Atomization inhalant prepared from interferon alpha and salbutamol sulfate
A technology of salbutamol sulfate and atomized inhalation, which is applied in the field of preparation of drugs for the treatment of viral pneumonia, and can solve the problems of mixed infection in children, insufficient system of viral pneumonia, statistical differences, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of the atomized inhalation of interferon alpha and albuterol sulfate
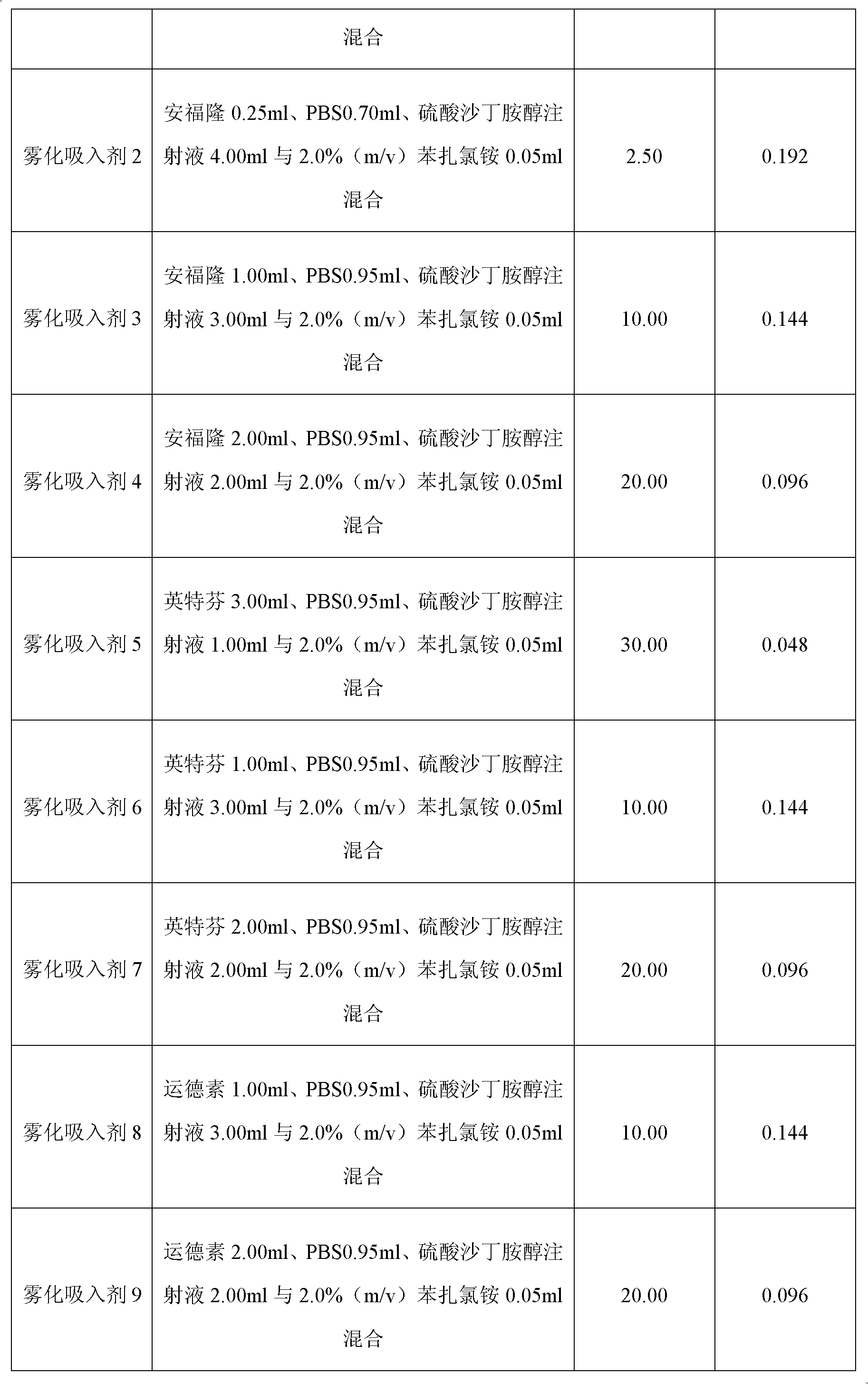
[0029] The nebulized inhalation of interferon alpha and albuterol sulfate was prepared according to the method in Table 1 below. Among them, "Anfulong" (trade name) is the recombinant human interferon α2b injection with a specification of 50 μg / ml / bottle produced by Tianjin Hualida Bioengineering Co., Ltd.; "Interfern" (trade name) is Shenyang Sansheng Pharmaceutical Co., Ltd. Recombinant human interferon α2a injection with a specification of 50 μg / ml / bottle produced by the limited liability company; Interferon α1b injection, "salbutamol sulfate injection" is produced by Jiangsu Yongda Pharmaceutical Co., Ltd., the specification is 0.48mg / 2ml / bottle; "PBS" is 25mmol / L disodium hydrogen phosphate containing 0.15mol / L NaCl- Sodium dihydrogen phosphate buffer (pH7.0). Unless otherwise specified in the subsequent embodiments, the above trade names have the same meaning as ...
Embodiment 2
[0033] Example 2: Detection of the purity of albuterol sulfate after mixing interferon alpha and salbutamol sulfate
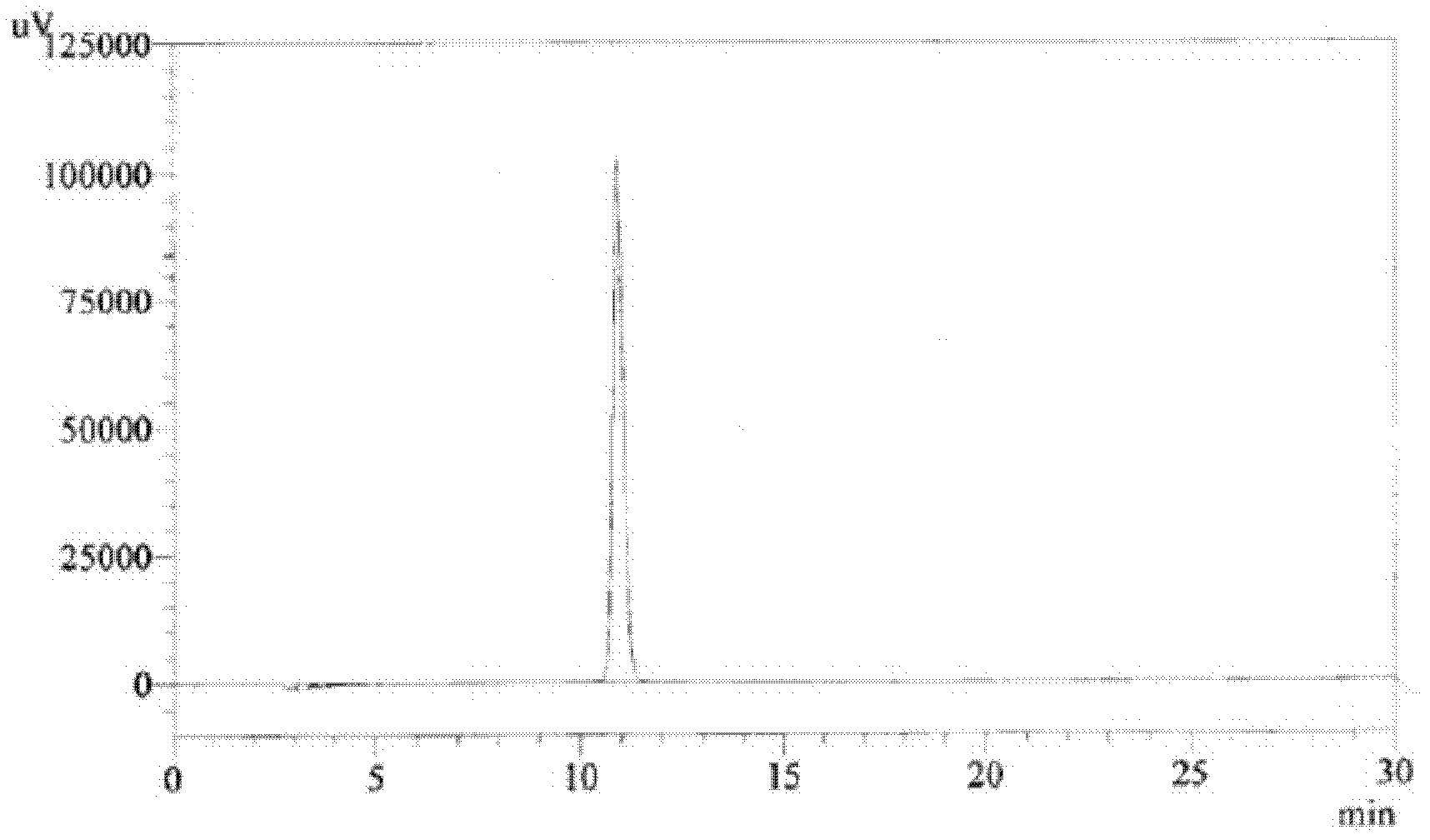
[0034] The aerosol inhalation of each interferon α and albuterol sulfate prepared by the method of Example 1 is carried out ultrafiltration with the Millipore ultrafiltration centrifuge tube with a molecular weight cut-off of 3000Da after placing the preset time, and the filtrate is treated with After diluting with deionized water to the same volume as before ultrafiltration and centrifugation, take 20 μl and put them on a DIKMAPlatisil C18 reversed-phase high-performance liquid chromatography column (5 μm filler particle size, column size 4.6×250mm), and perform chromatographic operation at a column temperature of 25°C. Phase 0.08mol / L sodium dihydrogen phosphate (phosphoric acid to adjust the pH value to 3.1±0.05)-methanol (85:15) elution, flow rate 1.0ml / min, detection wavelength 276nm. The measurement results are shown in Table 2 below.
[0035] Salbutamol...
Embodiment 3
[0038] Embodiment 3: detection of salbutamol sulfate content after mixing interferon alpha and salbutamol sulfate
[0039] The salbutamol sulfate reference substance (prepared by China Institute for the Control of Pharmaceutical and Biological Products, batch number 100328-200502) is dissolved and diluted with water respectively to a concentration of 0.06, 0.12, 0.24, 0.48, 0.72mg / ml and then presses the high performance liquid chromatography conditions of Example 2 Samples were loaded to determine the peak area of the main peak. Use the peak area (y) to perform linear regression on the concentration (x) to obtain a working curve, the equation is y=6900000x+5158, and the regression coefficient is r=0.9999.
[0040] The aerosolized inhalation of each interferon α and albuterol sulfate prepared by the method of Example 1 is carried out ultrafiltration centrifugation according to the method of Example 2 respectively after placing the preset time, and the filtrate is deionized w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com