Radix astragali particle and quality control method thereof
A quality control method and technology of Astragalus, which are applied in measuring devices, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems that the quality control of Astragalus granules is not accurate and reliable, and it is impossible to ensure clinical curative effect, etc. and retention, improving clinical effectiveness, changing the effect of decoction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Present embodiment is the astragalus granule obtained by the following method:
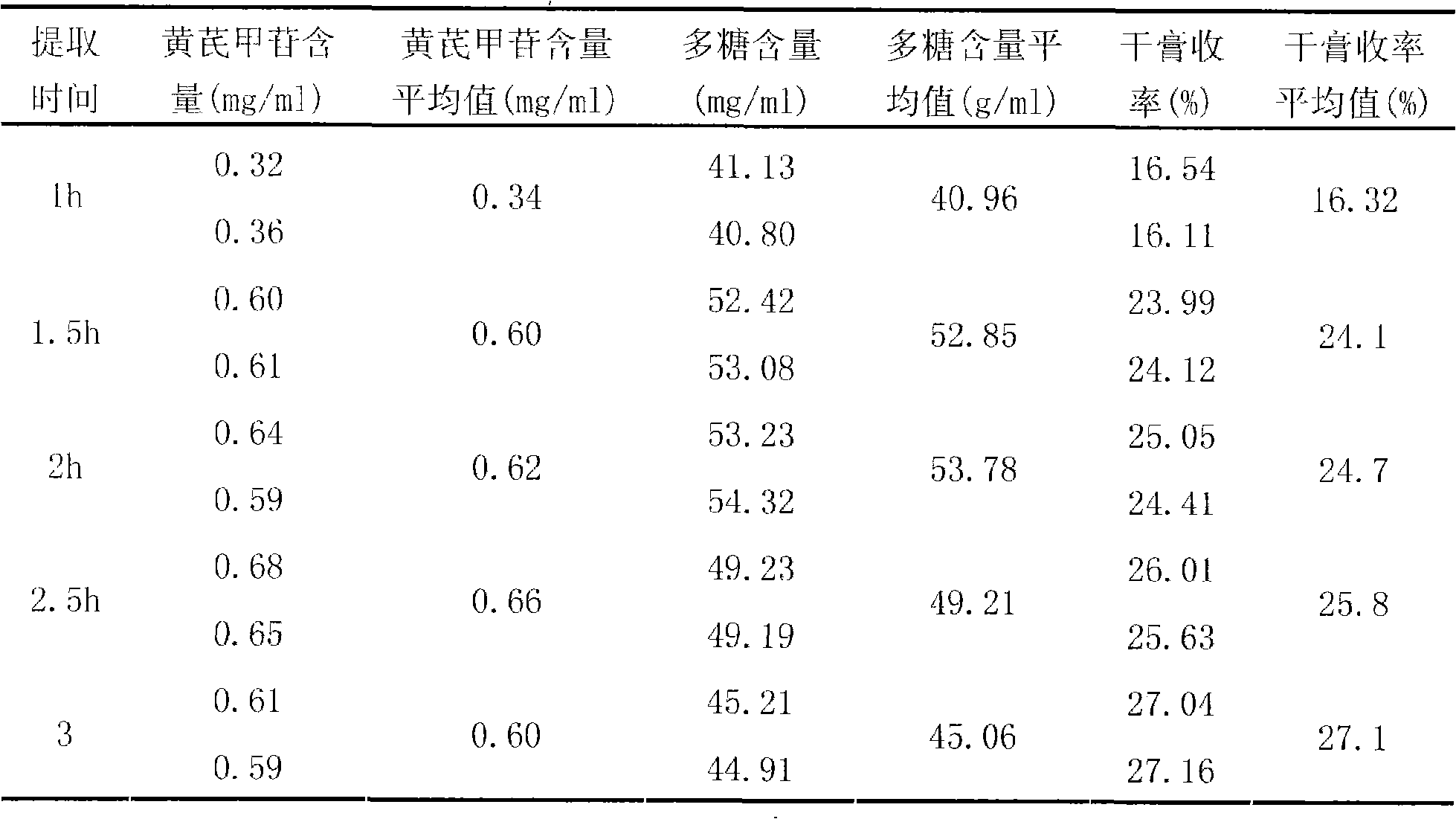
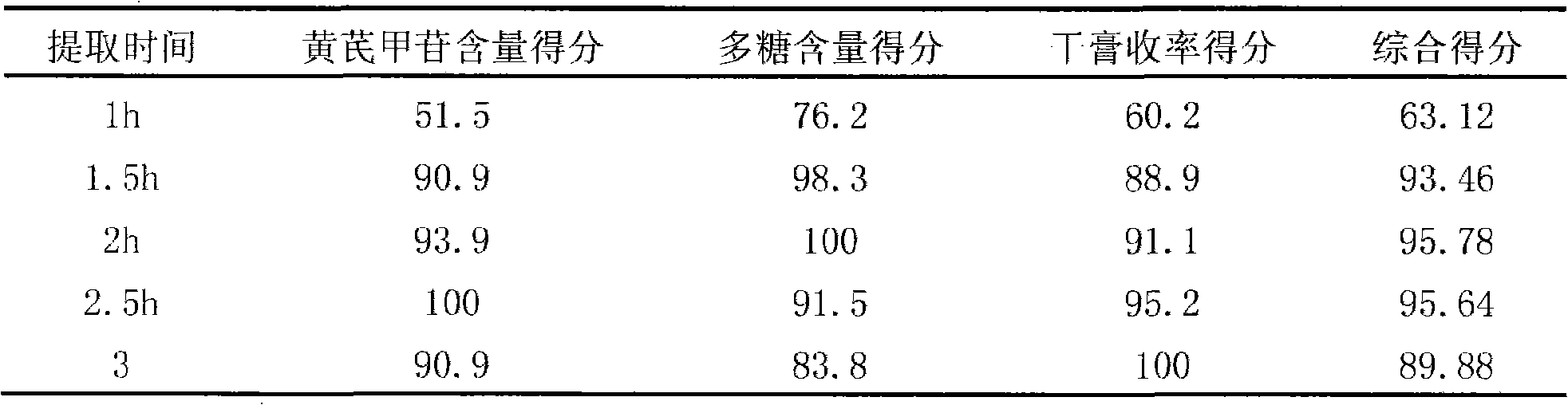
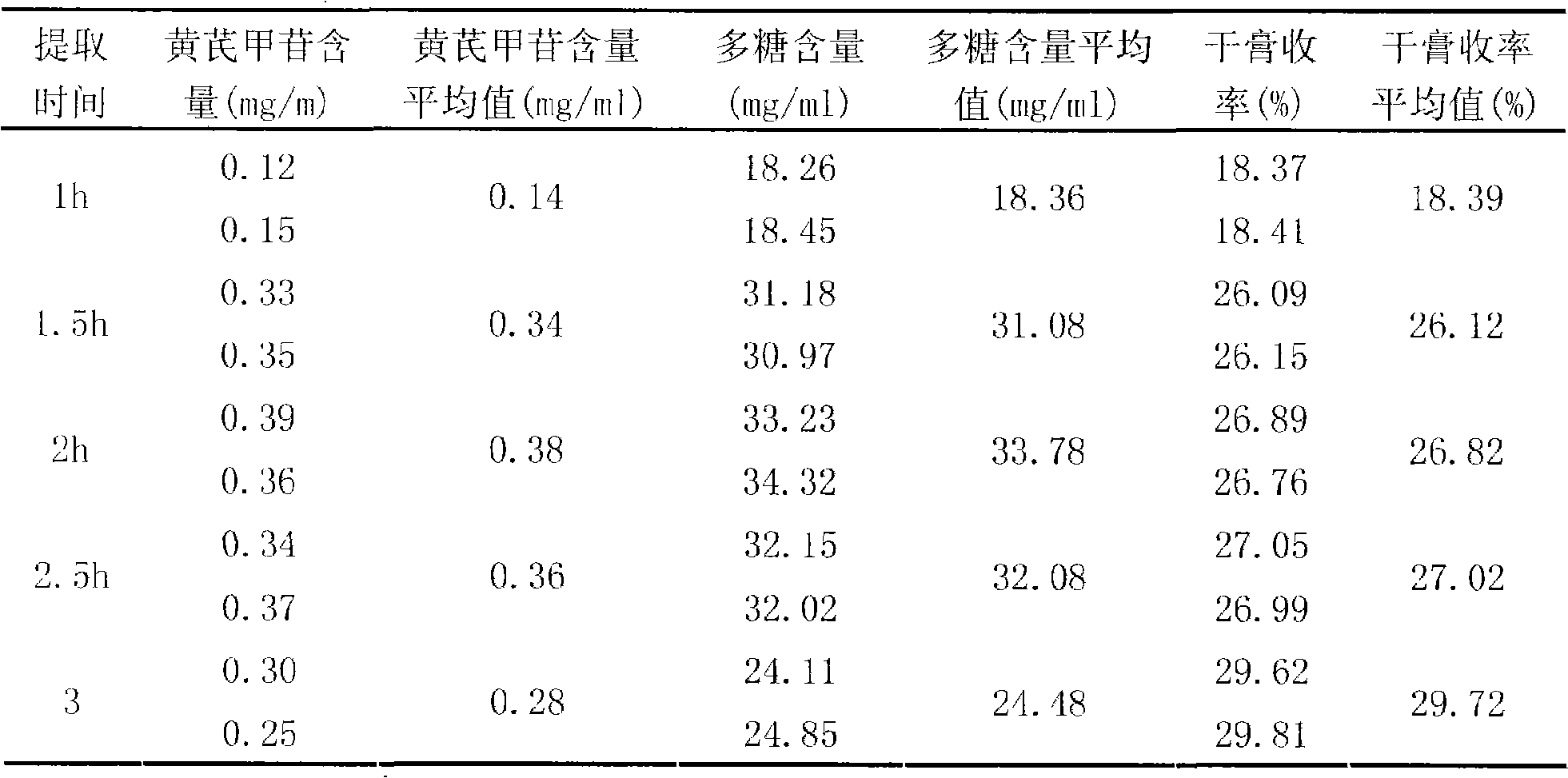
[0037] Take 1000g of astragalus and add water to decoct twice, decoct for 2 hours for the first time, decoct for 2 hours for the second time, combine the decoction, filter, concentrate the filtrate to a clear paste with a relative density of about 1.30 (70°C), add an appropriate amount of sucrose , made into 1000g of granules, dried, divided into 15g / bag, sterilized, and obtained.
Embodiment 2
[0039] Present embodiment is the astragalus granule obtained by the following method:
[0040] Take 1000g of Astragalus membranaceus, add water and decoct twice, decoct for 2.5 hours for the first time, and decoct for 1.5 hours for the second time, combine the decoction, filter, and concentrate the filtrate to a clear paste with a relative density of about 1.20 (80°C), add an appropriate amount of lactose , made into 1000g of granules, dried, divided into 10g / bag, sterilized, and obtained.
Embodiment 3
[0042] Present embodiment is the astragalus granule obtained by the following method:
[0043] Take 1000g of Radix Astragali, add water and decoct twice, decoct for 1.5 hours for the first time, decoct for 2.5 hours for the second time, combine the decoction, filter, and decompress the filtrate (pressure -0.06mpa~-0.07mpa, temperature 55~60℃ ) concentrated to a clear paste with a relative density of about 1.25 (75°C), adding an appropriate amount of xylitol to make 1000 g of granules, drying, subpackaging into 10 g / bag, and sterilizing to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com