Application of benzopyran alkaloid
A benzopyran alkaloid, benzopyran biological technology, applied in the field of medicine to achieve the effect of weakening the shrinkage ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
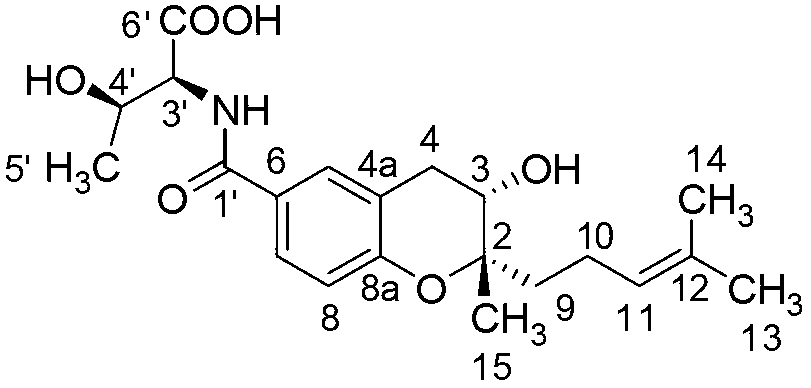
[0021] Example 1 Separation and purification of benzopyran alkaloid (compound 1)
[0022] Take the S.xiamenisis strain for plate culture (30 liters) for 7 days. After collecting and chopping the plate agar, extract it with a mixed solvent of equal volume ethyl acetate:methanol:formic acid=80:15:5 (volume ratio) 3 times, 12 hours each time. The extracts were combined and concentrated to obtain 12 grams of the total extract. Take the total extract (6 grams), dissolve it with an appropriate amount of chloroform-methanol mixed solvent, add 10 grams of 200-300 mesh silica gel G (product of Qingdao Ocean Chemical Group Co., Ltd.) On the column, chloroform-methanol [100:1, 50:1, 20:1, 10:1, 5:1, (volume ratio) to methanol] gradient elution, the polarity of the elution solvent can be increased by increasing the methanol in chloroform Gradient increase in the amount of each eluted fraction received 200 ml respectively, and the combined fractions were detected according to thin layer ...
Embodiment 2
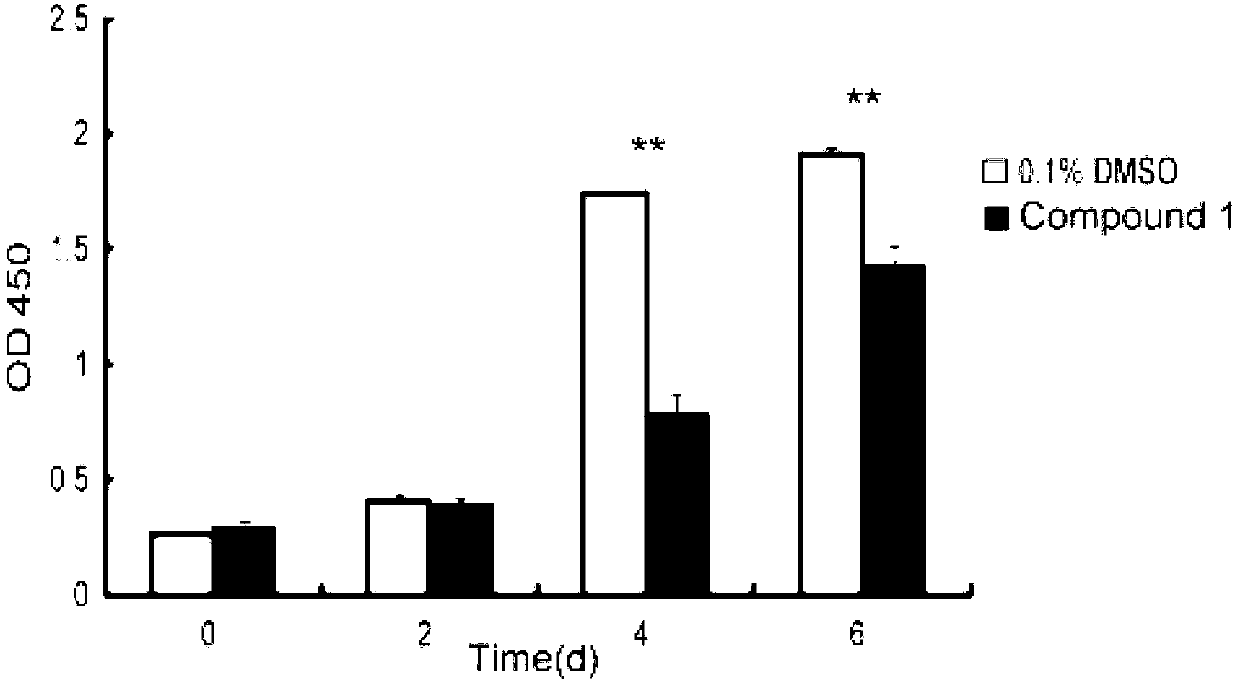
[0027] Example 2 Benzopyran alkaloids inhibit the proliferation and viability of normal human skin fibroblasts
[0028] The materials are as follows:
[0029] Cells: normal human skin fibroblasts (NHSF cells); drug: compound 1 obtained from the above examples.
[0030] Method: NHSF cells grown to 70-80% confluence were digested with 0.25% trypsin and seeded in 96-well plate, 1×10 per well 3 cells. After 24 hours, the medium was changed and the drug was added. The final concentration of compound 1 in the drug treatment group was 30 μg / ml, and the solvent DMSO concentration was 1 / 10000. The control group was DMSO, and the final concentration was 1 / 10000. The culture medium and drugs were changed every two days. Cell viability was measured on 0d, 2d, 4d, and 6d after adding the drug. The measurement method is aspirating out the medium, adding 100 μl of complete medium and 10 μl of CCK-8 reagent to each well, and incubating in the incubator for 60 minutes to measure the light ...
Embodiment 3
[0032] Example 3 Benzopyran alkaloids block the adhesion experiment of human normal skin dermis-derived fibroblasts and human monocytes / macrophages
[0033] The materials are as follows:
[0034] Cells: normal human skin fibroblasts (NHSF cells); human monocyte / macrophage (Human acute monocytic leukemia cell line, THP-1); drug: compound 1 obtained from the above examples.
[0035] Method: NHSF cells were inoculated into 24-well plates, and 500 μl of cell suspension was inoculated in each well at a density of 1×10 5 / ml, fully confluent after 3 days of culture. Centrifuge THP-1 cells 24 hours before co-cultivation and resuspend with DMEM plus 10% FBS and 1% double antibody. Suspended to make THP-1 cell density 5×10 4 / ml. Aspirate the supernatant of NHSF cells, wash once with PBS, add 500 μl of THP-1 suspension to each well, add 318 to each well of the drug treatment group to make the final concentration 30 μg / ml, the solvent DMSO concentration is 1 / 10000, and the control gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com