Choline fenofibrate film-controlled enteric slow-release pellet capsule
A technology of choline fenofibrate and sustained-release pellets, which can be used in medical preparations with non-active ingredients, metabolic diseases, organic active ingredients, etc., and can solve problems such as decline, membrane aging and release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Choline fenofibrate enteric-coated sustained-release pellet capsules with common ball core
[0036] 1. Prescription
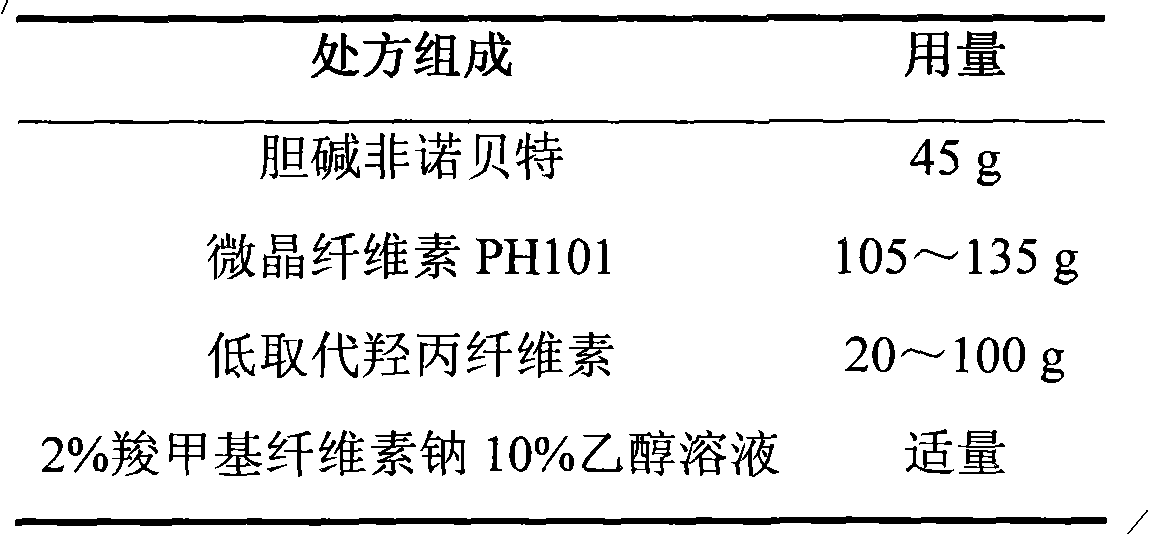
[0037] 1. Pill core prescription (1000 capsules)
[0038]
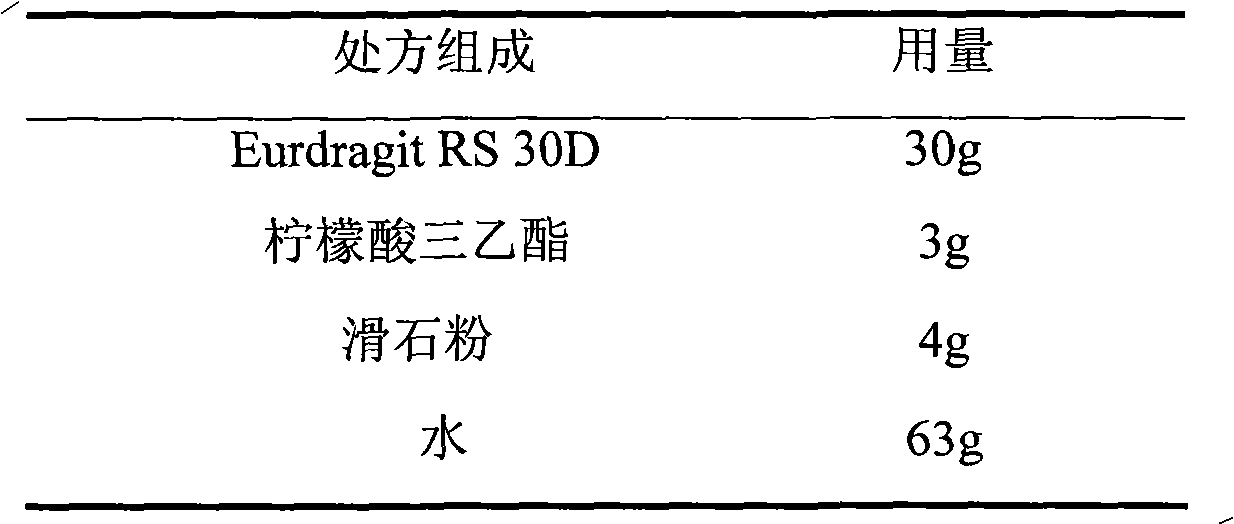
[0039] 2. Prescription of sustained-release film coating solution
[0040]
[0041]
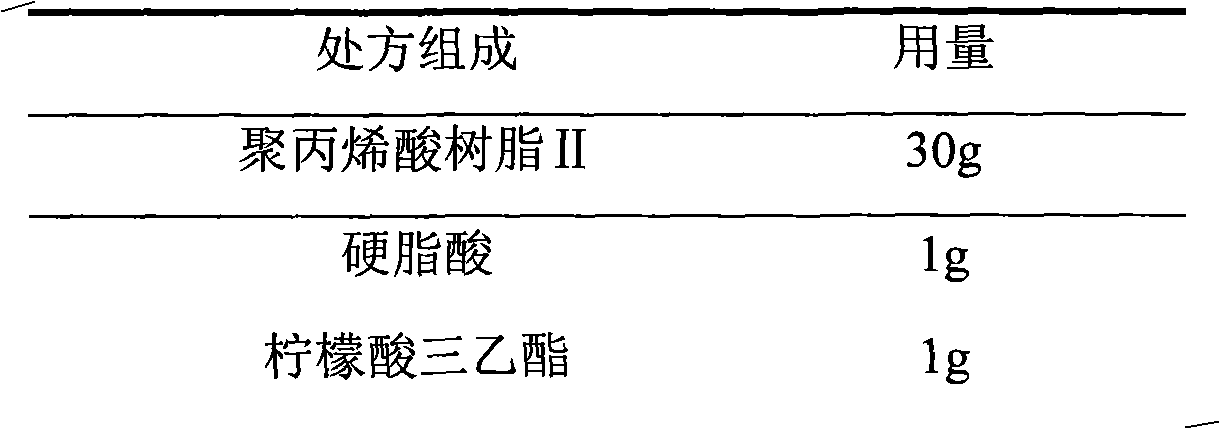
[0042] 3. Prescription of enteric coating solution
[0043]
[0044] 4. No. 0 stomach-soluble gelatin capsule shell 1000 capsules
[0045] Second, the preparation process:
[0046] 1. Ball core preparation process:
[0047] (1) Choline fenofibrate is passed through a 60 mesh sieve;
[0048] (2) Take choline fenofibrate and microcrystalline cellulose PH101 of prescription quantity, put in wet granulator and mix uniformly;
[0049] (3) 2% carboxymethylcellulose sodium 10% ethanol solution to make soft materials;
[0050] (4) Extrude on the extruder, the screen aperture is 1.0mm, and the extrusion speed is 20-30rpm;
[0051] (5) spheronization, the spheronization speed is 900~1000rpm,...
Embodiment 2
[0079] Example 2 Choline fenofibrate enteric-coated sustained-release pellet capsule containing 10% low-substituted hydroxypropyl cellulose
[0080] 1. Prescription
[0081] 1. Pill core prescription (1000 capsules)
[0082]
[0083] 2. Prescription of sustained-release film coating solution: same as in Example 1
[0084] 3. Prescription of enteric coating film coating solution: same as Example 1
[0085] 4. No. 0 stomach-soluble gelatin capsule shell 1000 capsules
[0086] Second, the preparation process:
[0087] 1. Ball core preparation process:
[0088] (1) Choline fenofibrate is passed through a 60 mesh sieve;
[0089] (2) Choline fenofibrate, microcrystalline cellulose PH101, and low-substituted hydroxypropyl cellulose are taken by weighing the prescription amount, and mixed uniformly in a wet granulator;
[0090] (3) 2% carboxymethylcellulose sodium 10% ethanol solution to make soft materials;
[0091] (4) Extrude on the extruder, the screen aperture is 1.0mm,...
Embodiment 3
[0113] Example 3 Choline fenofibrate enteric-coated sustained-release pellet capsules containing 20% low-substituted hydroxypropyl cellulose
[0114] 1. Prescription
[0115] 1. Pill core prescription (1000 capsules)
[0116]
[0117] 2. Prescription of sustained-release film coating solution: same as in Example 1
[0118] 3. Prescription of enteric coating film coating solution: same as Example 1
[0119] 4. No. 0 stomach-soluble gelatin capsule shell 1000 capsules
[0120] Second, the preparation process:
[0121] 1. Ball core preparation process:
[0122] (1) Choline fenofibrate is passed through a 60 mesh sieve;
[0123] (2) Choline fenofibrate, microcrystalline cellulose PH101, and low-substituted hydroxypropyl cellulose are taken by weighing the prescription amount, and mixed uniformly in a wet granulator;
[0124] (3) 2% carboxymethylcellulose sodium 10% ethanol solution to make soft materials;
[0125] (4) Extrude on the extruder, the screen aperture is 1.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com