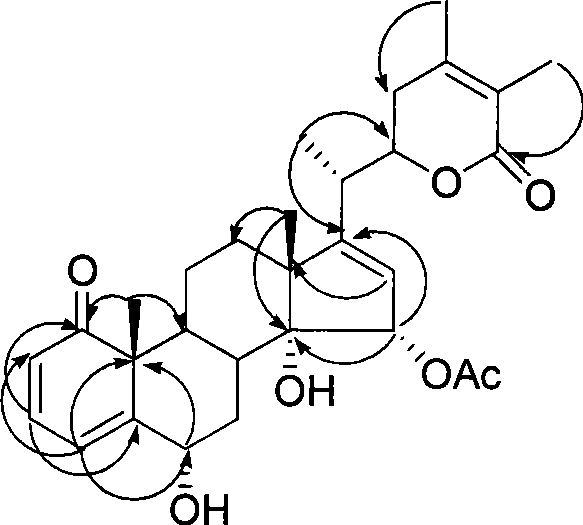
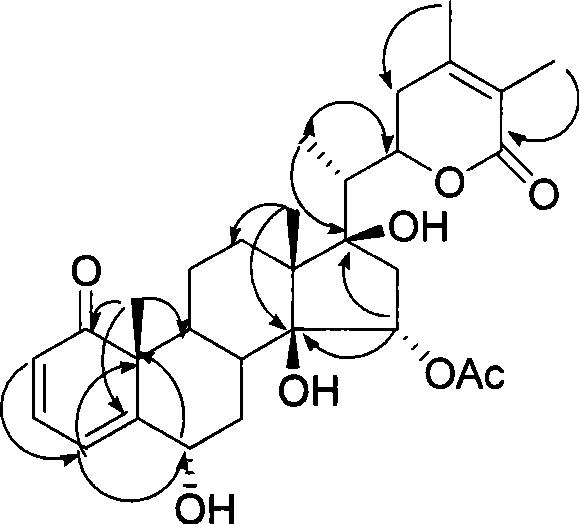
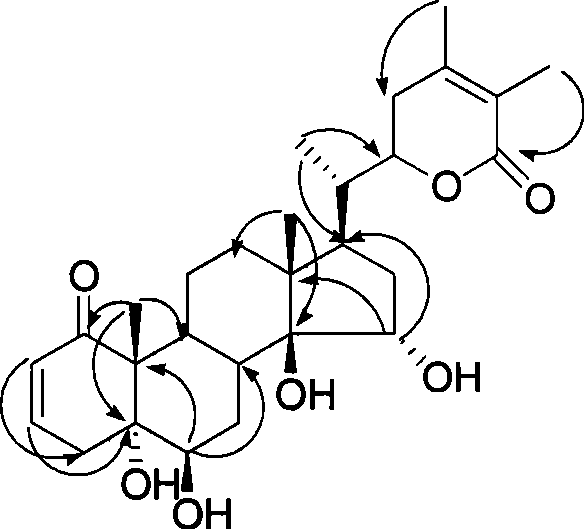
Immunosuppressant containing Withanolide type compound
A technology of immunosuppressants and compounds, which is applied in the field of immunosuppressants containing Withanolide-type compounds, can solve unclear problems, and achieve the effect of simple separation method, low cost and low toxicity immunosuppressive activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Total Withanolide compound screening sample preparation
[0046] 1. Physalis Total Withanolide Compound Screening Sample Preparation
[0047] Physalis 500g was extracted three times with 85% ethanol under reflux, concentrated, passed through macroporous adsorption resin (D101 type), first washed with 10% distilled water, then eluted with 75% ethanol, and concentrated under reduced pressure at the eluted part of 75% ethanol to get it out of the body Biological evaluation sample 1 (54 g).
[0048] 2. Sample preparation for screening of total withanolide compounds in Sophora oleifera:
[0049] 500g of bitter root was refluxed and extracted three times with 85% ethanol, concentrated, passed through macroporous adsorption resin (D101 type), washed with distilled water first, then eluted with 60% ethanol, and the 60% ethanol eluted part was concentrated under reduced pressure to be sent to in vitro biology Sample 2 (90 g) was evaluated.
Embodiment 2
[0050] Example 2 Separation of Withanolide-type compounds in Physalis
[0051] The aboveground part of Physalis allelengi was collected from Nanchuan County, Chongqing City, and was collected and identified as Physalis allelengi, a small plant of Solanaceae, by Mr. Yi Sirong of Chongqing Botanical Garden. The specimens are deposited in the Shanghai Modernization Research Center of Traditional Chinese Medicine.
[0052] Physalis aerial part (5.0Kg), with 3 times the amount of ethanol cold soaking extraction 3 times, each time for 2 days, combined filtrate, concentrated under reduced pressure to obtain extract (1.8kg), dissolved with a small amount of water, extracted with chloroform to obtain chloroform Fraction (197g), D-101 macroporous resin on the chloroform fraction, eluted with 25%, 75%, and 95% ethanol respectively to obtain 3 fractions (Fr.A-C). Fr.B (24.4g) was subjected to MCI column chromatography (MCI gel CHP 20P H 2 O / Me 2 CO 1:1) yielded 4 sites (Fr.B.1-B.4). F...
Embodiment 3
[0111] Example 3 Separation of Withanolide-type compounds in Radix Kulu
[0112] The aboveground part of Physalis angulata L was collected from Nanchuan County, Chongqing City, and was collected and identified as Physalis angulata L by Mr. Yi Sirong of Chongqing Botanical Garden.
[0113] 5.0Kg of the above-ground part of Physalis angulata L was extracted three times with 85% methanol to obtain 0.9Kg of extract, separated by polyamide column chromatography, 100% distilled water eluted part, 60% methanol eluted part, 80% methanol Elution part (containing chlorophyll), 60% methanol elution part (921g) silica gel column chromatography (petroleum ether / acetone 5:1-1:1) obtains four parts (part A-D), and part A passes ODS (C- 18) Column chromatography (methanol / water 85:15) and Sephadax LH-20 purification (chloroform / methanol) to obtain compounds Physagilin A (135 mg) and Withagulatin A (124 mg); part B was subjected to ODS (C-18) column chromatography (methanol / water 78:22) and S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com