Skeleton type roxithromycin sustained release pellet capsule
A technology of roxithromycin and sustained-release pellets, which is applied in the direction of medical preparations of non-active ingredients, organic active ingredients, oil/fat/wax non-effective ingredients, etc., and can solve the problem that the slow-release pellets cannot float and release Poor stability, long production process, etc., to achieve the effect of improving bioavailability, delaying release, and prolonging residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Taking the production of 1000 skeleton-type roxithromycin sustained-release pellet capsule products of the present invention as an example, the used bulk drug and auxiliary materials and their weight ratios are:
[0037] Roxithromycin 150g
[0038] Octadecanol 4.29g
[0039] Microcrystalline Cellulose 38.57g
[0040] Anhydrous sodium carbonate 4.29g
[0041] Carbomer 974P 4.29g
[0042] Mannitol 12.86g
[0043] In the present embodiment, the weight percent distribution ratio of each raw material is:
[0044] Roxithromycin 70%
[0045] Stearyl Alcohol 2%
[0046] Microcrystalline Cellulose 18%
[0047] Anhydrous Sodium Carbonate 2%
[0048] Carbomer 974P 2%
[0049] Mannitol 6%
[0050] Its preparation method is as follows:
[0051] Roxithromycin was crushed through a 120-mesh sieve, stearyl alcohol was crushed through a 80-mesh sieve, each component was weighed according to the above ratio, mixed evenly, and about 80 g of purified water was added to prepare a...
Embodiment 2
[0055] Taking the production of 1000 skeleton type roxithromycin sustained-release pellet capsule products of the present invention as an example, used bulk drug and adjuvant and weight ratio thereof are:
[0056] Roxithromycin 150g
[0057] Octadecanol 5g
[0058] Microcrystalline Cellulose 62.5g
[0059] Carbomer 974P 5g
[0060] Anhydrous sodium carbonate 2.5g
[0061] Mannitol 25g
[0062] In the present embodiment, the weight percent distribution ratio of each raw material is:
[0063] Roxithromycin 60%
[0064] Stearyl Alcohol 2%
[0065] Microcrystalline Cellulose 25%
[0066] Carbomer 974P 2%
[0067] Anhydrous Sodium Carbonate 1%
[0068] Mannitol 10%
[0069] Its preparation method is identical with embodiment 1. The prepared skeleton-type roxithromycin sustained-release pellet capsules each contain 150 mg of roxithromycin.
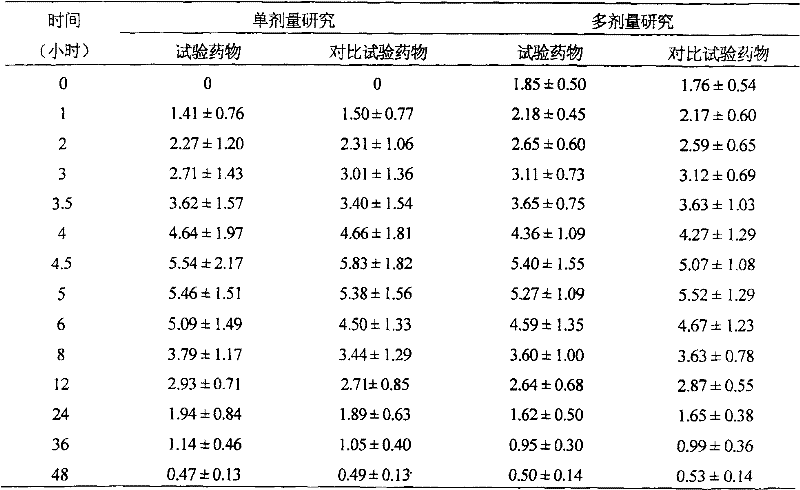
[0070] Determination of the release rate, the assay method was the same as in Example 1, and the measurement results: the release r...
Embodiment 3
[0072] Taking the production of 1000 skeleton-type roxithromycin sustained-release pellet capsule products of the present invention as an example, the used bulk drug and auxiliary materials and their weight ratios are:
[0073] Roxithromycin 150g
[0074] Octadecanol 10g
[0075] Microcrystalline Cellulose 20g
[0076] Carbomer 974P 10g
[0077] Anhydrous sodium carbonate 2g
[0078] Mannitol 8g
[0079] In the present embodiment, the weight percent distribution ratio of each raw material is:
[0080] Roxithromycin 75%
[0081] Stearyl Alcohol 5%
[0082] Microcrystalline Cellulose 10%
[0083] Carbomer 974P 5%
[0084] Anhydrous Sodium Carbonate 1%
[0085] Mannitol 4%
[0086] Its preparation method is identical with embodiment 1. The prepared skeleton-type roxithromycin sustained-release pellet capsules each contain 150 mg of roxithromycin.
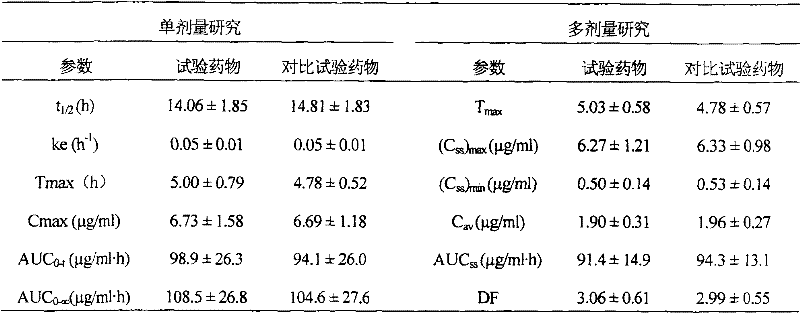
[0087] Determination of the release rate, the assay method was the same as in Example 1, and the measurement results: the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com