Patents
Literature
57 results about "Biological evaluation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
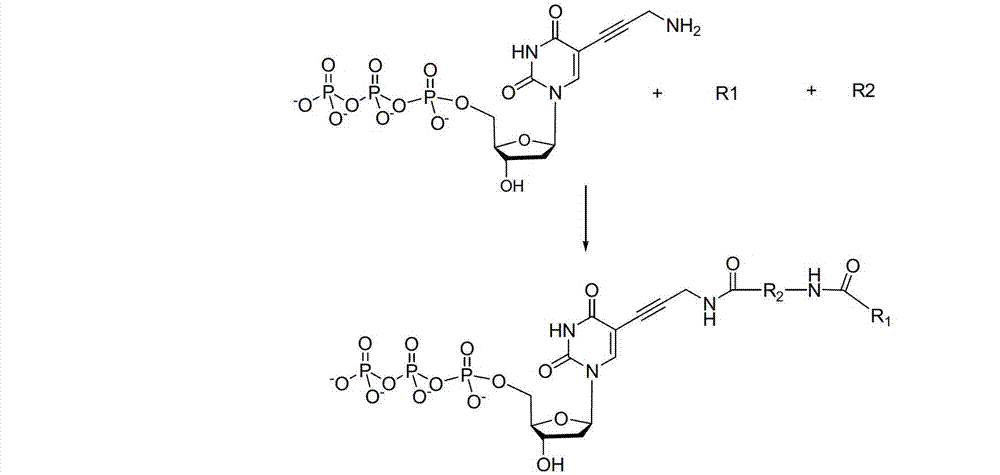
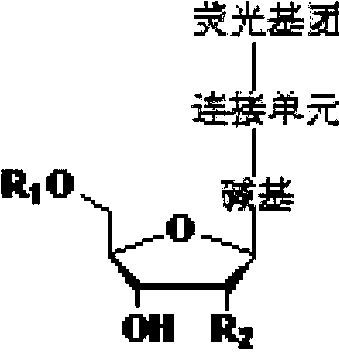
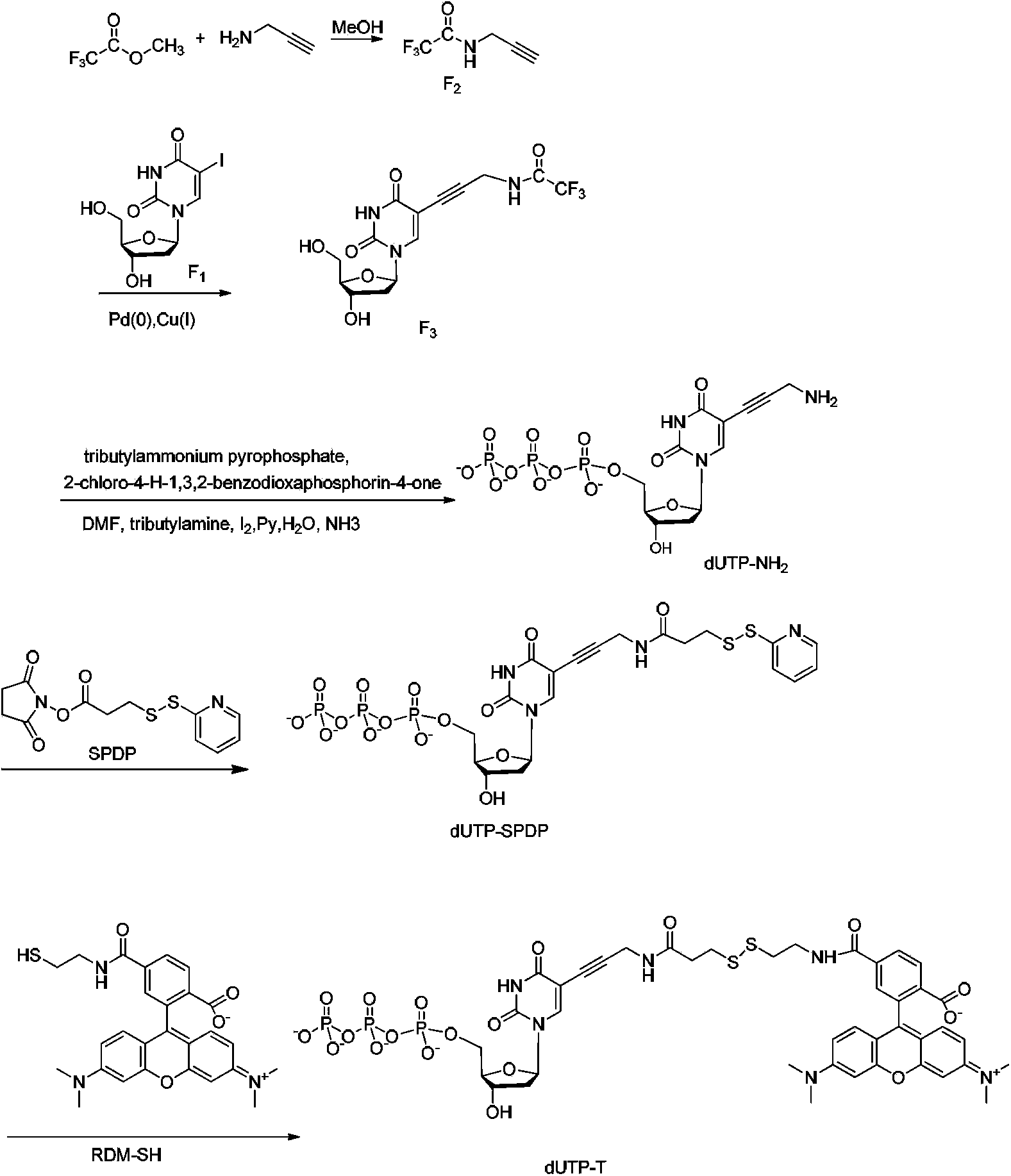
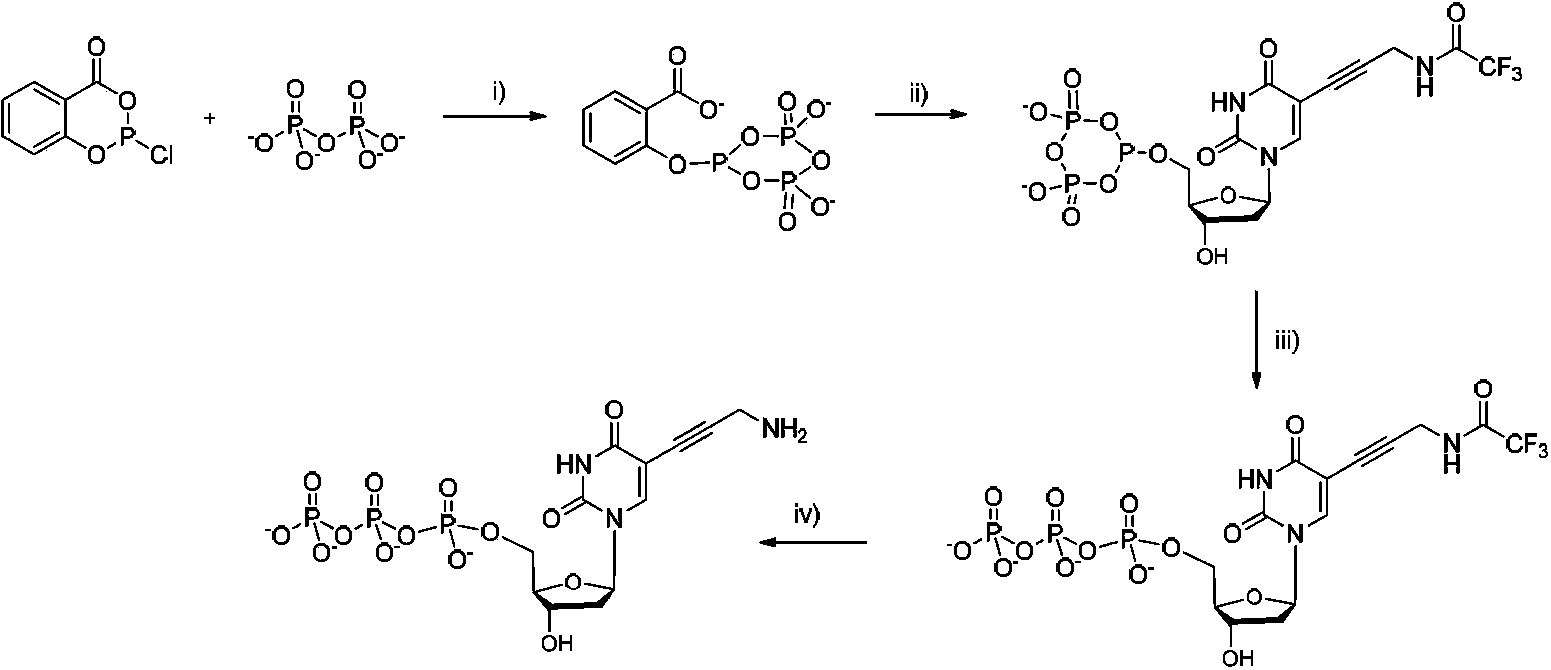
Reversible terminal and synthesis and use in DNA synthesis sequencing thereof
ActiveCN103087131ARaw materials are easy to obtainSugar derivativesMicrobiological testing/measurementChemical reactionHigh energy
The invention discloses a reversible terminal and synthesis and use in DNA synthesis sequencing thereof. The structural formula of the reversible terminal is described according to formula (I), wherein R1 is fluorescein and R2 is a connection unit. The cracking reversible terminal of the invention is available for DNA synthesis sequencing. At the same time, as synthesis required material is easily available and synthesis processes are all conventional chemical reactions, the reversible terminal disclosed by the invention is applicable for large-scale promotion and has good practical prospect due to a biological evaluation result showing that the reversible terminal is capable of totally satisfying biochemical reaction requirements of high-energy sequencing.
Owner:SHANGHAI JIAO TONG UNIV
Four-color fluorescence labeling reversible terminal and use thereof in DNA (Deoxyribonucleic Acid) sequencing
ActiveCN103484106ARaw materials are easy to obtainSugar derivativesMicrobiological testing/measurementChemical reactionBifunctional
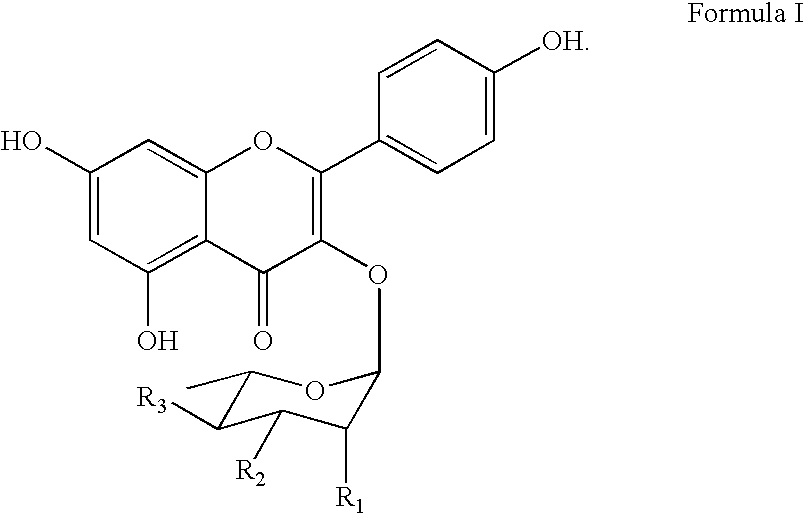
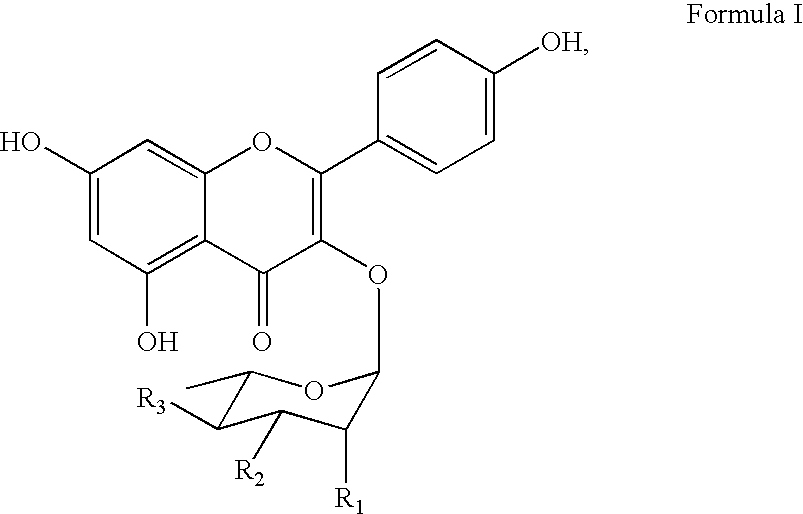
The invention discloses a four-color fluorescence labeling reversible terminal and a use thereof in DNA (Deoxyribonucleic Acid) sequencing. The structural formula of the reversible terminal is as shown in a formula (I) in the specification, wherein R1 is triphosphate; R2 is H or OH; the basic group is U, C, A, G or derivatives thereof; the connecting unit is a bifunctional compound which is breakable under a mild condition; the fluorescence group is one selected from a combination of BODIPY, fluorescein, rhodamine, coumarin, xanthene, cyanin, pyrene, phthalocyanine, alexa, squarene dye and an energy transferring dye, and derivatives thereof. The reversible terminal provided by the invention can be used for DNA single-molecule sequencing; simultaneously, raw materials required by the synthesis of the reversible terminal provided by the invention are simple and easy to get and the synthesis process of the reversible terminal is completely involved with conventional chemical reactions, so that the four-color fluorescence labeling reversible terminal can be popularized and utilized to a large scale; biological evaluation results indicate that the reversible terminal is capable of completely meeting the biochemical reaction requirements of high-flux sequencing and has a good practical prospect.
Owner:SHANGHAI JIAO TONG UNIV
Medical care disinfection treatment device
InactiveCN109589437AImprove uniformityDisinfect evenlyLavatory sanitoryChemicalsEngineeringAir blower
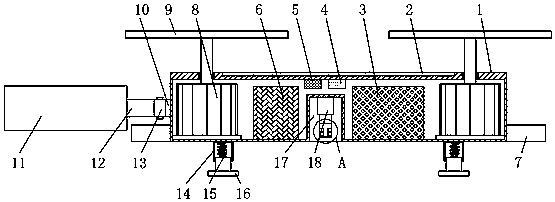
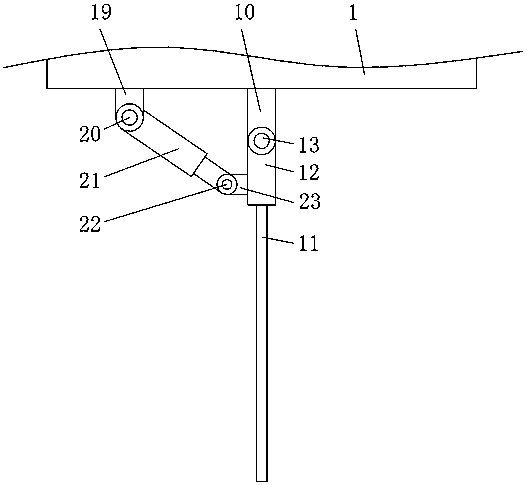
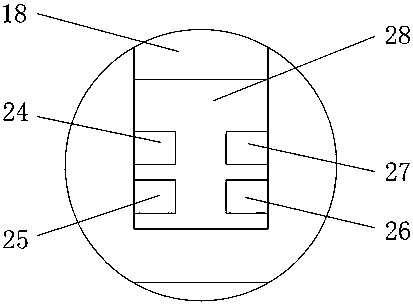
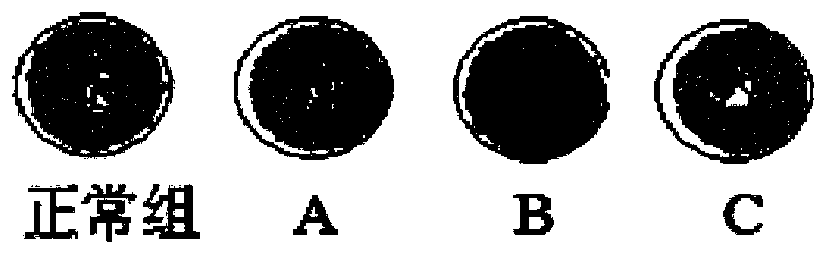
The invention discloses a medical care disinfection treatment device, and relates to the technical field of medical instruments. The device comprises a box body; bearings are clamped on both the frontand rear sides of the inner wall of the box body; a rotating shaft is sleeved in both the two bearings; a cam is engaged on the outer surface of the rotating shaft; and a driven gear is fixedly connected with one end of the back of the rotating shaft. By arranging a driving device, the rotating shaft, the cam, a sterilization net box, a telescopic device and a connecting plate, the medical care disinfection treatment is capable of carrying out drying through hot air blown by a hot air blower, thereby improving drying uniformity of medical instrument disinfection; by adoption of an object surface disinfection effect monitoring, common microorganisms such as bacteria and fungi can be killed, and germs and spores can be killed, thereby effectively improving the efficiency of disinfection anddrying to meet the biological evaluation criteria, and greatly meeting the needs of clinical use and laboratory.
Owner:青岛山大齐鲁医院
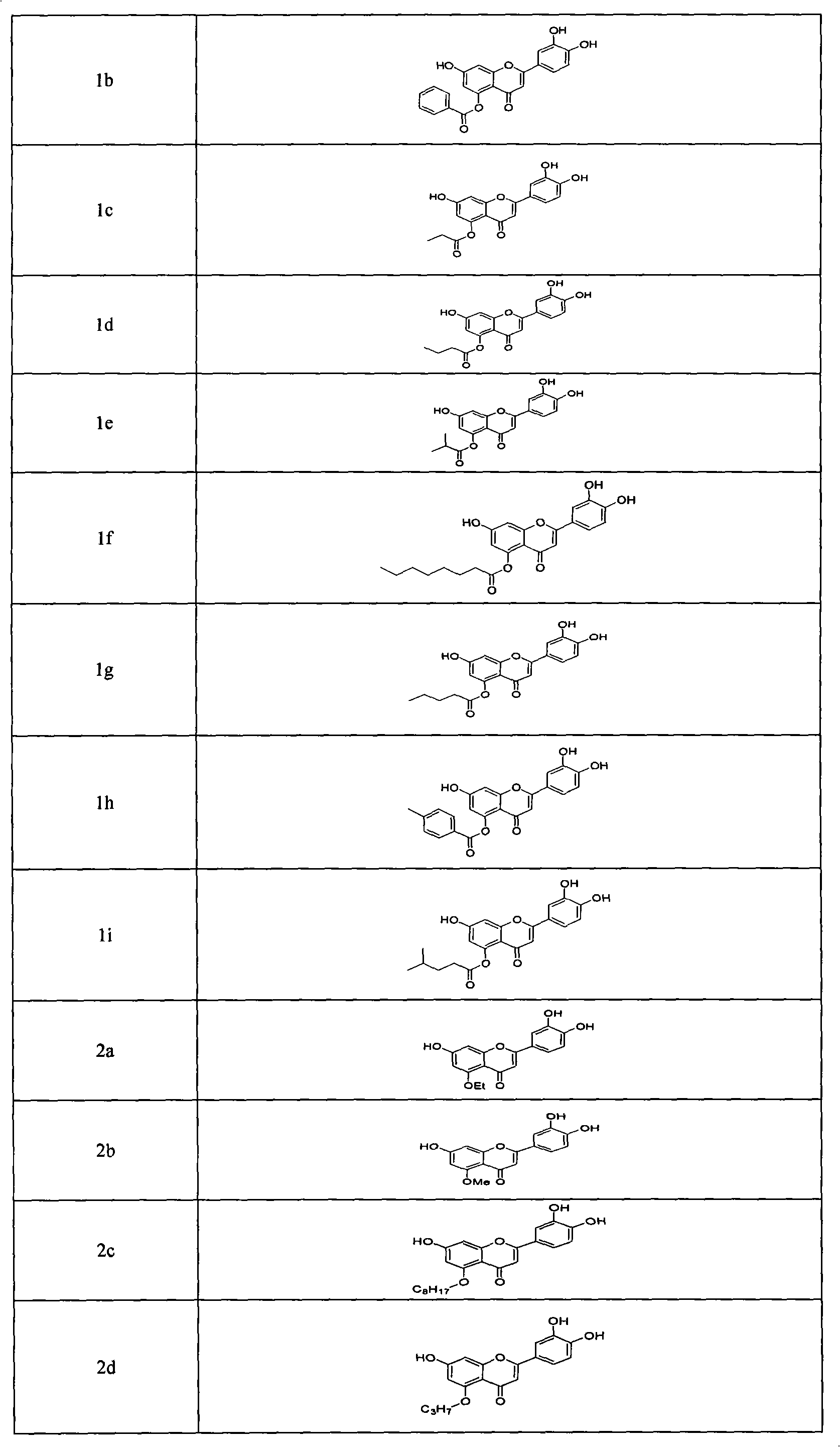
Flavonoid derivatives and application thereof in preparing medicinal composition
The invention belongs to the field of medicinal chemistry, and relates to flavonoid derivatives and application thereof in preparing a medicinal composition for treating diseases related with dopamine transport protein. The flavonoid derivatives of the invention have a structure of a formula (I). A biological evaluation result shows that a compound of the invention represents better activation function on the dopamine transport protein compared with the prior art, can adjust the transport of dopamine by promoting the transport function of the dopamine transport protein, maintain extracellular dopamine concentration balance, and can be prepared into dopamine transport protein agonist used for treating mental diseases and neurological diseases. R1 is selected from hydroxy, alkoxyl of C1 to C8, ester group of C1 to C8, glucoside and halogen; R2 is selected from hydrogen, C7-hydroxy, C7-alkoxyl, C7-ester and C7-halogen respectively; and R3 is selected from the hydrogen respectively, or contain one or a plurality of substituent groups on a benzene ring, wherein the substituent groups are selected from the hydroxy, alkoxyl, ester group, aryl (heteroaryl), the hydrogen and glycoside group respectively.
Owner:FUDAN UNIV
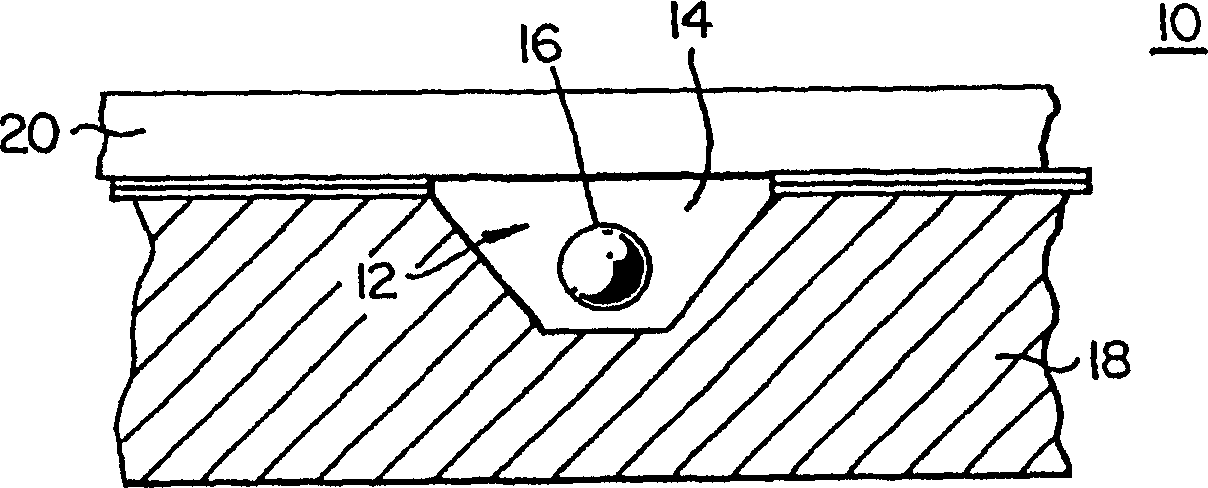
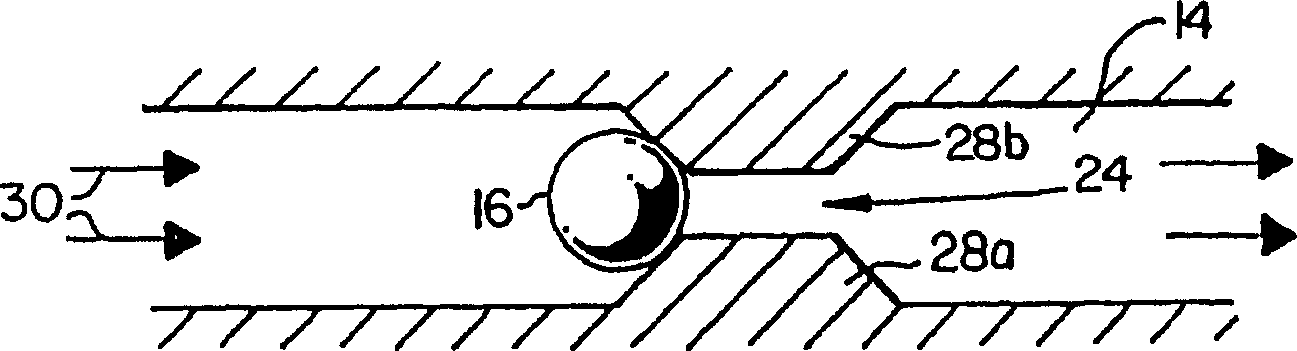
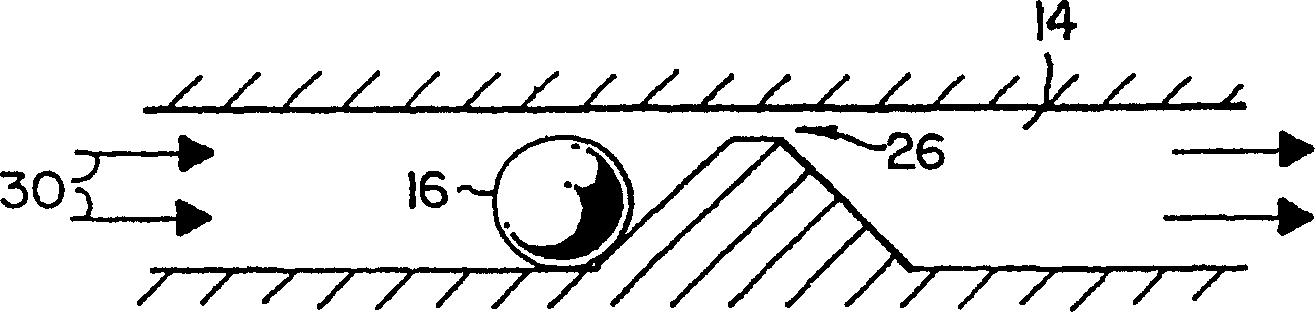
Microfluidic channel embryo and/or oocyte handling, analysis and biological evaluation
InactiveCN1441652AEliminate processingEasy to collectBioreactor/fermenter combinationsBiological substance pretreatmentsChannel geometrySurvivability
Microfluidic embryo scaled channels (14) for handling and positioning embryos provide the opportunity to evaluate and treat embryos in improved manners. Fluid flow is used to move and position embryos within microfluidic channels and channel geometrics may be used to place embryos at specific locations. Surface properties and compliance (deformation) properties of embryos are evaluated as a predictor of viability. The microfluidic channels provide the opportunity for fine controls of pressure to conduct various evalutions at forces slightly below which damage to embryos is known to occur. Measurement of the distance and / or which embryos roll in a same pressure gradient microfluidic channel provides information, with healthy embryos traveling slower or a shorter distance as they demonstrate more stiction to channel walls. Positioned at a constriction (14a, 14b, 24, 26), health embryos also appear to deform less than unhealthy embryos that are more readily pulled into a constriction. In addition, healthy embryos appear to resume their shape better. Fluid from microfluidic channels is easily collected downstream without altering the embryo environment, providing a better opportunity for chemical analysis of fluid chemical analysis than convention manual handling and sampling techniques. Zona pellucida removal of mammalian embryos is achieved as embryos are moved through flow to a precise location where lysing agent can be washed over the embryo to achieve zona removal. Cumulus removal is realized with a series of constrictions to cut cumulus followed by fluid flows to remove cut cumulus from the embryo.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
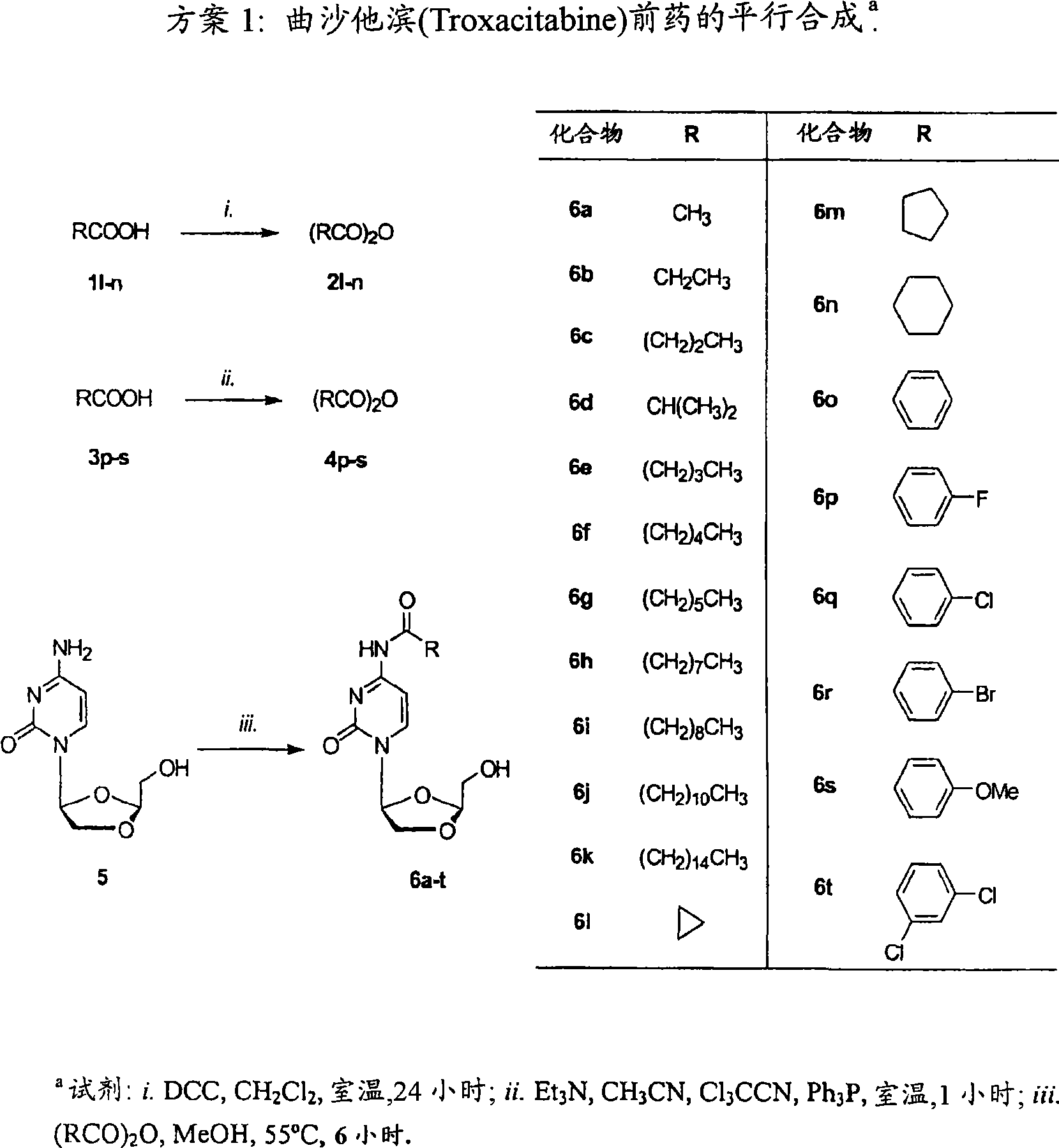
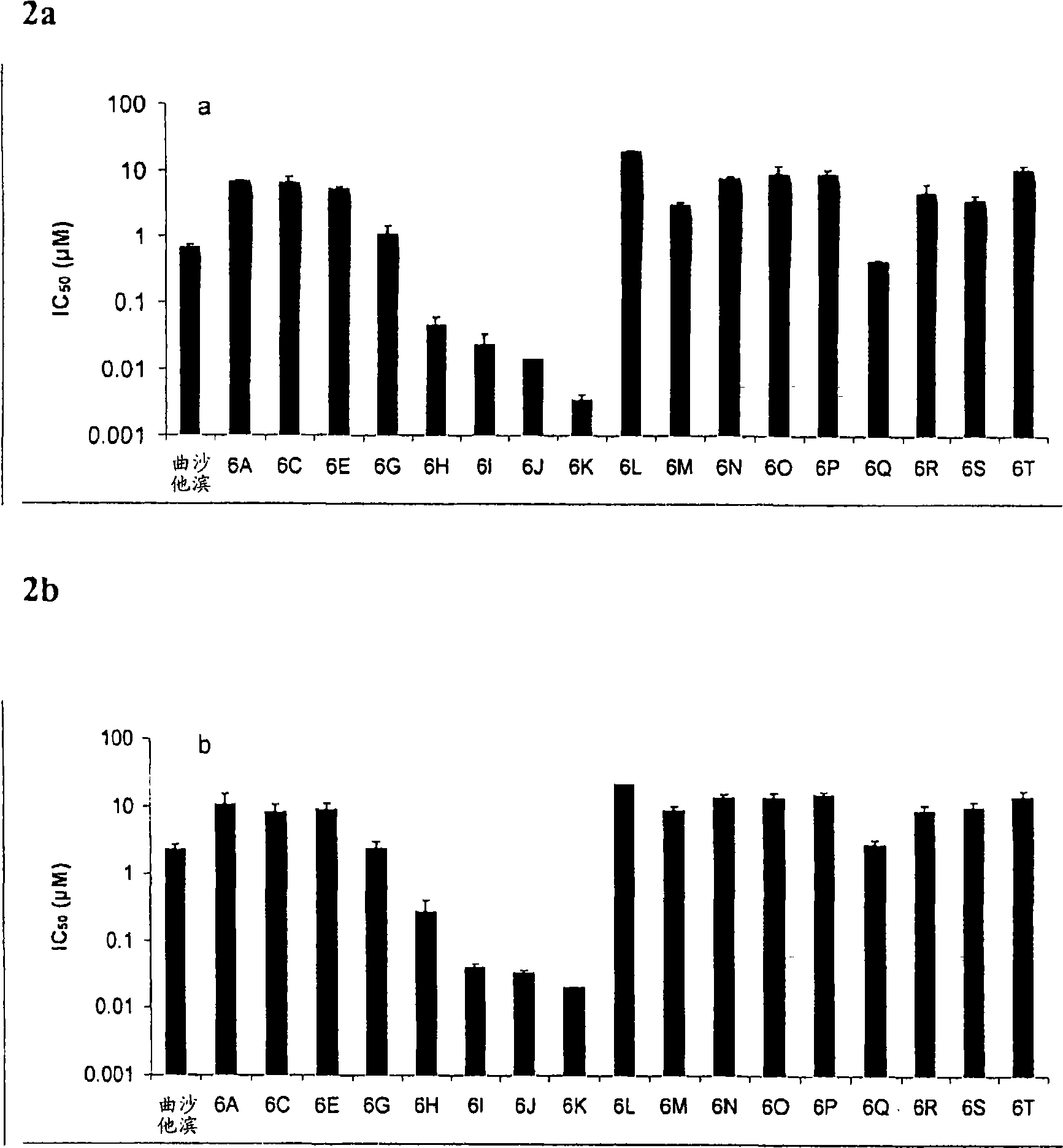
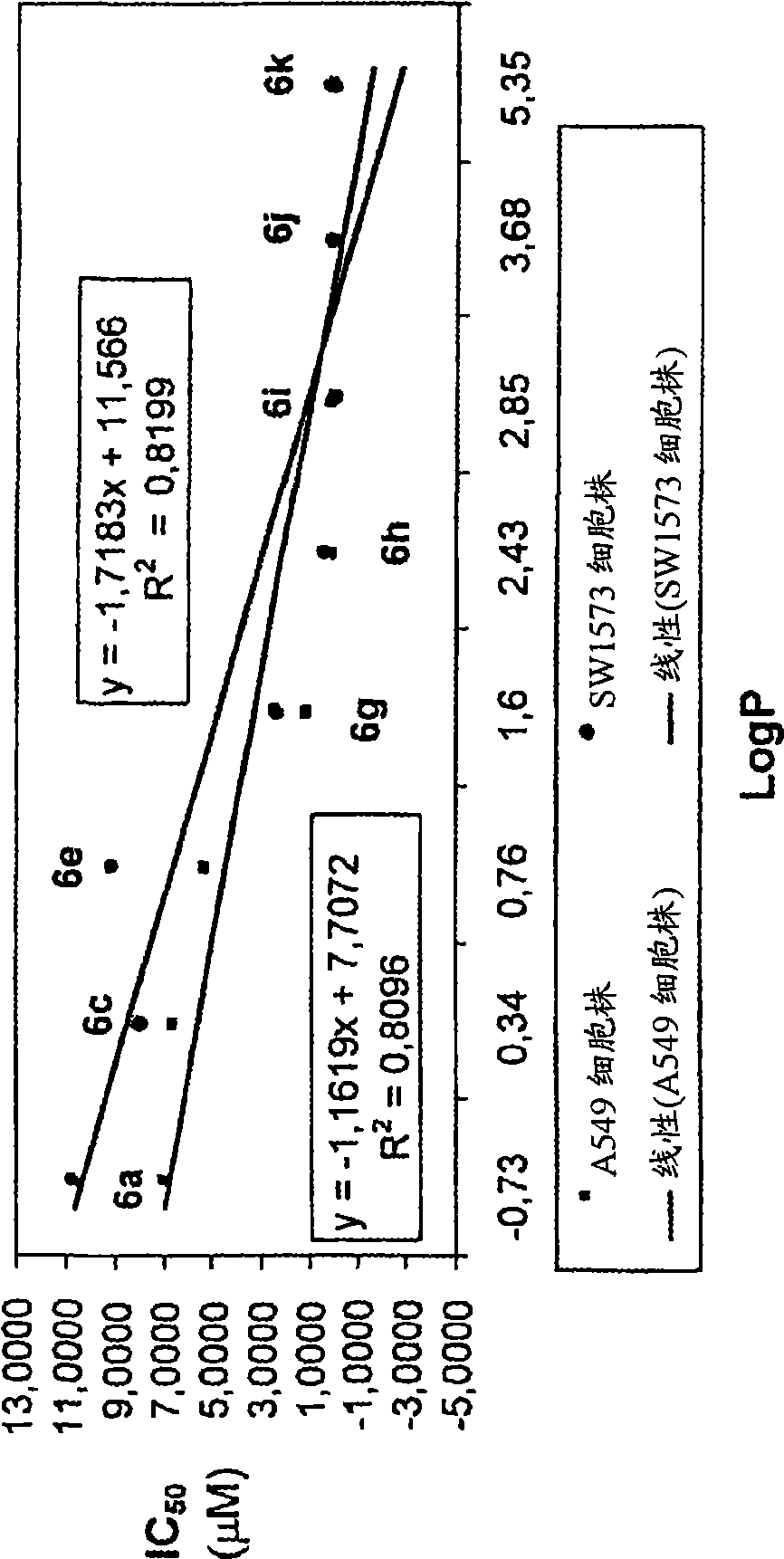
L-OddC prodrugs for cancer
ActiveCN101534835AImprove bioavailabilityUnique activityOrganic active ingredientsDigestive systemAnticarcinogenWilms' tumor
The main drawback in the use of most nucleoside anticancer agents originates from their hydrophilic nature, of which property requires a high and frequent dosage for an intravenous administration. Unlike other nucleoside anti-tumor agents, troxacitabine appears to predominantly enter tumor cells by passive diffusion rather then by using nucleoside transporters, although this may be model dependent. Accordingly, in the present work, a small library of twenty troxacitabine prodrugs has been synthesized using a parallel approach in order to evaluate the relationship between the lipophilicity of the prodrugs and their antitumor activity. Biological evaluation of the prodrugs on two non-small cell lung cancer cell lines (A549 and SW1573) and in pancreatic cell lines clearly showed better antitumor activity than that of troxacitabine, with IC50 values in the nanomolar range.
Owner:UNIV OF GEORGIA RES FOUND INC
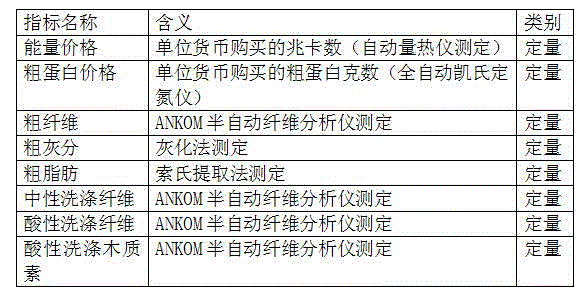
Biological evaluation method for forage
InactiveCN104313120AIncrease profitRealize the demonstration application of industrializationMicrobiological testing/measurementMaterial analysisCrude fibreIn vitro digestion
An biological evaluation method for forage belongs to the field of forage technology. The method is as below: determining the total energy, crude protein, crude fiber, crude ash, crude fat, neutral detergent fiber, acid detergent fiber and acid detergent lignin of a non conventional forage by using an analytic method of applied chemistry, and then calculating the digestive energy, metabolic energy and net energy of the non conventional forage and evaluating; determining the digestive energy and metabolism energy indexes of animals by using an animal in vivo digestion experiment, and evaluating the nutritional value of the non conventional forage; determining digestive energy and metabolic energy indexes of animals by using an animal in vitro digestion experiment can, and evaluating the nutritional value of the non conventional forage. Applied chemical analysis and animal in vivo and in vitro digestion experiments are combined to establish a nutritional value evaluation system for non conventional forage resources. The method can comprehensively, systematically and accurately evaluate common non conventional forage resources in North china. The nutritional value evaluation system for non conventional forage resources can be utilized to establish an efficient and low-cost forage technology system and realize industrialized demonstration application.
Owner:沈阳爱特杰牧业有限公司
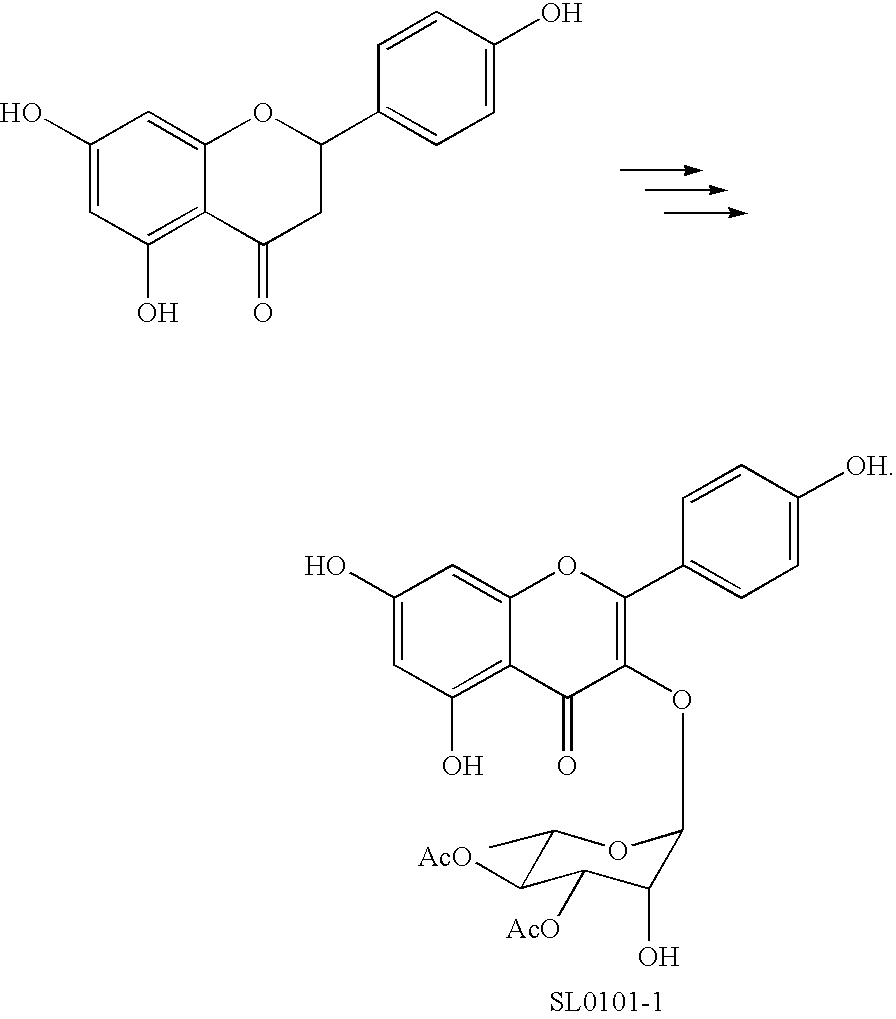
Synthesis of inhibitors of p90Rsk
The synthesis of the naturally occurring kaempferol glycoside SLO1O1-1, as well as analogs thereof, has been accomplished, as has its biochemical evaluation. SLO1O1-1 exhibits selective and potent p90 Rsk inhibitory activity at nanomolar concentrations without inhibiting the function of upstream kinases such as MEK, Raf, or PKC. The synthetic scheme of the invention verified the structural assignment of the natural product and has provided access to material sufficient for detailed biological evaluation.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
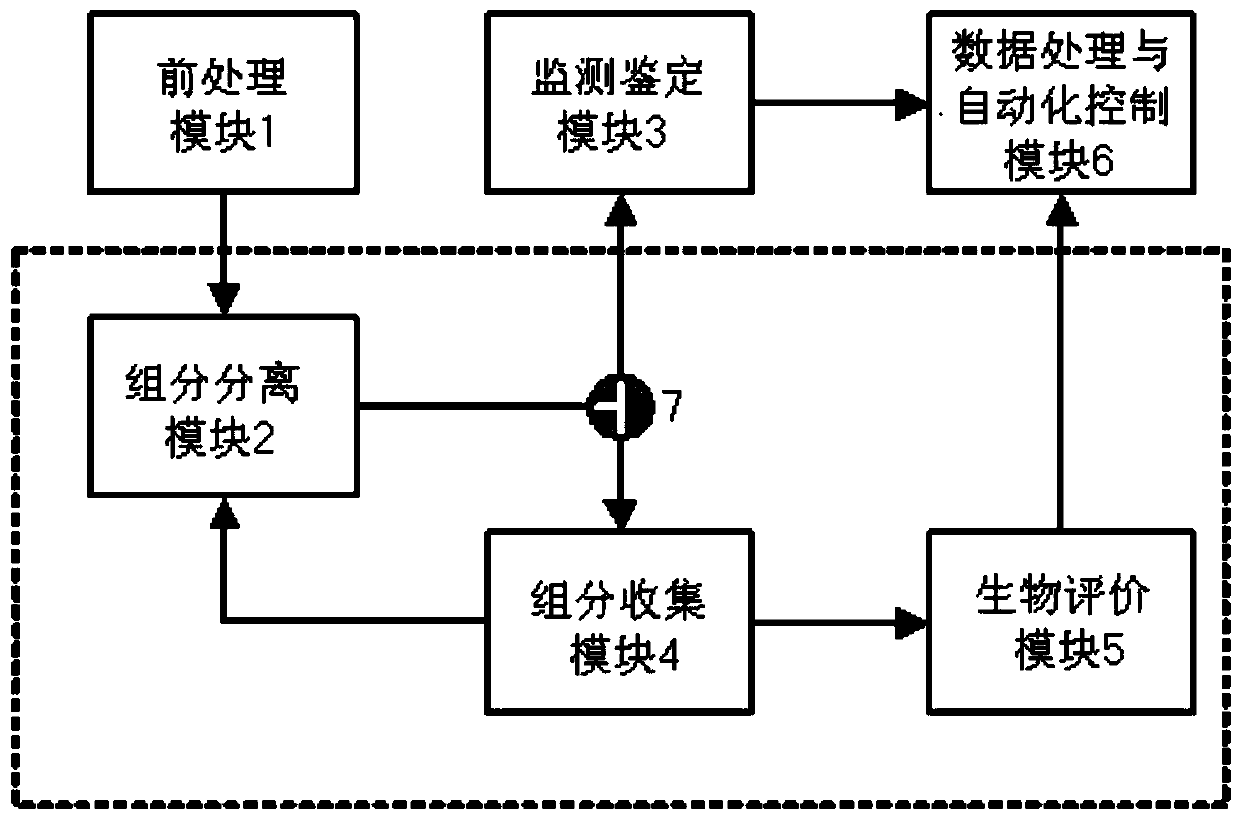
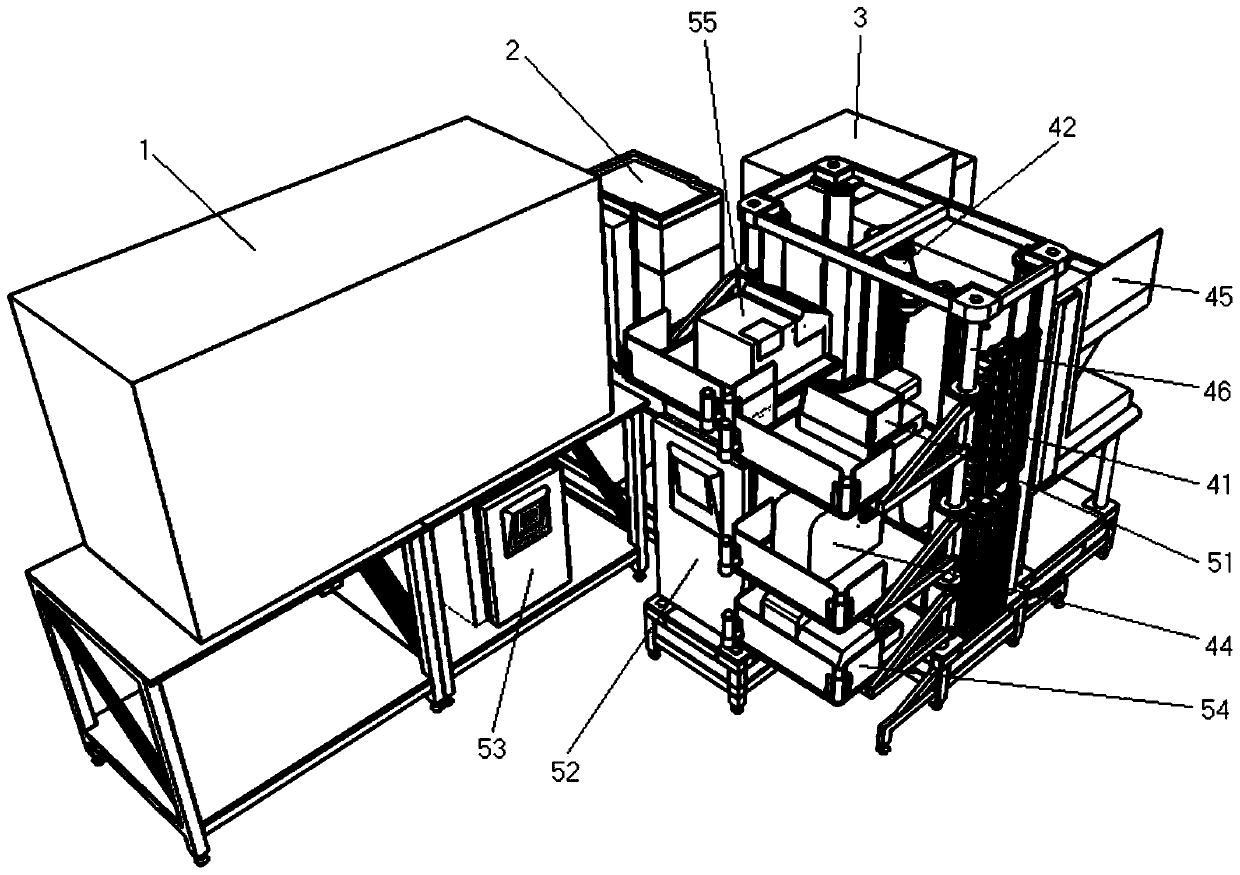
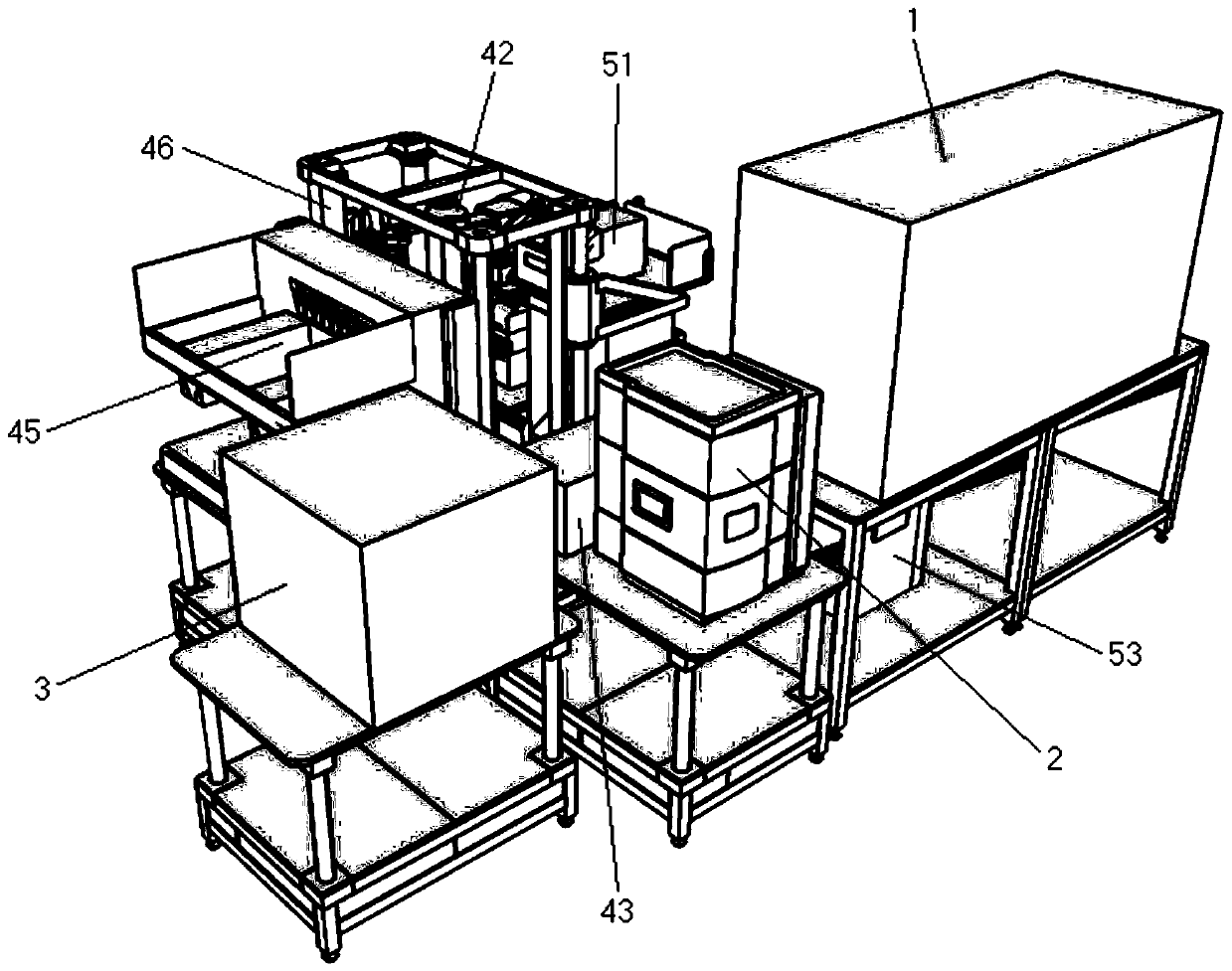
Full-automatic biological evaluation and chemical analysis integrated machine and method
ActiveCN110333364AImprove reliabilityImprove accuracyComponent separationAutomatic controlHigh-Throughput Screening Methods
The invention relates to a full-automatic biological evaluation and chemical analysis integrated machine and method. The integrated machine comprises a pretreatment module used for enriching, concentrating and purifying a to-be-analyzed sample, a component separation module used for separating various compounds in a mother liquor to be analyzed, a monitoring and identifying module used for carrying out real-time monitoring and collecting of chromatographic signals and quantitative detection of suspicious compounds on effluent, a component collecting module used for collecting, decreaming, redissolving and transferring the effluent, a biological evaluation module used for finishing cell culture and detection of cytotoxicity effects and toxic targets, and a data processing and automatic control module used for collecting, sorting and analyzing comprehensive data. According to the integrated machine and method, efficient, stable and normalized standard operation conditions are provided for toxicity screening of compounds in a complex system; the reliability of experimental data is improved while the labor cost required by detection is reduced; and the method is a relatively high-practicality technical method for high-throughput screening work.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Antioxidant compound having anti atherosclerotic effect and preparation thereof
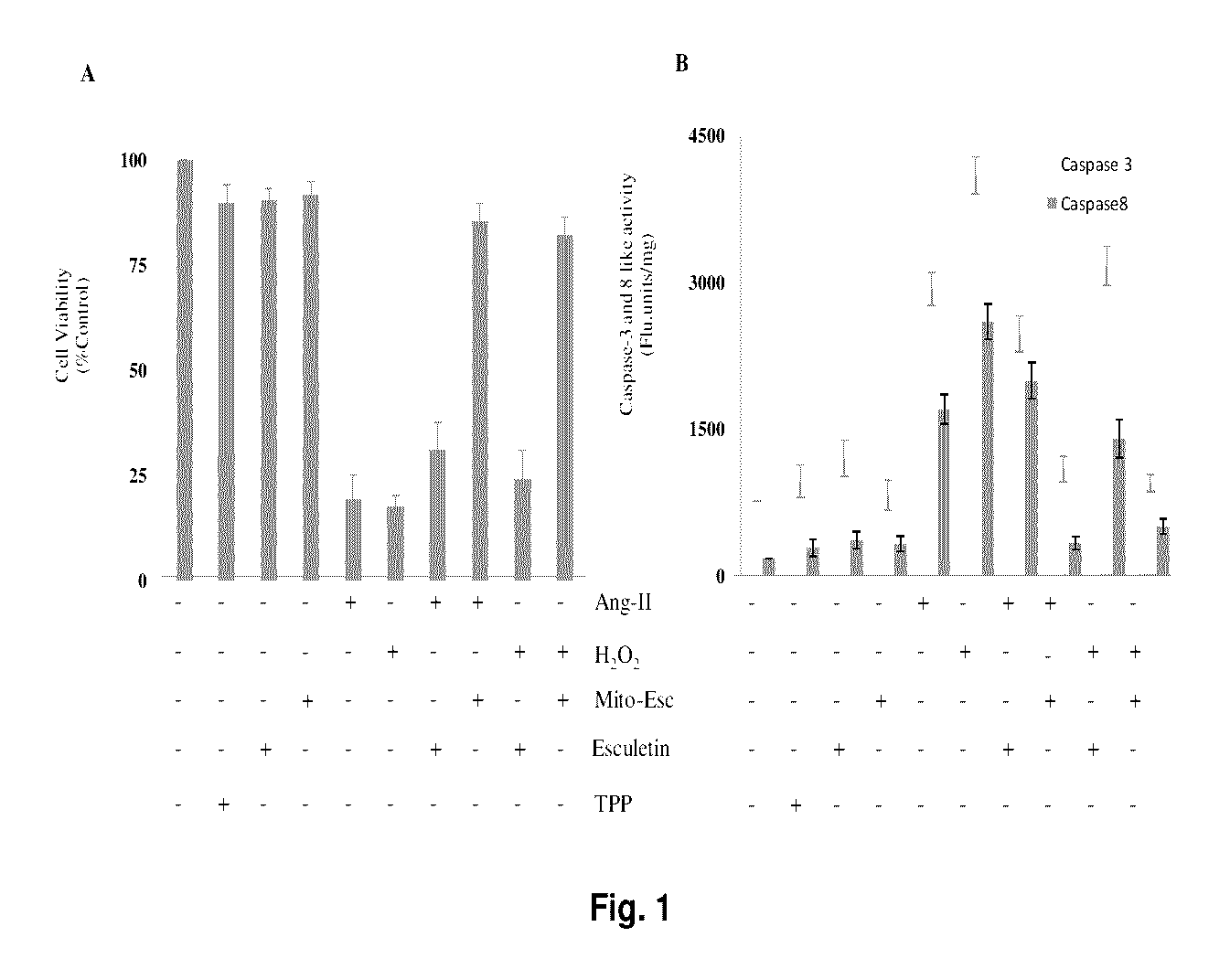
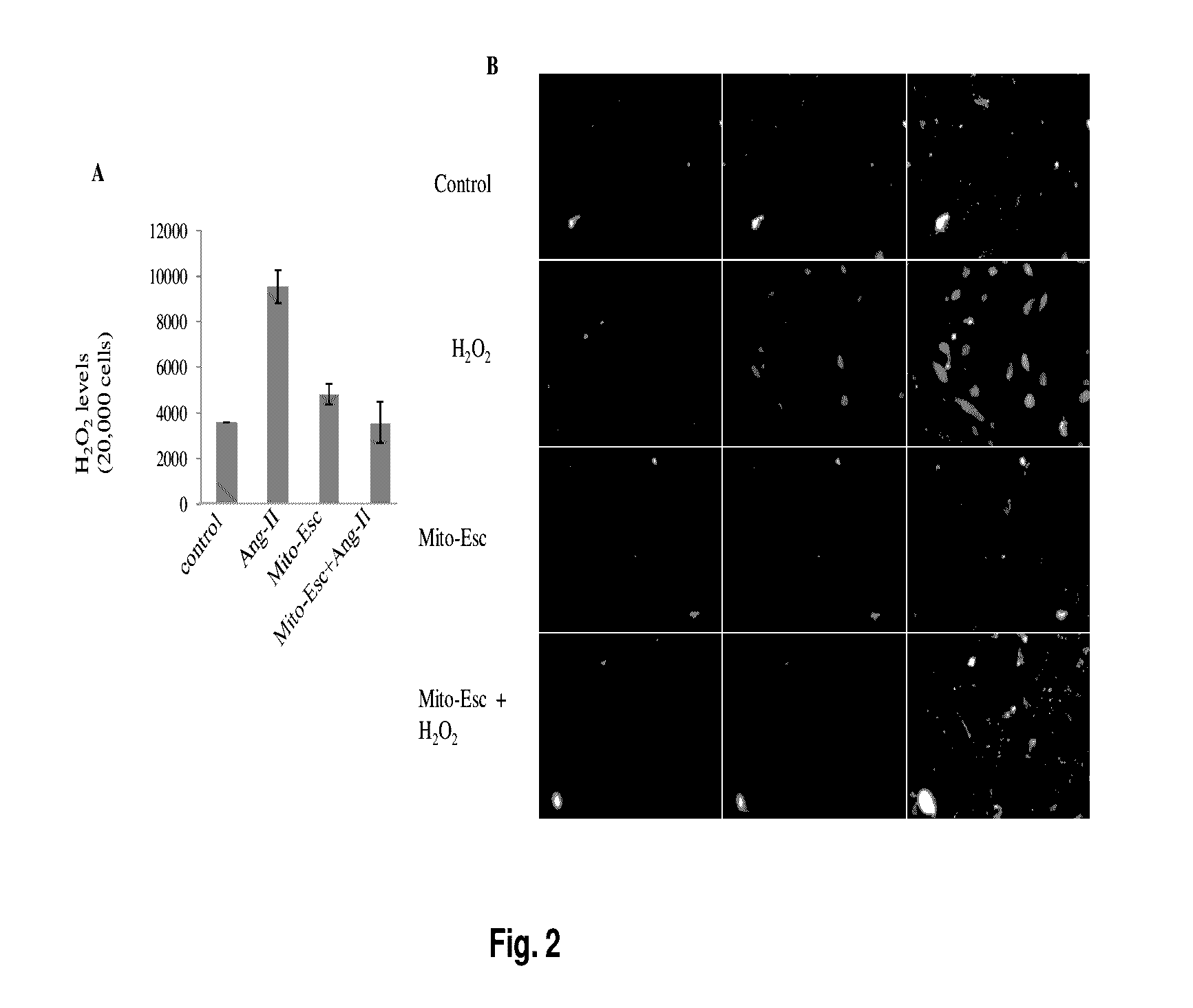
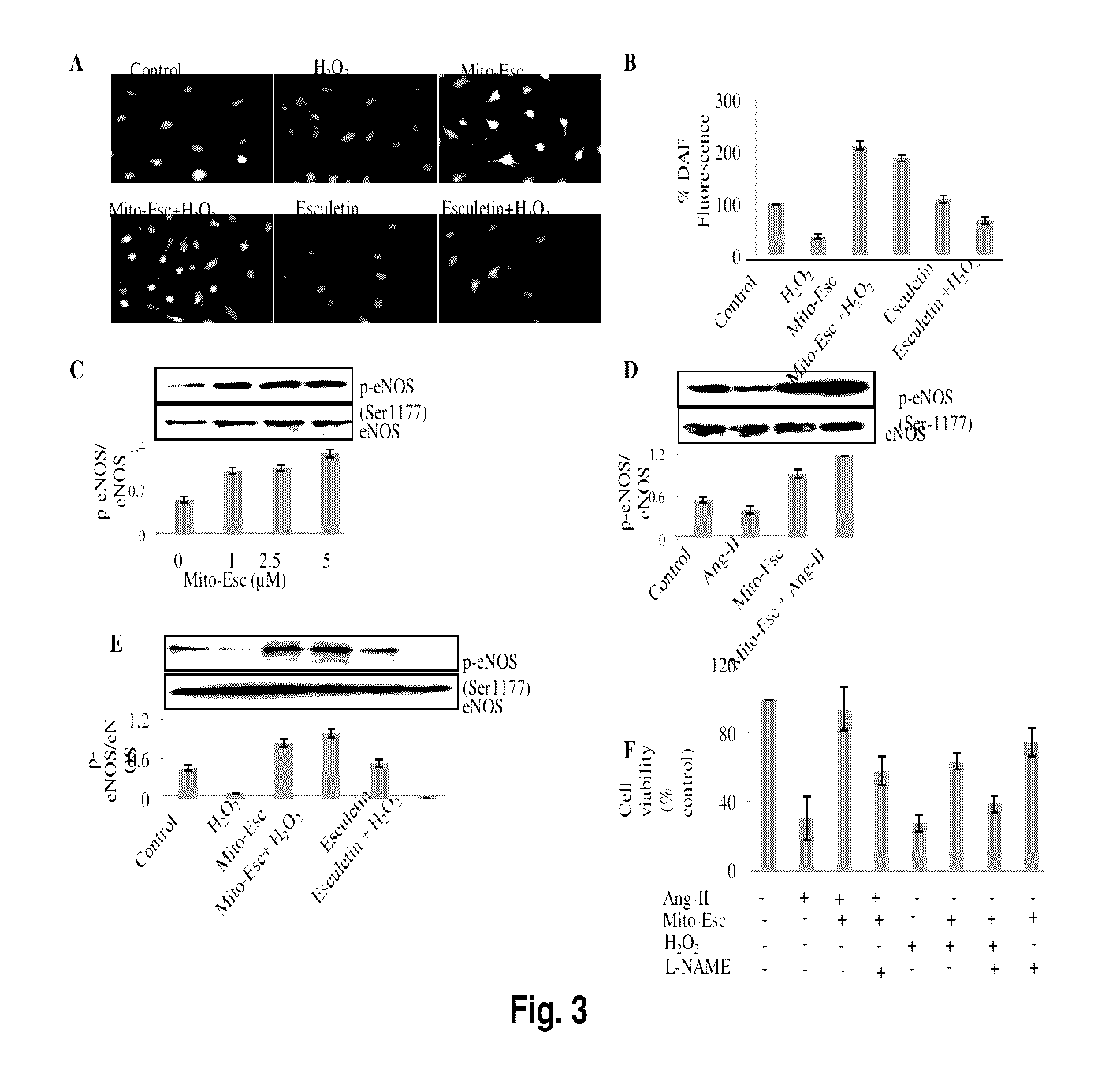
ActiveUS9580452B2Increased phosphorylationsAttenuated Ang-II-induced atherosclerosisPhosphorous compound active ingredientsPhosphorus organic compoundsBlood vesselAnti atherosclerotic
Owner:COUNCIL OF SCI & IND RES
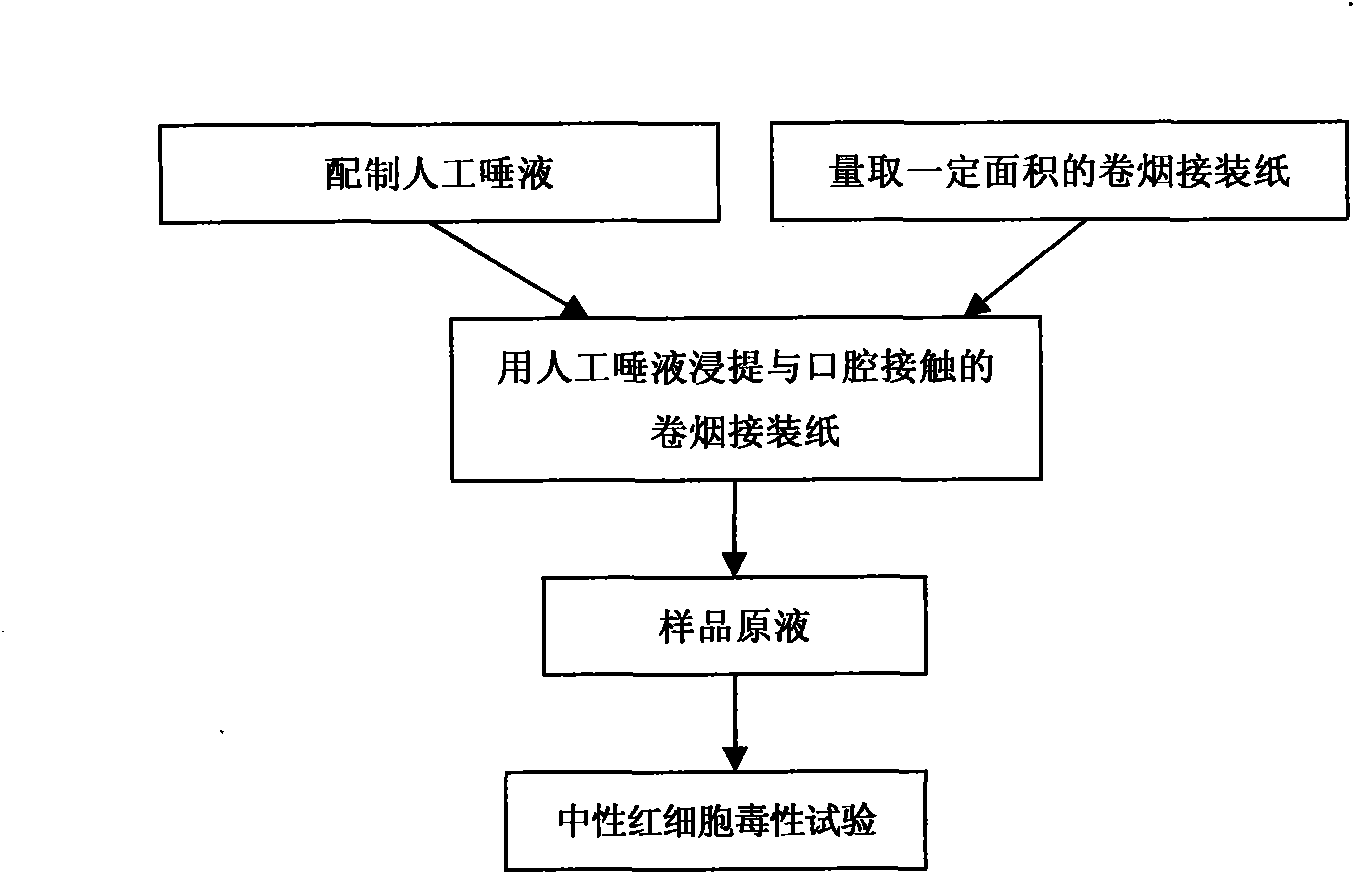
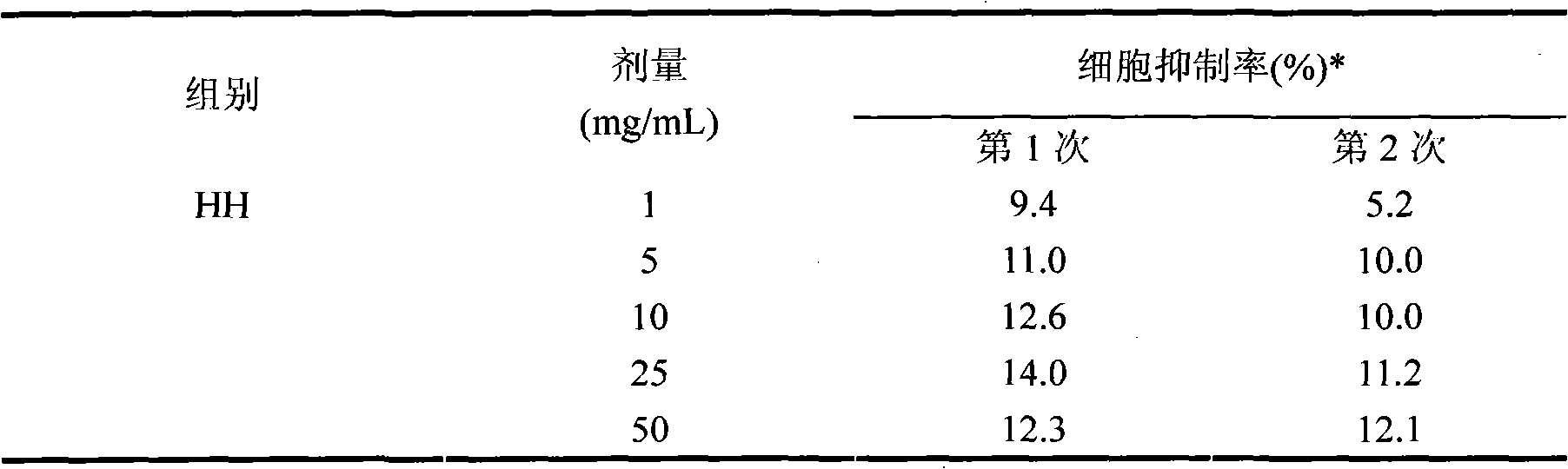
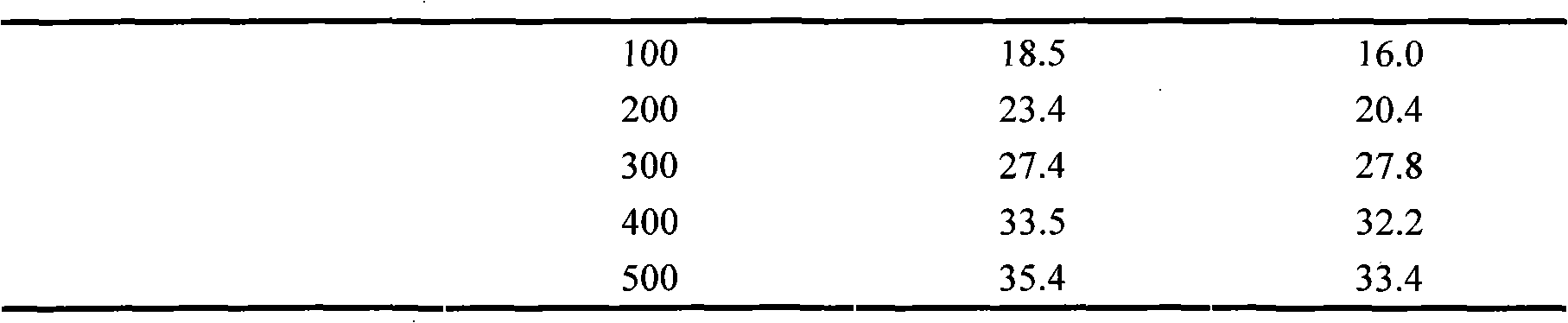
Neutral erythrocyte toxicity testing method for biological evaluation of cigarette tipping paper
InactiveCN102156188AAppropriate dose rangePractical methodBiological testingWater bathsRed blood cell
The invention relates to a neutral erythrocyte toxicity testing method for biological evaluation of a piece of cigarette tipping paper and belongs to the technical field of tobaccos. The method concretely comprises the following steps of: taking artificial saliva as tipping paper leach liquor; measuring and taking the tipping paper with a certain area, shearing to a strip with a length and a width of 1cm and 6cm and placing in a 50-250ml conical flask with a ground glass stopper for later use; pouring the leach liquor into the conical flask according to a proportion of the tipping paper area to the leach liquor of 24-36cm<2> / ml and putting back the stopper; placing the conical flask in a water-bath vibrator at a temperature of 36-38 DEG C, vibrating and leaching for 5-180min and preparing a sample stock solution; carrying out a neutral erythrocyte toxicity test, wherein doses of the sample stock solution per ml of nutrient solution successively are 1mg, 5mg, 10mg, 25mg, 50mg, 100mg, 200mg, 300mg, 400mg, 500mg; and evaluating the security of the tipping paper according to the result of the neutral erythrocyte toxicity test. The method provided by the invention has the advantages that the actual situation of the contact between the tipping paper and a mouth is effectively simulated to prepare a sample; the dose range of the sample stock solution for the neutral erythrocyte toxicity test is suitable; and the method is practical, simple and convenient.
Owner:YUNNAN RES INST OF TOBACCO SCI
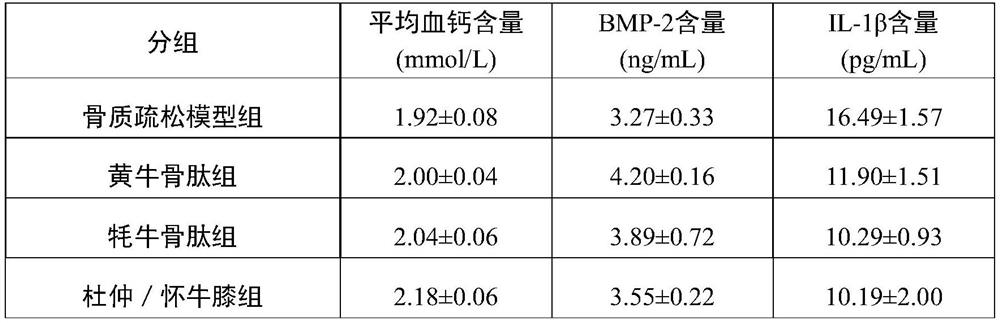
Bovine bone peptide composition and application thereof in preparation of medicine for regulating intestinal flora and preventing and treating osteoporosis
PendingCN114042146AIncrease weightPromote body lengthHydrolysed protein ingredientsDigestive systemGut floraPharmaceutical Substances
The invention relates to the technical field of bioactive peptides, in particular to a bovine bone peptide composition and application thereof in preparation of drugs for regulating intestinal flora and preventing and treating osteoporosis, and the bovine bone peptide composition provided by the invention comprises bovine bone peptide, eucommia ulmoides and achyranthes bidentata. The bovine bone peptide composition provided by the invention can be used for preventing and treating osteoporosis, promoting growth and development of thighbone, regulating calcium and phosphorus metabolism, promoting bone formation and reducing bone resorption. Through animal in-vivo biological evaluation, the bovine bone peptide composition provided by the invention can change the intestinal flora diversity and species community composition of mice, so that the disordered intestinal flora tends to be normal, and bone metabolism is regulated, thereby improving osteoporosis.
Owner:ANHUI GUOTAI BIOTECHNOLOGY CO LTD
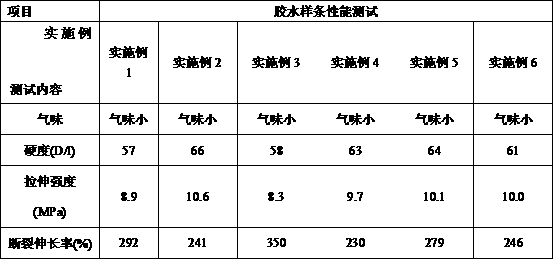
Low-odor and high-toughness ultraviolet curing adhesive and preparation method thereof
InactiveCN109929503ASmall smellImprove toughnessNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesAdhesiveEthylene Oxide Sterilization
A low-odor and high-toughness ultraviolet curing adhesive comprises the following raw materials in percentage by weight: 25-50% of acrylic modified polyurethane resin, 45-70% of an acrylic monomer diluent, 15-45% of a toughening monomer, 2-3% of a silane coupling agent, 2-3% Of a photoinitiator, 0-0.2% of a polymerization inhibitor and 0.1-0.5% of other auxiliaries; compared with the prior art, the low-odor and high-toughness ultraviolet curing adhesive has the beneficial effects of being small in odor, high in toughness, resistant to ethylene oxide sterilization and damp-heat aging, and qualified in biological evaluation performance, and can be used for bonding among common polymer materials such as PVC, PC, ABS and k-resin used in medical instruments.
Owner:HENAN TUOREN MEDICAL DEVICE GRP
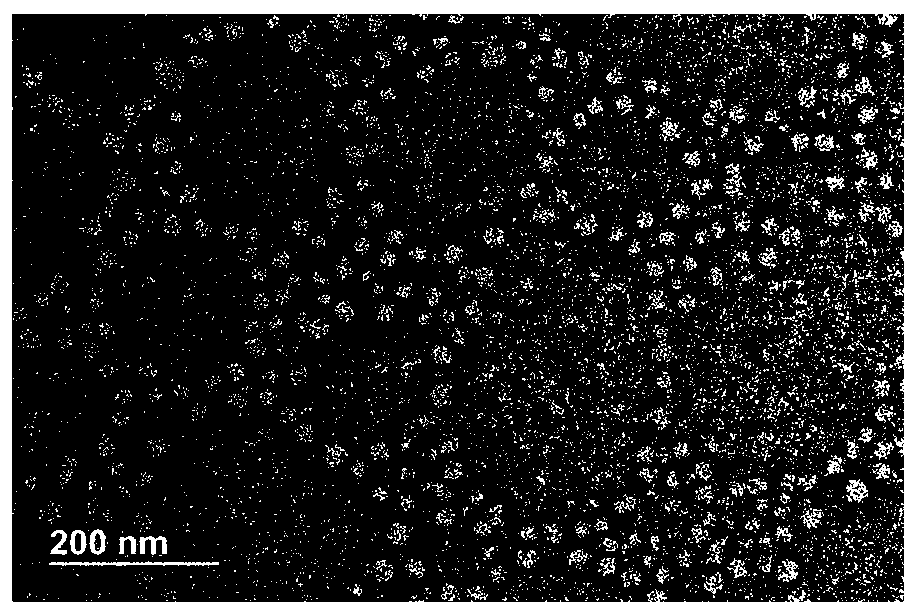
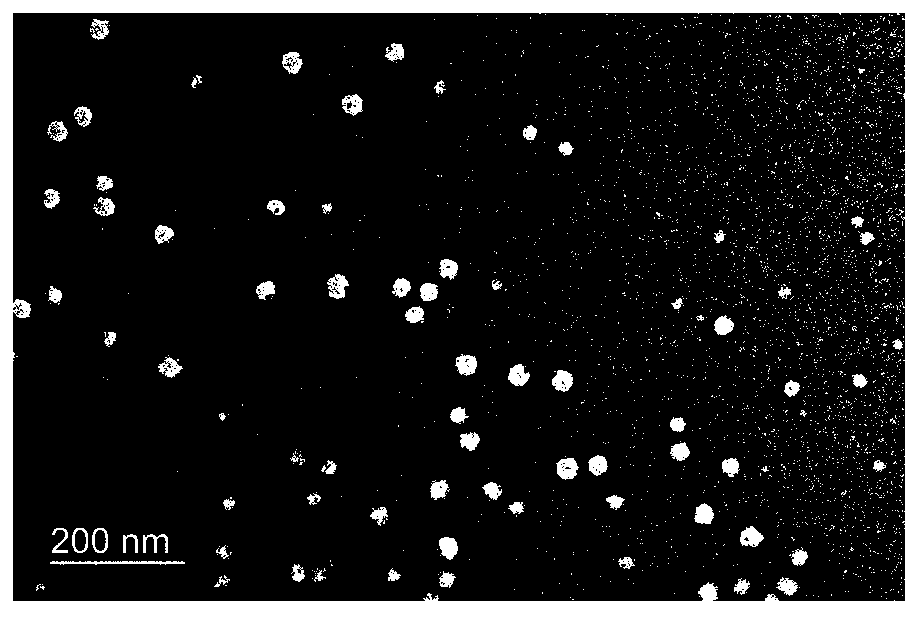
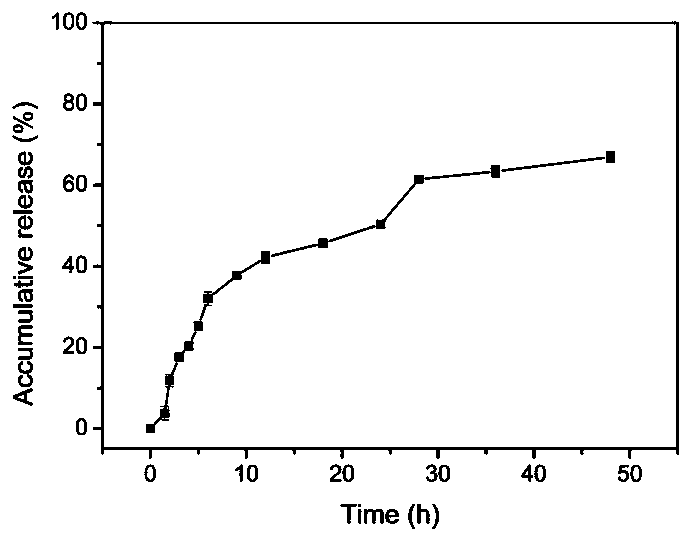
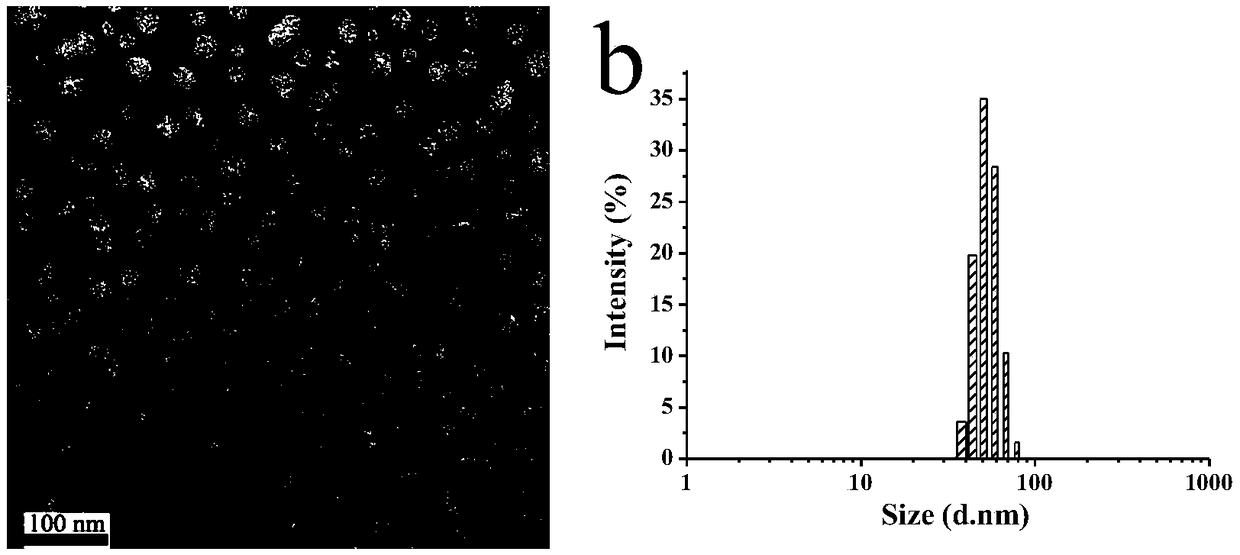
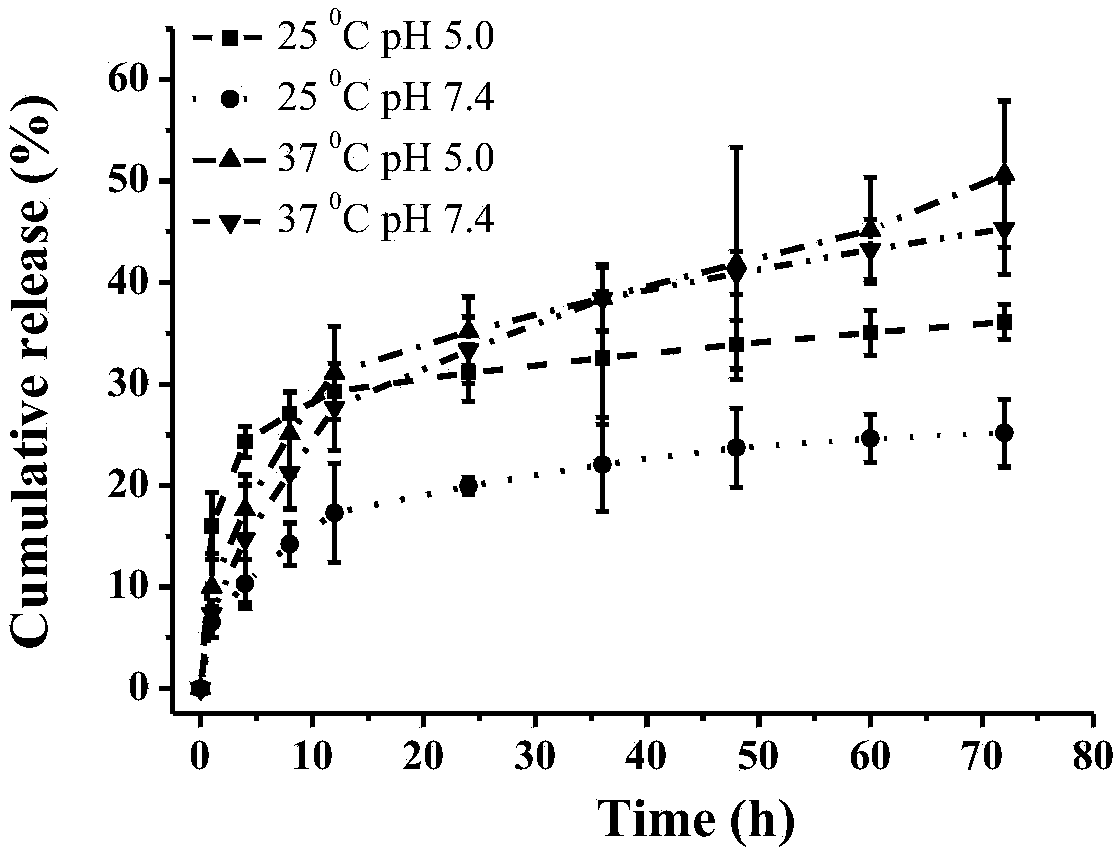
Method for preparing novel polyethylene glycol-poly gamma butyrolactone di-block copolymer nano drug carrying microsphere
InactiveCN110478332AGood biocompatibilityPrecise particle size controlOrganic active ingredientsPharmaceutical non-active ingredientsCarrying capacityMicrosphere
The invention discloses a method for preparing a novel polyethylene glycol-poly gamma butyrolactone (PEG-PBL) di-block copolymer nano drug carrying microsphere. The provided PEG-PBL copolymer copolymer nano drug carrying microsphere has a small particle size, is narrowly distributed, has a high drug carrying capacity, can be slowly released for a long time, and can reduce the drug delivery frequency. In addition, according to results of biological evaluation, it is proved that the polyethylene glycol-poly gamma butyrolactone di-block copolymer nano drug carrying microsphere has excellent biocompatibility and anticancer activity. The provided novel polyethylene glycol-poly gamma butyrolactone di-block copolymer nano drug carrying microsphere has a simple preparation method, is easily produced on a large scale, and has broad application prospects in the field of drug slow release.
Owner:QINGDAO UNIV OF SCI & TECH
Water environment detection device for water biological evaluation
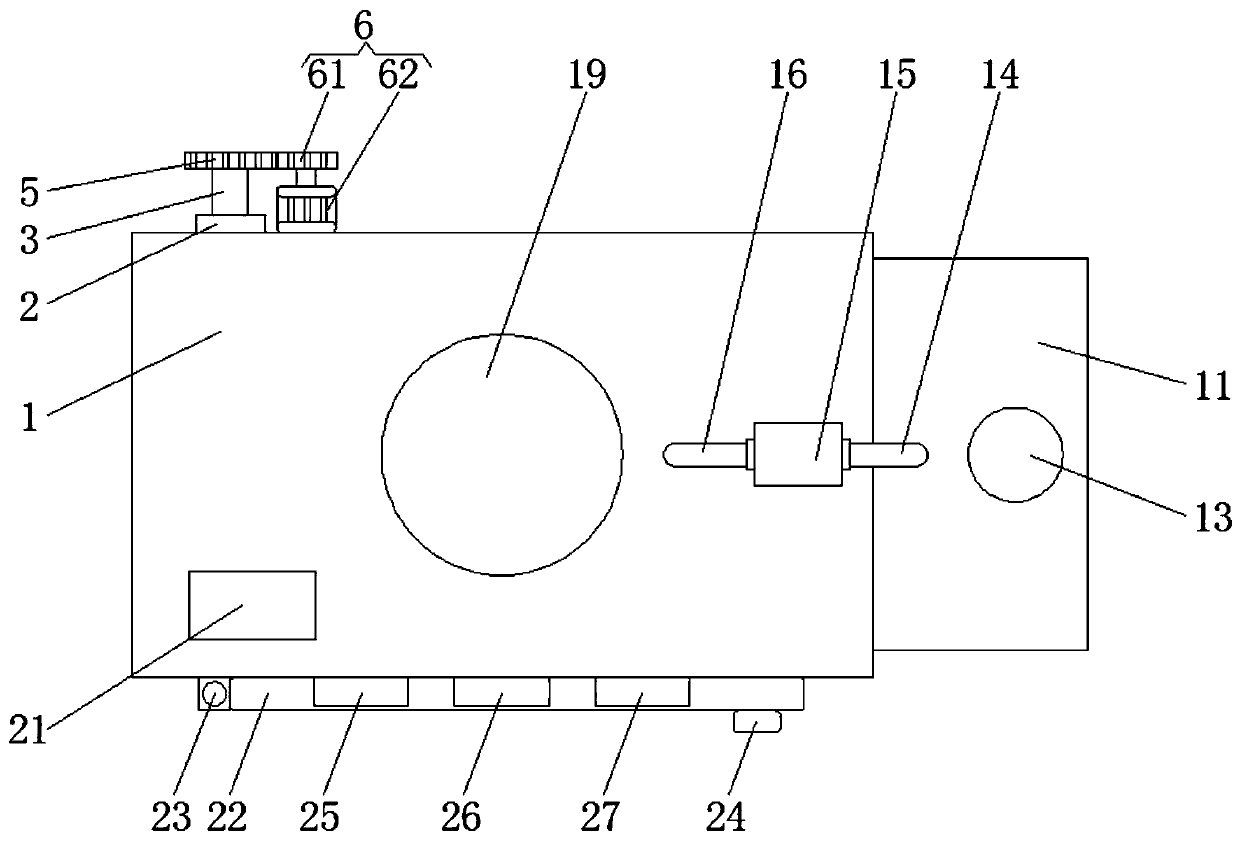
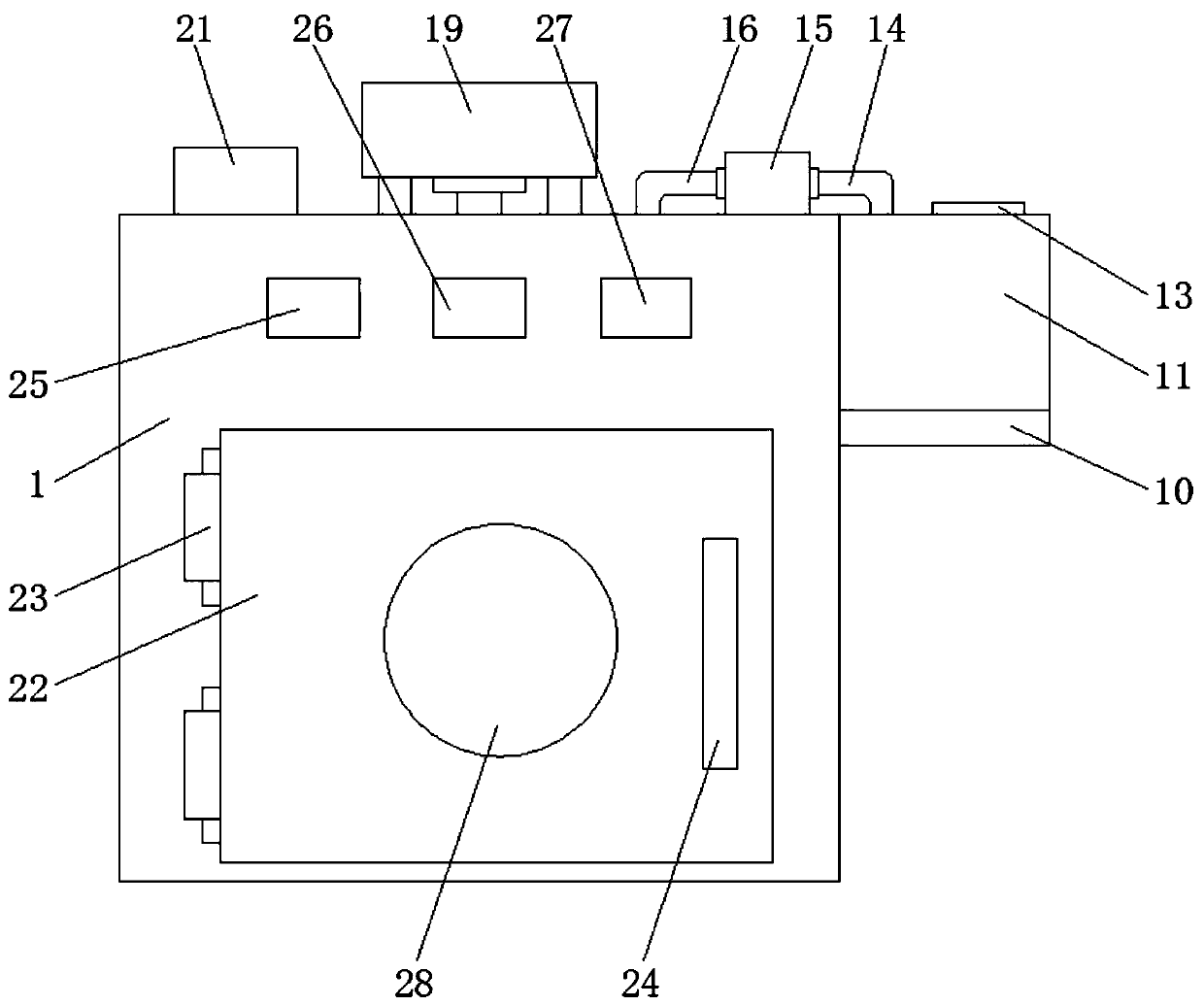
InactiveCN108333319AMeet regulatory needsGuarantee the safety of lifeTransmission systemsTesting waterElectric machineryEngineering
The invention discloses a water environment detection device for water biological evaluation. The water environment detection device comprises a support seat, wherein the top end of an inner cavity ofthe support seat is sequentially and fixedly connected with a wireless signal emitter and a 4G communication module from left to right; a motor is fixedly arranged at the periphery of the bottom of the inner cavity of the support seat; an output shaft of the motor is fixedly connected with blades; the middle end of the bottom of the support seat is provided with a groove. The motor is fixedly arranged at the periphery of the bottom of the inner cavity of the support seat; the output shaft of the motor is fixedly connected with the blades; meanwhile, through the effects of a first connecting rod, a first moving joint, a second connecting rod, the 4G communication module, a fourth connecting rod, a third moving joint, a second electric telescopic rod, a second moving joint and a third connecting rod, the regulation requirements of people on the deflecting plate deflection angle can be met; the detection device can reach any water region to be detected; the detection range is increased;meanwhile, the life safety of detection personnel is also guaranteed.
Owner:李丽芳
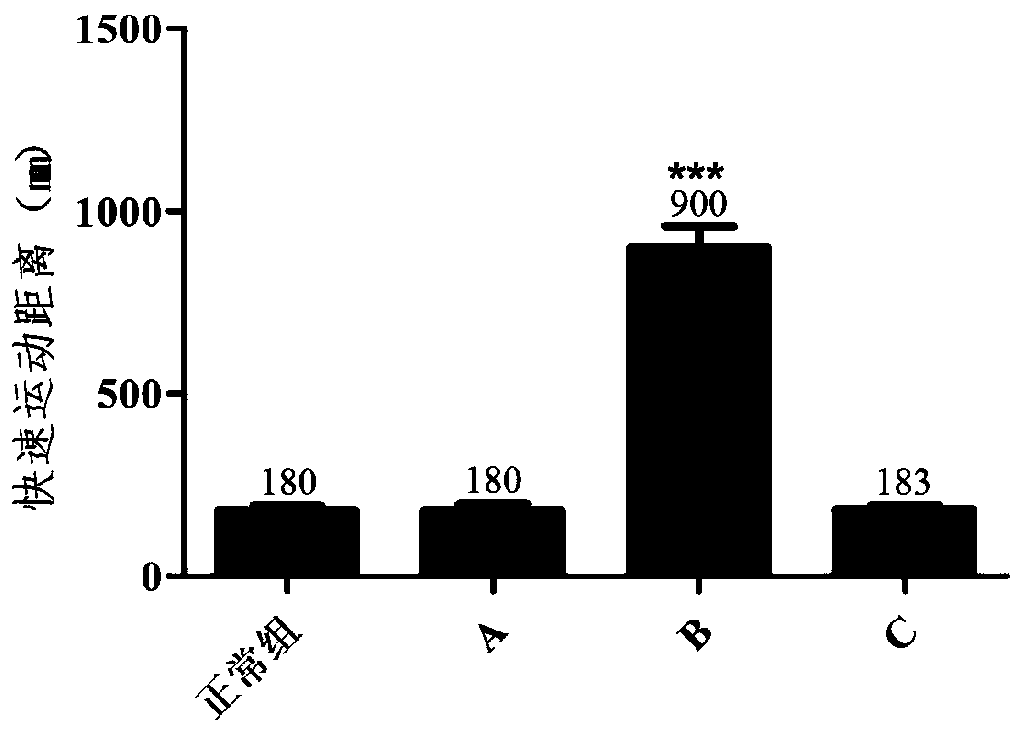
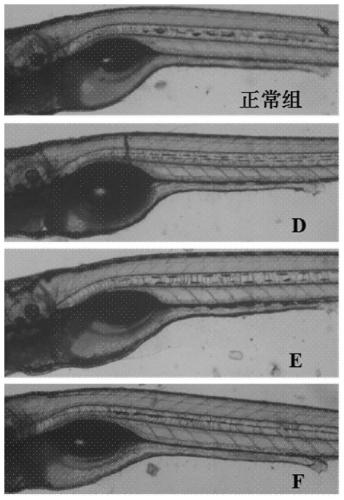
Method for rapidly detecting on-site catering safety
The invention relates to a method for rapidly detecting on-site catering safety, and the method comprises the following steps: constructing a zebra fish toxicity evaluation model, and evaluating the eating safety of catering food by detecting a zebra fish movement function, a cardiovascular function or an intestinal peristalsis function. According to the rapid detection method established by the invention, based on the thinking model of overall biological evaluation, the safety of catering food is integrally evaluated by qualitatively or quantitatively observing the transportation behavior, heartbeat and blood flow and diarrhea conditions of zebra fish, and the detection thought is not limited by the toxic influence of a traditional chemical detection method on a single component. The detection method provided by the invention is good in universality, can systematically and comprehensively evaluate the biological safety of diet in catering industries including restaurants, fast food restaurants, snack shops, canteens and the like, and has the advantages of quickness, convenience, reliability, good predictability and the like which cannot be compared by a traditional detection method.
Owner:南京新环检测科技有限公司
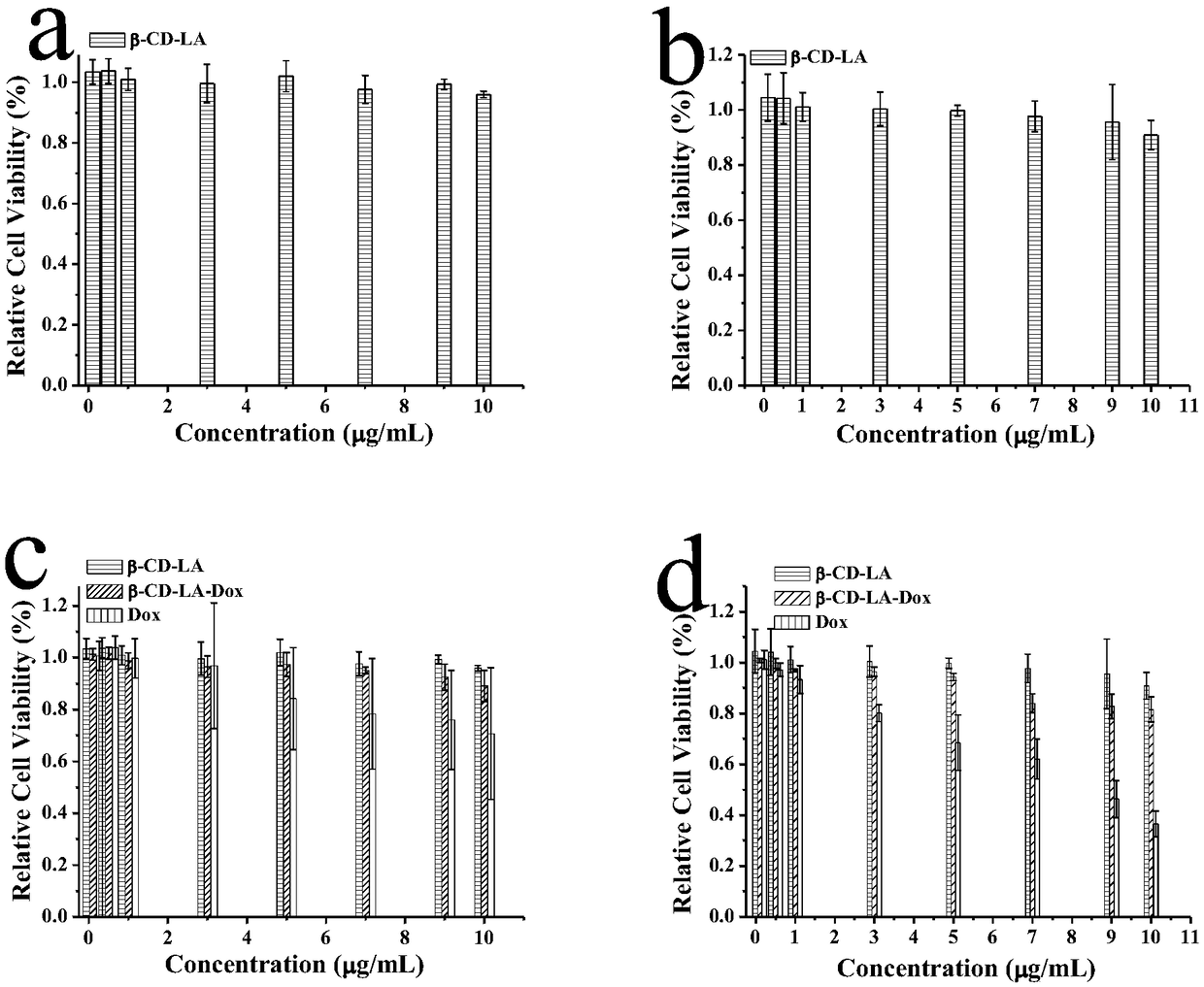
Synthesis of beta-CD-LA (cyclodextrin-linoleic acid) molecules and application of aggregate formed by beta-CD-LA molecules as drug delivery system
InactiveCN109125263APH responsivePH-responsive release propertiesOrganic active ingredientsEmulsion deliverySolubilityBiocompatibility Testing
The invention relates to synthesis of beta-CD-LA (cyclodextrin-linoleic acid), preparation of a beta-CD-LA micelle and a beta-CD-LA-Dox nanodrug and biological evaluation methods in vitro and in vivo,and belongs to the field of medicines. The preparation method comprises the following steps: firstly, LA activates a carboxylic acid group into a reactive carboxylic acid group under the action of DCC and NHS, and then is subjected to reaction with cyclodextrin, and beta-CD-LA molecules are generated. The beta-CD-LA molecules are self-assembled in an aqueous solution, and the micelle can be formed. Lipophilic anticancer drug doxorubicin is loaded into the beta-CD-LA micelle with an organic solvent volatilization method, and the beta-CD-LA-Dox nanodrug is formed. The beta-CD-LA micelle formedby the beta-CD-LA molecules has good biocompatibility, water solubility and stability. The beta-CD-LA-Dox nanodrug has slow pH-responsive release property and is capable of inducing apoptosis in cancer cells. Besides, the beta-CD-LA-Dox nanodrug can induce tumor cell apoptosis in tumor tissue and has anti-tumor effect, thereby having good application prospect.
Owner:BINZHOU MEDICAL COLLEGE
Synthesis and biological evaluation of 2',5'-dimethoxychalcone derivatives as microtubule-targeted anticancer agents
InactiveUS20110306775A1Readily apparentOrganic chemistryAntineoplastic agentsTerthiopheneBiologic DMARD
Disclosed are a serious of 2′,5′-dimethoxychalcone derivatives for treating cancer, wherein 2,5-dimethoxyacetophenone and methyl 4-formylbenzoate are condensed to form 4-carboxyl-2′,5′-dimethoxychalcone (compound 1), which is further reacted with alkyl halides or amines to synthesize the chalcone derivatives of compounds 2-17. In addition, 2,5-dimethoxyacetophenone is reacted with 5-formyl-2-thiophenecarboxylic acid to form compound 18 (3-(3-thiophene)carboxyl-1-(2,5-dimethoxyphenyl)prop-2-en-1-one). The synthesized 2′,5′-dimethoxychalcone derivatives can be acted as microtubule-targeted tubulin-polymerizing agents.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Method for detecting phosphorylation level of intracellular tyrosine
PendingCN111521587AEasy to operateShort detection cyclePreparing sample for investigationIndividual particle analysisImmunofluorescenceIntracellular
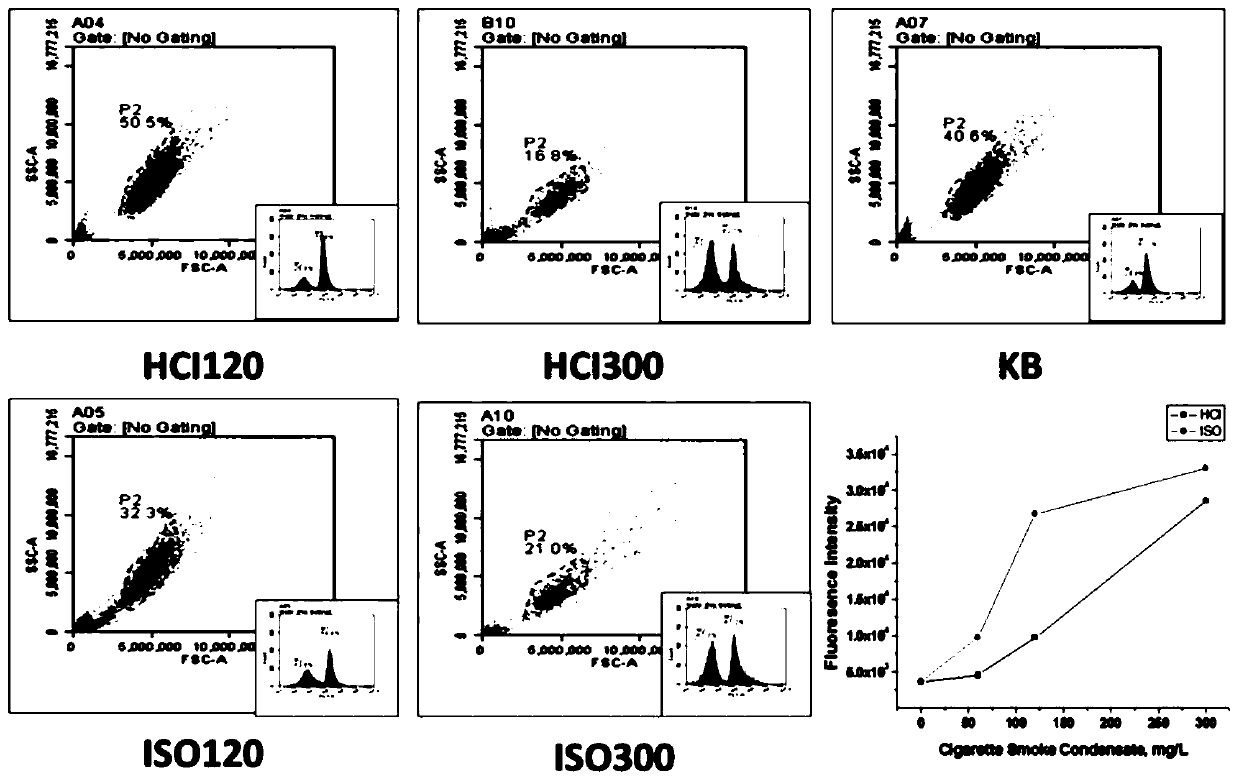
The invention discloses a method for detecting the phosphorylation level of intracellular tyrosine. The method comprises the following steps: preparing a smoke condensate, treating cells, carrying outimmunofluorescence labeling and sample treatment, and detecting. By researching the concentration level of active oxygen in cells after smoke exposure, the influence of smoke exposure on oxidative stress reaction of cells is evaluated, biological evaluation is performed on the safety of smoke components, and scientific reference is reversely provided for research, development and production of safe cigarettes.
Owner:SICHUAN BRANCH OF CHINA TOBACCO +2
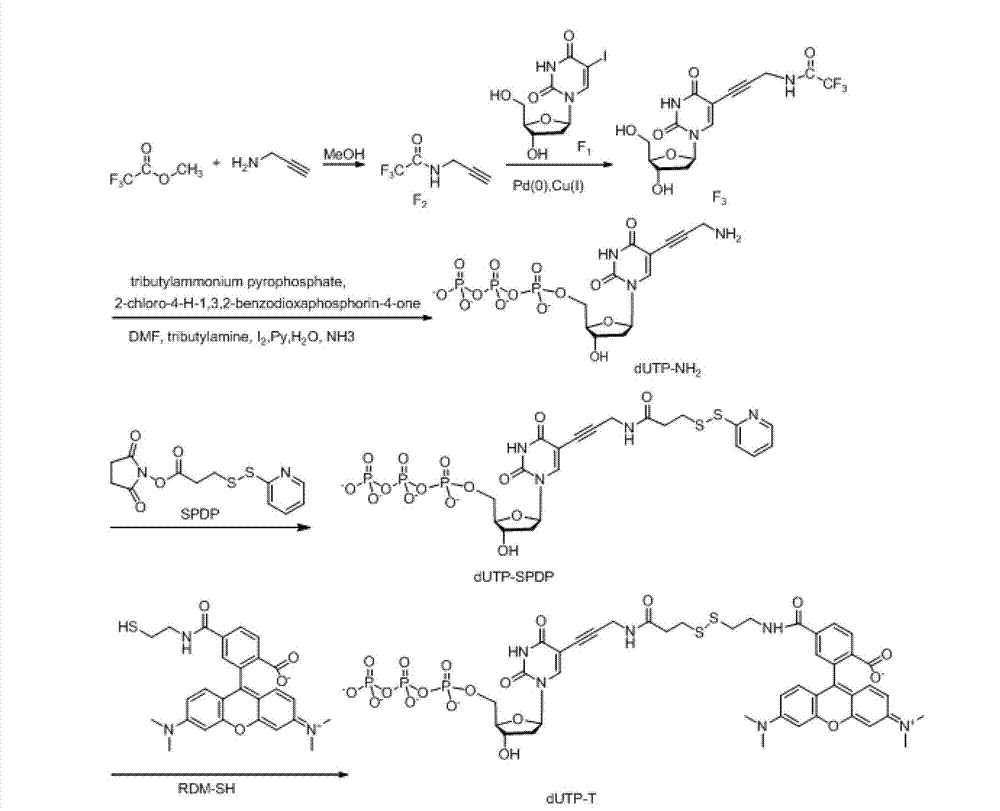
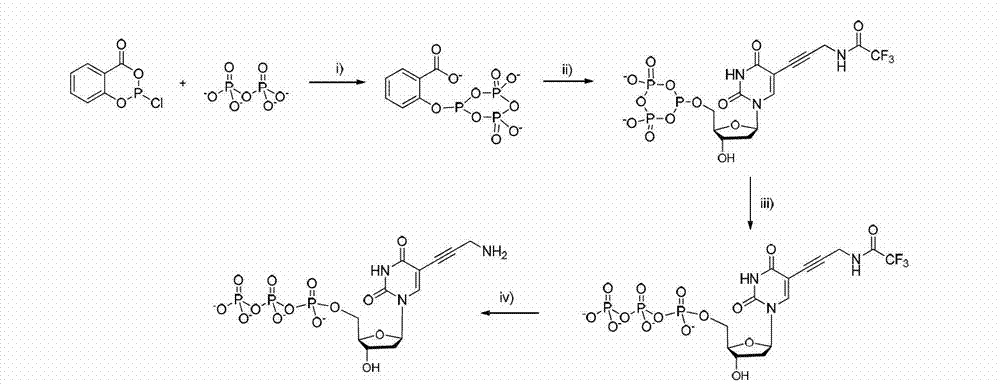
Radiotracer introduced [18F]fluoromethyl group targeting neuroinflammation for PET imaging and Synthesis of Radiotracer and its biological evaluation Method for Radiotracer
InactiveUS20160263258A1Overcome problemsHigh radiochemical yieldIsotope introduction to heterocyclic compoundsAntipyreticRadioactive tracerPet imaging
Disclosed are an [18F]fluoromethyl group-introduced radiotracer for brain neuroinflammation-targeting positron emission tomography (PET), the synthesis thereof, and a method for evaluating biological results using the same. In the method for the synthesis of an [18F]fluoromethyl group-introduced radiotracer for brain neuroinflammation-targeting positron emission tomography, a compound obtained by introducing triazolium triflate into normethyl-PBR28 is used as a precursor and a fluoromethyl group is labeled with fluorine-18 in a single step. The [18F]fluoromethyl group-introduced radiotracer for brain neuroinflammation-targeting positron emission tomography is synthesized by using a compound, obtained by introducing triazolium triflate into normethyl-PBR28, as a precursor and performing substitution with fluorine-18 in a single step.
Owner:BIO IMAGING KOREA
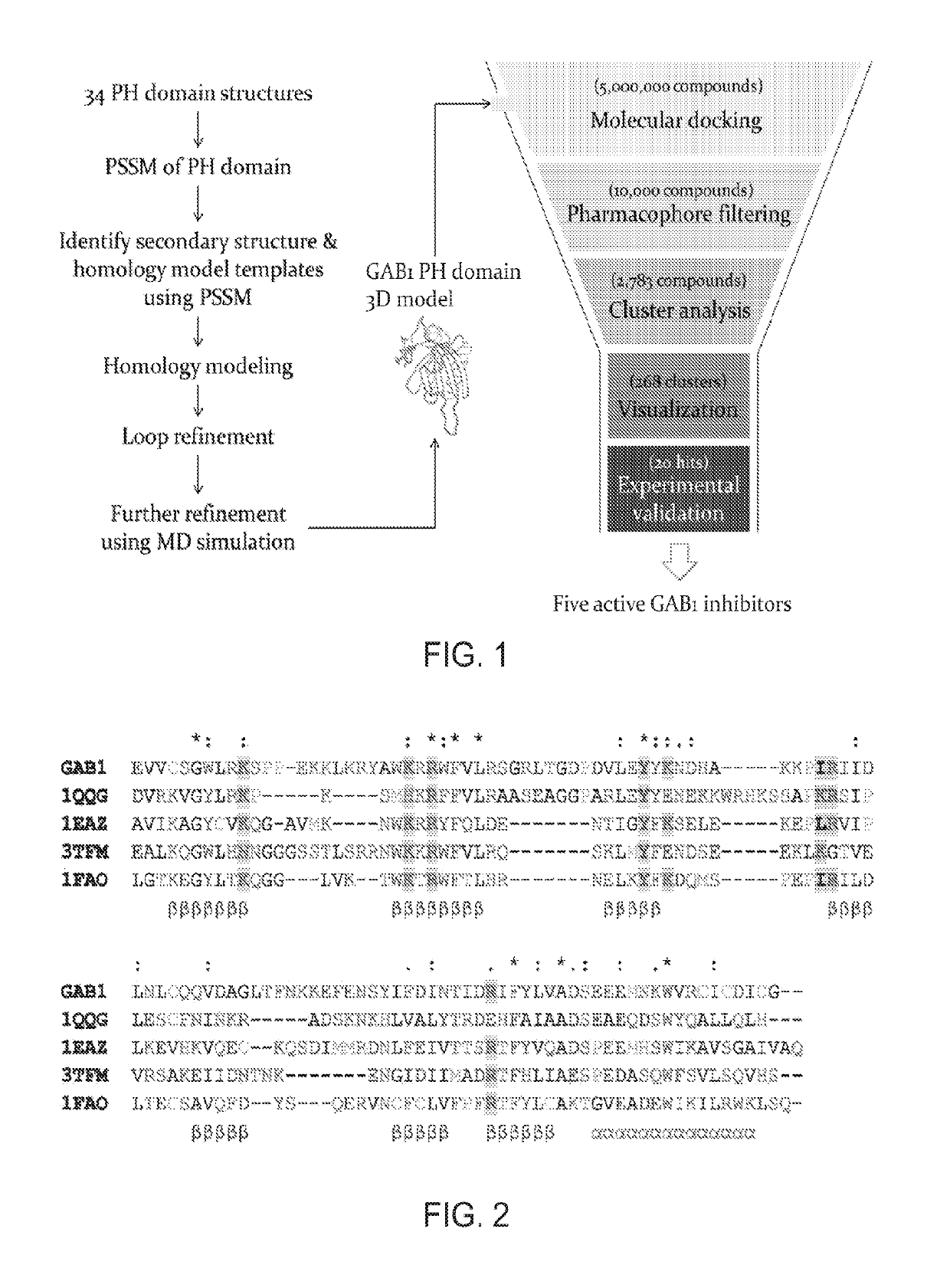
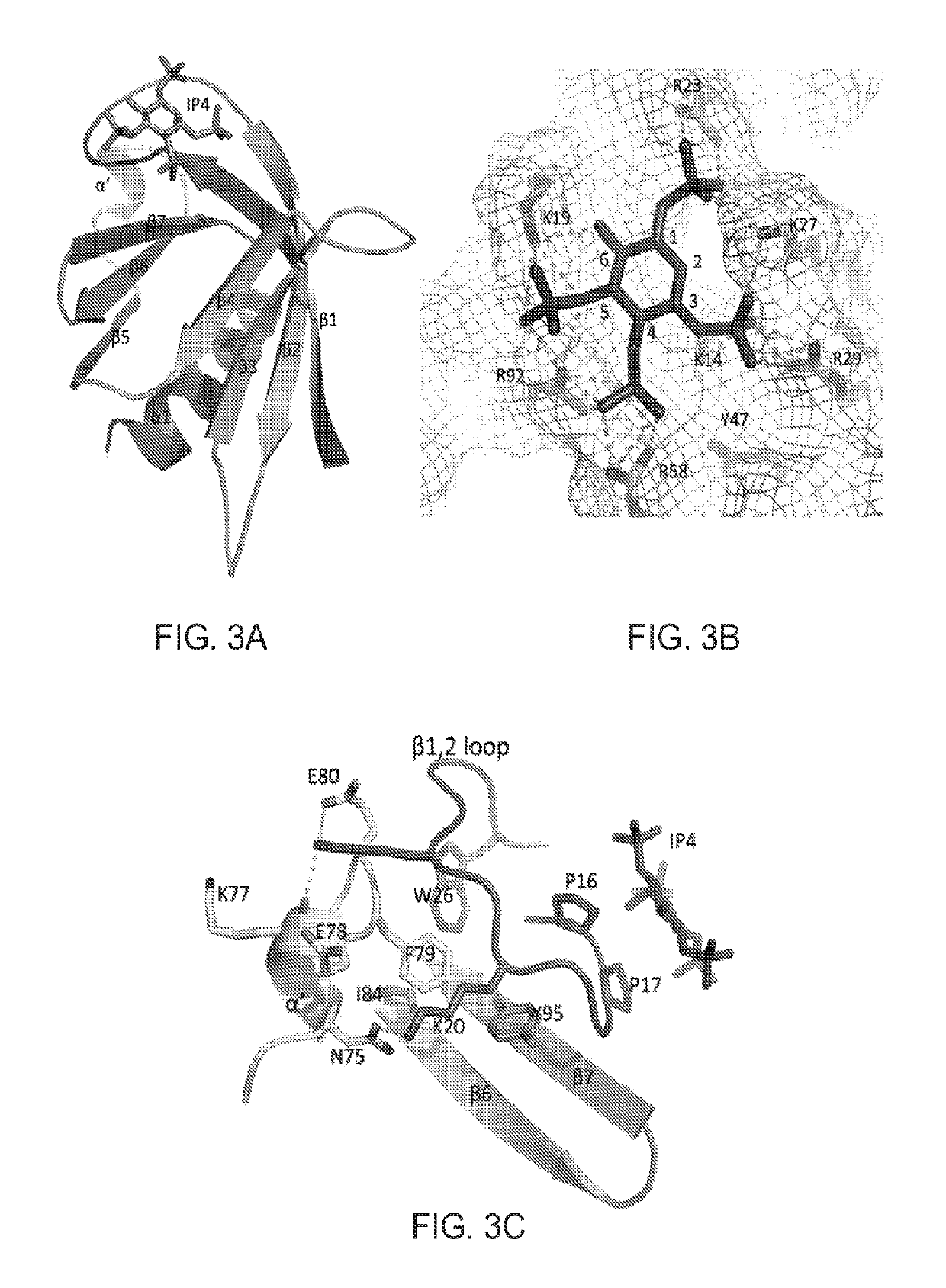
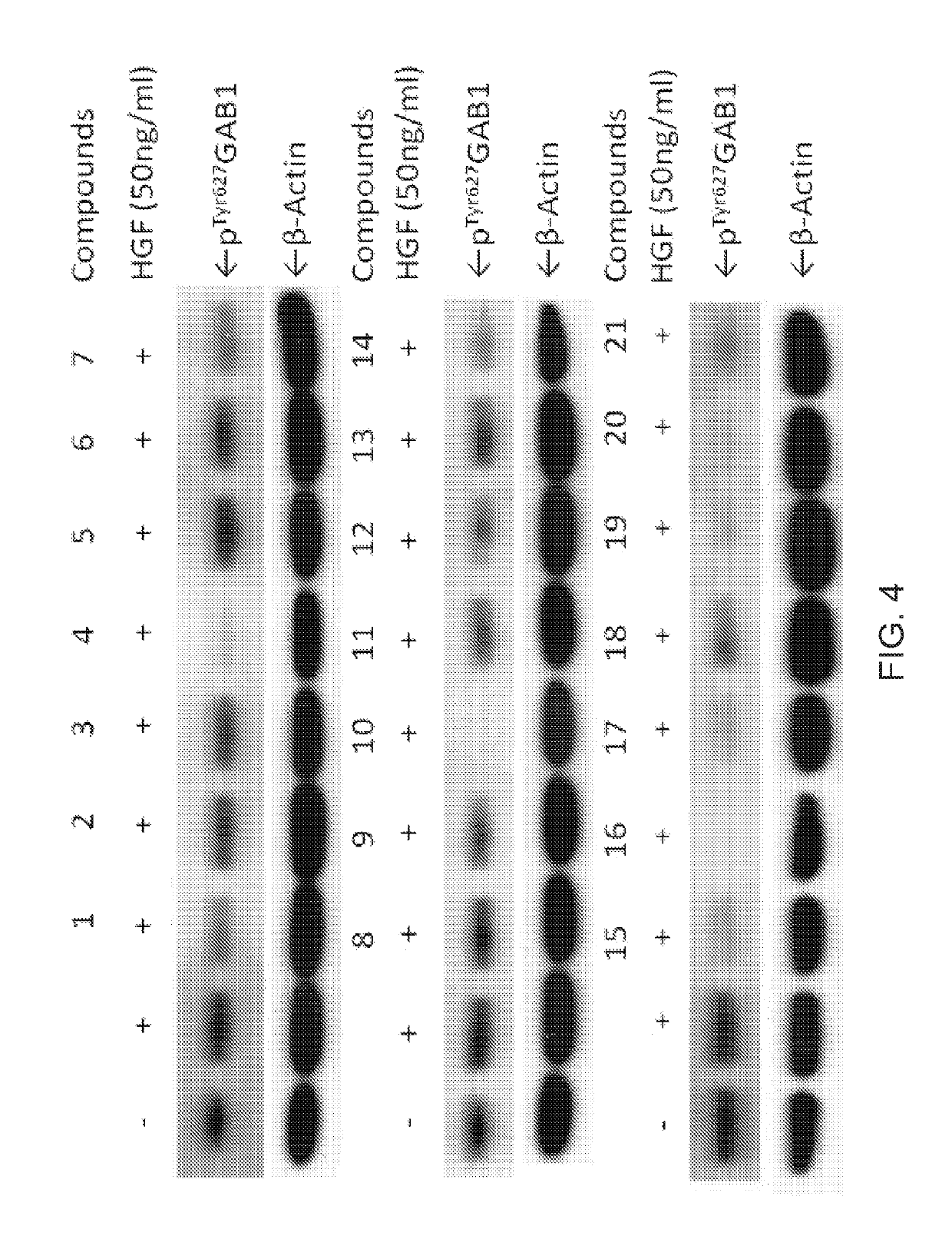
Inhibitors of GRB2-associated binding protein 1 (GAB1) and methods of treating cancer using the same
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Application of SLC2A1 expression to preparing kit for detecting fertility of oocytes
ActiveCN109825564ASimple and easy quality control meansMicrobiological testing/measurementBiologySlc2a1 gene
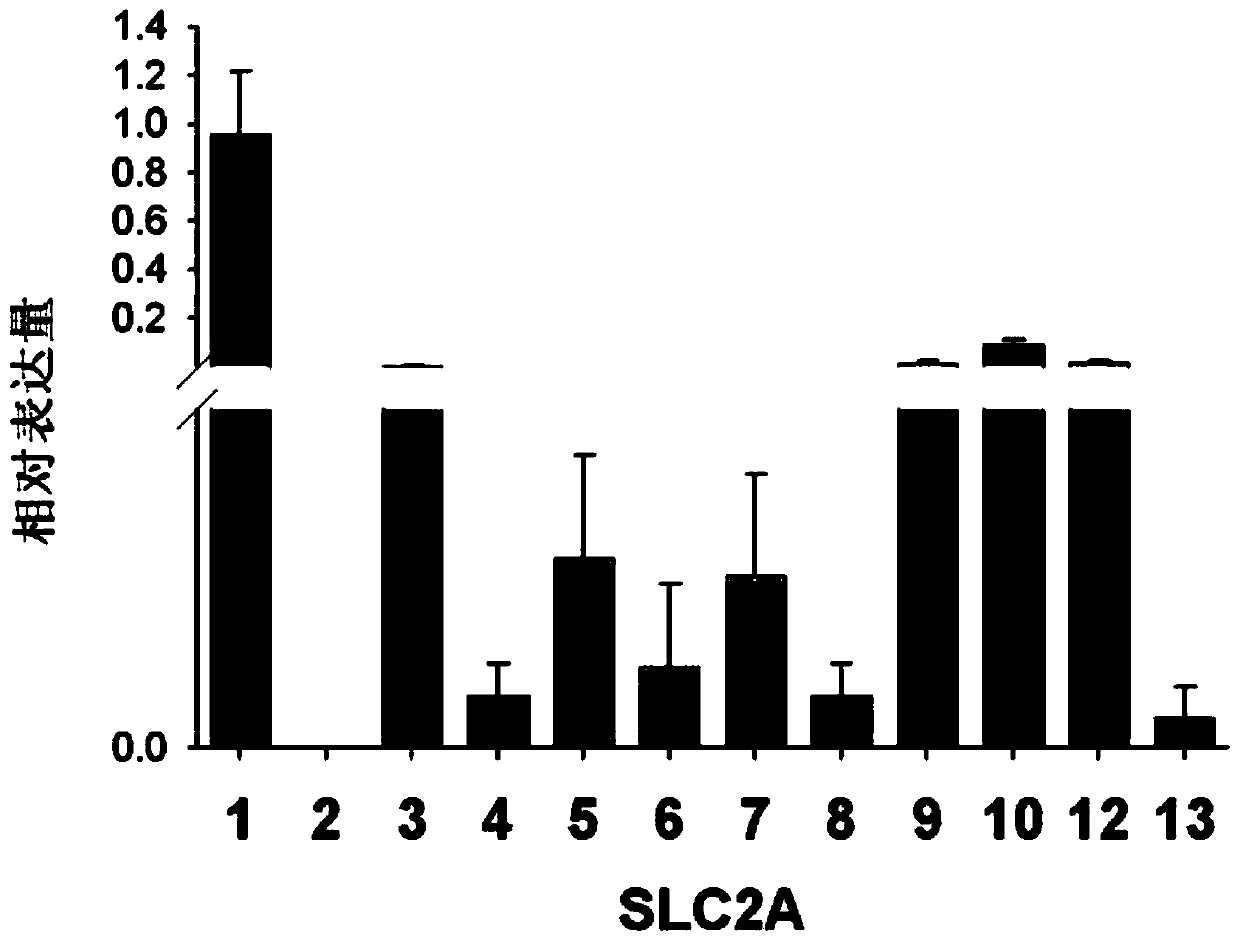
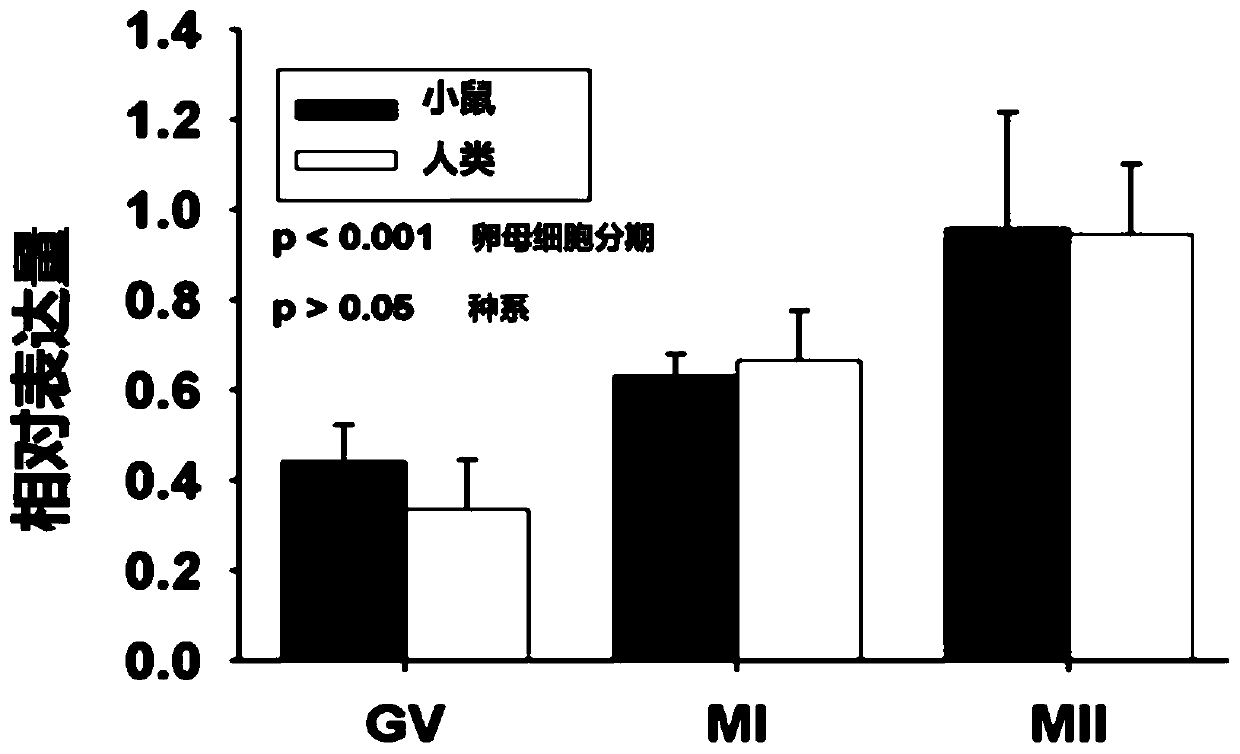
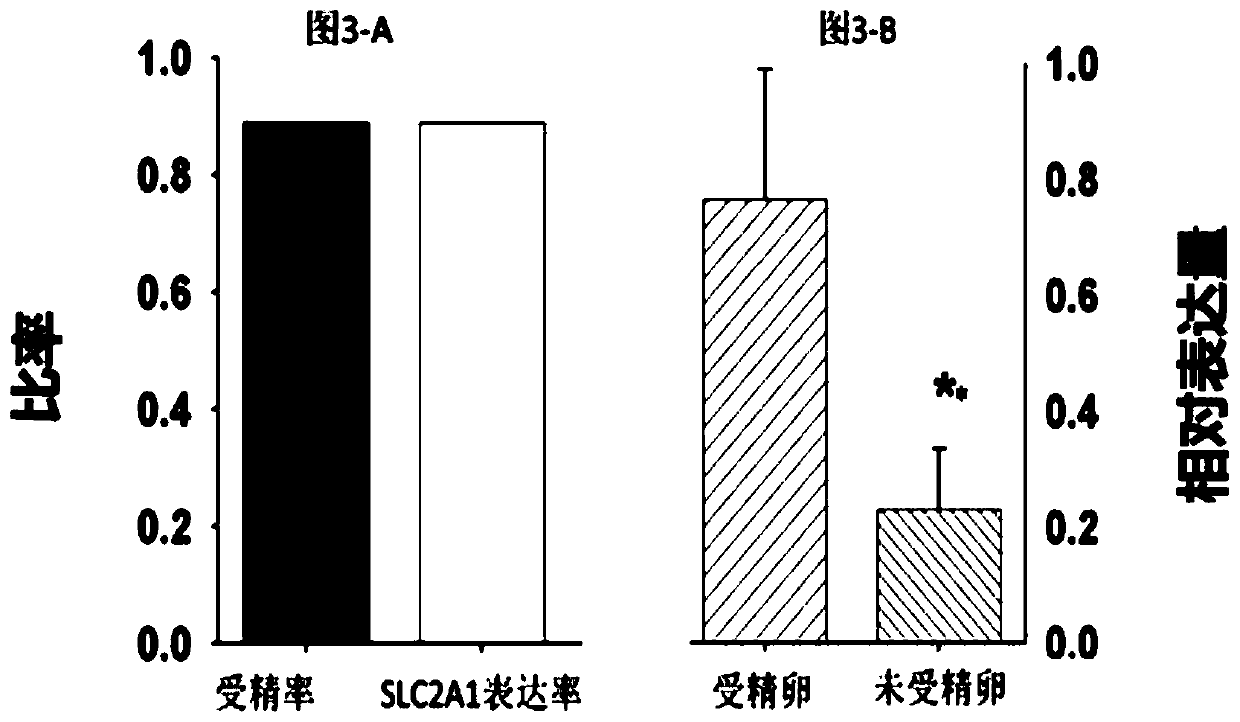
The invention discloses application of SLC2A1 expression to preparing a kit for detecting the fertility of oocytes, and a method for evaluating the physiological function of the oocytes, and belongs to the technical field of the cytology and biology. The application is uppermost characterized in that SLC2A1 is glucose transport protein the most expressed in the glucose transport protein family inthe oocytes, the SLC2A1 expression amount is in positive correlation with the fertility in the oocytes, the unfertilized oocytes present the missing and remarkable dropping of SLC2A1 genes, the kit for detecting the fertility of the oocytes detects and analyzes the expression of all the currently-known members in the same SLC2A family in the oocytes through the real-time fluorescent quantitative reverse transcription polypeptide chain reaction (qRT-PCR) method, it is found that the SLC2A1 is related to the quality and fertility of the oocytes, and the effective biological evaluation means is provided for controlling the quality of the assisted reproductive technology.
Owner:南京优而生物科技发展有限公司
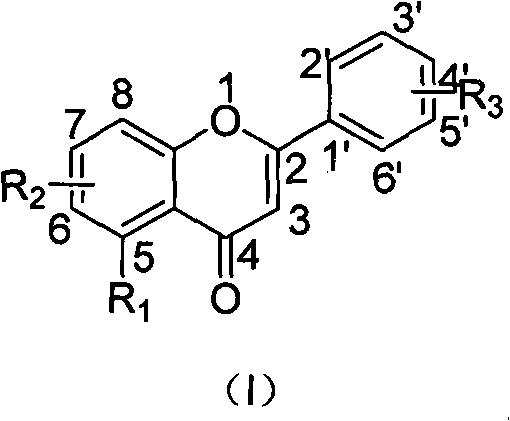
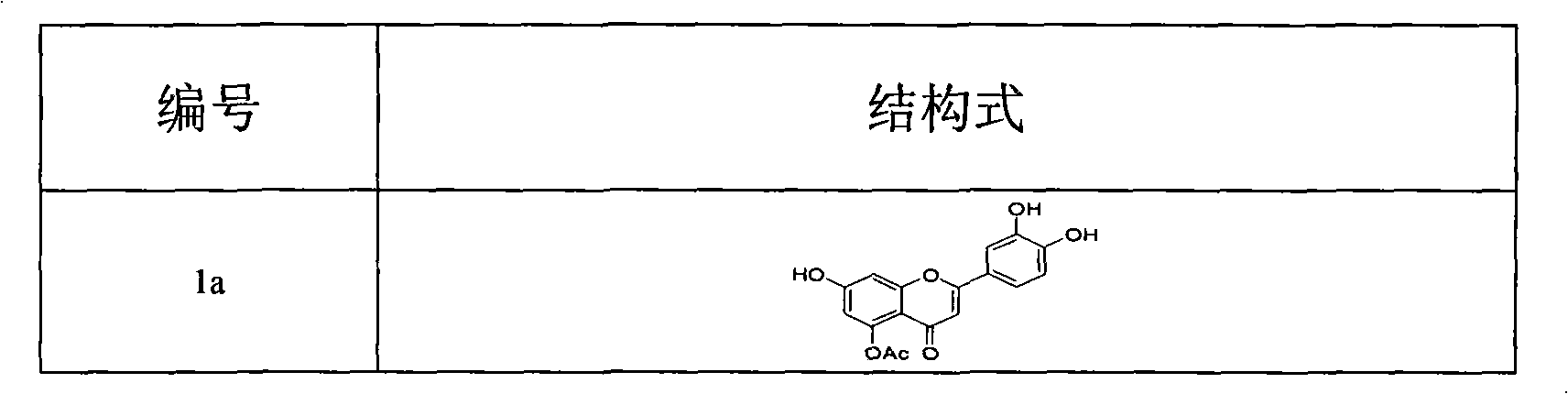
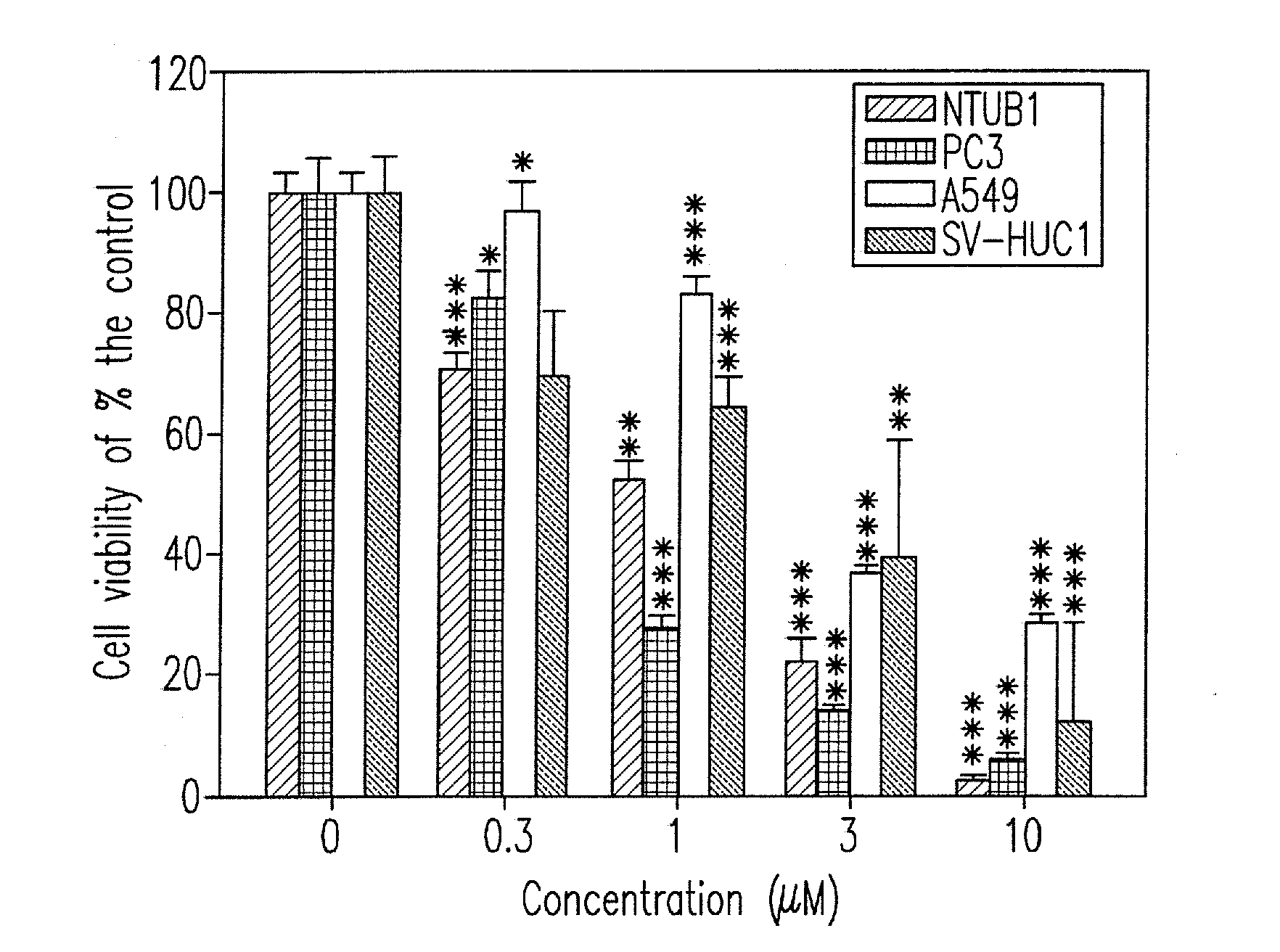
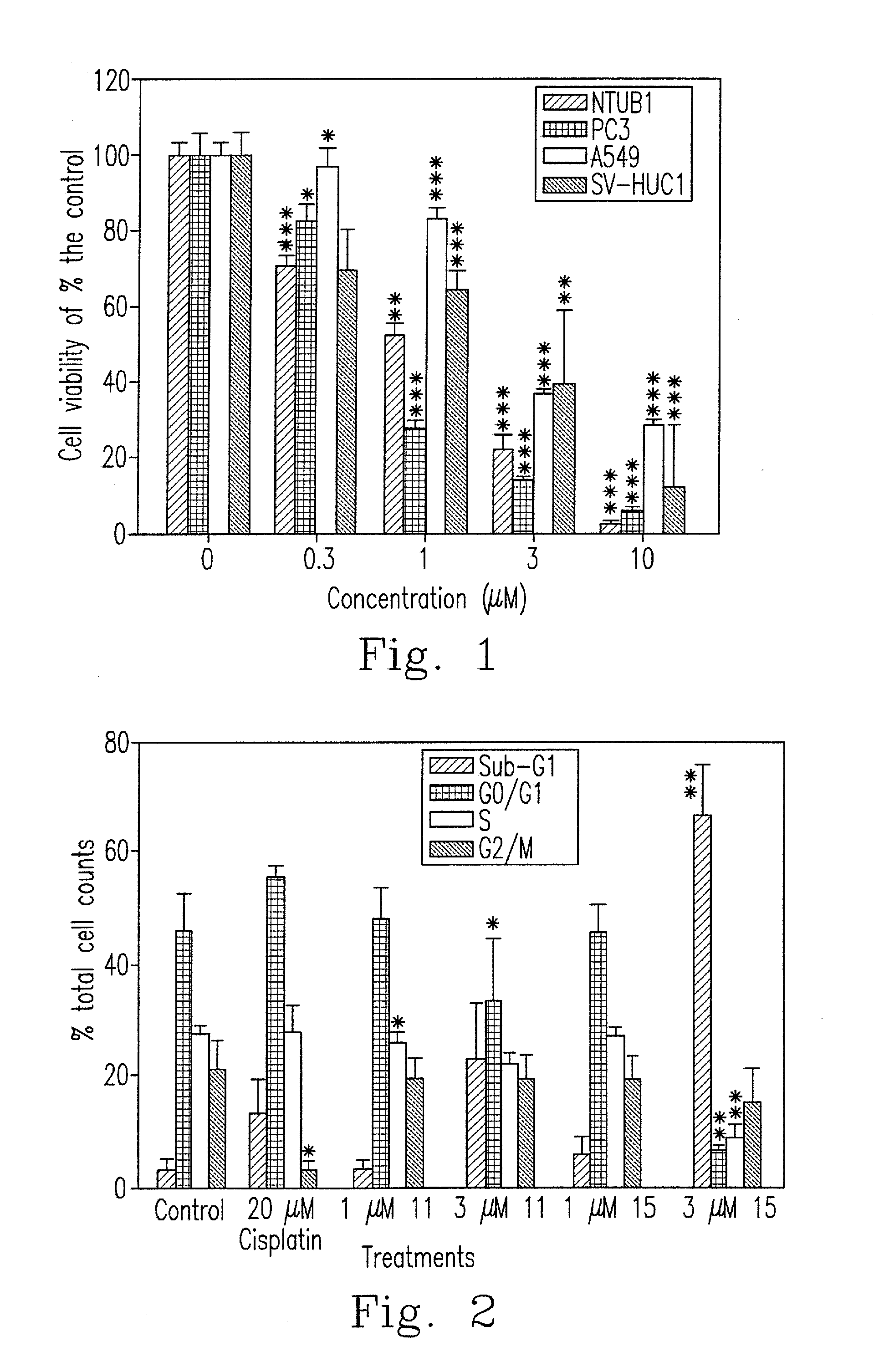
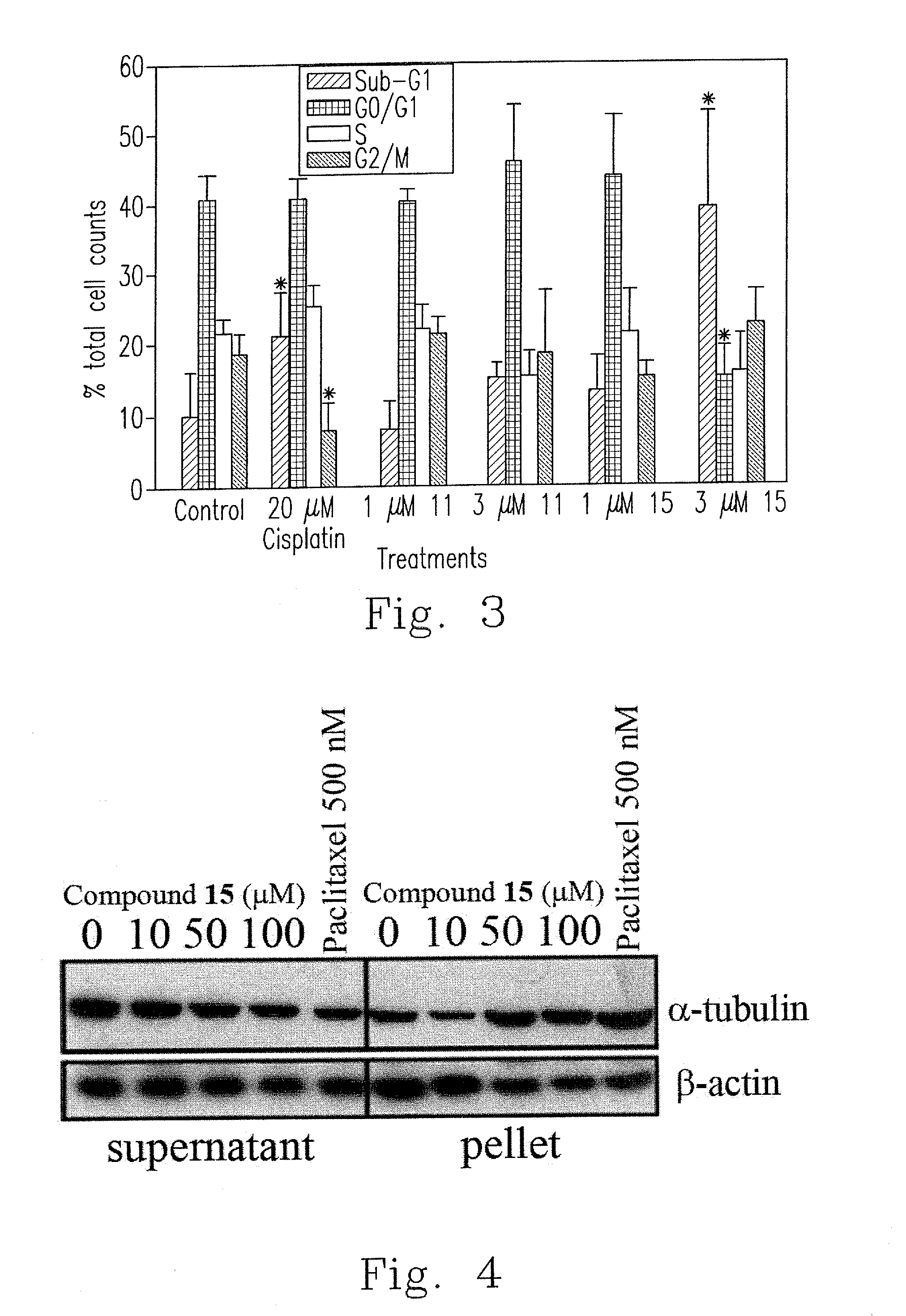
Design, synthesis, and biological evaluation of 1-methyl-1, 4-dihyrdoindeno[1,2-c]pyrazole analogues as potential anticaner agents targeting tubulin colchicine binding site
InactiveUS20170327468A1Strong specificityImprove effectivenessOrganic chemistryAnticarcinogenHuman leukemia
The invention discloses an indenopyrazole small-molecule tubulin inhibitor, which is characterized by having a structure represented by general formula I:wherein R represents NH2 or NHOH; the invention also discloses a preparation method of the indenopyrazole compound, or pharmaceutical salts thereof. The compound of the present invention is an indenopyrazole small-molecule tubulin inhibitor having a novel structure, and has very strong proliferation inhibition activity to human hepatocellular carcinoma (HepG2) cells, human prostate carcinoma (PC3) cells, human cervical carcinoma (HeLa) cells, human breast adenocarcinoma (MCF-7) cells, and human leukemia (K562) cells; the compound is similar to colchicine in mechanism of action, and thus capable of inhibiting tubulin polymerization; the compound is significant for enhancing the specificity and effectiveness of drugs, reducing toxic and side effects, preventing drug tolerance, and so on.
Owner:ACT PHARMA CO LTD
Preparation method and application of absorbable collagen sponge
InactiveCN112353997AGood biocompatibilityNot easy to spallPharmaceutical delivery mechanismAbsorbent padsLiver and kidneyFreeze-drying
The invention relates to a preparation method and application of absorbable collagen sponge and belongs to the technical field of biomedical materials. The collagen sponge is prepared through low-temperature extraction, freeze drying and electron beam irradiation sterilization, the triple helix structure of the collagen sponge is not damaged, and good biological activity is kept. Through biological evaluation and animal experiment research, the prepared sample has a remarkable hemostatic effect, and the degradation performance and the anti-adhesion effect of the collagen sponge remained in thebody after hemostasis can better meet the requirements. The sample has no obvious influence on blood routine examination, blood coagulation, blood biochemistry, immune organs, liver and kidney functions and the like. The product has great potential in the aspects of clinical hemostasis, wound healing, bone defect, nerve repair and the like.
Owner:HANGZHOU SINGCLEAN MEDICAL PROD
Simplified biological evaluation method of natural and artificial chemicals by using DNA injury index and apparatus therefor
InactiveUS20060099634A1Avoid passingAccurate detectionBioreactor/fermenter combinationsBiological substance pretreatmentsUltravioletIngested food
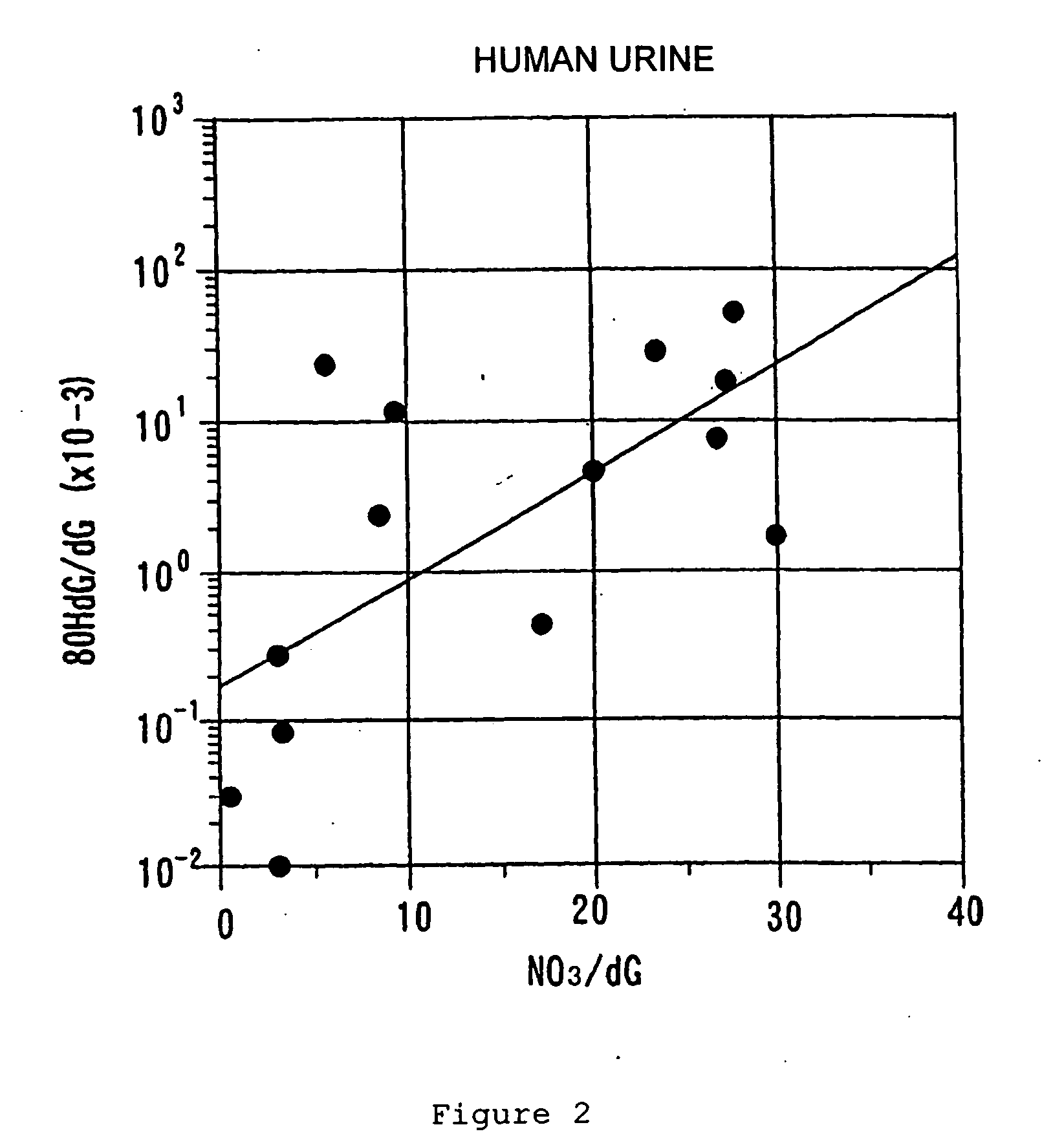
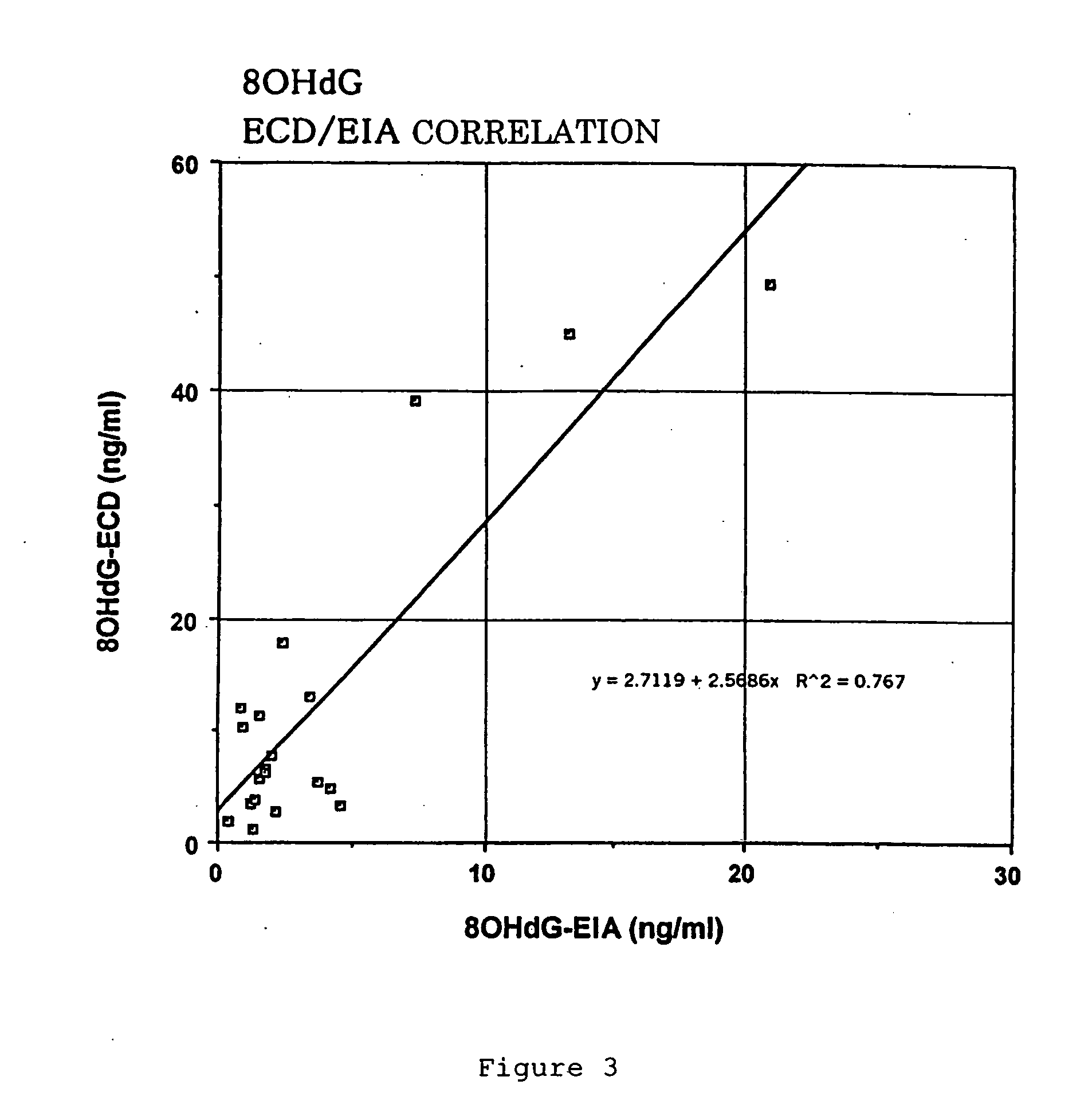
A biological evaluation method for rational and simply evaluating biological harmfulness or usefulness of a great number of natural and artificial chemicals, foods, etc. This method comprises adding a known amount of 2′-deoxyguanosine (dG) to a solution containing a test substance (drug, pesticide, functional food, etc.), optionally applying UV light and / or adding active oxygen generator, then quantifying 8-hydroxy-2′-deoxyguanosine (8OHdG) in the solution, and evaluating the toxicity or usefulness of the test substance according to the 8OhdG content (a higher 8OhdG content indicates a higher harmfulness of the test substance while a lower 8OhdG content indicates a lower harmfulness or an usefulness thereof) The invention also provide an apparatus for advantageously performing said biological evaluation method and an antioxidant preservative solution to be used in this apparatus.
Owner:TAS PROJECT
Method for training and improving space learning ability of mice
InactiveCN113940286ASpace Learning Ability ImprovementTaming and training devicesPhysical medicine and rehabilitationWater maze
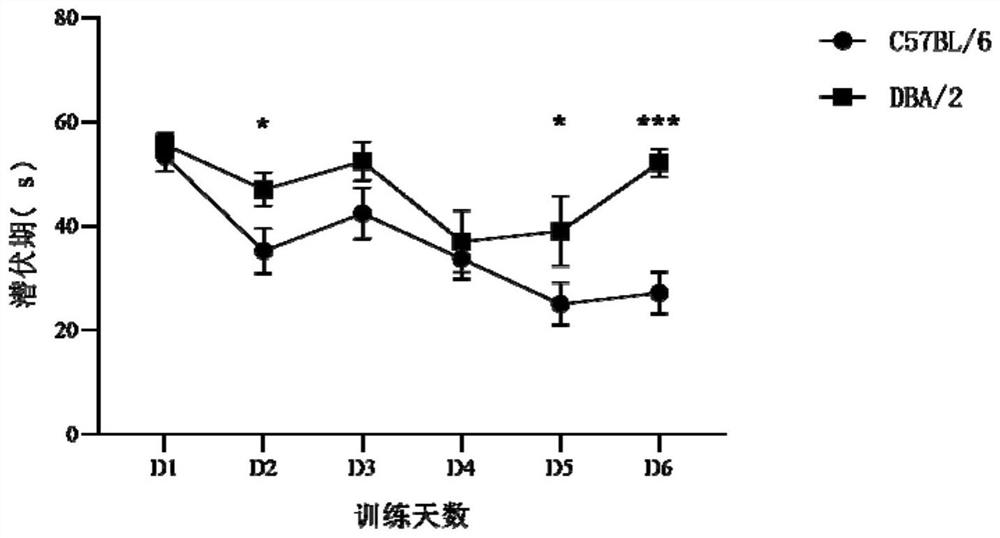
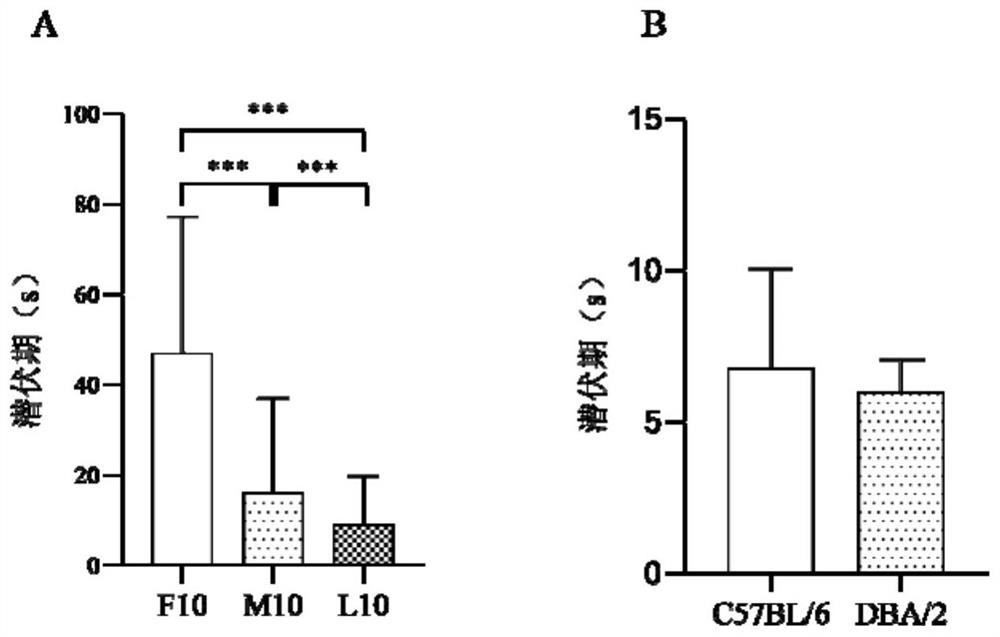
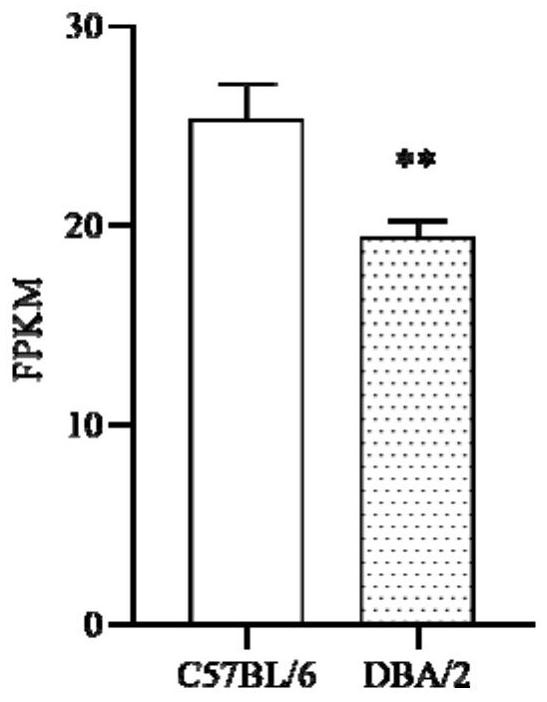
The invention provides a method for training and improving the space learning ability of mice. The method comprises the following steps: training the mice by using a water maze for 30 days twice a day, performing repeated measurement variance analysis on the incubation period of the DBA / 2 mice before an experiment and after the experiment, performing analysis on the incubation period of the DBA / 2 mice and the incubation period of the C57BL / 6 mice by using an independent sample t test, and realizing improvement effect evaluation of the space learning ability of the DBA / 2 mice. The invention also provides application of mouse Nr1d1 genes to improvement of the space learning ability of the mice. According to the invention, the improvement of Morris water maze training on the space learning ability of the DBA / 2 mice is doubly measured, and a molecular biological evaluation marker which can be used as a training or drug to improve the space learning ability of the mice is provided.
Owner:NANTONG UNIVERSITY
Biological detection method used for quality evaluation on and quality control over heat-clearing and detoxifying traditional Chinese medicines
ActiveCN106591414AGuarantee high quality and effectivenessObjective Quantitative Evaluation MethodMicrobiological testing/measurementMaterial analysisMedicineQuality control
The invention provides a biological detection method used for quality evaluation on and quality control over heat-clearing and detoxifying traditional Chinese medicines. The biological detection method comprises the steps of firstly adopting a method of anti-rabbit erythrocytea condensation outside the body to represent anti influenza virus activity of heat-clearing and detoxifying traditional Chinese medicines, then adopting a method of anti-mouse spleen swelling method inside the body to represent immune anti-inflammatory activity of heat-clearing and detoxifying traditional Chinese medicines, comparatively completely representing activity of anti-pathogenic microorganisms and anti-inflammatory-immune regulation of heat-clearing and detoxifying medicinal materials through the combination of identification experiments inside and outside the body, and identifying the quality of the medicinal materials through a biological evaluation method. The biological detection method used for the quality evaluation on and the quality control over the heat-clearing and detoxifying traditional Chinese medicines provides a more objective quantitative evaluation method for the clinical effectiveness of the medicinal materials, and guarantees high quality and effectiveness of the medicinal materials.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
A biological detection method for quality evaluation and quality control of heat-clearing and detoxifying traditional Chinese medicines
ActiveCN106591414BGuarantee high quality and effectivenessObjective Quantitative Evaluation MethodMicrobiological testing/measurementMaterial analysisMedicineQuality control
The invention provides a biological detection method used for quality evaluation on and quality control over heat-clearing and detoxifying traditional Chinese medicines. The biological detection method comprises the steps of firstly adopting a method of anti-rabbit erythrocytea condensation outside the body to represent anti influenza virus activity of heat-clearing and detoxifying traditional Chinese medicines, then adopting a method of anti-mouse spleen swelling method inside the body to represent immune anti-inflammatory activity of heat-clearing and detoxifying traditional Chinese medicines, comparatively completely representing activity of anti-pathogenic microorganisms and anti-inflammatory-immune regulation of heat-clearing and detoxifying medicinal materials through the combination of identification experiments inside and outside the body, and identifying the quality of the medicinal materials through a biological evaluation method. The biological detection method used for the quality evaluation on and the quality control over the heat-clearing and detoxifying traditional Chinese medicines provides a more objective quantitative evaluation method for the clinical effectiveness of the medicinal materials, and guarantees high quality and effectiveness of the medicinal materials.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
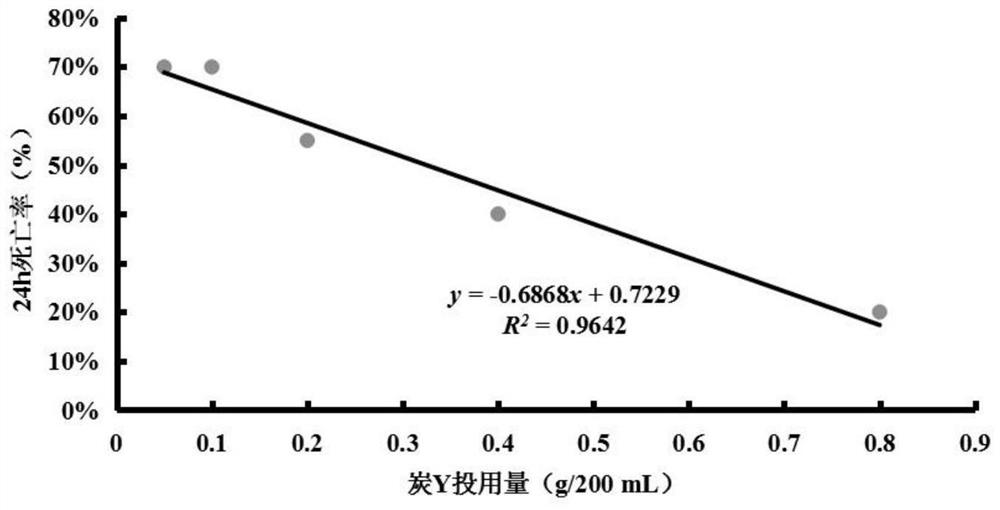
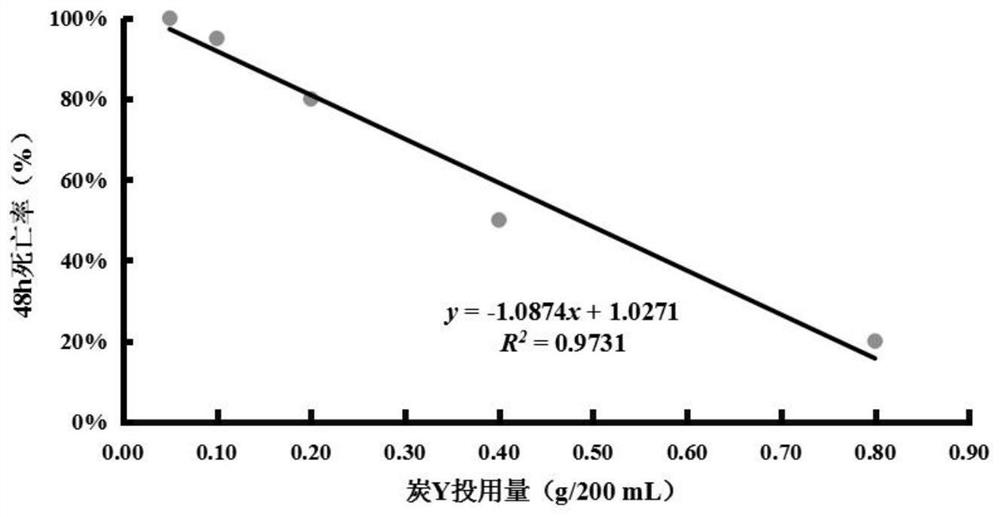
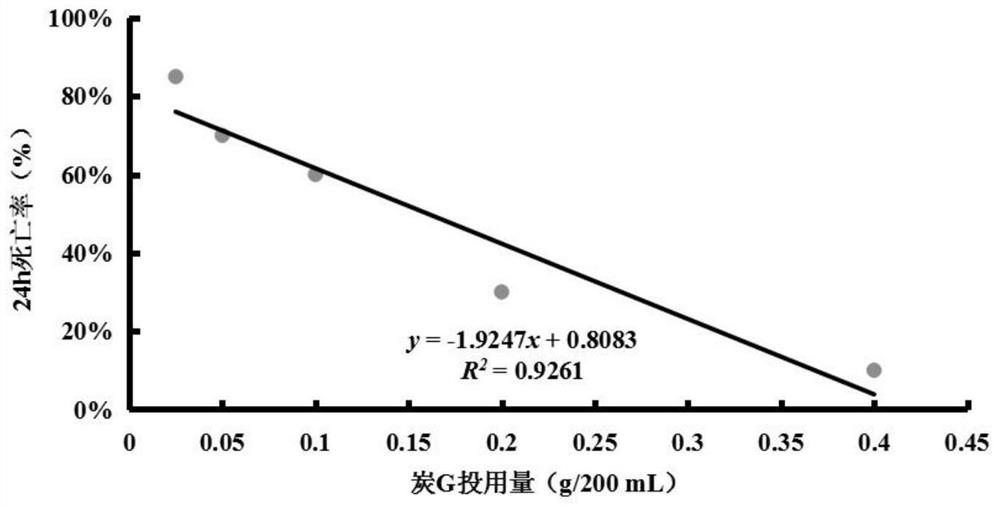
Biological evaluation method capable of quantitatively characterizing polycyclic aromatic hydrocarbon adsorption capacity of modified biochar in water body
PendingCN113075375AImprove adsorption capacityScientific guidanceTesting waterPolycyclic aromatic hydrocarbonDaphnia magna
The invention discloses a biological evaluation method capable of quantitatively characterizing the capacity of modified charcoal for adsorbing polycyclic aromatic hydrocarbon in a water body. The method comprises the following steps: firstly, establishing a linear regression equation reflecting a dose-effect relationship between the dosage of to-be-detected modified charcoal and the daphnia magna death rate; then selecting the dosage of the modified charcoal to be detected according to the death rate of ideal daphnia magna in the water body; or under the same dosage, comparing and evaluating the death rate of daphnia magna according to the death rate of daphnia magna. According to the method, the death rate of daphnia magna can be used as a biomarker for quantitatively characterizing the dosage of single carbon, and also can be used as a biological evaluation index for quantitatively characterizing and comparing the performance of repairing PAHs polluted water bodies by different carbons.
Owner:TIANJIN VOCATIONAL INST
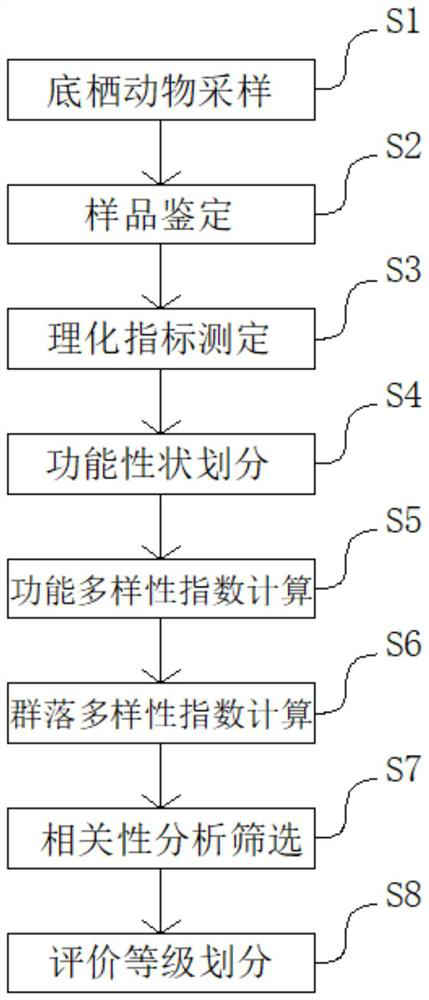
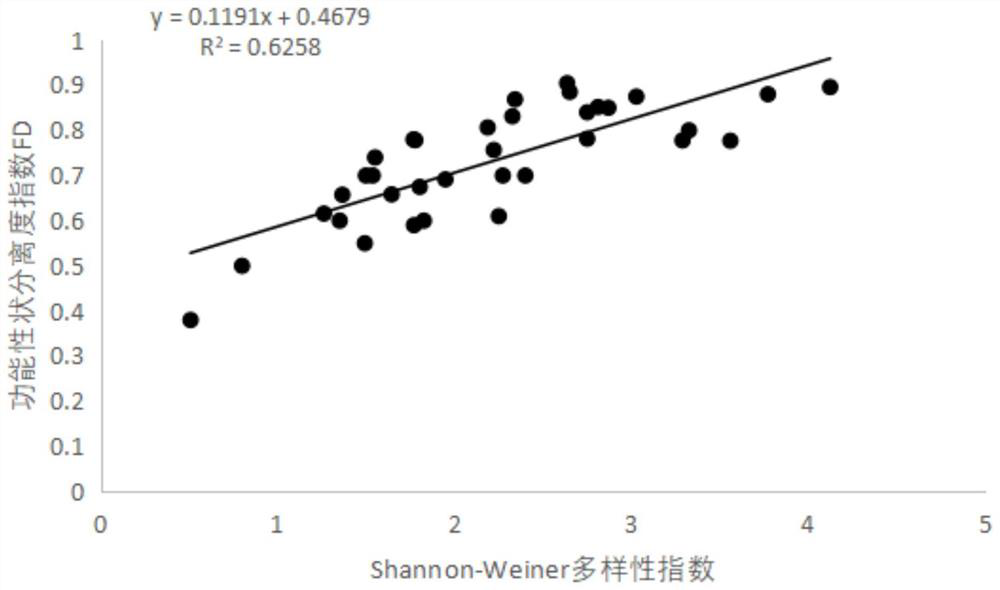
Stream water quality evaluation method based on comprehensive index of macrobenthonic animals
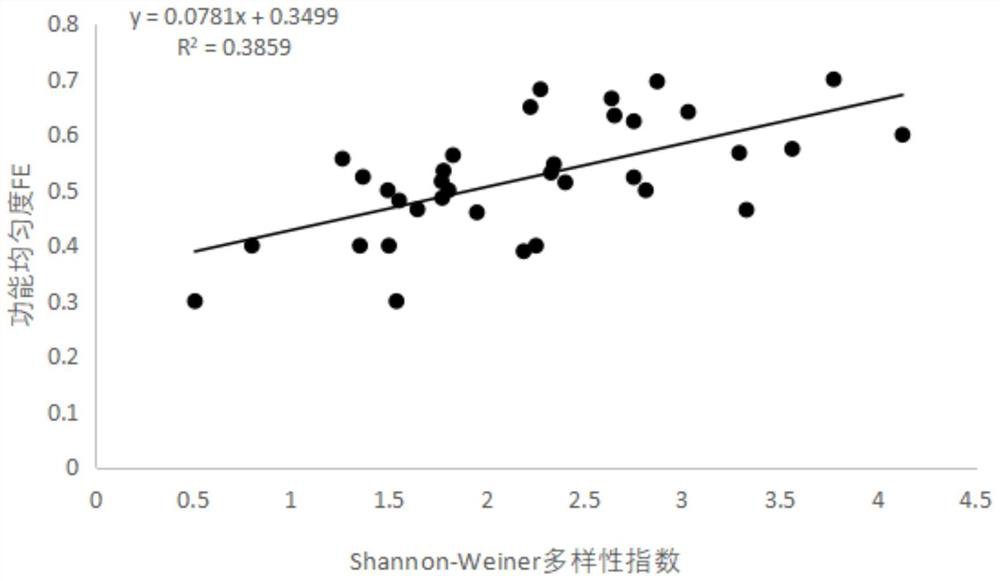
PendingCN114565246AAccurate evaluationIntuitive degree of pollutionClimate change adaptationResourcesDiversity indexWater quality
The invention discloses a stream water quality evaluation method based on comprehensive indexes of large benthonic animals, and relates to the technical field of water quality detection, and the method comprises the following steps: S1, sampling benthonic animals; s2, sample identification; s3, measuring physical and chemical indexes; s4, dividing functional characters; s5, calculating a functional diversity index; s6, calculating a community diversity index; s7, correlation analysis and screening; and S8, performing evaluation grade division. According to the stream water quality evaluation method, stream water quality biological evaluation is carried out in a mode of combining the Shannon-Wiener index H'with the functional diversity index and the physicochemical index, the evaluation method considers response of community structure and function change to environmental influence at the same time, and the structure and the function of a stream ecosystem can be comprehensively represented; and the stream water quality condition is relatively accurately evaluated.
Owner:SOUTH CHINA INST OF ENVIRONMENTAL SCI MEP
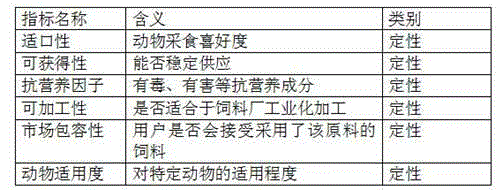
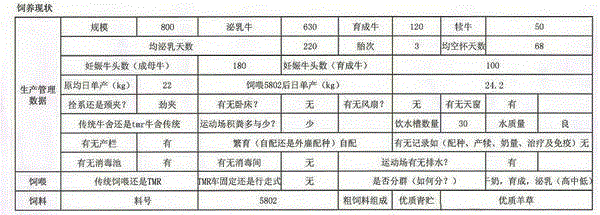
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Radiotracer introduced [18F]fluoromethyl group targeting neuroinflammation for PET imaging and Synthesis of Radiotracer and its biological evaluation Method for Radiotracer Radiotracer introduced [18F]fluoromethyl group targeting neuroinflammation for PET imaging and Synthesis of Radiotracer and its biological evaluation Method for Radiotracer](https://images-eureka.patsnap.com/patent_img/c78b5146-6fed-4a82-a821-b026d0e5054b/US20160263258A1-20160915-D00001.PNG)
![Radiotracer introduced [18F]fluoromethyl group targeting neuroinflammation for PET imaging and Synthesis of Radiotracer and its biological evaluation Method for Radiotracer Radiotracer introduced [18F]fluoromethyl group targeting neuroinflammation for PET imaging and Synthesis of Radiotracer and its biological evaluation Method for Radiotracer](https://images-eureka.patsnap.com/patent_img/c78b5146-6fed-4a82-a821-b026d0e5054b/US20160263258A1-20160915-D00002.PNG)
![Radiotracer introduced [18F]fluoromethyl group targeting neuroinflammation for PET imaging and Synthesis of Radiotracer and its biological evaluation Method for Radiotracer Radiotracer introduced [18F]fluoromethyl group targeting neuroinflammation for PET imaging and Synthesis of Radiotracer and its biological evaluation Method for Radiotracer](https://images-eureka.patsnap.com/patent_img/c78b5146-6fed-4a82-a821-b026d0e5054b/US20160263258A1-20160915-D00003.PNG)
![Design, synthesis, and biological evaluation of 1-methyl-1, 4-dihyrdoindeno[1,2-c]pyrazole analogues as potential anticaner agents targeting tubulin colchicine binding site Design, synthesis, and biological evaluation of 1-methyl-1, 4-dihyrdoindeno[1,2-c]pyrazole analogues as potential anticaner agents targeting tubulin colchicine binding site](https://images-eureka.patsnap.com/patent_img/2691e7e7-aae0-4a0e-ae8b-36f546f1bb13/US20170327468A1-20171116-D00001.png)
![Design, synthesis, and biological evaluation of 1-methyl-1, 4-dihyrdoindeno[1,2-c]pyrazole analogues as potential anticaner agents targeting tubulin colchicine binding site Design, synthesis, and biological evaluation of 1-methyl-1, 4-dihyrdoindeno[1,2-c]pyrazole analogues as potential anticaner agents targeting tubulin colchicine binding site](https://images-eureka.patsnap.com/patent_img/2691e7e7-aae0-4a0e-ae8b-36f546f1bb13/US20170327468A1-20171116-D00002.png)
![Design, synthesis, and biological evaluation of 1-methyl-1, 4-dihyrdoindeno[1,2-c]pyrazole analogues as potential anticaner agents targeting tubulin colchicine binding site Design, synthesis, and biological evaluation of 1-methyl-1, 4-dihyrdoindeno[1,2-c]pyrazole analogues as potential anticaner agents targeting tubulin colchicine binding site](https://images-eureka.patsnap.com/patent_img/2691e7e7-aae0-4a0e-ae8b-36f546f1bb13/US20170327468A1-20171116-C00001.png)