Tumor cell nucleus-targeted drug-loaded nanoparticles containing functional polypeptide-modified heptamethanocyanine dyes and preparation method thereof
A technology of Qijiachuan cyanine and functional polypeptide, which is applied in the field of medicine to achieve the effects of improving optical stability, improving photothermal performance, and compact structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
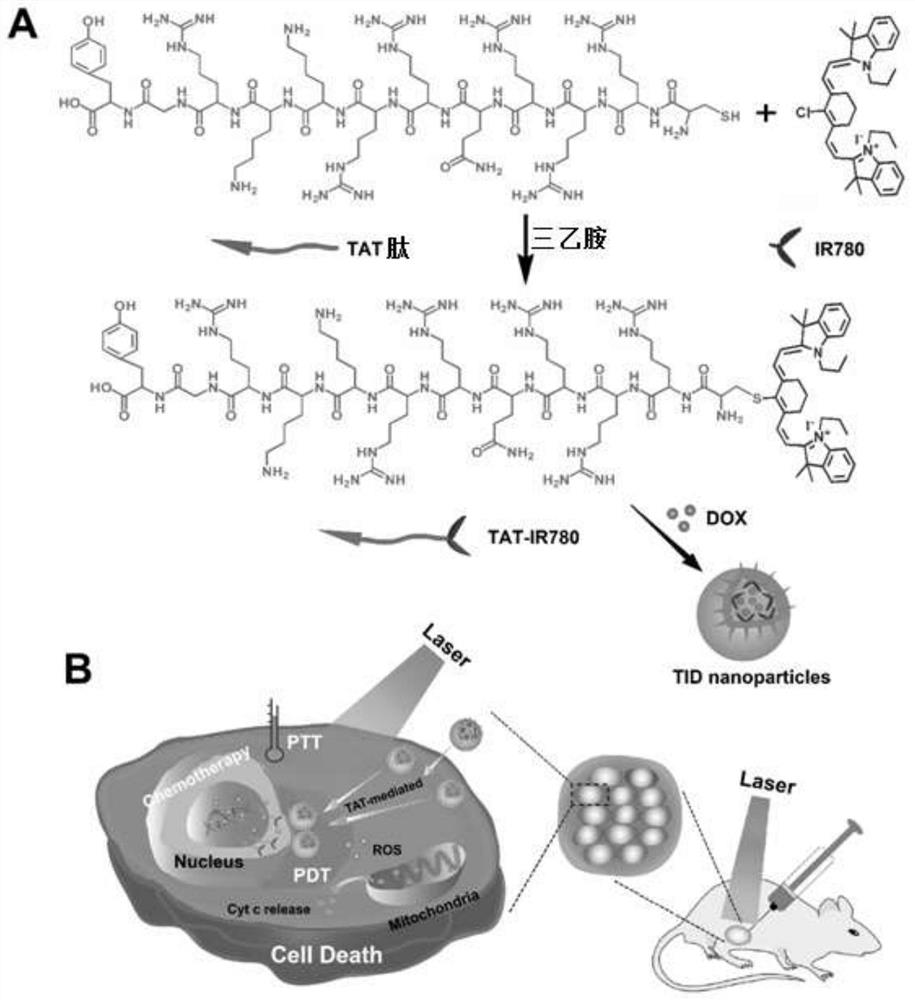
[0069] 1. Synthesis and characterization of TAT-IR780
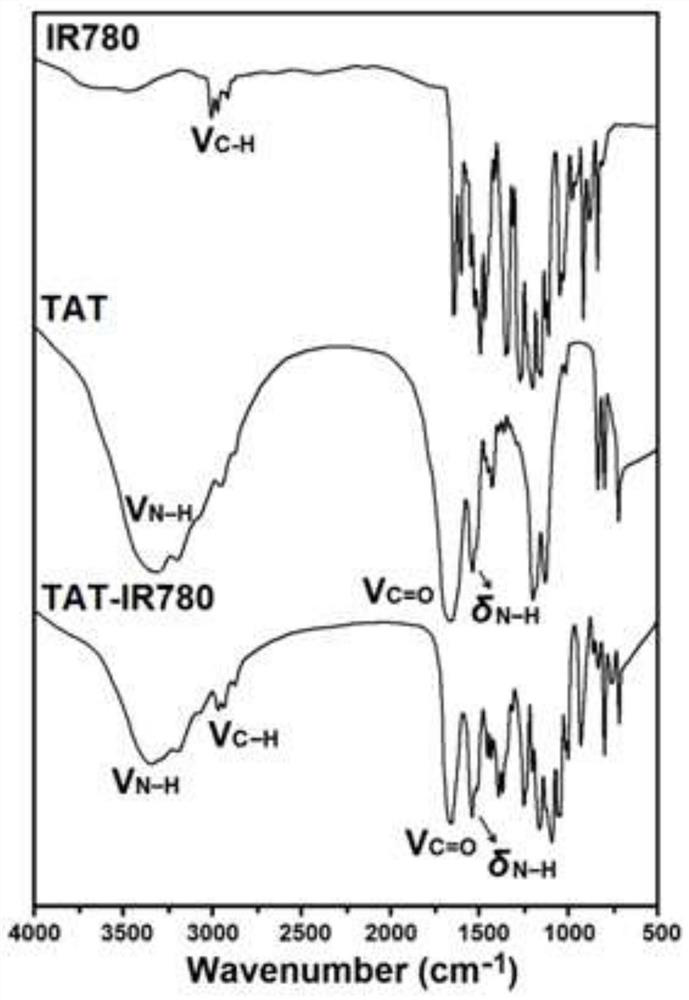
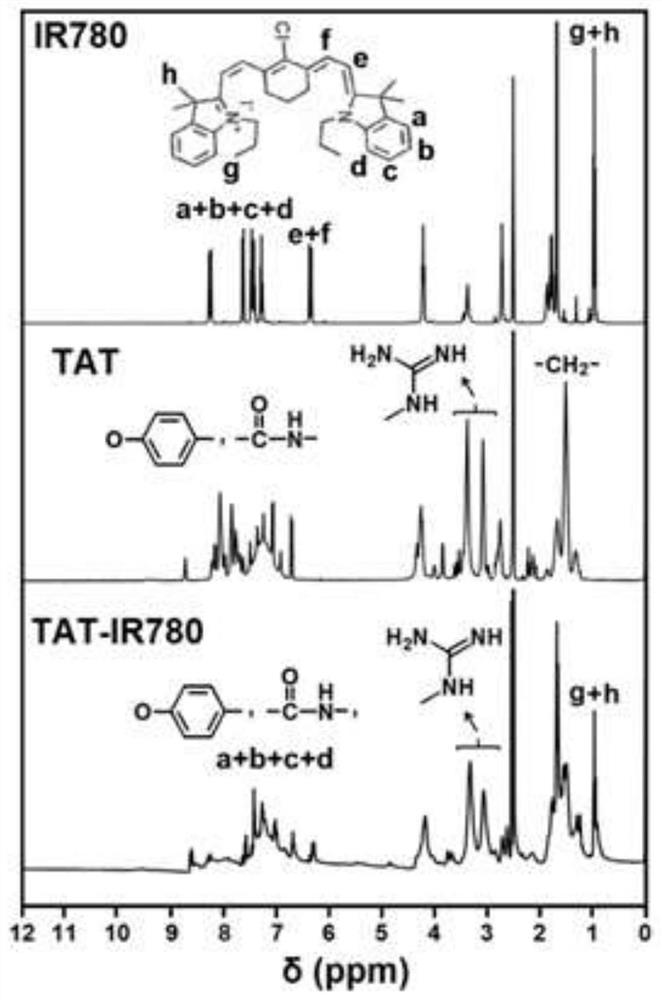
[0070] Dissolve TAT (120mg, 72μmol), IR780 (40mg, 60μmol) and triethylamine (15μL, 108μmol) in 12mL of dimethyl sulfoxide (DMSO), and let the reaction system flow with nitrogen gas, and stir the reaction at 40°C for 72h in the dark. , and then transfer the reaction solution to a dialysis bag (molecular weight cut-off of 500Da), and dialyze in ultrapure water for 24 hours, change the outer dialysate every 6 hours, remove unreacted TAT polypeptide, and obtain dark green powdery diisocyanate after freeze-drying. Joint product TAT-IR780. Synthetic route such as figure 1 Shown, the chemical structure of TAT-IR780 passes IR spectrum ( figure 2 ), 1 H NMR spectrum ( image 3 ) and mass spectrometry ( Figure 4 ) to characterize. The final synthesized TAT-IR780 obtained in the IR spectrum and 1 The H NMR spectrum has the characteristic peaks of IR780 and TAT at the same time. According to the molecular ion peak of the mas...
Embodiment 2
[0102] 1. Synthesis of penetrating peptide R9 coupled to IR780
[0103] R9 (sequence RRRRRRRRRC) polypeptide (110mg, 72μmol), IR780 (40mg, 60μmol) and triethylamine (15μL, 108μmol) were dissolved in 12mL dimethyl sulfoxide (DMSO), and the reaction system was ventilated with nitrogen gas flow, under dark conditions Stir and react at 40°C for 72 hours, then transfer the reaction liquid to a dialysis bag (molecular weight cut-off: 500 Da), and dialyze in ultrapure water for 24 hours, replace the outer dialysate every 6 hours, remove unreacted R9 polypeptide, and freeze-dry to obtain Dark green powder conjugate R9-IR780.
[0104] 2. Preparation and characterization of drug-loaded nanoparticle RID of R9-IR780 efficiently loaded with anthracycline chemotherapy drug DOX
[0105] Dissolve the anthracycline chemotherapy drug doxorubicin hydrochloride (DOX HCl) in methanol, add TEA according to 3 equivalents and stir for 8 hours, carry out desalination treatment, then rotary evaporate ...
Embodiment 3
[0107] 1. Synthesis of TAT-IR780
[0108] Dissolve TAT (120mg, 72μmol), IR780 (40mg, 60μmol) and triethylamine (15μL, 108μmol) in 12mL of dimethyl sulfoxide (DMSO), and let the reaction system flow with nitrogen gas, and stir the reaction at 40°C for 72h in the dark. , and then transfer the reaction solution to a dialysis bag (molecular weight cut-off of 500Da), and dialyze in ultrapure water for 24 hours, change the outer dialysate every 6 hours, remove unreacted TAT polypeptide, and obtain dark green powdery diisocyanate after freeze-drying. Joint product TAT-IR780.
[0109] 2. Preparation of drug-loaded nanoparticle TIE of TAT-IR780 efficiently loading anthracycline chemotherapy drug epirubicin (EPB)
[0110] Dissolve the anthracycline chemotherapeutic drug epirubicin hydrochloride (EPB·HCl) in methanol, add TEA according to 3 equivalents and stir for 8 hours, carry out desalination treatment, then remove the methanol by rotary evaporation, and then redissolve in DMSO, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com