Zileuton-containing membrane-controlled slow release pellets and preparation method thereof
A technology of sustained-release pellets and zileuton, which is applied in the field of film-controlled sustained-release pellets and its preparation, can solve the problems of drug safety hazards, large individual differences in absorption, and difficult control of reproducibility, and achieve inter-individual differences. The effect of small resistance, improvement of clinical effective rate, and avoidance of burst release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
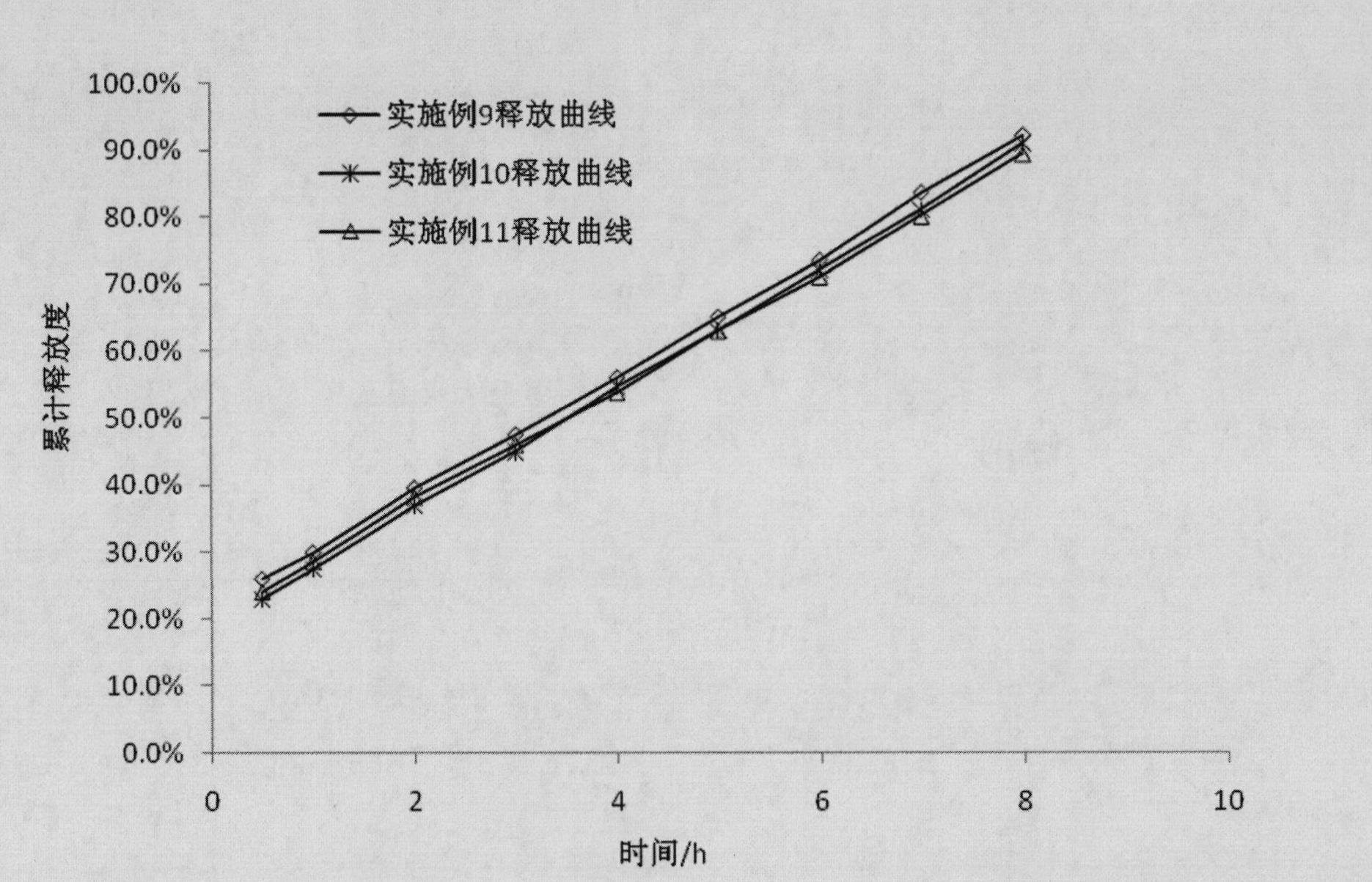
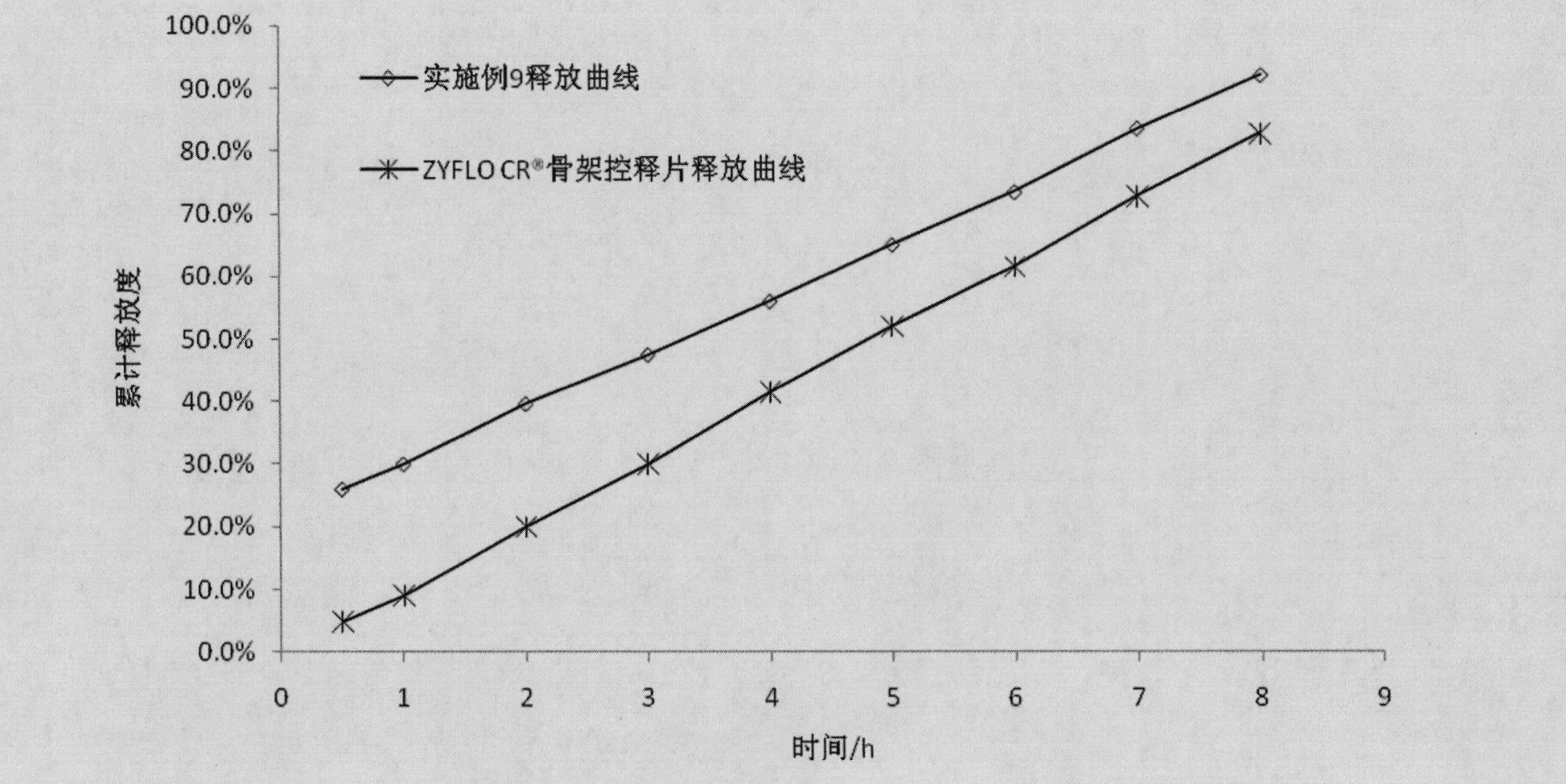
Examples
Embodiment 1
[0051] Embodiment 1 preparation containing pill core
[0052] Prescription: Zileuton 3000g
[0053] Sucrose (thinner) 1500g
[0054] Starch (binder) 500g
[0055] Preparation process: Weigh an appropriate amount of zileuton, sucrose, and starch, pass through an 80-mesh sieve respectively, mix evenly according to the prescription ratio, add an appropriate amount of 70% ethanol to prepare a soft material, and extrude through a sieve plate of an extruder to obtain a strip-shaped material. Put the strip material in the spheronizer, adjust the speed of 1500rpm and the spheronization time for 5 minutes to make the particles completely spheroidized, take out the pellets and dry them, heat-treat at 40°C for 12 hours, and then sieve the pellets with a size of 0.5-0.9mm to obtain the pellet core. Results The obtained pill-containing core was smooth and round in appearance, low in friability, and suitable for coating.
Embodiment 2
[0057] Preparation of pill-containing cores
[0058] Prescription: Zileuton 3000g
[0059] Microcrystalline cellulose (thinner) 1100g
[0060] Preparation process: the same as the preparation method in Example 1, wherein when the strip-shaped material is placed in a spheronizer for spheronization, the rotational speed is adjusted to 800 rpm and the spheronization time is 15 minutes.
Embodiment 3
[0062] Preparation of pill-containing cores
[0063] Prescription: Zileuton 3000g
[0064] Starch (thinner) 1250g
[0065] Povidone (adhesive) 200g
[0066] Preparation process: with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com