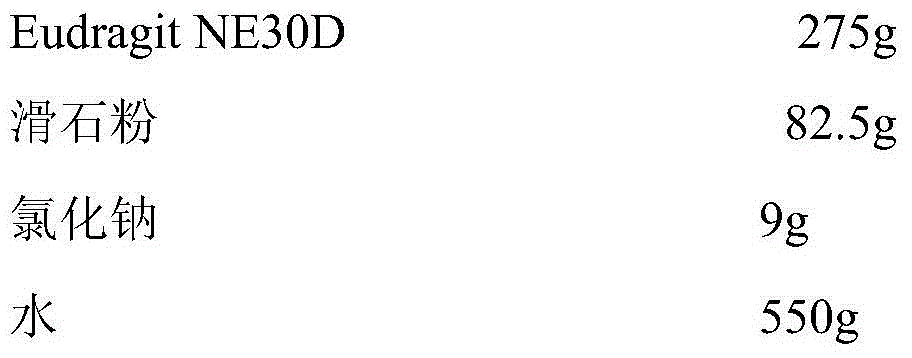Patents
Literature
55 results about "Sustained Release Pellet Capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
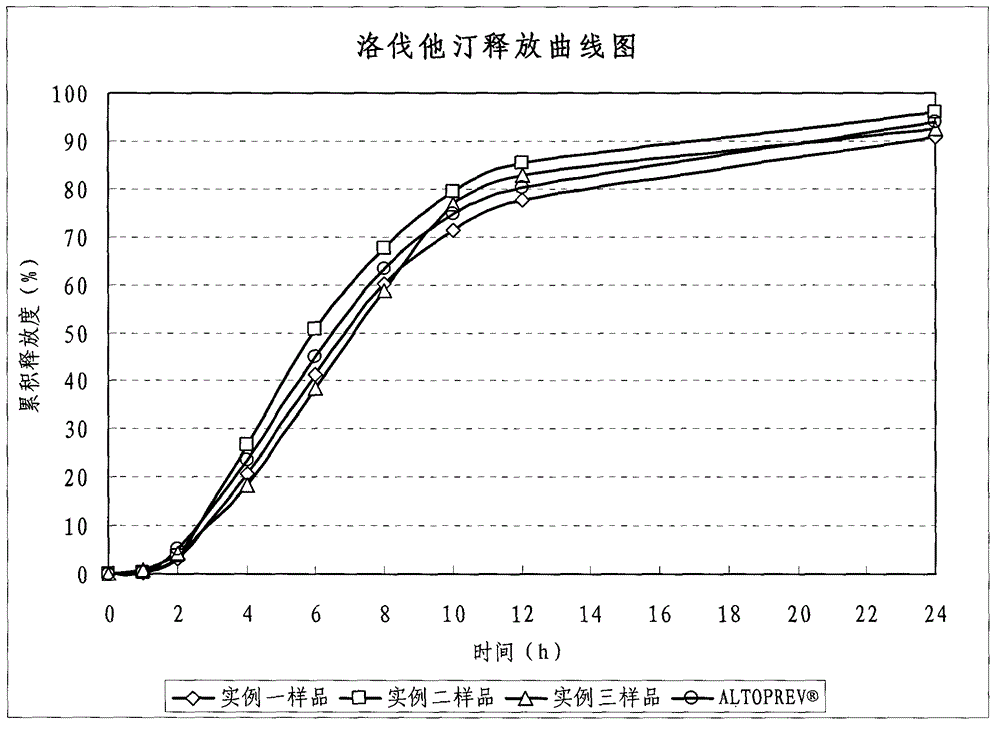
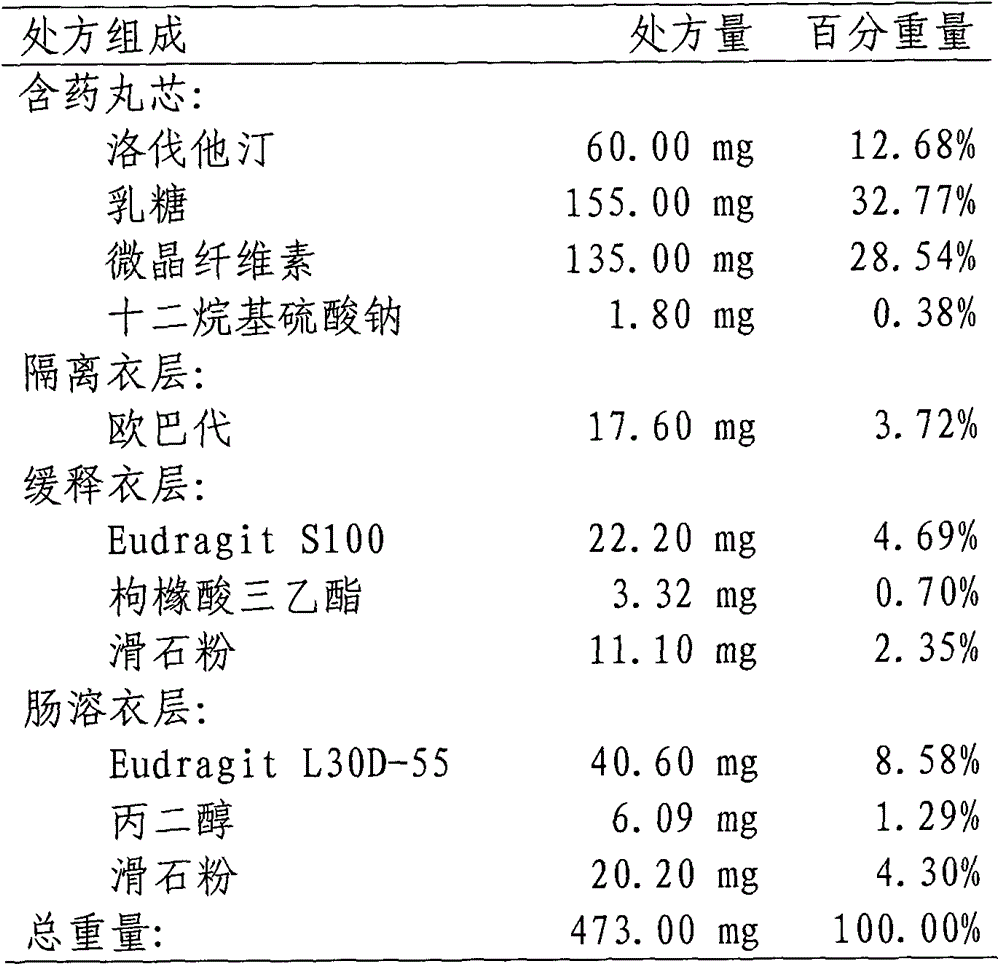
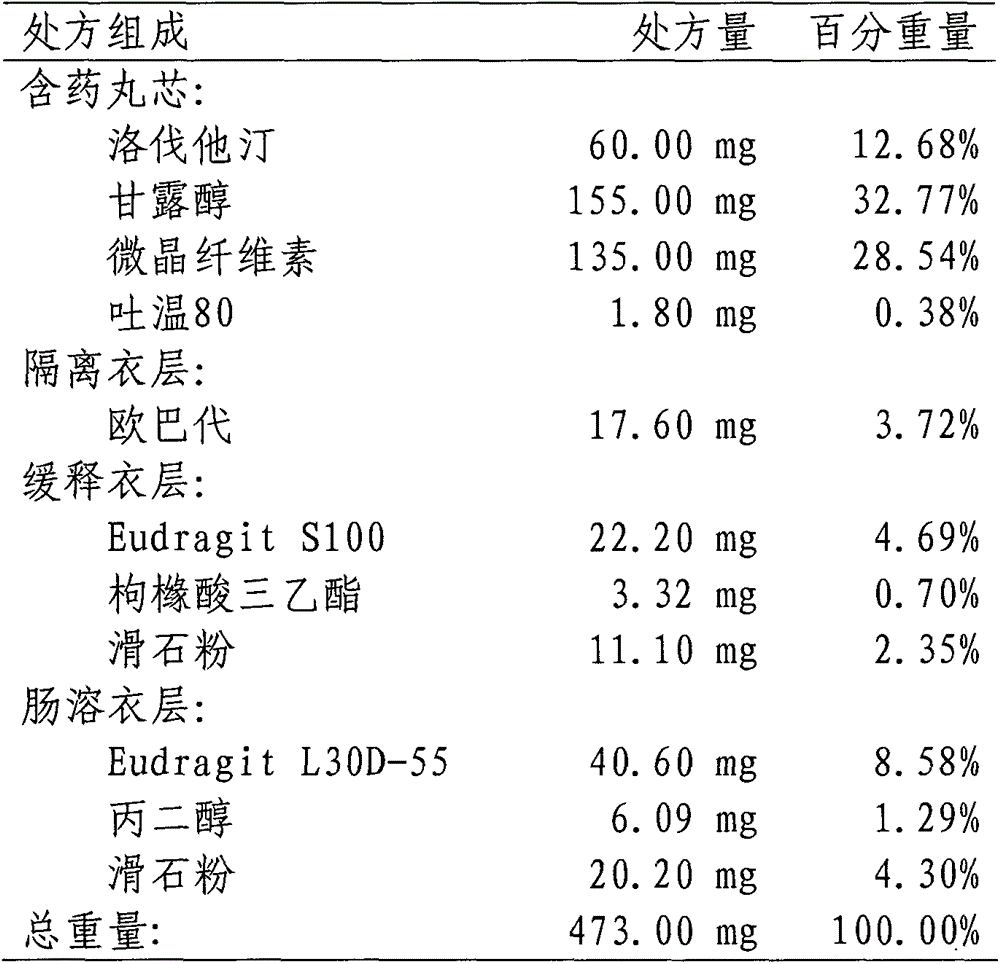
Lovastatin enteric coated sustained-release pellet capsule and preparation method thereof
InactiveCN103142552AUniform absorption rateSmall differences in individual bioavailabilityMetabolism disorderGranular deliverySustained release pelletsSide effect
The invention discloses a lovastatin-containing enteric coated sustained-release pellet capsule and a preparation method thereof. The lovastatin-containing enteric coated sustained-release pellet capsule comprises two parts, namely, an enteric coated sustained-release pellet and a hollow capsule, wherein the enteric coated sustained-release pellet comprises 55-86% of medicine-containing pellet core, 2-5% of isolation coating layer, 2-15% of a sustained-release coating layer and 10-25% of enteric-coated coating layer by weight. The prepared lovastatin enteric coated sustained-release pellet capsule is uniform in granule size and stable in medicine release; the medicine is not released in gastric acid but is slowly and constantly released in intestinal tracts and livers, has the characteristic of targeted medicine release, is small in irritation to gastrointestinal tracts, and can reduce the toxic and side effects of the medicine and reduce the number of the medicine administrations, so that the compliance of patients is improved; and meanwhile as the enteric coated sustained-release pellet preparation is composed of hundreds of pellets of uniform granule sizes, the sudden release of the whole preparation is not caused by the breakage of individual pellets, so that the medicine is safer than a sustained-release tablet, smaller in irritation to gastrointestinal tracts and more stable in blood concentration, and the clinical effectiveness and security are effectively improved.
Owner:广州科的信医药技术有限公司
Tobacco sweetened cooling agent sustained release capsules for filter sticks
ActiveCN102273733ANo premature lossWide variety of sourcesTobacco smoke filtersSweetnessCigarette filter
The invention discloses a sustained-release pellet capsule of a sweet cooling agent for a cigarette with a filter plug, belonging to the technical field of cigarettes. The sustained-release pellet capsule of the sweet cooling agent for the cigarette with the filter plug is prepared by the following steps of: (1) synthesizing corn mint oxime and N,2,3-trimethyl-2-isopropyl-butyrylamide; (2) compounding the corn mint oxime and the N,2,3-trimethyl-2-isopropyl-butyrylamide according to the proportion of (5-20): (1-10) to obtain a uniformly mixed white crystal compound; and (3) dropping. The invention has the advantages that the sustained-release pellet capsule prepared through synthesizing, compounding and dropping releases sweet substances only when the cigarette is smoked, therefore, effective components can not be lost in advance, and better fragrance keeping and remaining effects can be achieved. Meanwhile, the sweet and fragrant taste is unique and is coordinated with the smoke. In the process that the cigarette is smoked, the characteristic of a sweetener can be better embodied, the quality of the smoke is not remarkably influenced, and raw materials used in the invention have wide sources and low cost.
Owner:HUBEI CHINA TOBACCO IND +1
Acipimox film-controlled slow-release pellet capsule
ActiveCN103211785AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderMedicineLactose
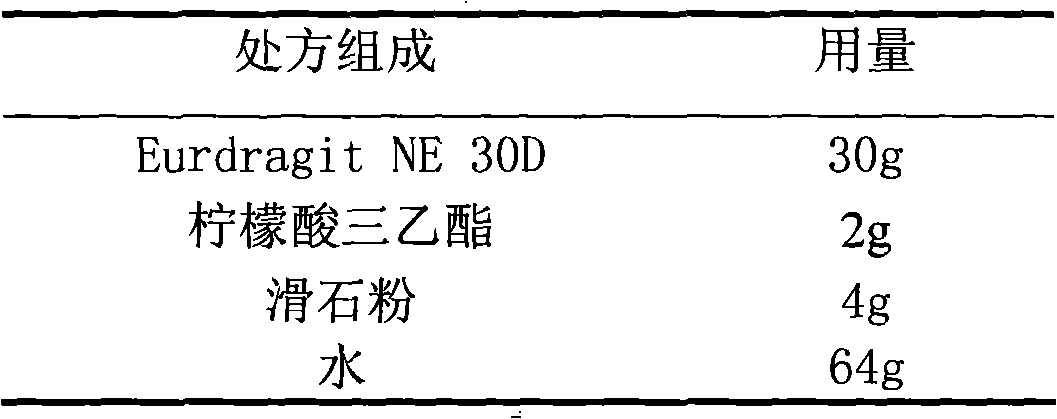
The invention relates to an acipimox film-controlled slow-release pellet capsule. A slow-release film of the acipimox film-controlled slow-release pellet utilizes Eurdragit NE 30D as a film-formation material. A pellet core of the acipimox film-controlled slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains pharmaceutically acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises the Eurdragit NE 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE 30D to triethyl citrate to talcum powder is 30: 2: 4 and coating weight gain is in a range of 20 to 39%. The acipimox film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the acipimox film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the acipimox film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept prior to expiration date.
Owner:北京天衡药物研究院有限公司
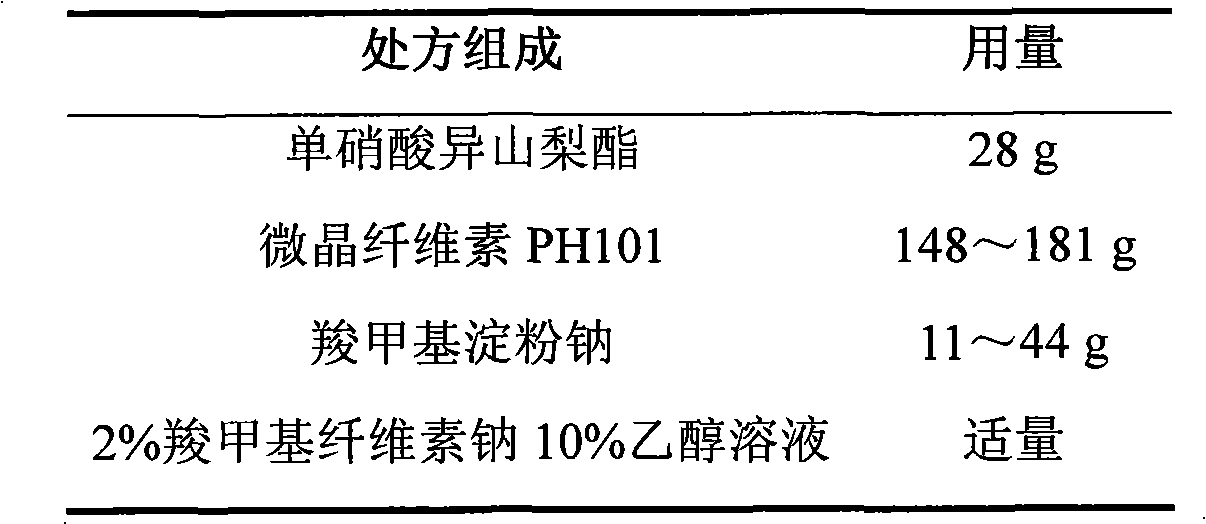
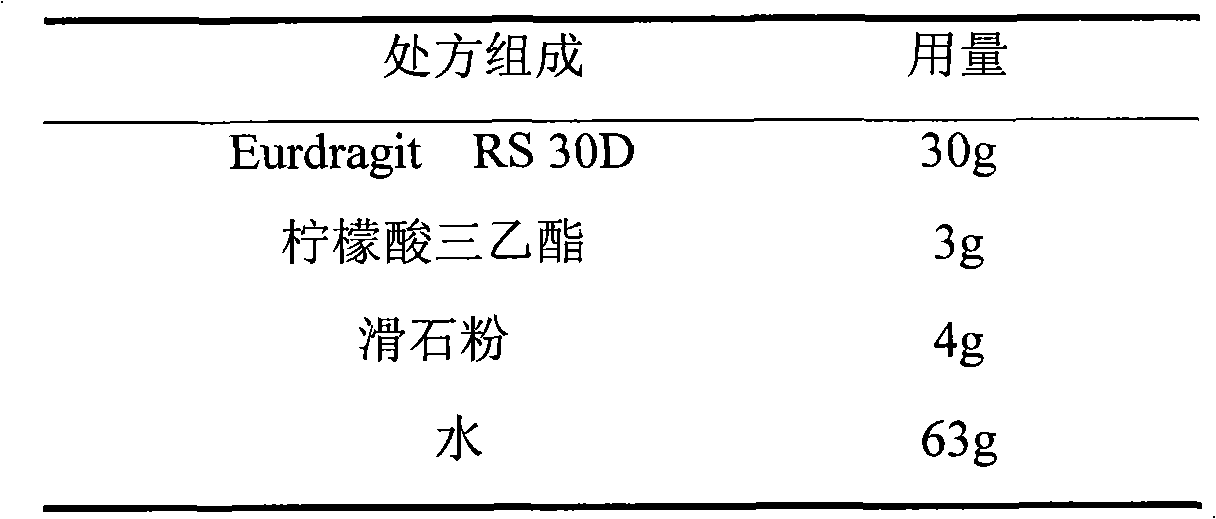
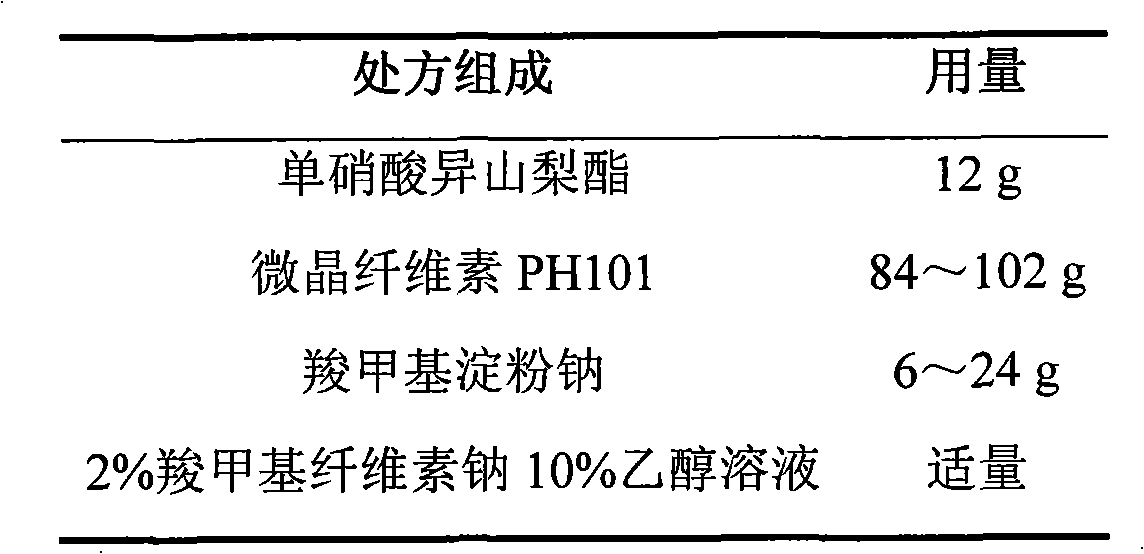
Isosorbide mononitrate sustained-release pellet, and isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it
ActiveCN103211768AAccelerated agingReduce permeabilityPharmaceutical non-active ingredientsGranular deliverySustained release pelletsMedicine
The invention relates to an isosorbide mononitrate sustained-release pellet, and an isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it. The sustained-release film of the sustained-release pellet adopts Eurdragit RS 30D as a film forming material, the core of the sustained-release pellet contains high-expansibility sodium carboxymethyl starch and a pharmaceutically-acceptable excipient commonly used for sustained-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of sodium carboxymethyl starch in the core of the sustained-release pellet is 5-20%. The sustained-release film of the sustained-release pellet includes the Eurdragit RS 30D, a plasticizer triethyl citrate and an anti-adherent talcum powder, the optimal ratio of the Eurdragit RS 30D to triethyl citrate to the talcum powder is 30:3:4, and the optimal coating weight gain is 19-38%. The core will obviously expand after absorbing water because of the containment of sodium carboxymethyl starch highly expanding after contacting with water, so the sustained-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:北京天衡药物研究院有限公司
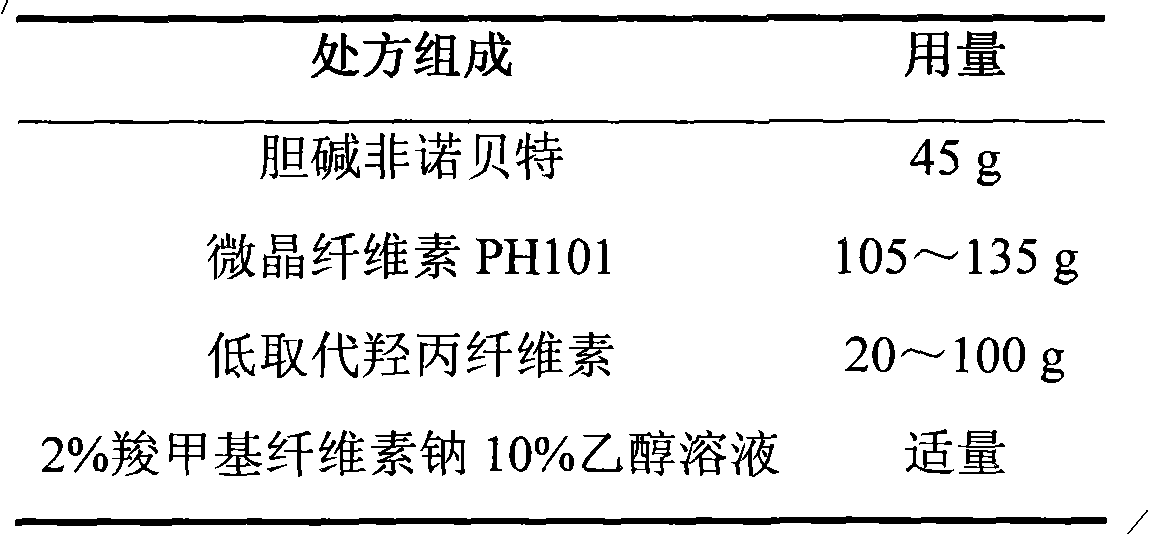
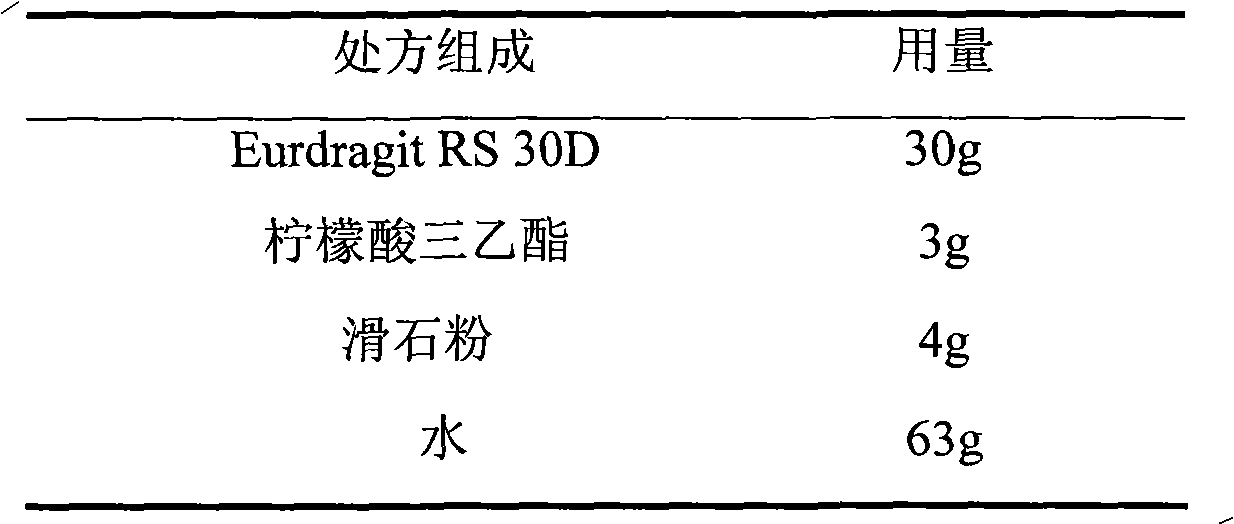
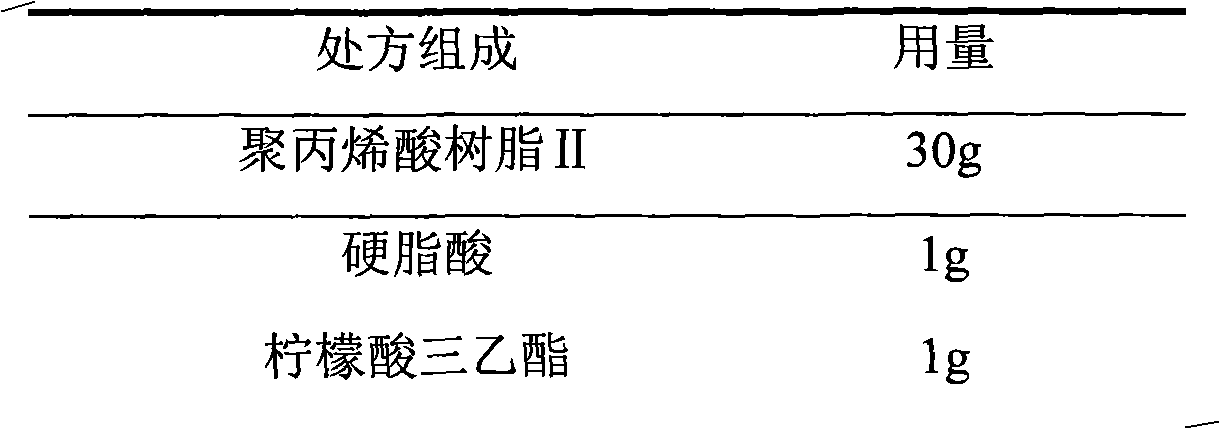
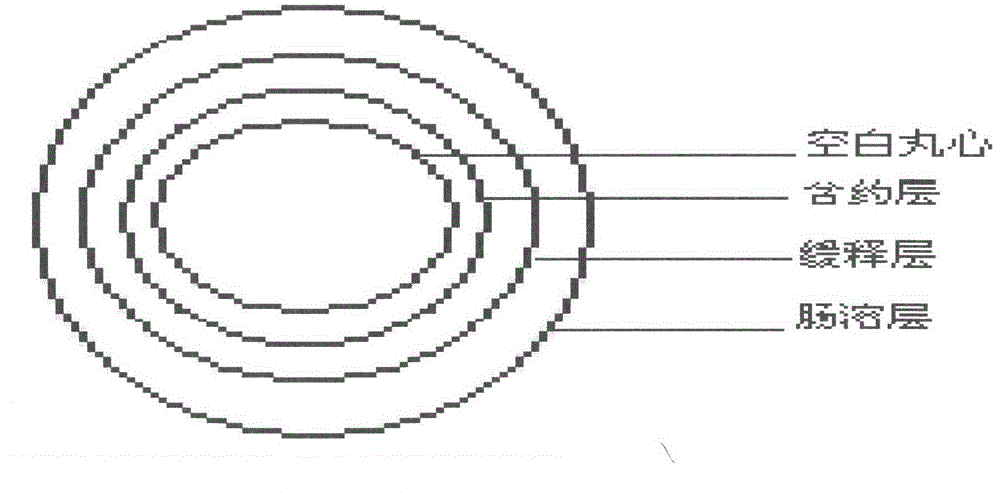
Choline fenofibrate film-controlled enteric slow-release pellet capsule
ActiveCN103211786AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderSustained release pelletsMedicine
The invention relates to a choline fenofibrate film-controlled enteric slow-release pellet capsule. A slow-release film of the choline fenofibrate film-controlled enteric slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the choline fenofibrate film-controlled enteric slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains a pharmaceutically acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of urdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 20 to 38%. The choline fenofibrate film-controlled enteric slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the choline fenofibrate film-controlled enteric slow-release pellet expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the choline fenofibrate film-controlled enteric slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Metformin hydrochloride enteric-coated sustained-release pellet and preparation method thereof
InactiveCN104434846AReduce dosageReduce the amount of feedOrganic active ingredientsMetabolism disorderSustained release pelletsMedicine
The invention belongs to the field of medicinal preparations, and discloses a metformin hydrochloride enteric-coated sustained-release pellet and a preparation method thereof. The preparation method comprises the steps of preparing a metformin hydrochloride active pellet core, coating a sustained-release layer and coating an enteric layer. The preparation method can be used for preparing the metformin hydrochloride enteric-coated sustained-release pellet which has attractive appearance, good fluidity and high bioavailability, can be slowly released in intestinal tracts and is mild in action and low in adverse reaction incidence by screening and optimizing the process.
Owner:CHINA PHARM UNIV
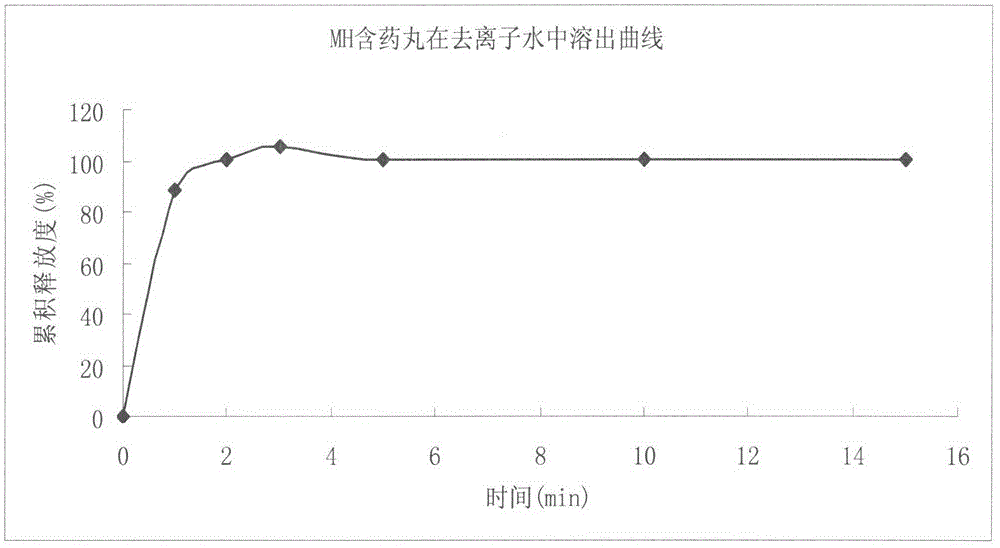
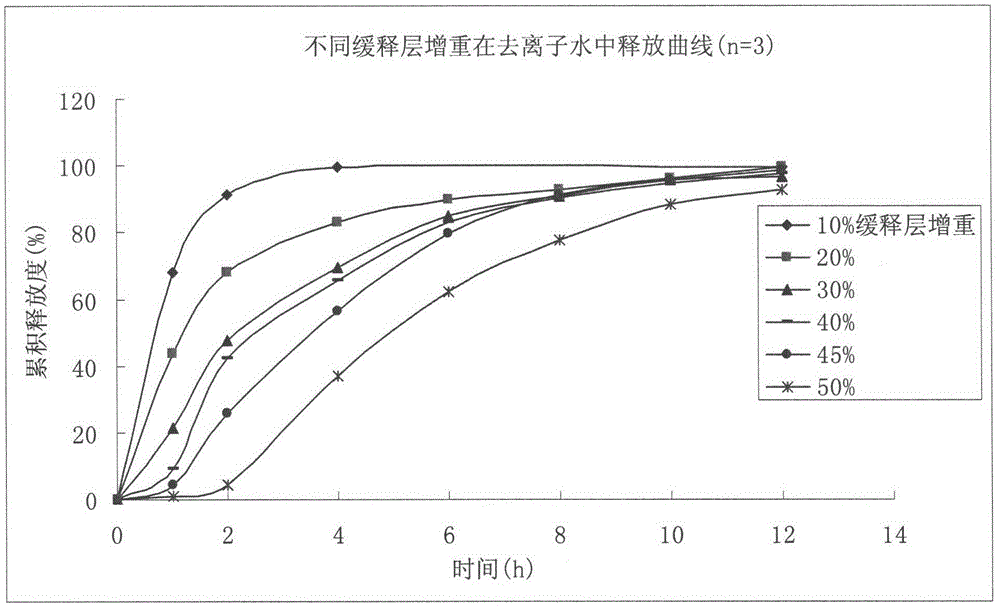
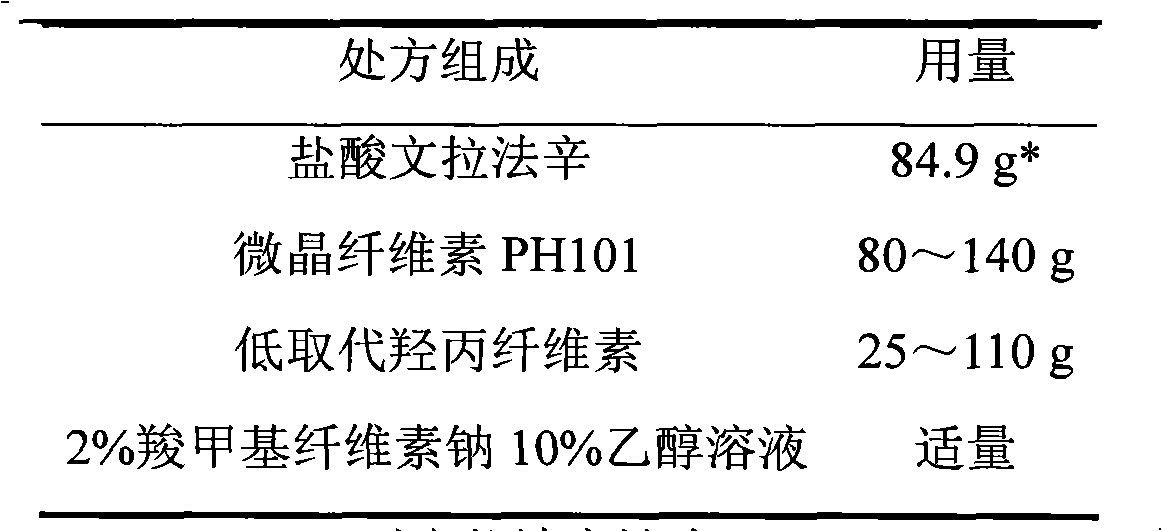
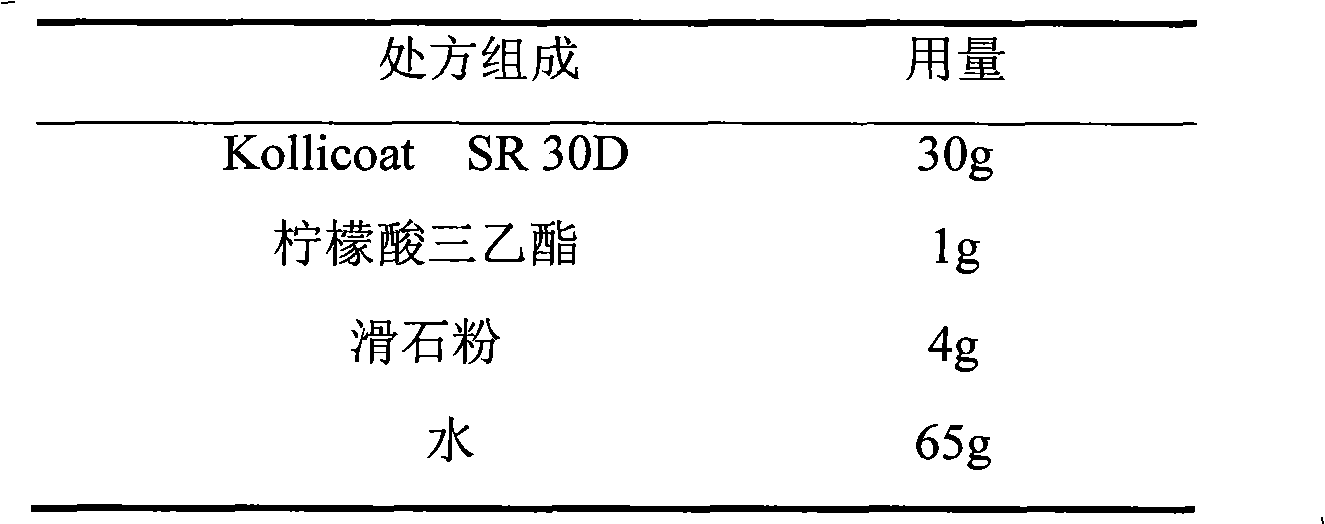
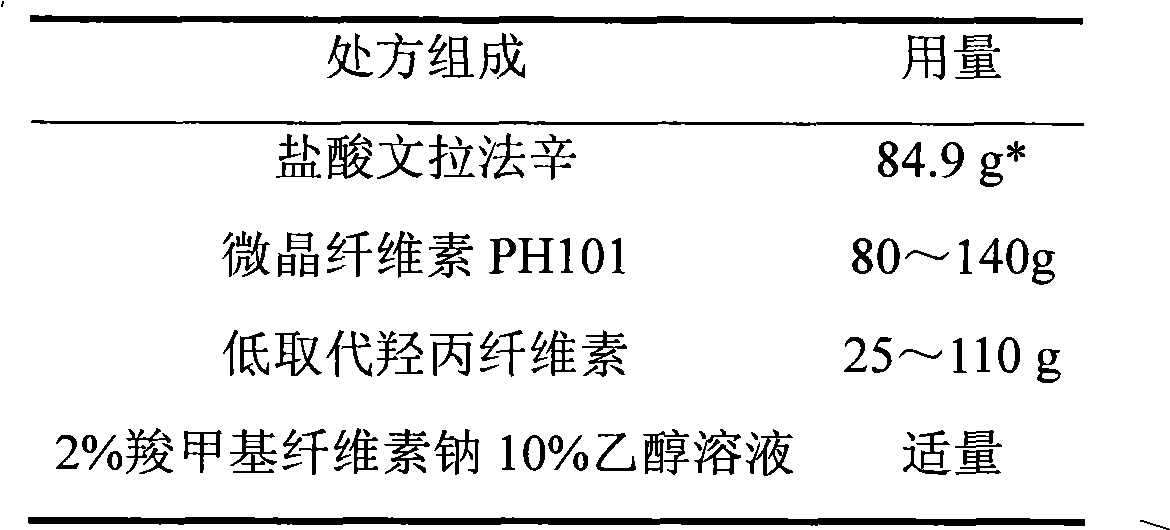
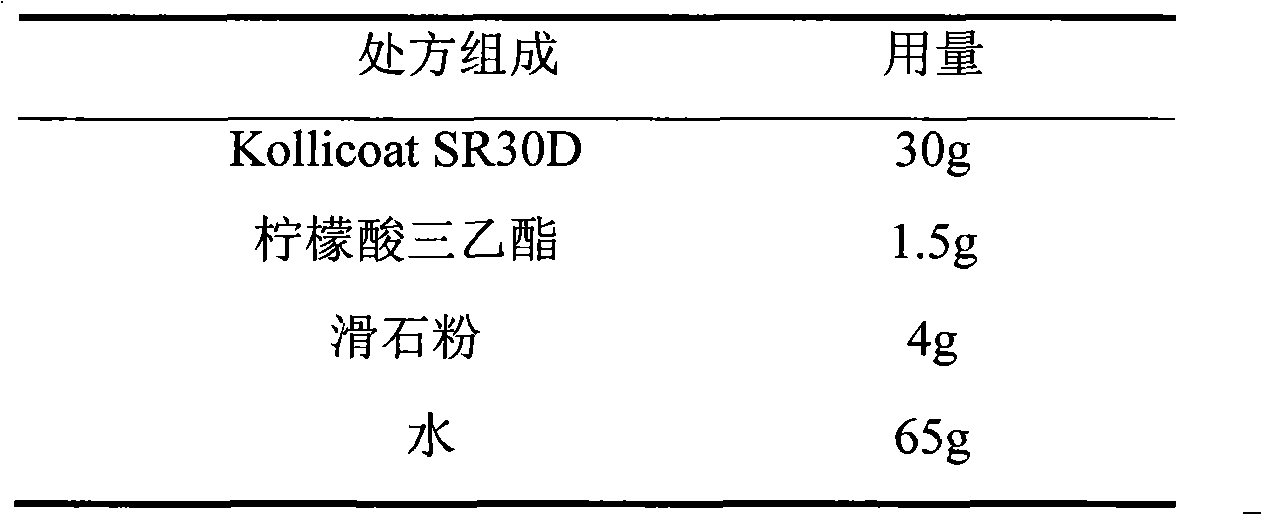
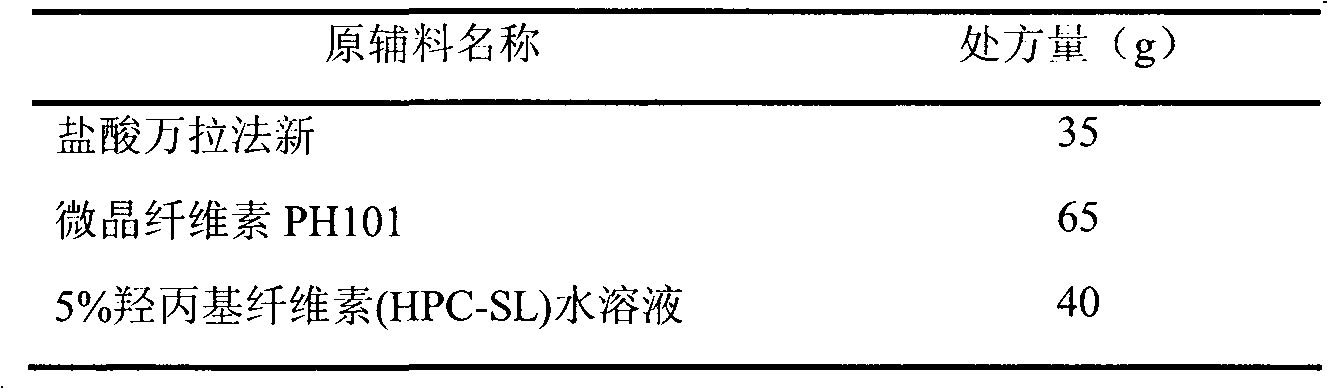
Venlafaxine hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211791AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsLow-substituted hydroxypropylcellulose
The invention relates to a venlafaxine hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the venlafaxine hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR30D as a film-formation material. A pellet core of the venlafaxine hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR30D to triethyl citrate to talcum powder is 30: 1: 4 and a film weight increasing ratio is in a range of 21 to 39%. The venlafaxine hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the venlafaxine hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the venlafaxine hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
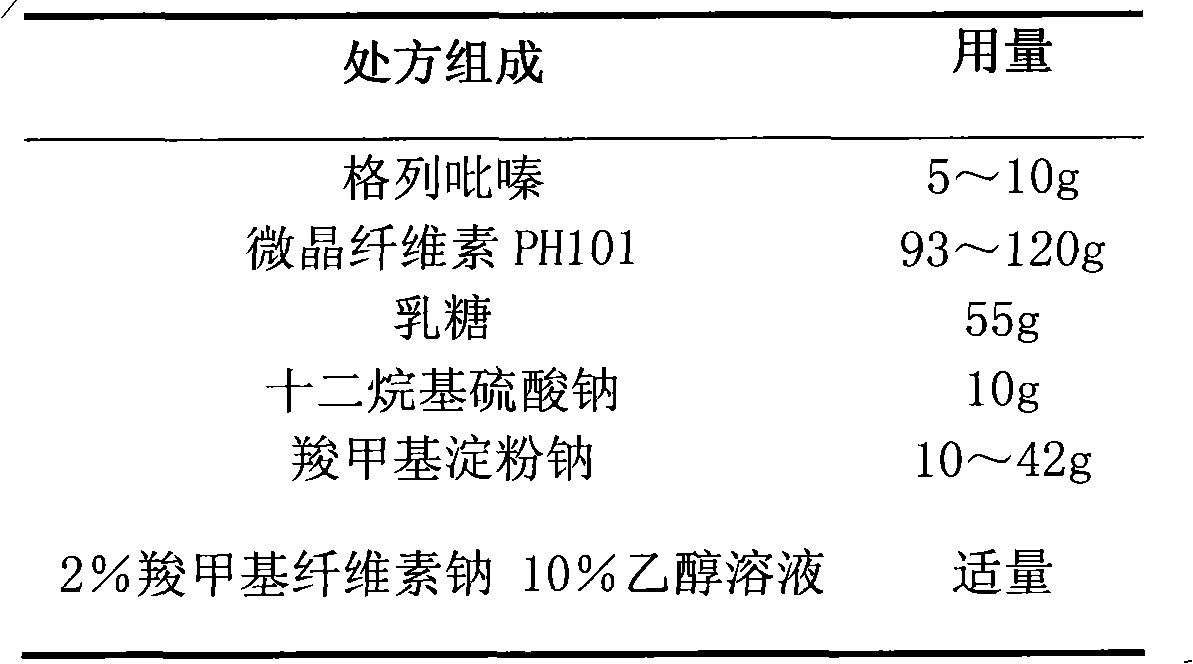
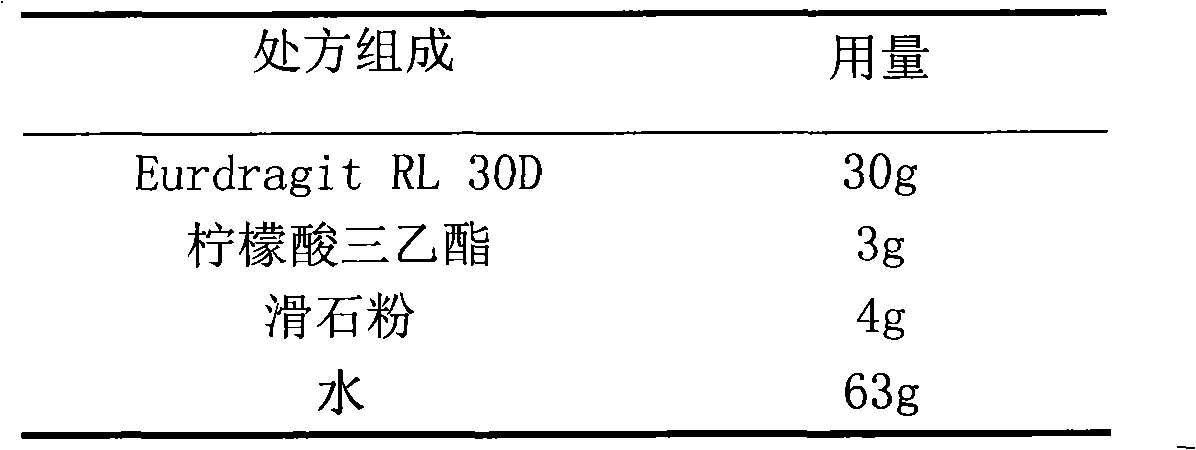
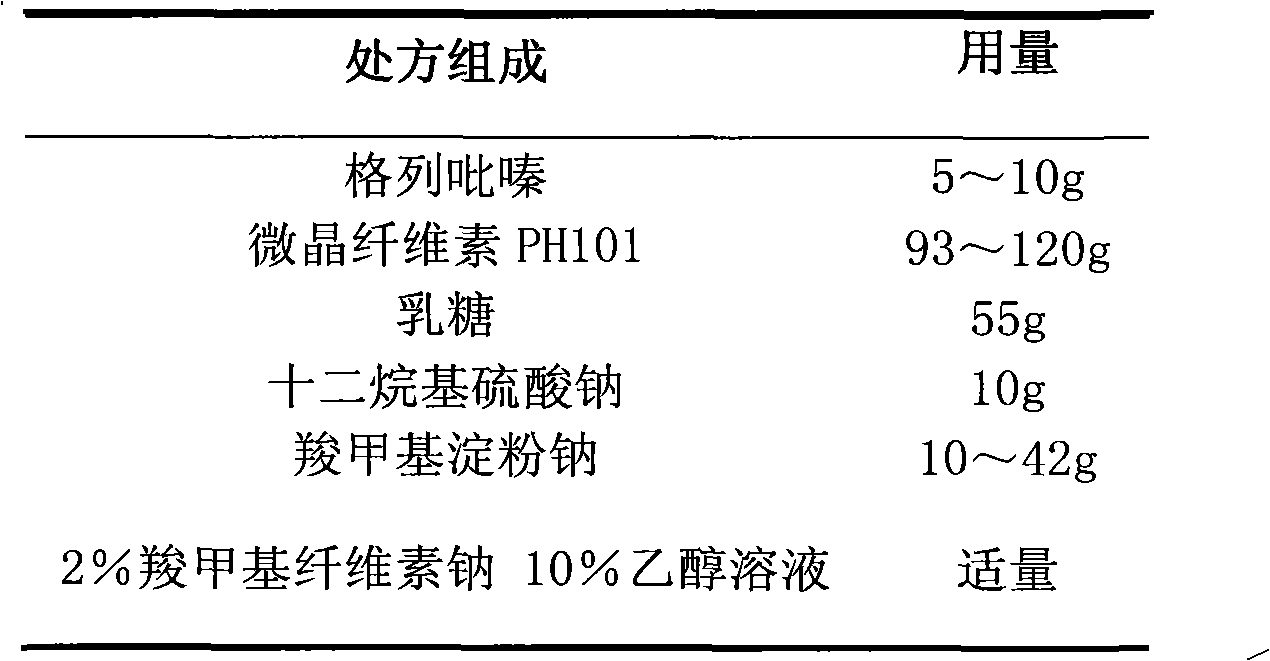
Glipizide film-controlled slow-release pellet capsule
ActiveCN103211787AAccelerated agingReduce permeabilityMetabolism disorderSulfonylurea active ingredientsSustained release pelletsMedicine
The invention relates to a glipizide film-controlled slow-release pellet capsule. A slow-release film of the glipizide film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the glipizide film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose, lactose and sodium dodecyl sulfate, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 17 to 36%. The glipizide film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the glipizide film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the glipizide film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:内蒙古天衡医院管理有限公司
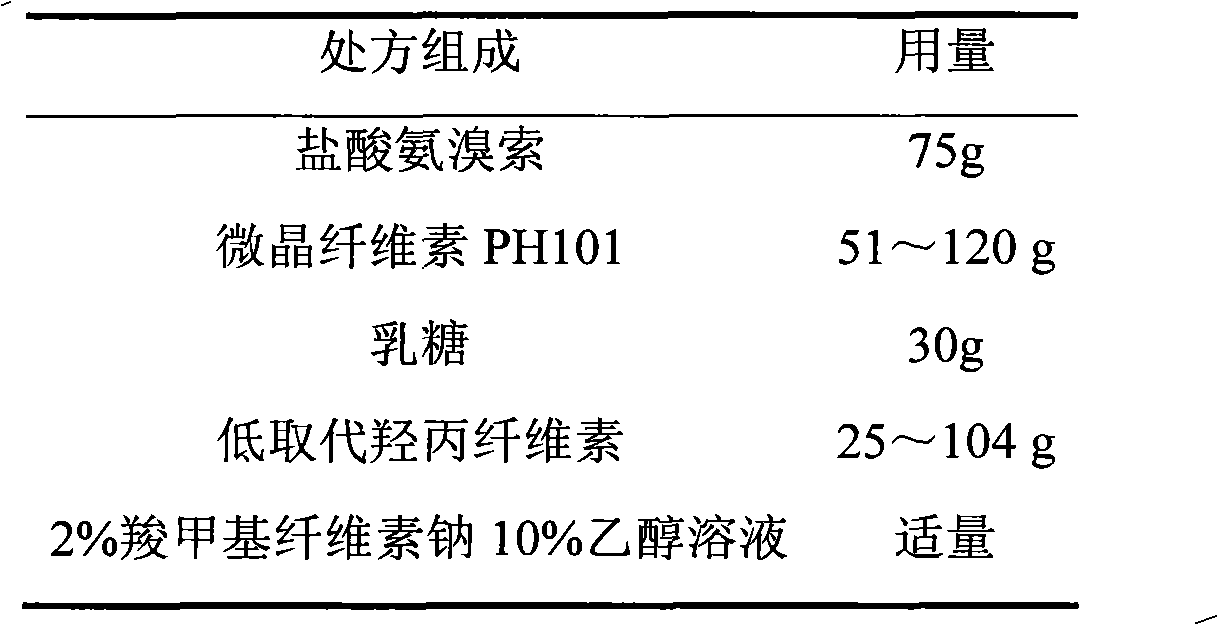
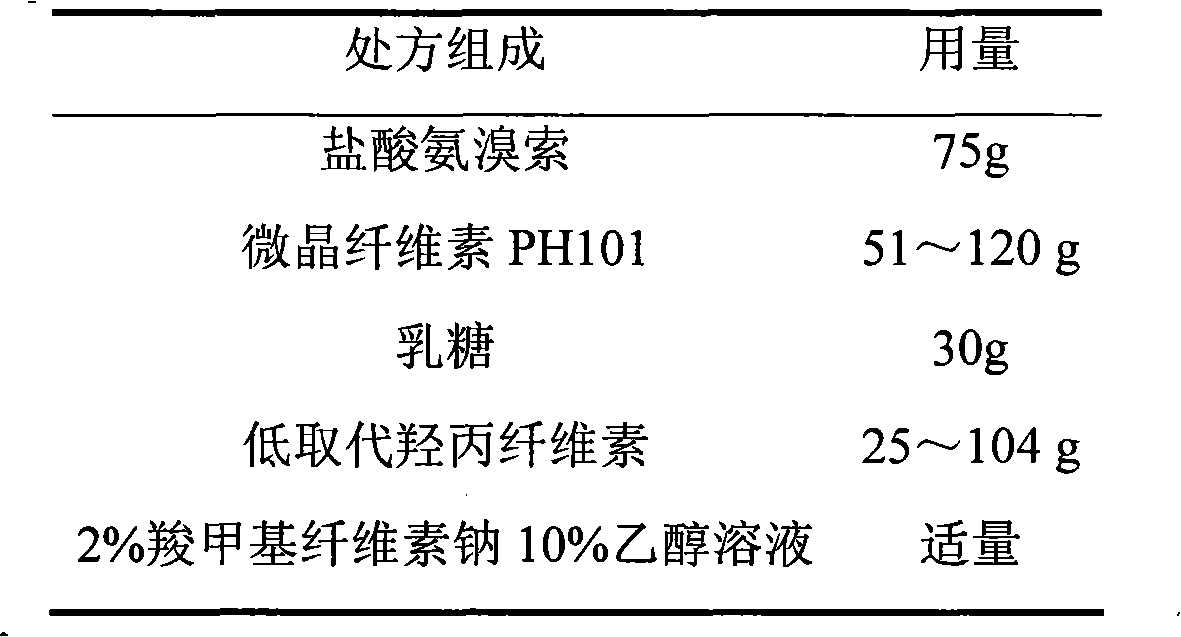
Ambroxol hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211789AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsLactose
The invention relates to an ambroxol hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the ambroxol hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR 30D as a film-formation material. A pellet core of the ambroxol hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR 30D to triethyl citrate to talcum powder is 30: 1.5: 4 and a film weight increasing ratio is in a range of 20 to 36%. The ambroxol hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the ambroxol hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the ambroxol hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
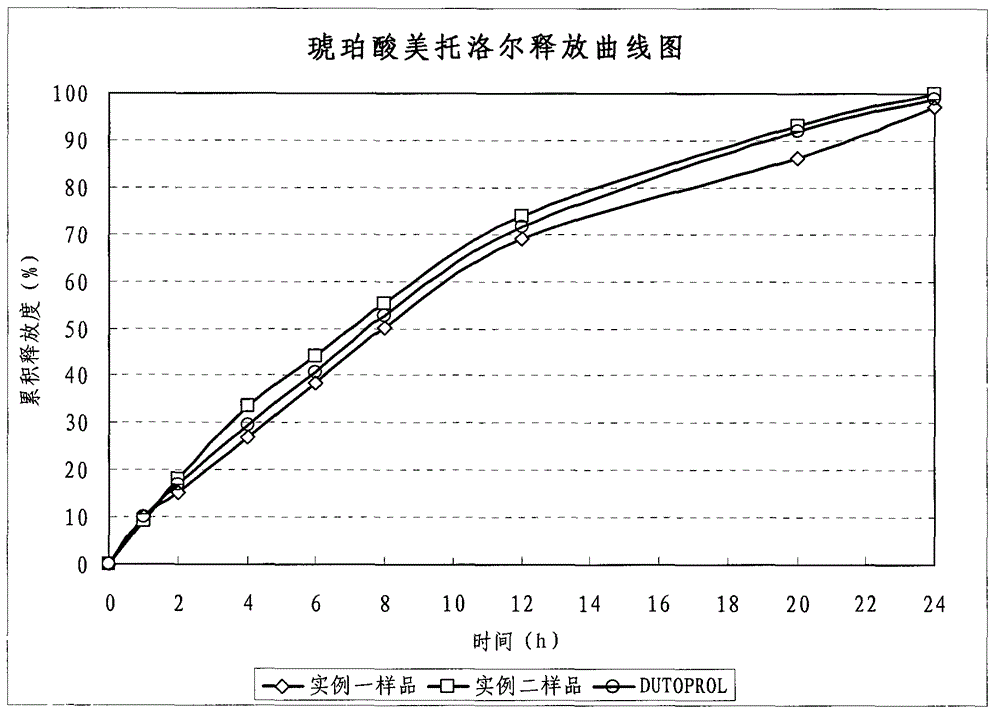
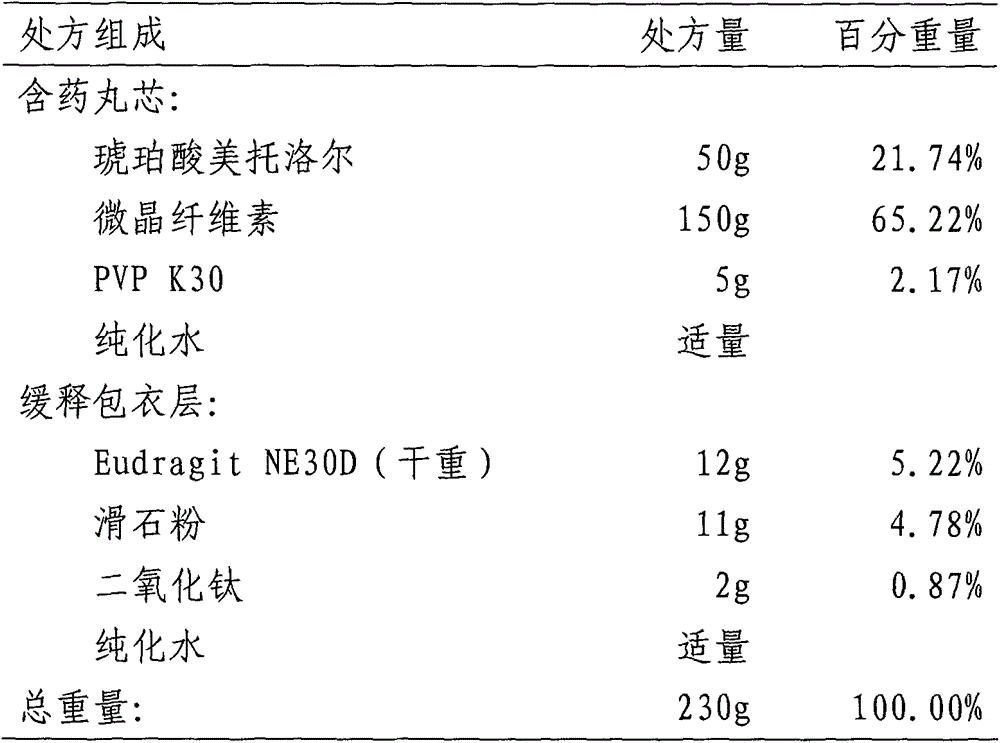
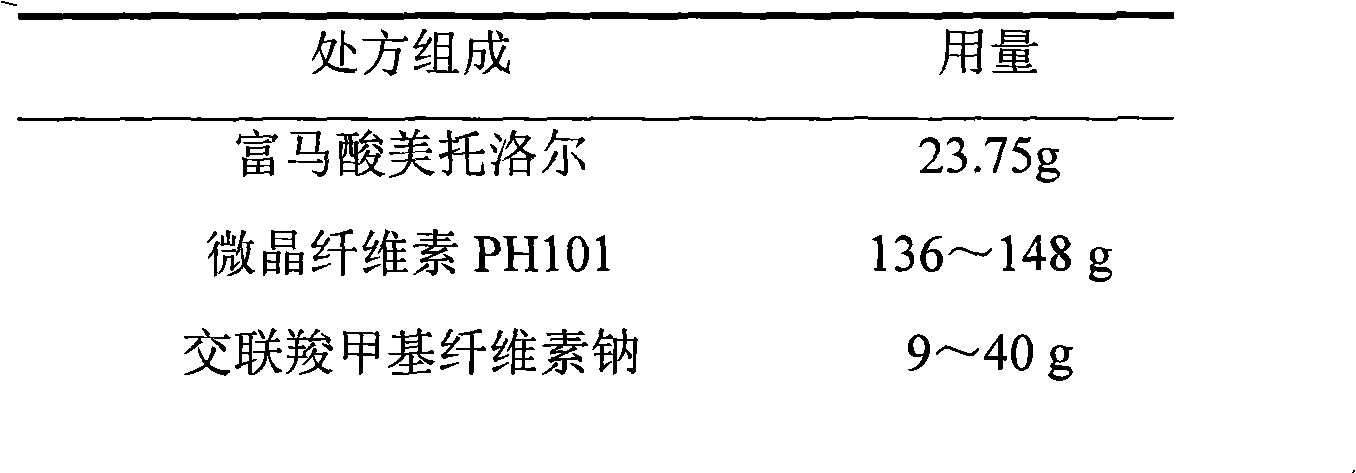
Metroprolol succinate hydrochlorothiazide sustained-release pellet capsule and preparation method thereof
InactiveCN103142618AUniform absorption rateSmall differences in individual bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSide effect
The invention discloses a metroprolol succinate hydrochlorothiazide sustained-release pellet capsule and a preparation method thereof. The metroprolol succinate hydrochlorothiazide sustained-release pellet capsule is prepared by mixing a metroprolol succinate sustained-release pellet and a hydrochlorothiazide quick-release pellet according to provided dose ratio, and filling into a hard capsule. The metroprolol succinate sustained-release pellet constantly releases the medicine for 24 hours, and the medicine is taken once a day, thereby retaining the lasting antihypertensive effect; the hydrochlorothiazide quick-release pellet is rapidly absorbed so as to achieve the diuresis and antihypertensive effect, and by combining the pellets, the antihypertensive effect is doubled, the toxic and side effects of the medicine are reduced, the number of the medicine administrations is reduced, and lasting and stable antihypertensive effects are achieved in vivo, so that the compliance of patients is improved; and meanwhile as the sustained-release pellet preparation is composed of hundreds of pellets of uniform granule sizes, the sudden release of the whole preparation is not caused by the breakage of individual pellets, so that the medicine is safer than a sustained-release tablet, smaller in irritation to gastrointestinal tracts and more stable in blood concentration, and the clinical effectiveness and security are effectively improved.
Owner:广州科的信医药技术有限公司
Skeleton Diclofenac Potassium Sustained-release Pellet Capsules and Production Process
ActiveCN102266292ALittle side effectsReduce the number of dosesOrganic active ingredientsAntipyreticSustained release pelletsDiclofenac Acid
The invention relates to matrix diclofenac potassium sustained-release pellet capsules and a production process thereof. The matrix diclofenac potassium sustained-release pellet capsules are prepared by filling matrix diclofenac potassium sustained-release pellets into gastric-soluble capsule shells. The matrix diclofenac potassium sustained-release pellets are prepared in one step by extrusion and rolling. The formula of the matrix diclofenac potassium sustained-release pellets comprises basic remedy diclofenac potassium 10-50%, matrix agent 1-20%, diluent 20-80%, antioxidant 0.1-5%, antisticking agent 0.1-5%, absorption promoting agent 1-10%, and any available wetting agent as balance, wherein the matrix agent is hydrophilic gel matrix agent, or hydrophilic gel matrix agent and erodiblematrix agent, or hydrophilic gel matrix agent and insoluble matrix agent. The matrix diclofenac potassium sustained-release pellet capsules provided by the invention have the advantages of high bioavailability, long in vivo holdup time, regular drug release, good in vivo (Beagle dogs) absorption and reproducibility, good sustained-release effect, simple production process, short production period, and no flying of dust during production, and are suitable for industrial production.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
Preparation method of diclofenac potassium sustained-release pellet capsule
ActiveCN101612140AStable blood concentrationSmall fluctuationOrganic active ingredientsAntipyreticSustained Release CapsuleDiclofenac Sodium
The invention relates to a preparation method of diclofenac potassium sustained-release pellet capsule. The preparation method sequentially comprises: preparing diclofenac potassium hormone pill by an extrusion-spheronization mothod, packing an isolation layer, packing a sustained-release layer and encapsulating the capsule, extruding the capsule into a stick on the condition that the set frequency of an extruder is 10-100Hz, and spheronizing the capsule for 3-30min on the condition that the frequency of a spheronization machine is 20-150Hz. In the invention, flying dust does not occur, the pollution is little and the production process meets the requirement of GMP; after the prepared diclofenac potassium sustained-release capsule is taken orally, the plasma concentration of the drug is gentle with small fluctuation and the capsule has good sustained-release effect.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
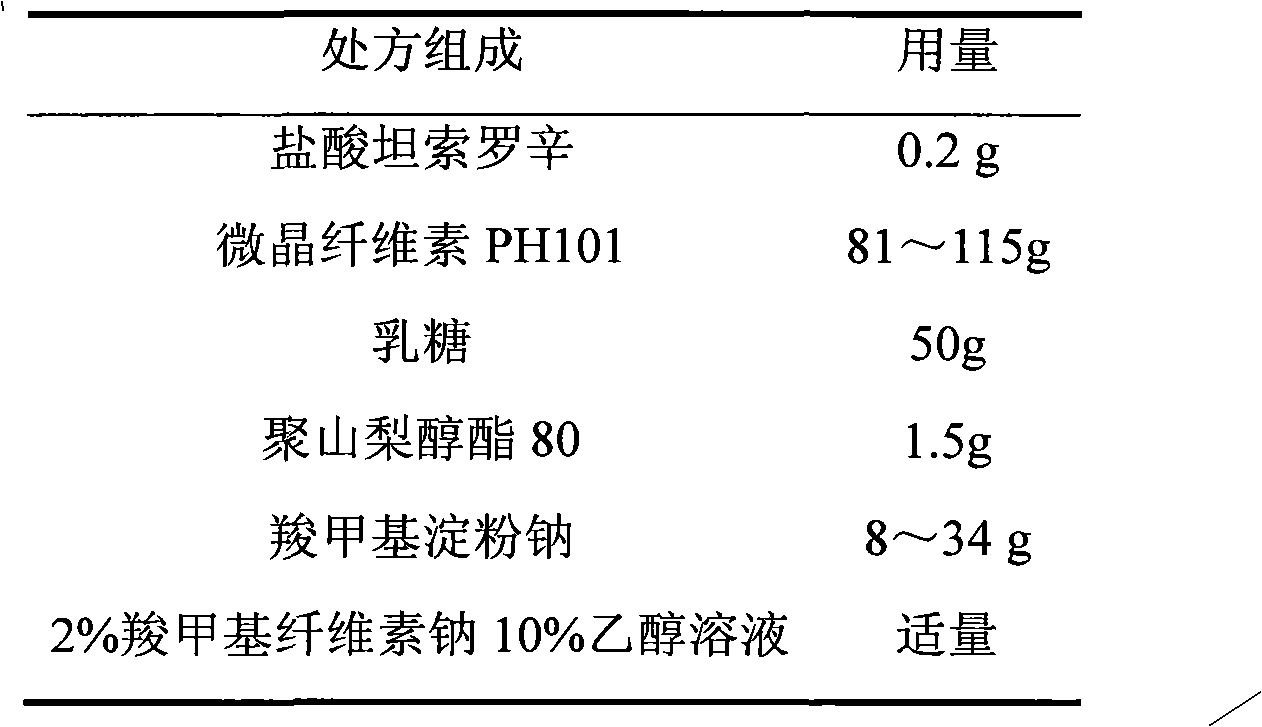
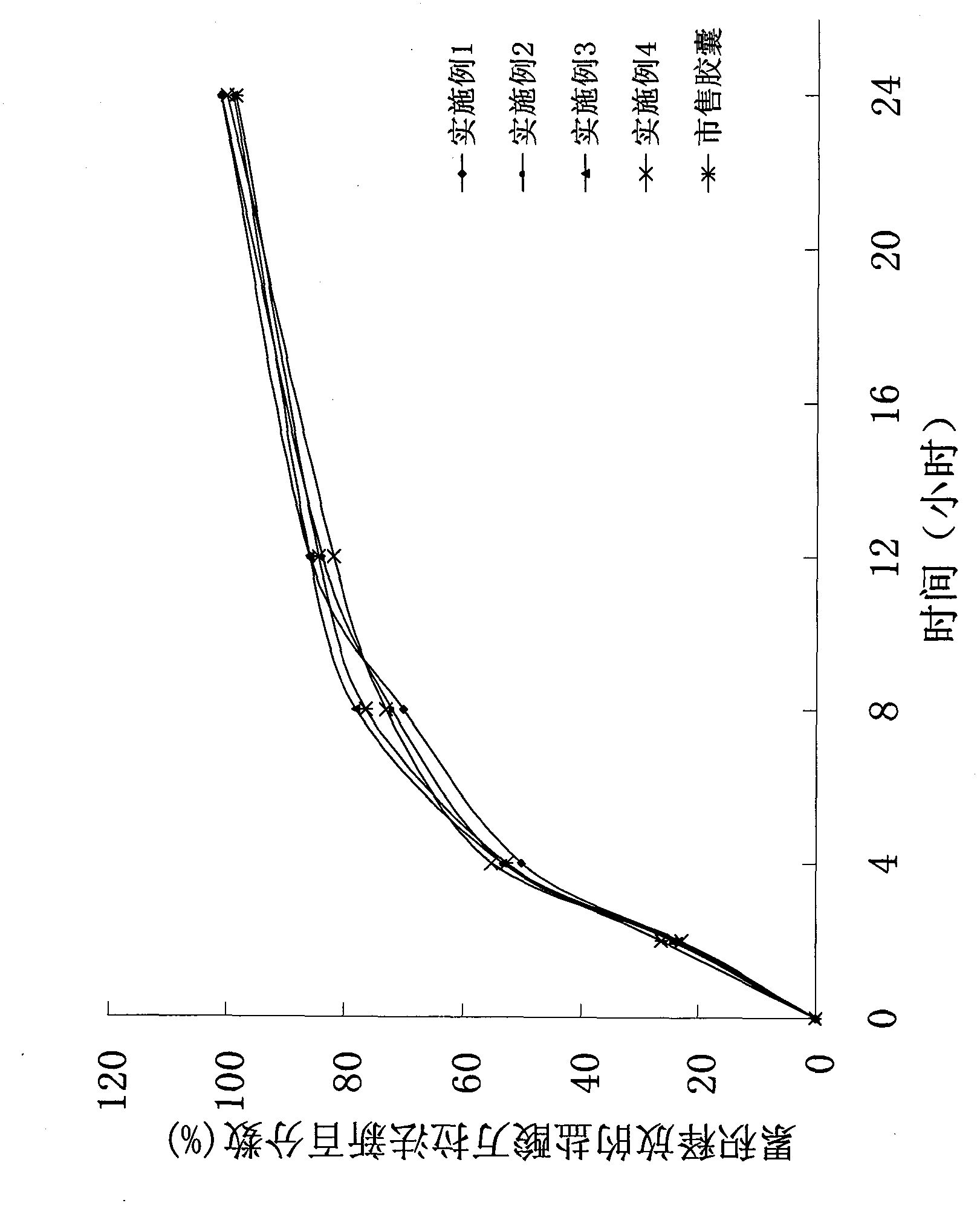
Tamsulosin hydrochloride sustained-release pellets and preparation method thereof
InactiveCN101695478BUrinary disorderPharmaceutical non-active ingredientsSustained release pelletsCaplet Dosage Form
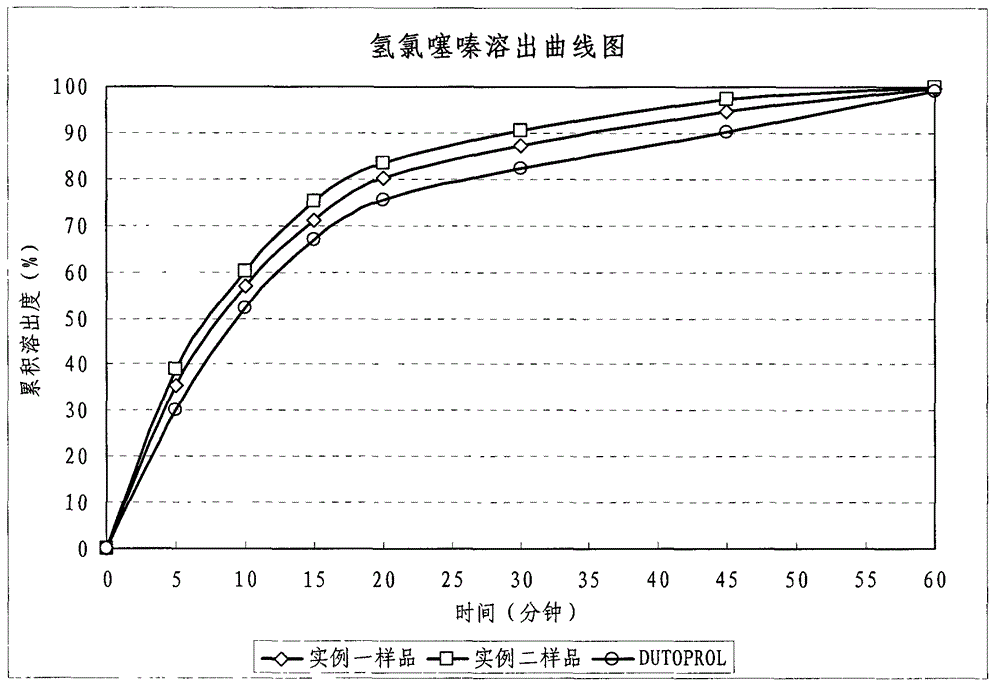
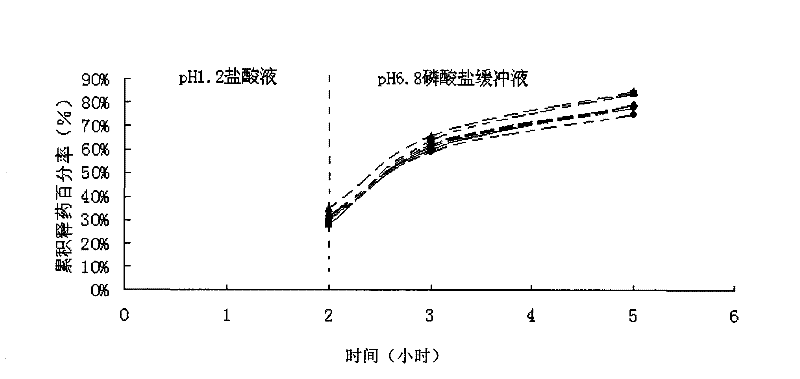
The invention discloses tamsulosin hydrochloride sustained-release pellets, which are prepared by coating medicament-containing blank pellets, wherein the blank pellets contain microcrystalline cellulose, soluble starch, lactose and croscarmellose sodium. Two layers of coating liquid are a Surelease sustained-release coating material and an enteric-coating material Kolicoat MAE 30DP respectively and the tamsulosin hydrochloride sustained-release pellets are capsules. The tamsulosin hydrochloride sustained-release pellets of the invention adopt the sustained-release coating material and the enteric-coating material to control the release of medicaments. The prepared tamsulosin hydrochloride sustained-release pellet capsules release less than 40 percent of medicaments in hydrochloric acid solution with a pH value of 1.2 in two hours and release 40 to 70 percent (about 60 percent) of medicaments in phosphate buffer solution with a pH value of 6.8 in three hours and over 70 percent (about90 percent) of medicaments in five hours, so the prepared tamsulosin hydrochloride sustained-release pellet capsules meet the requirements of quality standards. The invention discloses a preparation method of the tamsulosin hydrochloride sustained-release pellets.
Owner:JIANGSU UNIV +1
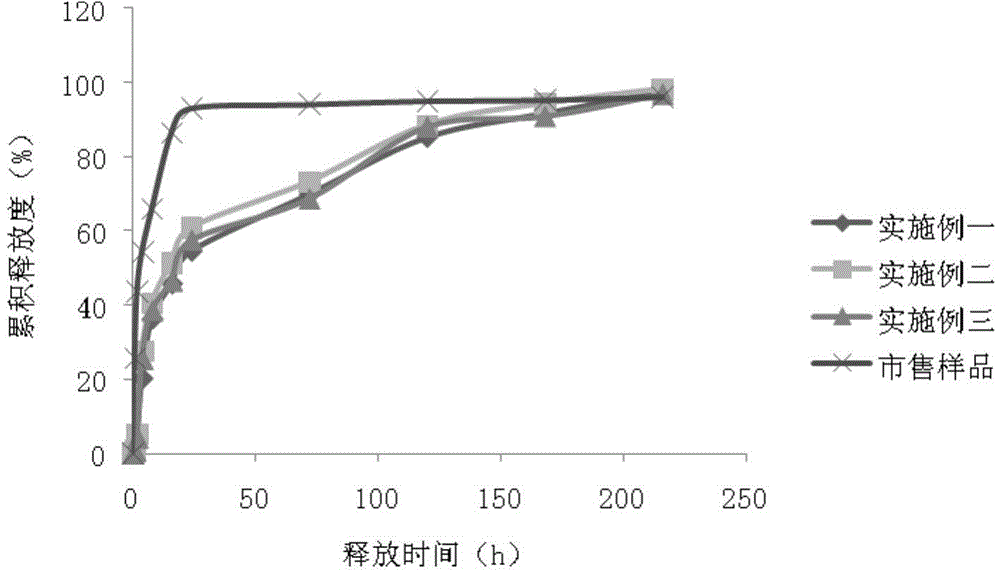
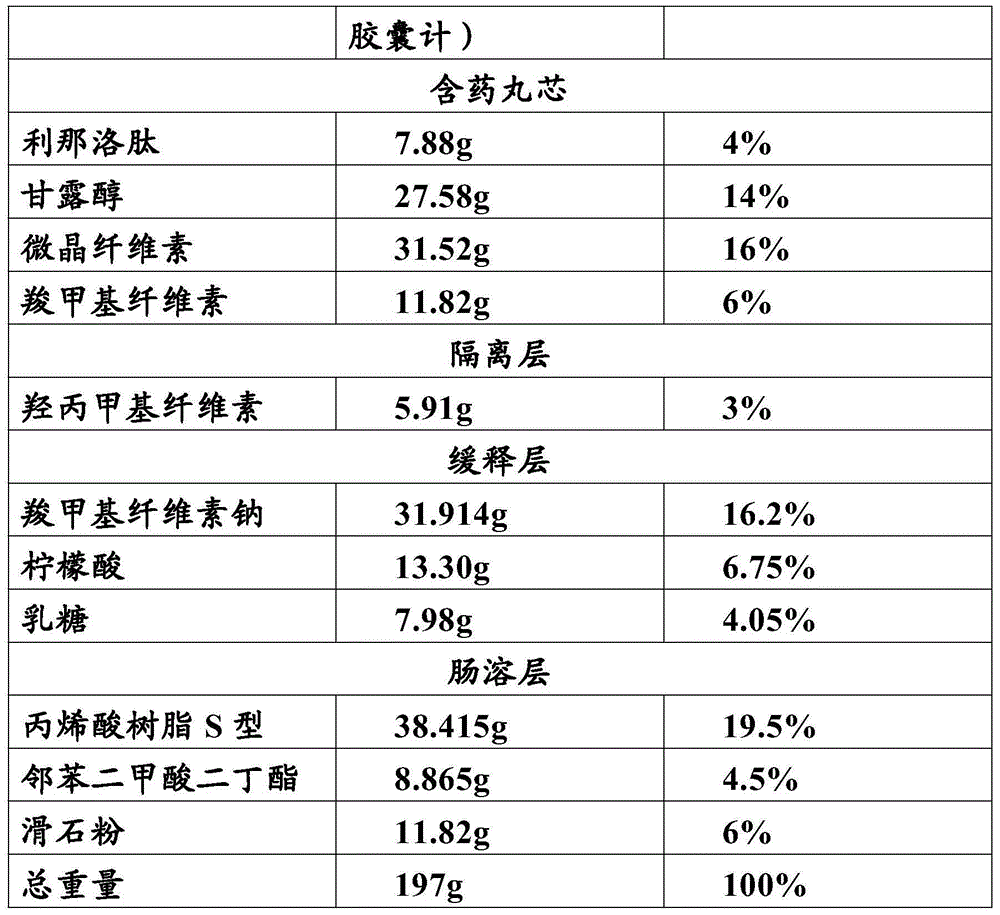
Linaclotide enteric controlled-release pellet capsule preparation and preparing method and application thereof
InactiveCN105412904AReduce stimulationDoes not affect burstPeptide/protein ingredientsDigestive systemControl releaseIsolation layer
The invention relates to a linaclotide enteric controlled-release pellet capsule preparation. The linaclotide enteric controlled-release pellet capsule preparation is composed of linaclotide enteric controlled-release pellets and a hollow capsule, wherein the linaclotide enteric controlled-release pellets comprise pellet cores, isolation layers, controlled-release layers and enteric layers. The invention further relates to a method for preparing the linaclotide enteric controlled-release pellet capsule preparation and application of the linaclotide enteric controlled-release pellet capsule preparation in preparing medicine for treating a constipation type irritable bowel syndrome.
Owner:HYBIO PHARMA
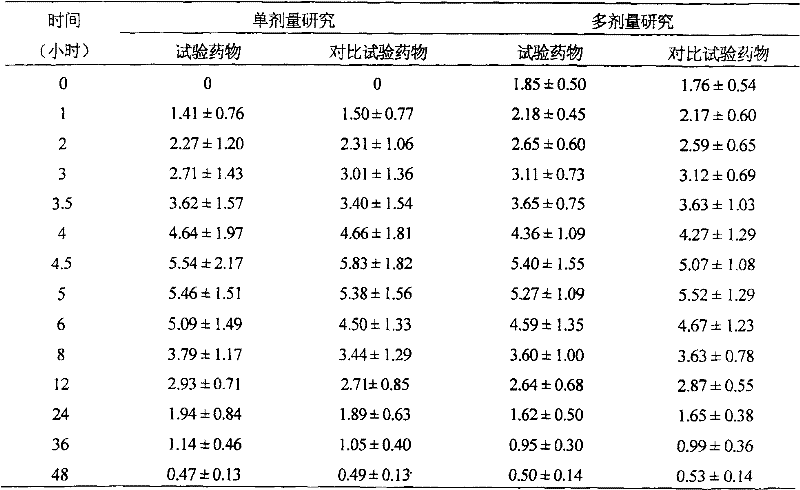
Venlafaxine hydrochloride pellet capsule and preparation method thereof
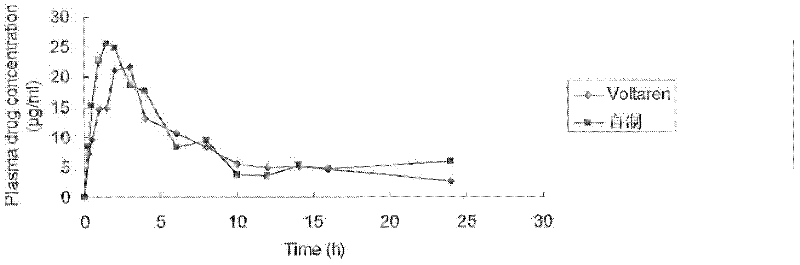
InactiveCN104644615AMaintain therapeutic blood levelsMaintain stabilityOrganic active ingredientsNervous disorderOral medicationPlasma concentration
The invention relates to a venlafaxine hydrochloride slow release capsule and a preparation method thereof. The capsule comprises a capsule shell and a medicated pellet placed in the capsule shell, and the medicated pellet comprises a medicated pellet core and a slow release layer wrapped outside the medicated pellet core. The venlafaxine hydrochloride slow release capsule is an oral administration slow release pellet capsule preparation with venlafaxine hydrochloride as an active medicine component, can be administrated once a day to realize 24h slow release and maintain the therapeutic plasma concentration, and accords requirements of stability, industrial scale production and the like.
Owner:TIANJIN YIYAO SCI & TECH
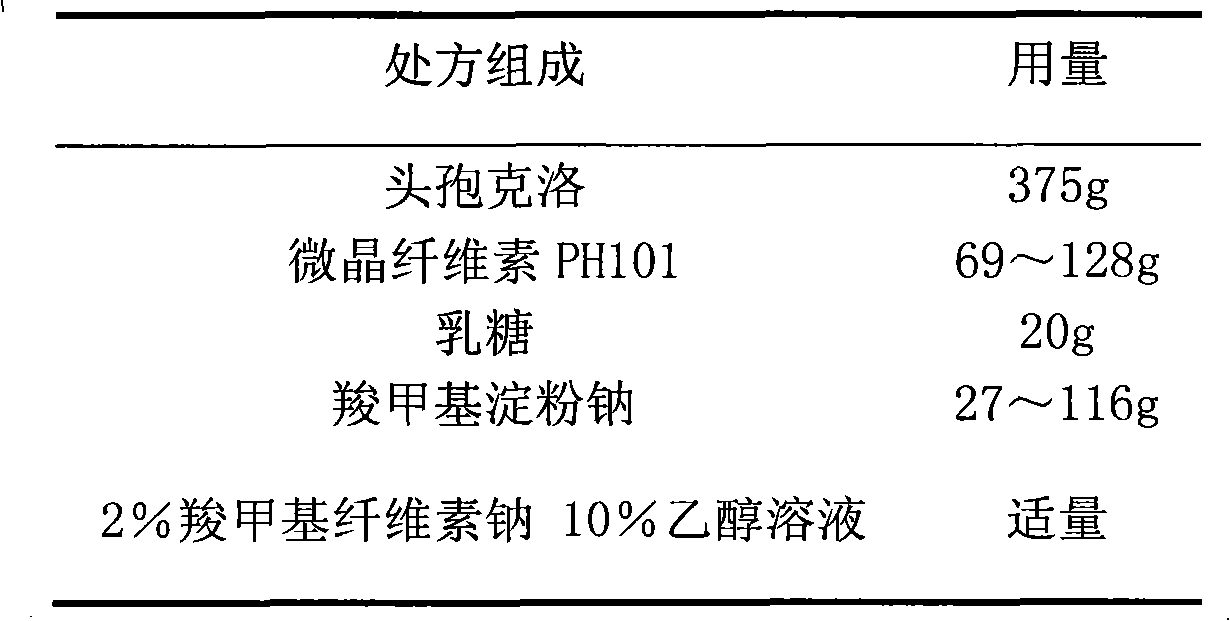
Cefaclor film-controlled slow-release pellet capsule
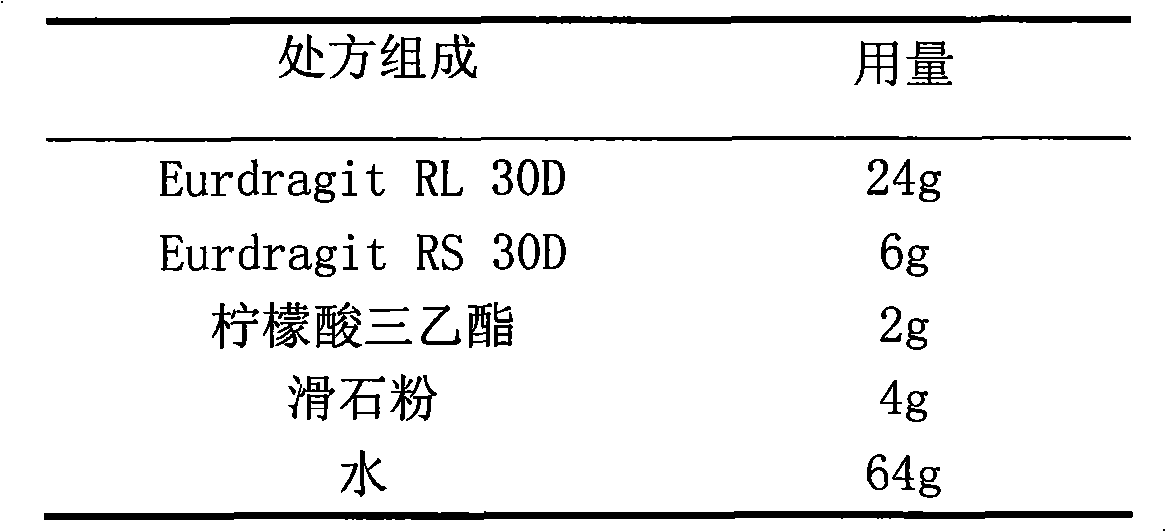
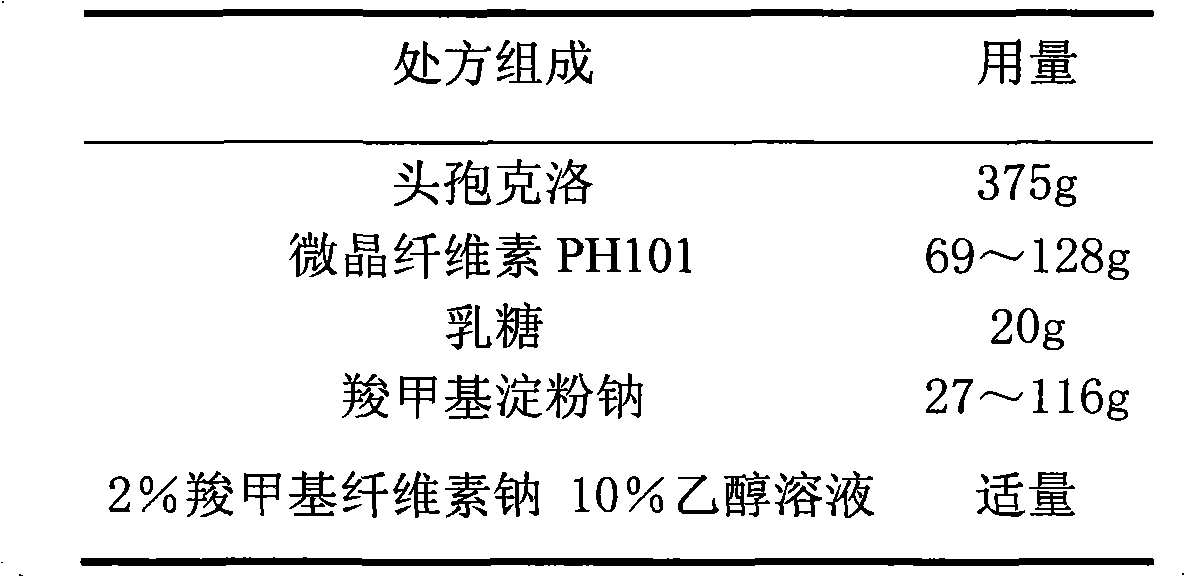
ActiveCN103211795AAccelerated agingReduce permeabilityAntibacterial agentsOrganic active ingredientsMedicineLactose
The invention relates to a cefaclor film-controlled slow-release pellet capsule. A slow-release film of the cefaclor film-controlled slow-release pellet utilizes a mixture of aqueous dispersion Eurdragit RL 30D and Eurdragit RS 30D as a film-formation material, wherein a weight ratio of Eurdragit RL 30D to Eurdragit RS 30D in the mixture is 4: 1. A pellet core of the cefaclor film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 5 to 20wt% of the sodium carboxymethyl starch. The slow-release film comprises the mixture of Eurdragit RL 30D and Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to Eurdragit RS 30D to triethyl citrate to talcum powder is 24: 6: 2: 4 and a film weight increasing ratio is in a range of 23 to 40%. The cefaclor film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the cefaclor film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the cefaclor film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
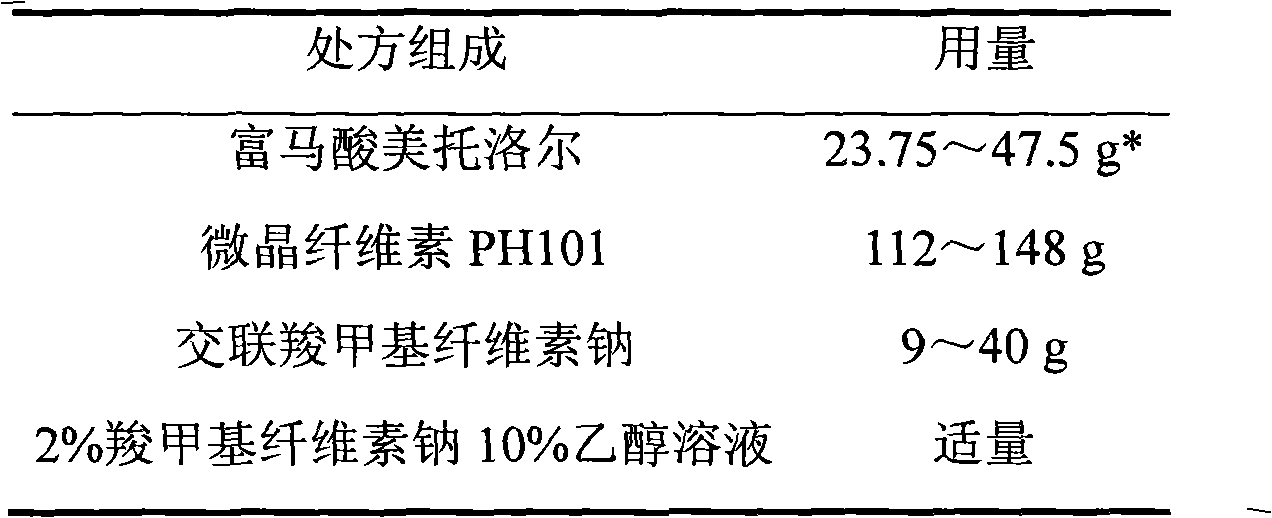
Metoprolol fumarate film-controlled slow-release pellet capsule
ActiveCN103211792AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderMetoprolol FumarateMedicine
The invention relates to a metoprolol fumarate film-controlled slow-release pellet capsule. A slow-release film of the metoprolol fumarate film-controlled slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the metoprolol fumarate film-controlled slow-release pellet contains croscarmellose sodium having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 5 to 20wt% of croscarmellose sodium. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 18 to 33%. The metoprolol fumarate film-controlled slow-release pellet comprises the pellet core containing croscarmellose sodium having high water expansibility and thus after absorbing water, the metoprolol fumarate film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the metoprolol fumarate film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
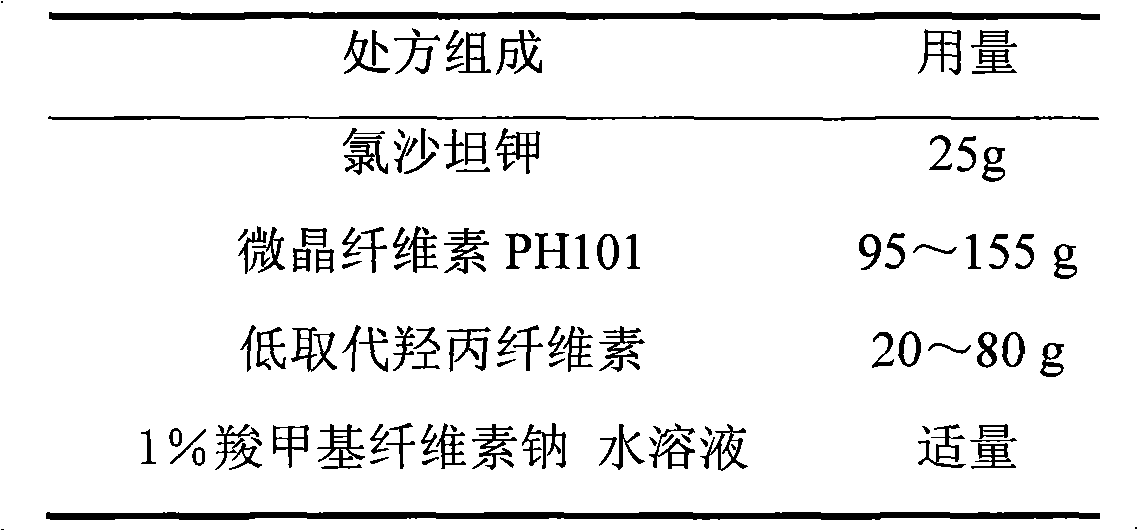
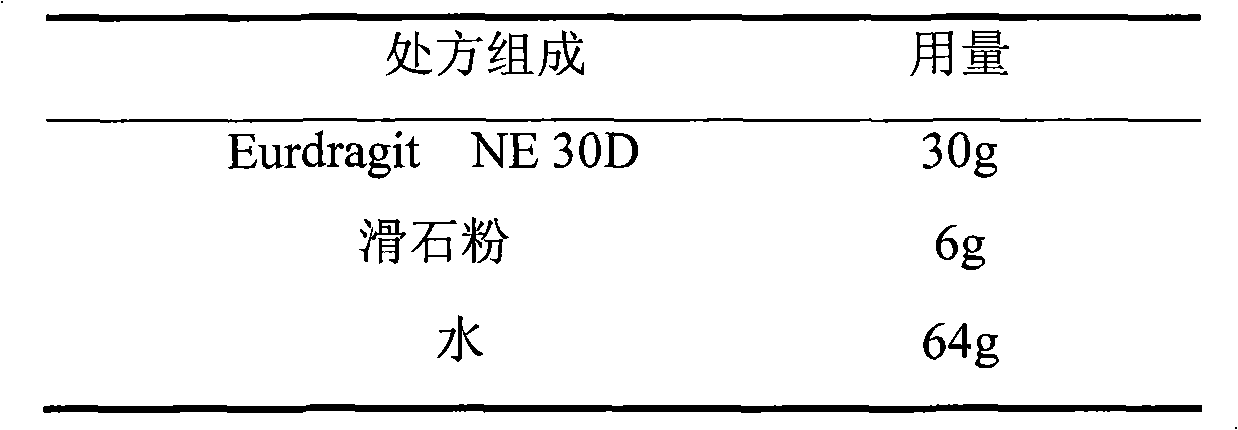
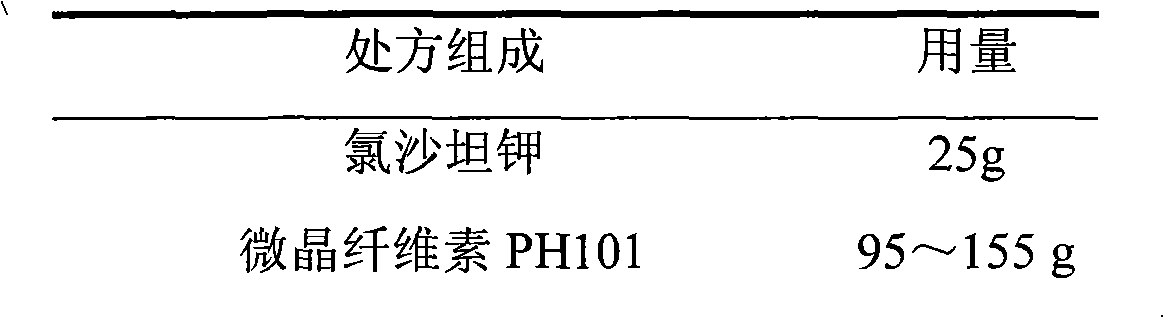
Losartan potassium membrane controlled-release pellet capsule
InactiveCN103211798AReduce permeabilityImprove permeabilityOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseMedicine
The invention relates to a losartan potassium membrane controlled-release pellet capsule. The controlled-release film of the pellet adopts Eurdragit NE 30D as a film forming material, the core of the controlled-release pellet contains high-expansibility low-substituted hydroxypropylcellulose and a pharmaceutically-acceptable excipient commonly used for controlled-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of the low-substituted hydroxypropylcellulose in the core of the controlled-release pellet is 10-40%. The controlled-release film of the controlled-release pellet includes the Eurdragit NE 30D and an anti-adherent talcum powder, the optimal ratio of the Eurdragit NE 30D to the talcum powder is 30:6, and the optimal coating weight gain is 19-36%. The core will obviously expand after absorbing water because of the containment of the low-substituted hydroxypropylcellulose highly expanding after contacting with water, so the controlled-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:内蒙古天衡医院管理有限公司
Ibuprofen sustained-release pellet capsule and preparation method thereof
ActiveCN110314152ALow content of related substancesLow in ibuprofen ethyl esterOrganic active ingredientsAntipyreticMedicineEthyl ester
The invention relates to an ibuprofen sustained-release pellet capsule and a preparation method thereof. The ibuprofen sustained-release pellet capsule is composed of a blank pellet core, a drug layerand a coating layer; the drug layer is composed of ibuprofen, histidine and a binder, and the content of the impurity ibuprofen ethyl ester is 0-0.03%. The pellet capsule not only can reduce the generation of ibuprofen impurities, but also has excellent damp-proof and anti-sticking properties.
Owner:西安圣雪沙药物开发有限公司
Sustained-release pellet capsule of sweet cooling agent for cigarette with filter plug
The invention discloses a sustained-release pellet capsule of a sweet cooling agent for a cigarette with a filter plug, belonging to the technical field of cigarettes. The sustained-release pellet capsule of the sweet cooling agent for the cigarette with the filter plug is prepared by the following steps of: (1) synthesizing corn mint oxime and N,2,3-trimethyl-2-isopropyl-butyrylamide; (2) compounding the corn mint oxime and the N,2,3-trimethyl-2-isopropyl-butyrylamide according to the proportion of (5-20): (1-10) to obtain a uniformly mixed white crystal compound; and (3) dropping. The invention has the advantages that the sustained-release pellet capsule prepared through synthesizing, compounding and dropping releases sweet substances only when the cigarette is smoked, therefore, effective components can not be lost in advance, and better fragrance keeping and remaining effects can be achieved. Meanwhile, the sweet and fragrant taste is unique and is coordinated with the smoke. In the process that the cigarette is smoked, the characteristic of a sweetener can be better embodied, the quality of the smoke is not remarkably influenced, and raw materials used in the invention have wide sources and low cost.
Owner:HUBEI CHINA TOBACCO IND +1
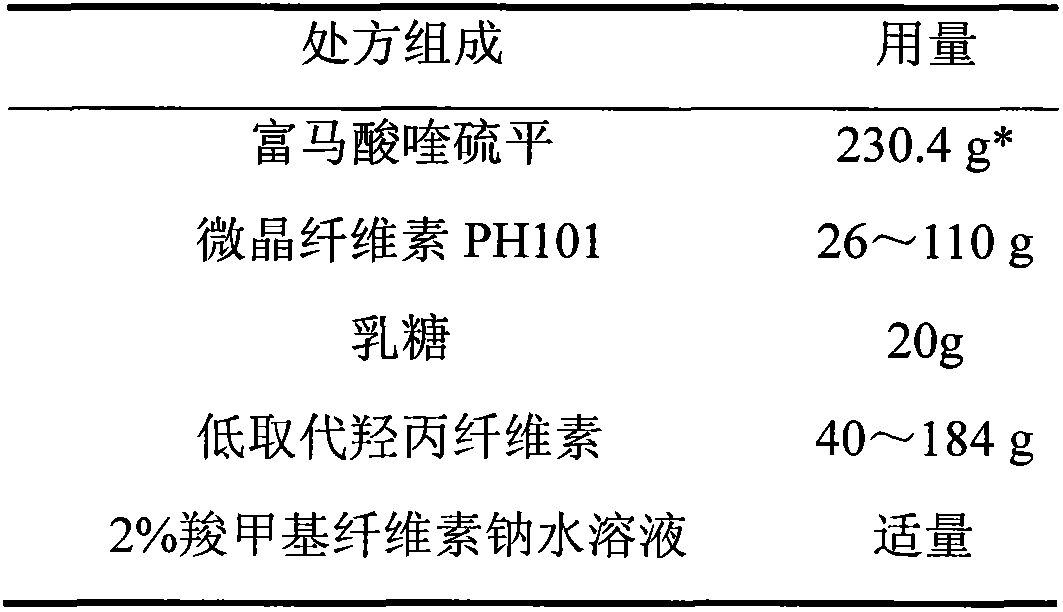
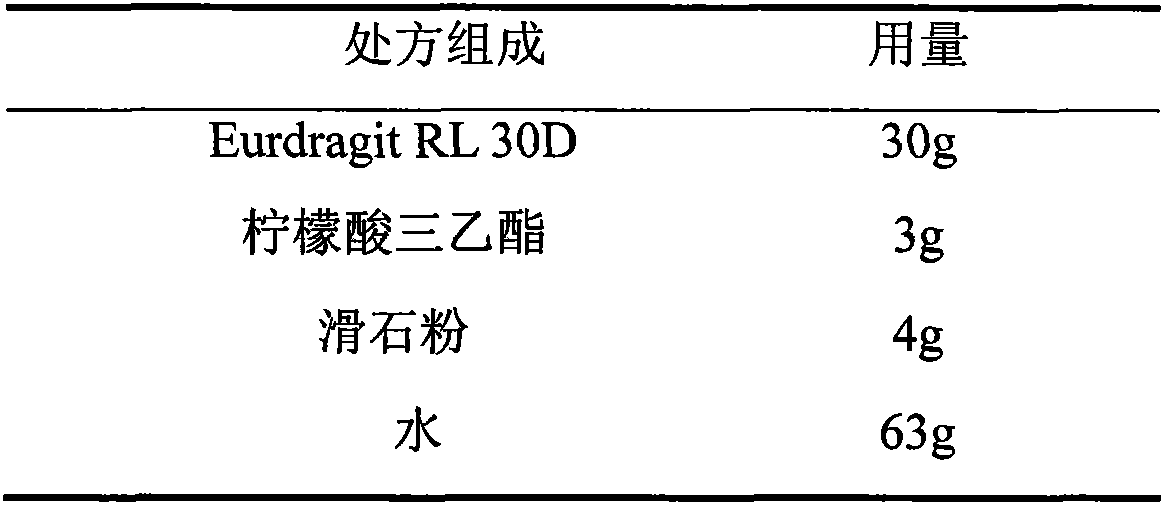
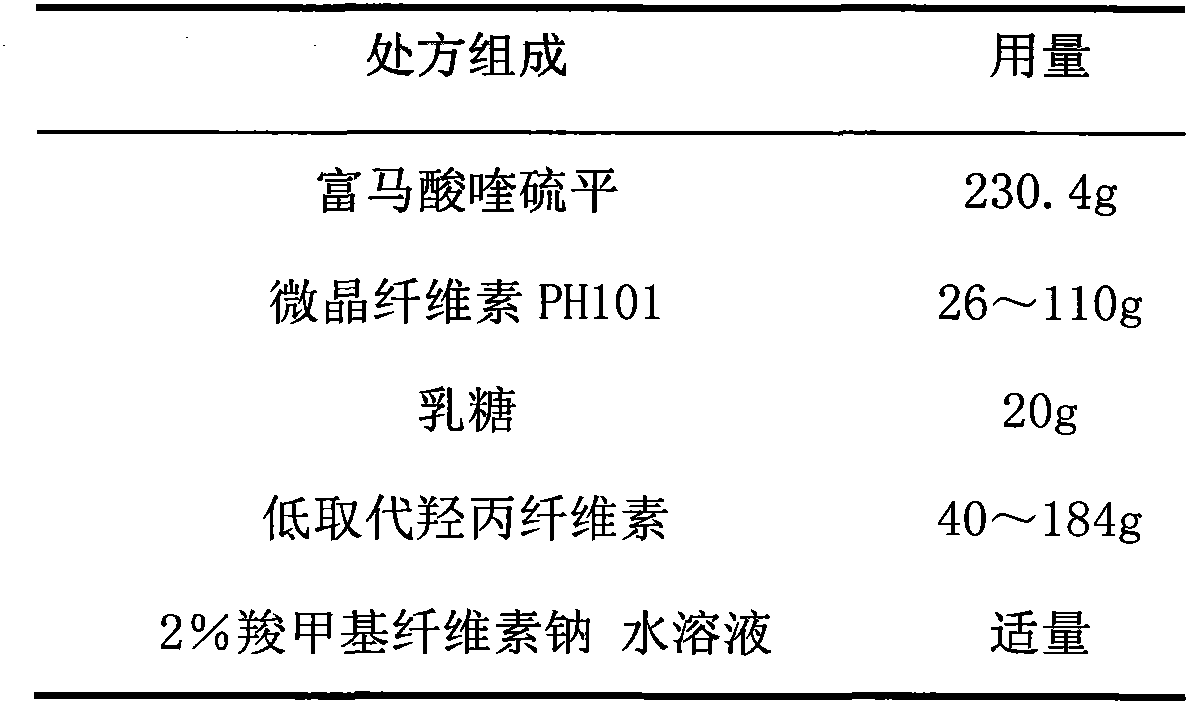
Quetiapine fumarate film-controlled slow-release pellet capsule
InactiveCN103211794AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsMedicine
The invention relates to a quetiapine fumarate film-controlled slow-release pellet capsule. A slow-release film of the quetiapine fumarate film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the quetiapine fumarate film-controlled slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 19 to 35%. The quetiapine fumarate film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the quetiapine fumarate film-controlled slow-release pellet expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the quetiapine fumarate film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:内蒙古天衡医院管理有限公司
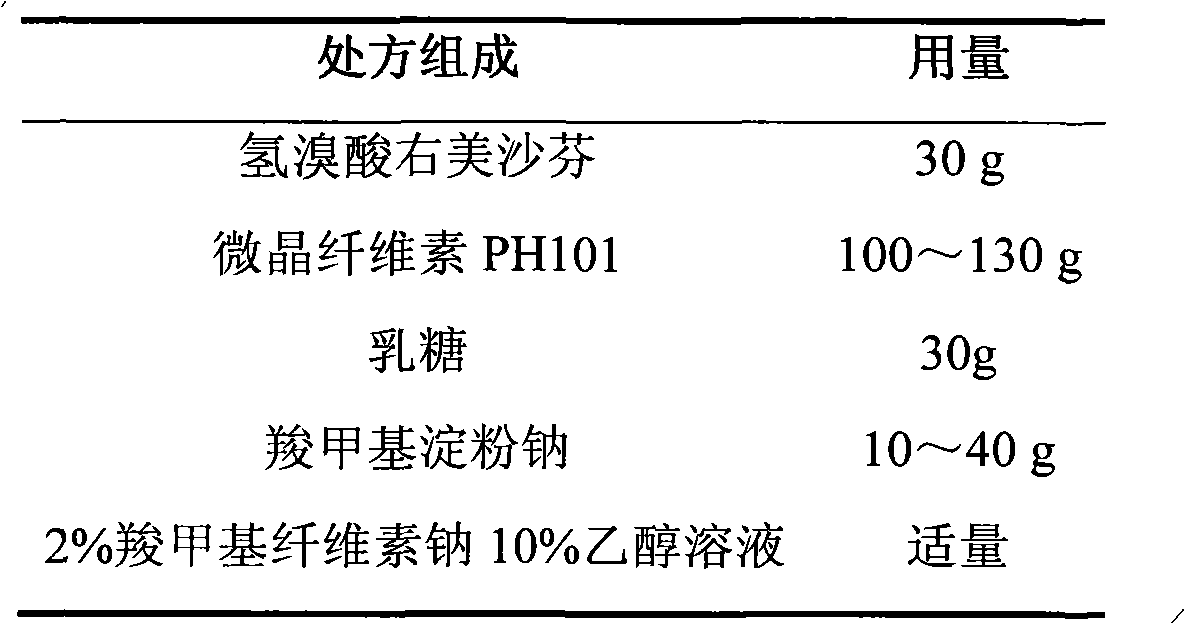
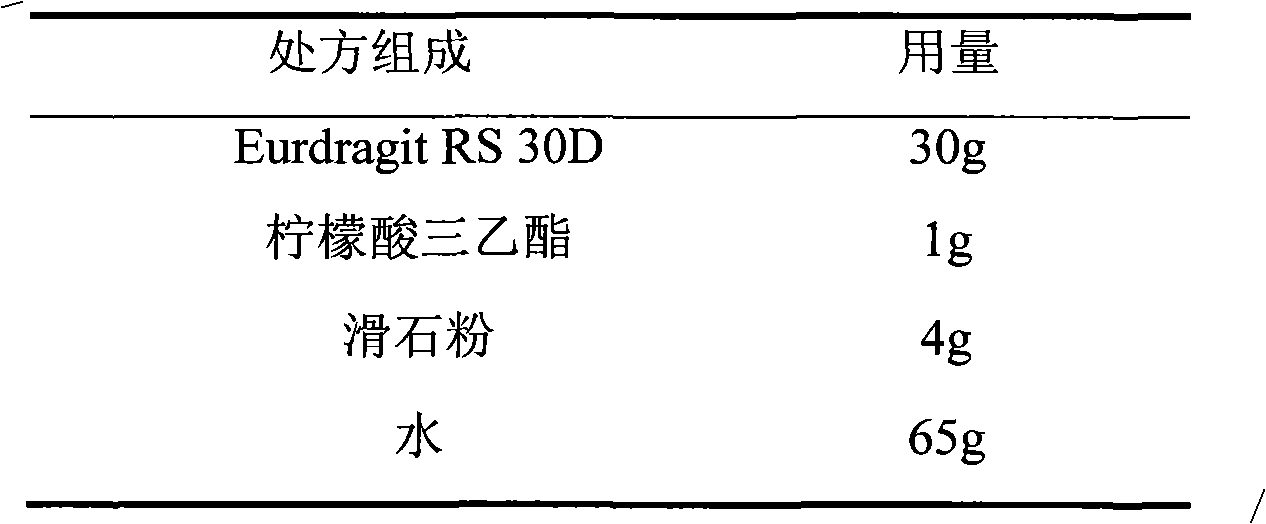
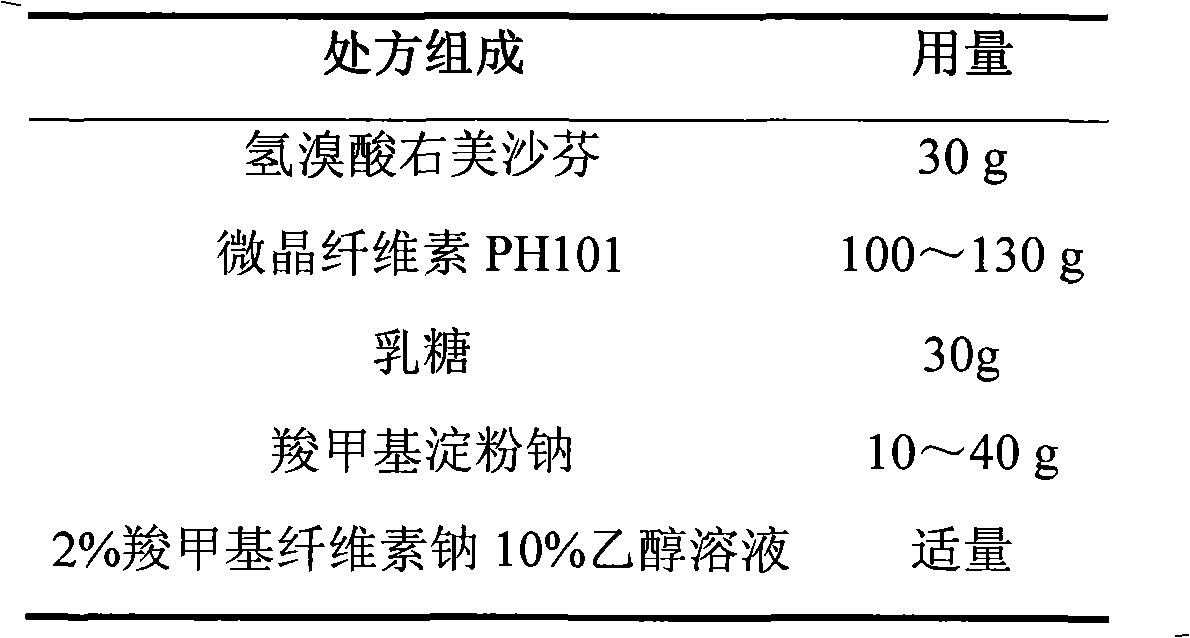
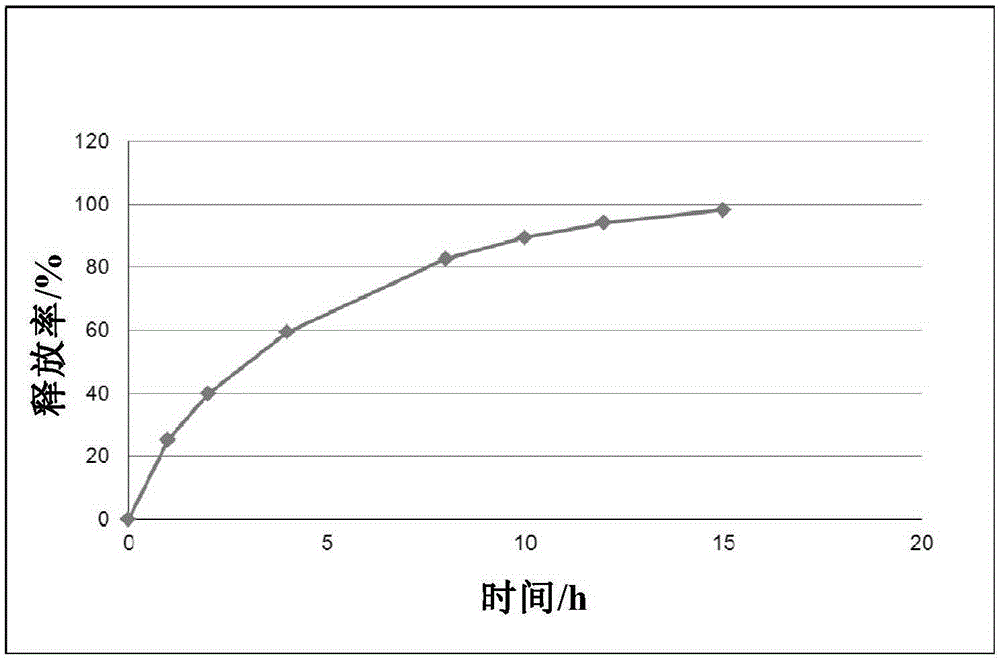
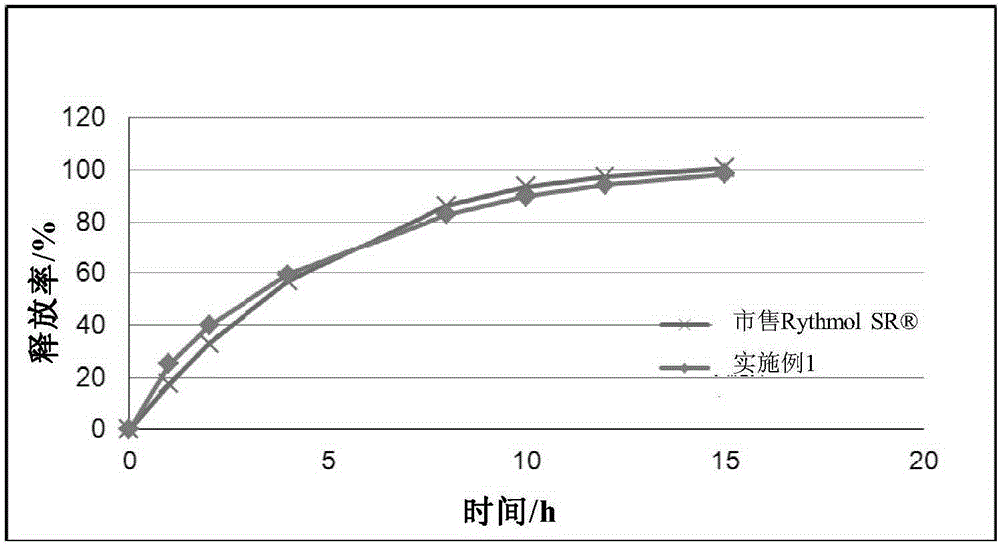
Dextromethorphan hydrobromide film-controlled slow-release pellet capsule
ActiveCN103211783AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsDextromethorphan Hydrobromide
The invention relates to a dextromethorphan hydrobromide film-controlled slow-release pellet capsule. A slow-release film of the dextromethorphan hydrobromide film-controlled slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the dextromethorphan hydrobromide film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RS 30D to triethyl citrate to talcum powder is 30: 1: 4 and coating weight gain is in a range of 16 to 35%. The dextromethorphan hydrobromide film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the dextromethorphan hydrobromide film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore diameter is increased; and permeability is improved and the permeability reduction caused by film aging is compensated. Therefore, in middle and later stages, the dextromethorphan hydrobromide film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept prior to expiration date.
Owner:北京天衡药物研究院有限公司
Propafenone hydrochloride sustained-release pellet capsule as well as preparation method and application thereof
InactiveCN105213349AStable drug releaseImprove liquidityOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSide effect
The invention provides a propafenone hydrochloride sustained-release pellet capsule as well as a preparation method and application thereof. The sustained-release pellet capsule consists of the following components in percentage by mass: 40-89.99% of propafenone hydrochloride, 10-50% of a filling agent and 0.01-10% of a binding agent. The propafenone hydrochloride sustained-release pellet capsule disclosed by the invention, on the basis of an extrusion-spheronization method, is successfully prepared by controlling the contents of various ingredients in a formula and by controlling conditions of a preparation process, and defects of the prior art which fails to prepare propafenone hydrochloride sustained-release pellets by virtue of the extrusion-spheronization method are overcome. Compared with a propafenone hydrochloride sustained-release micro-tablet capsule, the process of preparing the pellets by the extrusion-spheronization method is simple in step, and the process is capable of improving a production efficiency, significantly reducing differences between preparation batches and improving the stability of a sample; and the prepared propafenone hydrochloride sustained-release pellet capsule is stable in drug release, and the capsule is capable of improving medication compliance in patients and reducing toxic and side effects.
Owner:SALUS PHARMA TECH SHANGHAI
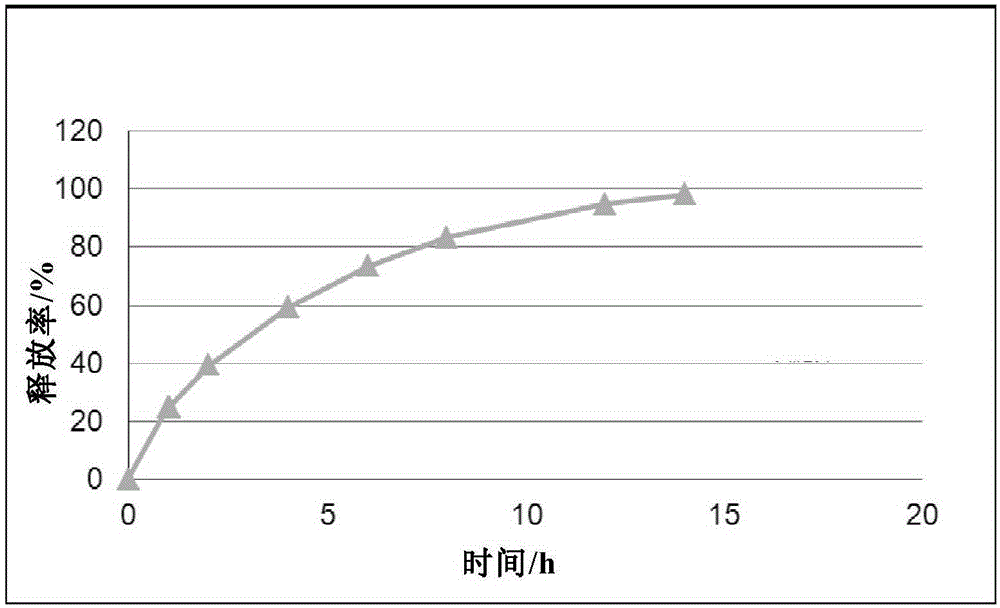
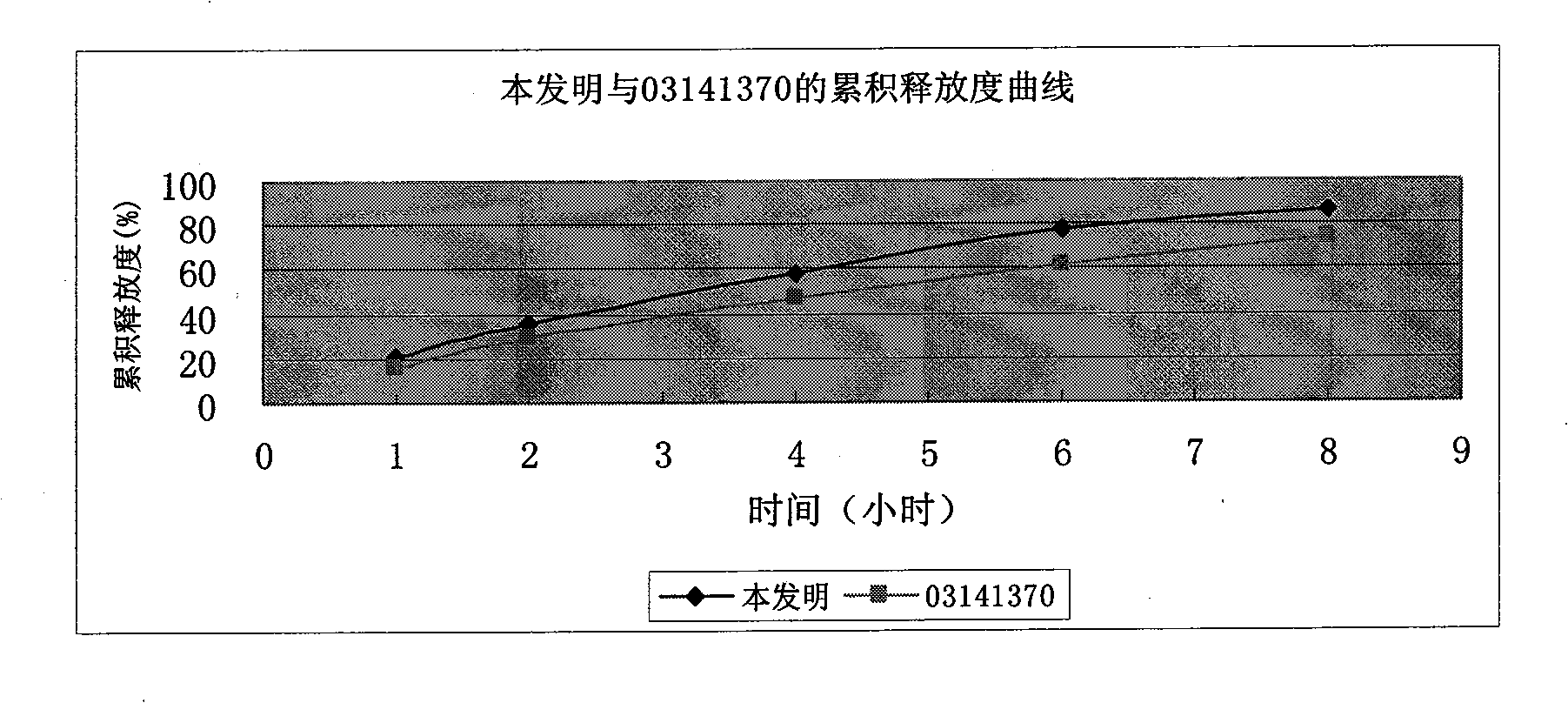
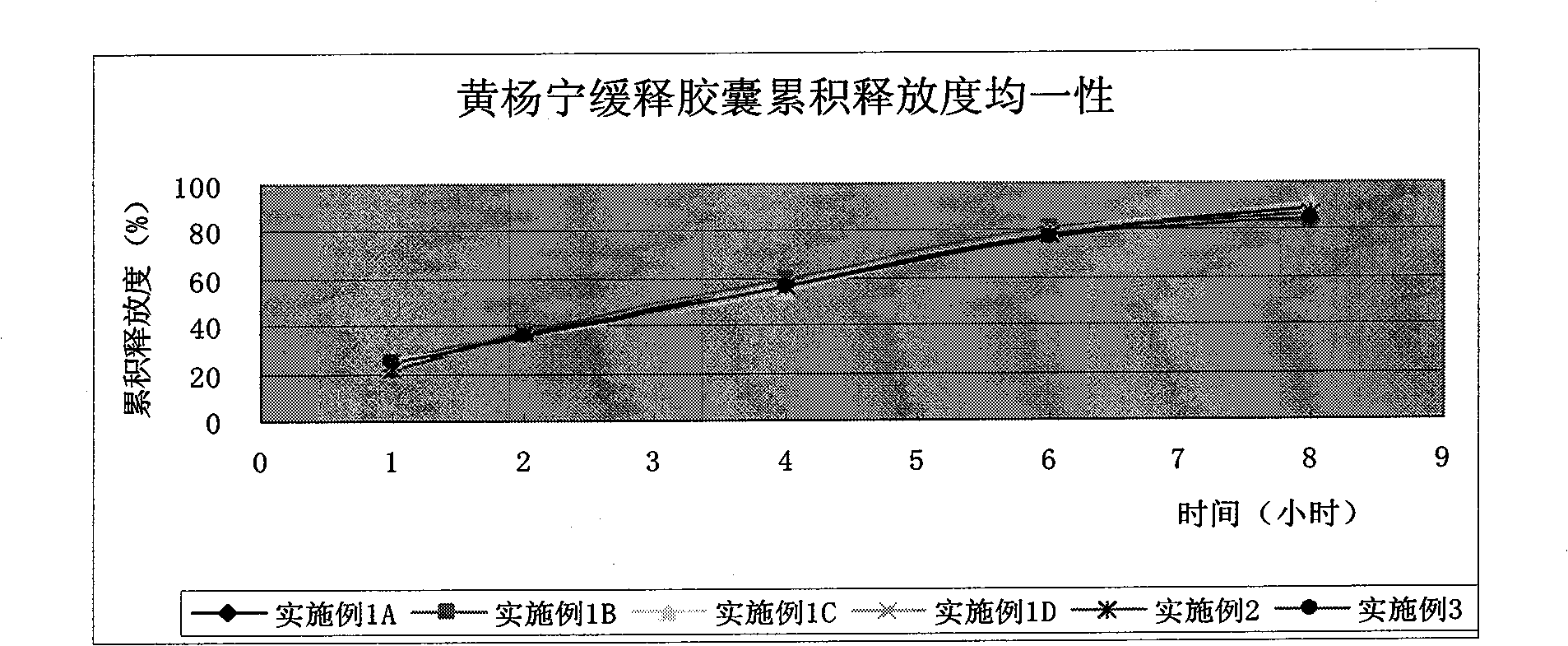
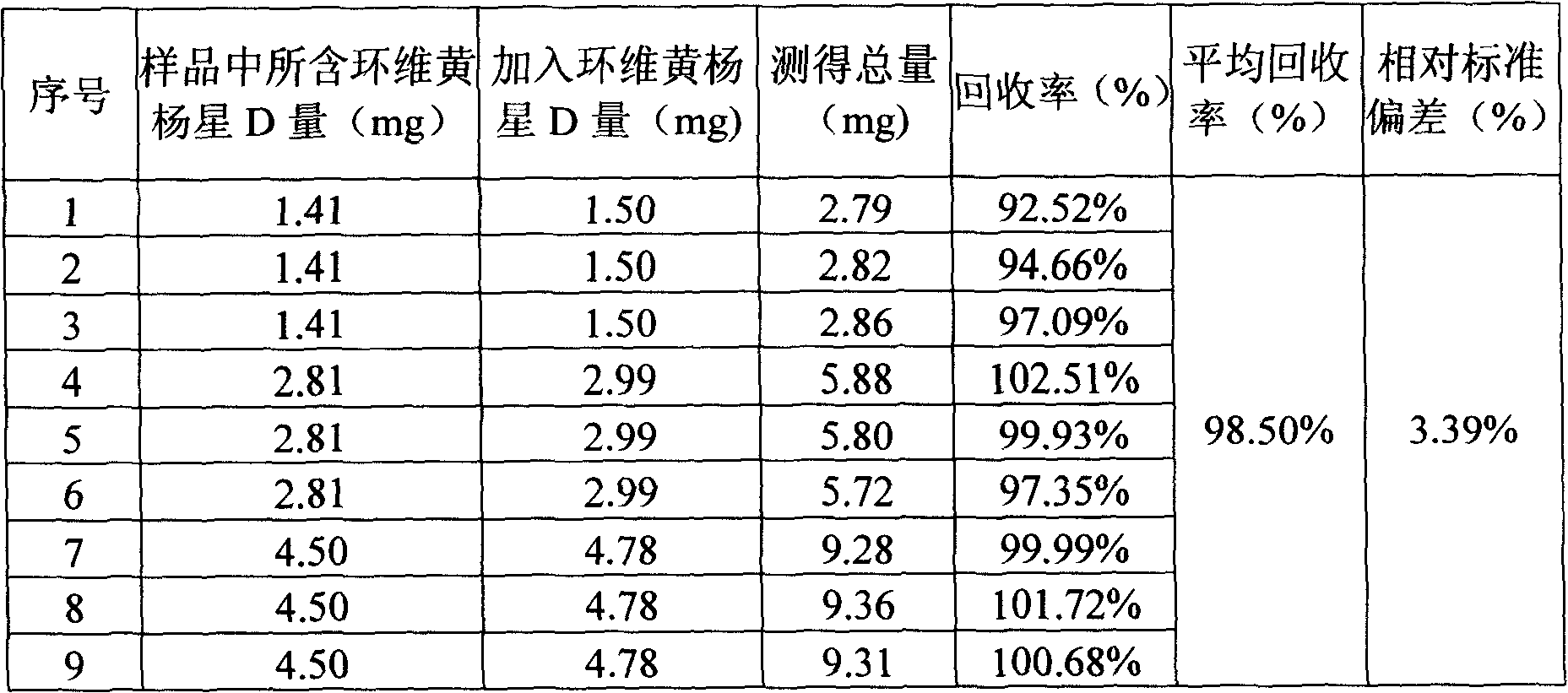
Cyclovirobuxine-D sustained-release micro-pill capsules and preparation method thereof
ActiveCN101491509AEasy to operateHigh yieldOrganic active ingredientsAerosol deliveryDibutyl sebacateAdhesive
The invention relates to a buxine slow-release micro-pill capsule and a method for preparing the same. A slow-release micro-pill consisting of a hollow pill core, a main drug layer using Cyclovirobuxine D as an active ingredient and slow-release preparation auxiliary material layer is encapsulated into a capsule to prepare the buxine slow-release micropill capsule; the hollow pill core, the Cyclovirobuxine D as a main drug and the slow-release preparation auxiliary materials comprise the following weight percent: 70 to 95 percent of the hollow pill core, 1 to 5 percent of the Cyclovirobuxine D and the 4 to 25 percent of the slow-release preparation auxiliary materials; and the slow-release preparation auxiliary materials comprise acrylic resin as an adhesive, ethyl cellulose as a coating material, dibutyl sebacate as a plasticizer and talcum powder as a lubricant for encapsulation. The method has good repeatability; and the buxine slow-release micro-pill capsule has even products, good slow release effect and high bioavailability.
Owner:HANGZHOU CONBA PHARMA
Nifedipine film-controlled slow-release pellet capsule
ActiveCN103211788AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsNifedipineEnvironmental engineering
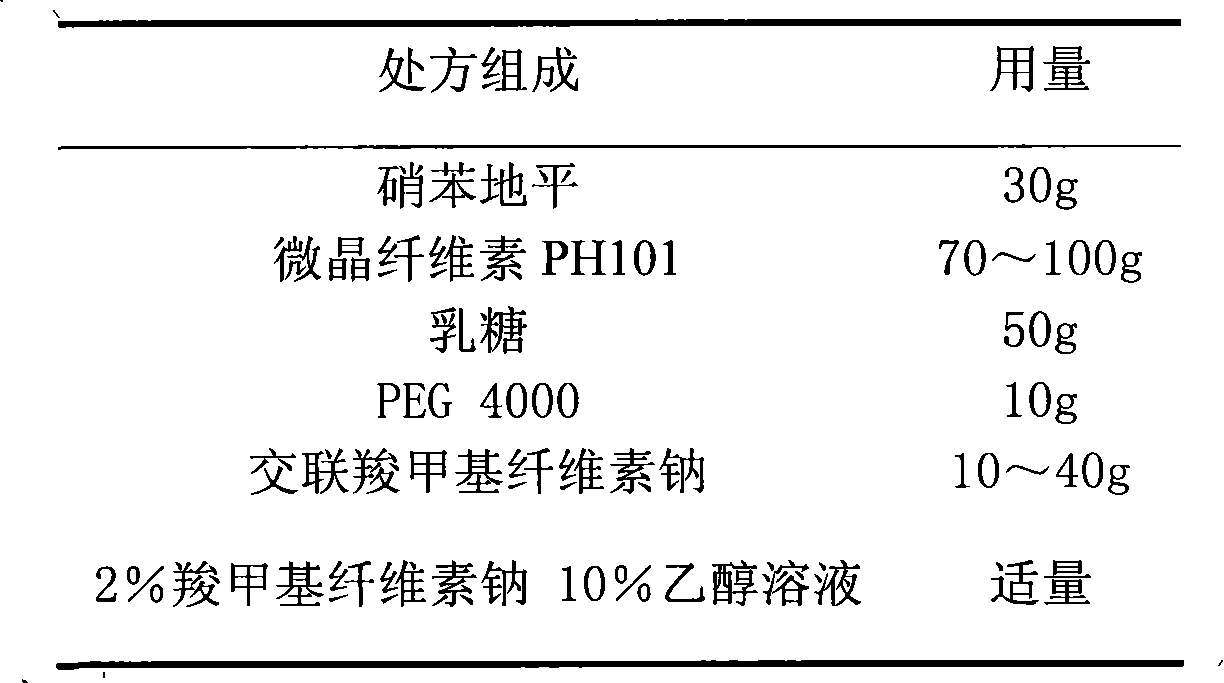
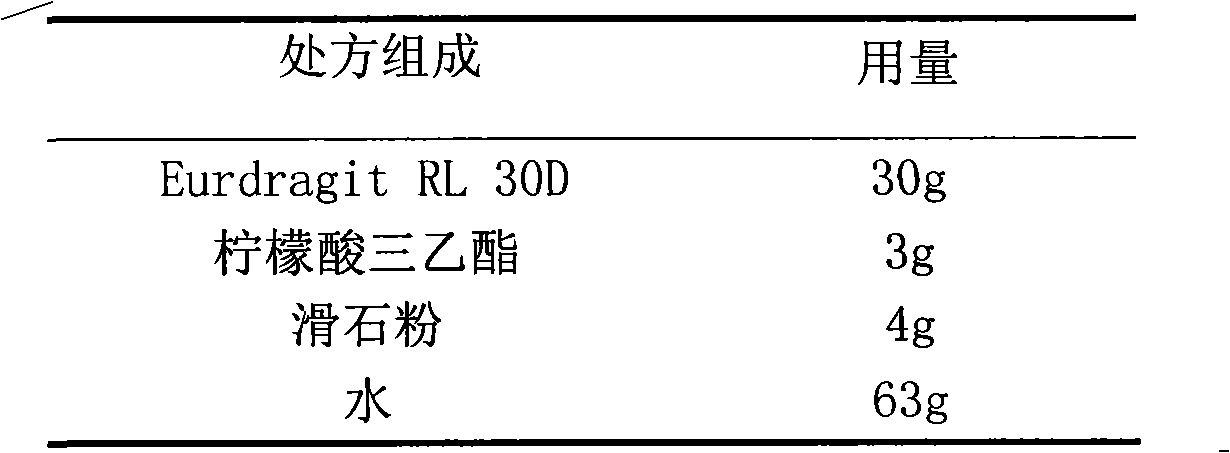
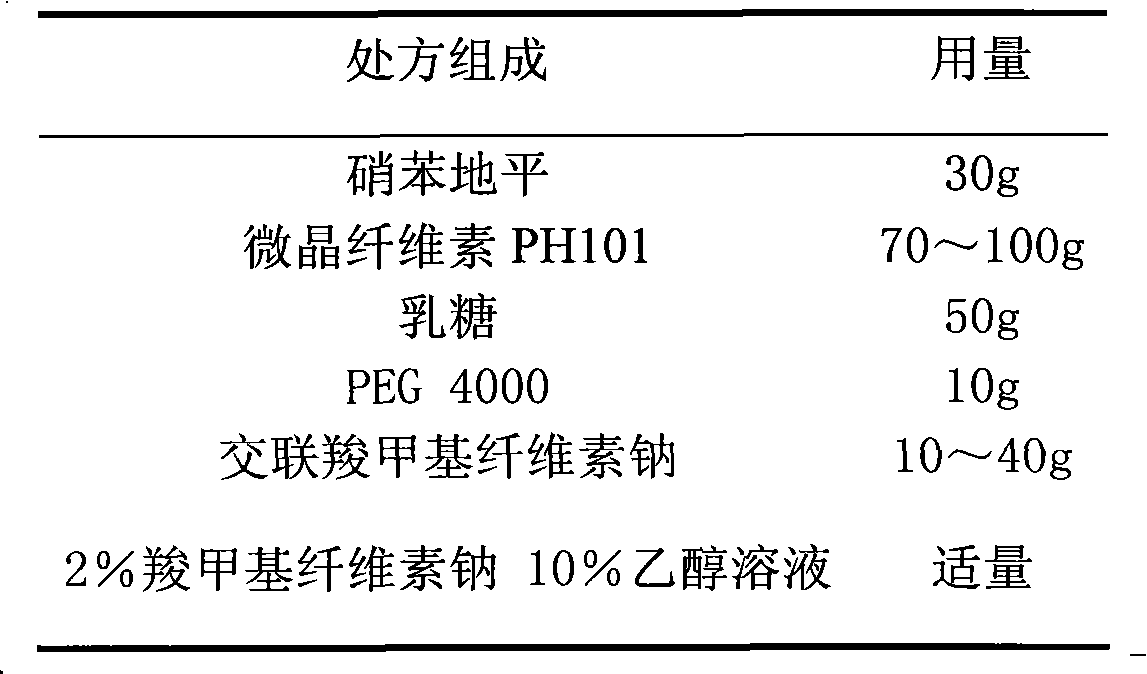
The invention relates to a nifedipine film-controlled slow-release pellet capsule. A slow-release film of the nifedipine film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the nifedipine film-controlled slow-release pellet contains croscarmellose sodium having high water expansibility. Excipients comprise microcrystalline cellulose and lactose as fillers and PEG 4000 as a solubilizer. The pellet core comprises 5 to 20wt% of croscarmellose sodium. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 21 to 35%. After absorbing water, the nifedipine film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, the nifedipine film-controlled slow-release pellet keeps stable release performances in the period of validity.
Owner:北京天衡药物研究院有限公司
Verapamil hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211797AImprove permeabilityReduce permeabilityPharmaceutical non-active ingredientsGranular deliveryVerapamil HydrochlorideExcipient
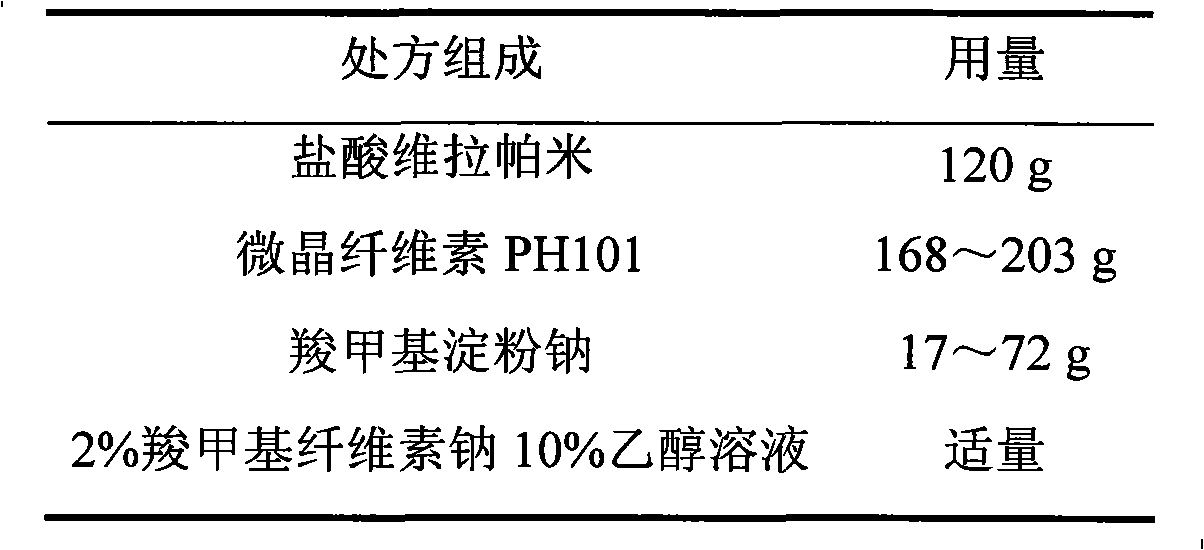
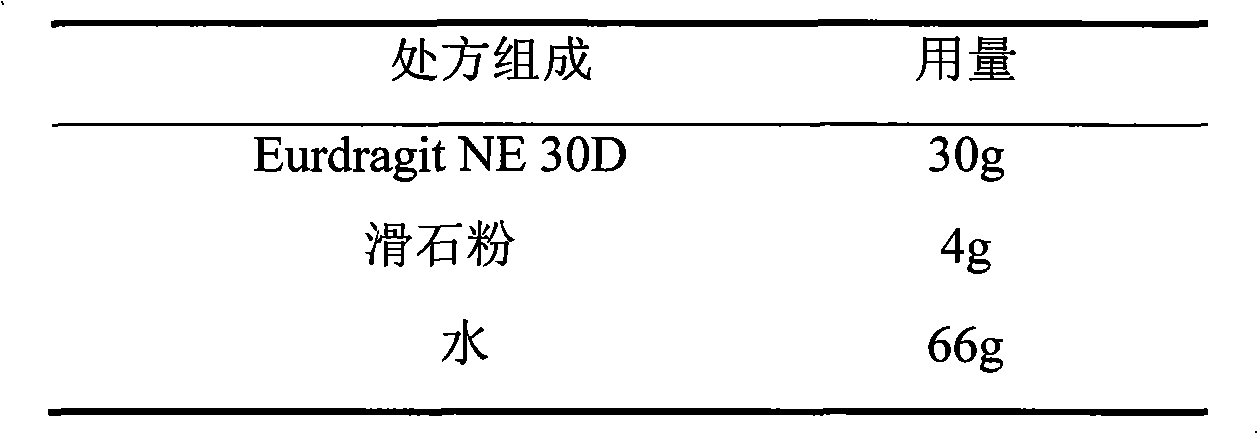
The invention relates to a verapamil hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the verapamil hydrochloride film-controlled slow-release pellet utilizes Eurdragit NE30D as a film-formation material. A pellet core of the verapamil hydrochloride film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit NE30D and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE30D to talcum powder is 30: 4 and a film weight increasing ratio is in a range of 19 to 35%. The verapamil hydrochloride film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the verapamil hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the verapamil hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Tamsulosin hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211790AAccelerated agingReduce permeabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsFiller ExcipientLactose
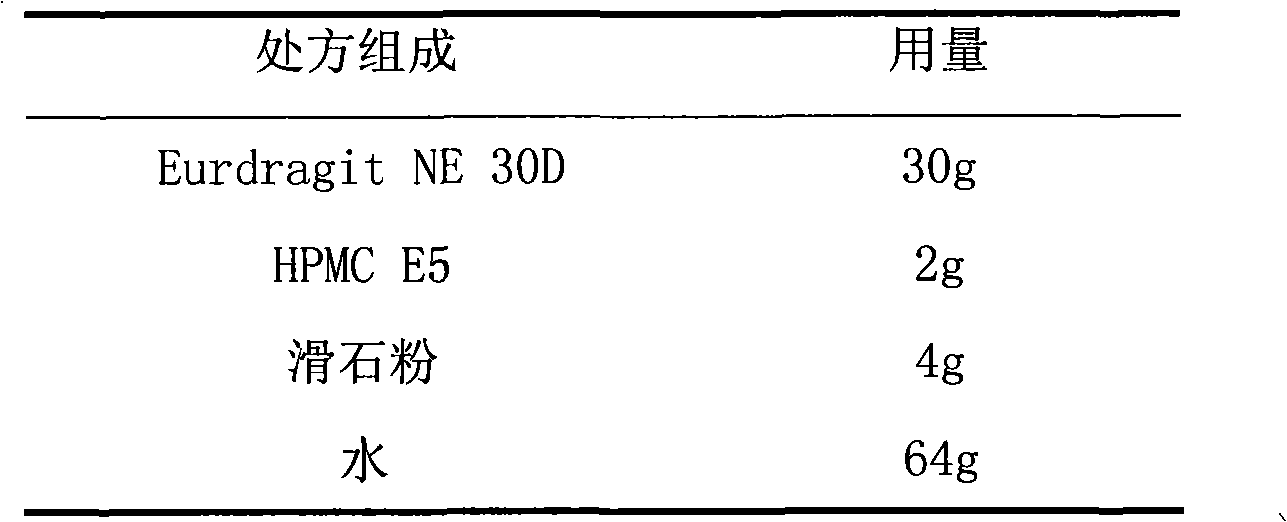
The invention relates to a tamsulosin hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the tamsulosin hydrochloride film-controlled slow-release pellet utilizes Eurdragit NE30D and HPMC E5 as film-formation materials. A pellet core of the tamsulosin hydrochloride film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose as fillers, and polysorbate 80 as a solubilizer; and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit NE30D, HPMC E5 and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE30D to HPMC E5 to talcum powder is 30: 2: 4 and a film weight increasing ratio is in a range of 19 to 36%. The tamsulosin hydrochloride film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the tamsulosin hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the tamsulosin hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Anti-depression slow-release capsule
InactiveCN103006618AGood reproducibilitySimple preparation processOrganic active ingredientsNervous disorderBlood concentrationOrganic solvent
The invention aims at providing an anti-depression slow-release capsule which is simple in preparation process, low in cost, small in pollution and high in safety. The slow-release capsule is formed by a drug-containing pill core, an isolating layer and a slow-release layer. The preparation method comprises the steps of adopting an extruding rounding method to prepare the drug-containing pill core, then coating a coating solution with the pill core together in a fluidized bed and drying to prepare the slow-release pellet. The prepared anti-depression slow-release pellet is further prepared into common capsules, and after being taken, the hold time of the effective blood concentration in the body is long and the adverse reaction is reduced. According to the invention, ethyl cellulose aqueous dispersion is adopted as the coating solution of the slow-release layer, plasticizer and antisticking agent are not needed to be added, the preparation method is simple and convenient, an organic solvent is not used in coating, the operation is simple and convenient, the cost is low, the safety is good, and no organic residue exists in the coating pellet, thereby effectively solving the problems of heavy pollution and low safety in coating of the organic solvent.
Owner:CHINA PHARM UNIV
Skeleton type roxithromycin sustained release pellet capsule
ActiveCN101224217BReduce releaseImprove bioavailabilityAntibacterial agentsOrganic active ingredientsRoxithromycinSustained release pellets
The invention provides a matrix-typed roxithromycin sustained release micropill capsule which is prepared by 60-75wt percent of roxithromycin, 2-5wt percent of waxy matrix material, 10-25wt percent of microcrystalline cellulose, lactose, cane sugar or xylose, 2-5wt percent of hydrophilic matrix material, 1-4wt percent of anhydrous sodium carbonate and 4-10wt percent of mannite according to the normal preparation method. The waxy matrix material is octodecyl alcohol, cetyl alcohol, octadecanoic acid, glycerin monostearate or hydrogenated castor oil. The hydrophilic matrix material is xanthan gum, alginic acid or carbomer 974P. The matrix material adopted in the invention delays the release of the drug, reduces the density of sustained release micropill, prolongs the staying time of the sustained release micropill in the stomach and intestinal tract and improves the bioavailability of the medicine. A comparison test between the medicine of the invention and the roxithromycin sustained release pill is carried out in eighteen testers and the test shows that the matrix-typed roxithromycin slow-release micropill capsule of the invention and the roxithromycin slow-release pill have bioequiavailability.
Owner:西安远大德天药业股份有限公司
Ketoprofen lansoprazole sustained-release pellets, as well as preparation method and preparation thereof
ActiveCN103432130AImprove stabilityImprove bioavailabilityOrganic active ingredientsAntipyreticSustained release pelletsIrritation
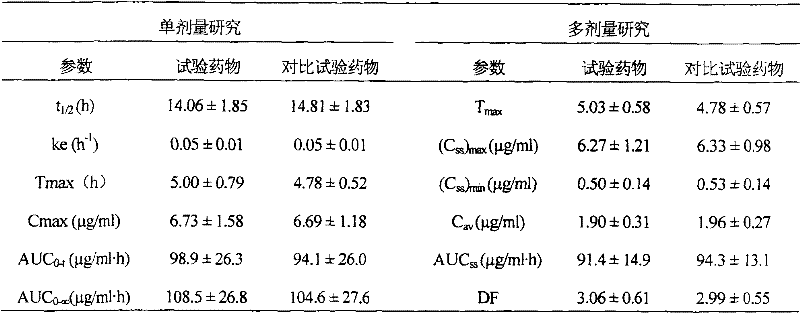
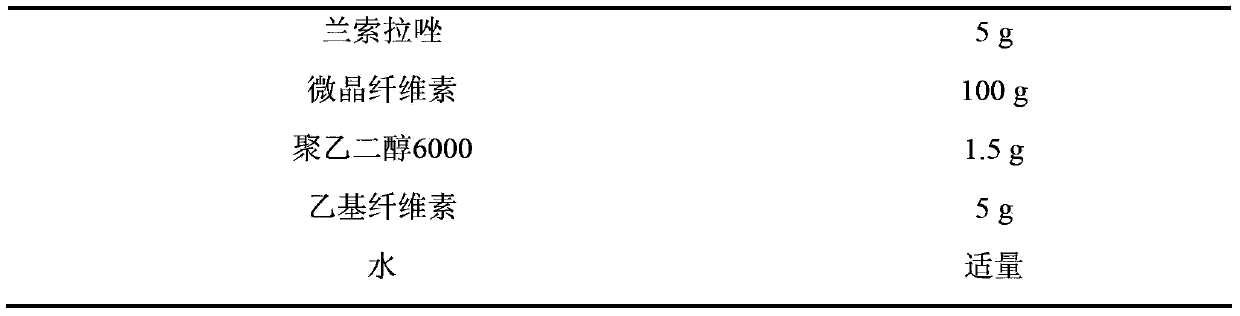
The invention discloses ketoprofen lansoprazole sustained-release pellets. The pellets comprise the following components in parts by weight: 30-50 parts of ketoprofen, 3-5 parts of lansoprazole, 60-100 parts of filler, 3-5 parts of retardant, and 0-2 parts of lubricant, wherein the filler is one or more of starch, saccharose, lactose and microcrystalline cellulose, the retardant is ethyl cellulose, and the lubricant is one or more of magnesium stearate, talcum powder and polyethylene glycol 6000. Compound sustained-release pellets prepared from ketoprofen and lansoprazole have the advantages of high bioavailability, small local irritation, uniform medicine absorption speed and the like; the sustained-release pellets are coated, so that the medicament stability can be improved, the bitter taste of the pellets can be effectively covered, and the compliance of patient administration can be strengthened; sustained-release pellet tablets or sustained-release pellet capsules which are further prepared from the sustained-release pellets or coated pellets are convenient to carry and take; ketoprofen lansoprazole sustained-release pellets prepared by adopting an extrusion spheronization method or centrifugal granulation method are simple in operation, easy to control quality, narrow in size distribution range, good in roundness and smooth in surface, and are suitable for further coating, subpackaging or preparing.
Owner:SOUTHWEST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com