Novel applications of tranilast
A disease and inflammasome technology, applied in the field of medicinal chemistry, can solve problems such as ineffective treatment, and achieve the effect of preventing and treating type 2 diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
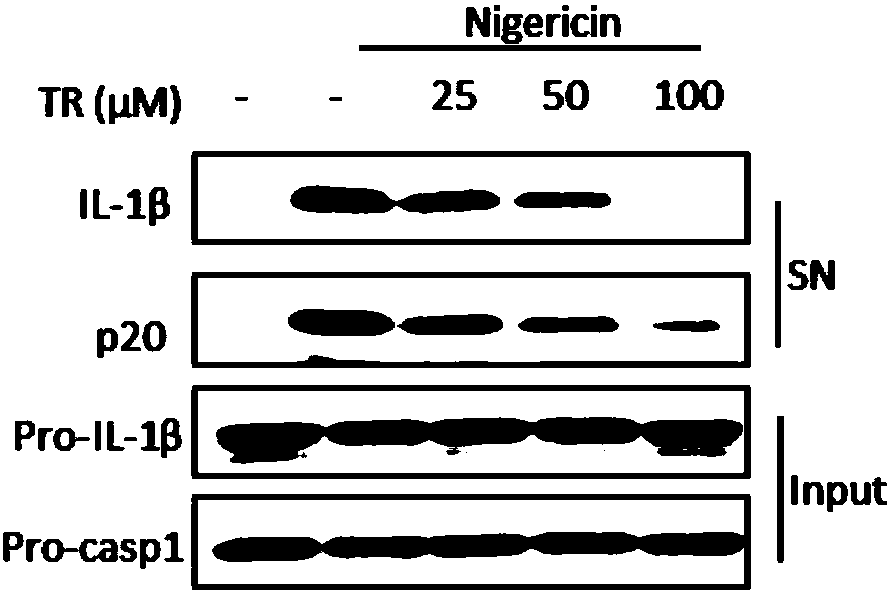
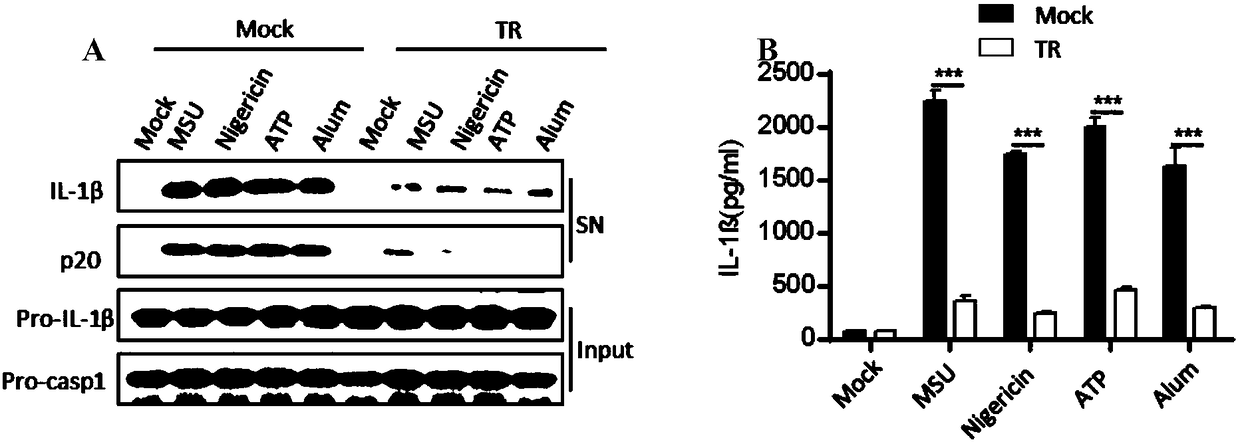
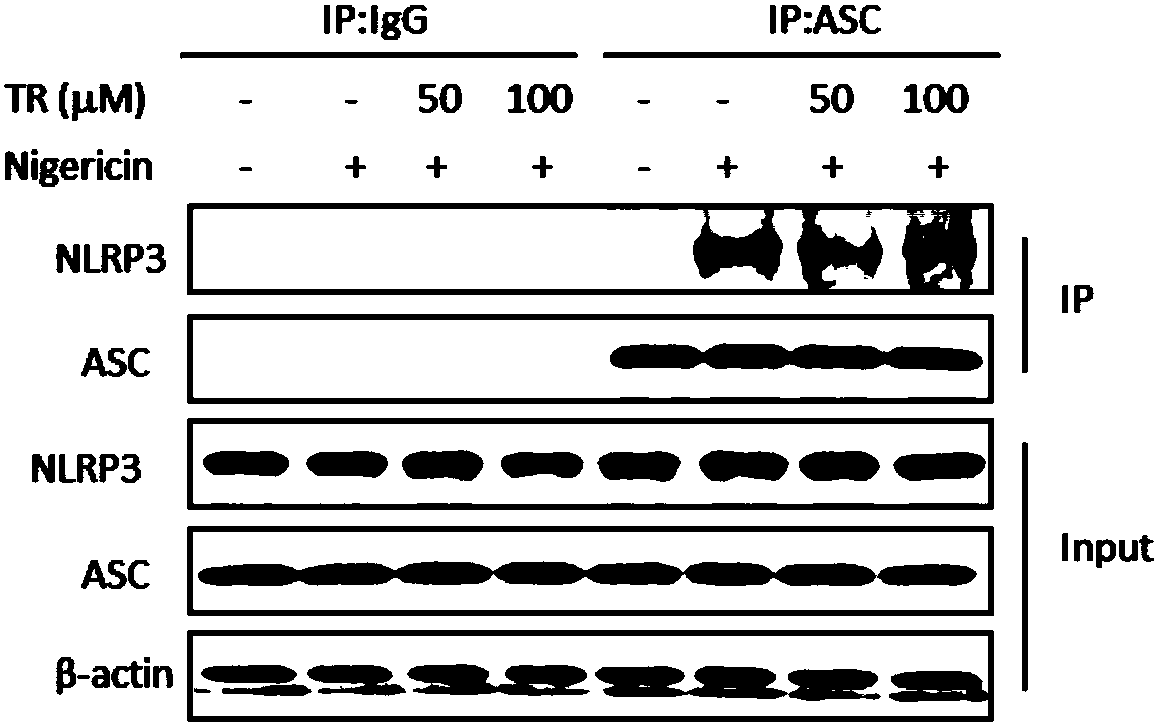
[0038] Example 1. TR inhibits the activation of macrophage NLRP3 inflammasome in vitro
[0039] 1. TR inhibits the secretion of IL-1β
[0040] 1. On the first day, the mouse bone marrow was taken, and cultured and differentiated in DMEM medium containing 20% L929 cell (mouse fibroblast) culture supernatant for 4 days;
[0041] 2. On the fifth day, divide the BMDM cells into twelve-well plates, each well 6×10 5 cells;
[0042] 3. On the sixth day, the supernatant was removed, and 500 μL of opti-MEM medium containing bacterial lipopolysaccharide (LPS) (50 ng / ml) was added to each well. After three hours of treatment, they were divided into five groups for different treatments, and each group had six replicates. Holes, the specific grouping process is as follows:
[0043] Group 1: Add 0.5 μL DMSO to each well for half an hour;
[0044] The second group: Add 0.5 μL DMSO to each well for half an hour, then add Nigericin to a final concentration of 5 μM, and then treat for hal...
Embodiment 2
[0092] Example 2, TR inhibits the acute inflammation induced by urate crystals
[0093] 1. TR inhibits urate crystal (MSU) accumulation-induced peritonitis
[0094] 1. Select 10-week-old wild mice and divide them into three groups, with three mice in each group. The specific grouping process is as follows:
[0095] The first group: 50 μL DMSO was injected intraperitoneally first, and 300 μL PBS was injected half an hour later;
[0096] The second group: 50 μL DMSO was injected intraperitoneally first, and MSU was injected half an hour later, and the injection dose was 0.5 mg per mouse;
[0097] The third group: 200mg / kg TR was injected intraperitoneally first, and MSU was injected half an hour later, and the injection dose was 0.5mg per mouse;
[0098] 2. After six hours, inject 10ml PBS into the abdominal cavity of each group of mice, and take the peritoneal lavage fluid and centrifuge;
[0099] 3. The supernatant of the peritoneal lavage fluid obtained in step 2 was used ...
Embodiment 3
[0114] Example 3, TR can alleviate metabolic disorder syndrome
[0115] 1. TR prevents high-fat food-induced metabolic disorders
[0116] 1. Select 8-week-old C57BL / 6J WT wild mice and divide them into four groups, with 9 mice in each group. sodium);
[0117] The second group: use 60% high-fat diet to feed the mice, and give 300 μ L placebo (sodium cellulose) by intragastric administration every day;
[0118] The third group: mice were fed with 60% high-fat food, and TR was given by intragastric administration every day at a dose of 25 mg / kg;
[0119] The fourth group: mice were fed with 60% high-fat food, and TR was administered orally every day at a dose of 50 mg / kg;
[0120] 2. Monitor the body weight and food intake of the mice, and record and test the blood sugar, glucose tolerance and insulin sensitivity of the mice. See the results in Figure 8 .
[0121] from Figure 8 The results showed that mice fed with 60% high-fat food had faster body weight gain than normal f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com