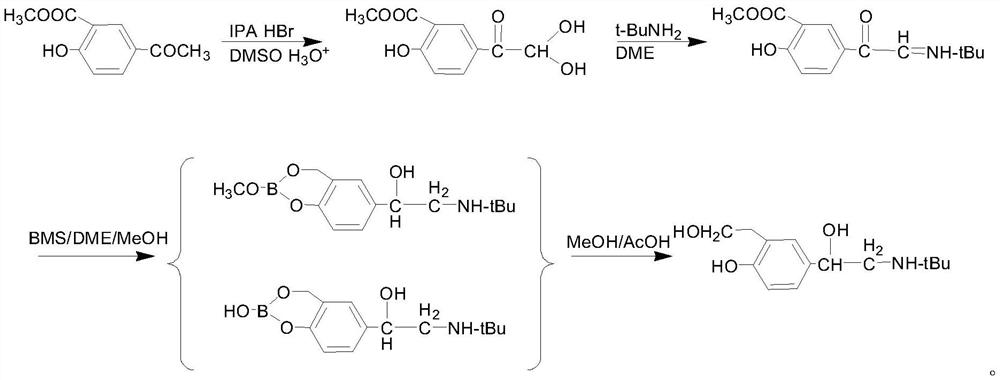
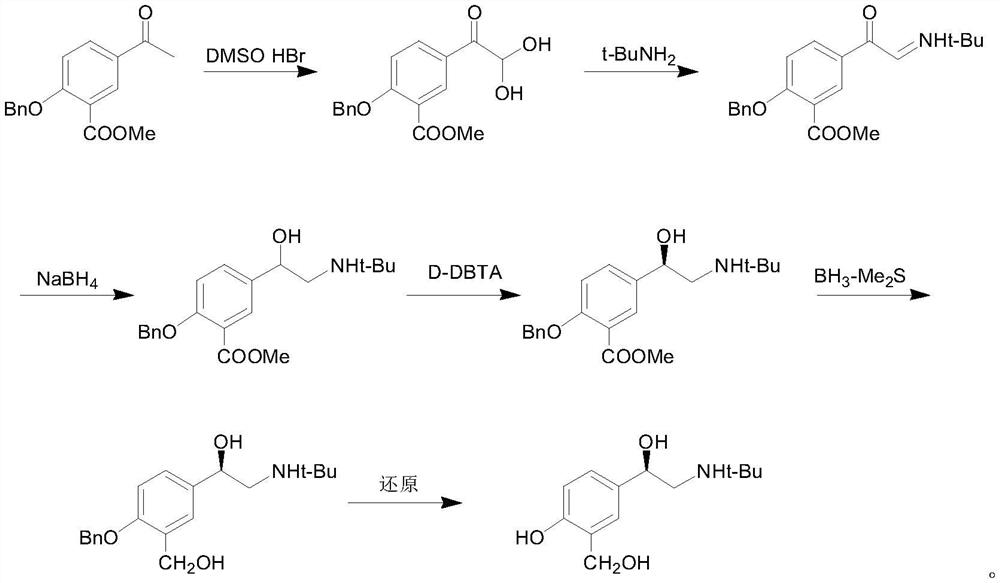
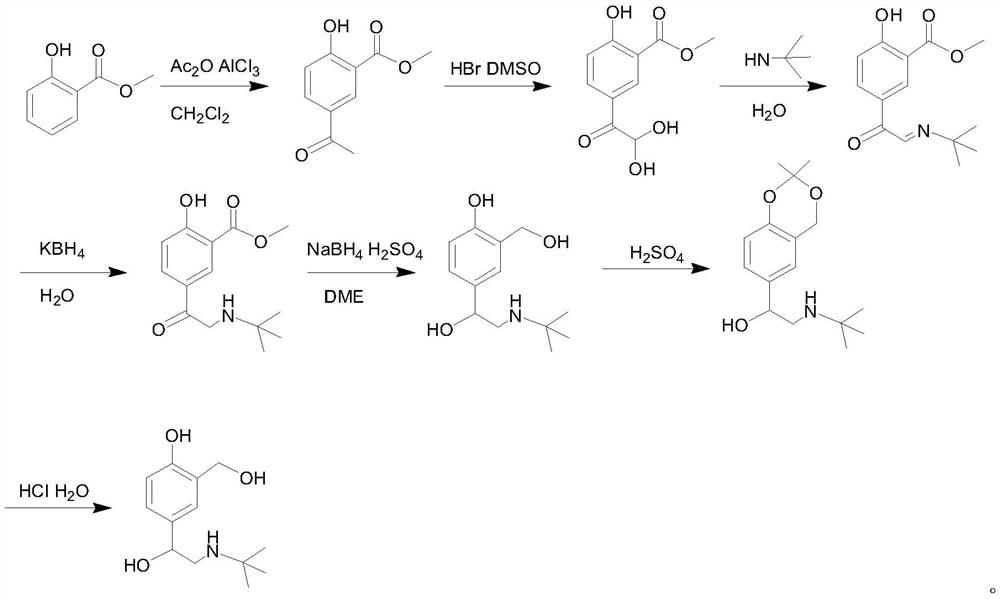
Preparation method of salbutamol sulfate
A technology of salbutamol sulfate and salbutamol, which is applied in the preparation of organic compounds, cyanide reaction preparation, chemical instruments and methods, etc., can solve problems such as unsuitable for laboratory operation, difficult to realize industrialization, and difficult chiral separation, so as to avoid low temperature Or high temperature reaction, easy manipulation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The preparation of embodiment 1 intermediate 1
Embodiment 1-1
[0073]
Embodiment 1-1-1
[0075] Under the protection of nitrogen, add 150mL of toluene, 20g of salicylic acid and 26g of tert-butylaminoacetyl chloride into the reaction flask, stir and dissolve, then raise the temperature to 55°C, slowly add 32mL of thionyl chloride and 48mL of diisopropyl Ethylamine, after the dropwise addition, reacted at 55°C, and no salicylic acid was detected by TLC. After the reaction is complete, add water to separate the layers, wash the organic phase with saturated sodium chloride solution, dry over anhydrous sodium sulfate, concentrate under reduced pressure to about 20 mL, cool down to 0-5°C, and filter to obtain 35.9 g of intermediate 1-1. Yield 91.8%, HPLC content 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com