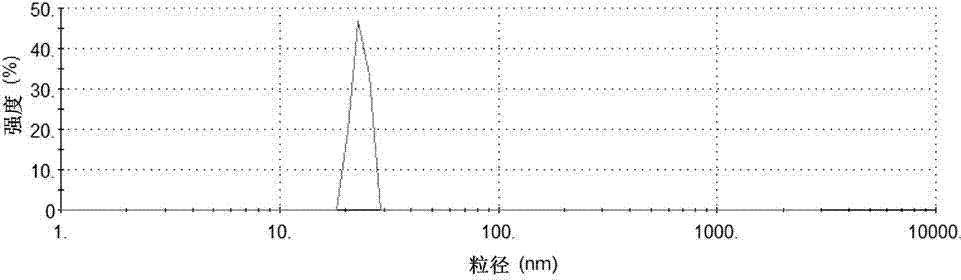
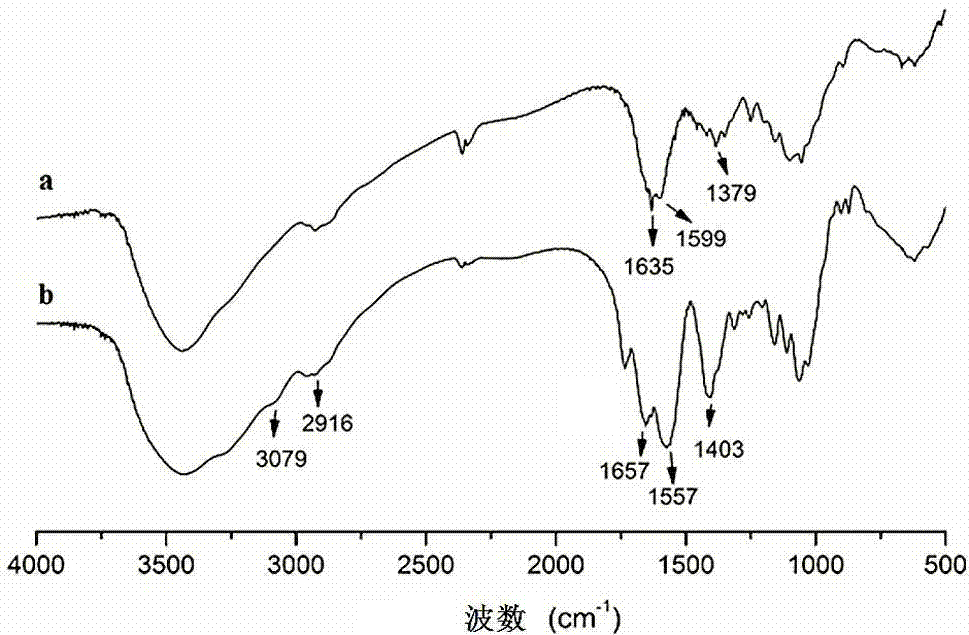
Low-density lipoprotein coupled N-succinyl chitosan nanoparticle vector, and preparation method and application thereof
A technology of succinyl chitosan and low-density lipoprotein, which is applied in the field of pharmaceutical preparations, can solve problems such as low encapsulation efficiency, difficulty in large-scale production, and high requirements for fat solubility, so as to reduce toxic and side effects, increase distribution, and slow release sex good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Frozen plasma was dissolved at 37°C, refrigerated and centrifuged at 9000rpm for 30 minutes, and the top white chylomicrons were carefully sucked off. Calculate the amount of dry potassium bromide that needs to be added by the Radding-Steining formula:
[0050]
[0051] in, d 0 and V 0 are the initial plasma density and initial volume, respectively, d 1 is the plasma density to be adjusted, and 0.312 is the specific volume of KBr (mL g -1 ), X It is the mass (g) of KBr to be added. According to d=m / v, the initial density of plasma is measured by weighing method d 0 , weigh a certain amount of dry KBr to adjust the plasma density to 1.3g·mL -1 . After the solid KBr is completely dissolved, inject 4 mL of plasma into the centrifuge tube with a syringe, and carefully spread along the tube wall to a density of 1.006 g·mL -1 3.5mL of NaCl solution, balanced by ultracentrifugation at 55000rpm for 4h, plasma lipoproteins have been separated after centrifugat...
Embodiment 2
[0055] Weigh 0.05g of Suc-CS and dissolve it in 50mL of distilled water, add 0.01g of doxorubicin hydrochloride (Dox·HCl) to fully dissolve, 500r·min -1 Preheat in a water bath at 30°C for 20min under magnetic stirring, and the concentration is 1.2mg·mL -1 The TPP aqueous solution was dropped into it drop by drop at a rate of 1 drop every 2 seconds until the solution just produced opalescence. Doxorubicin N-succinyl chitosan nanoparticles (Dox-SCS-NPs) were obtained by passing through a 0.22 μm microporous membrane. Take 5 mL of Dox-SCS-NPs prepared according to the optimal prescription, add 5 mL of PBS (pH 5.5) dissolved with 25 mg EDC·HCl and 5 mg NHS, stir at room temperature for 15 min, and adjust the pH of the solution to 7.4 to terminate the activation. Add 1 mL of LDL (dialyzed in borate buffer with pH 9.4) and stir at room temperature for 2 h, and filter through a 0.45 μm microporous membrane to obtain LDL-coupled doxorubicin N-succinyl chitosan polymer nanoparticles...
Embodiment 3
[0057] Weigh 0.1 g of Suc-CS and dissolve it in 50 mL of distilled water, add poloxamer 188 at a final concentration of 0.5%, and preheat in a water bath at 40 °C for 20 min under magnetic stirring at 500 r / min as the water phase. In addition, weigh egg yolk lecithin with a final concentration of 1.2% in a small beaker, add ethyl acetate and acetone (v / v=3:1) to dissolve phospholipids as the oil phase, and the volume ratio of oil to water is 1:5. The oil phase was quickly injected into the water phase under stirring, and the stirring was continued for 20 min after the white color of the solution faded. N-succinyl chitosan nanoparticles (SCS-NPs) were obtained by passing through a 0.22 μm microporous membrane. Take 5 mL of SCS-NPs prepared according to the optimal prescription, add 5 mL of PBS (pH 5.5) dissolved with 25 mg of EDC·HCl and 5 mg of NHS, stir at room temperature for 15 min, and adjust the pH of the solution to 7.4 to terminate the activation. Add 1mL of LDL (dialy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com