Linaclotide enteric controlled-release pellet capsule preparation and preparing method and application thereof
A technology of linaclotide enteric and sustained-release pellets, which can be applied to pharmaceutical formulations, peptide/protein components, and medical preparations containing active ingredients, etc., and can solve problems such as easy missed doses and ineffective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
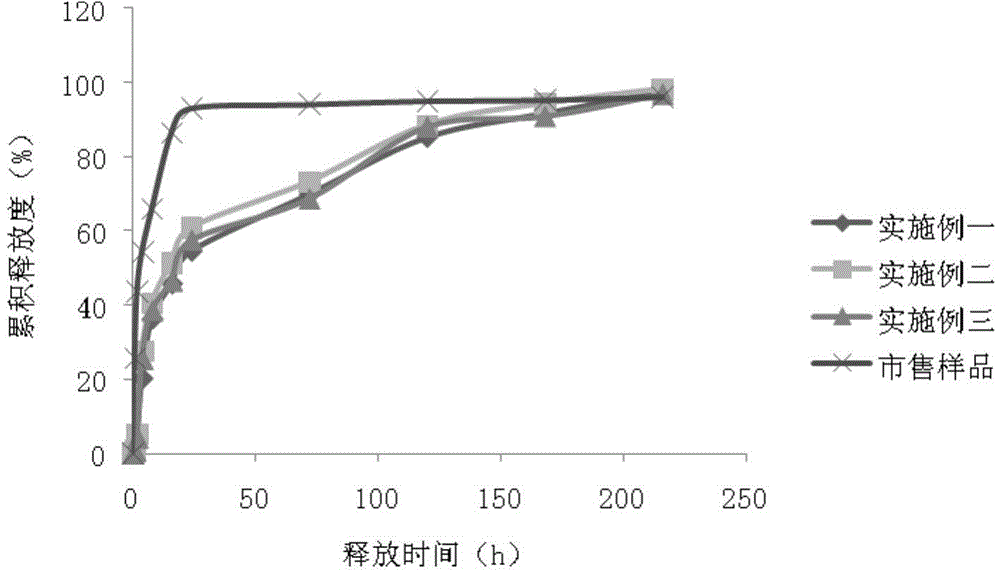
Image
Examples
Embodiment 1
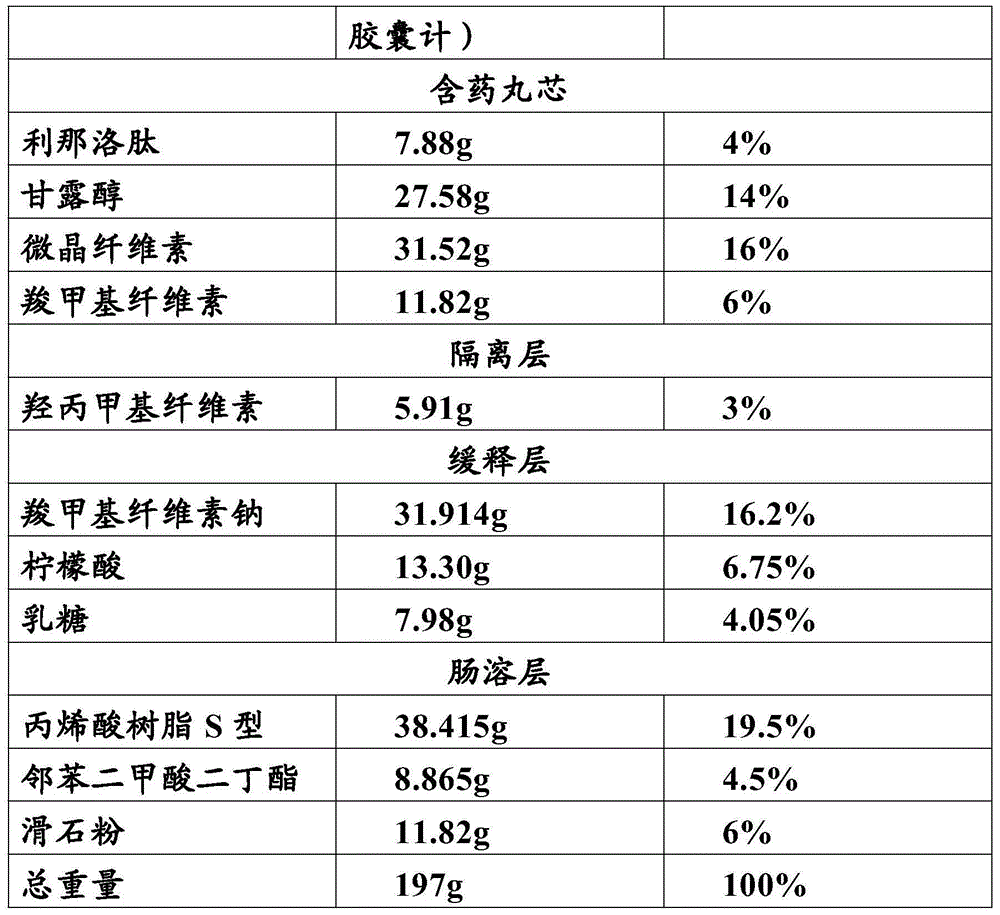
[0034] 1. Prescription
[0035]
[0036]
[0037] 2. Preparation process
[0038] Drug-containing pill core: Mix linaclotide, mannitol and microcrystalline cellulose evenly in an equal increasing method, add carboxymethyl cellulose solution dissolved in water to make a soft material, and put it in a multifunctional pill coating machine ( Purchased from Shenzhen Xinyite Technology Co., Ltd., the model is Mini250), extruded into a cylindrical strip, and then added to a multi-functional pellet coating machine and rolled into a 15-35-mesh drug-containing pellet core;
[0039] Isolation layer: Dissolve hydroxypropyl methylcellulose in water to prepare a 12% coating solution, then place the pill core in a multifunctional pill coating machine for coating, and then place the micropills wrapped in the isolation layer Dry in an electric blast drying oven (purchased from Shanghai Yiheng Scientific Instrument Co., Ltd., model DHG-9245A) at 45°C for 4 hours;
[0040] Sustained-rele...
Embodiment 2
[0045] 1. Prescription
[0046]
[0047] 2. Preparation process
[0048] Pill core with medicine: Mix linaclotide, starch and calcium chloride uniformly in equal increments, add hydroxypropyl methylcellulose solution dissolved in water to make a soft material, and put it in a multi-functional pill-making coating machine Extruded into cylindrical strips, then added to a multi-functional pellet coating machine and rounded to form a 15-35-mesh drug-containing pellet core;
[0049] Isolation layer: Dissolve polyvinylpyrrolidone in water to prepare a 12% coating solution, then place the pill core in a multifunctional pellet coating machine for coating, and then place the micropills wrapped in the isolation layer at 45°C Dry in an electric blast drying oven for 4 hours;
[0050] Sustained-release coating layer: gelatin, polyethylene glycol and silicon dioxide are dissolved in absolute ethanol to prepare a 10% coating solution, and the above-mentioned pellets are coated in a mul...
Embodiment 3
[0054] 1. Prescription
[0055]
[0056] 2. Preparation process
[0057] Drug-containing pill core: Mix linaclotide, dextrin and calcium hydrogen phosphate uniformly in an equal increasing method, add carboxymethyl cellulose solution dissolved in water to make a soft material, and put it in a multi-functional pill-making coating machine Extruded into cylindrical strips, then added to a multi-functional pellet coating machine and rounded to form a 15-35-mesh drug-containing pellet core;
[0058] Isolation layer: Dissolve ethyl cellulose in water, prepare 12% coating liquid, then place the pill core containing the pills in a multifunctional pellet coating machine for coating, then place the micropills wrapped by the isolation layer at 45 ℃ drying in an electric blast drying oven for 4 hours;
[0059] Sustained-release coating layer: Sodium alginate, citric acid and starch were dissolved in absolute ethanol to prepare a 10% coating solution, and the above-mentioned pellets w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com