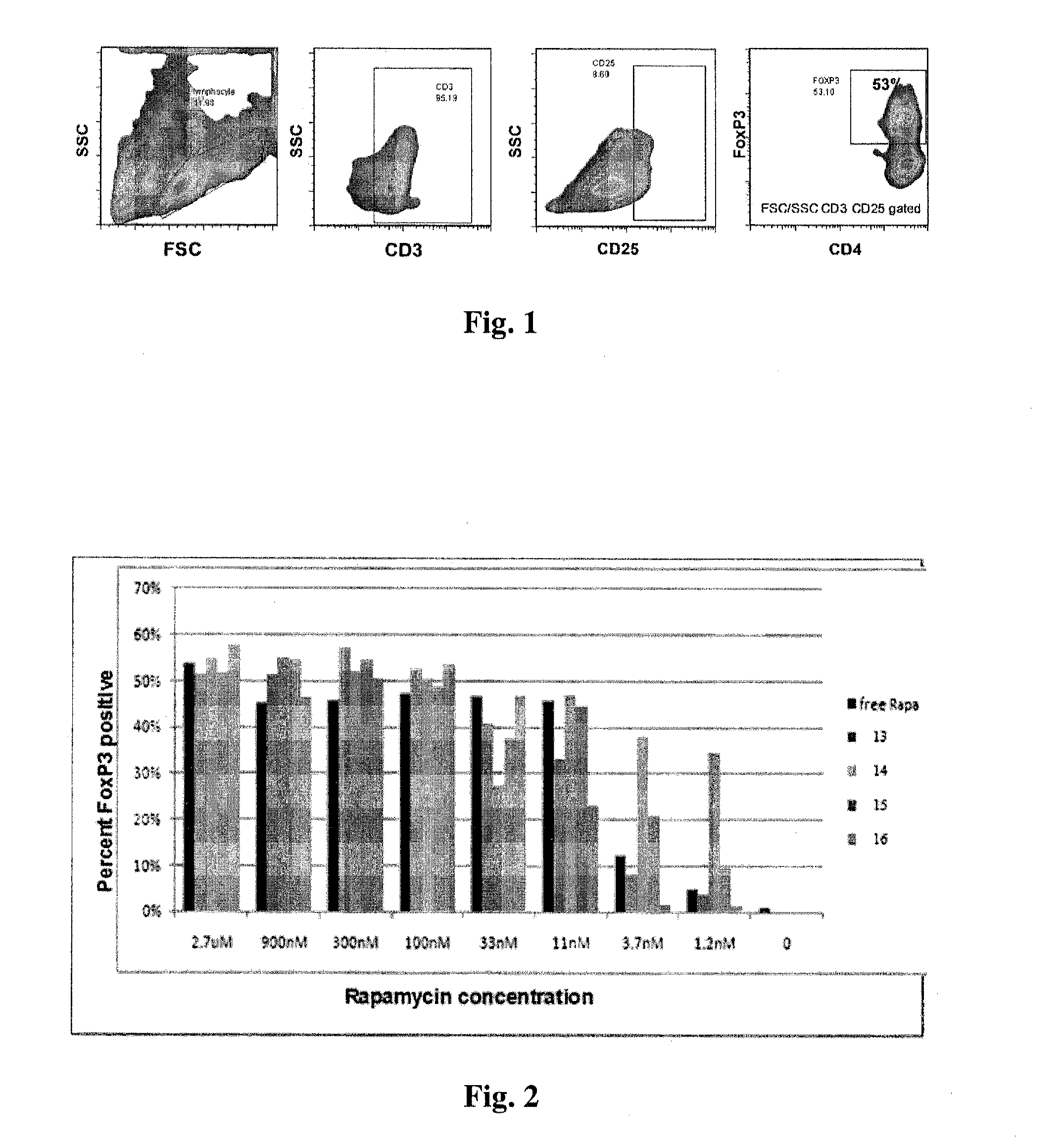
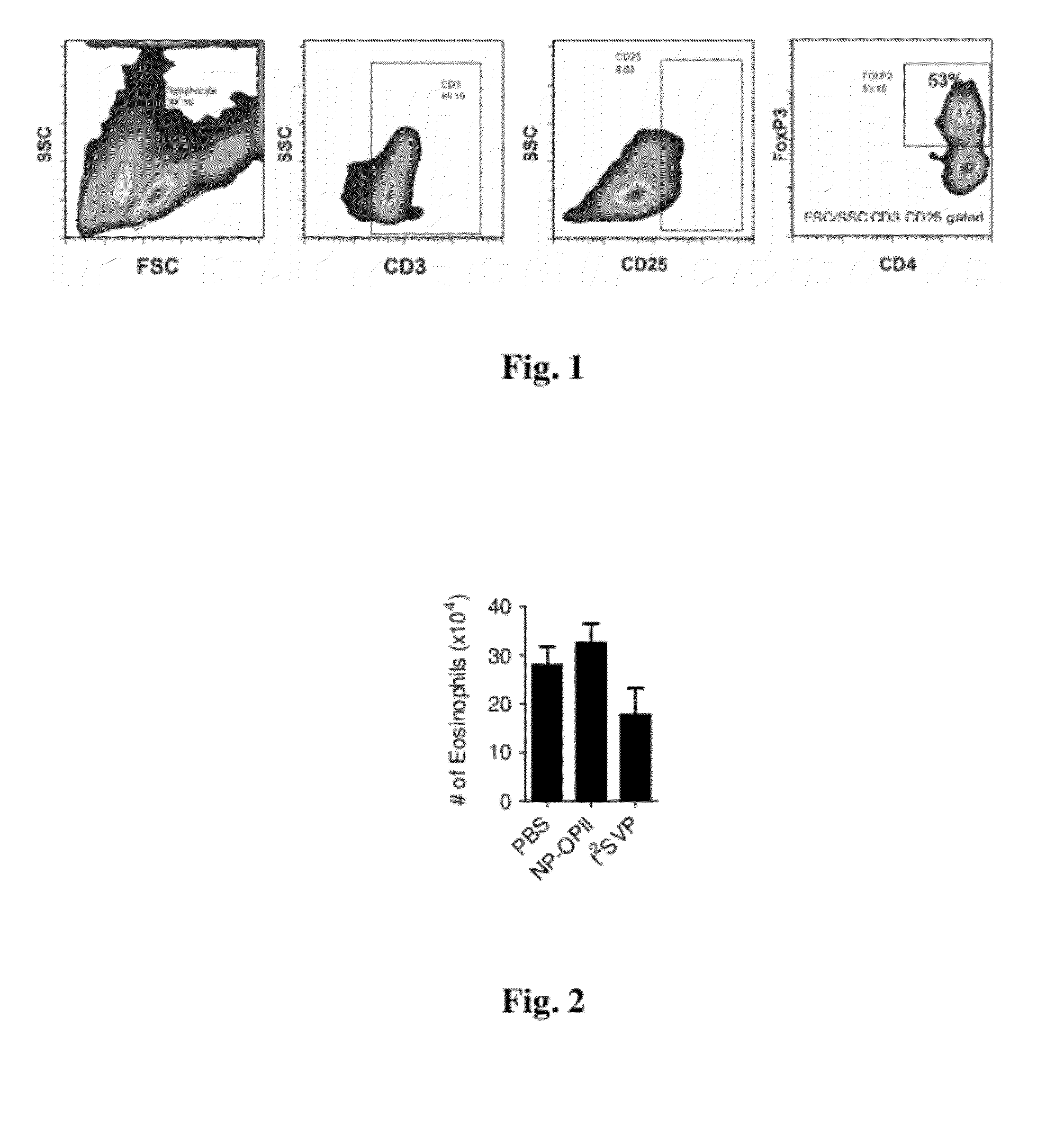
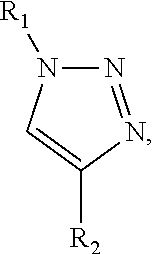
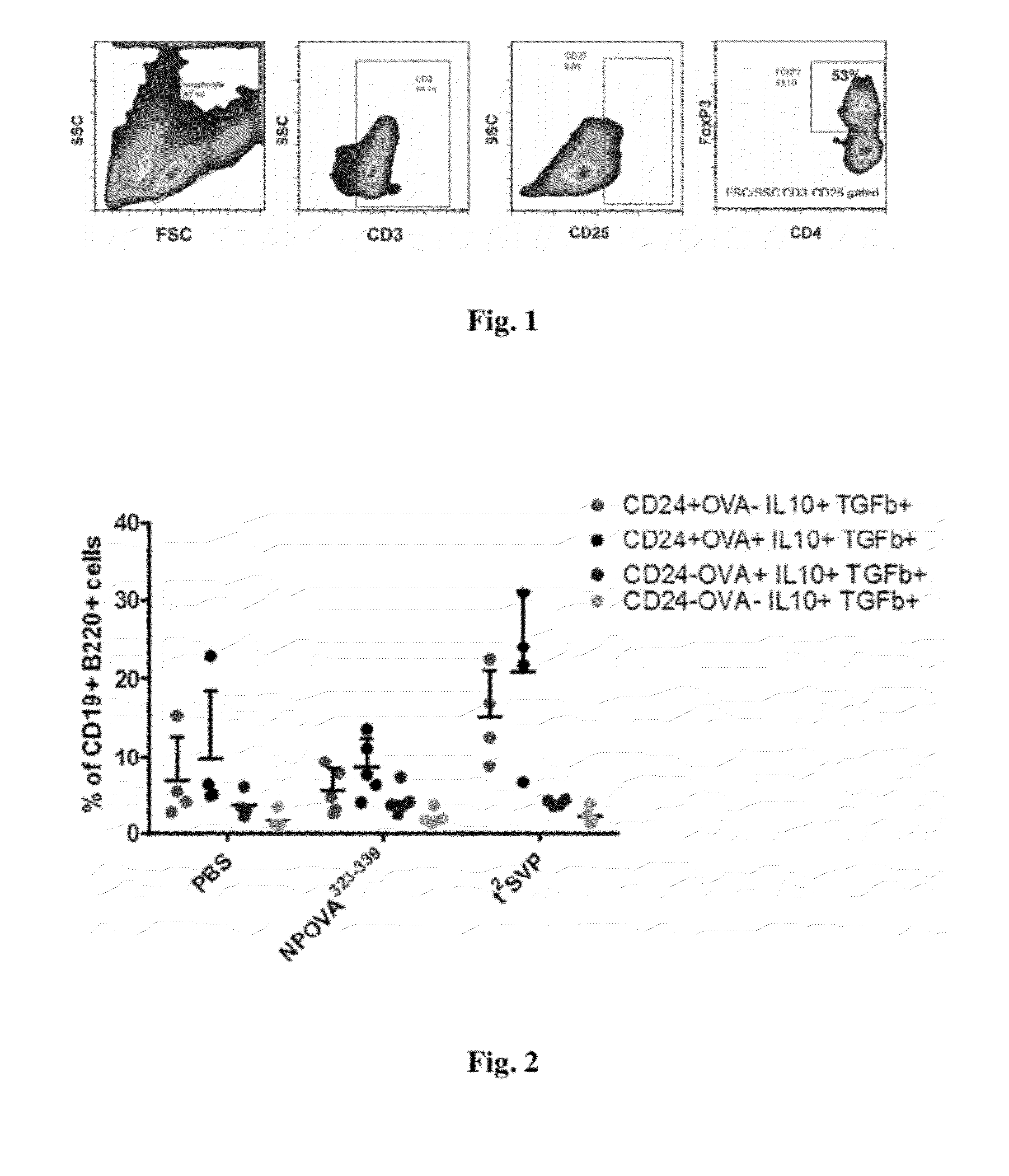
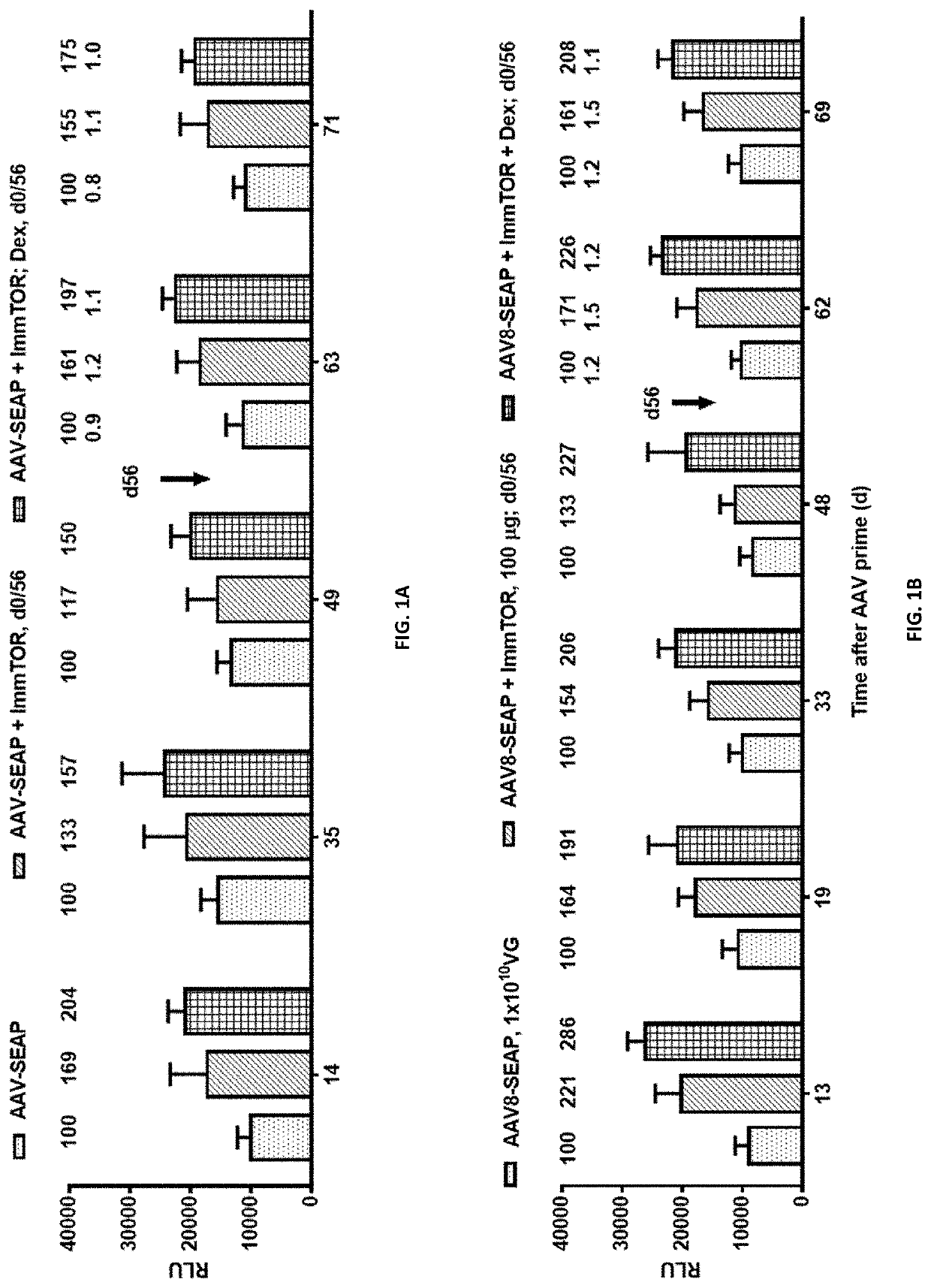
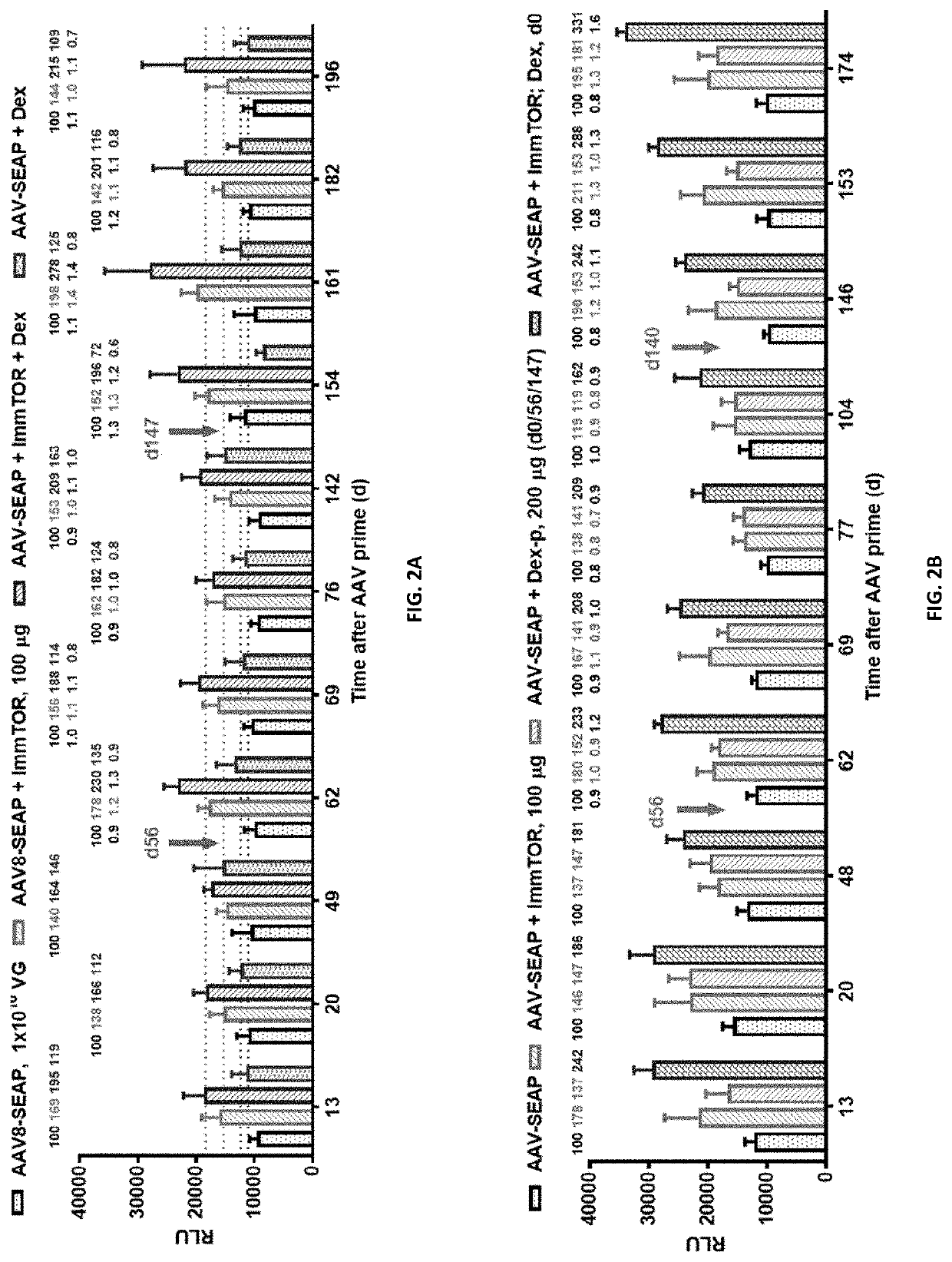
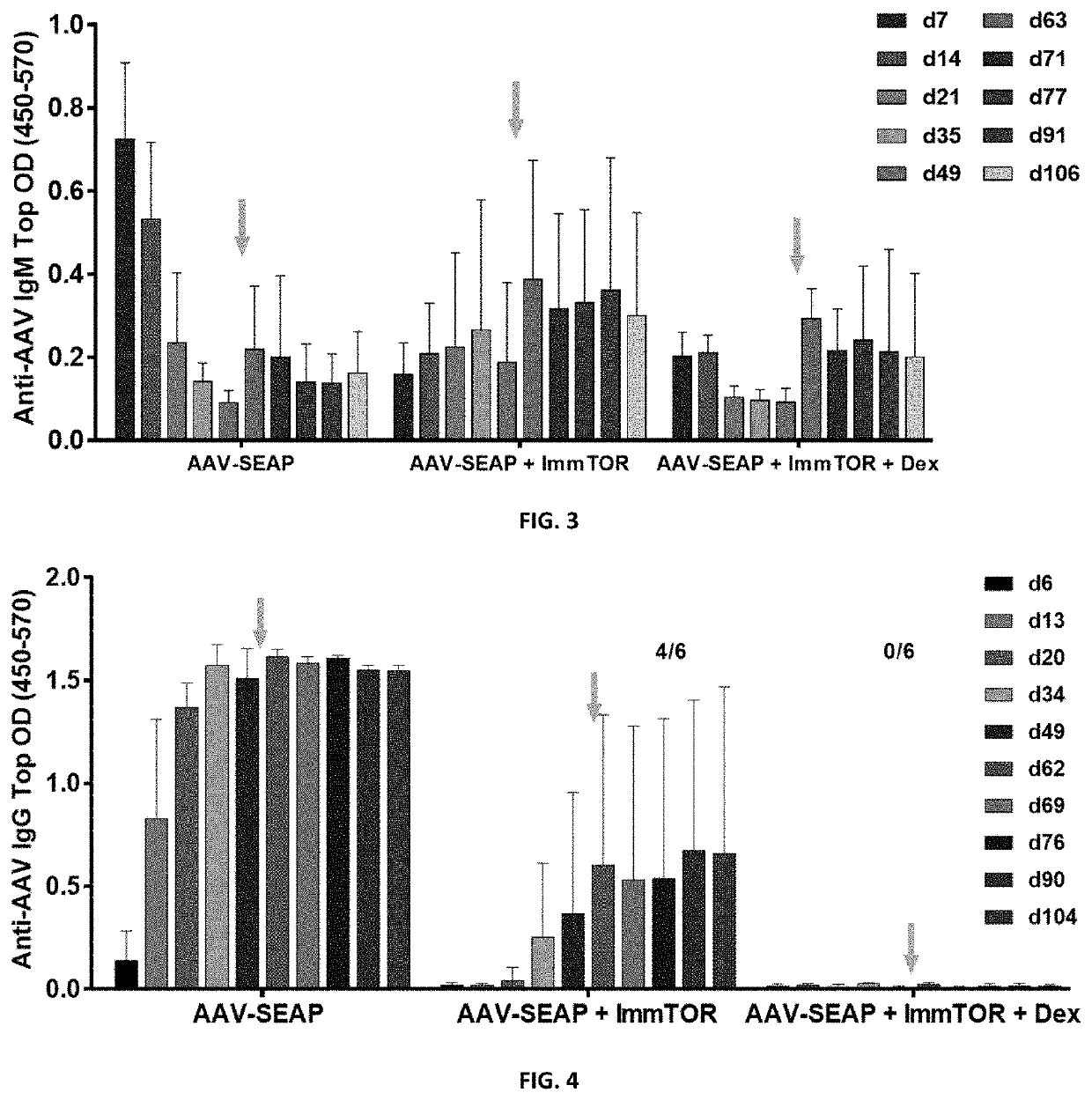
Patents
Literature
119 results about "IMMUNE SUPPRESSANTS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of Immunosuppressant. Immunosuppressant: An agent that can suppress or prevent the immune response. Immunosuppressants are used to prevent rejection of a transplanted organ and to treat autoimmune diseases such as psoriasis, rheumatoid arthritis, and Crohn's disease.
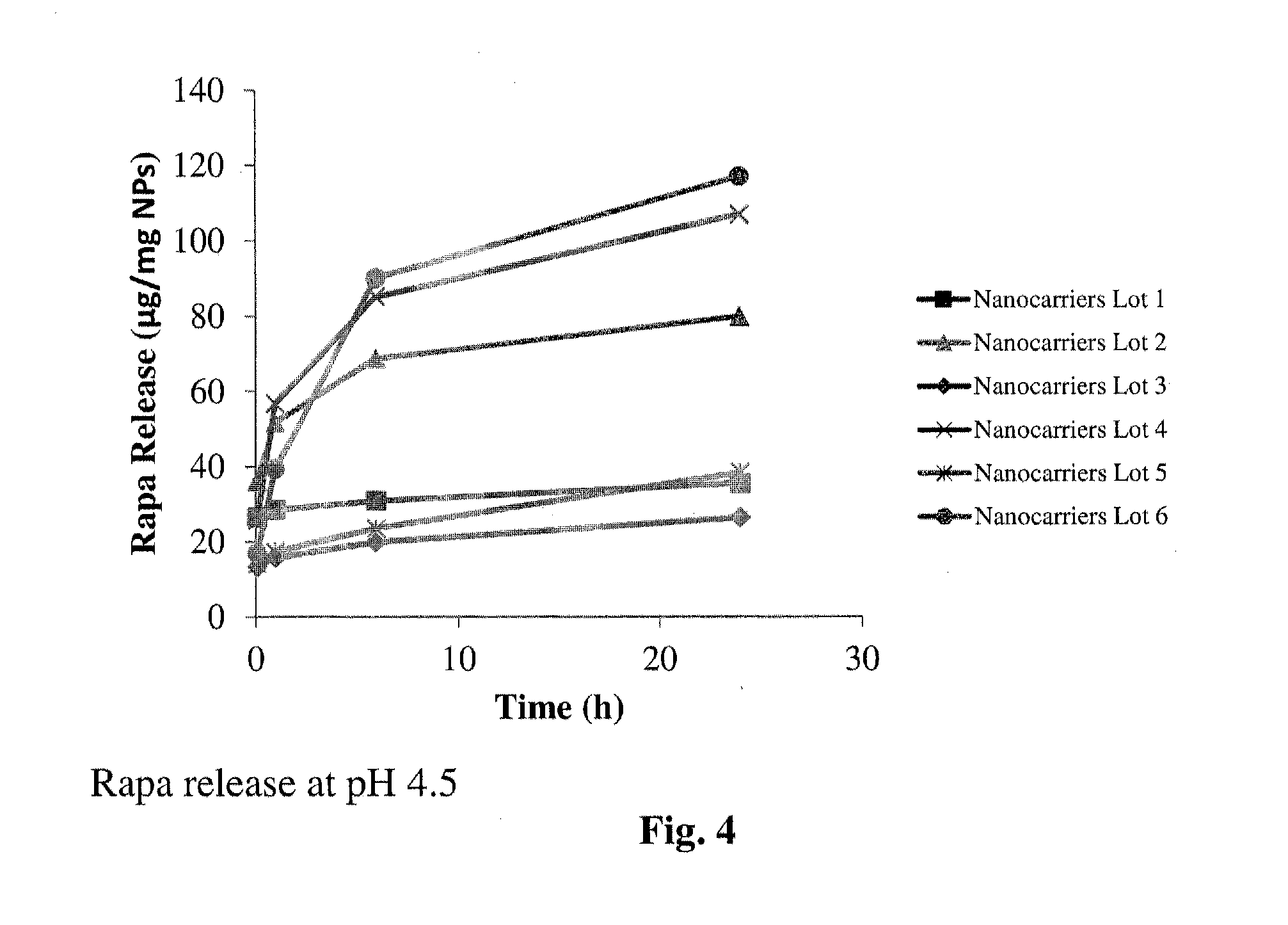
Controlled release of immunosuppressants from synthetic nanocarriers
InactiveUS20120301498A1Reduce in quantityReduce percentagePowder deliveryOrganic active ingredientsControlled releaseAntigen
Disclosed are synthetic nanocarrier compositions that provide controlled release of immunosuppressants as well as related methods. The synthetic nanocarrier compositions may also include antigen in some embodiments.
Owner:SELECTA BIOSCI
Method of treating transplant rejection
This invention relates to a method of treating transplant rejection comprising administering to a patient a pharmaceutical composition comprising an lck inhibitor and a calcineurin inhibitor or an immunosuppressant.
Owner:ABBOTT LAB INC
Tolerogenic synthetic nanocarriers to reduce immune responses to therapeutic proteins
ActiveUS20120276109A1Reduce frequencyHigh frequencyOrganic active ingredientsPowder deliveryAntigenIMMUNE SUPPRESSANTS
Disclosed are synthetic nanocarrier compositions, and related methods, comprising therapeutic protein APC presentable antigens and immunosuppressants that provide tolerogenic immune responses specific to therapeutic proteins.
Owner:SELECTA BIOSCI
Recombinant multiple domain fusion protein mitogens and use thereof for inducing enhancement or repression of antigen-specific immunity.
ActiveUS20100303811A1Increase heightVirusesPeptide/protein ingredientsIMMUNE SUPPRESSANTSAutoimmune responses
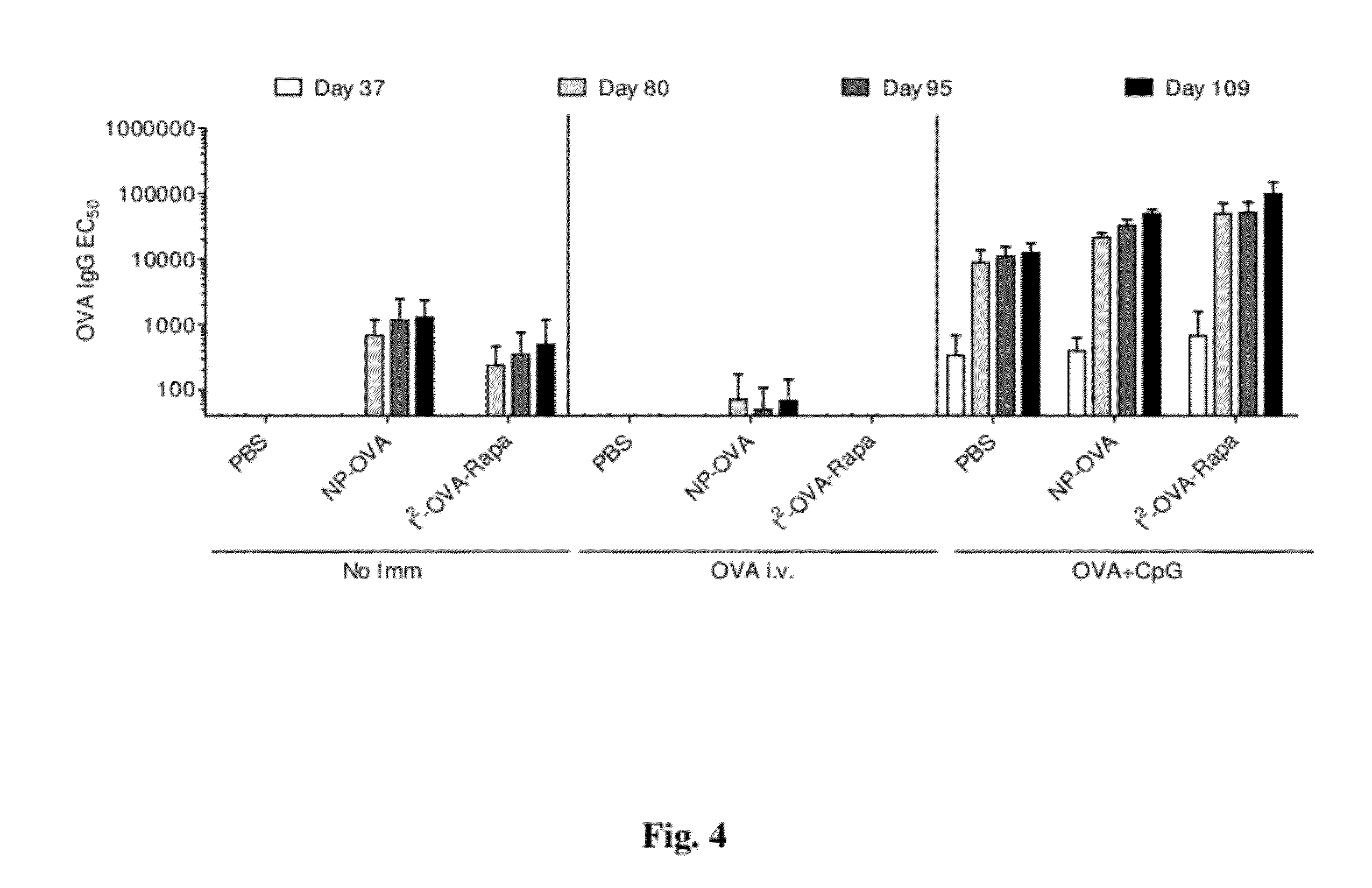
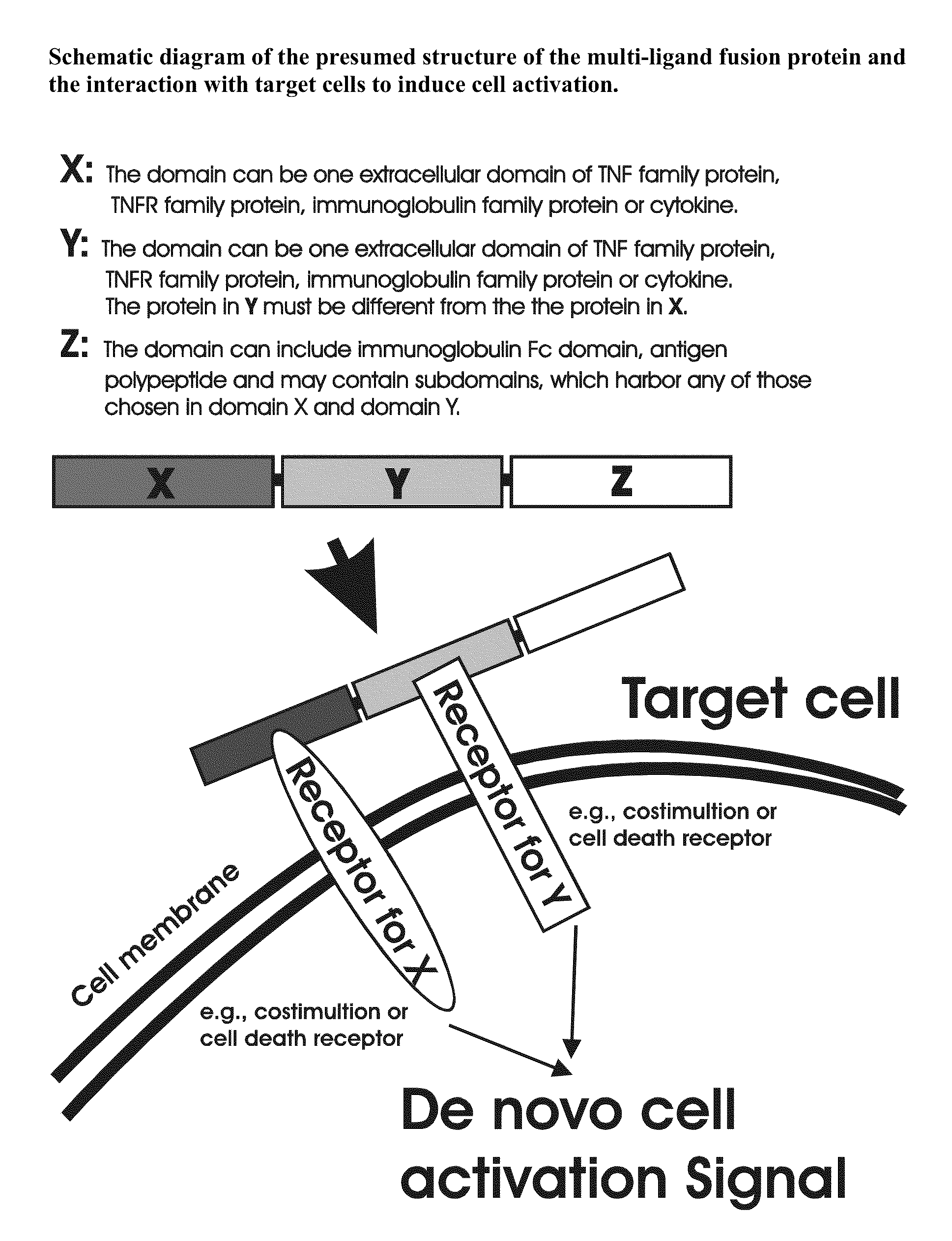
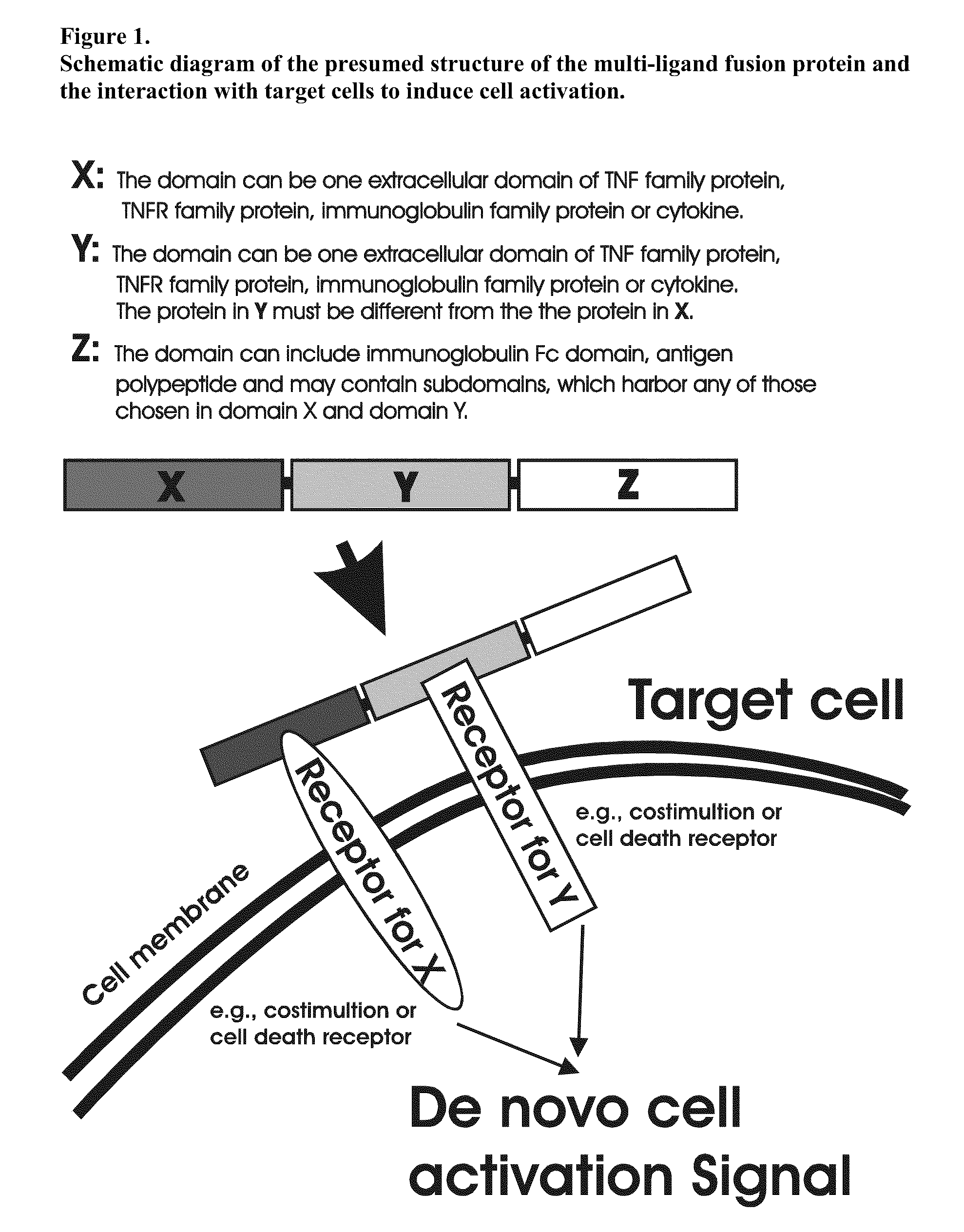
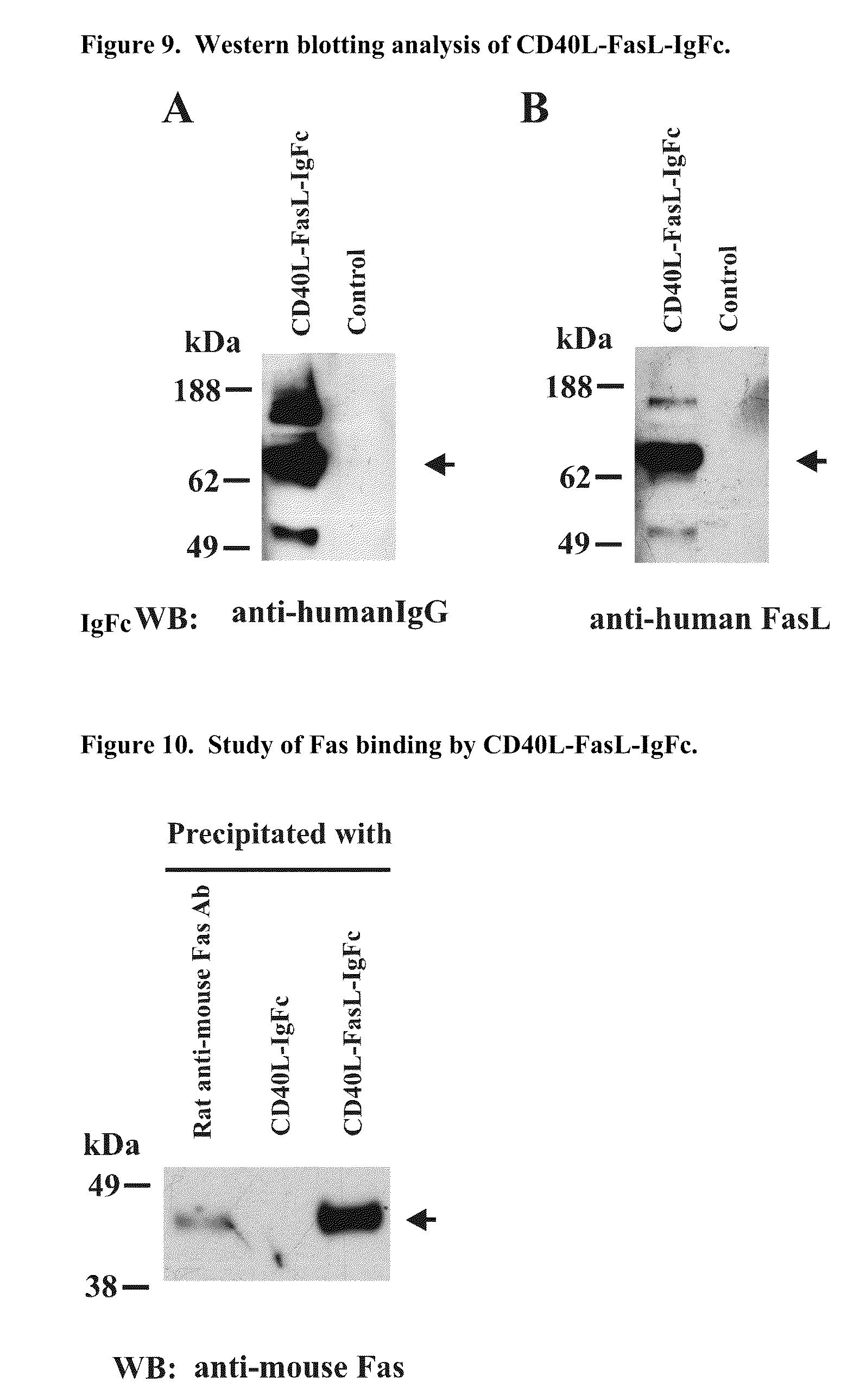
The invention relates to cell stimulatory fusion proteins and DNA sequences, vectors comprising at least two agonists of TNF / TNFR super family, immunoglobulin super family, cytokine family proteins and optional antigen combination. Instructions for use of these proteins and DNA constructs as immune adjuvants and vaccines for treatment of various chronic diseases such as viral infection are also provided. Additionally, the use of these protein and DNA constructs as immune suppressant for treatment of various chronic diseases, such as autoimmunity and organ transplant rejection, is also illustrated.
Owner:OCHI ATSUO
Tolerogenic synthetic nanocarriers for regulating innate immune responses
InactiveUS20120276160A1Suppressing antigen-specific activationReduce in quantityOrganic active ingredientsPowder deliveryB cellAntigen specific
Disclosed are synthetic nanocarrier methods, and related compositions, comprising administering B cell and / or MHC Class II-restricted epitopes of an antigen and immunosuppressants in order to reduce antigen-specific activation of innate immune cells.
Owner:SELECTA BIOSCI
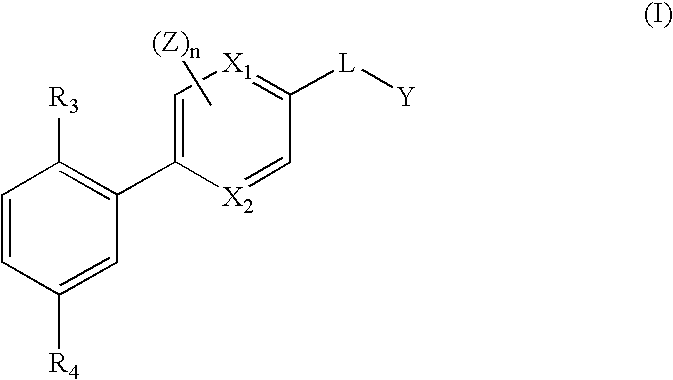
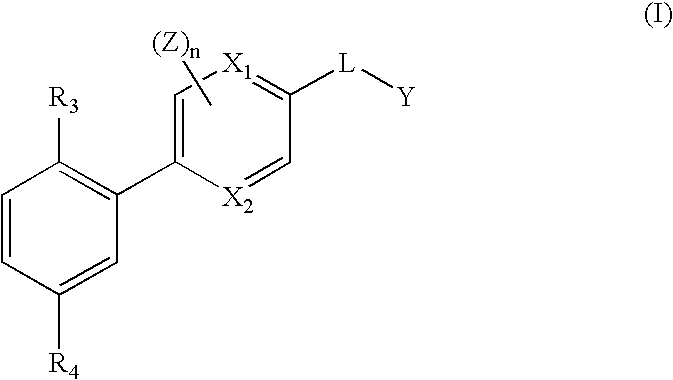
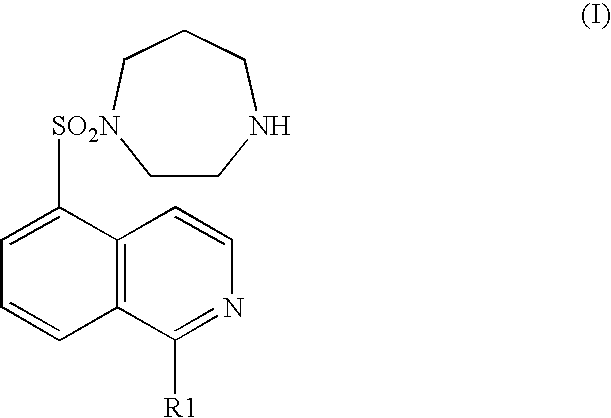
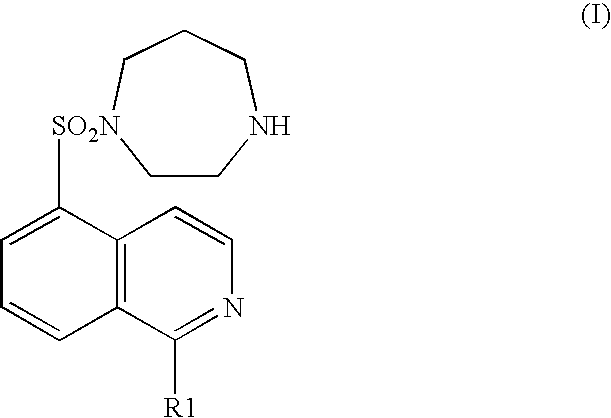
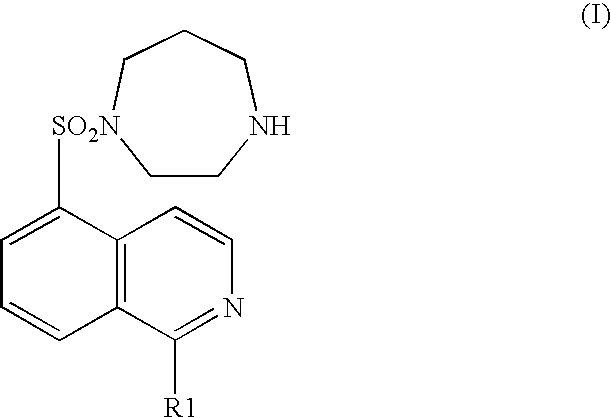
Pyridylphenyl compounds for inflammation and immune-related uses
The invention relates to compounds of structural formula (I): or a pharmaceutically acceptable salt, solvate, clathrate, or prodrug thereof, wherein Y, L, X1, X2, Z, R3, R4, and n are defined herein. These compounds are useful as immunosuppressive agents and for treating and preventing inflammatory conditions, allergic disorders, and immune disorders.
Owner:SYNTA PHARMA CORP
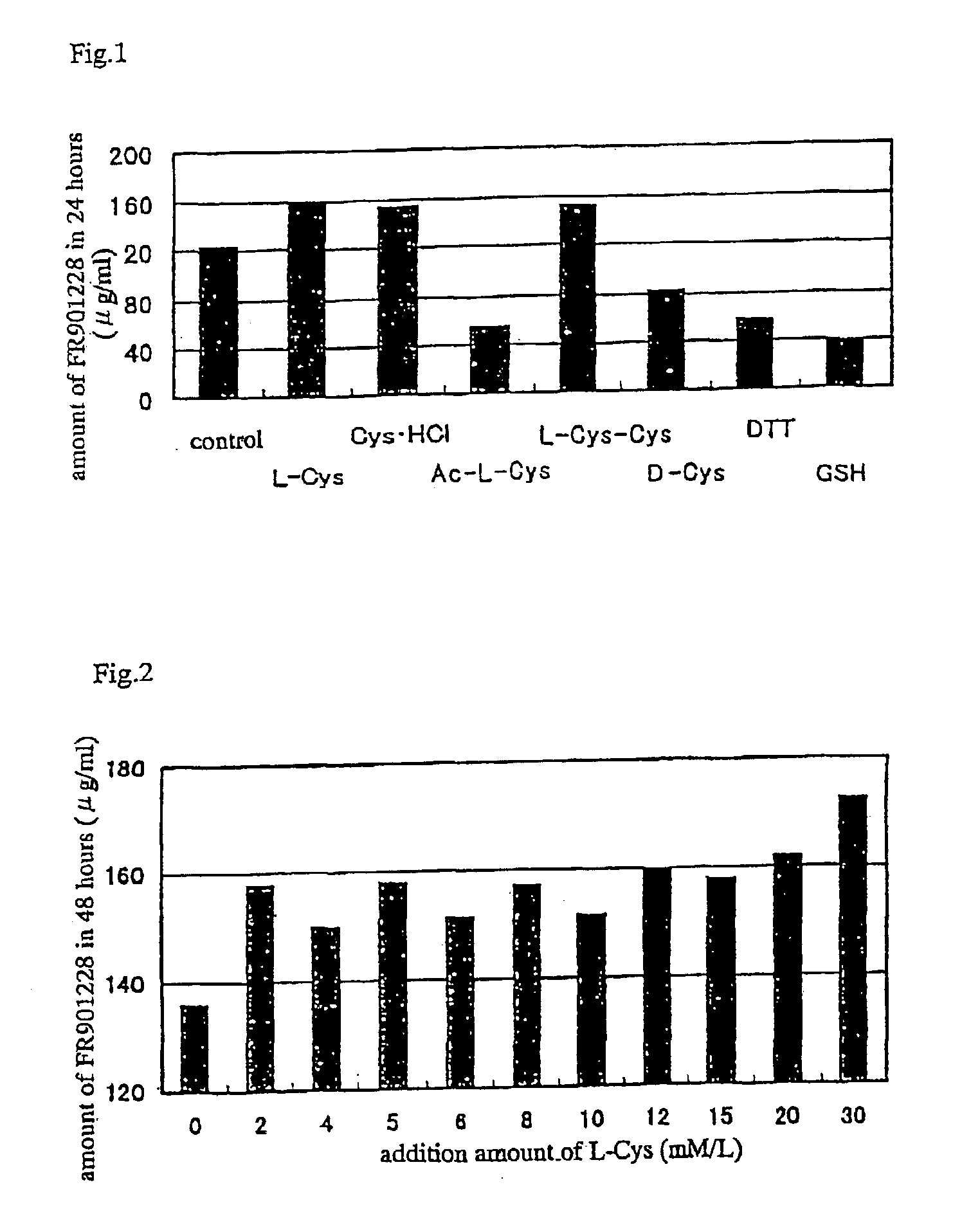
Method of producing FR901228
InactiveUS7396665B2Increase productionAntibacterial agentsMicroorganism based processesIMMUNE SUPPRESSANTSDipeptide
Owner:ASTELLAS PHARMA INC
Tolerogenic synthetic nanocarriers for inducing regulatory b cells
InactiveUS20120276133A1Reduce productionPowder deliveryOrganic active ingredientsEpitopeIMMUNE SUPPRESSANTS
Disclosed are synthetic nanocarrier methods, and related compositions, comprising B cell and / or MHC Class II-restricted epitopes and immunosuppressants in order to generate tolerogenic immune responses, such as the generation of antigen-specific regulatory B cells.
Owner:SELECTA BIOSCI
Pharmaceutical ointment formulations
A pharmaceutical ointment composition is provided comprising (a) a therapeutically effective amount of one or more active pharmaceutical ingredients comprising one or more macrolide related immunosuppressants or pharmaceutically acceptable salts or esters thereof; (b) an ointment base; and (c) an effective amount of one or more skin penetration enhancers capable of percutaneous delivery of the macrolide related immunosuppressant through the skin. Also provided is a process for its preparation and methods for delivering a macrolide related immunosuppressant or a pharmaceutically acceptable salt or ester thereof through the skin of a mammal in order to treat conditions situated on and beneath the skin.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Compositions and methods for tolerizing the immune system to allergens
InactiveUS20160263212A1Reduce development riskReduce riskHydroxy compound active ingredientsAllergen ingredientsIMMUNE SUPPRESSANTSBULK ACTIVE INGREDIENT
Compositions and methods can be used for tolerizing the immune system. The compositions can be physiologically acceptable and can include any of a wide variety of allergens that are designed to be administered in escalating doses to, for example, an infant. The compositions can include other active ingredients (e.g., one or more of a steroid, vitamin, mineral, vasodilator, hormone, decongestant, anticholinergic agent, leukotriene inhibitor, immunomodulator, mast cell stabilizer, expectorant, immune suppressant, anti-histamine, or anti-inflammatory agent) and / or a carrier.
Owner:STALLERGENES GREER PLC
Medicinal composition for prevention of or treatment for cerebrovascular disorder and cardiopathy
A pharmaceutical composition comprising at least one of components (a) and at least one of components (b) shown in below: (a) a compound represented by the general formula (I) (wherein R1 represents a hydrogen atom or a hydroxyl group) or an acid addition salt or hydrate thereof; and (b) an ameliorant of cerebral circulation, a vasodilator, a cerebral protecting drug, an brain metabolic stimulants, an anticoagulant, an antiplatelet drug, a thrombolytic drug, an amelirant of psychiatric symptom, a antihypertensive drug, an antianginal drug, a diuretic, a cardiotonic, an antiarrhythmic drug, an antihyperlipidemic drug, an immunosuppressant, or a pharmaceutically acceptable salt (except the components shown in (a)). It is useful as a preventive or remedy for cerebrovascular disorders and cardiac diseases.
Owner:ASAHI KASEI PHARMA
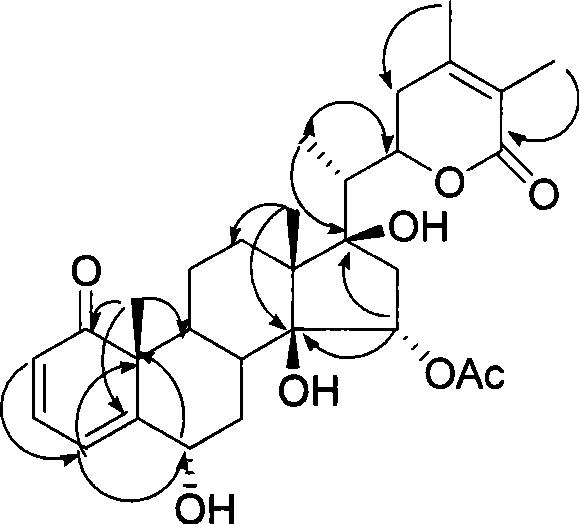
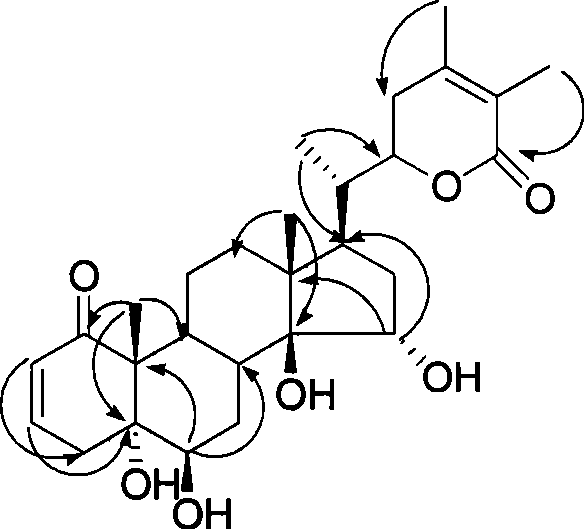
Immunosuppressant containing Withanolide type compound
InactiveCN101422467ALow toxicity immunosuppressive activitySimple separation methodOrganic active ingredientsAntipyreticDouble bondEther
The invention discloses an immunosuppressor which containing a compound shown in the following general formula I and / or the general formula II, or an optical isomer thereof; wherein, R1, R2, R3, R4, R5, R6 and R7 are selected from H, OH, O-acyl, OMe, OEt, O<n>Pr, OPr, F, Cl, Br, NH2, NO2 or CN; wherein, site 5 and site 6 are an epoxy bond or a double bond; in the general formula I, the ether linkages of site 14 and site 27 are connected or disconnected; in the general formula II, the sites 14 and 15 and the sites 16 and 17 are the epoxy bond or the double bond, or a plant extract containing the compound. The immunosuppressor has stronger immuno-suppression effect, generates repression function to T and B lymphocyte periods which are excessively proliferated, and can be used for curing the self immunological diseases and relevant diseases.
Owner:EAST CHINA UNIV OF SCI & TECH
Indoleamine 2,3-dioxygenase inhibitor and preparation method and applications thereof
The invention relates to an indoleamine 2,3-dioxygenase inhibitor with a structure represented by the formula (I) and a preparation method and applications thereof. The indoleamine 2,3-dioxygenase (IDO) inhibitor is the derivative of (Z)-N'-hydroxyl-N-phenyl formamidine, has an extremely high activity on inhibiting IDO, can effectively inhibit the activity of IDO, and can be used to inhibit the immunity inhibition of patients. The inhibitor can be widely used to treat or prevent cancer / tumor, viral infection, depression, neurodegenerative diseases, wounds, age related cataract, organ-graft refection, and autoimmune disease, and can be advantageously to be used as a novel immune-suppressor.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Heterocycle-aryl compounds for inflammation and immune-related uses
Owner:SYNTA PHARMA CORP
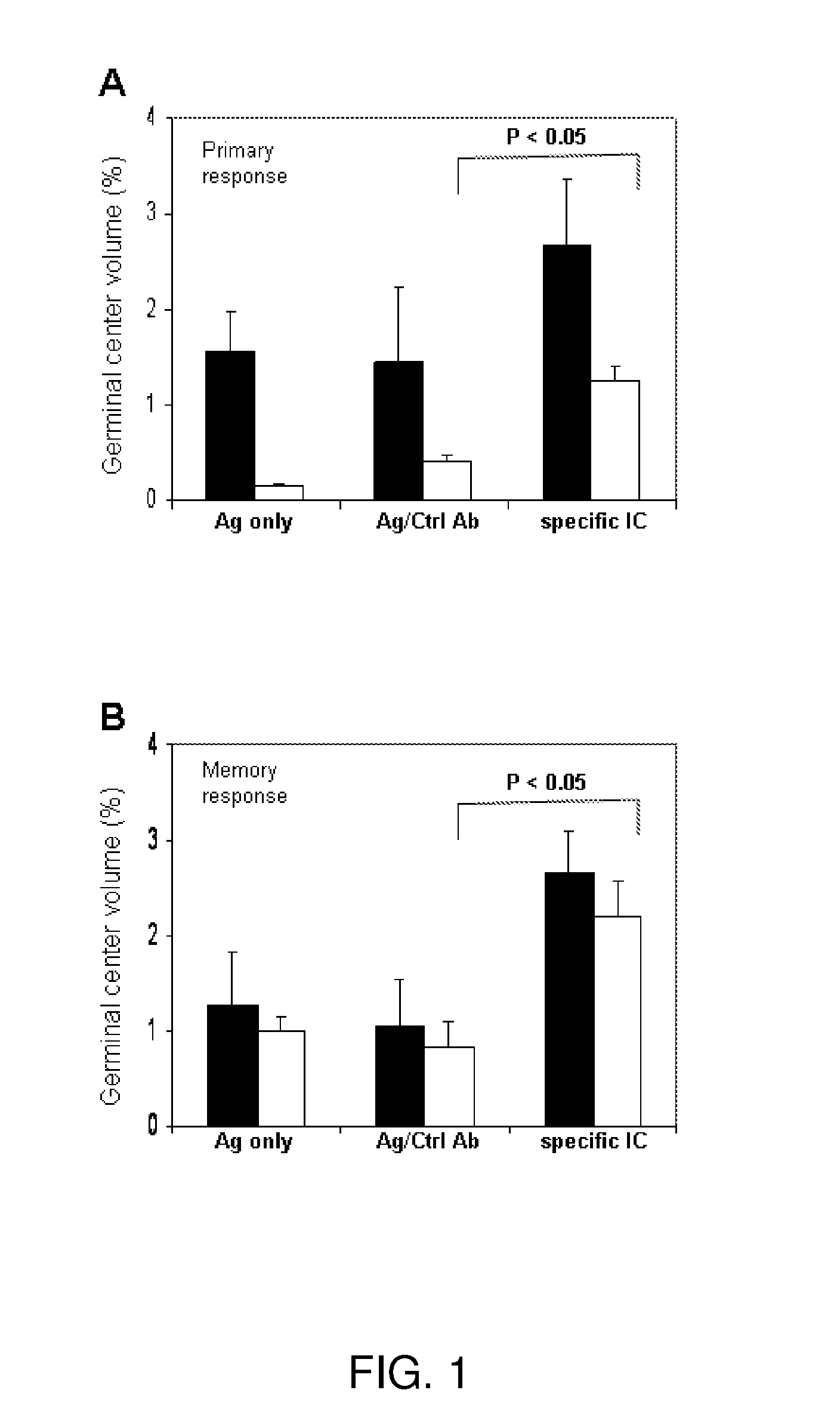
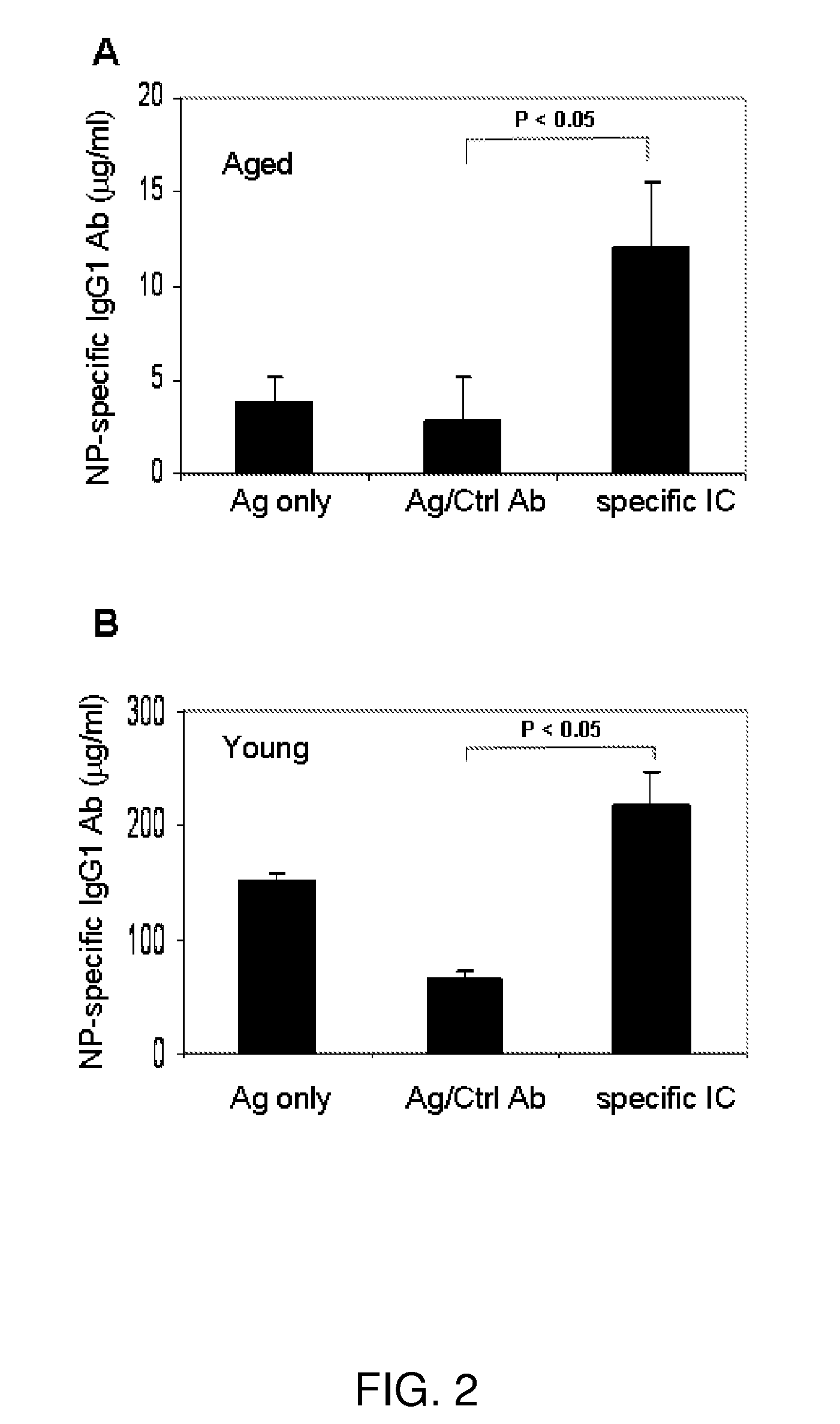
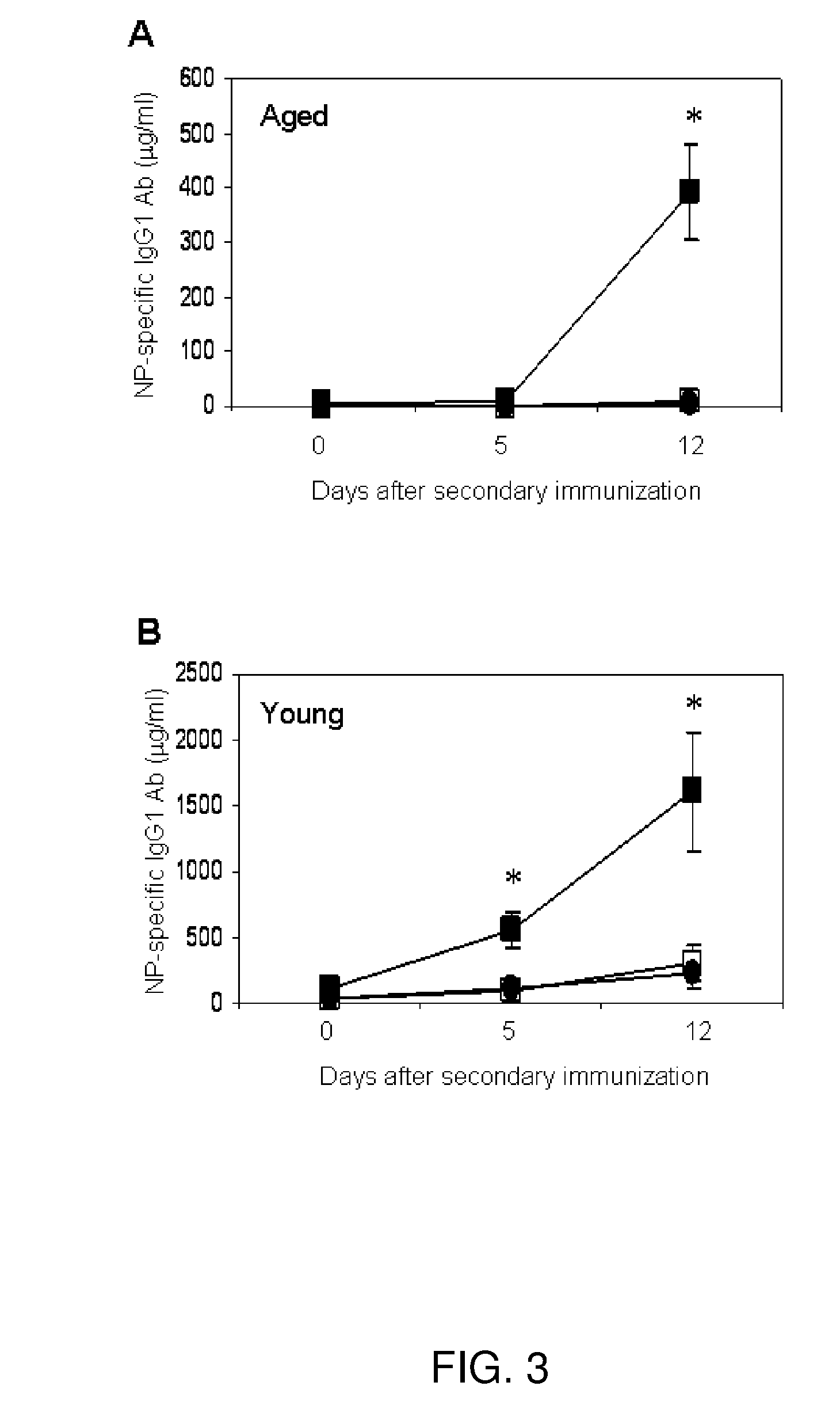
Immune complex vaccination as a strategy to enhance immunity in the elderly and other immune compromised populations
InactiveUS20080311135A1Reduced responseEnhance antibody responseAntibody ingredientsImmunoglobulinsImmune compromisedVaccination
The present invention generally concerns methods and compositions for improving the immune system of an individual that is an immune-compromised individual. In particular aspects, the immune-compromised individual is elderly or is immunosuppressed, such as from chemotherapy or immunosuppressants following organ or tissue transplantation. In specific embodiments, the invention relates to delivery to the immune-compromised individual of immune complexes harboring an antigen and an antibody that immunologically recognizes the antigen. The antigen may be viral, bacterial, or fungal, for example.
Owner:BAYLOR COLLEGE OF MEDICINE
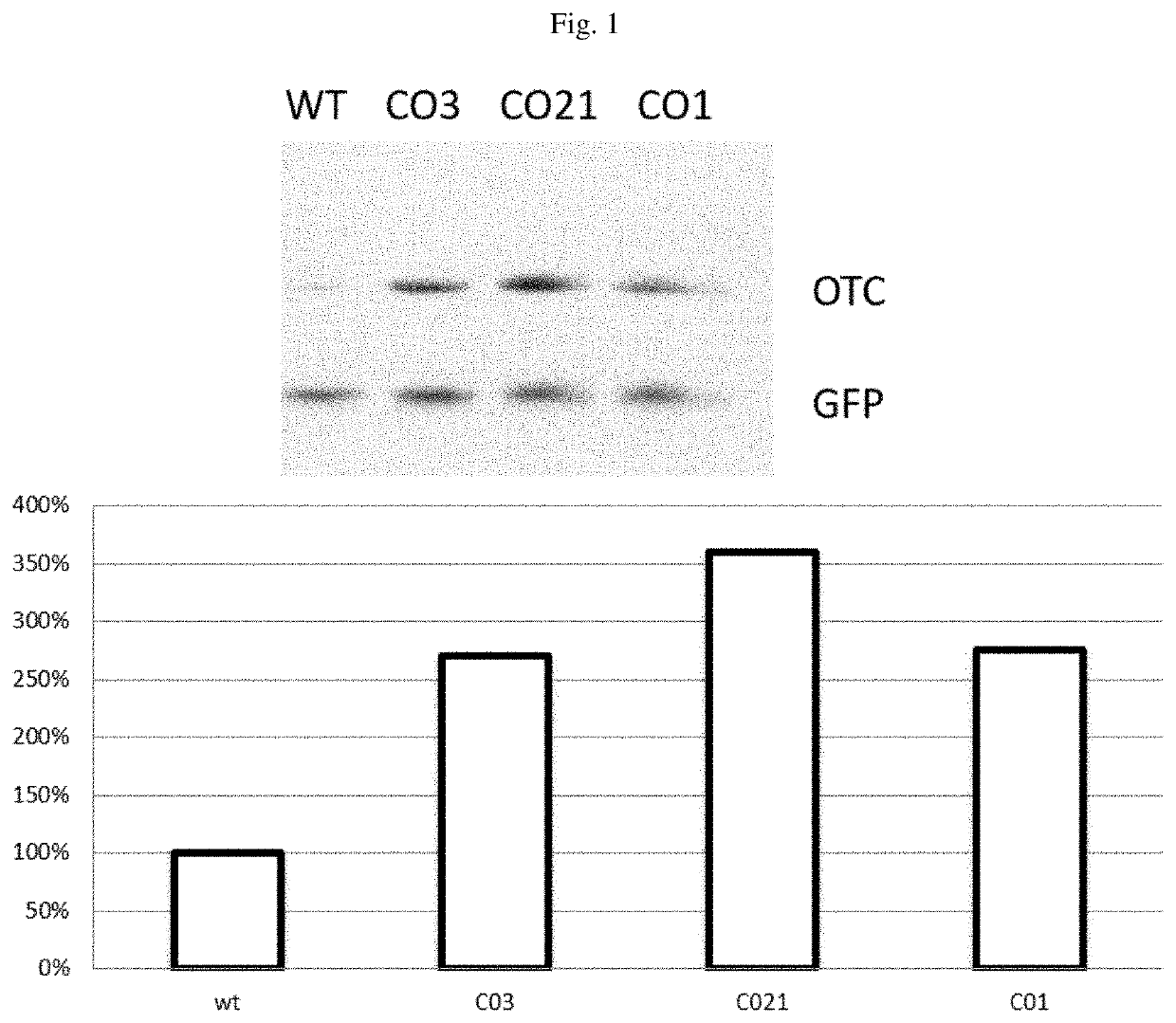
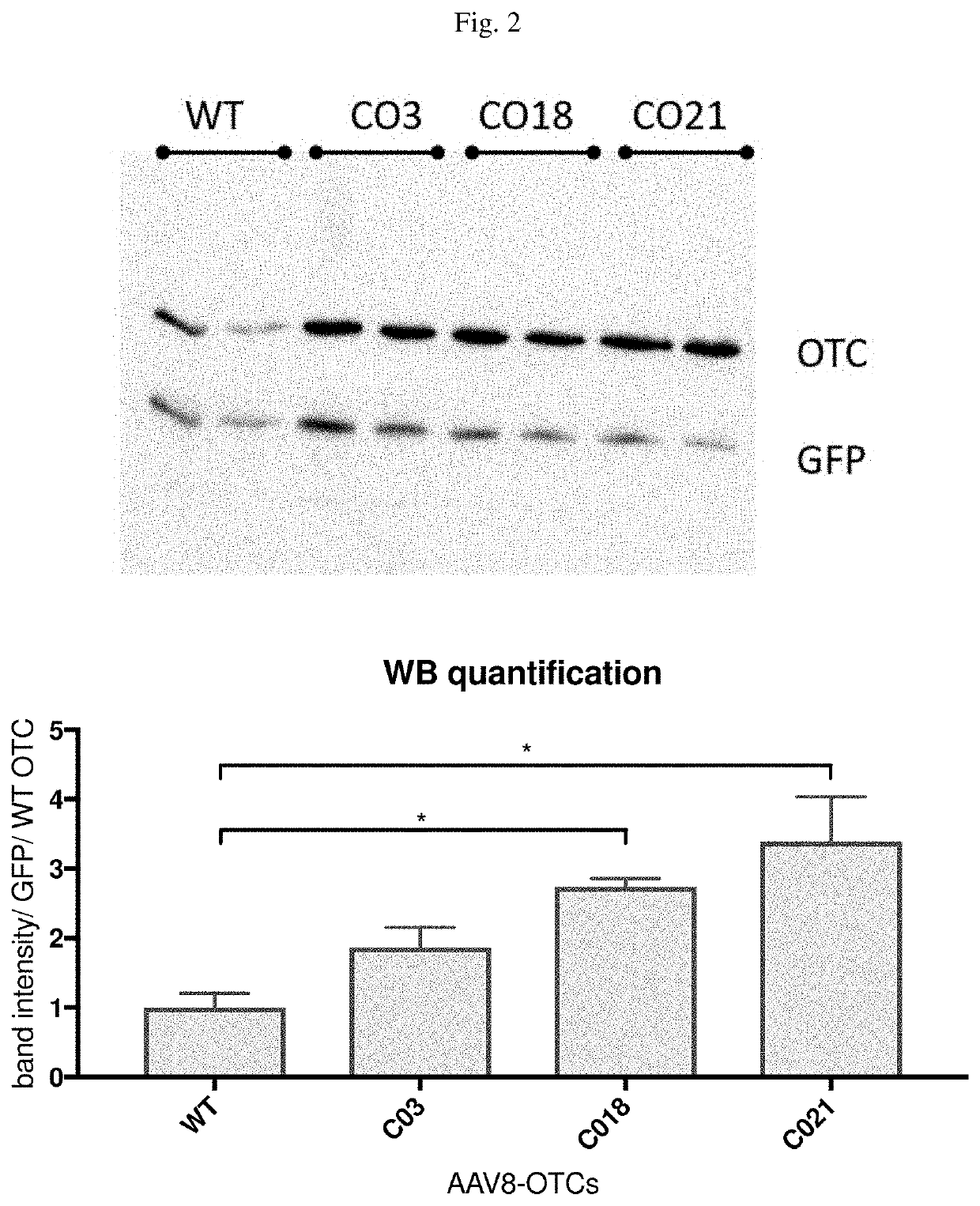
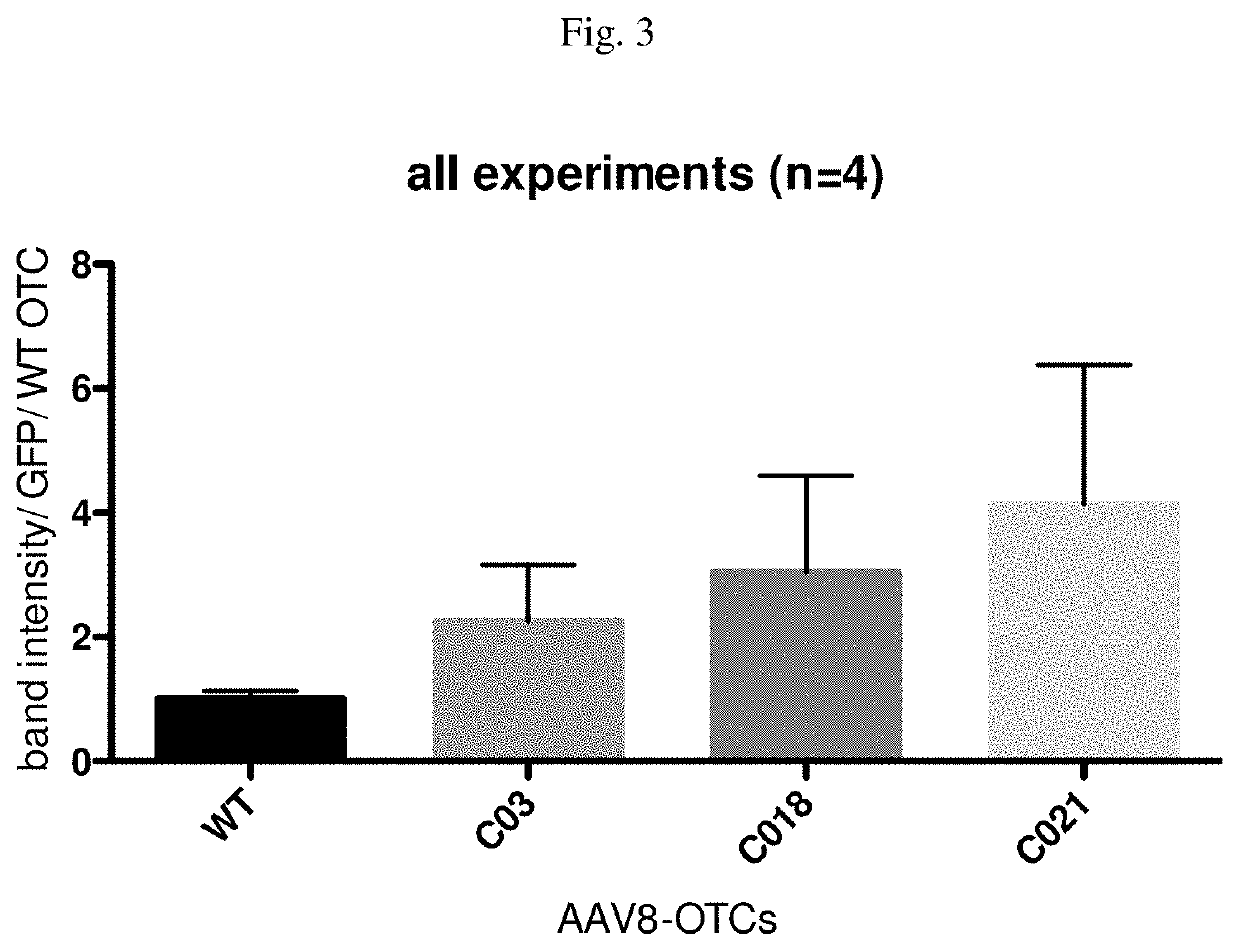
Methods and compositions of otc constructs and vectors
PendingUS20200038462A1Lower immune responseHigh expressionPowder deliveryOrganic active ingredientsIMMUNE SUPPRESSANTSNanocarriers
Provided herein are methods and compositions related to nucleic acids encoding ornithine transcarbamylase (OTC), such as nucleic acids comprising an OTC codon-optimized sequence, as well as related vectors, such as AAV vectors. Also, provided are methods for administering AAV vectors that comprise a sequence that encodes an enzyme associated with an urea cycle disorder and an expression control sequence, in combination with synthetic nanocarriers coupled to an immunosuppressant.
Owner:SELECTA BIOSCI
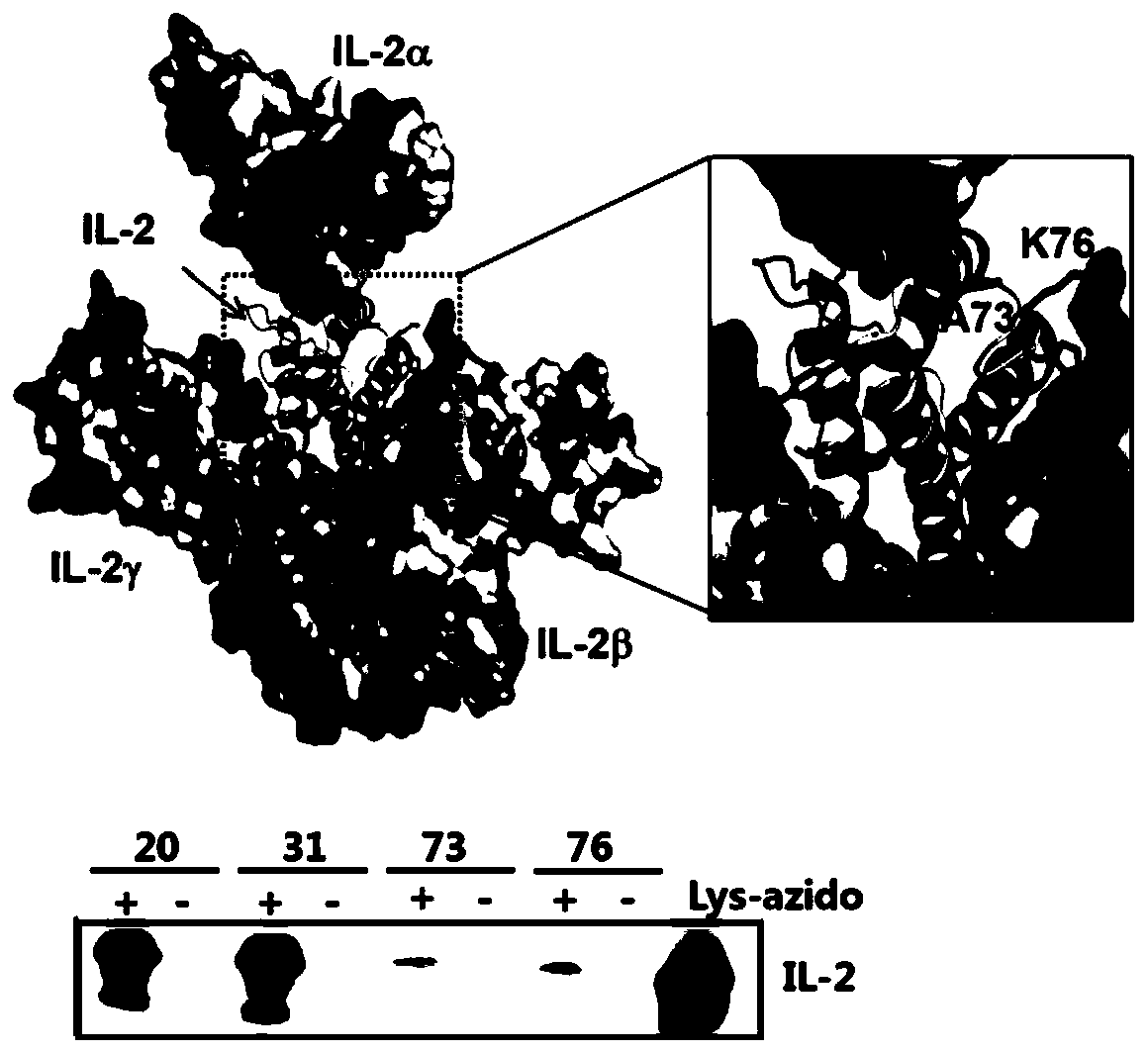
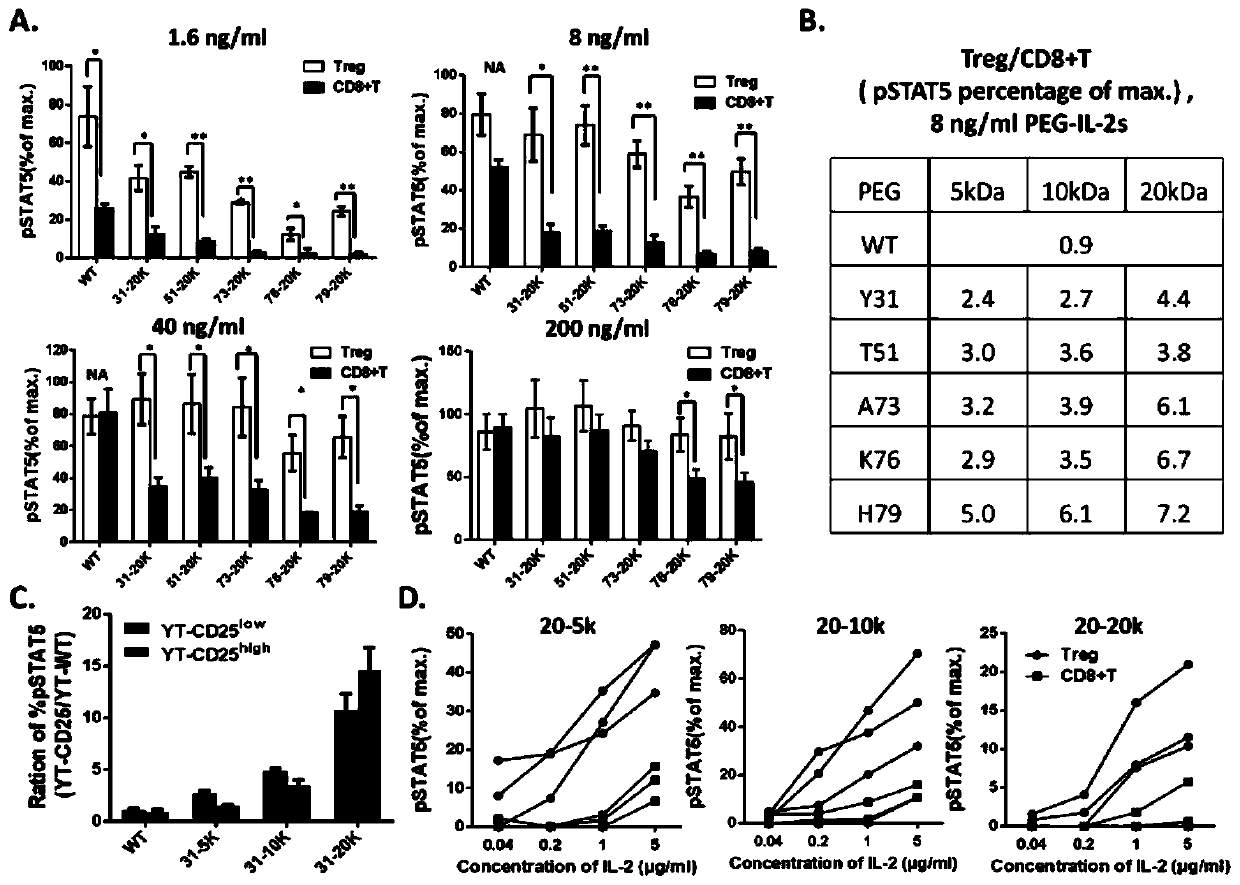
Long-acting interleukin-2 capable of realizing targeted regulation of T cell, and application of long-acting interleukin-2 for treating autoimmunity disease
ActiveCN110642934AExpanding Therapeutic WindowEffectivePeptide/protein ingredientsMetabolism disorderIMMUNE SUPPRESSANTSWhite blood cell
The invention relates to modification sites capable of enabling human interleukin-2 to carry out targeted activation on Treg, human interleukin-2 subjected to site-directed mutation on the modification sites, and human interleukin-2 subjected to site-directed modification on the modification sites. The modified long-acting interleukin-2 can carry out targeted activation regulation on T cells in awide therapeutic window, slightly and evenly does not activate other effector cells, and performs a long-acting systematic immunorepressive effect. The invention further relates to application of thecategory of site-directed mutation or modification interleukin-2, and purposes of the site-directed mutation or modification interleukin-2 for treating various autoimmunity diseases as a stable and long-acting immunosuppressor.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
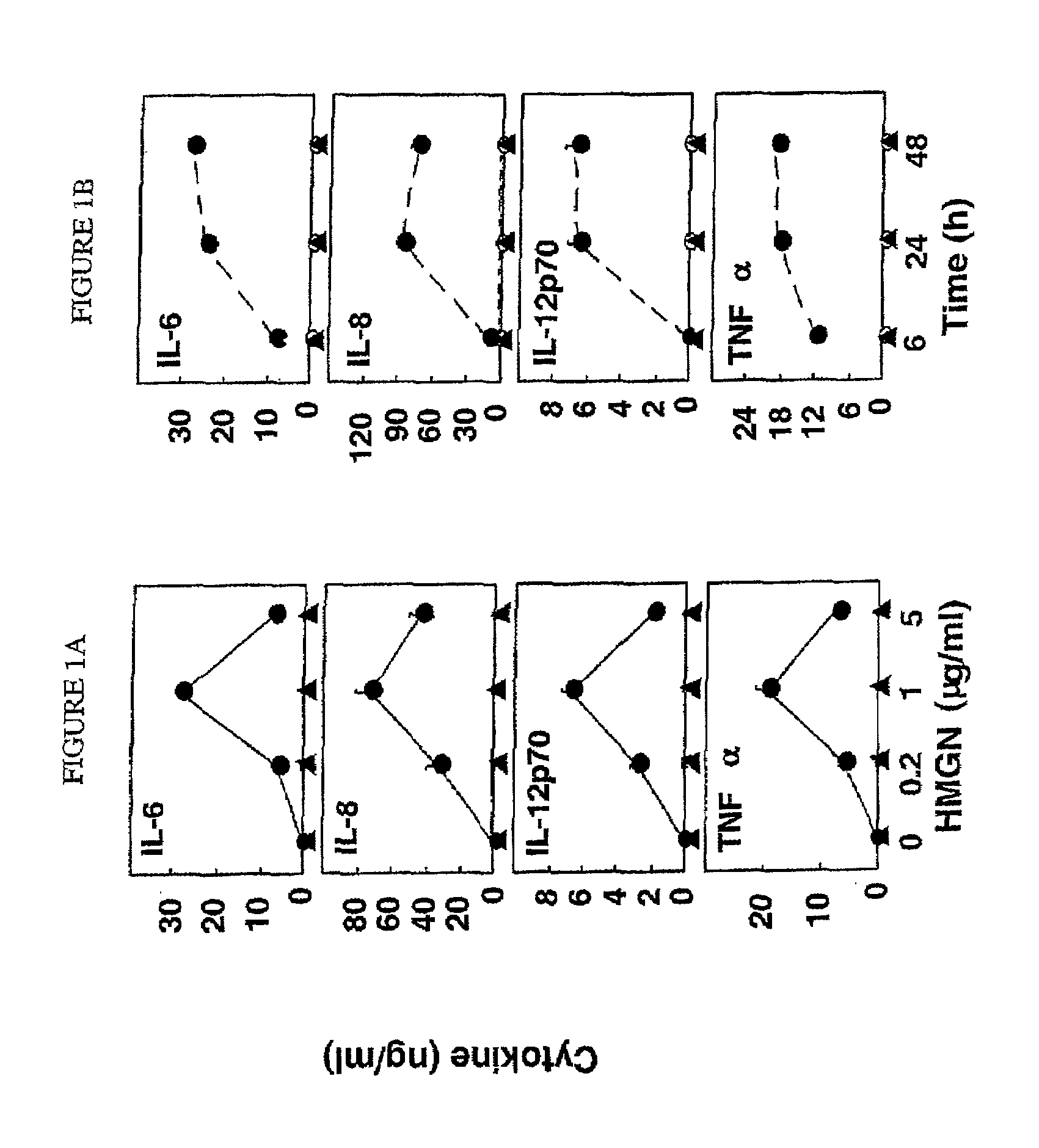
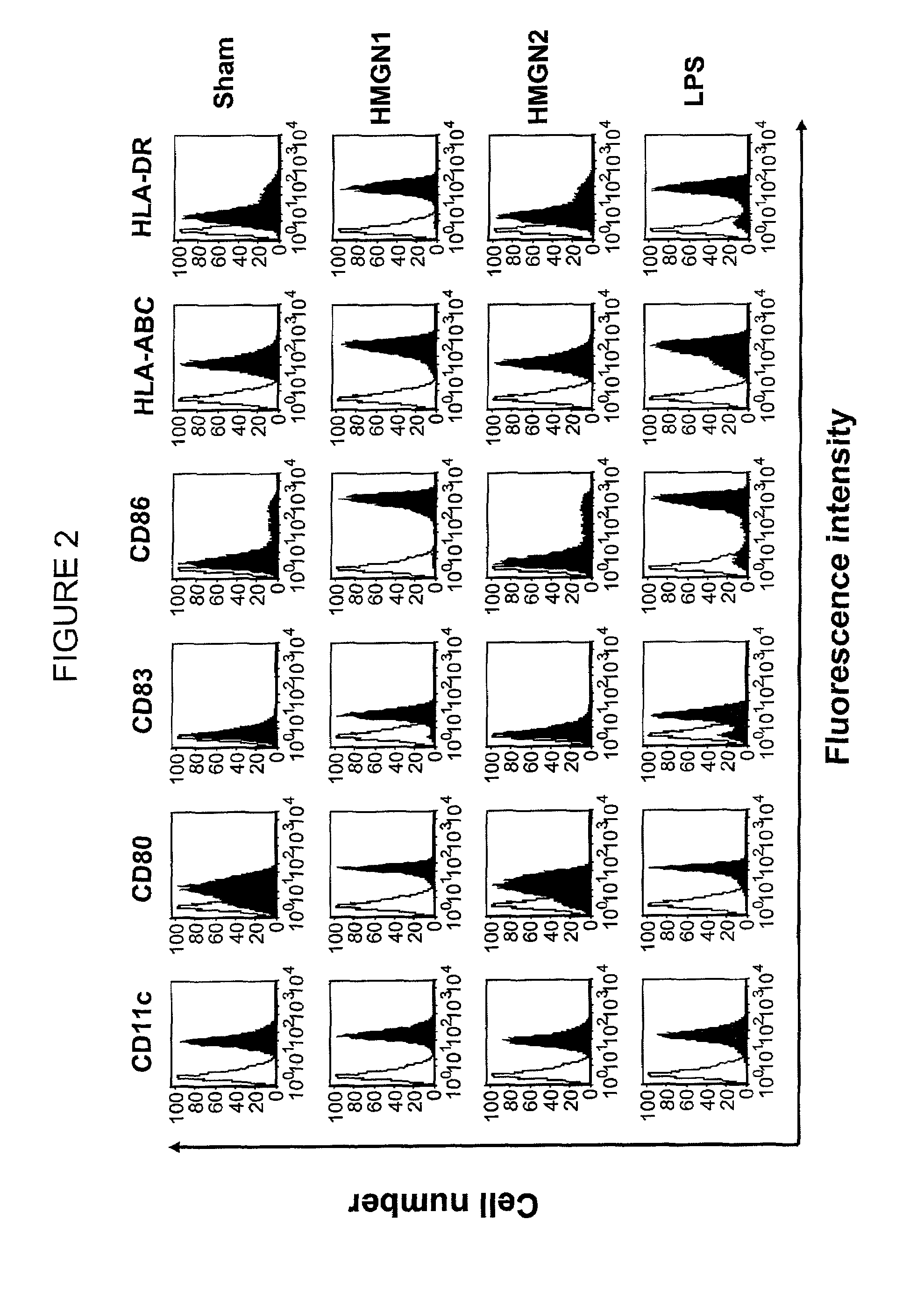
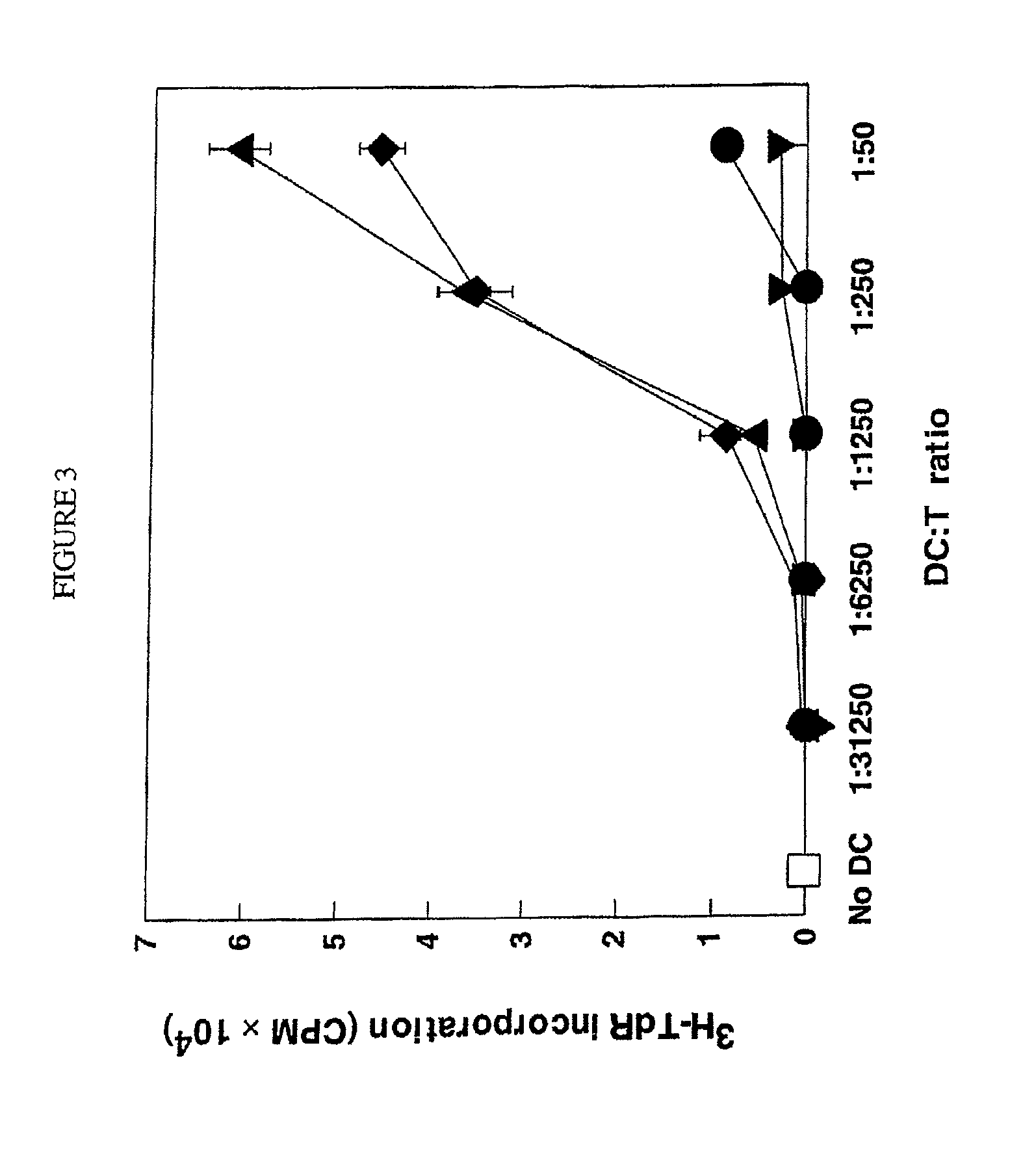
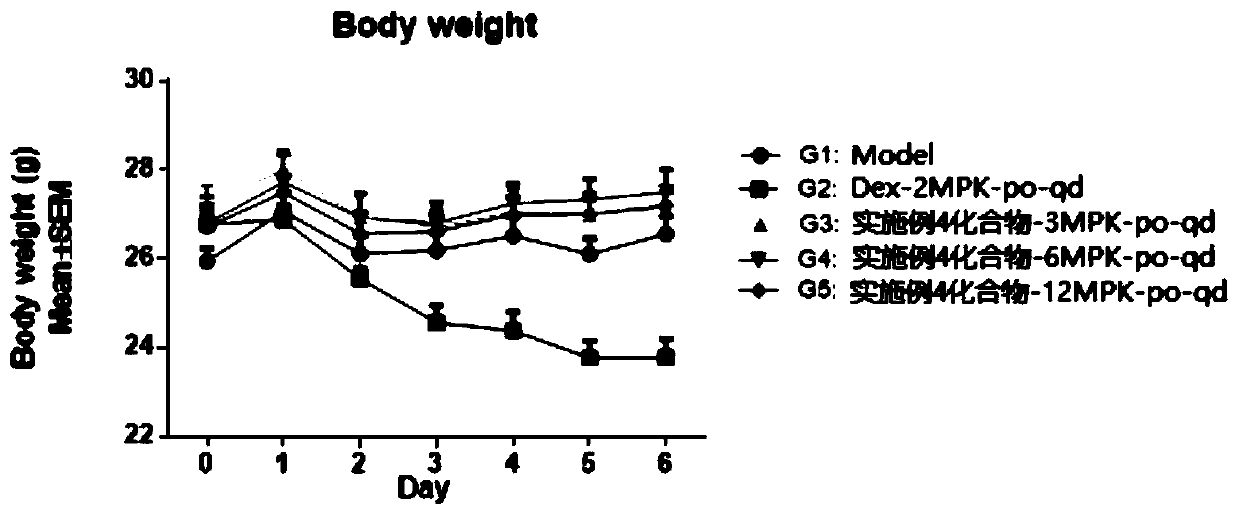
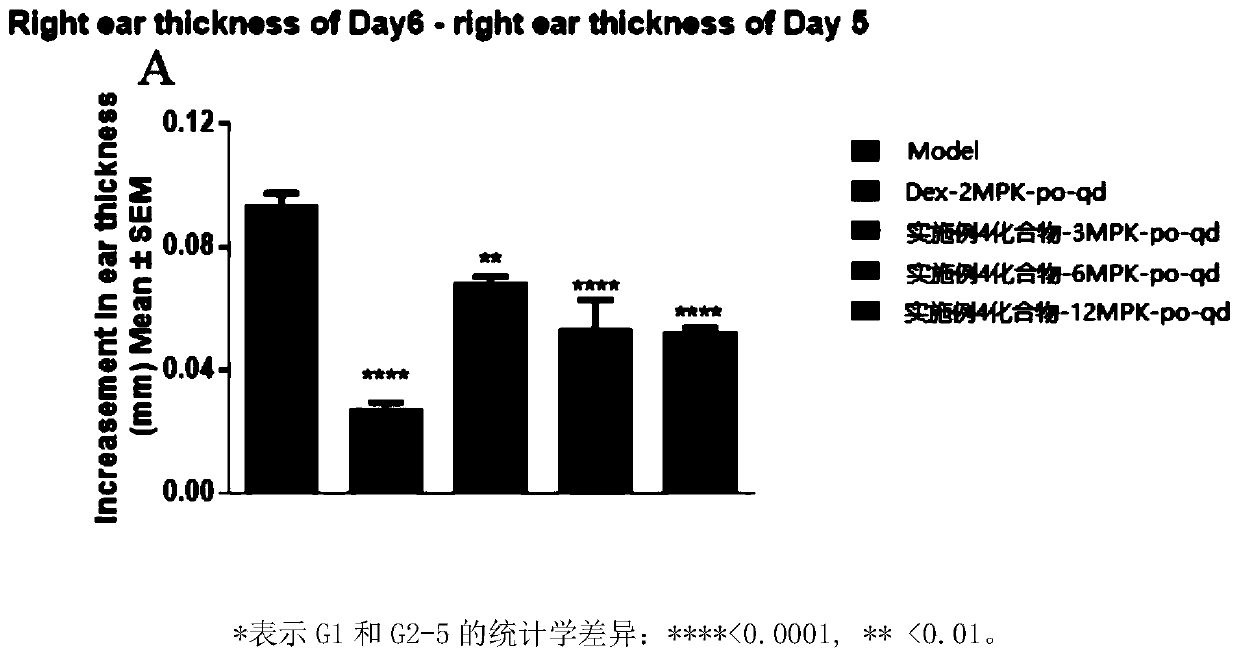
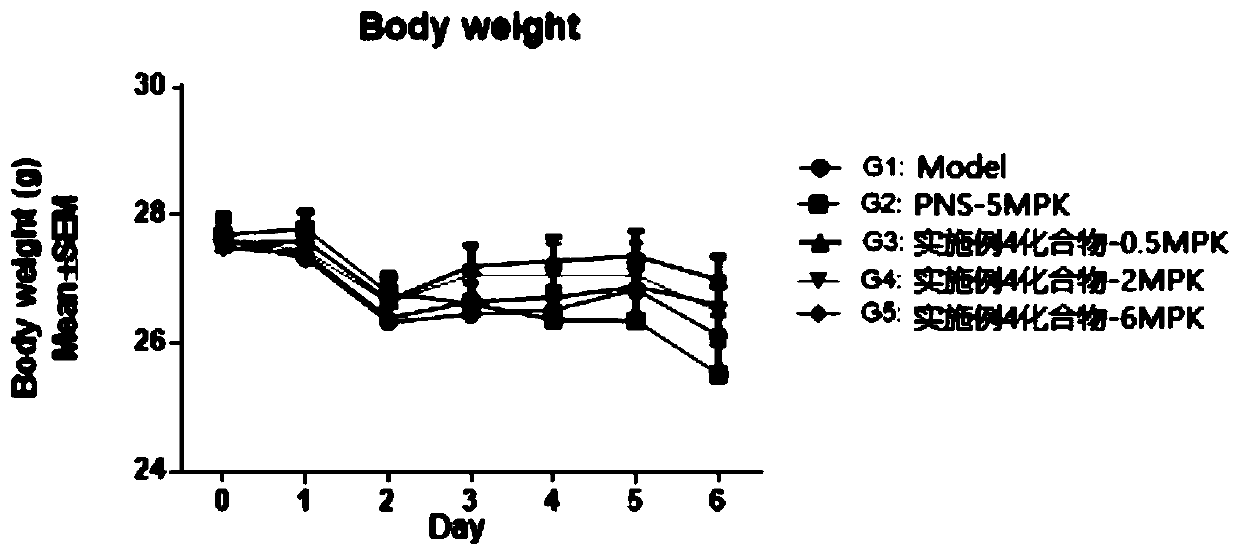
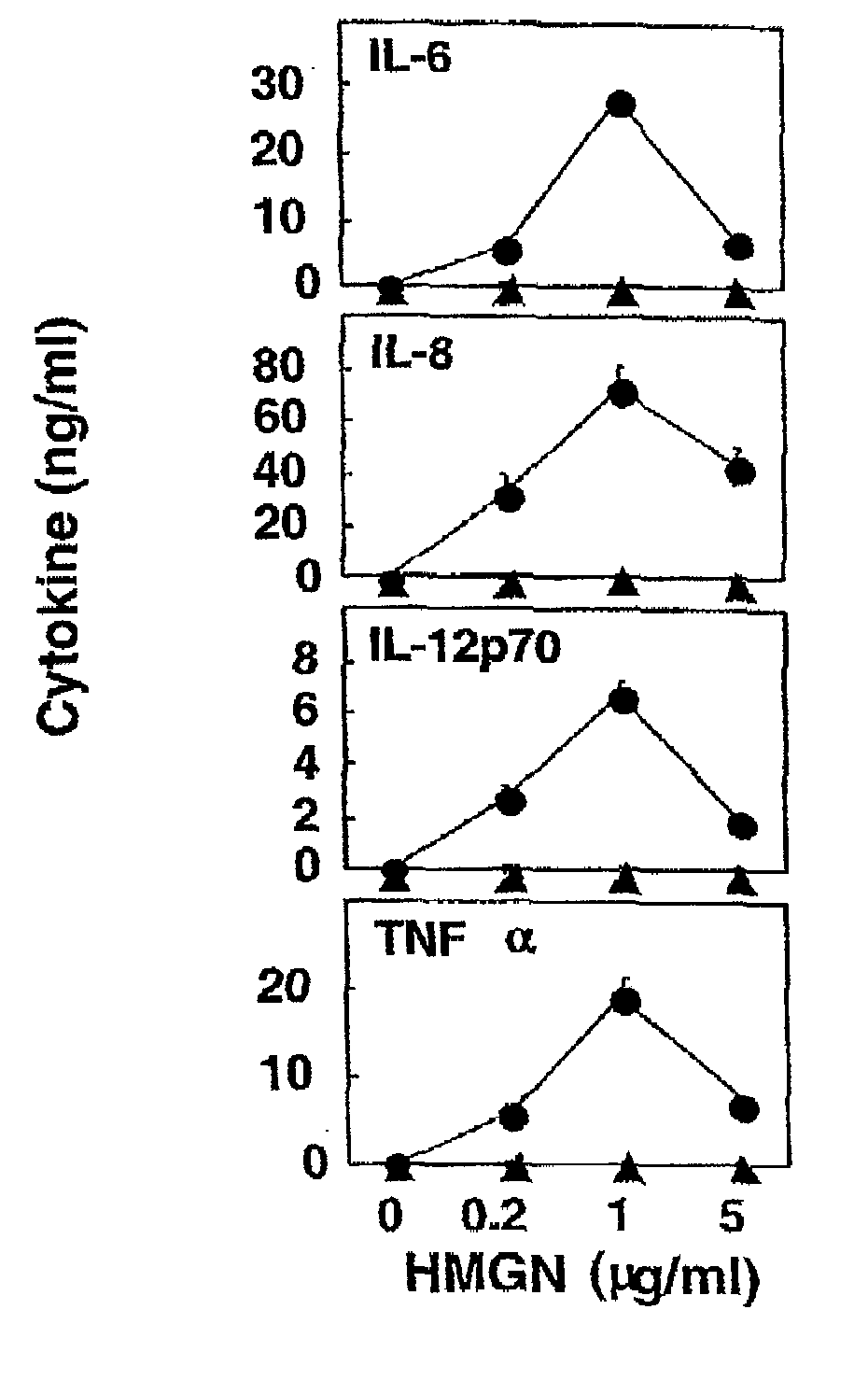
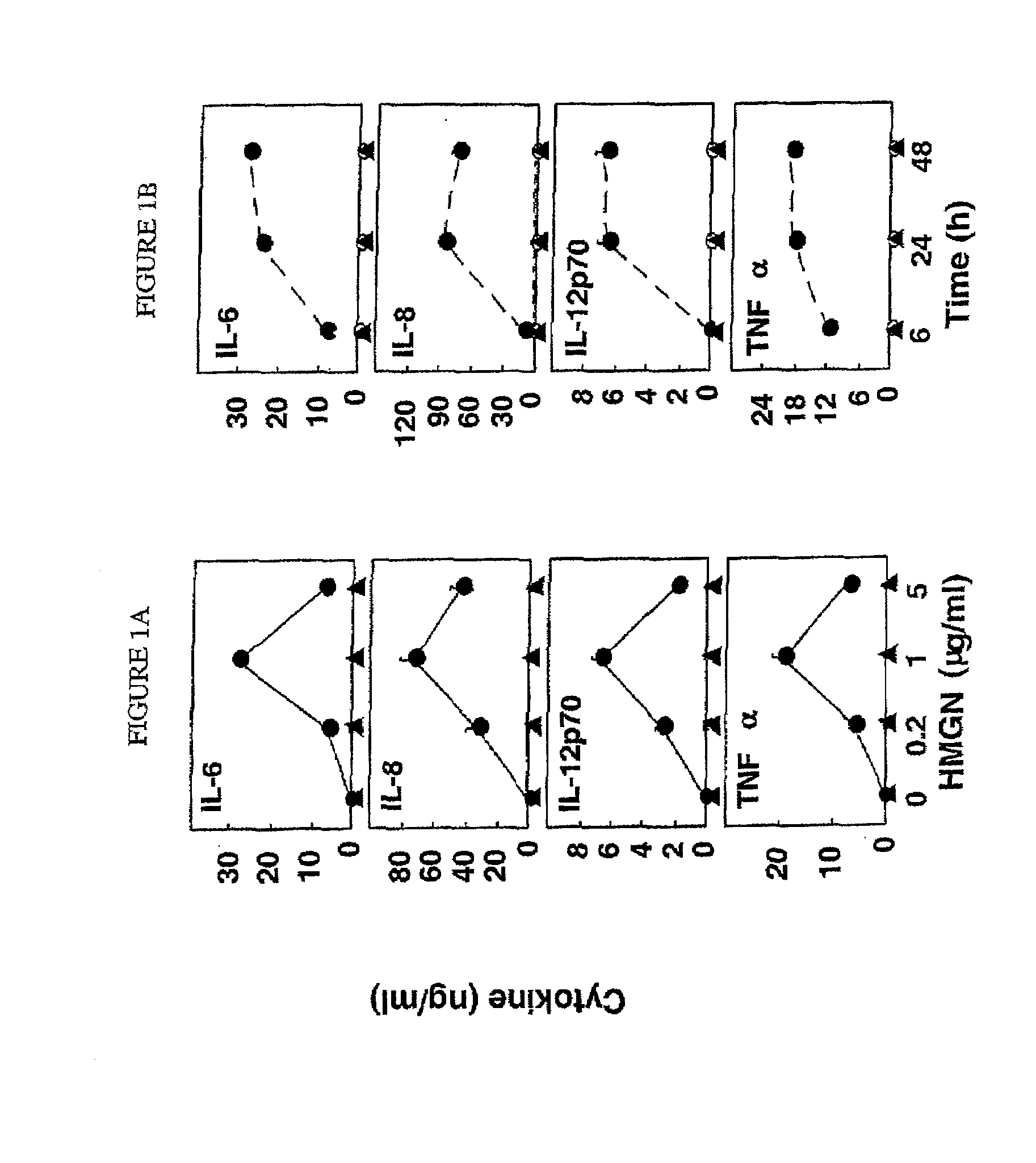
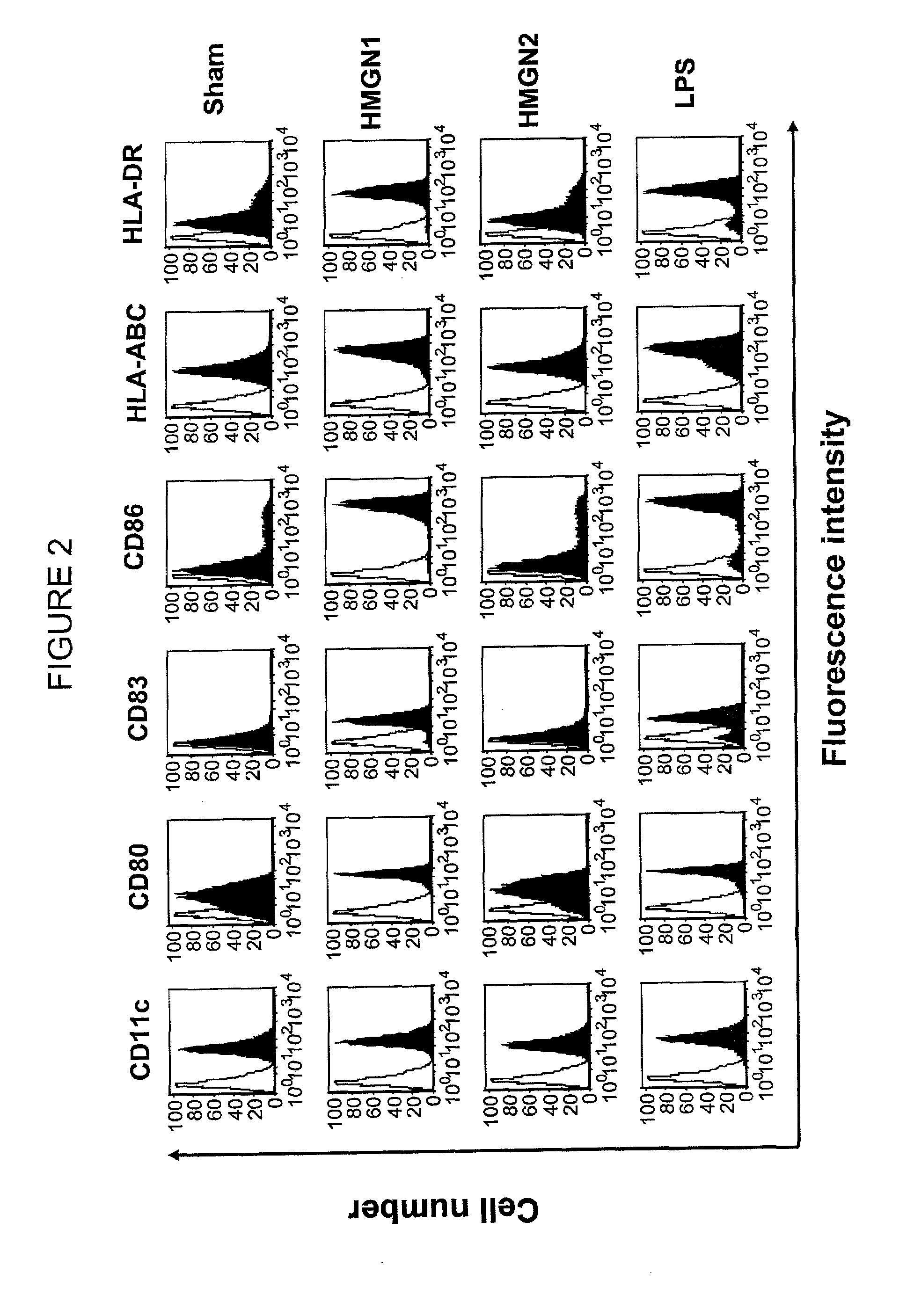
HMGN polypeptides as immune enhancers and HMGN antagonists as immune suppressants
Owner:UNITED STATES OF AMERICA
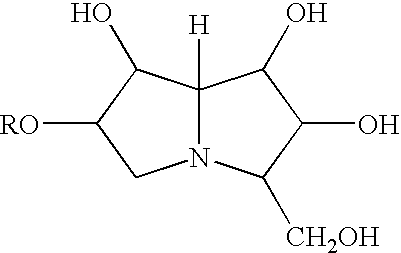
Immunomodulatory compositions
Isolated immunomodulatory (e.g. immunostimulatory) polyhydroxlated pyrrolizidine compounds having the formula are disclosed. In these compounds R is selected from hydrogen, straight or branched, unsubstituted or substituted, saturated or unsaturated acyl, alkyl (e.g. cycloalkyl), alkenyl, alkynyl and aryl groups. The compounds are useful in therapy and prophylaxis, including increasing the Th1:Th2 response ratio, hemorestoration, alleviation of immunosuppression, cytokine stimulation, treatment of proliferative disorders (e.g. cancer), vaccination, stimulation of the innate immune response and boosting of the activity of endogenous NK cells.
Owner:SUMMIT WALES +1
Treatment of graft rejection by administering a complement inhibitor to an organ prior to transplant
InactiveUS20160184391A1Easily penetrate organMinimize impactPeptide/protein ingredientsAntibody mimetics/scaffoldsIMMUNE SUPPRESSANTSAntiendomysial antibodies
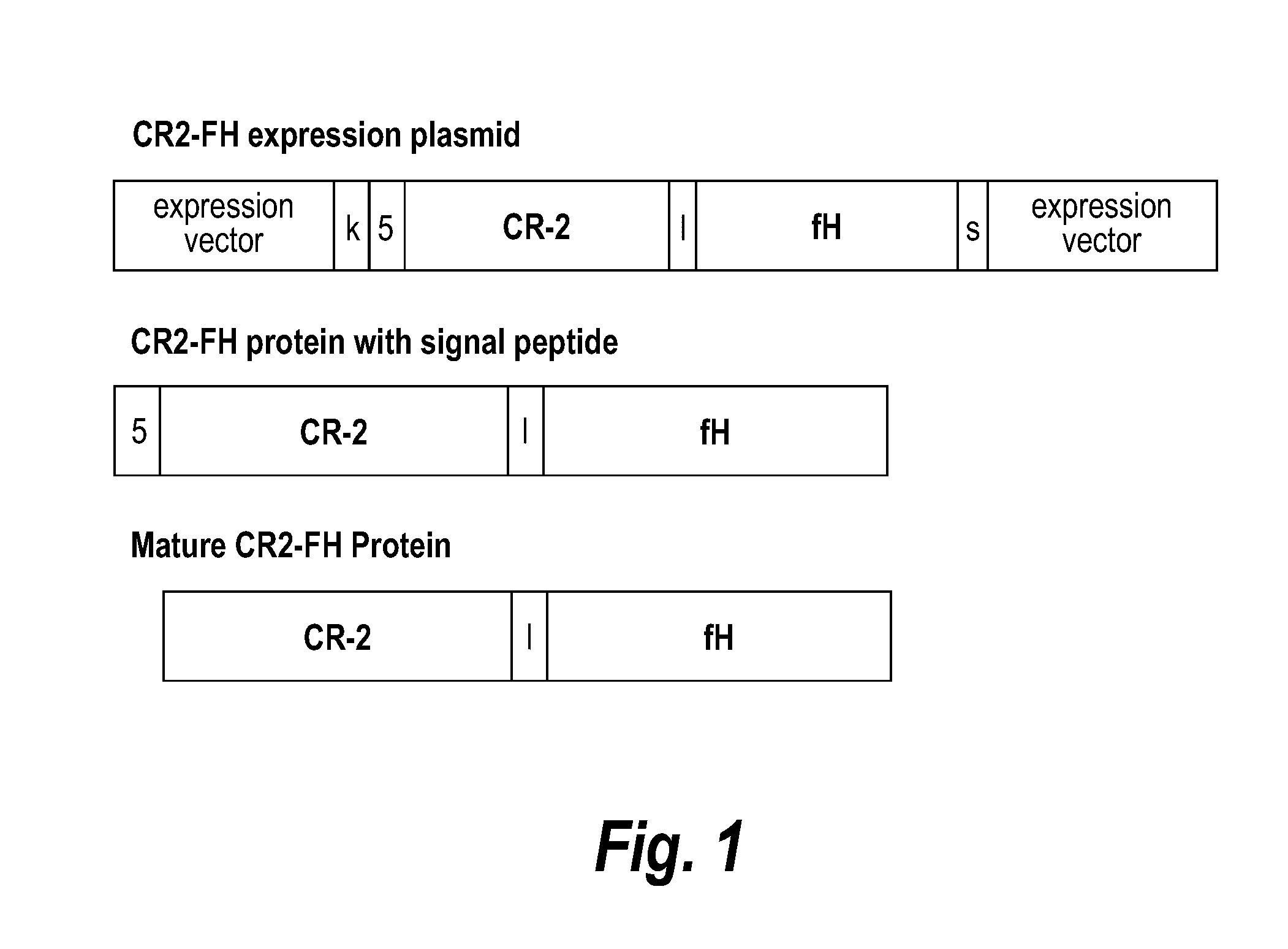
Methods of prolonging survival of a transplanted organ, as well as methods of preventing or attenuating rejection of a transplanted organ are provided. These methods involve contacting the organ with an inhibitor of complement activity (e.g., a complement inhibitor that has a maximum molecular weight of 70 kDa and / or a half-life shorter than 10 days, such as a CR2-FH fusion protein or a single chain anti-C5 antibody), prior to transplantation The methods also include administering to the allotransplant recipient an inhibitor of complement activity together with one or more immunosuppressants. A pretreatment with an alternative complement inhibitor was found to be effective in improving graft survival and decreasing ischemia-reperfusion injury in animal.
Owner:ALEXION PHARMA INC
Tetrahydroquinoline alkaloid Malaysiensin with immunosuppression activity and production method and application of tetrahydroquinoline alkaloid Malaysiensin with immunosuppression activity
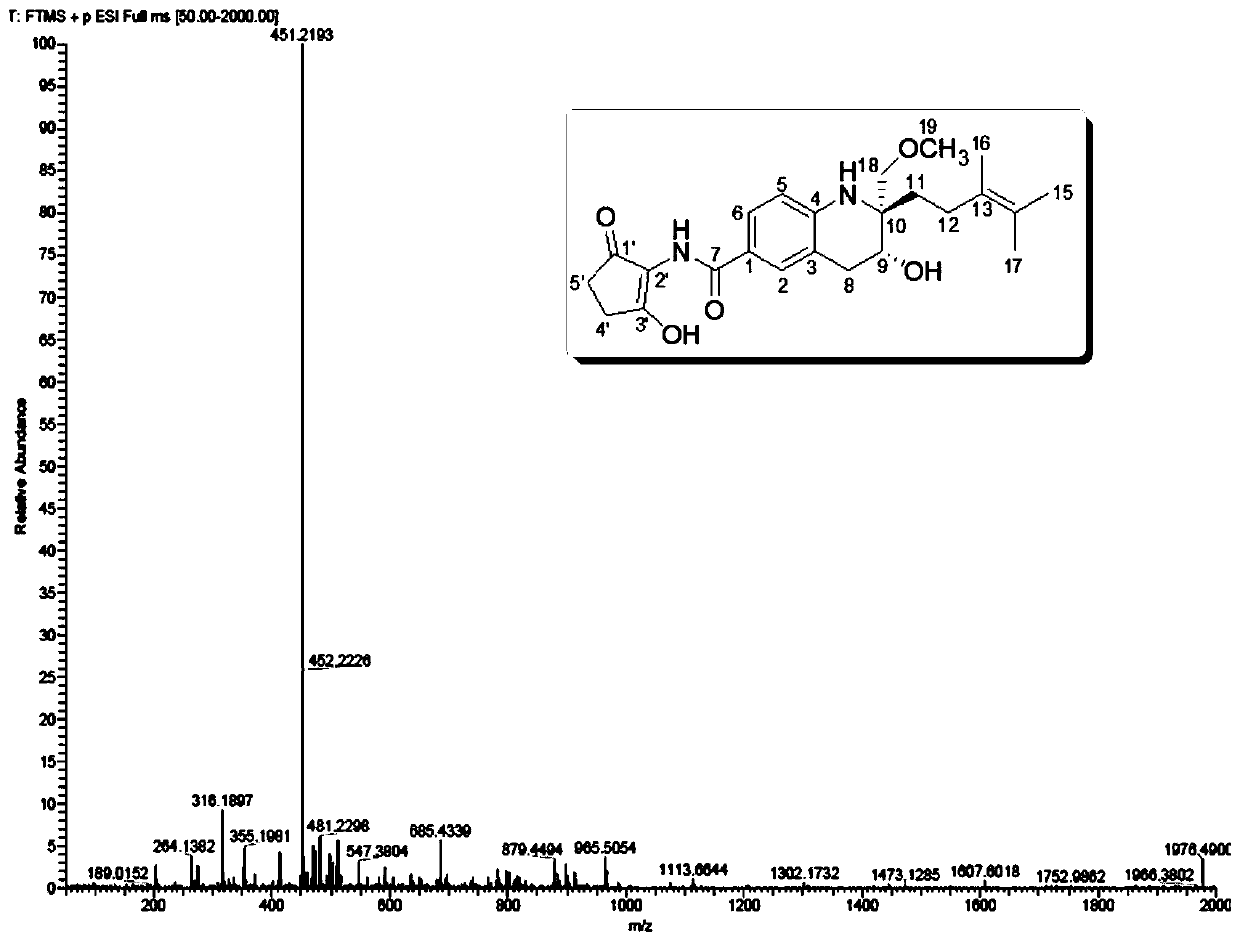
ActiveCN110724096APrevent proliferationOrganic chemistryImmunological disordersIMMUNE SUPPRESSANTSAutoimmune condition
The invention provides a tetrahydroquinoline alkaloid Malaysiensin with immunosuppression activity and a production method and application of the tetrahydroquinoline alkaloid Malaysiensin with the immunosuppression activity. The compound is obtained by conducting SFM solid medium fermentation on Streptomyces sp.DSM 4137 and separating and purifying a fermentation product. The compound can significantly inhibit ConA-induced proliferation of T cells, an immunosuppression effect of the compound is equivalent to that of an immunosuppressor cyclosporin A which is clinically and widely used, and action mechanism studies show that Malaysiensin exerts the immunosuppression activity for a targeted NFAT signal path, is an efficient immunosuppressor, and can be used for producing immunosuppressive medicines for organ transplantation and autoimmune diseases.
Owner:HAINAN UNIVERSITY
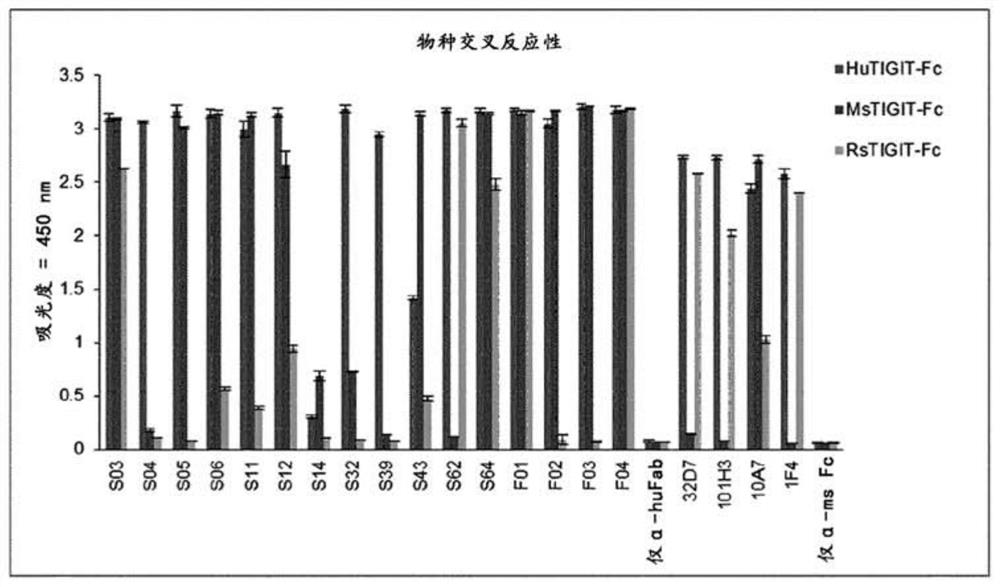
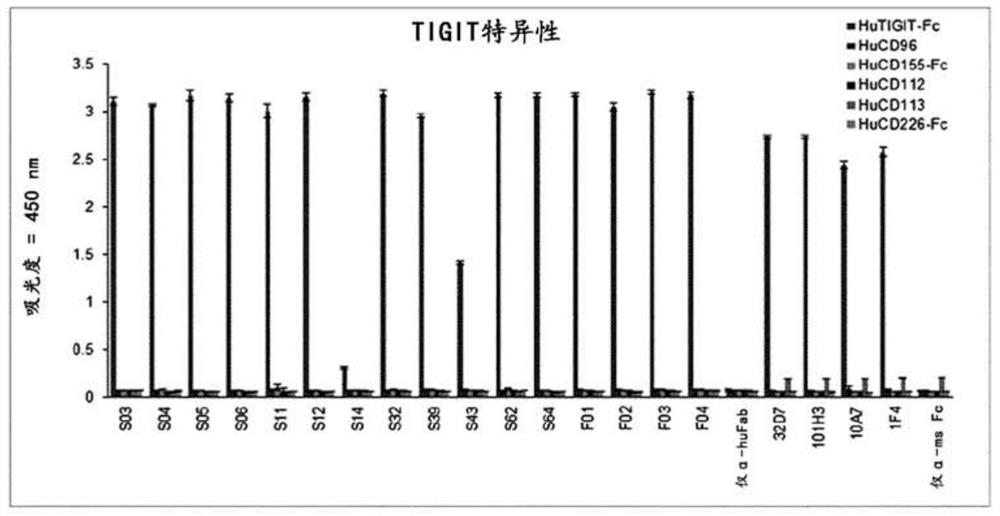
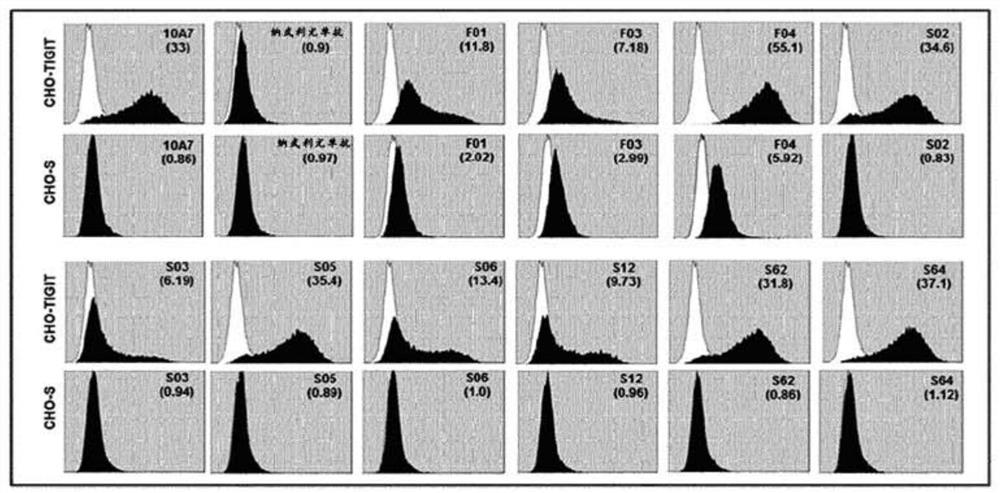
Anti-tigit antibodies and uses thereof
PendingCN112154155AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsIMMUNE SUPPRESSANTSAntigen Binding Fragment
Disclosed are a novel antibody specifically binding to the tumor-immunosuppressant, TIGIT (T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif [ITIM] domain) or an antigen-bindingfragment thereof, a nucleic acid encoding the antibody or the antigen-binding fragment thereof, a vector and a host cell including the nucleic acid, a method for producing the antibody or the antigen-binding fragment thereof, a pharmaceutical composition containing the antibody or the antigen-binding fragment thereof as an active ingredient, and uses of the pharmaceutical composition. The antibody specifically binding to TIGIT or the antigen-binding fragment thereof and the pharmaceutical composition containing the same as an active ingredient are preferably used for the treatment of cancer or tumors.
Owner:YUHAN
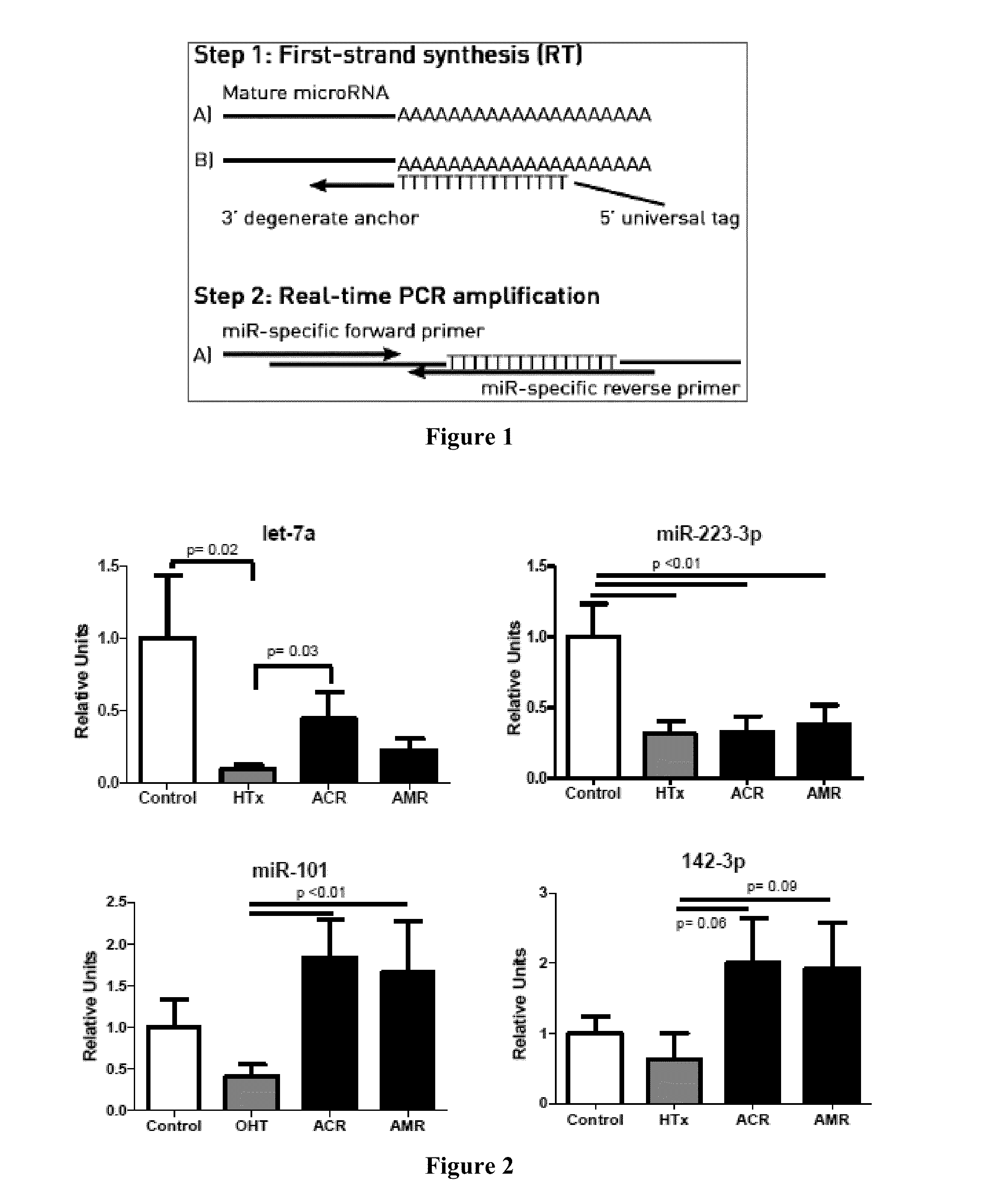
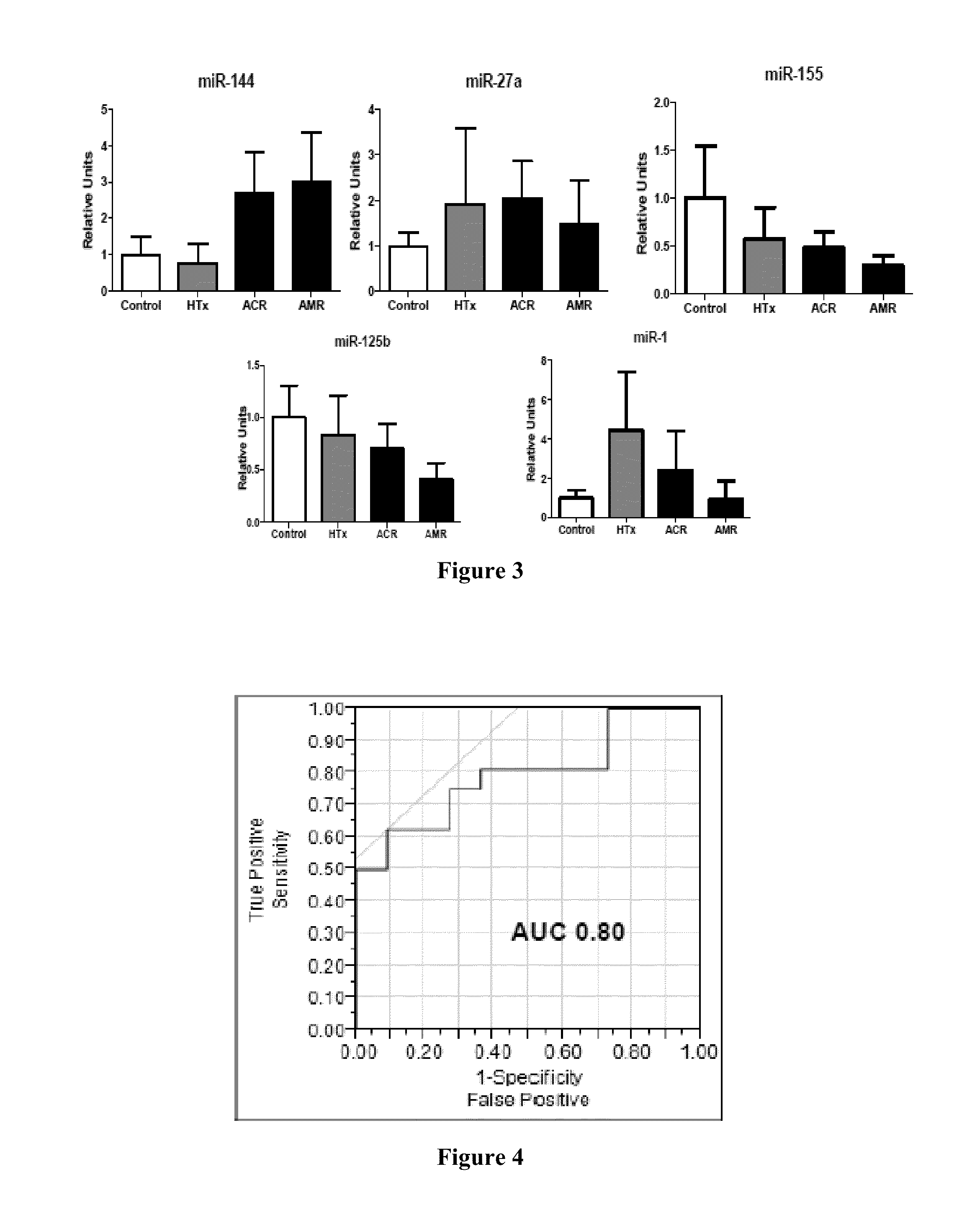
Circulating Non-coding RNA Profiles for Detection of Cardiac Transplant Rejection
InactiveUS20160138106A1Nucleotide librariesMicrobiological testing/measurementTransplant rejectionNon-coding RNA
The level of miRNAs in a sample from a patient who has received a transplant is assayed and used as an indicator for transplant rejection. Based on the measured level of the miRNAs, therapeutic intervention, such as an immunosuppressant therapy, may be started, adjusted, continued or discontinued.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods and compositions for attenuated Anti-viral transfer vector immune response
PendingUS20200390718A1High expressionOrganic active ingredientsDsDNA virusesIMMUNE SUPPRESSANTSNanocarriers
Owner:SELECTA BIOSCI
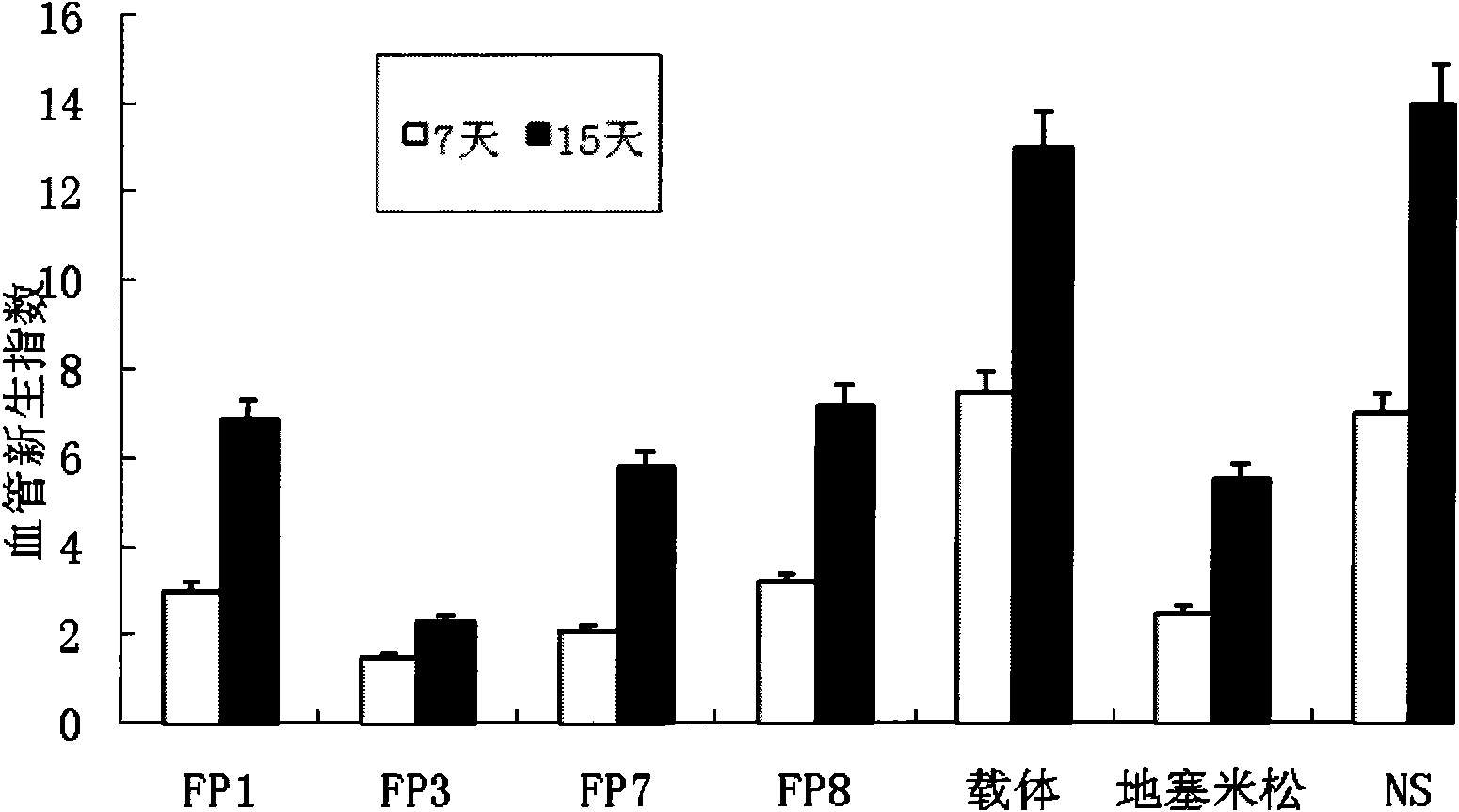
Application of VEGF acceptor fusion proteins in preparation of drugs for inhibiting growth of ocular surface neovascularization
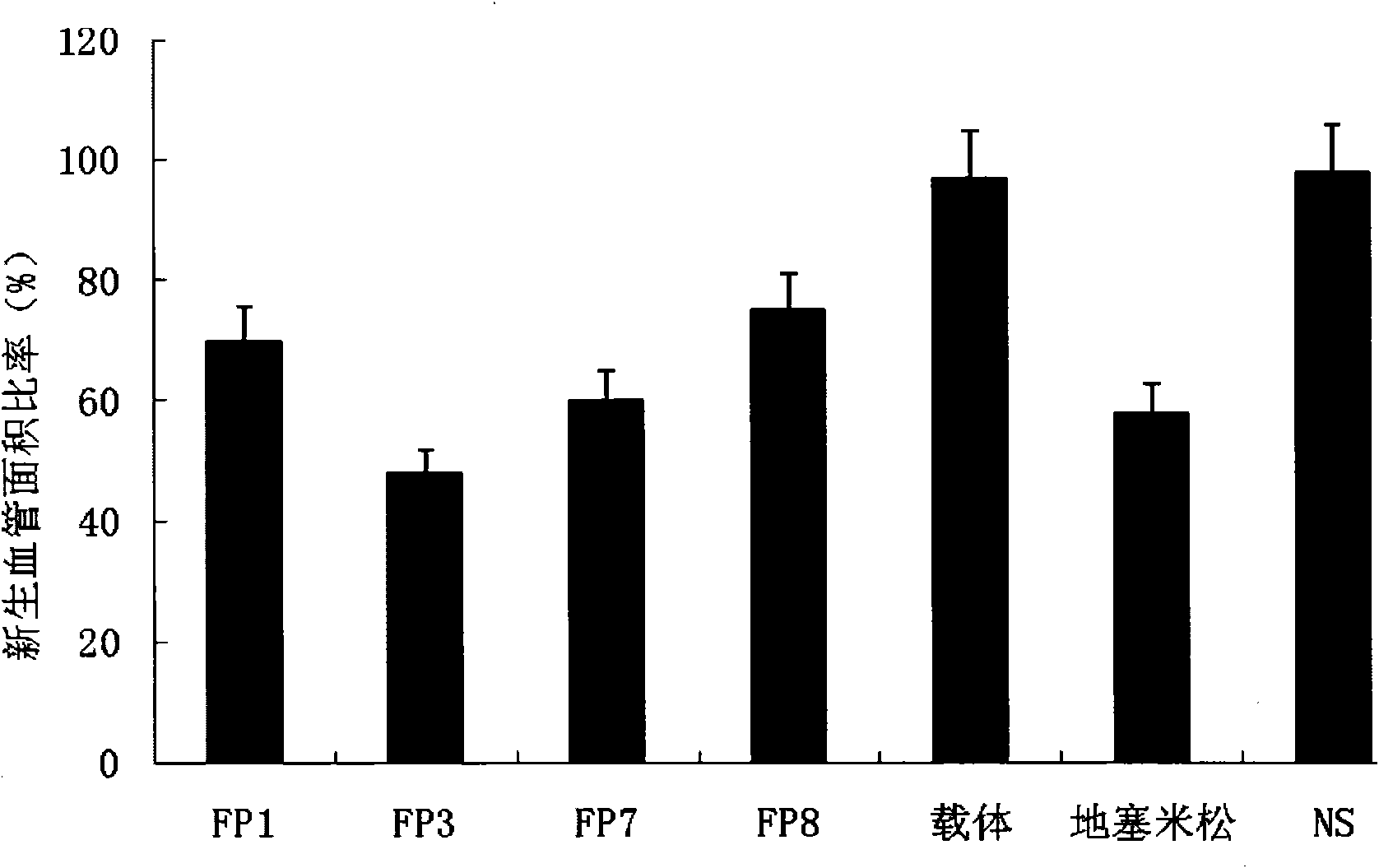
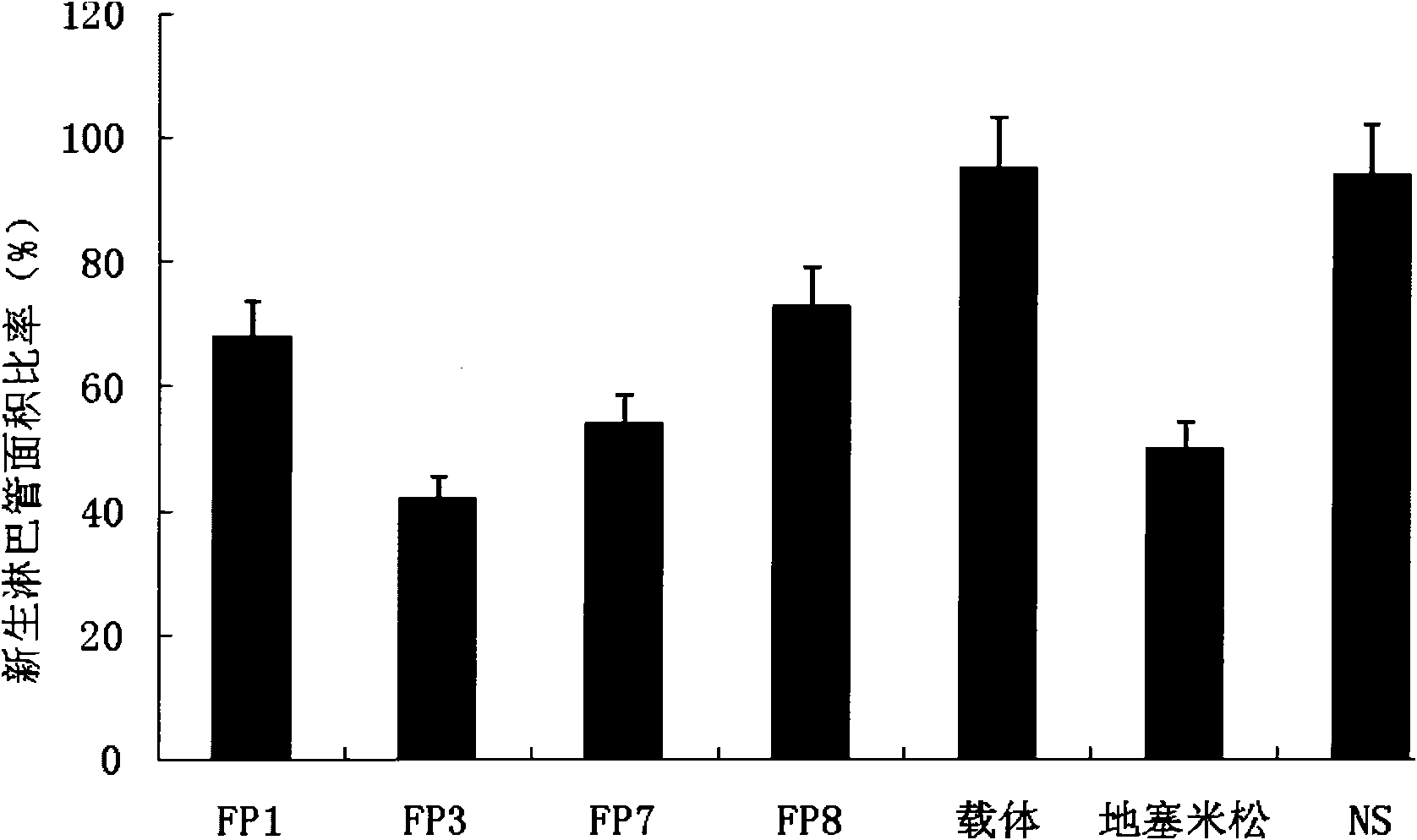
The invention relates to the application of VEGFR fusion proteins FP1, FP3, FP7 and FP8 in the preparation of drugs for treating ocular surface neovascularization and relevant diseases and a combined preparation of fusion proteins FP1, FP3, FP7, FP8 and an immunosuppressant, wherein the immunosuppressant is one or a combination selected from the group consisting of corticosteroid, rapamycin, dexamethasone and cyclosporine A; and amino acid sequences of FP1, FP3, FP7 and FP8 are respectively as shown in SEQ ID NO:1, 2, 3, 4.
Owner:CHENGDU KANGHONG BIOTECH
Novel anti-plasmodium falciparum epitope and vaccine containing the same
InactiveCN101298615AImproving immunogenicityHigh antigen recognition diversityBacteriaImmunoglobulinsEpitopePolynucleotide
The invention relates to the gene exploitation and application field of the biomedicine high-tech, in particular to an artificial antigen amino acid sequence which is provided with a plurality of epipositions and can be identified by blood serum of falciparum malaria sufferers and a polynucleotide sequence coded with the amino acid sequence. The invention also relates to a vaccine containing the amino acid sequence or the polynucleotide sequence. The invention further also relates to the application of the artificial antigen used for preparing the immunity preparation against the falciparum malaria parasite.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Dihydroartemisinin and steroid conjugates, and preparation method and application thereof
ActiveCN110407904AEasy to synthesizeGood chemical stabilityOrganic active ingredientsSenses disorderImmunologic disordersAutoimmune disease
The present invention discloses conjugates, having a general formula (I), obtained by condensation of dihydroartemisinin and steroids, or isomers or pharmaceutically acceptable salts or prodrug molecules thereof. The 10-position hydroxyl group of artemisinin and the 3-position hydroxyl group of the steroid compounds are condensed and linked by an ether bond. The invention discloses a preparation method of the compounds, and an application of the compounds in the treatment of autoimmune diseases. The dihydroartemisinin and steroid conjugates of the present invention are novel immunosuppressants, and can be used alone or in combination with other drugs to treat the human autoimmune diseases. The conjugates have the advantages of high curative effect, small toxicity and very broad applicationprospect.
Owner:YUN BAI YAO ZHENG WU TECH SHANGHAI CO LTD
Hmgn polypeptides as immune enhancers and hmgn antagonists as immune suppressants
ActiveUS20100021488A1Enhance antigen-specific immune responseAntibacterial agentsBiocideIMMUNE SUPPRESSANTSSpecific immunity
A method of enhancing an antigen-specific immune response in a host comprising administering to the host an HMGN polypeptide comprising at least one of HMGN1, HMGN3a, HMGN3b, HMGN4, Nsbpl, or a functional fragment thereof, in an amount effective to enhance an antigen-specific immune response; as well as a pharmaceutical composition comprising an HMGN polypeptide comprising at least one of HMGN1, HMGN3a, HMGN3b, HMGN4, Nsbpl, or a functional fragment thereof, and an antigen, or nucleic acids encoding such molecules; and related methods and compositions.
Owner:UNITED STATES OF AMERICA
Evaluation method and screening method for substance having action of activating/suppressing innate immunity, agent and food product for activating/suppressing innate immune mechanism and method for producing the same
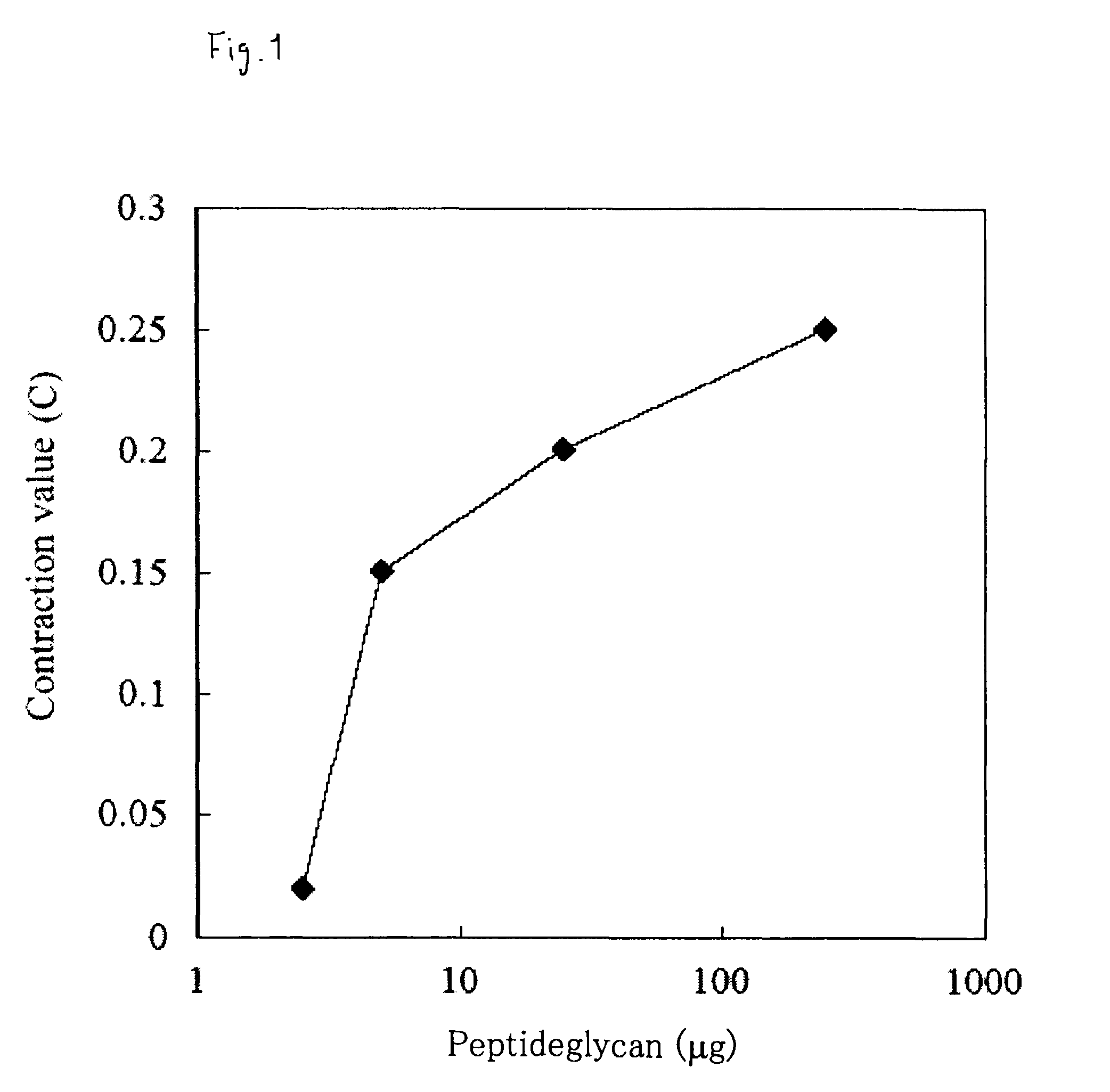
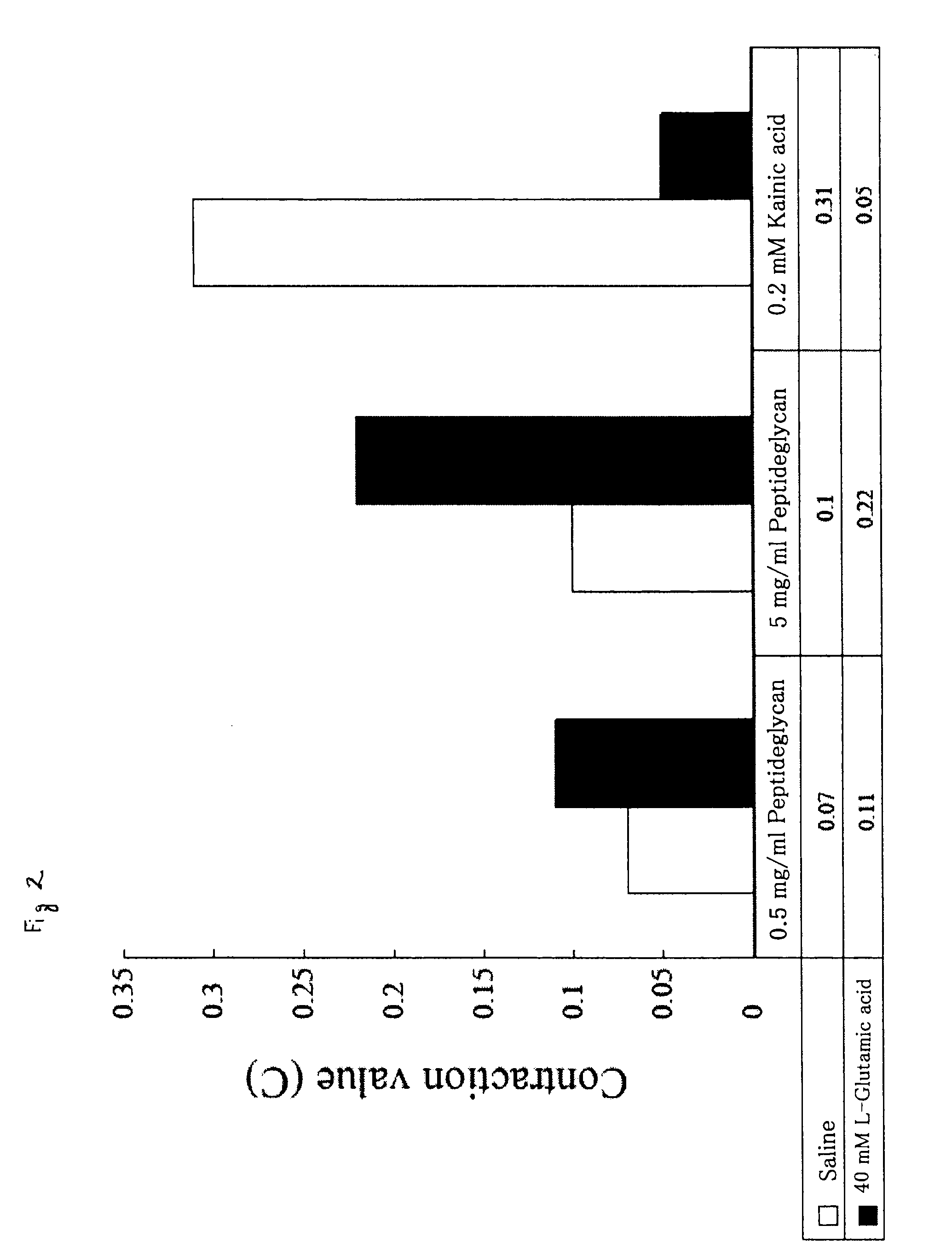
ActiveUS8313779B2Solve problemsPeptide/protein ingredientsMammal material medical ingredientsBiological bodySuppressor
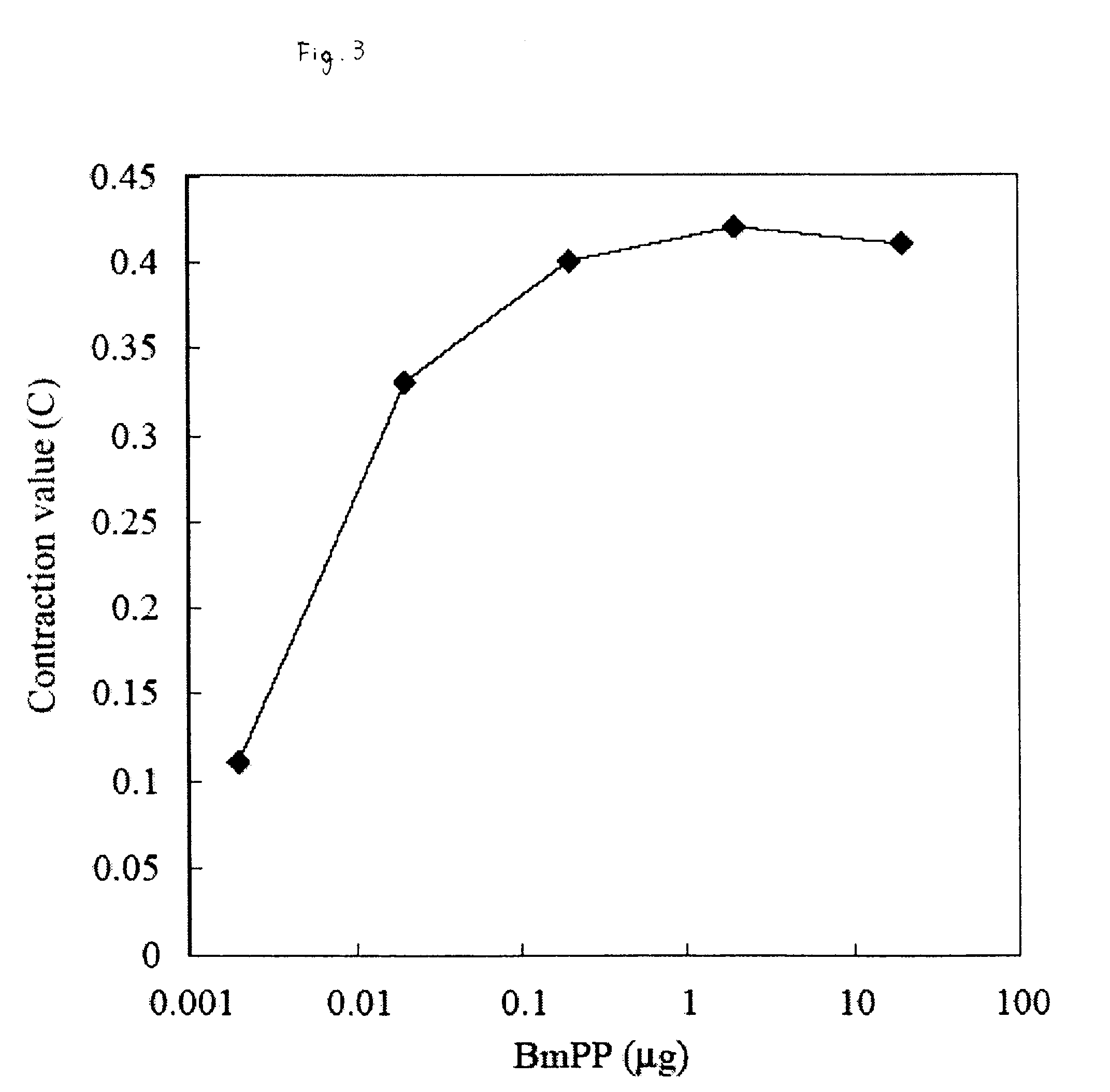
It is intended to provide an evaluation method and a screening method capable of eliminating a substance that disturbs in vivo kinetics in an individual and capable of simply and easily searching a substance having an action of activating / suppressing an innate immune mechanism without being affected by LPS derived from bacteria, which can be contaminated during the search, as well as a drug and a food for activating / suppressing the innate immune mechanism, and methods of producing the same. The present invention provides a method of evaluating or screening the substance having the action of activating / suppressing the innate immune mechanism using a muscular contraction of an organism having the innate immune mechanism as an indicator, and methods of producing the drug and the food for activating / suppressing the innate immune mechanism. Also, an innate immunity activator and the food having the action of activating the innate immune mechanism containing the substance having the action of contracting the muscle of the organism having the innate immune mechanism, and an innate immunity suppressor and the food having the action of suppressing the innate immunity containing the substance having the action of suppressing the contraction of the muscle of the organism having the innate immune mechanism are provided.
Owner:IMAGINE GLOBAL CARE CORP +1
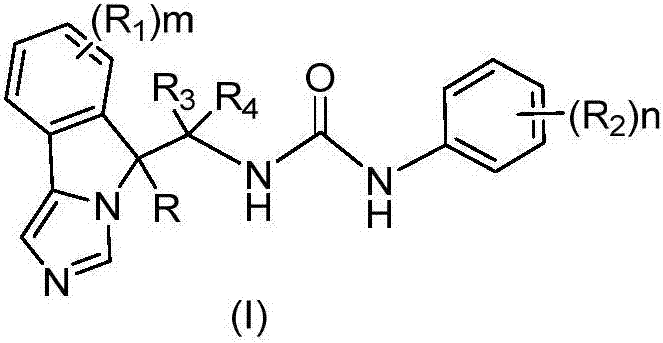
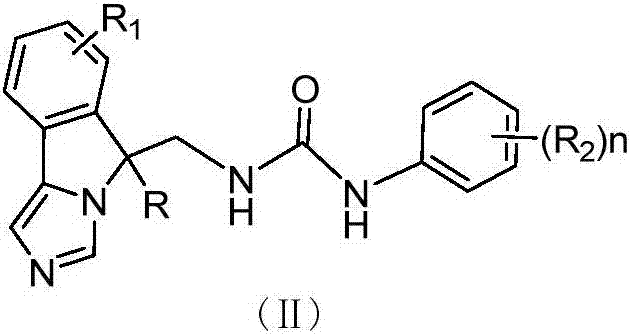
Fused imidazole derivative having IDO/TDO inhibition activity and having structure represented by formula (I), preparation method and applications thereof
The present invention relates to a fused imidazole derivative having IDO / TDO inhibition activity and having a structure represented by a formula (I), a preparation method and applications thereof. According to the present invention, the series of the fused imidazole derivatives have high IDO / TDO inhibition activity, can be widely used for treating or preventing cancers or tumors, viral infections, depression, neurodegenerative disorders, trauma, age-related cataract, organ transplant rejections or autoimmune diseases, can further be used to inhibit the immune suppression of patients, and are expected to be developed into the new generation of the immunosuppressive agent. The formula (I) is defined in the specification.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com