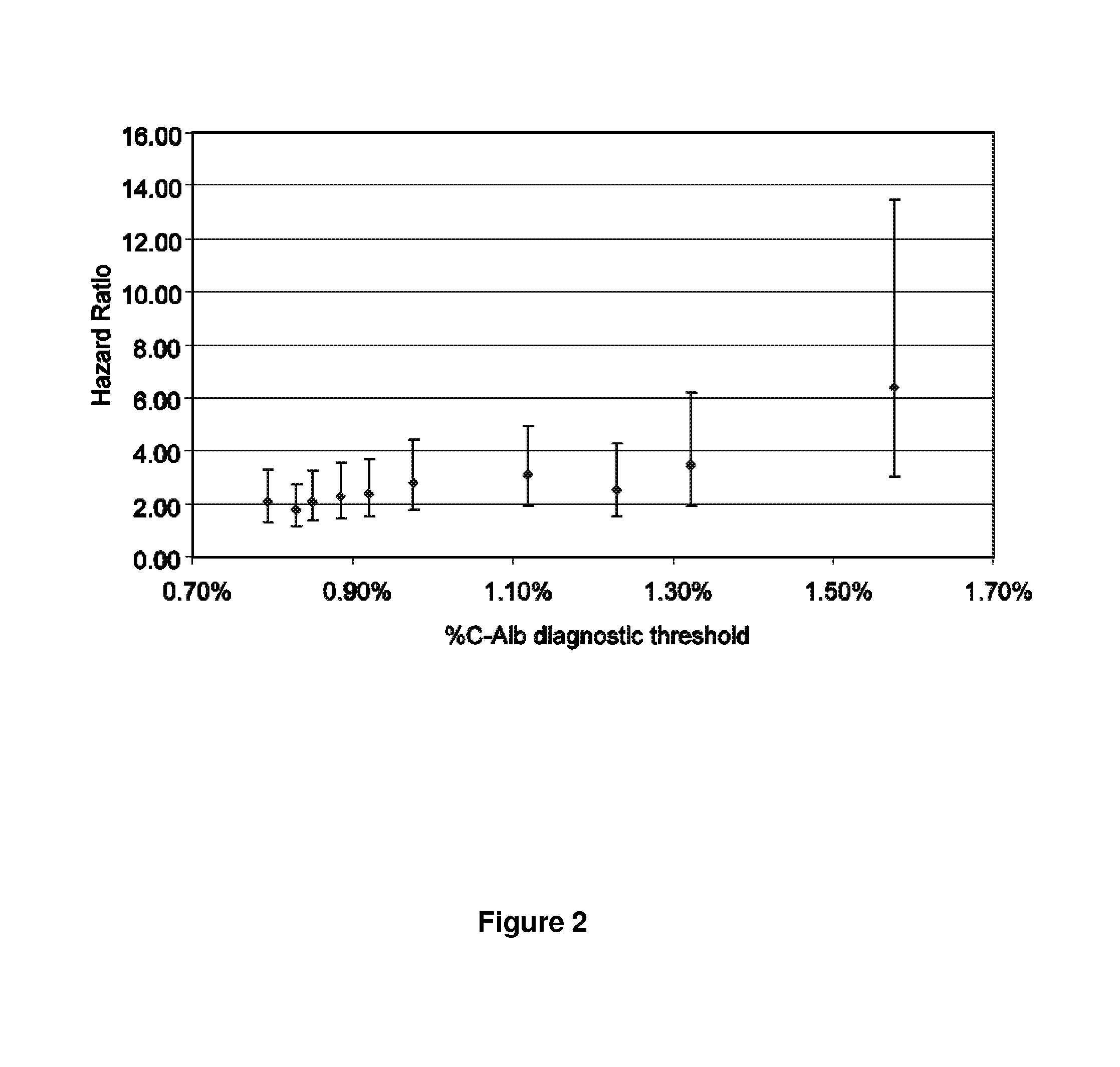
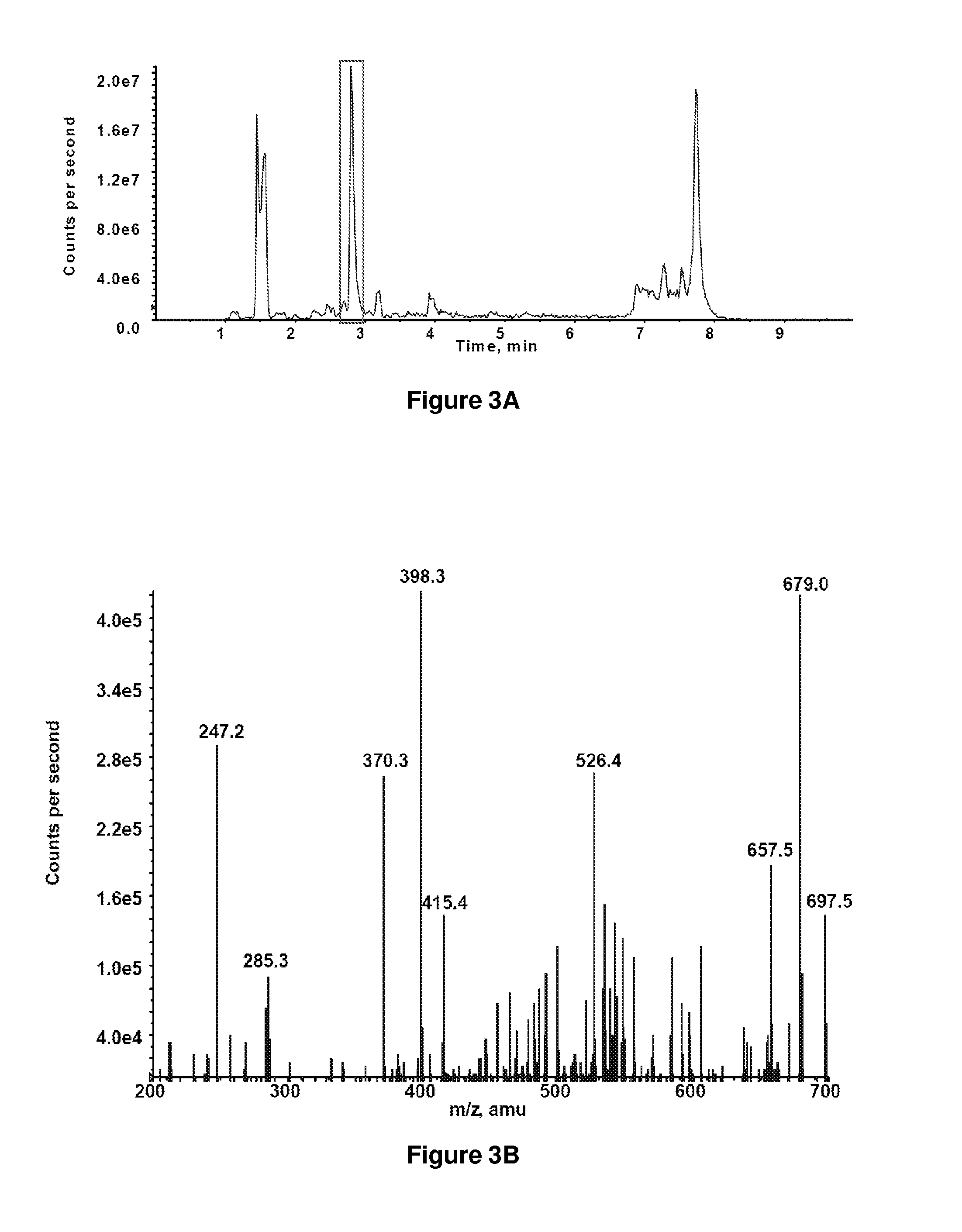
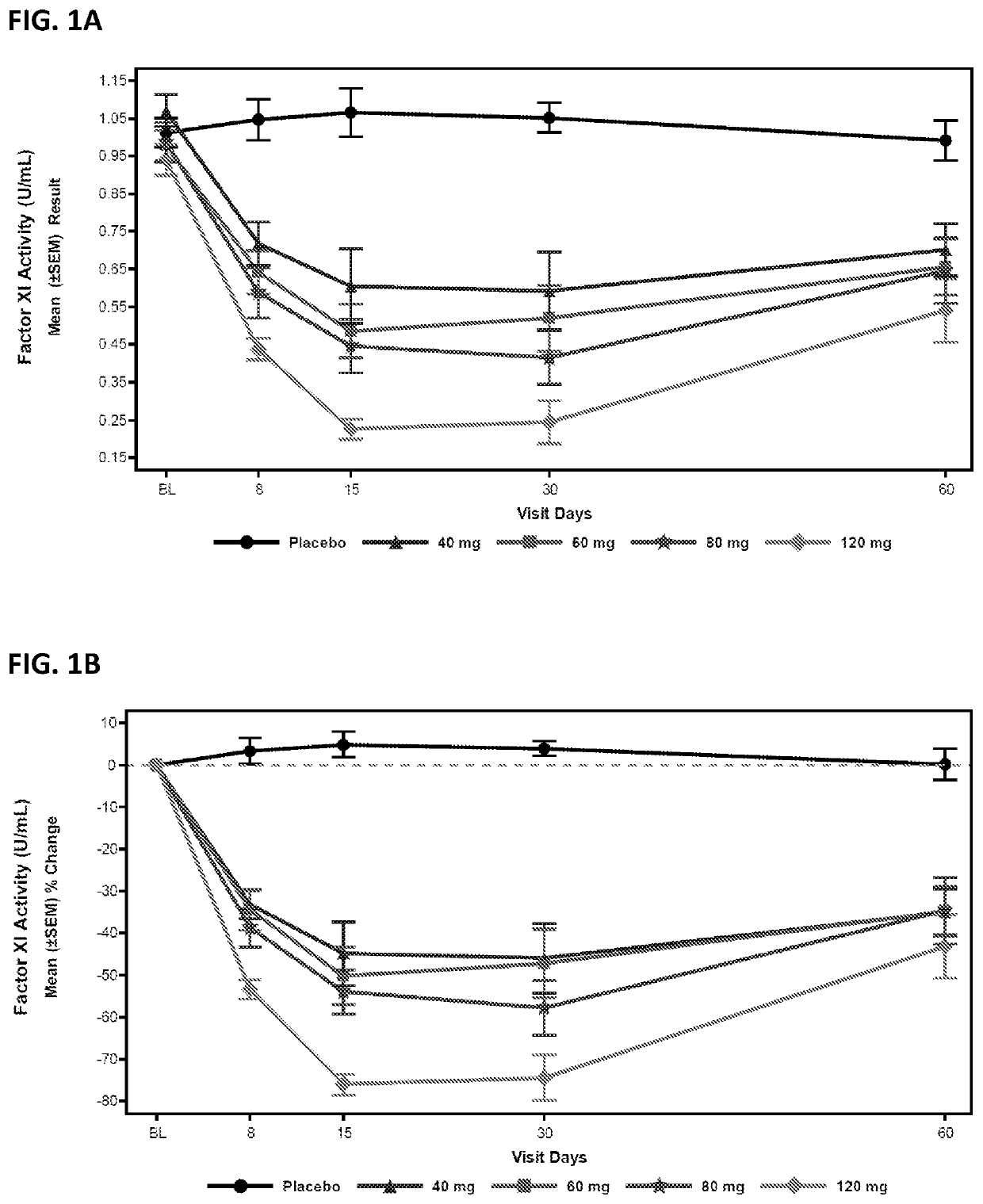
Patents
Literature
52 results about "End-stage kidney disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
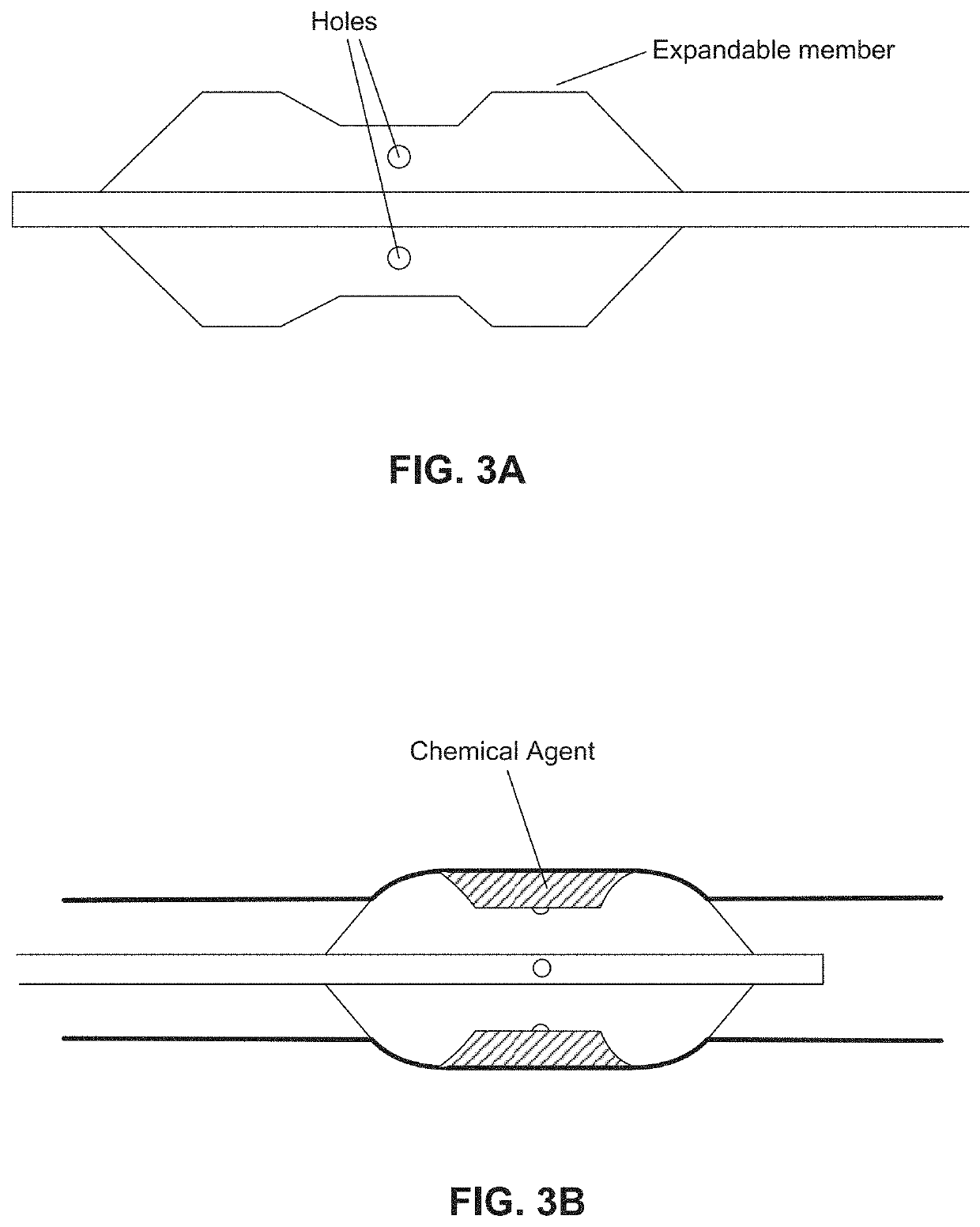
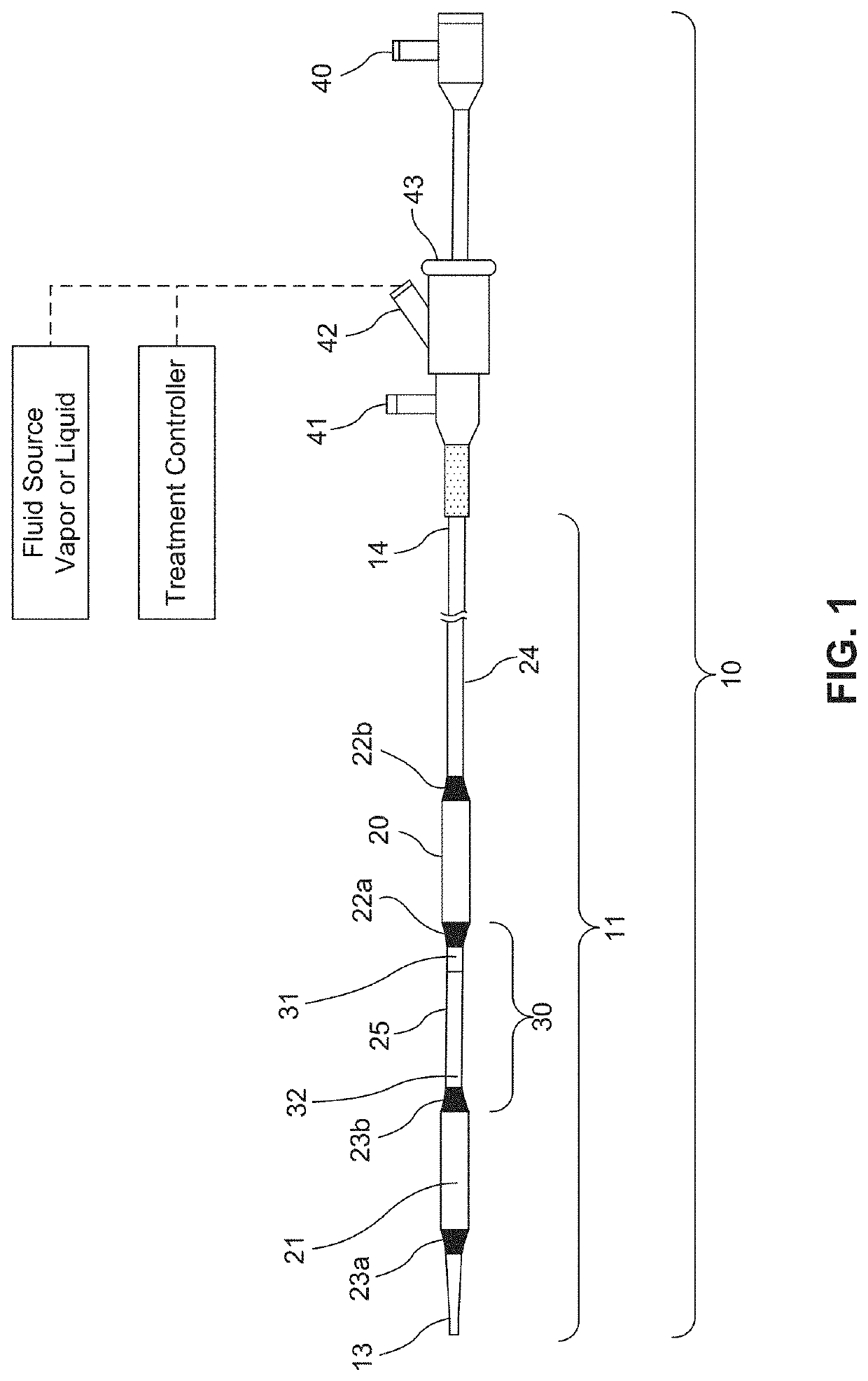
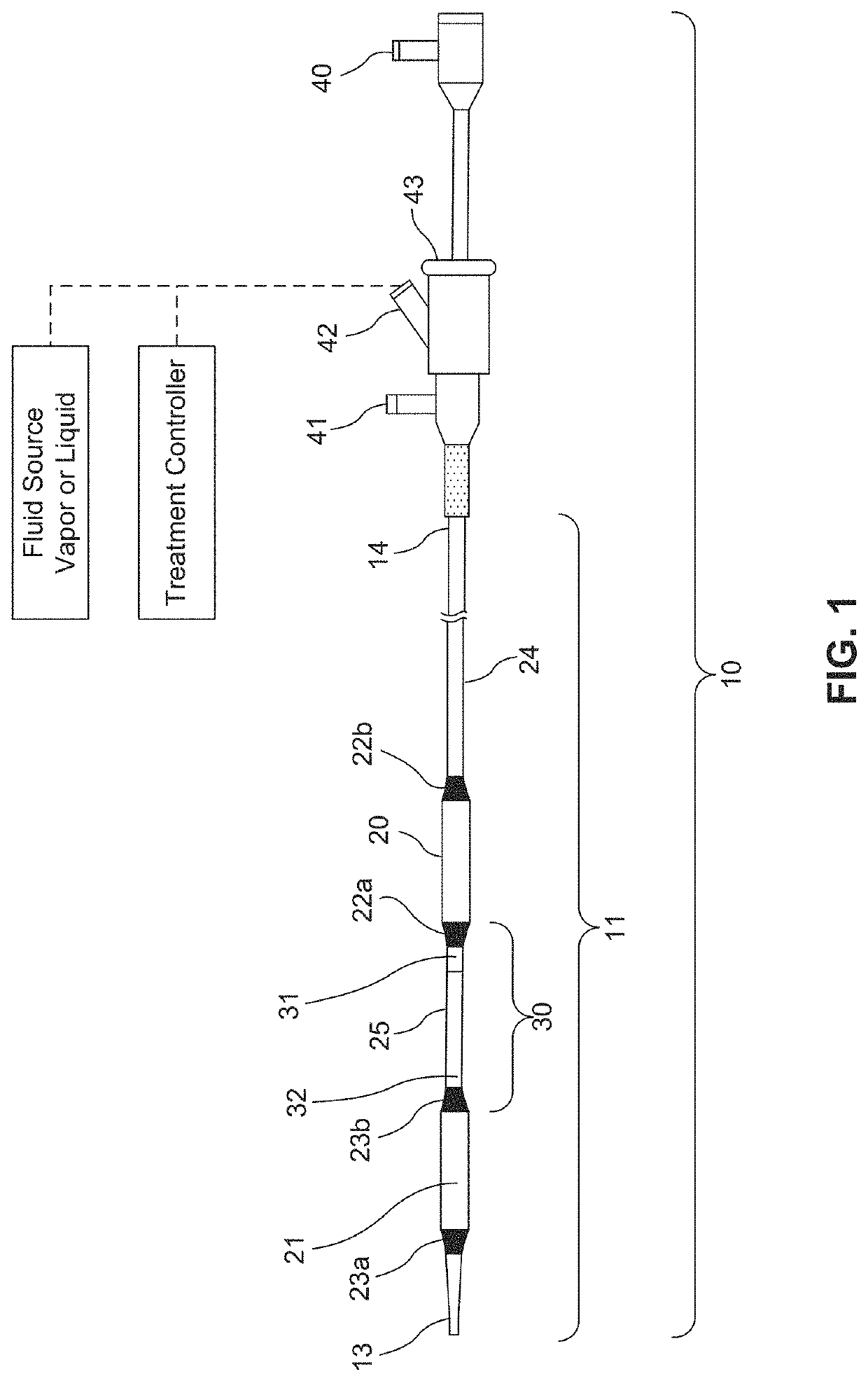
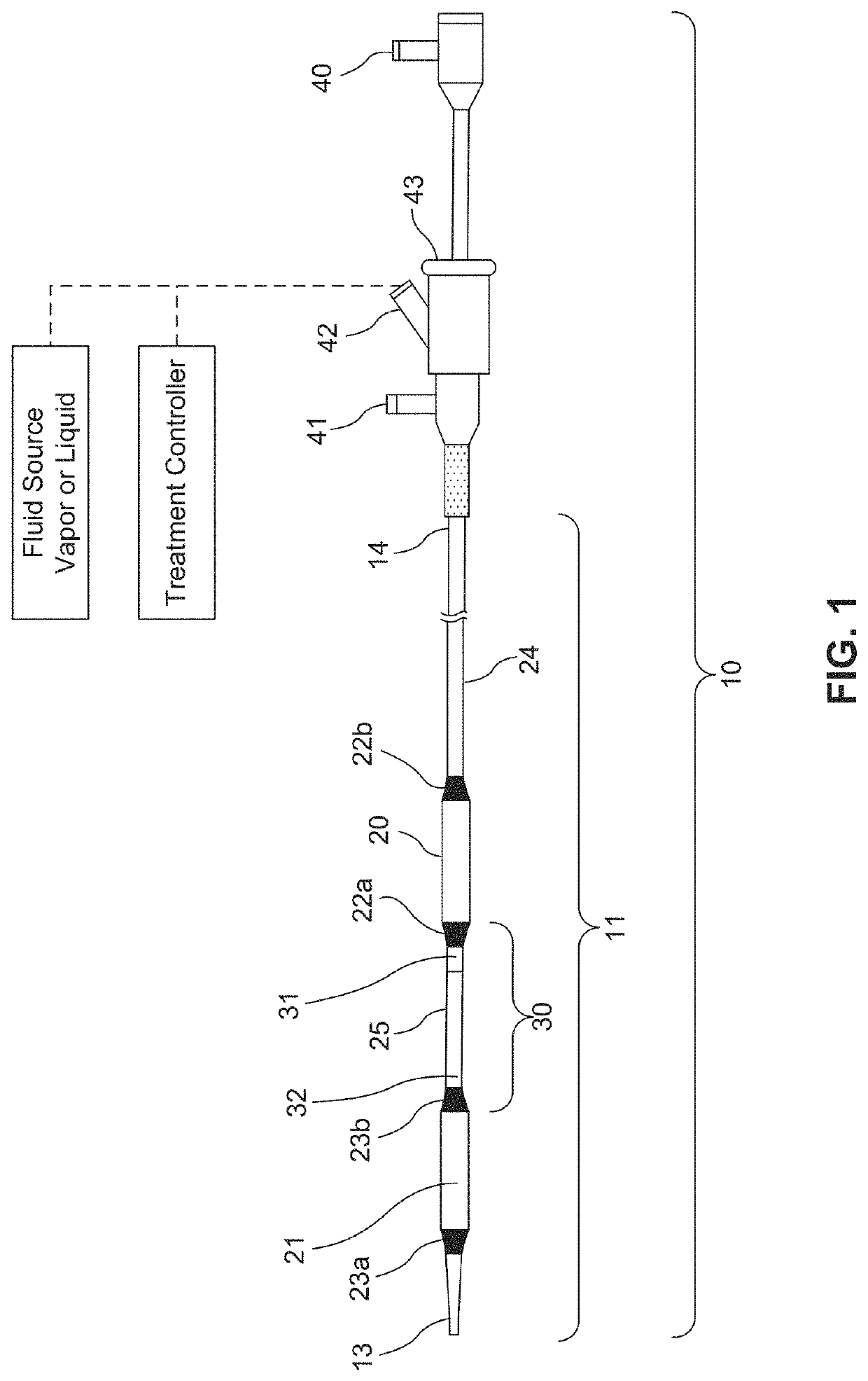
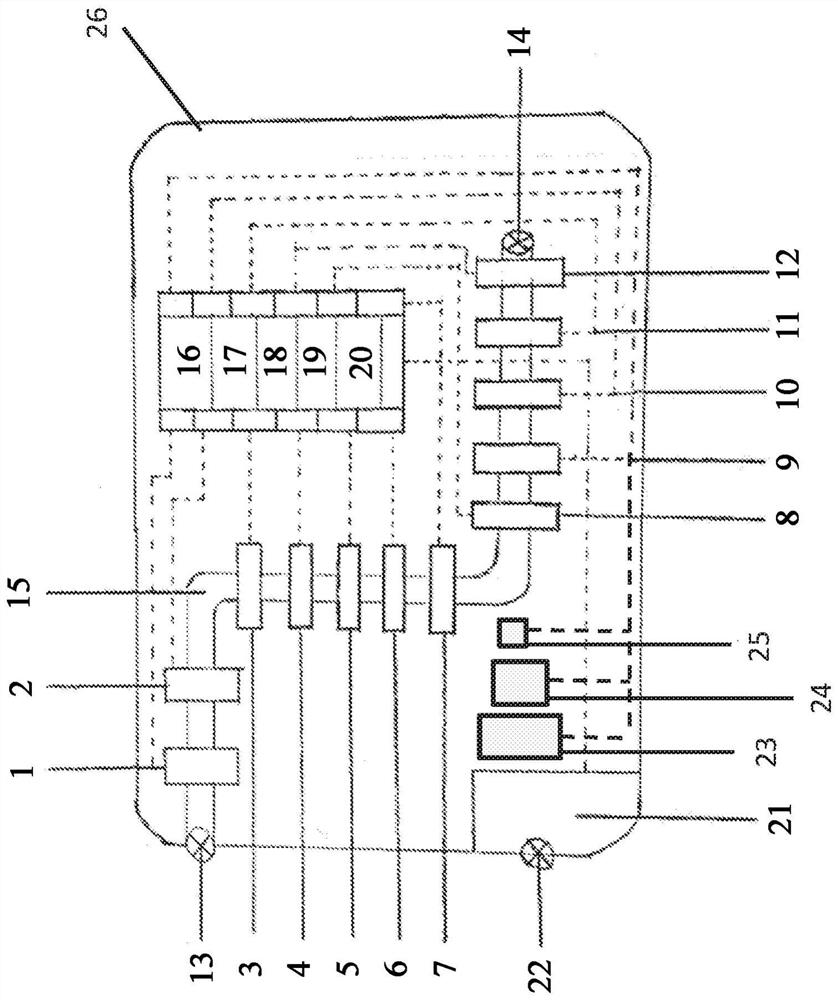
Fluid circuit for delivery of renal replacement therapies
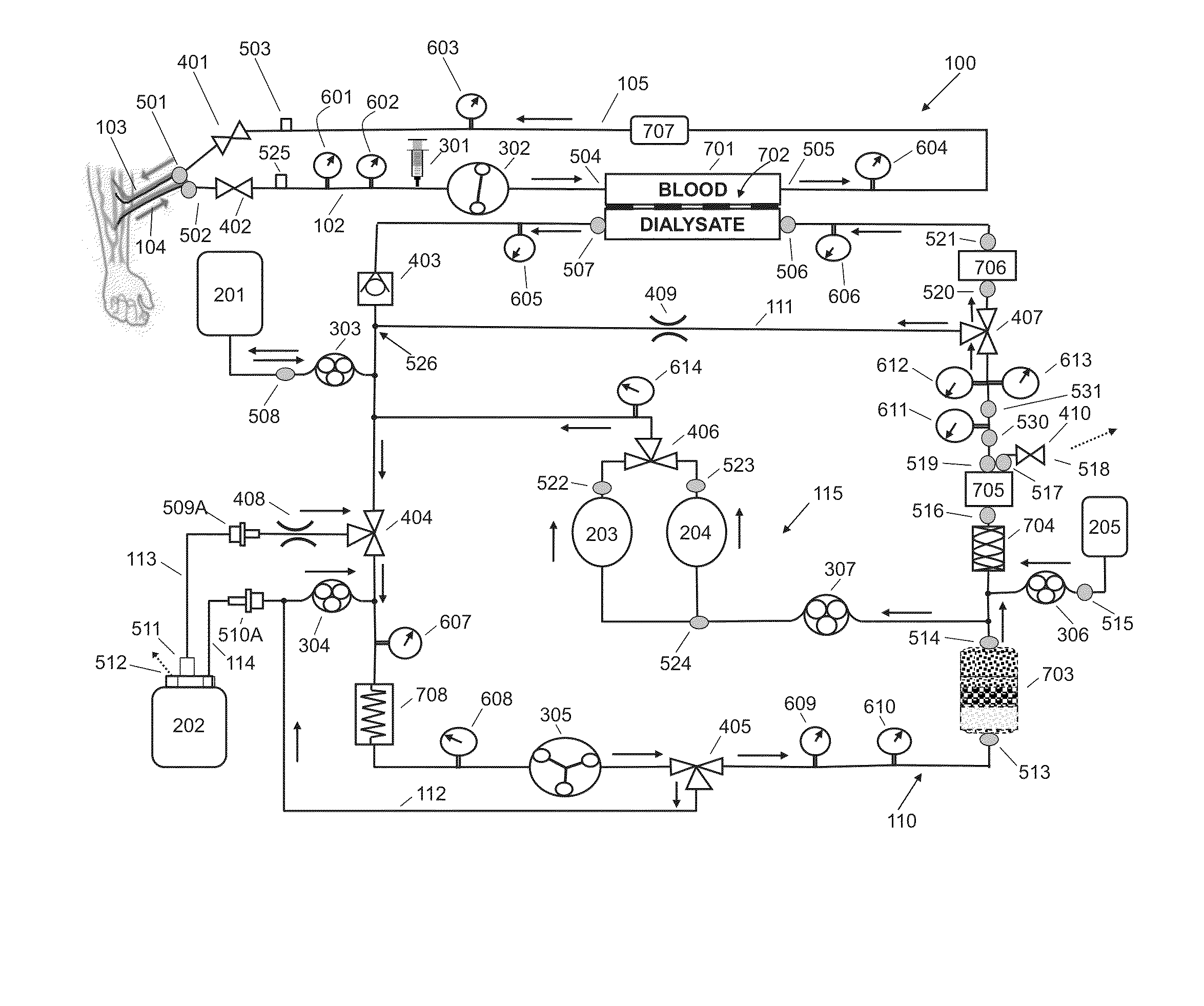
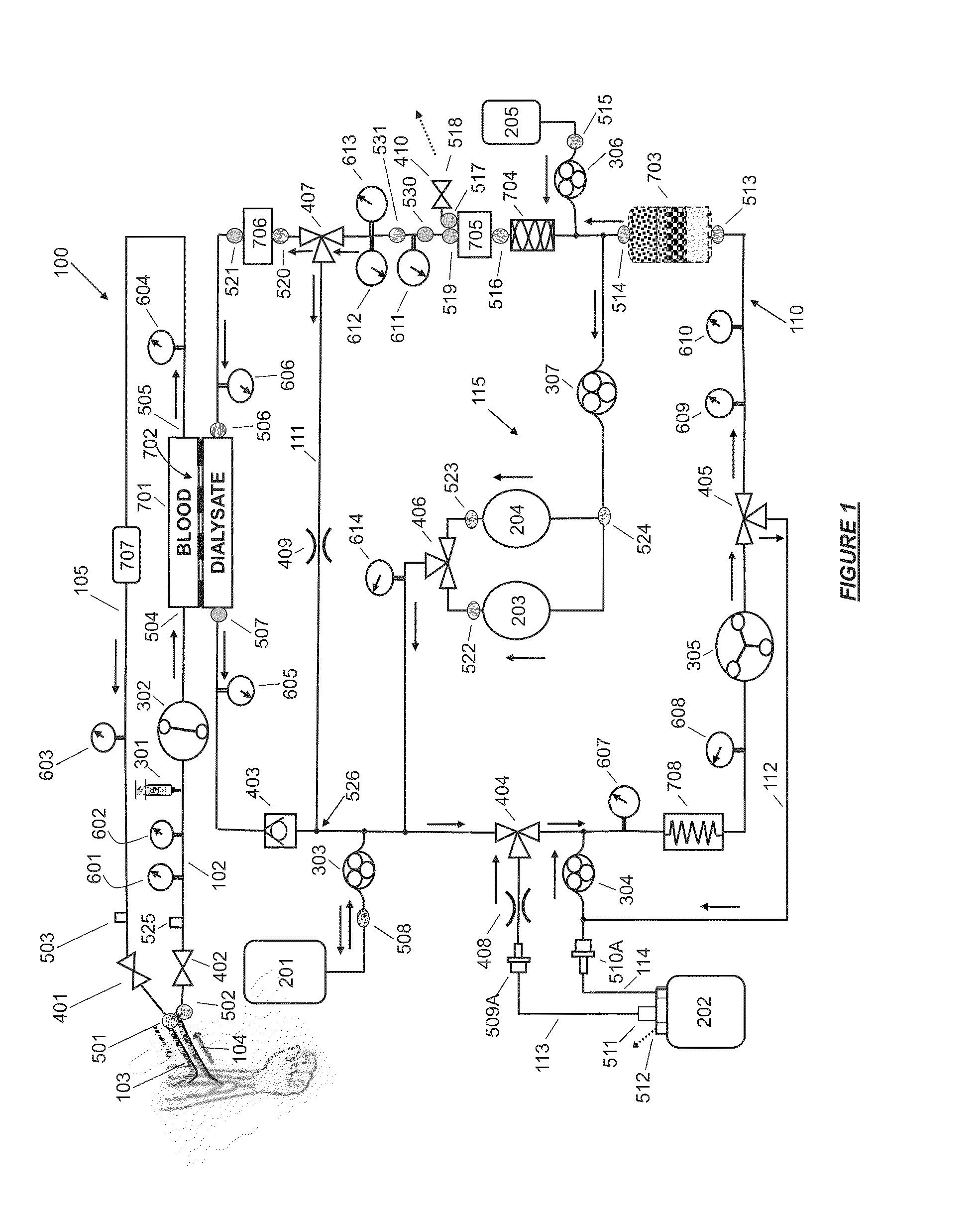
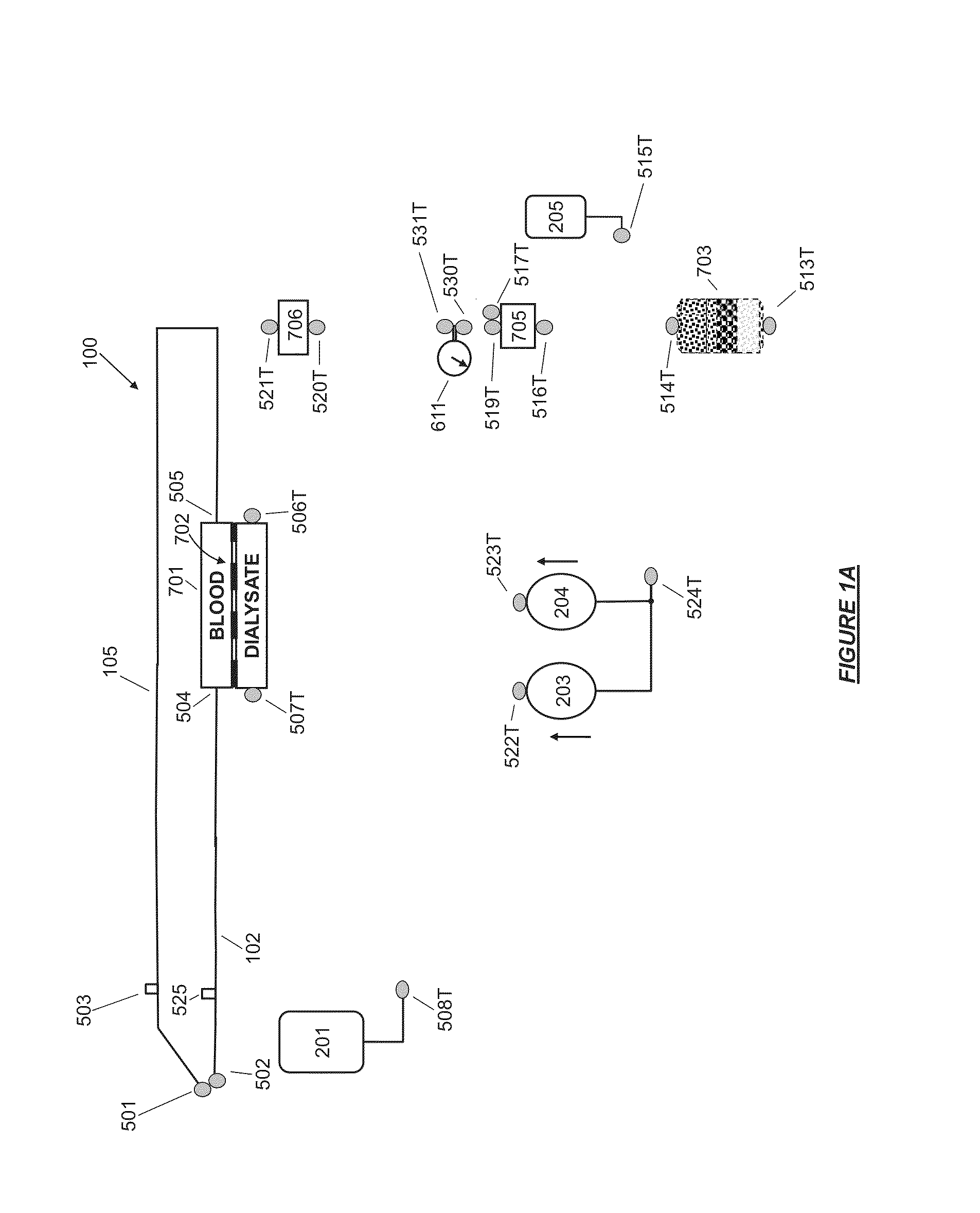
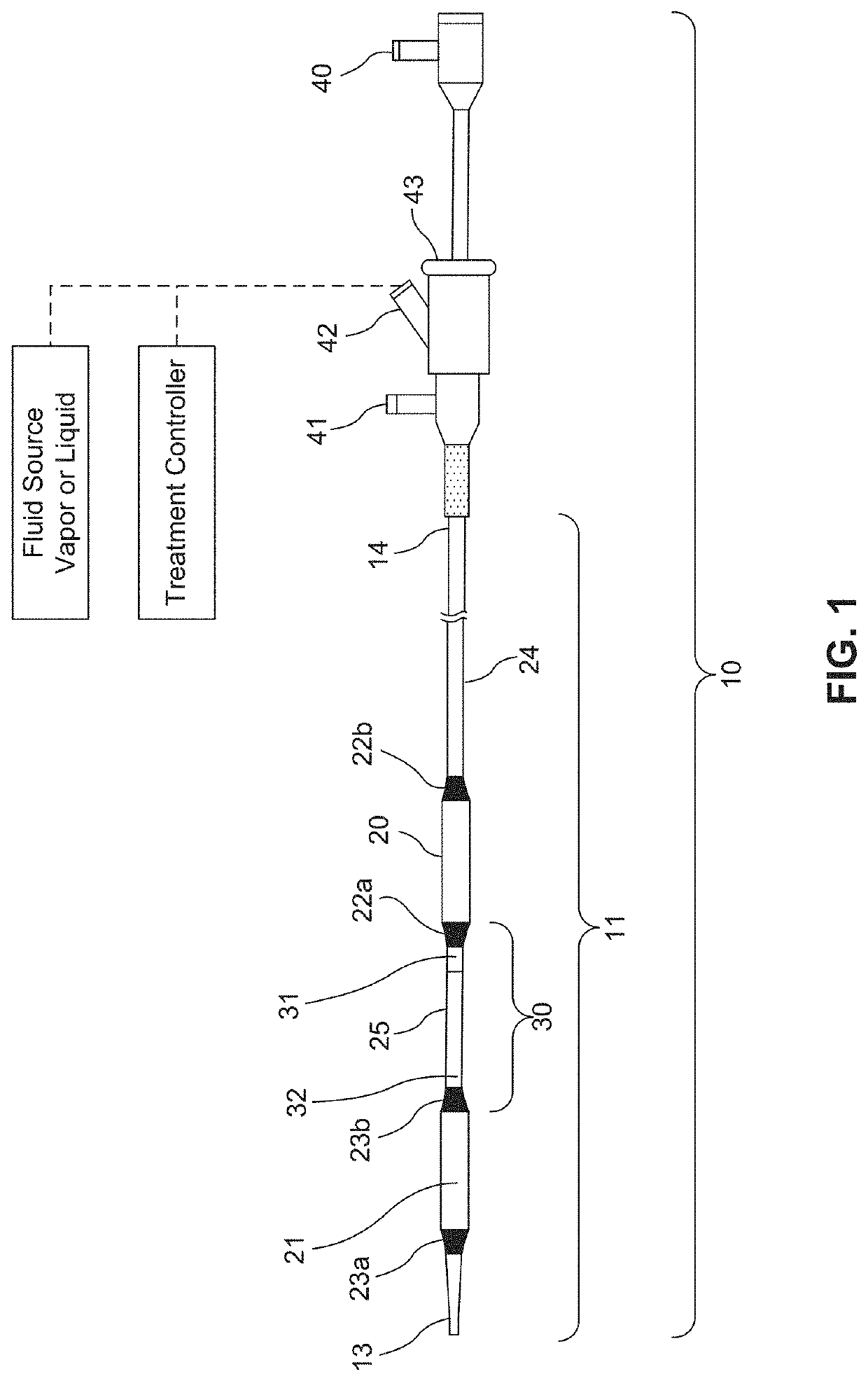
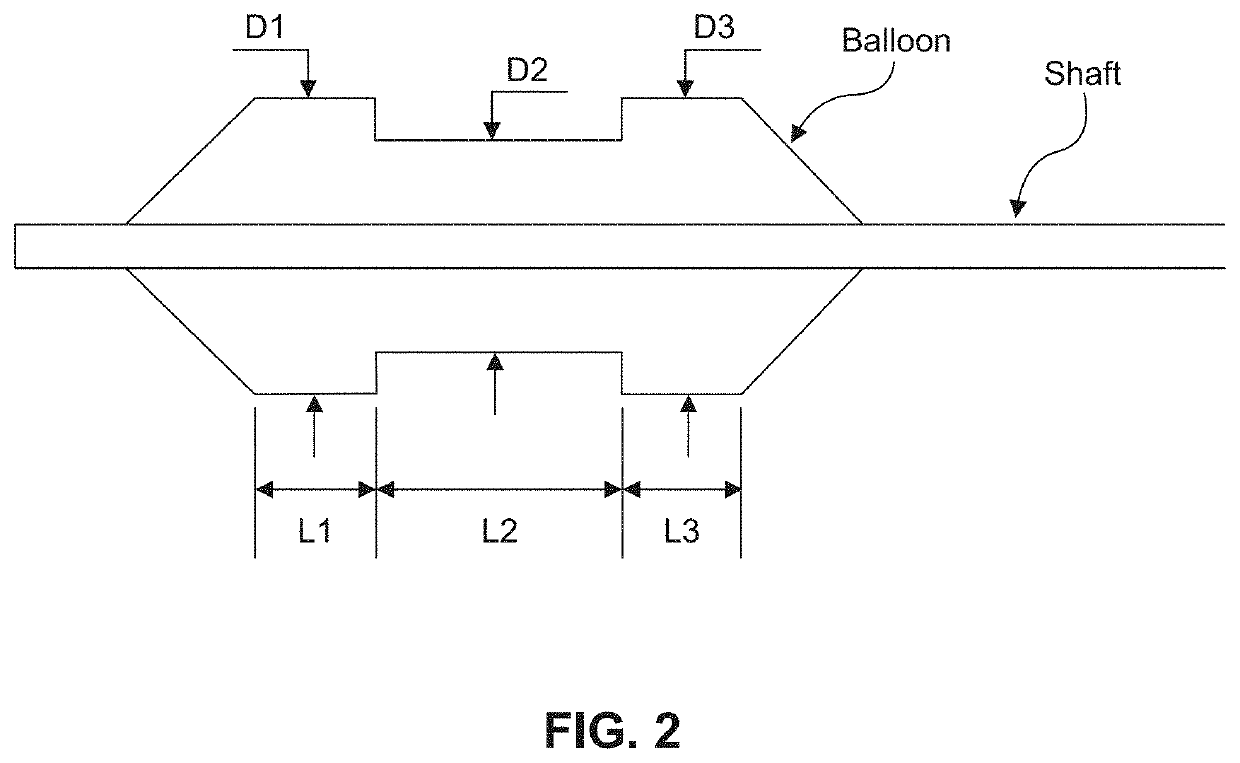
A flow loop for hemodialysis, hemodiafiltration and hemofiltration for the treatment of pathological conditions such as End Stage Renal Disease (ESRD) that has a controlled compliant flow path for preparing fluids required for a hemodialysis therapy session from water. The controlled compliant flow path modifies water into any one of a solution for priming a hemodialysis system, a physiologically compatible solution for contacting blood, a physiologically compatible solution for infusion to a subject, and a solution for blood rinse back to a subject. The controlled compliant flow path has a means for selectively metering in and metering out fluid from the flow path.
Owner:MOZARC MEDICAL US LLC
Ion binding compositions
ActiveUS20060024336A1Powder deliveryPharmaceutical non-active ingredientsEnd stage renal diseaseChronic renal insufficiency
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR INT AG
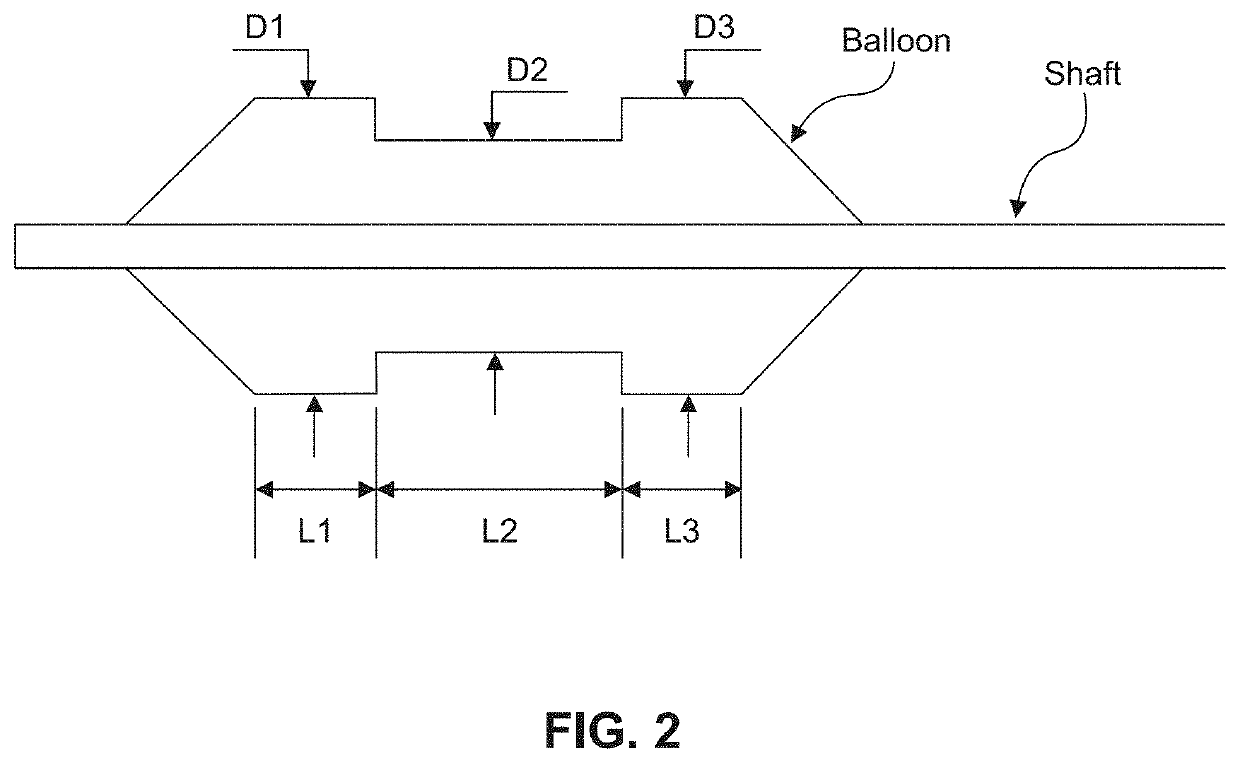
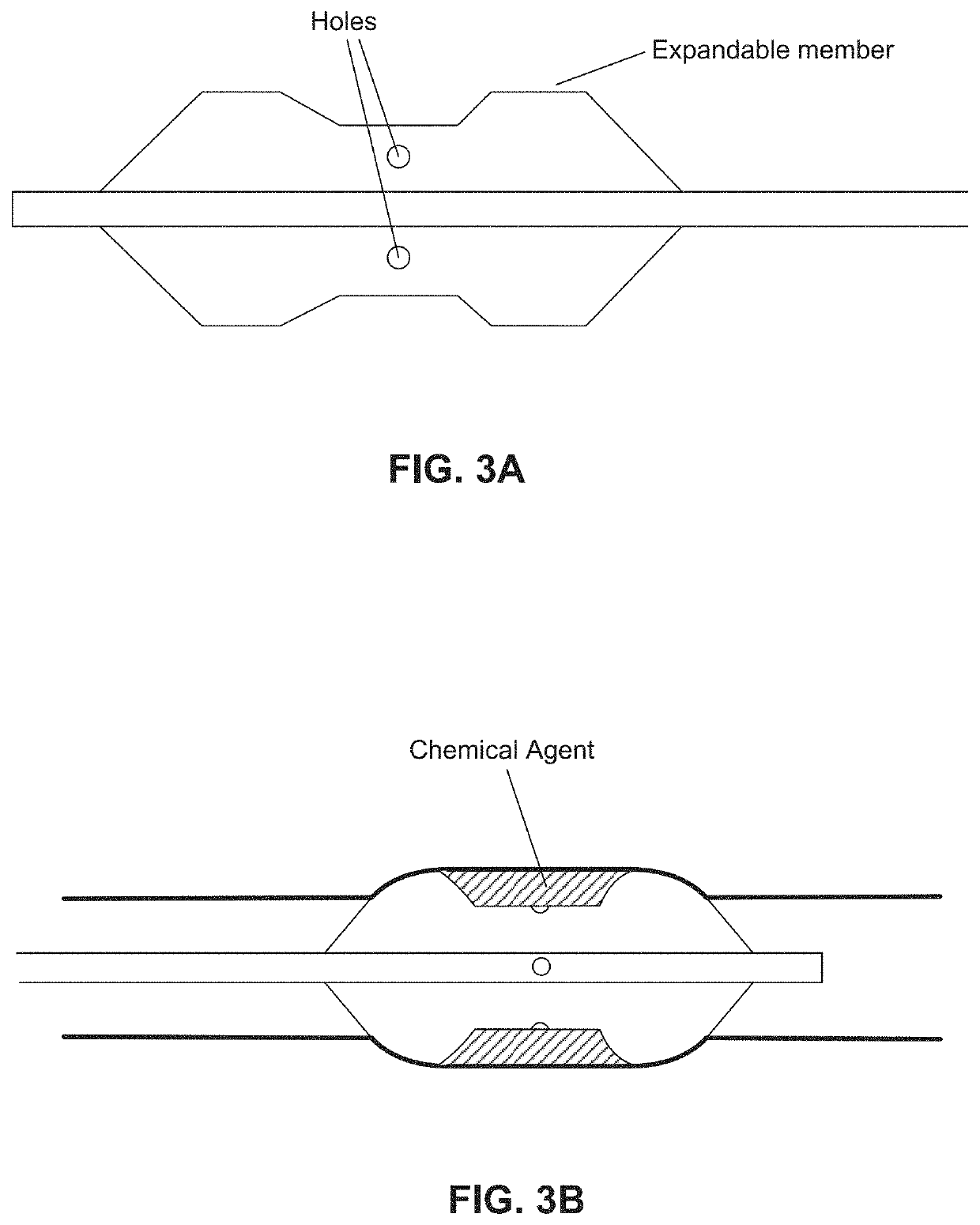
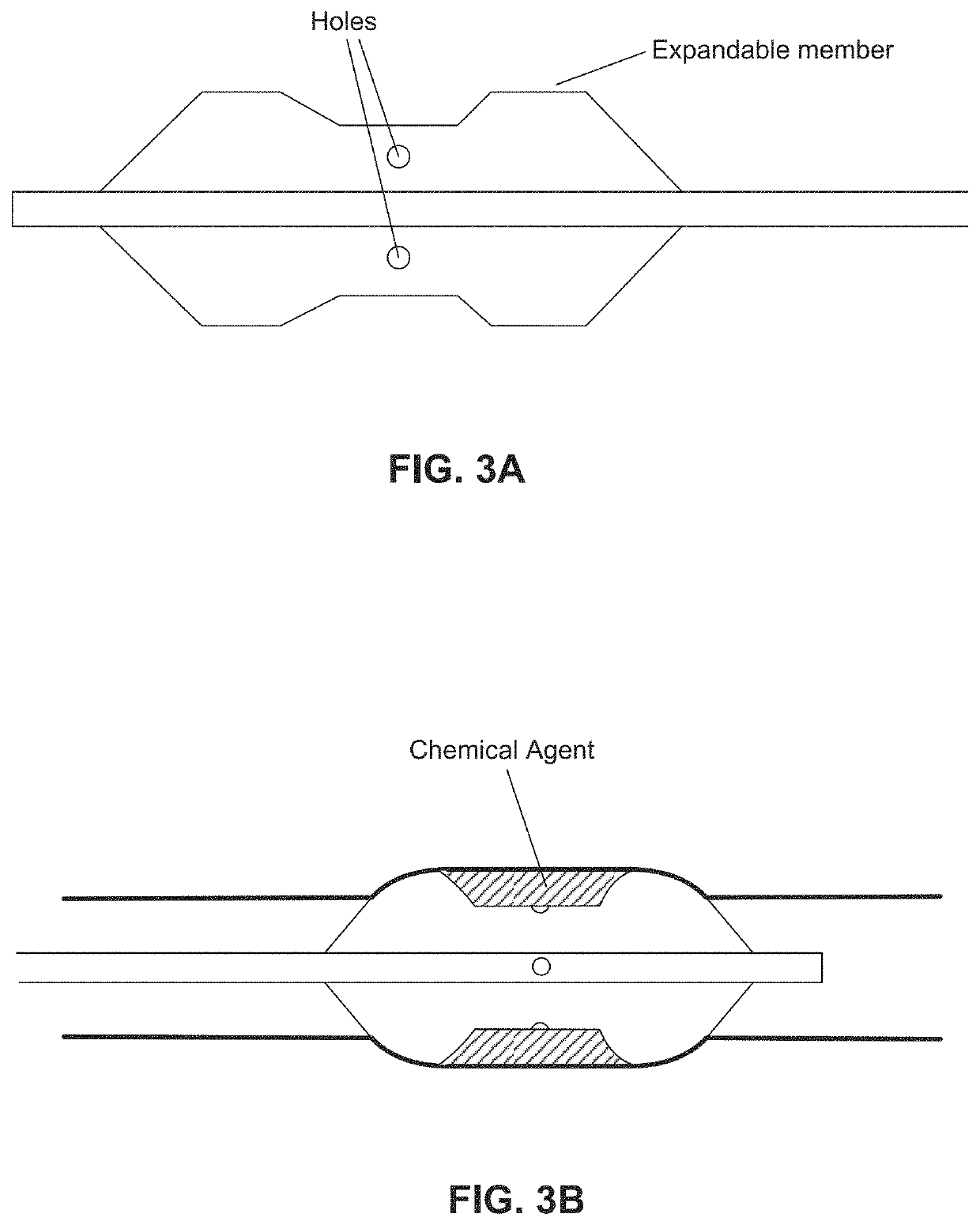
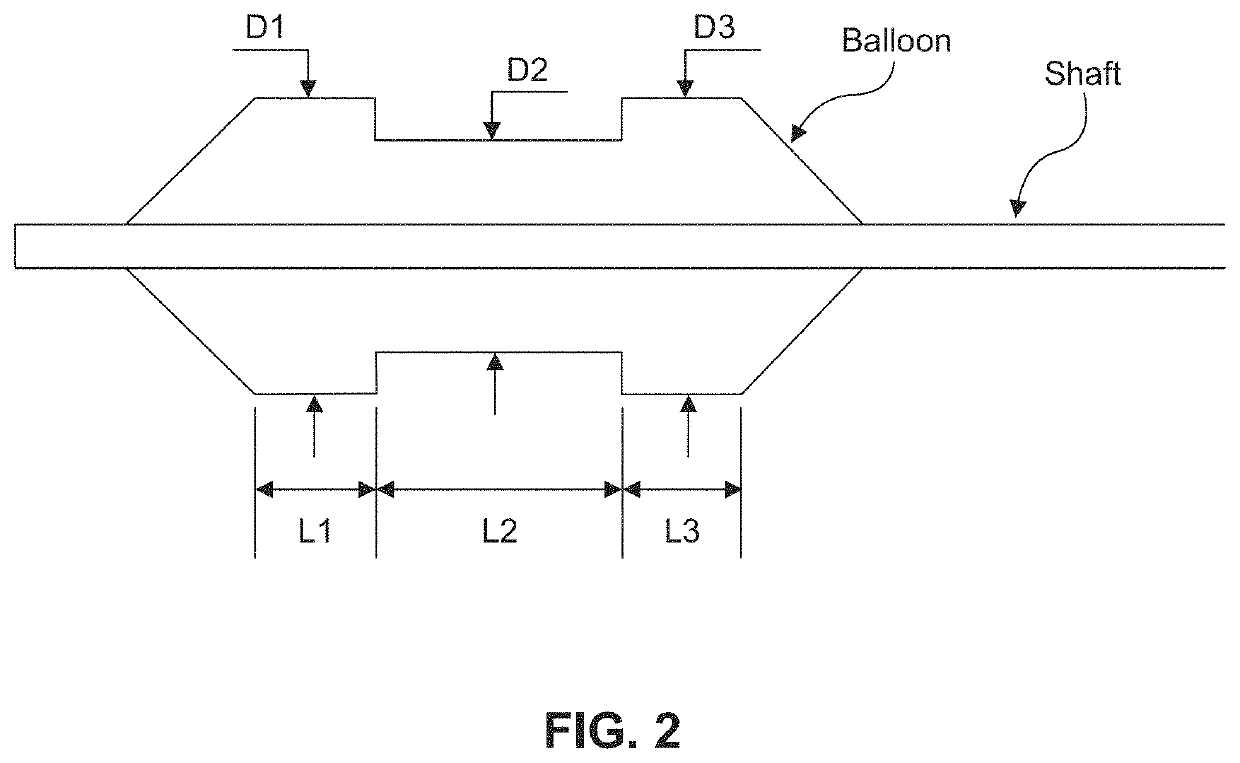
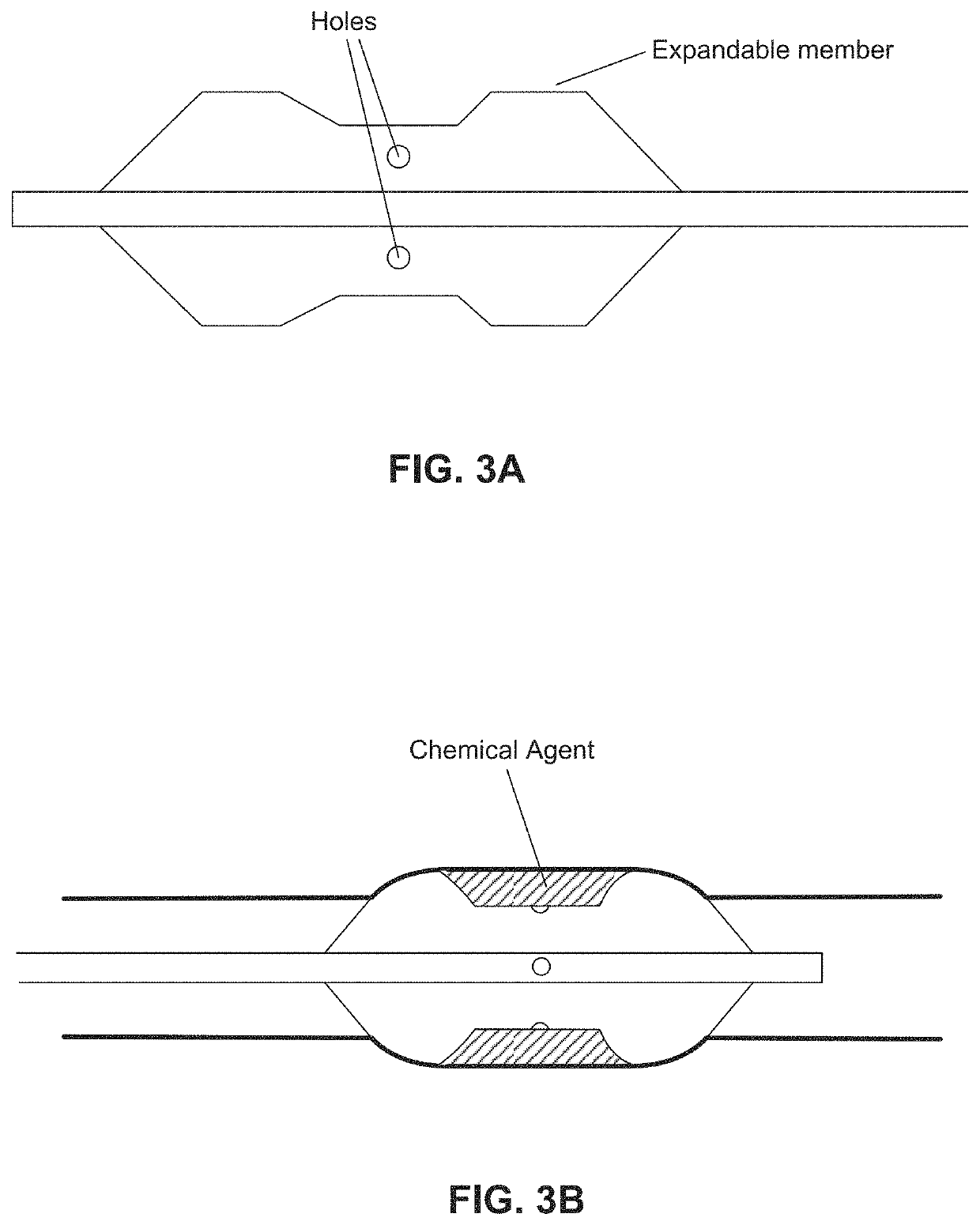
Chemical ablation and method of treatment for various diseases
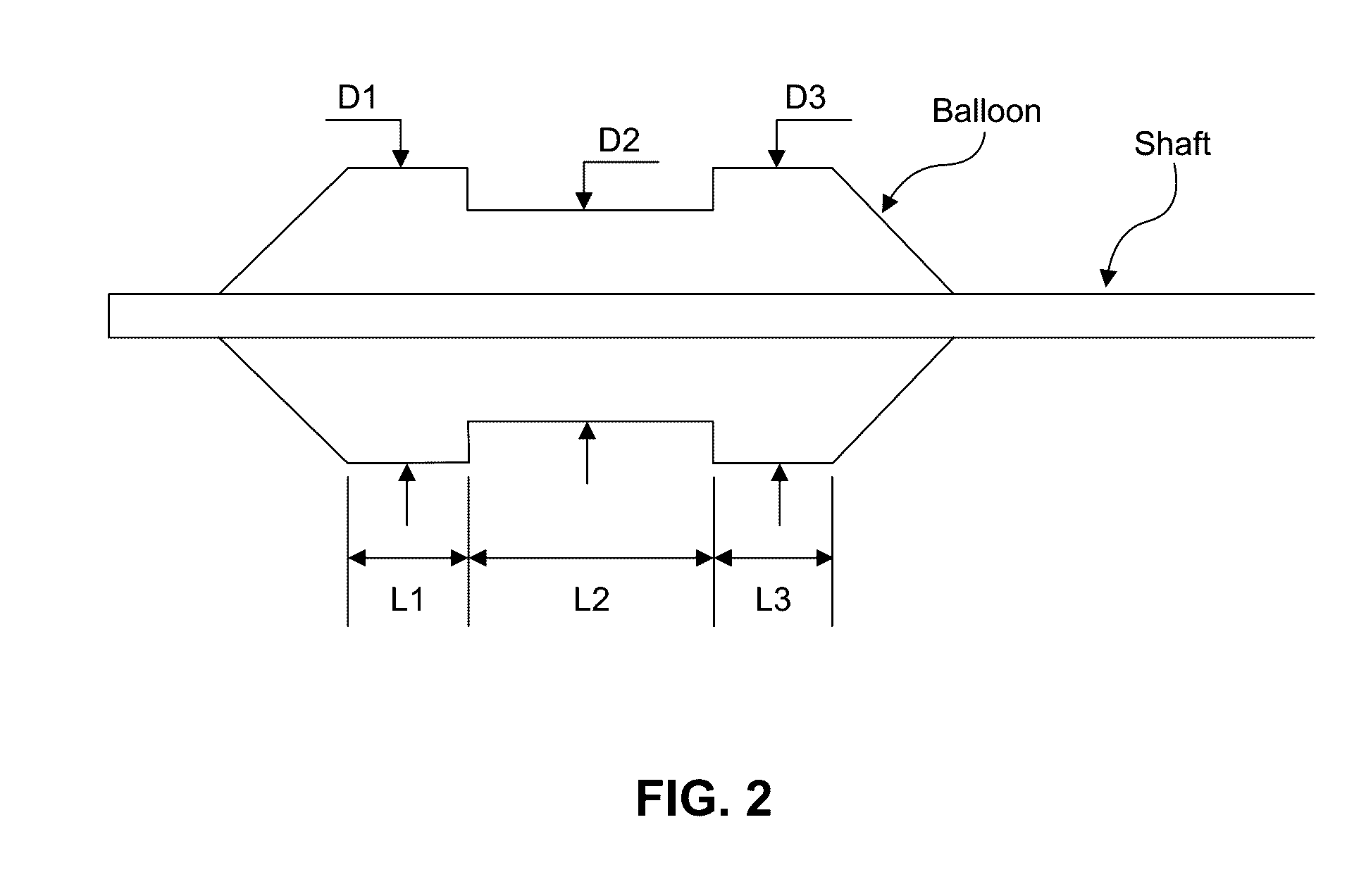
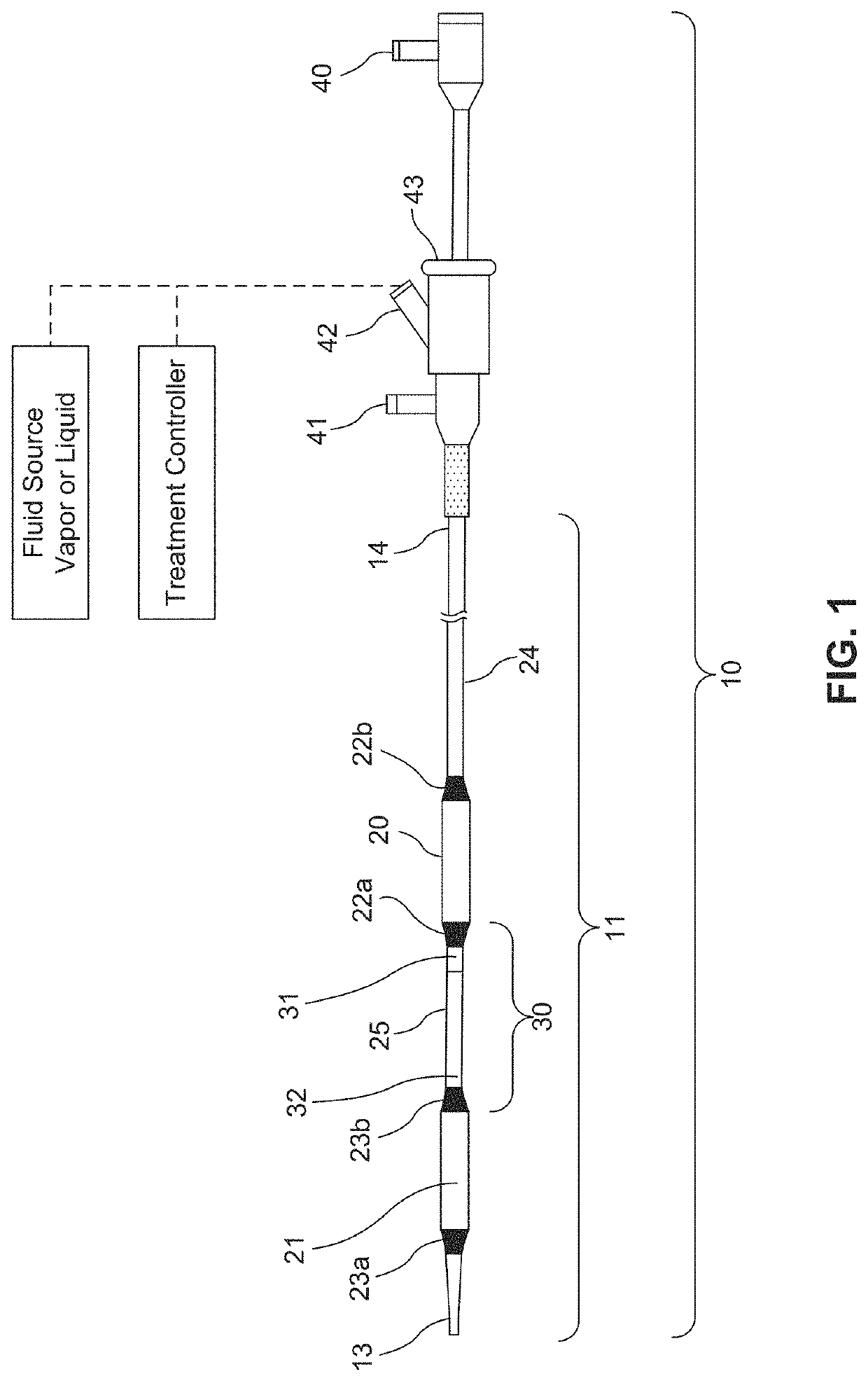
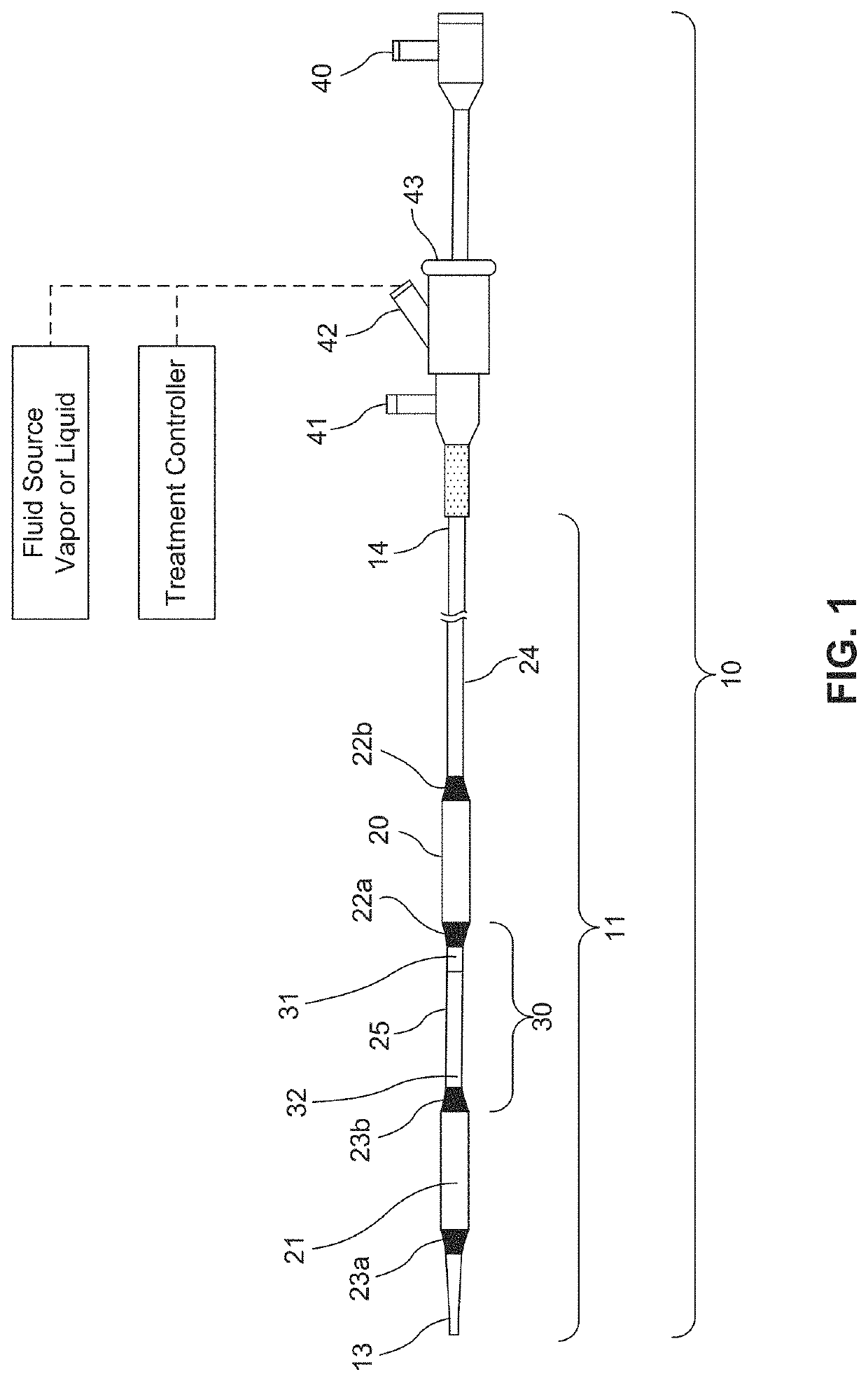
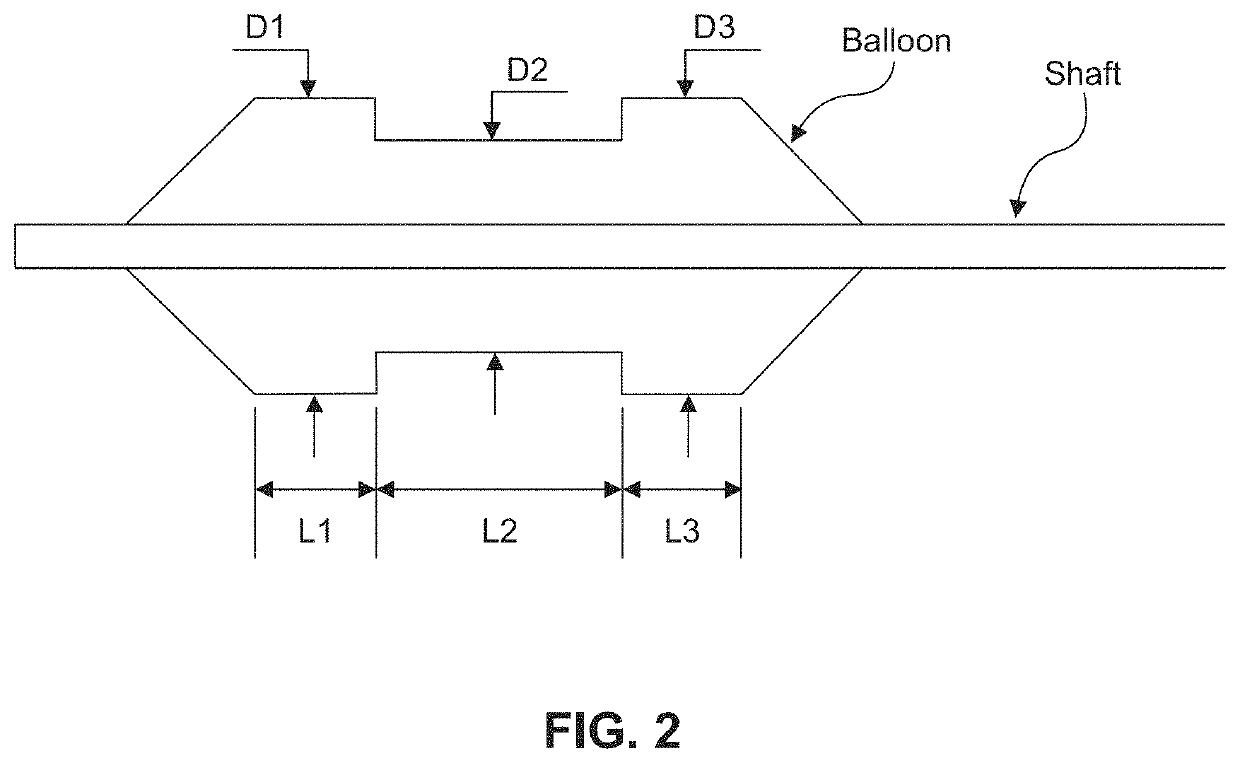
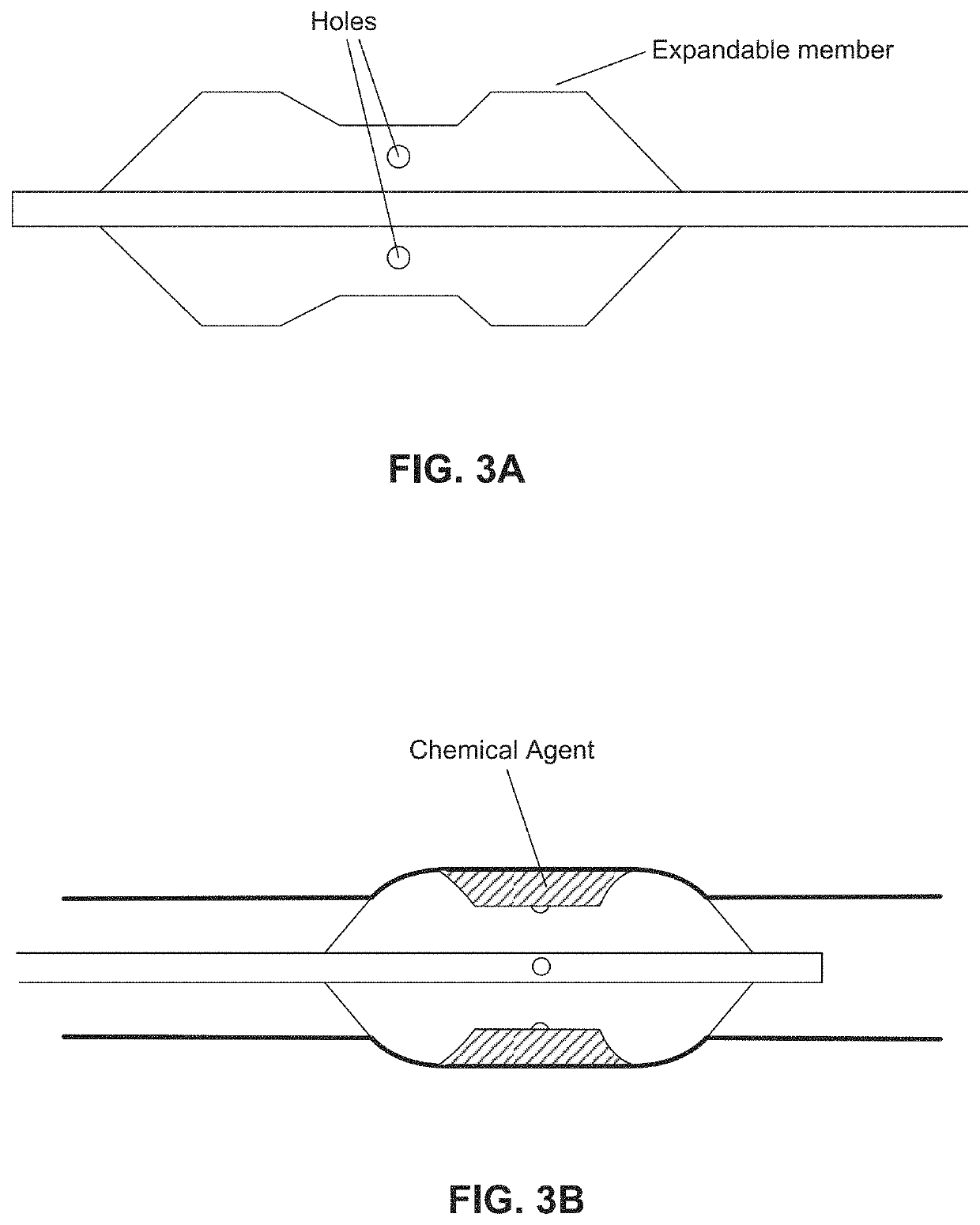
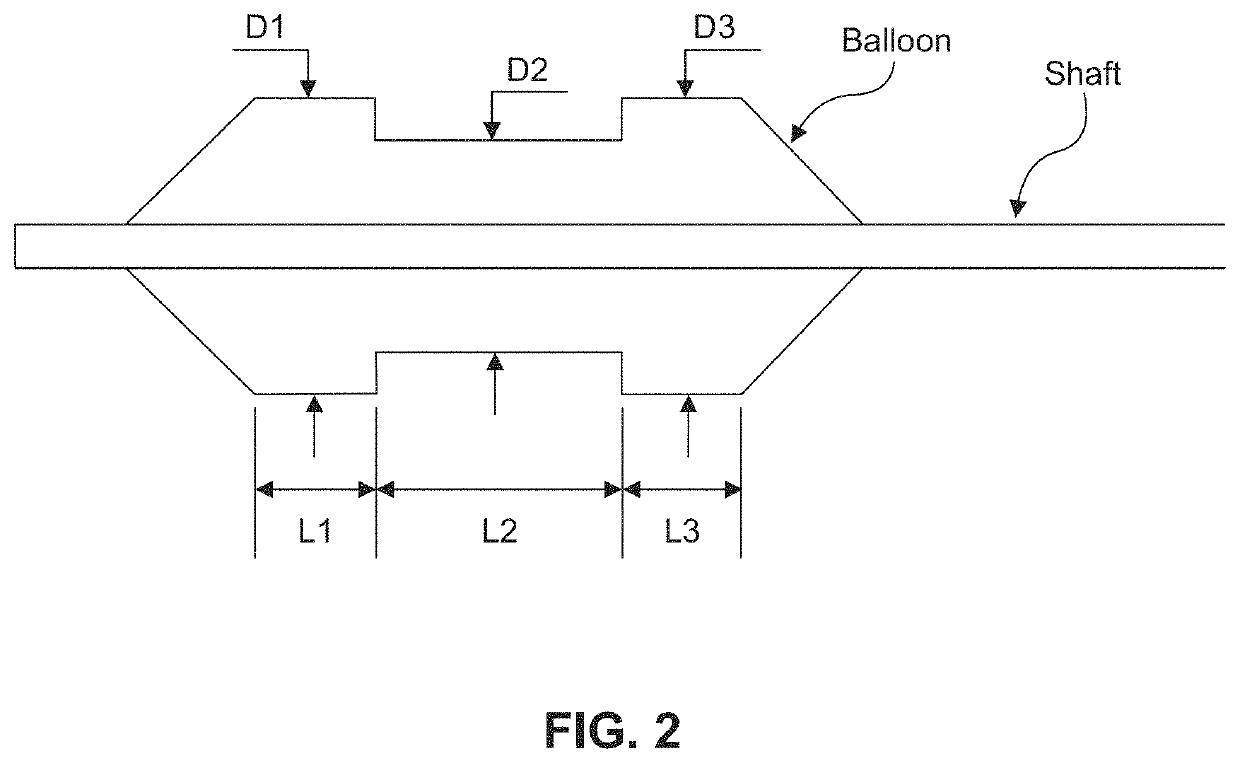
ActiveUS20160310200A1Improve treatment safetyImprove efficacyUltrasonic/sonic/infrasonic diagnosticsBalloon catheterAbnormal tissue growthDamages tissue
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, nonalcoholic fatty liver disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
Implantable human kidney replacement unit
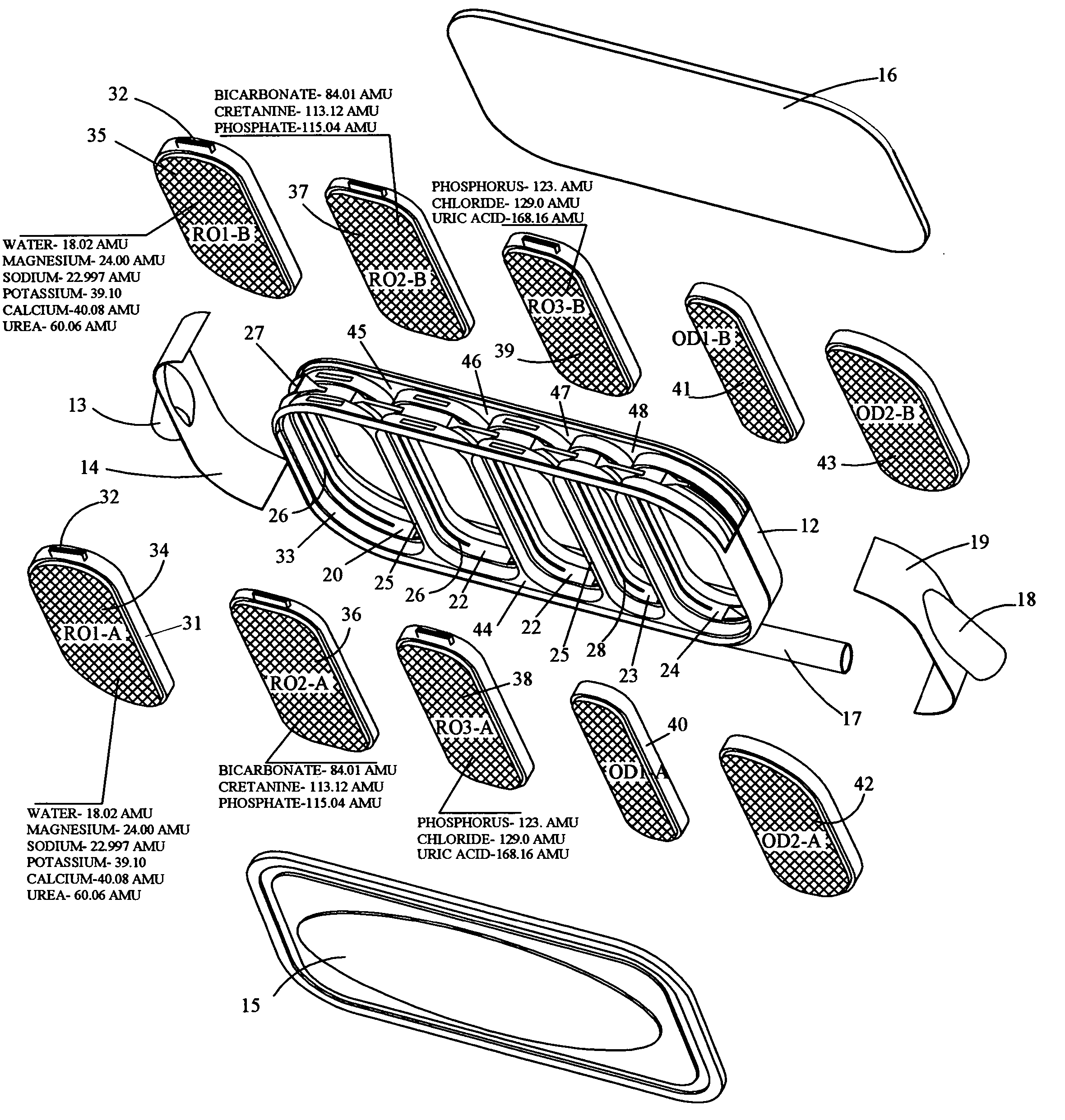
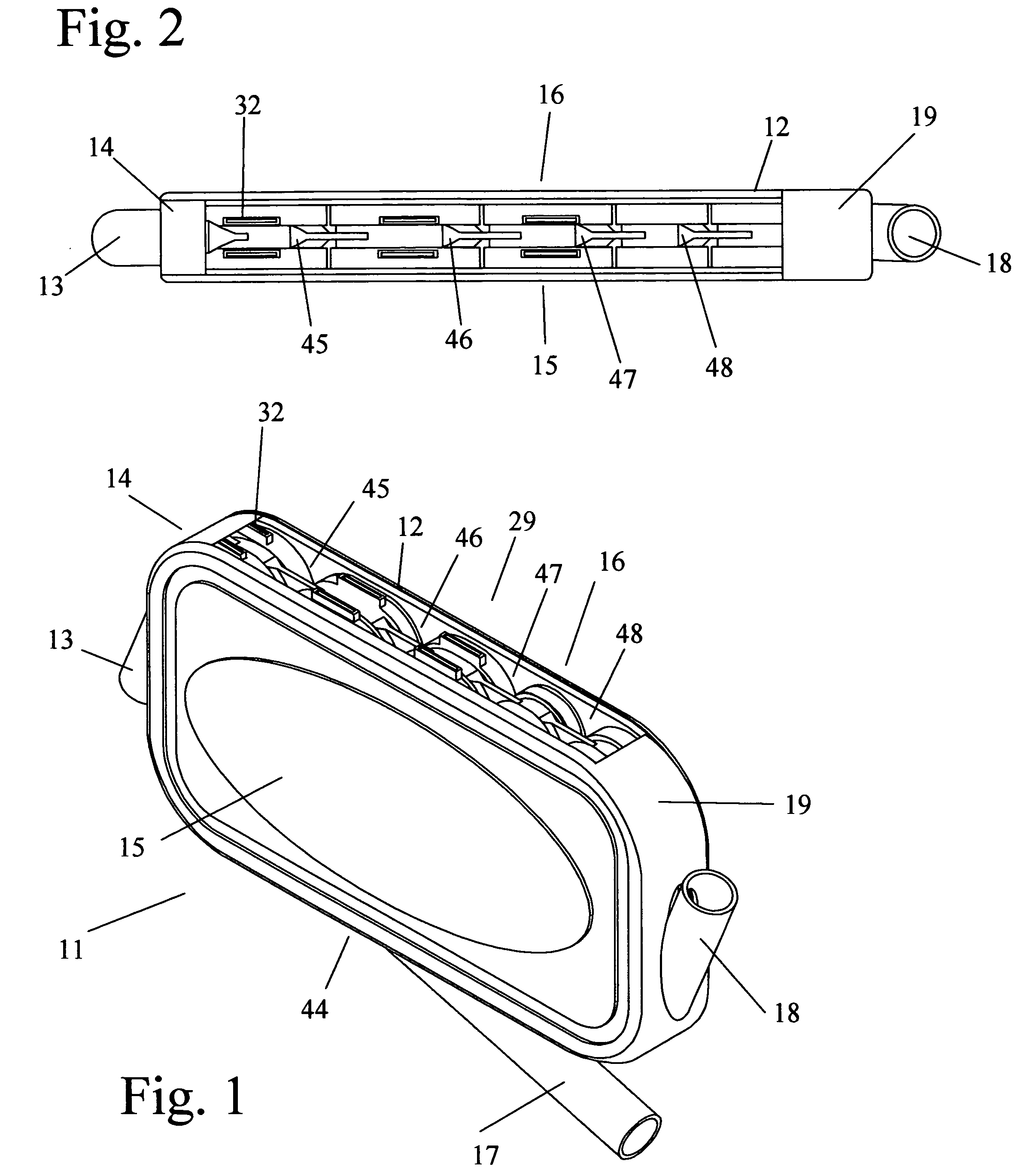
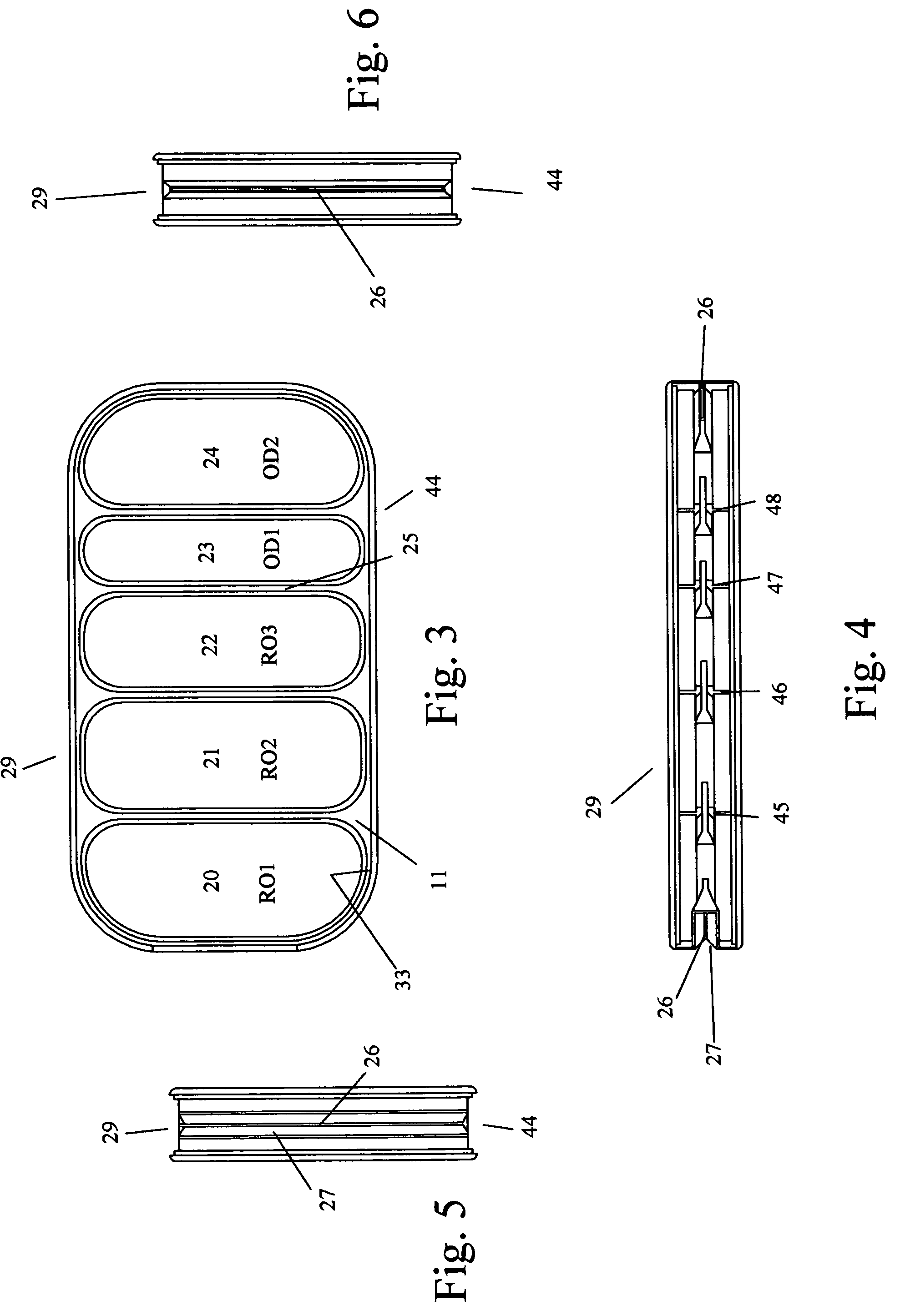
An implantable human kidney replacement unit. Fully functional self contained, providing patients with end stage renal disease the freedom of traveling and moving about normally. Replacing donor kidneys. Implanted in the flank with at least one inlet and outlet tube each, sutured to the iliac artery and vain, at least one urine tube to the ureter. The housing constructed of anti-coagulant bacteriostatic materials has a plurality of reverse-osmosis process chambers with semipemeable membranes through the unit, followed by osmosis-diffusion chambers and membranes. Blood from the artery enters the first of the chambers. Small molecules such as water, magnesium, sodium, potassium, calcium, urea etc. are extracted from the blood according to their weight in atomic mass units as blood wipes past the self-cleaning membrane cartridges in the chambers. Molecules are further separated and urea sent to the bladder with excess water and electrolytes. The remainder is channeled to at least one diffusion chamber and reabsorbed into the blood. The same process is repeated in the other chambers where selected larger molecules such as creatinine and phosphorus are excreted, and some diffused back into the blood.
Owner:LUDLOW ROLAND G
Compositions and methods for treating renal disease
ActiveUS20120195902A1Treating and reducing likelihoodDeleterious effectMetabolism disorderMicrobiological testing/measurementNephrosisEnd-stage kidney disease
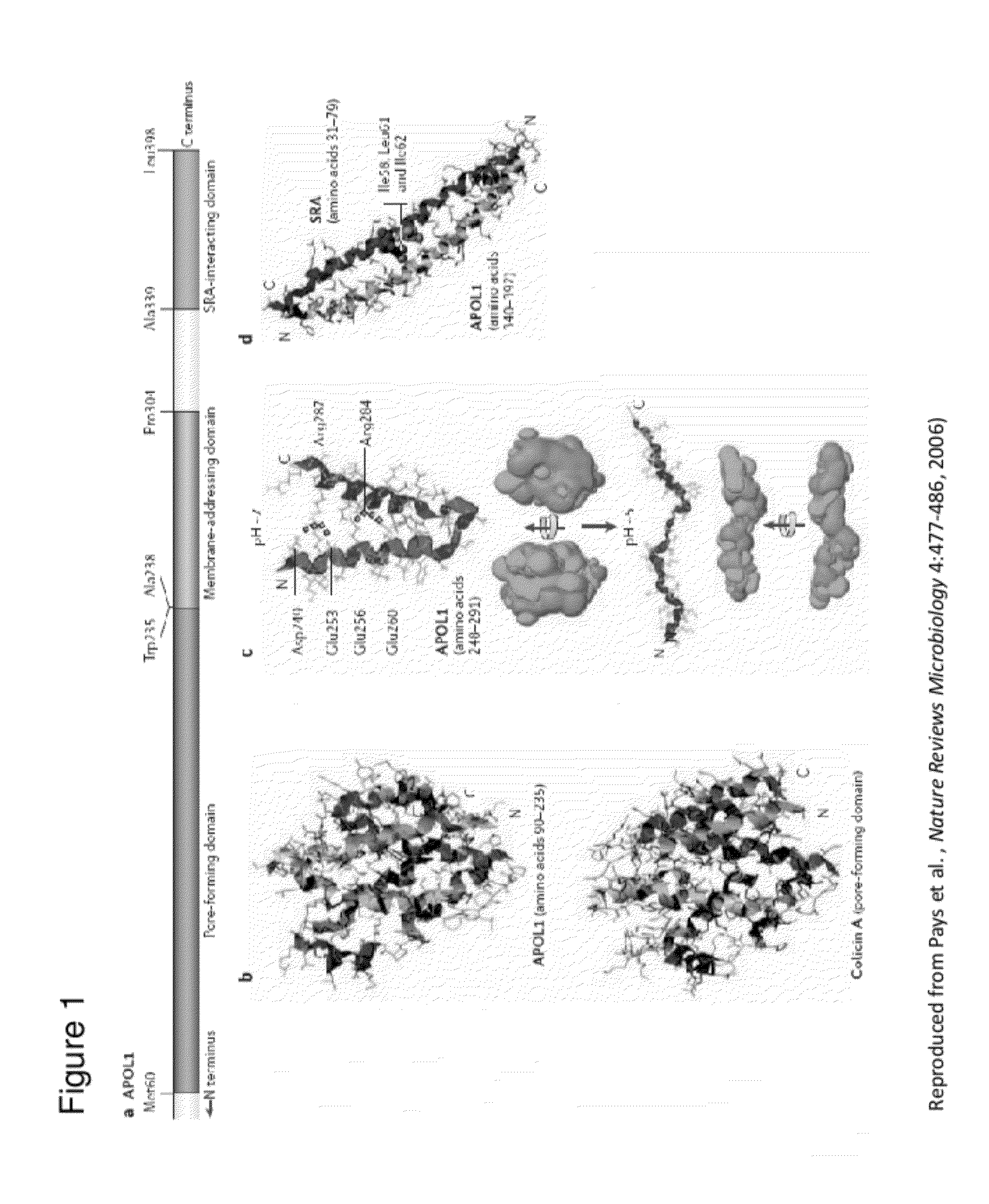
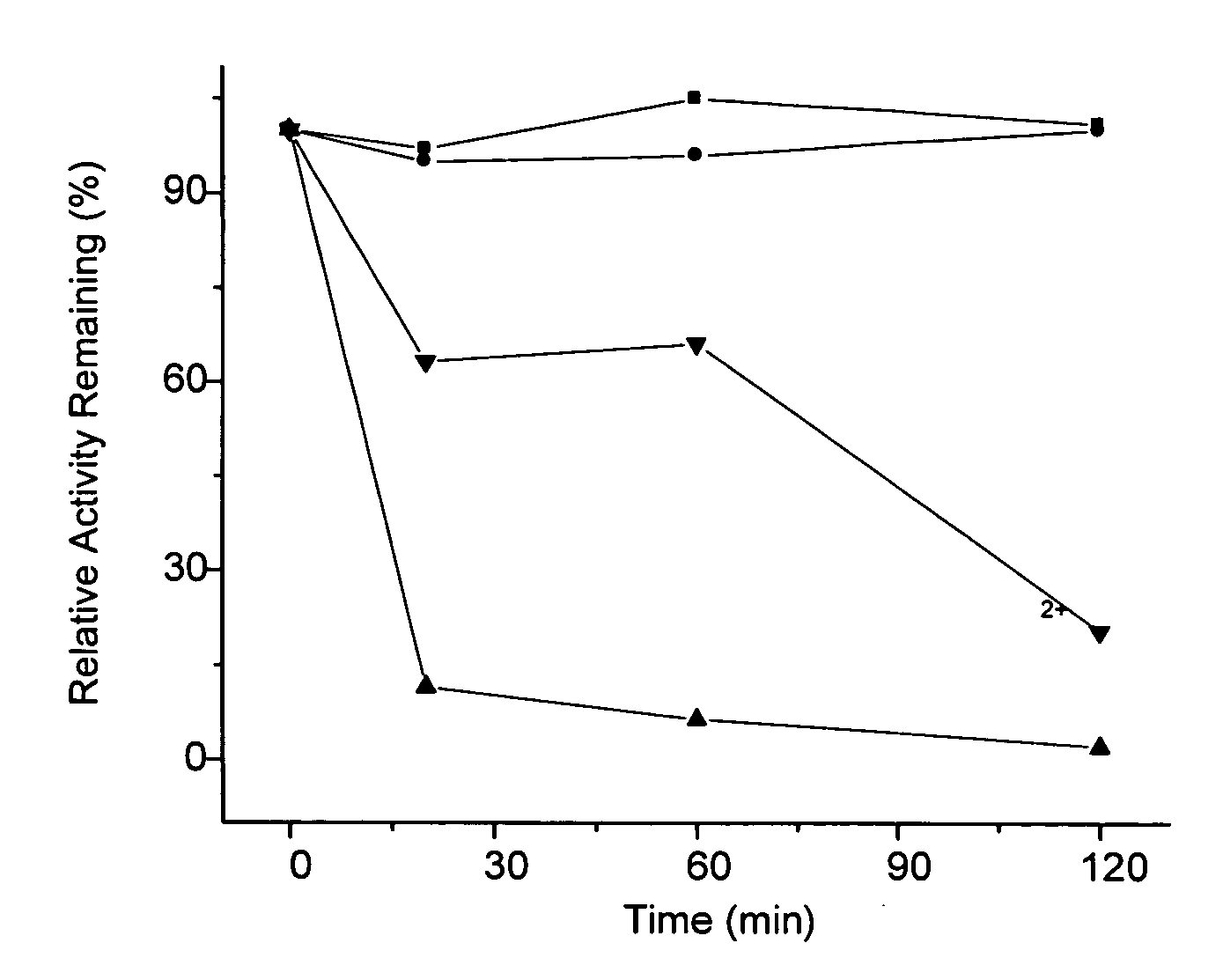
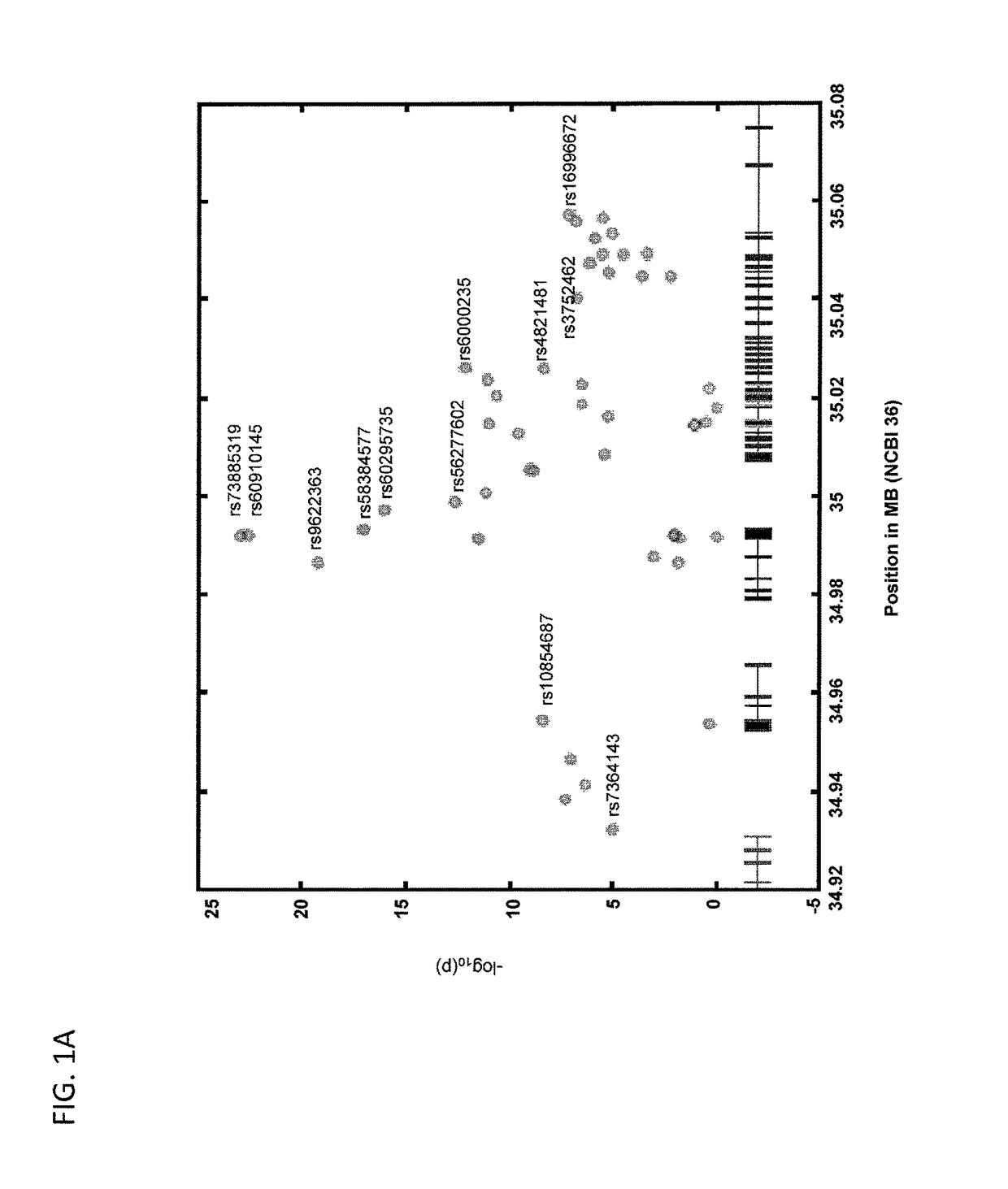
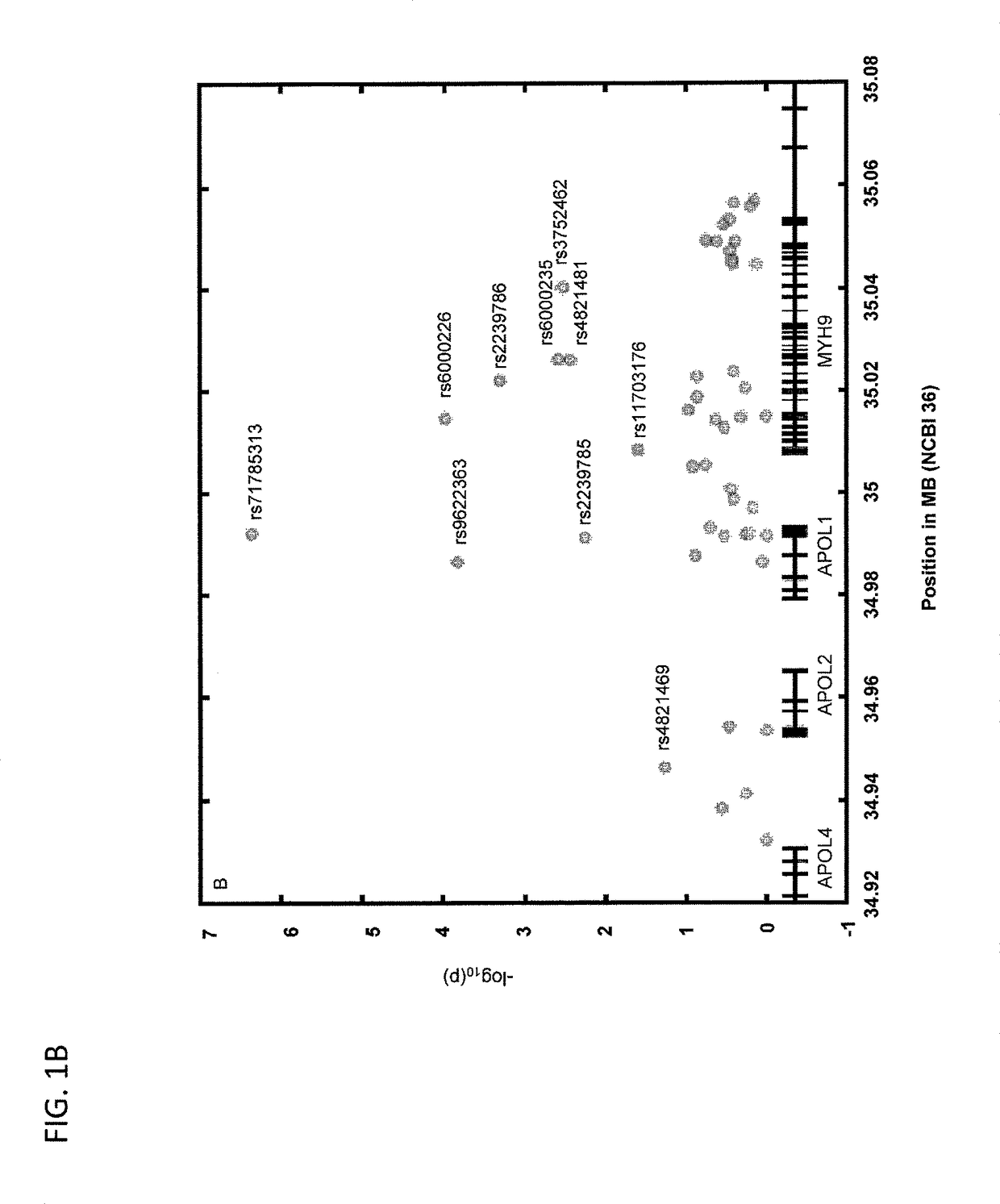
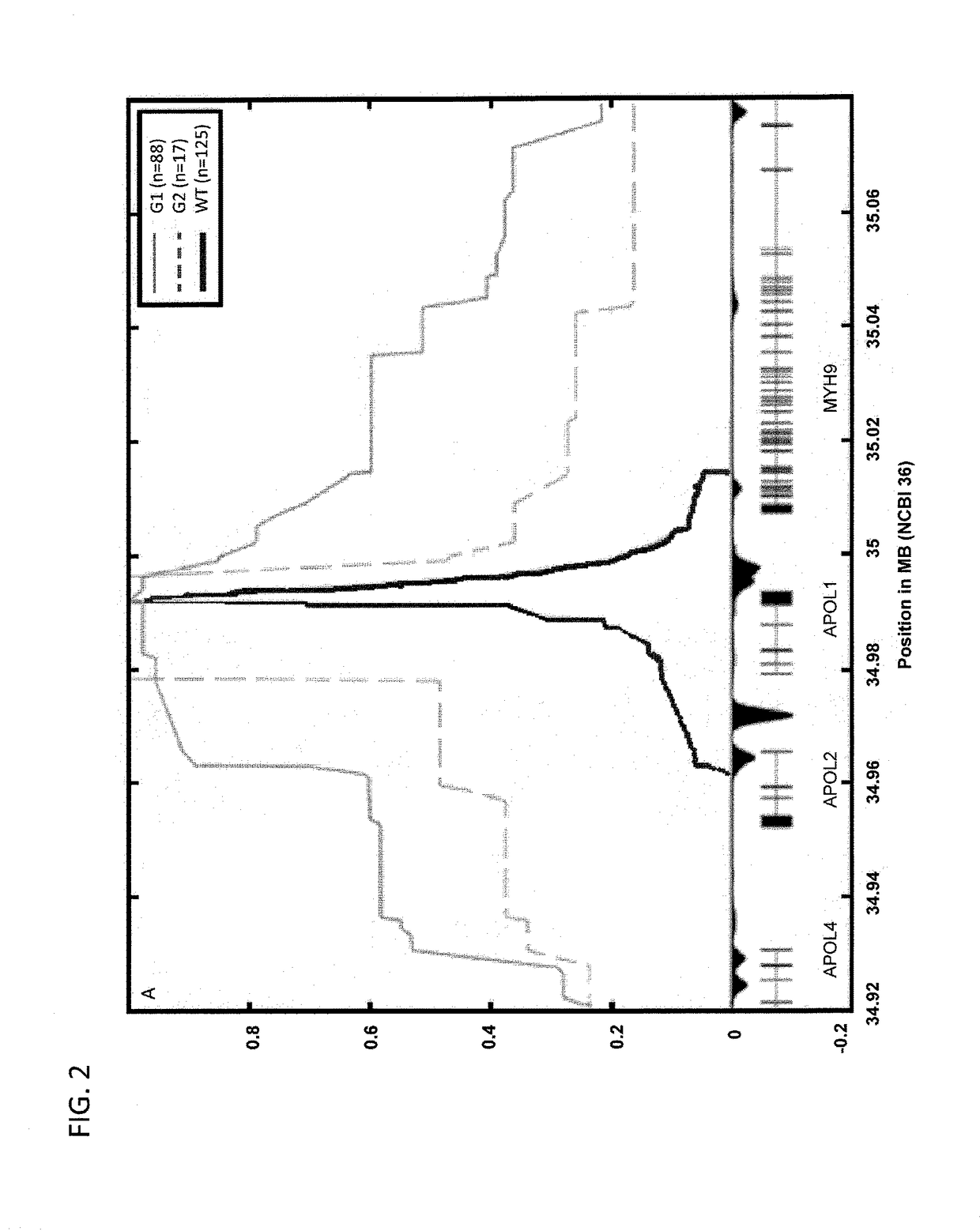
Compositions and methods are disclosed herein for treating or reducing the symptoms of a renal disease, such as focal segmental glomerulosclerosis (FSGS), hypertensive end-stage kidney disease (ESKD), and HIV-associated nephropathy (a distinct form of FSGS, also termed collapsing glomerulopathy). The compositions include the common variant of APOL1 and fragments thereof, as well as antibodies and fragments thereof that bind and neutralize pathogenic APOL1, nucleic acid molecules that encode the common variant of APOL1 and fragments thereof, and other compounds that bind and neutralize pathogenic APOL1. The methods of the invention include administering one or more of the compositions of the invention to a subject having or at risk of developing renal disease.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Compositions and methods for oxalate reduction
InactiveUS20070184118A1Reducing oxalateMaintain enzyme activityPowder deliveryPeptide/protein ingredientsUlcerative colitisEnd-stage kidney disease
The present invention comprises methods and compositions for the reduction of oxalate in humans. For example, the invention provides methods and compositions for the delivery of one or more oxalate-reducing enzymes embedded in particle compositions. The compositions of the present invention are suitable in methods of treatment or prevention of oxalate-related conditions including, but not limited to, hyperoxaluria, absorptive hyperoxaluria, enteric hyperoxaluria, primary hyperoxaluria, idiopathic calcium oxalate kidney stone disease (urolithiasis), vulvodynia, oxalosis associated with end-stage renal disease, cardiac conductance disorders, inflammatory bowel disease, Crohn's disease, ulcerative colitis, and patients who have undergone gastrointestinal surgery and bariatric surgery (surgery for obesity), and / or who have undergone antibiotic treatment.
Owner:OXTHERA INTPROP
Diagnostic polymorphisms for the ecnos promoter
InactiveUS20050084849A1Eliminate the effects ofDigital data processing detailsMicrobiological testing/measurementValvular diseaseProstate cancer
Disclosed are single nucleotide polymorphisms (SNIps) associated with breast cancer, lung cancer, prostate cancer, non-insulin dependent diabetes, end stage renal disease due to non-insulin dependent diabetes, hypertension, end stage renal disease F due to hypertension, myocardial infarction, colon cancer, hypertension, atherosclerotic peripheral vascular disease due to hypertension, cerebrovascular accident due to hypertension, cataracts due to hypertension, cardiomyopathy with hypertension, myocardial infarction due to hypertension, non-insulin dependent diabetes mellitus, atherosclerotic peripheral vascular disease due to non-insulin dependent diabetes mellitus, cerebrovascular accident due to non-insulin dependent diabetes mellitus, ischemic cardiomyopathy, ischemic cardiomyopathy with non-insulin dependent diabetes mellitus, myocardial infarction due to non-insulin dependent diabetes mellitus, atrial fibrillations without valvular disease, alcohol abuse, anxiety, asthma, chronic obstructive pulmonary disease. cholecystectomy, degenerative joint disease, end stage renal disease and frequent de-clots, end stage renal disease due to focal segmental glomerular sclerosis, end stage renal disease due to insulin dependent diabetes mellitus, or seizure disorder. Also disclosed are methods for using SNPs to determine susceptibility to these diseases; nucleotide sequences containing SNPs; kits for determining the presence of SNPs; and methods of treatment or prophylaxis based on the presence of SNPs.
Owner:VIRAL THERAPEUTICS
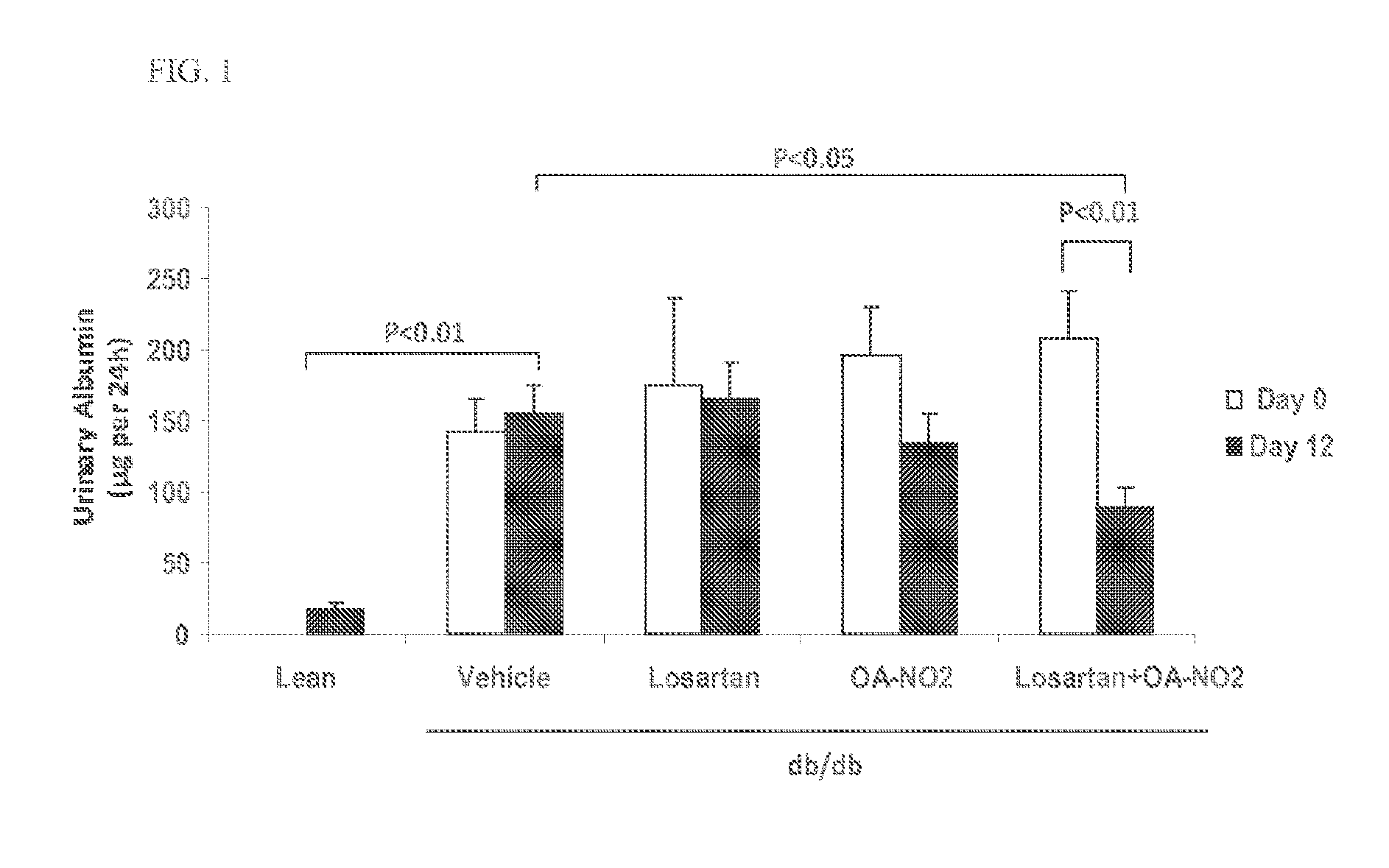
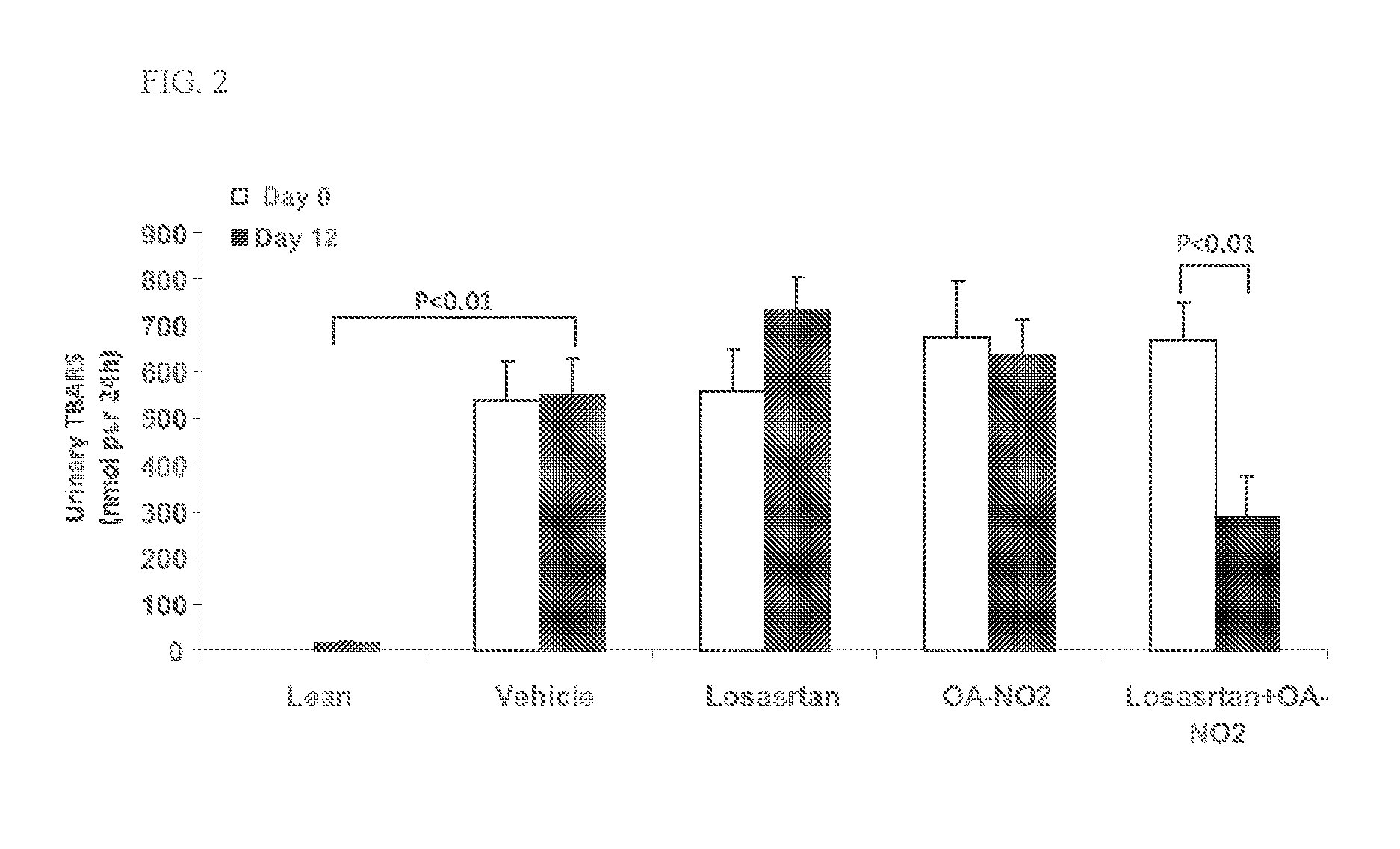
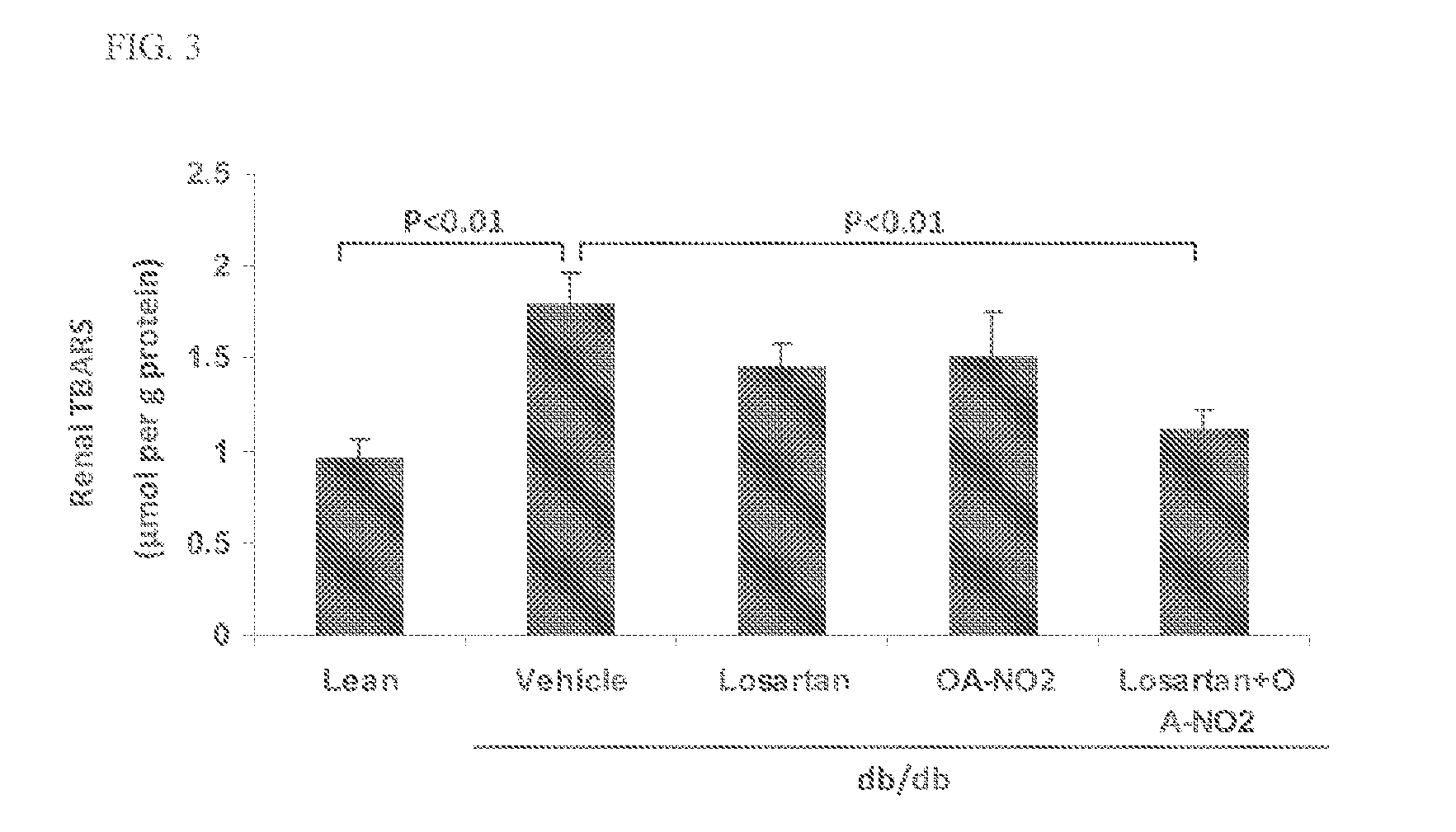
Combination therapy with nitrated lipids and inhibitors of the renin-angiotensin-aldosterone system
ActiveUS20140243380A1Reducing mRNA expressionHigh expressionBiocideUrinary disorderNephrosisEnd stage renal disease
The present technology provides compositions and methods for treating chronic kidney disease, end-stage renal disease, or diabetic nephropathy. The compositions comprise a nitrated lipid and an inhibitor of the renin-angiotensin-aldosterone system. The methods comprise administering a nitrated lipid in combination with an inhibitor of the renin-angiotensin-aldosterone system to a subject in need thereof, in an amount effective to treat diabetic nephropathy, chronic kidney disease, and / or end-stage renal disease. The use of a nitrated lipid with an inhibitor of the renin-angiotensin-aldosterone system exhibits a synergistic effect in treating chronic kidney disease and diabetic nephropathy.
Owner:UNIV OF UTAH RES FOUND
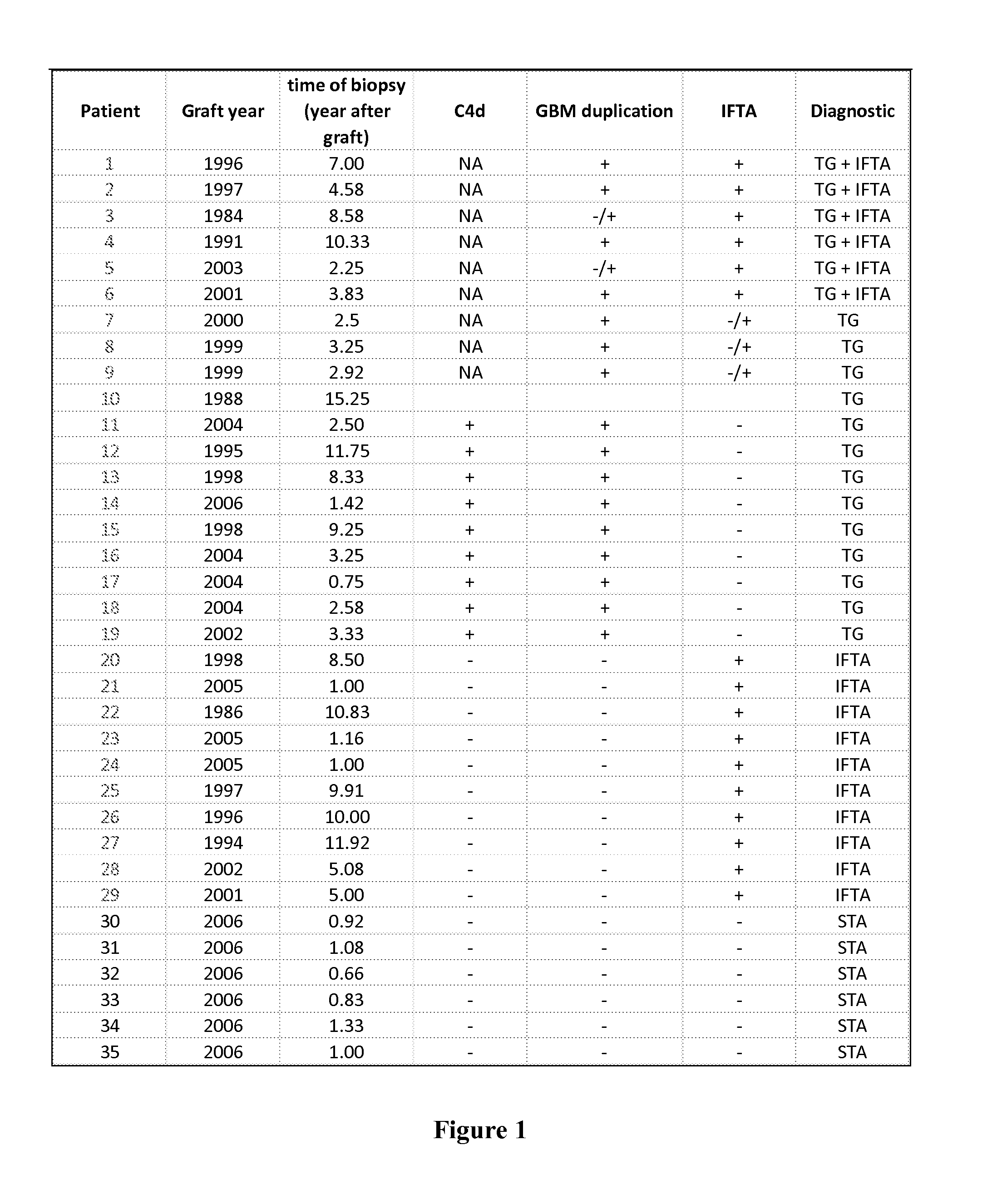
Biomarkers for the Diagnosis of Renal Allograft and Kidney Status
InactiveUS20120046181A1Solid foundationPeptide librariesLibrary screeningRenal transplantBiomarker (petroleum)
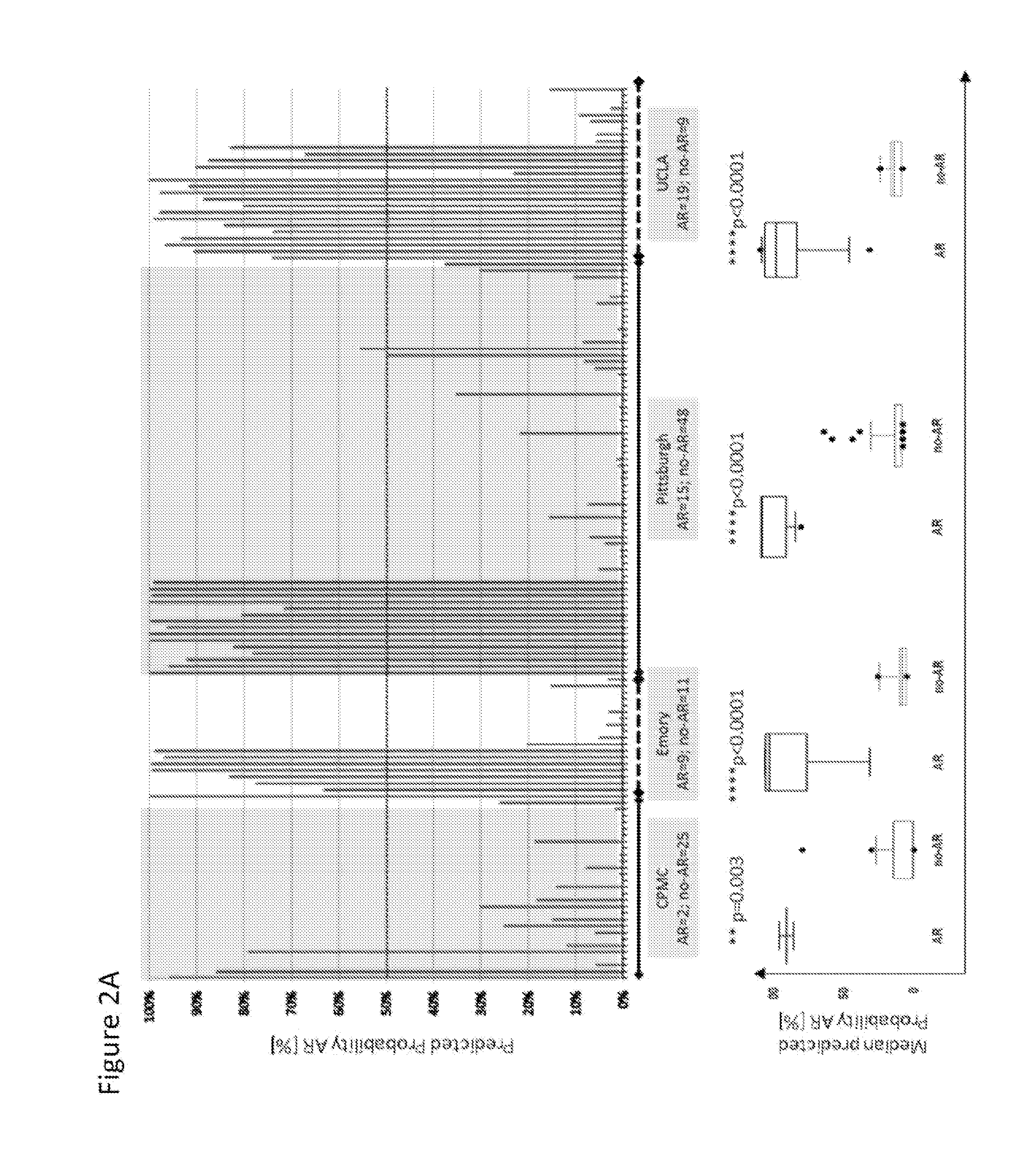
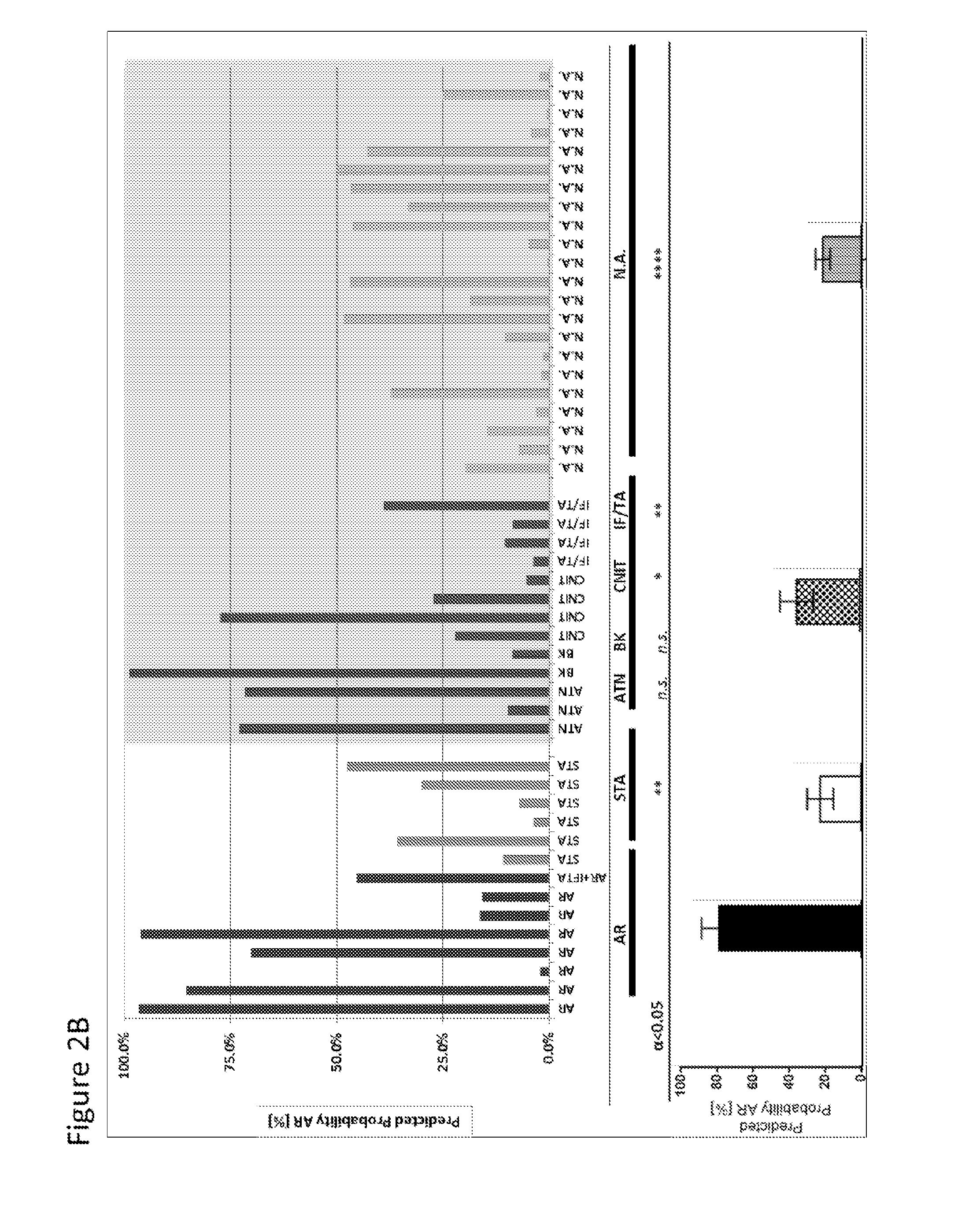
The present invention relates to the identification and use of protein biomarkers with clinical relevance to kidney status and chronic renal injury or disorder. In particular, the invention provides the identity of marker proteins which are recognized by antibodies present in patients suffering from end-stage renal disorder, stable renal transplant, renal transplant glomerulopathy (TG), and interstitial fibrosis and tubular atrophy (IFTA). Methods and kits are described for using these proteins in the study and diagnosis of chronic renal transplant injury, and in the selection and / or monitoring of treatment regimens.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
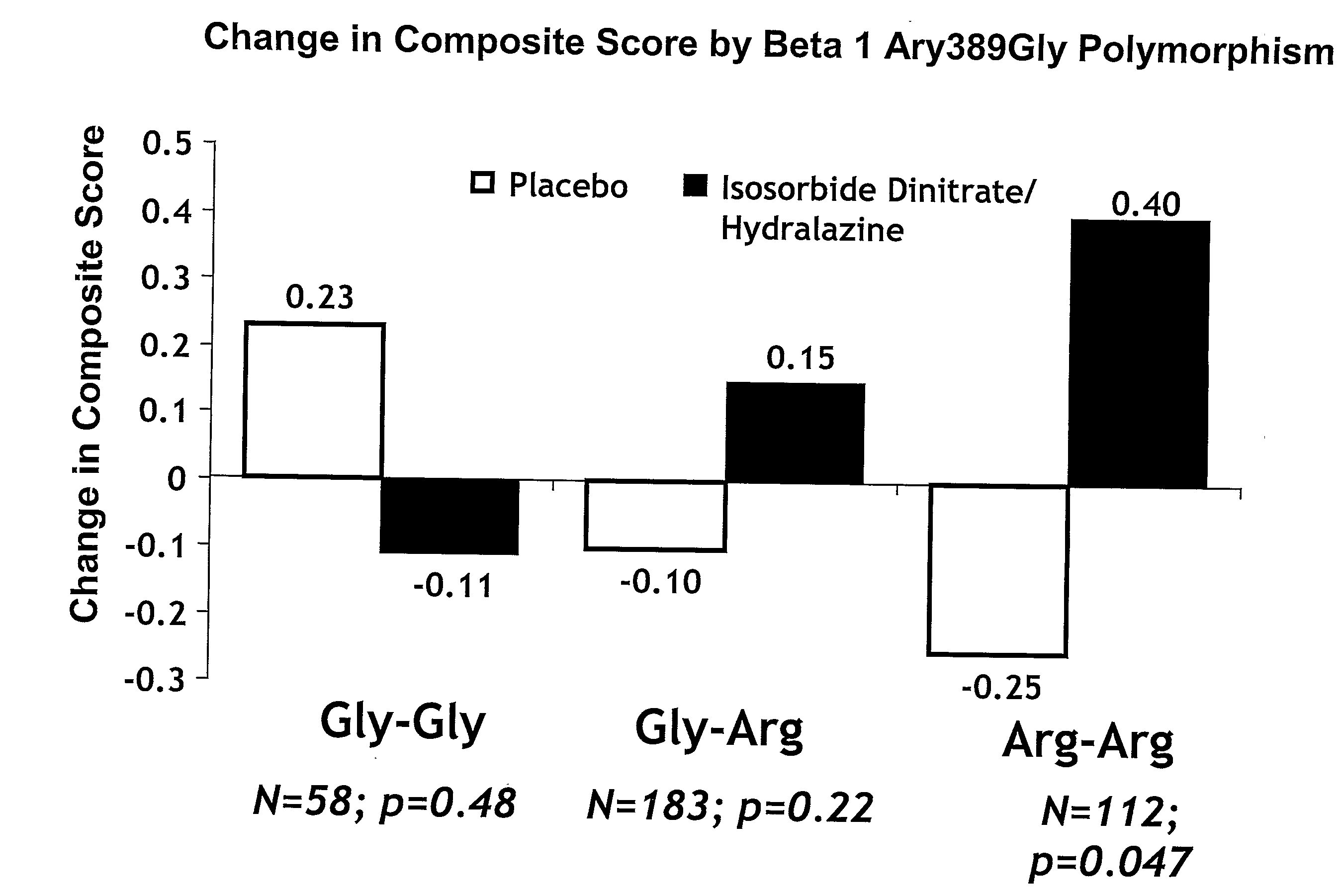
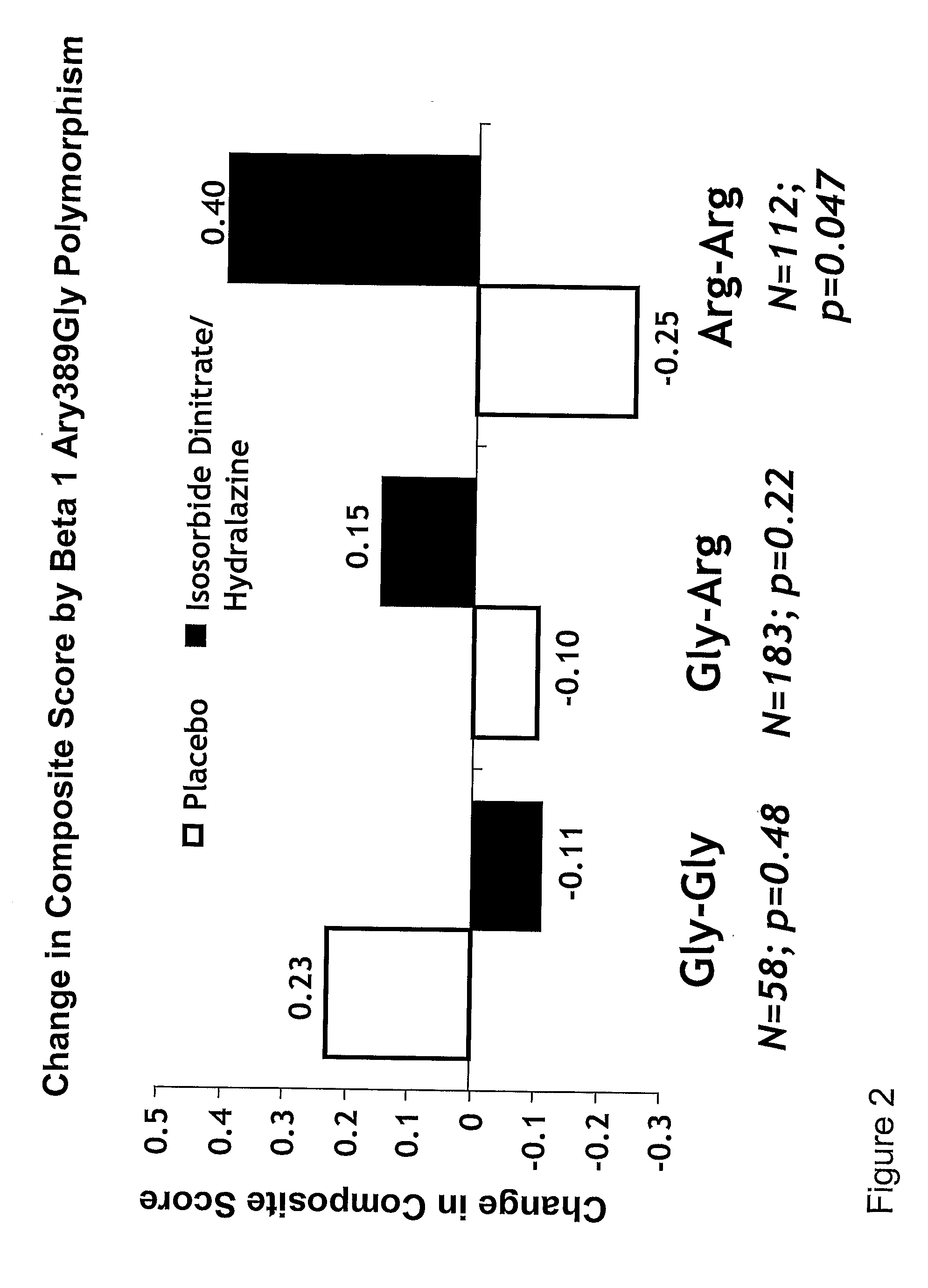
Genetic risk assessment in heart failure: impact of genetic variation of beta 1 adrenergic receptor gly389arg polymorphism
InactiveUS20090192128A1Reduce mortalityIncreased oxygen consumptionBiocideMicrobiological testing/measurementAntioxidantLeft ventricular size
The invention provides methods for (a) reducing mortality associated with heart failure; (b) improving oxygen consumption; (c) treating heart failure; (d) treating hypertension; (e) improving the quality of life in a heart failure patient; (f) inhibiting left ventricular remodeling; (g) reducing hospitalizations related to heart failure; (h) improving exercise tolerance; (j) increasing left ventricular ejection fraction; (k) decreasing levels of B-type natriuretic protein; (l) treating renovascular diseases; (m) treating end-stage renal diseases; (n) reducing cardiomegaly; (o) treating diseases resulting from oxidative stress; (p) treating endothelial dysfunctions; (q) treating diseases caused by endothelial dysfunctions; or (r) treating cardiovascular diseases; in a patient in need thereof, wherein the patient has a Arg389Arg polymorphism and / or a Gly389Gly polymorphism in the beta 1 adrenergic receptor gene, comprising administering to the patient (i) at least one antioxidant compound or a pharmaceutically acceptable salt thereof; (ii) at least one nitric oxide enhancing compound; and (iii) optionally the best current therapy for the treatment of cardiovascular diseases. In one embodiment the antioxidant is a hydralazine compound or a pharmaceutically acceptable salt thereof and the nitric oxide enhancing compound is isosorbide dinitrate and / or isosorbide mononitrate.
Owner:NITROMED +1
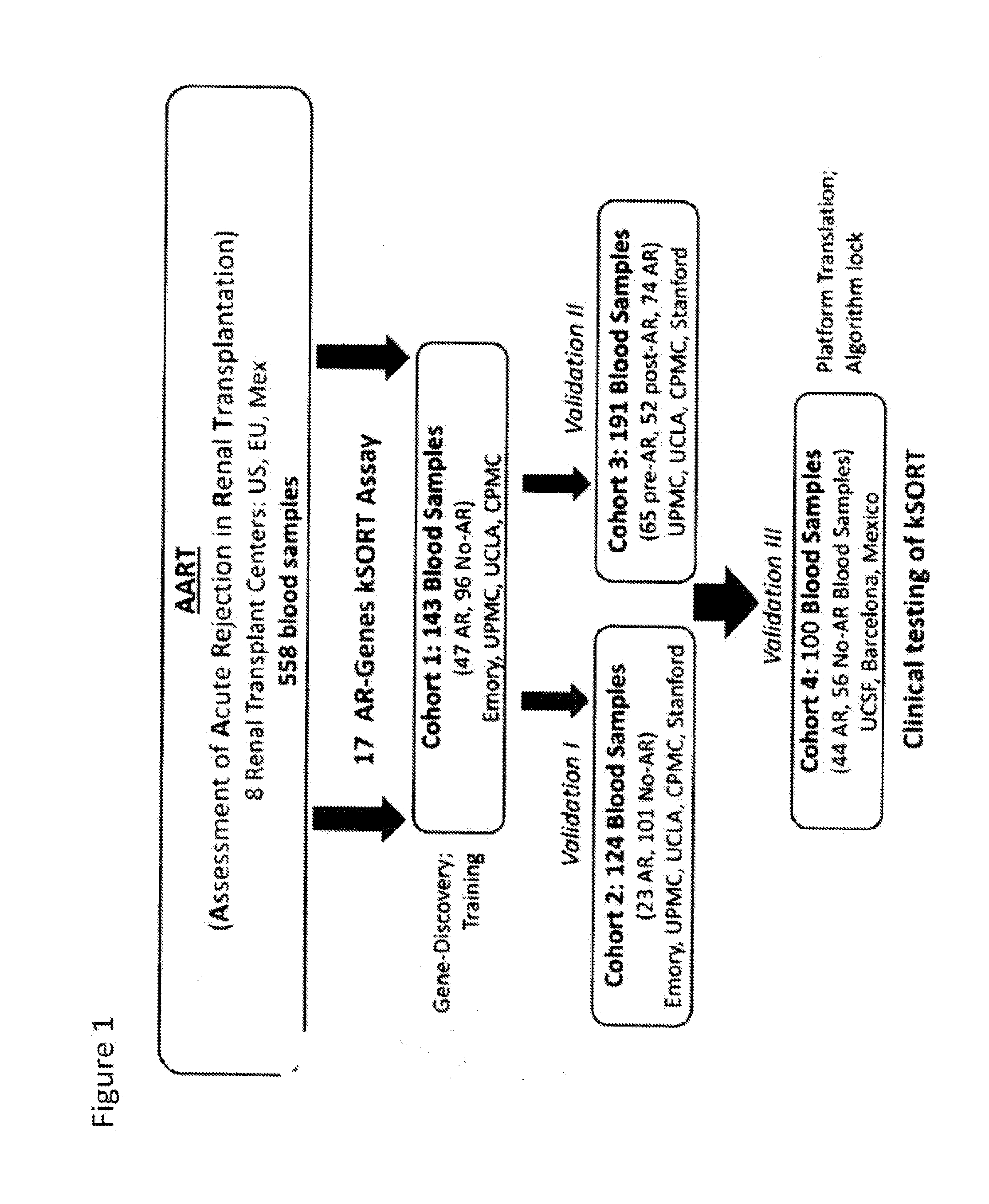
Compositions and methods for assessing acute rejection in renal transplantation
InactiveUS20160348174A1Microbiological testing/measurementUrinary disorderNephrosisEnd stage renal disease
Provided herein are methods, compositions, and kits for diagnosing acute rejection of renal transplants using the gene expression profile of sets of classifier genes. Such methods and compositions are independent of external confounders such as recipient age, transplant center, RNA source, assay, cause of end-stage renal disease, co-morbidities, immunosuppression usage, and the like.
Owner:IMMUCOR GTI DIAGNOSTICS
Chemical ablation and method of treatment for various diseases
PendingUS20200086093A1Improve treatment safetyImprove efficacyUltrasound therapyBalloon catheterEnd-stage kidney diseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, nonalcoholic fatty liver disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and / or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
Methods of predicting predisposition to or risk of kidney disease
ActiveUS20130079244A1Raise the possibilityMicrobiological testing/measurementLibrary screeningNephrosisNephropathy
Methods are disclosed herein for detecting a genetic predisposition to focal segmental glomerulosclerosis (FSGS) or hypertensive end-stage kidney disease (ESKD) or both in a human subject, e.g., by detecting the presence of at least one single nucleotide polymorphism (SNP) in an APOL1 gene, such as the C-terminal exon of an APOL1 gene. In a further embodiment, methods are disclosed for detecting resistance of a subject to a disease associated with Trypanosoma infection, e.g., by detecting at least one single nucleotide polymorphism (SNP) in an APOL1 gene, such as the C-terminal exon of an APOL1 gene. Also disclosed are methods for treating a subject infected with T. brucei. The methods include administering a therapeutically effective amount of an APOL1 protein including a S342G substitution, an I384M substitution, and / or a deletion of N388 and Y389 to the subject.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Chemical ablation and method of treatment for various diseases
ActiveUS10537375B2Improve efficacyImprove securityUltrasonic/sonic/infrasonic diagnosticsBalloon catheterEnd-stage kidney diseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, nonalcoholic fatty liver disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and / or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
Compounds and methods for inhibiting nhe-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders
ActiveUS20130274285A1Reduce interdialytic weight gainMore palatable dietBiocideAnimal repellantsNephrosisEnd stage renal disease
The present disclosure is directed to compounds and methods for treating irritable bowel syndrome, chronic kidney disease and end stage renal disease by administering to a subject in need thereof a compound or a pharmaceutically acceptable salt thereof, wherein the compound has the structure
Owner:ARDELYX
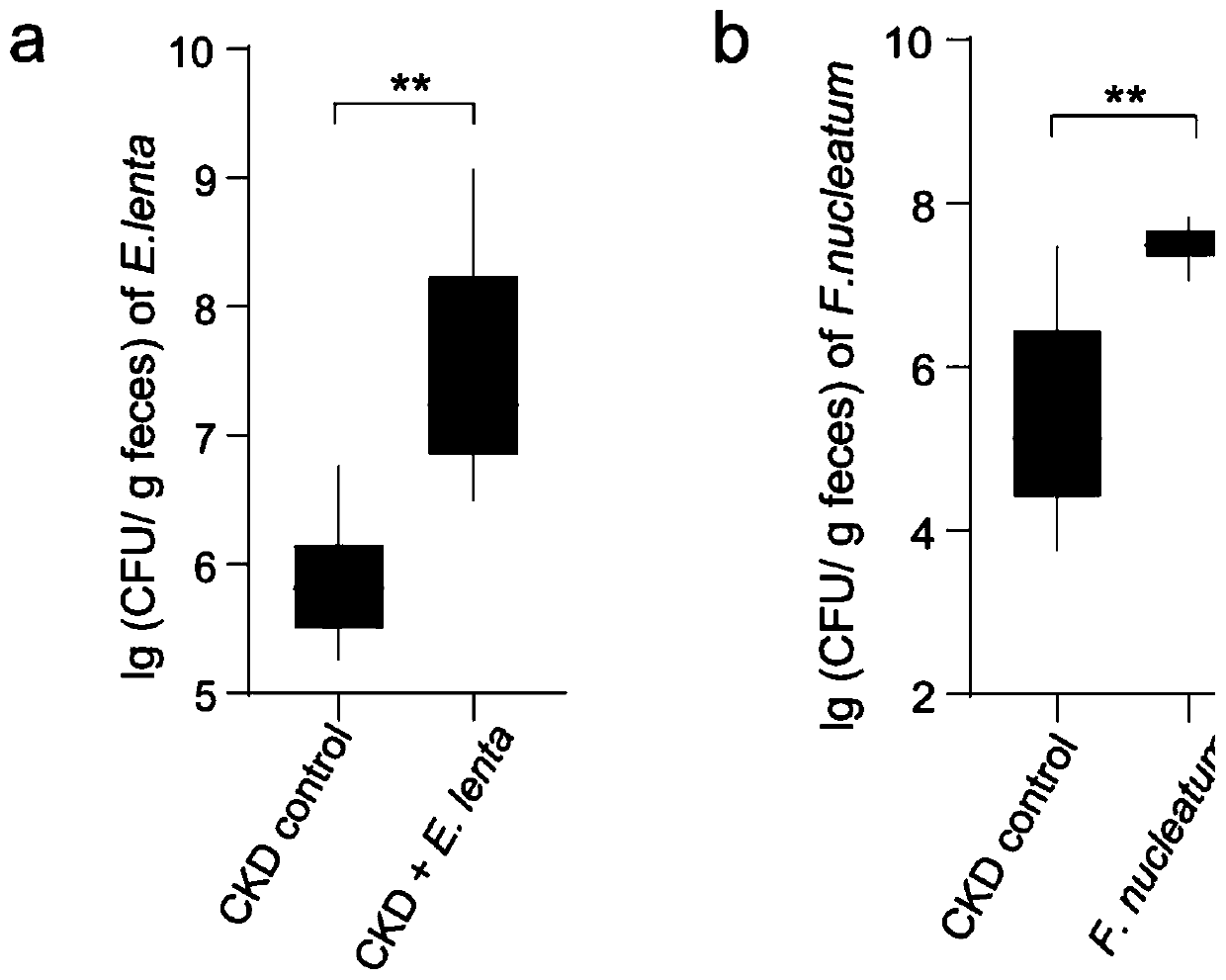
Biomarkers for end-stage renal disease and application thereof
The invention brings forward biomarkers for end-stage renal disease and an application thereof. The biomarkers for end-stage renal disease are Akkermansia muciniphila, Alistipes finegoldii, Alistipesshahii, Bacteroides fibrobacter, Bacteroides fragilis, Bifidobacterium dentium, Clostridium difficile, Clostridium saccharolyticum, Desulfovibrio Vulgaris, Eggerthella lenta, Enterococcus faecalis, Enterococcus faecium, Flavonifractor plautii, Fusobacterium nucleatum, butyric acid-producing enterobacteriaceae, Lactobacillus amylovorus, Lactobacillus casei, Lactobacillus fermentum, Lactobacillus plantarum, Streptococcus infantarius, Streptococcus thermophilus, butyrate-producing bacteria, Eubacterium rectal and Faecalibacterium prausnitzi. Whether a subject suffers from or is susceptible to end-stage renal disease can be effectively determined by determining these microbial markers in the intestinal flora of the subject.
Owner:深圳谱元科技有限公司
Chemical ablation and method of treatment for various diseases
PendingCN113040895ABalloon catheterHydroxy compound active ingredientsDiseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and / or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
Method of treatment for various diseases
PendingUS20210275784A1Improve efficacyImprove securityUltrasound therapyBalloon catheterEnd-stage kidney diseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, nonalcoholic fatty liver disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and / or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
Method of treatment for various diseases
PendingUS20210275786A1Improve efficacyImprove securityUltrasound therapyBalloon catheterEnd-stage kidney diseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, nonalcoholic fatty liver disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and / or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
Method of treatment for various diseases
PendingUS20210275785A1Improve efficacyImprove securityUltrasound therapyBalloon catheterEnd-stage kidney diseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, nonalcoholic fatty liver disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and / or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
Method of treatment for various diseases
PendingUS20210275787A1Improve efficacyImprove securityUltrasound therapyBalloon catheterEnd-stage kidney diseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, nonalcoholic fatty liver disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and / or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
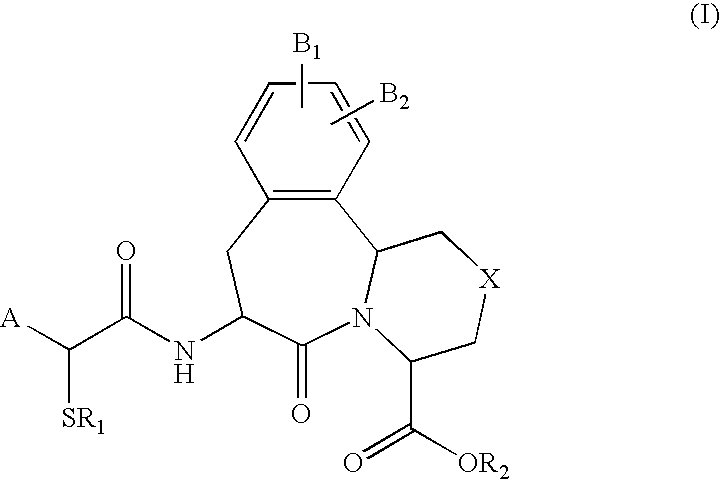
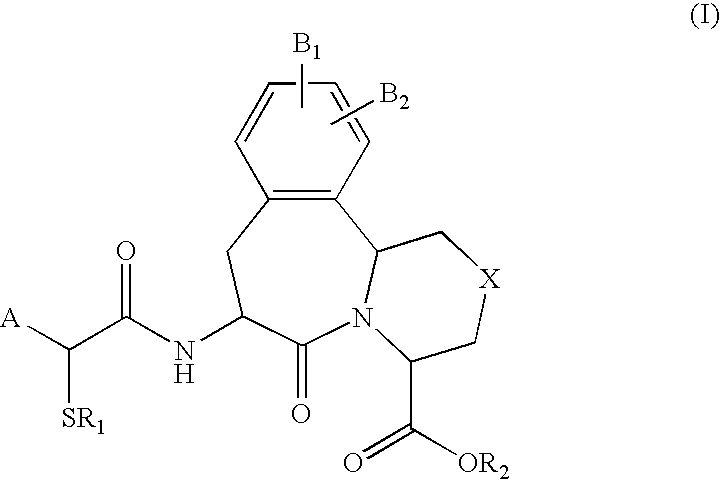
Use of vasopeptidase inhibitors in the treatment of metabolic diseases, nephropathy and advanced glycation end-product associated diseases
The invention describes and claims the use of vasopeptidase inhibitors of formula (I) for the treatment of nephropathy in diabetic or non-diabetic patients, including diabetic or non-diabetic nephropathy, glomerulonephritis, glomerular sclerosis, nephrotic syndome, hypertensive nephrosclerosis, microalbuminuria or end stage renal disease, or insulin resistance or of metabolic diseases associated with advanced glycation end-products, such as diabetic complications, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, cataracts, myocardial infarction and / or diabetic cardiomyopathy, or atherosclerosis or endothelial dysfunction.
Owner:SANOFI AVENTIS DEUT GMBH
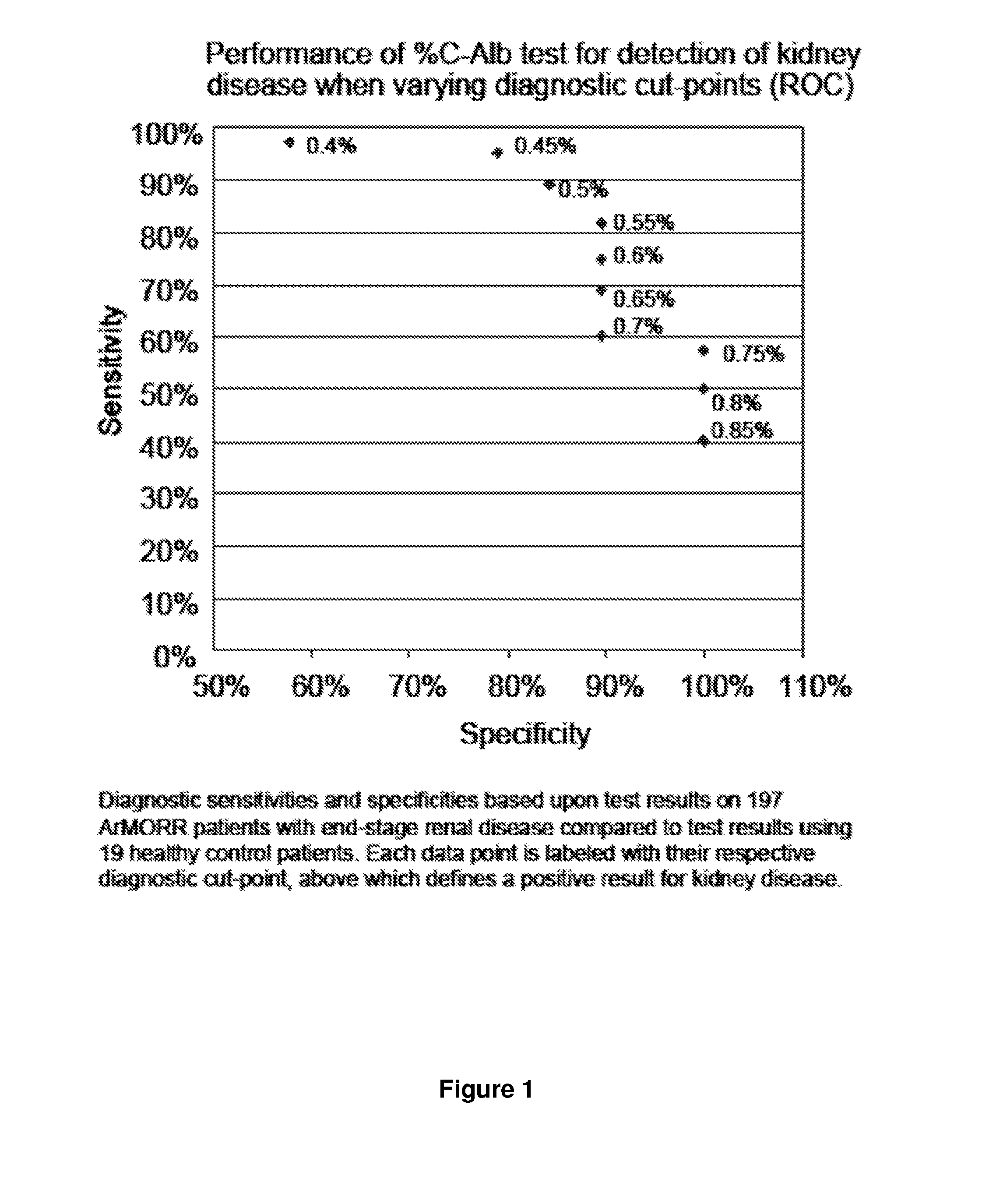
Diagnosis, prognosis, and treatment of kidney disease
ActiveUS20140228296A1Reduce the frequency of occurrenceReduce severityBiocideDipeptide ingredientsNephrosisProtein carbamoylation
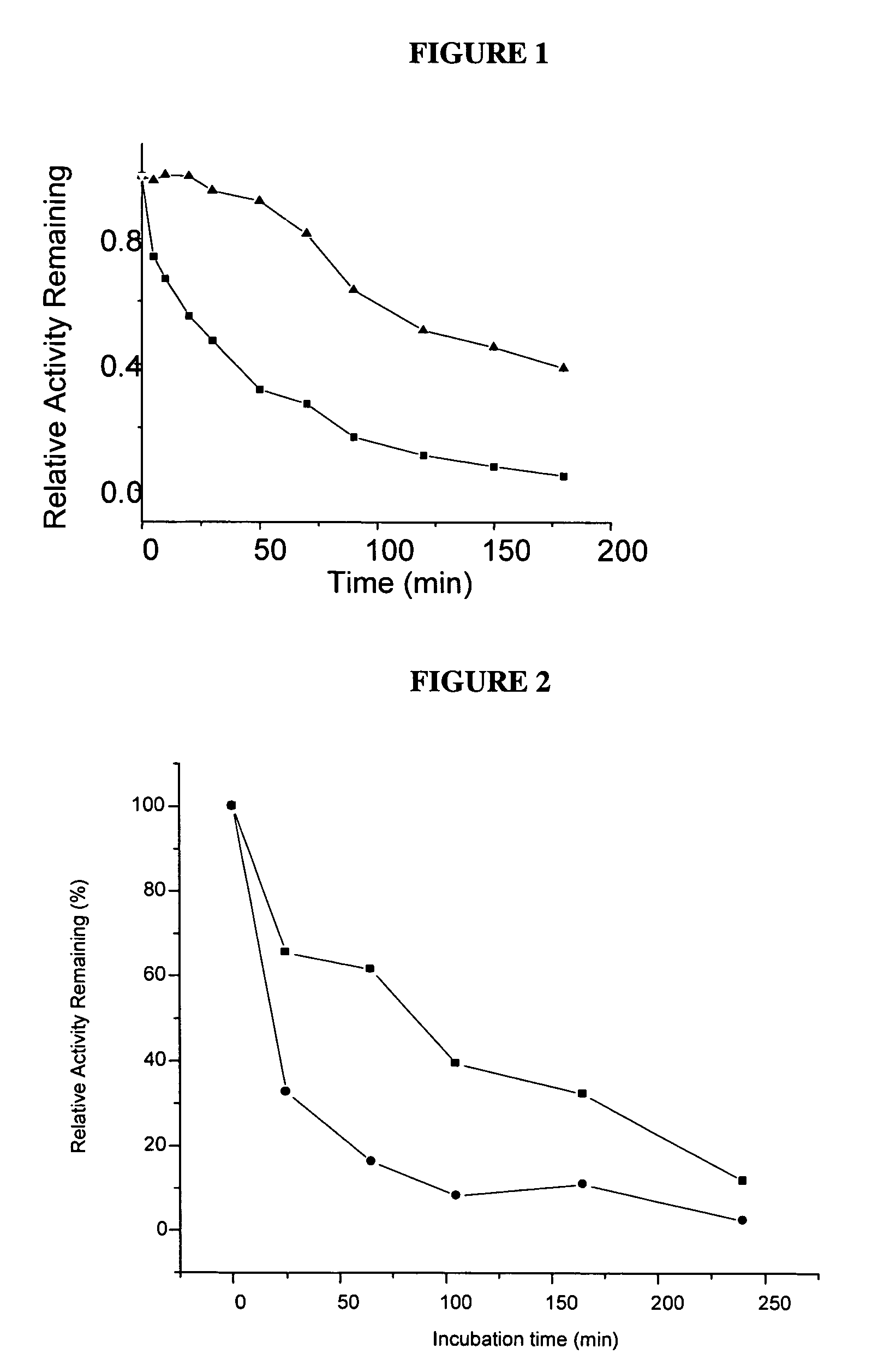
The present invention relates to the discovery that an increased fraction of albumin is carbamylated in patients suffering from kidney disease (e.g., end-stage renal disease) and that the fraction of carbamylated albumin is also correlated with increased disease severity, particularly risk of mortality. The present invention also relates to the discovery that free amino acids can reduce carbamylation of albumin. Based on these discoveries the present invention provides diagnostic and prognostic methods for patients suffering from, or suspected of suffering from kidney disease. The invention also provides methods for treating kidney disease by administration of a compound or composition that reduced protein carbamylation, such as free amino acids or dipeptides.
Owner:THE GENERAL HOSPITAL CORP +1
Kidney healing soup
The invention provides kidney healing soup which belongs to a Chinese herbal medicine soup for oral administration in traditional Chinese medicine and is suitable for treating various early-stage, medium-stage and end-stage kidney diseases. The kidney healing soup is prepared according to formula 1 as follows: 20g of semen plantaginis, 20g of pyrrosia lingua, 15g of grifola and 30g of pearl barley, or according to formula 2 as follows: 20g of poria cocos, 20g of euryale ferox salisb, 20g of white atractylodes rhizome, 20g of wolfberry fruit, 20g of astragalus membranaceus and 5g of cartialgenous; and all the raw materials are decocted with water for administration. The kidney healing soup is convenient to administrate, free of toxic and side effects and accurate in curative effect.
Owner:孔宪高
Compositions and methods for assessing acute rejection in renal transplantation
Provided herein are methods, compositions, and kits for diagnosing acute rejection of renal transplants using the gene expression profile of sets of classifier genes. Such methods and compositions are independent of external confounders such as recipient age, transplant center, RNA source, assay, cause of end-stage renal disease, co-morbidities, immunosuppression usage, and the like.
Owner:IMMUCOR GTI DIAGNOSTICS
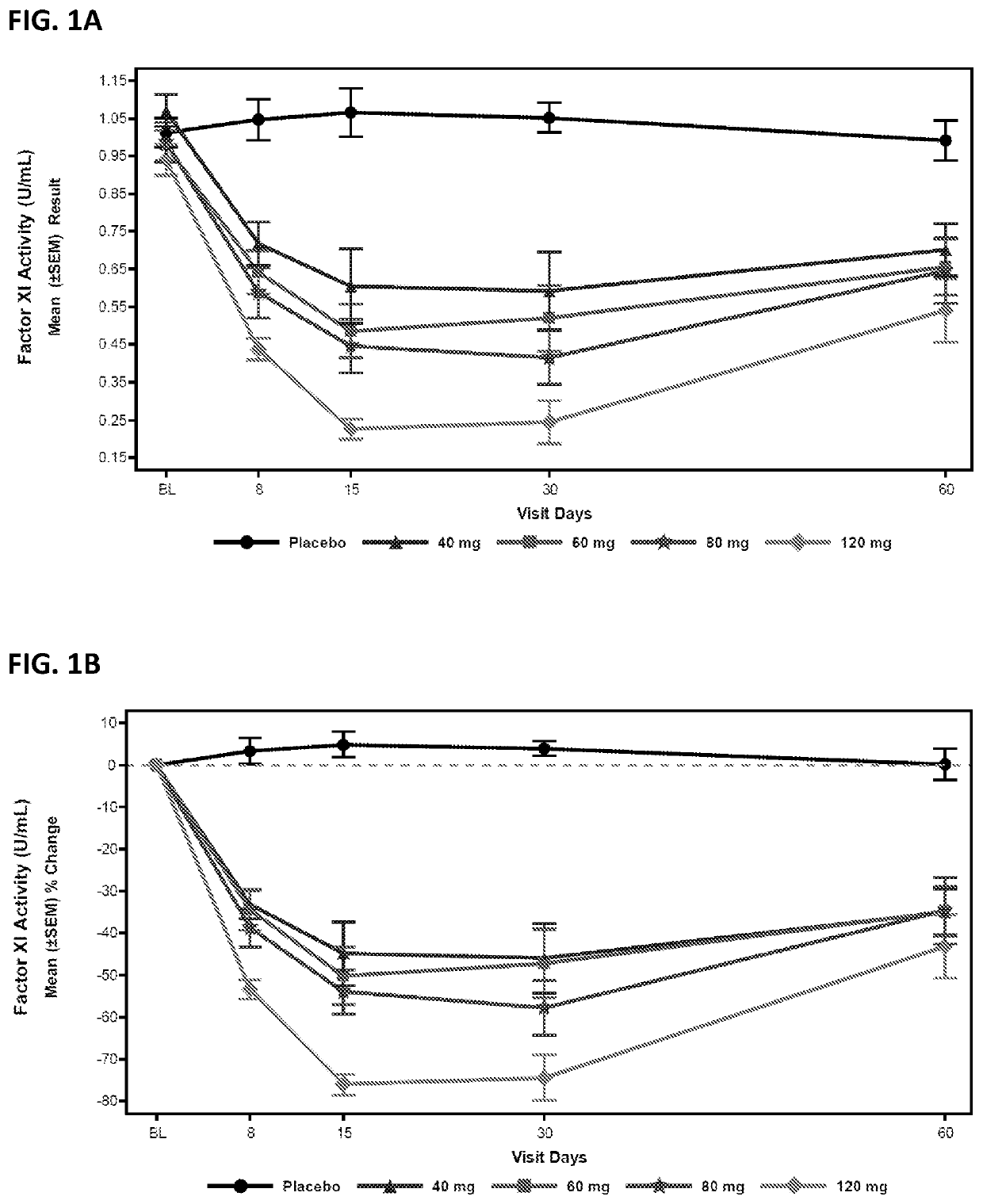
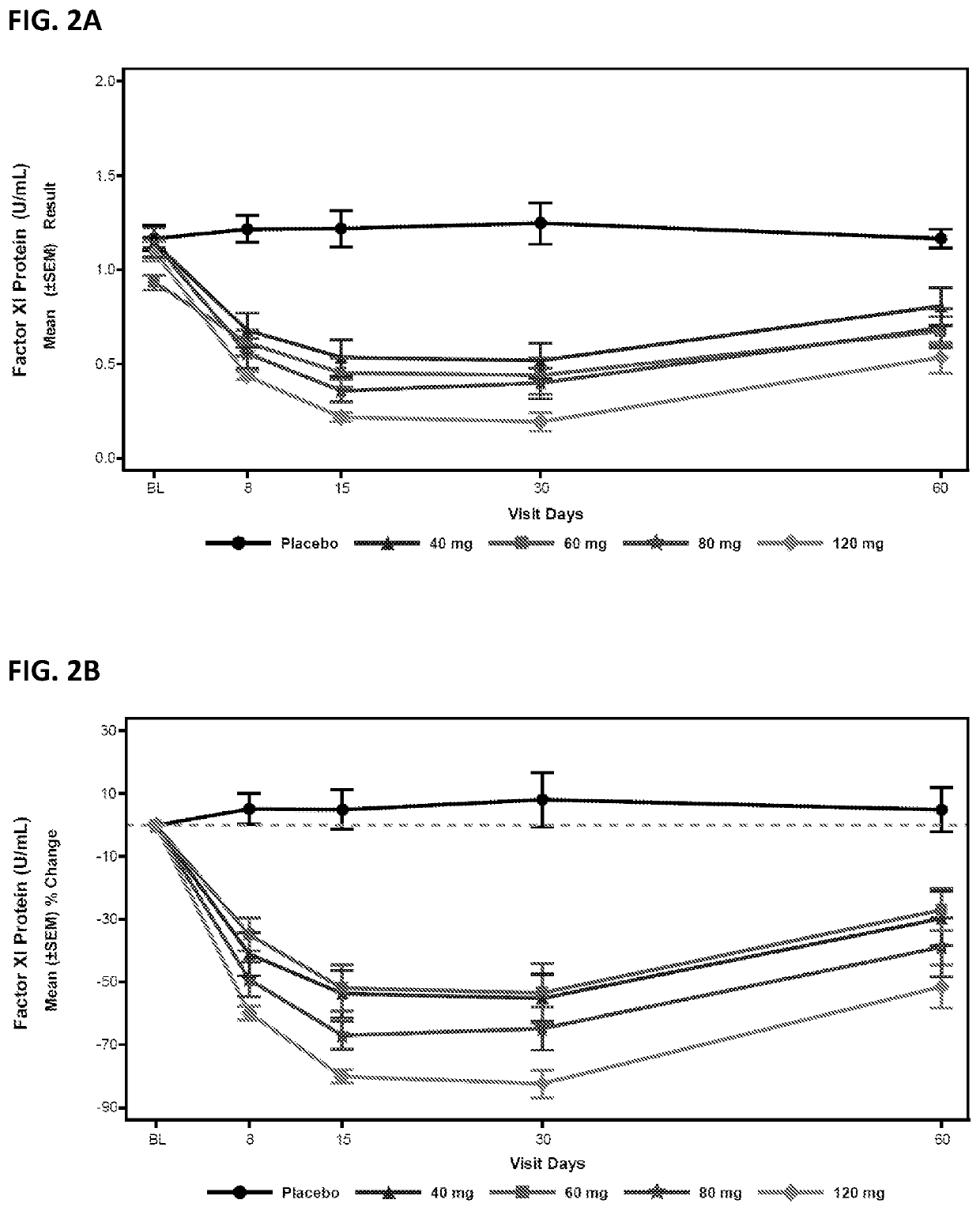
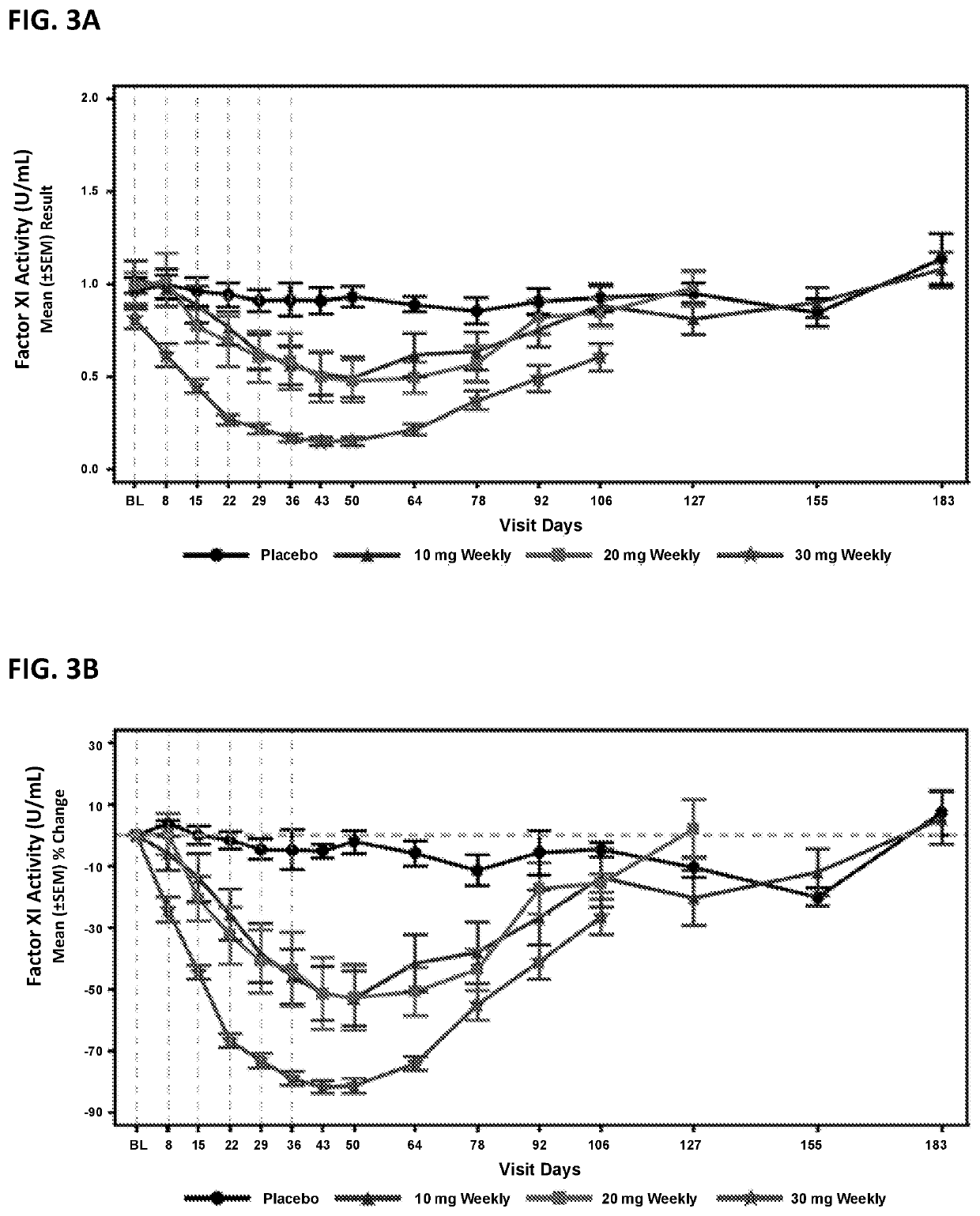
Compounds and methods for reducing fxi expression
ActiveUS20210087569A1Reduce the amount requiredReduce FXI protein activityCarbohydrate active ingredientsUrinary disorderDiseaseThrombus
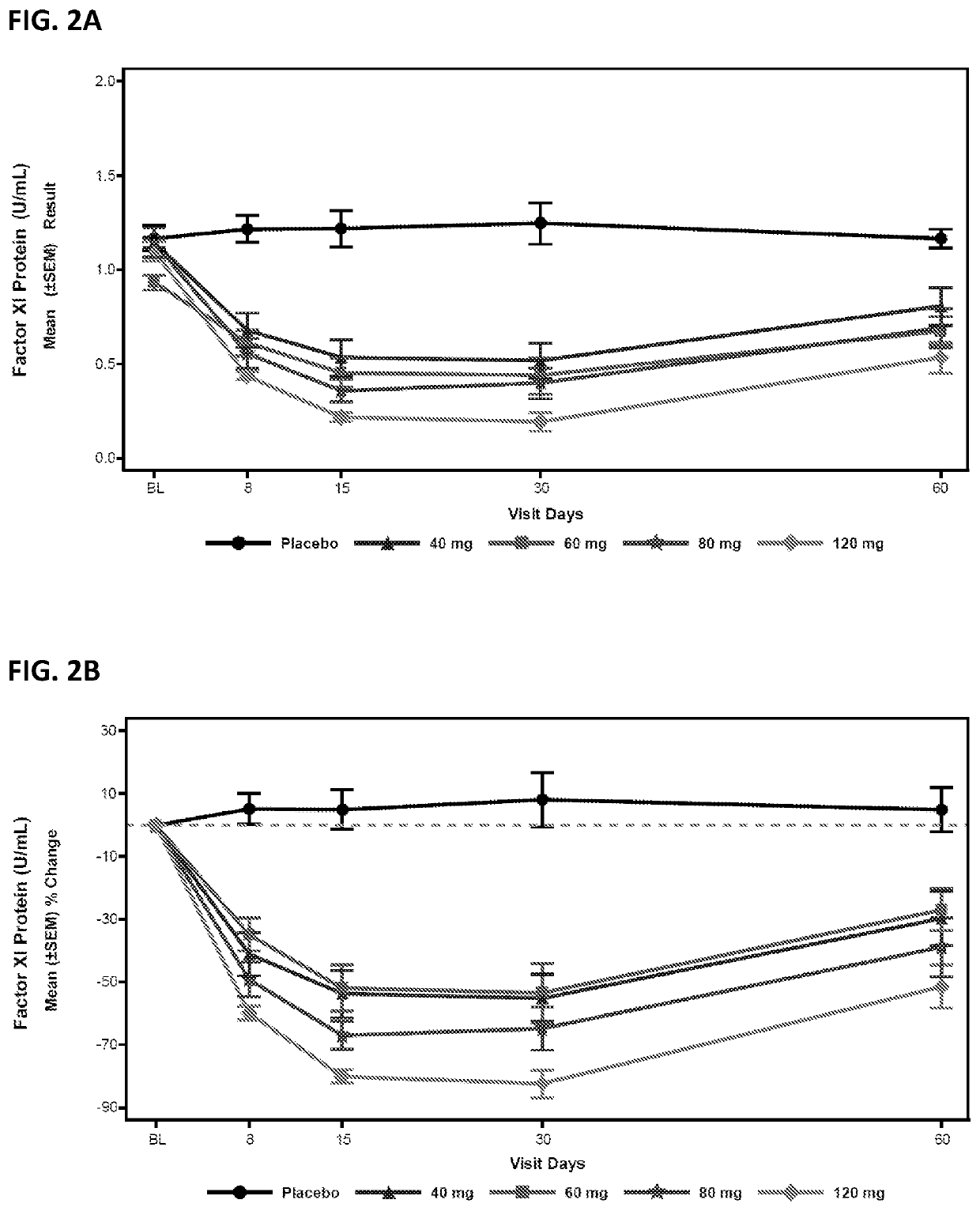
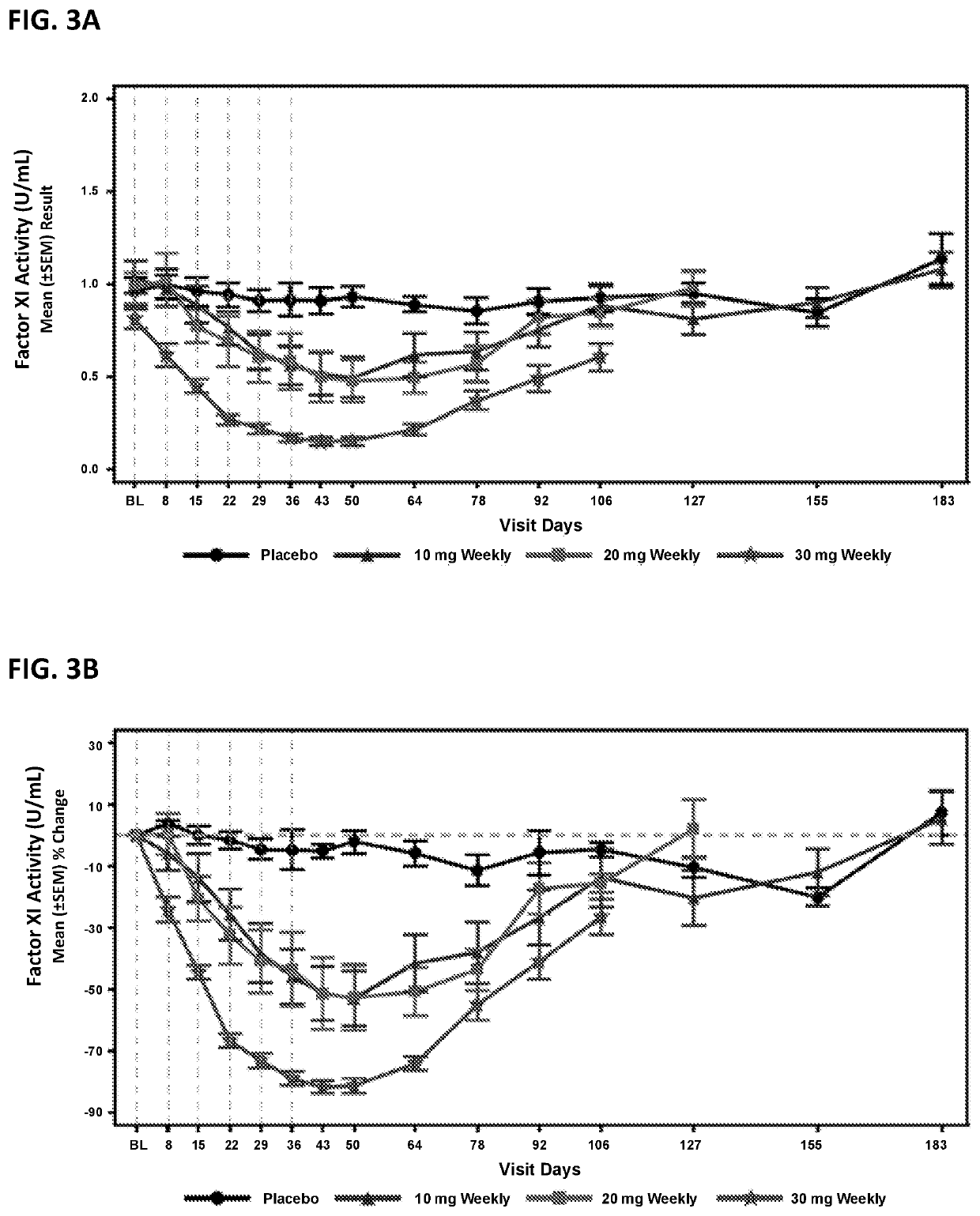
Provided are compounds, methods, and pharmaceutical compositions for reducing the amount or activity of FXI RNA in a cell or subject, and in certain instances reducing the amount of FXI protein in a cell or subject. Such compounds, methods, and pharmaceutical compositions are useful to prevent, treat, or ameliorate at least one symptom of a thromboembolic condition without a significant increase in a bleeding risk. Such thromboembolic conditions include deep vein thrombosis, venous or arterial thrombosis, pulmonary embolism, myocardial infarction, stroke, thrombosis associated with chronic kidney disease or end-stage renal disease (ESRD), including thrombosis associated with dialysis, or other procoagulant condition. Such symptoms include decreased blood flow through an affected vessel, death of tissue, and death.
Owner:IONIS PHARMA INC
Methods of predicting predisposition to or risk of kidney disease
ActiveUS9828637B2Raise the possibilityMicrobiological testing/measurementLibrary screeningNephrosisNephropathy
Methods are disclosed herein for detecting a genetic predisposition to focal segmental glomerulosclerosis (FSGS) or hypertensive end-stage kidney disease (ESKD) or both in a human subject, e.g., by detecting the presence of at least one single nucleotide polymorphism (SNP) in an APOL1 gene, such as the C-terminal exon of an APOL1 gene. In a further embodiment, methods are disclosed for detecting resistance of a subject to a disease associated with Trypanosoma infection, e.g., by detecting at least one single nucleotide polymorphism (SNP) in an APOL1 gene, such as the C-terminal exon of an APOL1 gene. Also disclosed are methods for treating a subject infected with T. brucei. The methods include administering a therapeutically effective amount of an APOL1 protein including a S342G substitution, an I384M substitution, and / or a deletion of N388 and Y389 to the subject.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Coated pharmaceutical compositions
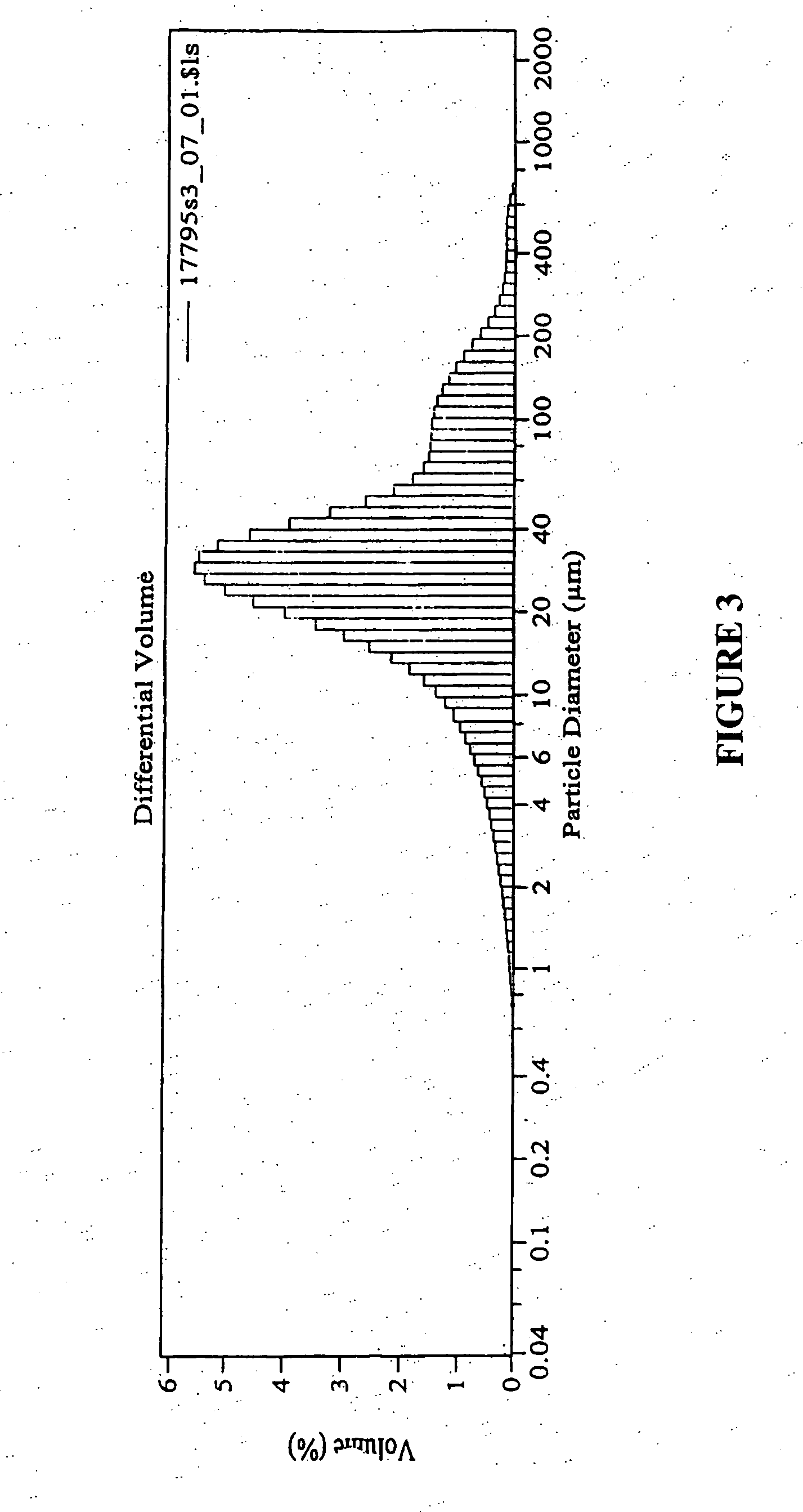
InactiveUS20150283170A1Granular deliverySynthetic polymeric active ingredientsEnd stage renal diseasePolymer
The present invention relates to polycarbophil coated crosslinked amine polymers and / or pharmaceutical compositions comprising polycarbophil coated crosslinked amine polymers. The polycarbophil coated crosslinked amine polymers have several therapeutic applications, including, but not limited to, hyperphosphatemia, chronic kidney disease and End-Stage Renal Disease.
Owner:GENZYME CORP
Sensor monitoring system for in-dwelling catheter based treatments
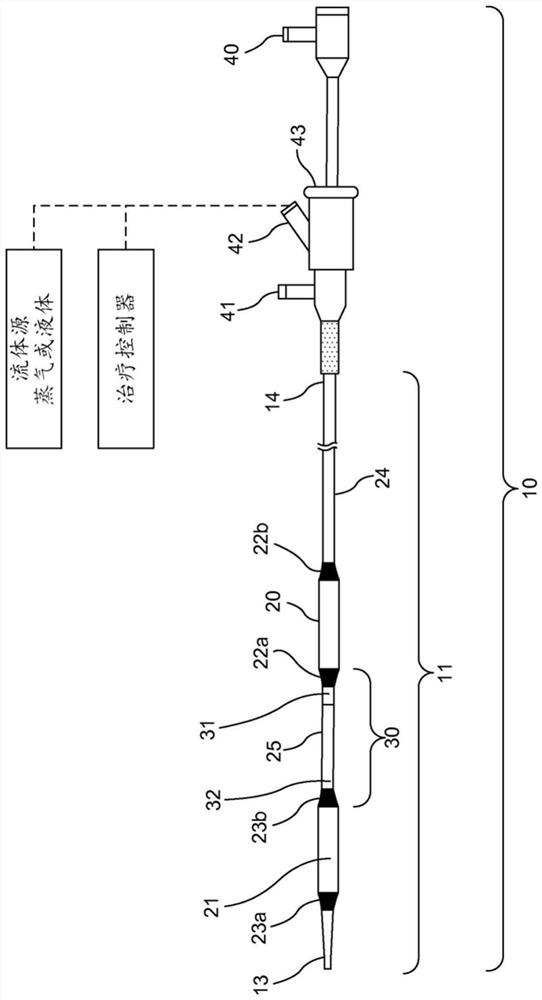
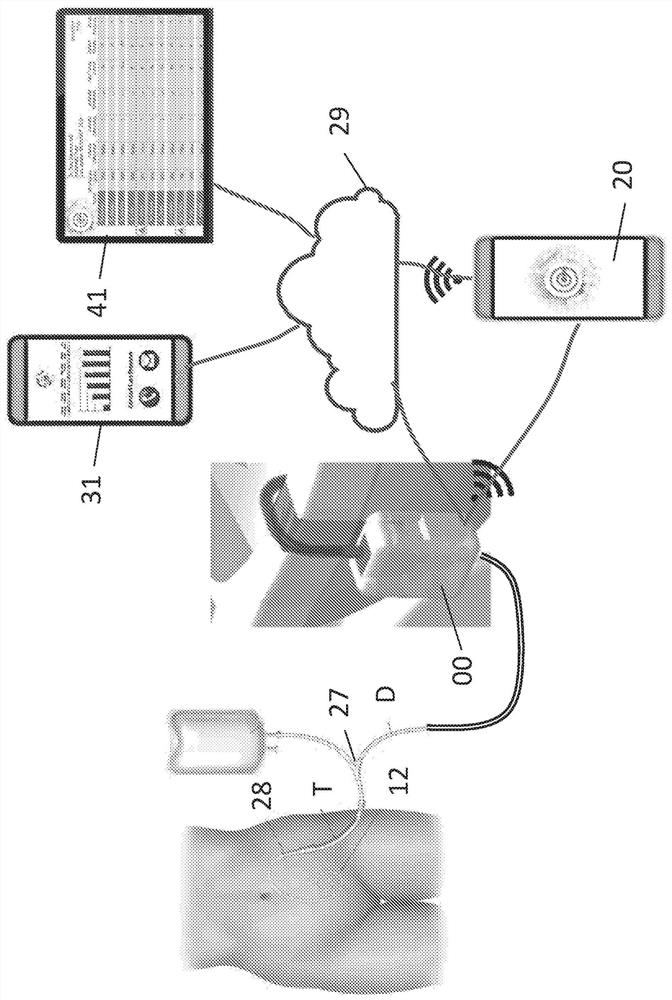
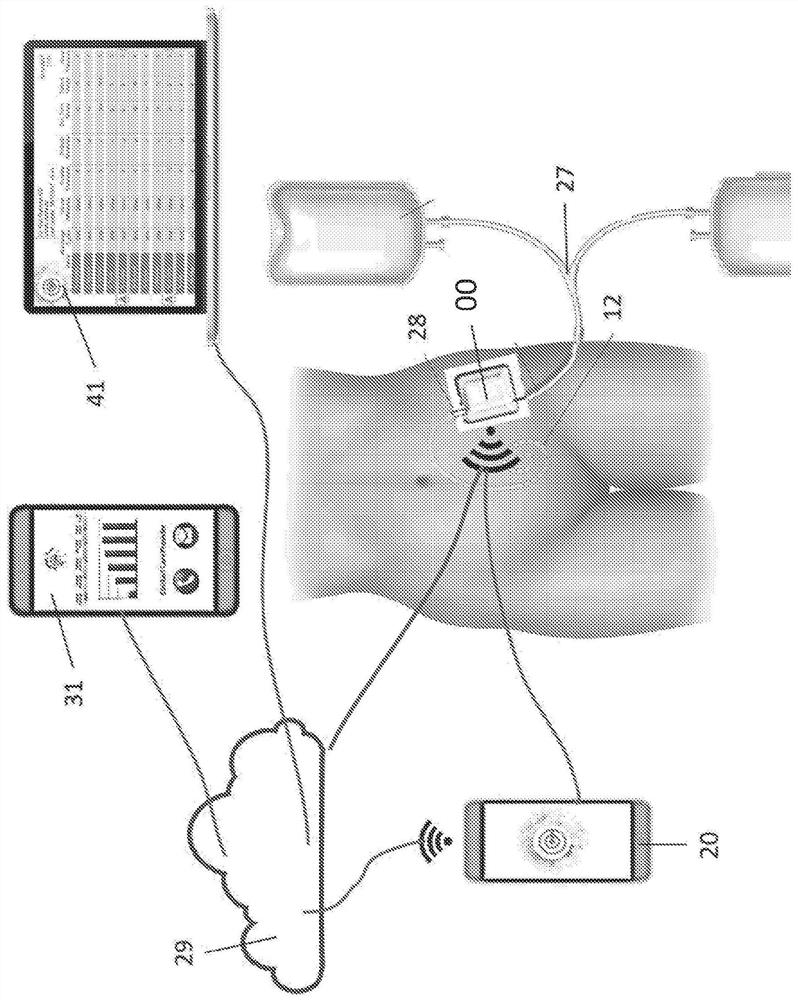
PendingCN111712196APrevent hospitalizationResolve complicationsDiagnostics using lightMedical devicesCentral venous lineEnd-stage kidney disease
A patient monitoring system may be used with catheters to monitor the infusion and drainage of any solution into the human body. The system may be used, for example, with in-dwelling catheters for peritoneal dialysis in end stage renal disease (ESRD) patients, urinary tract catheters, insulin pumps in diabetic patients, feeding tubes and central venous line catheters. The patient monitoring systemincludes one or more fluid pathways for infusing into and / or draining solutions out of the catheter, and one or more sensors to monitor the fluid. The patient monitoring system transmits the patientmonitoring data to a database, allowing data storage, processing, and access through graphical user interfaces to patients and providers via device applications or browser-based web access portals.
Owner:GASTROKLENZ INC
Compounds and methods for reducing FXI expression
ActiveUS11021710B2Reduce riskDecreased blood flowCarbohydrate active ingredientsUrinary disorderDiseaseThrombus
Provided are compounds, methods, and pharmaceutical compositions for reducing the amount or activity of FXI RNA in a cell or subject, and in certain instances reducing the amount of FXI protein in a cell or subject. Such compounds, methods, and pharmaceutical compositions are useful to prevent, treat, or ameliorate at least one symptom of a thromboembolic condition without a significant increase in a bleeding risk. Such thromboembolic conditions include deep vein thrombosis, venous or arterial thrombosis, pulmonary embolism, myocardial infarction, stroke, thrombosis associated with chronic kidney disease or end-stage renal disease (ESRD), including thrombosis associated with dialysis, or other procoagulant condition. Such symptoms include decreased blood flow through an affected vessel, death of tissue, and death.
Owner:IONIS PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com