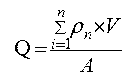Liposome gel preparation and preparation method and application thereof
A technology of liposome gel and liposome, which is applied in the direction of liposome delivery, antibody delivery, liquid delivery, etc. It can solve the problems of prominent toxic and side effects, affecting the metabolism and activity of skin tissue cells, etc., achieving less toxic and side effects, regulating Skin immune cytokines, the effect of improving poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
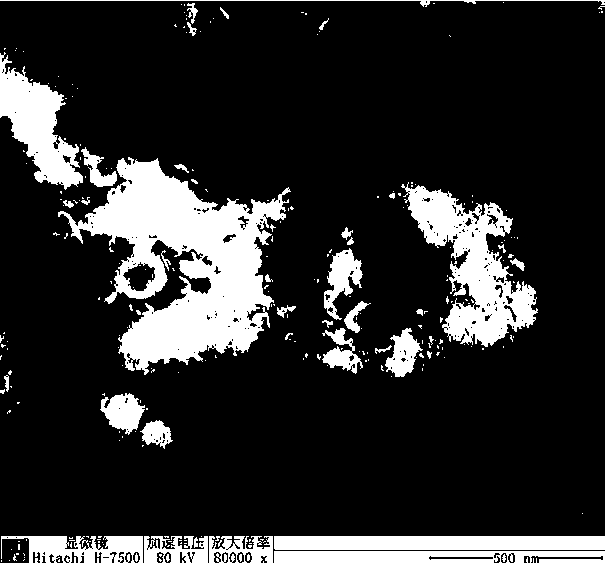
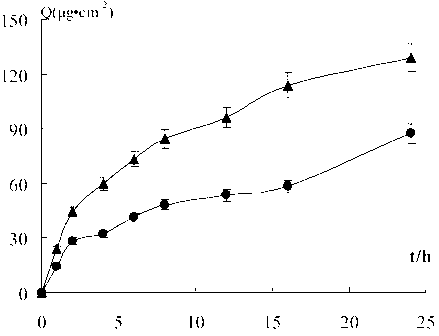
Image
Examples
Embodiment 1
[0036] Embodiment 1: Paeonol transdermal gel preparation
[0037] Formula: paeonol 40mg, egg yolk lecithin 400mg, ethanol 8mL, cholesterol 200mg, Avastin-PEG-DOPE 20mg, phosphate buffer (pH 7.4) 10mL, carbomer 934 3g, glycerin 0.3g.
[0038] Preparation method: Dissolve paeonol in ethanol, add egg yolk lecithin, cholesterol and Avastin-PEG-DOPE, heat and stir in a water bath at 50-60°C to dissolve, and form a clear solution. Slowly inject into phosphate buffer (pH 7.4) in liquid flow through a syringe needle with a fine pore size, magnetically stir, the stirring speed is controlled at 300rpm, and N is introduced during the injection process. 2 Remove most of the ethanol. The resulting liquid was ultrasonically pulverized 3 times, each time for 1 minute, at a frequency of 300 Hz. Add Carbomer 934 to the above liposome solution, after the swelling is complete, add glycerin, grind evenly, pack separately, apply on the mounting material, and sterilize to get the product.
Embodiment 2
[0039] Embodiment 2: Paeonol transdermal gel preparation
[0040] Formula: paeonol 42mg, hydrogenated soybean lecithin 400mg, ether 5mL, cholesterol 200mg, Avastin-PEG-DOPE 20mg, phosphate buffer (pH 7.4) 10mL, carbomer 980 2.5g, glycerol 0.3g.
[0041] Preparation method: Dissolve paeonol with ether, add hydrogenated soybean lecithin, cholesterol and Avastin-PEG-DOPE to dissolve and form a clear solution. Slowly inject into phosphate buffer (pH 6.5) in liquid flow through a syringe needle with a fine pore diameter, stir magnetically at a speed of 300 rpm, form a milky white liquid, and then ultrasonically pulverize 3 times, each time for 1 minute, with a frequency of 300 Hz. Add Carbomer 980 into the above liposome solution, after the swelling is complete, add glycerin, grind evenly, pack separately, apply on the mounting material, and sterilize to get the product.
Embodiment 3
[0042] Embodiment 3: Paeonol transdermal gel preparation
[0043] Formula: paeonol 4g, hydrogenated soybean lecithin 40g, ethanol 800mL, cholesterol 20g, Avastin-PEG-DOPE 2g, phosphate buffer (pH 7.4) 1 L, poloxamer 188 2Kg, glycerin 300g.
[0044] Preparation method: Dissolve paeonol in ethanol, add hydrogenated soybean lecithin, cholesterol and Avastin-PEG-DOPE, heat and stir in a water bath at 50-60°C to dissolve, and form a clear solution. Slowly inject into phosphate buffer (pH 7.4) in liquid flow through a micropipeline with a fine pore size, magnetically stir, the stirring speed is controlled at 250rpm, and N is introduced during the injection process. 2 Remove most of the ethanol. The obtained liquid was high-pressure emulsified three times under the condition of 10 bar to obtain a liposome solution. Add poloxamer 188 into the above liposome solution, after the swelling is complete, add glycerin, grind evenly, dispense, apply on the mounting material, and sterilize...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com