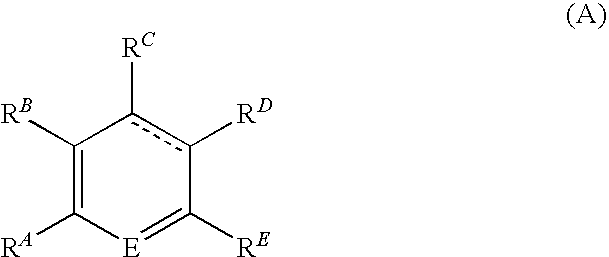
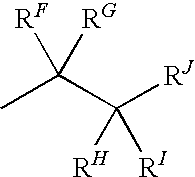
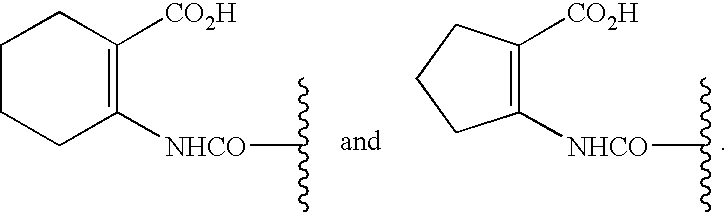
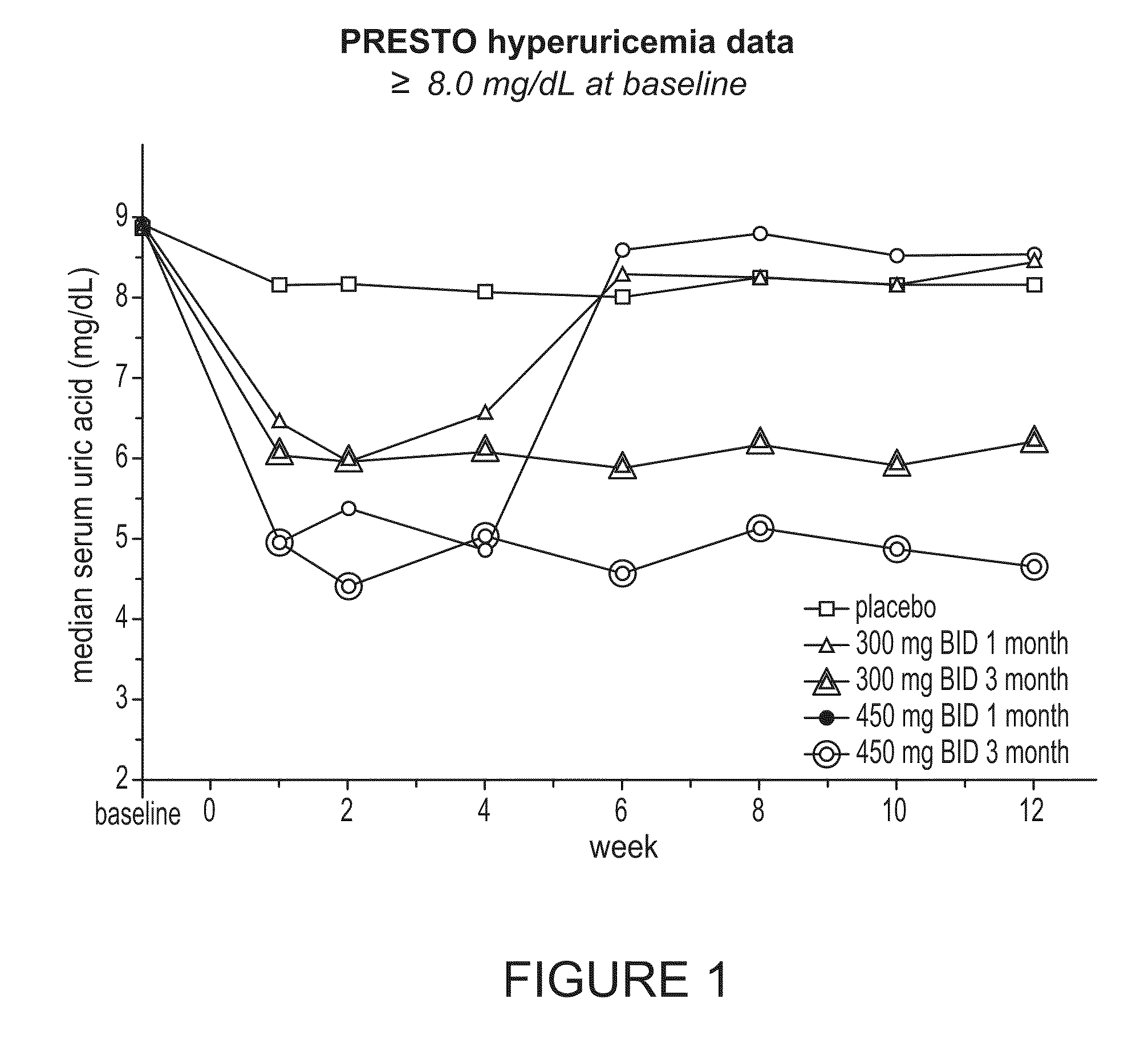
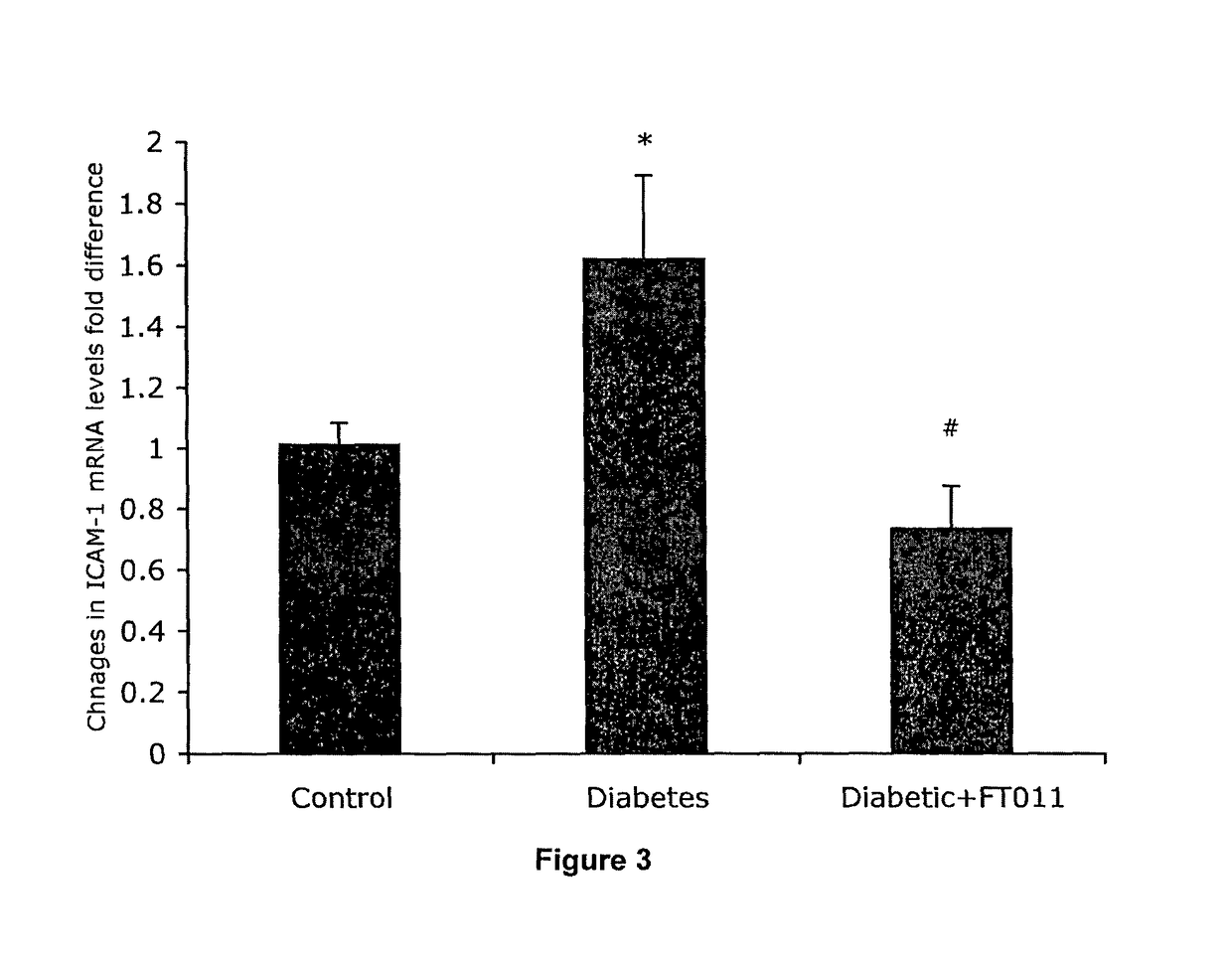
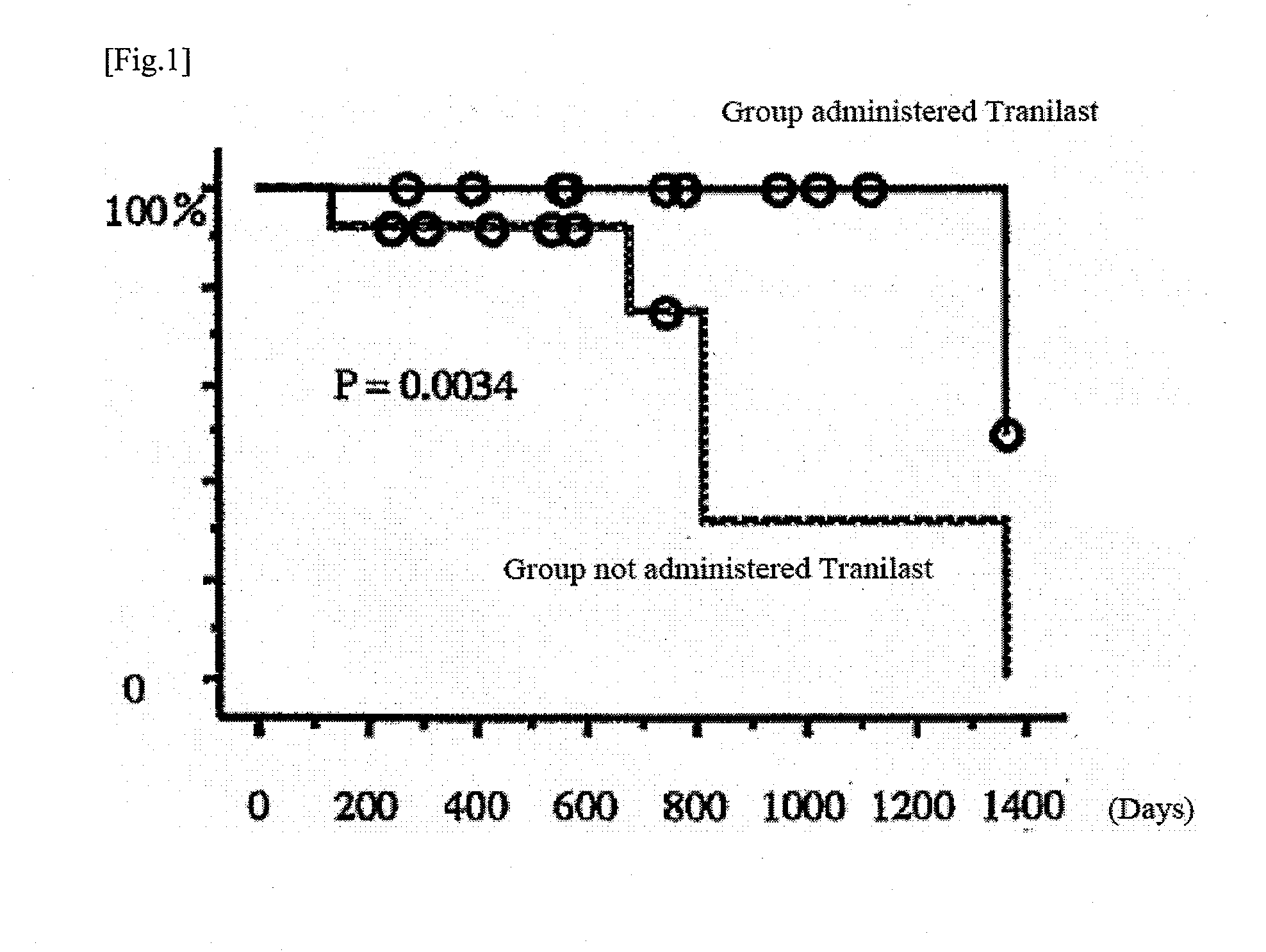
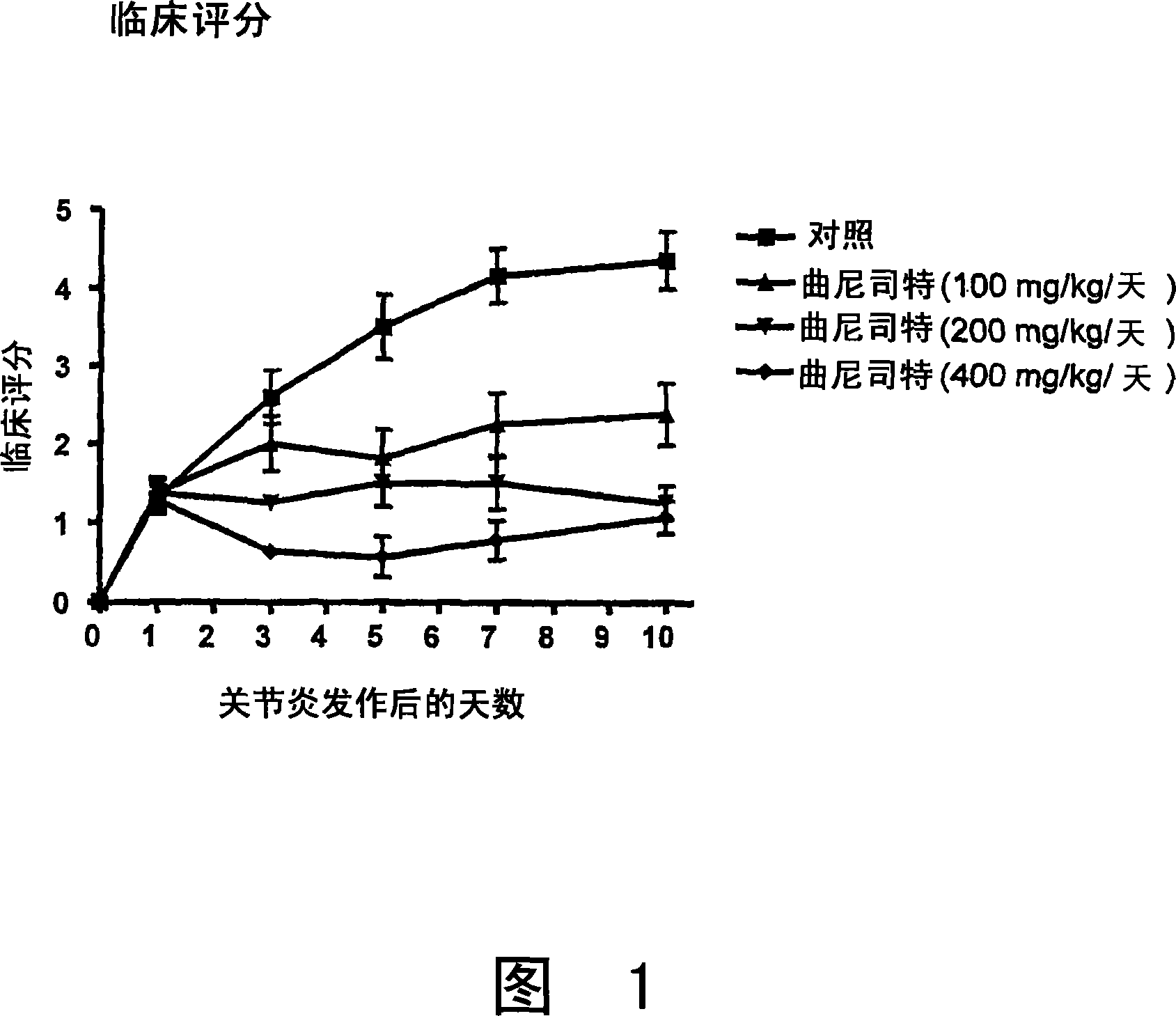
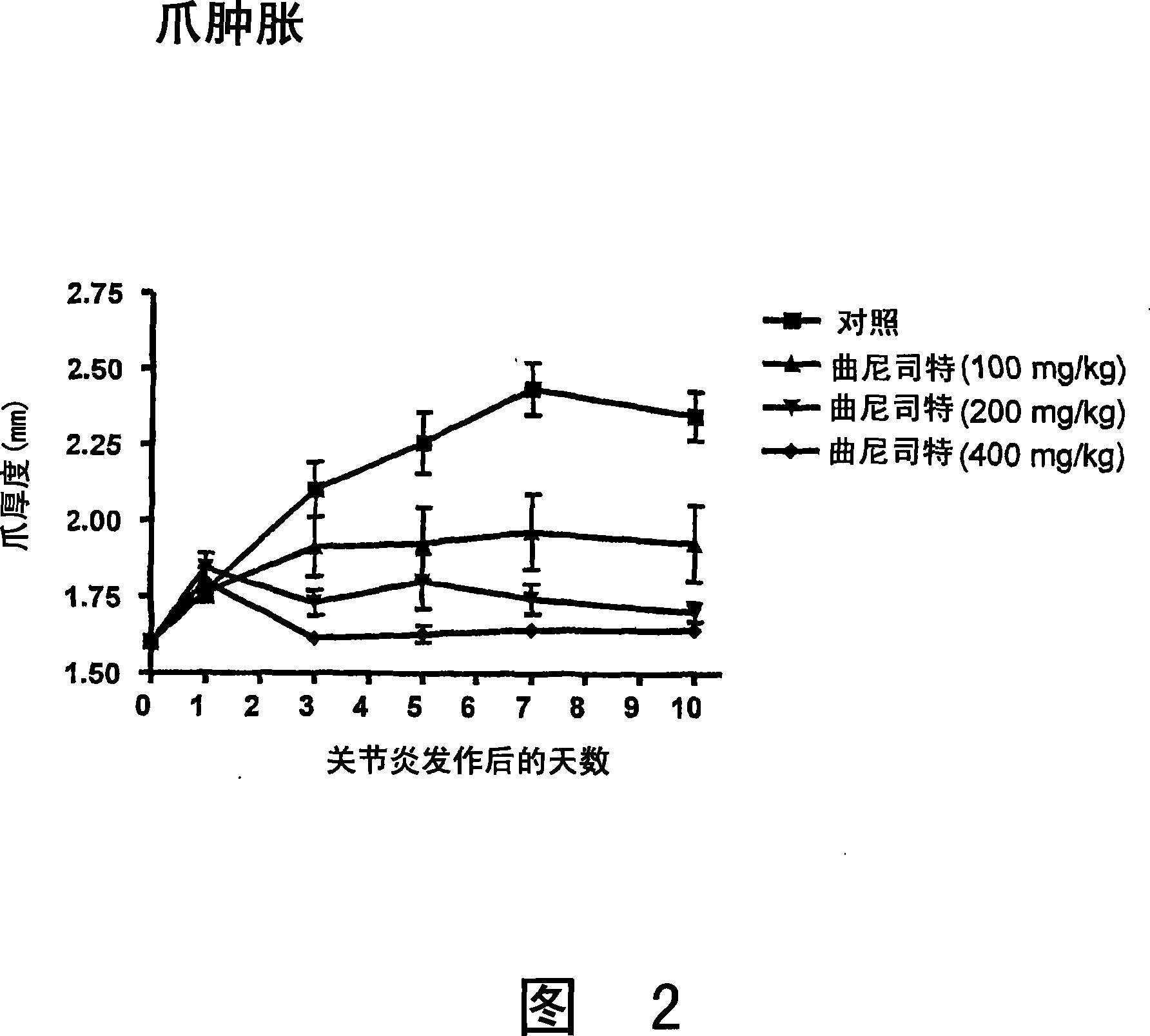
Patents
Literature
47 results about "Tranilast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tranilast (INN, brand name Rizaben) is an antiallergic drug. It was developed by Kissei Pharmaceuticals and was approved in 1982 for use in Japan and South Korea for bronchial asthma. Indications for keloid and hypertrophic scar were added in the 1980s.
Combination therapy of arthritis with tranilast
InactiveUS20100158905A1Good treatment effectAntibacterial agentsSalicyclic acid active ingredientsCombined Modality TherapyCOX-2 inhibitor
Combination therapy is disclosed herein for the treatment an arthritic condition (e.g. rheumatoid arthritis, osteoarthritis or psoriatic arthritis). The therapies disclosed herein comprise administering tranilast or an analogous compound in combination with a pharmaceutical agent, such as a non-steroidal anti-inflammatory drug, a disease-modifying drug, a COX-2 inhibitor, an antibiotic, an analgesic or combination thereof.
Owner:NUON THERAPEUTICS INC
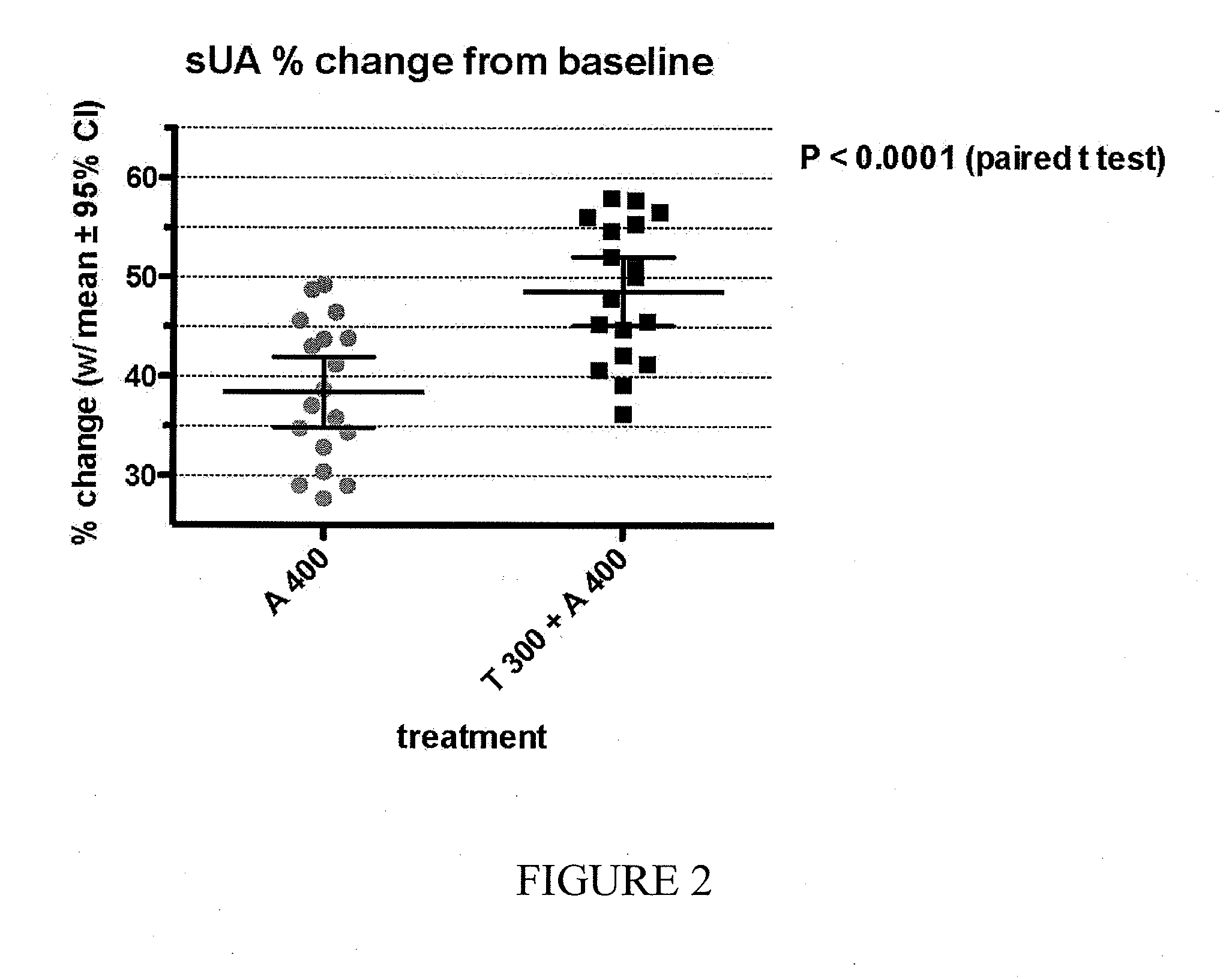
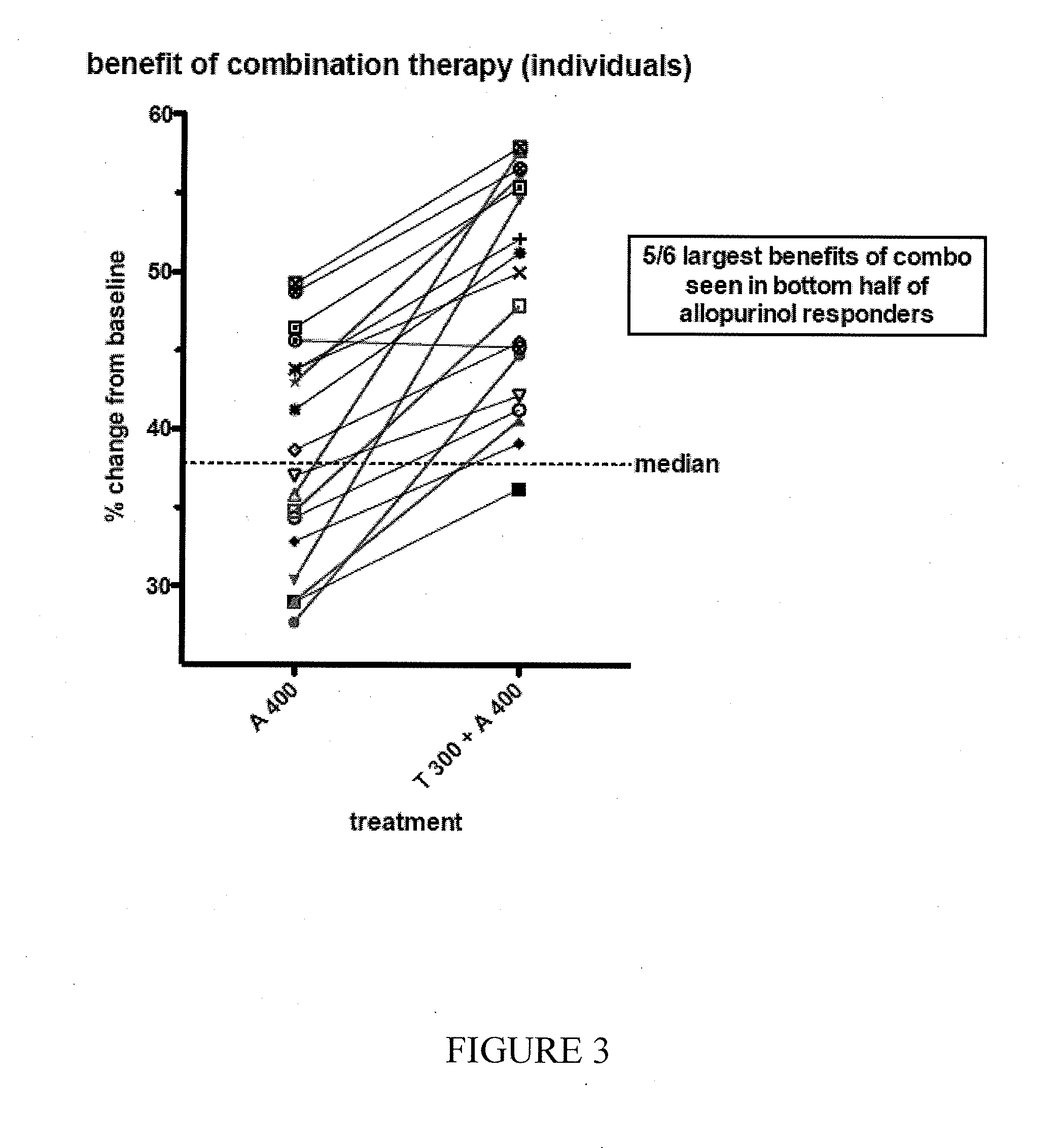
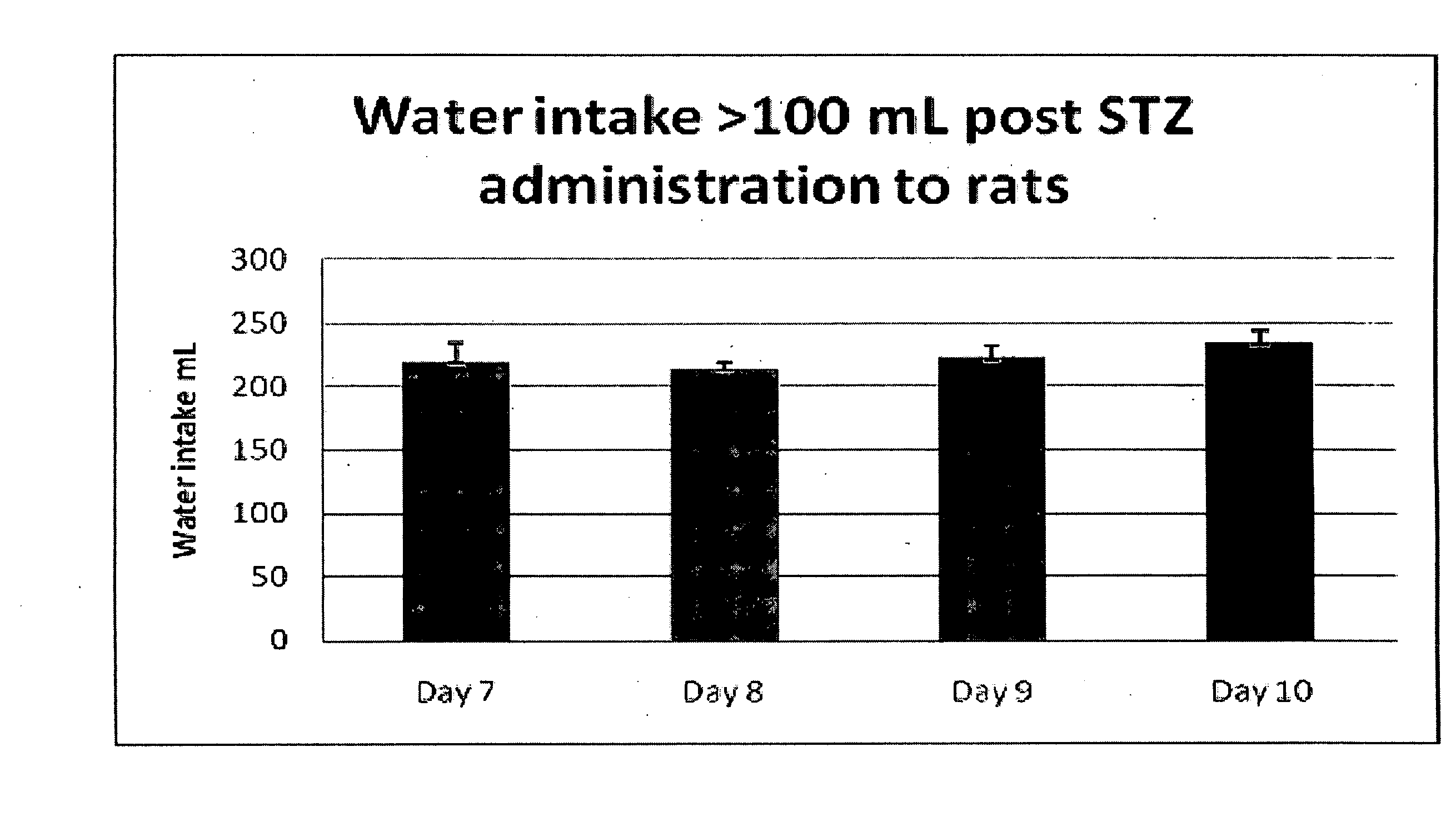
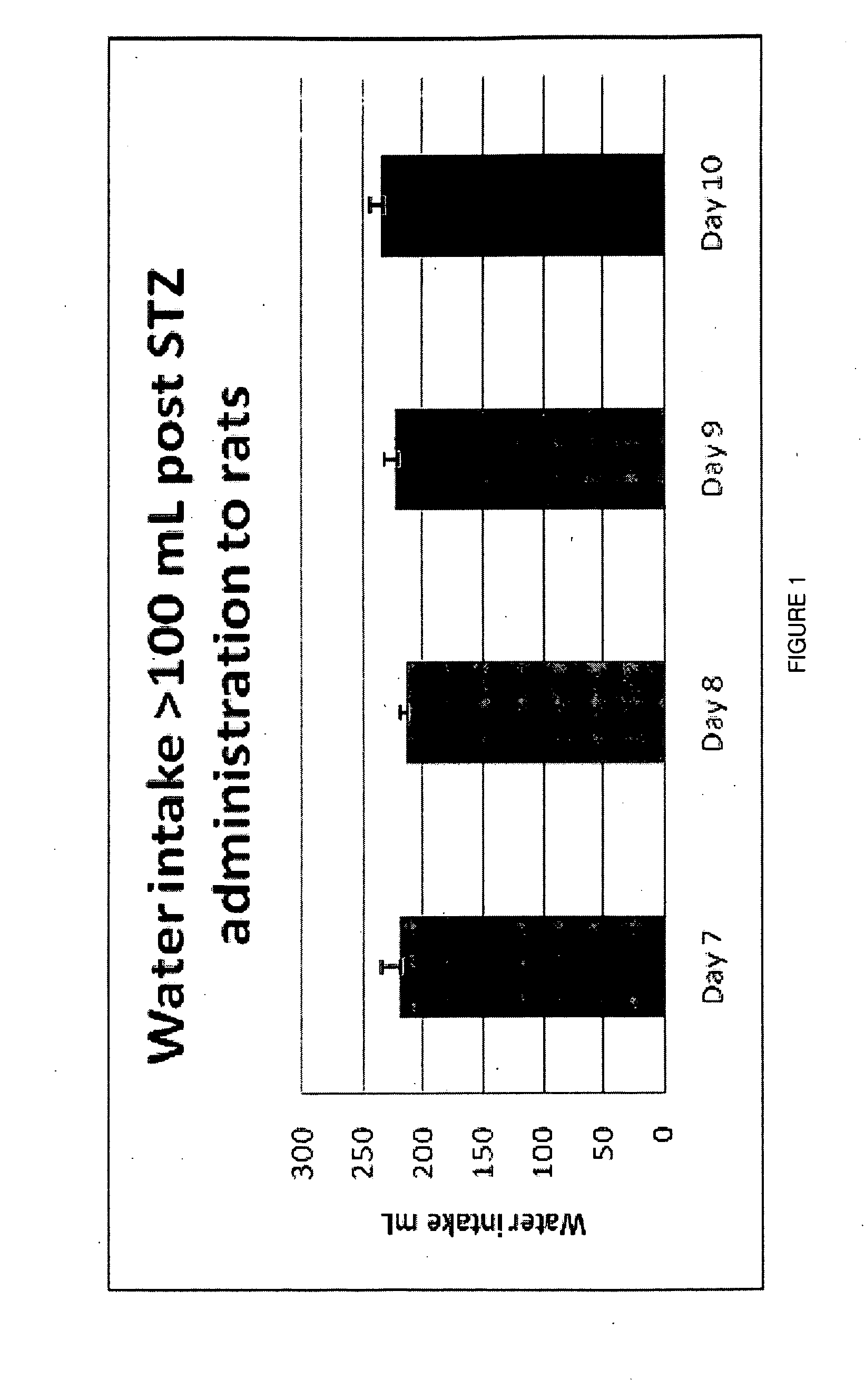
Combination formulations of tranilast and allopurinol and methods related thereto
InactiveUS20110136835A1Increasing serum uric acid lowering effectivenessOrganic active ingredientsBiocideAllopurinolTranilast
Disclosed is a pharmaceutical composition comprising tranilast or a pharmaceutically acceptable salt thereof and allopurinol or a pharmaceutically acceptable salt thereof, wherein the amount by weight of said allopurinol or pharmaceutically acceptable salt thereof in said composition is greater than the amount by weight of said tranilast or pharmaceutically acceptable salt thereof in said composition.
Owner:NUON THERAPEUTICS INC
Treatment of neuropathic pain
A method of decreasing neuropathic pain in a mammal, comprising administering to said mammal an effective amount of tranilast for a period of time sufficient to decrease pain.
Owner:NUON THERAPEUTICS INC
Pharmaceutical product containing tranilast
InactiveUS20070197648A1Improve light resistanceBiocideOrganic active ingredientsMedicineDrug product
Owner:ROHTO PHARM CO LTD
Bracket for controlling releasing and elution of tranilast medicament coating
InactiveCN101181650AEvenly distributedNo crackStentsOrganic active ingredientsTectorial membranePercent Diameter Stenosis
The invention relates to a tranilast medicine coat controlled release eluting bracket, pertaining to the field of medicine and medical appliance. The invention takes tranilast as the active component;three layers of a medicine loading layer, a controlled release layer and a protecting film layer are at the surface of the bracket. The bracket coat is evenly distributed on the surface of the bracket without crack and exfoliating phenomena; the original morphological structure can be kept after expanding. Proved by animal experiment result, the invention can effectively restrain the coronary hyperplasia and restenosis and markedly reduce the function of restraining the blood vessel endothelial cell at the same time; compared with the single coat or double coat structure of the existing drug-eluting bracket, the bracket of the invention can prevent the restenosis of the bracket, and has the functions of prventing bracket thrombus from being formed and promoting the early reparation of thebracket endothelium.
Owner:SHANGHAI PUTUO DISTRICT CENT HOSPITAL
Therapeutic Compounds
Substituted cinnamoyl anthranilate compounds exhibiting anti-fibrotic activity; or derivatives thereof, analogues thereof, pharmaceutically acceptable salts thereof, and metabolites thereof; with the proviso that the compound is not Tranilast.
Owner:CERTA THERAPEUTICS PTY LTD
Methods of treating eye diseases associated with inflammation and vascular proliferation
ActiveUS9839640B2Decreasing plasma clearance rateImprove stabilityAntibacterial agentsSenses disorderBenzoic acidVascular proliferation
Methods for treating eye diseases associated with inflammation and / or vascular proliferation in subjects are disclosed. The methods include administering therapeutically effective amounts of a tranilast compound, in particular (E)-2-[[3-(3-Methoxy-4-propargyloxy)phenyl)-1-oxo-2-propenyl]amino]benzoic acid or (E)-2-[[3,4-Bis(difluoromethoxy)phenyl)-1-oxo-2-propenyl]amino]benzoic acid or pharmaceutically acceptable salts or solvates thereof.
Owner:OCCURX
Pharmaceutical composition for prevention of progress of intestinal constriction associated with crohn's disease
InactiveUS20100113597A1Inhibit progressBiocideOrganic active ingredientsIntestinal StrictureBULK ACTIVE INGREDIENT
The present invention provides an oral pharmaceutical composition for inhibiting the progression of intestinal stricture associated with Crohn's disease. That is, the present invention relates to a pharmaceutical composition for inhibiting the progression of intestinal stricture associated with Crohn's disease which comprises as an active ingredient N-(3,4-dimethoxycinnamoyl)anthranilic acid (generic name: tranilast) or a pharmaceutically acceptable salt thereof. The present invention can provide a pharmaceutical composition useful as an agent for inhibiting the progression of intestinal stricture associated with Crohn's disease for medical therapy.
Owner:KISSEI PHARMA
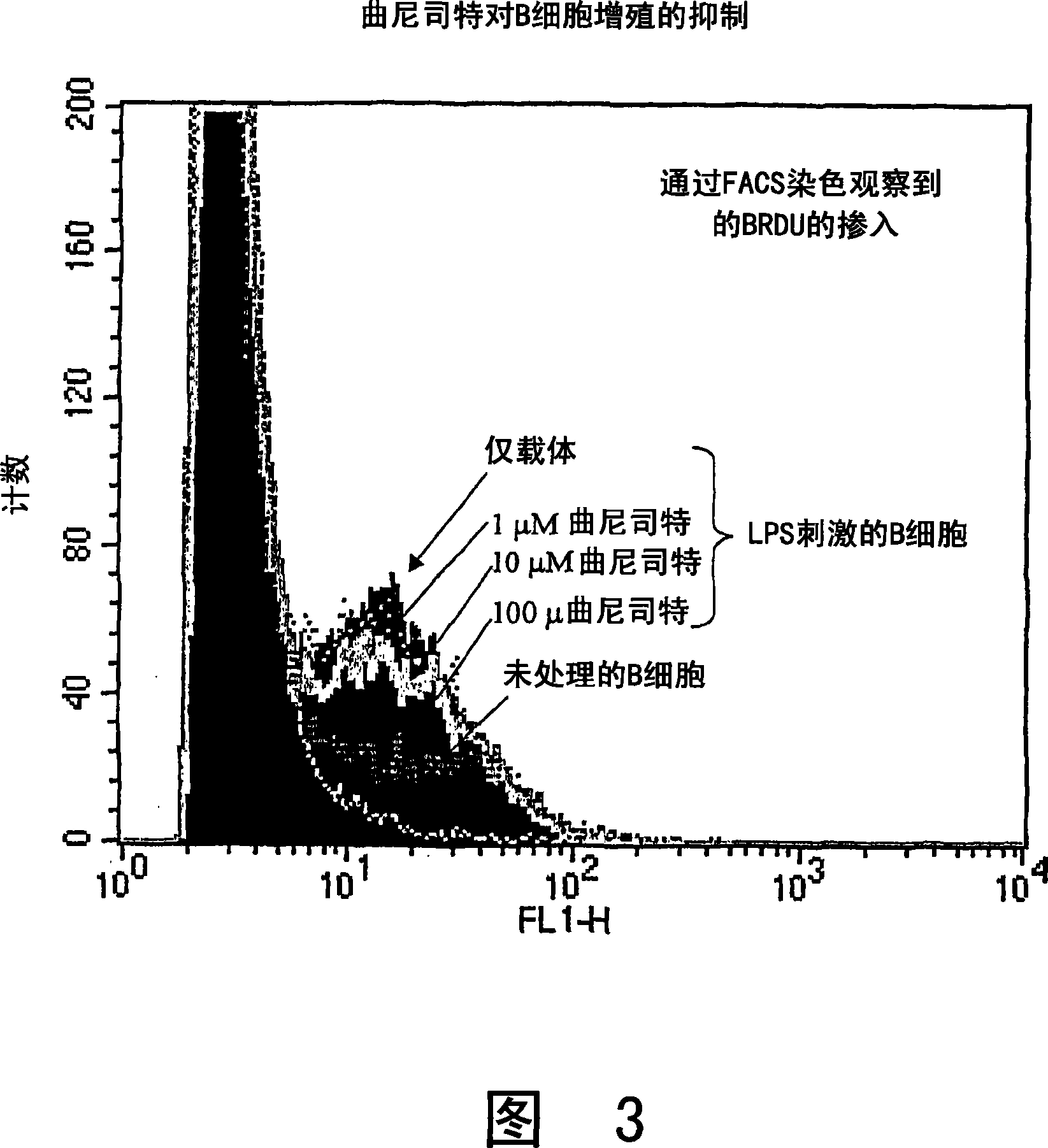
Method of modulating b cell functioning
Owner:太平洋治疗学控股有限公司
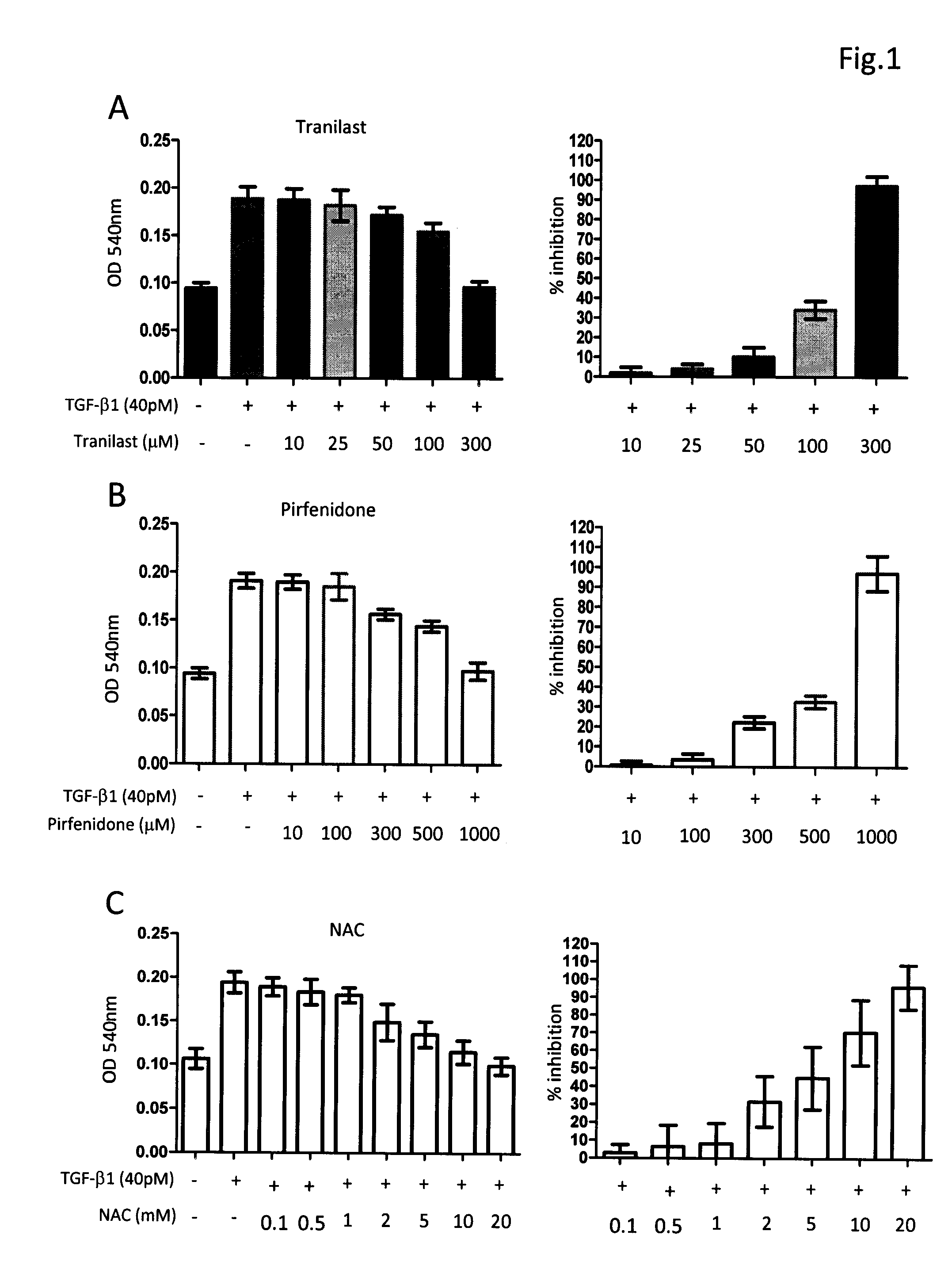
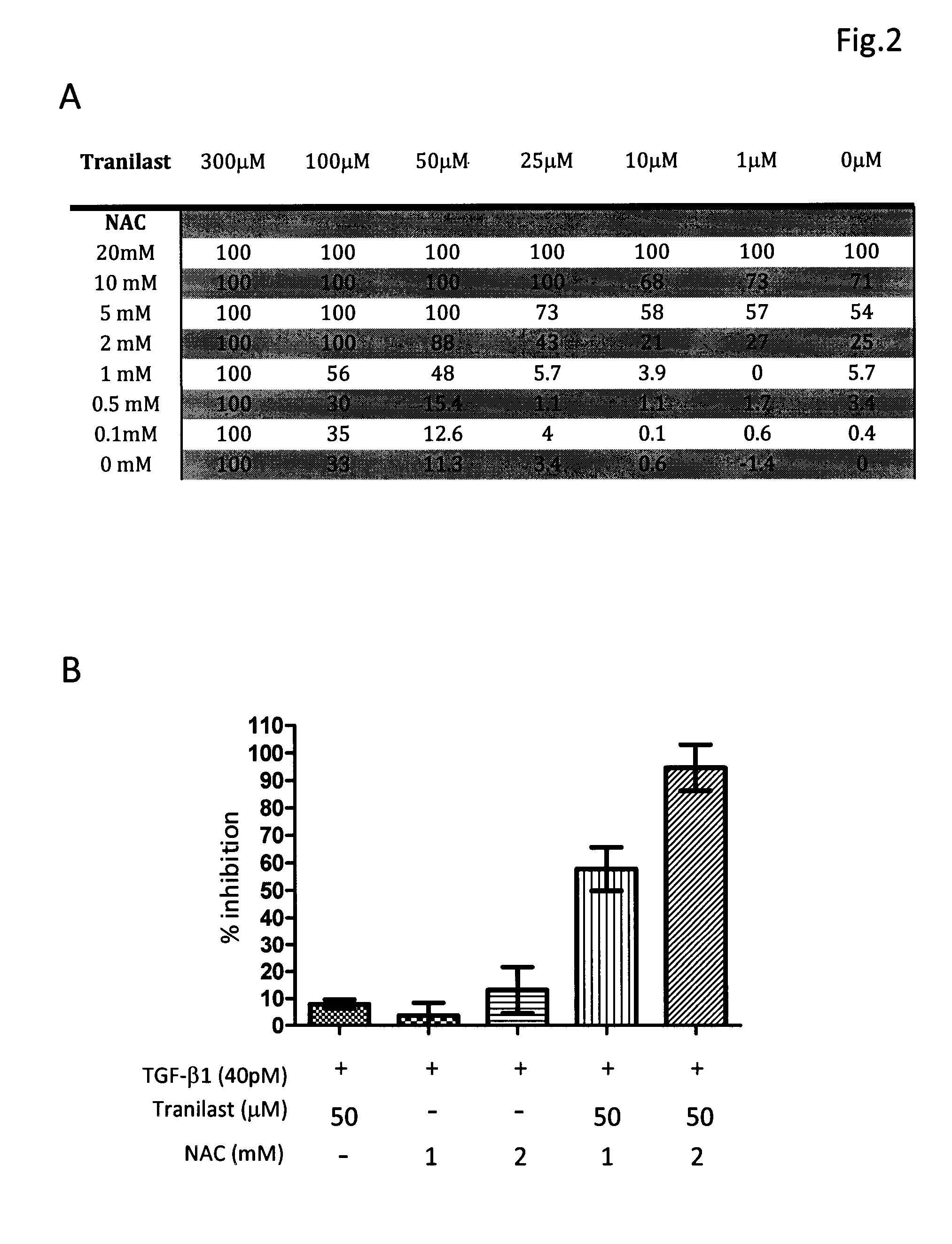
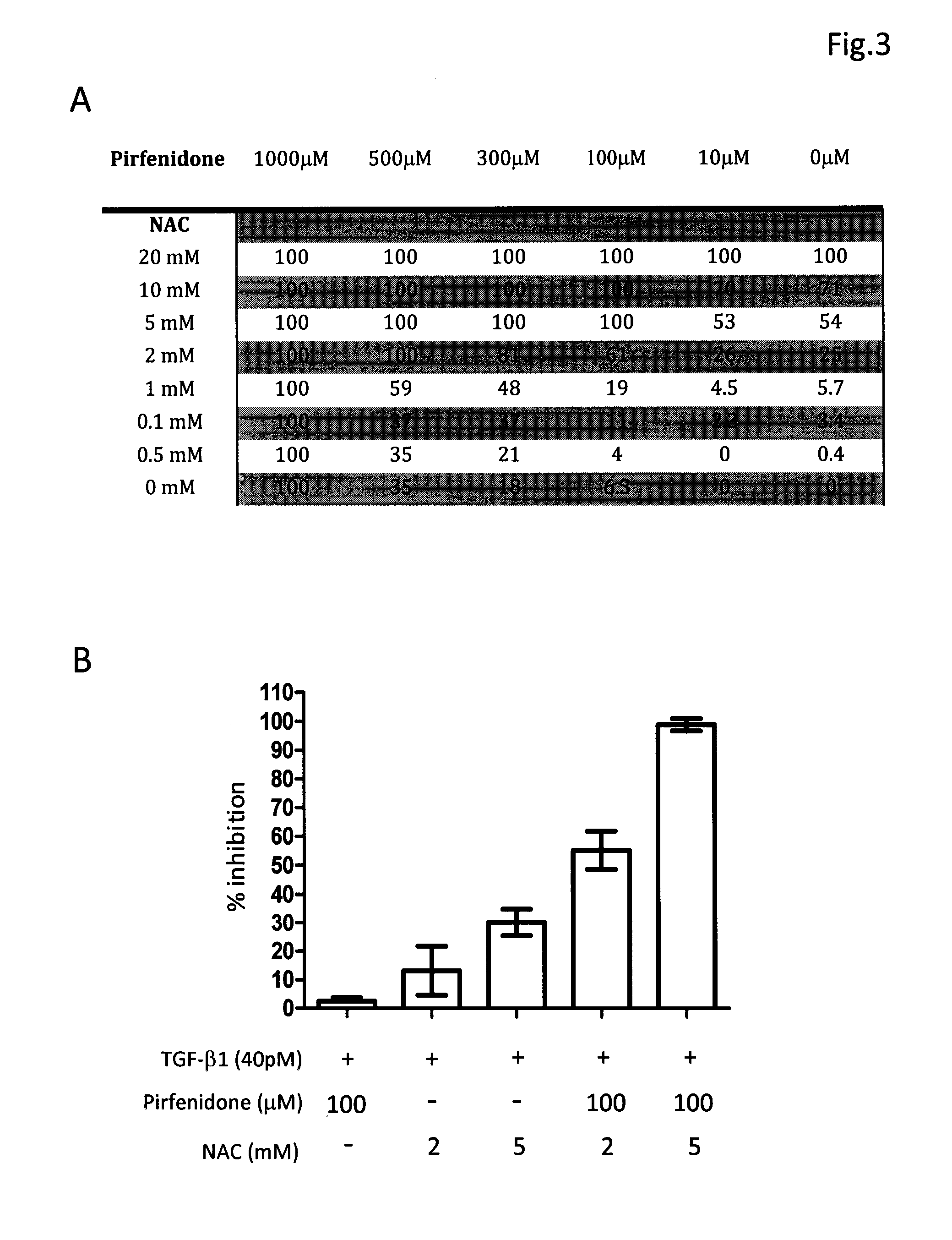
Composition and method for treating fibrosis
InactiveUS20110288134A1Treating and preventing and reducing fibroproliferative disorderDelay disease progressionBiocidePeptide/protein ingredientsMetaboliteFibrosis
The present invention relates, in general, to fibroproliferative disorders, and, in particular, to a method of treating, preventing or reducing fibroproliferative disorders by administering to a mammal in need a composition comprising pharmacologically effective doses of a cytokine modifier, such as tranilast or pirfenidone, and an anti-oxidant which is a precursor of glutathione, such as N-acetyl-cysteine, or their pharmaceutically acceptable derivatives, salts, metabolites, or structural or functional analogues thereof.
Owner:FORGE THERAPEUTICS INC
Synthesis method of tranilast
InactiveCN104693063AHigh purityEmission reductionOrganic compound preparationCarboxylic acid amides preparationMalonic acidBenzaldehyde
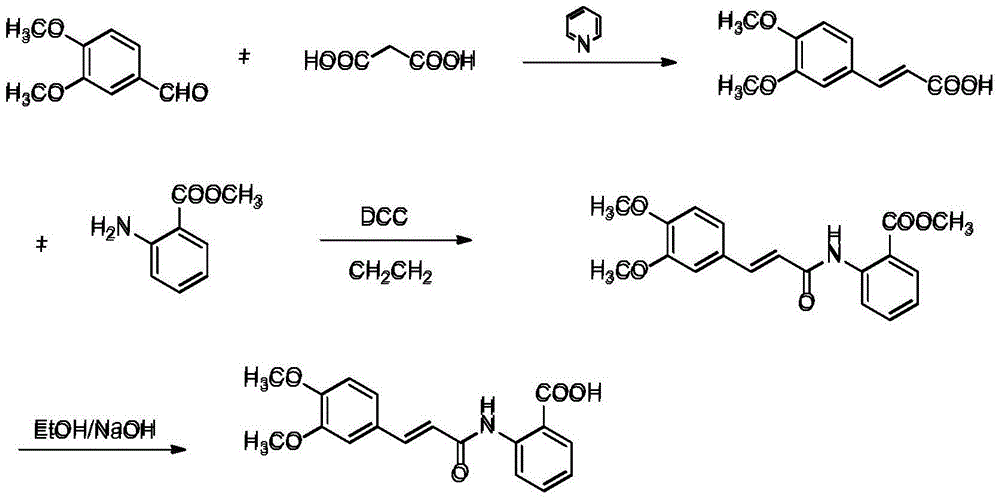
The invention provides a method for preparing tranilast. The method is that 3, 4-dimethyl benzaldehyde is used as a starting material subjected to condensation reaction with malonic acid by pyridine catalyzing to synthesize 3, 4-dimethoxycinnamic acid; the 3, 4-dimethoxycinnamic acid is condensed with methyl anthranilate to generate tranilast methyl ester; the tranilast methyl ester is hydrolyzed through sodium hydroxide to obtain tranilast. The total yield of tranilast is up to 67%. The method is short in process line, simple to operate, high in yield, low in production cost, stable, reliable, and simple to operate; the high-purity tranilast raw medicine can be obtained with high yield; the production cost can be completely reduced; the emission of pollutants can be decreased.
Owner:药大制药有限公司
Treatment of neuropathic pain
A method of decreasing neuropathic pain in a mammal, comprising administering to said mammal an effective amount of tranilast for a period of time sufficient to decrease pain.
Owner:NUON THERAPEUTICS PTY LTD
Drug-enhanced adhesion prevention
ActiveUS20050106229A1Inhibition formationOrganic active ingredientsLiposomal deliveryDelivery vehicleWhole body
The present invention includes methods for the inhibition of post-operative adhesion formation between tissue surfaces in a body cavity having been subjected to a surgical procedure, which methods involve administering Tranilast, or an analog thereof, directly to tissue surfaces in the body cavity in amounts and under conditions effective to inhibit formation of adhesions, and to delivery vehicles and compositions suitable for use for local, non-systemic administration of a drug to the body and directly to tissue within a body cavity having been subjected to a surgical procedure.
Owner:ETHICON INC
Preventive therapeutic composition for muscular fatigue, pulled muscle and disease attributed thereto
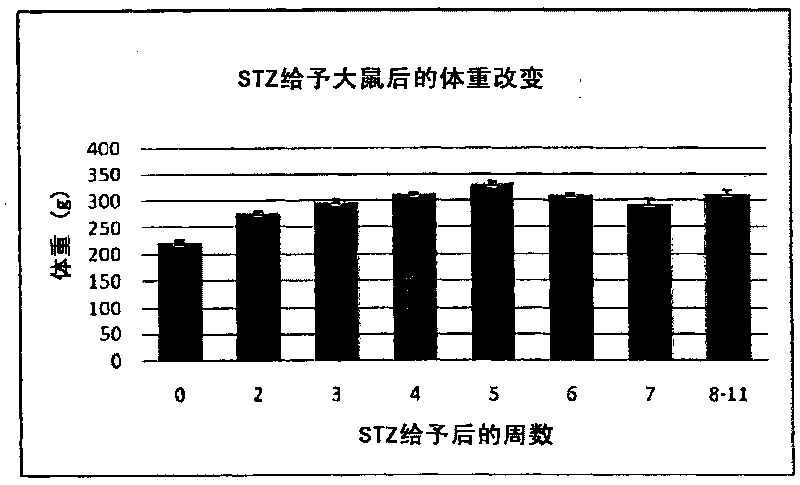
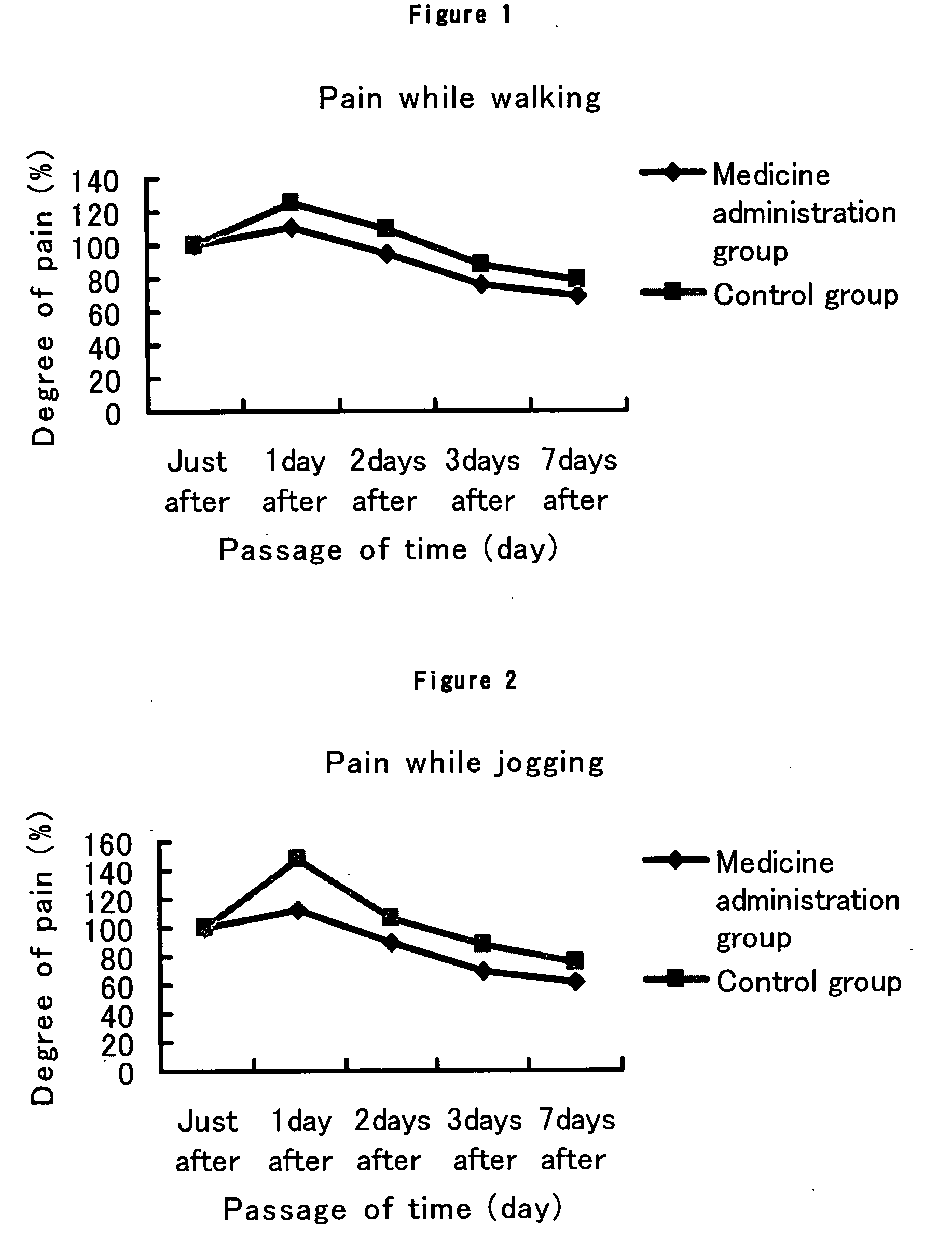
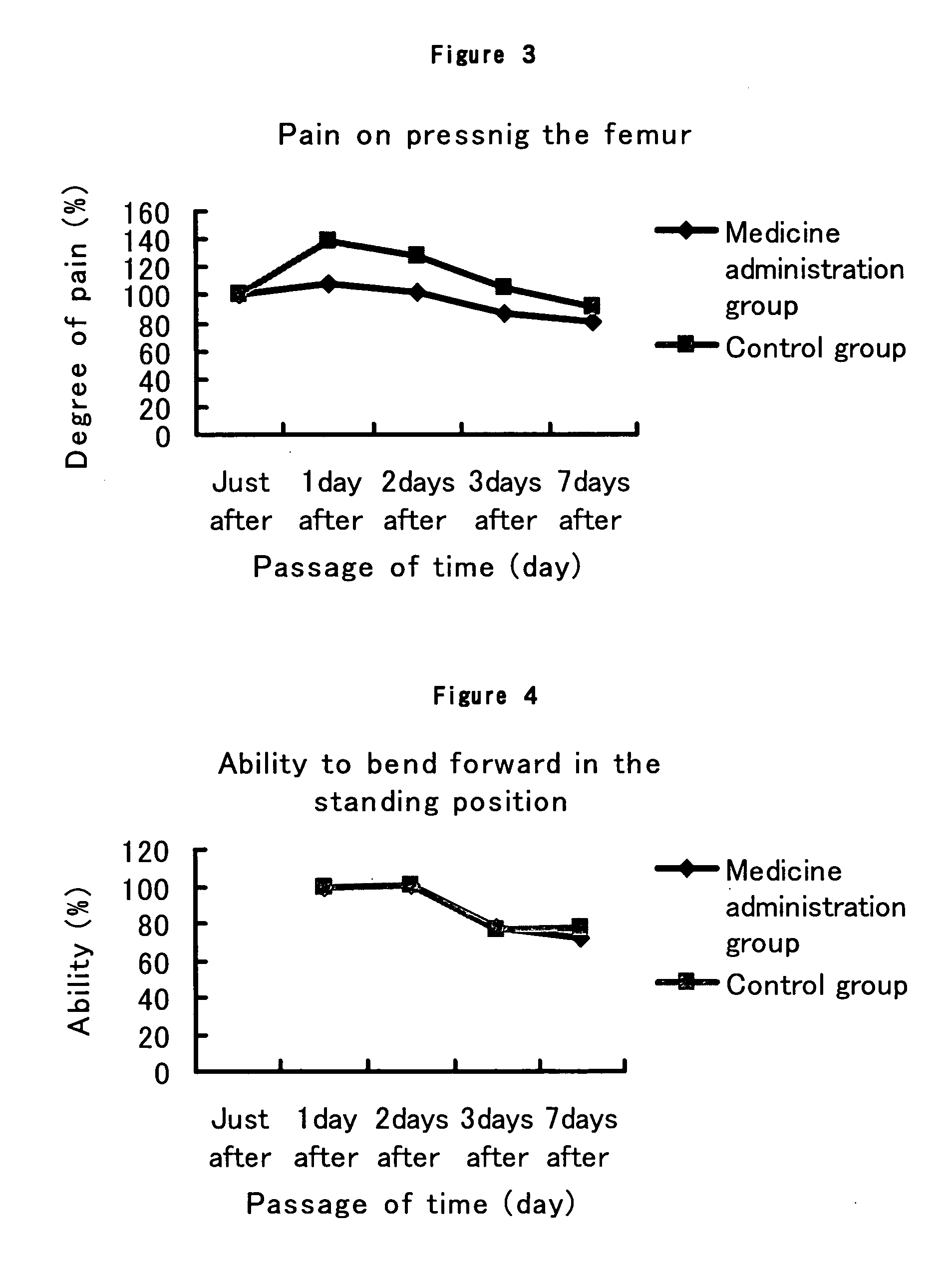
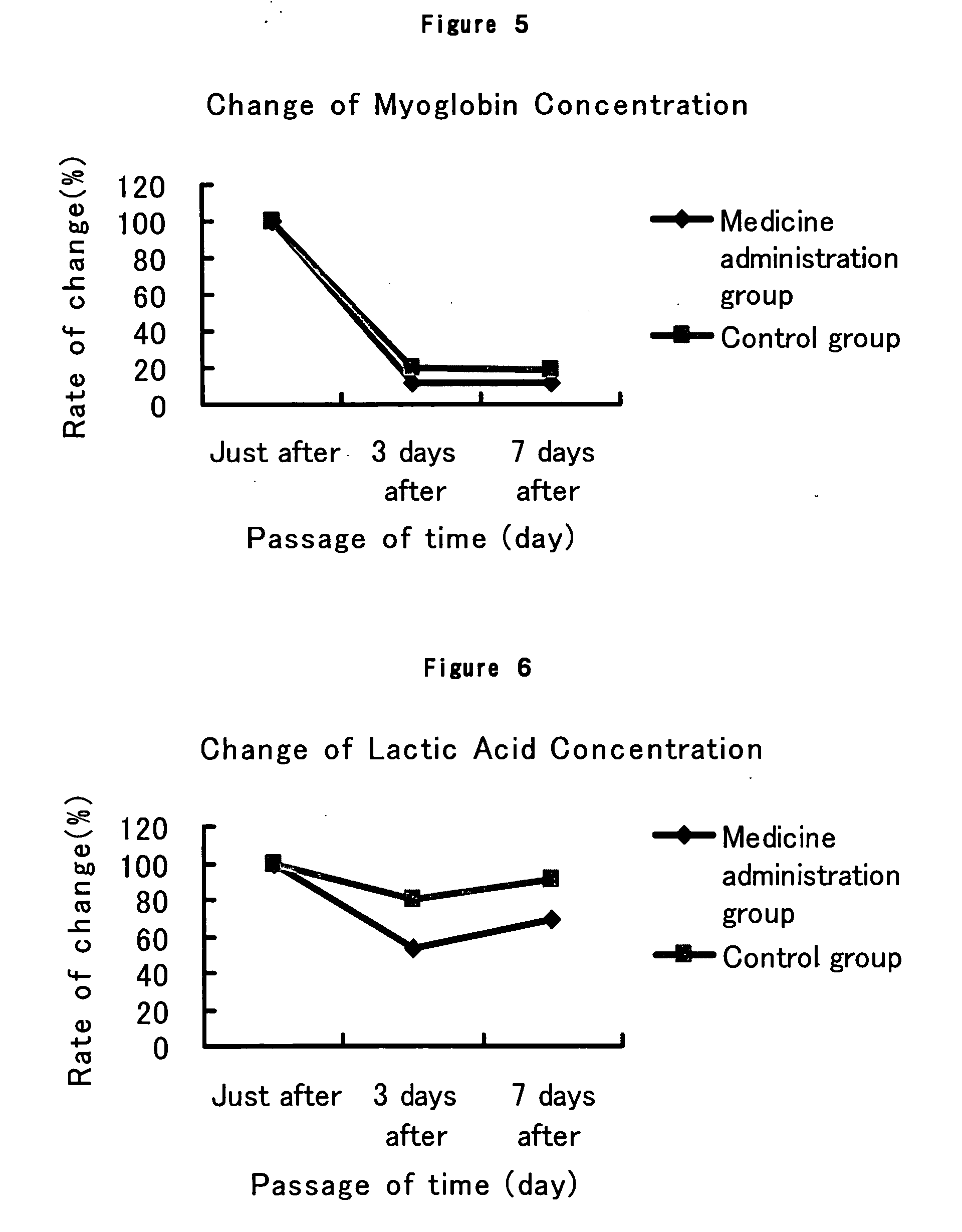
The present invention provides a composition for the inhibition of and promotion of recovery from muscular fatigue or muscular damage and diseases associated therewith, in particular, muscular fatigue or muscular damage caused by exercise stress and diseases associated therewith, and a composition for the prevention or treatment of myofibrosis during the restoration at the damaged part associated with or incidental to surgical injury or surgical procedure, which are characterized by comprising as an active ingredient N-(3,4-dimethoxy-cinnnamoyl)anthranilic acid (generic name: tranilast) or a pharmaceutically acceptable salt thereof or a pharmaceutically acceptable solvate thereof, and a method for use thereof.
Owner:FUKUSHIMA KAZUMASA +1
Mixture of betamethasone and tranilast with a transdermal gel for scar treatment
ActiveUS9173940B1Suppressing collagen synthesisInhibit expressionOrganic active ingredientsPeptide/protein ingredientsBetamethasone valerateOleic Acid Triglyceride
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Prescription and preparation method for tranilast gel and ointment
InactiveCN104784101AIncrease drug concentrationFully absorbedOrganic active ingredientsAerosol deliveryTherapeutic effectAugmented Ointment
Belonging to the field of medical technologies, the invention relates to a prescription and preparation method for topical gel and ointment dosage forms containing tranilast component, and therapeutic effects of the dosage forms in keloid and hypertrophic scar fields. The topical gel and ointment contain tranilast, a cosolvent, a substrate, a humectant, a penetration enhancer, a preservative, a pH regulator, purified water and other ingredients. The preparation method includes the steps of: 1. taking a prescribed amount of the substrate, adding a proper amount of purified water, performing full swelling at 20-80DEG C, adjusting the pH to less than 5, adding a prescribed amount of humectant, and conducting heat preservation to obtain A; 2. taking a prescribed amount of tranilast, the cosolvent, the preservative, and a proper amount of purified water, carrying out heating dissolving at 20-80DEG C, and performing heat preservation to obtain B; and 3. Pouring B into A, conducting heat preservation and swelling, and stirring the mixture evenly, thus obtaining the products. The tranilast gel and ointment provided by the invention have the pharmacological activity of treating hypertrophic scars and keloids.
Owner:药大制药有限公司
Drug-enhanced adhesion prevention
InactiveUS20050106230A1Inhibition formationOrganic active ingredientsSurgical drugsDelivery vehicleWhole body
The present invention includes methods for the inhibition of post-operative adhesion formation between tissue surfaces in a body cavity having been subjected to a surgical procedure, which methods involve administering Tranilast, or an analog thereof, directly to tissue surfaces in the body cavity in amounts and under conditions effective to inhibit formation of adhesions, and to delivery vehicles and compositions suitable for use for local, non-systemic administration of a drug to the body and directly to tissue within a body cavity having been subjected to a surgical procedure.
Owner:ETHICON INC
Oral compound pharmaceutic preparation containing tranilast and salbutamol
InactiveCN101683330AOrganic active ingredientsPharmaceutical delivery mechanismOral treatmentBULK ACTIVE INGREDIENT
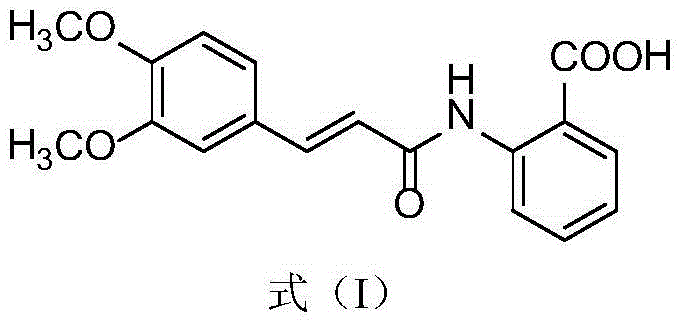
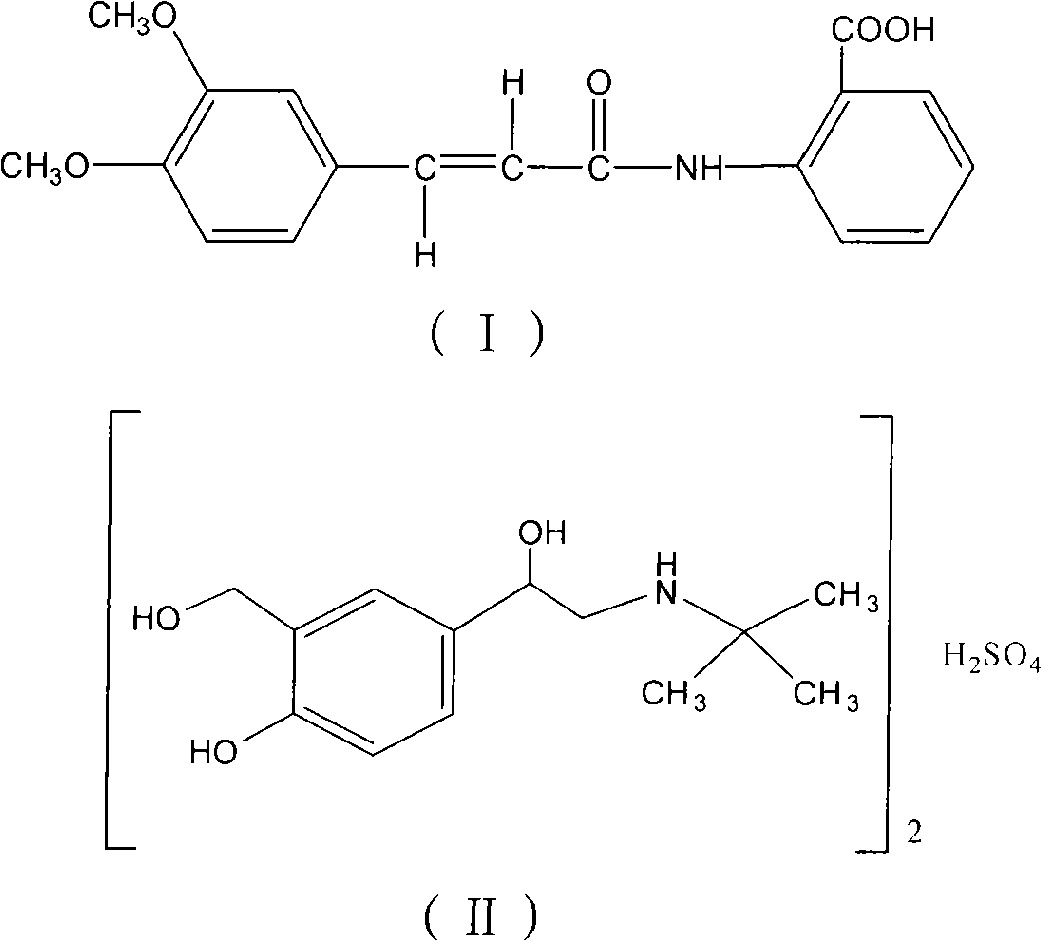
The invention relates to an oral compound pharmaceutic preparation for treating asthma, which contains tranilast shown as the formula (I) and salbutamol sulfate shown as the formula (II) or other pharmaceutically allowed active ingredients. The invention also relates to the application of compounds shown as formulas (I) and (II) or pharmaceutically allowed salts thereof in the preparation of an oral therapeutical agent for treating asthma.
Owner:沈阳三川医药科技有限公司
Composite gel containing traditional Chinese medicine composition and calcium ion and preparation method thereof
ActiveCN104117063AGood biocompatibilityGood anti-adhesionOrganic active ingredientsSurgical drugsCyclodextrinTraditional medicine
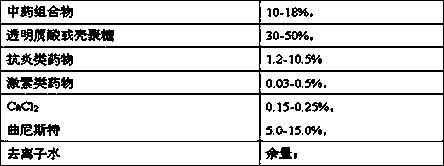
The invention relates to a composite gel containing a traditional Chinese medicine composition and calcium ion and a preparation method thereof. The composite gel containing the traditional Chinese medicine composition and calcium ion is composed of the following substances in percent by weight: 10-18% of the traditional Chinese medicine composition, 30-50% of hyaluronic acid or chitosan, 1.2-10.5% of a salt-resistant medicine, 0.03-0.5% of a hormone medicine, 0.15-0.25% of CaCl2, 5.0-15.0% of tranilast and the balance deionized water. The traditional Chinese medicine composition is obtained though clathration of ligusticum wallichii, radix salviae miltiorrhizae and sanguis draxonis by cyclodextrin. The composite gel containing the traditional Chinese medicine composition and calcium ion has relatively good postoperative antistick effect.
Owner:THE FIRST AFFILIATED HOSPITAL OF XINXIANG MEDICAL UNIV
Tranilast compositions and cocrystals
ActiveUS20150119428A1Useful in treatmentBiocideUrea derivatives preparationTreatment useNicotinamide
Mew tranilast complexes and new tranilast cocrystals are disclosed. These include all tranilast nicotinamide complex, a 1:1 tranilast nicotinamide cocrystal, a 1:1 tranilast saccharin complex, a 1:1 tranilast saccharin cocrystal, a 1:1 tranilast gentisic acid complex, a 1:1 tranilast gentisic acid cocrystal, a 1:1 tranilast salicylic acid complex, a 1:1 tranilast salicylic acid cocrystal, a 1:1 tranilast urea complex, a 1:1 tranilast urea cocrystal, a 1:1 tranilast 4-amtnoben2oic acid complex, a 1:1 tranilast 4-am!nobers2oic acid cocrystal, a 1:1 tranilast 2,4-di′hydroxybenzoic acid complex and a 1:1 tranilast 2,4-dihydroxybenzoic acid cocrystal. Also disclosed are pharmaceutical compositions containing a tranilast complex or cocrystal of the invention and a pharmaceutically acceptable carrier. Methods of treatment using the tranilast complexes and cocrystais as well as the pharmaceutical compositions are disclosed.
Owner:NUFORMIX LTD
Eye drop containing tranilast and its preparing process
ActiveCN1857199AImprove stabilityLess irritatingOrganic active ingredientsSenses disorderIrritationMedical prescription
The present invention discloses the recipe and preparation process of eye drop containing tranilast for treating allergic conjunctivitis. The recipe contains also supplementary material, including meglumine, citric acid, PVP-K30 and nipalgin. The medicine of the present invention has high stability and no thrill on eyes.
Owner:药大制药有限公司
Methods of treating eye diseases associated with inflammation and vascular proliferation
ActiveUS20130310386A1Inhibit progressPreventing eye diseaseAntibacterial agentsBiocideBenzoic acidVascular proliferation
Methods for treating eye diseases associated with inflammation and / or vascular proliferation in subjects are disclosed. The methods include administering therapeutically effective amounts of a tranilast compound, in particular (E)-2-[[3-(3-Methoxy-4-propargyloxy)phenyl)-1-oxo-2-propenyl]amino]benzoic acid or (E)-2-[[3,4-Bis(difluoromethoxy)phenyl)-1-oxo-2-propenyl]amino]benzoic acid or pharmaceutically acceptable salts or solvates thereof.
Owner:OCCURX
Method of modulating b cell functioning
InactiveUS20100041756A1Cell from functioningAntibacterial agentsBiocideMetaboliteAutoimmune condition
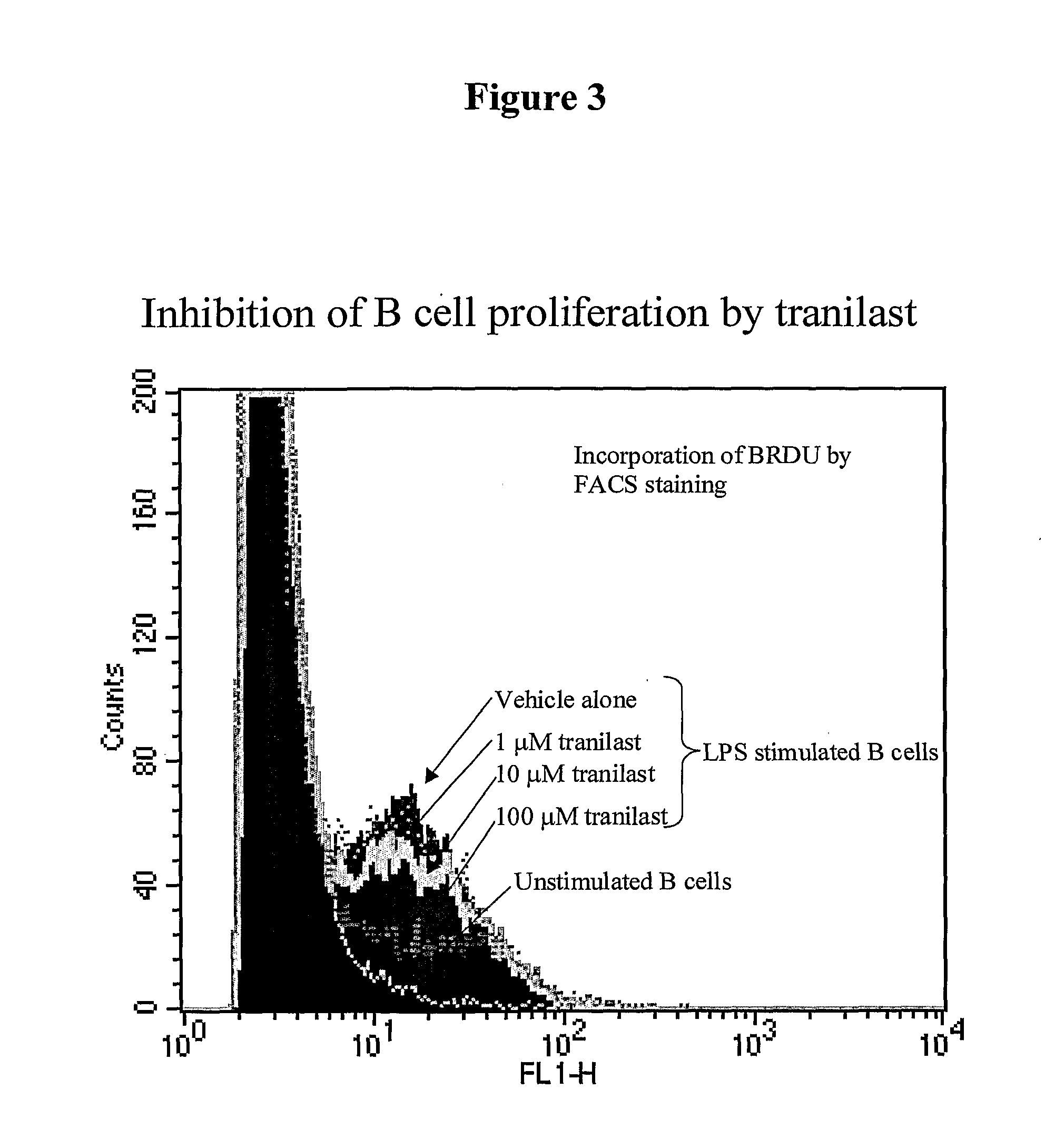
The present invention relates generally to a method of modulating cellular functioning. More particularly, the present invention relates to a method of modulating B cell functioning, for example B cell proliferation, utilising an IDO-mediated tryptophan metabolite as herein defined (particular examples of such IDO-mediated tryptophan metabolites include 3-hydroxykynurenic acid, 3-hydroxyanthranilic acid, picolinic acid, quinolinic acid and tranilast). The method of the present invention is useful, inter alia, in the treatment and / or prophylaxis of conditions characterised by aberrant, unwanted or otherwise inappropriate B cell functioning such as antibody production, autoimmune conditions and B cell proliferation and neoplasias. In a related aspect, the present invention is directed to a method of therapeutically and / or prophylactically treating rheumatoid arthritis via the administration of the above-mentioned compounds.
Owner:NUON THERAPEUTICS PTY LTD
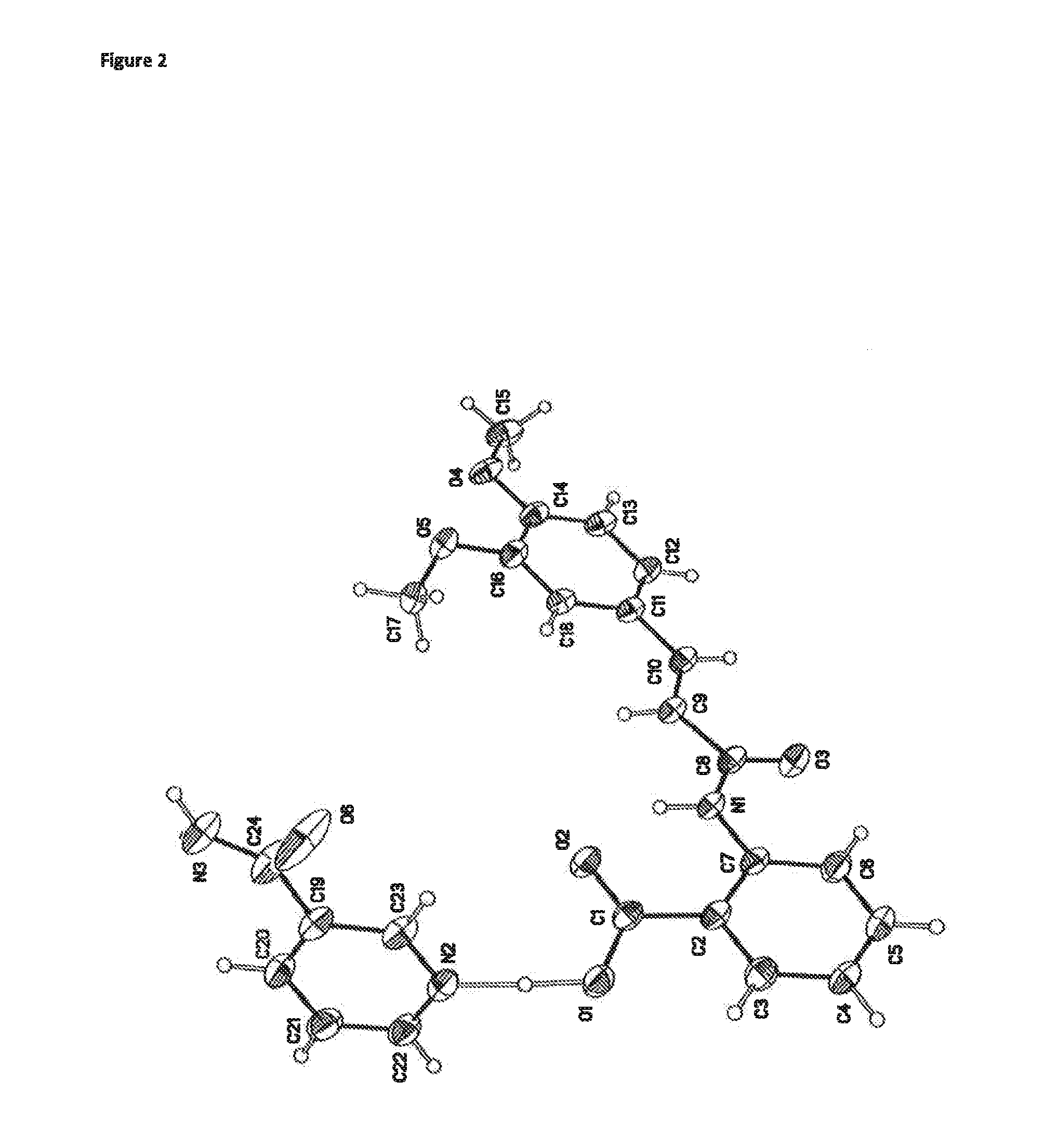
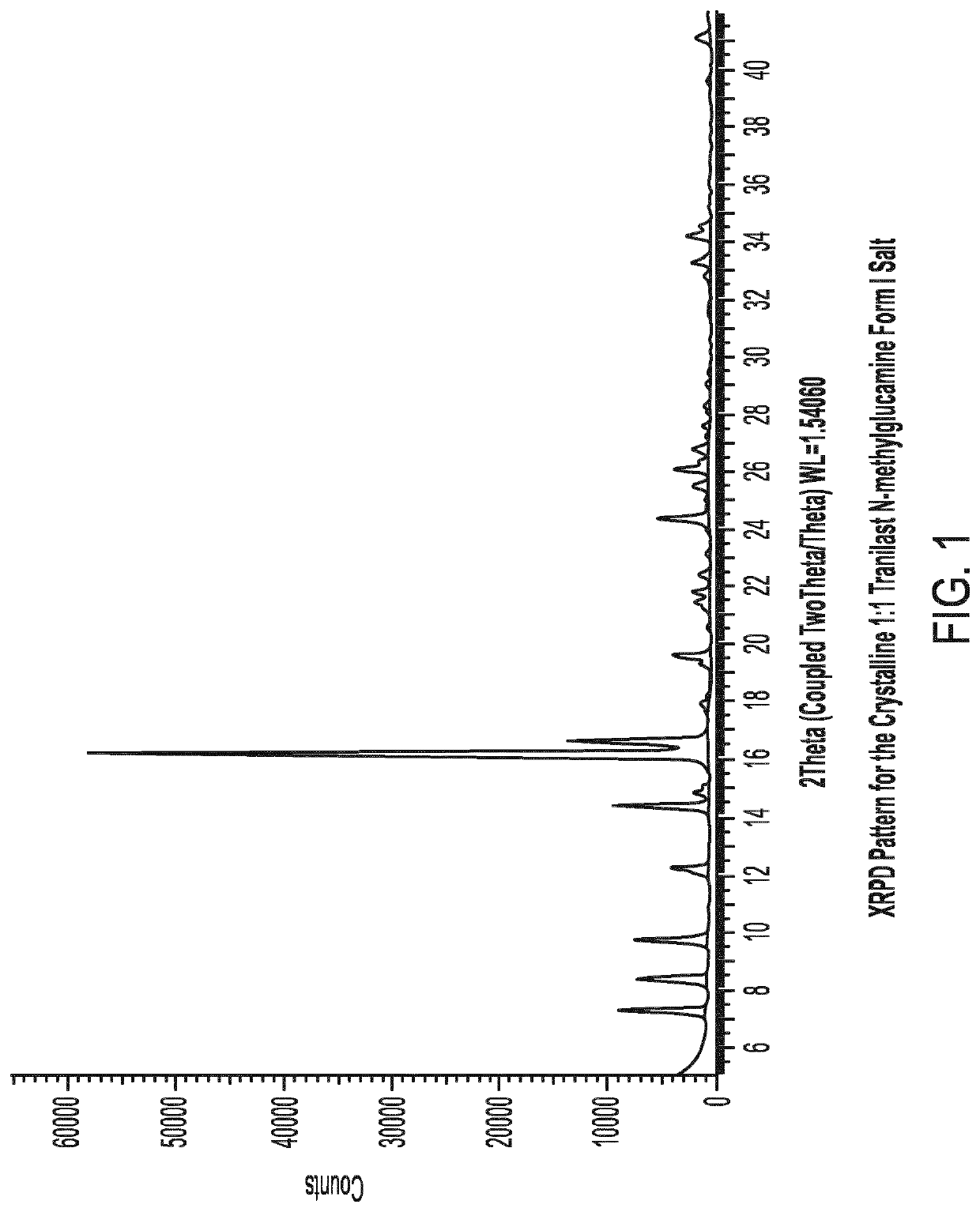
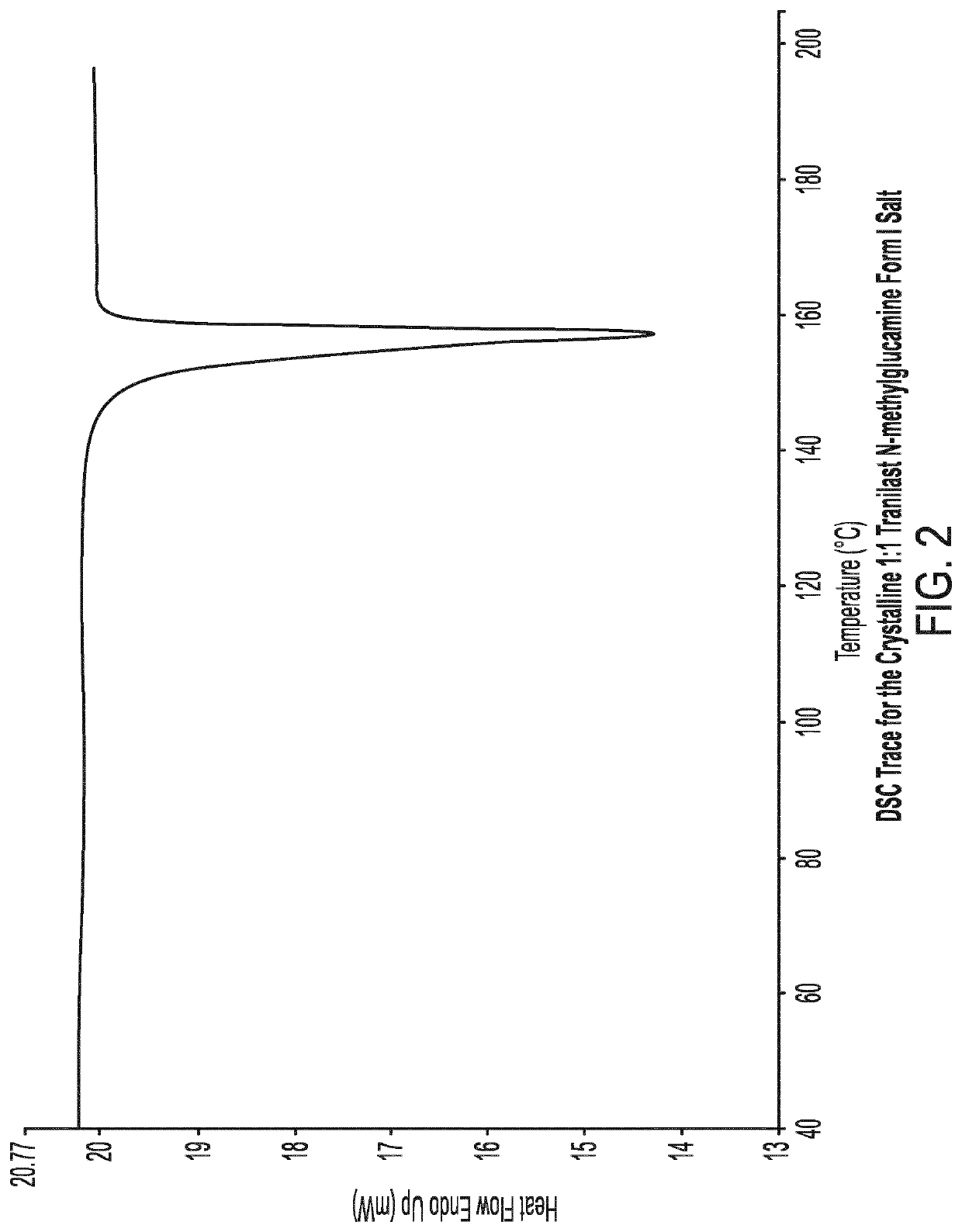
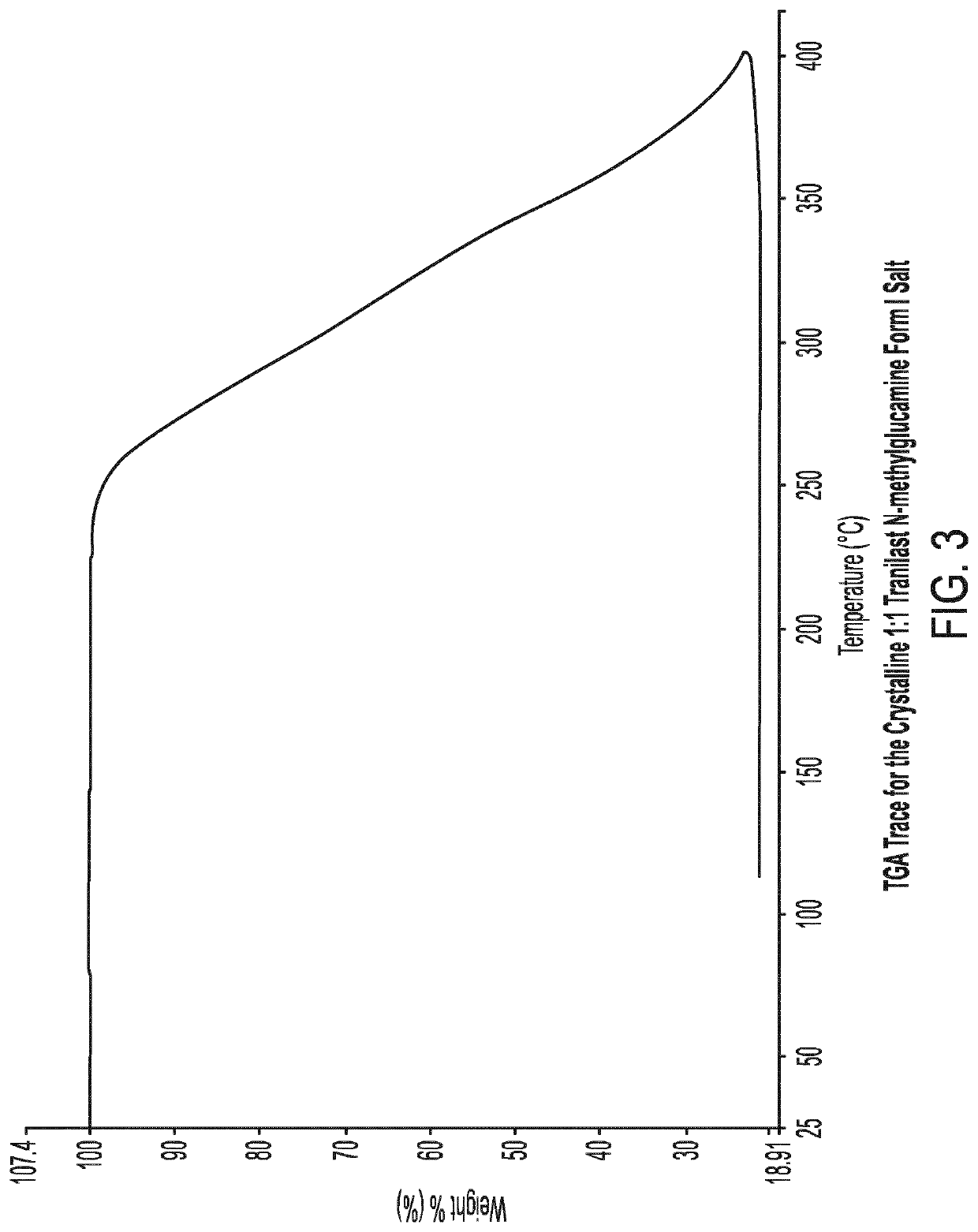
Crystalline tranilast salts and their pharmaceutical use
ActiveUS11078155B2Improved propertyUseful in treatmentOrganic active ingredientsOrganic chemistry methodsPharmaceutical medicinePharmaceutical Substances
The invention relates to crystalline tranilast salts. The crystalline tranilast salts, their preparation and their characterization are described and shown in the figures. The invention relates to pharmaceutical compositions containing a crystalline tranilast salt of the invention and a pharmaceutically acceptable carrier. The invention also relates to methods of treatment and the use of a therapeutically effective amount of a crystalline tranilast salt of the invention for treatment. The invention also relates to a method of preparing a liquid pharmaceutical composition comprising the step of dissolving a crystalline tranilast salt of the invention in a pharmaceutically acceptable solvent and to liquid pharmaceutical compositions prepared according to that method.
Owner:NUFORMIX TECH LTD
Application of tranilast in preparation of medicament for treating tuberculosis
InactiveCN106361737AImprove complianceReduce formationAntibacterial agentsOrganic active ingredientsClinical efficacyAtrophic scarring
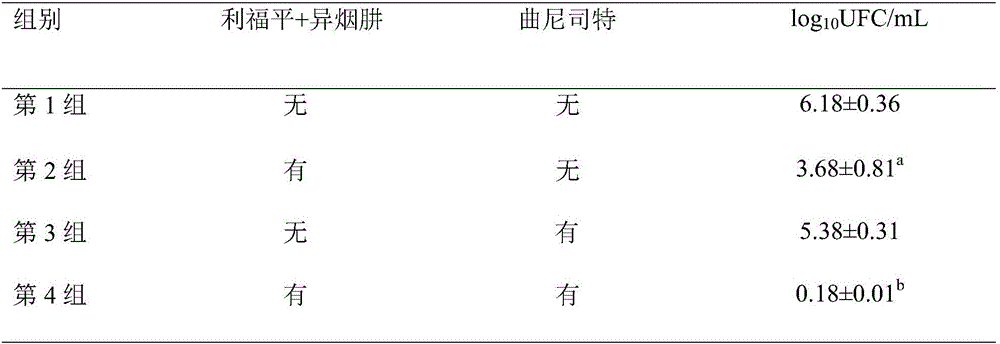
The invention discloses application of tranilast-combined anti-tuberculosis medicaments in preparation of a medicament for treating tuberculosis, and at least two of the tranilast-combined anti-tuberculosis medicaments (isonicotinyl hydrazine, rifampicin, pyrazinoic acid amide and ethambutol) are adopted. After the medicament combination is applied for 50 days, a patient can feel that skin damage is obviously alleviated; after the medicament combination is applied for 3 to 4 months, skin damage of the patient almost completely disappears with a speed obviously greater than that of a control group of pure anti-tuberculosis treatment, so that the anti-tuberculosis treatment course is greatly shortened, tissue damage such as atrophic scar formation and pigmentation can be reduced, and no obvious adverse reaction exists, which indicates that the tranilast-combined classical anti-tuberculosis medicaments have a synergistic anti-tuberculosis effect, are capable of obviously shortening the anti-tuberculosis treatment course and enhancing the anti-tuberculosis curative effect when used for resisting the tuberculosis, are good in toleration and relatively high in compliance of patients, free from obvious adverse reaction, and relatively good in clinical curative effect and security, and are expected to become necessary medicaments for combined treatment of the tuberculosis.
Owner:JIANGSU PROVINCE HOSPITAL
Mixture of Betamethasone and Tranilast with a Transdermal Gel for Scar Treatment
ActiveUS20150306221A1Suppressing collagen synthesisInhibit expressionOrganic active ingredientsBiocideBetamethasone valerateSolvent
The present disclosure relates to a synergistic mixture of betamethasone valerate and tranilast combined with a transdermal gel using skin permeation enhancement for application in scars for reducing or preventing the abnormal scar formation, specially keloids and hypertrophic scars. The disclosed synergistic mixture may exhibit permeation enhancing properties. The transdermal gel may include a mixture of silicone, phosphatidylcholine, pracaxi oil, and seje oil; one or more oils having essential fatty acids, behenic acid, oleic acid; one or more skin lipids; a butter having linoleic acid and linolenic acid; and solvents as transdermal penetration enhancers.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Novel applications of tranilast
InactiveCN108938617AReduced mature secretionConvenient treatmentOrganic active ingredientsNervous disorderDiseaseStimulant
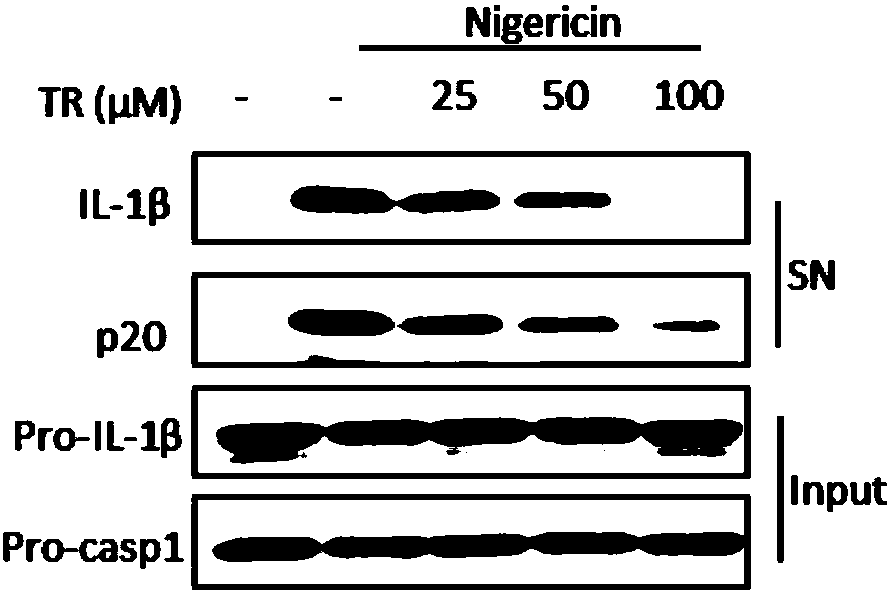
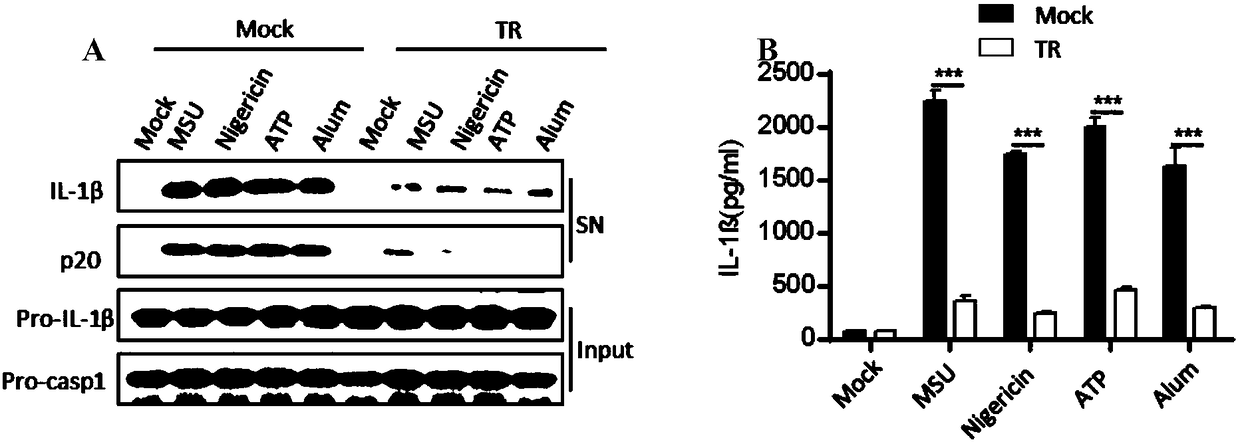
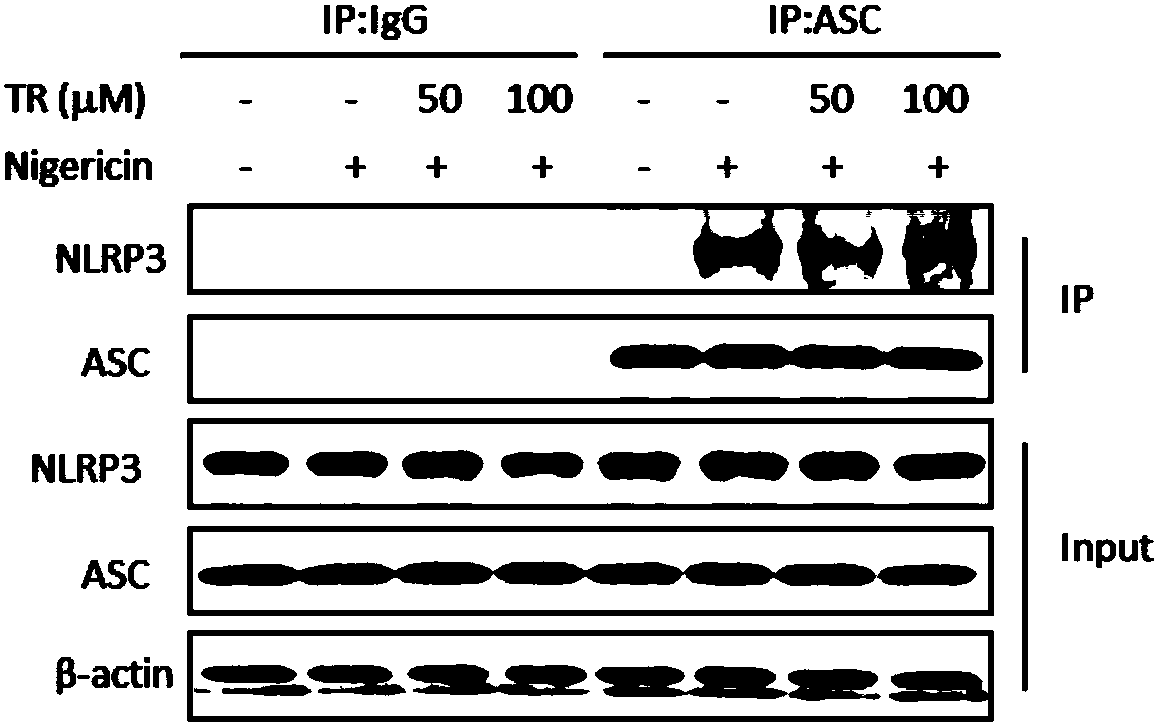
The invention belongs to the field of pharmaceutical chemistry, and provides novel applications of tranilast, and especially applications of tranilast in preparation of medicines used for treating NLRP3 inflammasome related diseases. It is shown by experiments that tranilast is capable of reducing IL-1beta and IL-18 maturation secretion, blocking obesity development, and preventing type II diabetes through specific inhibition on NLRP3 inflammasome activation, and treating peritonitis and gout caused by NLRP3 inflammasome stimulant urate crystal (MSU) accumulation, and Muckle Wells syndromes caused by NLRP3 mutation preferably at the same time.
Owner:UNIV OF SCI & TECH OF CHINA
Compound tranilast orally disintegrating tablet formulation and preparation method thereof
InactiveCN101375843ASolve the respective defects in pharmacological actionEasy to takeOrganic active ingredientsPharmaceutical non-active ingredientsOrally disintegrating tabletBioavailability
The invention aims to provide a compound oral disintegrating tablet preparation which is composed of tranilast and salbutamol sulfate, and a preparation method thereof. The adopted specific technical proposal for achieving the purpose of the invention is as follows: the compound tranilast oral disintegrating tablet preparation is characterized in that the formula of the preparation is composed of the following components by parts by weight (by 1000 tablets): 80.0g of tranilast, 2.4g of salbutamol sulfate, 5-30g of disintegrant, 10-80g of filling agent, 5-25g of effervescing agent, 0.5-20g of flavoring agent and 0.3-3g of lubricant. The compound oral disintegrating tablet preparation which is prepared by the method can solve the shortcomings of the two drugs on the pharmacological effects during the treatment process of bronchial asthma and allow the compound oral disintegrating tablet preparation to relieve the asthma instantly and maintain and consolidate the effect; furthermore, the compound oral disintegrating tablet preparation has convenient administration, rapid onset of action, high bioavailability and good taste. The preparation method of the preparation has simple steps and low cost.
Owner:SHANGHAI NORMAL UNIVERSITY
Application of inhibitor of nlrp3 inflammasome in preparation of medicine for treating pituitary adenoma and medicine for treating pituitary adenoma
ActiveCN111214662BGrowth inhibitionInhibit the secretion of hormonesOrganic active ingredientsAntineoplastic agentsPharmaceutical drugInflammasome
The invention discloses the application of an inhibitor of NLRP3 inflammasome in the preparation of medicine for treating pituitary adenoma and the medicine for treating pituitary adenoma. NLRP3 inflammasome inhibitors are MCC950 or tranilast. Drugs containing NLRP3 inflammasome inhibitors may enable the treatment of pituitary adenomas. NLRP3 inflammasome inhibitors bring good news to patients with pituitary adenomas.
Owner:武汉叶风生物科技有限公司
Amyloid fiber formation limiter or inhibitor
PendingUS20170035717A1Prevent or treat amyloid plaquesOrganic active ingredientsNervous disorderDiseasePharmacometrics
The object of the present invention is to provide a formulation with the effect of effectively suppressing or inhibiting amyloid fibril formation by the dissolution, elimination (discharge), etc. of amyloid fibril formation in vivo.If an agent for suppressing or inhibiting an amyloid fibril formation comprising tranilast or a pharmacologically acceptable salt thereof as an active ingredient is administered by a method such as oral administration, amyloid fibril formation can be effectively suppressed or inhibited in vivo as a result of effects such as amyloid fibril dissolution or elimination (discharge). Therefore, it is possible to prevent or treat amyloid plaques, in which amyloid fibrils formed by the aggregation of amyloid protein have been deposited, and to prevent or treat diseases arising from amyloid fibril deposition, that is, diseases arising from the deposited amyloid fibrils themselves and diseases that cause dysfunction of organs or tissues as a result of amyloid fibril deposition.
Owner:TAKEMURA TSUKASA +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com