A biological detection method for quality evaluation and quality control of heat-clearing and detoxifying traditional Chinese medicines
A heat-clearing, detoxifying, and quality-evaluating technology, applied in biochemical equipment and methods, microbial determination/inspection, measuring devices, etc., can solve the problems of non-medicinal ingredients and low bioavailability, and achieve high-quality and effective results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of reference substance solution: Take appropriate amount of quercetin, quercetin and gallic acid reference substance, weigh them accurately, add methanol to make a mixed solution containing 15 µg of quercetin, 15 µg of quercetin and 20 µg of gallic acid per 1 ml, Instantly.
[0033] Preparation of the test sample: Take an appropriate amount of the test sample, mix it evenly, take about 0.1g, weigh it accurately, put it in a 25mL measuring bottle, add 10mL water to dissolve it (ultrasound can be used to help dissolve it if necessary), add methanol to dilute to Scale, filter (0.45μm microporous membrane), take the subsequent filtrate, that is.
[0034] 1. Chromatographic conditions
[0035] Mobile phase: A: B = acetonitrile: 0.1% phosphoric acid aqueous solution (gradient elution program as shown in Table 1), column temperature: 35 degrees, flow rate: 1.0ml / min, wavelength: 254nm.
[0036]
[0037] Accurately draw the mixed reference solution (quercetin 1...
Embodiment 2
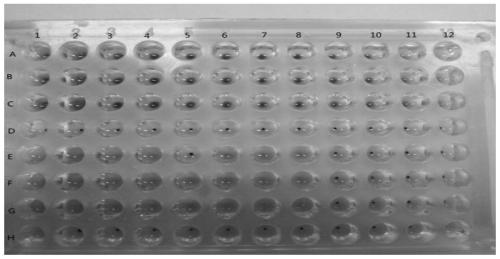
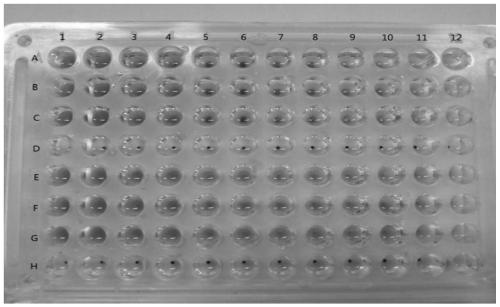
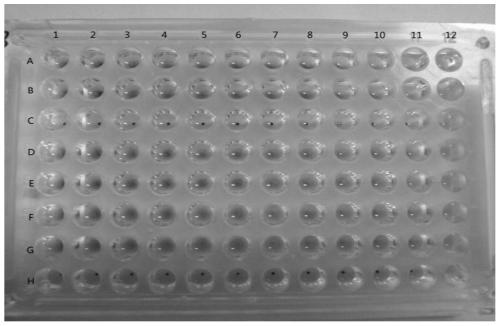
[0040] Example 2 Characterization of anti-influenza virus activity of heat-clearing and detoxifying traditional Chinese medicines by anti-rabbit erythrocyte aggregation method
[0041] Alsever's solution: Take 0.5g of citric acid monohydrate, 8.0g of trisodium citrate dihydrate, 20.5g of glucose, and 4.2g of sodium chloride, add water to dissolve and dilute to 1L (pH about 6.2), dispense Bacteria, let cool, and store at 4°C for later use.
[0042] Preparation of 1% erythrocyte suspension: Take 8 mL of rabbit blood, mix it with an equal volume of Alfred’s solution, wash with an appropriate amount of PBS for 3 times, and wash at 2000 r min for the last time. -1 Centrifuge for 10 minutes, take 1 mL of packed red blood cells, make 100 mL of 1% red blood cell suspension with PBS, and store at 4°C for later use.
[0043] Phosphate buffered saline (PBS): Take 1.10g of anhydrous disodium hydrogen phosphate, 0.32g of anhydrous sodium dihydrogen phosphate, and 8.50g of sodium chloride...
Embodiment 3
[0057] Example 3 The method of resisting splenomegaly in mice to characterize the immune and anti-inflammatory activity of traditional Chinese medicines for clearing away heat and detoxification
[0058] Weighed 48 SPF grade C57 female mice (13-20 g), shaved their back hair with a shaver to form a skin area with a size of about 2 cm × 3 cm, and raised them in single cages. According to body weight, they were randomly divided into 6 groups, 8 in each group, respectively blank group (NG), model group (MG), high-dose group of alcohol extract of Angelica dahurica (BH, 0.112g / mL), low dose of alcohol extract of Angelica dahurica group (BL, 0.056g / mL), high-dose monkey earring water extract group (HH, 0.256g / mL) and monkey earring water extract low-dose group (HL, 0.128g / mL).
[0059] The blank group smeared 80 mg of Vaseline on the back of the mice regularly every day, and gavaged the mice with normal saline at the same time (the dosage was 0.04 mL·g -1 ), the model group applied ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com