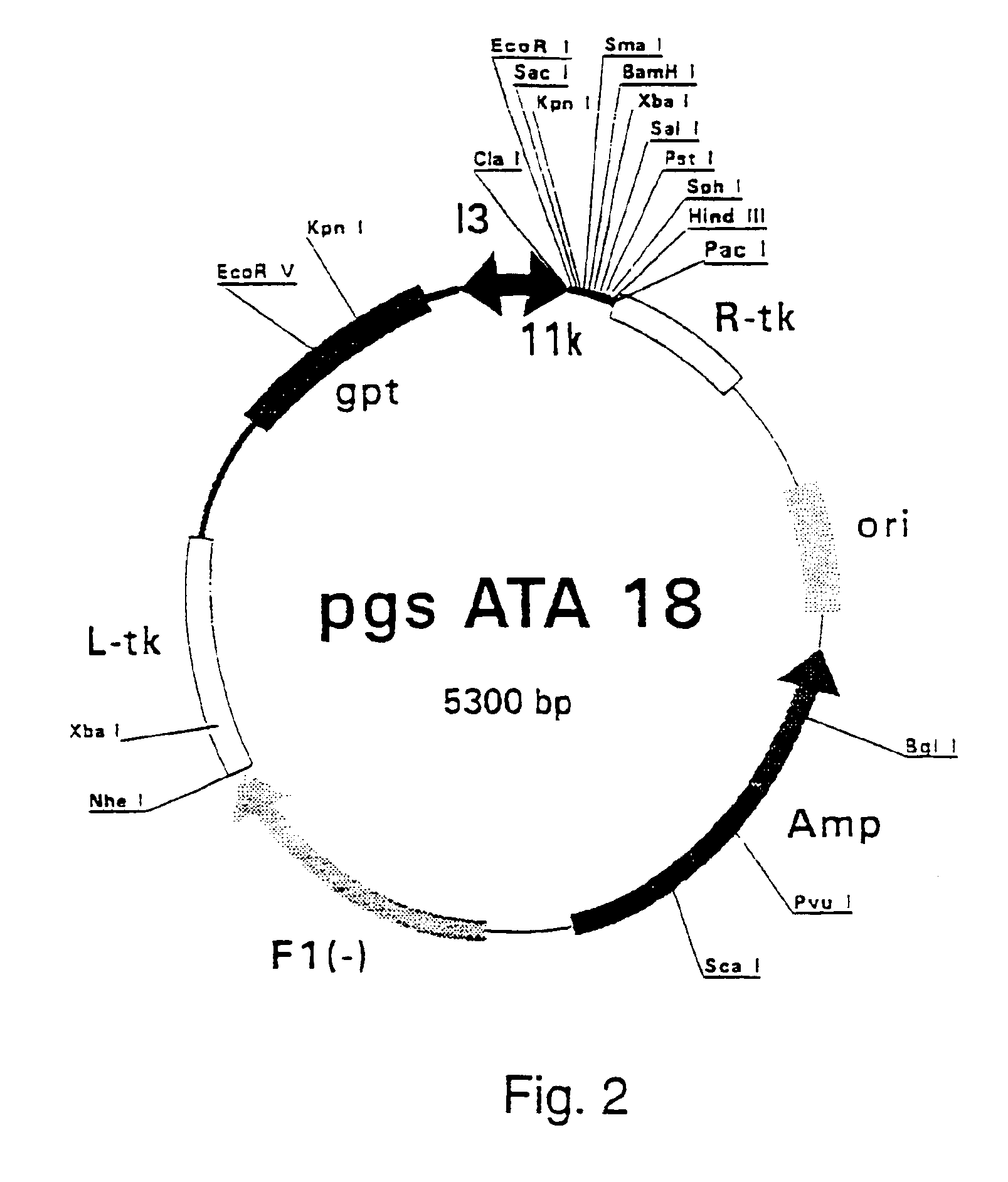
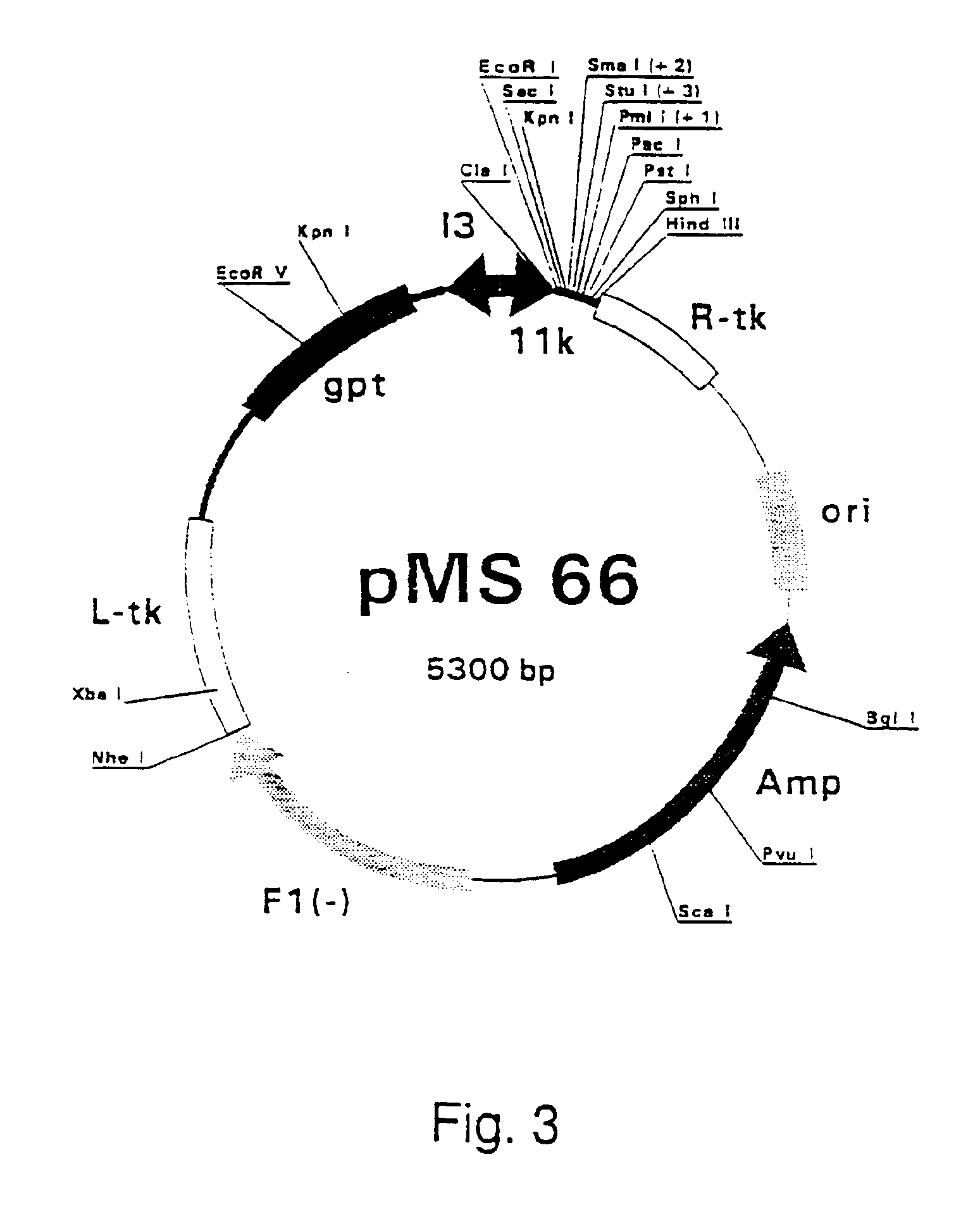
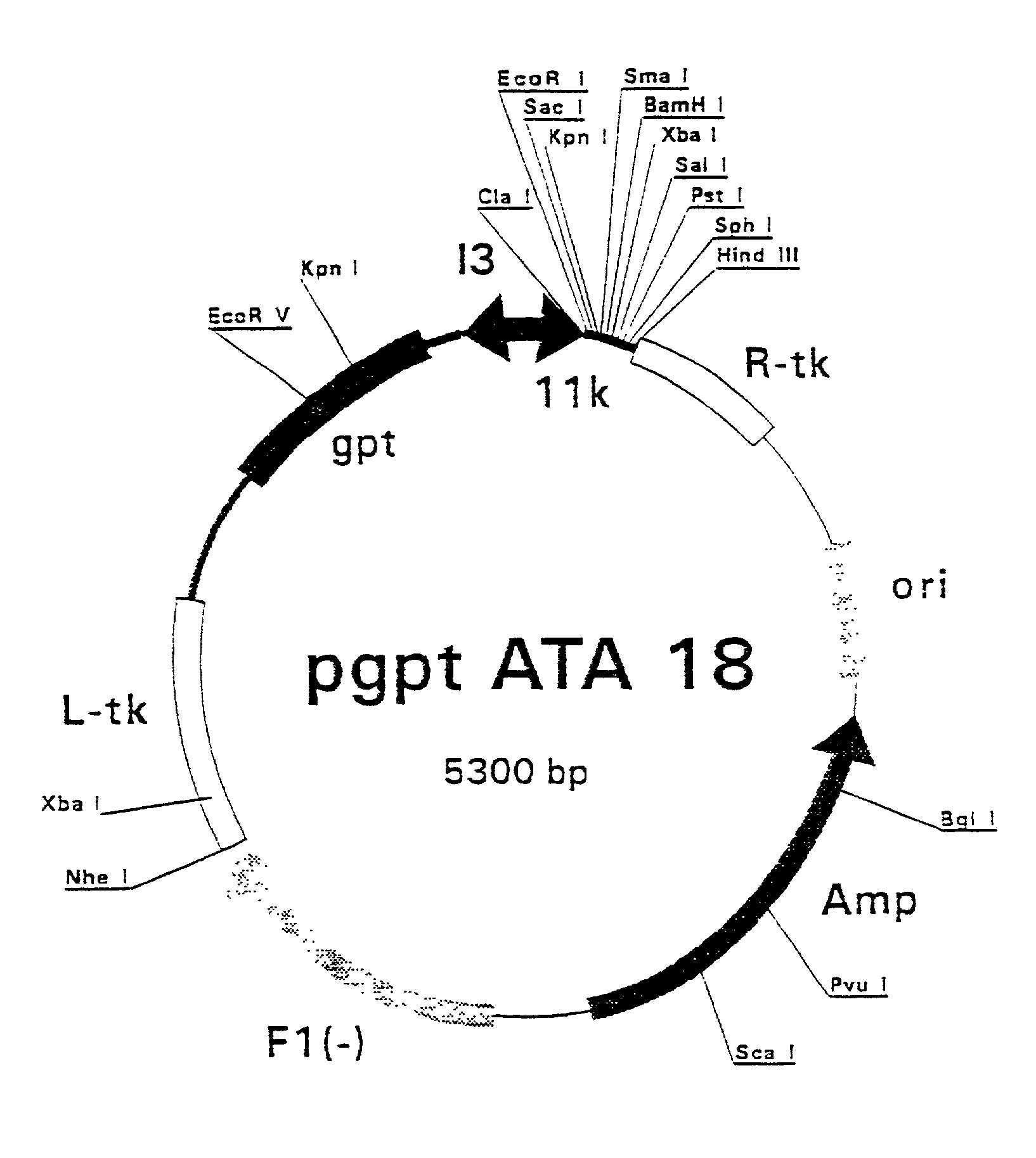
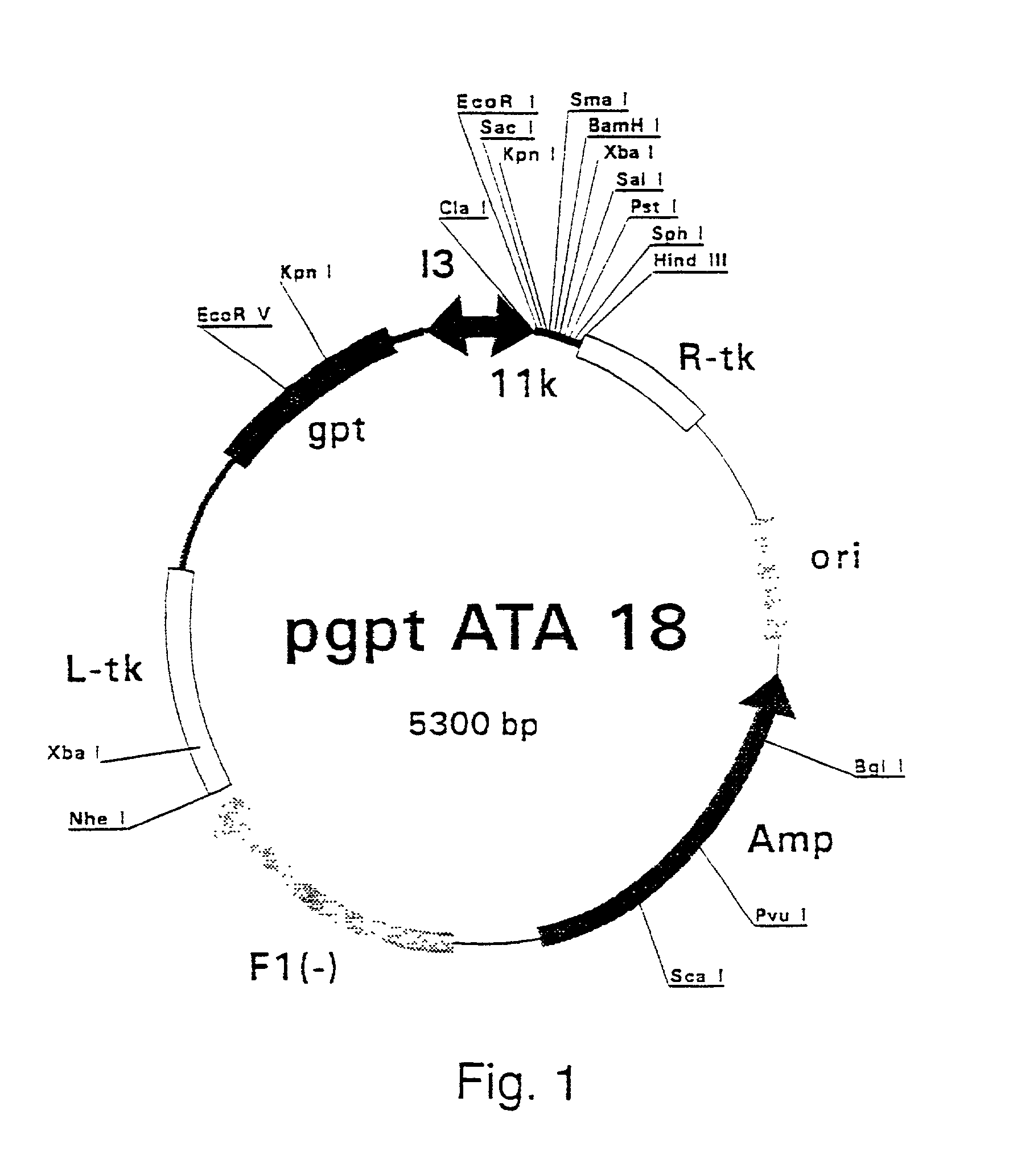
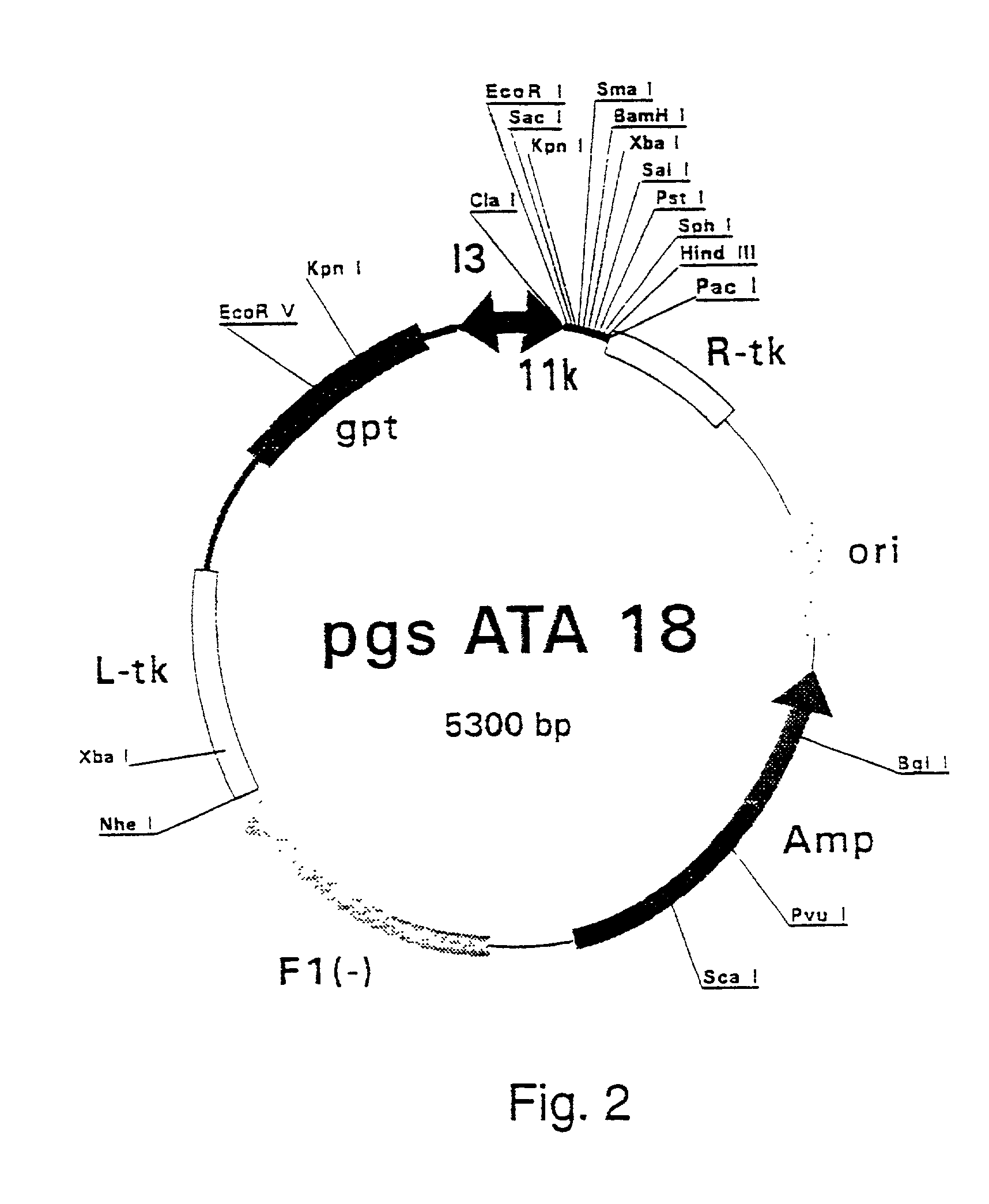
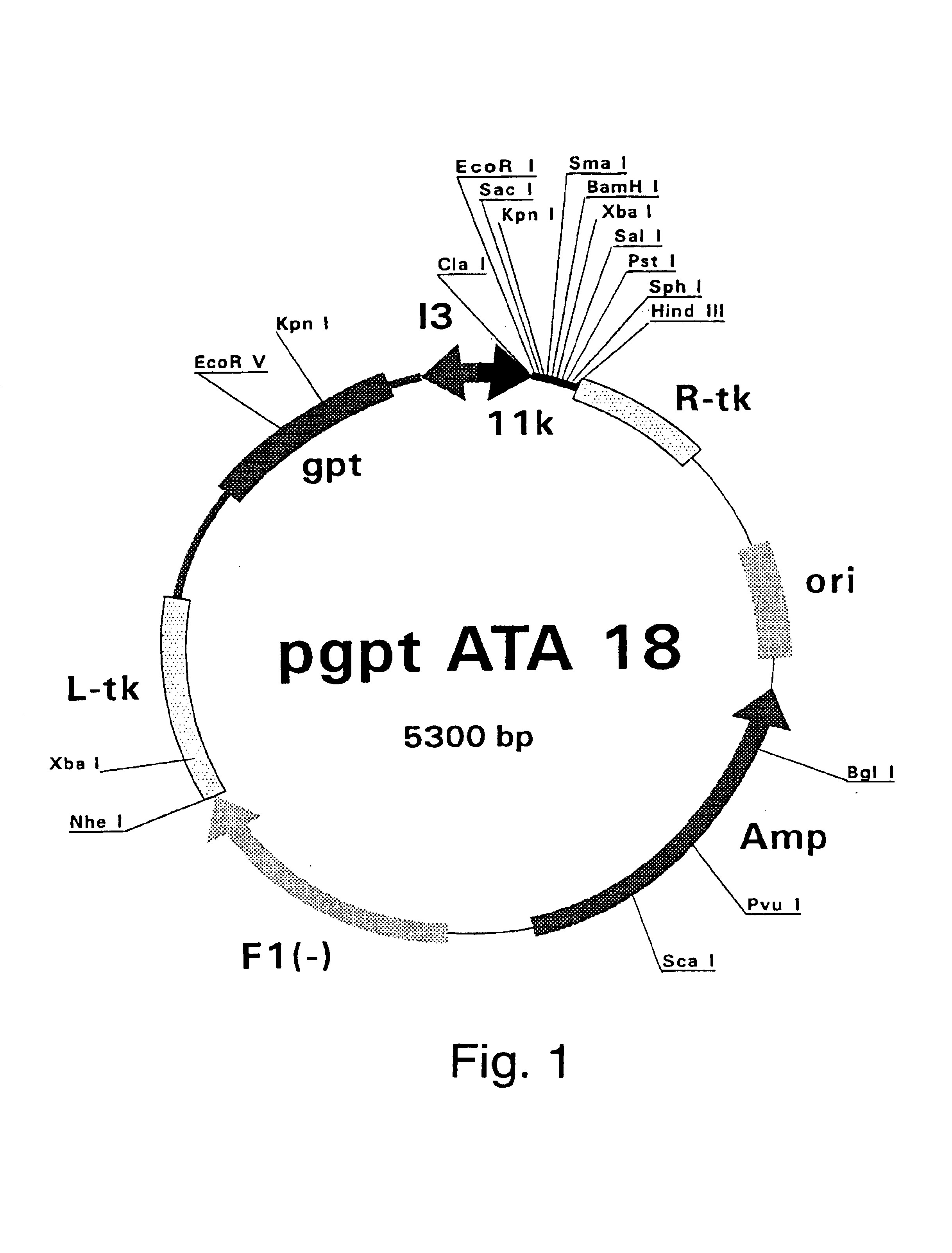
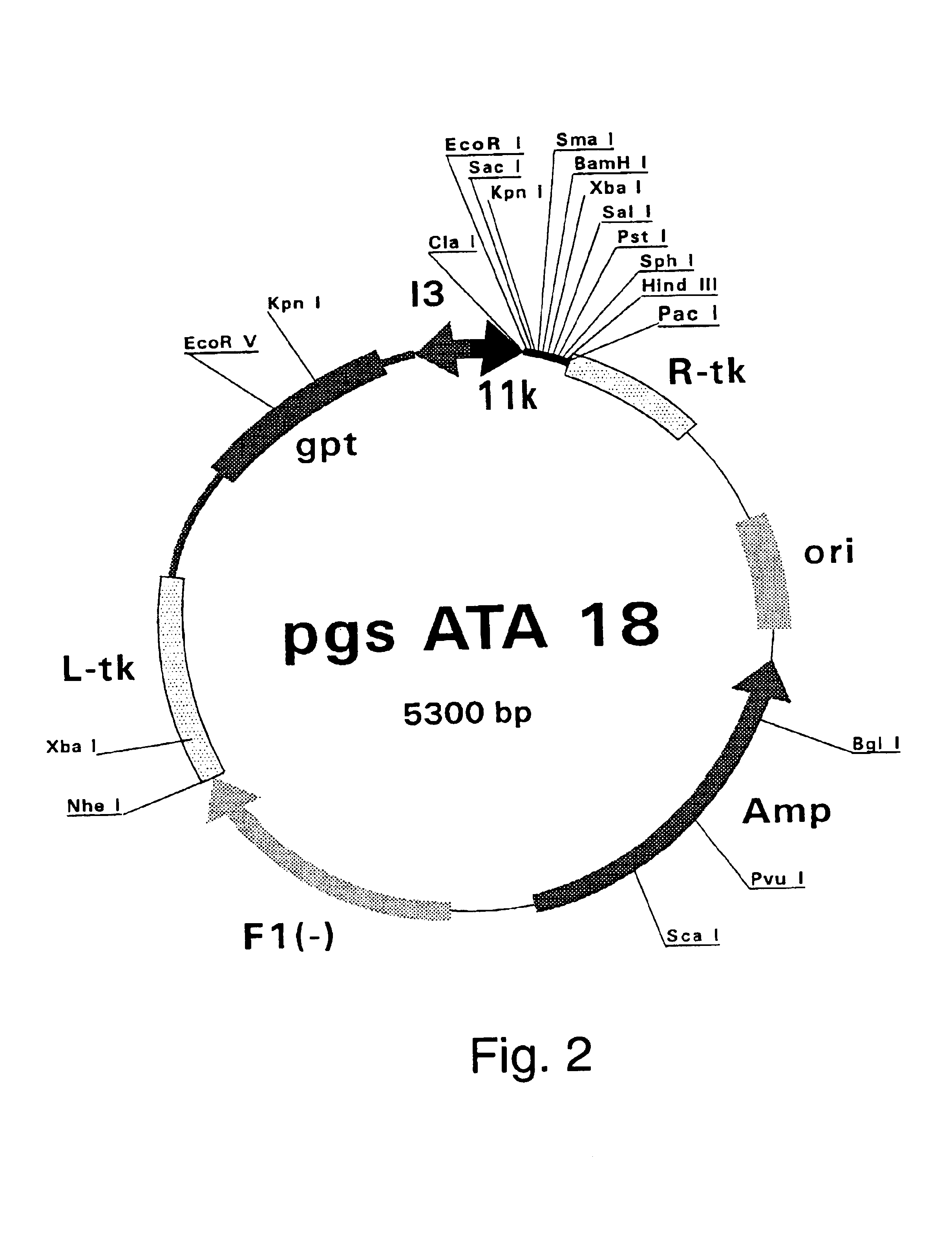
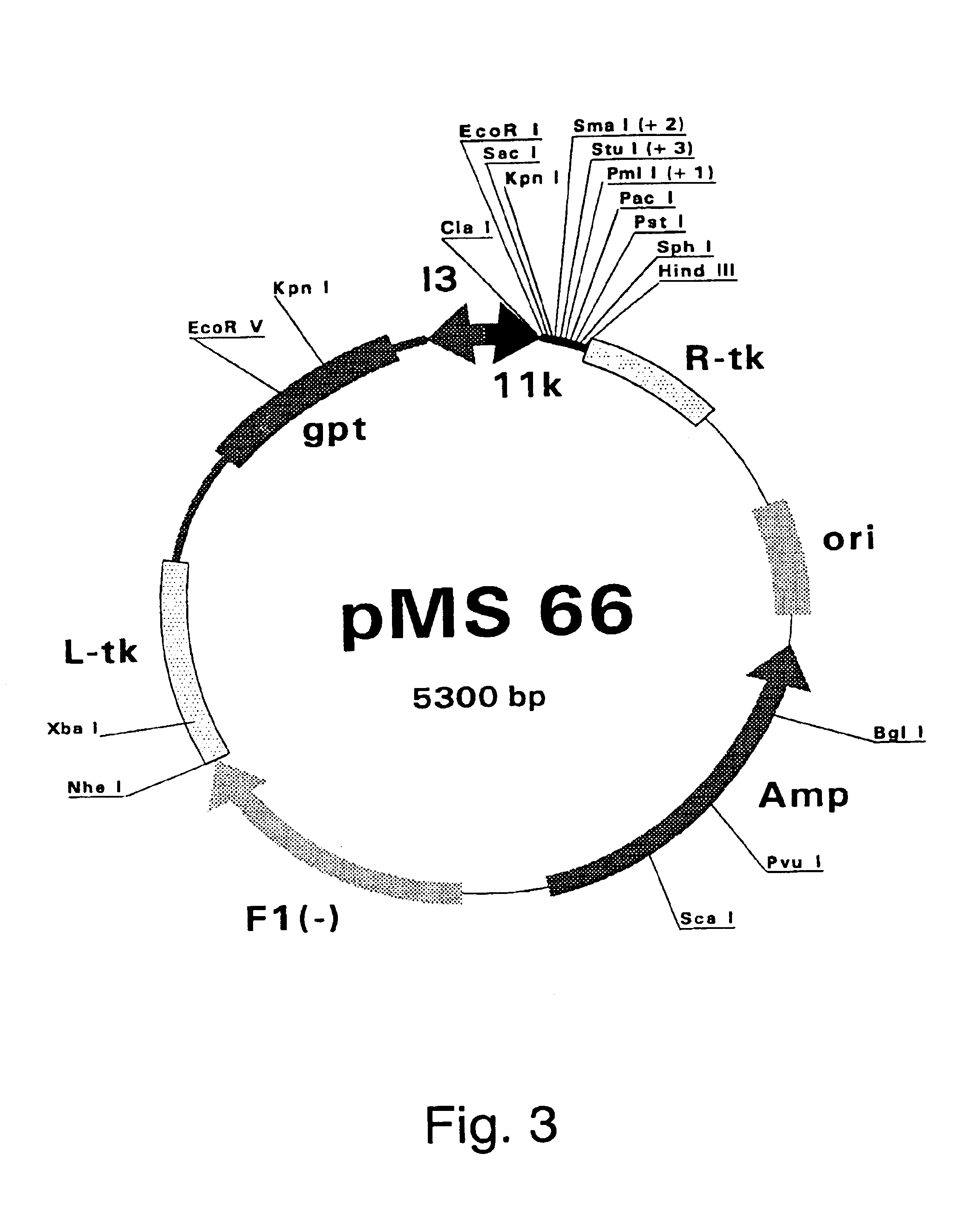
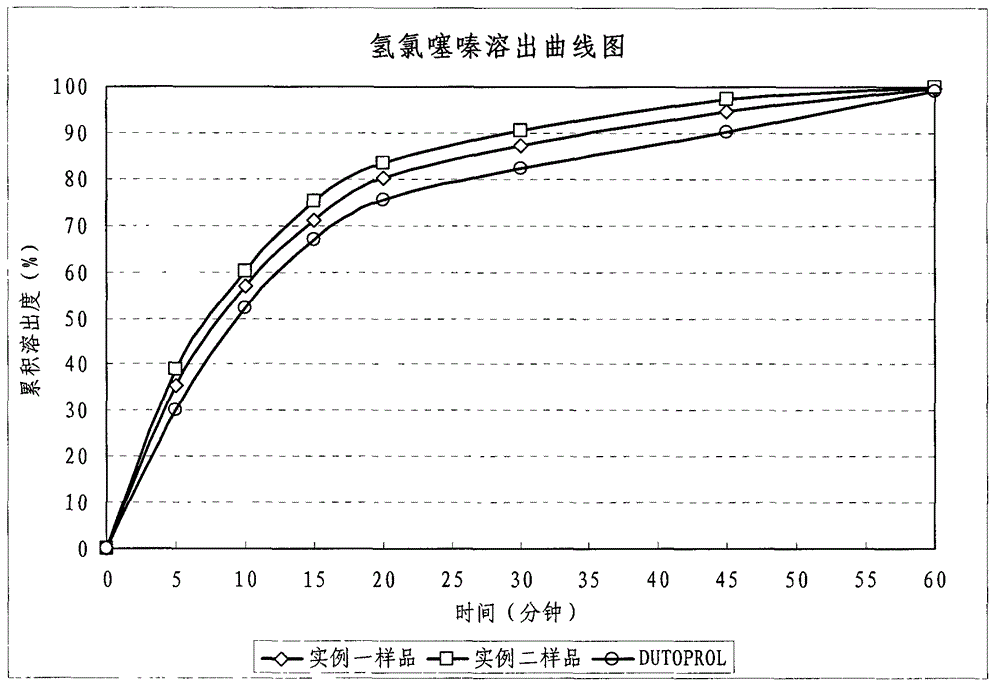
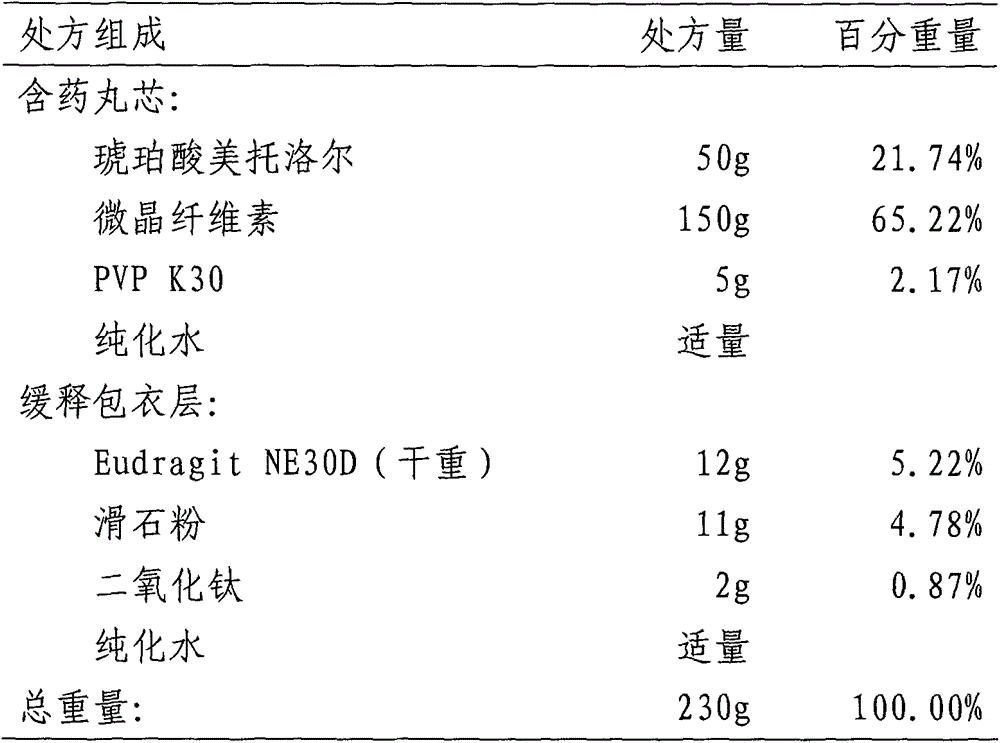
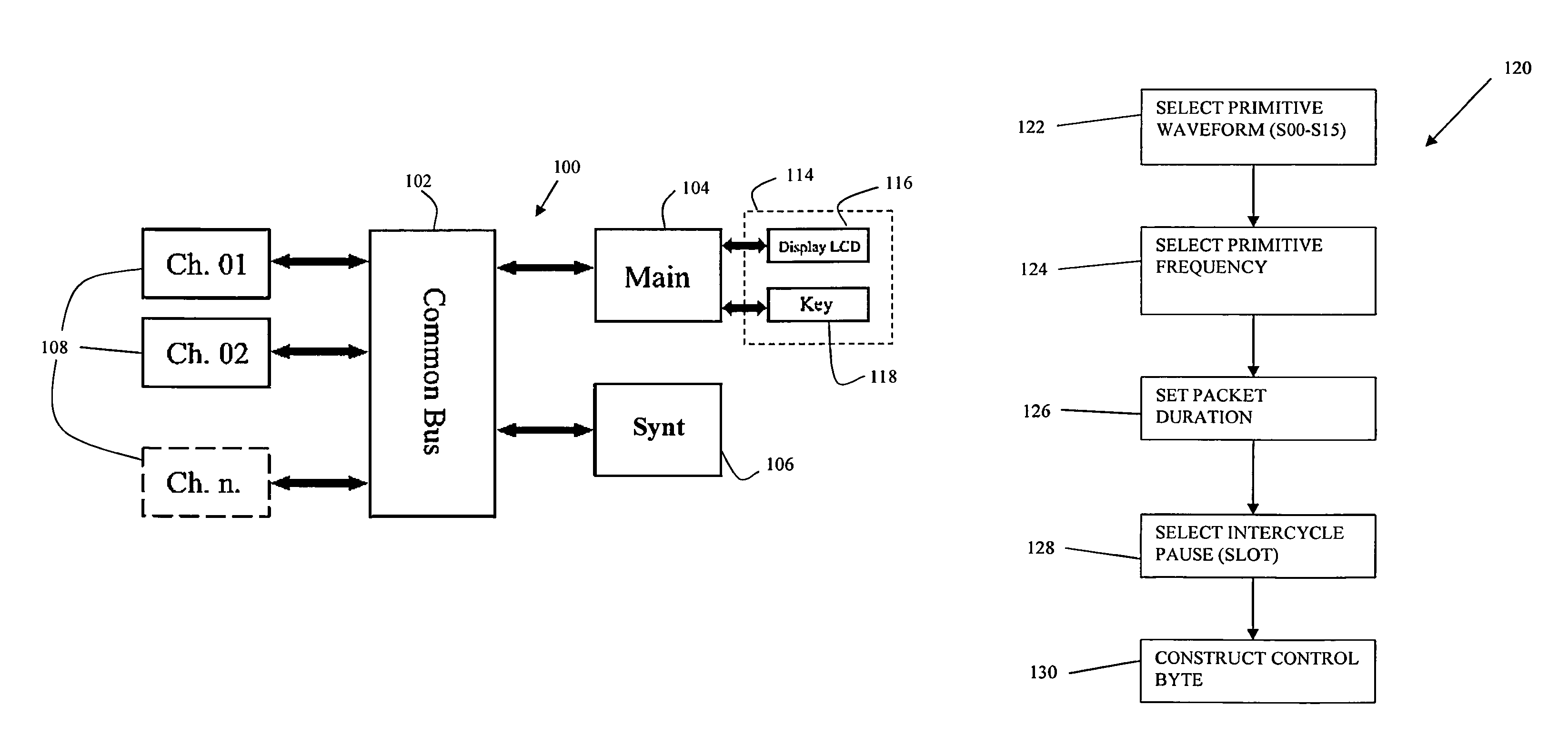
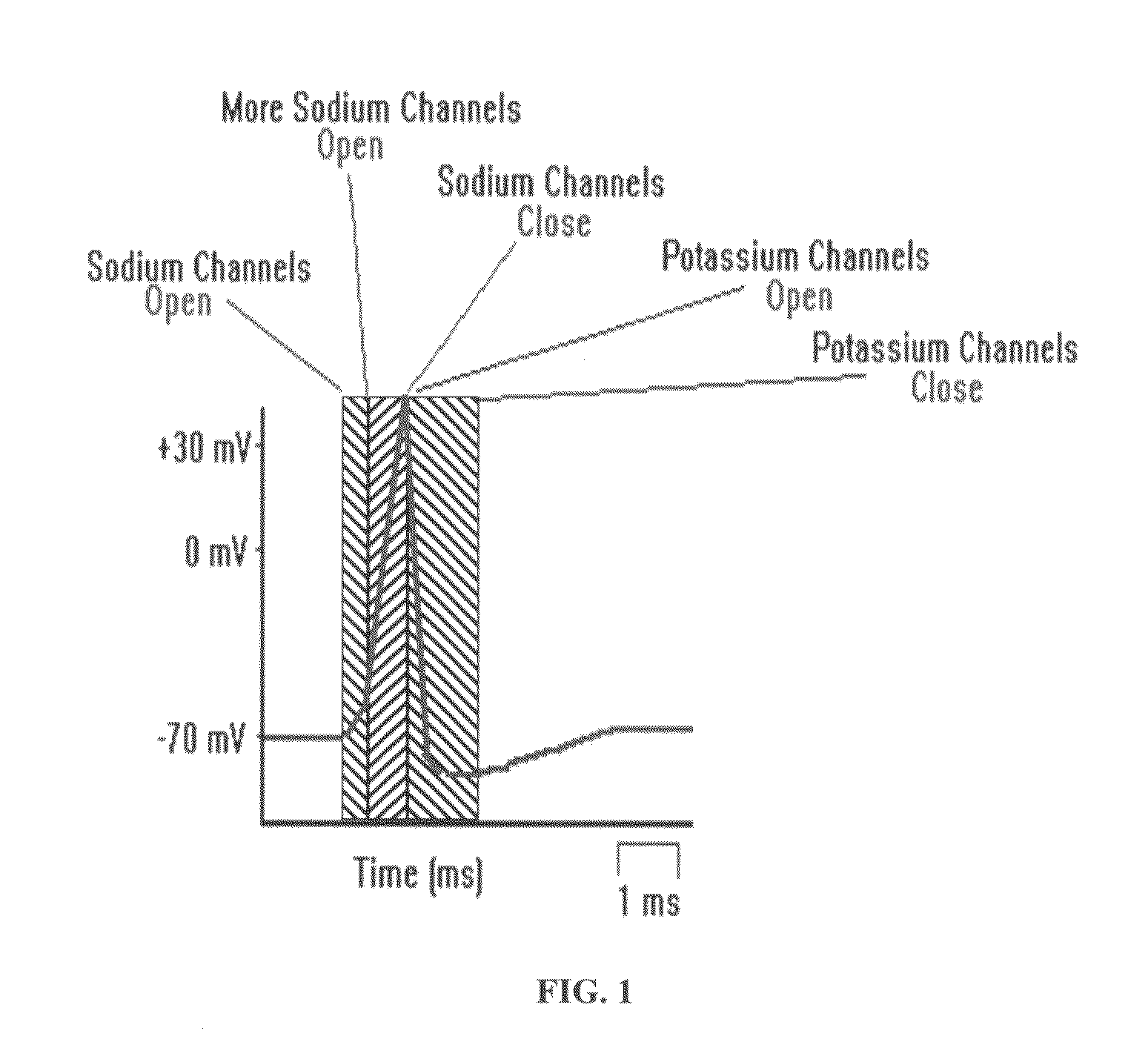
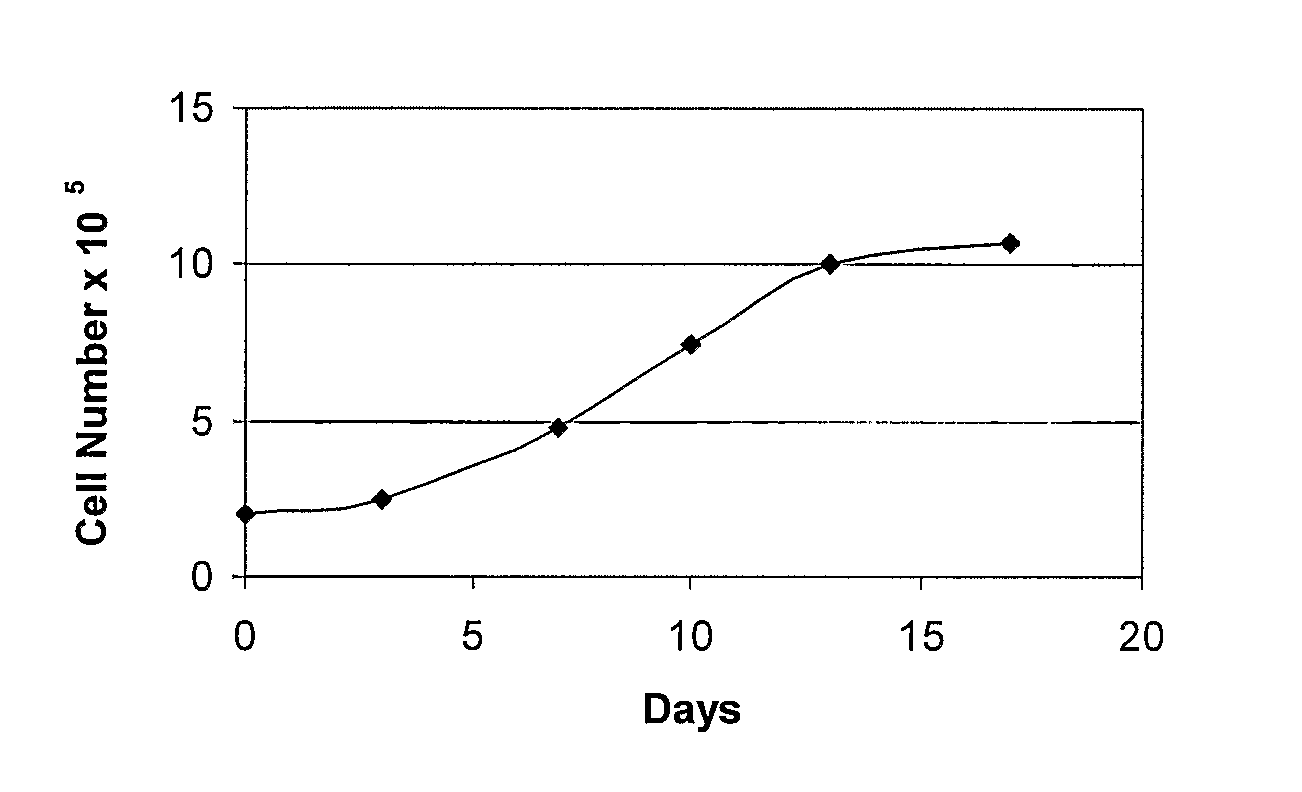
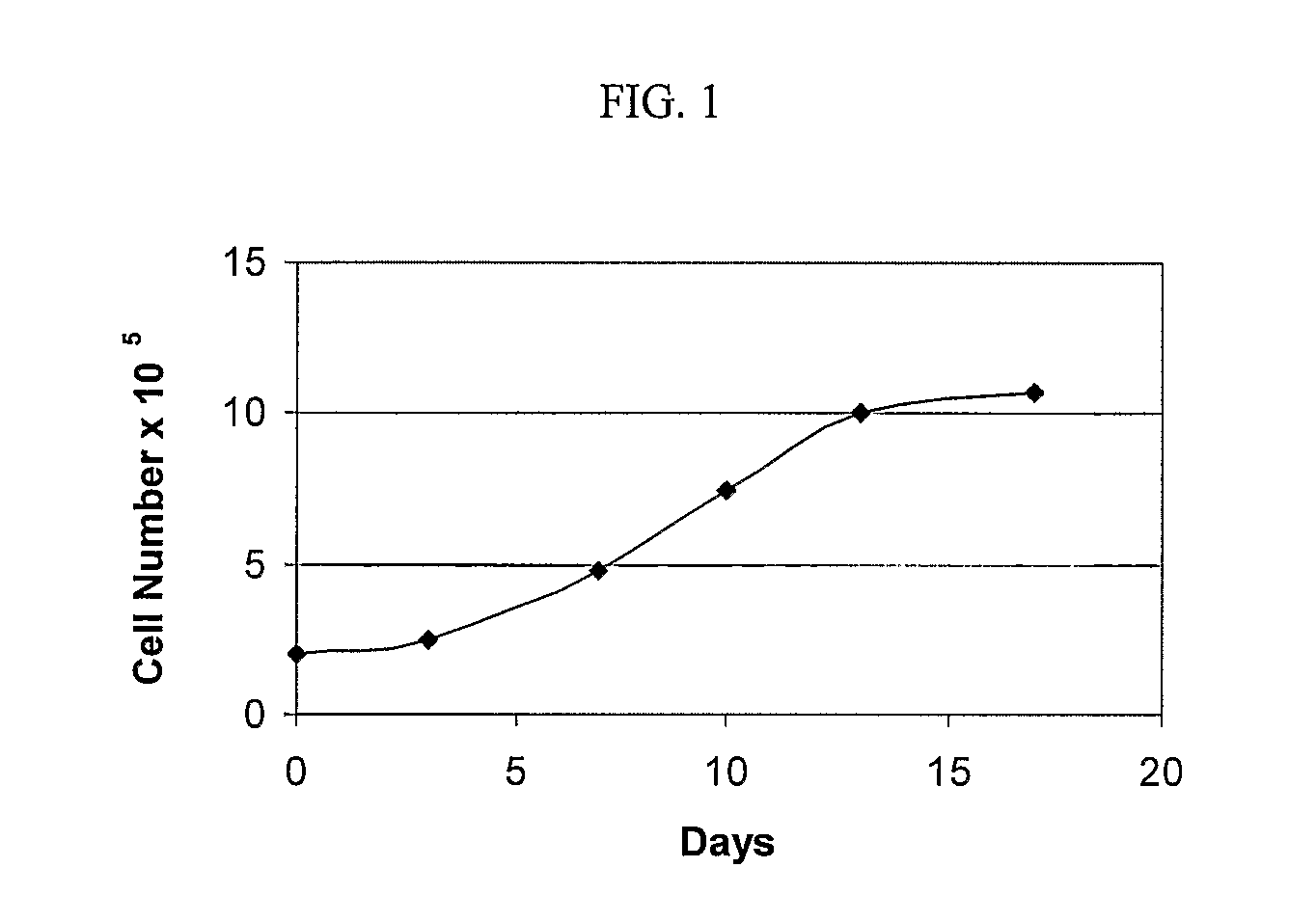
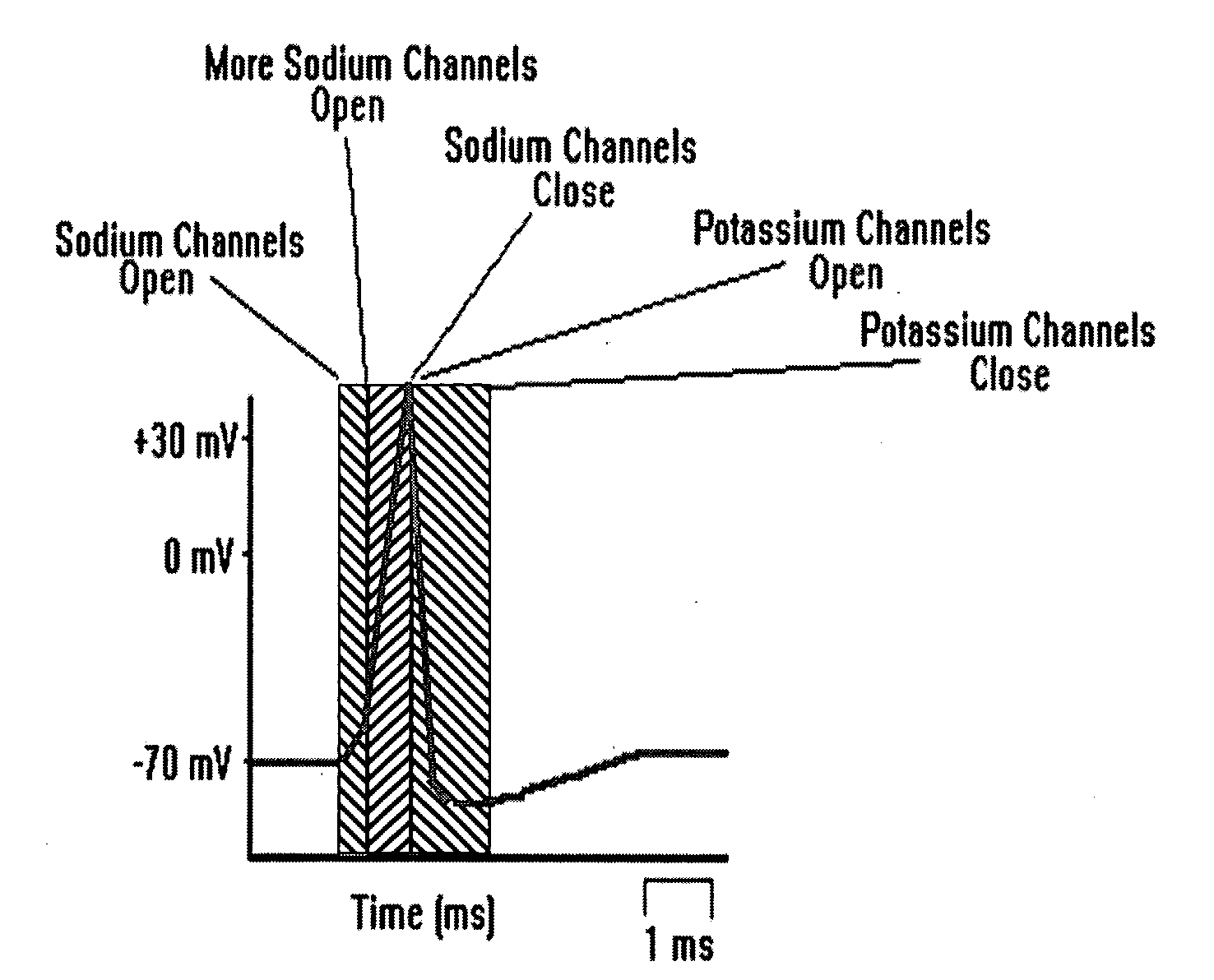
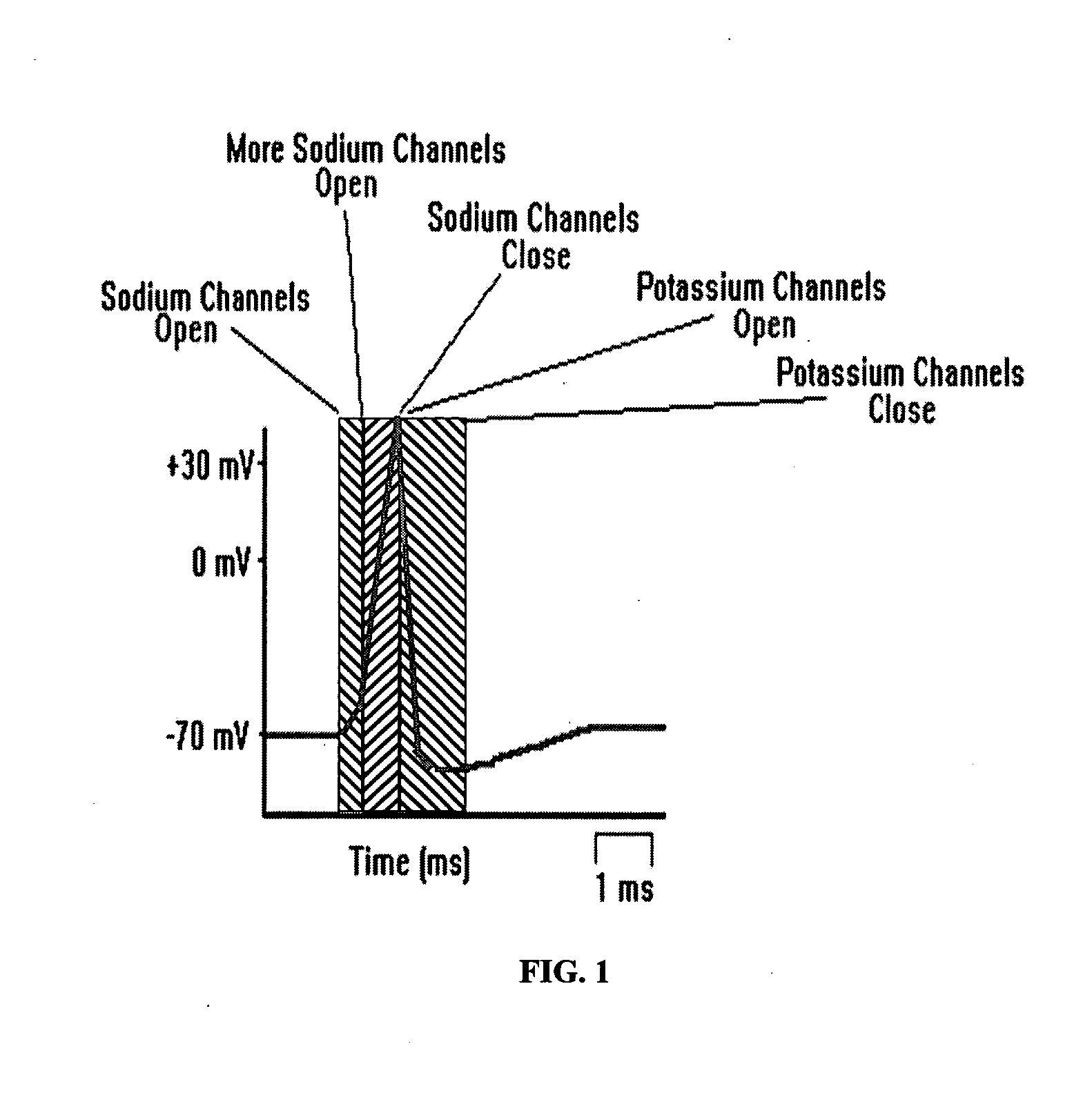
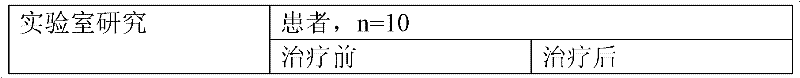Patents
Literature
52 results about "Clinical effectiveness" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Clinical Effectiveness and Decision Science (CEDS) program seeks to fill clinical information gaps by producing valid, trustworthy, and useful new evidence comparing the effectiveness of different clinical options. In situations where there already is adequate evidence,...
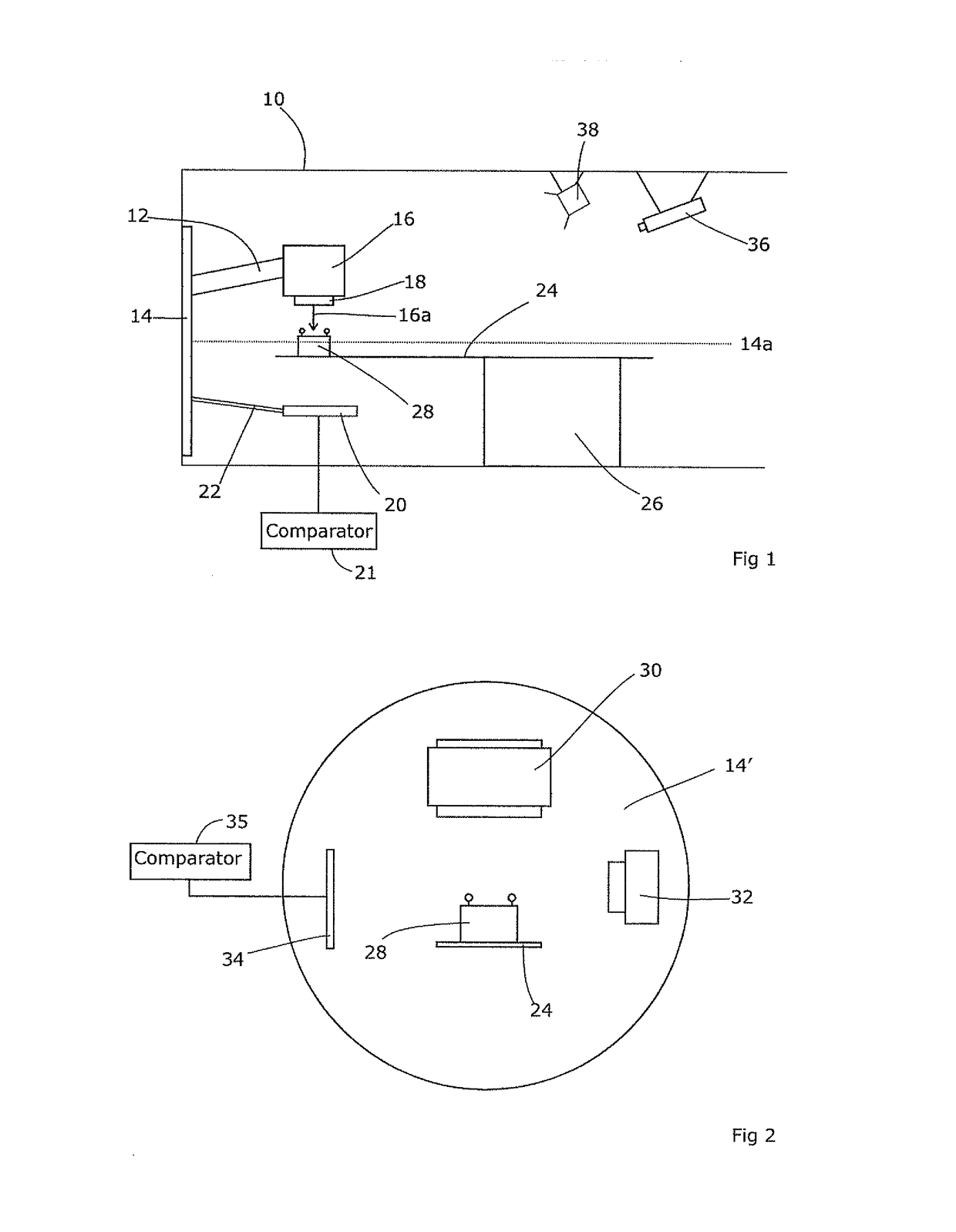
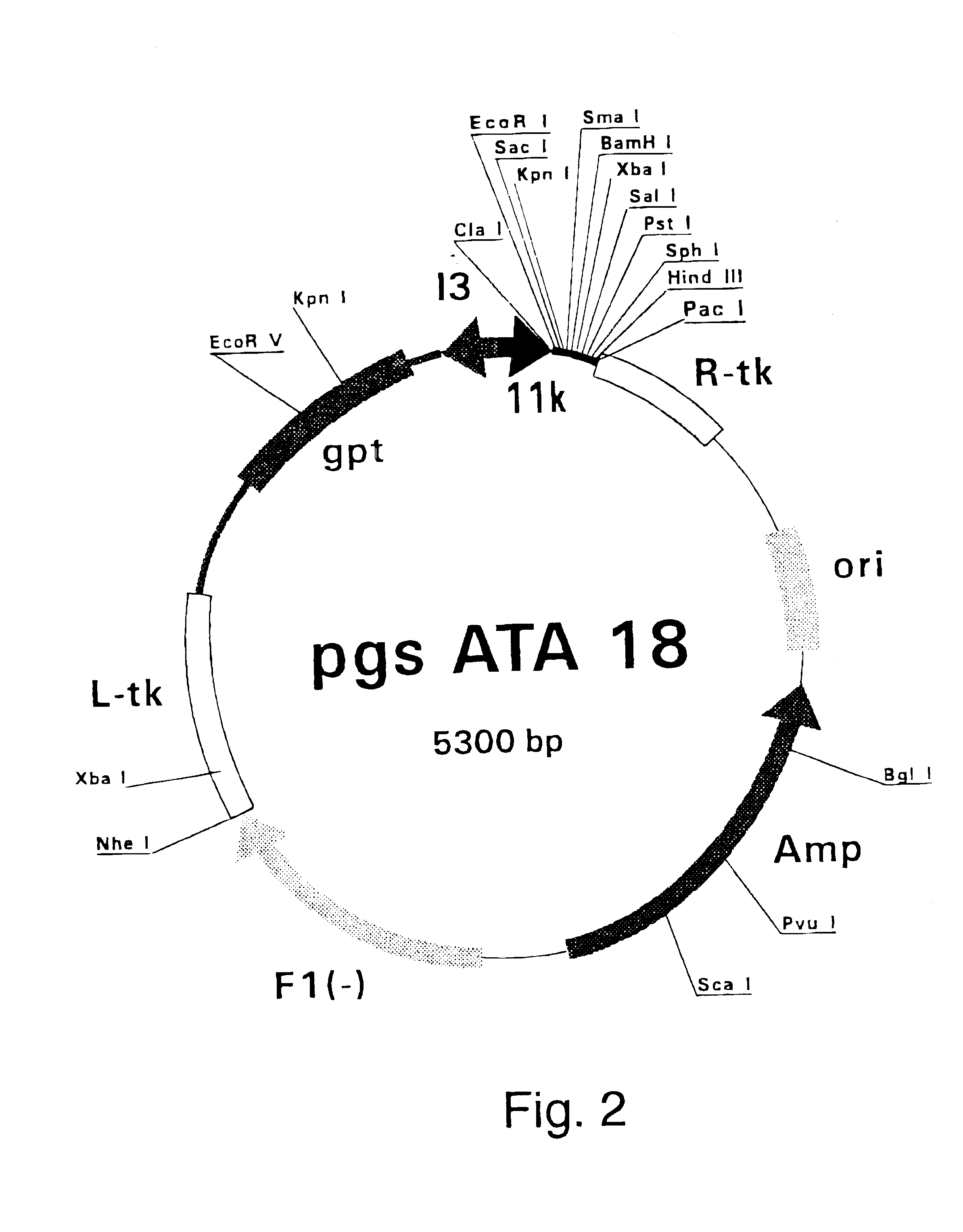
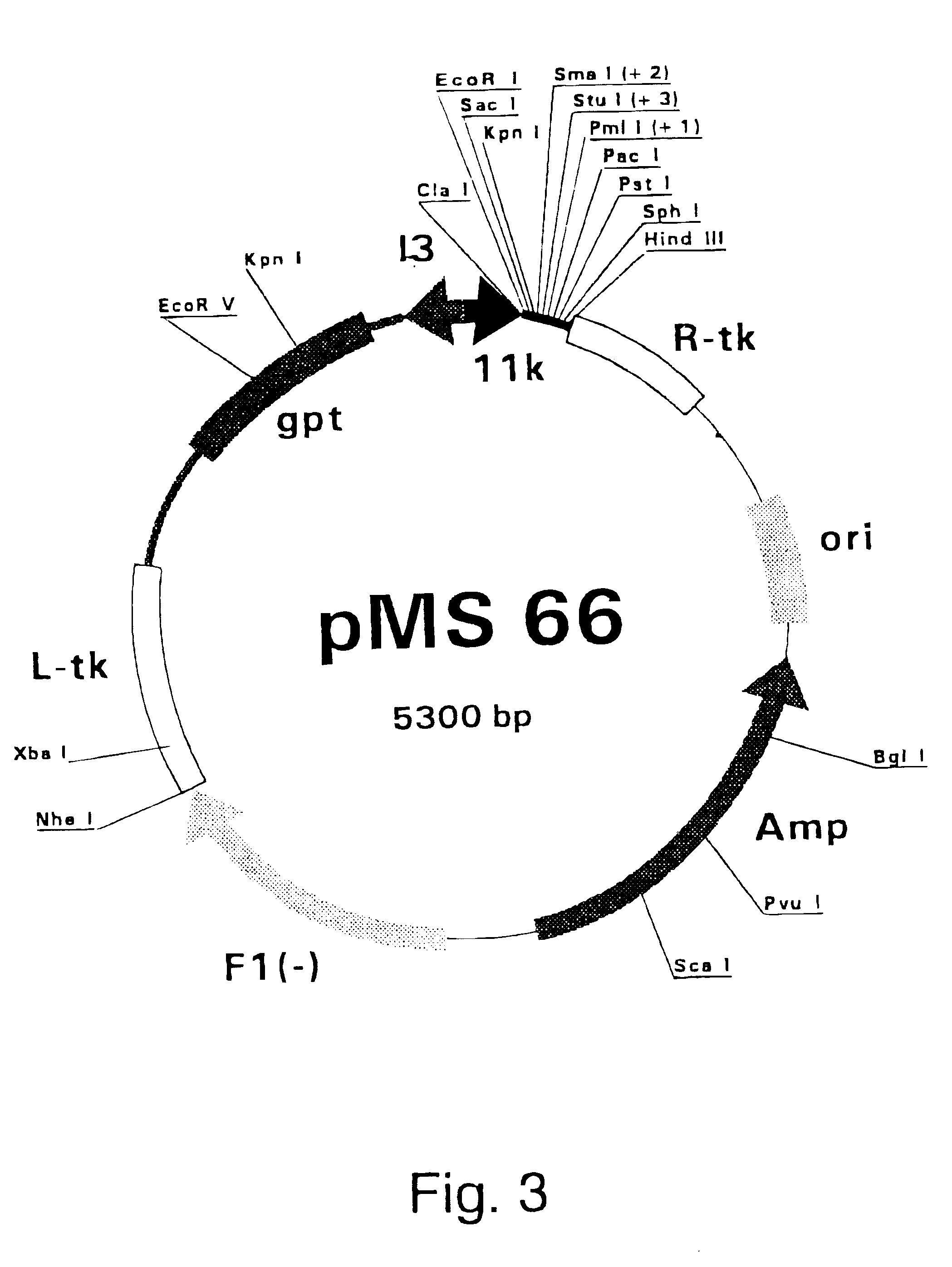
Radiotherapeutic apparatus
ActiveUS7907699B2Easy to practiceGood correlationRadiation beam directing meansInstrumentsData setComputer science
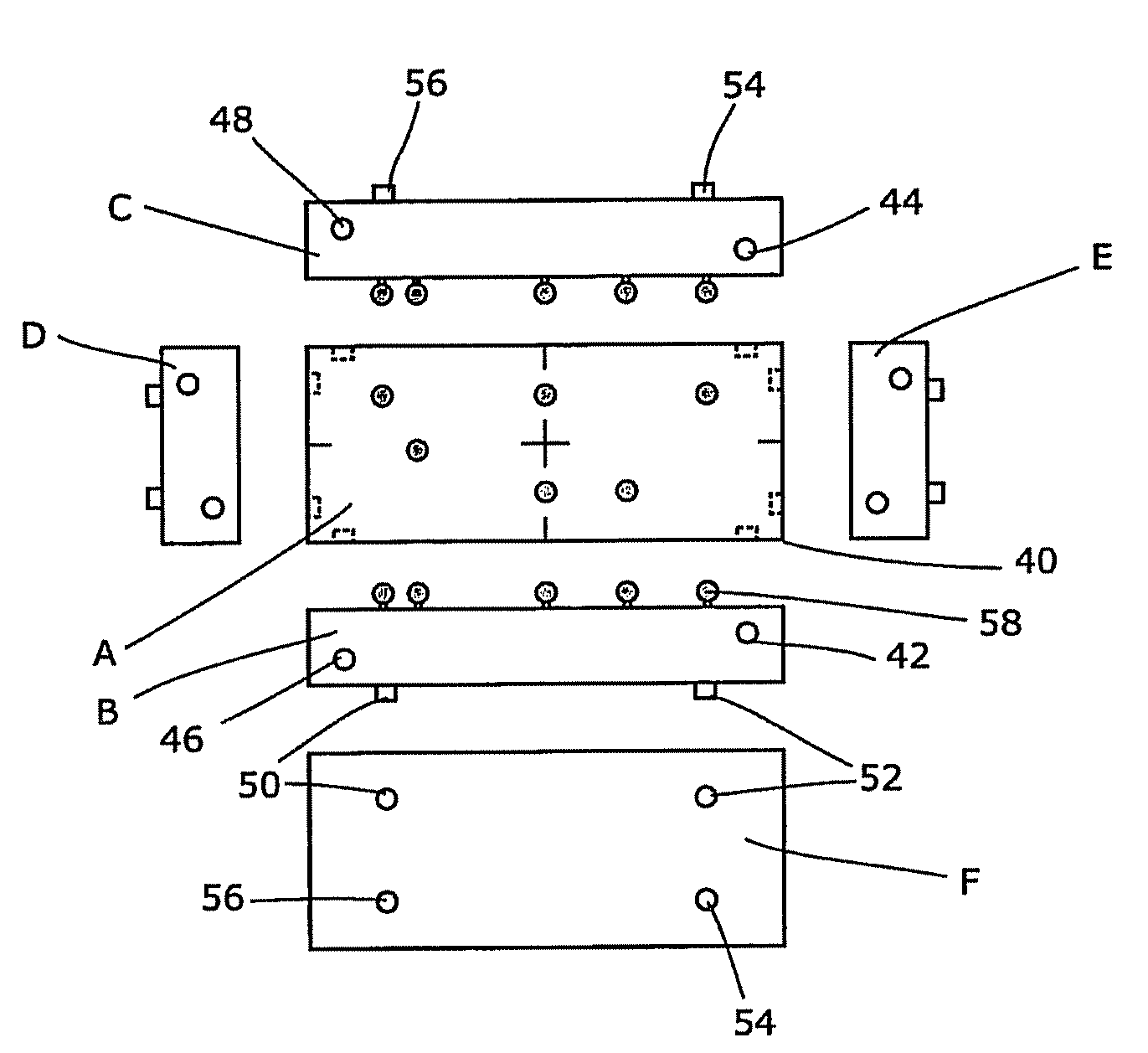
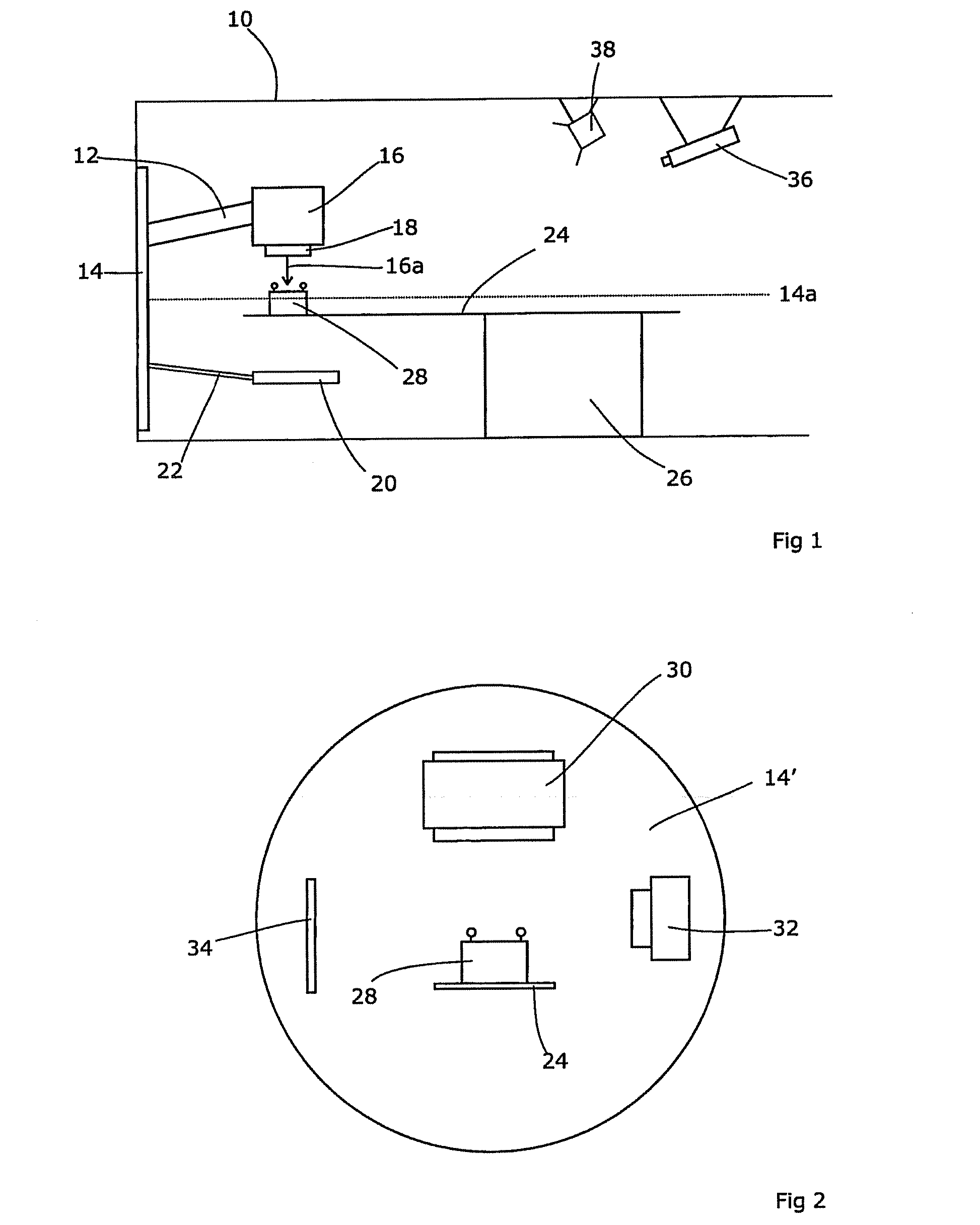
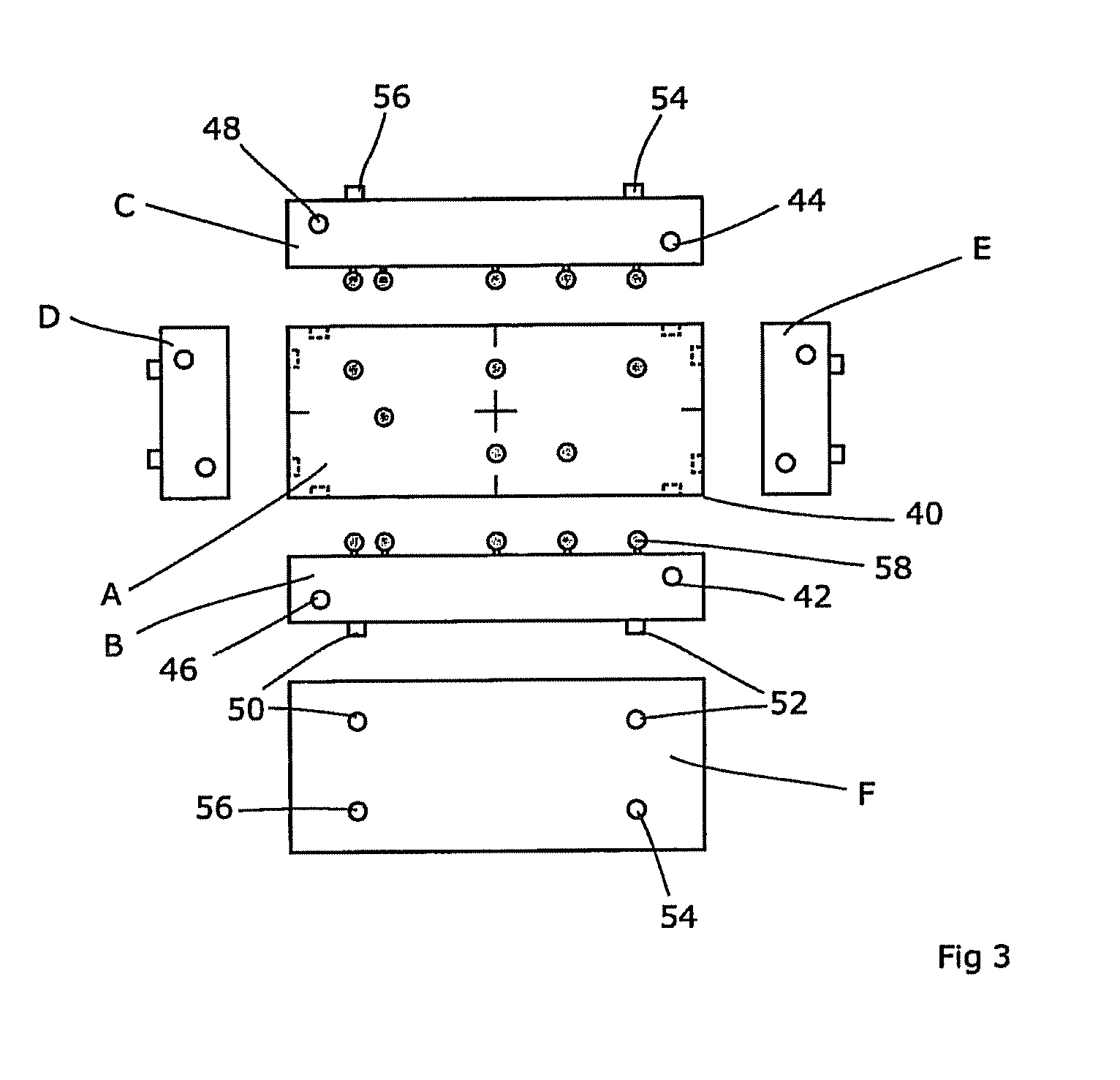
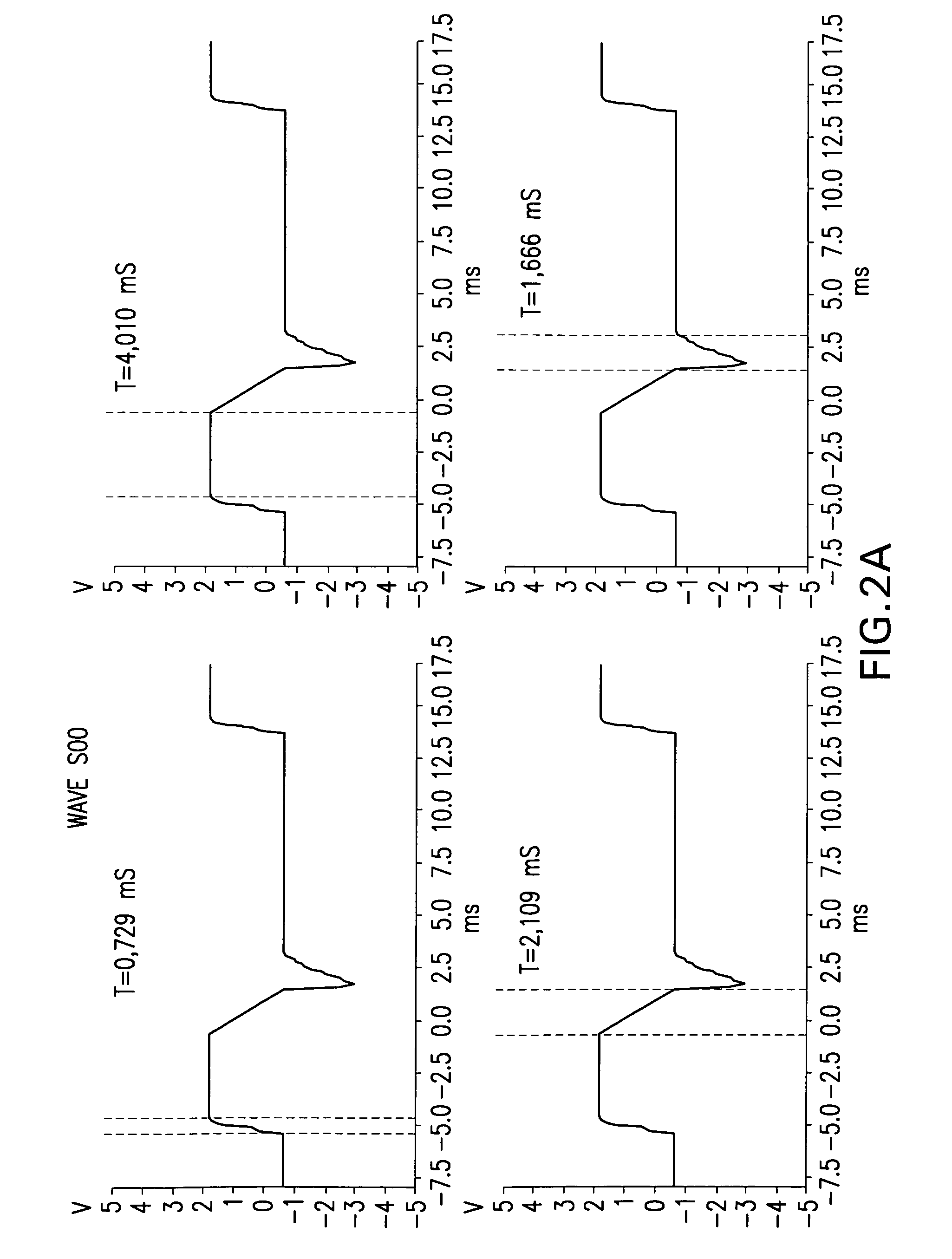
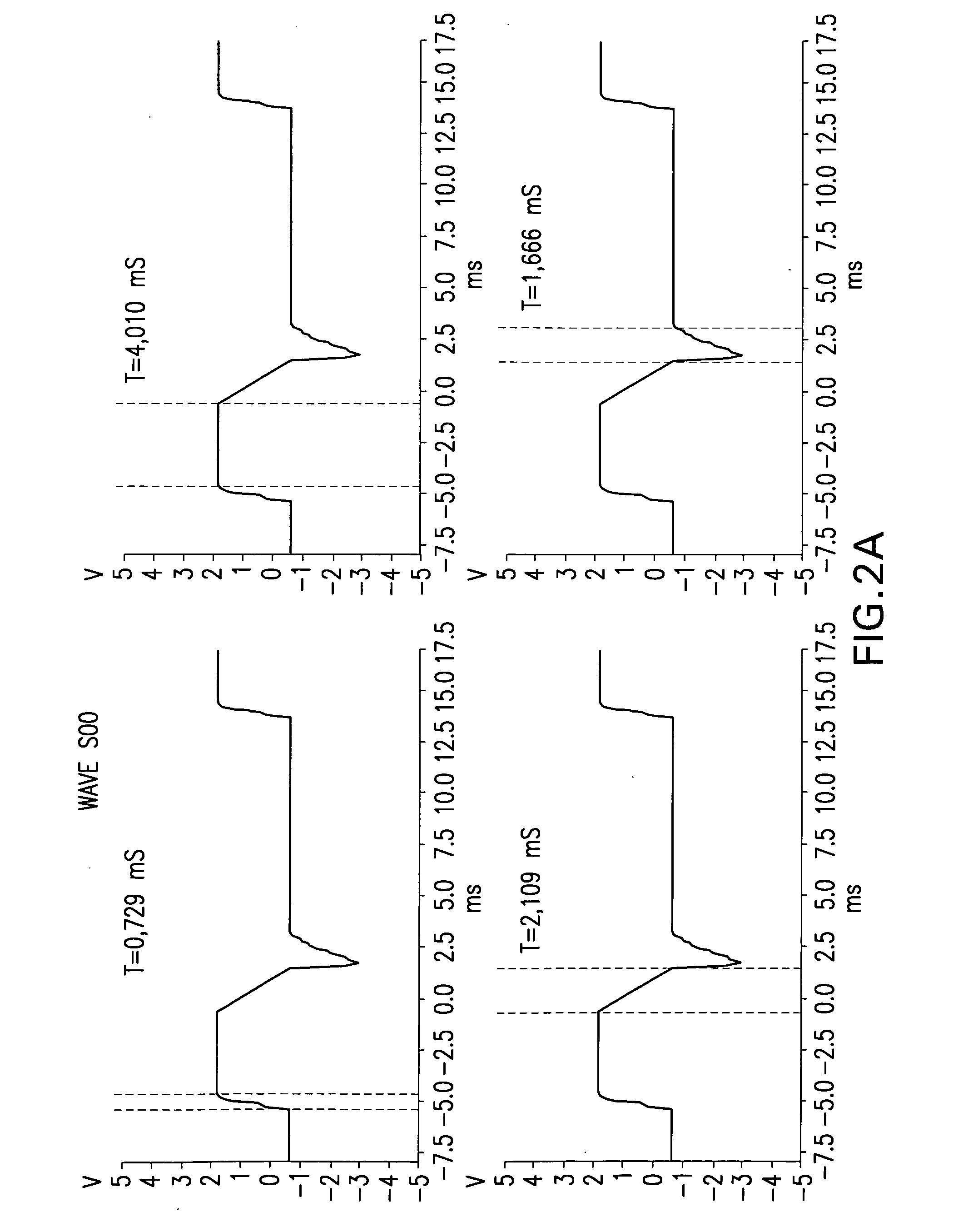
A reference phantom includes CT-imageable detail together with light-reflective spheres. This item can be placed on a patient table in a known location, following which the diagnostic source can be activated to detect the phantom position relative to the isocentre and camera employed to detect the PSS position. A synthetic image of the phantom can be used for comparison with the CT dataset. This allows improved correlation of the source and the patient support, enable further steps to be taken in enhancing the clinical effectiveness of the apparatus. In-use variations of the isocentre location can be corrected in real time by adjustment of the patient support. Thus, as the isocentre moves, the patient can be moved so as to track the moving isocentre. The linac arm could also be designed differently, as the existing design constraint (that isocentre movement must be limited as far as possible) could potentially be relaxed in order to achieve other aims.
Owner:ELEKTA AB
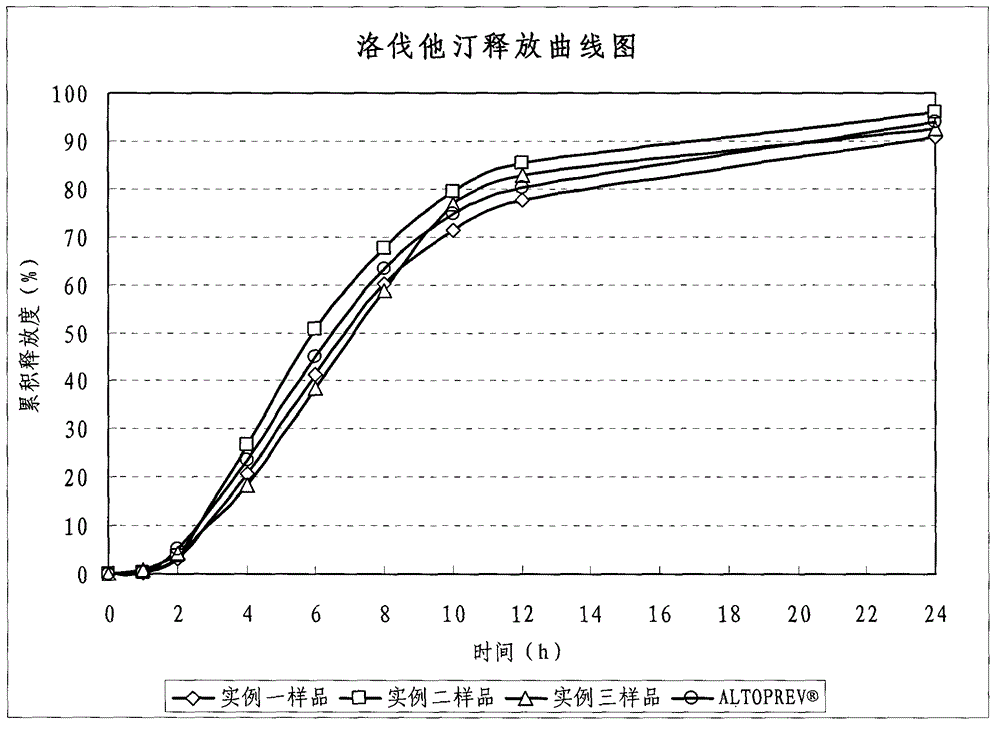
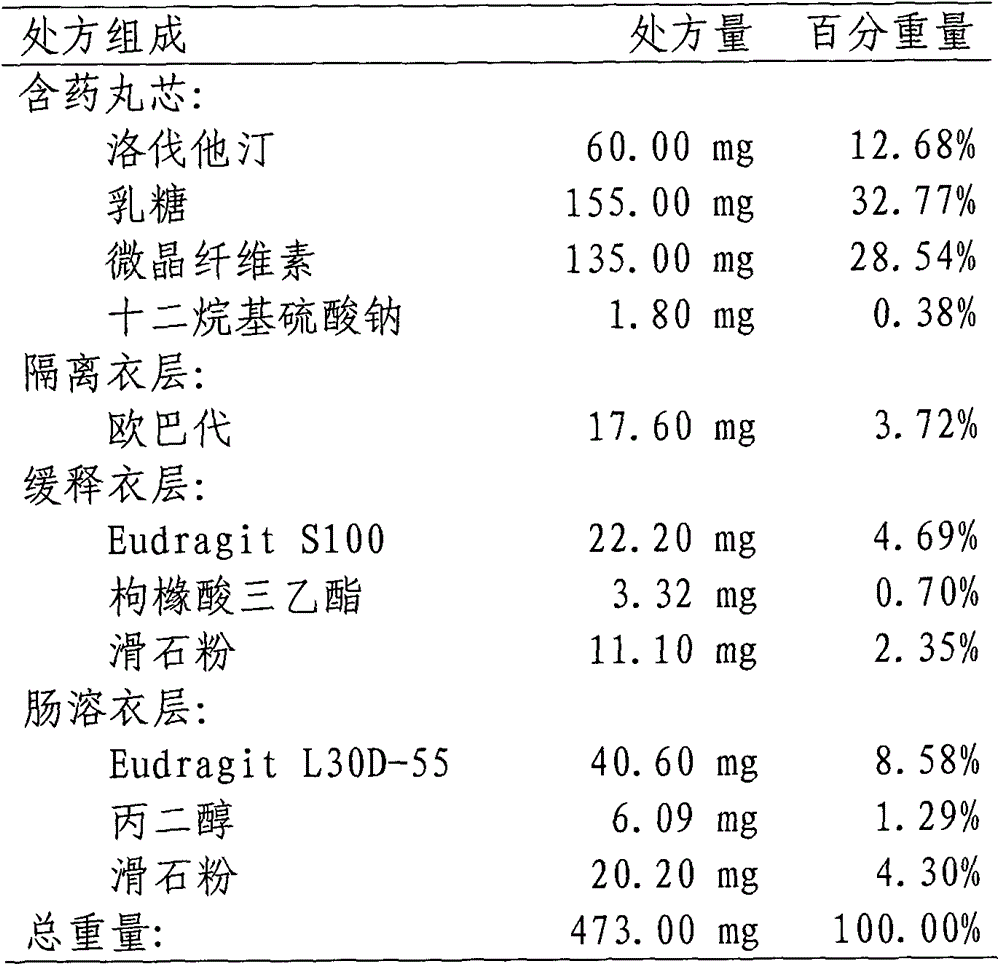
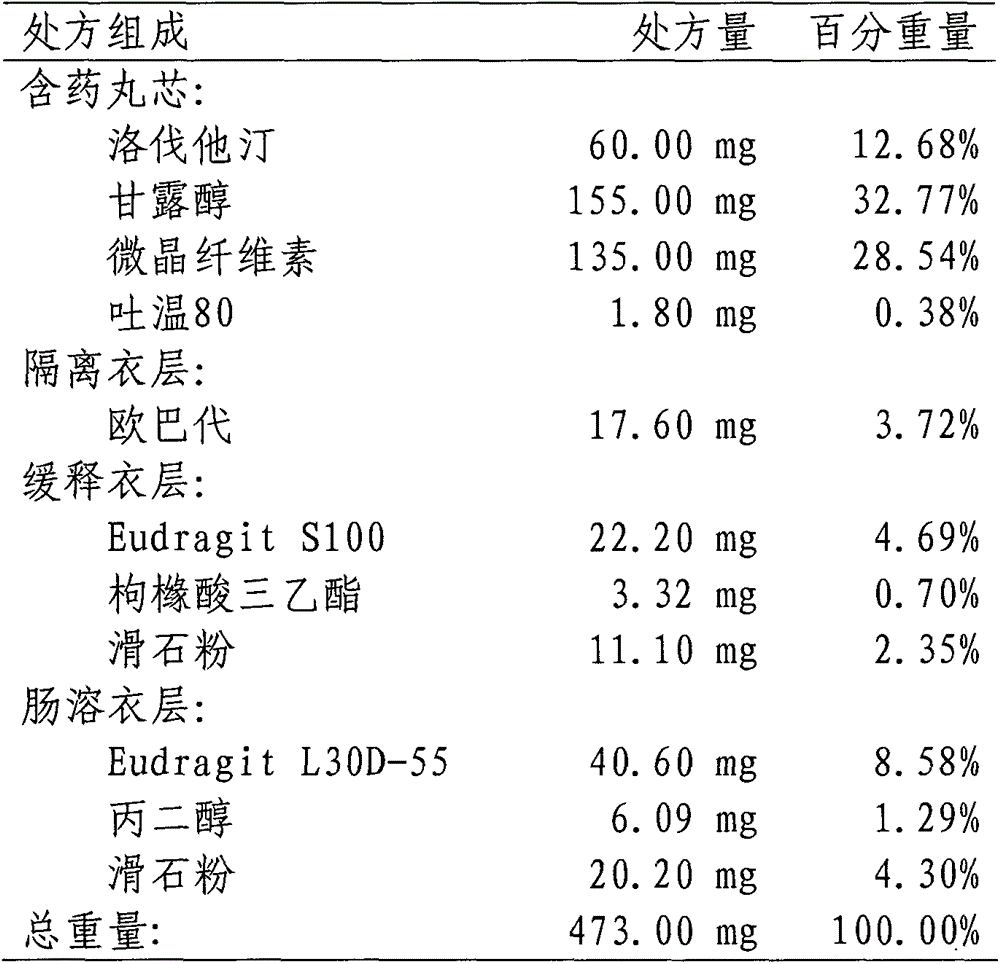
Lovastatin enteric coated sustained-release pellet capsule and preparation method thereof
InactiveCN103142552AUniform absorption rateSmall differences in individual bioavailabilityMetabolism disorderGranular deliverySustained release pelletsSide effect
The invention discloses a lovastatin-containing enteric coated sustained-release pellet capsule and a preparation method thereof. The lovastatin-containing enteric coated sustained-release pellet capsule comprises two parts, namely, an enteric coated sustained-release pellet and a hollow capsule, wherein the enteric coated sustained-release pellet comprises 55-86% of medicine-containing pellet core, 2-5% of isolation coating layer, 2-15% of a sustained-release coating layer and 10-25% of enteric-coated coating layer by weight. The prepared lovastatin enteric coated sustained-release pellet capsule is uniform in granule size and stable in medicine release; the medicine is not released in gastric acid but is slowly and constantly released in intestinal tracts and livers, has the characteristic of targeted medicine release, is small in irritation to gastrointestinal tracts, and can reduce the toxic and side effects of the medicine and reduce the number of the medicine administrations, so that the compliance of patients is improved; and meanwhile as the enteric coated sustained-release pellet preparation is composed of hundreds of pellets of uniform granule sizes, the sudden release of the whole preparation is not caused by the breakage of individual pellets, so that the medicine is safer than a sustained-release tablet, smaller in irritation to gastrointestinal tracts and more stable in blood concentration, and the clinical effectiveness and security are effectively improved.
Owner:广州科的信医药技术有限公司
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
InactiveUS20040126395A1SsRNA viruses positive-senseViral antigen ingredientsHcv treatmentDisulphide bonds
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E2 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:MAERTENS GEERT +2
Radiotherapeutic apparatus
ActiveUS20090238338A1Reduce uncertaintySmall volumeRadiation beam directing meansInstrumentsData setComputer science
A reference phantom includes CT-imageable detail together with light-reflective spheres. This item can be placed on a patient table in a known location, following which the diagnostic source can be activated to detect the phantom position relative to the isocentre and camera employed to detect the PSS position. A synthetic image of the phantom can be used for comparison with the CT dataset. This allows improved correlation of the source and the patient support, enable further steps to be taken in enhancing the clinical effectiveness of the apparatus. In-use variations of the isocentre location can be corrected in real time by adjustment of the patient support. Thus, as the isocentre moves, the patient can be moved so as to track the moving isocentre. The linac arm could also be designed differently, as the existing design constraint (that isocentre movement must be limited as far as possible) could potentially be relaxed in order to achieve other aims.
Owner:ELEKTA AB
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
InactiveUS7101561B2Different reactivityEasy to prepareSsRNA viruses positive-sensePeptide/protein ingredientsHcv treatmentDisulphide bonds
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E2 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:GENIMMUNE NV
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
InactiveUS20030147918A1Reduce the possibilityHigh sensitivitySsRNA viruses positive-sensePeptide/protein ingredientsHcv treatmentDisulphide bonds
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E2 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment
Owner:GENIMMUNE NV
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:INNOGENETICS NV
Method for quantitatively analyzing myocardium acoustic contrast image
InactiveCN101536919ABlood flow measurement devicesInfrasonic diagnosticsPattern recognitionMathematical model
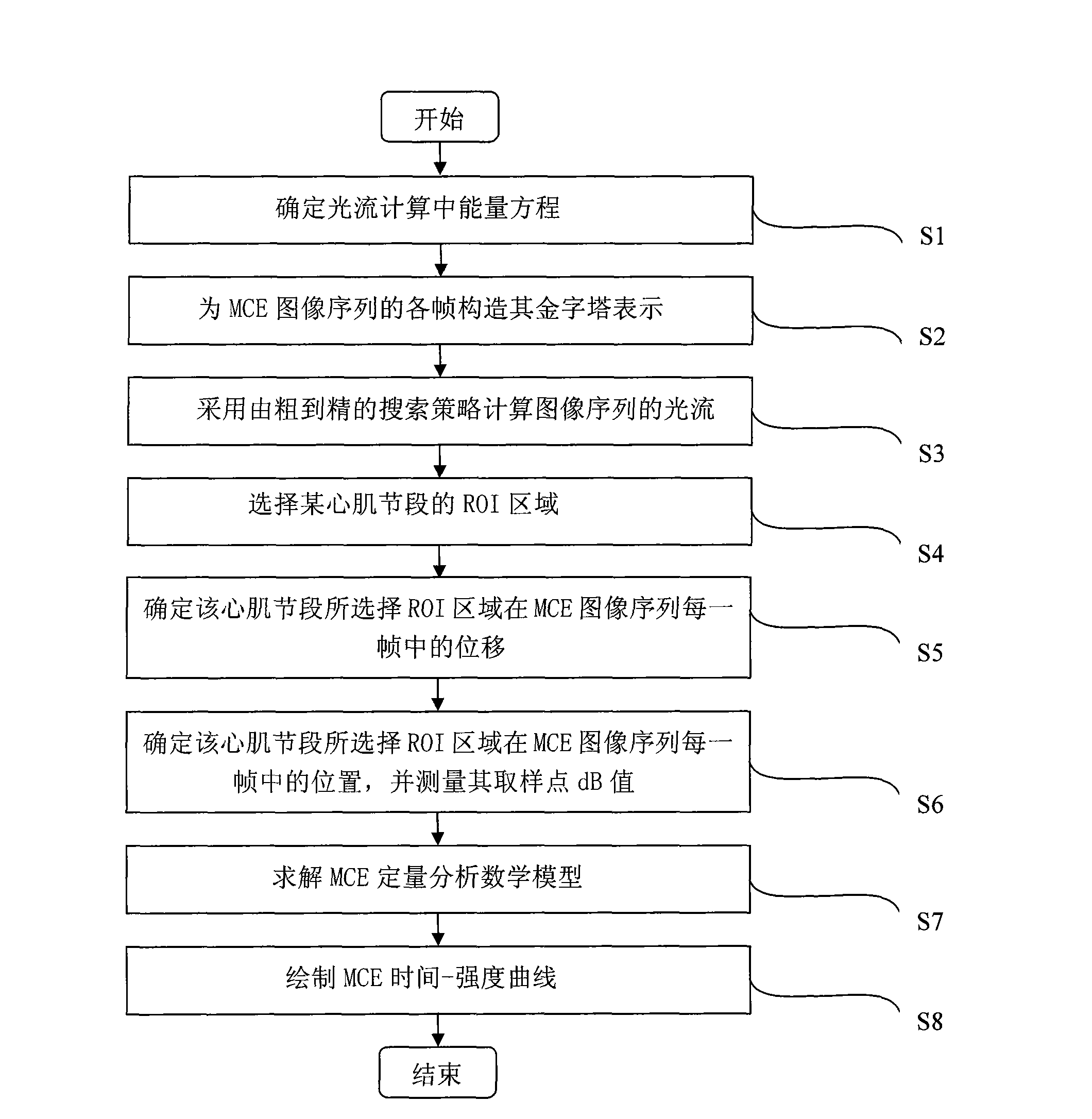
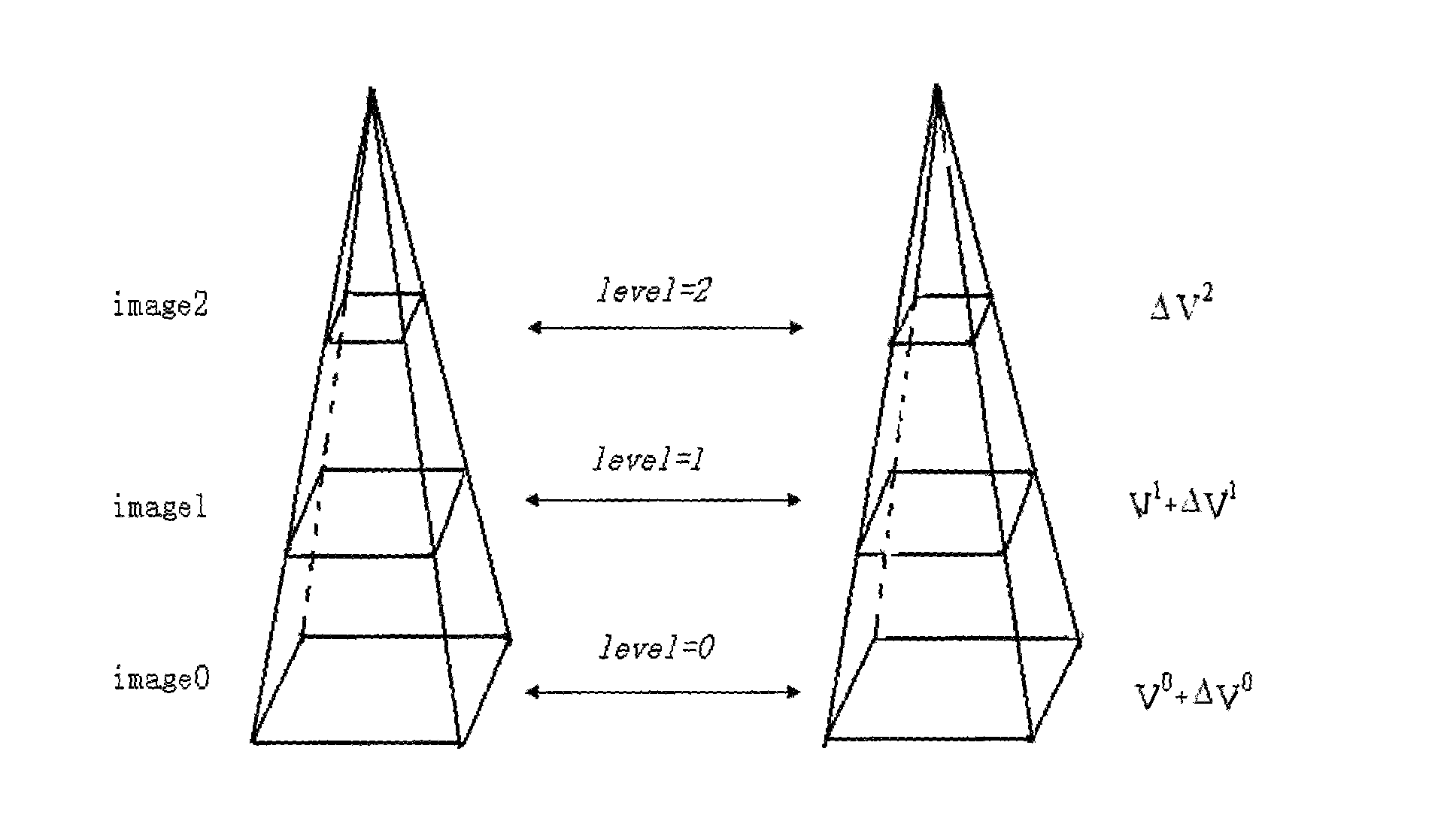
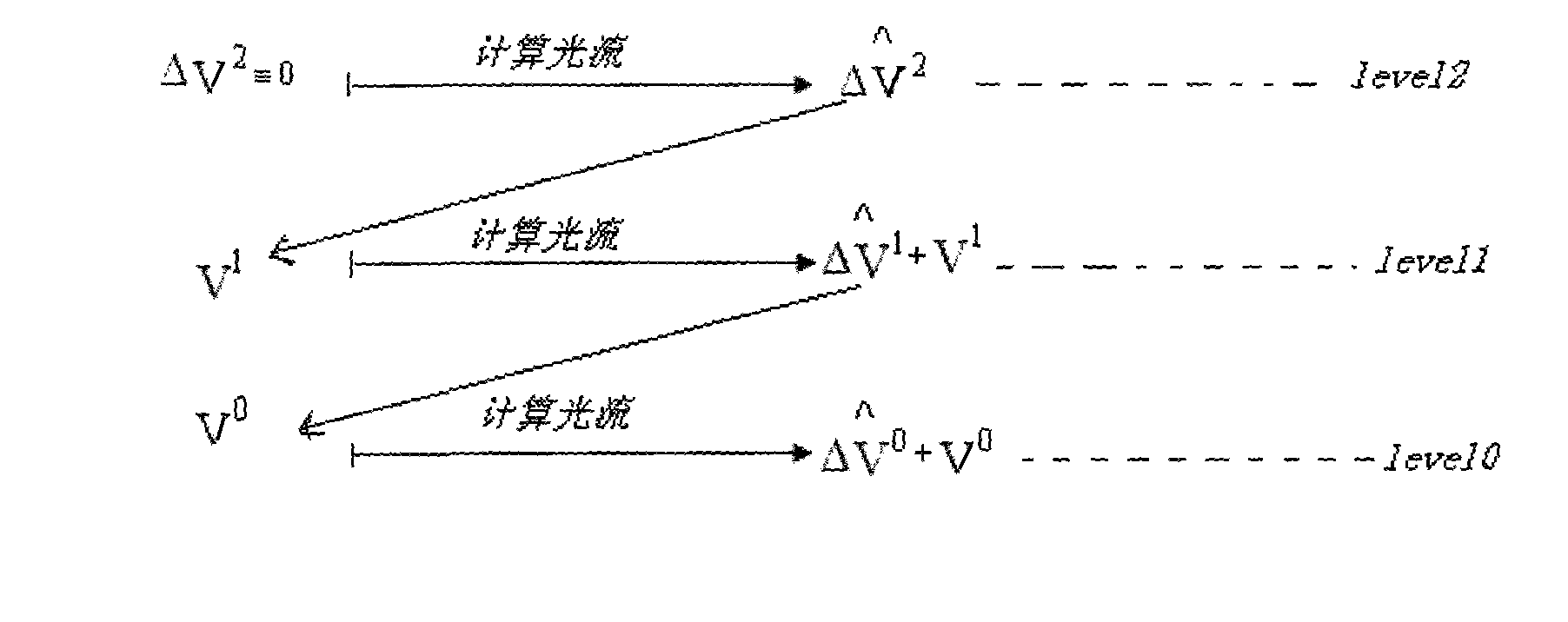
The invention relates to a method for quantitatively analyzing a myocardium acoustic contrast image, which belongs to the technical field of medical image analysis and processing. The method comprises the following steps: determining an energy equation in optical flow calculation; constructing pyramid expressions of each frame of a myocardium acoustic contrast image sequence; calculating an optical flow field of the image sequence by adopting a searching policy from roughness to precision; selecting an ROI area of a myocardium segment; determining displacements of the selected ROI area of the myocardium segment in each frame of the myocardium acoustic contrast image sequence; determining positions of the selected ROI area of the myocardium segment in each frame of the myocardium acoustic contrast image sequence and measuring dB values of sampling points of the positions; determining unknown parameters in a quantitative analysis mathematical model of the myocardium acoustic contrast; and drawing a time-intensity curve of the myocardium acoustic contrast. The method achieves the full automation of the quantitative analysis of the myocardium acoustic contrast image and has accurate analysis result and high clinical effectiveness and objectivity.
Owner:SHANDONG UNIV
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E1 / E2 characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:INNOGENETICS NV
Anti-rheumatism traditional Chinese medicine quality evaluation method based on bioavailability
PendingCN105543328AInhibitory biological potencyInhibitory activityMicrobiological testing/measurementBiological testingInflammatory factorsRheumatism
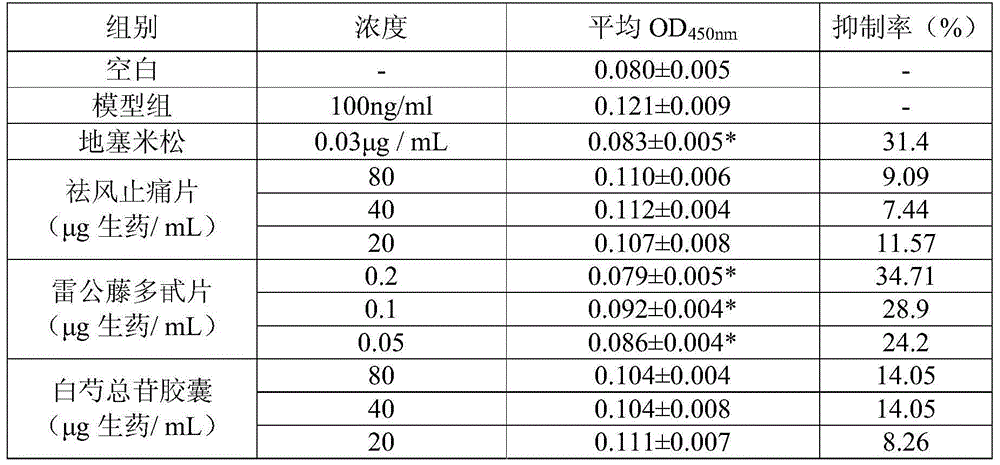
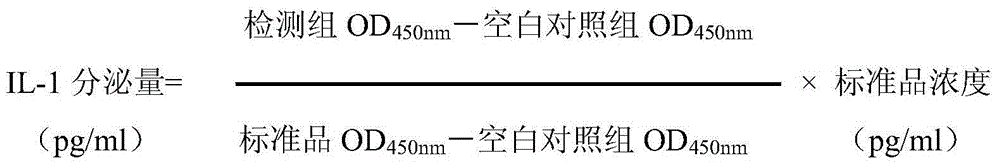
The present invention relates to a traditional Chinese medicine quality evaluation method based on anti-rheumatism bioavailability. According to the quality evaluation method, bioavailability is adopted as an evaluation index, and particularly bioavailability for chondrocyte protection, bioavailability for COX-2 activity inhibition, and bioavailability for inflammatory factor secretion inhibition are adopted as the quality evaluation indices, wherein the bioavailability for chondrocyte protection is the bioavailability for chondrocyte NO secretion inhibition, and the inflammatory factor is TNF-alpha or IL-1. According to the present invention, the quality evaluation method can be adopted as the effective complement of the traditional chemical detection method, directly reflects the clinical effectiveness, and is the quality control and detection method which truly meets the traditional Chinese medicine theory, reflects the clinical application characteristics of the traditional Chinese medicine, and ensures the safety and the effectiveness of the traditional Chinese medicine.
Owner:周亚伟
Pharmaceutical composition comprising a proton pump inhibitor and a prebiotic for the treatment of ulcerous lesions of the stomach and duodenum
InactiveCN102333534ARepair ulcerContinuous removalAntibacterial agentsHydroxy compound active ingredientsDiseaseSide effect
A pharmaceutical composition comprising proton pump inhibitors and prebiotics is proposed for the treatment of gastric and duodenal ulcer, this allowing effective ulcer treatment and eradication of H. pylori from the gastric and duodenal mucosa to be carried out without using wide-spectrum antibiotics. The comprehensive treatment of ulcer disease associated with a helicobaterial infection using a pharmaceutical composition of a PPI and a prebiotic makes it possible, in conditions of an elevated pH of the stomach contents, actively to stimulate the growth of lactobacilli in the upper sections of the gastrointestinal tract, including the duodenum, and substantially to increase the titre of lactobacilli, which are antagonists of H. pylori, which greatly improves the effectiveness of the ulcer treatment.; The high clinical effectiveness and safety resulting from the synergistic action of the proton pump inhibitor and the prebiotic in the upper sections of the gastrointestinal tract, the absence of side effects and recurrences, and also the level of compliance to the combined treatment provided by the claimed pharmaceutical composition indicate that the proposed formulation is a promising new means for the treatment of gastric and duodenal ulcer. Use of the claimed composition dramatically reduces the number of recurrences of the illness.
Owner:亚历山大·弗拉基米罗维奇·季科夫斯基
Radix astragali particle and quality control method thereof
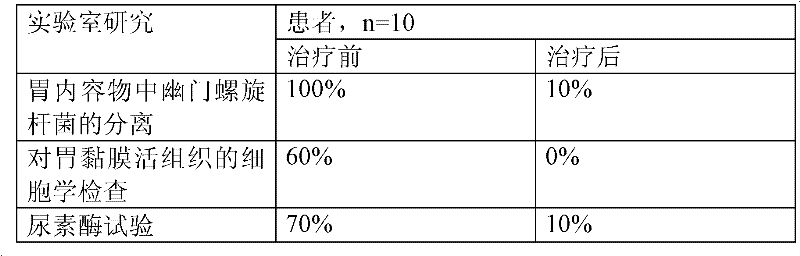
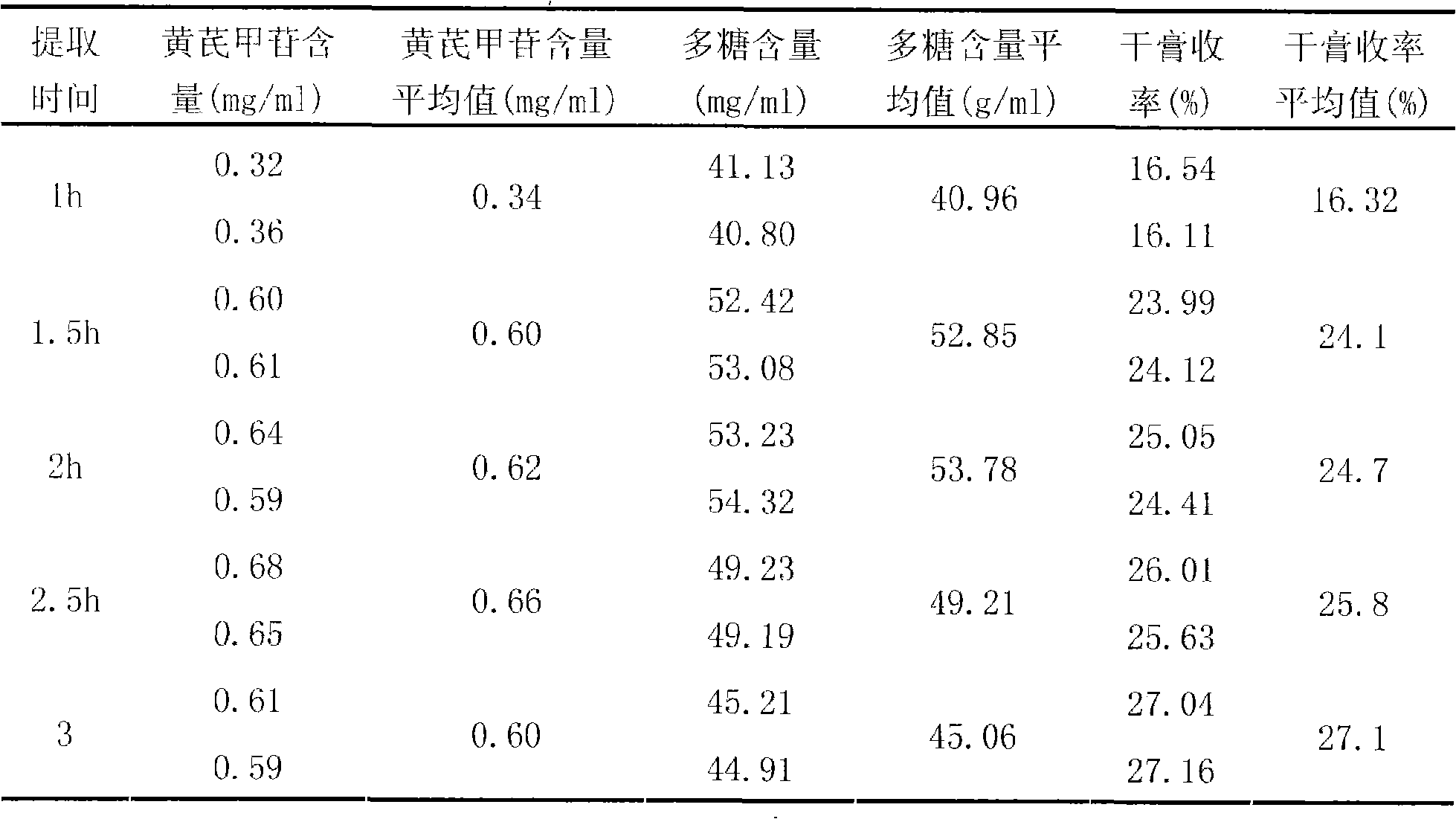
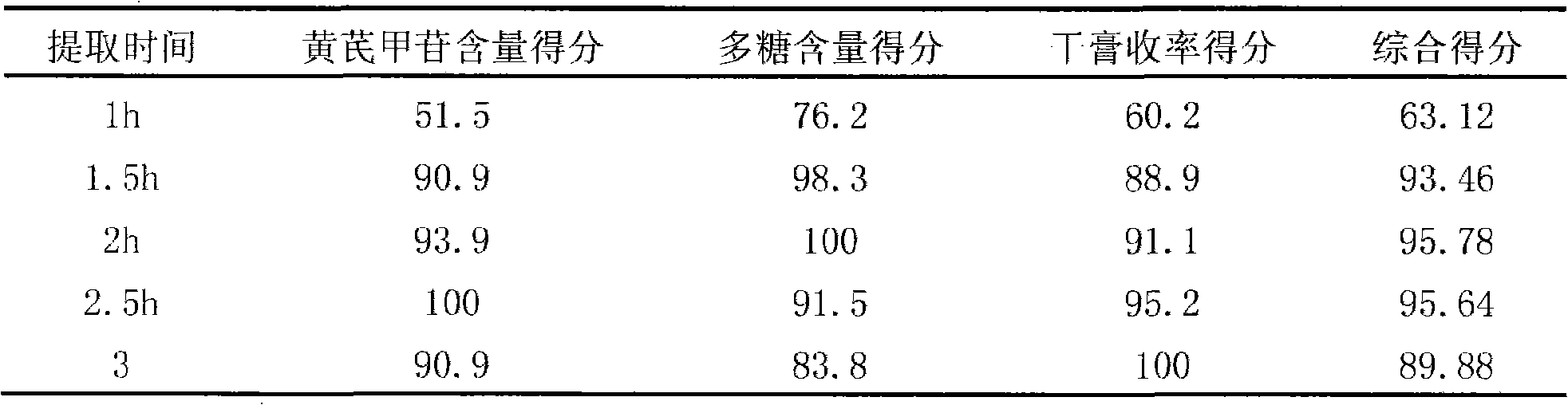
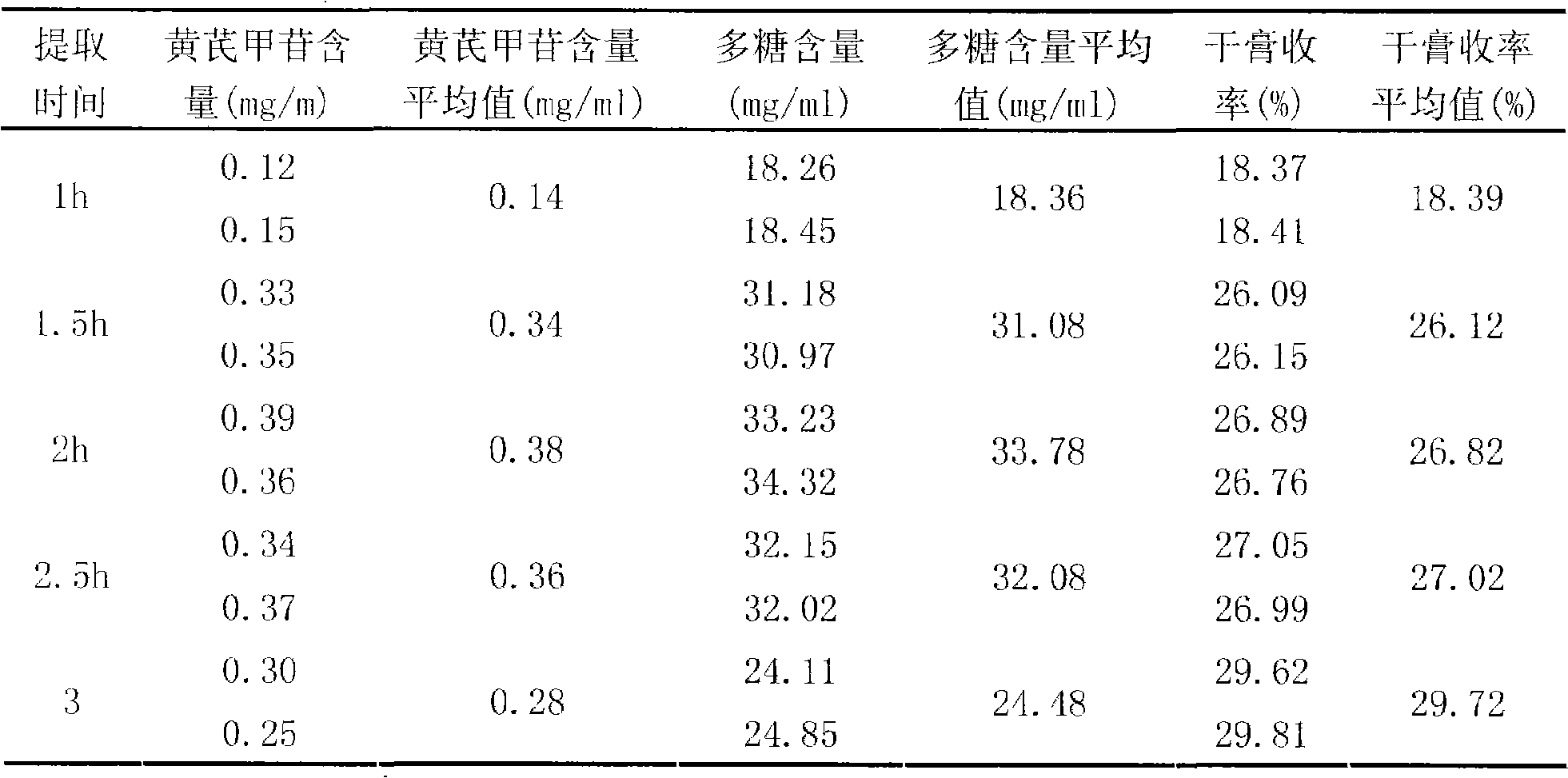
InactiveCN101342230AImprove clinical effectivenessPromote dissolutionComponent separationUrinary disorderAstragalus polysaccharideAlcohol
The invention discloses a raidx astragali particle. The radix astragali particle is prepared by a following method: the medicinal materials of the radix astragali are decocted with water and extracted twice; the medicinal materials of the radix astragali are decocted for 1.5 to 2.5hours every time; decoctions are put together and filtered; filtrate is concentrated into a clear paste with relative density of 1.20 to 1.30 (70 to 80 DEG C); auxiliary materials are added and mixed evenly; then the medicinal materials of the radix astragali and the auxiliary materials are processed by granulation, drying, sub-package and sterilization; and the raidx astragali particle is made. The radix astragali particle is prepared by the new method and the procedures of alcohol precipitation is reduced. That decoction time is changed is beneficial to the dissolution and preservation of effective components. As a result, the effective components of the medicinal materials of the radix astragali, including astragalus polysaccharides and total saponin, and the like, are reserved in the radix astragali particle as much as possible, thus greatly improving the content of the effective components so as to improve the clinical effectiveness of the raidx astragali. Meanwhile, production period is greatly shortened; energy consumption is decreased; and cost is lowered. The invention also provides a quality control method of the raidx astragali particle.
Owner:SICHUAN BAILI PHARM CO LTD
Preparation technology of Campsis grandiflora freshly-squeezed spray-dried granule decoction pieces
InactiveCN107320508AReduce volumeQuality improvementGranular deliveryPlant ingredientsEconomic benefitsCurative effect
The invention discloses a preparation technology of Campsis grandiflora freshly-squeezed spray-dried granule decoction pieces. According to the technology, after Campsis grandiflora medicinal materials are collected, sorting, rinsing and fresh squeezing are conducted, and a decoction is obtained from filtering; water is added into dregs for stirring, and filtering and squeezing are conducted; after obtained decoctions are mixed, the mixture is subjected to vacuum concentration and spray-dried, spray-dried products are collected, the spray-dried products are smashed and mixed with the firstly squeezed decoction to obtain granules, and after granulation, the granules are vacuum-packed. According to the technology, Campsis grandiflora granule decoction pieces are obtained, and polar and non-polar effective components in the medicinal materials are kept to the utmost extent; the granule decoction pieces have the advantages of being good in liquidity, easy to store and transport, convenient to make as a prescription, controllable in quality, reliable in curative effect and of great importance in guaranteeing clinical effectiveness of traditional Chinese medicine; popularization and implementing of the technology will have significant social meaning and economic benefit.
Owner:WUHU TIANCHENG PUYANG TRADITIONAL CHINESE MEDICINE TECH CO LTD
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E2 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:INNOGENETICS NV (NL)
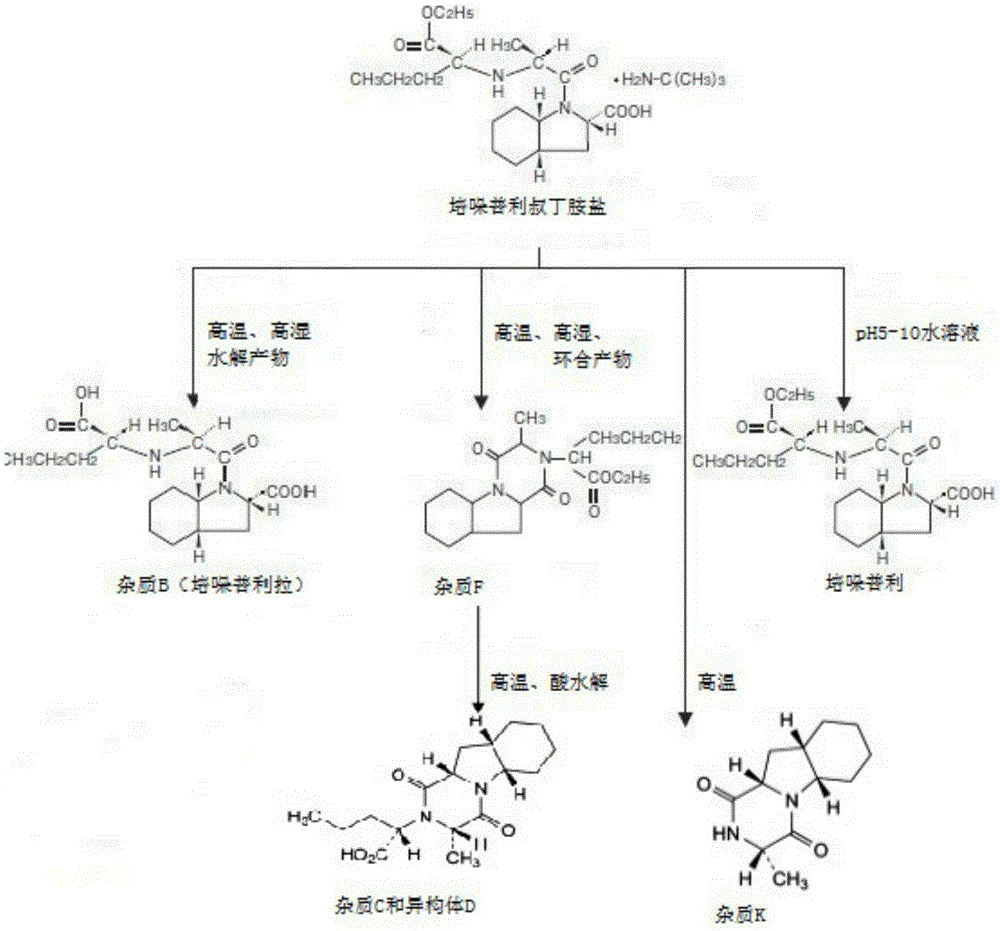
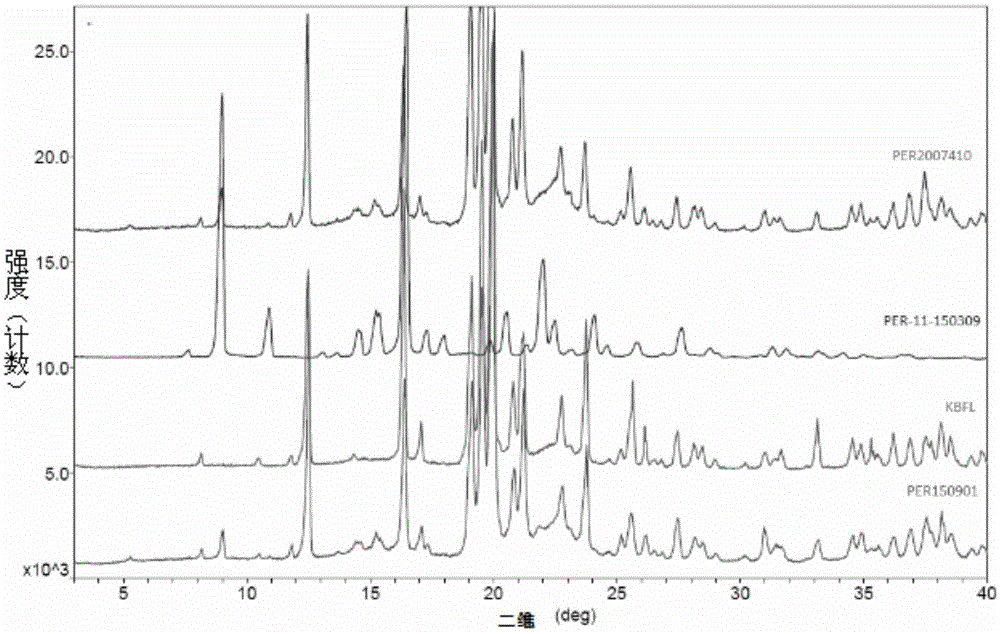
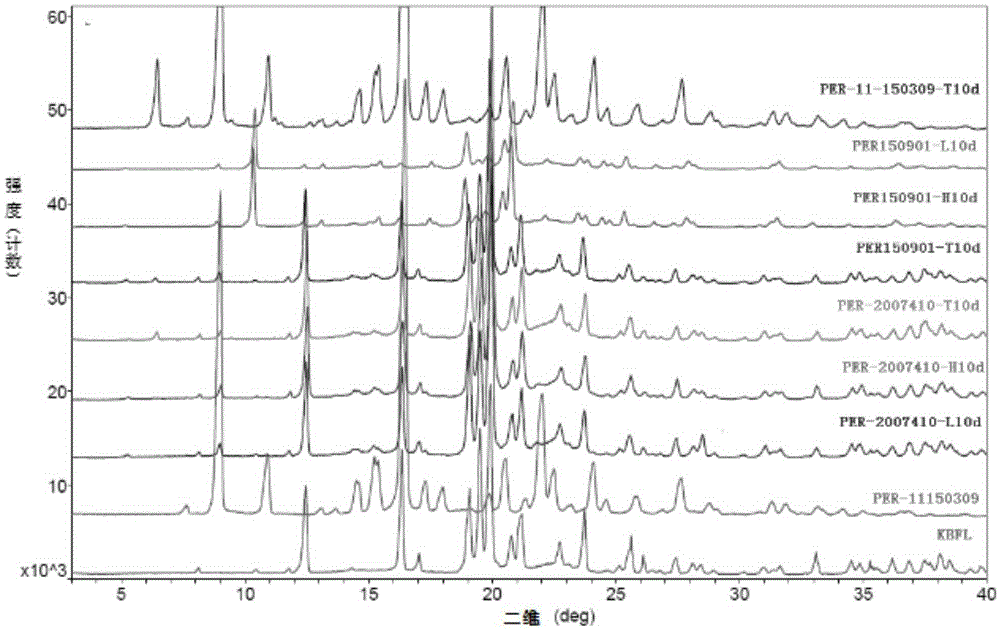
Stable alpha-crystalline form perindopril tert-butylamine tablet and preparation method thereof
ActiveCN105395497AReduce sizePiece weight smallDipeptide ingredientsPharmaceutical non-active ingredientsMagnesium stearateDissolution
The invention provides a stable alpha-crystalline form perindopril tert-butylamine tablet, which consists of perindopril tert-butylamine, lactose, microcrystalline cellulose and magnesium stearate. A preparation method, by virtue of a powder direct compression process, comprises the following steps: firstly, sieving and mixing the lactose and the microcrystalline cellulose, and drying the lactose and the microcrystalline cellulose; respectively sieving and mixing some lactose and microcrystalline cellulose with perindopril tert-butylamine; finally, adding the magnesium stearate and mixing; sampling and detecting, tabletting and packaging an obtained tablet with an aluminum-plastic foamed mask, wherein the interior of the aluminum-plastic foamed mask contains a drying agent and the exterior of the aluminum-plastic foamed mask is covered by a composite film bag. The perindopril tert-butylamine tablet prepared by the method disclosed by the invention has no significant change with published products in character, dissolution rate, moisture, content, crystalline form as well as dissolution behaviors in mediums at different pH values, and the perindopril tert-butylamine tablet is more stable than the published products in production and storage stability, so that the clinical effectiveness and safety of the medicine during taking are guaranteed. The perindopril tert-butylamine tablet preparation method provided by the invention is simple, and the production cost of the medicine is reduced.
Owner:NINGBO MENOVO TIANKANG PHARMA CO LTD
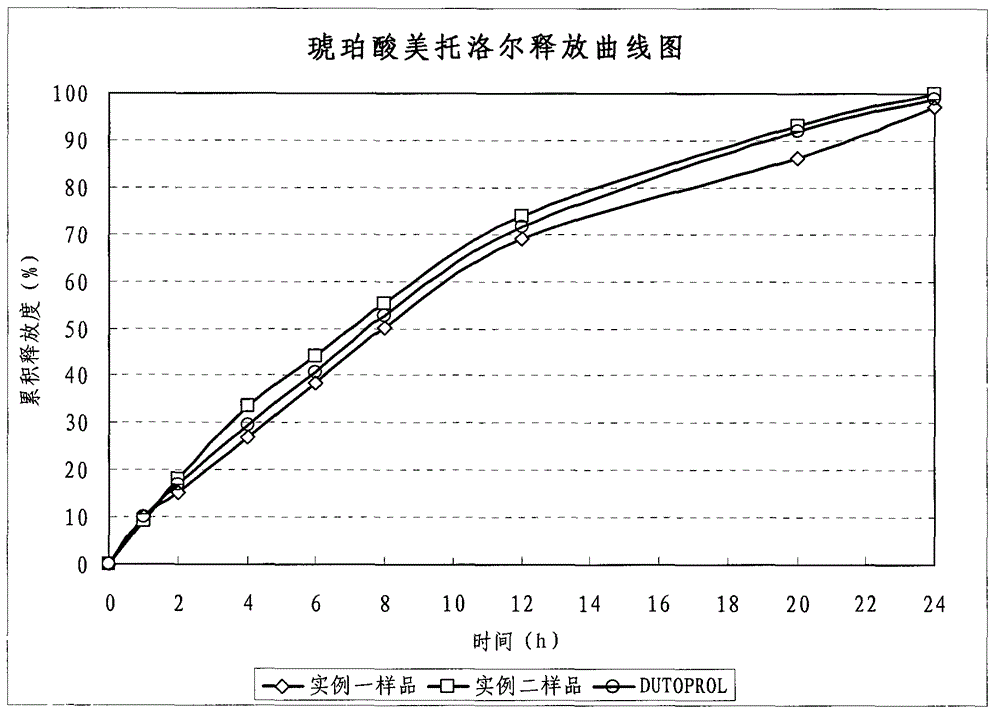
Metroprolol succinate hydrochlorothiazide sustained-release pellet capsule and preparation method thereof
InactiveCN103142618AUniform absorption rateSmall differences in individual bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSide effect
The invention discloses a metroprolol succinate hydrochlorothiazide sustained-release pellet capsule and a preparation method thereof. The metroprolol succinate hydrochlorothiazide sustained-release pellet capsule is prepared by mixing a metroprolol succinate sustained-release pellet and a hydrochlorothiazide quick-release pellet according to provided dose ratio, and filling into a hard capsule. The metroprolol succinate sustained-release pellet constantly releases the medicine for 24 hours, and the medicine is taken once a day, thereby retaining the lasting antihypertensive effect; the hydrochlorothiazide quick-release pellet is rapidly absorbed so as to achieve the diuresis and antihypertensive effect, and by combining the pellets, the antihypertensive effect is doubled, the toxic and side effects of the medicine are reduced, the number of the medicine administrations is reduced, and lasting and stable antihypertensive effects are achieved in vivo, so that the compliance of patients is improved; and meanwhile as the sustained-release pellet preparation is composed of hundreds of pellets of uniform granule sizes, the sudden release of the whole preparation is not caused by the breakage of individual pellets, so that the medicine is safer than a sustained-release tablet, smaller in irritation to gastrointestinal tracts and more stable in blood concentration, and the clinical effectiveness and security are effectively improved.
Owner:广州科的信医药技术有限公司
Apparatus and method for quick pain suppression
ActiveUS8380317B2Good reproducibilityImprove effectivenessElectrotherapyArtificial respirationMedicineChronic pain
Apparatus and methods for quick acute and chronic pain suppression, particularly useful and effective towards high-grade pains and / or pains resistant to other analgesic drugs such as opiates. One apparatus and method generate synthetic “non-pain” information strings of great clinical effectiveness, allowing high reproducibility of the clinical result. Synthesis of the strings occurs by combining novel geometries of complex waveforms in a sequence, perceived as “self” and “non-pain” by the CNS.
Owner:MARINEO GIUSEPPE
Stable perindopril indapamide tablet and preparation technology
ActiveCN106620644AReduce sizePiece weight smallOrganic active ingredientsDipeptide ingredientsDissolutionSilicon dioxide
The invention discloses a stable perindopril indapamide tablet and a preparation technology. The pharmaceutical composition is composed of the following components: perindopril tert-butylamine, indapamide, lactose, hydroxypropyl cellulose, sodium carboxymethyl starch, silicon dioxide, and magnesium stearate. Powder is directly pressed to prepare the pharmaceutical composition. The properties, dissolution rate, water content, content, crystal form, and dissolving-out behavior in different medium with different pH values of the prepared perindopril indapamide tablet are similar with those of commercial products. Furthermore, the dissolution curve shows that the difference of tablets in a batch and the difference of tablets in different batches is prominently less than that of commercial products. Compared with the commercial products, the stability of the provided tablet is better in production and storage. The clinical effectiveness and safety are guaranteed. Moreover, the preparation technology is simple, and the production cost is reduced.
Owner:杭州新诺华医药有限公司
Medicament for resisting influenza and preparation method and application thereof
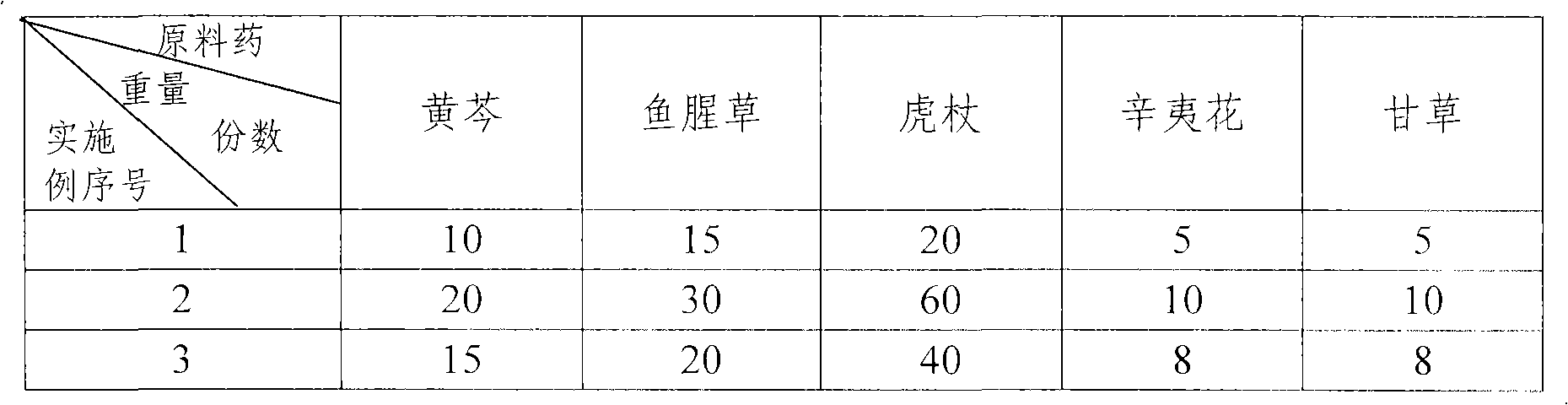
InactiveCN101791338AMedicallyReduce typesDispersion deliveryAntiviralsBaical Skullcap RootHouttuynia
The invention discloses a Chinese medicinal composition for resisting influenza in particular H1N1 influenza A, and a preparation method thereof. The Chinese medicinal composition for resisting the influenza is characterized by being prepared from the following raw materials in part by weight: 10 to 20 parts of baical skullcap root, 20 to 60 parts of giant knotweed rhizome, 5 to 10 parts of flos magnoliae liliflorae, and 5 to 10 parts of licorice. In order to further improve the curative effect, the Chinese medicinal medicament also comprises 15 to 30 parts of heartleaf houttuynia herb in part by weight. The medicament is prepared according to the following formula principles of: reasonable formula selection, clinical effectiveness, abundant resources, economical efficiency and practicability, and convenient popularization. Thus, the medicament reduces the types of used Chinese medicinal materials as much as possible, and under the condition that the influenza happens, particularly the H1N1 influenza A is pandemic, the Chinese medicinal composition can be popularized on a national scale to effectively prevent and control the spread of the influenza with the minimum economic cost and resource cost.
Owner:KMS MEDITECH
Cardiac failure resisting medicine LCZ696 oral sustained-release pellets and preparation method thereof
InactiveCN106176681ASmall toxicityGood sustained release effectMicrocapsulesCardiovascular disorderSustained release pelletsAdhesive
The invention belongs to the field of medicine, and particularly relates to cardiac failure resisting medicine LCZ696 oral sustained-release pellets and a preparation method thereof. The sustained-release pellets comprise pellet cores prepared from, by weight, 2-55 parts of LCZ696 medicine, 15-90 parts of diluent and 1-15 parts of adhesive and a coating prepared from, by weight, 1-35 parts of sustained-release materials, 0.1-5 parts of pore-foaming agent, 0.1-5 parts of plasticizer and 0.1-25 parts of antisticking agent on the outer portions of the pellet cores. The cardiac failure resisting medicine LCZ696 oral sustained-release pellets have the advantages that LCZ696 can be slowly released in the body by means of the sustained-release pellets, the plasma concentration is more stable, and clinical effectiveness and safety are effectively improved. The cardiac failure resisting medicine LCZ696 oral sustained-release pellets are suitable for symptoms caused by the cardiac failure, medicine release is stable, the effect is good, irritation to the gastrointestinal tract is small, and absorption is good after oral medication. The preparation method of the sustained-release pellets is easy to operate, the production cost is low, efficiency is high, and the sustained-release pellets are suitable for large-scale production.
Owner:TAILITE MEDICINE HUBEI
Application of Chinese herbal medicine shrubby sophora extract to preparation of weight losing and lipid lowering medicament or medicament with lipase activity suppression effect
The invention discloses application of Chinese herbal medicine shrubby sophora extract to preparation of a weight losing and lipid lowering medicament or a medicament with lipase activity suppression effect, solves the problems of low efficiency and high side effect in the conventional clinical weight losing medicament, particularly traditional Chinese medicine, and provides the weight losing andlipid lowering medicament which has the advantages of clear mechanism, scientific target point, small side effect and high clinical effectiveness.
Owner:BEIJING WBL PEKING UNIV BIOTECH
Materials and Methods for Minimally-Invasive Administration of a Cell-Containing Flowable Composition
InactiveUS20080118561A1Reduce smooth muscle cell proliferation, occlusive thrombosis, intimal hyperplasia, restenosisPowder deliveryBiocideSequelaIntimal proliferation
The disclosed invention is based on the discovery that a cell-based therapy can be used to treat, ameliorate, manage and / or reduce the progression of clinical sequelae associated with vascular interventions or cardiovascular diseases, particularly occlusive thrombosis, restenosis, intimal hyperplasia, inflammation and vasodilation. The invention further benefits from the additional discovery that a heretofore undescribed implantable flowable composition is capable of sustaining a confluent population of sufficiently viable cells which can be effectively administered via a minimally-invasive surgical procedure without diminishing the clinical effectiveness or the viability of the cells. The disclosed invention can be used to treat vasculature as well as non-vascular tubular structures such as a fallopian tube.
Owner:SHIRE REGENERATIVE MEDICINE INC
Apparatus and method for quick pain suppression
ActiveUS20090076568A1Patient compliance is goodGood clinical efficacyExternal electrodesMedicineChronic pain
Apparatus and methods for quick acute and chronic pain suppression, particularly useful and effective towards high-grade pains and / or pains resistant to other analgesic drugs such as opiates. One apparatus and method generate synthetic “non-pain” information strings of great clinical effectiveness, allowing high reproducibility of the clinical result. Synthesis of the strings occurs by combining novel geometries of complex waveforms in a sequence, perceived as “self” and “non-pain” by the CNS.
Owner:MARINEO GIUSEPPE
Zileuton-containing membrane-controlled slow release pellets and preparation method thereof
InactiveCN102429872AThe drug release rate is stable and controllableThe release rate is ideal and easy to controlOrganic active ingredientsGranular deliveryMedicineMembrane configuration
The invention discloses zileuton-containing membrane-controlled slow release pellets and a preparation method thereof. The zileuton-containing membrane-controlled slow release pellets contain quick release coated pellets and slow release coated pellets in a weight part ratio of 1:3-1:7, wherein the coated pellets consist of zileuton-containing medicated pellet cores and coatin layers wrapped outside the medicated pellet cores; and the weight part ratio of the medicated pellet cores to the coating layer is 5:1-15:1. The drup release rate of the zileuton-containing membrane-controlled slow release pellets is stable and controllable, and the clinical effectiveness is obviously improved; meanwhile, adverse effect occurrence of the gastrointestinal tract is also greatly reduced; and the pellets have a simple preparation process and high repeatability and are suitable for scale-up production.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD
Burnt and scalded skin caring liquid and preparation method thereof
InactiveCN103751248APain relief works quicklyPain reduction or eliminationAntibacterial agentsHydroxy compound active ingredientsChlorhexidine AcetateMedicine
The invention discloses burnt and scalded skin caring liquid, which is prepared from the following materials in parts by weight: 100 parts of white sesame seed oil, 4-10 parts of Concha Margaritifera, 2-12 parts of Radix Lithospermi, 2-9 parts of borneol, and 0.1-2 parts of chlorhexidine acetate. The burnt and scald skin caring liquid has the advantages of quick pain relieving action, strong anti-infection effect and short treatment course to burnt and scald, can solve the problem of bacterial drug resistance, and has no scar left for healing up of burnt and scald with degree of deep II or below. The Chinese and western integrated formulation has good clinical effectiveness to burnt and scald, and effectively solves the problem of bacterial drug resistance in burnt and scald. The invention also discloses a preparation method of the burnt and scalded skin caring liquid.
Owner:GUANGZHOU MINGWEI CHINESE MEDICINE RES & DEV CO LTD
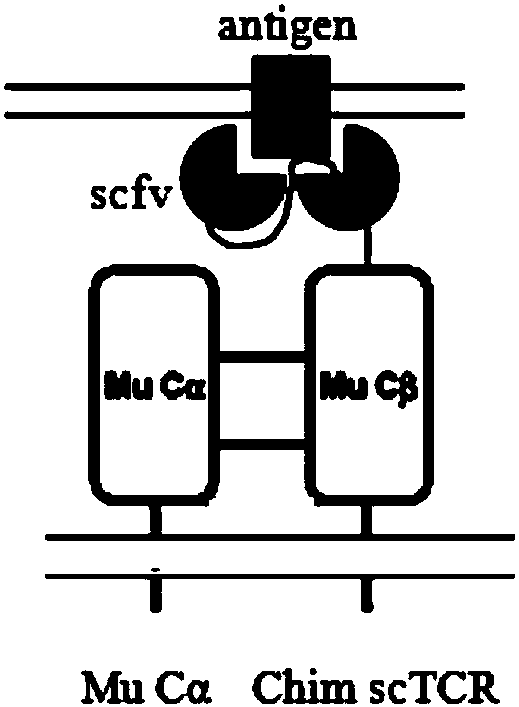
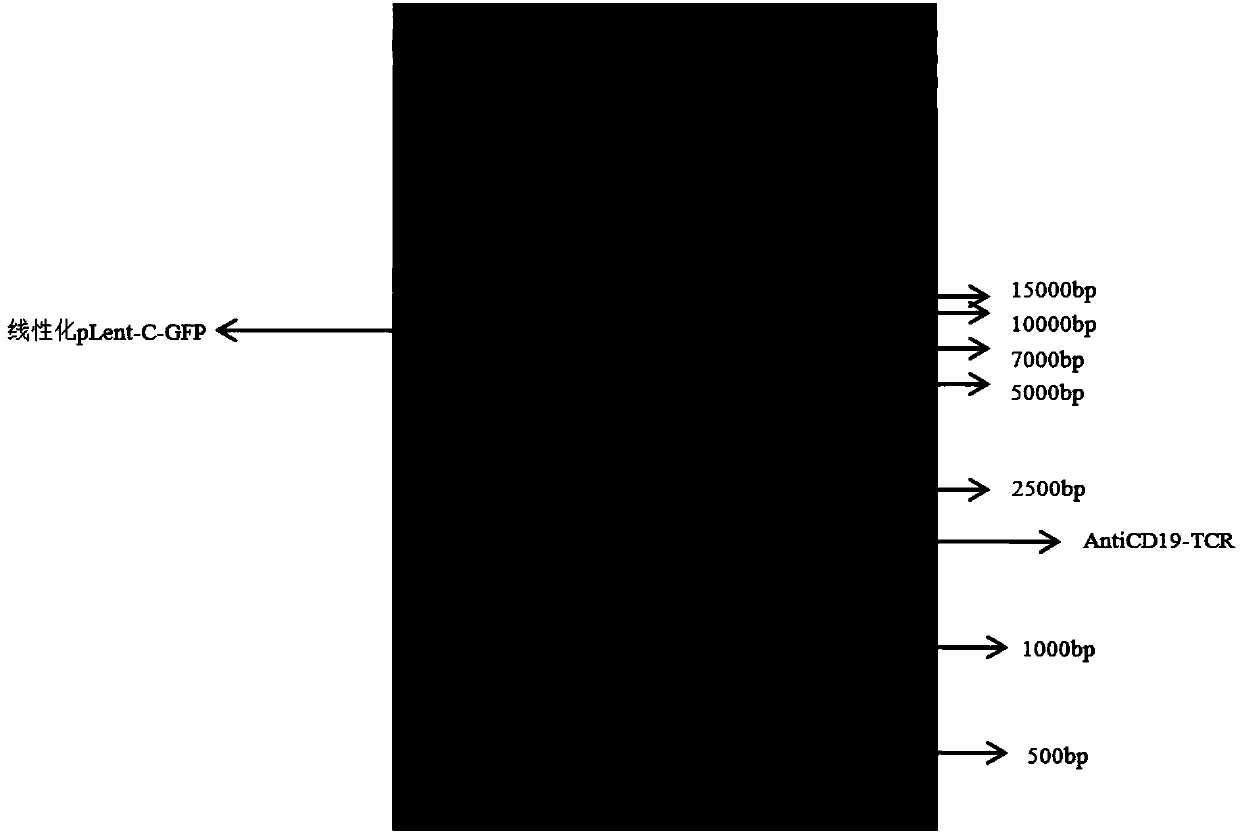
TCR gene targeted to CD19, preparation method, plasmid with TCR gene, kit and application
ActiveCN107916269AImprove effectivenessImprove securityImmunoglobulin superfamilyAntibody mimetics/scaffoldsGene targetsNucleotide sequencing
The invention discloses a safe and efficient TCR gene targeted to CD19. The TCR gene is characterized in that a nucleotide sequence of the TCR gene is SEQ ID NO.1, and the TCR gene is formed by CD8leader, CD19scFv, TRBC, T2A, CD8leader, myc-tag and TRAC which are sequentially connected in series. The invention also discloses a preparation method of the TCR gene, a plasmid with the TCR gene, a kitand an application. The TCR gene disclosed by the invention has the advantages that clinical effectiveness and safety of a TCR technology are improved, and fussy experimental operations are also avoided.
Owner:上海兴瑞一达生物科技有限公司
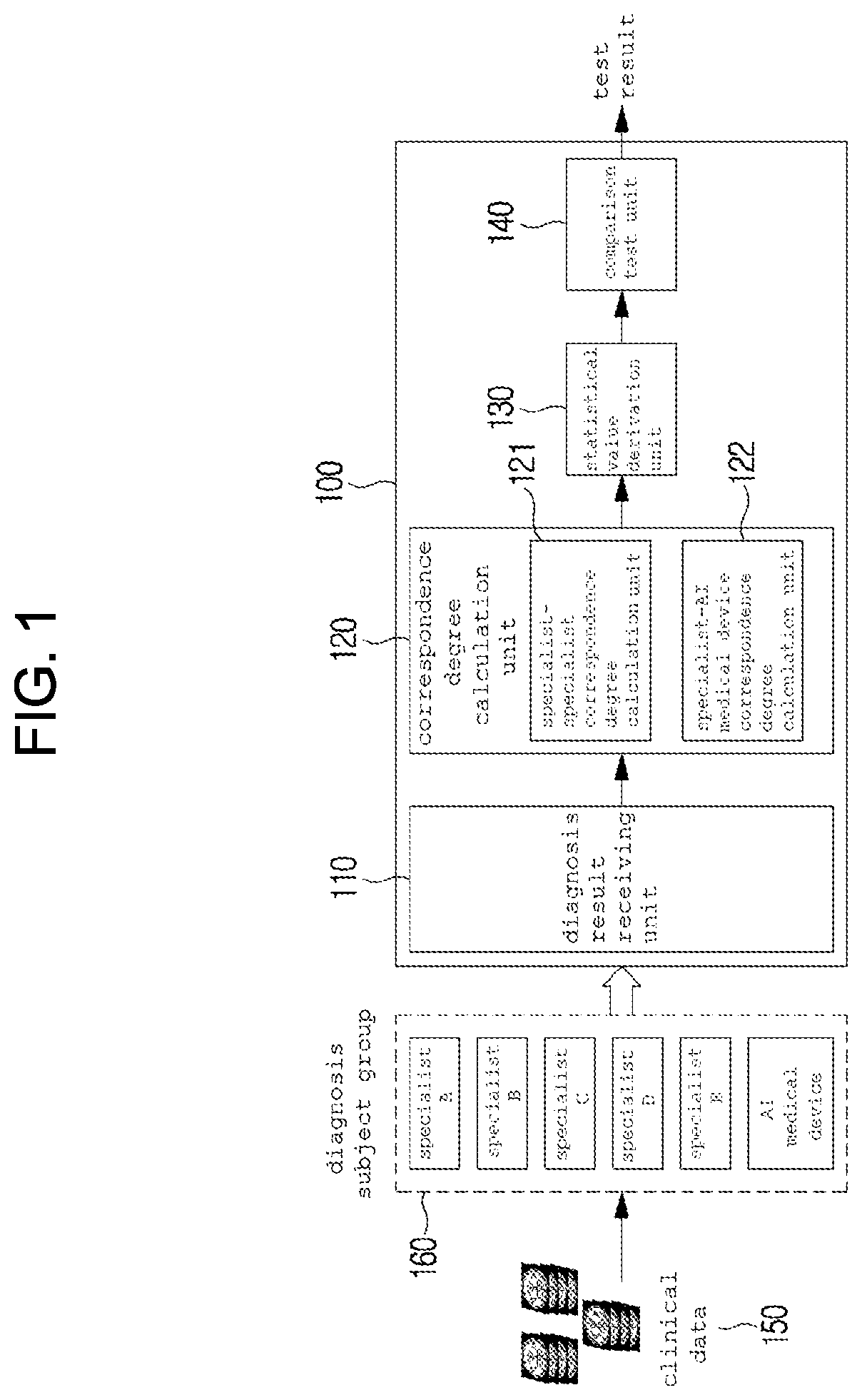
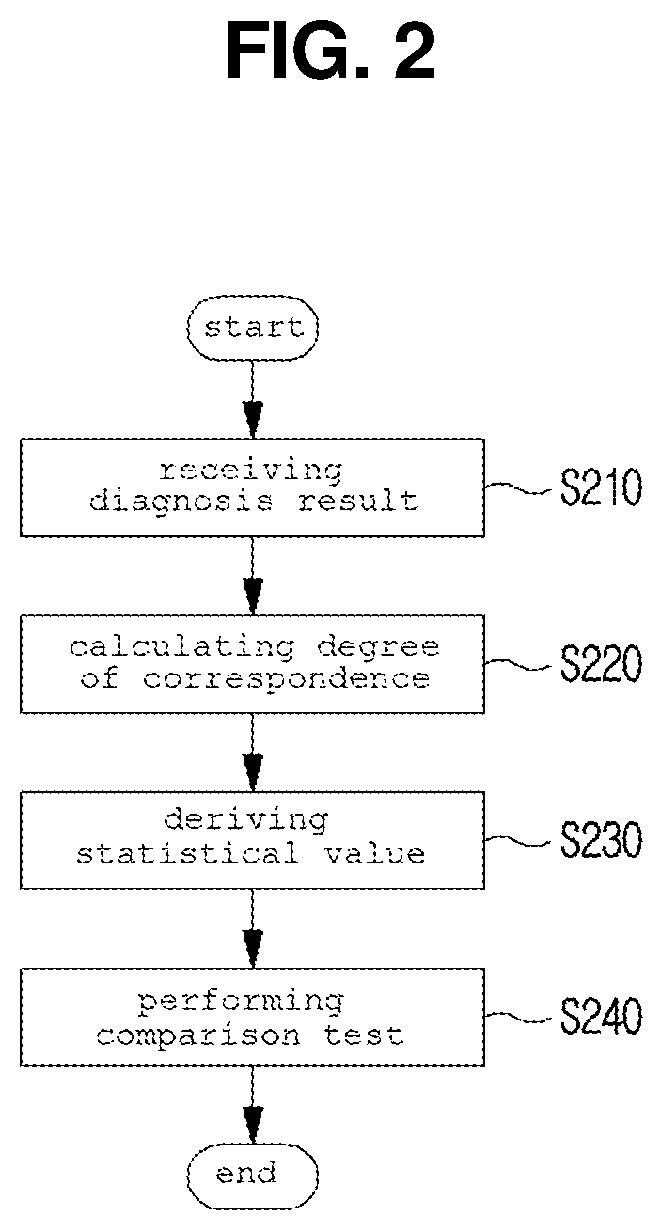
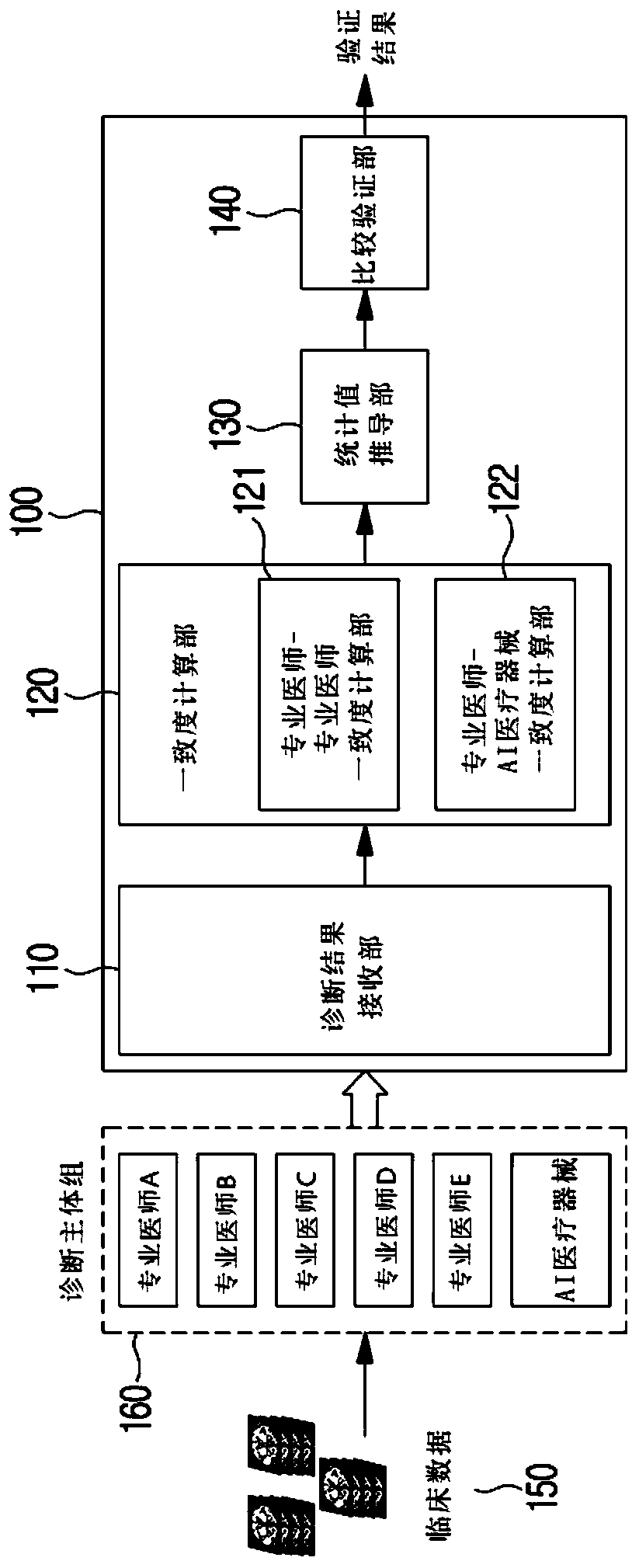
Method and system for clinical effectiveness evaluation of artificial intelligence based medical device
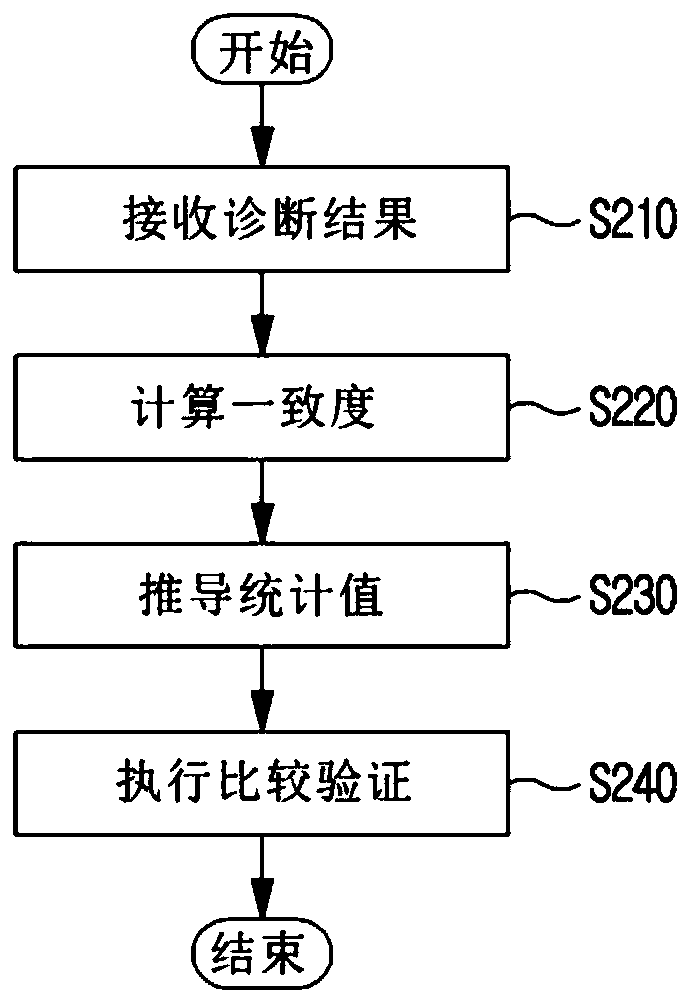
InactiveUS20190371464A1Verify clinical effectivenessImprove reliabilityMedical data miningMedical automated diagnosisComparative testMedicine
There is disclosed a method and system for clinical effectiveness evaluation of artificial intelligence based medical devices. The clinical effectiveness evaluation system includes a diagnosis result receiving unit receiving diagnosis results for arbitrary clinical data from a plurality of specialists and the artificial intelligence based medical device; a correspondence degree calculation unit calculating a degree of correspondence between the received diagnosis results; a statistical value derivation unit deriving a predetermined statistical value on the basis of the calculated degree of correspondence; and a comparison test unit performing a comparison test on the artificial intelligence based medical device using the derived statistical value.
Owner:JLK INSPECTION
Method and system for clinical effectiveness evaluation of artificial intelligence based medical device
InactiveCN110547763AImprove reliabilityMedical data miningMedical automated diagnosisData miningEvaluation system
Owner:株式会社JLK英思陪胜
Environment-friendly production process for high-purity montmorillonite
ActiveCN103073010BStrong water absorptionEasy to separate and purifySilicon compoundsEnvironmental resistanceEmulsion
Owner:乔敏 +2
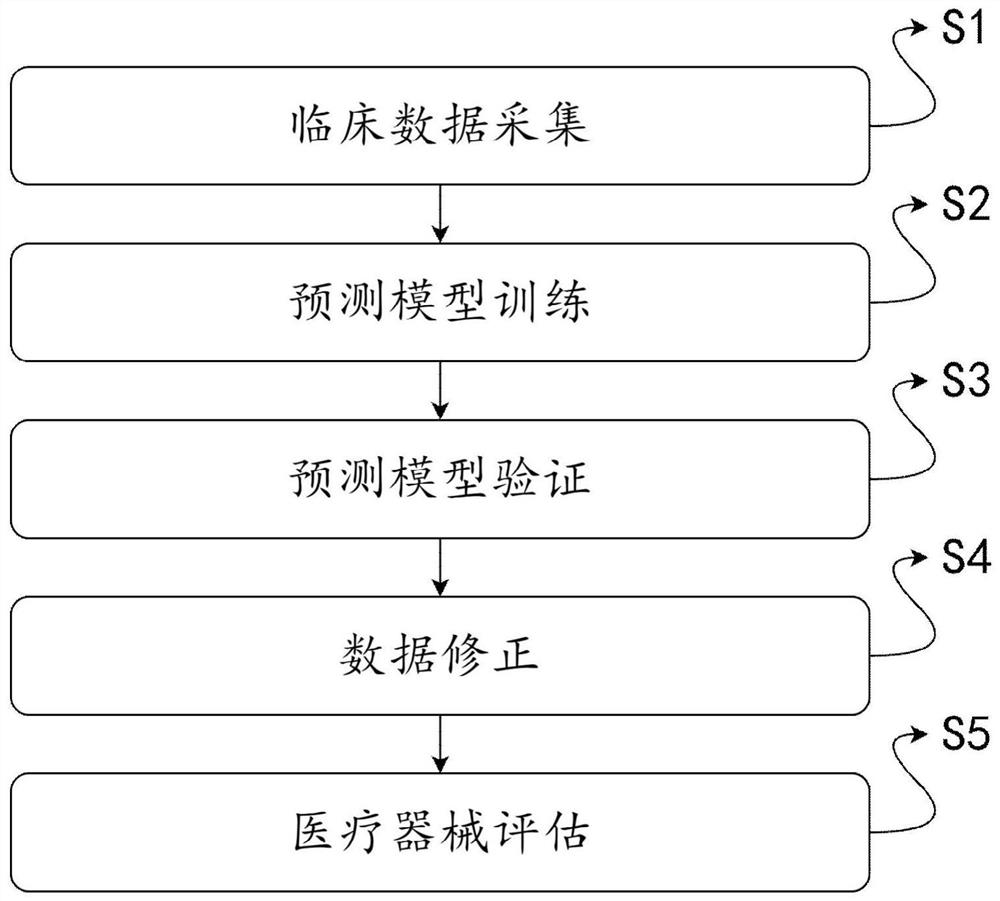
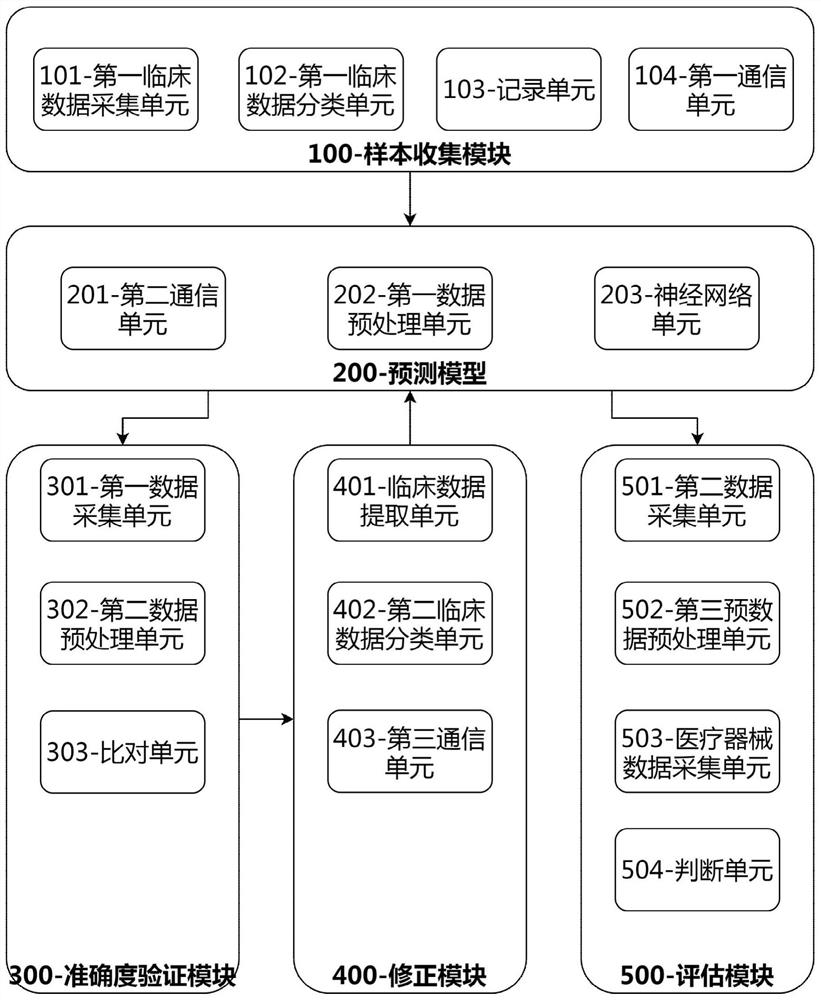
Clinical effectiveness evaluation method and system of medical apparatus based on artificial intelligence
ActiveCN113066549AAvoid false resultsEvaluation results are simpleMedical data miningCharacter and pattern recognitionEvaluation resultMedicine
The invention discloses a clinical effectiveness evaluation method and system for medical instruments based on artificial intelligence, and relates to the technical field of clinical effectiveness evaluation of the medical instruments, and the system comprises a sample collection module, a prediction model, an accuracy verification module, a correction module and an evaluation module. The method comprises the steps: collecting clinical data of patients in each medical center to obtain enough samples, inputting the samples into the prediction model to be trained, adopting the prediction model to evaluate the effectiveness of the medical instrument based on artificial intelligence in a clinical experiment, and obtaining the evaluation result. Only the prediction model needs to be trained in the whole process, and in the assessment process, no professional physician is needed for assessment, so the whole assessment process is simple; the problems that the efficiency of the evaluation process is low, the evaluation result is inaccurate due to the influence of factors such as the professional degree of doctors and the like when the medical instruments adopting the artificial intelligence technology are subjected to effectiveness evaluation at present, and doctors of different professions need to evaluate different types of medical instruments adopting the artificial intelligence technology are solved.
Owner:青岛瑞斯凯尔生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com