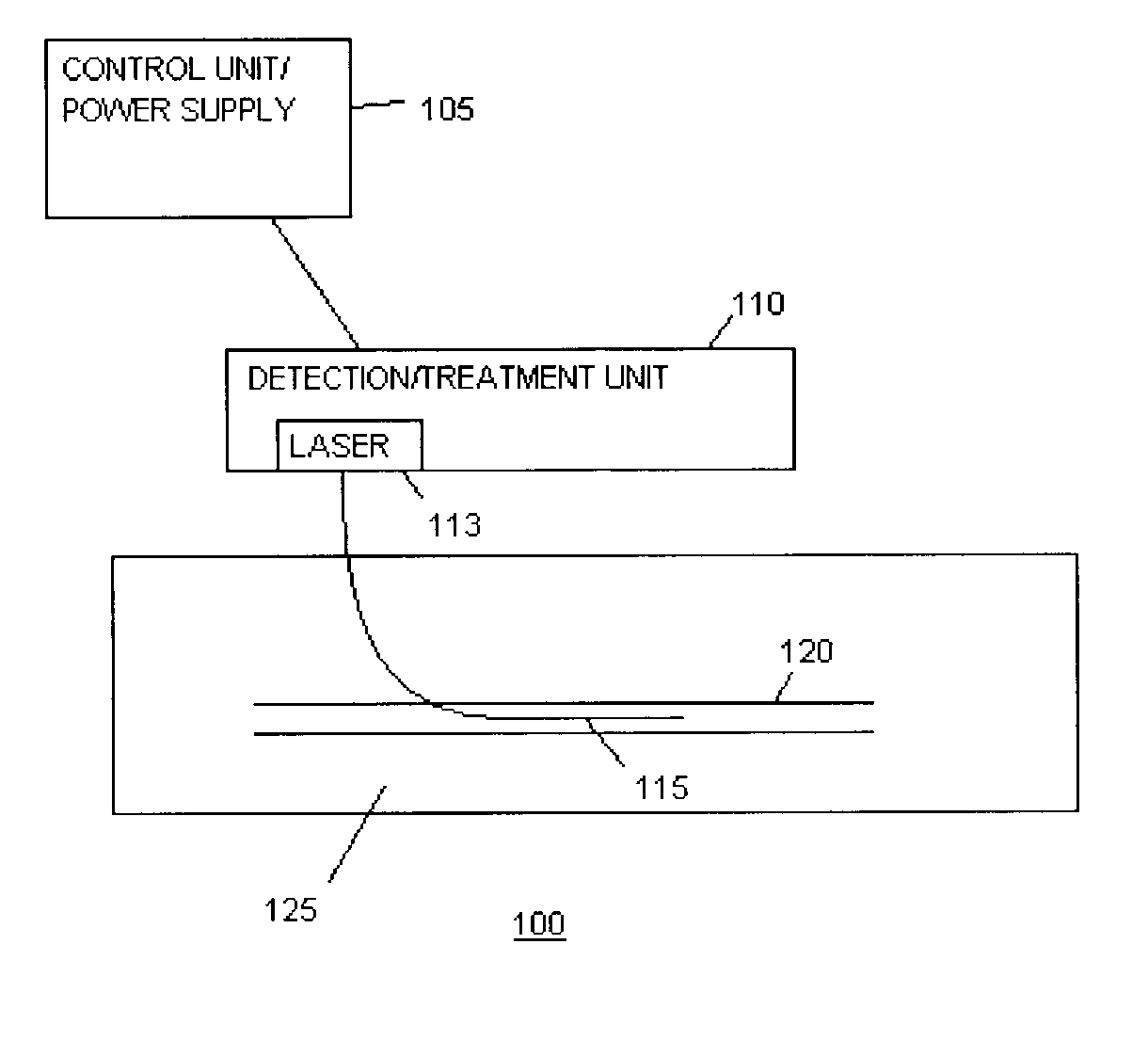
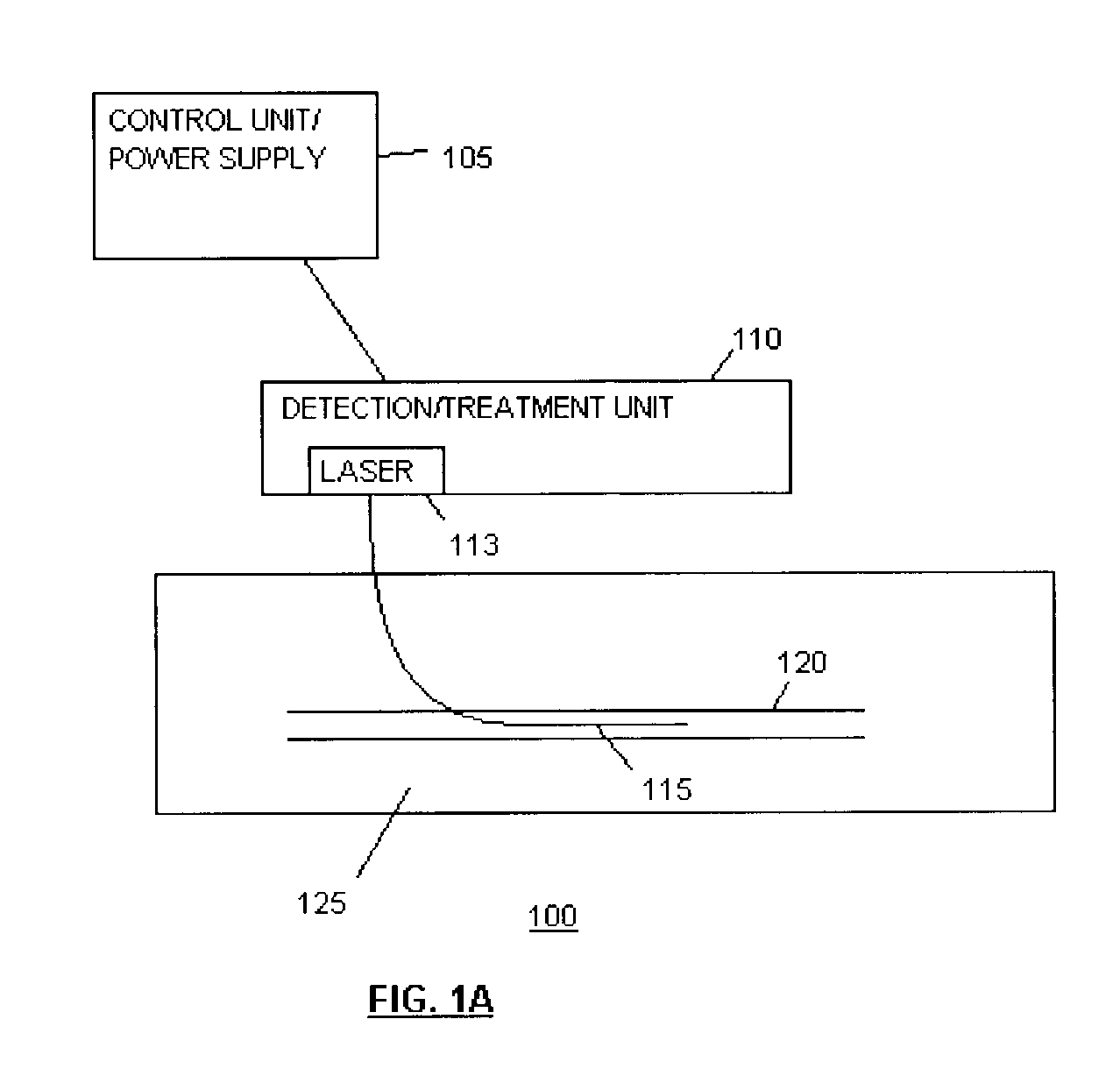
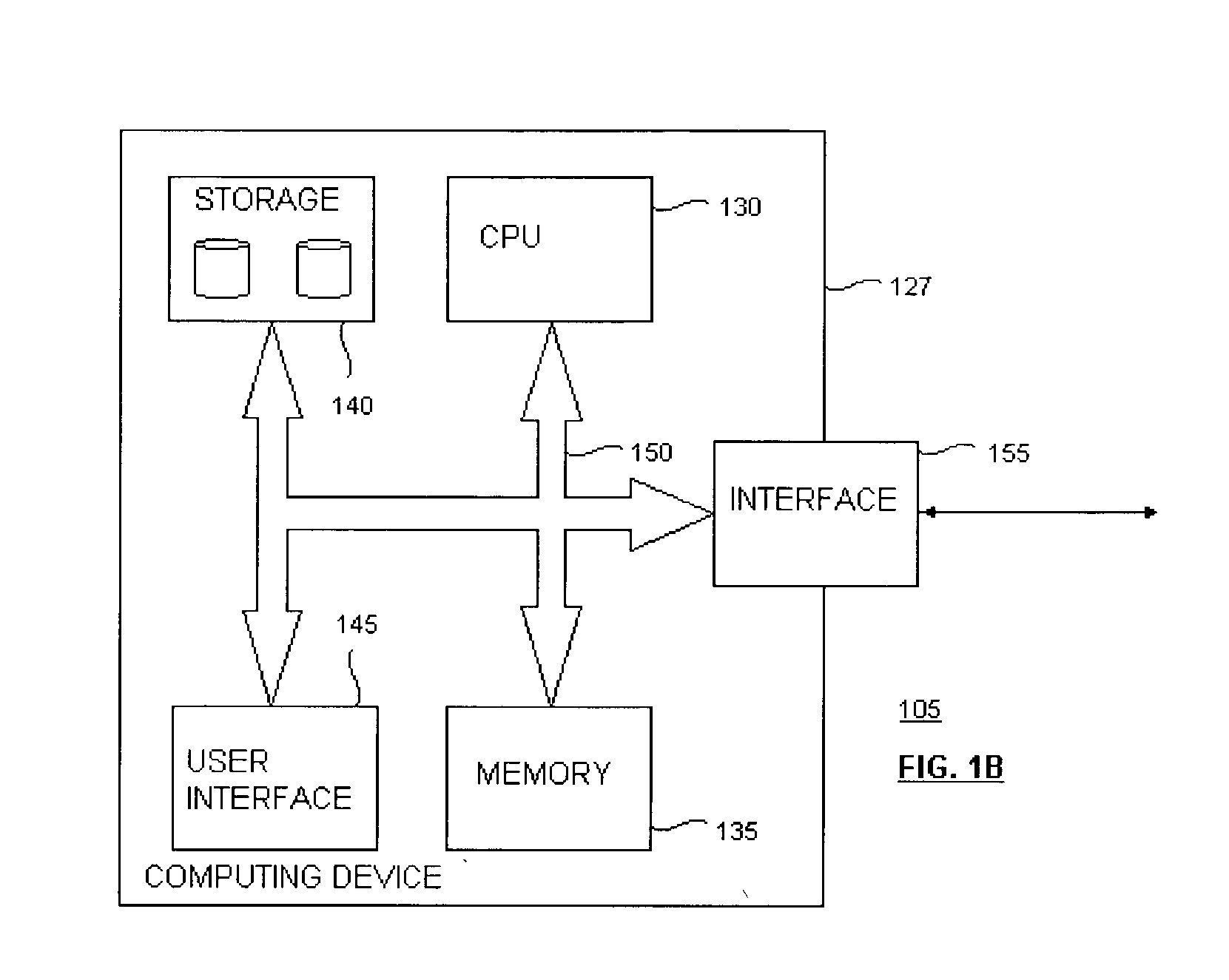
Methods and devices for detection and therapy of atheromatous plaque
a technology of atheromatous plaque and detection method, which is applied in the direction of macromolecular non-active ingredients, diagnostics using spectroscopy, energy modified materials, etc., can solve the problem of unacceptably high lack of density in the fibrous cap of the atheromatous plaque, and not always preventing the incidence of acute coronary syndrom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Purification of Photosensitizer Compositions
[0279] A photosensitizer composition comprising chlorine.sub.6 ("c.sub.c6") coupled to maleylated-albumin) was prepared for optimal targeting to macrophages of a vulnerable plaque animal model system.
[0280] Results
[0281] Four photosensitizer compositions were studied (i.e., two BSA-c.sub.e6 conjugates and their maleylated counterparts). The N-hydroxy succinimide (NHS) ester of c.sub.e6was prepared by reacting approximately 1.5 equivalents of dicyclohexylcarbodiimide and approximately 1.5 equivalents of NHS with approximately 1 equivalent of c.sub.e6 (Porphyrin Products, Logan, Utah) in dry DMSO. After standing in the dark at room temperature for approximately 24 hours, the NHS ester was frozen in aliquots for further use. BSA (Sigma Chemical Co, St Louis, Mo.) (approximately 2.times.50 mg) was dissolved in NaHCO.sub.3 buffer (0.1 M, pH 9.3, approximately 3 ml), and approximately 30 .mu.l and approximately 120 .mu.l of c.sub...
example 2
Macrophage-Targeting of Photosensitizers
[0289] The photosensitizer composition comprising chlorin.sub.e6 coupled to maleylated-albumin described in Example 1 was shown to accumulate in the macrophage-rich plaques of an animal model system that are analogous to vulnerable plaques in humans. Thus, methods of the present invention provide highly specific intravascular detection and therapy of vulnerable plaques.
[0290] Cell Culture
[0291] J774.A1 (J774) and RAW 264.7 mouse macrophage-like cell lines, together with EMT-6 mouse mammary fibrosarcoma cells, were obtained from ATCC (Rockville, Md.). Cells were grown in RPMI 1640 media containing HEPES, glutamine, 10% fetal calf serum (FCS), 100 U / ml penicillin and 100 .mu.g / ml streptomycin. They were passaged by washing with phosphate buffered saline (PBS) without Ca.sup.2+ and Mg.sup.2+ and by adding trypsin-EDTA to the plate for 10 minutes at 37.degree. C.
[0292] Rabbits
[0293] Male New Zealand white rabbits weight 2.5-3.0 kg (Charles River B...
example 3
[0309] An intravascular fluorescence catheter that efficiently localized a fluorescence signal from a vulnerable plaque in the rabbit coronary (although not limited to rabbit) through flowing blood was developed. In addition, a therapeutic intravascular light delivery system was developed that illuminated the vulnerable plaques through flowing blood with the appropriate wavelength, fluence and fluence rate of light, achieving the desired therapeutic effect.
[0310] Results
[0311] PDT in rabbit aorta was demonstrated to be possible in vivo in living rabbits through flowing blood without undue harm to the rabbits and with no short-term toxicity. The same parameters were used as above (photosensitizer composition, dose and time interval) in order to be able to correlate treatment effects with previously determined dye localization in plaque lesions. Animals (one atherosclerotic and one normal rabbit, each injected with Mal-BSA-c.sub.e6 24 hours previously; and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com