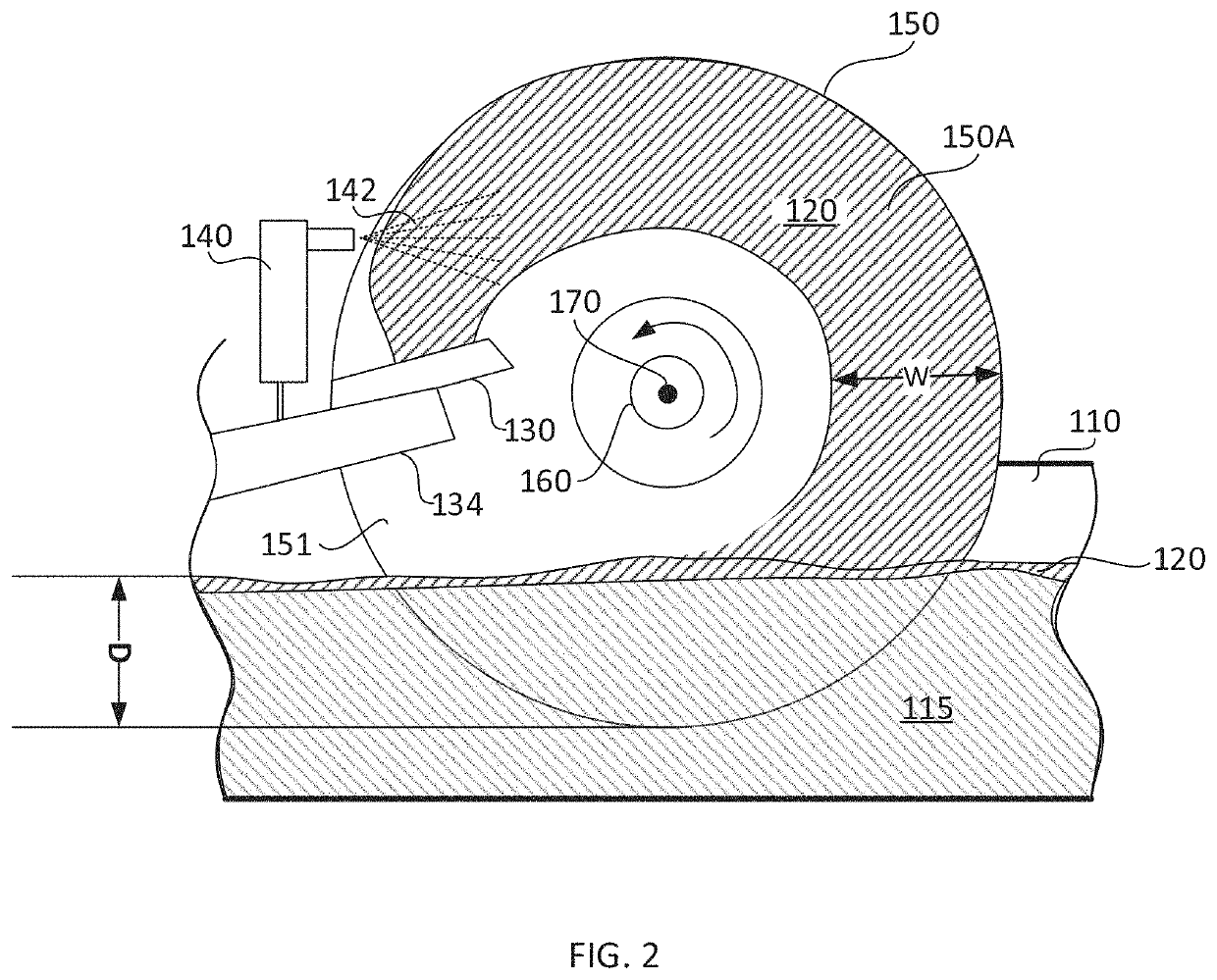
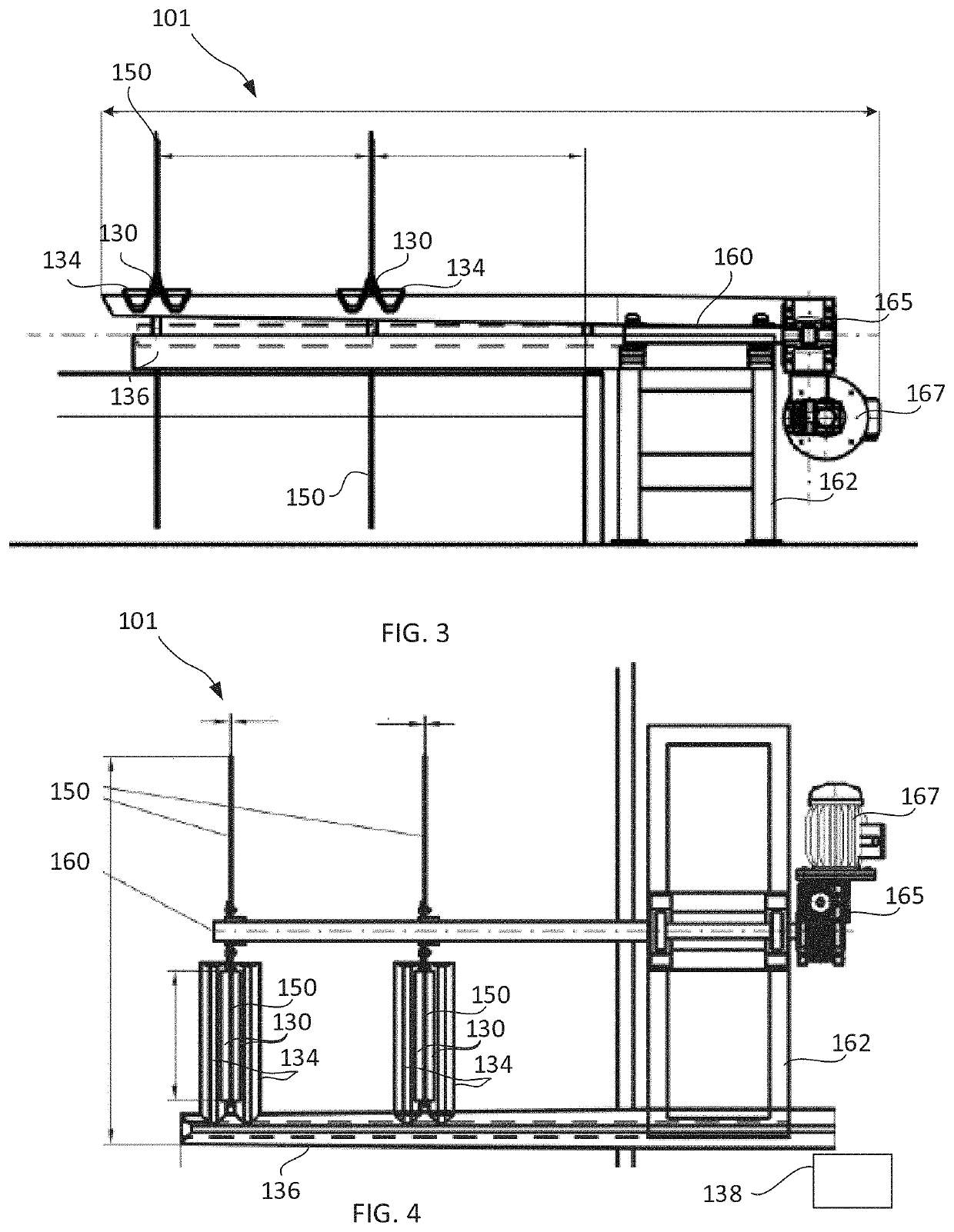
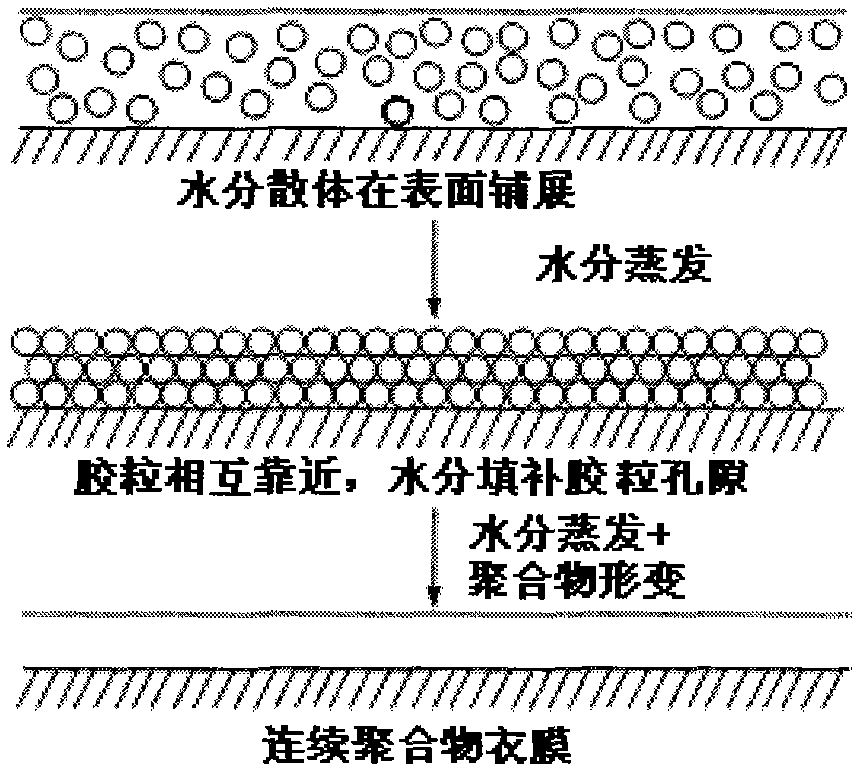
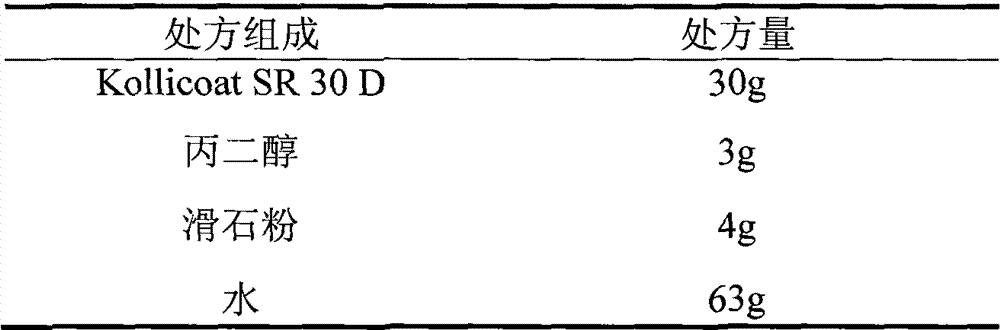
Patents
Literature
43results about How to "Small residual" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
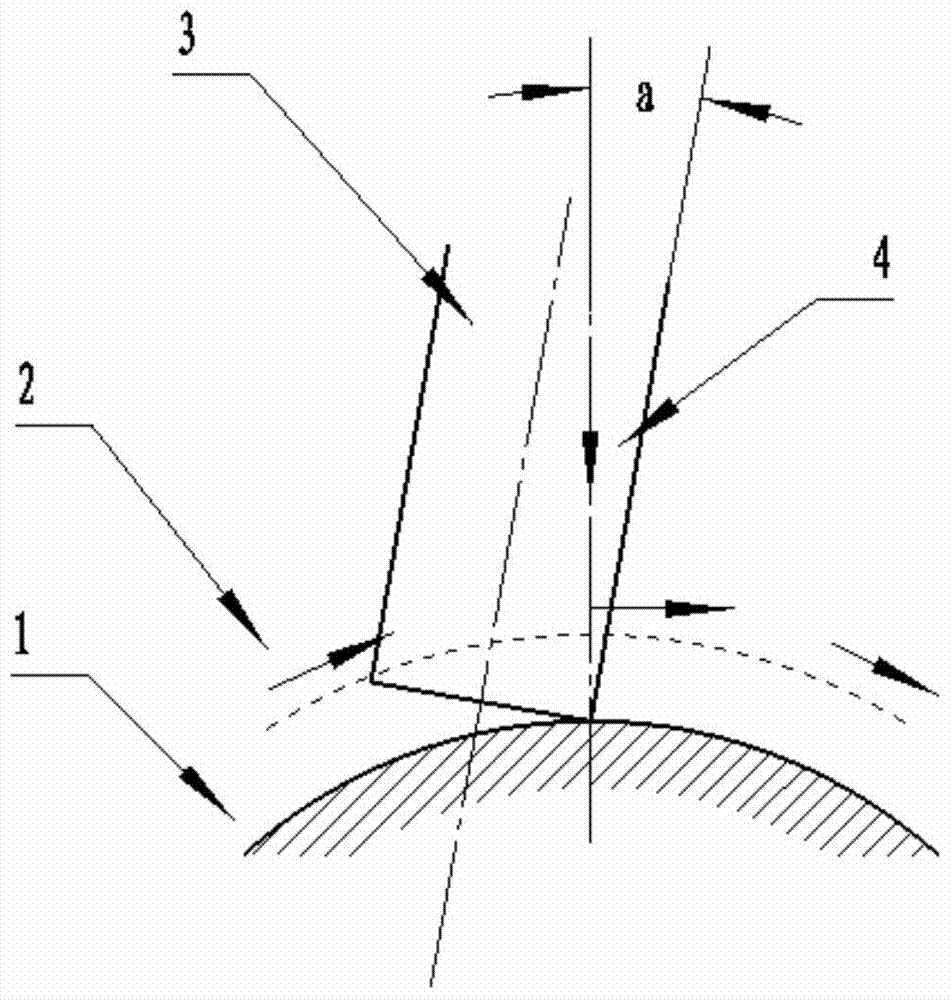
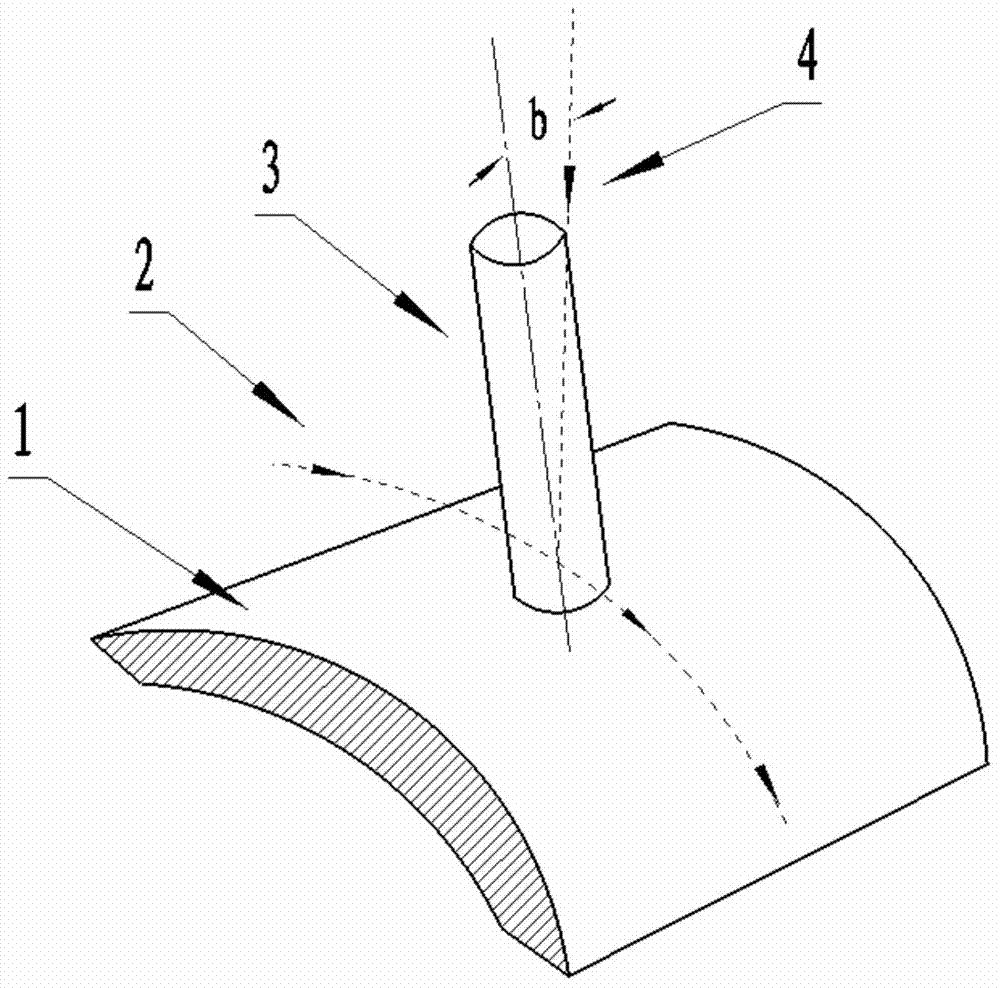
Multi-axis curved surface type numerically-controlled method for machining complicated curved surface part
ActiveCN103537743AImprove cutting efficiencySmall residualAutomatic control devicesFeeding apparatusNumerical controlMilling cutter
The invention discloses a multi-axis curved surface type numerically-controlled method for machining a complicated curved surface part. According to the method disclosed by the invention, the axis of a cutter can be arranged according to the directions of longitude and latitude normal lines for forming a curved surface, an inclined angle is adjusted to generate a cutter path, and the machining efficiency and the part surface smoothness can be improved. The numerically-controlled method can be realized by the following technical scheme: a part is fixed in a rotary center of a five-shaft machine tool; a curved surface is reconstructed according to the curvature change, the normal line direction of the curved surface and a machining path; a space change of an axis vector of the cutter is controlled by adjusting the front inclined angle and the side inclined angle of a cutter shaft of a flat-bottom end mill so that the cutter always keeps that a cutter tip participates in curved surface cutting in the five-axis continuous cutting process; a curved surface projection machining manner is adopted in the programming process so that the mill can carry out multi-axis linked milling machining according to the curvature change of the curved surface; the axis of the cutter is similar with a relatively small front inclined angle alpha overlapped to the normal line direction of the cutter path and a side inclined angle is set as 0; the axis of the cutter is kept vertical to the direction of the machining path to implement a five-axis linked machining numerical control procedure.
Owner:四川泛华航空仪表电器有限公司
Indoor disinfectant and preparation method thereof
The invention discloses an indoor disinfectant and a preparation method thereof and relates to the technical field of disinfectants. The indoor disinfectant comprises the following components: 90-110 parts of Chinese herbal medicine extract, 40-50 parts of borneol, 30-40 parts of bamboo carbon powder, 20-30 parts of essential oil components, 20-30 parts of nano-zinc oxide, 10-15 parts of fatty alcohol-polyoxyethylene ether, 2-10 parts of a cationic surfactant, 2-8 parts of a buffering agent, 2-4 parts of a solubilizer and 40-50 parts of deionized water. The indoor disinfectant disclosed by the invention has the characteristics of being zero in toxic or side effect, zero in residue and low in irritation and the like, has high safety, can be directly contacted with skin when sprayed in air, is non-irritating to skin, has broad spectrum bactericidal performance and high stability and has excellent effect of killing bacteria, fungi and moulds.
Owner:HEFEI HUAGAI BIOTECH CO LTD
Acipimox film-controlled slow-release pellet capsule
ActiveCN103211785AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderMedicineLactose
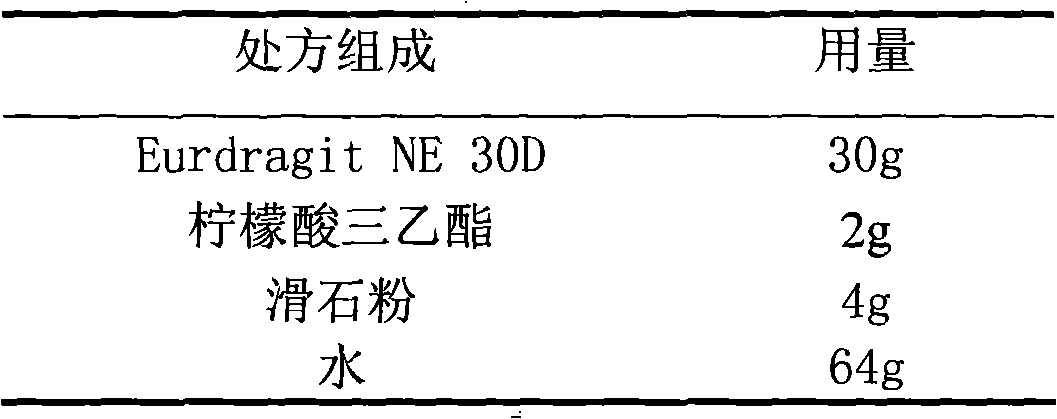
The invention relates to an acipimox film-controlled slow-release pellet capsule. A slow-release film of the acipimox film-controlled slow-release pellet utilizes Eurdragit NE 30D as a film-formation material. A pellet core of the acipimox film-controlled slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains pharmaceutically acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises the Eurdragit NE 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE 30D to triethyl citrate to talcum powder is 30: 2: 4 and coating weight gain is in a range of 20 to 39%. The acipimox film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the acipimox film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the acipimox film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept prior to expiration date.
Owner:北京天衡药物研究院有限公司
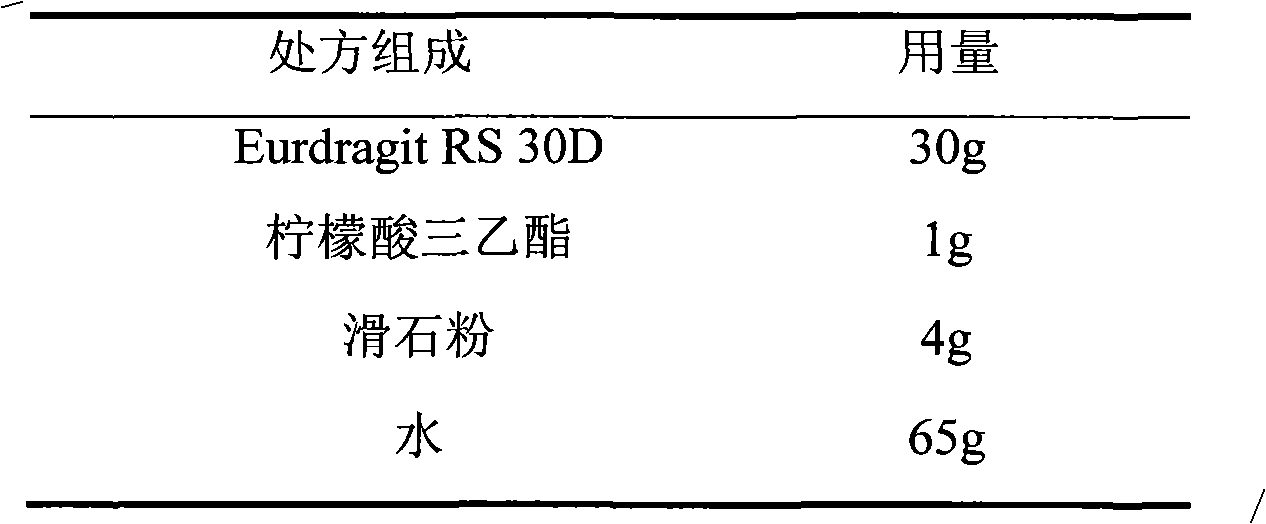
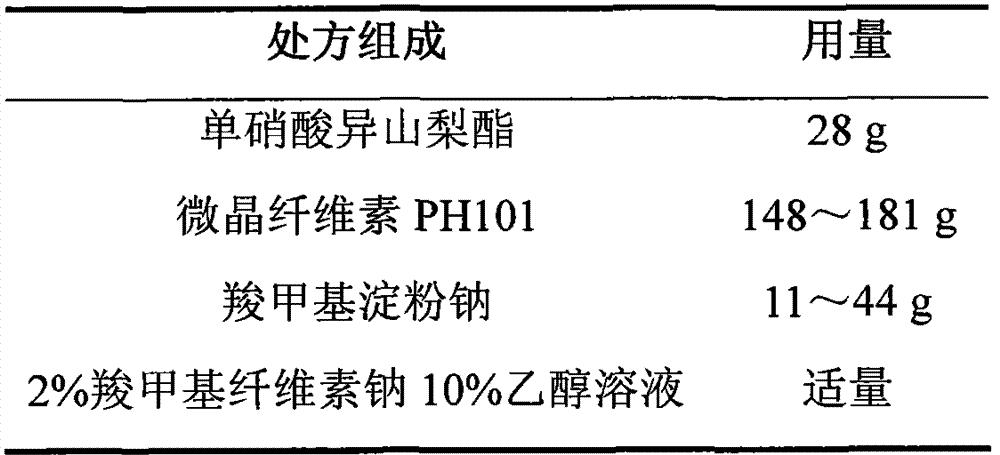
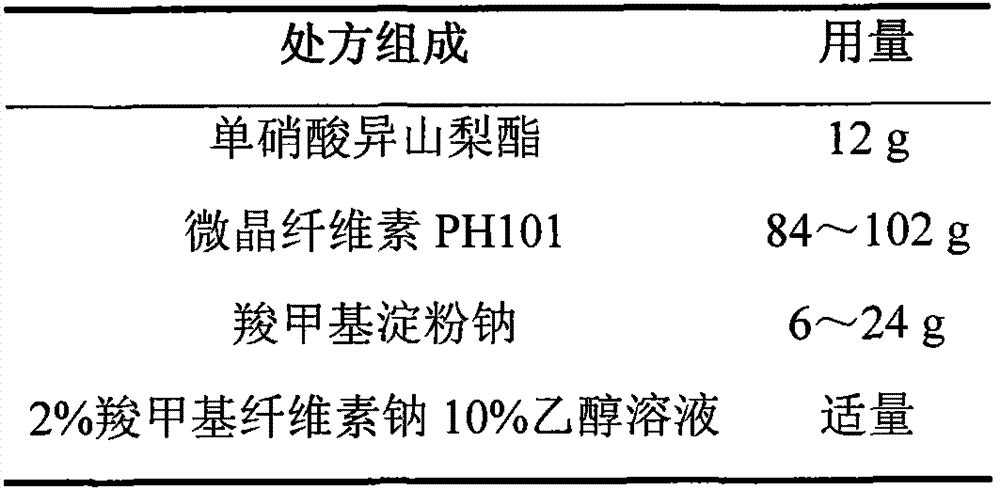
Isosorbide mononitrate sustained-release pellet, and isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it
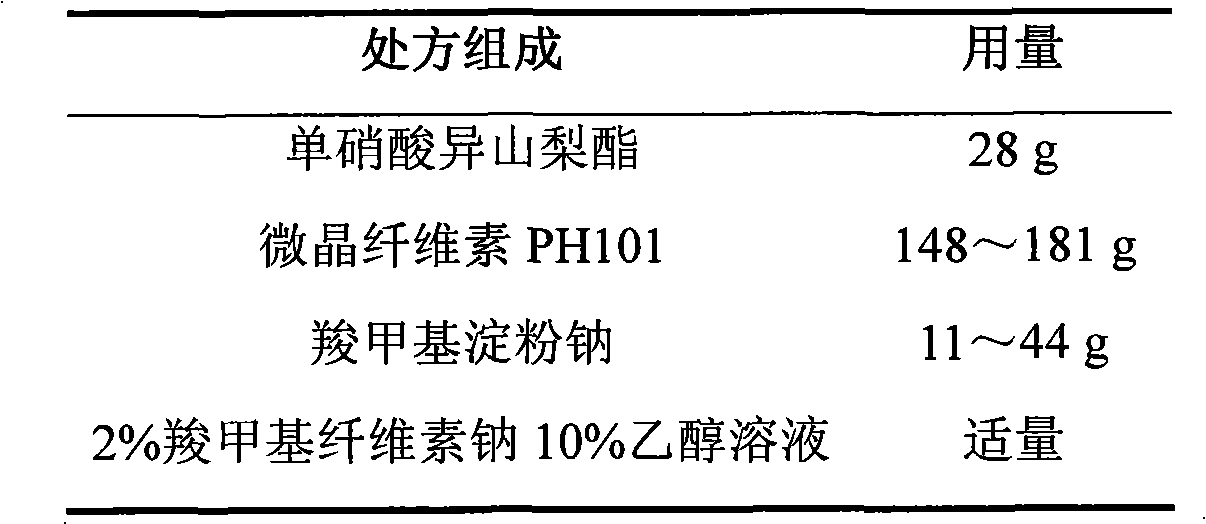
ActiveCN103211768AAccelerated agingReduce permeabilityPharmaceutical non-active ingredientsGranular deliverySustained release pelletsMedicine
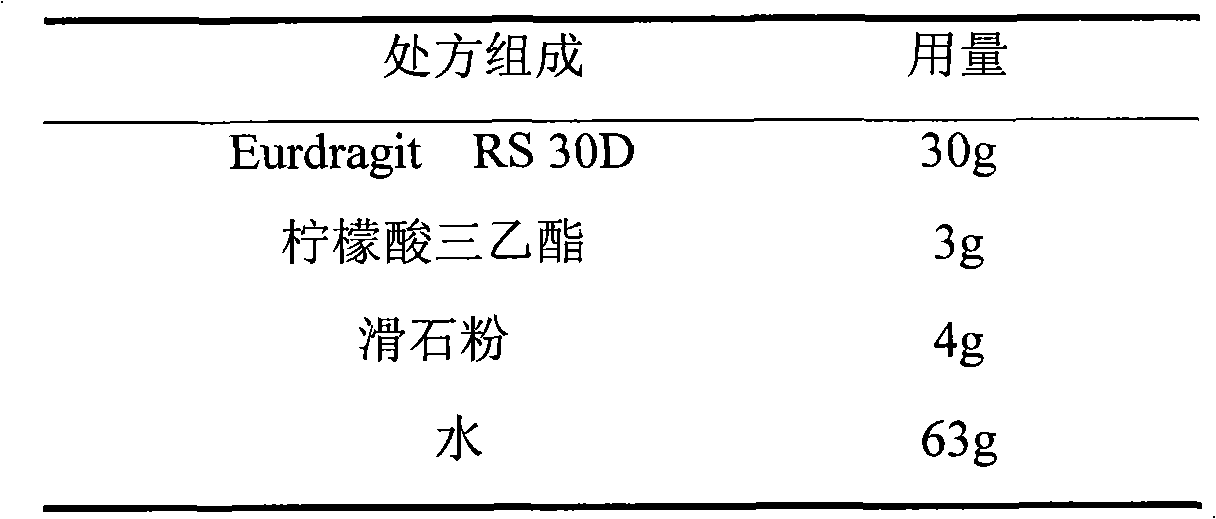
The invention relates to an isosorbide mononitrate sustained-release pellet, and an isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it. The sustained-release film of the sustained-release pellet adopts Eurdragit RS 30D as a film forming material, the core of the sustained-release pellet contains high-expansibility sodium carboxymethyl starch and a pharmaceutically-acceptable excipient commonly used for sustained-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of sodium carboxymethyl starch in the core of the sustained-release pellet is 5-20%. The sustained-release film of the sustained-release pellet includes the Eurdragit RS 30D, a plasticizer triethyl citrate and an anti-adherent talcum powder, the optimal ratio of the Eurdragit RS 30D to triethyl citrate to the talcum powder is 30:3:4, and the optimal coating weight gain is 19-38%. The core will obviously expand after absorbing water because of the containment of sodium carboxymethyl starch highly expanding after contacting with water, so the sustained-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:北京天衡药物研究院有限公司
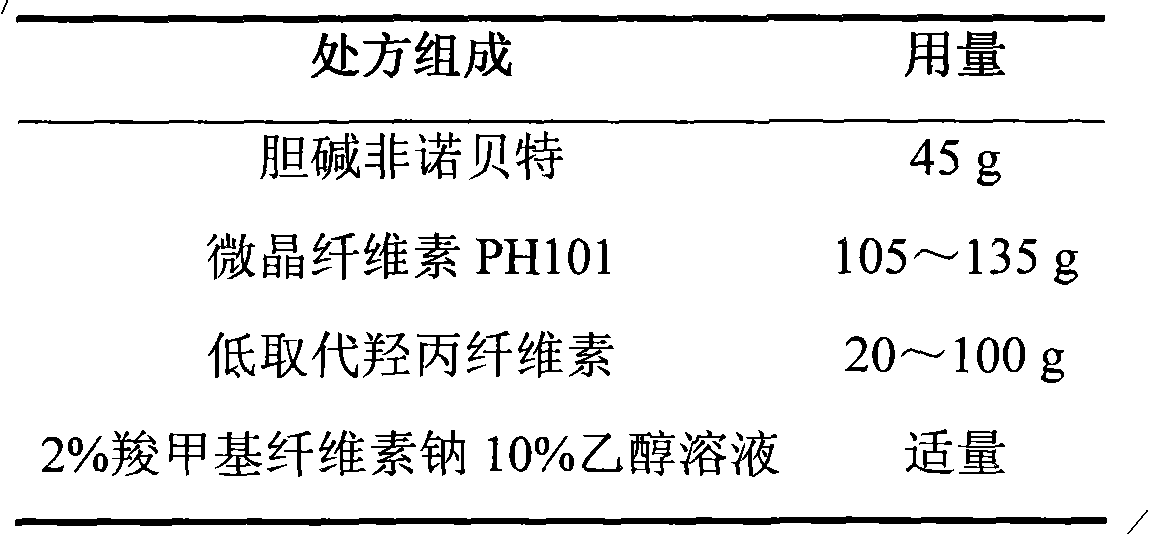
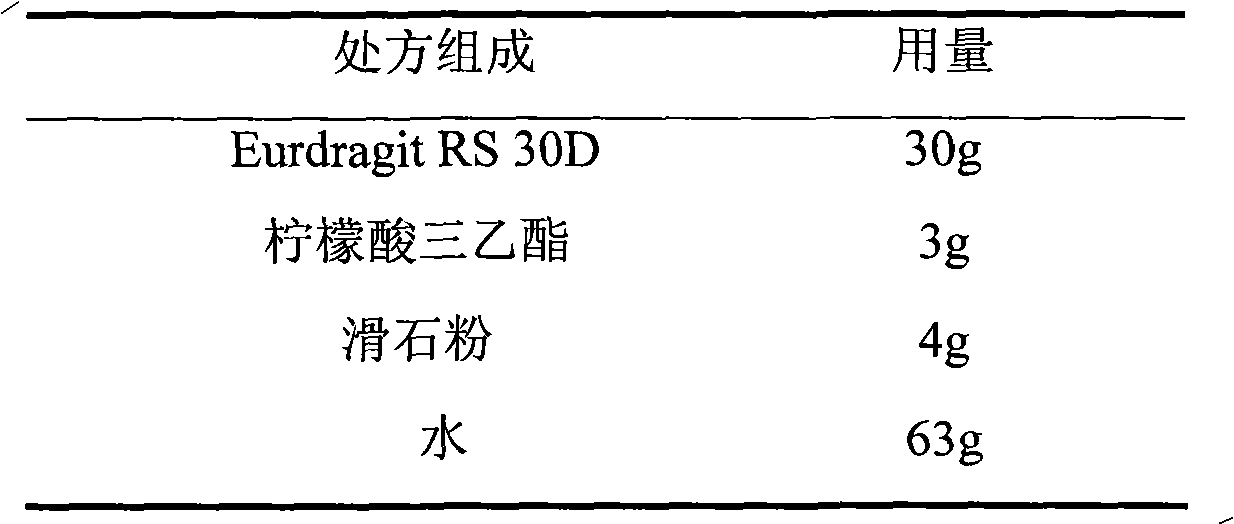
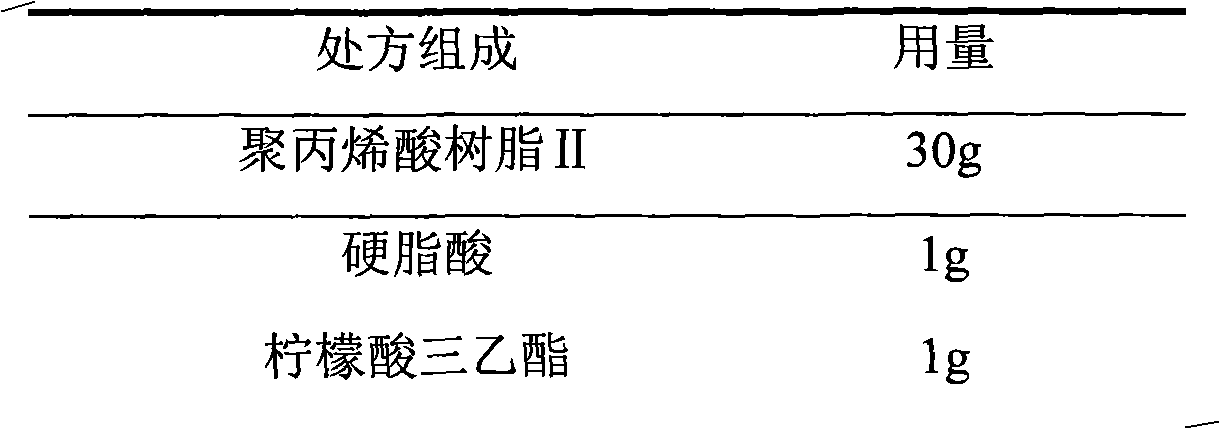
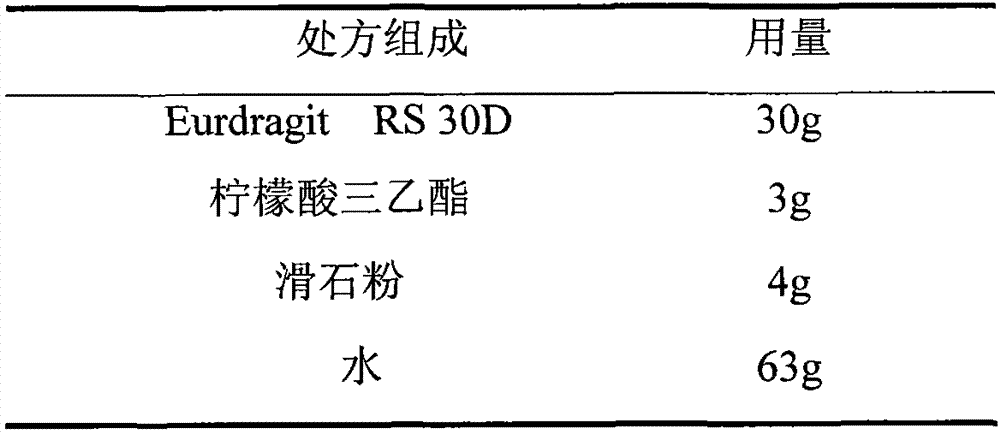
Choline fenofibrate film-controlled enteric slow-release pellet capsule
ActiveCN103211786AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderSustained release pelletsMedicine
The invention relates to a choline fenofibrate film-controlled enteric slow-release pellet capsule. A slow-release film of the choline fenofibrate film-controlled enteric slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the choline fenofibrate film-controlled enteric slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains a pharmaceutically acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of urdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 20 to 38%. The choline fenofibrate film-controlled enteric slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the choline fenofibrate film-controlled enteric slow-release pellet expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the choline fenofibrate film-controlled enteric slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
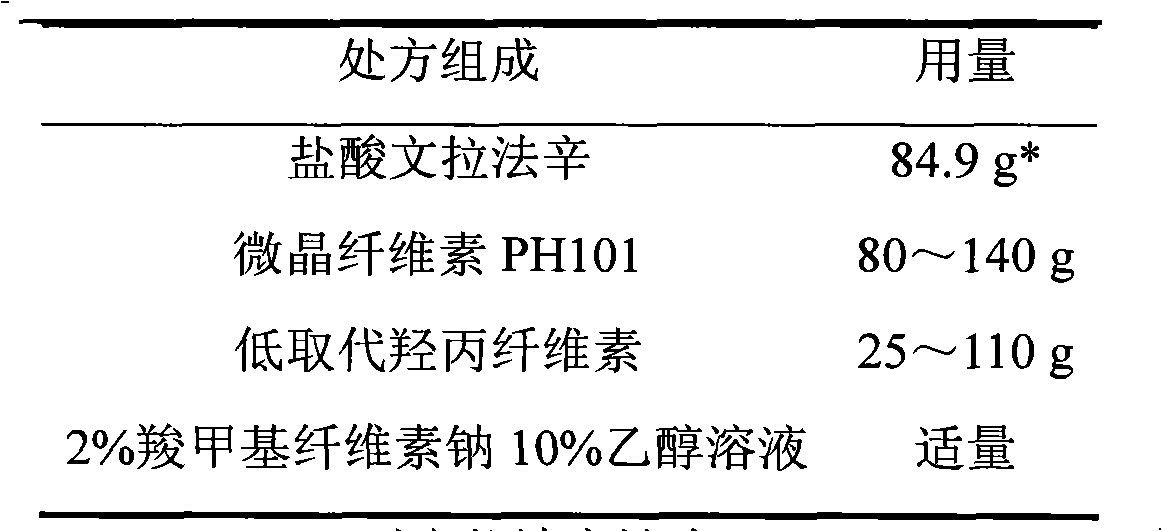
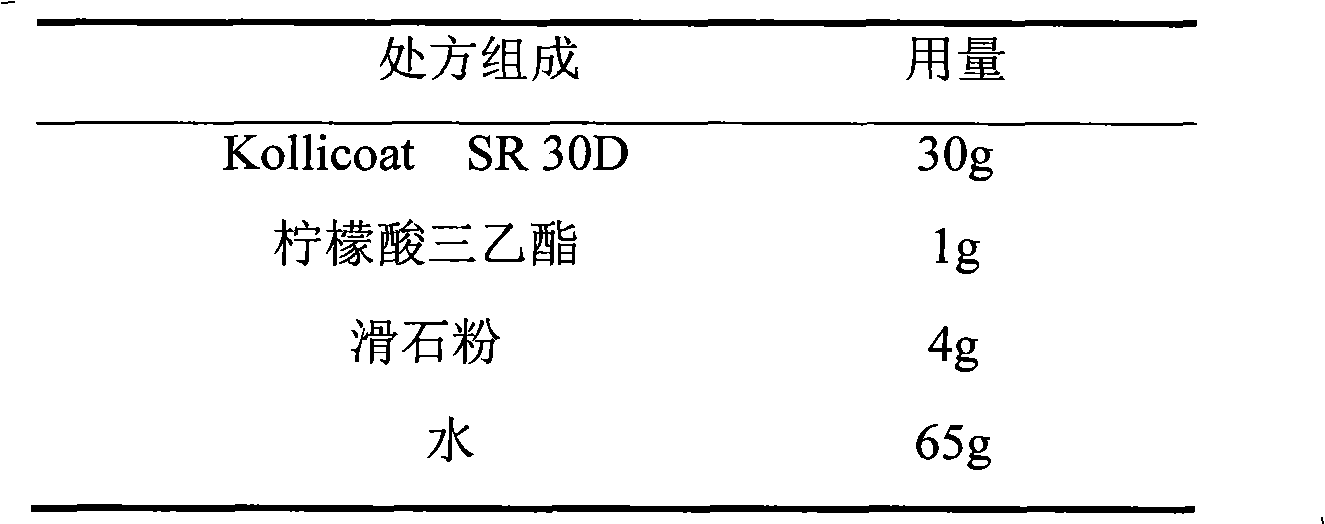
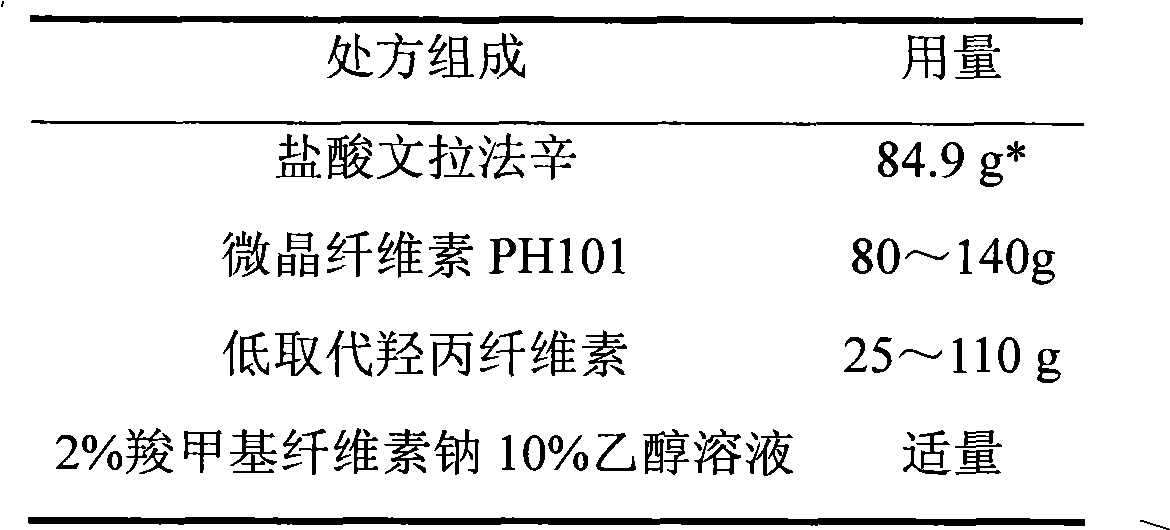
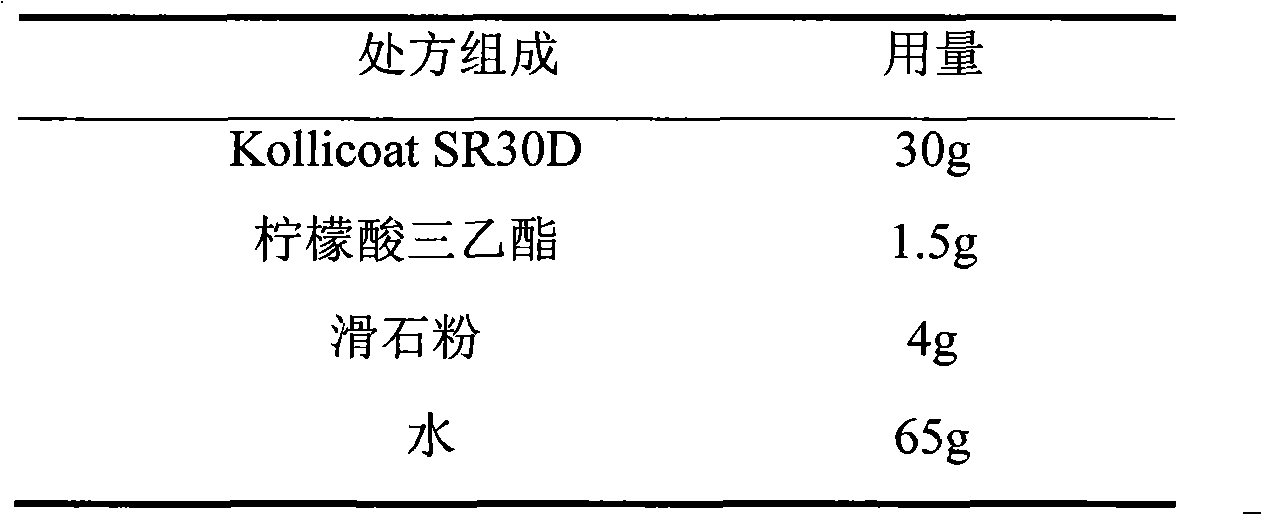
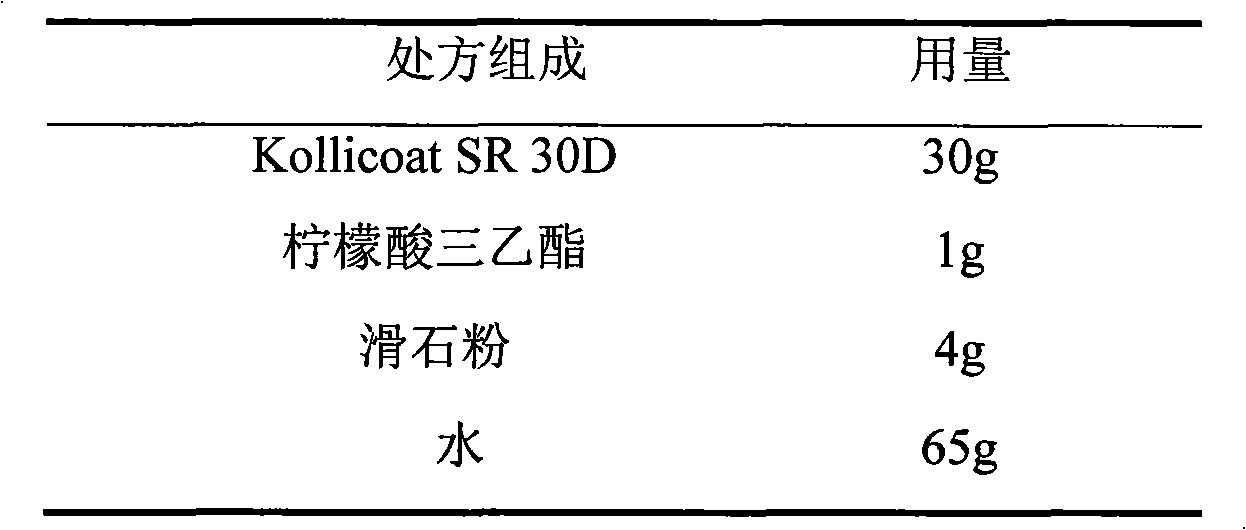
Venlafaxine hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211791AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsLow-substituted hydroxypropylcellulose
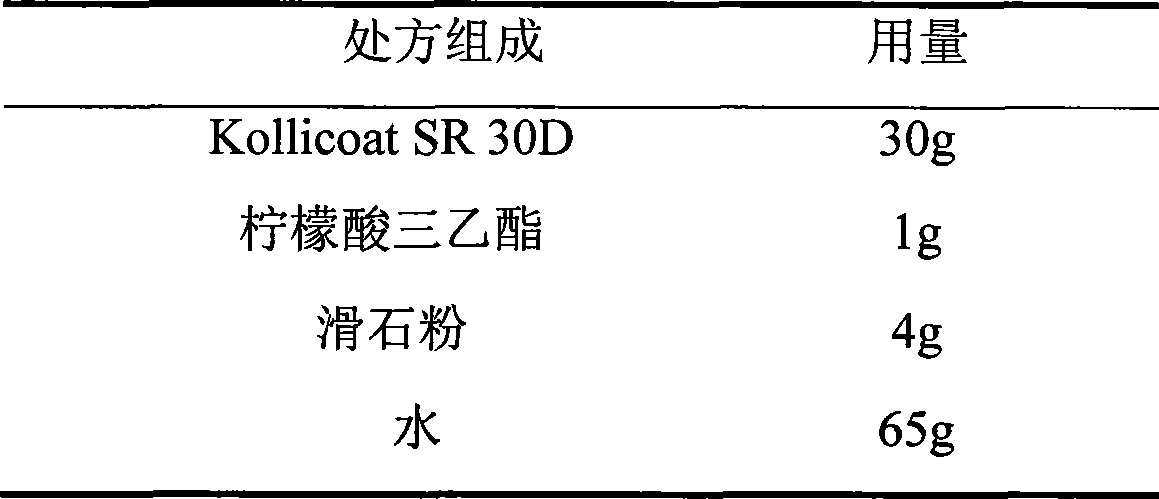
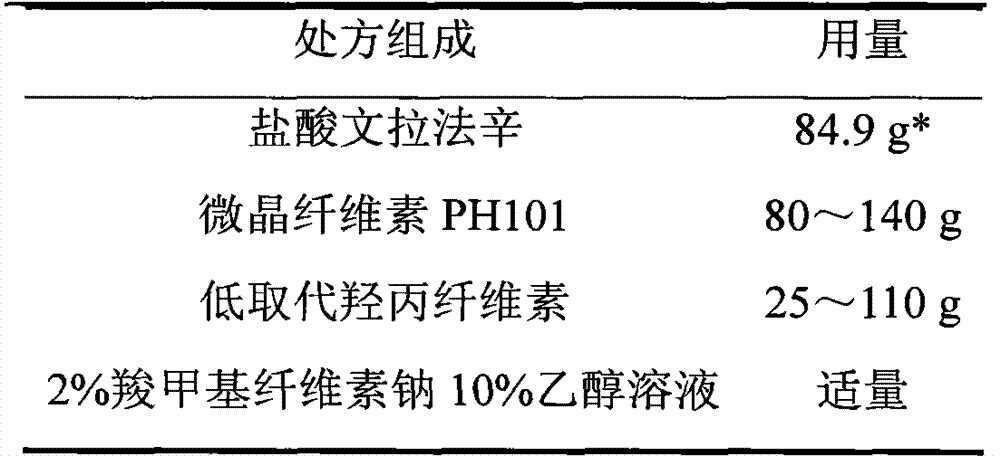
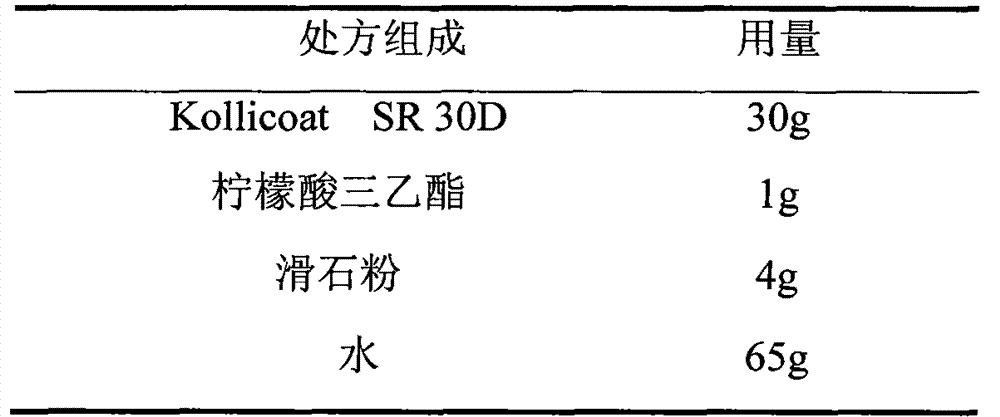
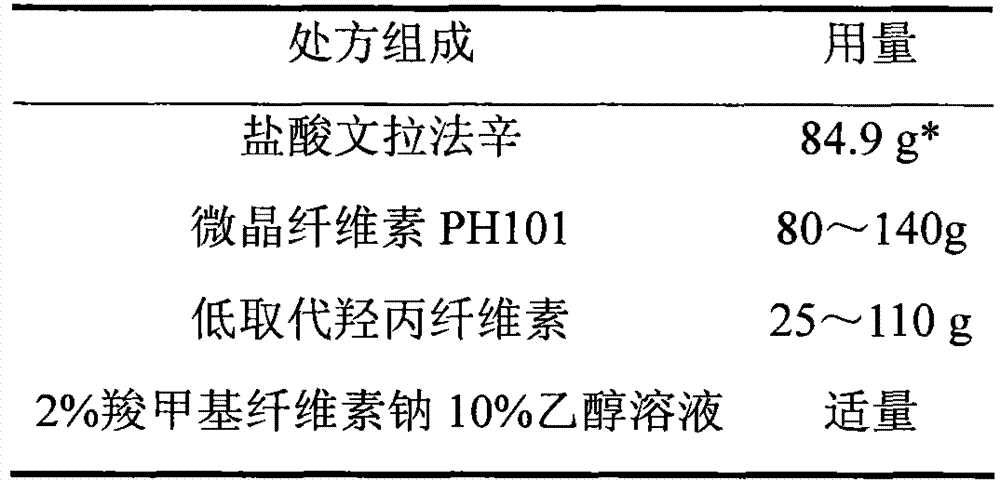
The invention relates to a venlafaxine hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the venlafaxine hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR30D as a film-formation material. A pellet core of the venlafaxine hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR30D to triethyl citrate to talcum powder is 30: 1: 4 and a film weight increasing ratio is in a range of 21 to 39%. The venlafaxine hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the venlafaxine hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the venlafaxine hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
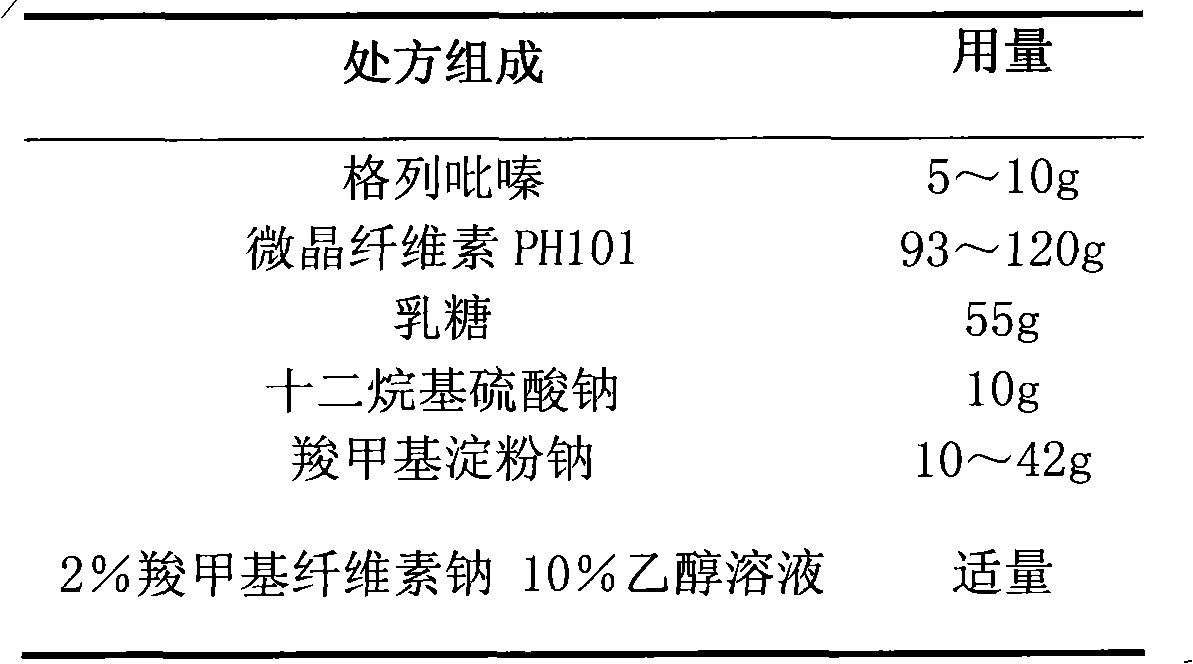
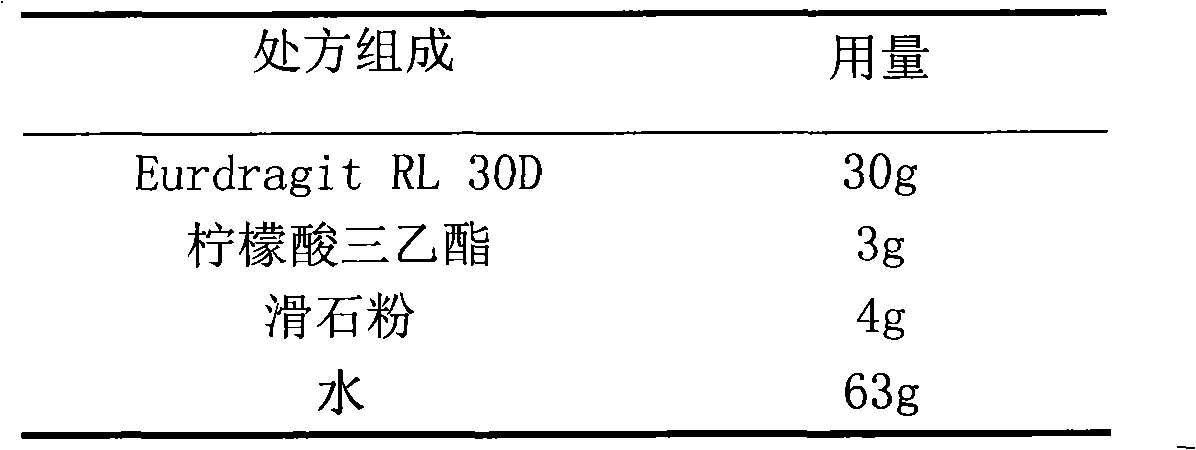
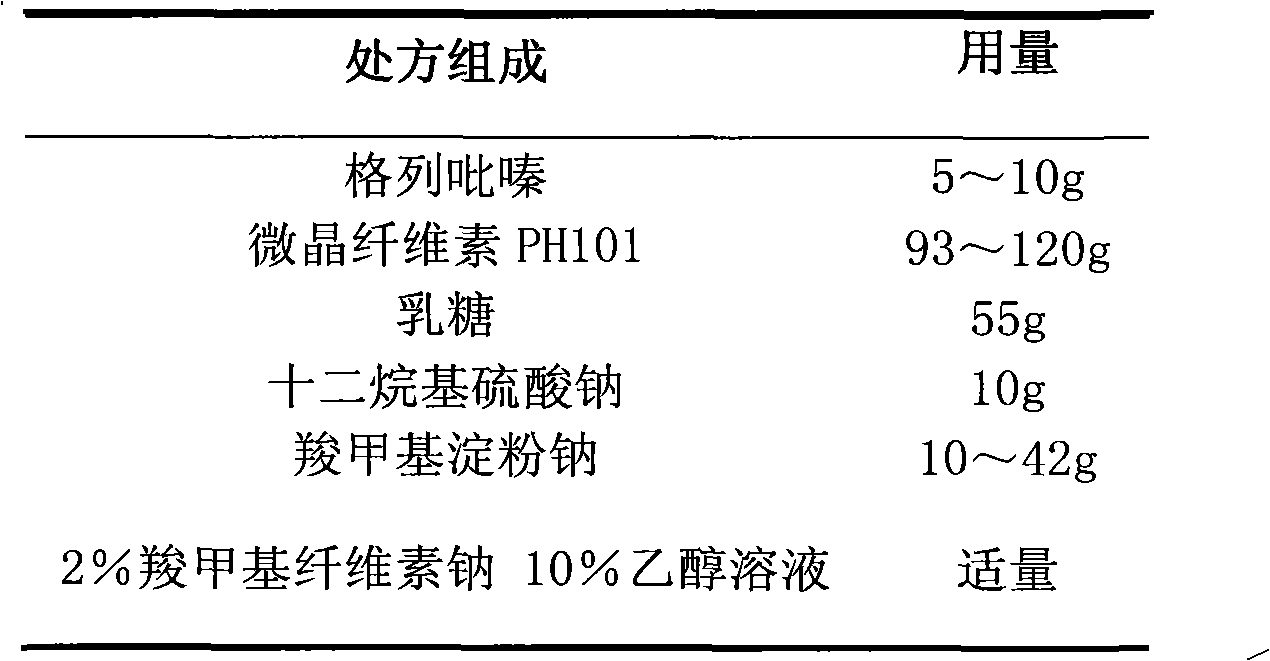
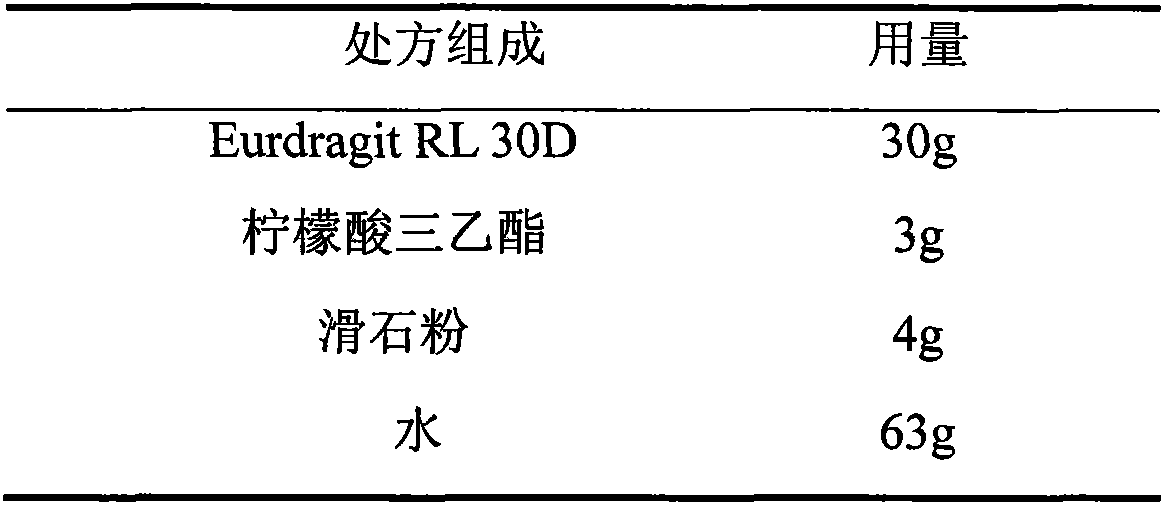
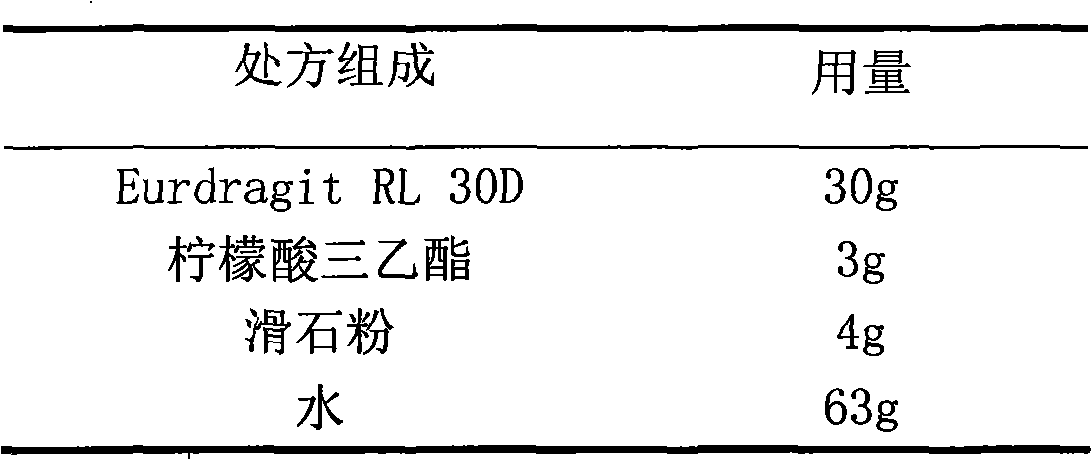
Glipizide film-controlled slow-release pellet capsule
ActiveCN103211787AAccelerated agingReduce permeabilityMetabolism disorderSulfonylurea active ingredientsSustained release pelletsMedicine
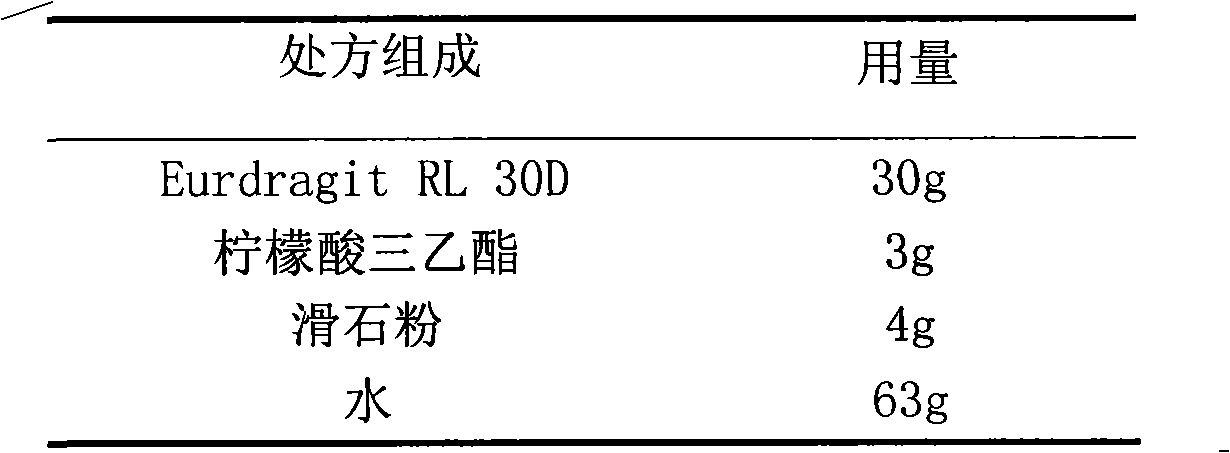
The invention relates to a glipizide film-controlled slow-release pellet capsule. A slow-release film of the glipizide film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the glipizide film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose, lactose and sodium dodecyl sulfate, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 17 to 36%. The glipizide film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the glipizide film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the glipizide film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:内蒙古天衡医院管理有限公司
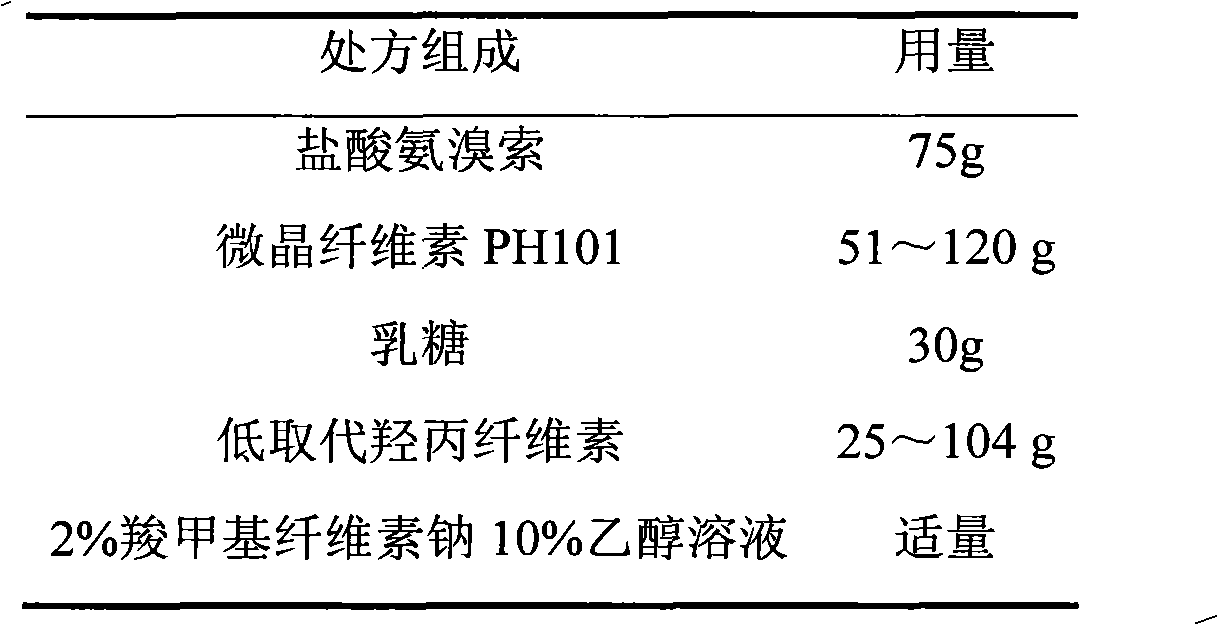
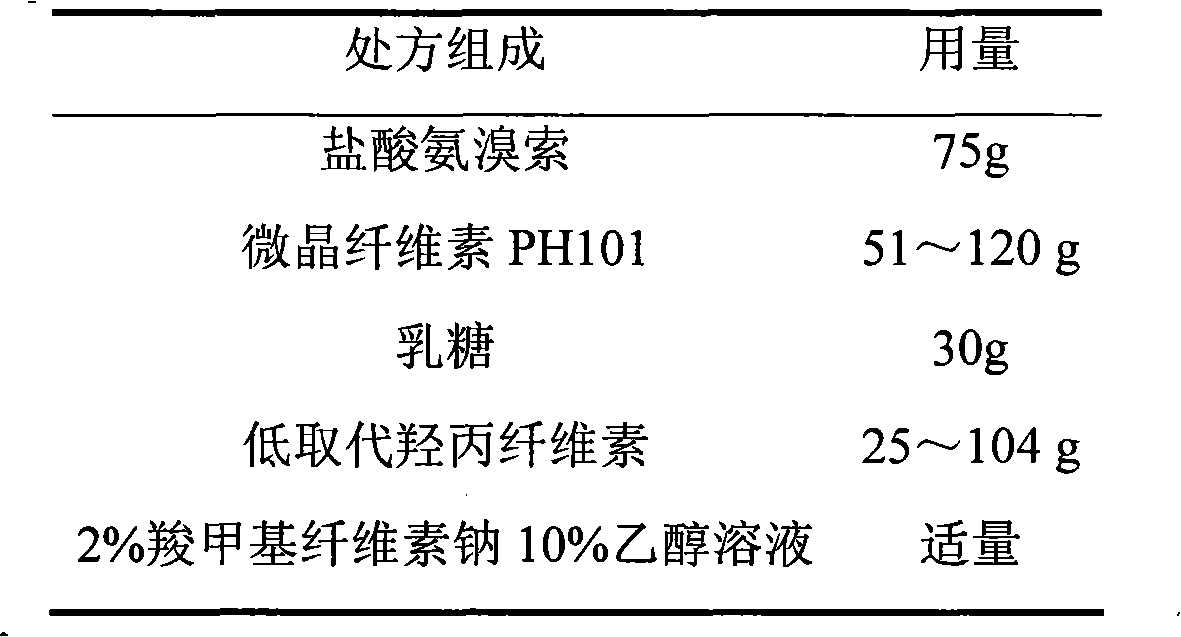
Ambroxol hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211789AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsLactose
The invention relates to an ambroxol hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the ambroxol hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR 30D as a film-formation material. A pellet core of the ambroxol hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR 30D to triethyl citrate to talcum powder is 30: 1.5: 4 and a film weight increasing ratio is in a range of 20 to 36%. The ambroxol hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the ambroxol hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the ambroxol hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
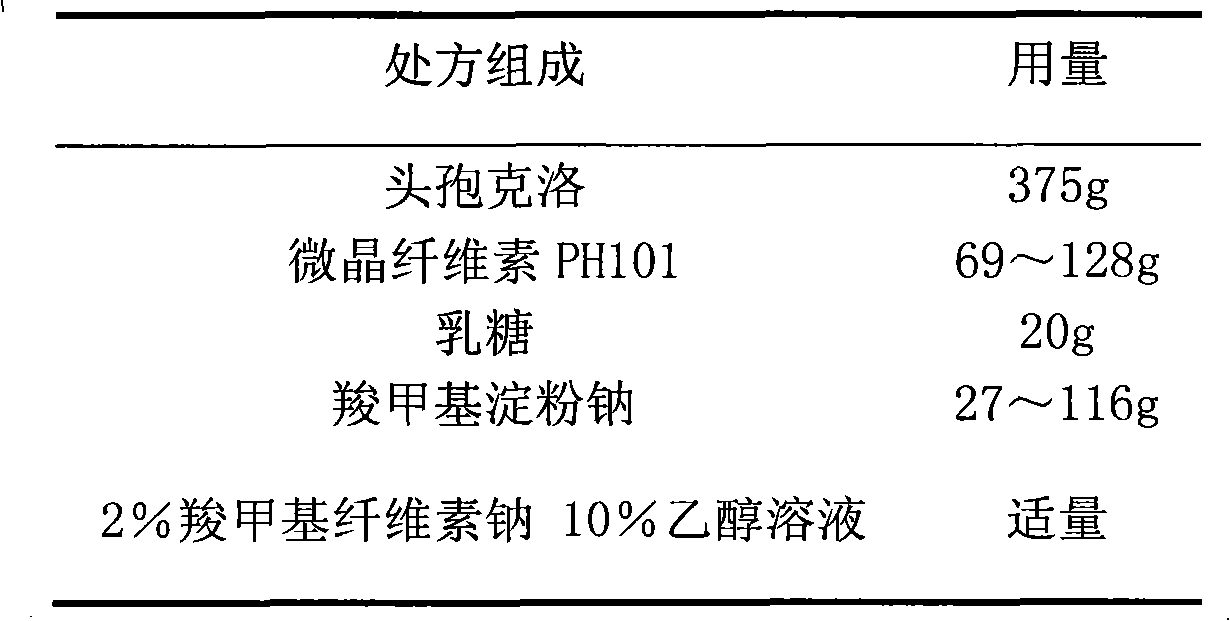
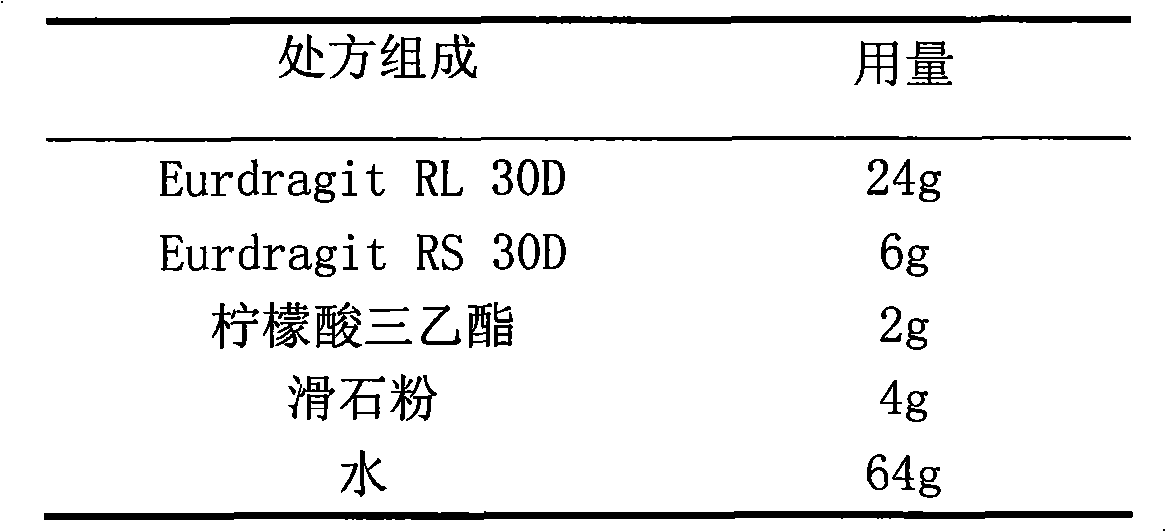
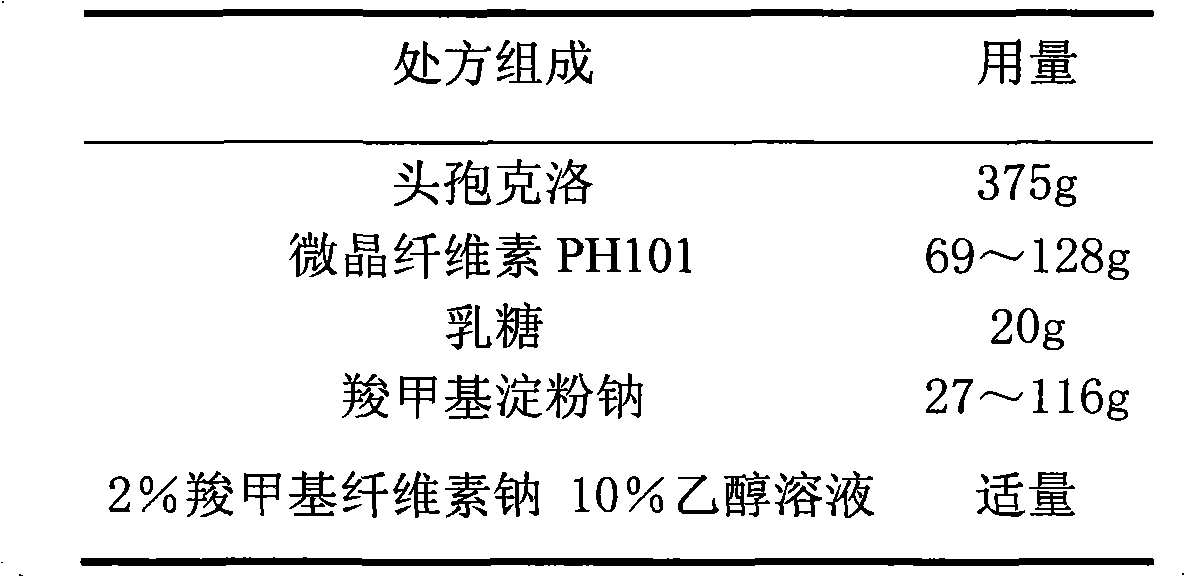
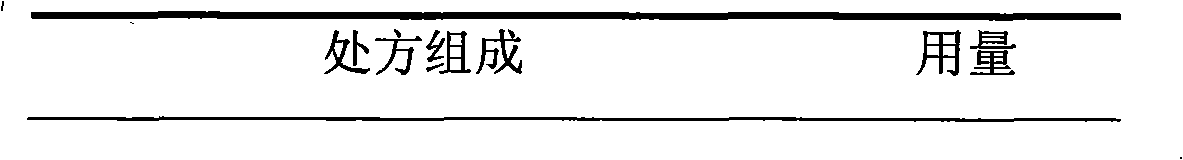
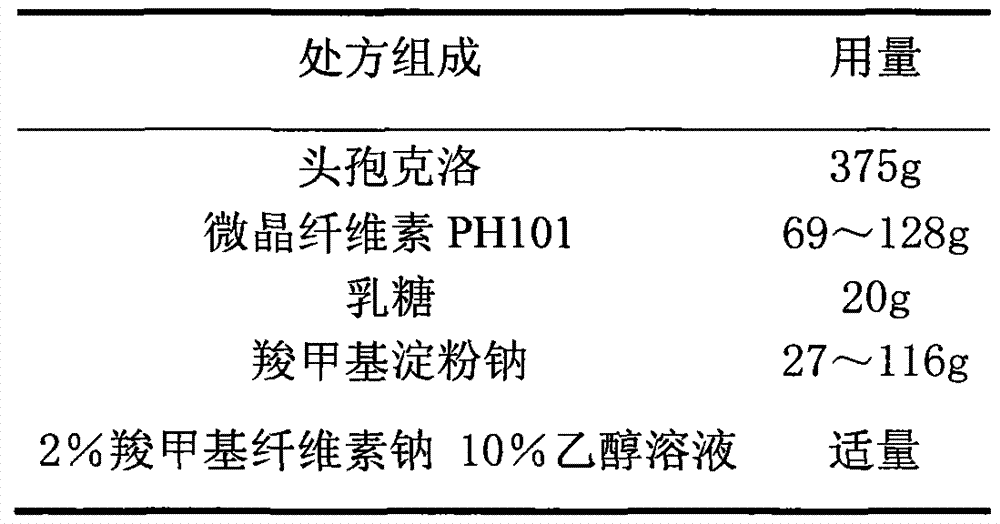
Cefaclor film-controlled slow-release pellet capsule
ActiveCN103211795AAccelerated agingReduce permeabilityAntibacterial agentsOrganic active ingredientsMedicineLactose
The invention relates to a cefaclor film-controlled slow-release pellet capsule. A slow-release film of the cefaclor film-controlled slow-release pellet utilizes a mixture of aqueous dispersion Eurdragit RL 30D and Eurdragit RS 30D as a film-formation material, wherein a weight ratio of Eurdragit RL 30D to Eurdragit RS 30D in the mixture is 4: 1. A pellet core of the cefaclor film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 5 to 20wt% of the sodium carboxymethyl starch. The slow-release film comprises the mixture of Eurdragit RL 30D and Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to Eurdragit RS 30D to triethyl citrate to talcum powder is 24: 6: 2: 4 and a film weight increasing ratio is in a range of 23 to 40%. The cefaclor film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the cefaclor film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the cefaclor film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
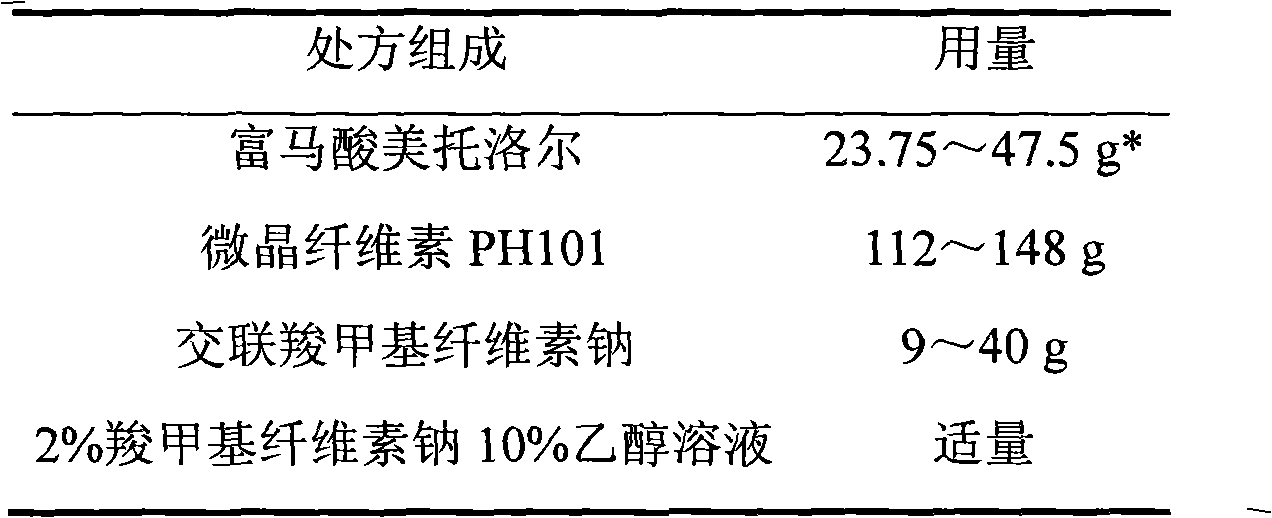
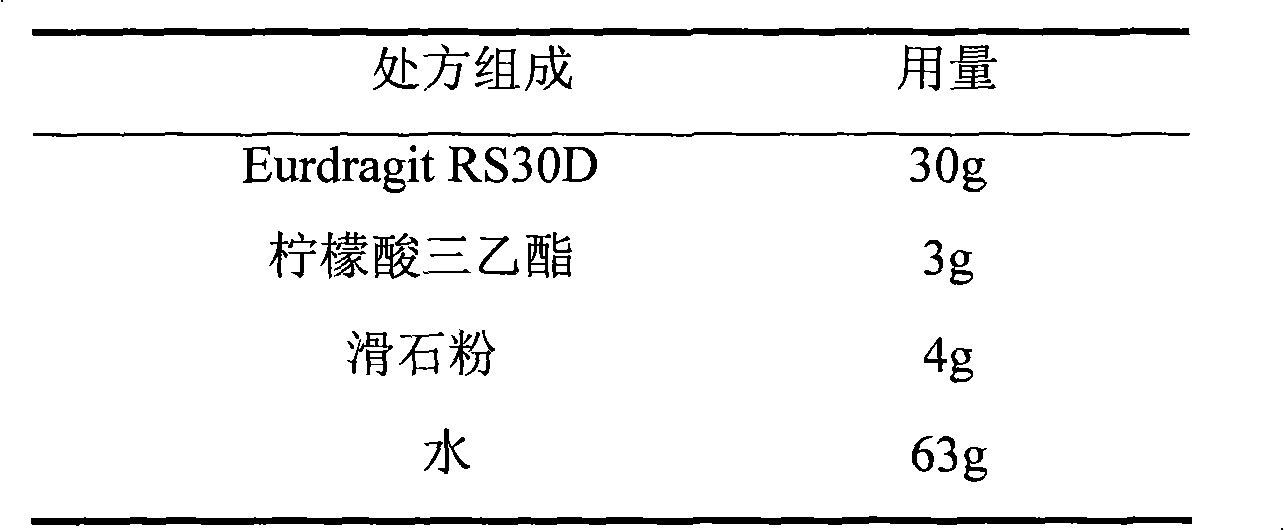
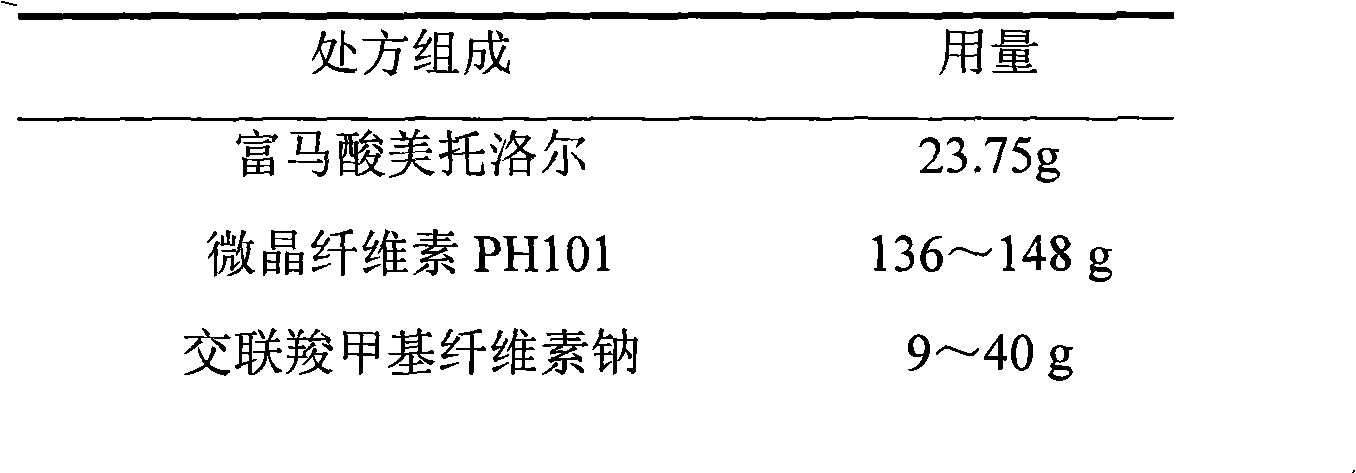
Metoprolol fumarate film-controlled slow-release pellet capsule
ActiveCN103211792AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderMetoprolol FumarateMedicine
The invention relates to a metoprolol fumarate film-controlled slow-release pellet capsule. A slow-release film of the metoprolol fumarate film-controlled slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the metoprolol fumarate film-controlled slow-release pellet contains croscarmellose sodium having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 5 to 20wt% of croscarmellose sodium. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 18 to 33%. The metoprolol fumarate film-controlled slow-release pellet comprises the pellet core containing croscarmellose sodium having high water expansibility and thus after absorbing water, the metoprolol fumarate film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the metoprolol fumarate film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
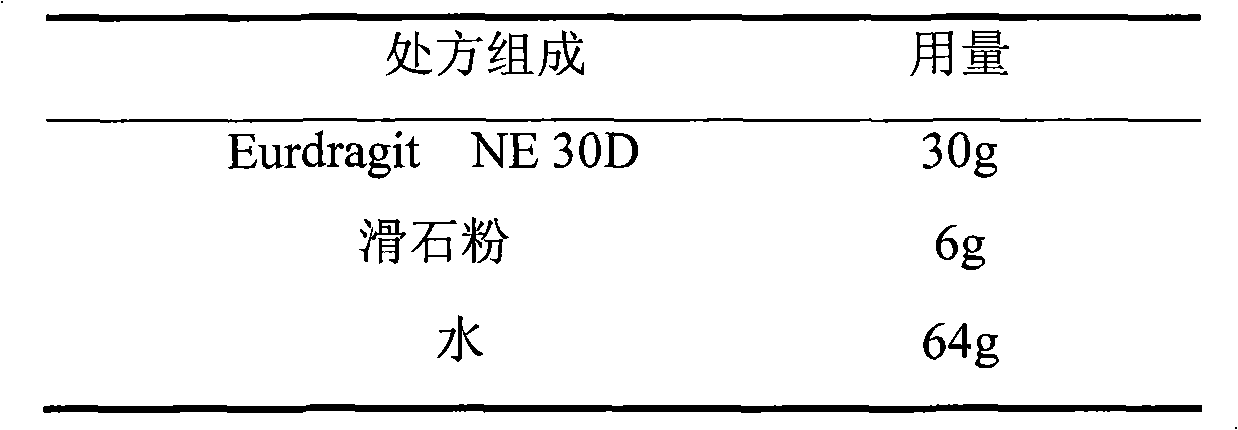
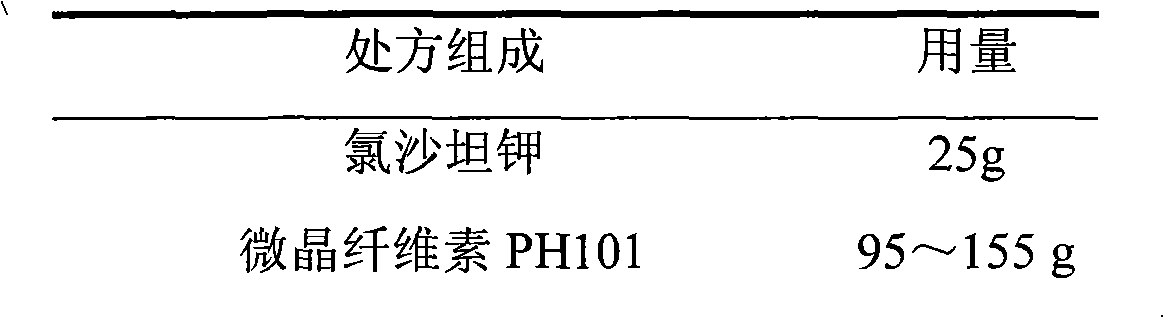
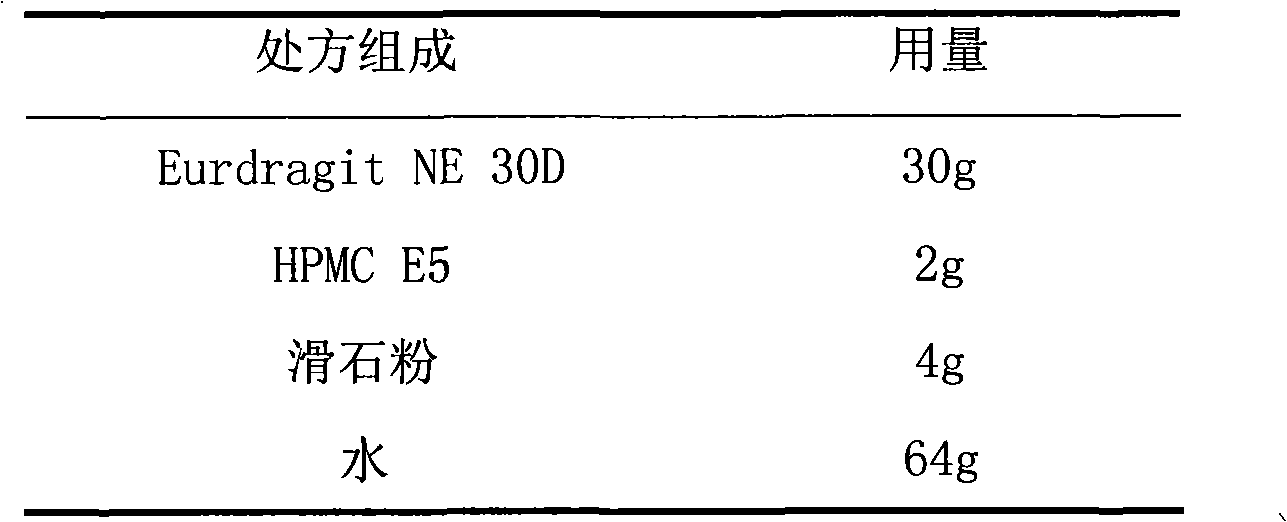
Losartan potassium membrane controlled-release pellet capsule
InactiveCN103211798AReduce permeabilityImprove permeabilityOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseMedicine
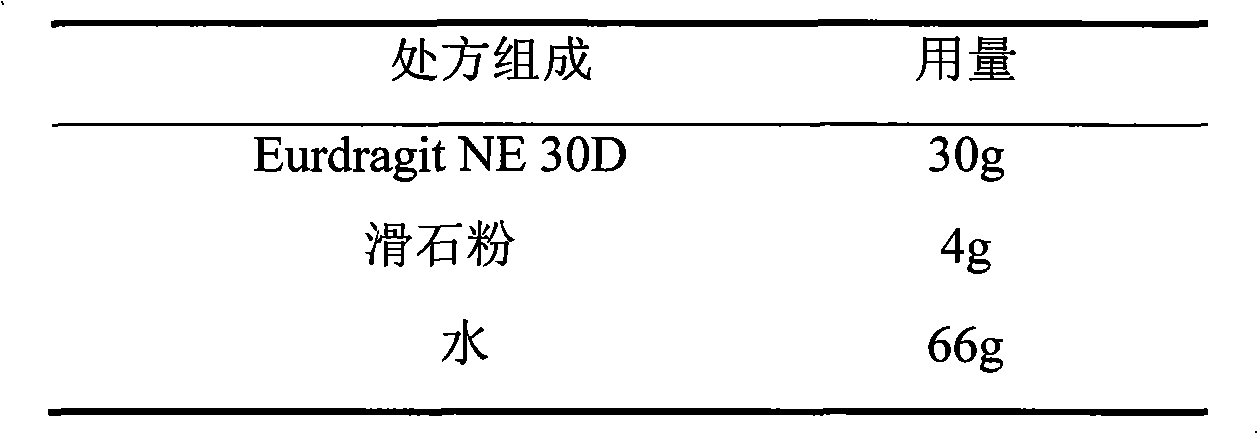
The invention relates to a losartan potassium membrane controlled-release pellet capsule. The controlled-release film of the pellet adopts Eurdragit NE 30D as a film forming material, the core of the controlled-release pellet contains high-expansibility low-substituted hydroxypropylcellulose and a pharmaceutically-acceptable excipient commonly used for controlled-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of the low-substituted hydroxypropylcellulose in the core of the controlled-release pellet is 10-40%. The controlled-release film of the controlled-release pellet includes the Eurdragit NE 30D and an anti-adherent talcum powder, the optimal ratio of the Eurdragit NE 30D to the talcum powder is 30:6, and the optimal coating weight gain is 19-36%. The core will obviously expand after absorbing water because of the containment of the low-substituted hydroxypropylcellulose highly expanding after contacting with water, so the controlled-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:内蒙古天衡医院管理有限公司
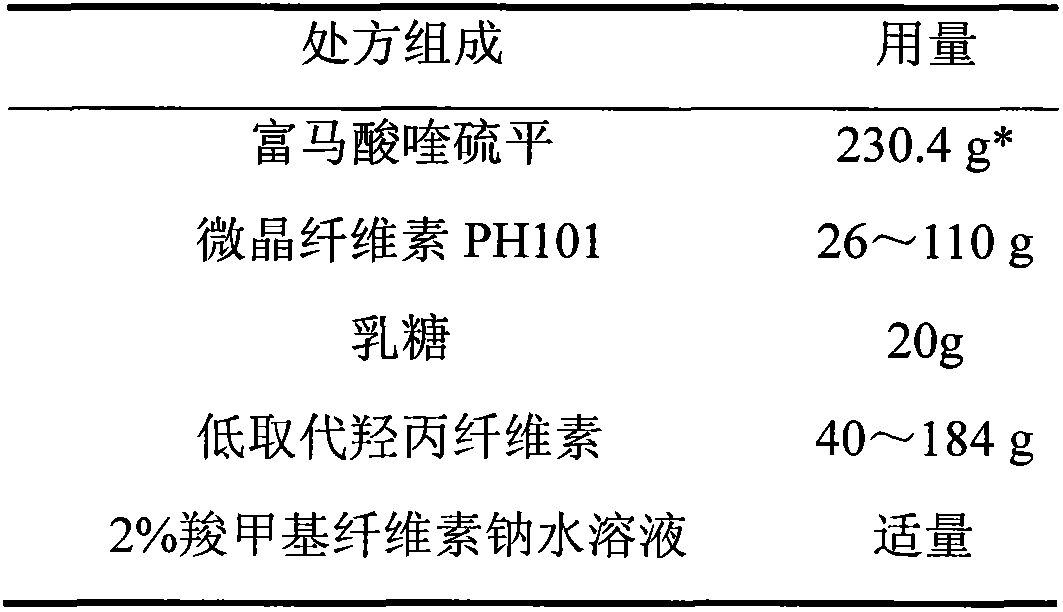
Quetiapine fumarate film-controlled slow-release pellet capsule
InactiveCN103211794AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsMedicine
The invention relates to a quetiapine fumarate film-controlled slow-release pellet capsule. A slow-release film of the quetiapine fumarate film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the quetiapine fumarate film-controlled slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 19 to 35%. The quetiapine fumarate film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the quetiapine fumarate film-controlled slow-release pellet expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the quetiapine fumarate film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:内蒙古天衡医院管理有限公司
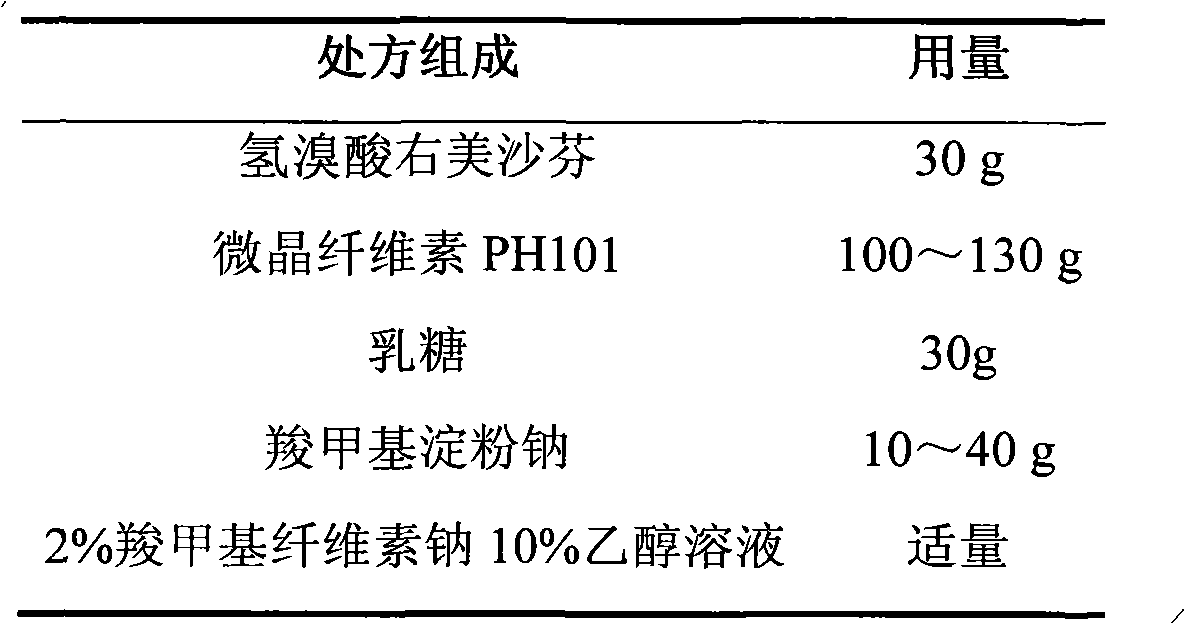
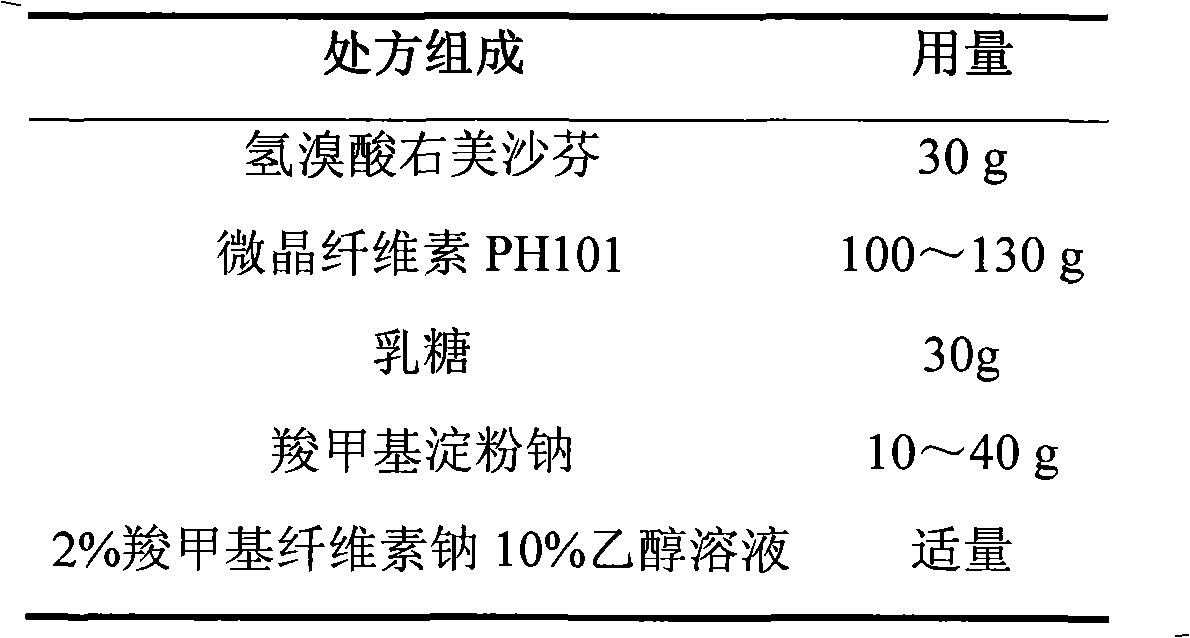
Dextromethorphan hydrobromide film-controlled slow-release pellet capsule
ActiveCN103211783AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsDextromethorphan Hydrobromide
The invention relates to a dextromethorphan hydrobromide film-controlled slow-release pellet capsule. A slow-release film of the dextromethorphan hydrobromide film-controlled slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the dextromethorphan hydrobromide film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RS 30D to triethyl citrate to talcum powder is 30: 1: 4 and coating weight gain is in a range of 16 to 35%. The dextromethorphan hydrobromide film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the dextromethorphan hydrobromide film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore diameter is increased; and permeability is improved and the permeability reduction caused by film aging is compensated. Therefore, in middle and later stages, the dextromethorphan hydrobromide film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept prior to expiration date.
Owner:北京天衡药物研究院有限公司
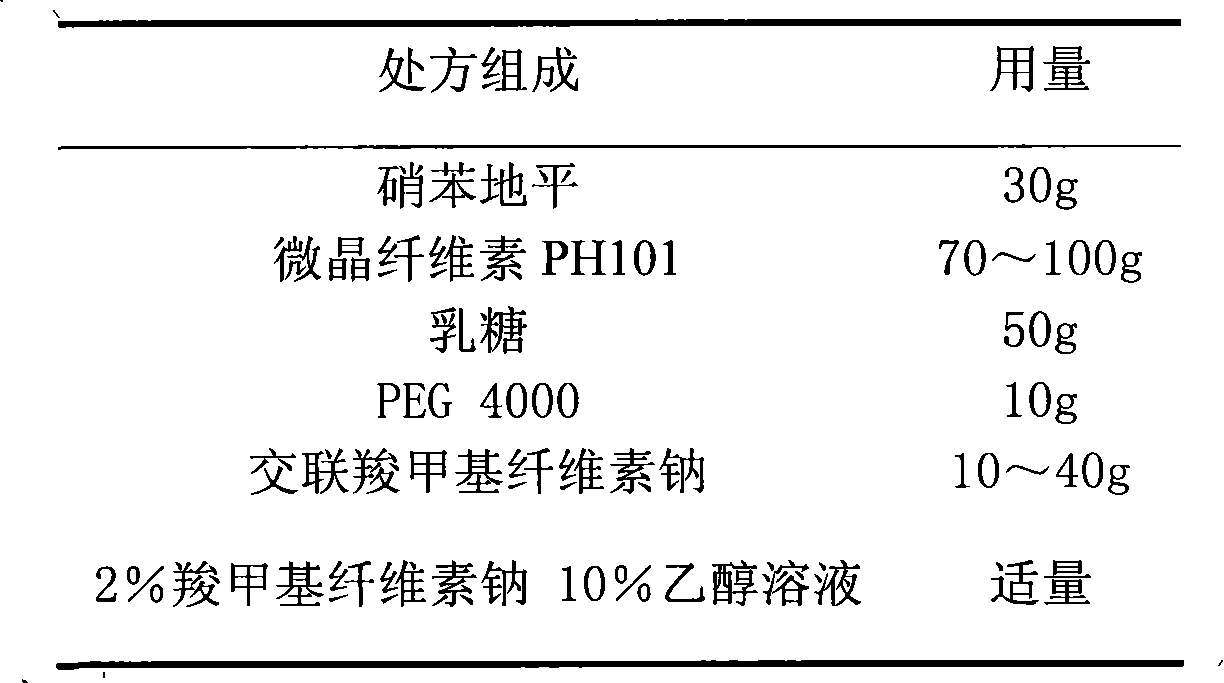
Nifedipine film-controlled slow-release pellet capsule
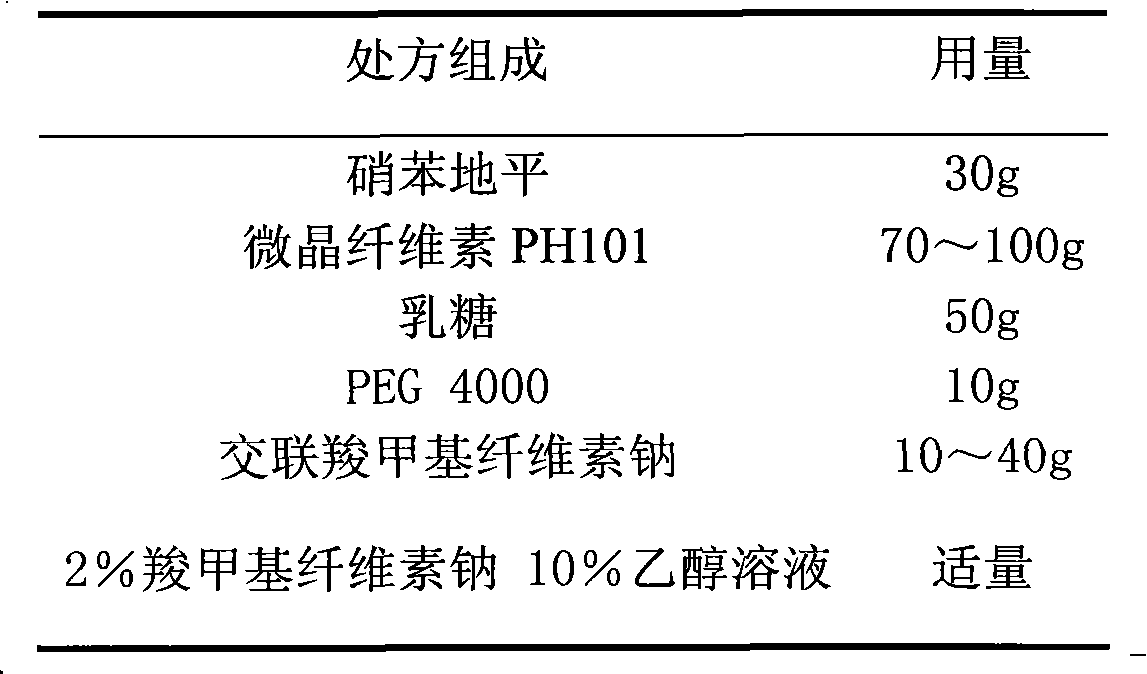
ActiveCN103211788AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsNifedipineEnvironmental engineering
The invention relates to a nifedipine film-controlled slow-release pellet capsule. A slow-release film of the nifedipine film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the nifedipine film-controlled slow-release pellet contains croscarmellose sodium having high water expansibility. Excipients comprise microcrystalline cellulose and lactose as fillers and PEG 4000 as a solubilizer. The pellet core comprises 5 to 20wt% of croscarmellose sodium. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 21 to 35%. After absorbing water, the nifedipine film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, the nifedipine film-controlled slow-release pellet keeps stable release performances in the period of validity.
Owner:北京天衡药物研究院有限公司
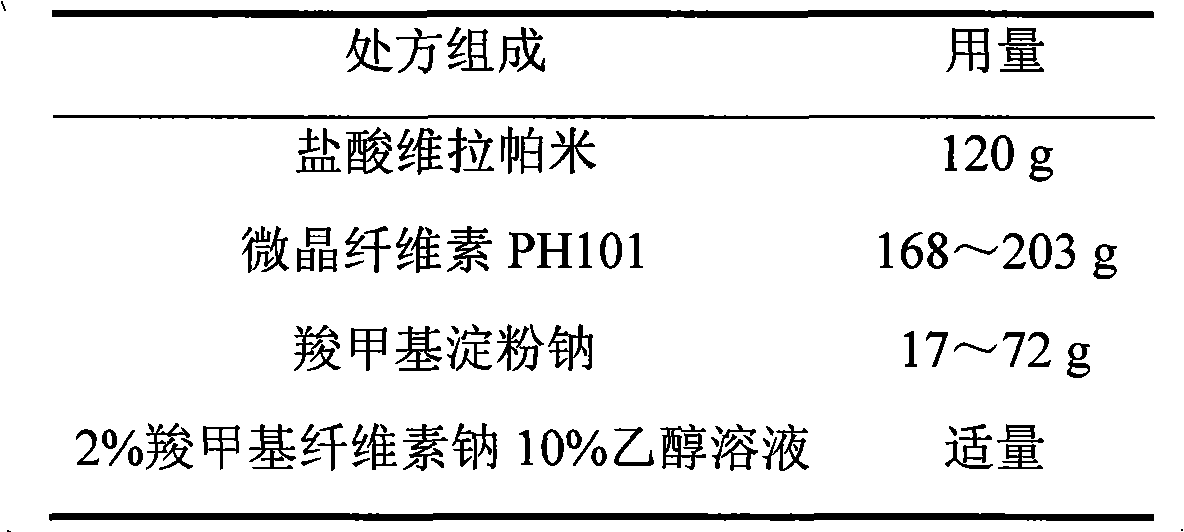
Verapamil hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211797AImprove permeabilityReduce permeabilityPharmaceutical non-active ingredientsGranular deliveryVerapamil HydrochlorideExcipient
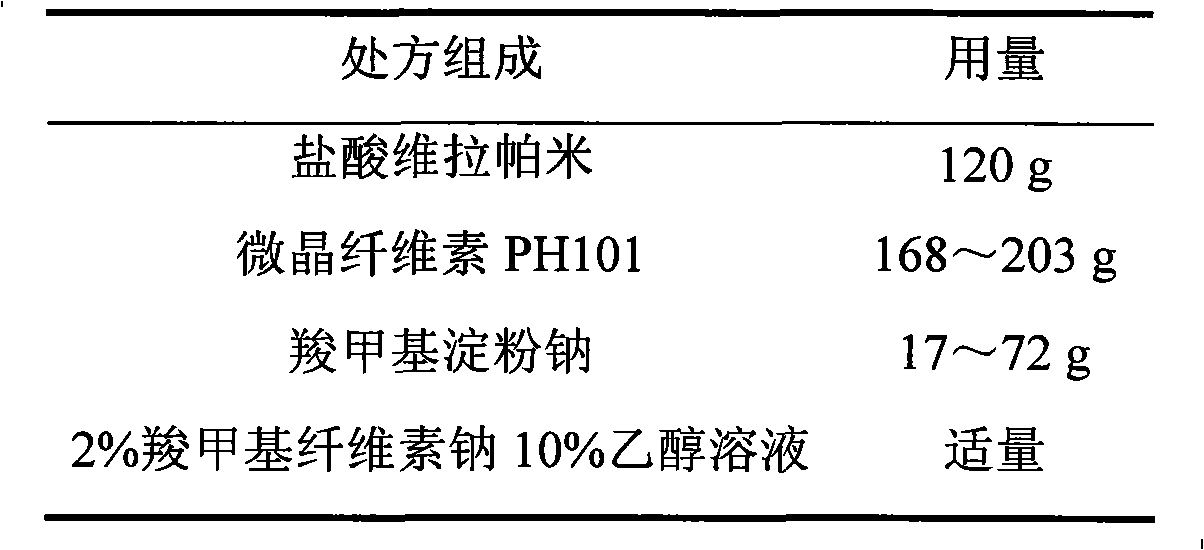
The invention relates to a verapamil hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the verapamil hydrochloride film-controlled slow-release pellet utilizes Eurdragit NE30D as a film-formation material. A pellet core of the verapamil hydrochloride film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit NE30D and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE30D to talcum powder is 30: 4 and a film weight increasing ratio is in a range of 19 to 35%. The verapamil hydrochloride film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the verapamil hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the verapamil hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
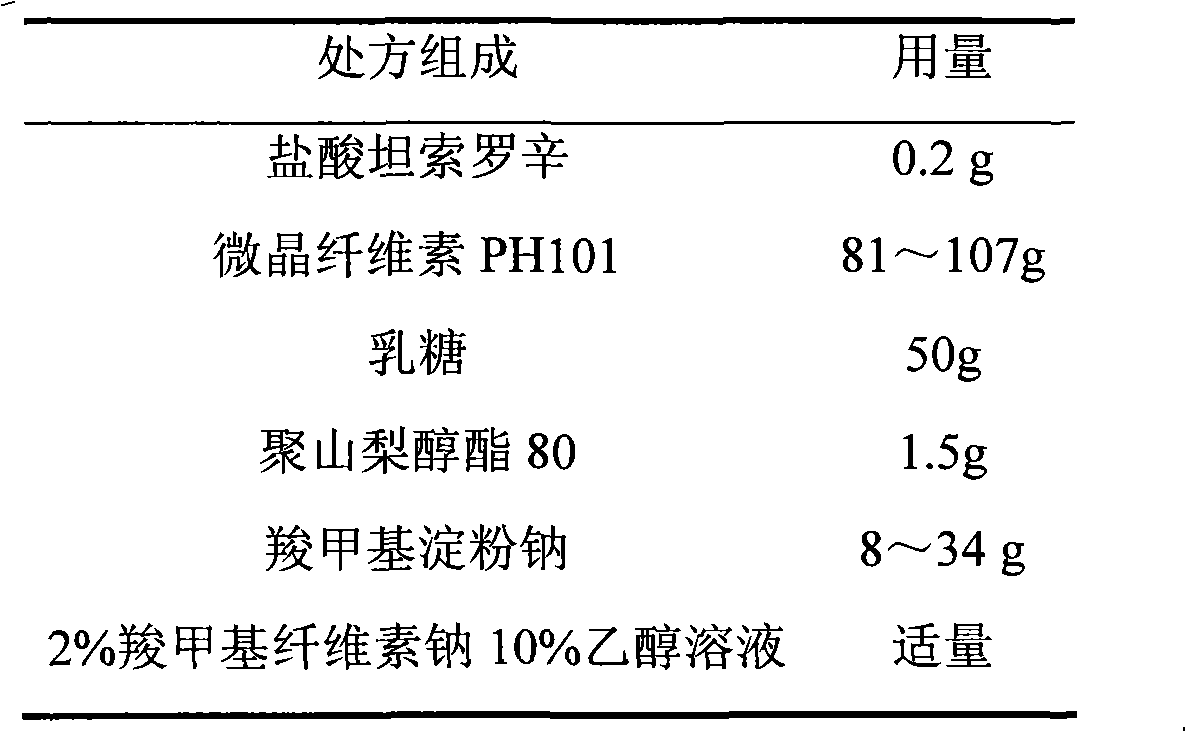
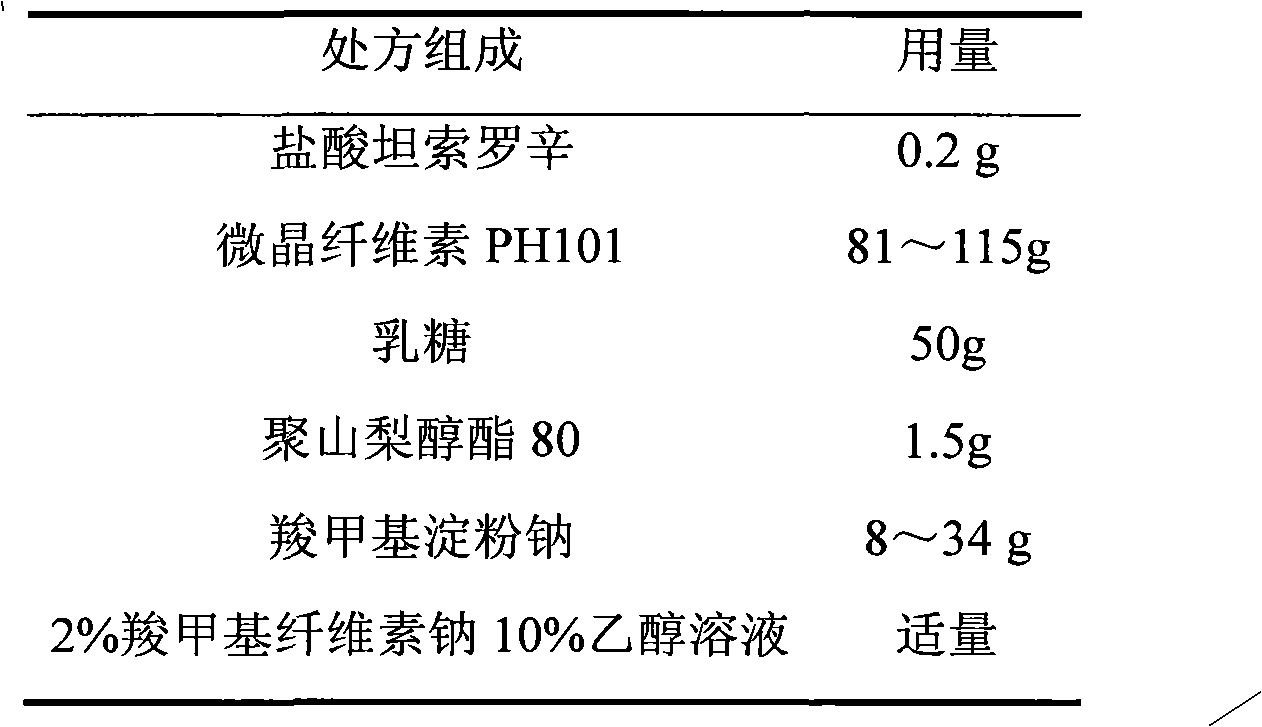
Tamsulosin hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211790AAccelerated agingReduce permeabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsFiller ExcipientLactose
The invention relates to a tamsulosin hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the tamsulosin hydrochloride film-controlled slow-release pellet utilizes Eurdragit NE30D and HPMC E5 as film-formation materials. A pellet core of the tamsulosin hydrochloride film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose as fillers, and polysorbate 80 as a solubilizer; and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit NE30D, HPMC E5 and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE30D to HPMC E5 to talcum powder is 30: 2: 4 and a film weight increasing ratio is in a range of 19 to 36%. The tamsulosin hydrochloride film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the tamsulosin hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the tamsulosin hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
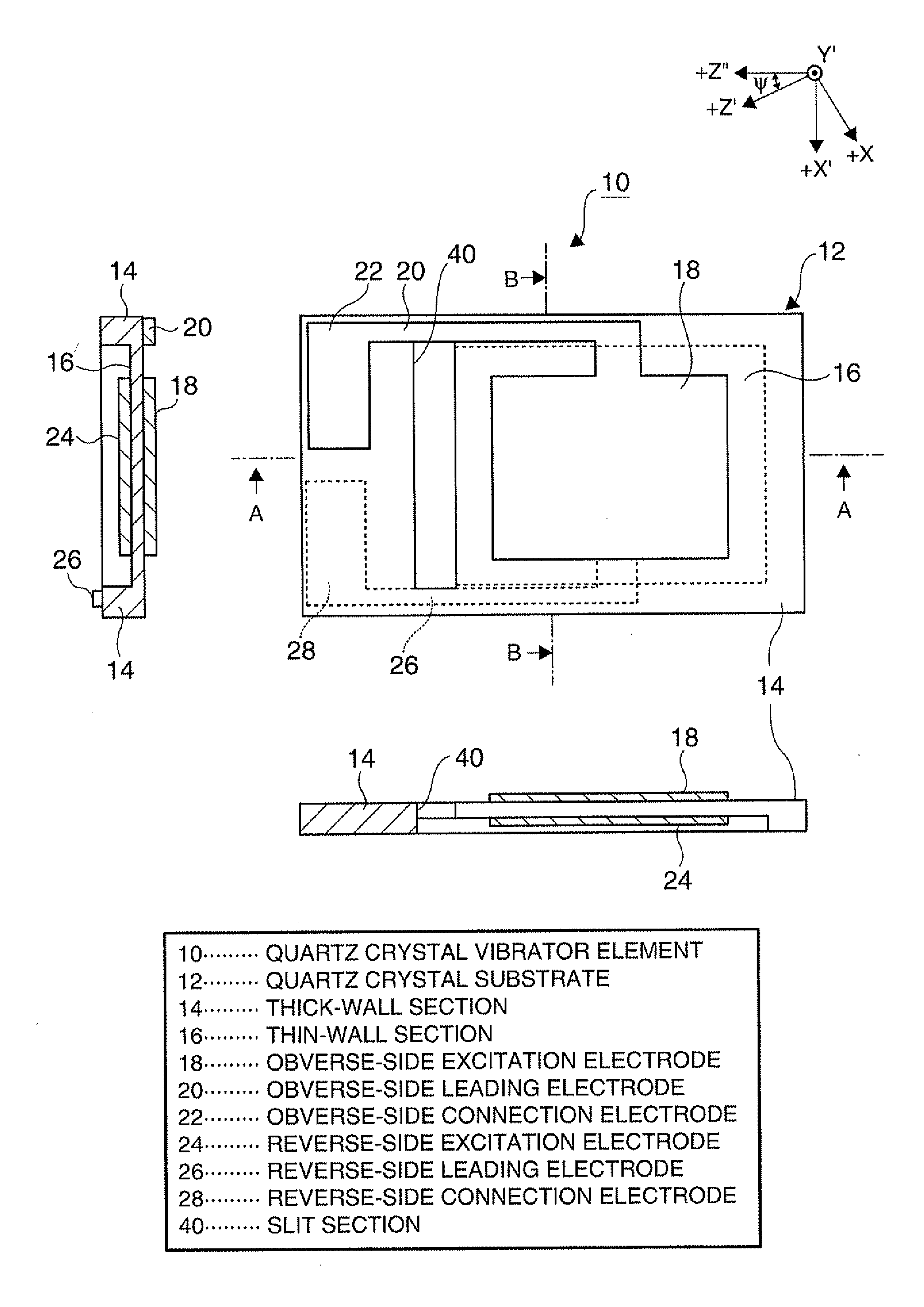
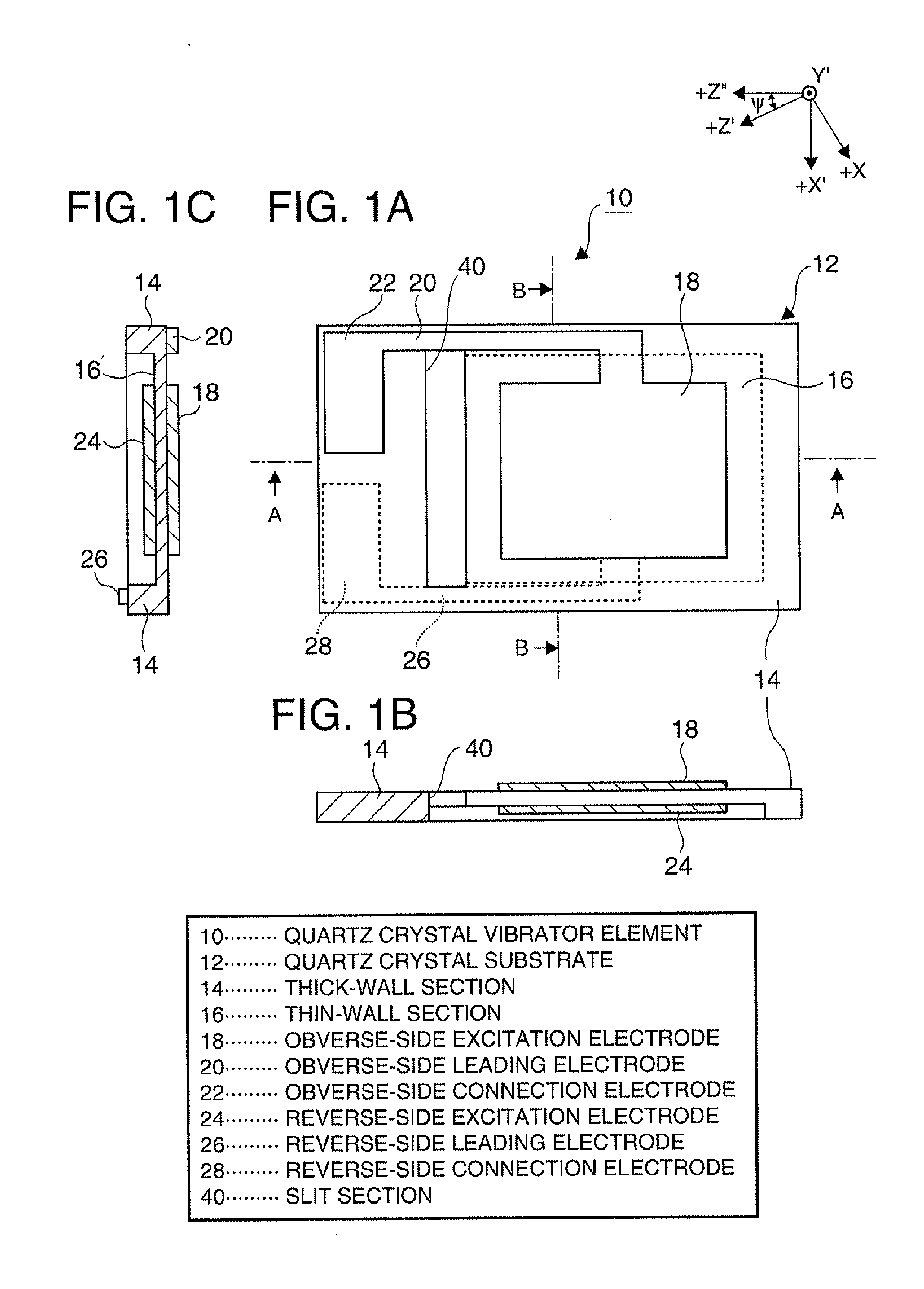
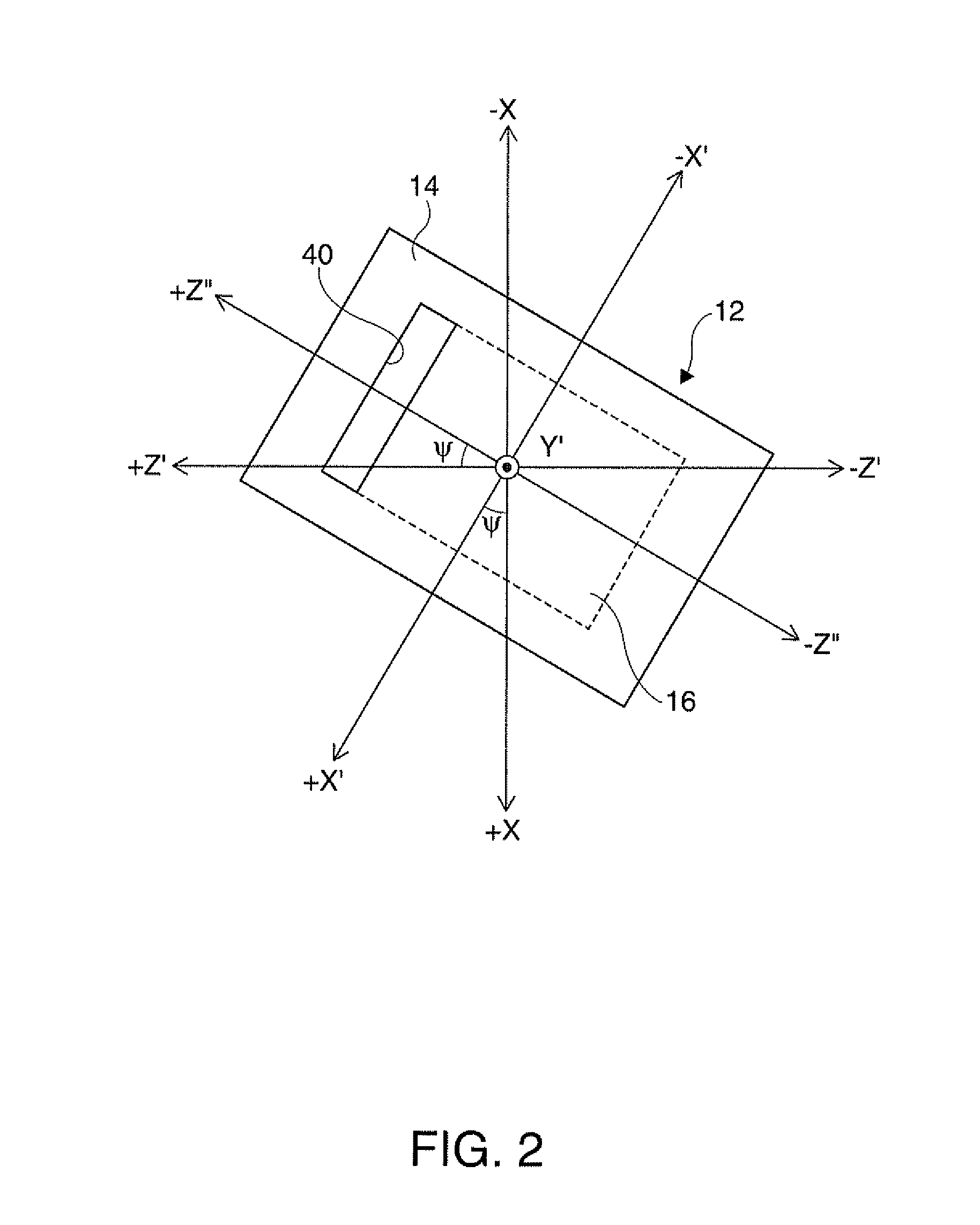
Piezoelectric vibrator element and method of manufacturing the same
InactiveUS20120025675A1Prevent protrusionSmall sizePiezoelectric/electrostrictive device manufacture/assemblyImpedence networksThin walledEtching
A piezoelectric vibrator element provided by performing wet etching on a piezoelectric substrate includes: a thin-wall section including a vibrating section; a thick-wall section thicker than the thin-wall section; and a slit section penetrating in a thickness direction, wherein the slit section is disposed in an area intervening between the thin-wall section and the thick-wall section.
Owner:SEIKO EPSON CORP
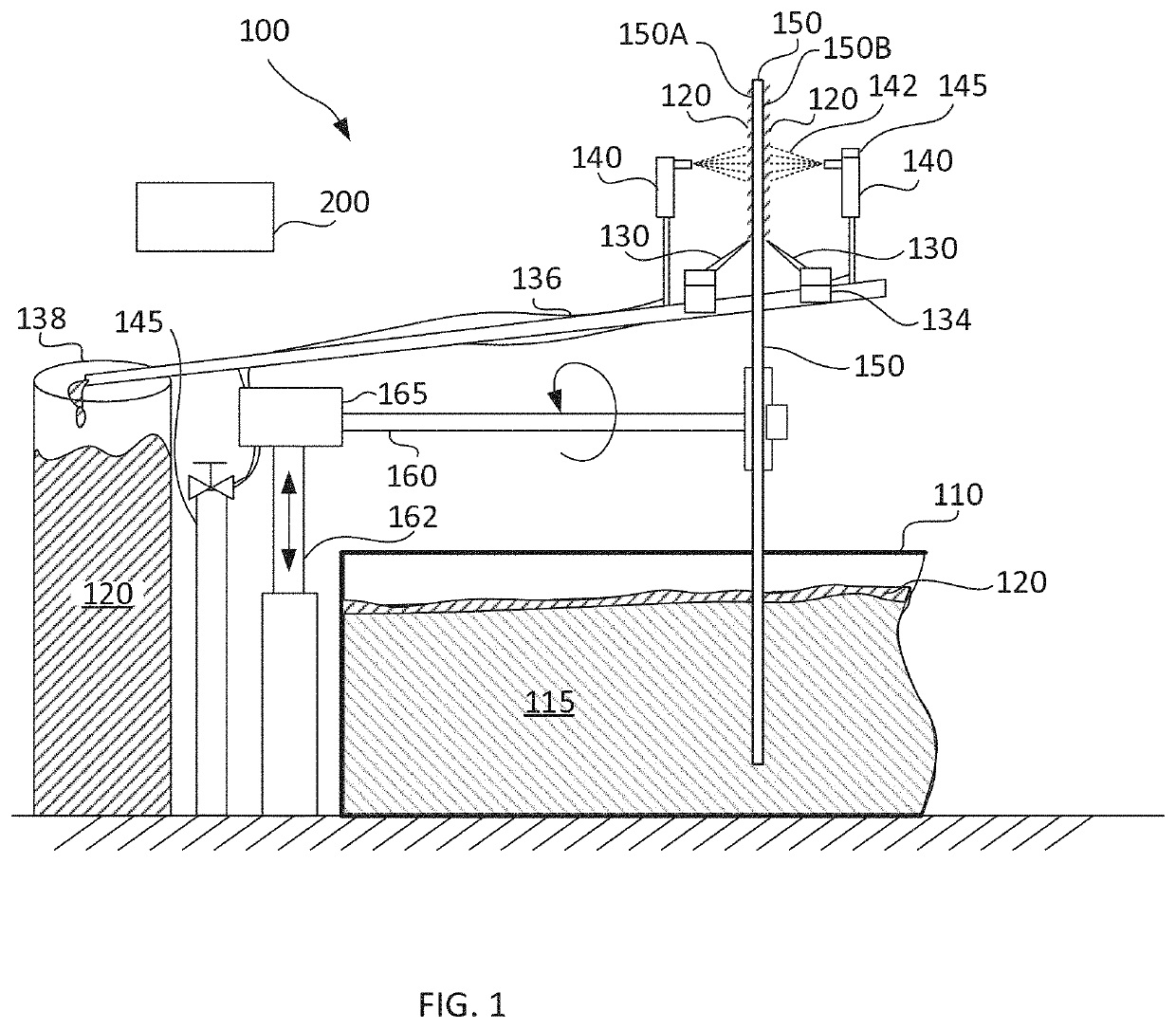
System and method for harvesting aquatic plants
ActiveUS10925212B2Stable conditionLower potentialWater cleaningHarvestersEngineeringEnvironmental engineering
A harvesting system for harvesting aquatic plants in or floating on a culture medium a harvesting bed configured to be circulated in and out of the culture medium. An actuator circulates the harvesting bed, a scraper positioned against a surface of the harvesting bed scraps the aquatic plants on the harvesting bed and a channel transports the aquatic plants removed by the scraper toward a collection vat. The collection vat receives the aquatic plants transported via the channel.
Owner:HINOMAN LTD
Method for improving the anti-aging performance of film-controlled sustained-release pellets coated with aqueous dispersion
ActiveCN103006569BCompensation for permeability changesEliminates the effects of over-tighteningPharmaceutical non-active ingredientsGranular deliveryControl releasePresent method
The invention relates to a method for improving the anti-aging performance of film-controlled sustained-release pellets coated with water dispersion, in particular to a method for improving the film-controlled slow-release pellets coated with water dispersion by adopting high-swellability pellet cores. A method for releasing the anti-aging properties of pellets, the core of which contains high expansibility auxiliary materials. The method can be used to prepare film-controlled slow-release micropills that can maintain stable release performance within the validity period of the drug without being limited by storage time. The invention also provides the use of the polymer swelling agent for improving the anti-aging performance of the film-controlled sustained-release pellets coated with the water dispersion. In addition, the invention provides a film-controlled sustained-release pellet coated with an anti-aging performance, and the pellet core is an expandable pellet core containing a polymer expander.
Owner:北京天衡药物研究院有限公司
Method for separating and purifying tanshinone IIA from radix salviae miltiorrhizae by using polyamide gel
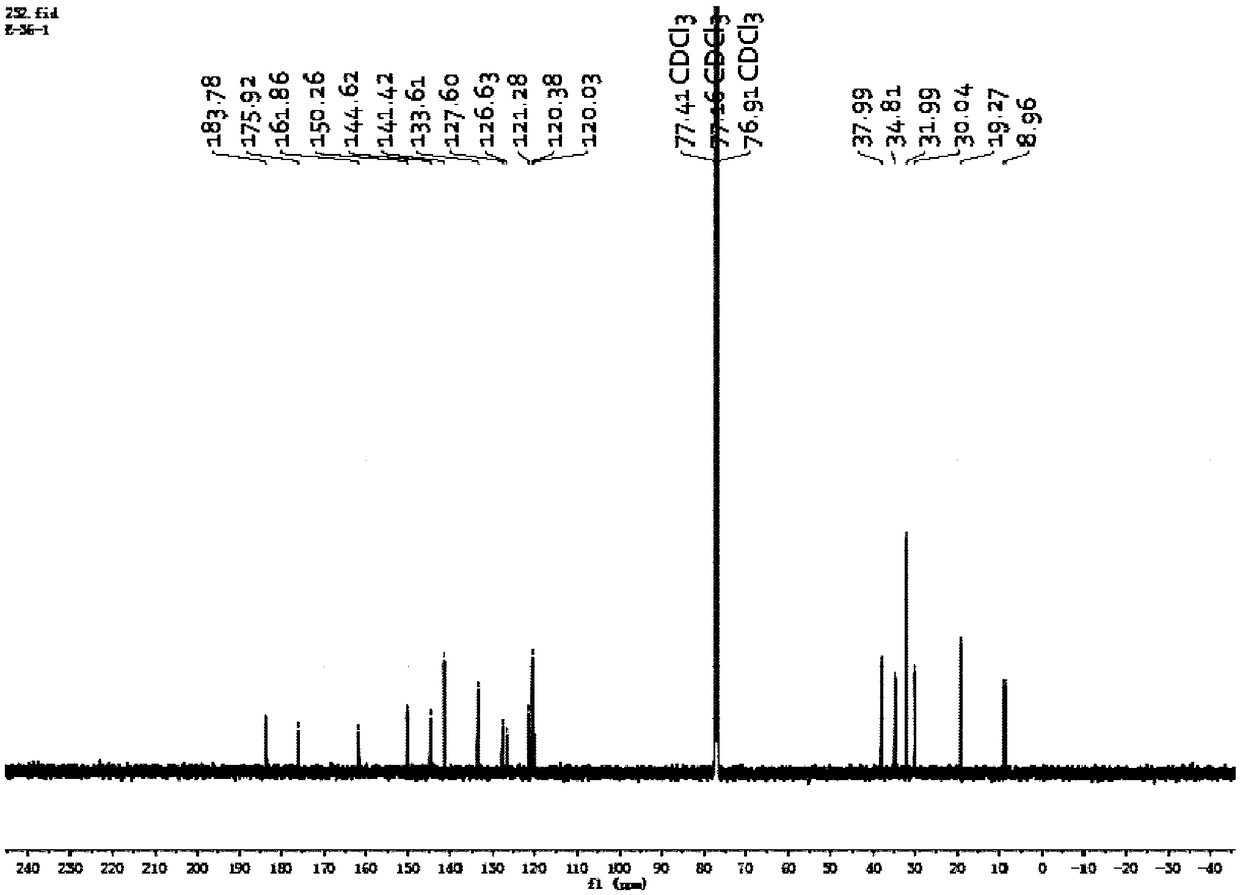
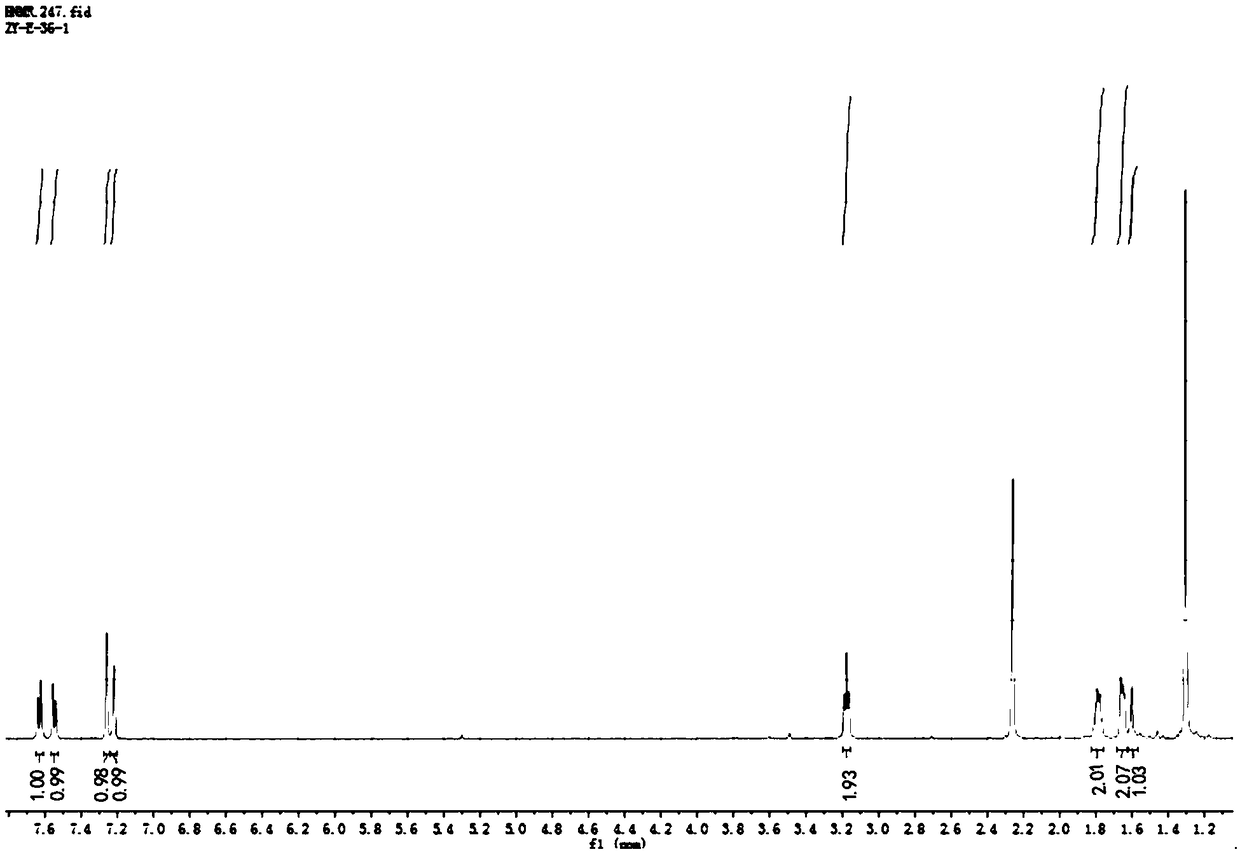
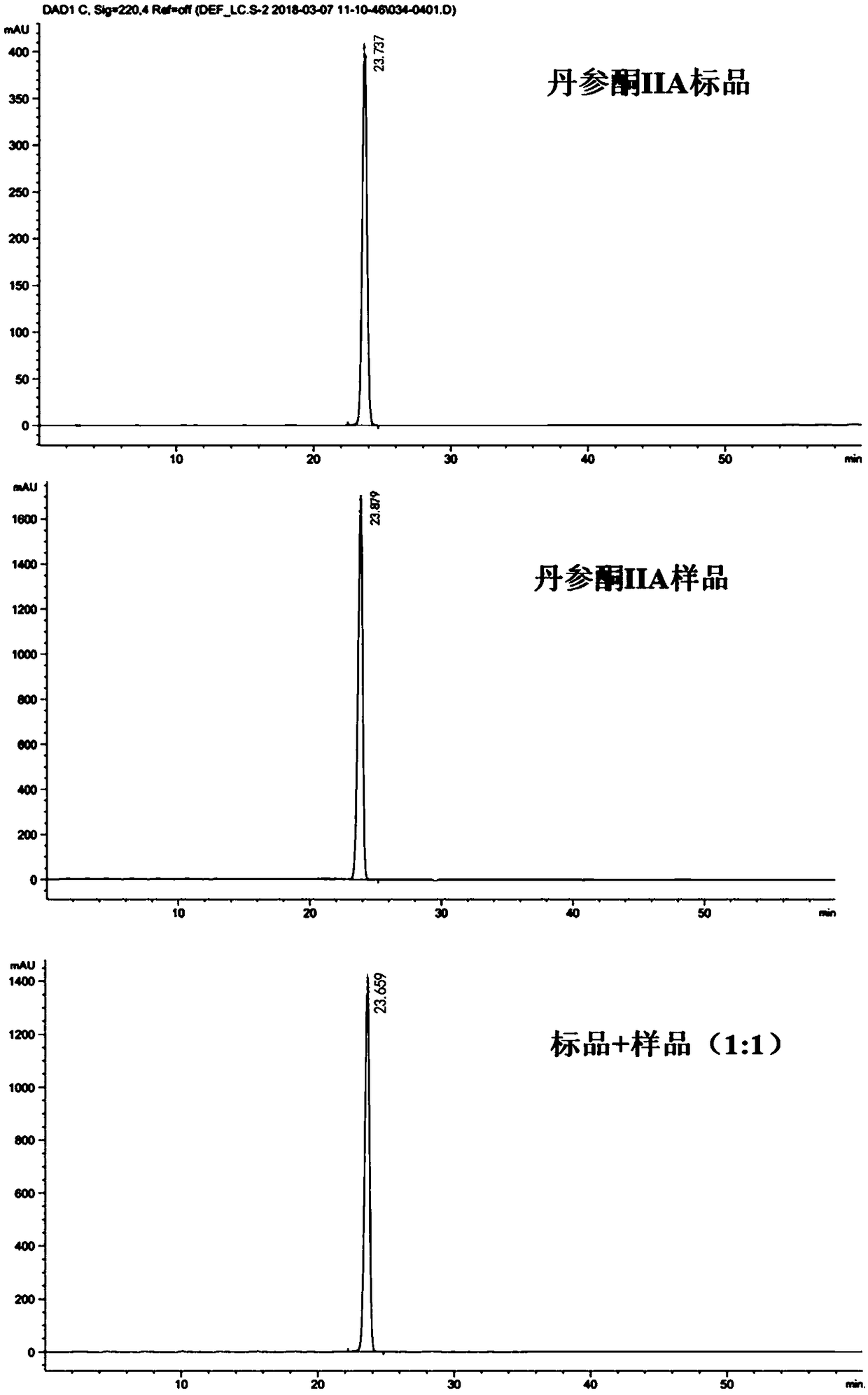
The invention relates to a method for separating and purifying tanshinone IIA from radix salviae miltiorrhizae by using polyamide gel, which belongs to the technical field of the development of traditional Chinese medicines. The method adopts petroleum ether and vinyl acetate as extraction agnets, utilizes a polyamide gel column to separate extract rich in a target substance, and utilizes ethanolto perform gradient elution separation to obtain a compound tanshinone IIA, wherein the extraction separation purity is about 93 percent. The extraction process is less in solvent residue, safe and reliable; and by utilizing the polyamide gel, the cost is relatively low, the yield and purity of the obtained tanshinone IIA are relatively high, and the method is an efficient method for extracting and separating the tanshinone IIA, and provides scientific evidence for reasonably utilizing the separation extraction technology of the tanshinone IIA.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Powdered ink for electronic photographic development and preparation method thereof
InactiveCN106940516ALower fusing temperatureGuaranteed heat-resistant storageDevelopersWaxCharge control
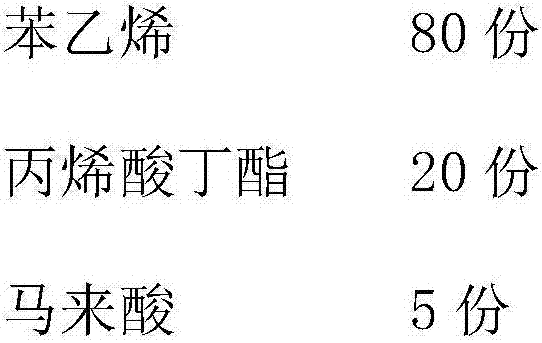
The invention provides powdered ink for electronic photographic development and a preparation method thereof. The powdered ink comprises, by weight, 100 parts of a vinyl resin monomer, 5 to 30 parts of a linear polyester, 1 to 20 parts of a pigment, 1 to 20 parts of wax, 0.5 to 4 parts of a charge control agent, 0.1 to 3 parts of an additive and 2.5 to 4 parts of a polymerization initiator. The powdered ink for electronic photographic development is advantageous for reducing the fixation temperature and the energy consumption of the apparatus and does not cause high temperature shift and caking agglomeration. The preparation method of the powdered ink can produce powdered ink particles containing the linear polyester by a simple suspension polymerization method so that powdered ink performances are improved. The preparation method realizes preparation of polyester resin powdered ink by a chemical method without use of an emulsifier.
Owner:天津市合成材料工业研究所有限公司
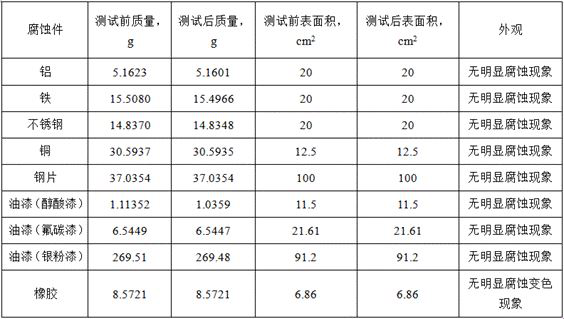
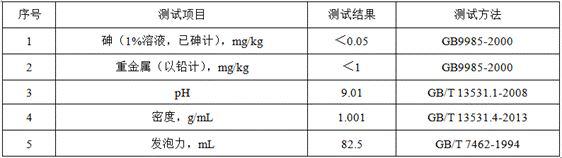
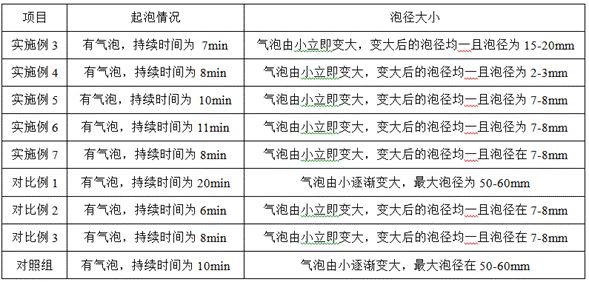
A kind of gas leak detection agent and its preparation method and application
ActiveCN114526871BSmall residualPrecise positioningDetection of fluid at leakage pointSodium metasilicateGlycerol
The invention relates to the technical field of gas leak detection agents, and proposes a gas leak detection agent and its preparation method and application. The gas leak detection agent includes component A, component B and component C, component A and component B, The mass ratio of component C is (39‑68): (47‑63): (35‑48), component A includes the following components by weight: 30‑45 parts of deionized water, 1‑3 n-heptanoic acid part, 1-3 part of dodecanedibasic acid, 6.5-16 part of triethanolamine, 0.3-0.5 part of borate, 0.2-0.5 part of benzotriazole; Component B comprises the following components by weight: 45-60 parts of deionized water, 0.56-1 part of foaming agent, 1.44-2 parts of propylene glycol; component C includes the following components by weight: 30-40 parts of deionized water, 0.1-0.5 parts of guar gum, five 0.1-0.5 parts of sodium metasilicate in water, 4.8-7.0 parts of glycerin. Through the above technical solution, the problem of inaccurate location of leak point and easy residue of the gas leak detection agent in the prior art is solved because of its large bubble diameter.
Owner:河北华油天然气有限责任公司
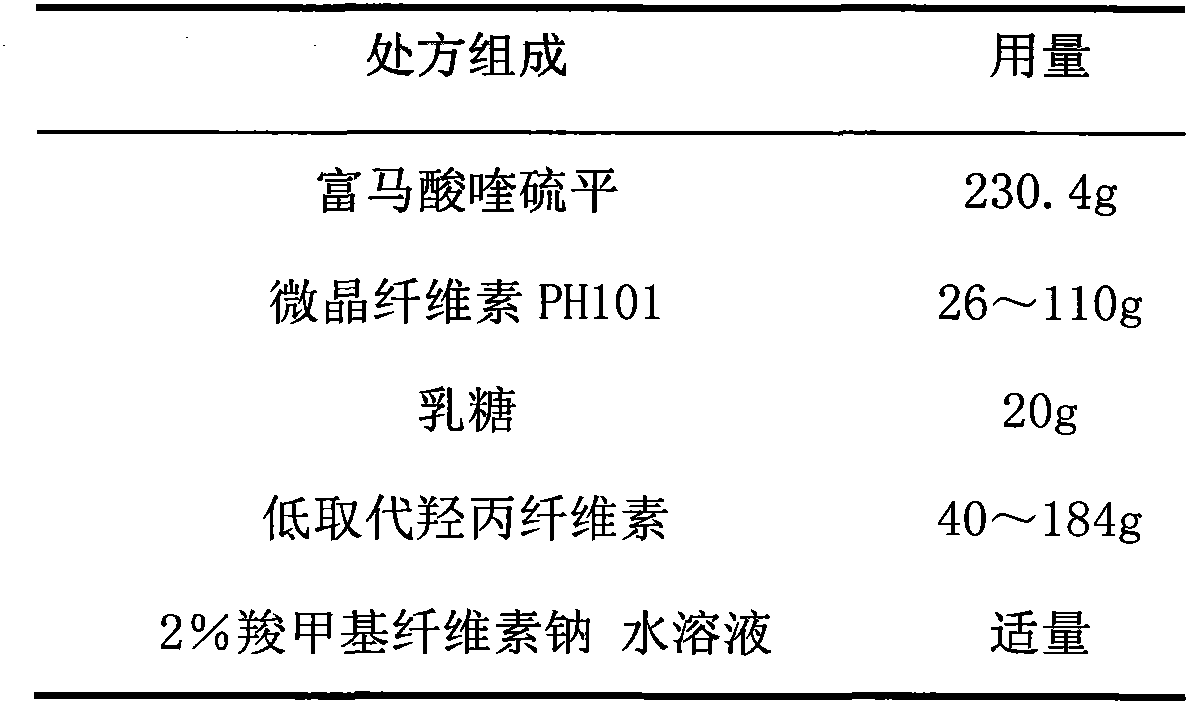
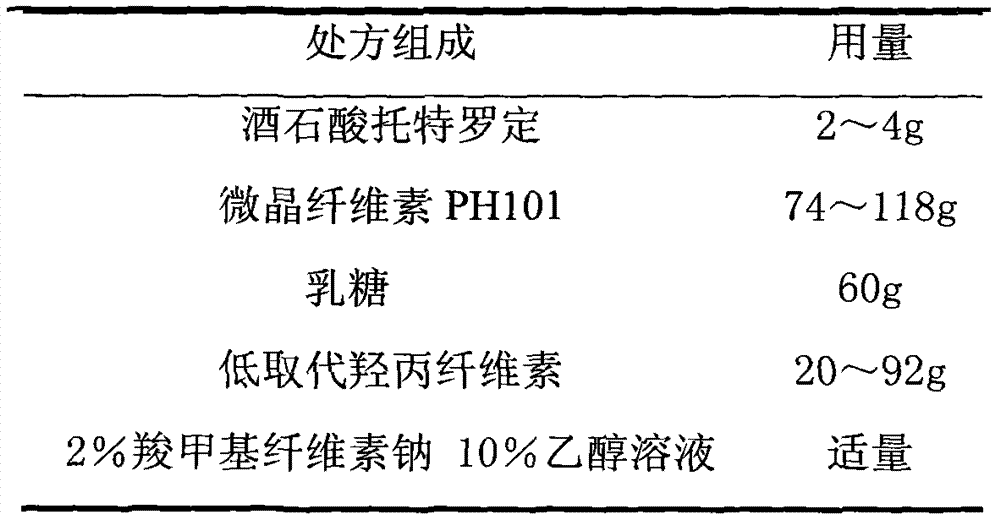
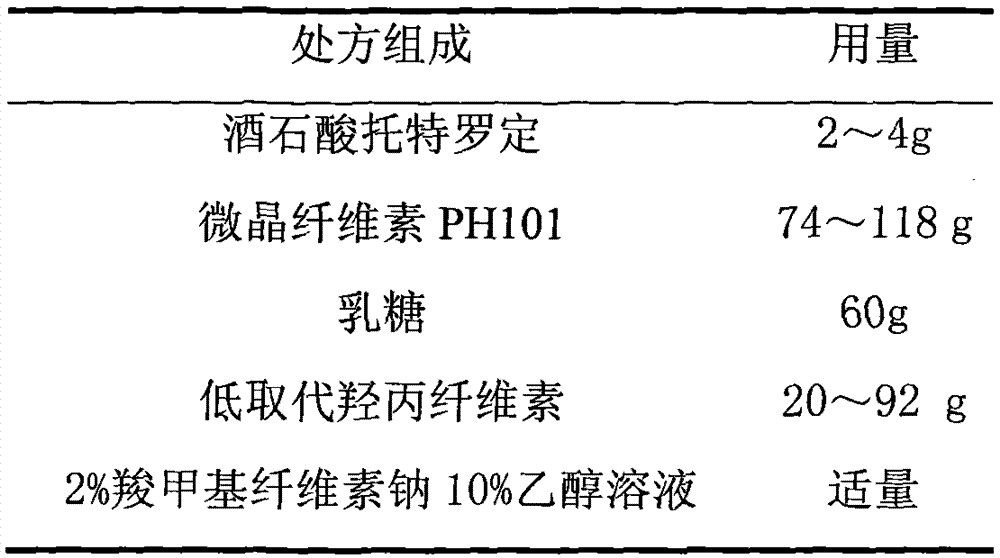
Tolterodine Tartrate Film-controlled Sustained-release Pellet Capsules
ActiveCN103211784BAccelerated agingReduce permeabilityOrganic active ingredientsUrinary disorderSustained release pelletsLow-substituted hydroxypropylcellulose
The present invention relates to a kind of tolterodine tartrate film-controlled slow-release pellet capsule, the slow-release film of its pellet adopts the mixture of water dispersion Eurdragit RL 30D:Eurdragit RS 30D=9:1 weight ratio as film-forming material, The ball core contains low-substituted hydroxypropyl cellulose with high expansibility, and excipients commonly used in pharmaceutically acceptable sustained-release pellets. The excipients are preferably microcrystalline cellulose and lactose, wherein the low-substituted hydroxypropyl cellulose in the ball core Propylene cellulose accounts for 10-40% by weight of the ball core. The sustained-release coating film comprises a mixture of water dispersion film-forming materials Eurdragit RL 30D and Eurdragit RS 30D, plasticizer triethyl citrate and anti-sticking agent talc, the ratio of which is preferably Eurdragit RL 30D: Eurdragit RS 30D: citric acid Triethyl ester:talc powder=27:3:2:4, and the coating weight gain is preferably 19-34%. Because the ball core contains low-substituted hydroxypropyl cellulose with high water swelling, it will swell obviously after absorbing water, resulting in the expansion of the slow-release coating film, the thickness becomes thinner, the pore size of the water-permeable micropores becomes larger, and the permeability is improved. It has become better and compensated for the decrease in permeability caused by membrane aging, so that the release rate in the middle and late stages is basically constant, and the residual at the end is small, and it can always maintain a stable release performance within the validity period.
Owner:北京天衡药物研究院有限公司
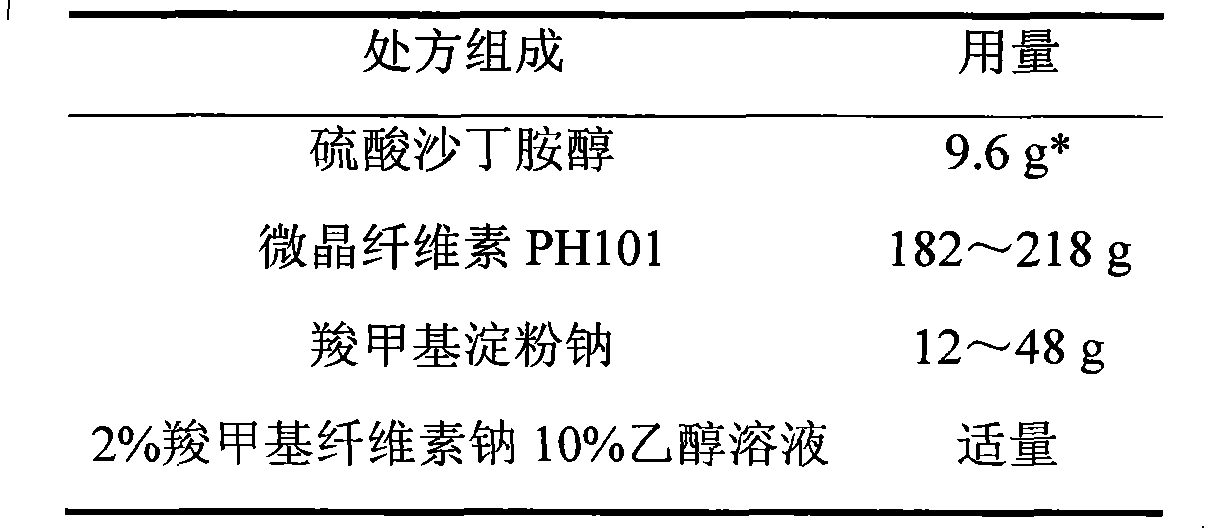
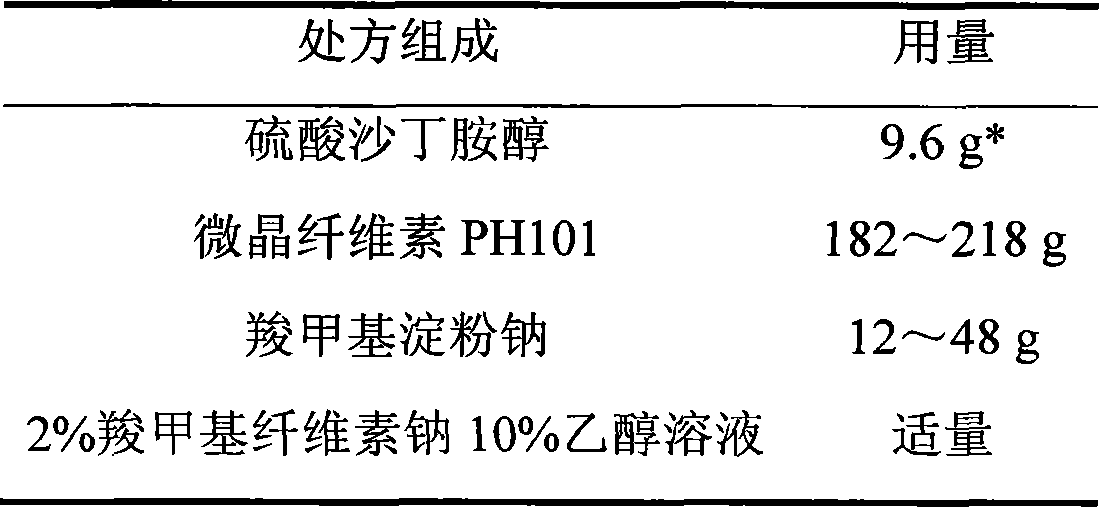
Salbutamol sulfate film-controlled slow-release pellet capsule
ActiveCN103211796AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineExcipient
The invention relates to a salbutamol sulfate film-controlled slow-release pellet capsule. A slow-release film of the salbutamol sulfate film-controlled slow-release pellet utilizes Kollicoat SR30D as a film-formation material. A pellet core of the salbutamol sulfate film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 5 to 20wt% of the sodium carboxymethyl starch. The slow-release film comprises Kollicoat SR30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR30D to triethyl citrate to talcum powder is 30: 1: 4 and a film weight increasing ratio is in a range of 19 to 38%. The salbutamol sulfate film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the salbutamol sulfate film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the salbutamol sulfate film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
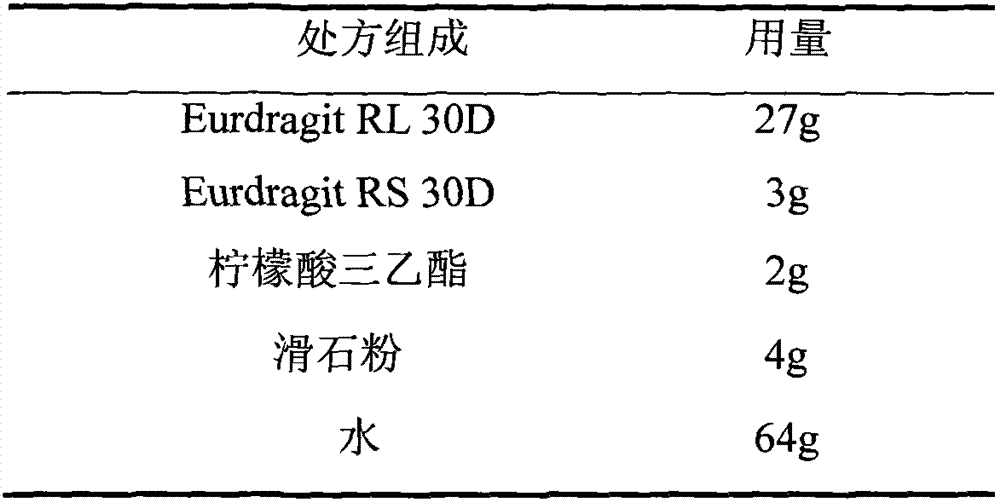
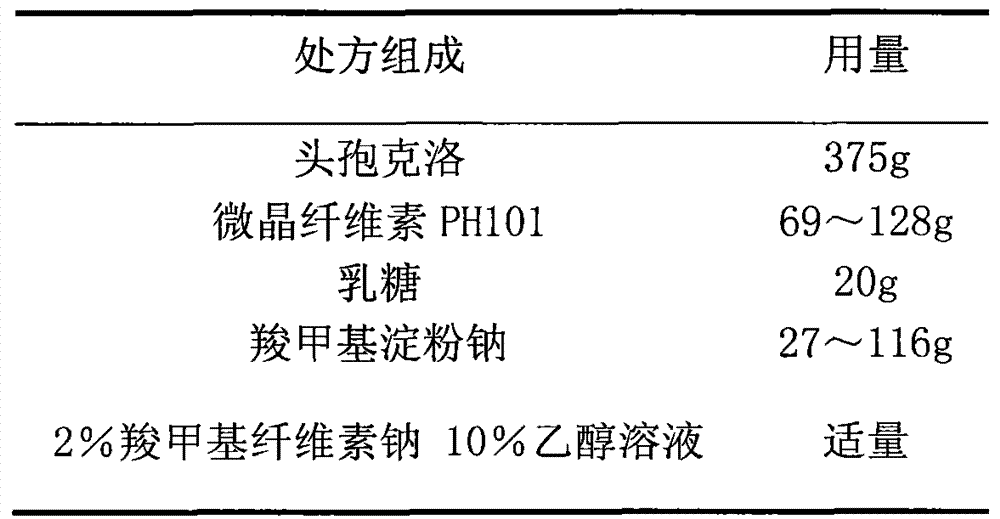
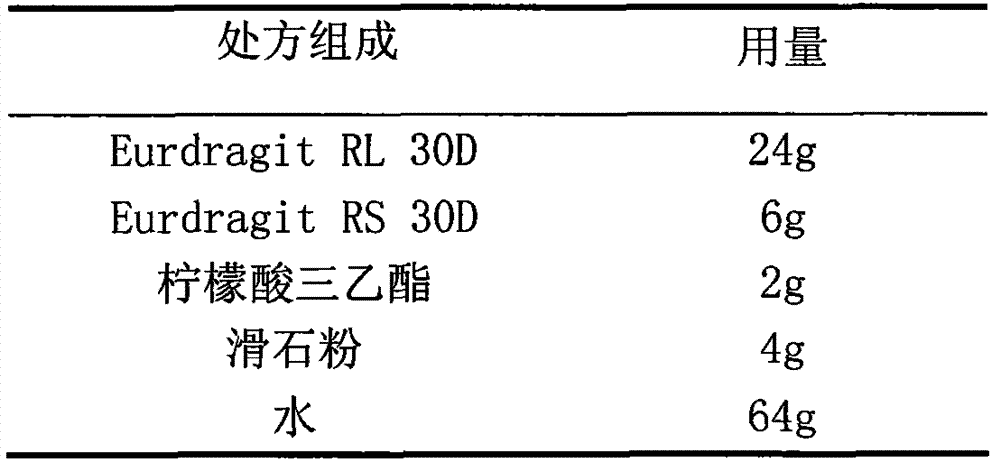
Cefaclor film-controlled sustained-release pellets and capsules
ActiveCN103211795BAccelerated agingReduce permeabilityAntibacterial agentsOrganic active ingredientsSustained release pelletsTalc
The invention relates to a cefaclor film-controlled sustained-release pellet capsule, the sustained-release film of the pellet adopts the mixture of water dispersion Eurdragit RL 30D:Eurdragit RS 30D=4:1 (weight ratio) as the film-forming material, the pellet The core contains high-expandability sodium carboxymethyl starch, and the commonly used excipients of pharmaceutically acceptable sustained-release pellets. The excipients are preferably microcrystalline cellulose and lactose, wherein the sodium carboxymethyl starch in the ball core is It accounts for 5-20% of the weight of the ball core. The sustained-release coating film comprises a mixture of water dispersion film-forming materials Eurdragit RL 30D and Eurdragit RS 30D, a plasticizer triethyl citrate and an anti-sticking agent talc, and the ratio is preferably Eurdragit RL 30D: Eurdragit RS 30D: citric acid Triethyl ester: talcum powder = 24:6:2:4, and the coating weight gain is preferably 23-40%. Because the ball core contains sodium carboxymethyl starch, which has high water swelling property, it will swell obviously after absorbing water, resulting in the expansion of the slow-release coating film, the thickness becomes thinner, the pore size of the water-permeable micropores becomes larger, and the permeability becomes better. , which compensates for the decrease in permeability caused by membrane aging, so that the release rate in the middle and late stages is basically constant, and the residue in the end stage is small, which can always maintain a stable release performance within the validity period.
Owner:北京天衡药物研究院有限公司
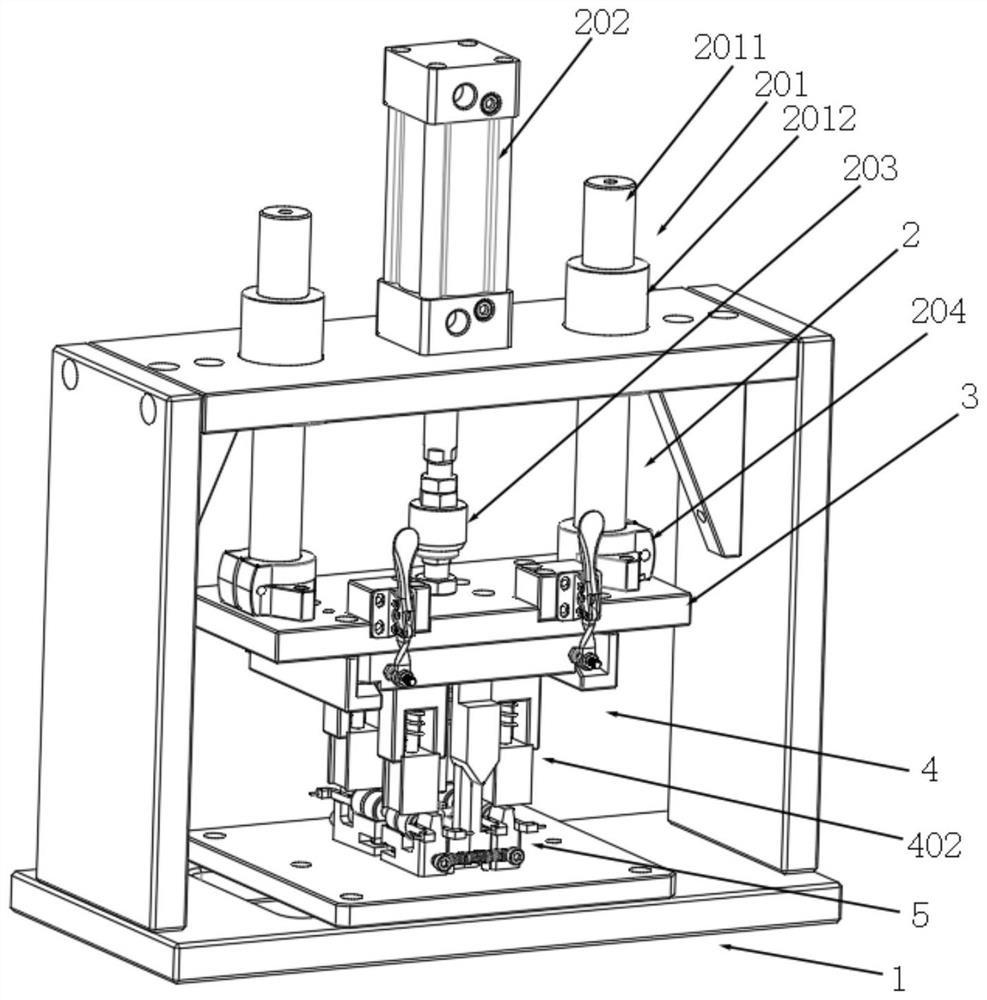
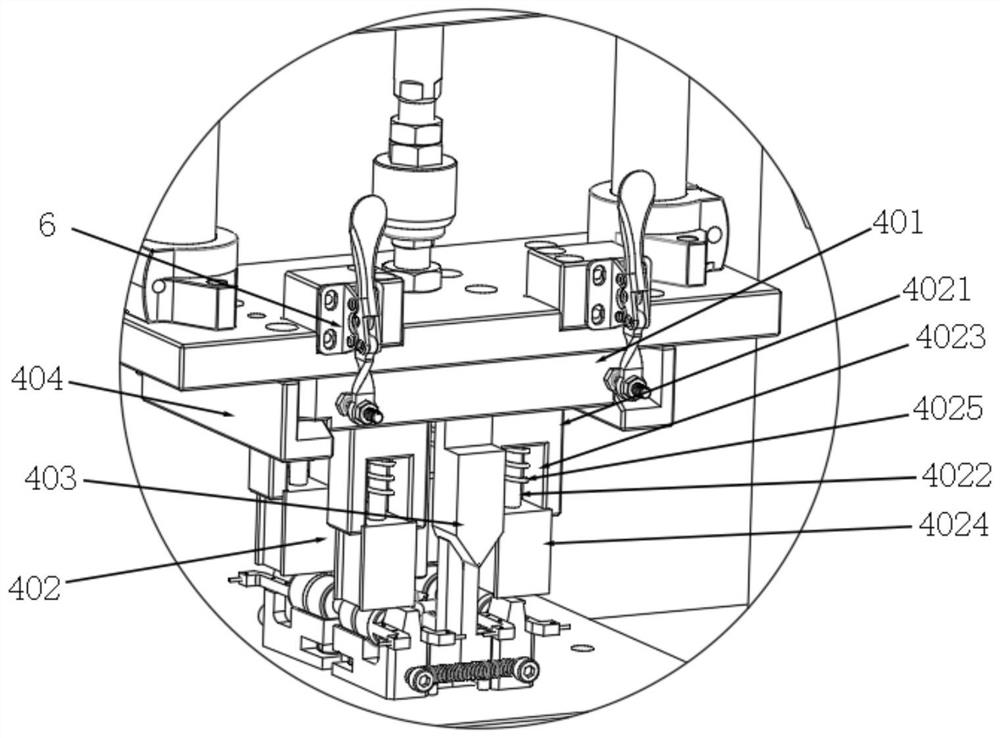
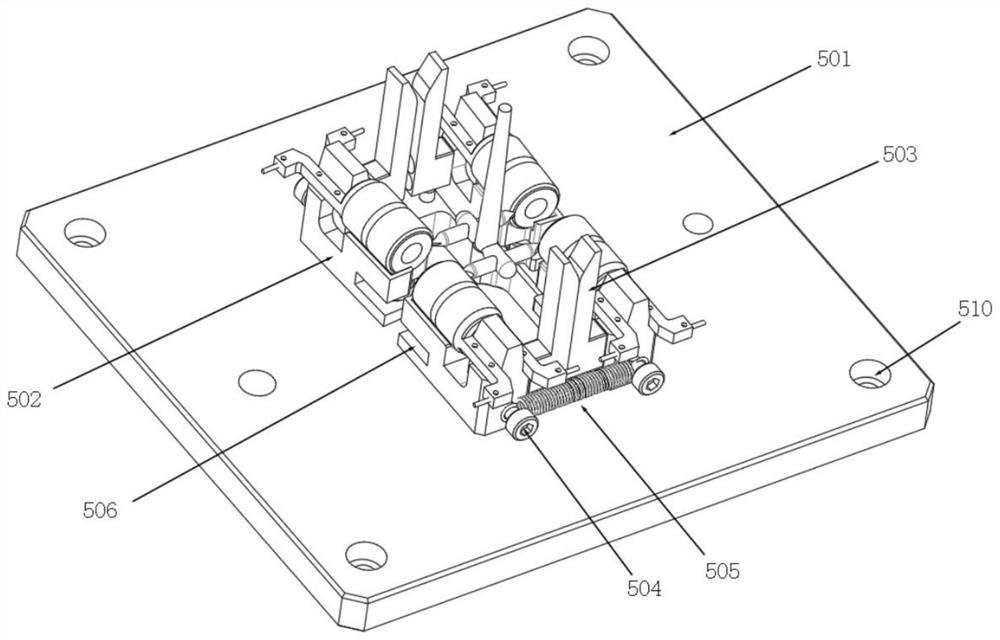
A punching collector ring nozzle device
The invention discloses a punching collector ring nozzle device, comprising a base, a flushing mechanism that moves up and down is installed on the top of the base, a fixed plate is matched and connected to the lower end of the flushing mechanism, and a limited separation mechanism is matched to the lower end of the fixed plate. The bottom of the base is provided with a bearing mechanism corresponding to the limit separation mechanism. The limit separation mechanism is used to press and separate the workpieces to be processed on the bearing mechanism. In this way of separating the product from the nozzle, there is no punching knife, and there is no problem that the punching knife is too close to the product, which directly avoids damage to the product, and through this method of punching the nozzle, the nozzle of the product is naturally broken, and the entire ring The nozzle material will fall off, and the residual margin is very small, which avoids the increase of secondary processing in the subsequent process.
Owner:XUZHOU YUNTAI AUTOMOBILE ELECTRICAL APPLIANCE
Salbutamol Sulfate Film-controlled Sustained Release Pellet Capsules
ActiveCN103211796BAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsTalc
The invention relates to a salbutamol sulfate film-controlled sustained-release pellet capsule. The sustained-release film of the pellet adopts Kollicoat SR30D as a film-forming material, and the pellet core contains high-expandability sodium carboxymethyl starch, and pharmaceutically acceptable The commonly used excipients of the sustained-release pellets, the excipient is preferably microcrystalline cellulose, wherein, the sodium carboxymethyl starch in the pellet core accounts for 5-20% of the weight of the pellet core. The sustained-release coating film comprises Kollicoat SR30D, plasticizer triethyl citrate and anti-sticking agent talcum powder, and its ratio is preferably Kollicoat SR 30D: triethyl citrate: talcum powder=30:1:4, coating weight gain Preferably it is 19 to 38%. Because the ball core contains sodium carboxymethyl starch, which has high water swelling property, it will swell obviously after absorbing water, resulting in the expansion of the slow-release coating film, the thickness becomes thinner, the pore size of the water-permeable micropores becomes larger, and the permeability becomes better. , which compensates for the decrease in permeability caused by membrane aging, so that the release rate in the middle and late stages is basically constant, and the residue in the end stage is small, which can always maintain a stable release performance within the validity period.
Owner:北京天衡药物研究院有限公司
Venlafaxine Hydrochloride Film-controlled Sustained Release Pellet Capsules
ActiveCN103211791BAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsControlled release
Owner:北京天衡药物研究院有限公司
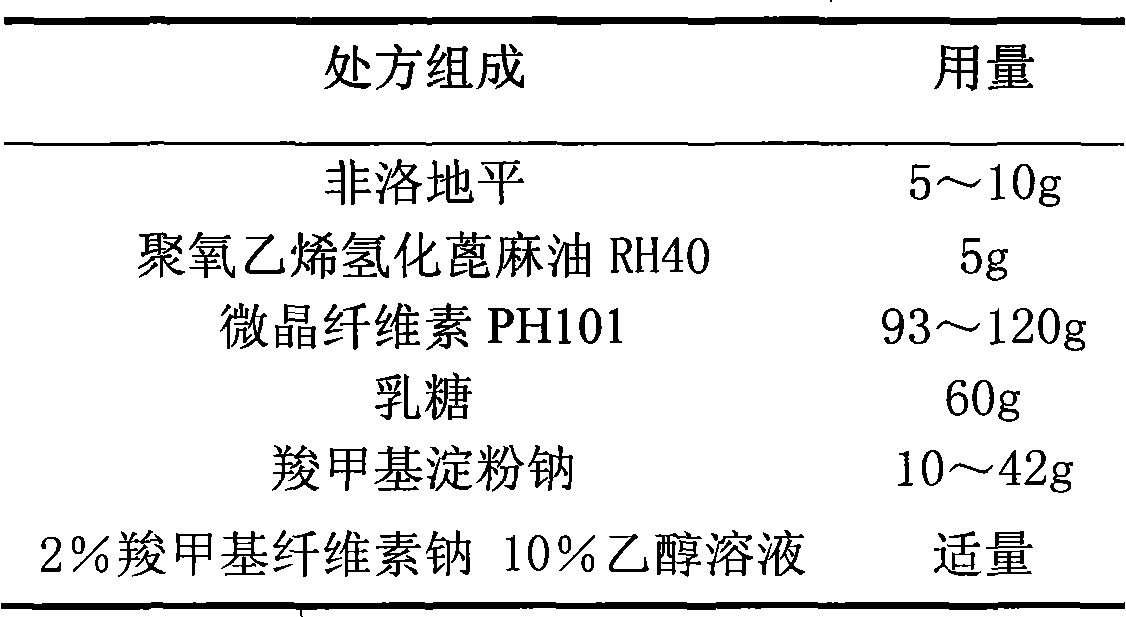
Felodipine film-controlled slow-release pellet capsule
ActiveCN103211793AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical delivery mechanismSolventSustained Release Pellet Capsule
The invention relates to a felodipine film-controlled slow-release pellet capsule. A slow-release film of the felodipine film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the felodipine film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose as fillers and polyoxyethylene hydrogenated castor oil RH40 as a solubilizer, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 20 to 36%. The felodipine film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the felodipine film-controlled slow-release pellet expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the felodipine film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
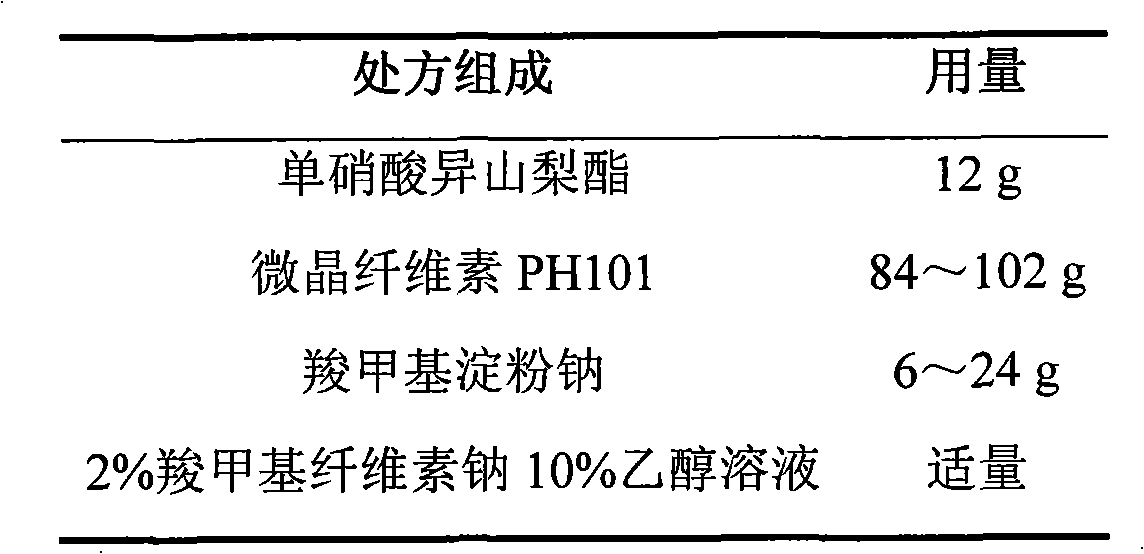
Isosorbide mononitrate sustained-release pellets and Isosorbide mononitrate immediate-release-sustained-release pellets capsules using the same
ActiveCN103211768BAccelerated agingReduce permeabilityPharmaceutical non-active ingredientsGranular deliverySustained release pelletsMedicine
The invention relates to an isosorbide mononitrate sustained-release pellet, and an isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it. The sustained-release film of the sustained-release pellet adopts Eurdragit RS 30D as a film forming material, the core of the sustained-release pellet contains high-expansibility sodium carboxymethyl starch and a pharmaceutically-acceptable excipient commonly used for sustained-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of sodium carboxymethyl starch in the core of the sustained-release pellet is 5-20%. The sustained-release film of the sustained-release pellet includes the Eurdragit RS 30D, a plasticizer triethyl citrate and an anti-adherent talcum powder, the optimal ratio of the Eurdragit RS 30D to triethyl citrate to the talcum powder is 30:3:4, and the optimal coating weight gain is 19-38%. The core will obviously expand after absorbing water because of the containment of sodium carboxymethyl starch highly expanding after contacting with water, so the sustained-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:北京天衡药物研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com