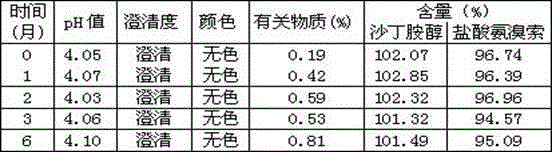
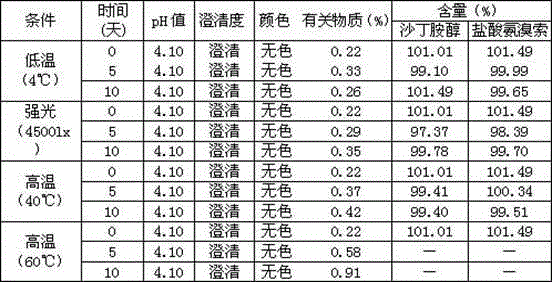
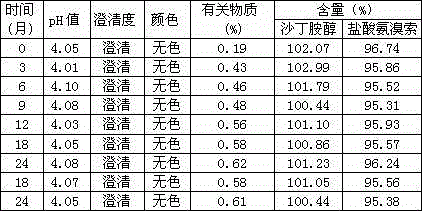
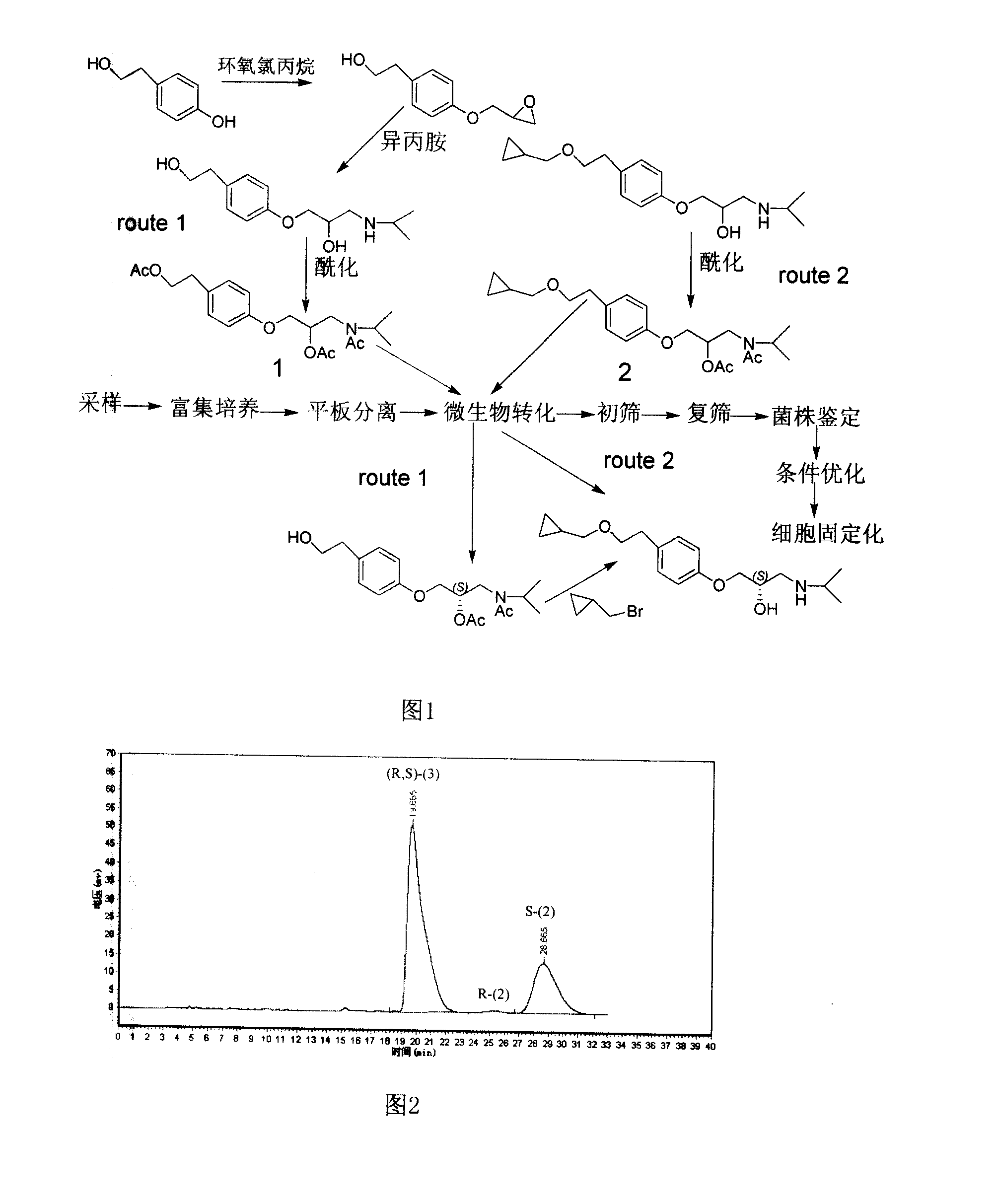
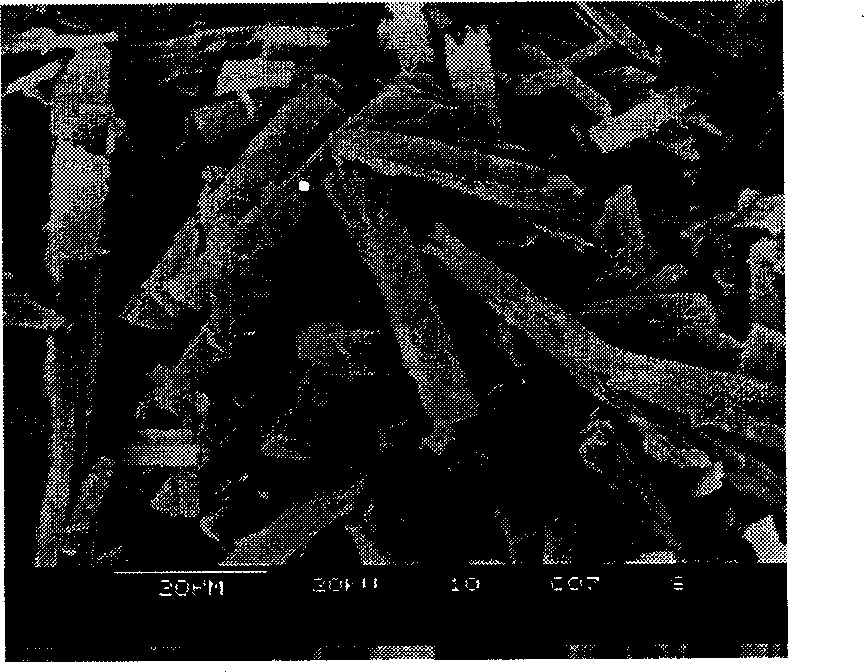
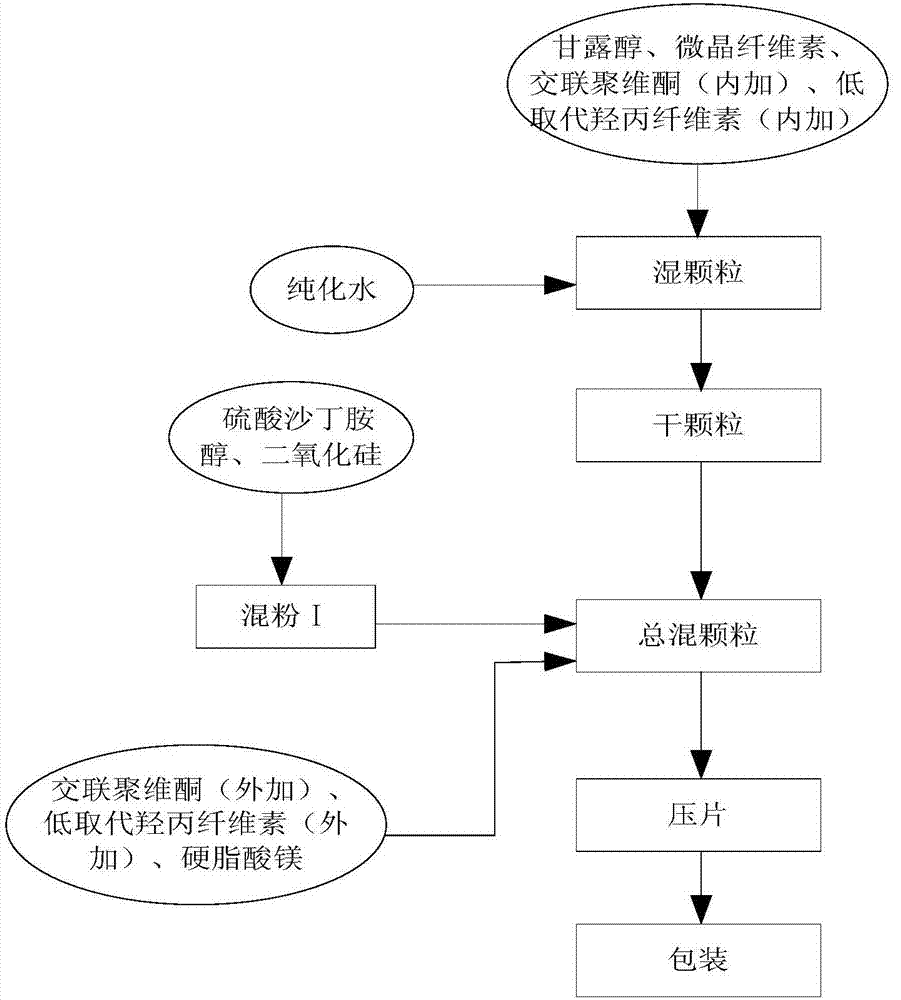
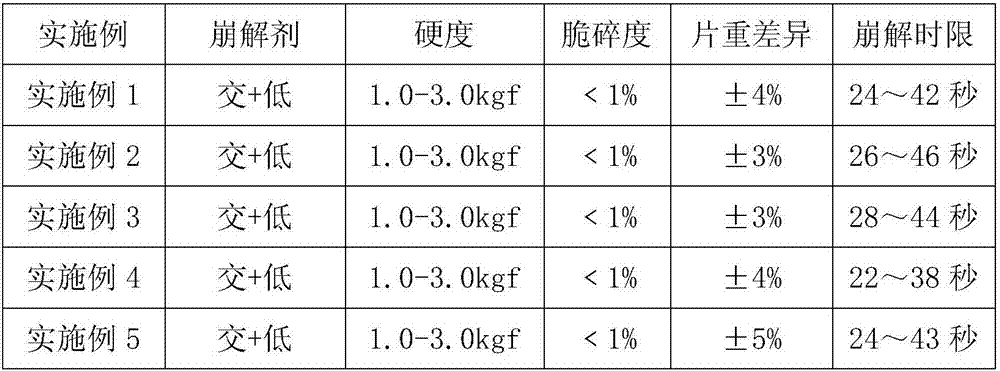
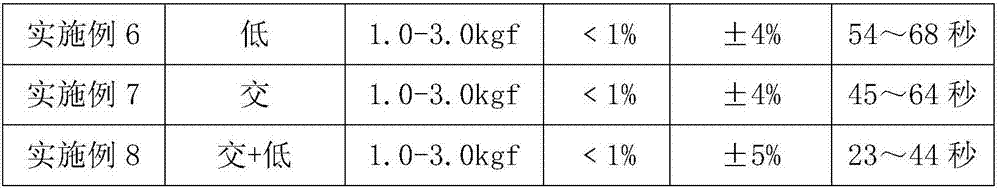
Patents
Literature
108 results about "Salbutamol sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
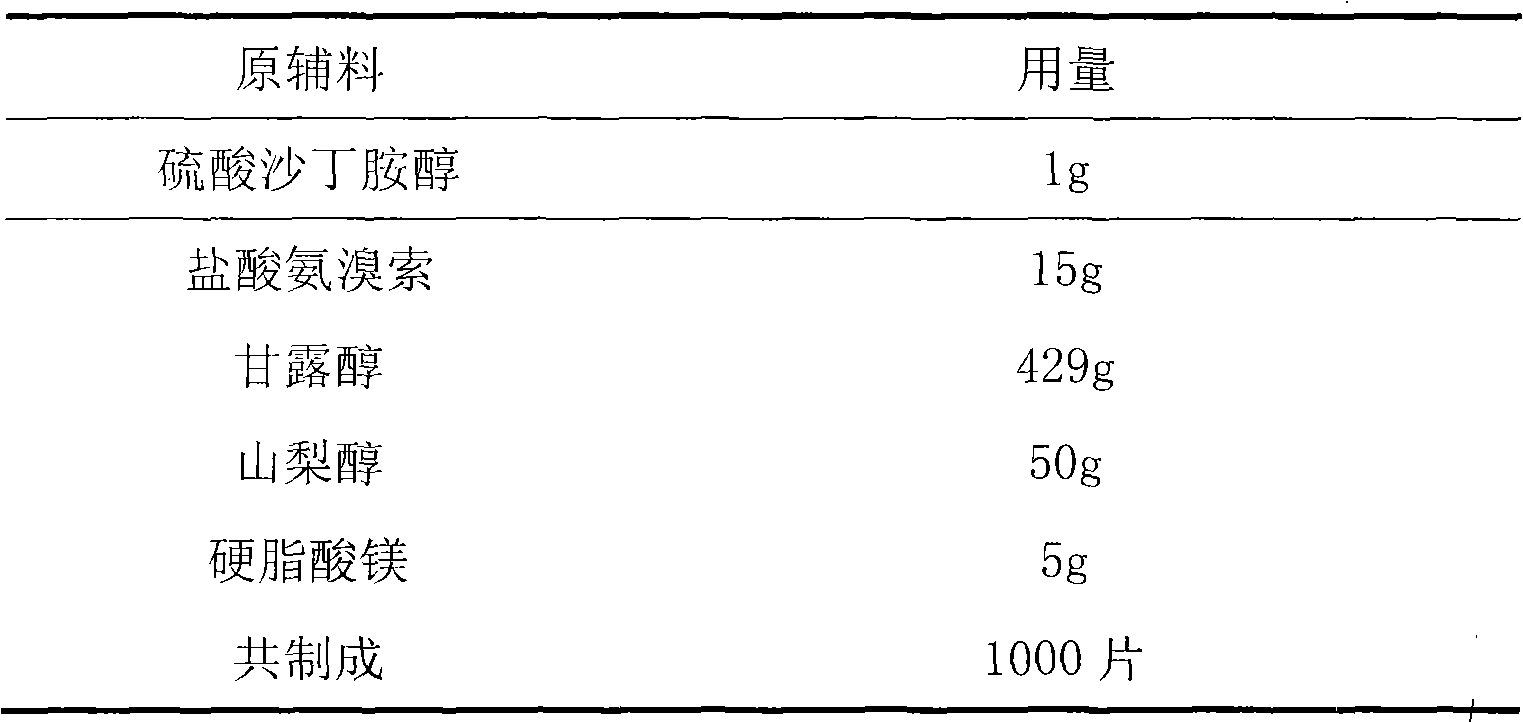
Oral solution containing ambroxol hydrochloride and salbutamol sulfate
InactiveCN104622855ASimple prescriptionGuaranteed stabilityOrganic active ingredientsPharmaceutical delivery mechanismDiseaseMedicine
The invention provides an oral solution containing ambroxol hydrochloride and salbutamol sulfate, and belongs to the technical field of medicines. An auxiliary material of the oral solution mainly comprises preservatives and corrigents. The oral solution is used for treating respiratory system diseases such as acute and chronic bronchitis, asthmatoid bronchitis and bronchial asthma, has a simple prescription, a good medication effect and a good taste, and is quick in response.
Owner:CP PHARMA QINGDAO CO LTD
Saccharomycete with stereoselectivity lipase liveness and application in producing S- type betaxolol hydrochloride with biological split method thereof
InactiveCN101220336ALow priceMild reaction conditions for production conversionFungiHydrolasesBacterial strainSalbutamol sulfate
The invention discloses a yeast which has the stereoselective lipase activity and the application of the yeast in the preparation of an S-type betaxolol hydrochloride by using a biological separation method. The method selects one yeast with the stereoselective lipase activity by screening from the soil, utilizes an immobilized cell which is obtained by immobilizing the wet bacteria or sodium alginate-activated carbon-polyethylenimine as an enzyme preparation, carries out an enantiomer separation to a substrate which contains acyl group and prepares the S-type betaxolol hydrochloride. The conversion method is simple, the cost is low and the stereoselectivity is better. The usage of the bacterial strain can carry out the chiral separation of Beta-receptor blocker of betaxolol etc., ephedrine hydrochloride, epinephrine, levodropropizine, salbutamol sulfate, captopril, zofenopril and other compounds which contain hydroxy group or acyl group at the chiral center, thereby having important application value for promoting the development process of the chiral drugs of China.
Owner:ZHENGZHOU UNIV
Particulate materials
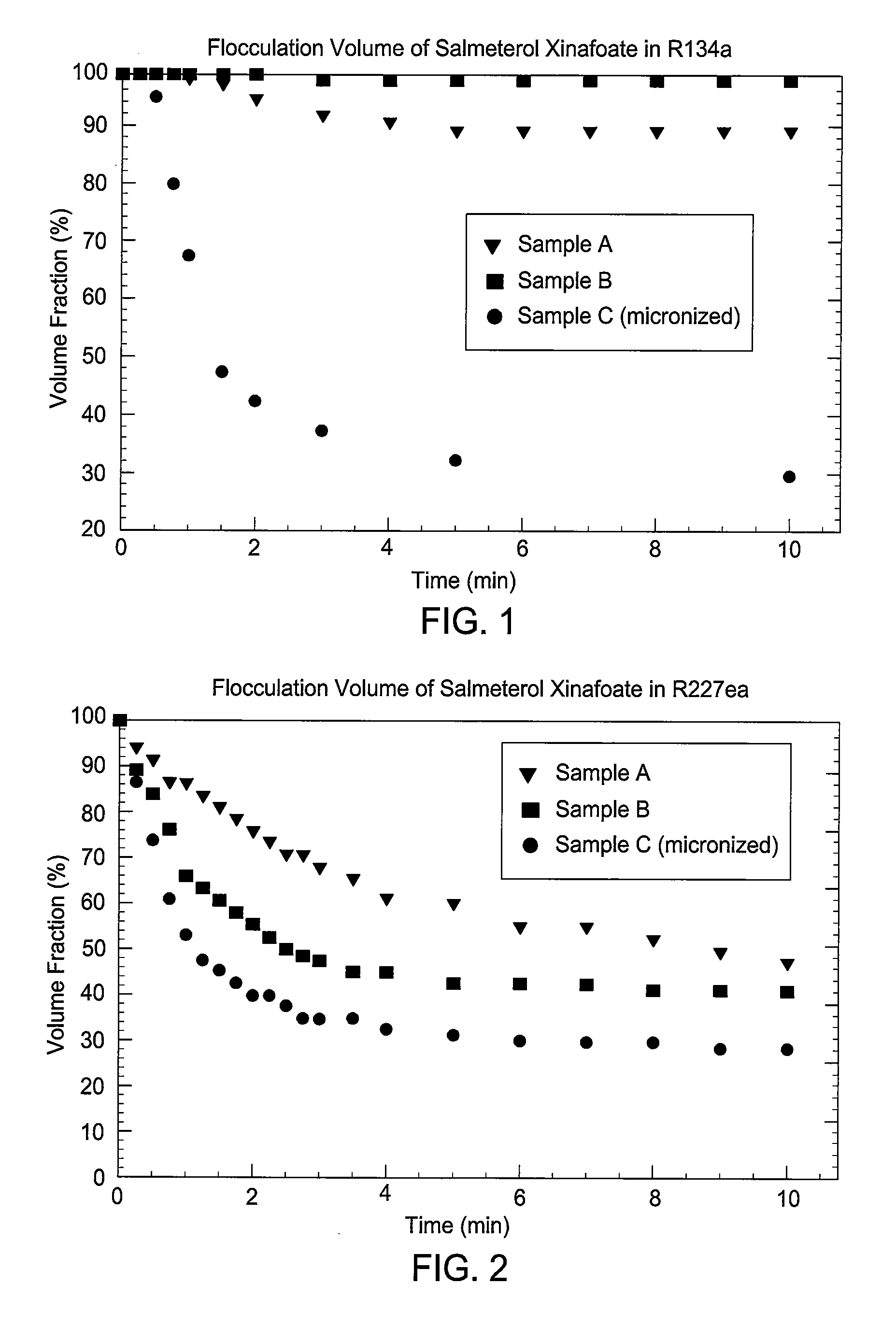
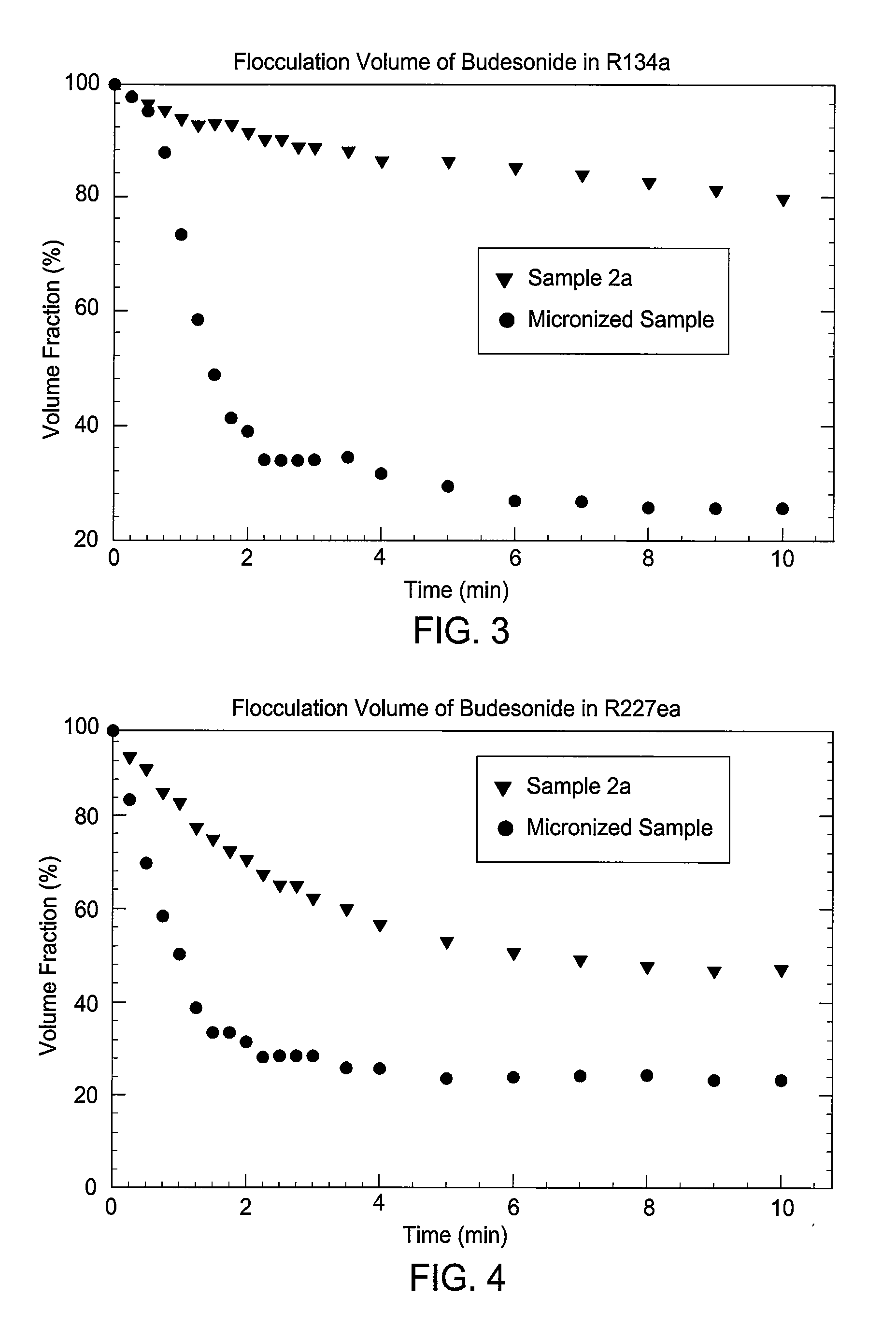
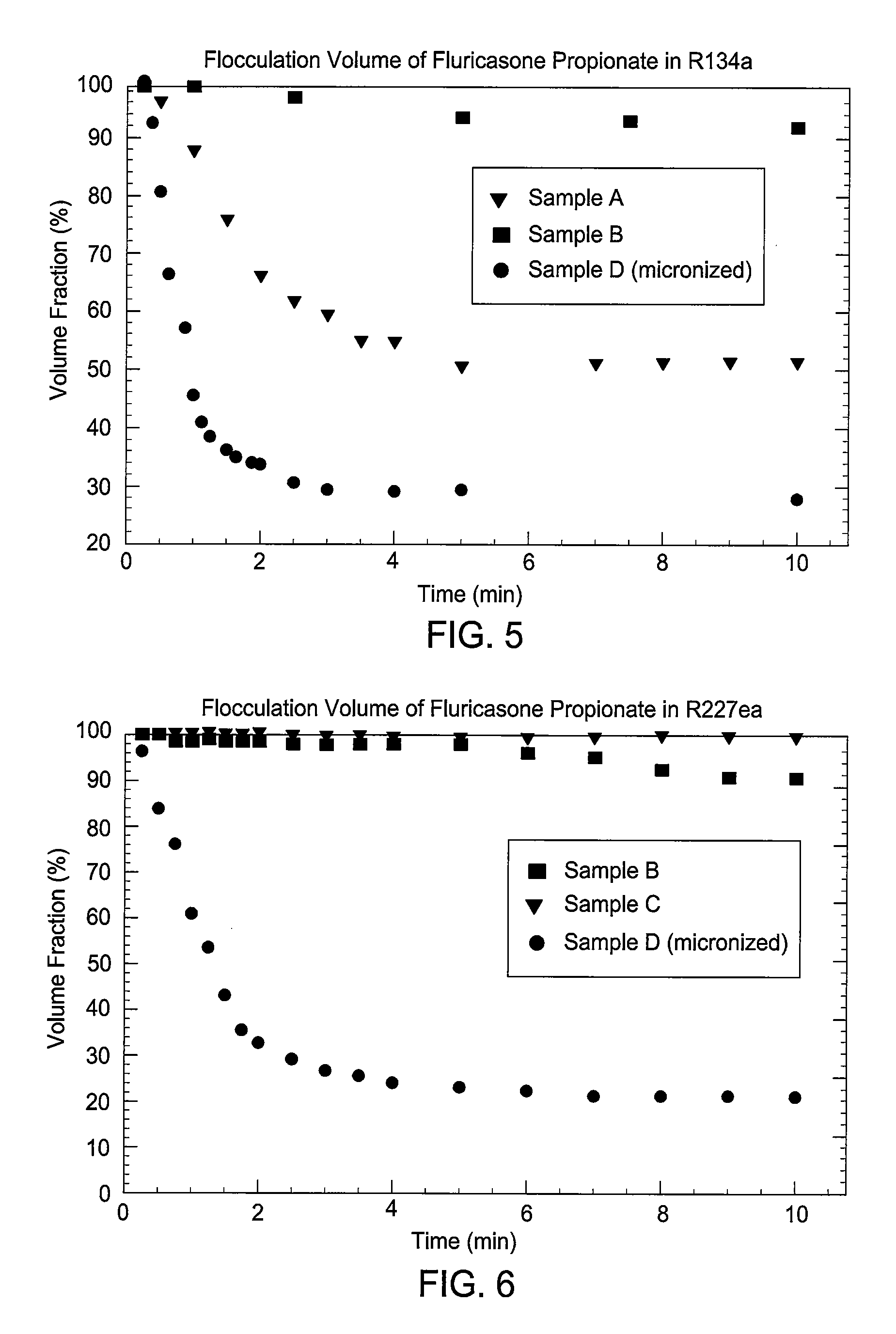
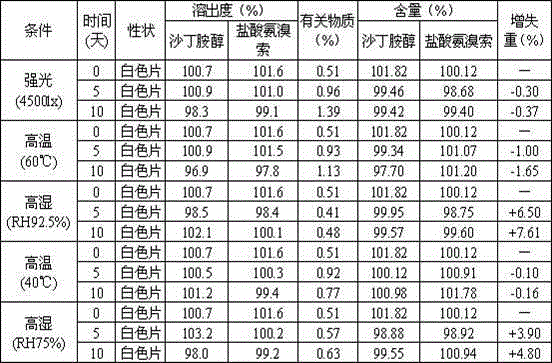
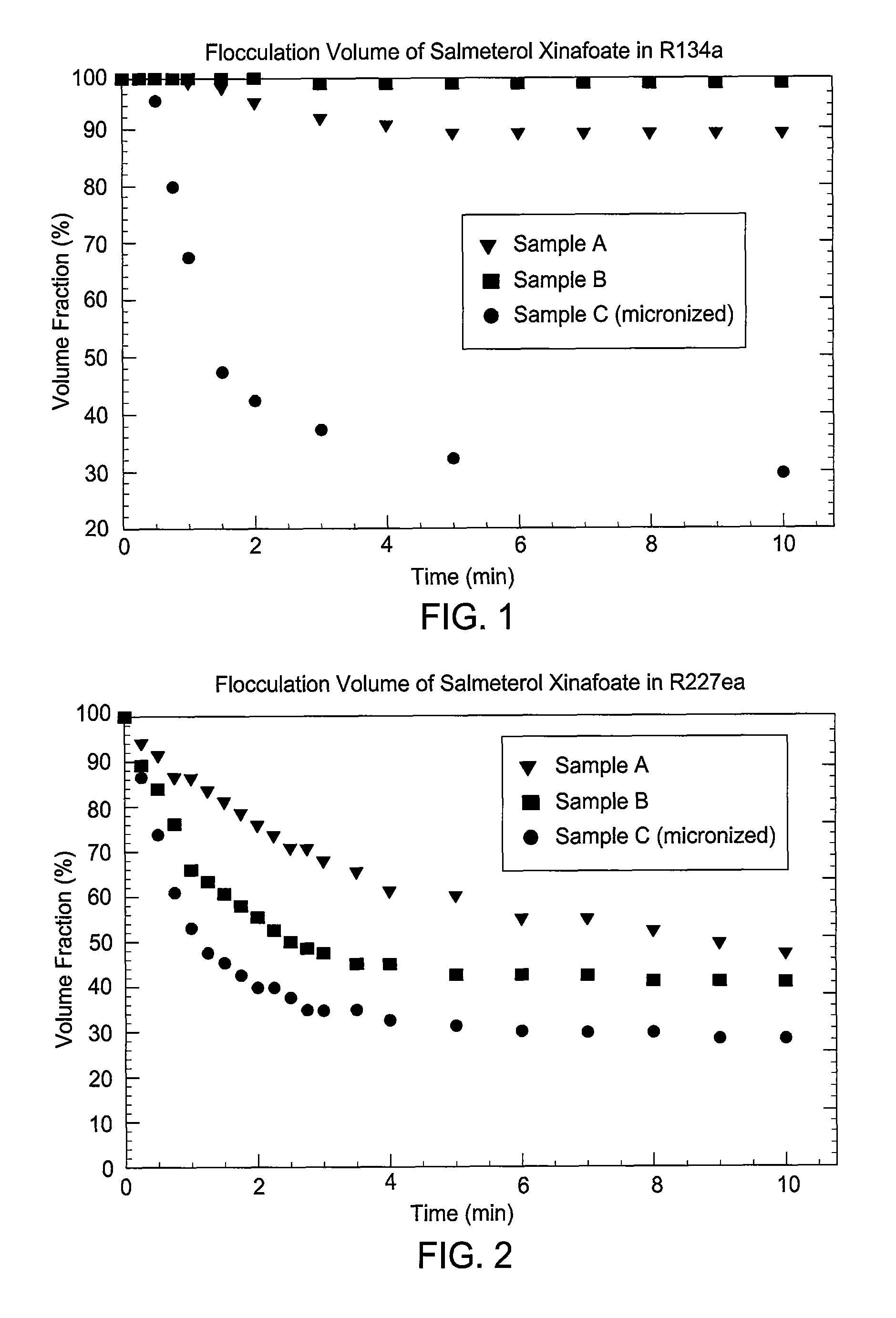
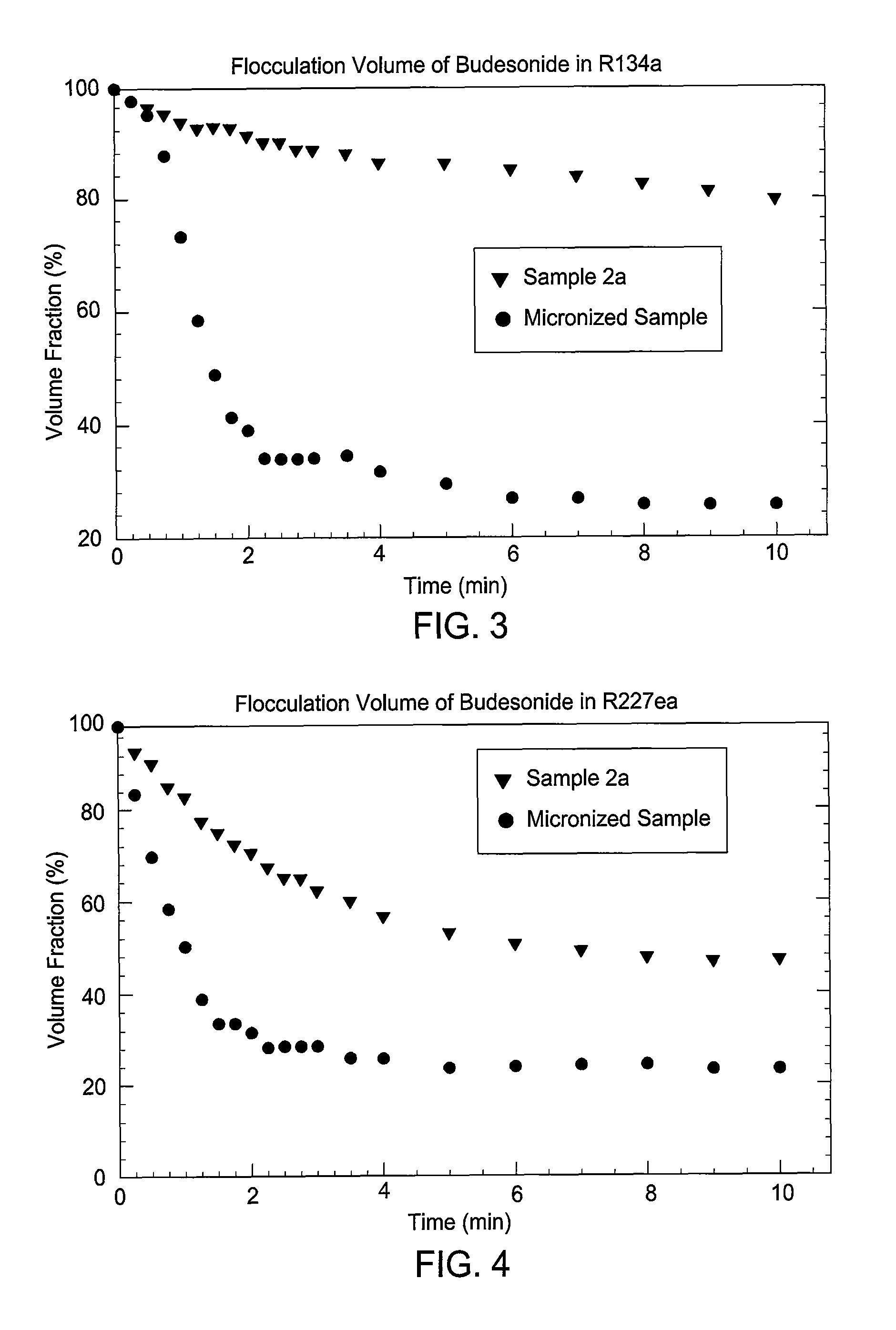
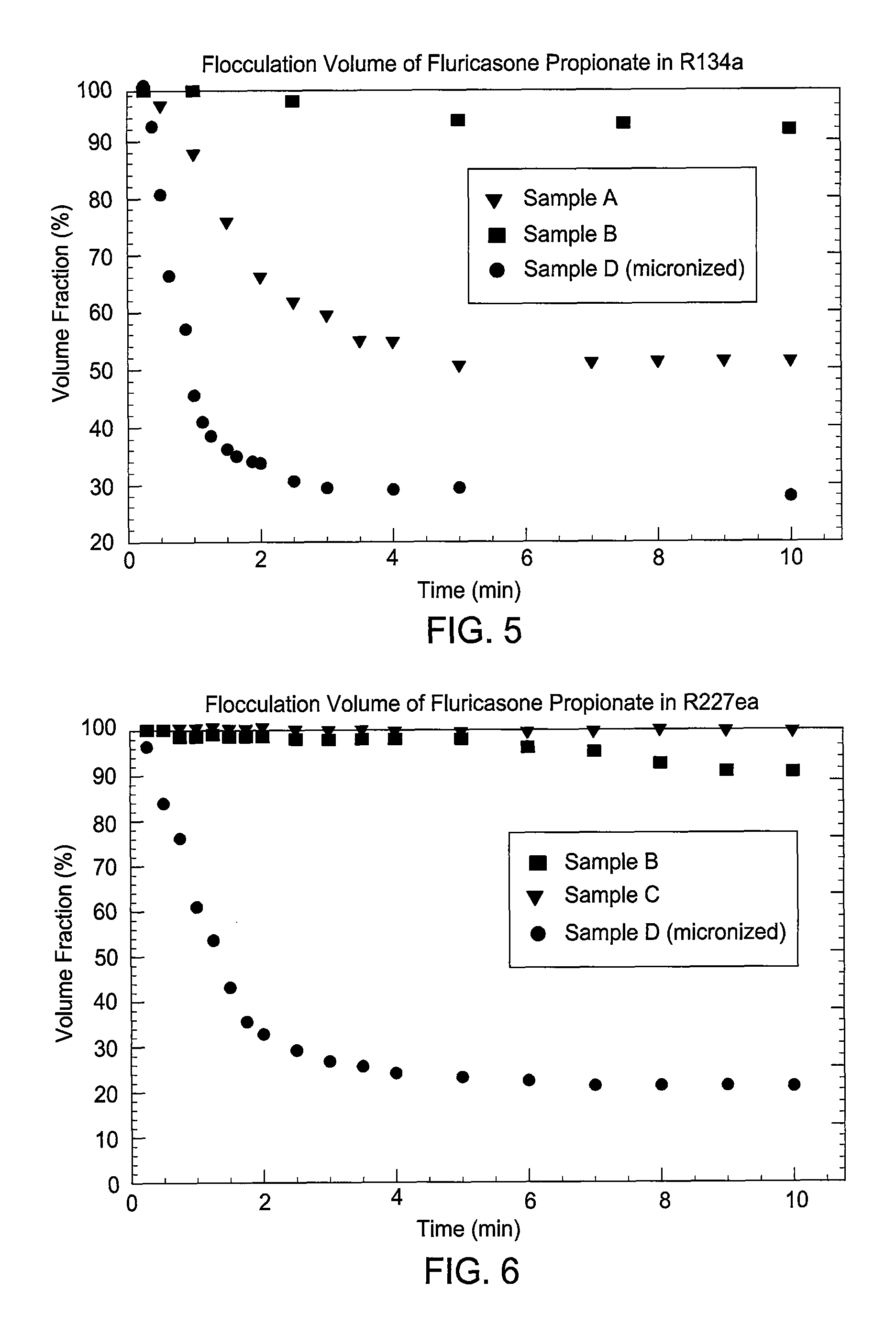
Embodiments of the invention relate to particles of active substances, methods for preparing the particles, formulations containing the particles, and metered dose inhalers containing the particles or formulations. In one embodiment, an inhaler contains an aerosol formulation containing a particulate active substance of non-micronized, solid particles having a mass median aerodynamic diameter of less than 10 μm. The particles may be suspended in a nonsolvent hydrofluorocarbon fluid vehicle (e.g., HFA 134a or 227ea) at a concentration within a range from about 0.2% w / v to about 5% w / v. The formulation exhibits a flocculation volume of about 85% or greater about 1 minute after mixing the particulate active substance and the vehicle. The particulate active substance may contain salmeterol xinafoate, budesonide, salbutamol sulfate, dihydroergotamine mesylate, risperidone-(9-hydroxy)-palmitate, bromocriptine mesylate, or derivatives thereof. In some examples, the active substance is dihydroergotamine mesylate.
Owner:NEKTAR THERAPEUTICS INC
Pharmaceutical composition for preventing and treating COVID-19 and preparation method of pharmaceutical composition
ActiveCN111265500AGood treatment effectImprove toleranceOrganic active ingredientsDispersion deliveryBULK ACTIVE INGREDIENTSalbutamol sulfate
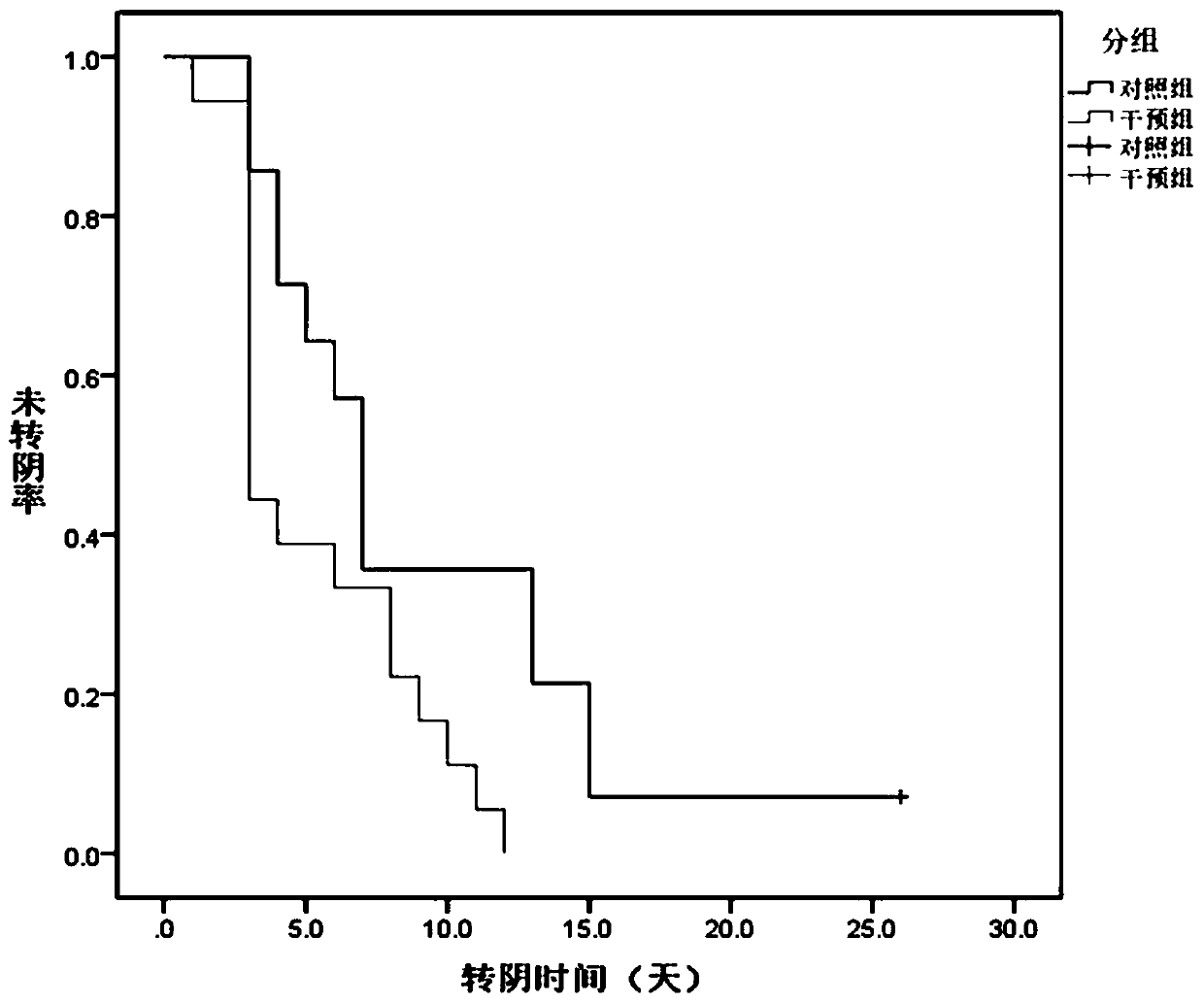
The invention belongs to the technical field of medicines. The invention relates to a pharmaceutical composition, in particular to a pharmaceutical composition for preventing and treating COVID-19 (diseases caused by novel coronaviruses) and a preparation method thereof. The pharmaceutical composition comprises active ingredients of mycobacterium vaccae for injection and salbutamol sulfate, and the weight ratio of the mycobacterium vaccae for injection to the salbutamol sulfate is 1: (50-200). The medicine prepared from the medicine composition can adopt an aerosol inhalation treatment mode, and through short-term aerosol inhalation, the compliance of a patient can be improved by providing the medicine with a specific purpose for the patient, so that the patient can take the medicine conveniently, and the medical cost is reduced. The pharmaceutical composition for preventing and treating COVID-19 disclosed by the invention has the advantages that the COVID-19 can be effectively prevented and treated; severe or critical symptoms of patients are relieved, nucleic acid negative conversion time is shortened by about 4 days averagely, treatment time is correspondingly shortened, other respiratory virus infections can be prevented and treated nonspecifically, and a new way and scheme are provided for clinical prevention and treatment of COVID-19 and respiratory virus infections.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGXI MEDICAL UNIV
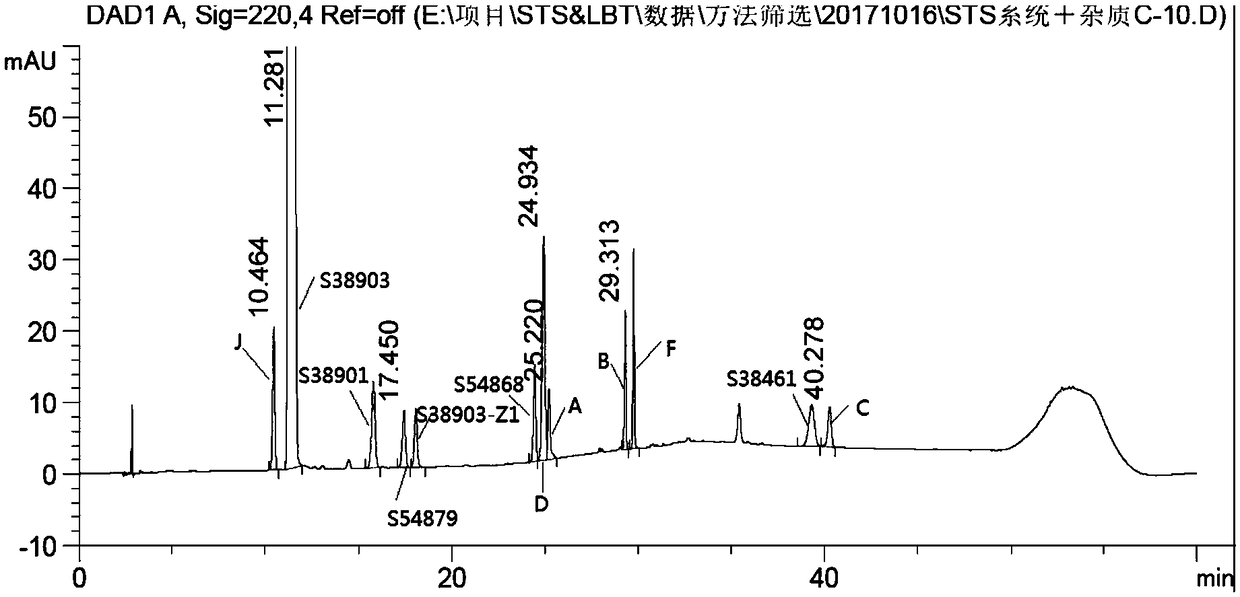
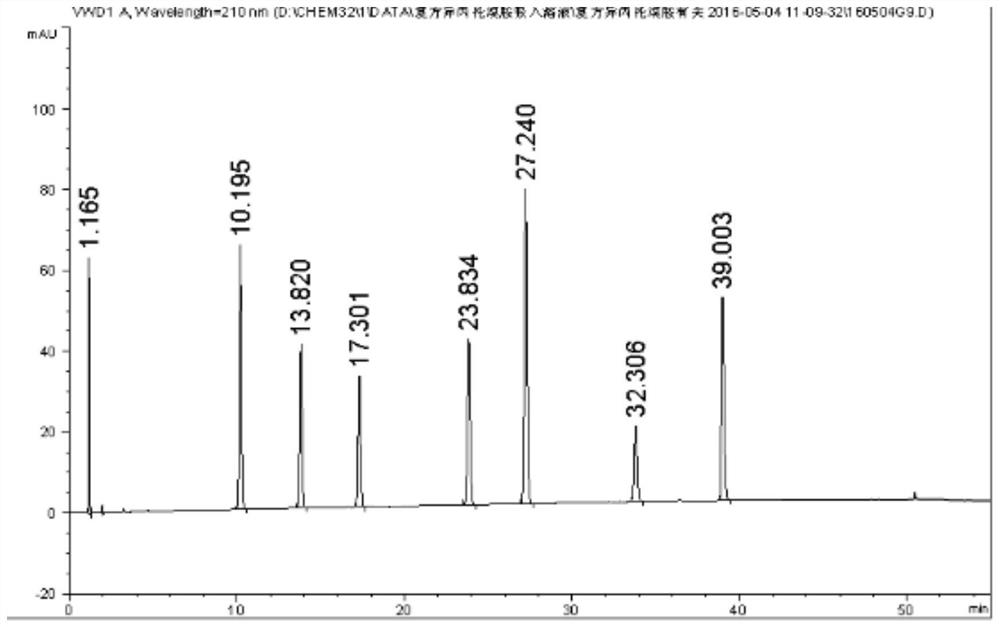
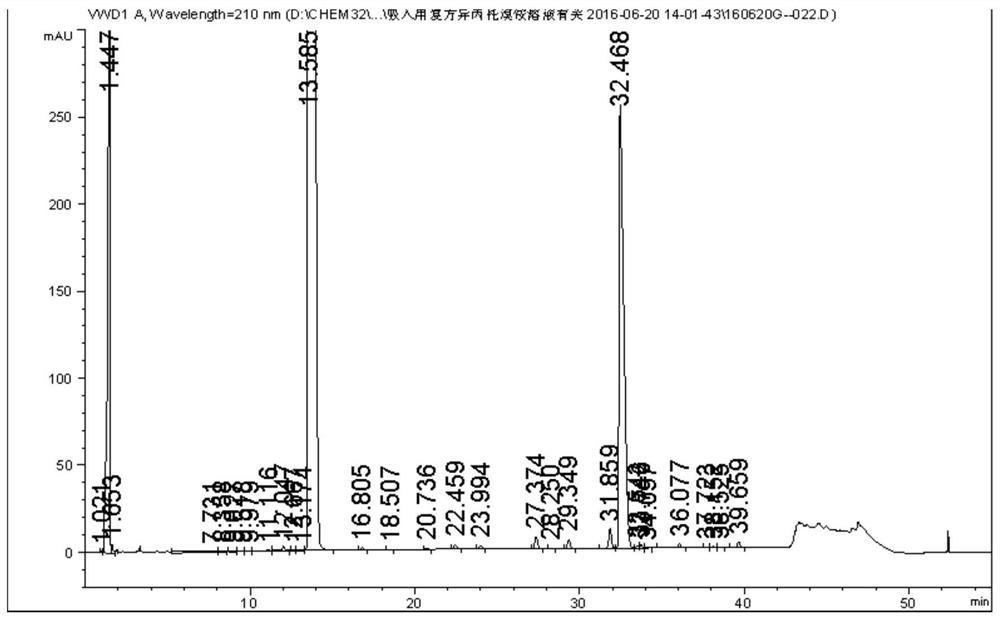
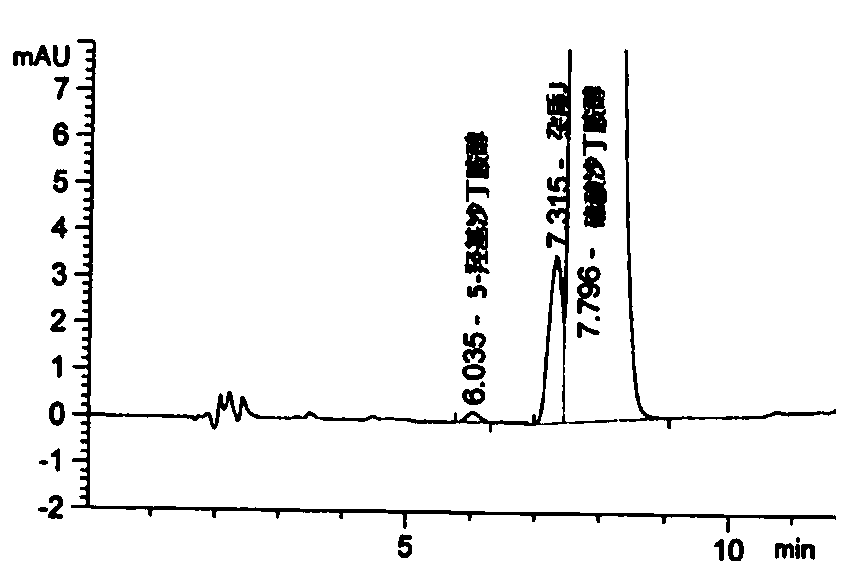
Detection method of salbutamol sulfate-related substances
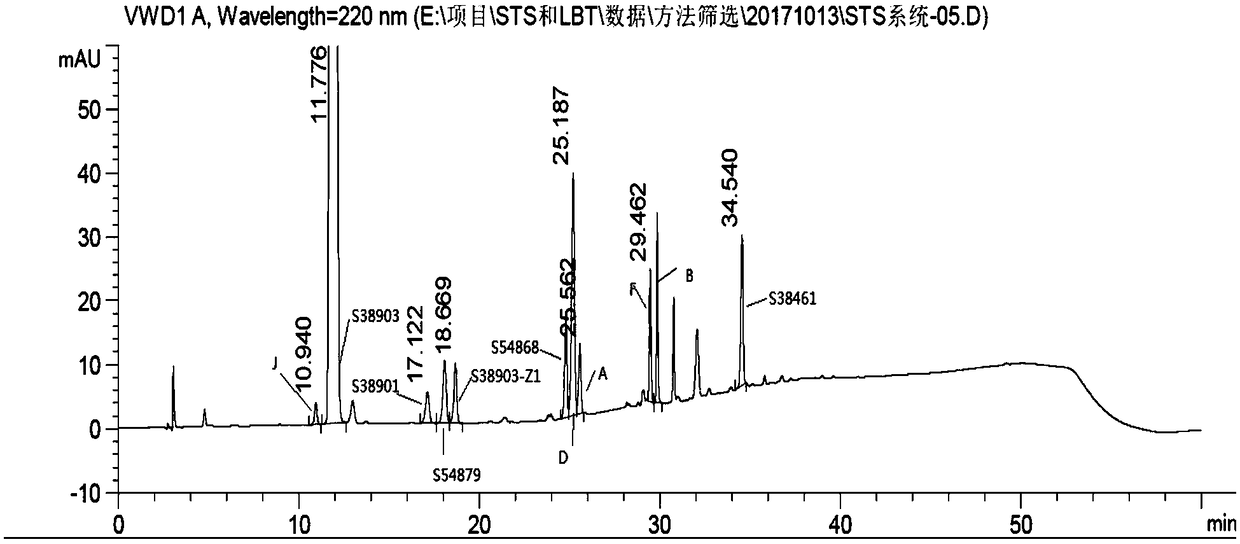
ActiveCN108627597AEfficient separationColumn depressionComponent separationGradient elutionAcetonitrile
The invention discloses a detection method of salbutamol sulfate-related substances. The detection method qualitatively or quantitatively detects the salbutamol sulfate-related substances with high performance liquid chromatography; detection conditions comprise a chromatographic column: octyl-bonded silica gel column, a detection wavelength: 210-400 nm, and mobile phases comprising a mobile phaseA and a mobile phase B, wherein the mobile phase A is an aqueous phase containing a buffer solution and having pH of 1.5-4.0, further having pH of 2.0-3.0; the mobile phase B mainly comprises methanol or acetonitrile; the mobile phases are eluted by gradient elution; and detection steps are as follows: (1) preparing a test substance solution and a reference substance solution; and (2) respectively sampling and detecting the test solution and the reference solution. The detection method of the salbutamol sulfate-related substances adopted by the invention can ensure effective separation of therelated substances.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
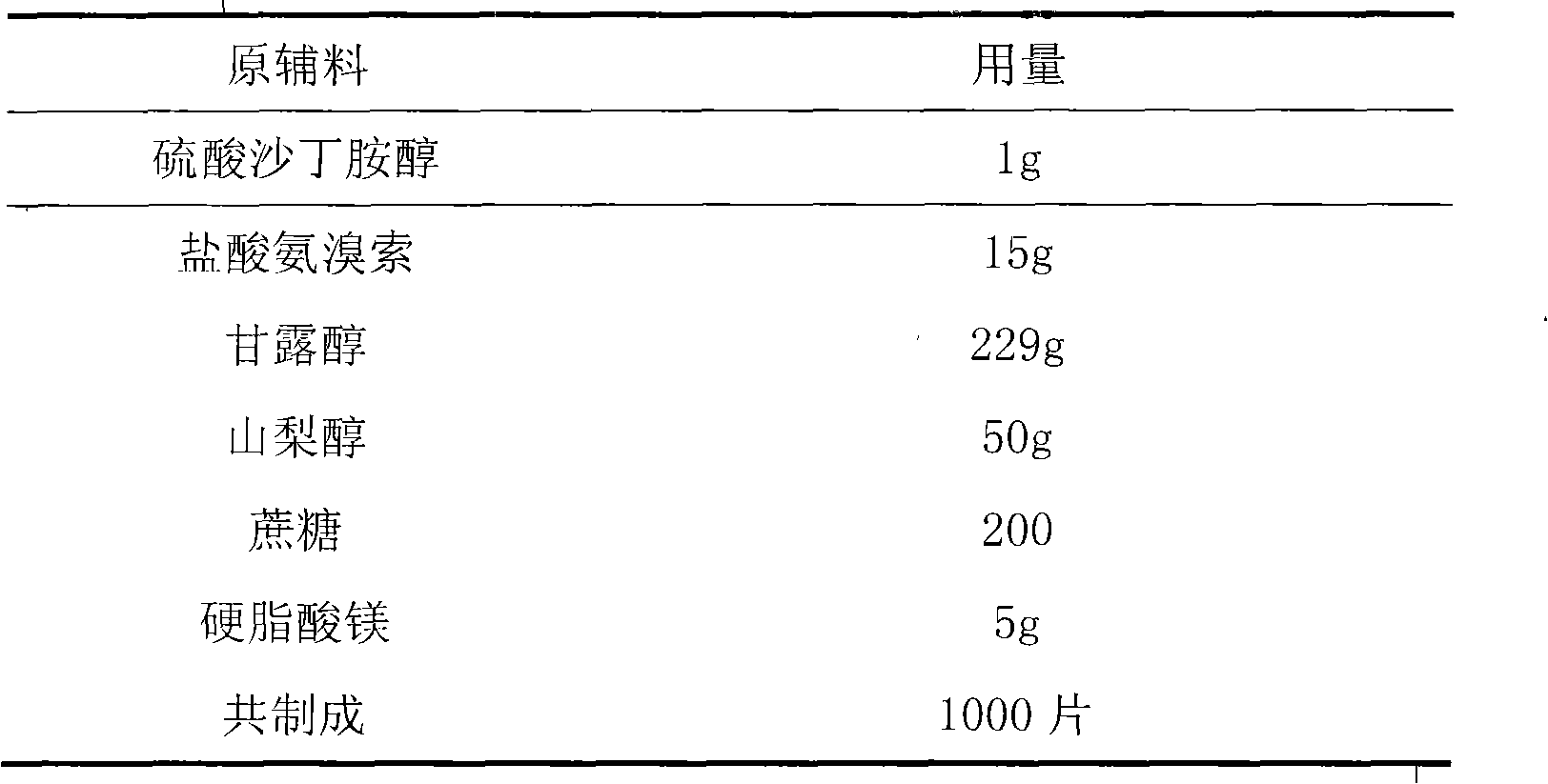
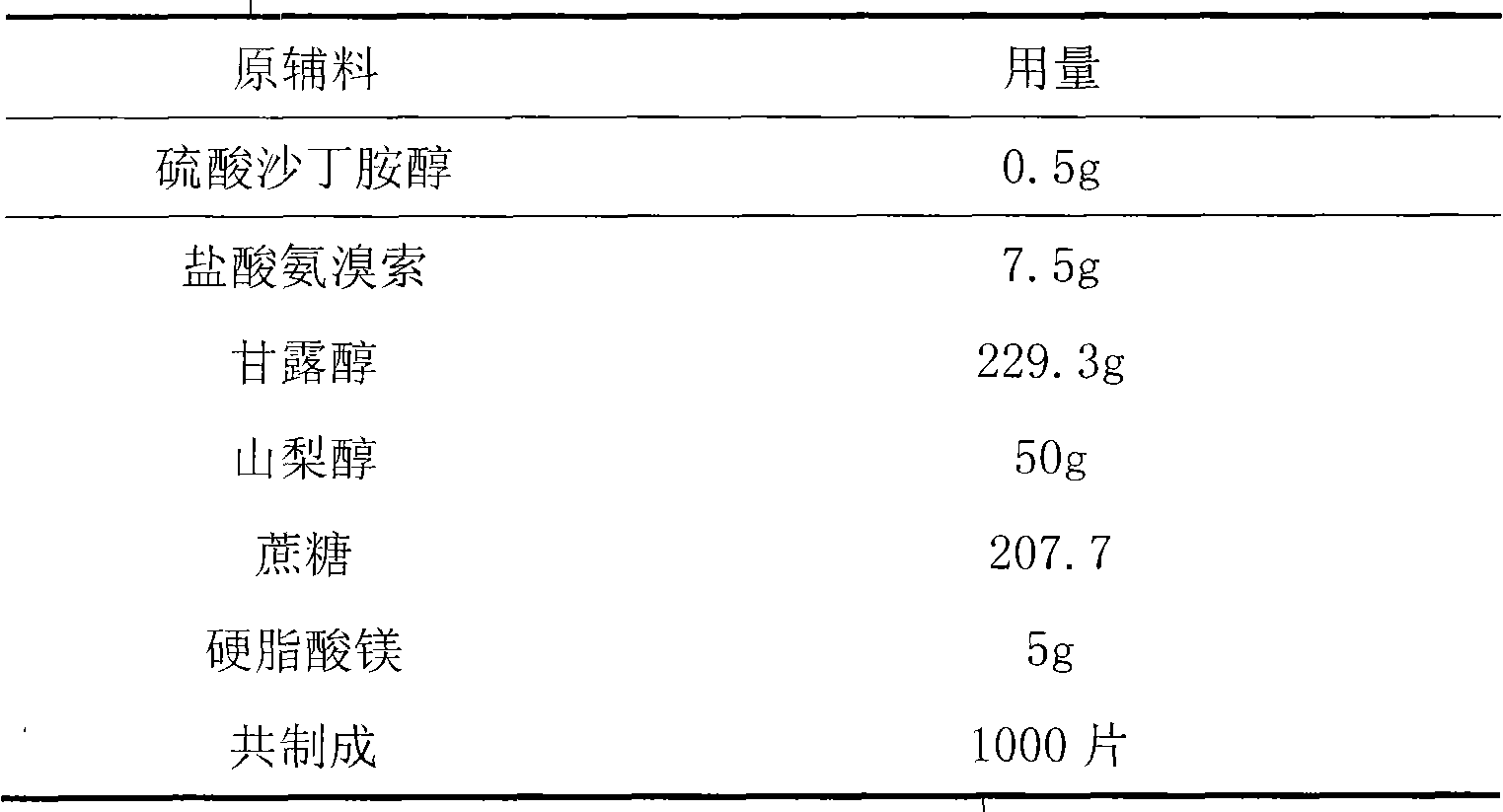
Tablet containing ambroxol hydrochloride and salbutamol sulfate
InactiveCN104622854AMethod Prescription is simpleSimple prescriptionOrganic active ingredientsPharmaceutical non-active ingredientsAsthmatic bronchitisDisease
The invention provides a tablet containing ambroxol hydrochloride and salbutamol sulfate, and belongs to the technical field of medicines. The tablet provided by the invention mainly comprises the following auxiliary materials: a filler, a disintegrating agent, a lubricant and an adhesive. The tablet provided by the invention is capable of treating diseases of a respiratory system, such as acute and chronic bronchitis, asthmatic bronchitis and bronchial asthma; sputum is easy to cough up when trachea is expanded; the tablet is simple in prescription, and good in curative effect; and the preparation technology is suitable for industrialized mass production.
Owner:CP PHARMA QINGDAO CO LTD
Particulate materials
Embodiments of the invention relate to particles of active substances, methods for preparing the particles, formulations containing the particles, and metered dose inhalers containing the particles or formulations. In one embodiment, an inhaler contains an aerosol formulation containing a particulate active substance of non-micronized, solid particles having a mass median aerodynamic diameter of less than 10 μm. The particles may be suspended in a nonsolvent hydrofluorocarbon fluid vehicle (e.g., HFA 134a or 227ea) at a concentration within a range from about 0.2% w / v to about 5% w / v. The formulation exhibits a flocculation volume of about 85% or greater about 1 minute after mixing the particulate active substance and the vehicle. The particulate active substance may contain salmeterol xinafoate, budesonide, salbutamol sulfate, dihydroergotamine mesylate, risperidone-(9-hydroxy)-palmitate, bromocriptine mesylate, or derivatives thereof. In some examples, the active substance is dihydroergotamine mesylate.
Owner:NEKTAR THERAPEUTICS INC
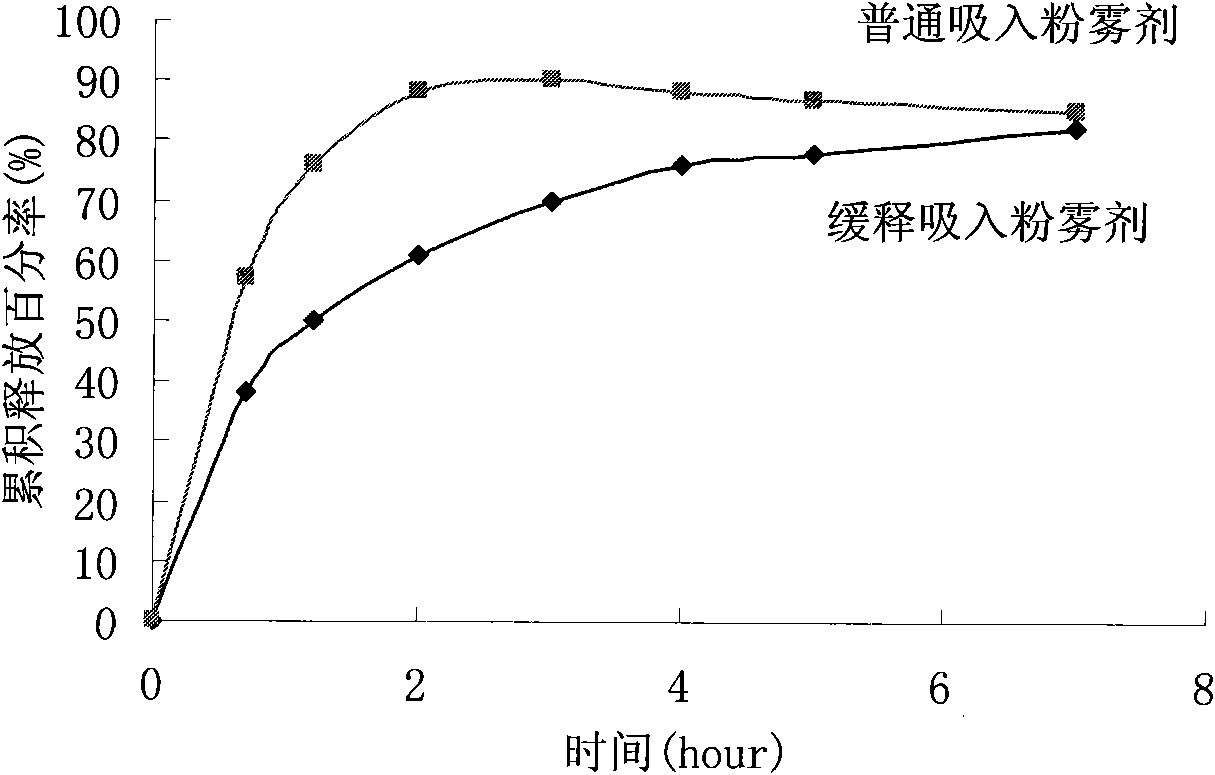
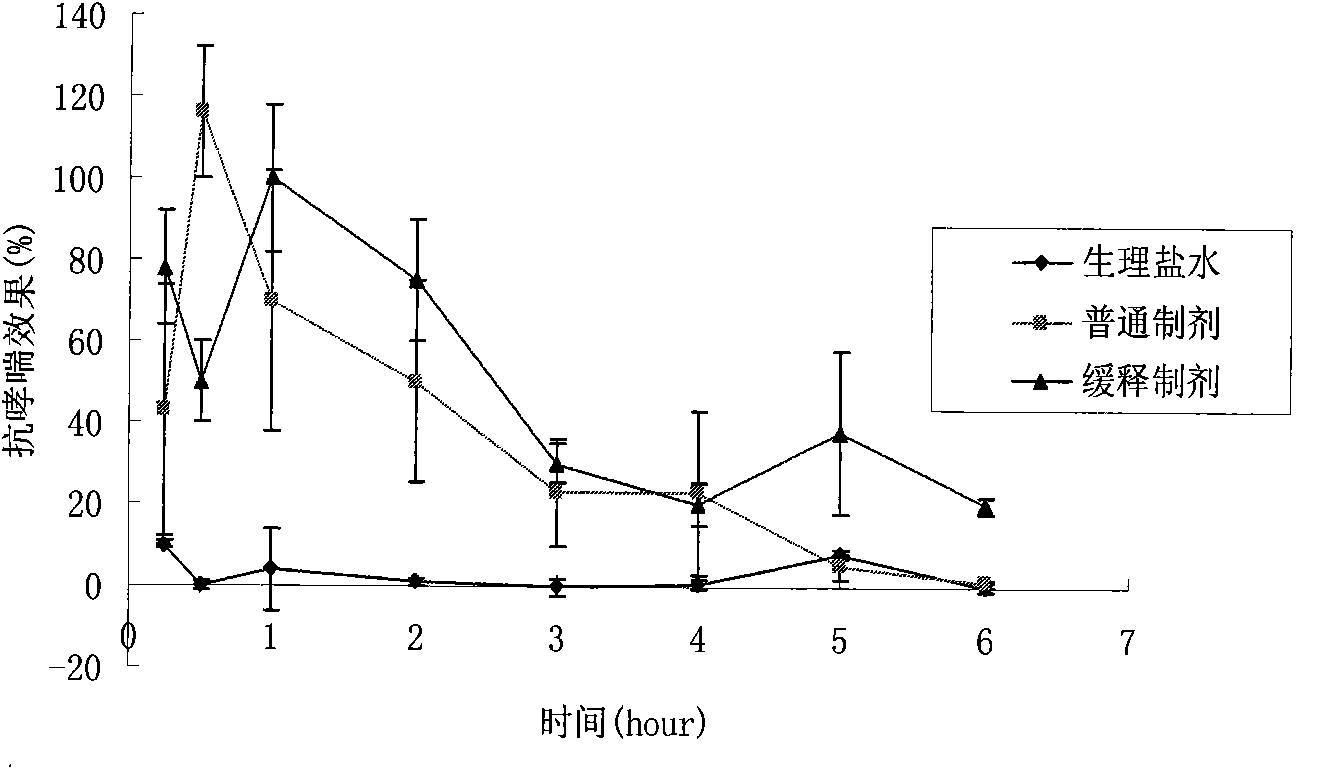
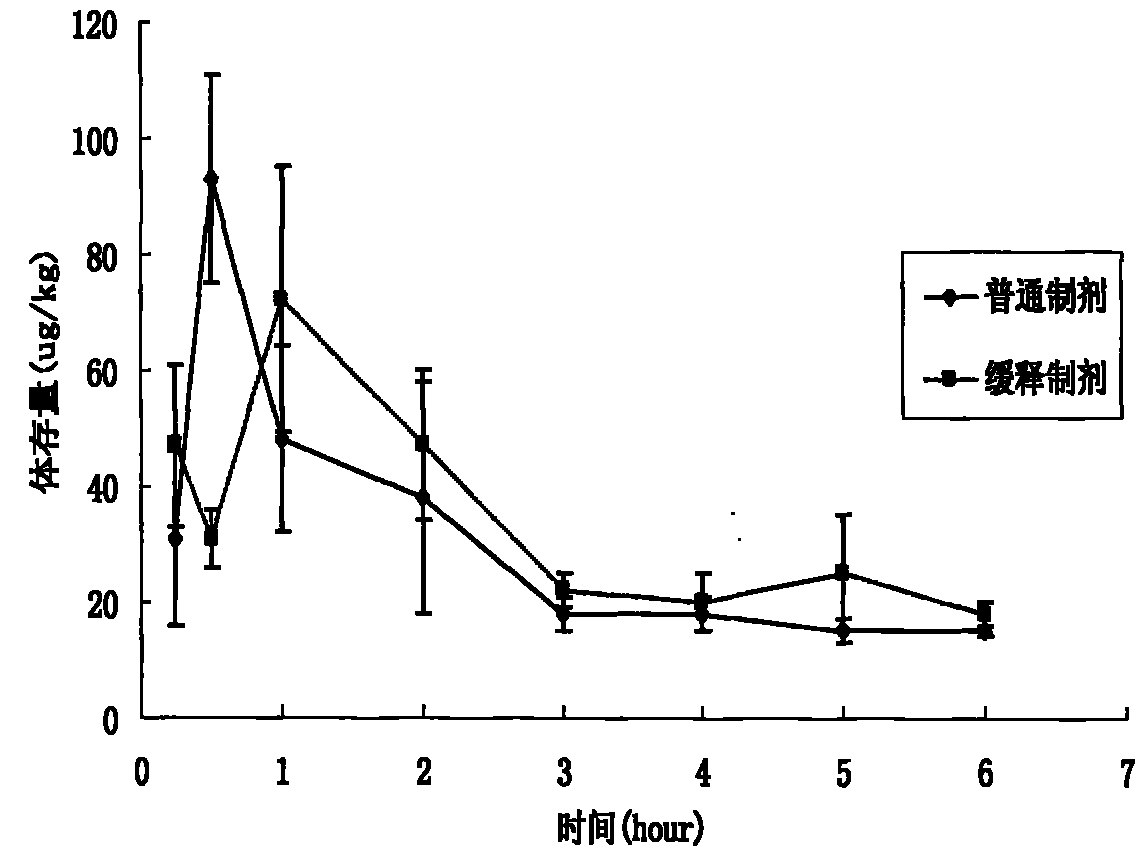
Salbutamol sulfate sustained-release aerosol of micropowder for inspiration and preparation method thereof
The invention relates to the field of medicinal preparations, in particular to salbutamol sulfate sustained-release aerosol of micropowder for inspiration and a preparation method thereof. The invention is characterized in that the salbutamol sulfate sustained-release aerosol of micropowder for inspiration consists of salbutamol sulfate, polyhydroxyl sugar alcohol, a release sustaining material and amino acid. On the basis of the conventional aerosol of micropowder for inspiration, the sustained-release aerosol of micropowder for inspiration is prepared by adding certain materials. The obtained product has in-bronchus sustained-release performance, has high dissolubility, high bulk density, good angle of repose and high atomization performance and can prevent absorbing moisture.
Owner:CHINA PHARM UNIV
Synthetic method for superfine sulfuric acid and salbutamol
InactiveCN1566076ASimple operation processReduce manufacturing costOrganic compound preparationAmino-hyroxy compound preparationOrganic solventSalbutamol sulfate
The invention relates to a synthetic method for superfine sulfuric acid and salbutamol through using salbutamol and sulfuric acid as reactants, dissolving salbutamol into inert organic solvent, controlling the process operation parameters of the reaction, separating out salbutamol sulfate crystallization, filtering, scouring and drying to obtain the end product.
Owner:NANOMATERIALS TECH PTE +1
Pharmaceutical compounds and compositions
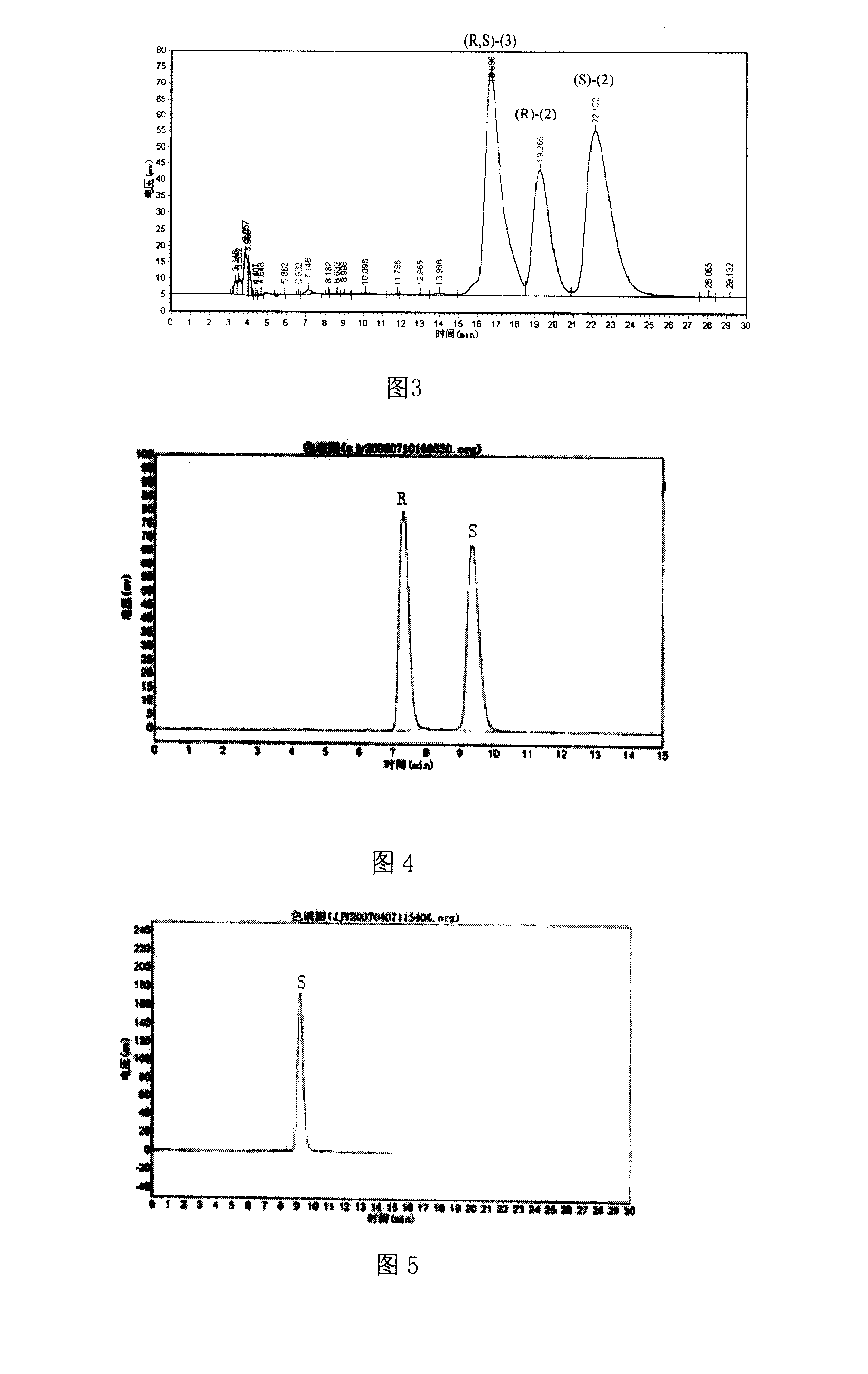
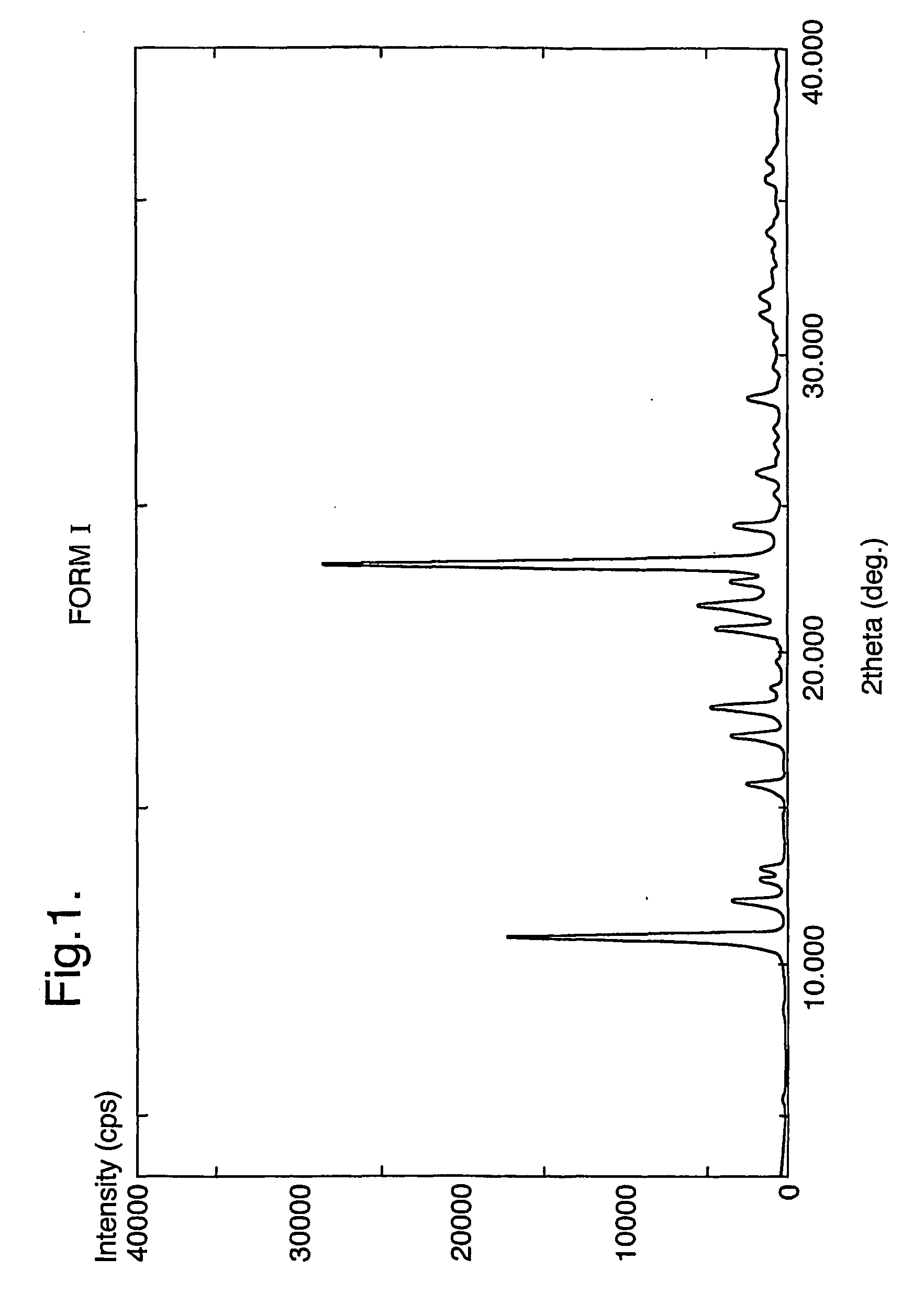
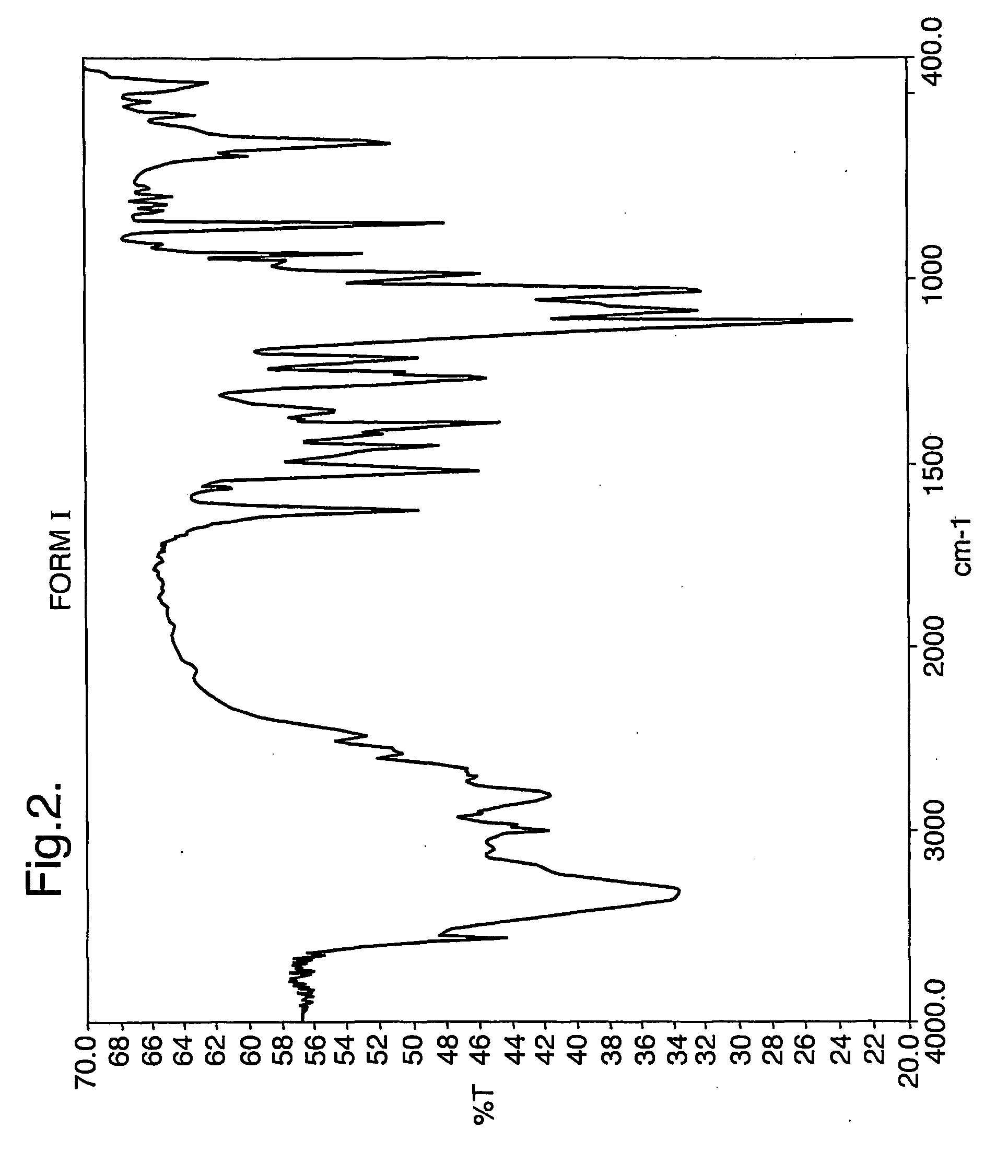
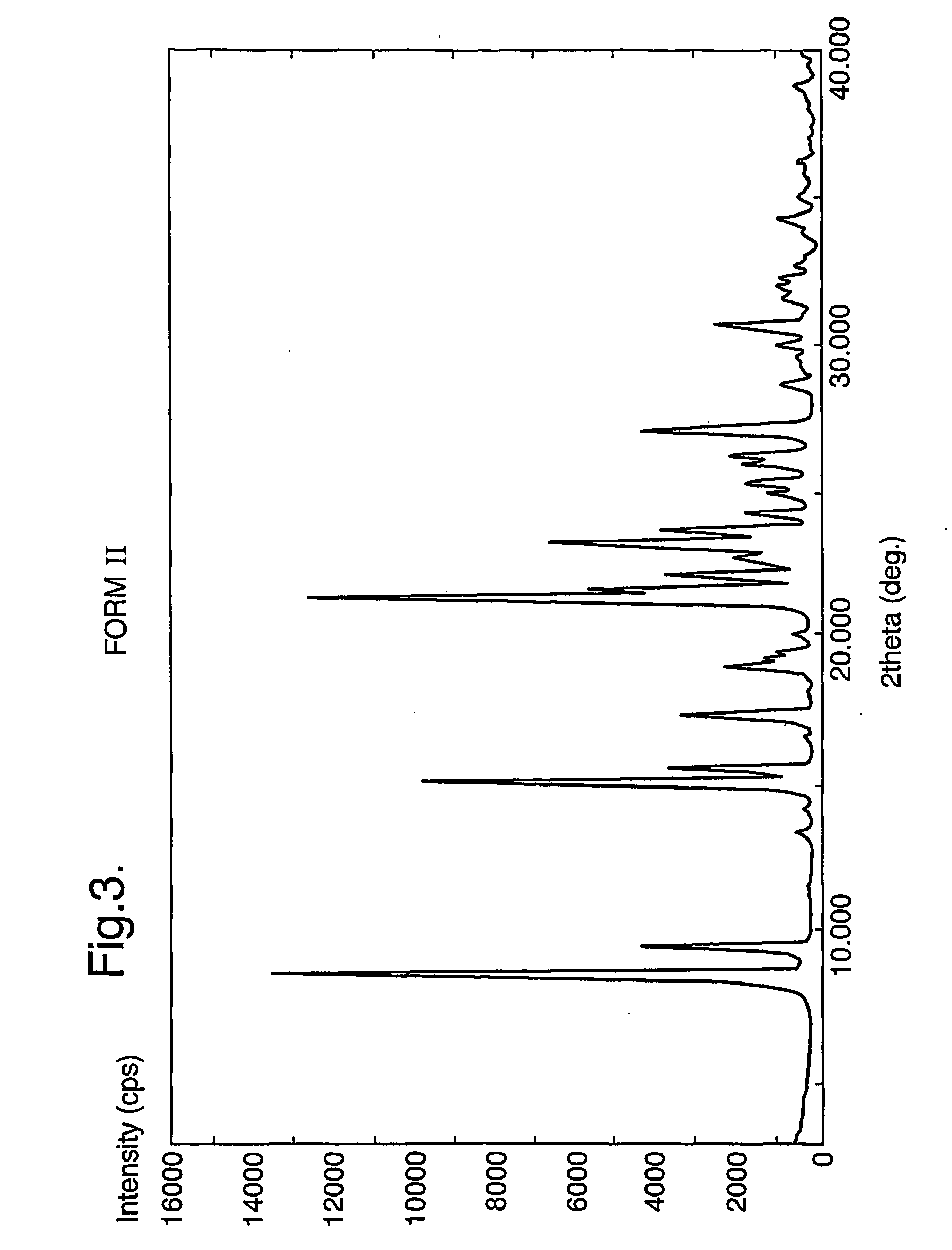
The invention provides three polymorphic forms of crystalline levosalbutamol sulphate designated herein as Forms I, II and III. Crystalline levosalbutamol sulphate Form I is characterised by a powder XRD pattern with peaks at 10.8, 11.9, 13.0, 18.3, 28.5±0.2 degrees 2 theta. Crystalline levosalbutamol sulphate Form II is characterised by a powder XRD pattern with peaks at 8.7, 9.6, 15.2, 15.7, 19.1, 27.2, 30.7±0.2 degrees 2 theta. Crystalline levosalbutamol sulphate Form III is characterised by a powder XRD pattern with peaks at 5.5, 6.9, 7.3, 18.7±0.2 degrees 2 theta. Processes for making the new polymorphic forms and pharmaceutical compositions comprising them are also provided. A pharmaceutical composition comprises a therapeutically effective isomer of salbutamol or a salt, solvate, ester, derivative or polymorph thereof, a glucocorticoid and a pharmaceutically acceptable carrier or excipient and optionally one or more other therapeutic agents. Preferably the composition is an aerosol formulation comprising the drugs, a propellant and optionally one or more other ingredients, such as a surfactant, cosolvent, or bulking agent. Alternatively, DPI or inhalation suspensions may be used.
Owner:CIPLA LTD
Buccal tablets taking salbutamol sulfate and ambroxol hydrochloride as main active ingredients and preparation method thereof
InactiveCN101810590ASlow onsetReduced bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsTreatment effectOlder people
The invention relates to buccal tablets taking salbutamol sulfate and ambroxol hydrochloride as main active ingredients and a preparation method thereof, and aims to provide a new preparation, namely salbutamol sulfate and ambroxol hydrochloride buccal tablets, for the vast number of patients and medical workers. The preparation has quick response, can improve the bioavailability of medicaments, give full play to the treatment effects of the medicaments and reduce adverse reactions, is convenient for children to swallow and for old people to take with reduced dosage, and is convenient to carry, store and transport. The preparation method is simple and suitable for mass production. In the method, the buccal tablets are prepared by adding some specific types of auxiliary materials in certain proportions into the salbutamol sulfate and ambroxol hydrochloride serving as the active ingredients according to the conventional technique of pharmaceutical engineering.
Owner:北京利乐生制药科技有限公司
Pills taking salbutamol sulfate and ambroxol hydrochloride as main active ingredients and preparation method thereof
InactiveCN101810589AImprove bioavailabilityIncrease blood concentrationOrganic active ingredientsPill deliveryOlder peopleCurative effect
The invention relates to pills taking salbutamol sulfate and ambroxol hydrochloride as main active ingredients and a preparation method thereof, and aims to provide a new preparation, namely salbutamol sulfate and ambroxol hydrochloride pills, for the vast number of patients and medical workers. The preparation can improve the bioavailability of medicaments, give full play to the treatment effects of the medicaments and reduce adverse reactions, is convenient for children to swallow and for old people to take with reduced dosage, and is convenient to carry, store and transport. The preparation method is simple and suitable for mass production. In the method, the pills are prepared by adding some specific types of auxiliary materials in certain proportions into the salbutamol sulfate and ambroxol hydrochloride serving as the active ingredients according to the conventional technique of pharmaceutical engineering.
Owner:北京利乐生制药科技有限公司
Method for synchronously detecting five related substances in compound ipratropium bromide solution for inhalation
ActiveCN111721845AMeet the requirementsAvoid replacementComponent separationAgainst vector-borne diseasesPhosphateSilica gel
The invention relates to a method for synchronously detecting five related substances in a compound ipratropium bromide solution for inhalation, and belongs to the technical field of drug quality determination methods. High performance liquid chromatography is adopted for detection, a chromatographic column with alkyl bonded silica gel is adopted as a filler, a phosphate buffer solution containingsodium heptanesulfonate is used as a mobile phase A, an organic phase is used as a mobile phase B, and a detection wavelength is 205-300nm. The detection method provided by the invention can effectively separate ipratropium bromide and salbutamol sulfate from other impurities, and has characteristics of simple operation, a comprehensive result, accuracy, reliability and strong specificity.
Owner:LUNAN PHARMA GROUP CORPORATION
Alendronate sodium powder inhalation used for respiratory drug delivery and preparation method and application thereof
ActiveCN105640924AAvoid side effectsSimple manufacturing processPowder deliveryOrganic active ingredientsSide effectStimulant
The invention provides alendronate sodium powder inhalation used for respiratory drug delivery and a preparation method and application thereof. Alendronate sodium powder inhalation includes single-component alendronate sodium micro powder and does not include auxiliary materials. In the production and preparation process of the alendronate sodium micro powder, other auxiliary materials or solvents are not involved, and alendronate sodium raw material medicine is directly smashed to prepare the alendronate sodium micro powder. The alendronate sodium powder inhalation can improve the breathing function of a chronic obstructive pulmonary disease (COPD) rat by about 40% and has a better effect than salbutamol sulfate with a common usage amount, and drug resistance of salbutamol sulfate and other beta2 receptor stimulants can be eliminated. The powder inhalation mainly makes sedimentation in the trachea, bronchia, bronchiole and other pulmonary respiratory ducts to directly take effect after drug-delivery inhalation, the diastolic function of the respiratory ducts becomes effective rapidly, and the drug effect lasts for a long time; the alendronate sodium powder inhalation enters pulmonary alveoli, is low in absorption and blood-entering amount, can avoid systematic side effects and is suitable for being developed to be a drug for treating COPD and asthma.
Owner:杭州东博医药科技开发有限公司
Preparation method of salbutamol sulfate aerosol inhalation solution
InactiveCN104224760AImprove product qualityOrganic active ingredientsPharmaceutical delivery mechanismForeign matterFiltration membrane
The invention discloses a preparation method of a salbutamol sulfate aerosol inhalation solution. The preparation method comprises the following steps: S1, adding 700 thousand ml of water for injection in a hermetic preparation tank for later use; S2, dissolving benzalkonium chloride with hot water for injection in advance, adding a benzalkonium chloride solution into a preparation barrel, and uniformly stirring; S3, adding water for nitrogen filling injection to 320 thousand ml and stirring for 15 minutes to be uniformly stirred; S4, adding water for nitrogen filling injection to full dose, and stirring for 15 minutes to be uniformly stirred; S5, filtering with a 0.35 mu m polyether sulfone microporous filtration membrane until the solution is clarified; S6, sampling and testing the content and pH value of an intermediate, covering the preparation barrel, and introducing nitrogen into the liquid medicine to protect; and S7, filtering the liquid medicine in a liquid storage barrel by a 0.22 mu m polyether sulfone microporous filtration membrane, sampling and examining visible foreign matters, and inputting the qualified visible foreign matters into a liquid storage container for encapsulation. The production quality of the salbutamol sulfate aerosol inhalation solution is effectively improved.
Owner:SHANGHAI XINYI JINZHU PHARMA
Process for preparing micropowdered salbutamol sulfate
InactiveCN1451648ANarrow particle size distributionParticle size controllableOrganic compound preparationAmino-hyroxy compound preparationPolymer scienceGranularity
A process for preparing the micropowdered salbutamol sulfate includes mixing the solution of raw material containing salbutamol sulfate and acetone in filler layer of rotating filler bed, recrystallizing, filtering, washing and drying. Its advantages are high granularity uniformity, and controllable average granularity.
Owner:BEIJING UNIV OF CHEM TECH +1
Process for preparing salbutamol sulfate orally disintegrating tablets
InactiveCN106913551AImprove liquidityGood molding effectOrganic active ingredientsPharmaceutical non-active ingredientsPrillOrally disintegrating tablet
The invention discloses a process for preparing salbutamol sulfate orally disintegrating tablets. The process is characterized by comprising the steps of S1, weighing the main drug salbutamol sulfate and auxiliary materials according to a ratio; S2, mixing salbutamol sulfate with a lubricant to prepare mixed powder I; S3, mixing a filler with an internally added disintegrant, and adding an adhesive to prepare wet particles; S4, drying the wet particles to obtain dry particles; S5, conducting size stabilization on the dry particles; S6, adding an externally added disintegrant, the lubricant and the mixed powder I to the particles prepared in S5 to be mixed, so that total blended particles are obtained; S6, tabletting and weighing the total blended particles; and S7, packaging qualified tablets obtained in S6. The process is simple, the cost is low, large-scale production can be achieved, the problem that in the prior art, the stability of the thermosensitive crude drug salbutamol sulfate can be affected by high temperature is solved, and the hardness, the friability, the slice heavy difference and the disintegration time of the prepared salbutamol sulfate orally disintegrating tablets all meet requirements.
Owner:CHONGQING CONQUER PHARML
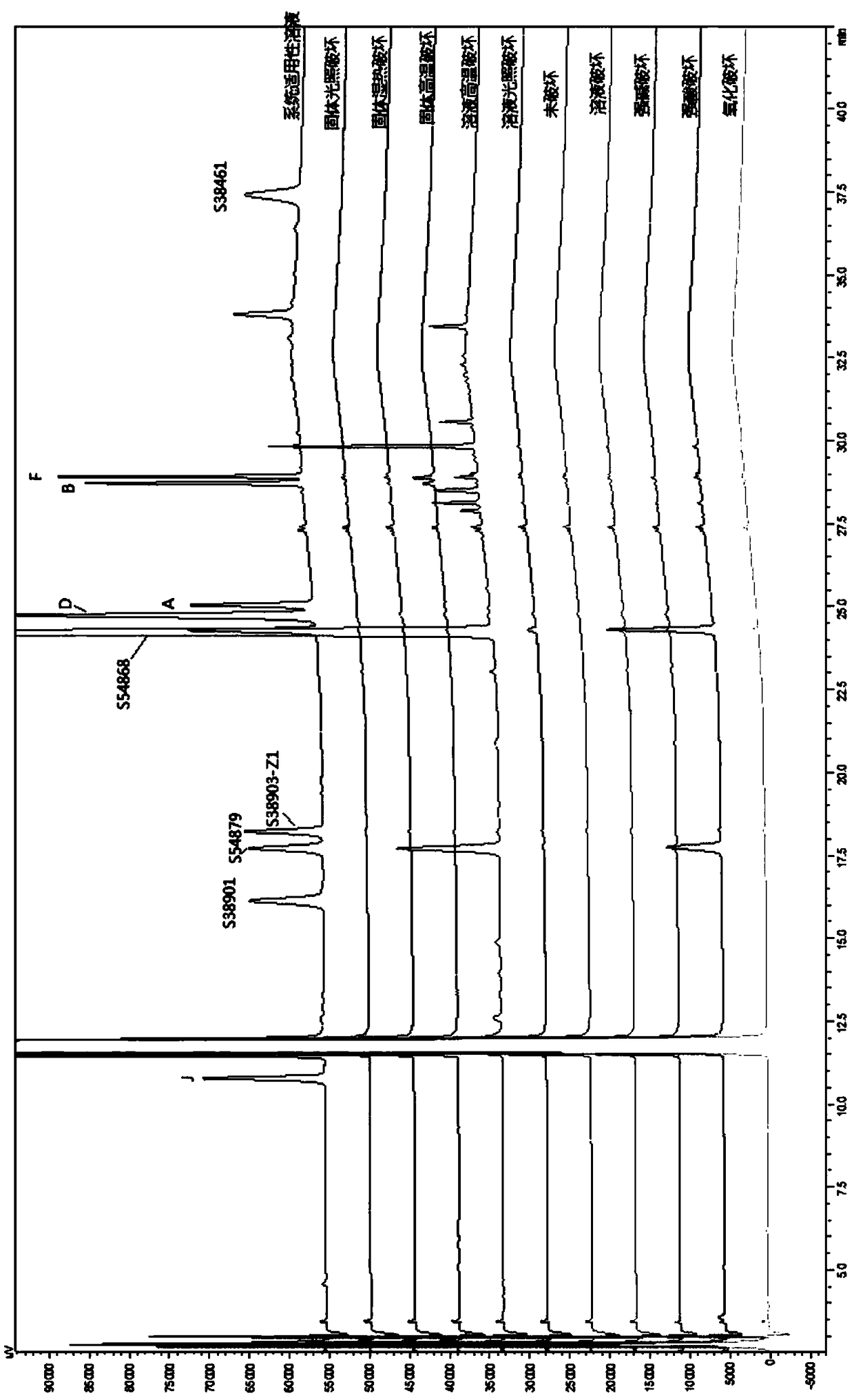
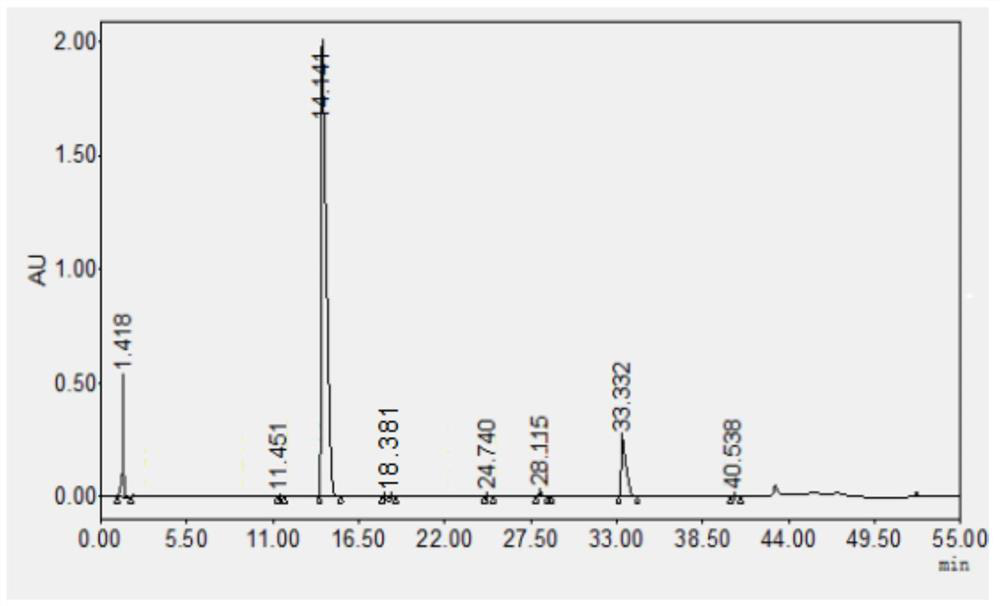
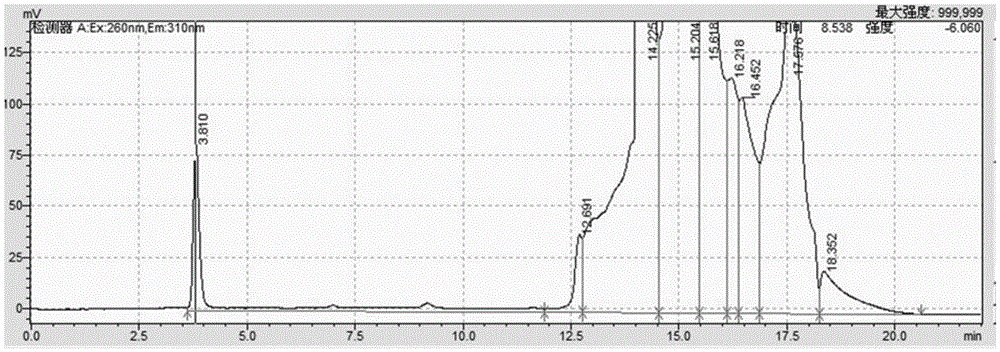
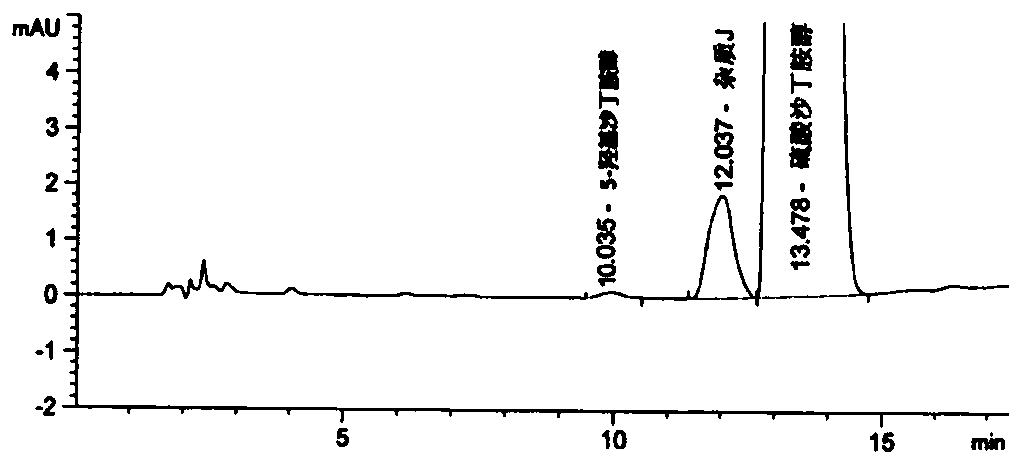
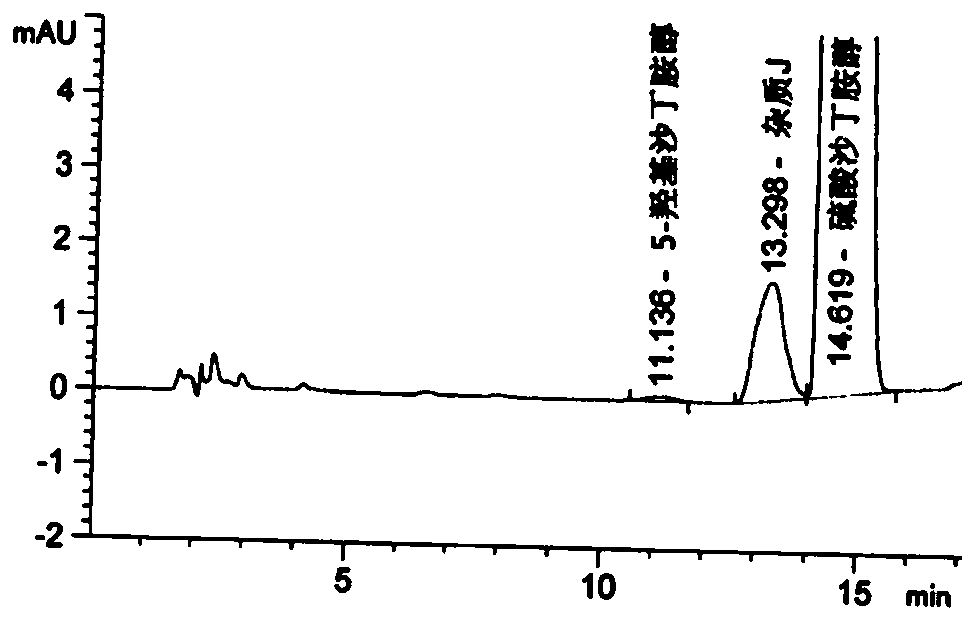
Detection method for related substances of salbutamol sulfate solution used for inhalation
InactiveCN110632205AImprove quality controlEfficient separationComponent separationPhosphateGradient elution
The invention discloses a detection method for related substances of salbutamol sulfate solution used for inhalation. A high performance liquid chromatography method is adopted for performing qualitative or / and quantitative detection on the related substances of the salbutamol sulfate solution used for inhalation, and liquid chromatography detection conditions comprise that a C8 chromatographic column is adopted, mobile phases comprise a mobile phase A and a mobile phase B, wherein the mobile phase A is phosphate buffer solution, the mobile phase B is mixed solution of acetonitrile and methanol with the volume ratio of (40-65):(60-35), and the mobile phases adopt a gradient elution method. By adopting the detection method disclosed by the invention, a main drug and other related impuritiesin the salbutamol sulfate solution used for inhalation can be effectively detetected, and the detection method has specificity and stability indication capability and also can detect a new impurity 5-hydroxy salbutamol at the same time.
Owner:SICHUAN PURITY PHARM CO LTD
Salbutamol sulfate aerosol inhalation solution, and preparation process and application thereof
PendingCN112402400APromote atomizationImprove thermal stabilityOrganic active ingredientsDispersion deliveryMedicineAerosolize
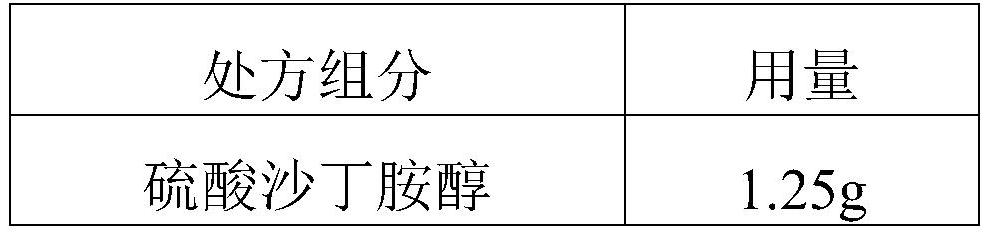
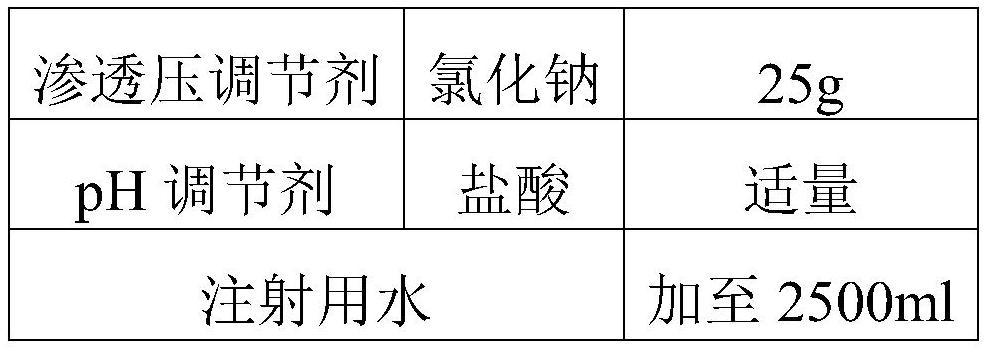
The invention discloses a salbutamol sulfate aerosol inhalation solution, and a preparation process and application thereof. The salbutamol sulfate solution is prepared from the following components:salbutamol sulfate, an osmotic pressure regulator, a pH regulator and water for injection, wherein the pH value of the salbutamol sulfate solution is 3.0 to 5.0. The salbutamol sulfate solution has good atomization performance and thermal stability.
Owner:SHANGHAI SINE PHARMA LAB
Medicine composition for preventing and treating asthma
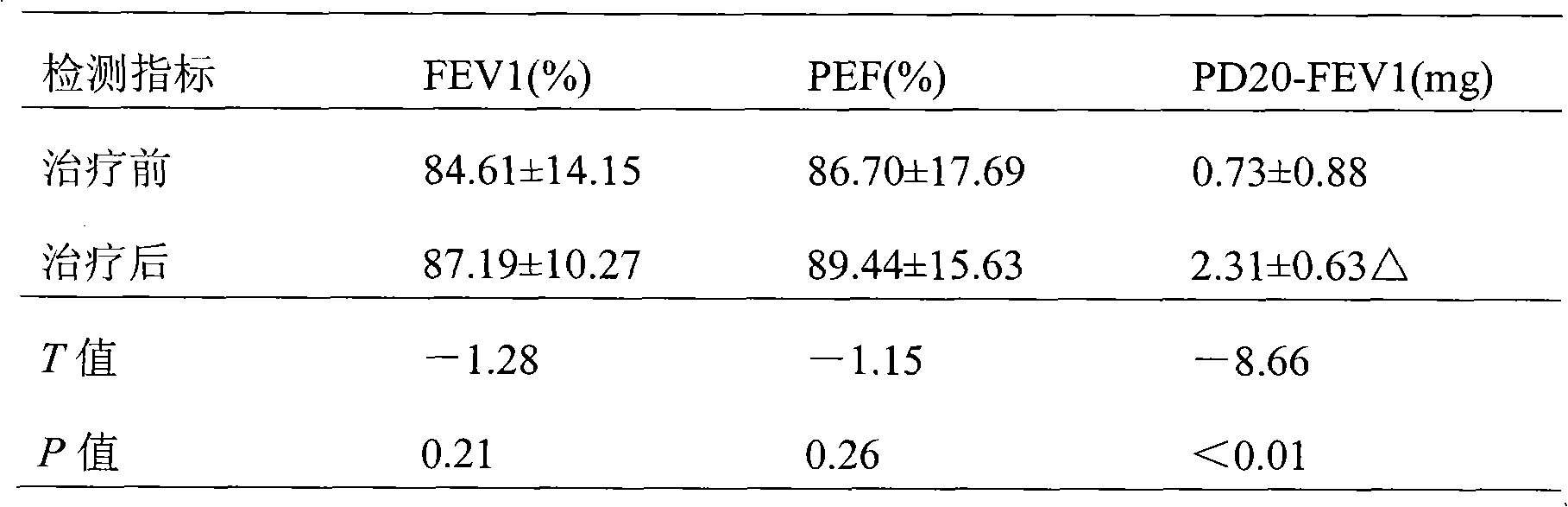
InactiveCN101829154AAvoid affecting reactivity assay resultsPrevent drinkingOrganic active ingredientsBacteria material medical ingredientsAirway responsivenessPatients symptoms
The invention discloses a medicine composition for preventing and treating asthma, containing active components of 1 percent by weight of inactivated mycobacterium phlei F.U.36 and 1000-2000 percent by weight of salbutamol sulfate. The medicine composition is used for treating patients suffering from mild to moderate bronchial asthma and according with the 2006GINA diagnosis standard, measuring the lung function (FEV1%, PEF%) of the patients before and after treatment and the airway reactivity (PD20-FEV1), observing the symptoms of the patients and untoward effects of medicaments, and the like, and the difference of the FEV1% and the PEF% has no statistical significance as comparison before and after the treatment; the PD20-FEV1 after the treatment is obviously increased (p is smaller than 0.01) than that before the treatment; the provocation test negative conversion ratio is 88.9% (24 cases); and after the treatment, the symptoms of the patients are obviously relieved or disappear. Results show that the medicine composition can rapidly lower the airway reactivity and relieve the symptoms of asthma.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGXI MEDICAL UNIV
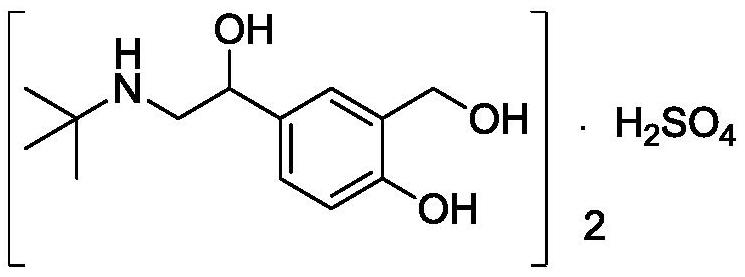
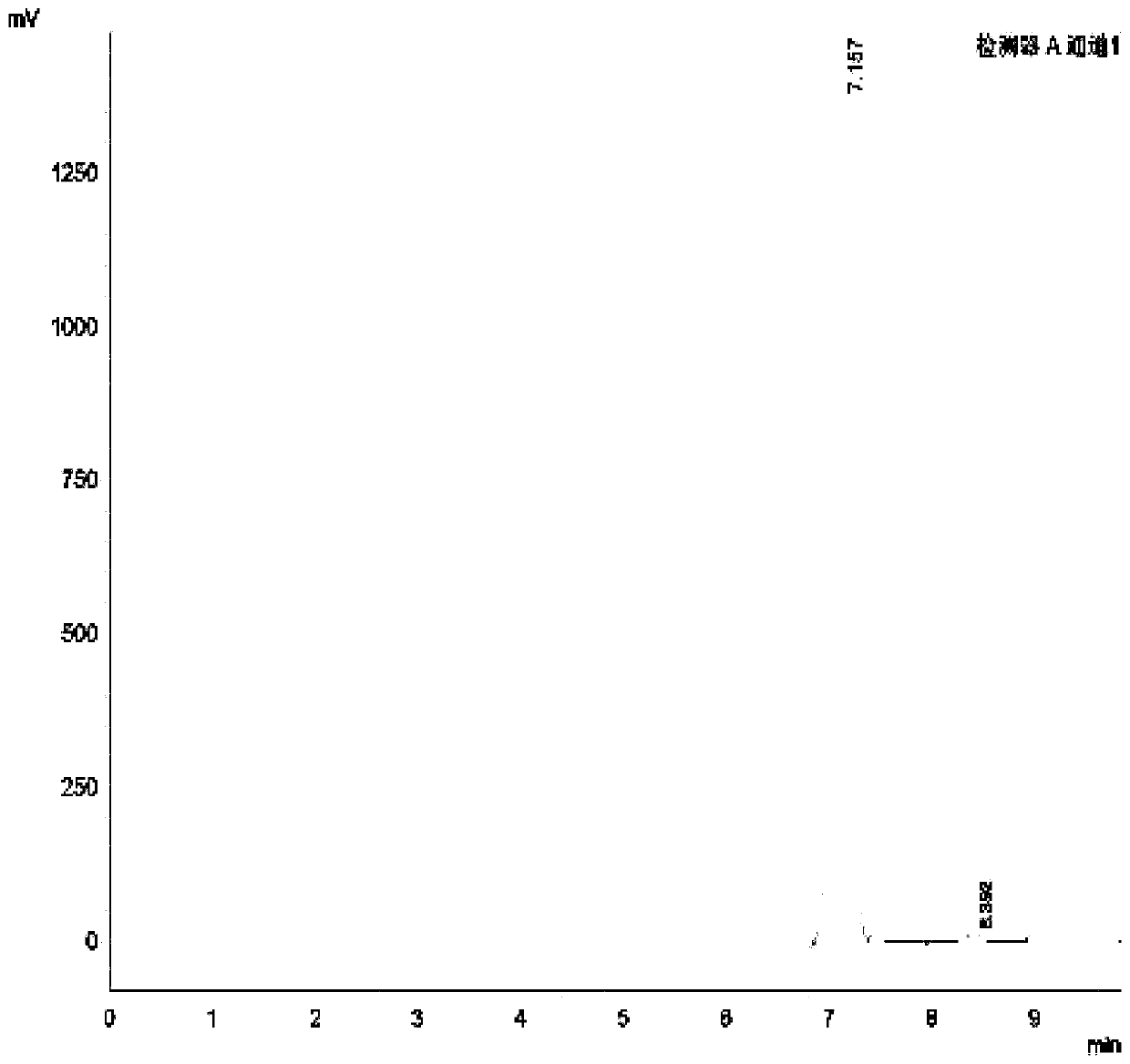
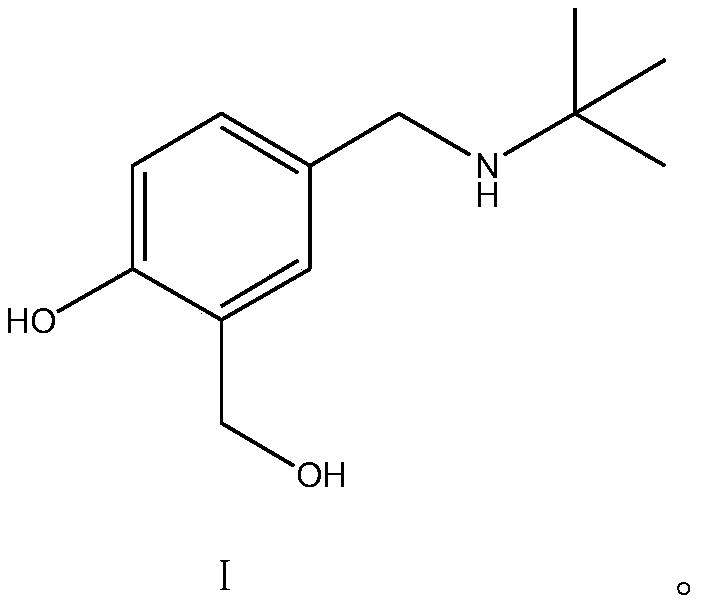
Salbutamol sulfate impurity and preparation method thereof
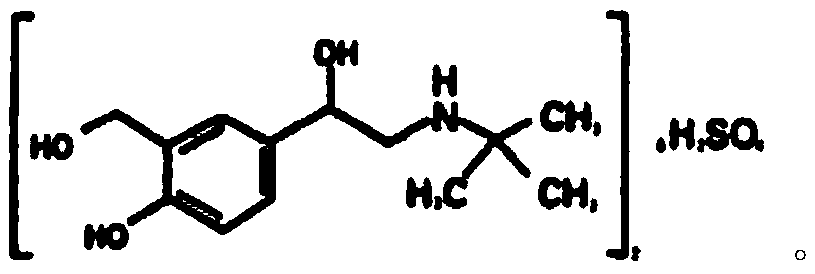
PendingCN110981740ASimple and fast operationThe reaction conditions are mild and controllableOrganic compound preparationAmino-hyroxy compound preparationPhenolSalbutamol sulfate
The invention discloses a salbutamol sulfate impurity and a preparation method thereof, the chemical name of the salbutamol sulfate impurity is (4-[(tert-butylamino) methyl]-2-(hydroxymethyl) phenol),and the salbutamol sulfate impurity is prepared by carrying out Schiff base reaction on a compound shown as a formula II and tert-butylamine under the action of a dehydrating agent to obtain a compound shown as a formula III, and carrying out reduction reaction on the compound shown in a formula III under the action of a reduction catalyst to prepare the compound shown in a formula I, namely (4-[(tert-butylamino) methyl]-2-(hydroxymethyl) phenol). The preparation method of the salbutamol sulfate impurity disclosed by the invention is simple and convenient to operate, mild and controllable inreaction condition, good in reaction repeatability, high in product yield and high in purity; an impurity reference substance meeting the requirements is provided for quality control of salbutamol sulfate, and the impurity reference substance can be used for quality research of salbutamol sulfate bulk drugs and drugs thereof, so that the salbutamol sulfate bulk drugs and the drugs thereof meet related substance standards.
Owner:ANHUI HEALSTAR PHARM CO LTD
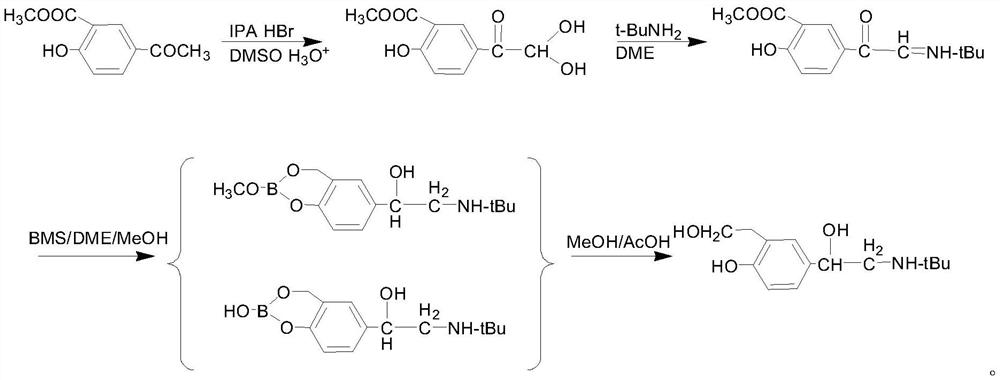
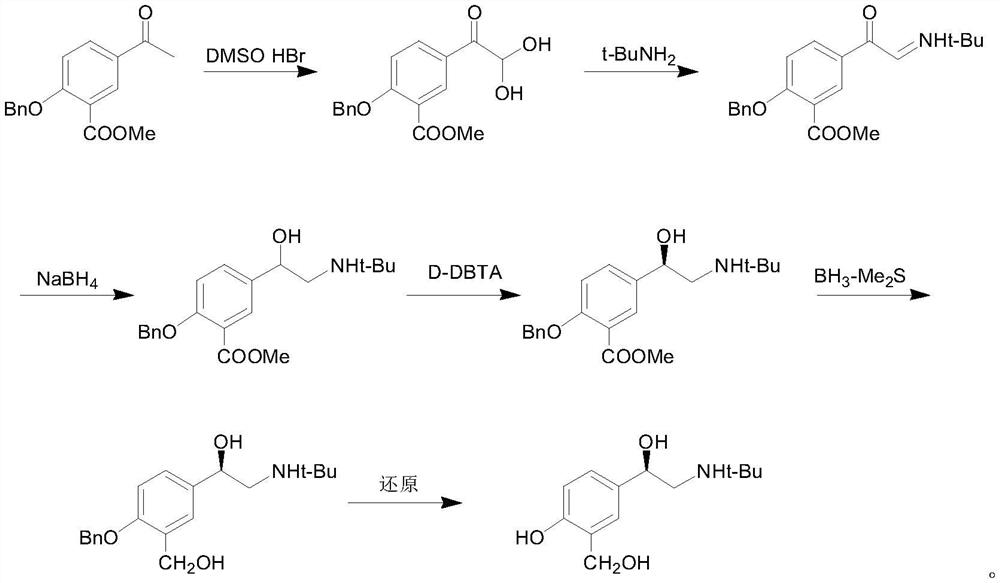
Preparation method of salbutamol sulfate
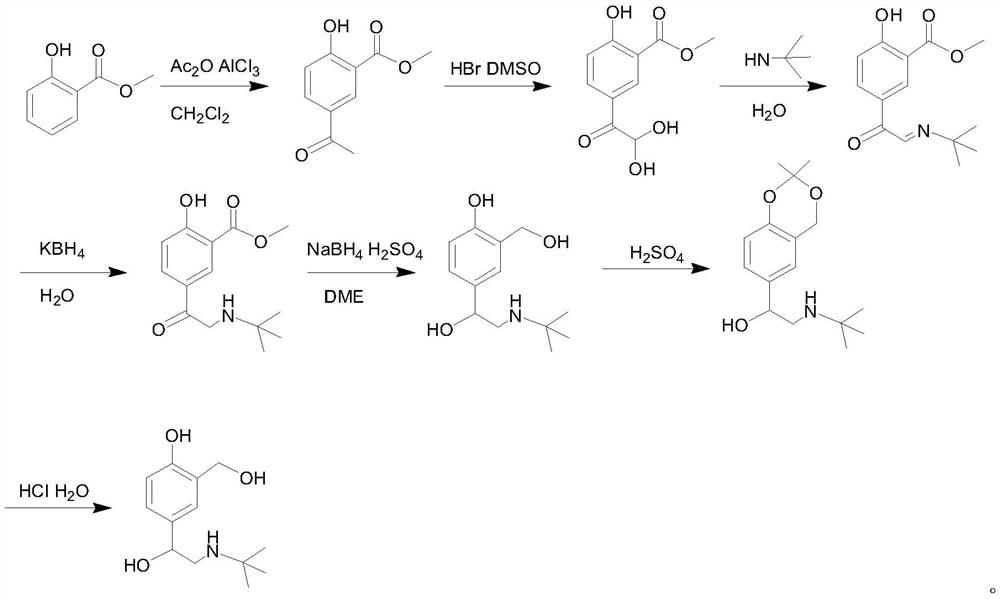
PendingCN113121369AHigh yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisPtru catalyst
The invention provides a preparation method of salbutamol sulfate, and relates to the technical field of chemical synthesis. The preparation method of salbutamol sulfate comprises the following steps: (a) carrying out reaction on salicylic acid and a tert-butyl amino acetyl halogenating reagent under the action of a chlorinating agent to generate an intermediate 1; (b) reacting the intermediate 1 under the action of Lewis acid to generate an intermediate 2; (c) reacting the intermediate 2 under the action of an acid reagent to generate an intermediate 3; (d) reacting the intermediate 3 with a reducing agent under the action of a catalyst to generate salbutamol; and (e) reacting salbutamol with sulfuric acid to generate salbutamol sulfate. The raw materials are simple and easy to obtain, the reaction condition is mild, the reaction operation is simple, the reaction process is easier to control, and the safety coefficient is high; used raw materials, solvents and the like are environment-friendly; and the yield and purity of salbutamol sulfate are high, and a process route capable of industrially producing a product with higher quality is provided.
Owner:TIANJIN PHARMA GROUP CORP
Oral compound pharmaceutic preparation containing tranilast and salbutamol
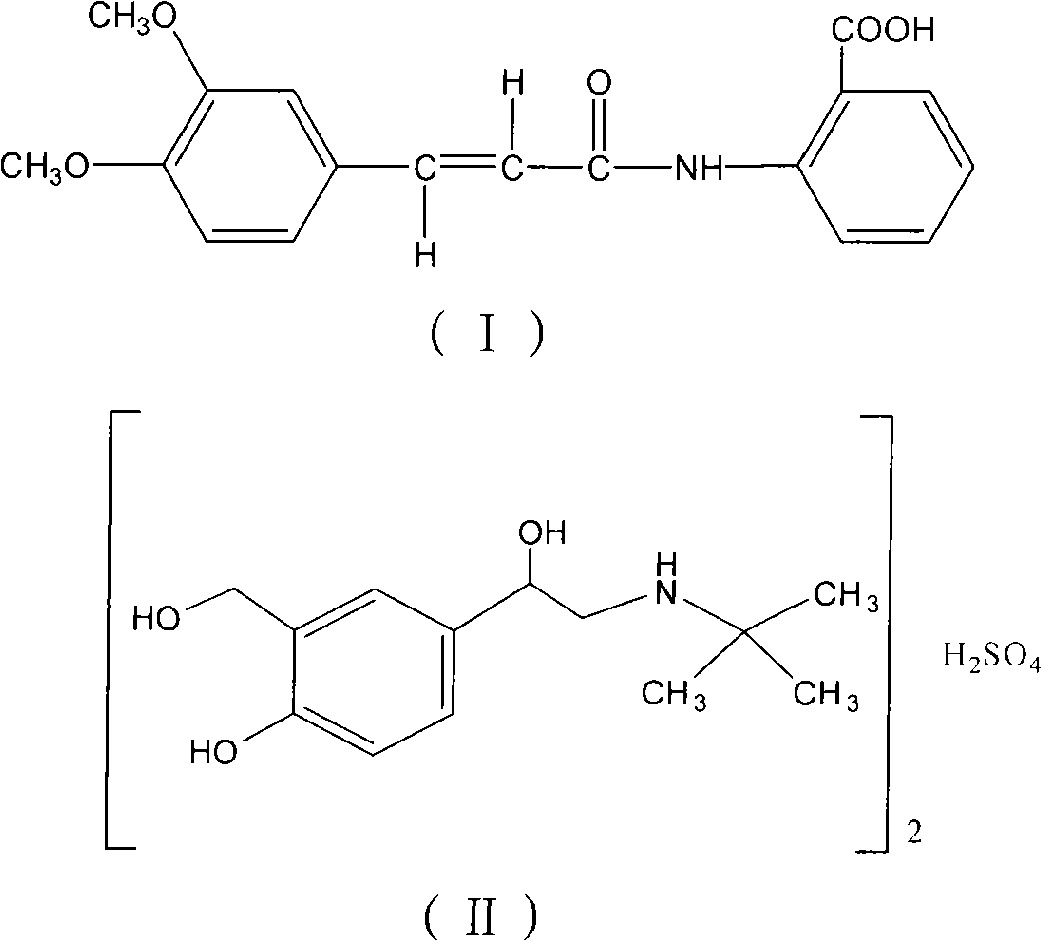
InactiveCN101683330AOrganic active ingredientsPharmaceutical delivery mechanismOral treatmentBULK ACTIVE INGREDIENT
The invention relates to an oral compound pharmaceutic preparation for treating asthma, which contains tranilast shown as the formula (I) and salbutamol sulfate shown as the formula (II) or other pharmaceutically allowed active ingredients. The invention also relates to the application of compounds shown as formulas (I) and (II) or pharmaceutically allowed salts thereof in the preparation of an oral therapeutical agent for treating asthma.
Owner:沈阳三川医药科技有限公司
Salbutamol sulfate oral cavity disintegration tablets and preparation method thereof
InactiveCN111228227AReduce usageLow costOrganic active ingredientsPill deliveryPharmaceutical AidsTableting
The invention provides salbutamol sulfate oral cavity disintegration tablets and a preparation method thereof. The preparation method comprises the following steps of weighing a raw material namely salbutamol sulfate and auxiliary materials, performing screening, premixing the raw material with the auxiliary materials, and performing tabletting, wherein a powder direct tabletting technology is adopted for tabletting. The preparation method disclosed by the invention has the following advantages that the direct tabletting technology is adopted, fewer working procedures are adopted, equipment issimple, and commercial production is easy; after the particle diameter of the auxiliary materials is controlled, the fluidity and the compressibility of granules are greatly improved; after the technology is improved, the impurity level is lower than that of an original product; and the safety of a patient is improved, and potential safety hazards are avoided.
Owner:CHONGQING CONQUER PHARML
Inhalation powder spray for treating respiratory diseases such as chronic obstructive pulmonary disease and asthma
InactiveCN101428011ASignificant effectGood effectPharmaceutical delivery mechanismRespiratory disorderRespiratory tract diseaseObstructive Pulmonary Diseases
Owner:李虎山
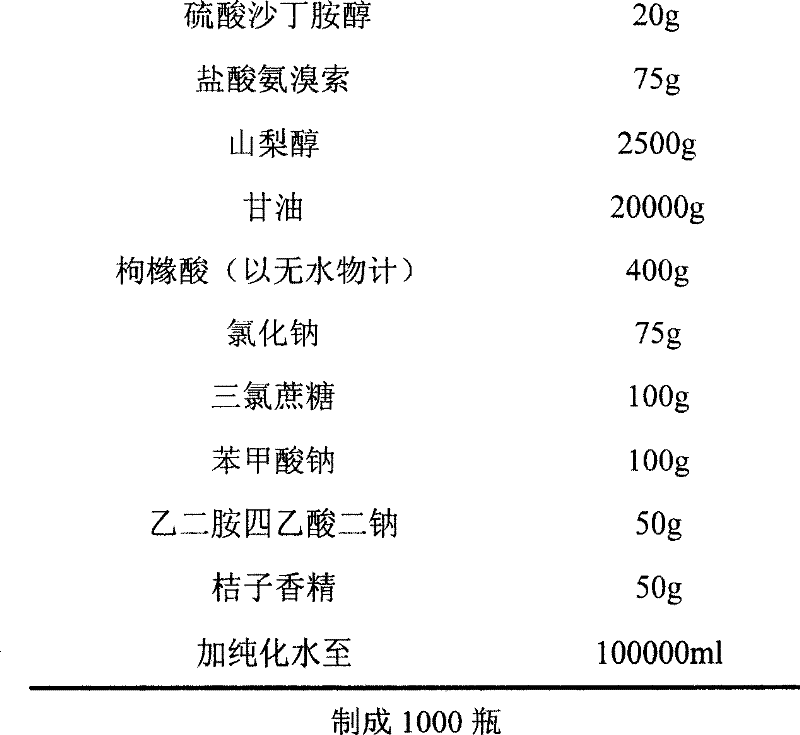
Liquid composite containing salbutamol sulfate and ambroxol hydrochloride
ActiveCN101204386BOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantBULK ACTIVE INGREDIENT
The invention discloses a drug composition taking salbutamol sulfate and ambroxol hydrochloride as active ingredient, also the composition contains a stabilizer, a PH modifying agent and a flavoring agent. However, the composition does not contain an antioxidant or a metal ion complexing agent. The drug composition is used for curing respiratory diseases such as bronchitis and asthma, etc.
Owner:万特制药(海南)有限公公司
Atomization inhalant prepared from interferon alpha and salbutamol sulfate
ActiveCN102430112AGood treatment effectOrganic active ingredientsPeptide/protein ingredientsAdjuvantAntiviral drug
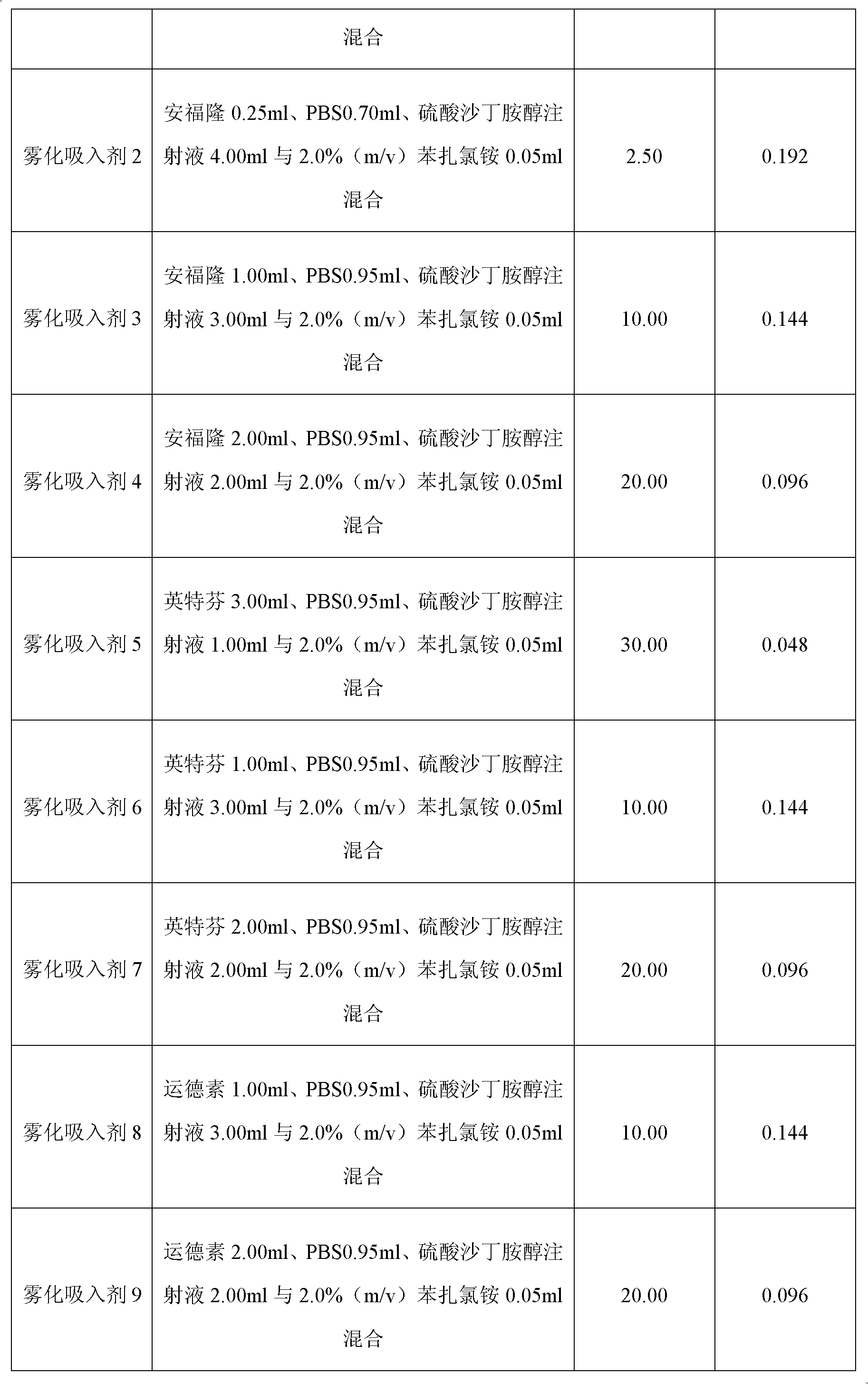
The invention belongs to the field of composites of antiviral medications, which relates to an atomization inhalant prepared from interferon alpha and salbutamol sulfate. The atomization inhalant comprises the interferon alpha with a curative effective dose, the salbutamol sulfate and a pharmaceutically acceptable adjuvant with sufficient quantity. Preferentially, the single dose comprises 2.5-30mug of the interferon alpha, 0.048-0.192mug of the salbutamol sulfate and the pharmaceutically acceptable adjuvant with the sufficient quantity, and more preferentially, the single dose comprises 10-20mug of the interferon alpha, 0.096-0.144mug of the salbutamol sulfate and the pharmaceutically acceptable adjuvant with the sufficient quantity. Compared with treating viral pneumonia by singly usingthe interferon alpha or the salbutamol sulfate, the curative effect can be obviously improved on treating the viral pneumonia by using the atomization inhalant prepared from the interferon alpha and the salbutamol sulfate.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
Western medicine composition for treating chronic bronchitis
InactiveCN106668044AReduce generationAchieve full recoveryOrganic active ingredientsRespiratory disorderWestern medicineNalidixic acid
The invention discloses a western medicine composition for treating chronic bronchitis. The western medicine composition for treating chronic bronchitis is prepared from the following main raw materials in parts by weight: 4-9 parts of tenacissoside H, 3-10 parts of pristimerin, 7-15 parts of nalidixic acid, 2-6 parts of trihydroxyflavone, 12-17 parts of benzoylpaeoniflorin, 8-9 parts of picroside, 1-4 parts of myricetin, 0.7-1.5 parts of andrographolide and 0.1-0.5 part of salbutamol sulfate. According to the western medicine composition disclosed by the invention, by virtue of synergistic effects of all the medicines, the aim of comprehensive rehabilitation is achieved, both symptoms and root causes are treated, the treatment course is short, the effect can be taken quickly, toxic and side effects are less, and the cost is extremely low.
Owner:郑州莉迪亚医药科技有限公司
Ambroxol salbutamol lipid solid dispersion
InactiveCN105496992AGood effectDispersion quality is stableOrganic active ingredientsRespiratory disorderVitamin E AcetateOctanoic Acids
The invention relates to an ambroxol salbutamol lipid solid dispersion and a preparing method thereof, belonging to the field of medicinal preparations. The lipid solid dispersion contains ambroxol hydrochloride, salbutamol sulfate and butyl hydroxy anisol, a lipid carrier is a mixture of 25% of laurin, 30% of coconut oil, 15% of polyethylene glycol octanoic acid / caprin, 20% of vitamin E acetate and 10% of PEG200, a solid carrier is a mixture of 30% of aerosol and 70% of corn starch, and the ambroxol salbutamol lipid solid dispersion is stable in quality, obvious in effect and capable of effectively treating diseases of the respiratory system such as acute and chronic bronchitis and asthma.
Owner:CP PHARMA QINGDAO CO LTD
Preparing method of ambroxol salbutamol oral liquid
InactiveCN105496991AGood effectQuality improvementOrganic active ingredientsDispersion deliveryDiseaseMANNITOL/SORBITOL
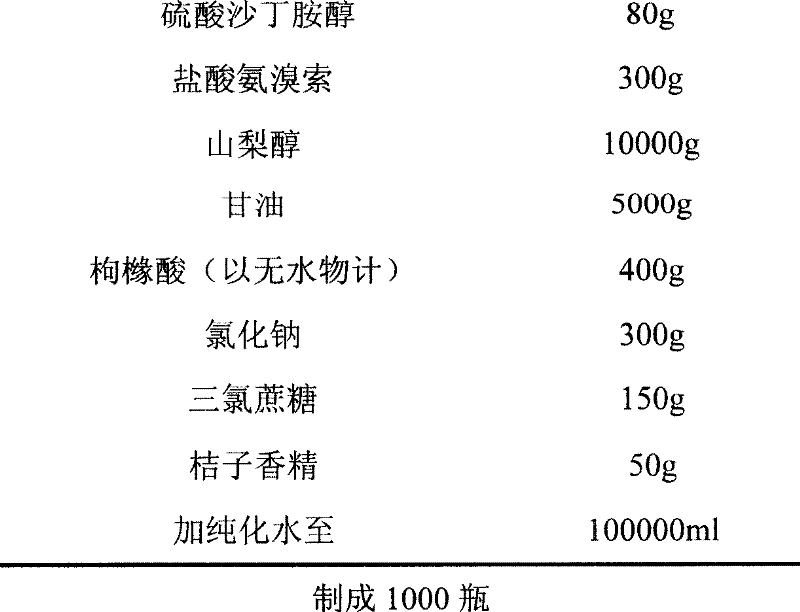
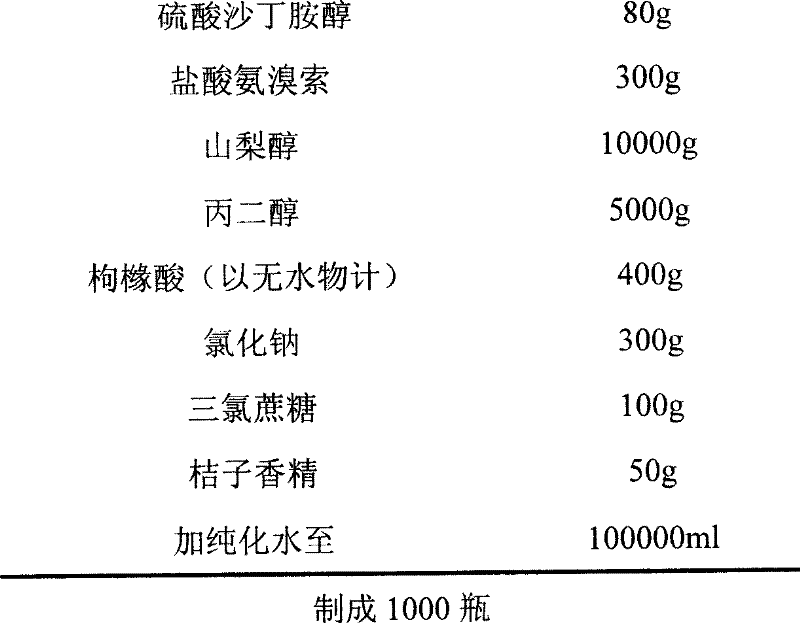
The invention relates to an ambroxol salbutamol oral liquid and a preparing method thereof, belonging to the field of medicinal preparations. The oral liquid contains components such as ambroxol hydrochloride, salbutamol sulfate, propylene glycol, glycerol, methyl parahydroxybenzoats, sodium benzoate, mannitol, Steviosin and flavoring orange essence, is stable in quality, obvious in effect and capable of effectively treating diseases of the respiratory system such as acute and chronic bronchitis and asthma.
Owner:CP PHARMA QINGDAO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com