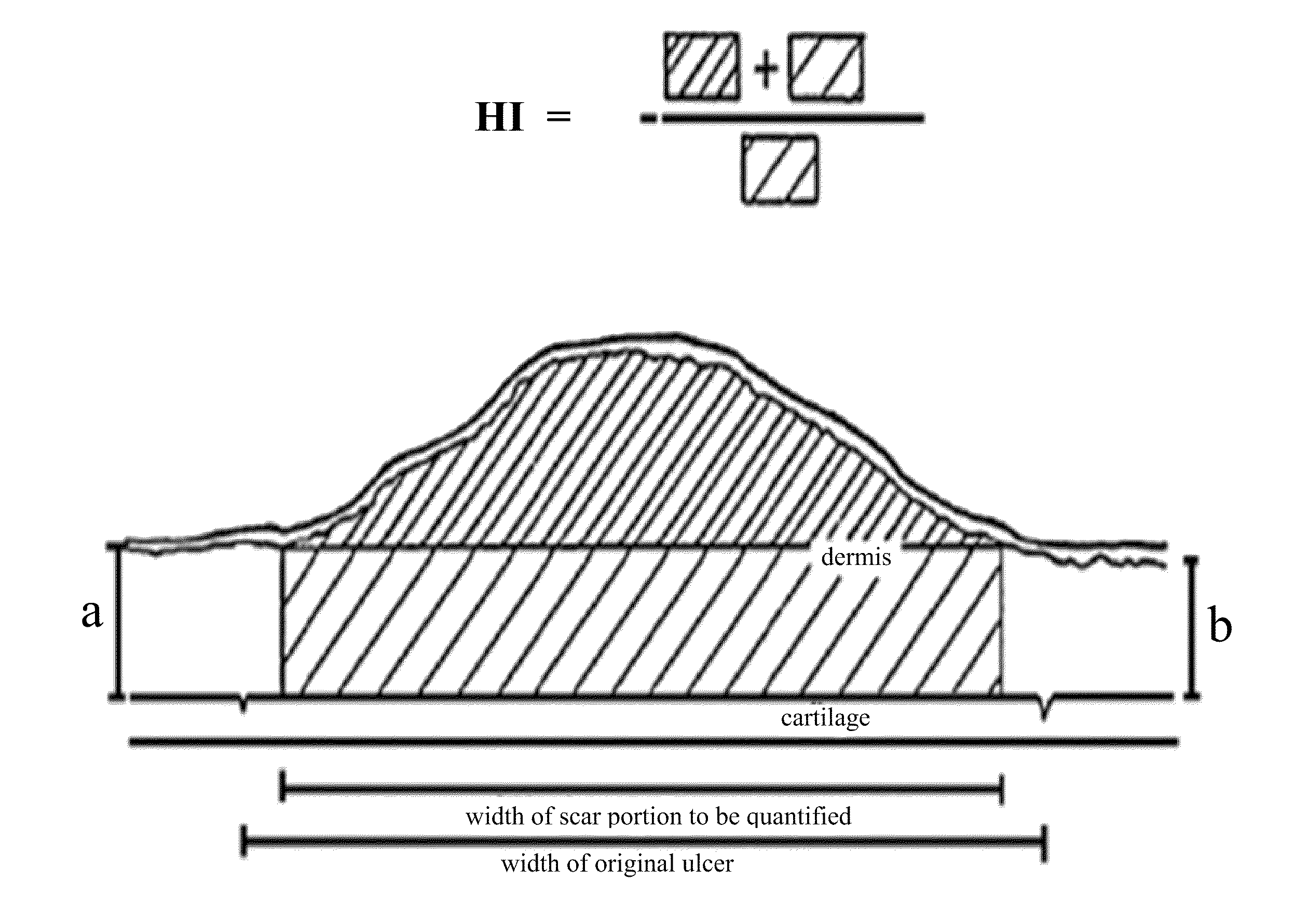
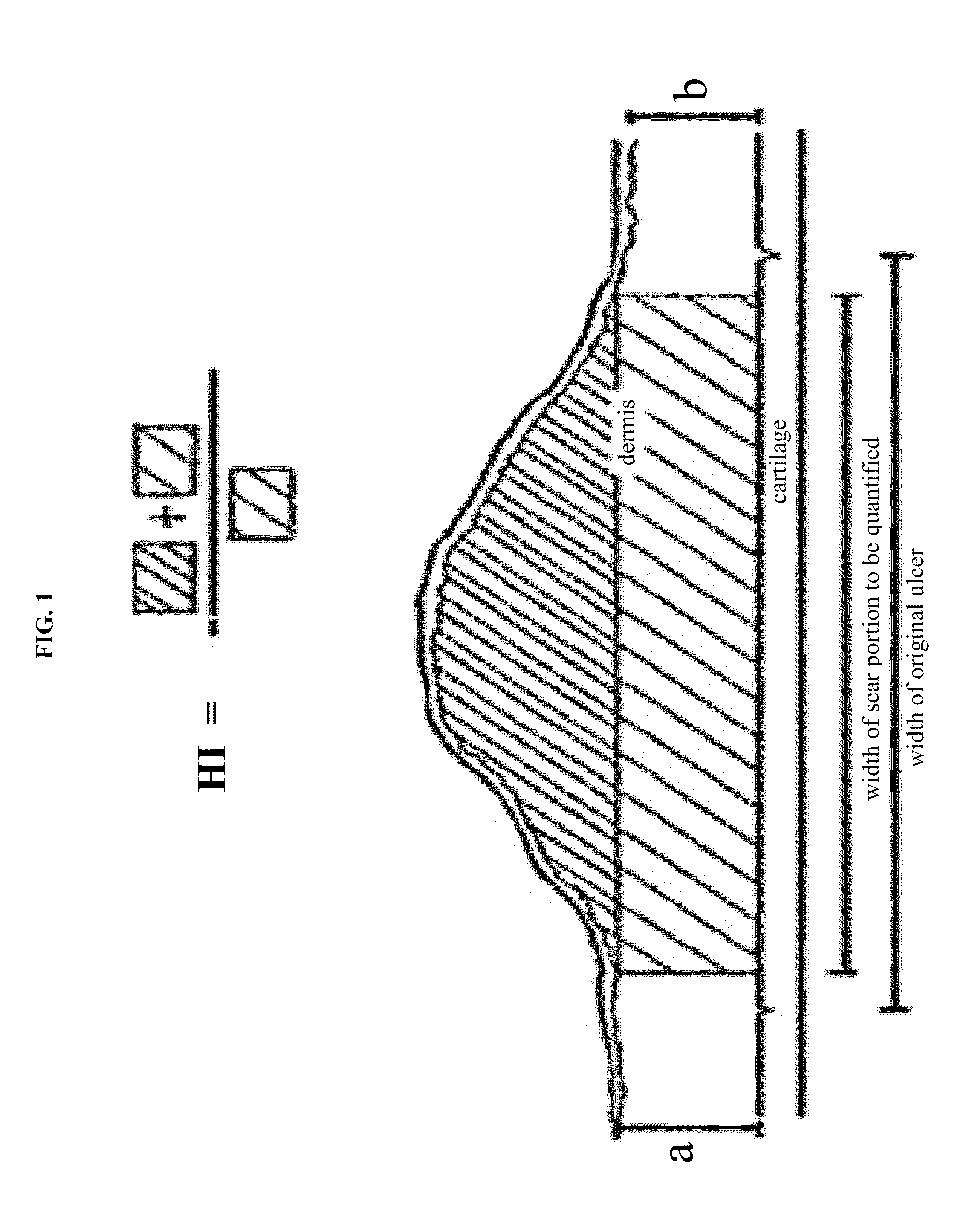
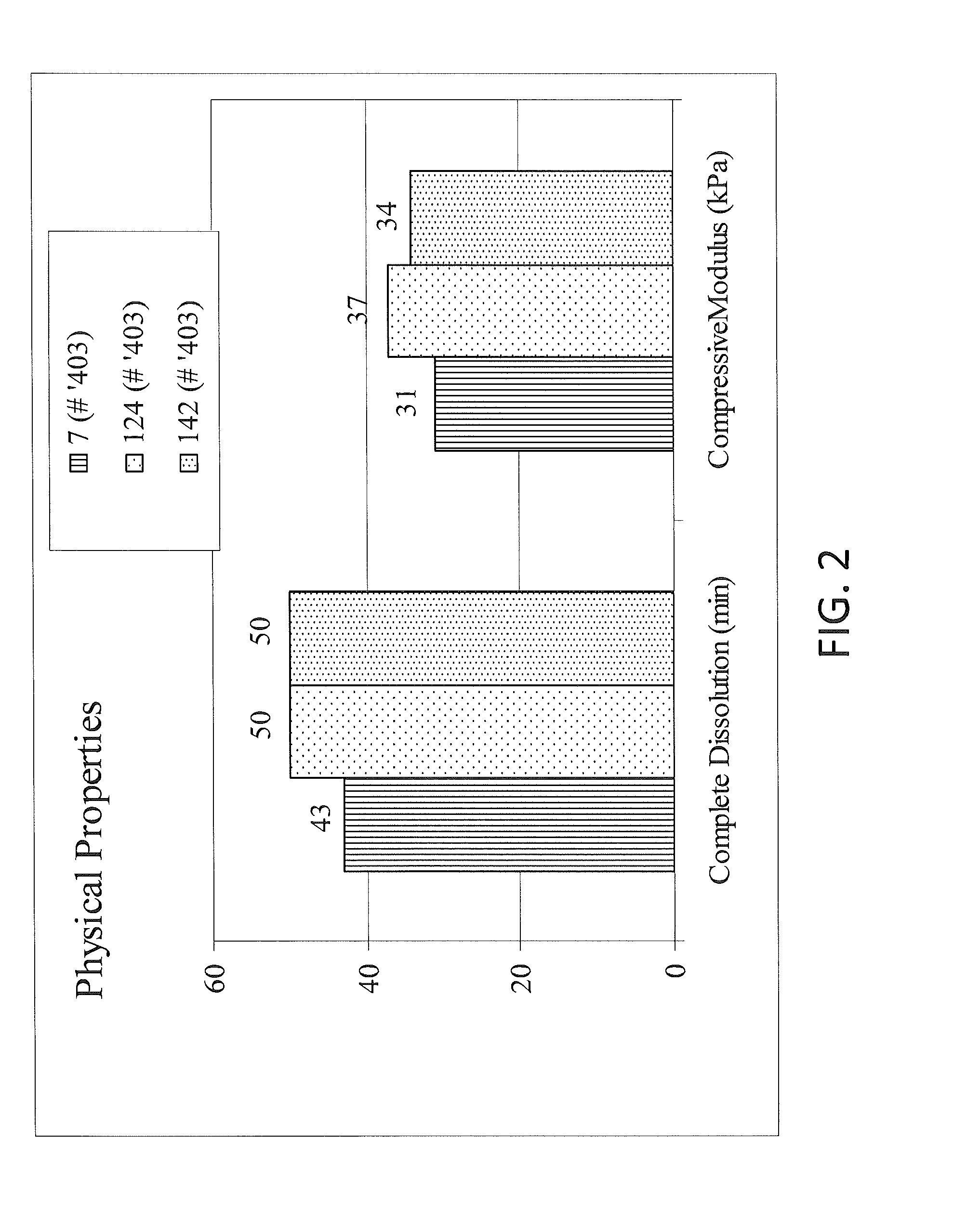
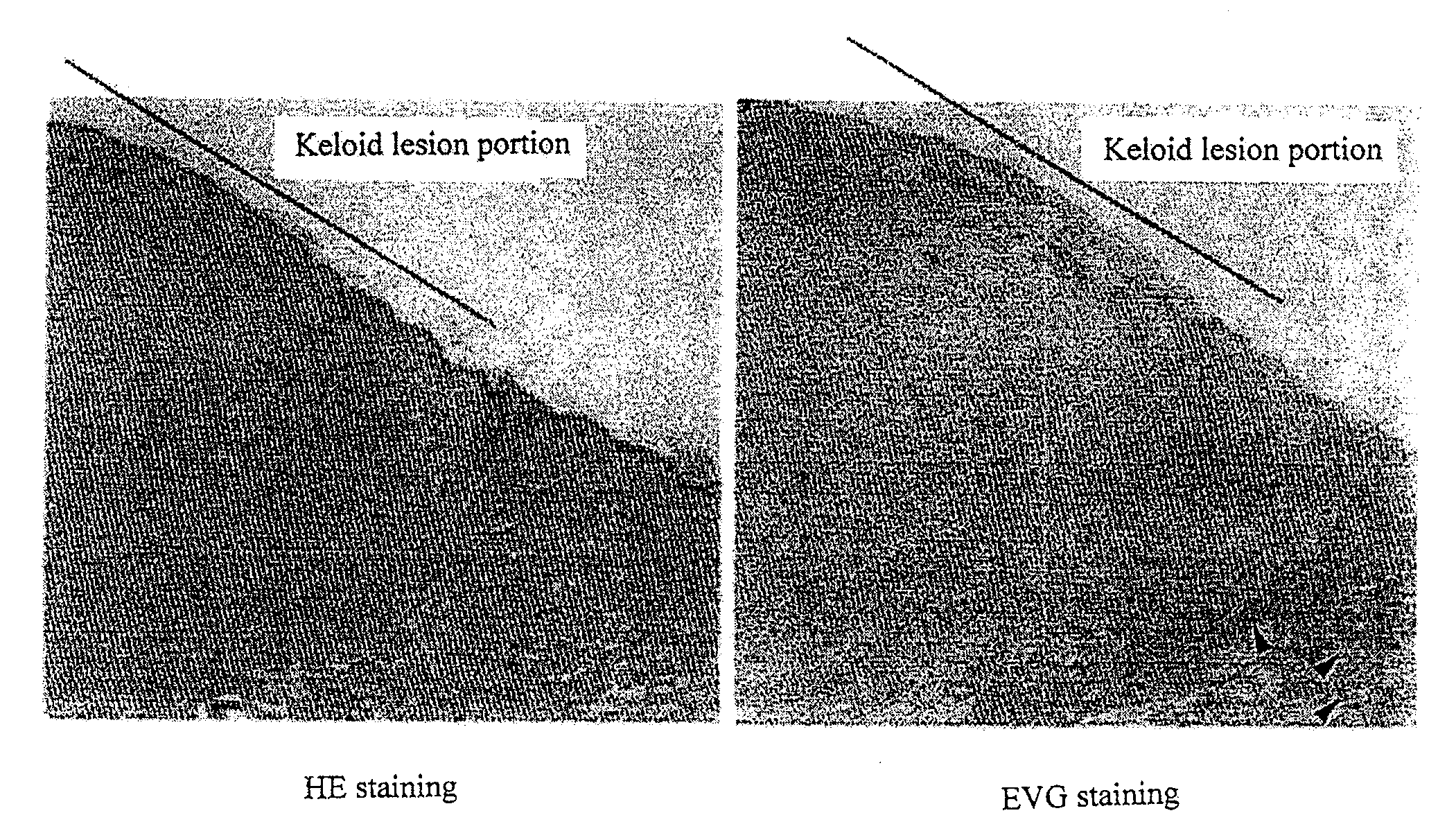
Patents
Literature
119 results about "Hypertrophic scars" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
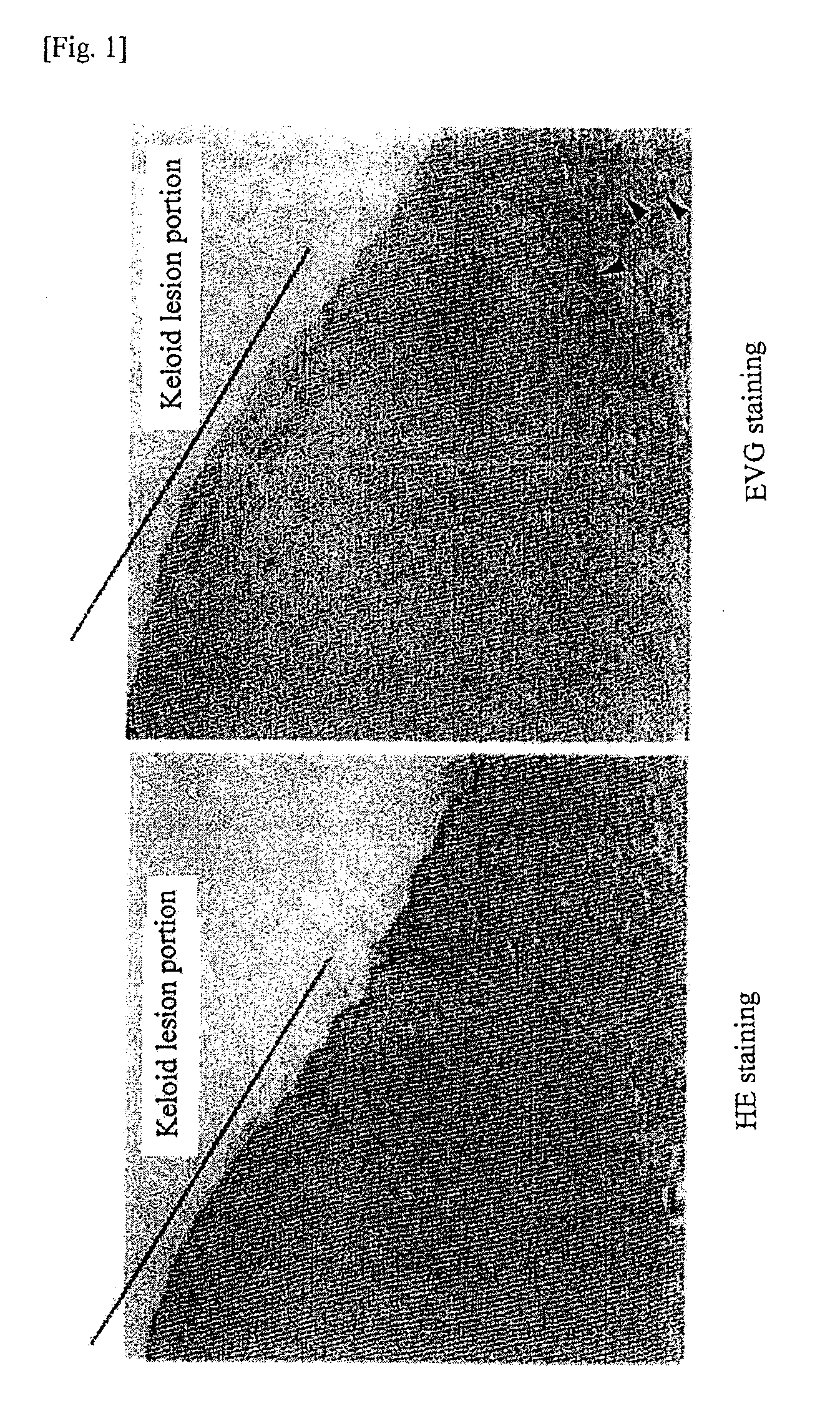
A hypertrophic scar is a thickened, wide, often raised scar that develops where skin is injured. Scars are common during the wound healing process, but a hypertrophic scar is a result of an abnormal response to a trauma or injury.
Methods for scar prevention
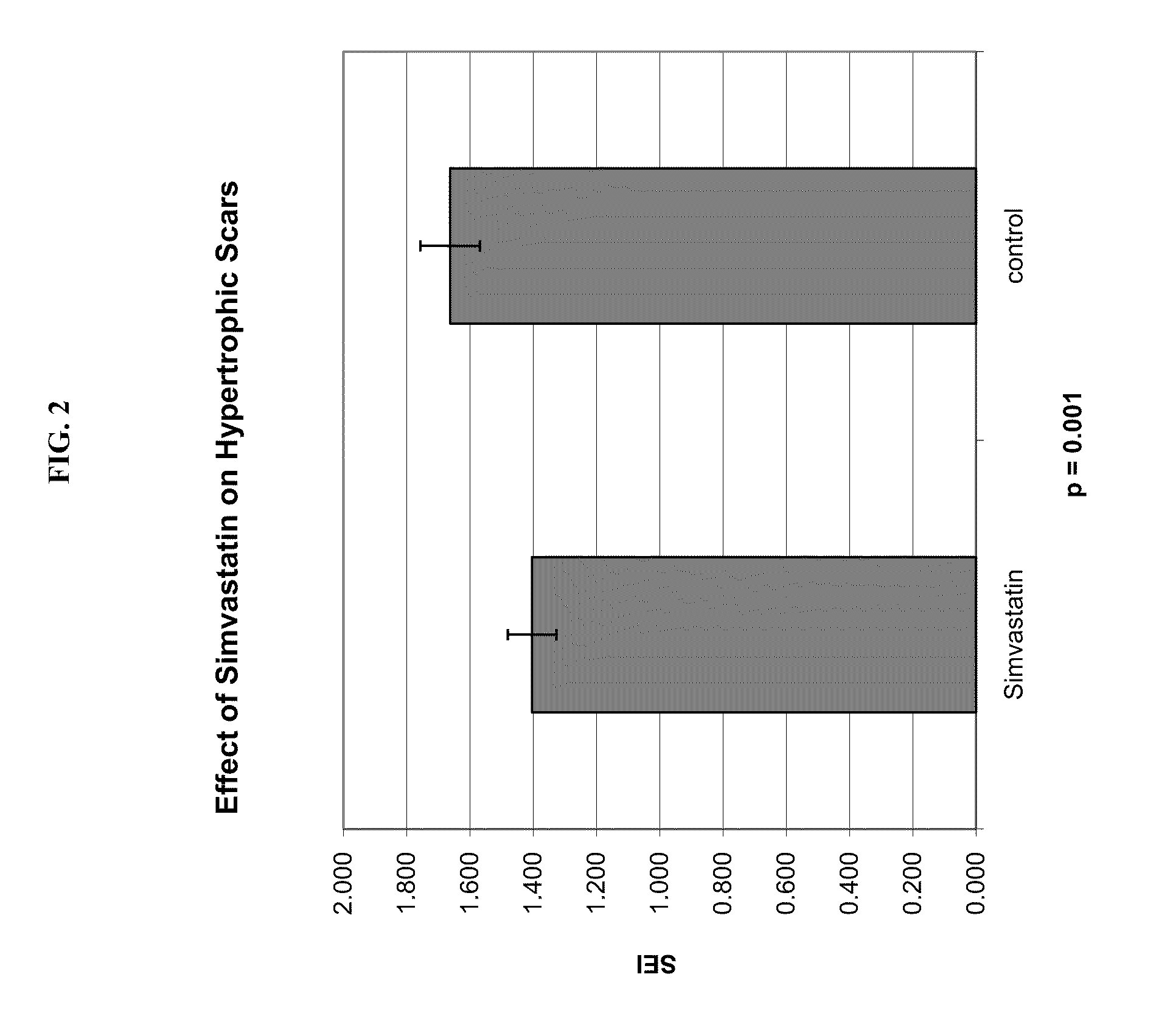
Provided herein are compositions and methods for preventing or reducing scar formation (e.g., hypertrophic scars). For example, provided herein are methods of administrating HMG-CoA-inhibiting agents for preventing or reducing scar formation.
Owner:NORTHWESTERN UNIV
Pharmaceutical composition containing ghrp-6 to prevent and eliminate fibrosis and other pathological deposits in tissues
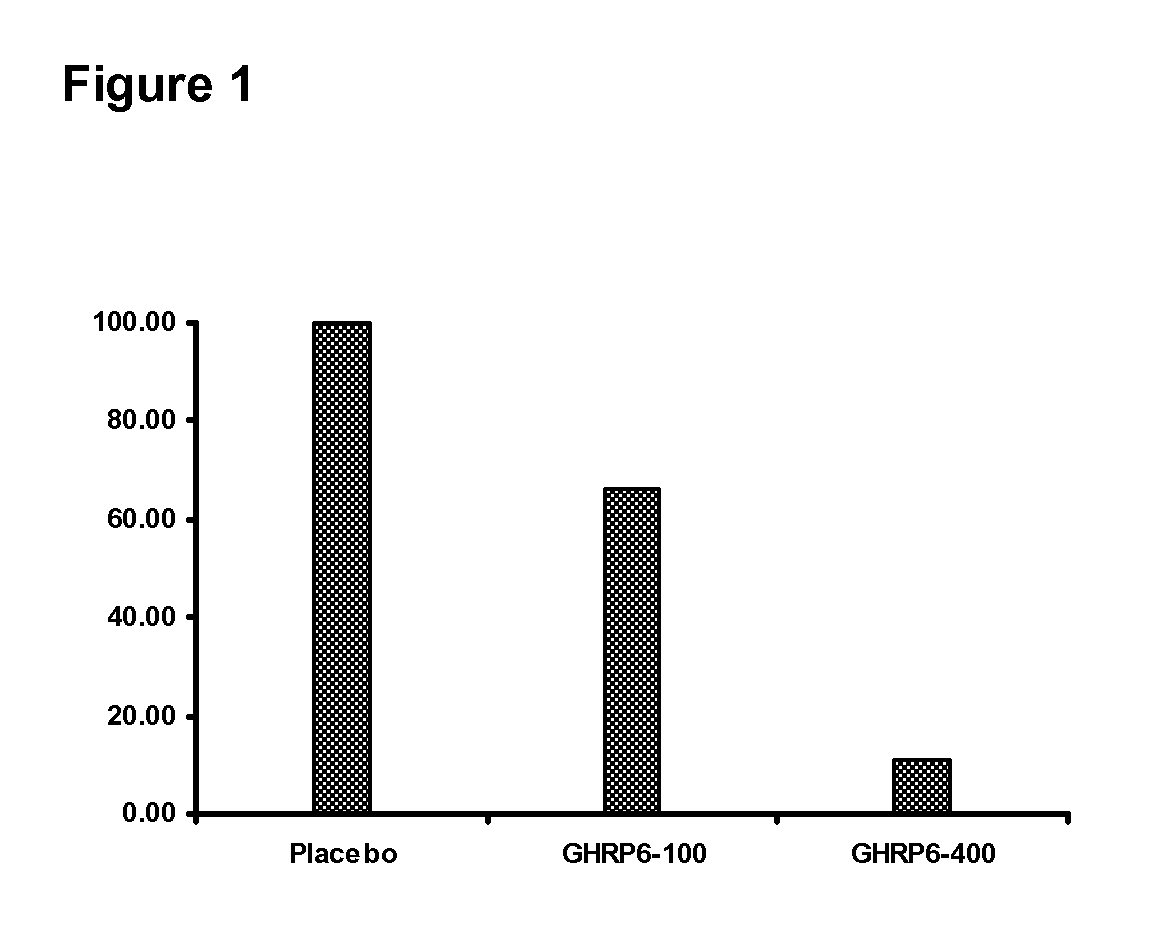
The present invention is related to the use of secretagogue peptides repeatedly administered as part of a pharmaceutical composition that prevent and eradicate the deposition of pathological fibrotic material in parenchymal tissues of internal organs like the liver, lungs, esophagus, small intestine, kidneys, blood vessels, joints, and other systemic forms of cutaneous fibrosis of any etiopathogenesis. Additionally, these peptides prevent and eradicate deposition of amiloid and hyaline materials in any of their correspondent chemical forms and tissue manifestations in the brain, cerebellum, blood vessels, liver, intestines, kidneys, spleen, pancreas, joints and the skin, among others. By this way, cellular, tissular and organ dysfunctions generated by these depositions are corrected. The peptides of the present invention are infiltrated or topically applied, contributing to prevent and eradicate keloids and hypertrophic scars in the skin, derived as sequelae of burns and other cutaneous trauma.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Methods and compositions for wound treatment
The present invention provides compositions and methods useful in the treatment of wounds, particularly in reducing or preventing scar formation, particularly hypertrophic scar or keloid formation. The invention thus further provides methods of treatment, including methods useful in hypertrophic scar or keloid revision as well as prophylactic, scar inhibiting methods.
Owner:TIF MANAGEMENT
Liposome gel preparation and preparation method and application thereof
ActiveCN102697705AImprove poor water solubilityImprove stabilityAerosol deliveryOintment deliveryGel preparationLipid particle
The invention provides a liposome gel preparation for treating hypertrophic scars. The liposome gel preparation comprises liposome, gel material and glycerol, wherein the liposome is encapsulated with paeonol, and the surface of the liposome is bonded with vascular endothelial growth factor (VEGF) antibodies through polyethylene glycol. The invention provides a preparation method and application of the gel preparation at the same time. The gel preparation is encapsulated by using a material such as phospholipid with good physiological compatibility with the human body, so that the problems of poor water solubility and low stability of the paeonol are effectively solved. The VEGF antibodies are used for modifying the surface of the liposome, lipid particles enter the dermis through the epidermis by using the permeation promoting effect of liposome carriers and are directionally bonded with VEGF receptors of the pathological change skin part, and the encapsulated medicament is released at the same time, so that positioned retention of the medicament in the scar hyperplasia part is realized. Compared with other reported scar hyperplasia resistant medicament preparations or treatment schemes, the invention has the advantages that the liposome gel preparation is low in relapse rate, reduces the occurrence rate of the toxic or side effect and can be applied to any scar hyperplasia part of the body.
Owner:GUANGDONG PHARMA UNIV
Preventives/remedies for thickened scar, keloid or chronic arthritic diseases
InactiveUS7300916B2Good control effectImproving effect on progressive proliferation of collagen fibersPeptide/protein ingredientsAntipyreticErythropoietin AntibodyErythropoietin receptor
A pharmaceutical preparation for preventing and / or treating hypertrophic scars, keloid or chronic arthritic diseases comprising as an effective component an erythropoietin antagonist. More specifically, there is provided a pharmaceutical preparation for preventing and / or treating hypertrophic scars, keloid or chronic arthritic diseases comprising as an effective component an erythropoietin antagonist such as an anti-erythropoietin antibody, an erythropoietin receptor protein, etc. This pharmaceutical preparation has excellent prophylactic and / or therapeutic effects on collagenous hyperproliferation such as hypertrophic scars, keloid, etc., or chronic arthritic diseases such as rheumatoid arthritis, etc.
Owner:YASUDA YOSHIKO
Macromolecular compound latex scar paste applied to inhibiting discomforts such as pruritus
InactiveCN103340843AProlong the action timeGood effectHydroxy compound active ingredientsAntipyreticPolymethyl methacrylateMethyl salicylate
The invention discloses a macromolecular compound latex scar paste used for controlling subjective discomforts such as local pruritus and fever caused by hypertrophic scar after wound healing of burn. The latex comprises the following components of hydroxypropyl guar gum powder, polymethyl methacrylate-chitin, methyl salicylate, heparin sodium, allantoin, menthol, camphor and borneol. Animal experiments and human body tests verify that the macromolecular compound latex (scar paste) is exact in itch-relieving effect, and can be used for controlling subjective discomforts such as local pruritus and fever caused by hypertrophic scar.
Owner:CHONGQING MJ MEDICAL DEVICES
Wound dressing and preparation method thereof
InactiveCN105769442AImprove breathabilitySmall sizeNon-adhesive dressingsPlastersFiberWound dressing
Provided are a wound dressing and a preparation method thereof.The wound dressing is composed of a silicone membrane layer and an electrospun nanofiber layer.The preparation method comprises the steps that silicone, liquid paraffin, normal hexane and span-80 are mixed to be uniform, the mixed solution is cast in a polytetrafluoroethylene mold through a membrane casting method, after a membrane is formed through drying, a silicone membrane is soaked in ethyl alcohol, the composition of paraffin is removed, and the silicone membrane with a pore structure is obtained; eletrospinning is conducted on the silicone membrane to form a layer of biodegradable polymer nanofibers through an eletrospinning method, after drying is conducted, radiation sterilization is conducted, and a finished product is obtained.The wound dressing prepared through the method has good moisture retention and air permeability, the wound can be kept moist, hypertrophic scars are prevented, body tissue generation can be promoted by means of the unique support function of the nanofiber layer, and wound healing is accelerated.
Owner:GUANGZHOU DIANFANG BIOTECH CO LTD
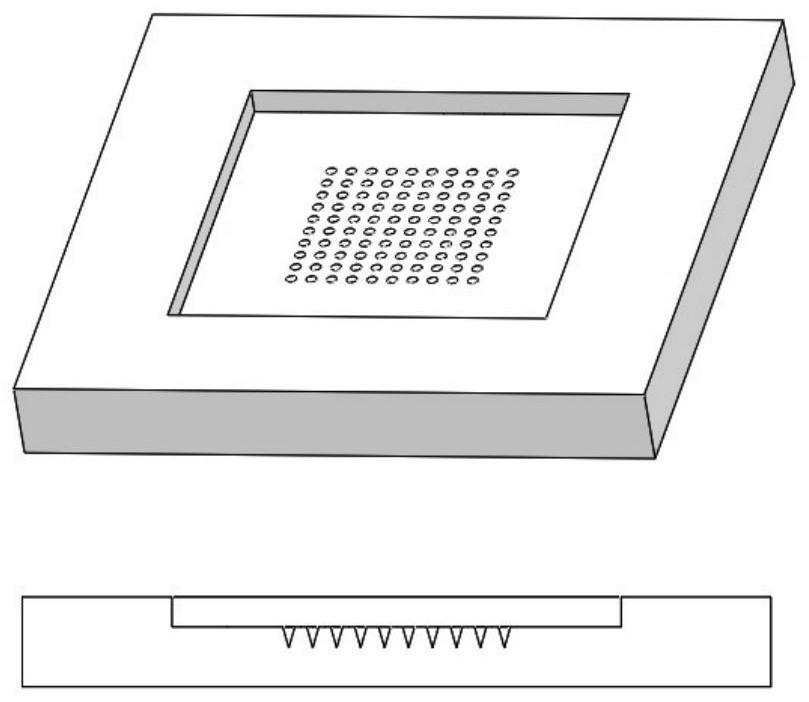
Microneedle patch for scar treatment and preparation method thereof
InactiveCN112999297AHave mechanical strengthEasy to useOrganic active ingredientsMicroneedlesPolyvinyl alcoholPolyethylene glycol
The invention provides a microneedle patch for scar treatment and a preparation method thereof, the microneedle patch comprises a substrate and a needle body arranged on the surface of the substrate, the needle body comprises an anti-scar active component and a matrix material, the anti-scar active component comprises onion extract, cannabis sativa leaf extract, allantoin and heparin sodium in a mass ratio of 1: (0-1): (0-0.5): (0-0.02); the anti-scar active component mainly comprises an onion extract, the substrate also comprises a matrix material, and the matrix material comprises but not limited to hyaluronic acid, sodium hyaluronate, polyvinyl alcohol, polyvinylpyrrolidone, polyethylene glycol, sodium carboxymethyl cellulose, hydroxypropyl-beta-cyclodextrin, sodium alginate, polylactic acid, chitosan and glucan; the microneedle patch for scar treatment has certain mechanical strength, is convenient to use and good in stability, can realize minimally invasive painless effect when acting on skin, and provides a new and effective method for treatment of hypertrophic scars at any part of a body.
Owner:上海行渝生物科技有限公司
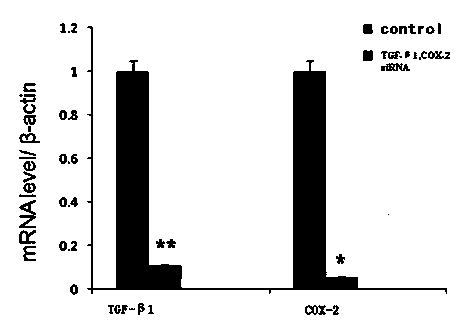
siRNA molecular composition and application thereof in treatment on pathological scars
InactiveCN104174032APromote apoptosisGood treatment effectGenetic material ingredientsDermatological disorderMolecular compositionMedicine
The invention relates to a siRNA molecular composition and application thereof in treatment on hypertrophic scars and keloids. The siRNA molecular composition contains siRNA molecules with the sequence of 5'-CCCAAGGGCUACCAUGCCAACUUCU-3' and siRNA molecules with the sequence of 5'-GGUCUGGUGCCUGGUCUGAUGAUGU-3'. The siRNA molecular composition is proved to be capable of promoting in-vitro fibroblast apoptosis of the hypertrophic scars and keloids and have a good treatment effect on the hypertrophic scars in an animal model.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Biological beautifying preparation containing autologous stroma cells
ActiveCN104173252AImprove wrinklesAcne scar repairCosmetic preparationsToilet preparationsBlood plasmaAcne scarring
The invention provides a method for preparing a biological beautifying preparation containing autologous stroma cells. The method comprises the following steps: acquiring autologous plasma from autologous peripheral blood and culturing to acquire autologous stroma cells; and adding the autologous stroma cells into the autologous plasma to prepare the biological beautifying preparation. The invention simultaneously provides the biological beautifying preparation prepared from the autologous stroma cells and the autologous plasma, which is added with growth factors and anti-oxidation factors, and is capable of removing skin wrinkles, repairing acne scarring, repairing and improving burns, trauma and hypertrophic scars, resisting senescence and the like.
Owner:蔡贤芬
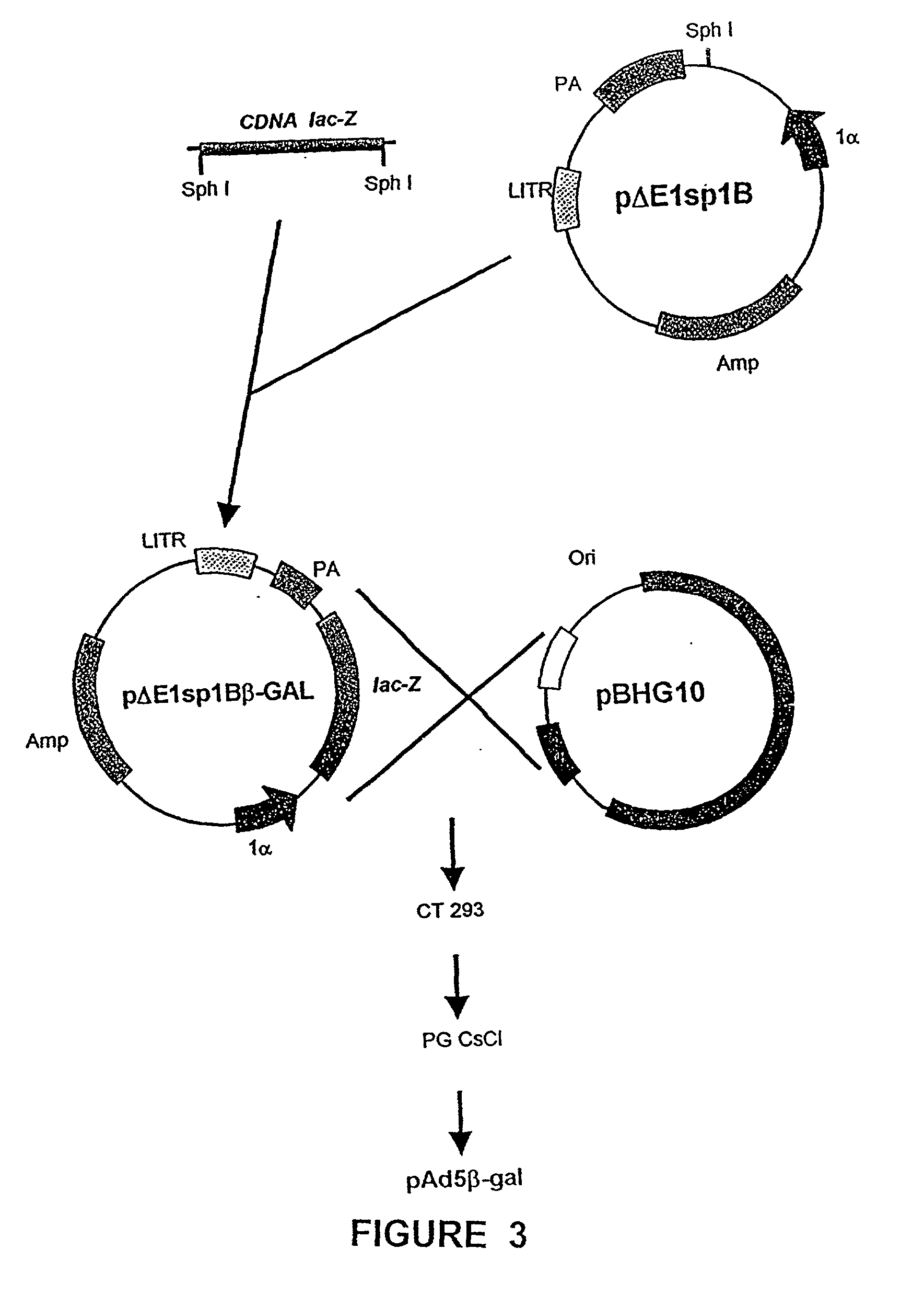
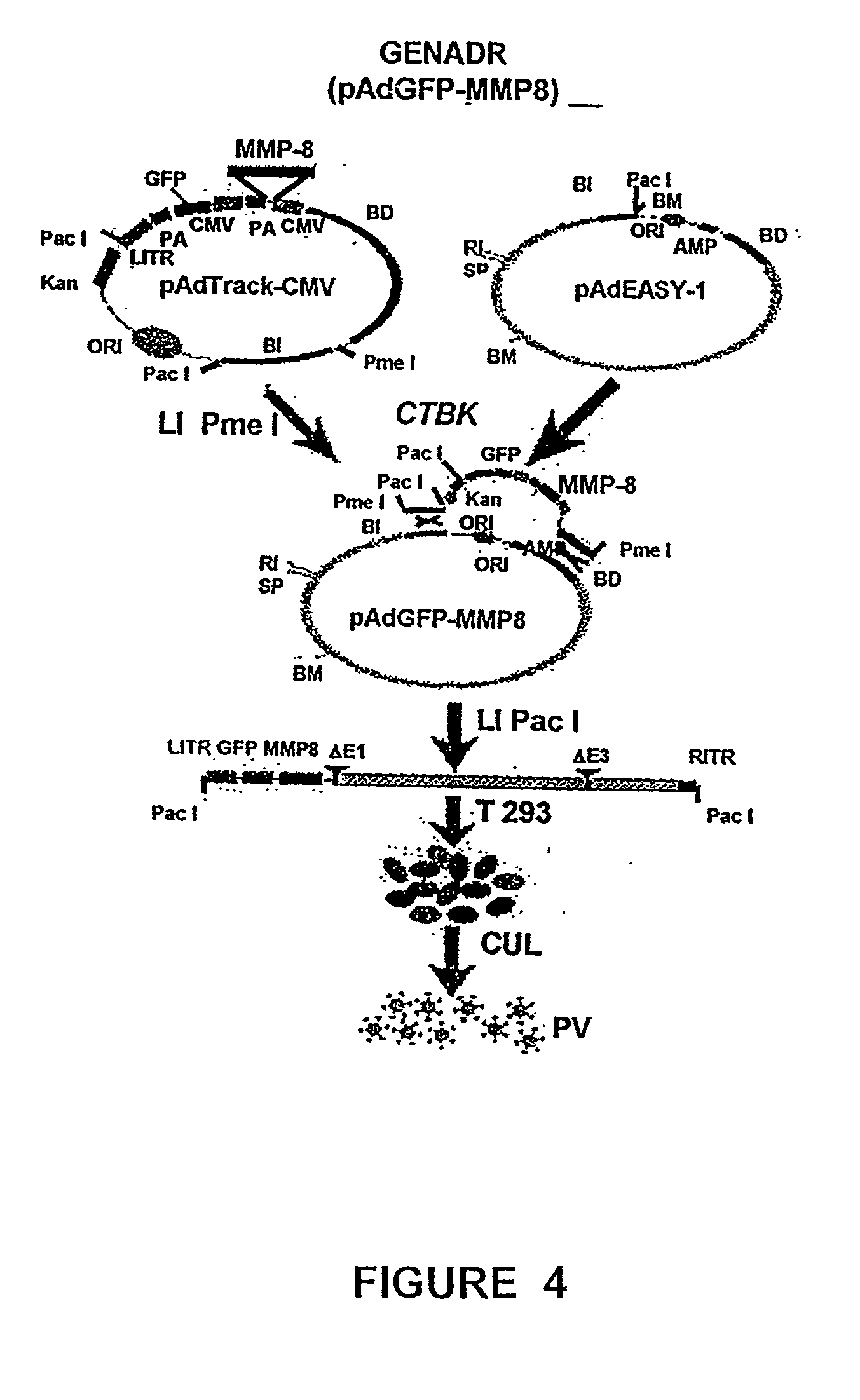
Recombinant adenoviral vectors and their utility in the treatment of various types of fibrosis: hepatic, renal, pulmonary, as well as hypertrophic scars
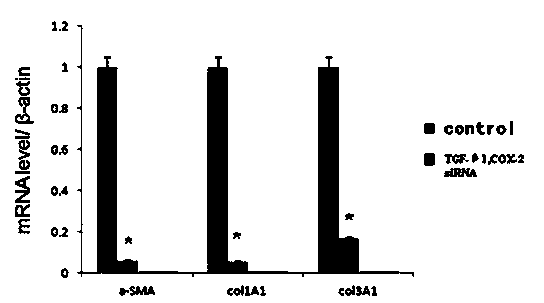
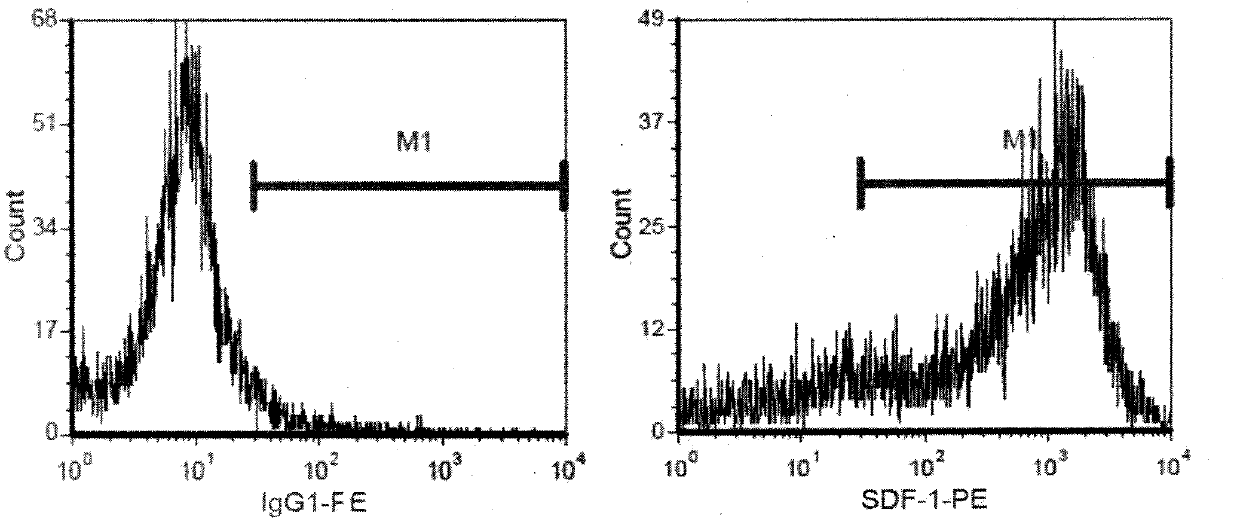
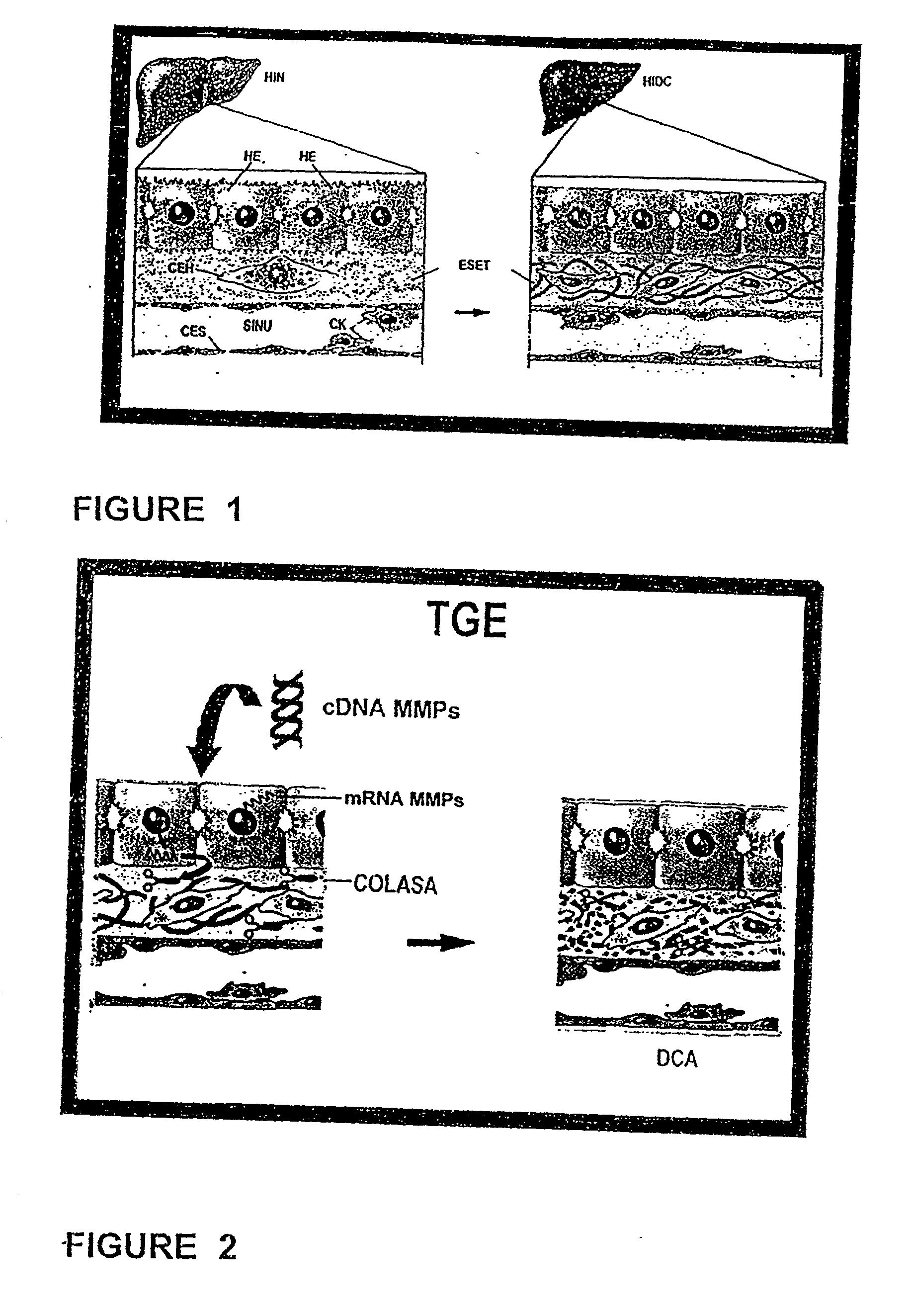
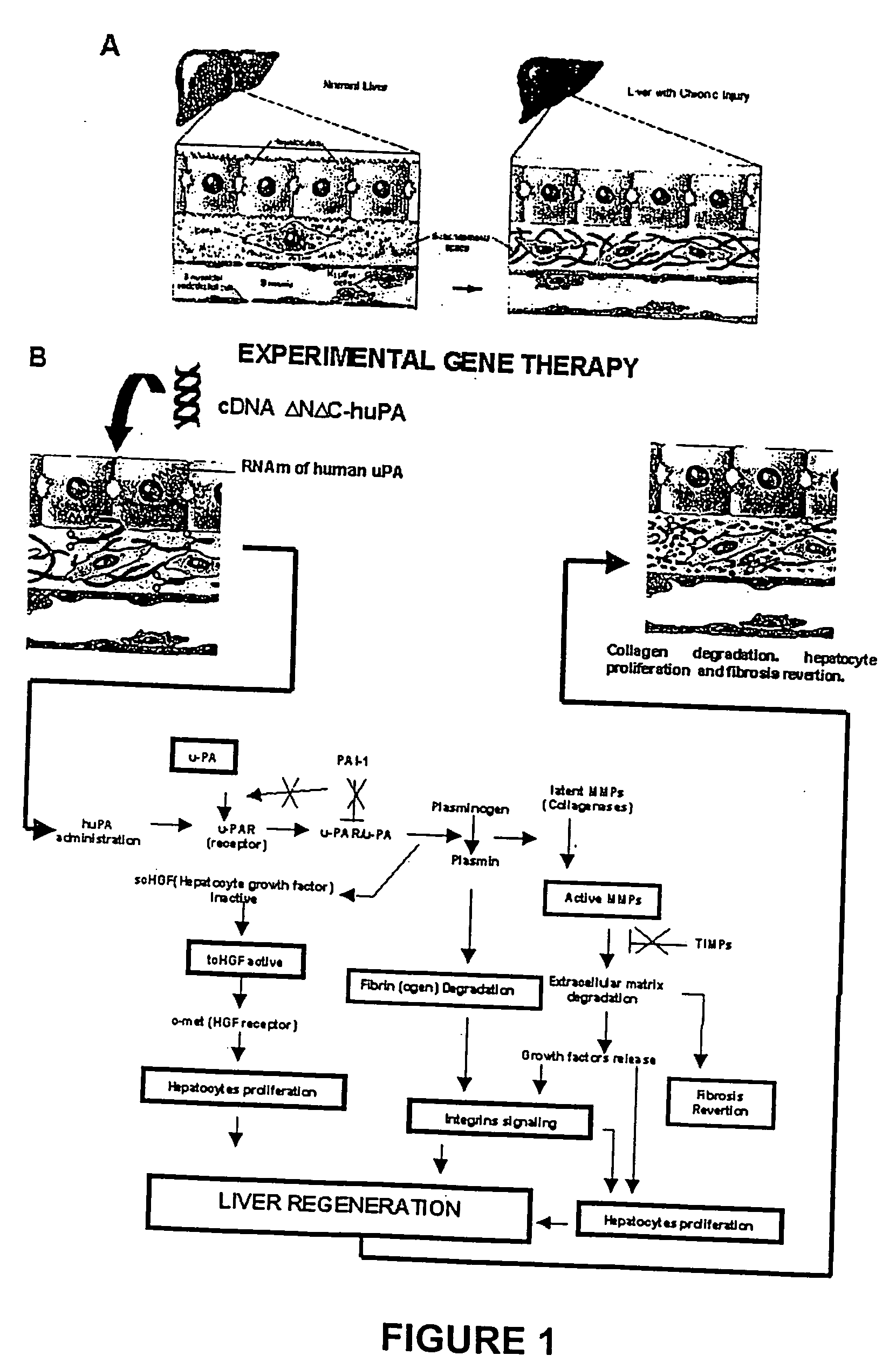
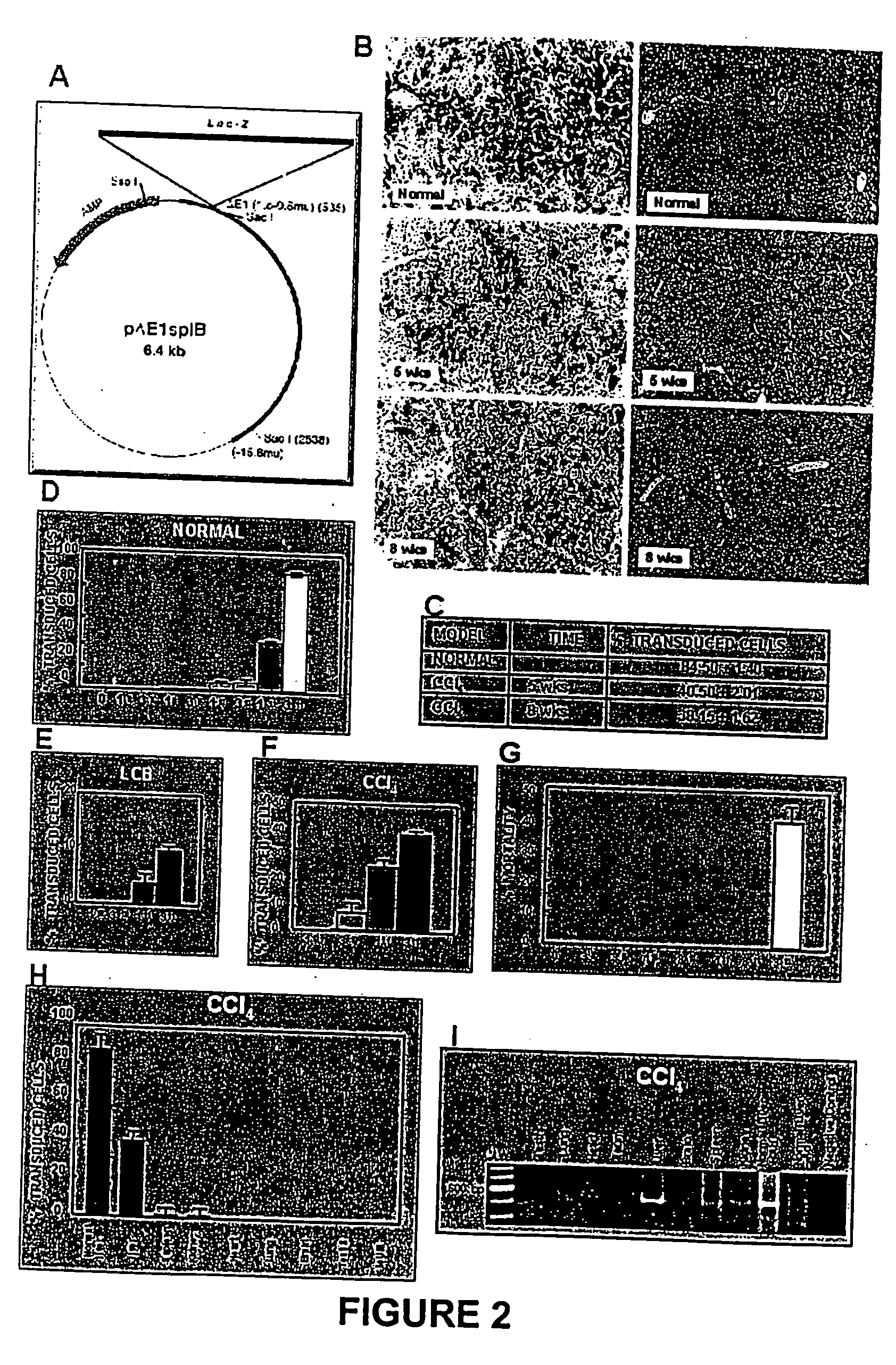
<heading lvl="1">SUMMARY OF THE INVENTION < / heading> The use of gene therapy for the treatment of different kinds of fibrosis in human beings is disclosed. The purpose is the use of "therapeutic2 genes specifically directed to target organs to revert and / or prevent the development of the fibrosis process. The potential application of gene therapy to patients with fibrosis and / or cirrhosis will depend to a large extent on the successful delivery of genes which encode for therapeutic proteins to livers with severe fibrosis and that these genes which encode for proteins human MMP-8 active and latent, MMP-1, MMP-2, MMP-9 and MMP-13; human uPA wild type and / or modified (or its truncated version), the truncated receptor for TGF-beta type II and Smad-7 can be directed by adenovirus and / or other recombinant vectors that cannot transduce (infect) others organs. The recombinant adenoviruses (AdR) are vectors highly efficient for the transduction of therapeutic genes to diverse target cells. We have proved that they can carry genes to cirrhotic livers. The delivery of therapeutic genes through such adenoviral vectors and other recombinant vectors could also be performed using cationic and anionic liposomes (DOTMA). Therefore, we propose the use of this patent to be applied in the same manner to: Renal fibrosis Pulmonary fibrosis Hypertrophic and keloid scars (skin fibrosis), and Other kinds of fibrosis.
Owner:TGT LAB DE C V
Medicinal composition for treating burn and scald and preparation method thereof
ActiveCN102228623AMaintain ecological normalityInhibitory necrosisDermatological disorderPlant ingredientsSecond-Degree BurnBarbed Skullcap Herb
The invention discloses a medicinal composition for treating burn and scald. The medicinal composition comprises the following bulk drugs in percentage by weight: 13 percent of garden burnet, 10 percent of rhizoma bletillae, 10 percent of callicarpa undiflora, 27 percent of rhubarb, 15 percent of giant knotweed, 10 percent of barbed skullcap herb and 15 percent of gromwell root, wherein the medicaments are settled and crushed into fine powder and then uniformly mixed to obtain a mixture; the mixture is immersed in sesame oil in a ratio of 1:(6-8) to obtain a second mixture; and the second mixture is sieved to filter off residues and is bottled for later use. The medicinal composition has the advantages of powerful anti-infection, pain relieving and impervious functions, no irritation to a wound surface, capability of promoting repairing and healing of the wound surface of a cut, locally flat healed wound surface, no scar on the healed wound surface of the first, second and third degree burns and scalds, short treatment period, no irritation phenomenon, no toxic or side effect, low treatment cost, convenience in operation, easiness in change of a medical prescription and convenience in nursing. The medicinal composition can be used for reducing and treating hypertrophic scar of deep second degree burn and superficial third degree burn, promoting the healing of the wound surface and is an external pure traditional Chinese medicine for treating the burn and scald without being limit by medical conditions during operation and implementation.
Owner:彭卫新
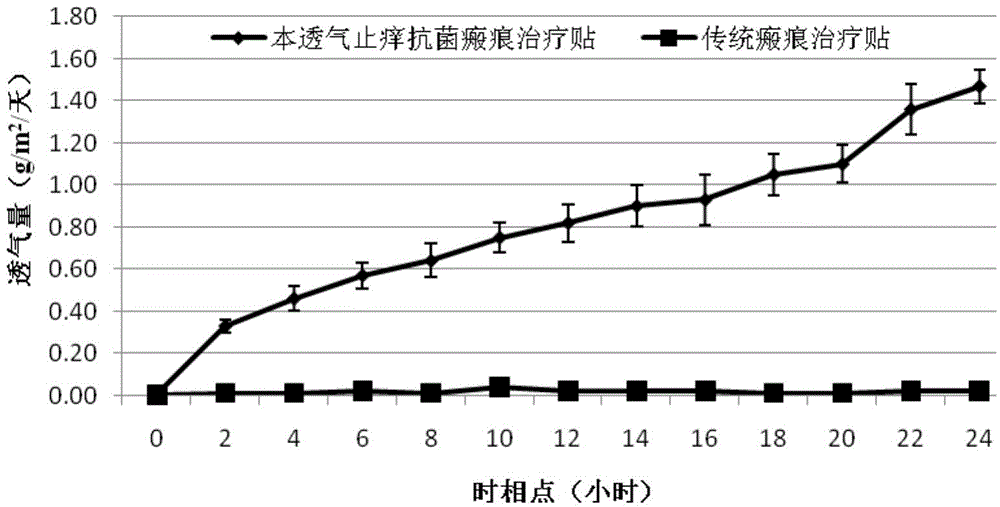
Breathable itching-relieving antisepsis scar treating paster and preparation method thereof
ActiveCN103550254AHydroxy compound active ingredientsInorganic active ingredientsMedicineBULK ACTIVE INGREDIENT
The invention belongs to the technical field of treating skin scars, and particularly relates to a breathable itching-relieving antisepsis scar treating paster and a preparation method thereof. The technical problem to be solved is to provide a new choice for treating a hypertrophic scar. According to the technical scheme, the breathable itching-relieving antisepsis scar treating paster is prepared from the following active ingredients in percentage by weight: 97.9% of silicone, 1% of mint, 1% of borneol, 0.05% of heparin sodium, and 0.05% of nanosilver. The invention further provides a preparation method for the breathable itching-relieving antisepsis scar treating paster. The treating paster provides a new choice for treating the scar.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
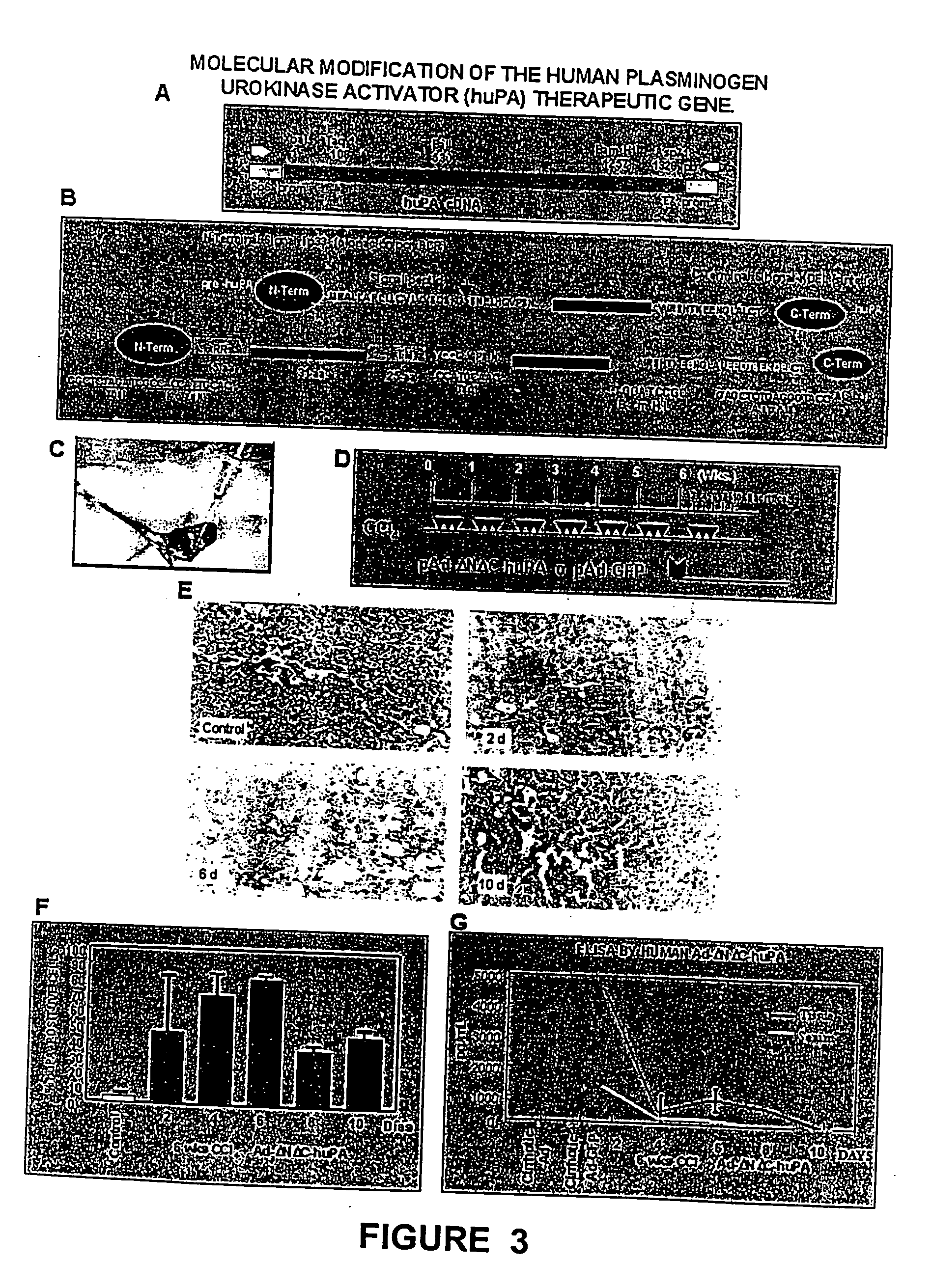
Recombinant viral and non-viral vectors containing the human urokinase plasminogen activator gene and its utilization in the treatment of various types of hepatic, pulmonary, pancreatic and cardiac fibrosis and hypertrophic scars
InactiveUS20040097455A1Reestablishment of liver functionFacilitated DiffusionPeptide/protein ingredientsGenetic material ingredientsCardiac fibrosisCause of death
Hepatic cirrhosis is considered a severe health problem in Mexico, since it is the third mortality cause in working-age people and there is no 100% effective treatment. Cirrhosis is characterized by an exacerbated increase of collagen in liver parenchyma, replacing the hepatocytes and thus provoking liver failure. This is one of the reasons why we have used a gene therapy through specific delivery to cirrhotic livers of the gene of human urokinase plasminogen activator (huPA), which activates mechanisms that induce the degradation of excess cellular matrix and stimulate hepatocyte proliferation, obtaining thus a fast re-establishment of the liver function. In the instant invention, the modified human uPA gene was inserted in the adenoviral vector (pAd-DeltahuPA), because it is not secreted and does not provoke hypercoagulation or spontaneous internal bleedings. Moreover, data from the bio-distribution essay with an adenoviral vector with reporter gene beta-gal have shown liver specificity as the target organ of the vector. Using ELISA, huPa protein was detected in liver homogenates (4500 pg / ml) in animals treated with pAd-DeltahuPA and was also intracellularly detected through immunochemistry in liver cuts (80% positive cells). huPa induced a dramatic fibrosis reduction (85%) on day 10 of vector administration, compared to control cirrhotic rats and 55% hepatocyte proliferation increase. Liver function tests (ALT, AST, alkaline phosphatase and bilirubin) dropped to nearly normal levels and hepatocyte proliferation was observed. Because of the two beneficial event cascades, gene therapy with modified huPA can be developed as a definite potential treatment for patients with liver cirrhosis.
Owner:TGT LAB DE C V
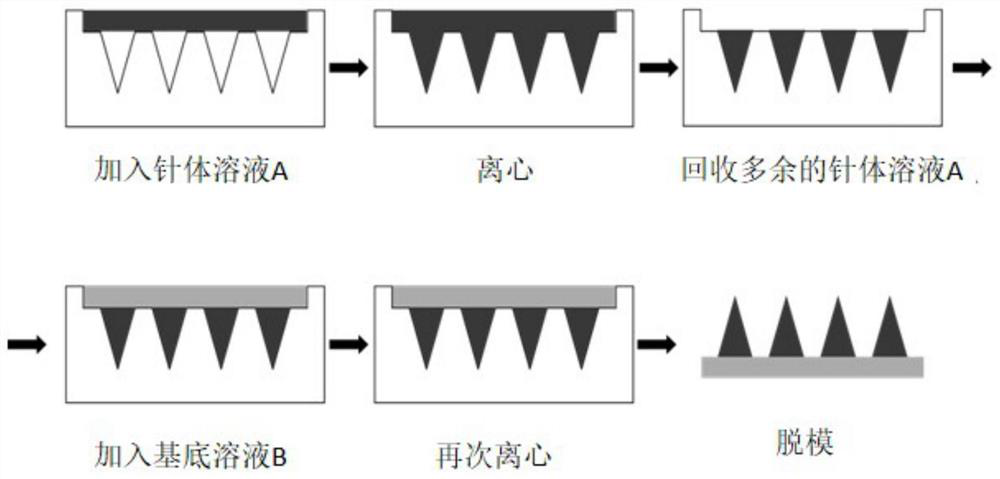
Microneedle for hypertrophic scars and preparation method for microneedle
The invention provides a microneedle for hypertrophic scars and a preparation method for the microneedle. The preparation method comprises the following steps of adopting crystalline macromolecules which serve as first polymers, casting a first solution into a die with a micropore array, and performing vacuumizing to obtain a die of which micropores are filled with the first solution; adding a second solvent into the die of which the micropores are filled with the first solution, and performing vacuumizing to obtain the microneedle for hypertrophic scars. Therefore, the prepared microneedle is hard enough to penetrate through scar tissues, and moreover, a water-soluble medicament as well as an oil-soluble medicament can be supported by the microneedle.
Owner:海南德拉米克投资有限公司
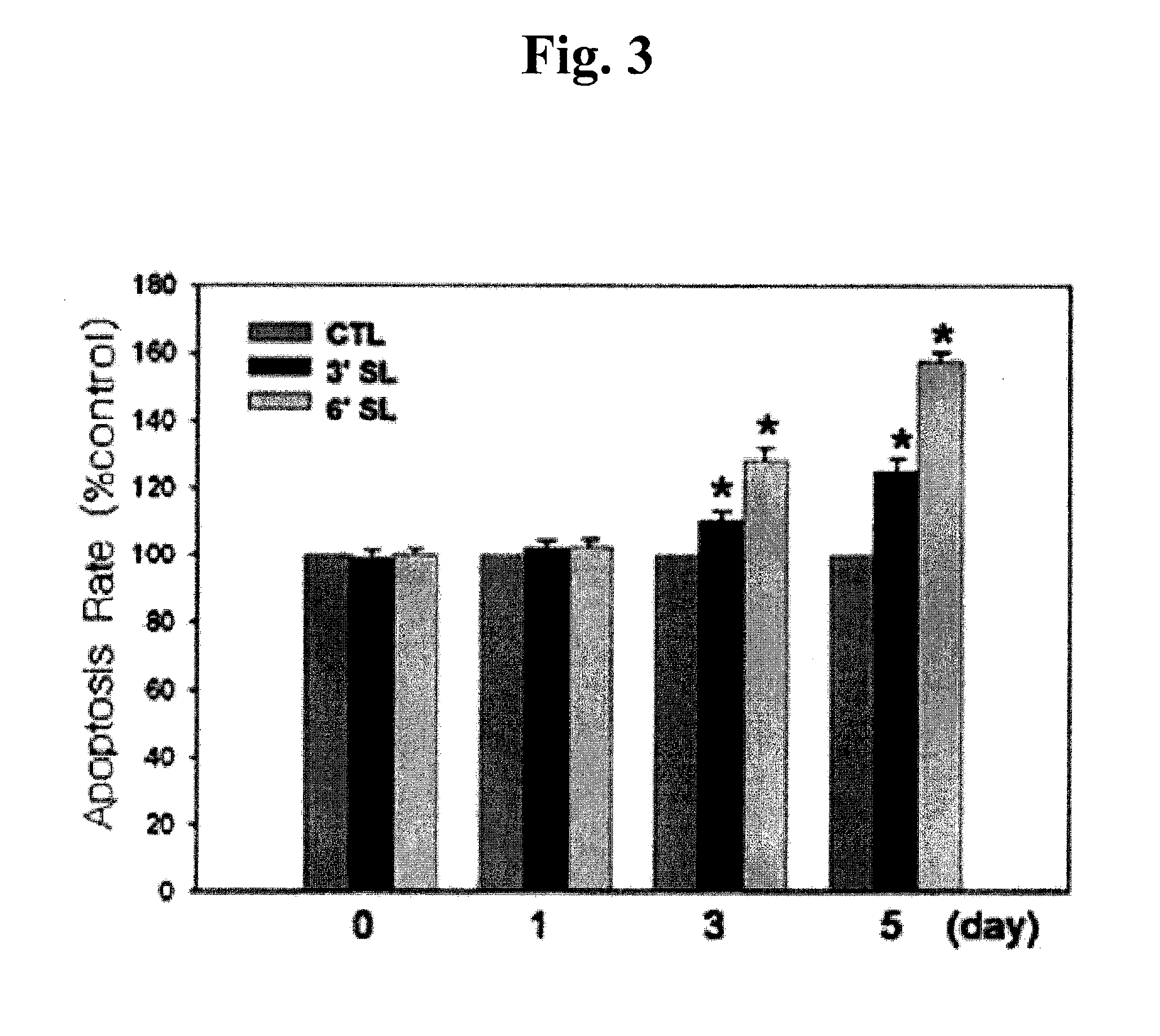
Composition for Prevention or Treatment of Hypertrophic Scars or Keloids
ActiveUS20120122814A1Preventing and treating hypertrophic scar and keloidPreventing and treating keloidBiocideCosmetic preparationsSialic acidKeloid
The present invention relates to a composition for preventing or treating hypertrophic scar or keloid, comprising as an active ingredient a compound represented by the following general formula I:S-(MS)p-(MS)q (I)wherein S represents sialic acid, and (MS)p and (MS)q independently represent a monosaccharide residue.The composition of the present invention inhibits proliferation of keloid fibroblasts and induces apoptosis of keloid fibroblasts, thereby effectively preventing or treating keloid. The active ingredient used in this invention is a natural compound or its derivative or isomer and therefore very safe to human.
Owner:BENEBIOSIS
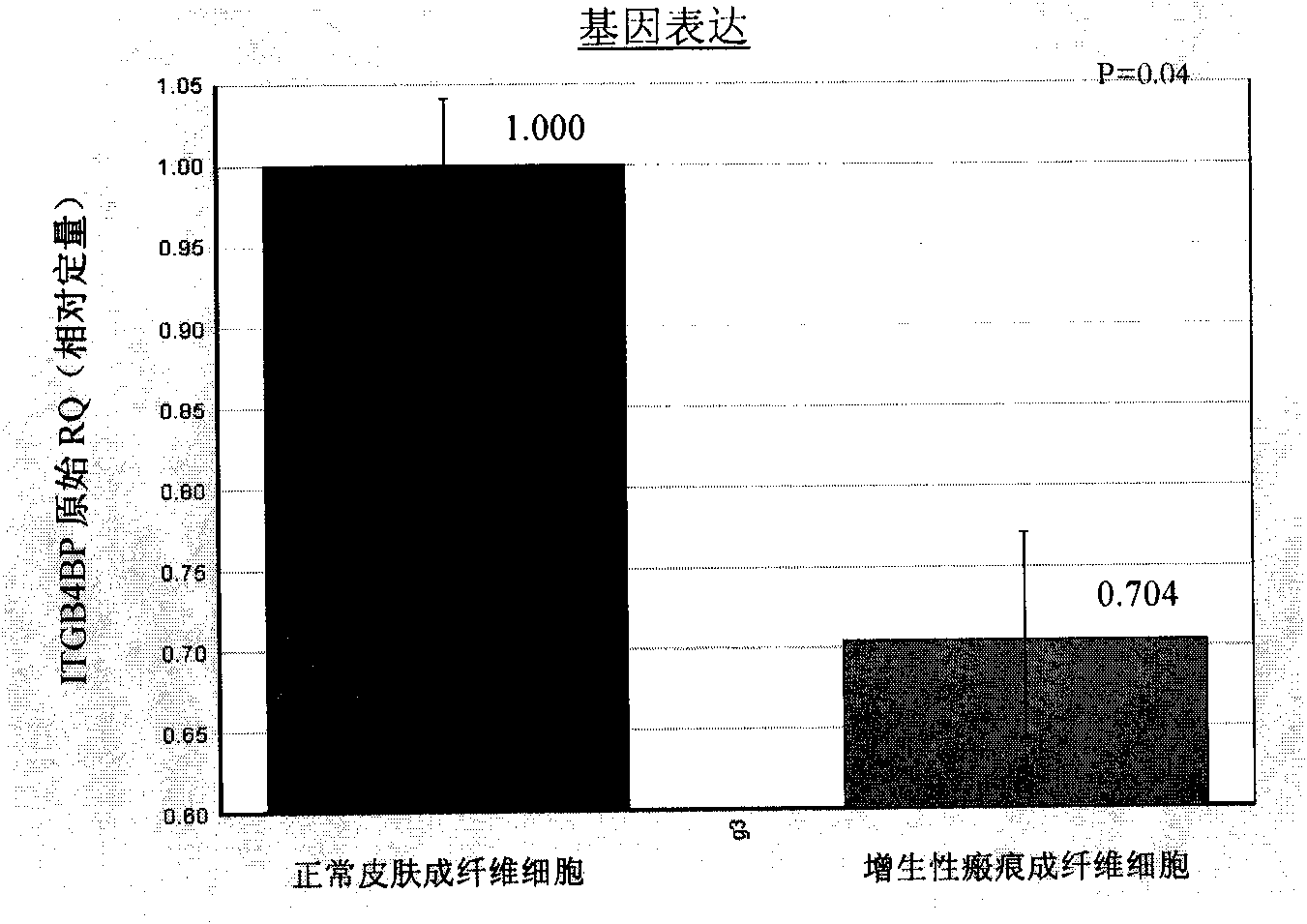
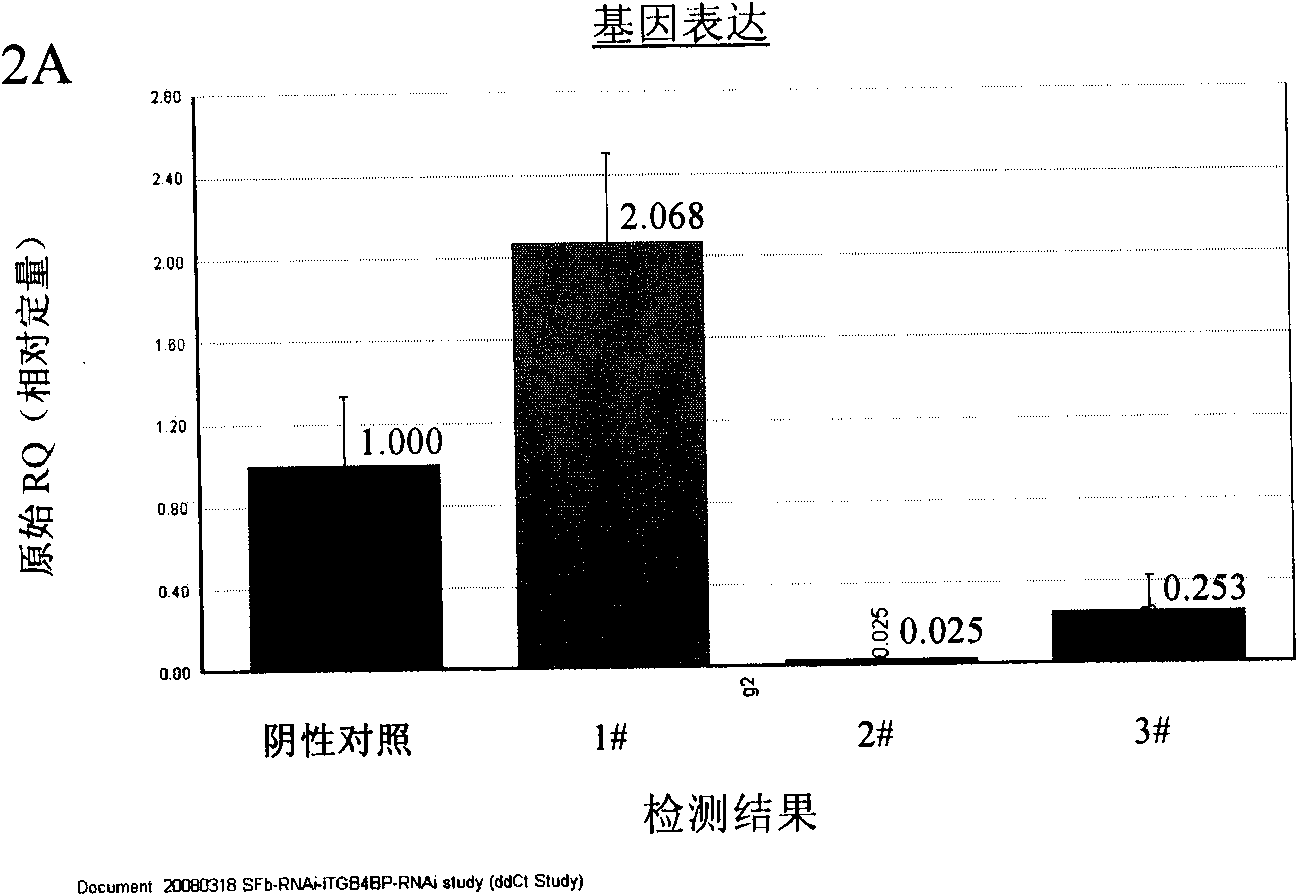
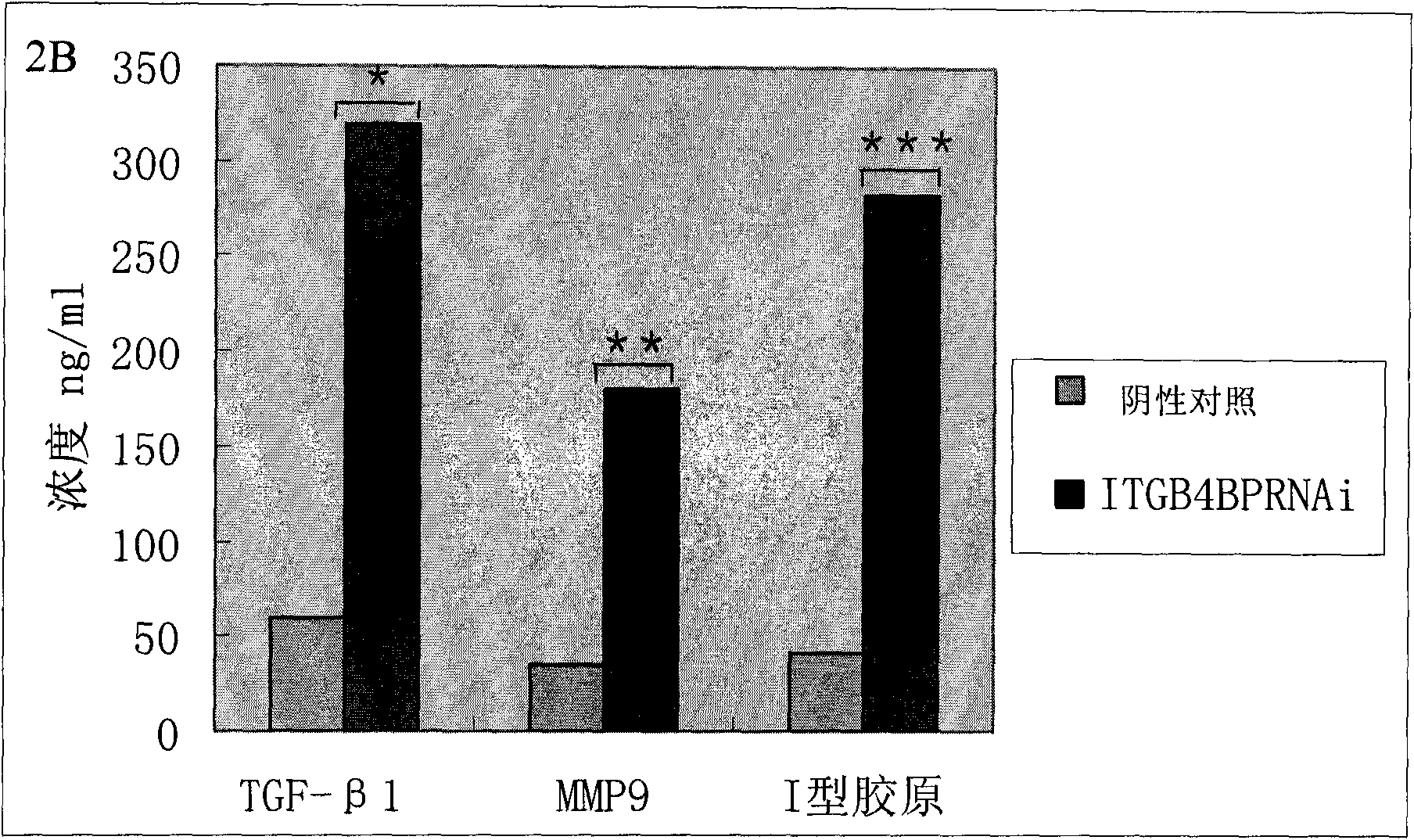
ITGB4BP and derivates thereof used for preventing and/or treating hypertrophic scar and fibrosis lesion
The invention relates to the new applications of integrin beta 4 binding protein (ITGB4BP) and derivates thereof, or gene recombinant expression vector expressing the ITGB4BP or derivates thereof in preventing and / or treating hypertrophic scar or fibrosis lesion; wherein, the amino acid sequence of the ITGB4GB is SEQ ID NO 1; the derivates of the ITGB4BP are protein derivates retaining the combining site and the functional site of the ITGB4BP (SEQ ID NO 1). The application is realized by applying the ITGB4BP or derivates thereof, or gene recombinant expression vector expressing the ITGB4BP orderivates thereof, or the drug combination or the kit of the invention on subjects with an effective dose in treatment. The drug combination or the kit contains the ITGB4BP or derivates thereof, or gene recombinant expression vector expressing the ITGB4BP or derivates thereof and an officinal excipient or vectors and the like with an effective dose in treatment. Preferably, the subjects are humanbeings or animals.
Owner:SOUTHWEST HOSPITAL OF CHONGQING
Application of benzopyran alkaloid
ActiveCN102764253AReduced shrinkage performanceBlocking adhesionOrganic active ingredientsDermatological disorderBenzopyranMedicine
The invention relates to the technical field of medicines, in particular to application of a benzopyran alkaloid. The application of the benzopyran alkaloid to the preparation of medicines for restraining formation of hypertrophic scars is involved; the benzopyran alkaloid has the structural formula which is shown in specifications; and the benzopyran alkaloid can restrain proliferation and viability of normal human skin fibroblast, block the adhesion of normal human skin fibroblast (NHSF) and human mononuclear / macrophage (THP-1), and weaken contractility of the normal human skin fibroblast (NHSF).
Owner:上海盛通康维药业有限公司
Preparation method and application of capsaicin-collagen sponge
ActiveCN104490760AImproves transdermal penetrationAvoid first pass effectOrganic active ingredientsPharmaceutical delivery mechanismFreeze-dryingEthylic acid
The invention relates to the technical field of external medicines for hypertrophic scars, and particularly relates to a preparation method and an application of capsaicin-collagen sponge. The preparation method comprises the following steps: 1 preparing collagen powder from tendo calcaneus; 2 extracting the collagen powder with pepsin, so as to obtain an extract liquid; 3 centrifugally collecting supernate; 4 separating out collagen by adding sodium hydroxide, and washing, so as to prepare high-purity collagen; 5 redissolving the collagen with ethylic acid; and 6 evenly mixing the collagen liquid, glycerinum and capsaicin, and then carrying out freeze-drying, so as to obtain the capsaicin-collagen sponge. The capsaicin-collagen sponge prepared by the method is applied to tissue trauma repair and inhibition of the hypertrophic scars in the tissue trauma repair process, and has the advantage of good percutaneous permeability; the drug liver first-pass effect can be avoided; and the thrill to the skin can be reduced.
Owner:GUANGDONG MEDICAL UNIV
Composition for preventing and treating hypertrophic scars, and drug and application thereof
InactiveCN104940232AAvoid formingEasy to makeAerosol deliveryInanimate material medical ingredientsMedicinePreservative
The invention discloses a composition for preventing and treating hypertrophic scars, and a drug and application thereof. The composition comprises the following components in parts by weight: 200-250 parts of silicone oil, 100-150 parts of glycerinum, 40-80 parts of white oil, 2-15 parts of cetostearyl alcohol, 10-25 parts of tween 80, 2-15 parts of stearic acid, and 1-10 parts of carbomer. The composition for preventing and treating hypertrophic scars disclosed by the invention is simple to prepare, low in cost, free from preservative, steady in physicochemical property, exquisite, uniform and long in expiration date; when being used, the composition is directly coated on a wound; therefore, the composition is convenient and practical; pruritus at an affected part in the treatment process can be obviously lightened; therefore, the original colour of skin can be restored; chromatosis is avoided; obvious scars do not exist; formation of hypertrophic scars after operations, burns, scalds and trauma can be effectively prevented; and the composition has good curative effect on formed hypertrophic scars.
Owner:CHANGSHA DARUIQI IND
Agent for Treating Skin Aging and Scars
InactiveUS20110028699A1Sufficient effectCosmetic preparationsPeptide/protein ingredientsCutaneous laxityKeloid
It is to provide an agent for preventing and treating skin aging, or an agent for treating skin scar that can exert a sufficient effect. An agent for preventing and treating skin aging comprising bFGF for treating aging of the skin that is administered intradermally or subcutaneously, or an agent for treating skin scar comprising bFGF for treating scar of skin that is administered intradermally or subcutaneously is utilized. Preferred examples of aging of skin include skin wrinkle, pigmented spot, sagging skin, rough skin, skin thinning, decrease of skin viscoelasticity, etc., and preferred examples of scar include keloid, hypertrophic scar, scar contracture, etc.
Owner:LABO JUVERSA
Hydrogel adapted for treatment of acute dermal wounds
The present invention provides compositions and methods useful in the treatment of wounds, particularly in reducing or preventing scar formation, particularly hypertrophic scar or keloid formation. The invention thus further provides methods of treatment, including methods useful in hypertrophic scar or keloid revision as well as prophylactic, scar inhibiting methods.
Owner:HALSCION
Moist exposed burn ointment and preparation method thereof
InactiveCN102793854AReduce financial burdenEasy to prepareAerosol deliveryOintment deliveryInfection rateSweat gland
The invention relates to an external ointment, in particular to a moist exposed burn ointment and a preparation method thereof. The moist exposed burn ointment is characterized by being prepared from the following raw materials in parts by weight: 30 parts of giant knotweed, 40 parts of gromwell, 30 parts of radix angelicae, 30 parts of hyacinth bletilla, 30 parts of baikal skullcap root, 30 parts of honey-suckle stem, 30 parts of astragalus root, 30 parts of phellodendron bark, 50 parts of gallnut, 30 parts of bee wax and 1000 parts of sesame oil. According to the invention, the preparation method is simple, and the cost of a patent medicine is low, thus the economic burden of patients is relieved; and the therapy shows that a wound has slight pain, the infection rate is low, the progressive sloughing area of the stasis tissue is reduced, a shallow wound can be healed without scarring, an extensive burn does not need skin grafting, malformed sarcomas and hypertrophic scars can not be formed after healing, and the growth of the sweat gland and fine hair can be effectively facilitated.
Owner:陈惠娟
Silica gel dressing film and production method thereof
ActiveCN105288701AImprove complianceSolve the problem of easy falling offAbsorbent padsBandagesPolymer scienceTreatment effect
The invention belongs to the technical field of medical patch beauty materials, and particularly relates to a silica gel dressing film which comprises an upper layer and a lower layer, namely a self-adhering silica gel layer and an anti-adhering silica rubber layer which are integrated through high-temperature sulfurization. The produced corrugated silica gel dressing film has the outstanding advantages of being high in softness and compliance, so that the problem that the silica gel dressing film is liable to fall off when in use can be solved; the silica gel dressing film can be closely and seamlessly adhered to the body surface without being impacted by body movement, can be applied to the surface of a scar for a long time, can be prevented from falling off automatically, is more convenient to use, and is beneficial to improvement of the treating effect on hypertrophic scars.
Owner:SHANGHAI NUOYUE MEDICAL TECH
Radical therapeutic agent for keloid and hypertrophic scar
InactiveUS20110008312A1Image obtainedMore inhibitory effectPeptide/protein ingredientsPeptidesKeloidHypertropic
An effective therapeutic agent for keloids and / or hypertrophic scars is provided. Specifically, an elastic fiber regenerating agent consisting of chondroitinase ABC derived from Proteus vulgaris and a therapeutic agent for keloids and / or hypertrophic scars comprising the regenerating agent as an active ingredient are provided.
Owner:SUZUKI SHIGEHIKO +1
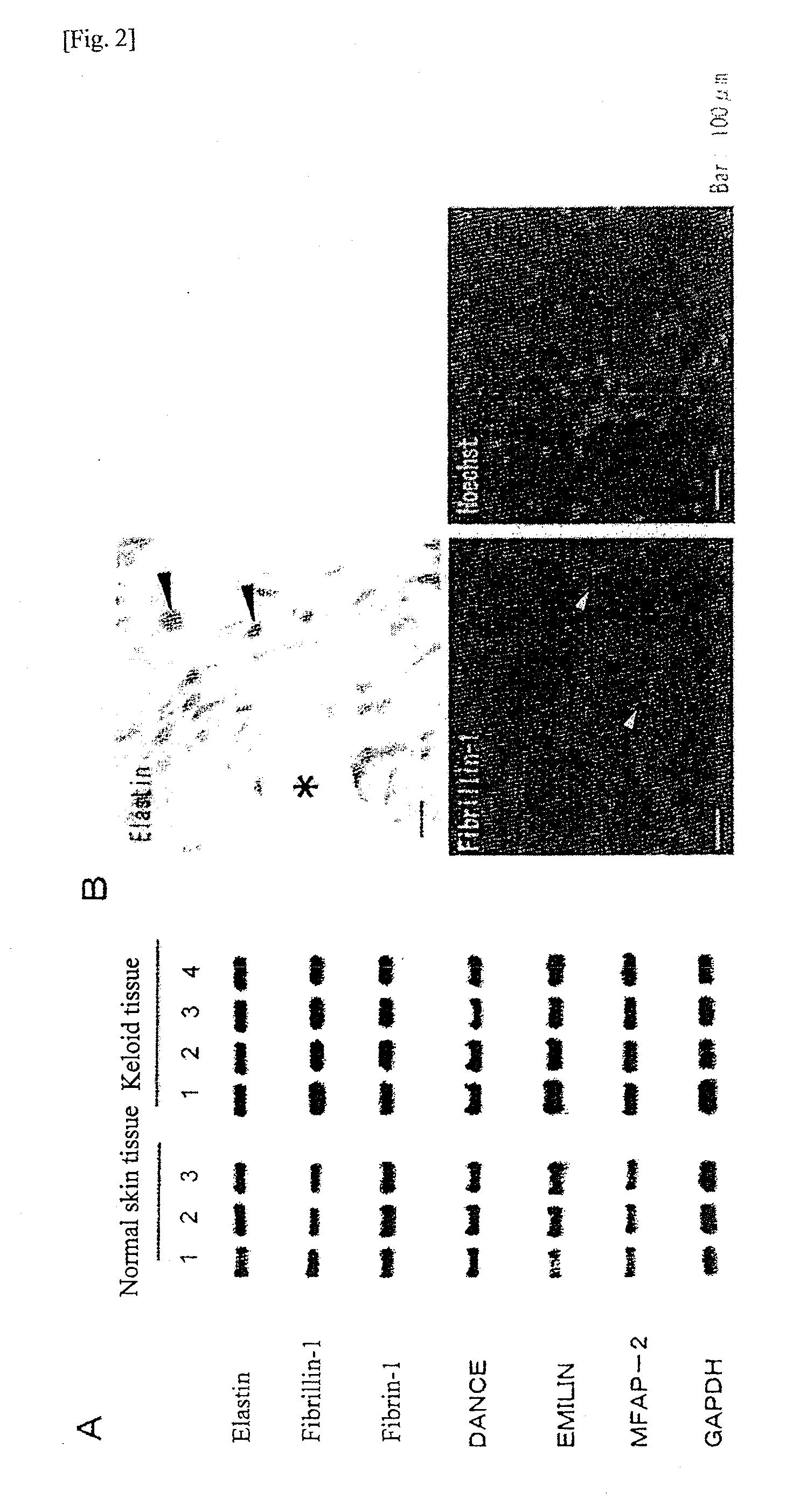
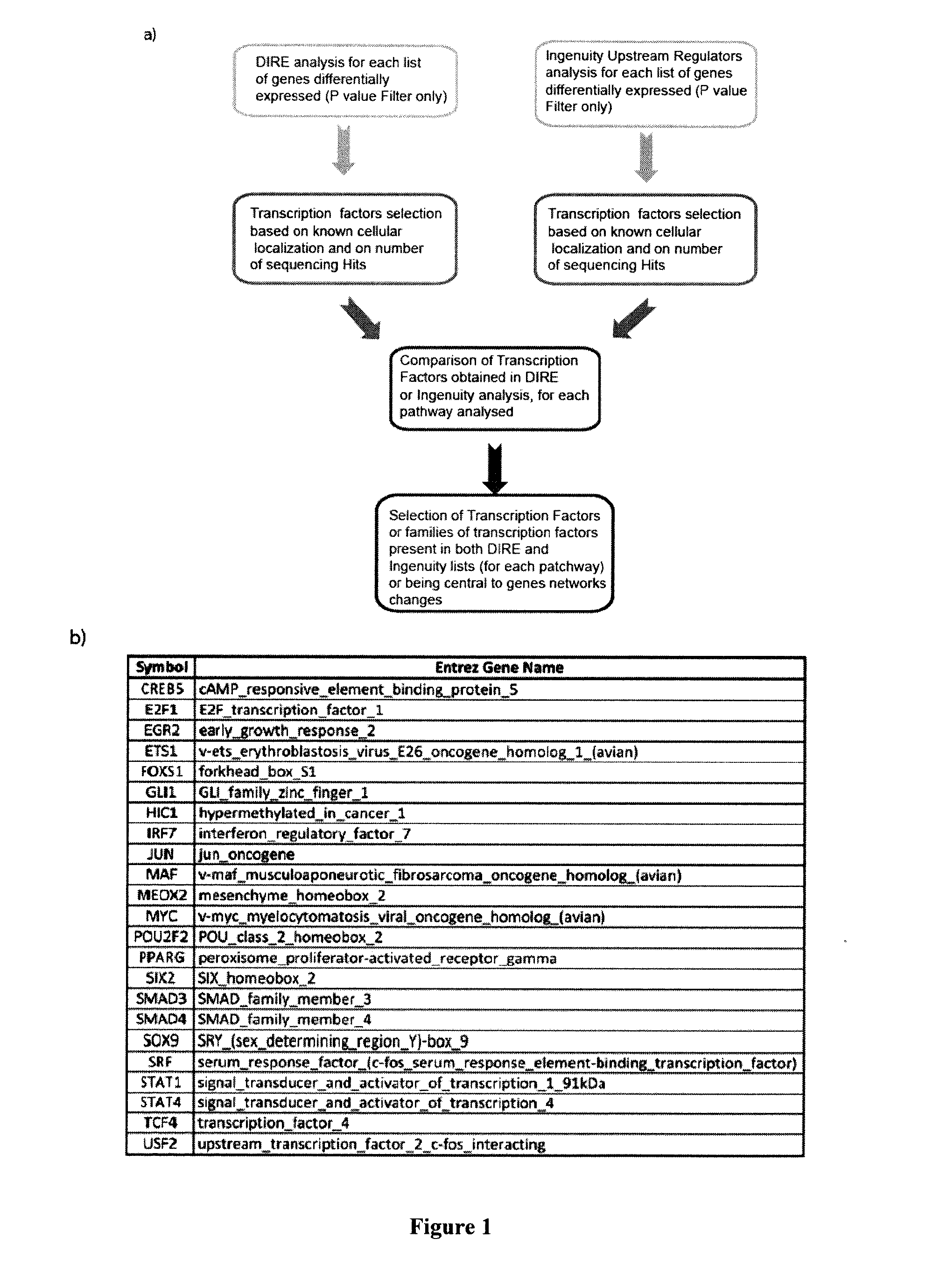
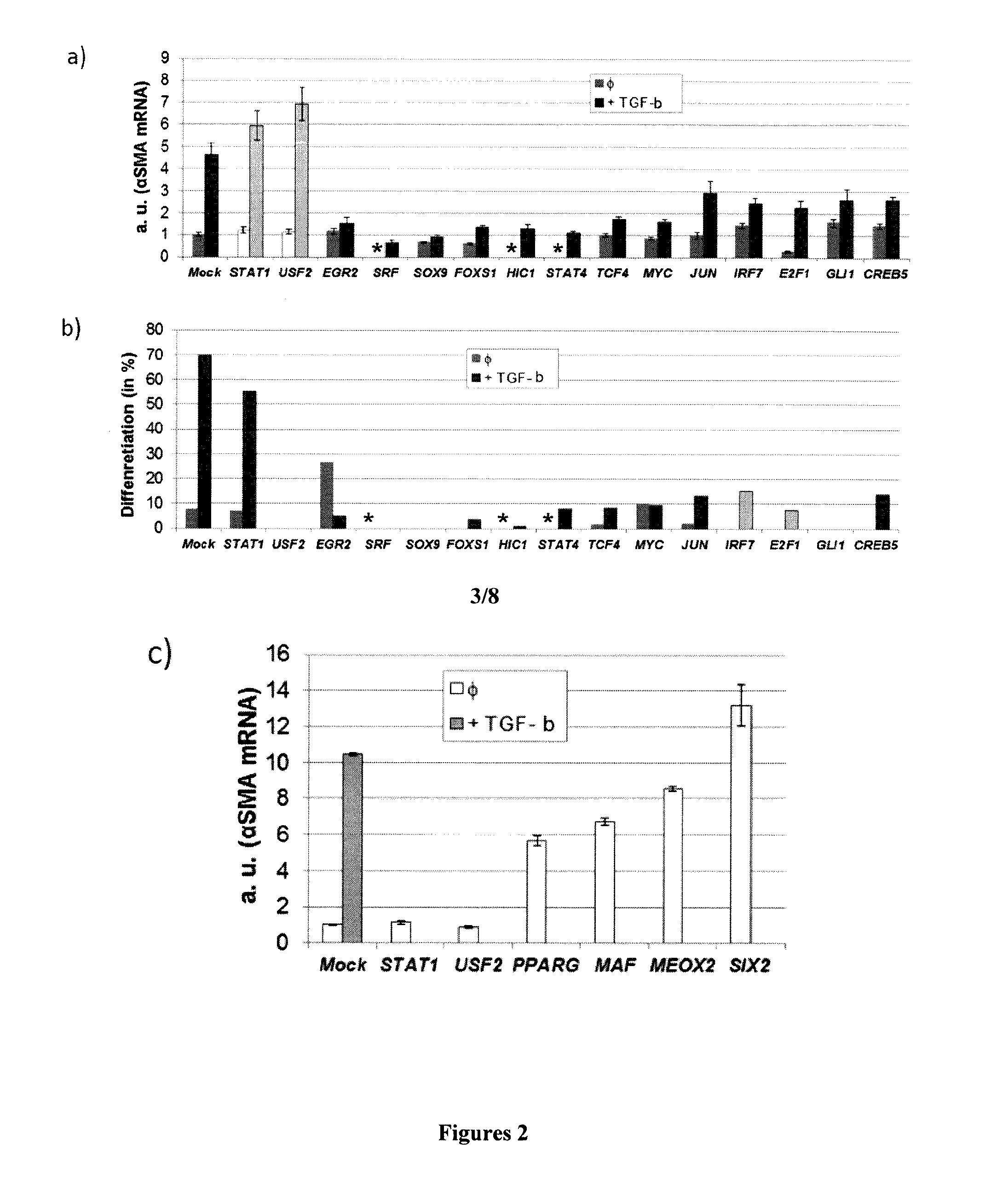
Molecular targets for the prevention and/or treatment of fibrosis, hypertrophic scars or keloids
InactiveUS20160168565A1High expressionEnhances the activity of at least one geneOrganic active ingredientsPeptidesKeloidIRF7
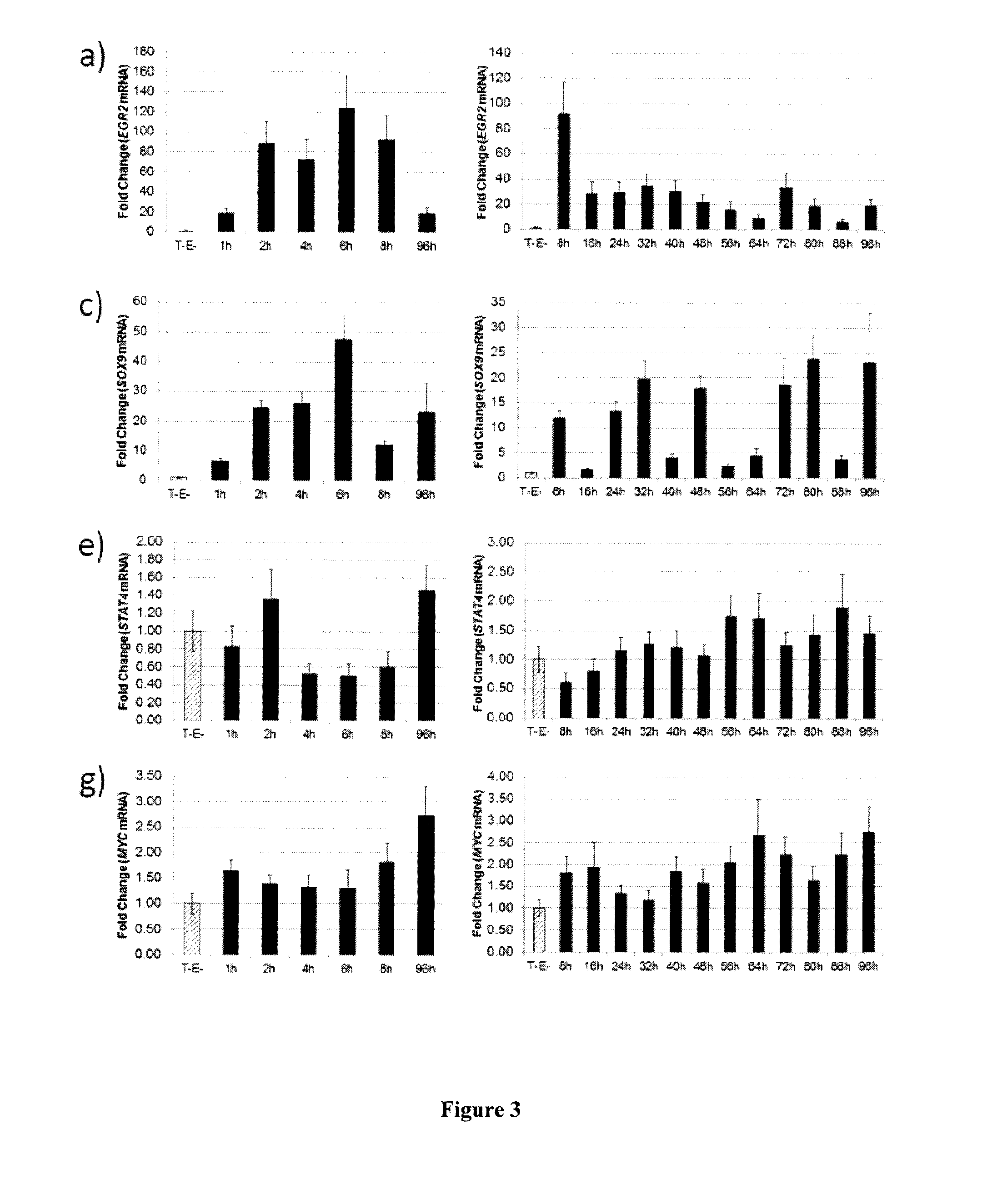
The present invention relates to a therapeutic compound comprising: an agent that inhibits the activity of at least one gene selected from the group consisting of HIC1, FOXS1, CREB5, IRF7, POU2F2, STAT4, TCF4, and / or an agent that enhances the activity of at least one gene selected from the group consisting of MAF, MEOX2, SIX2.
Owner:ECOLE NORMALE SUPERIEURE +4
Mixture of betamethasone and tranilast with a transdermal gel for scar treatment
ActiveUS9173940B1Suppressing collagen synthesisInhibit expressionOrganic active ingredientsPeptide/protein ingredientsBetamethasone valerateOleic Acid Triglyceride
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Prescription and preparation method for tranilast gel and ointment
InactiveCN104784101AIncrease drug concentrationFully absorbedOrganic active ingredientsAerosol deliveryTherapeutic effectAugmented Ointment
Belonging to the field of medical technologies, the invention relates to a prescription and preparation method for topical gel and ointment dosage forms containing tranilast component, and therapeutic effects of the dosage forms in keloid and hypertrophic scar fields. The topical gel and ointment contain tranilast, a cosolvent, a substrate, a humectant, a penetration enhancer, a preservative, a pH regulator, purified water and other ingredients. The preparation method includes the steps of: 1. taking a prescribed amount of the substrate, adding a proper amount of purified water, performing full swelling at 20-80DEG C, adjusting the pH to less than 5, adding a prescribed amount of humectant, and conducting heat preservation to obtain A; 2. taking a prescribed amount of tranilast, the cosolvent, the preservative, and a proper amount of purified water, carrying out heating dissolving at 20-80DEG C, and performing heat preservation to obtain B; and 3. Pouring B into A, conducting heat preservation and swelling, and stirring the mixture evenly, thus obtaining the products. The tranilast gel and ointment provided by the invention have the pharmacological activity of treating hypertrophic scars and keloids.
Owner:药大制药有限公司
Putrescine slow-release topical formulations
InactiveUS20190298688A1Effective penetrationEfficient deliveryCosmetic preparationsOrganic active ingredientsWound healingVitamin C
The present invention describes slow-release compositions for the effective delivery of active ingredients such as putrescine and Vitamin C into the skin. These compositions may be used in a variety of cosmetic and therapeutic applications including for promoting wound healing, for reducing or preventing the formation of hypertrophic scar tissue, for reducing or preventing skin irritation and inflammation and / or for reducing or preventing skin's signs of aging.
Owner:VIVIER CANADA
Method and composition for the treatment of scars
InactiveUS7731983B2Prevent and reduce scarringIncrease in sizeBiocideCosmetic preparationsAdditive ingredientBiomedical engineering
A method and composition for treating healed wounds including hypertrophic scars so as to prevent scarring or reduce the size and improve the appearance of scars comprises applying to the healed wound or scar a composition comprising a fluid, film-forming carrier which may contain one or more active topical ingredients, the carrier hardening to a solid, tangible, membrane juxtaposed to the skin. Novel compositions using the film-forming carrier are also useful to treat a variety of adverse skin disorders, and internal physiological conditions.
Owner:STUDIN JOEL R
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com