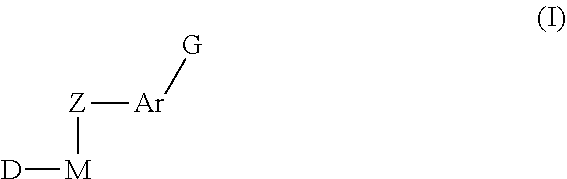Patents
Literature
124 results about "VEGF receptors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
VEGF receptors are receptors for vascular endothelial growth factor (VEGF). There are three main subtypes of VEGFR, numbered 1, 2 and 3. Also, they may be membrane-bound (mbVEGFR) or soluble (sVEGFR), depending on alternative splicing.
Anti-human VEGF receptor Flt-1 monoclonal antibody
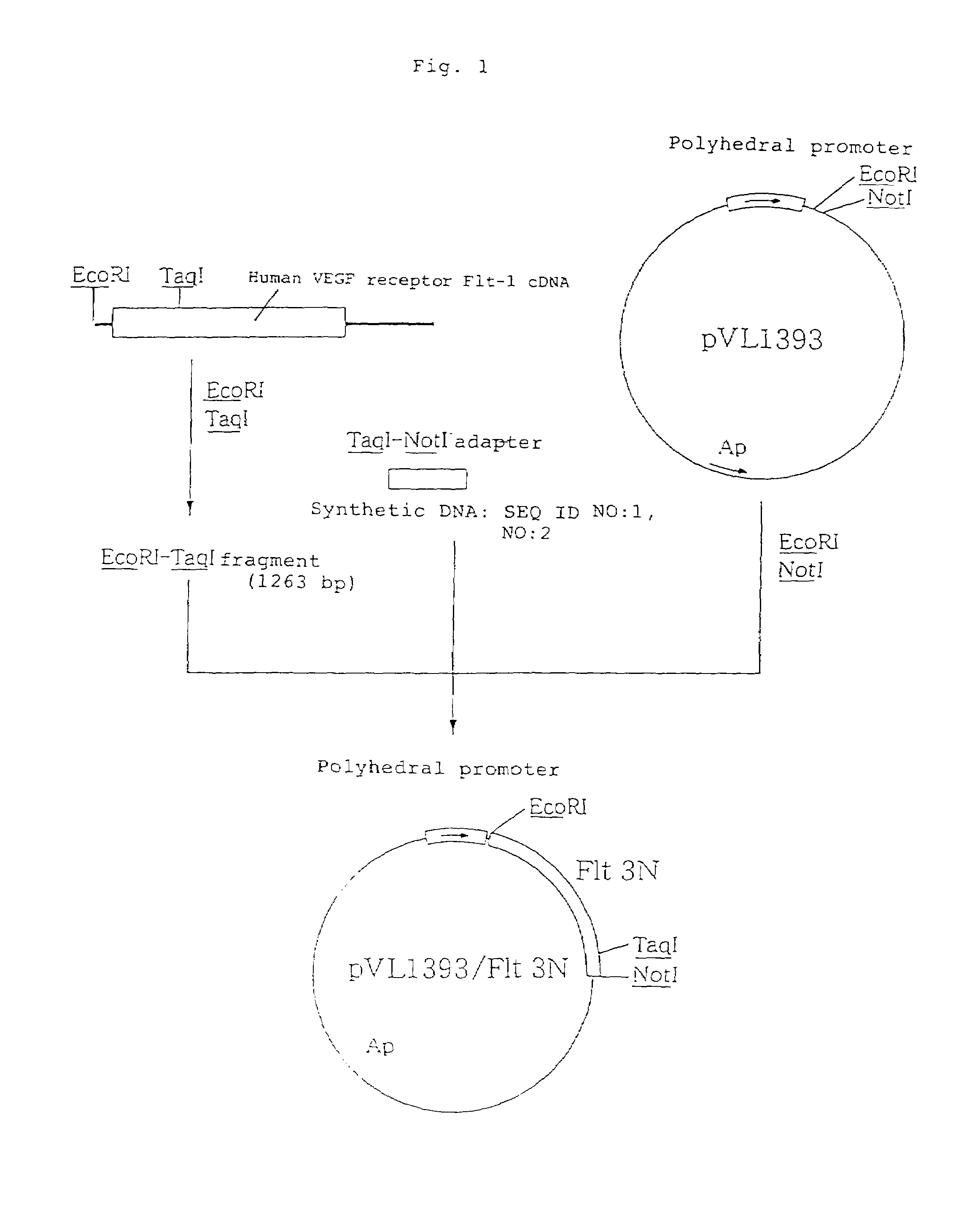
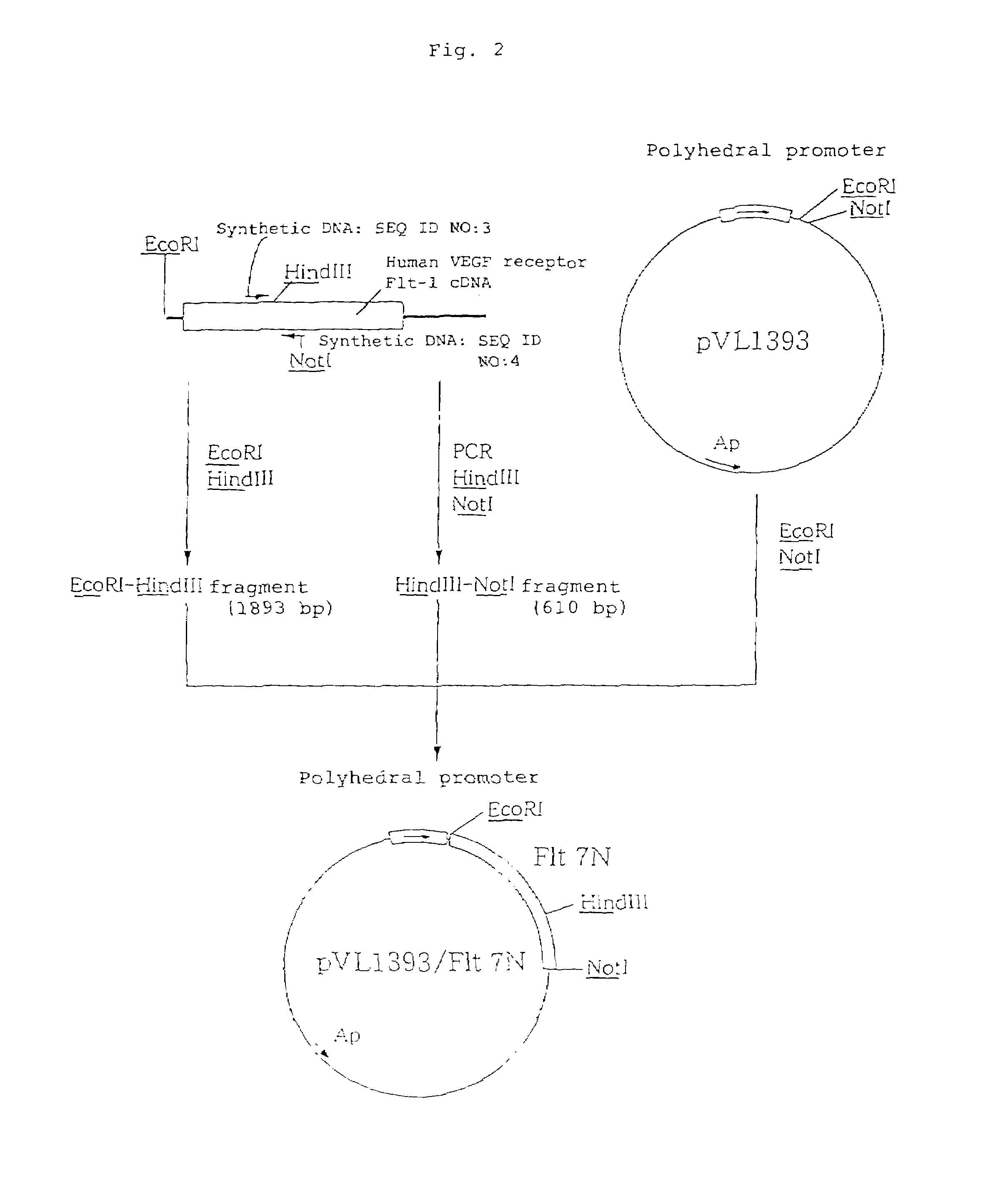
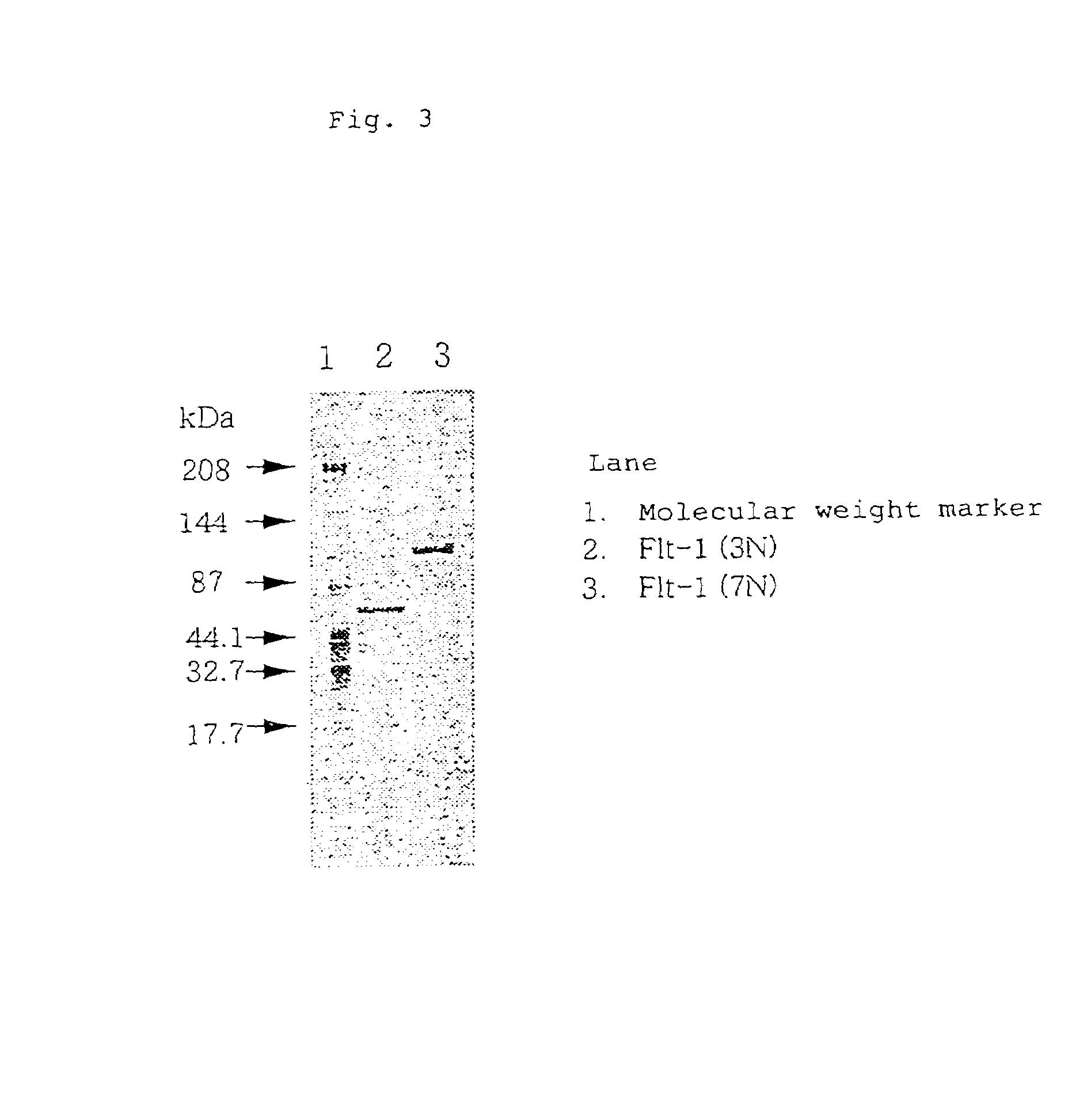
The present invention provides an antibody or peptide which immunologically reacts with human VEGF receptor Flt-1 and cells in which human VEGF receptor Flt-1 is expressed on the cell surface and an antibody or peptide which inhibits binding of human VEGF to human VEGF receptor Flt-1. It also provides a means for the diagnosis or treatment of diseases in which their morbid states progress by abnormal angiogenesis, such as proliferation or metastasis of solid tumors, arthritis in rheumatoid arthritis, diabetic retinopathy, retinopathy of prematurity, psoriasis, and the like.
Owner:KYOWA HAKKO KIRIN CO LTD
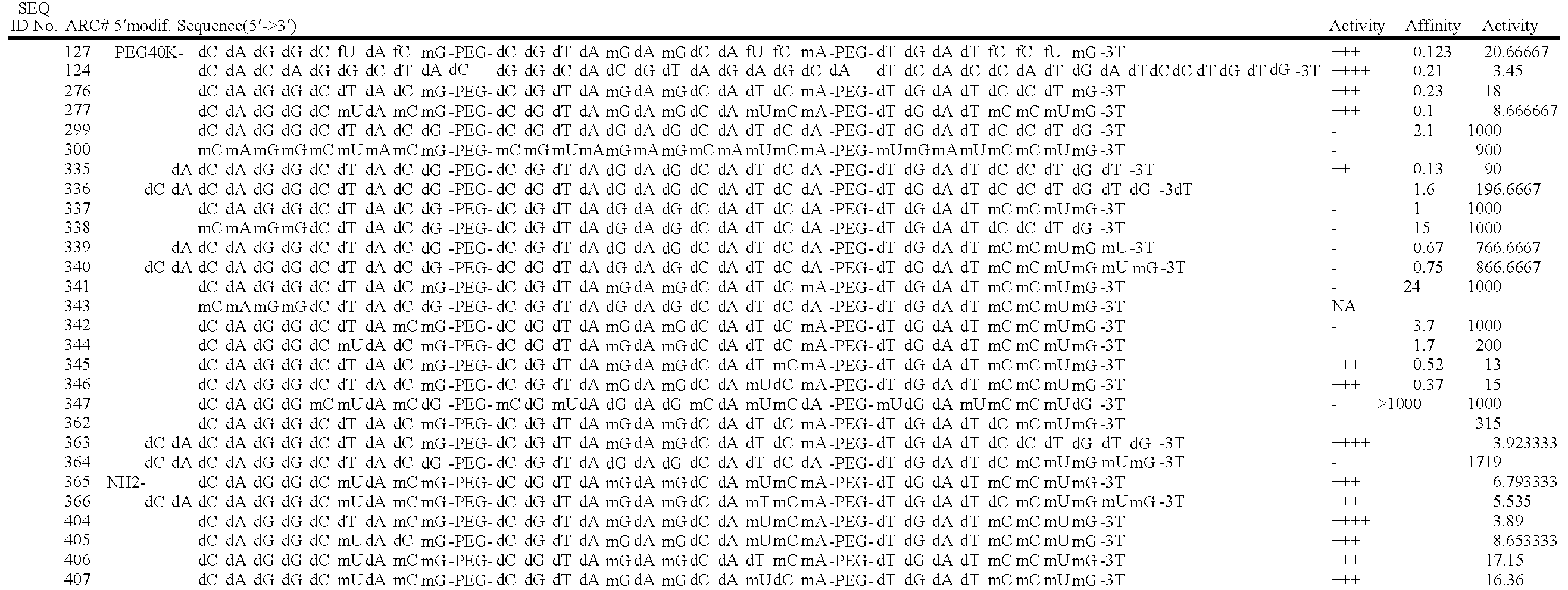
Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics
Materials and methods are provided for producing and using aptamers useful as oncology therapeutics capable of binding to PDGF, PDGF isoforms, PDGF receptor, VEGF, and VEGF receptor or any combination thereof with great affinity and specificity. The compositions of the present invention are particularly useful in solid tumor therapy and can be used alone or in combination with known cytotoxic agents for the treatment of solid tumors. Also disclosed are aptamers having one or more CpG motifs embedded therein or appended thereto.
Owner:ARCHEMIX CORP
Inhibitors of VEGF receptor and HGF receptor signaling
ActiveUS20070004675A1Promote motilityPromote invasionBiocideOrganic chemistryVEGF receptorsHGF Receptor
The invention relates to the inhibition of VEGF receptor signaling and HGF receptor signaling. The invention provides compounds and methods for inhibiting VEGF receptor signaling and HGF receptor signaling. The invention also provides compositions and methods for treating cell proliferative diseases and conditions
Owner:MIRATI THERAPEUTICS INC
Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics
Materials and methods are provided for producing and using aptamers useful as oncology therapeutics capable of binding to PDGF, PDGF isoforms, PDGF receptor, VEGF, and VEGF receptor or any combination thereof with great affinity and specificity. The compositions of the present invention are particularly useful in solid tumor therapy and can be used alone or in combination with known cytotoxic agents for the treatment of solid tumors. Also disclosed are aptamers having one or more CpG motifs embedded therein or appended thereto.
Owner:ARCHEMIX CORP
Inhibitors of protein tyrosine kinase activity
This invention relates to compounds that inhibit protein tyrosine kinase activity. In particular the invention relates to compounds that inhibit the protein tyrosine kinase activity of growth factor receptors, resulting in the inhibition of receptor signaling, for example, the inhibition of VEGF receptor signaling and HGF receptor signaling. More particularly, the invention relates to compounds, compositions and methods for the inhibition of VEGF receptor signaling and HGF receptor signaling. The invention also provides compositions and methods for treating cell proliferative diseases and conditions.
Owner:METHYLGENE
Inhibitors of VEGF receptor and hgf receptor signaling
ActiveUS20060287343A1Invasive growth is enhancedPromote invasionBiocideOrganic chemistryCompound (substance)VEGF receptors
The invention relates to the inhibition of VEGF receptor signaling and HGF receptor signaling. The invention provides compounds and methods for inhibiting VEGF receptor signaling and HGF receptor signaling. The invention also provides compositions and methods for treating cell proliferative diseases and conditions
Owner:METHYLGENE
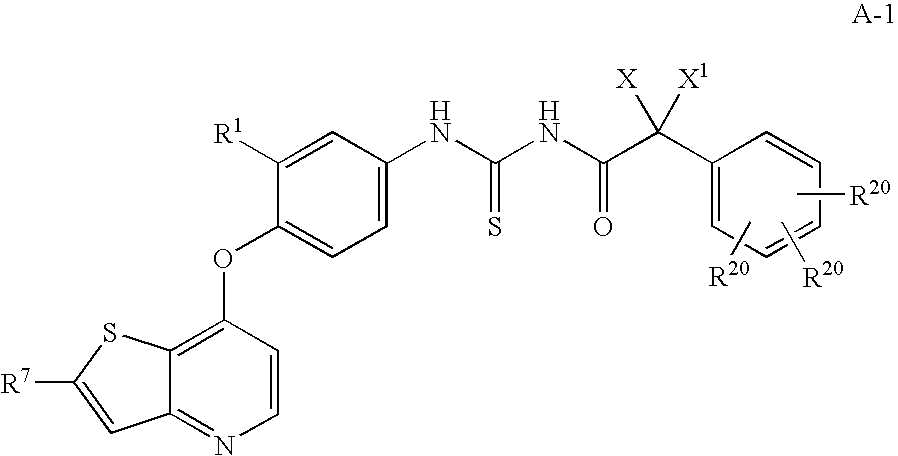
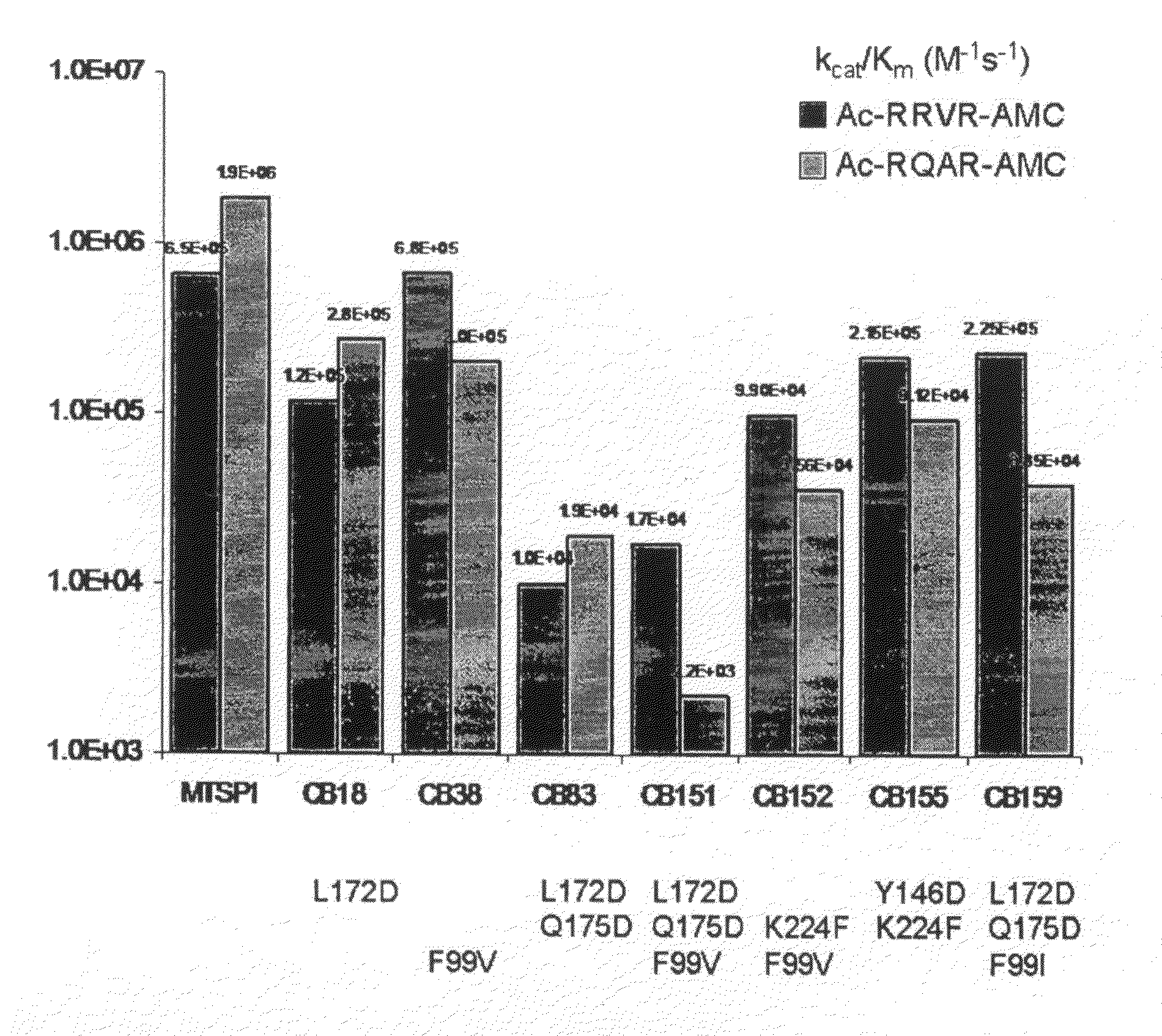
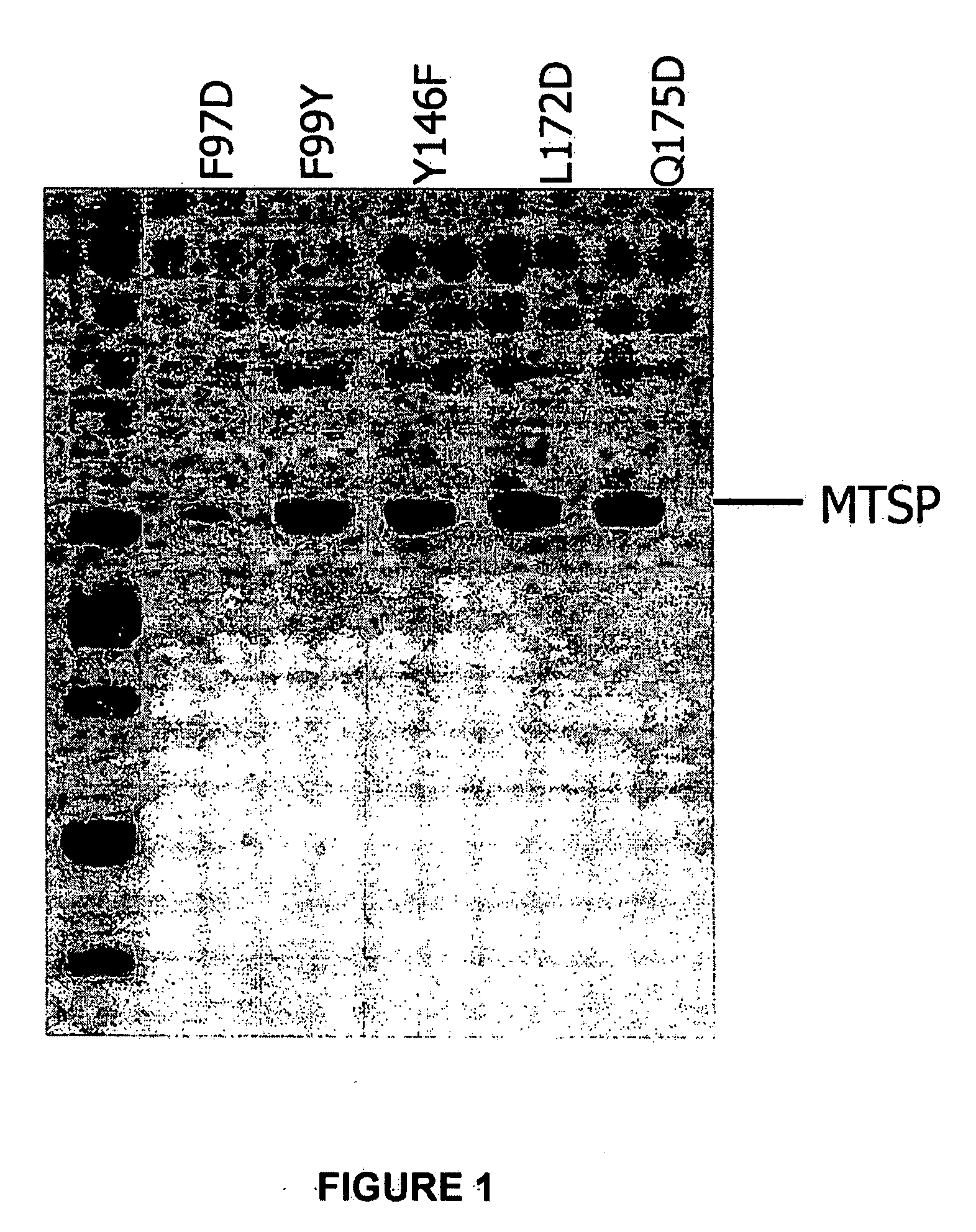
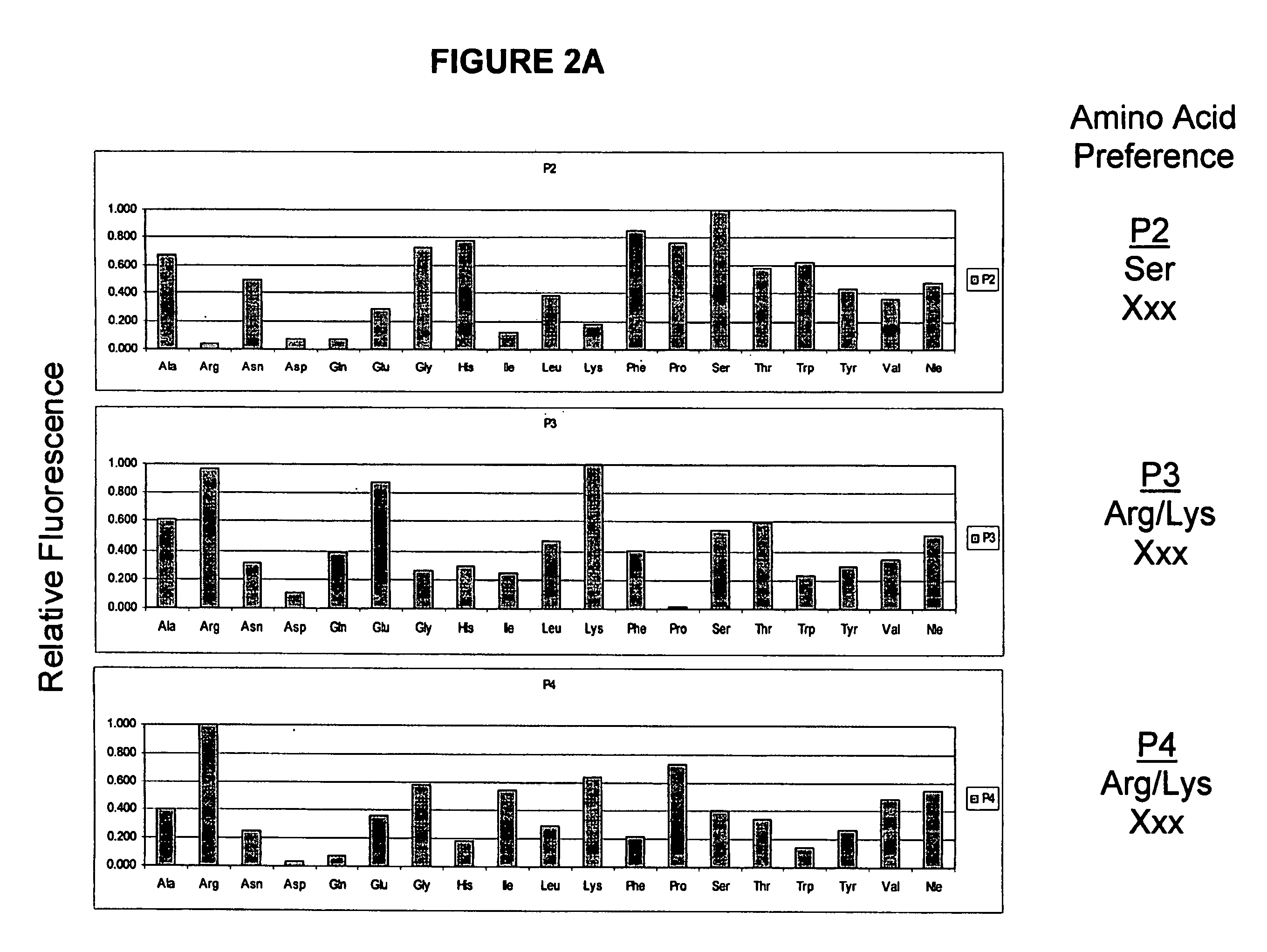
Cleavage of VEGF and VEGF receptor by wildtype and mutant MT-SP1
MT-SP1 mutein proteases with altered specificity for the target molecules they cleave can be used to treat human diseases, such as cancer. Cleaving VEGF or VEGFR at certain substrate sequences with wild-type and mutein MT-SP1 proteases can be used to treat pathologies associated with angiogenesis.
Owner:VERTEX PHARMA INC
VEGF receptor fusion proteins, their pharmaceutical compositions and therapeutic applications for the eye diseases
InactiveUS20090264358A1Improve stabilityImprove securitySenses disorderPeptide/protein ingredientsDiabetic retinopathyVEGF receptors
Vascular endothelial growth factor (VEGF) receptor fusion protein comprising Ig domain 2 of Flt-1 and Ig domains 3, or Ig domain 2 of Flt-1 and Ig domain 3 and 4 of KDR, the gene encoding the fusion protein, the pharmaceutical composition containing the fusion protein and the pharmaceutical use of the fusion protein are provided. The fusion protein can be used for treatment of eye disorders involving angiogenesis such as diabetic retinopathy.
Owner:CHENGDU KANGHONG BIOTECH
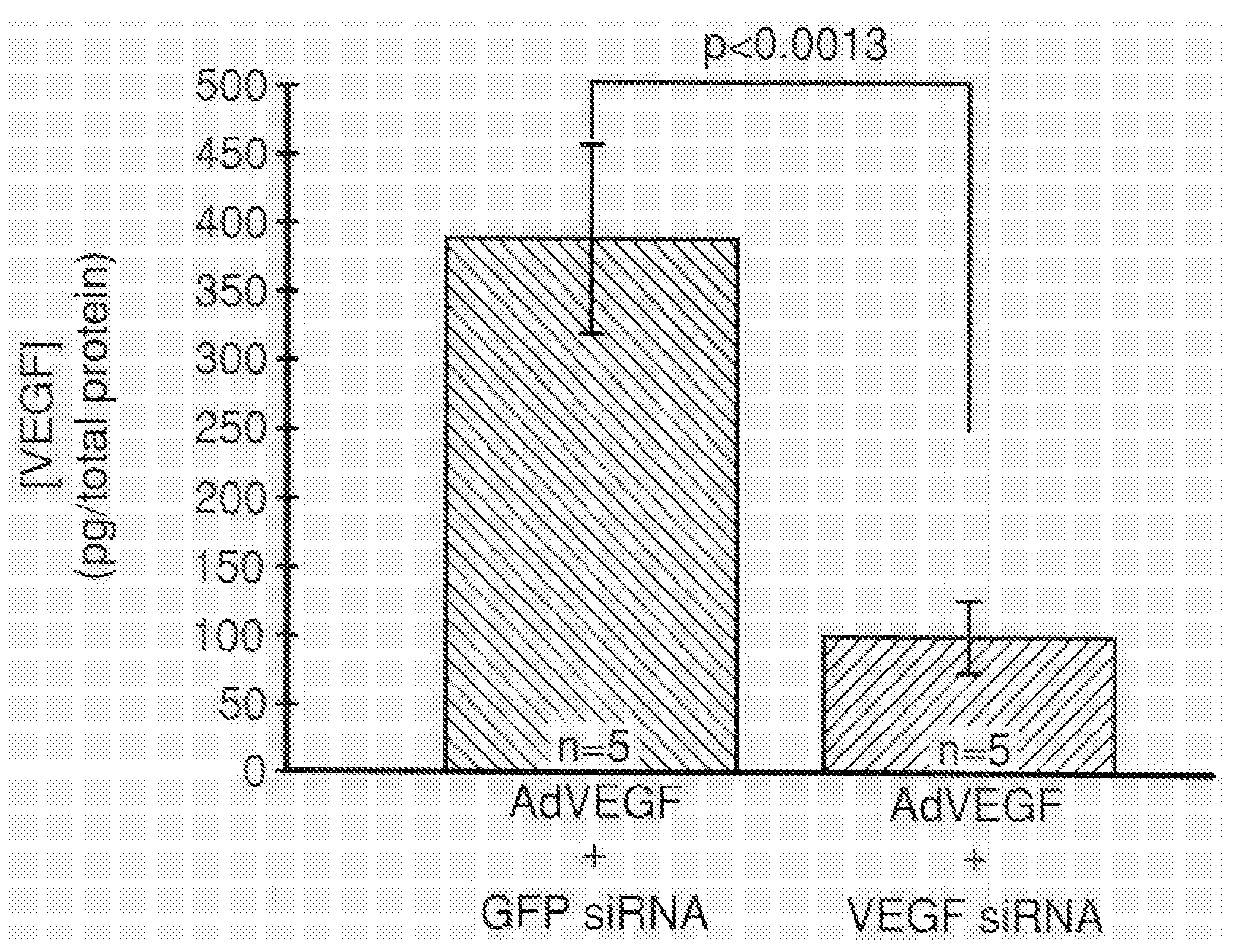
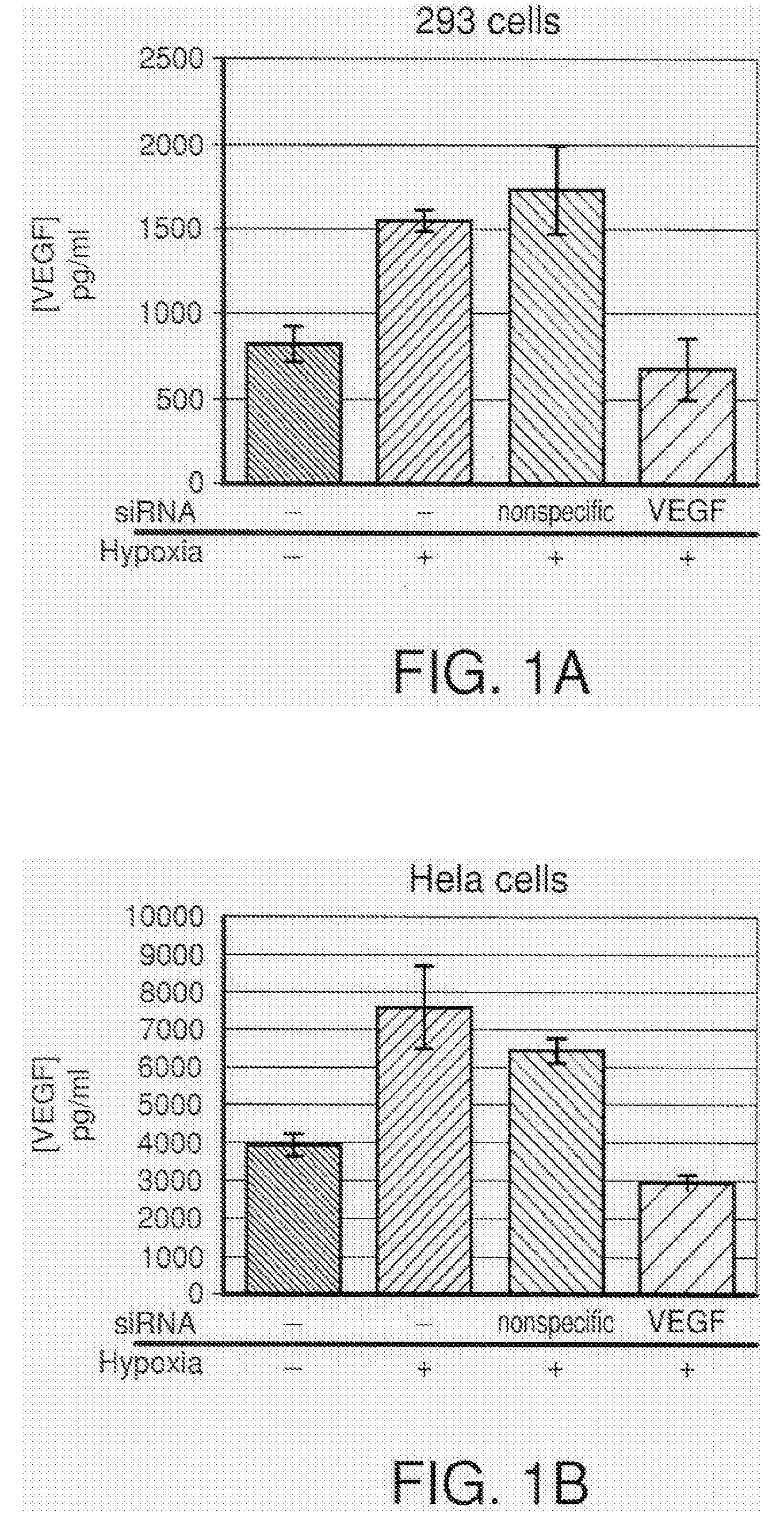
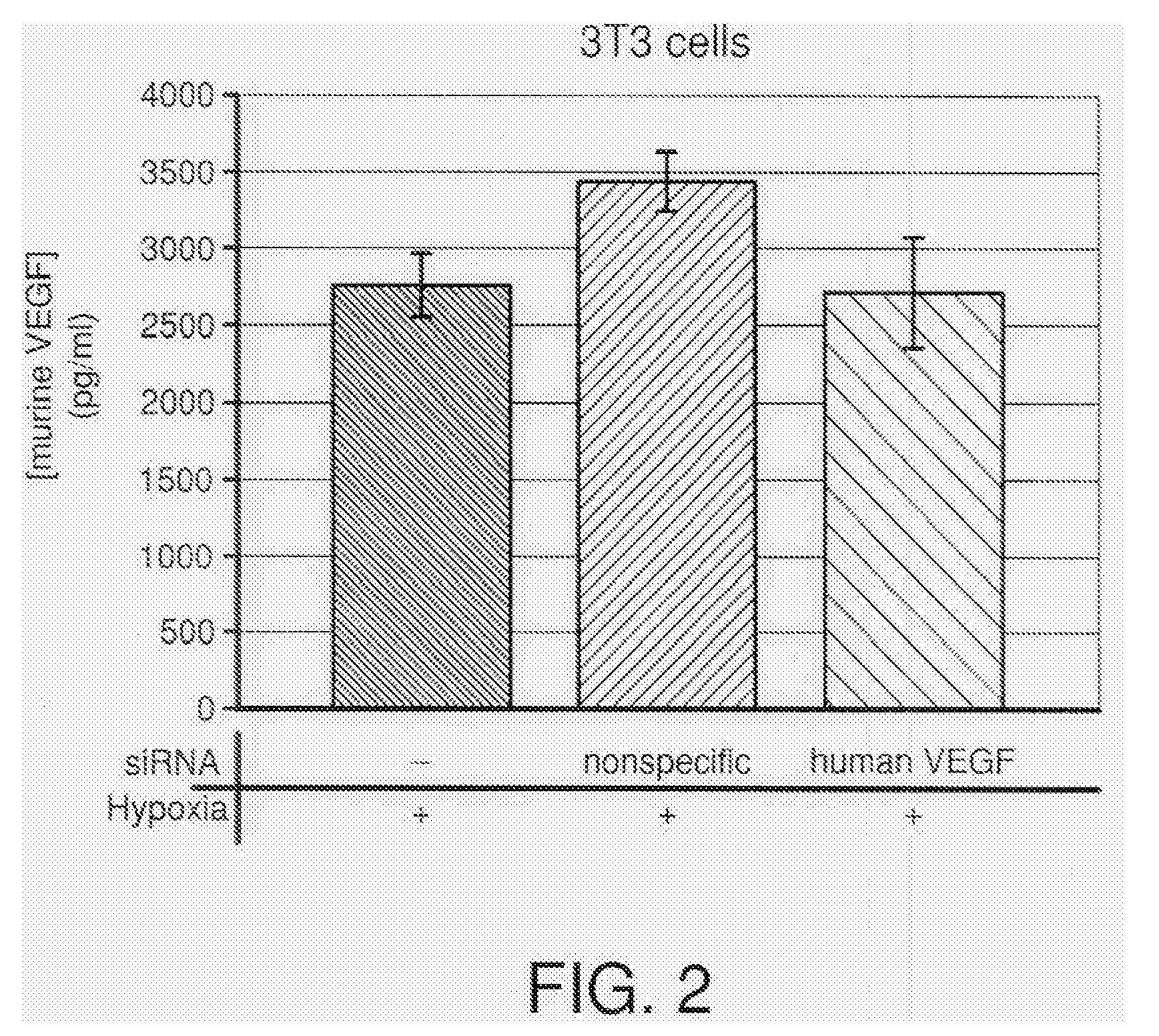
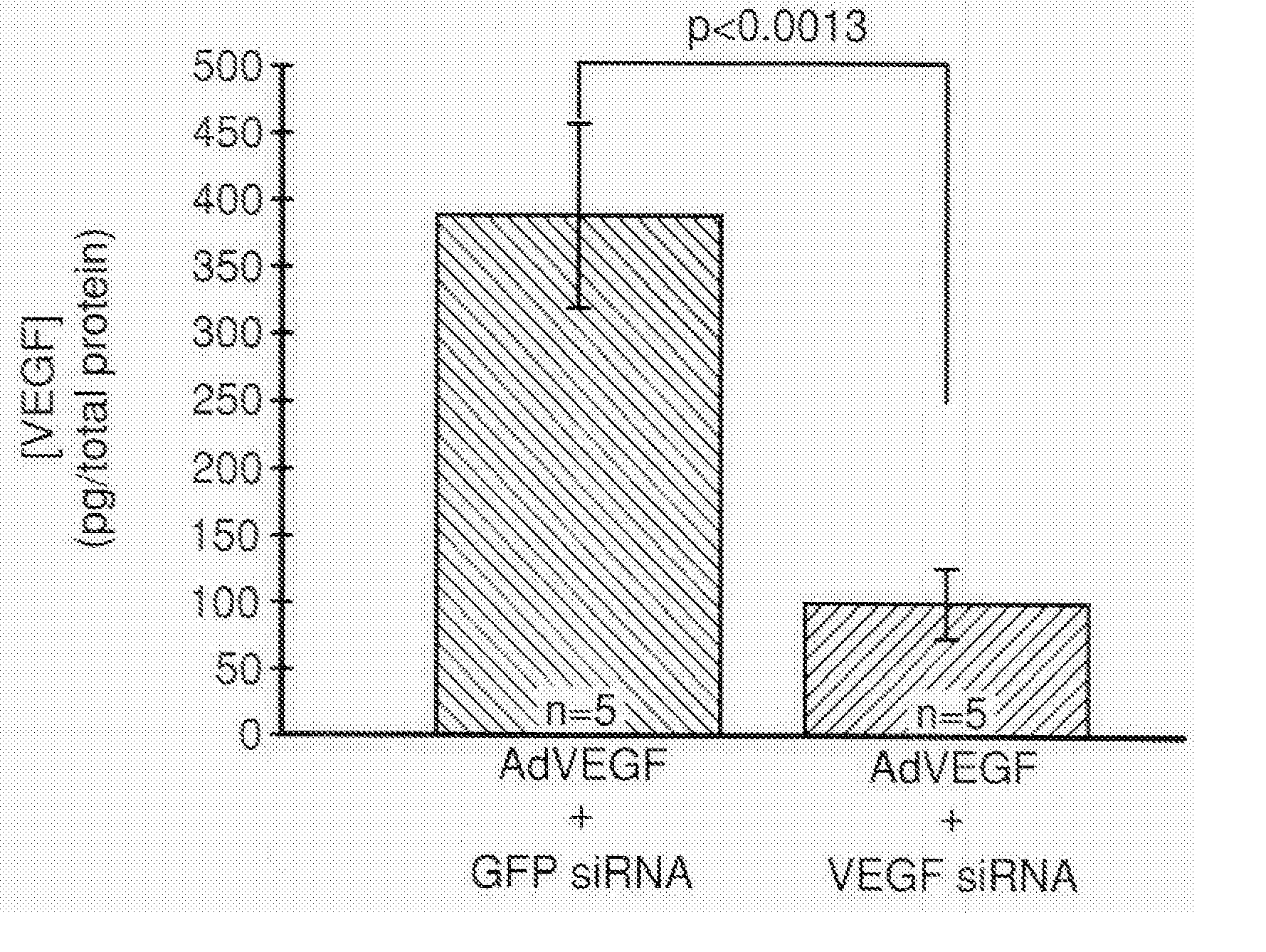
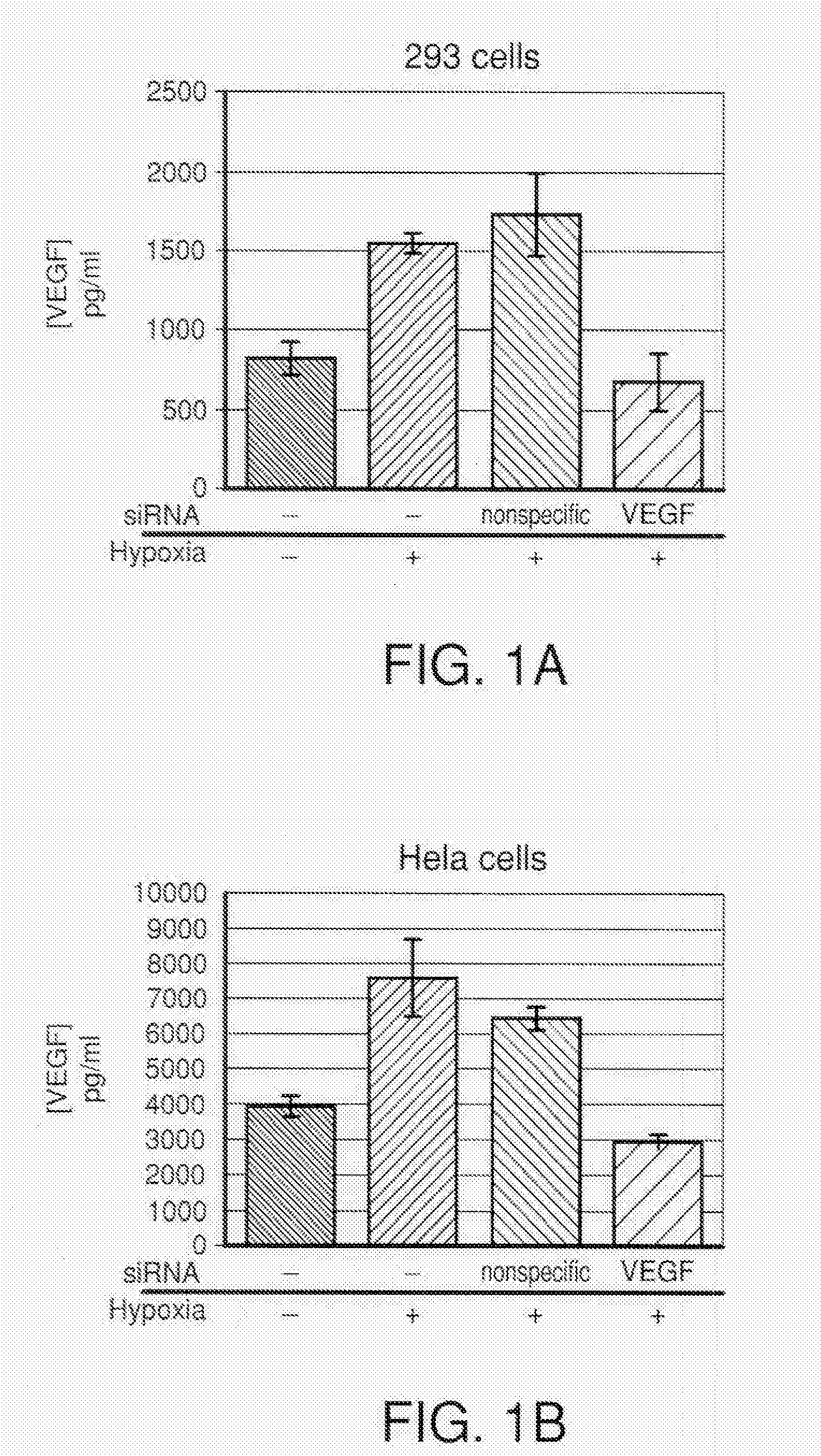
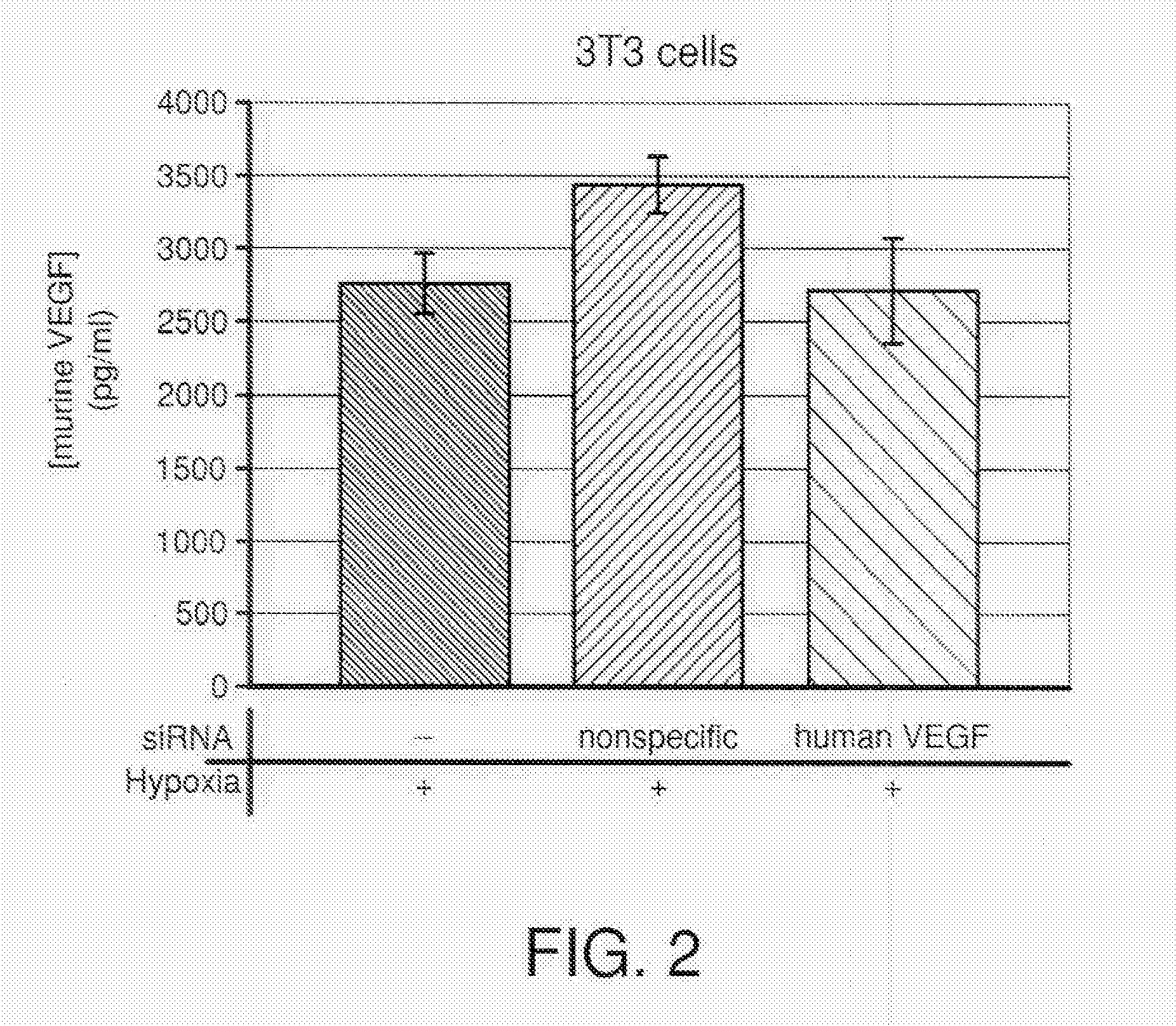
COMPOSITIONS AND METHODS FOR siRNA INHIBITION OF ANGIOGENESIS
RNA interference using small interfering RNAs which are specific for the vascular endothelial growth factor (VEGF) gene and the VEGF receptor genes Flt-1 and Flk-1 / KDR inhibit expression of these genes. Diseases which involve angiogenesis stimulated by overexpression of VEGF, such as diabetic retinopathy, age related macular degeneration and many types of cancer, can be treated by administering the small interfering RNAs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Inhibitors of VEGF receptor and HGF receptor signaling
ActiveUS20060074056A1Promote motilityPromote invasionBiocideOrganic chemistryVEGF receptorsHGF Receptor
The invention relates to the inhibition of VEGF receptor signaling and HGF receptor signaling. The invention provides compounds and methods for inhibiting VEGF receptor signaling and HGF receptor signaling. The invention also provides compositions and methods for treating cell proliferative diseases and conditions
Owner:METHYLGENE
Cleavage of VEGF and VEGF receptor by wildtype and mutant MT-SP1
MT-SP1 mutein proteases with altered specificity for the target molecules they cleave can be used to treat human diseases, such as cancer. Cleaving VEGF or VEGFR at certain substrate sequences with wild-type and mutein MT-SP1 proteases can be used to treat pathologies associated with angiogenesis.
Owner:VERTEX PHARMA INC
COMPOSITIONS AND METHODS FOR siRNA INHIBITION OF ANGIOGENESIS
RNA interference using small interfering RNAs which are specific for the vascular endothelial growth factor (VEGF) gene and the VEGF receptor genes Flt-1 and Flk-1 / KDR inhibit expression of these genes. Diseases which involve angiogenesis stimulated by overexpression of VEGF, such as diabetic retinopathy, age related macular degeneration and many types of cancer, can be treated by administering the small interfering RNAs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
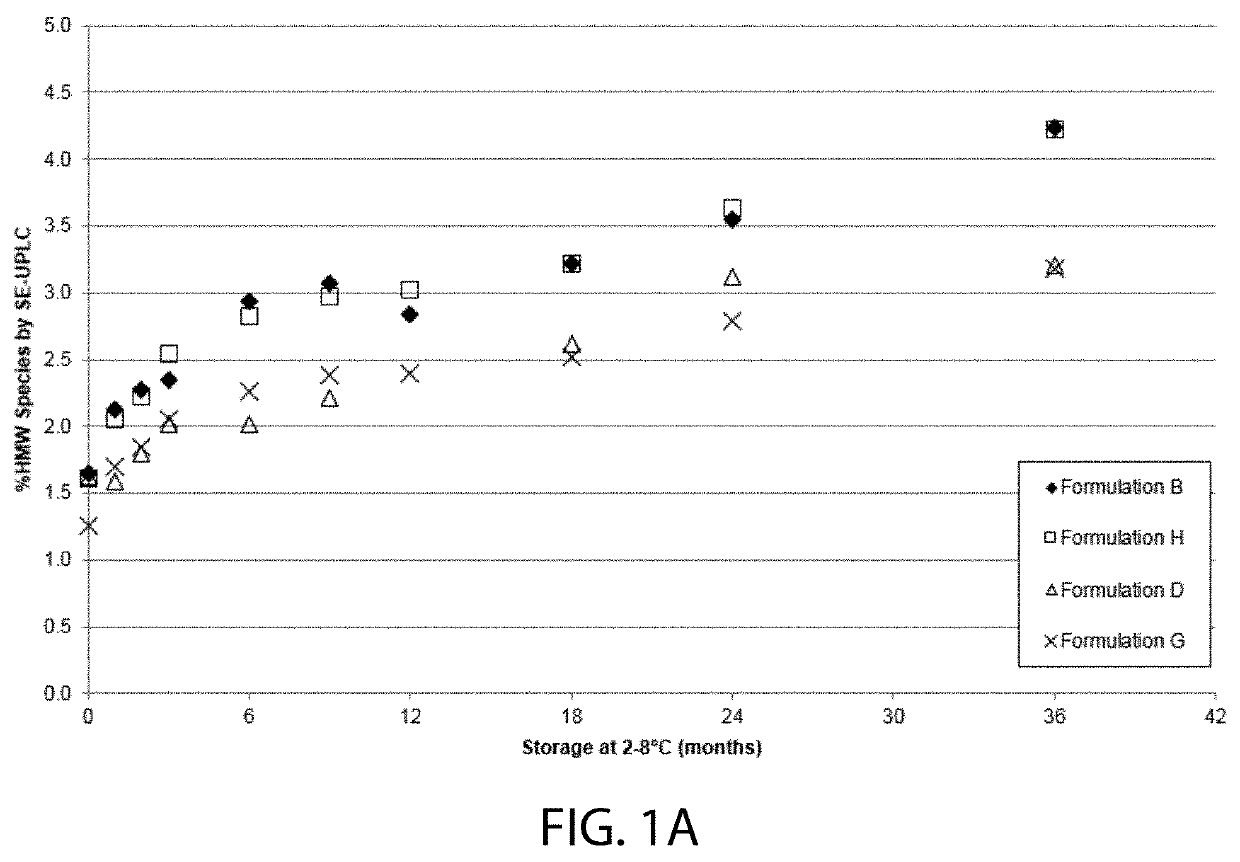
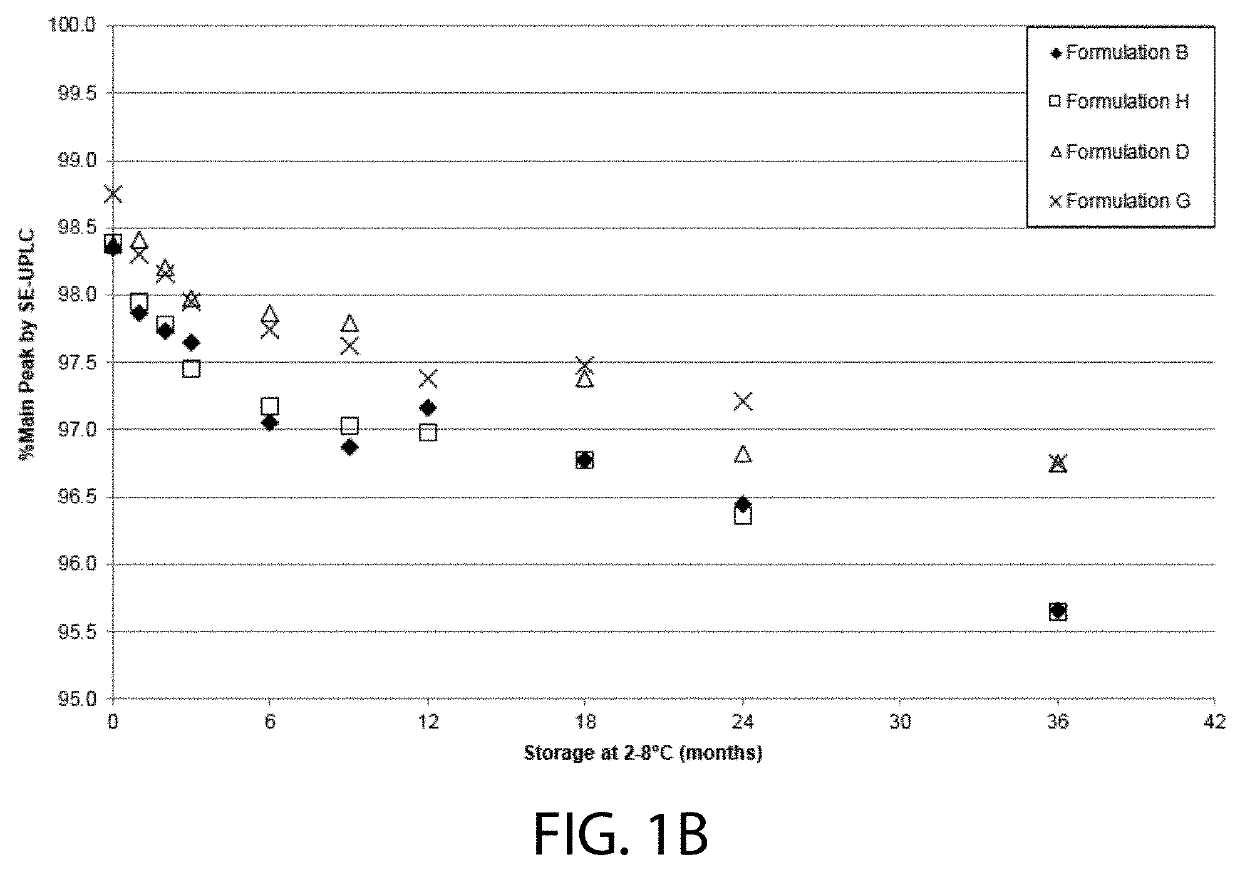
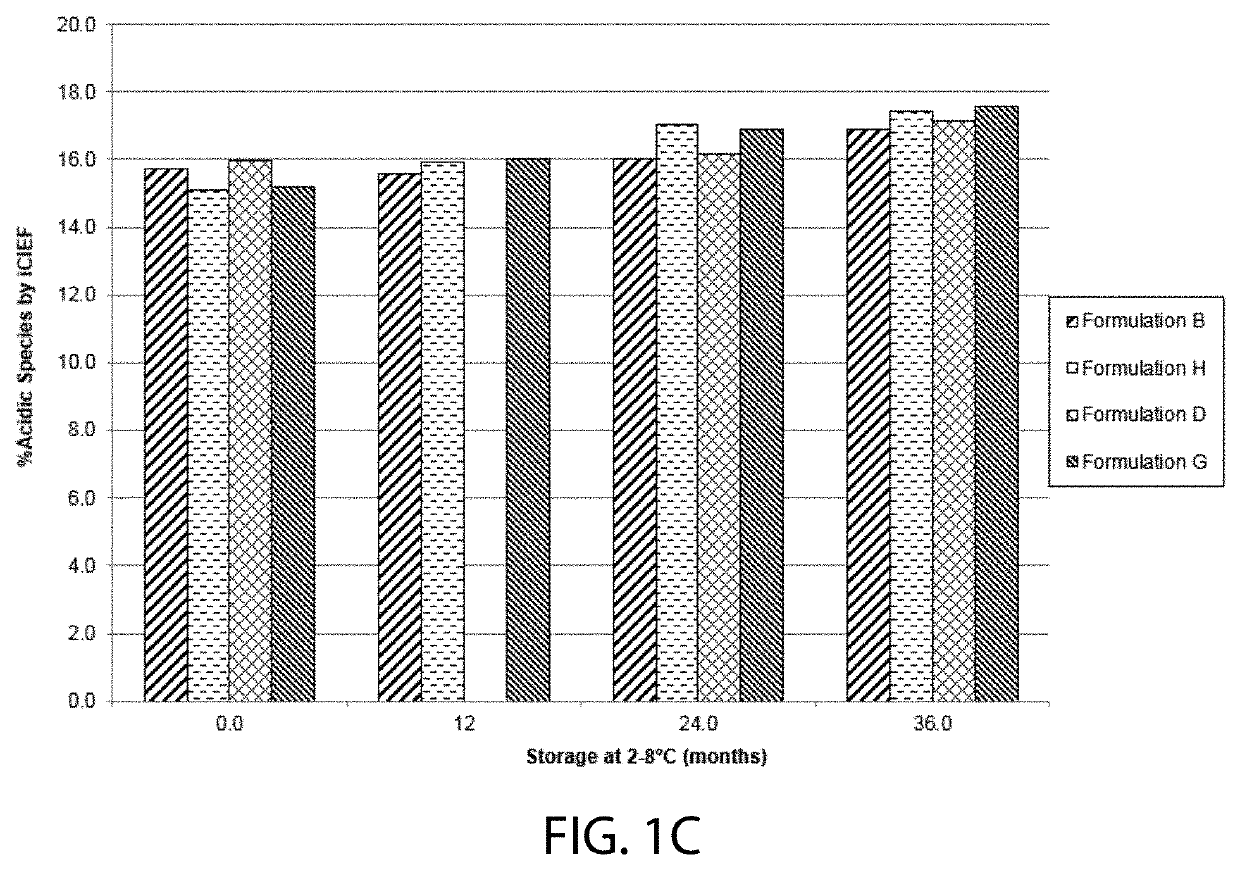
High concentration VEGF receptor fusion protein containing formulations
ActiveUS20190343918A1Long-term manufacturing and storage stabilityReduce dosageSenses disorderPeptide/protein ingredientsHigh concentrationVEGF receptors
The present invention provides ophthalmic formulations having high concentrations of vascular endothelial growth factor (VEGF) receptor fusion protein and high stability during storage. Methods for treating angiogenic eye disorders using the high concentration formulations are also provided.
Owner:REGENERON PHARM INC
Application of fusion protein of VEGF receptor for treating disease of eye
ActiveCN1915427AExcellent eye disease treatment effectImprove stabilitySenses disorderPeptide/protein ingredientsDiseaseDiabetes retinopathy
An application of the receptor fusion protein VEGF in treating eye diseases including the age associated macula lutea lesion, retinosis of diabetic, xanthelasma of diabetic, etc is disclosed.
Owner:CHENGDU KANGHONG BIOTECH
Cell Lines That Secrete Anti-Angiogenic Antibody-Scaffolds and Soluble Receptors and Uses Thereof
ActiveUS20120141573A1Stable duplexPrevent proliferationSenses disorderPeptide/protein ingredientsDiseaseSingle-Chain Antibodies
The invention provides nucleic acid and polypeptide sequences encoding antibody based scaffolds such as full antibodies, antibody Fab fragments, single chain antibodies, soluble VEGF receptor-Fc fusion proteins, and / or anti-angiogenic PDGF receptors. Also encompassed are cell lines encoding such anti-angiogenic antibody scaffolds, VEGF receptors, and / or PDGF receptors. The invention also provides encapsulated cell therapy devices that are capable of delivering such anti-angiogenic antibody scaffolds, VEGF receptors, and / or PDGF receptors as well as methods of using these devices to deliver the anti-angiogenic antibody scaffolds, VEGF receptors, and / or PDGF receptors to medically treat disorders in patients, including ophthalmic, vascular, inflammatory, and cell proliferation diseases.
Owner:NEUROTECH USA
Inhibitors of Protein Tyrosine Kinase Activity
This invention relates to compounds that inhibit protein tyrosine kinase activity. In particular the invention relates to compounds that inhibit the protein tyrosine kinase activity of growth factor receptors, resulting in the inhibition of receptor signaling, for example, the inhibition of VEGF receptor signaling. The invention also provides compounds, compositions and methods for treating cell proliferative diseases and conditions and opthalmological diseases, disorders and conditions.
Owner:METHYLGENE
Inhibitors of Protein Tyrosine Kinase Activity
InactiveUS20130096136A1Inhibit angiogenesisInhibit tyrosine kinase activityBiocideOrganic chemistryDiseaseKinase activity
The present invention provides new compounds and methods for treating a disease responsive to inhibition of kinase activity, for example a disease responsive to inhibition of protein tyrosine kinase activity, for example a disease responsive to inhibition of protein tyrosine kinase activity of growth factor receptors, for example a disease responsive to inhibition of receptor type tyrosine kinase signaling, or for example, a disease responsive to inhibition of VEGF receptor signaling.
Owner:METHYLGENE
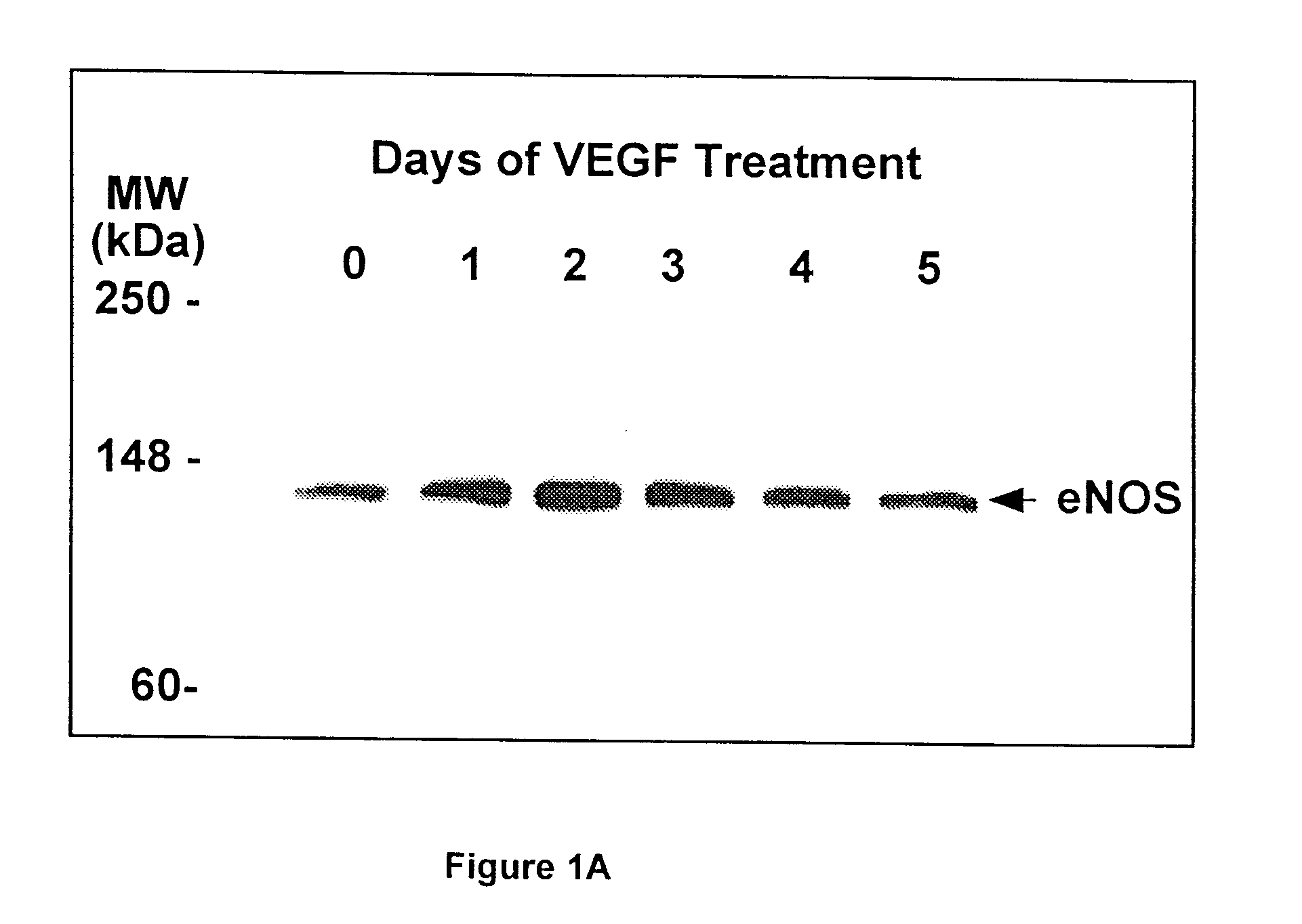
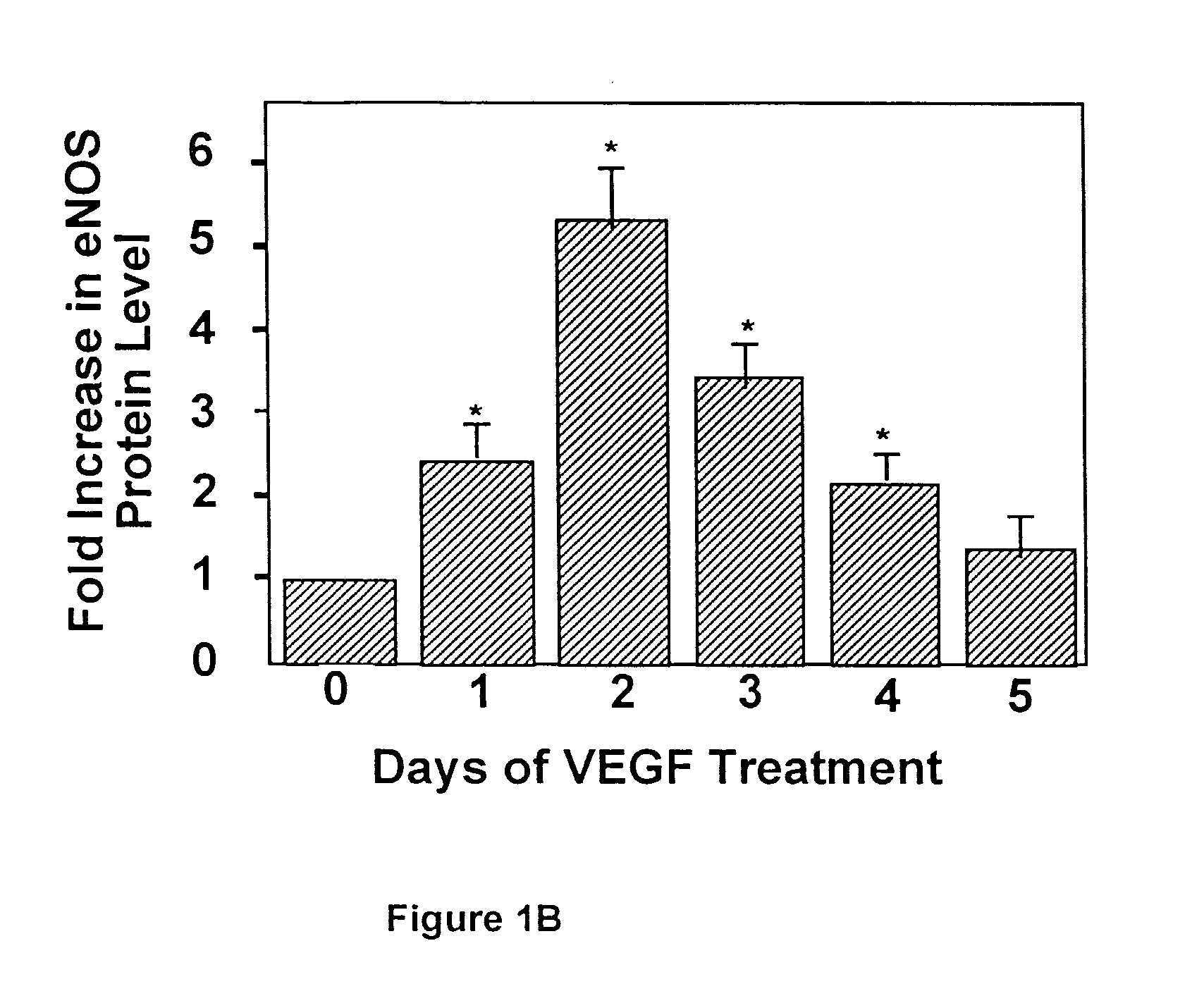
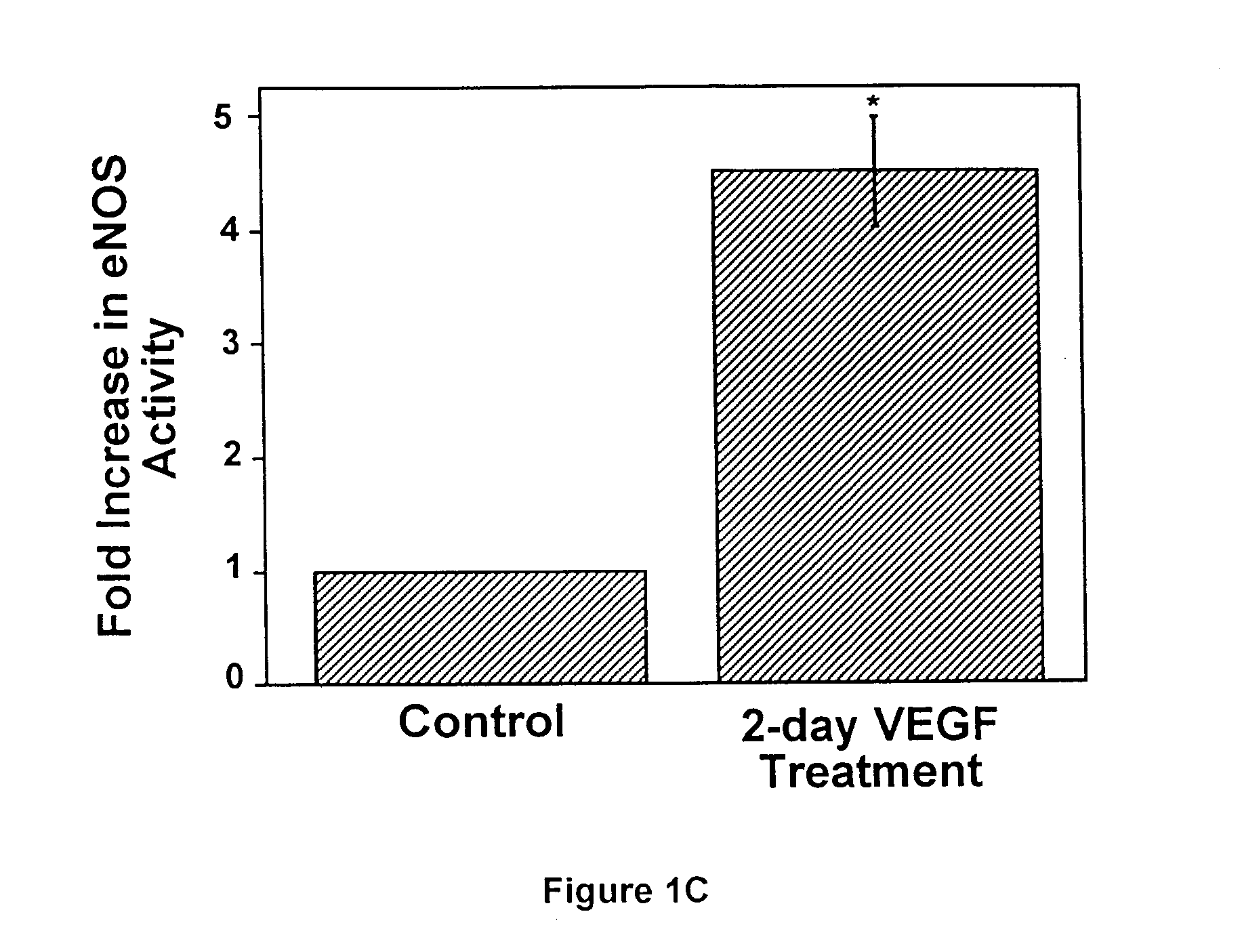
Modulation of eNOS activity and therapeutic uses thereof
InactiveUS7078382B1Effective activityIncrease productionPeptide/protein ingredientsPeptide preparation methodsVEGF receptorsAngina
The present invention provides uses of VEGF or VEGF receptor agonists for the up-regulation of eNOS expression and activity. VEGF and VEGF receptor agonists are useful in the treatment of or prevention from hypertension, diabetes, angina, thrombosis, atherosclerosis, heart failure, and other conditions or disorders wherein nitric oxide is an important regulator.
Owner:GENENTECH INC
Soluble inhibitors of vascular endothelial growth factor and use thereof
InactiveUS20080261867A1Reduce spreadSenses disorderFungiVascular endothelial growth inhibitorVEGF receptors
Owner:CHILDRENS MEDICAL CENT CORP
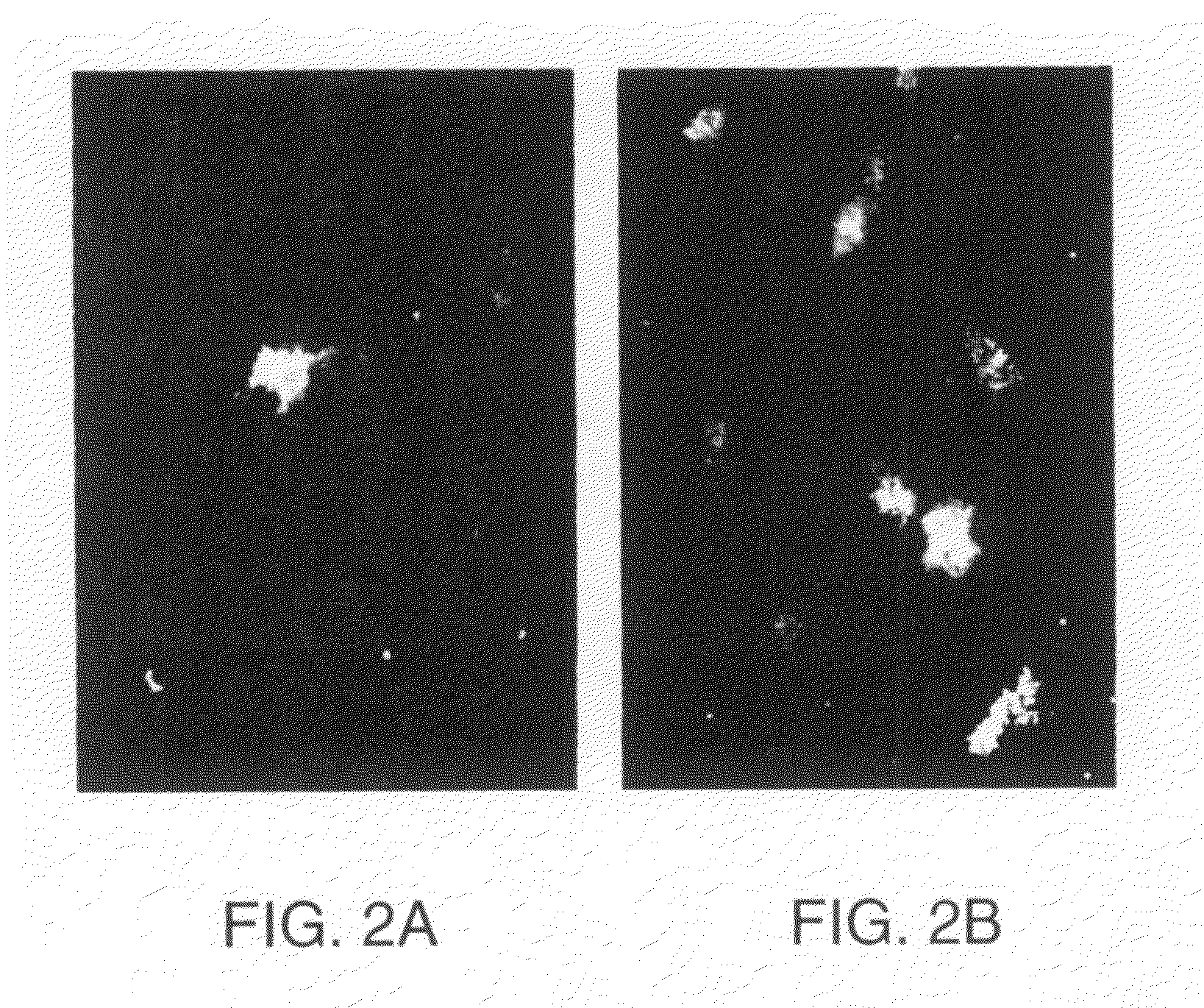
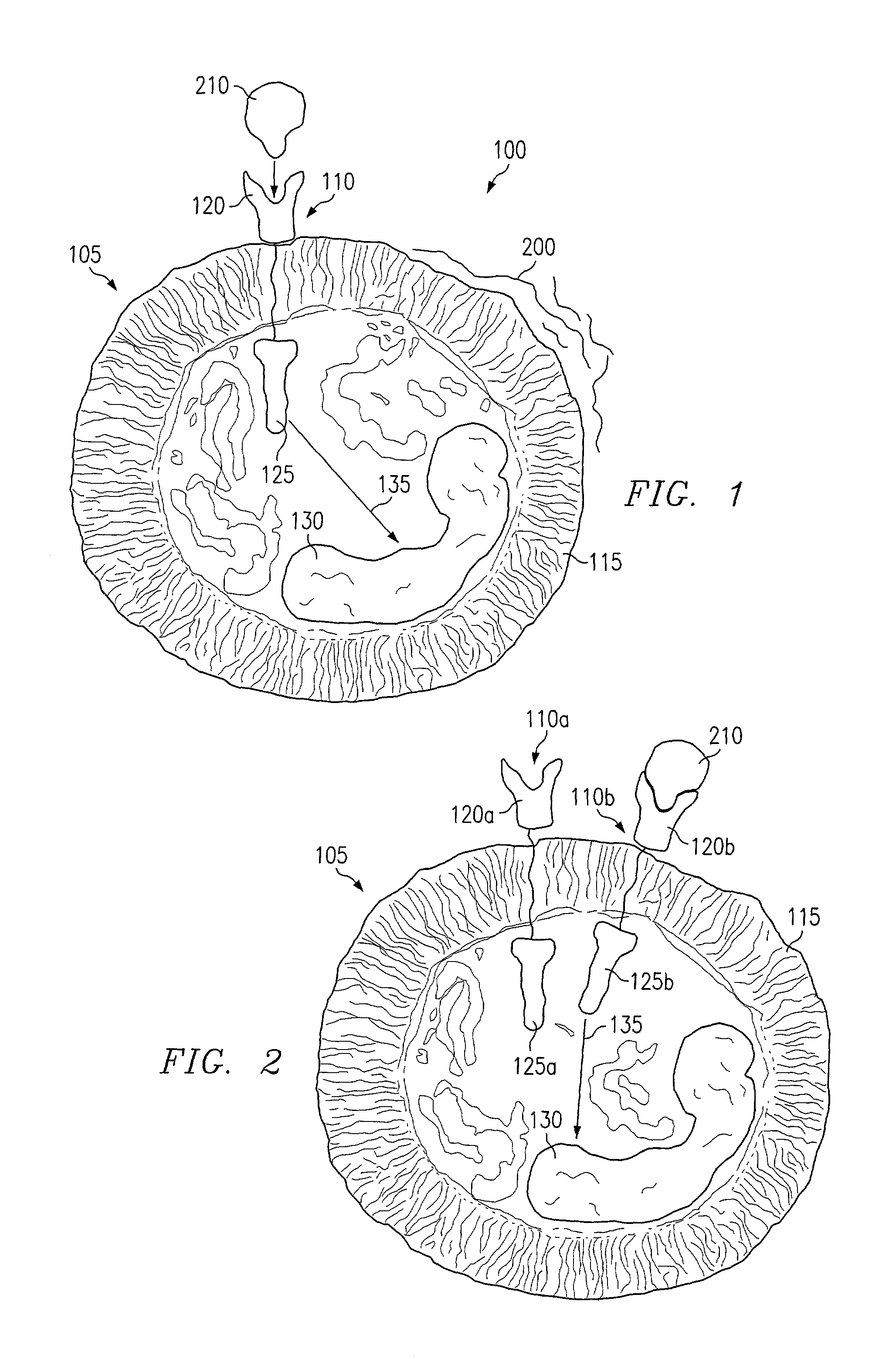
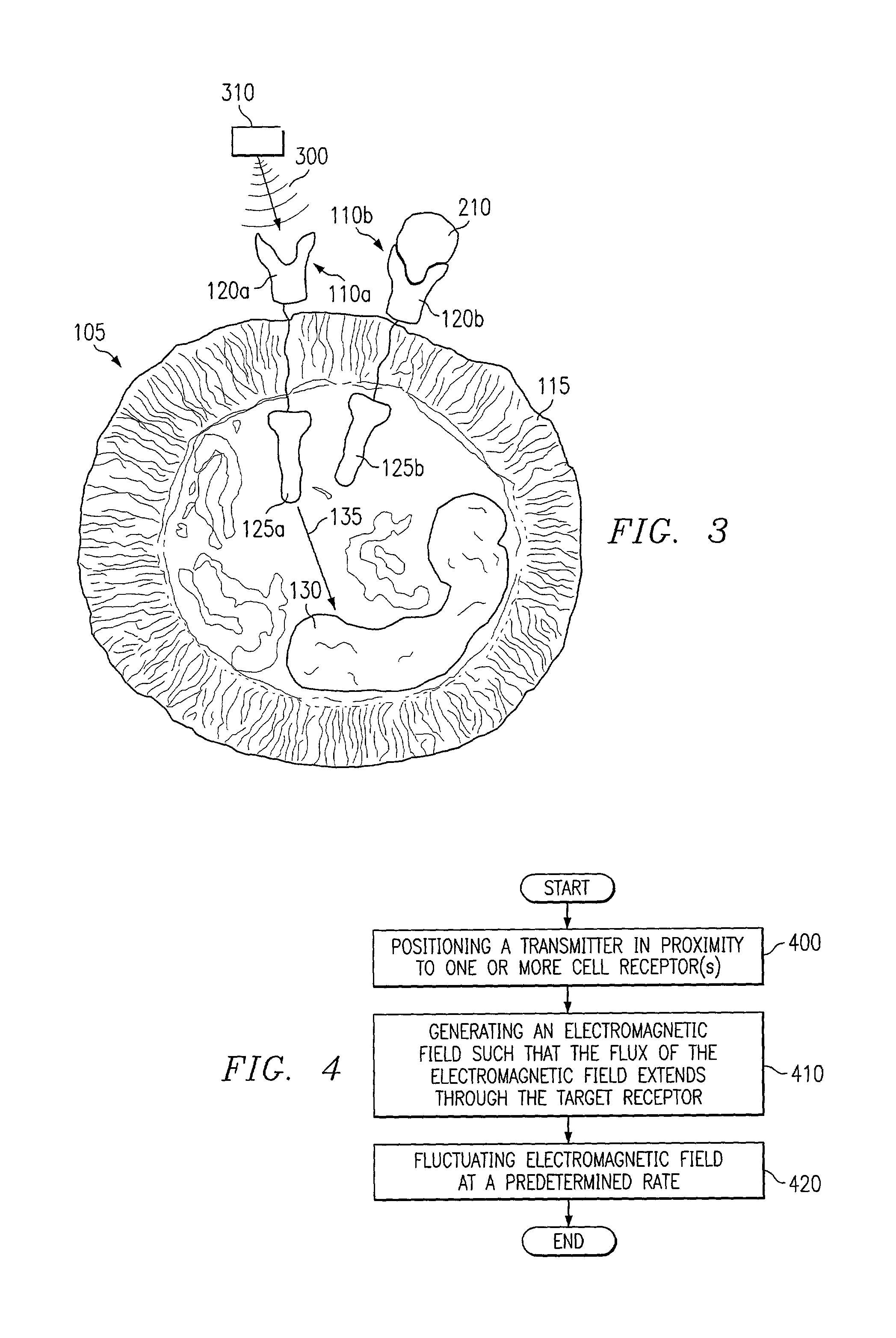
Methods of stimulating cell receptor activity using electromagnetic fields
InactiveUS7089060B1Disadvantages and reduced eliminatedPromote healingElectrotherapyMagnetotherapy using coils/electromagnetsVEGF receptorsVascular endothelial growth factor
A method for activating a vascular endothelial growth factor (VEGF) receptor of one or more cells includes positioning an electromagnetic field generator in proximity to a VEGF receptor such that the flux of an electromagnetic field generated by the electromagnetic field generator will extend through the VEGF receptor. The method also includes generating an electromagnetic field, using the electromagnetic field generator, having a rate of fluctuation that activates the VEGF receptor.
Owner:AMEI TECH
Inhibitors of vascular endothelial growth factor (VEGF) receptors and methods of use thereof
InactiveUS20130071397A1Inhibitory activityInhibiting the activity of the VEGF receptorSenses disorderPeptide/protein ingredientsVEGF receptorsAnti vegf
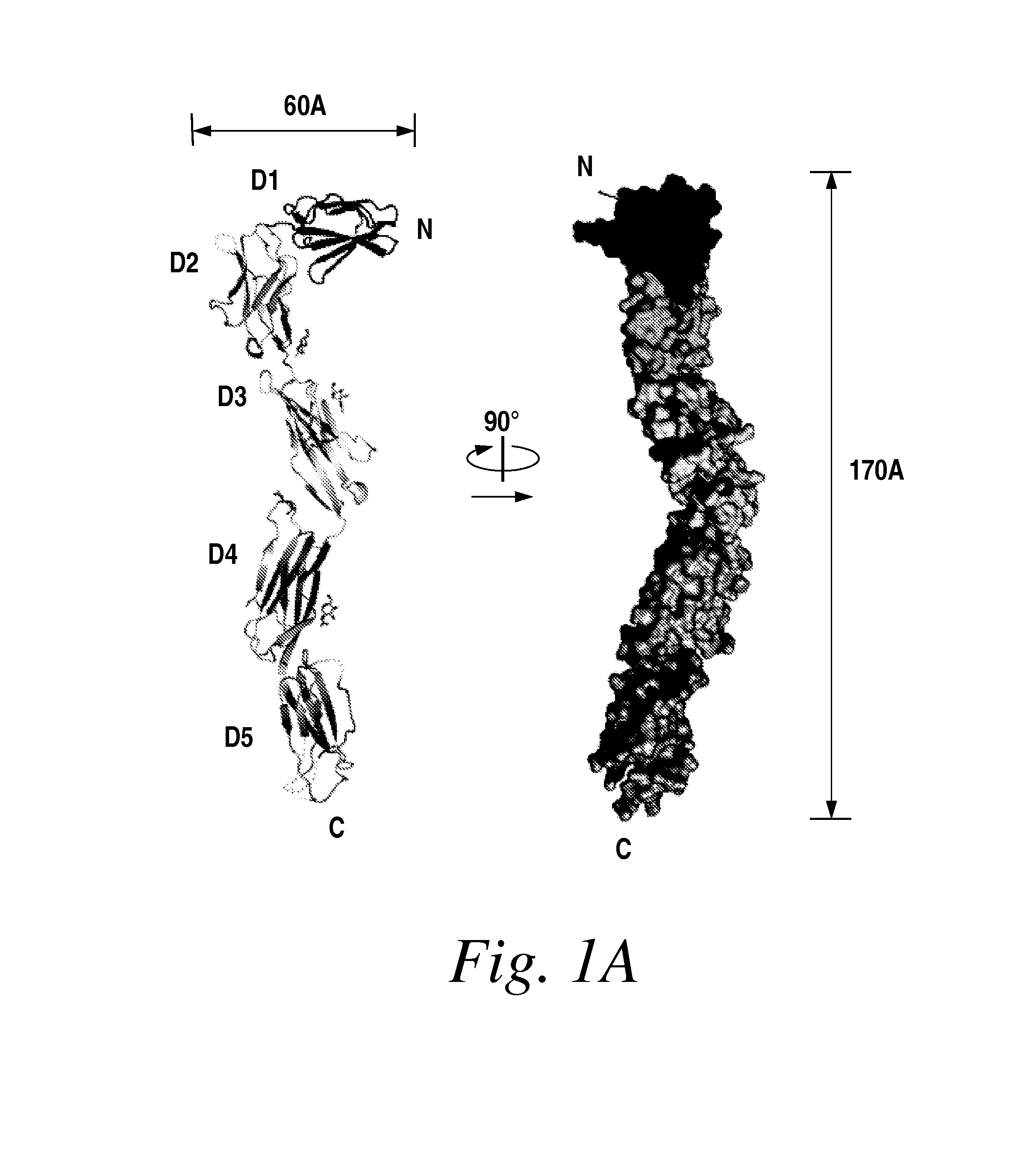
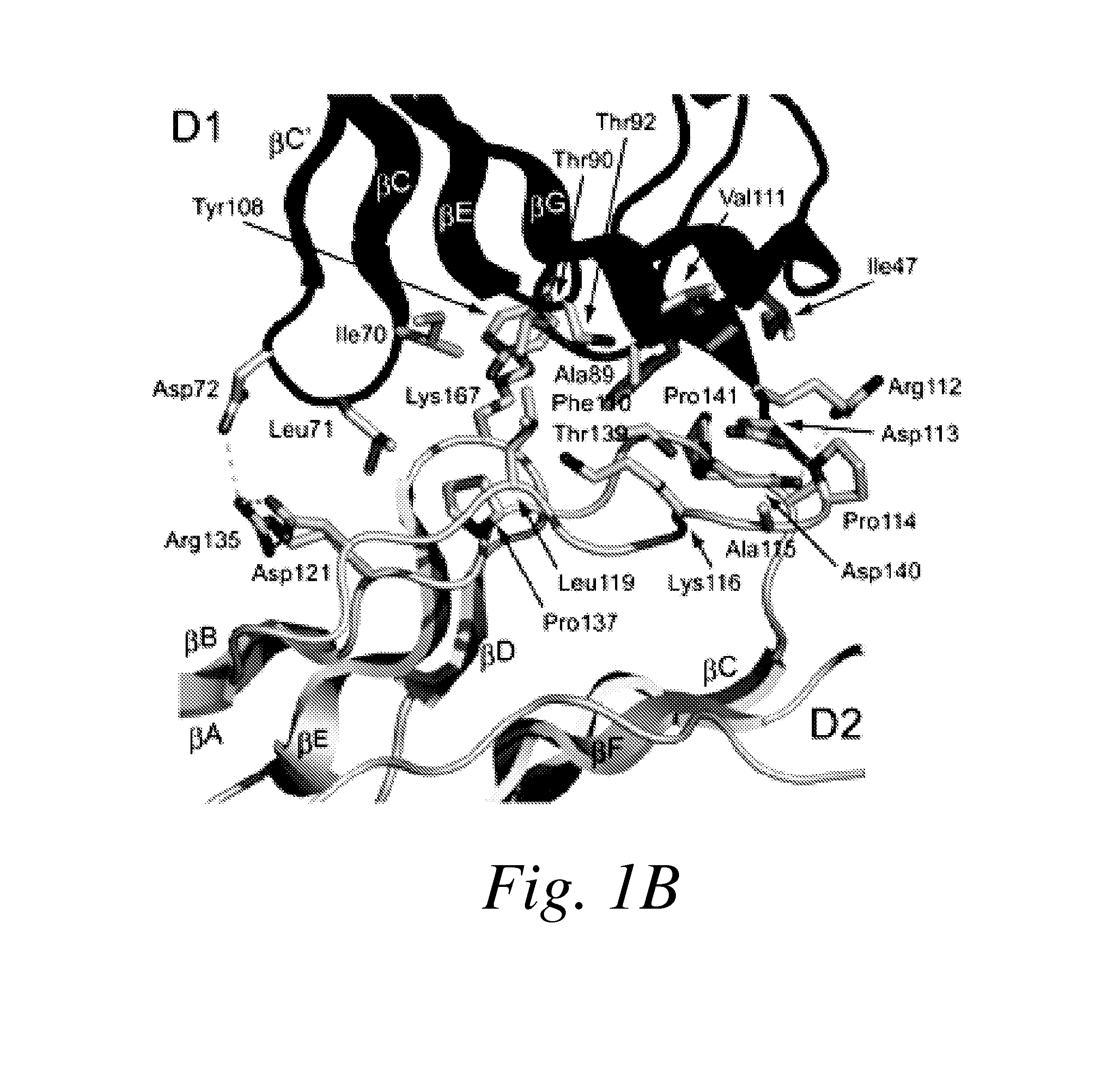
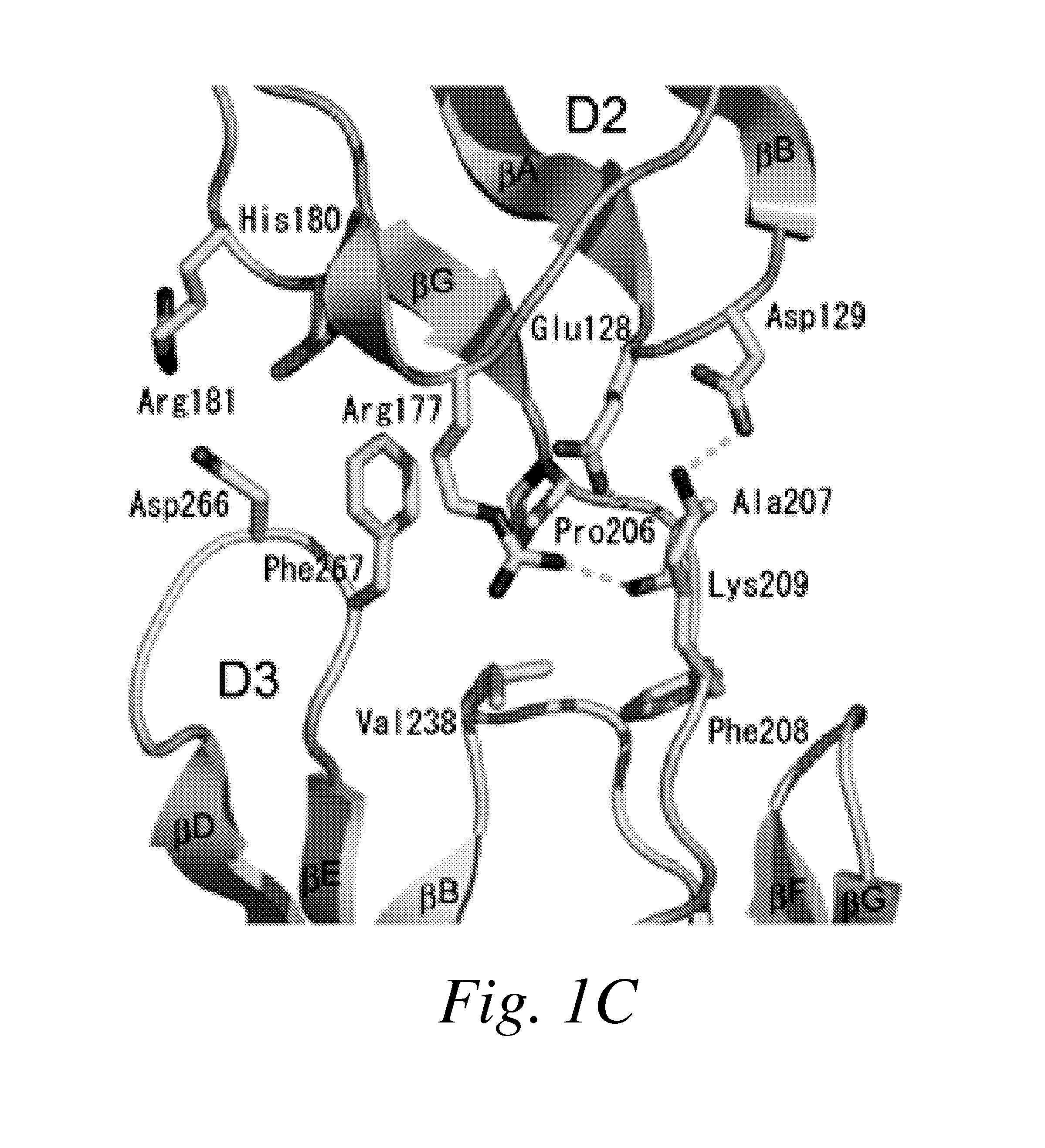
The present invention provides moieties that bind to the most membrane-proximal Ig-like domain of the ectodomain (D7) of vascular endothelial growth factor (VEGF) receptors, wherein the moieties antagonize the activity of the VEGF receptor.
Owner:YALE UNIV
Inhibitors of protein tyrosine kinase activity
ActiveUS20090264440A1Promote motilityPromote invasionBiocideSenses disorderKinase activityProtein-Tyrosine Kinases
This invention relates to compounds that inhibit protein tyrosine kinase activity. In particular the invention relates to compounds that inhibit the protein tyrosine kinase activity of growth factor receptors, resulting in the inhibition of receptor signaling, for example, the inhibition of VEGF receptor signaling and HGF receptor signaling. More particularly, the invention relates to compounds, compositions and methods for the inhibition of VEGF receptor signaling and HGF receptor signaling. The invention also provides compositions and methods for treating cell proliferative diseases and conditions.
Owner:MIRATI THERAPEUTICS INC
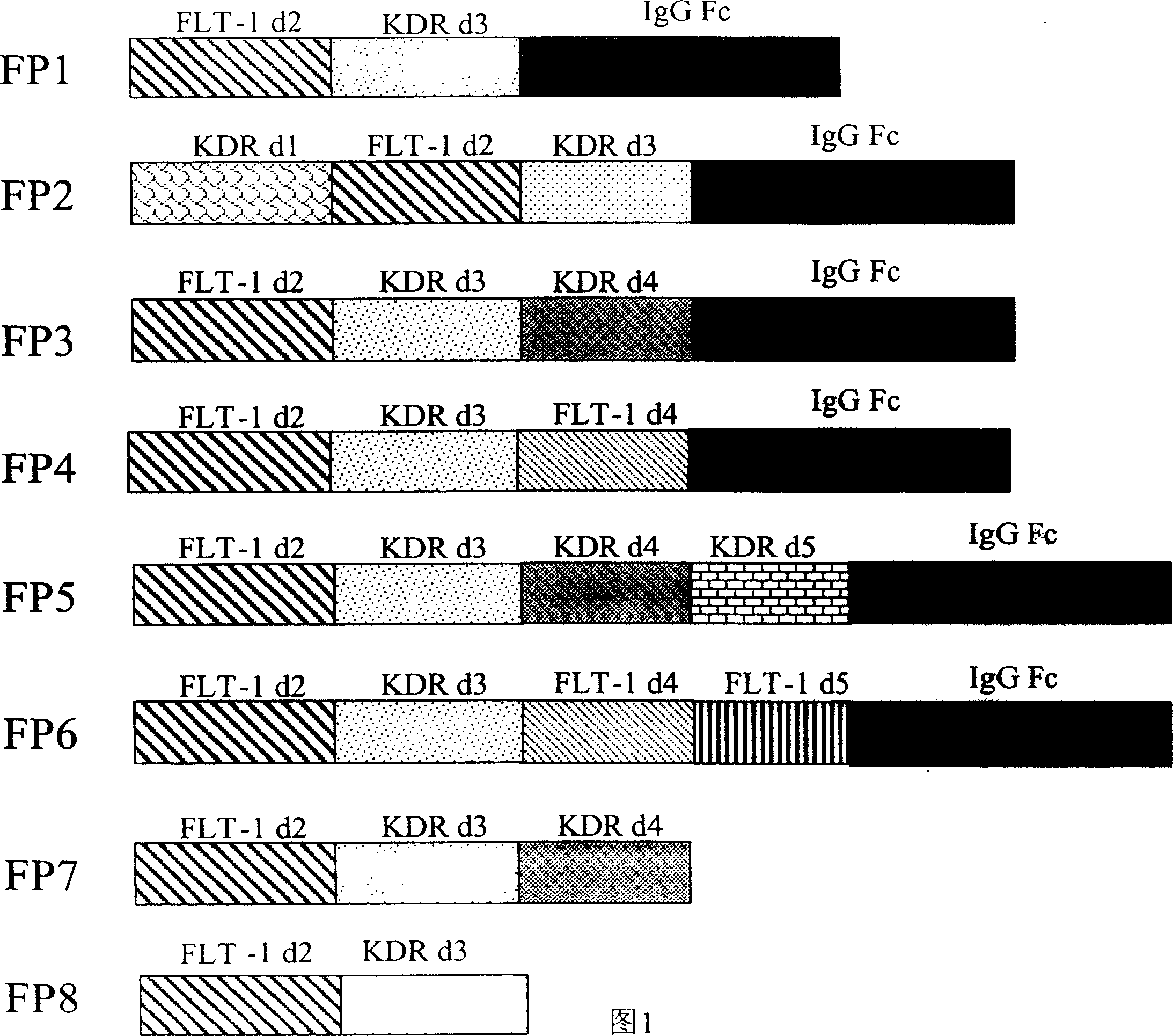
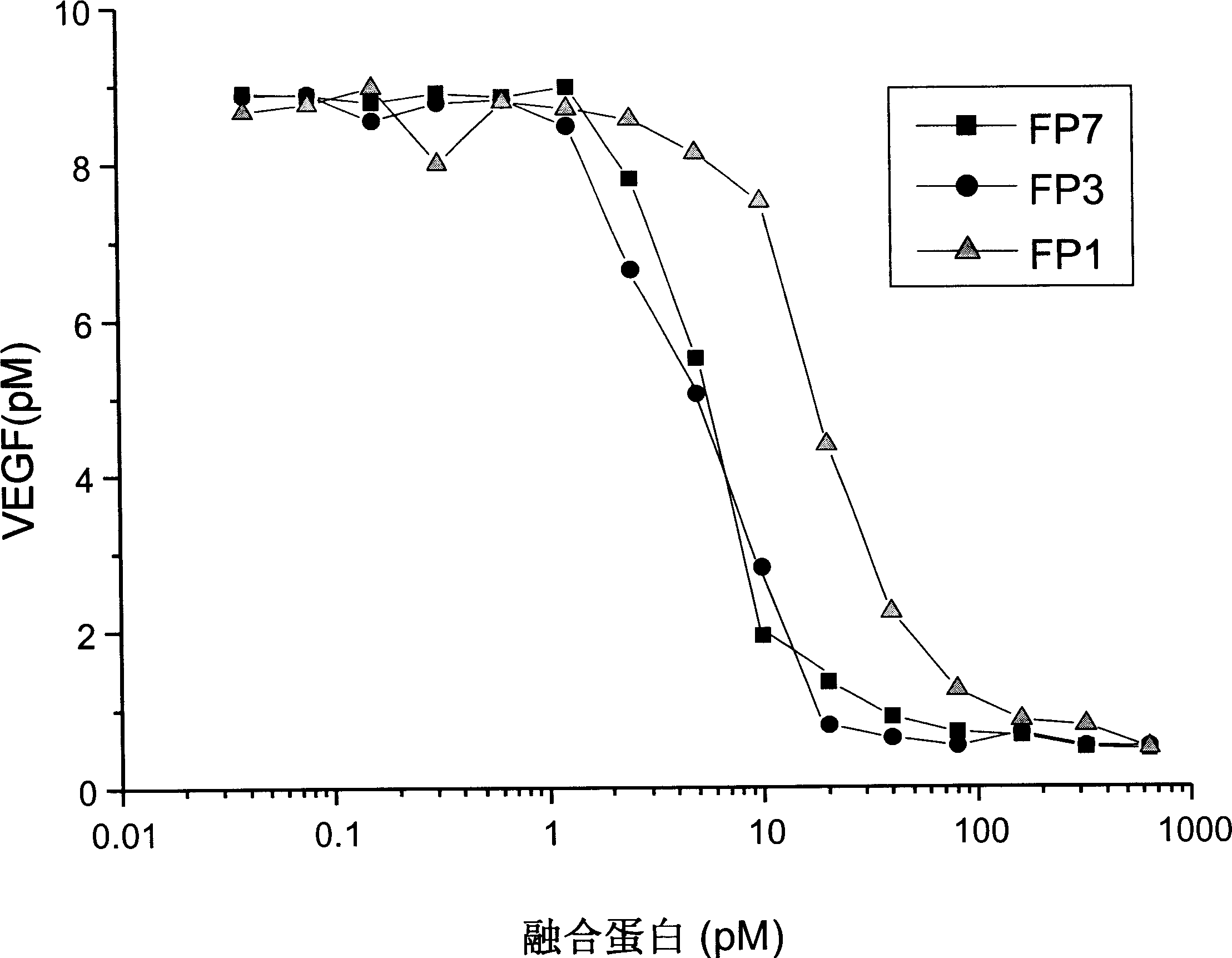
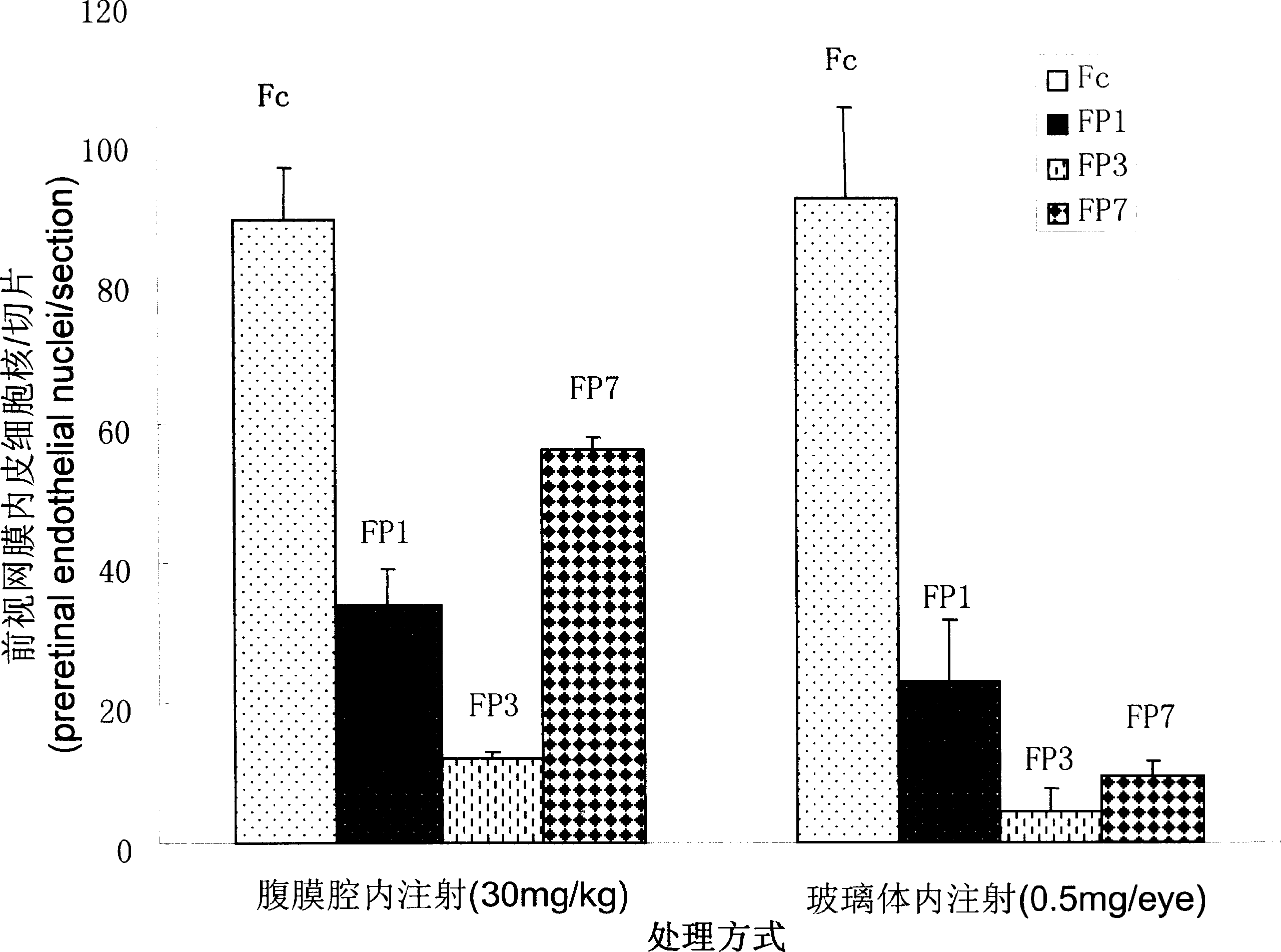
Application of VEGF acceptor fusion proteins in preparation of drugs for inhibiting growth of ocular surface neovascularization
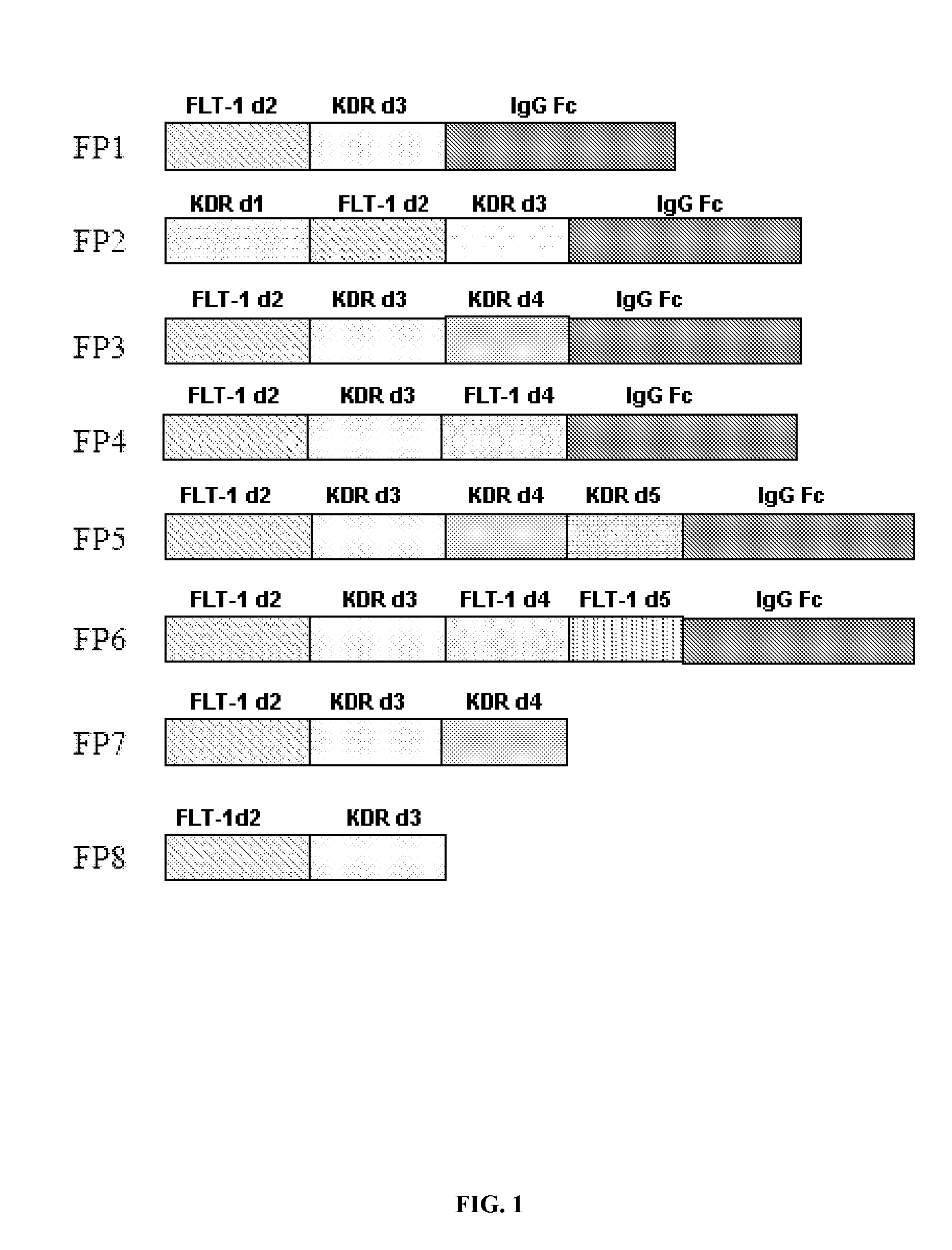
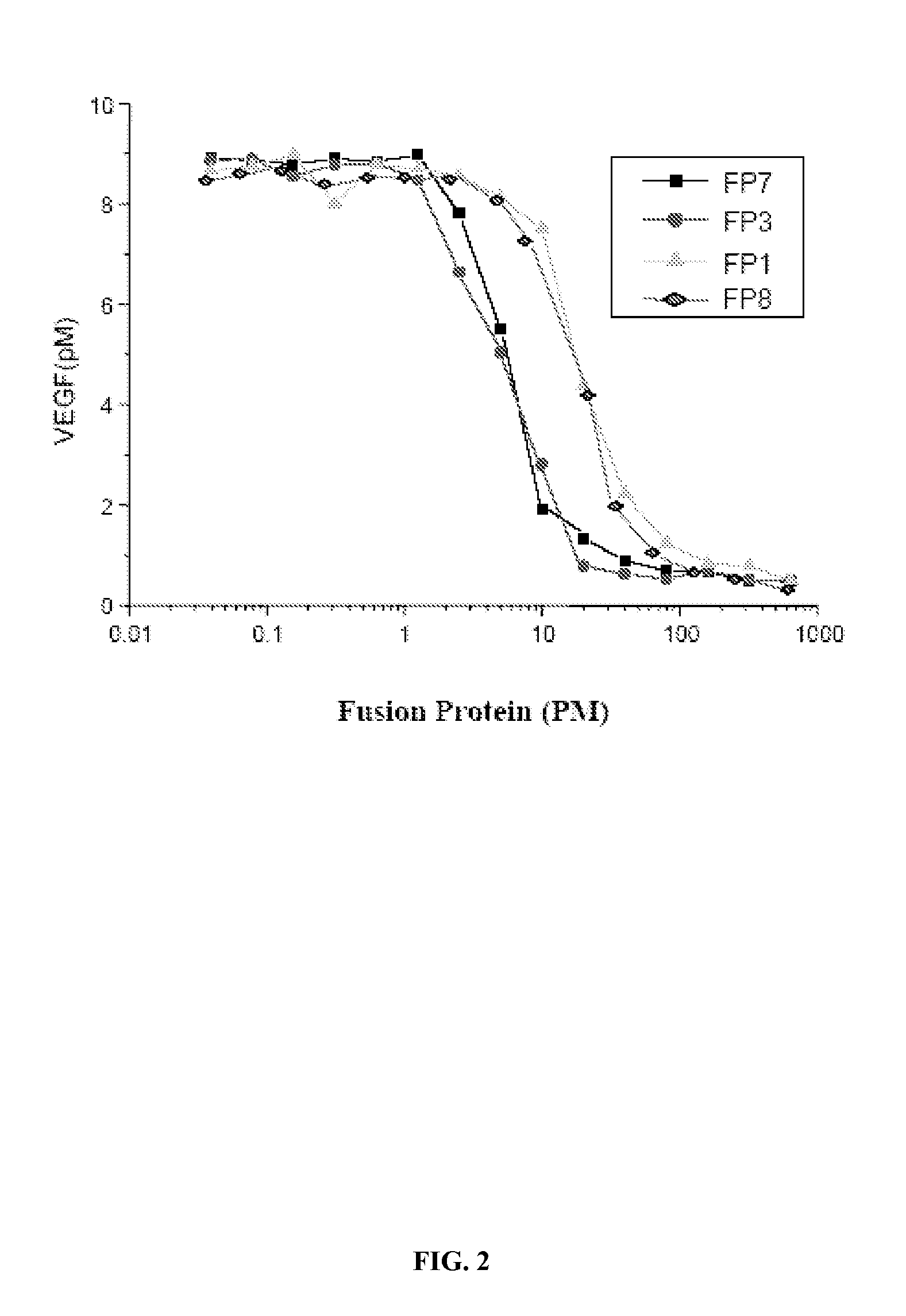
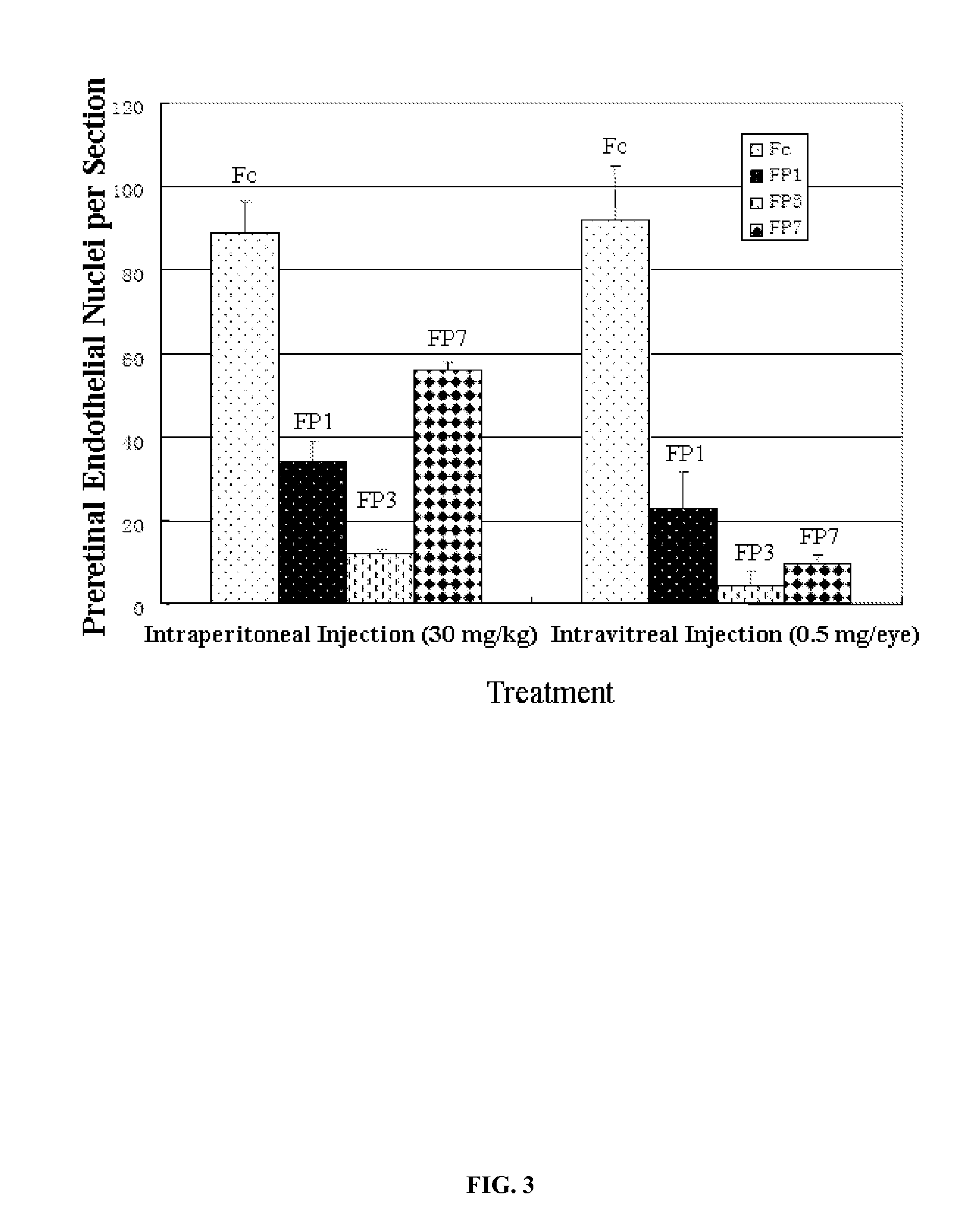
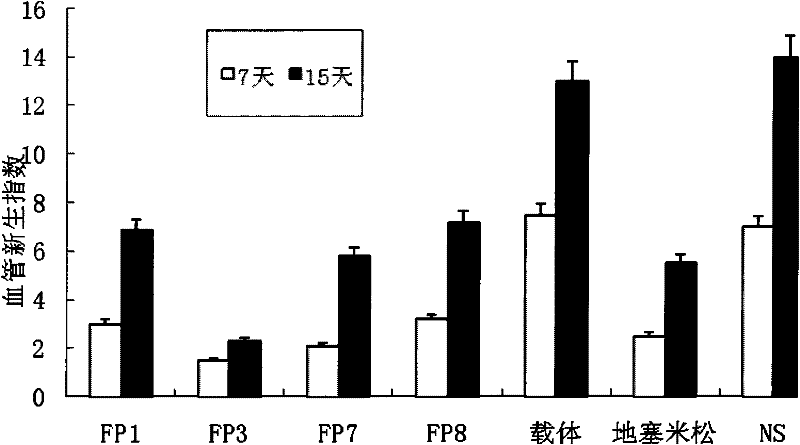
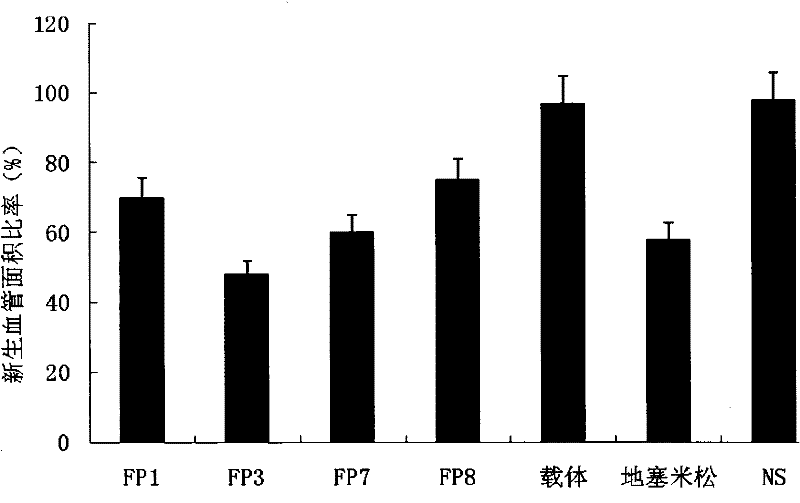
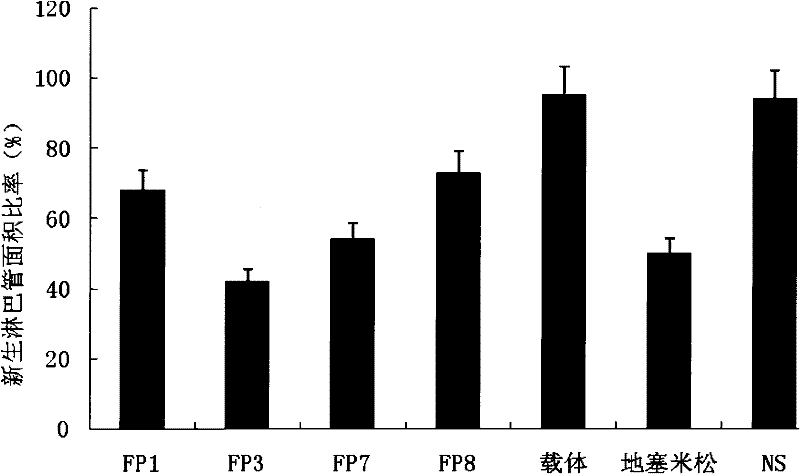
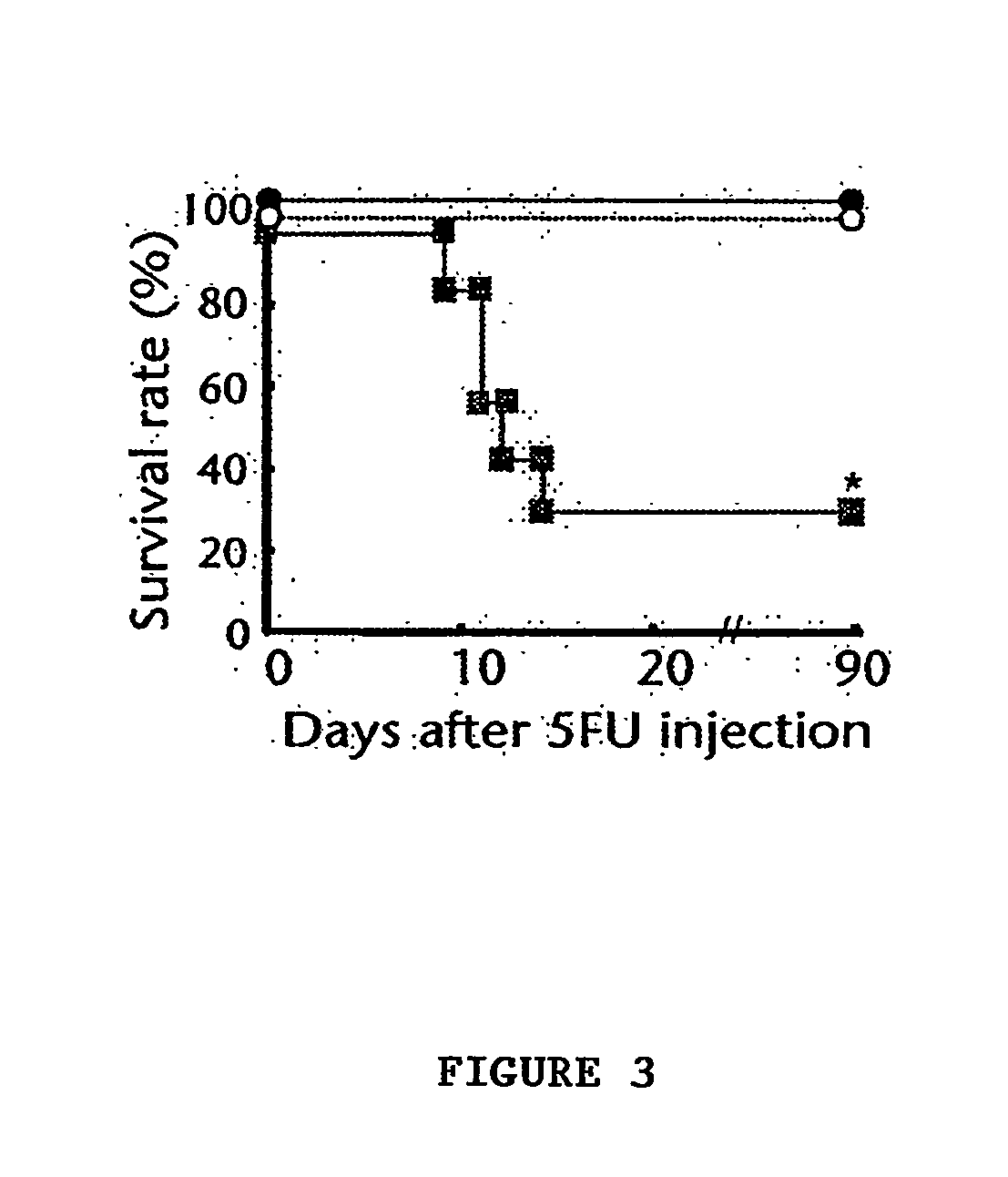
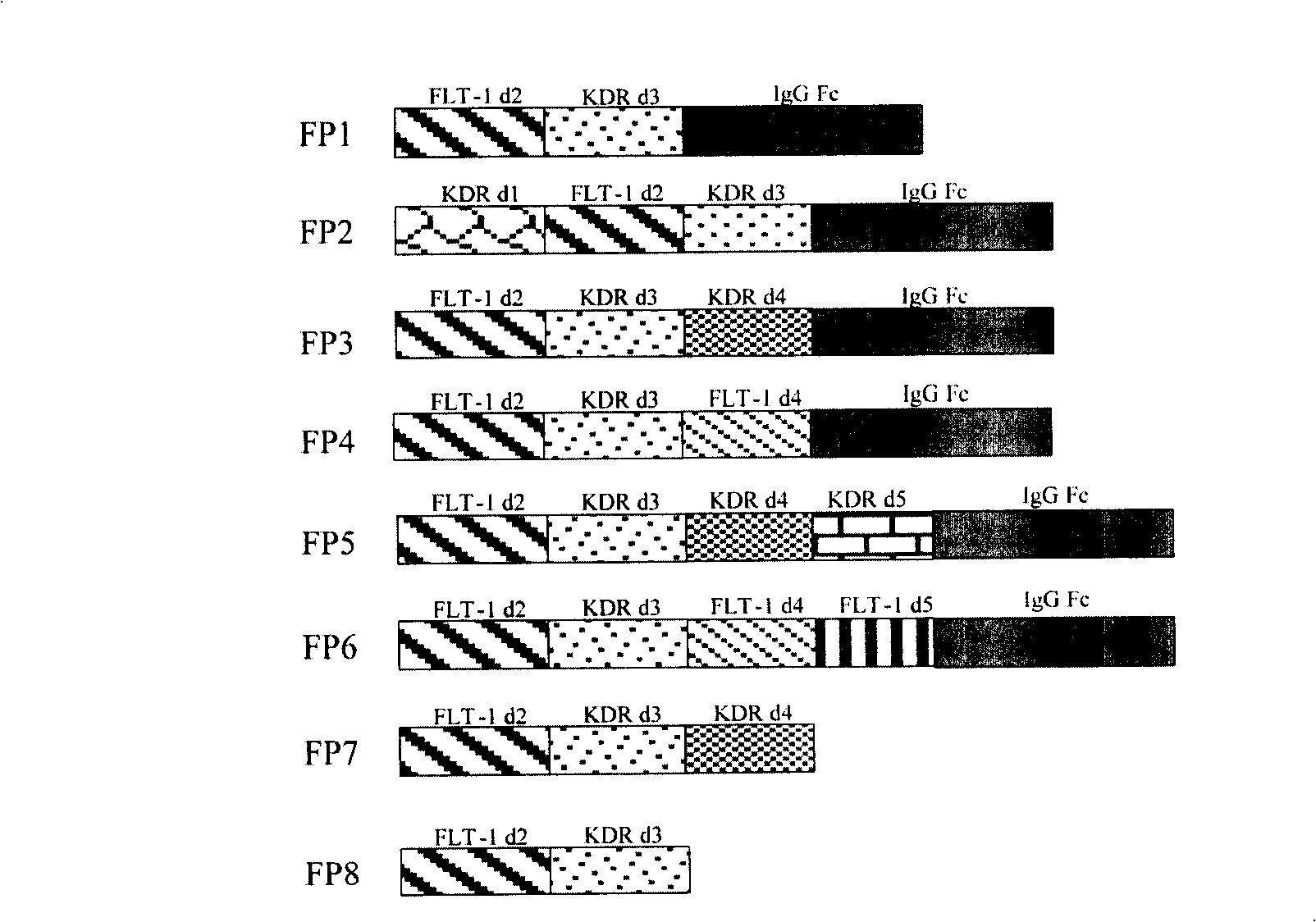
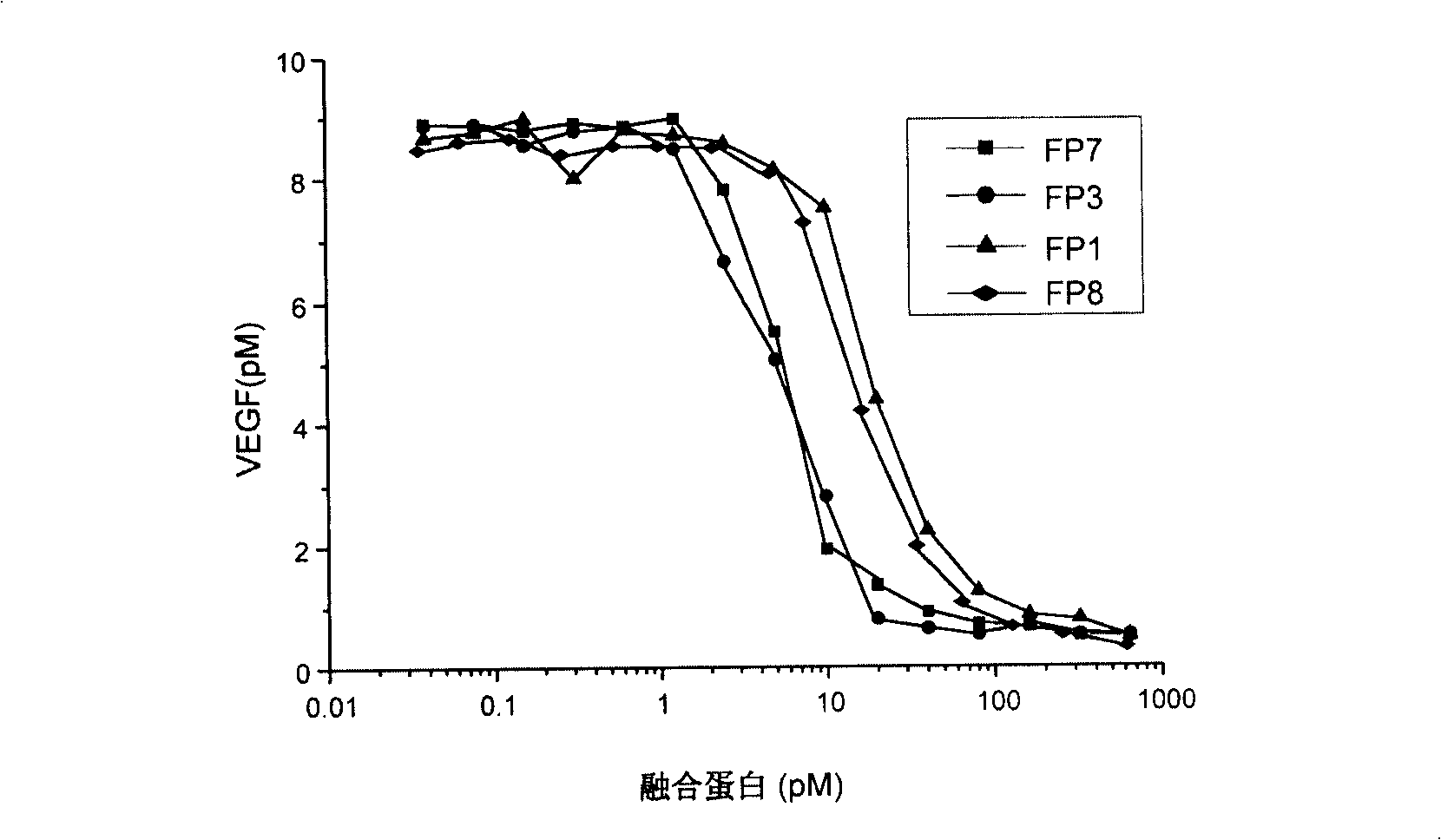
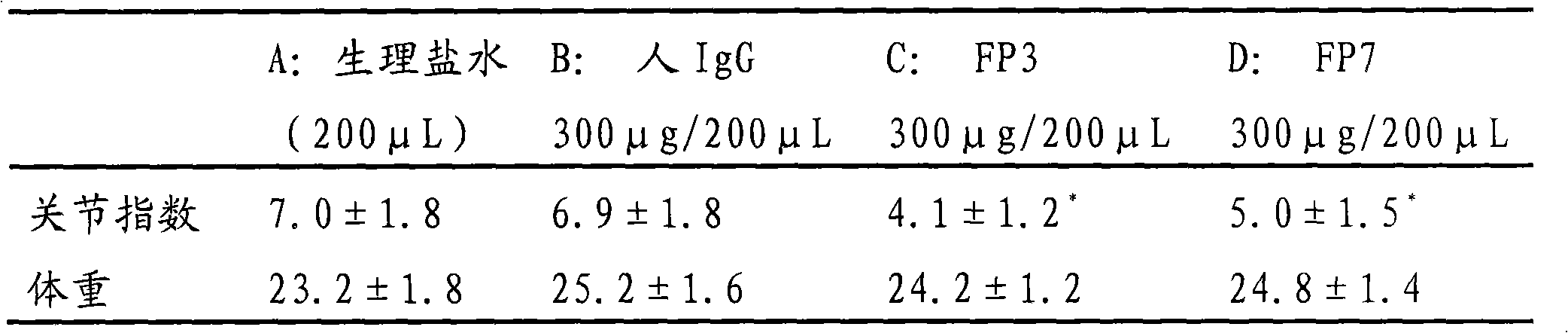
The invention relates to the application of VEGFR fusion proteins FP1, FP3, FP7 and FP8 in the preparation of drugs for treating ocular surface neovascularization and relevant diseases and a combined preparation of fusion proteins FP1, FP3, FP7, FP8 and an immunosuppressant, wherein the immunosuppressant is one or a combination selected from the group consisting of corticosteroid, rapamycin, dexamethasone and cyclosporine A; and amino acid sequences of FP1, FP3, FP7 and FP8 are respectively as shown in SEQ ID NO:1, 2, 3, 4.
Owner:CHENGDU KANGHONG BIOTECH
Inhibitors of protein tyrosine kinase activity
InactiveUS20110077240A1Inhibit angiogenesisInhibit tyrosine kinase activityBiocideSenses disorderDiseaseKinase activity
This invention relates to compounds that inhibit protein tyrosine kinase activity. In particular the invention relates to compounds that inhibit the protein tyrosine kinase activity of growth factor receptors, resulting in the inhibition of receptor signaling, for example, the inhibition of VEGF receptor signaling. The invention also provides compounds, compositions and methods for treating cell proliferative diseases and conditions and opthalmological diseases, disorders and conditions.
Owner:METHYLGENE
STABLE LIQUID FORMULATION OF FUSION PROTEIN WITH IgG Fc DOMAIN
ActiveUS20160376342A1Long-term stabilityReduce storage conditionsSenses disorderPeptide/protein ingredientsDiseaseVEGF receptors
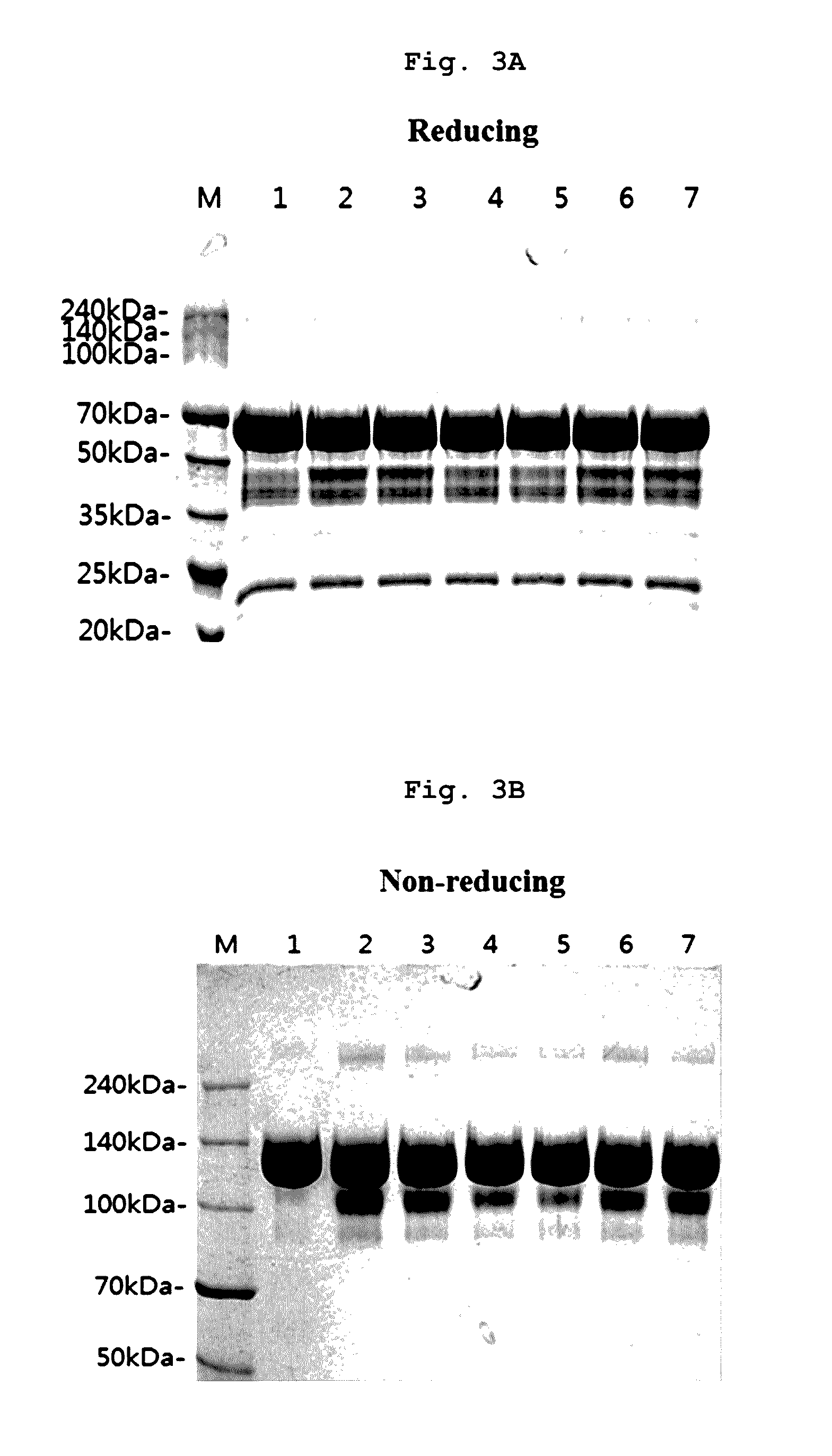
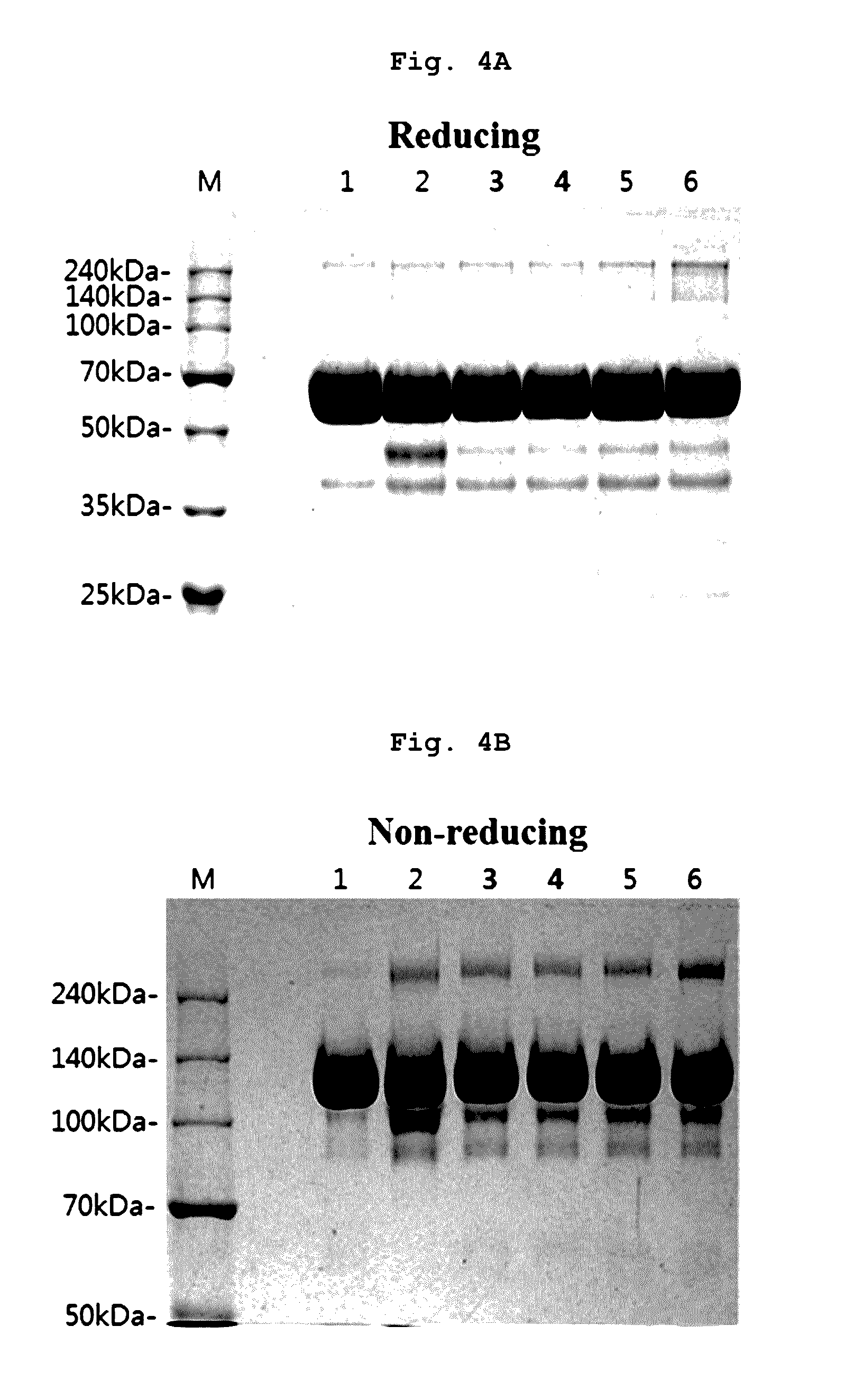
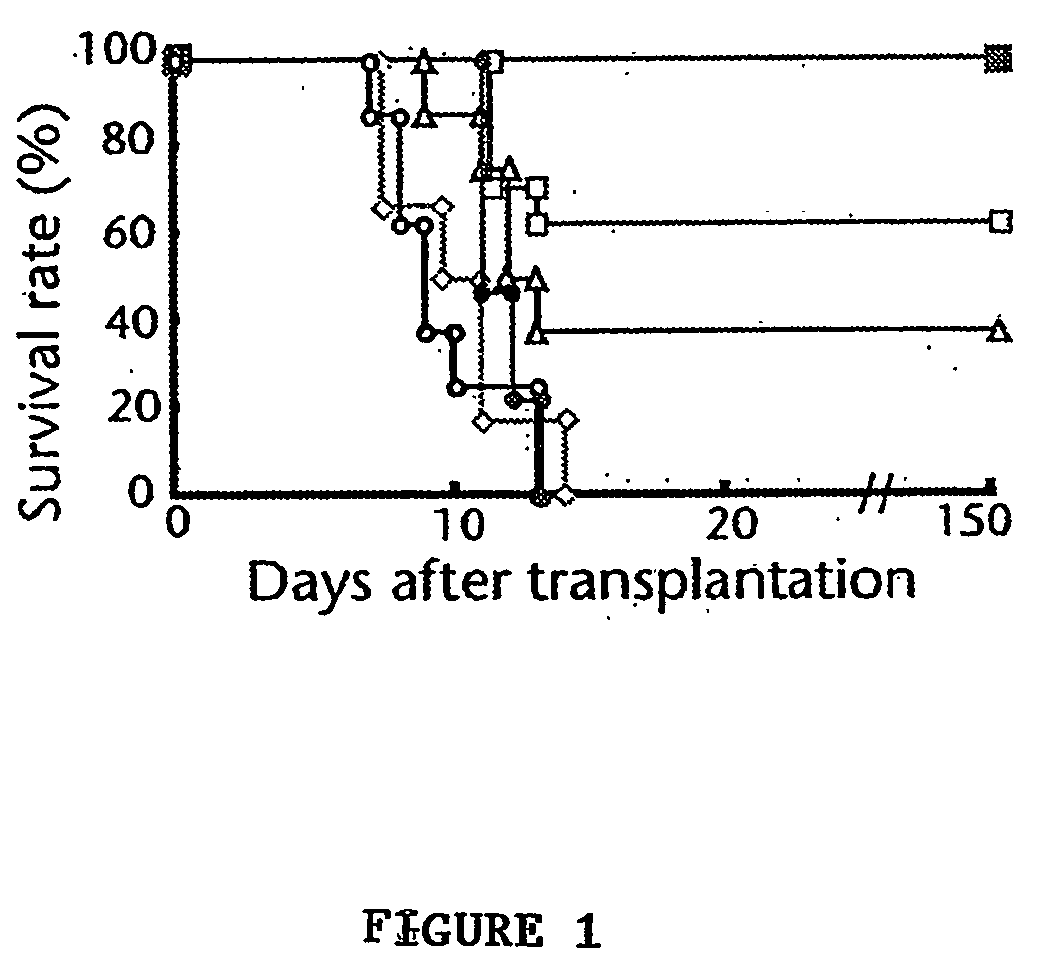
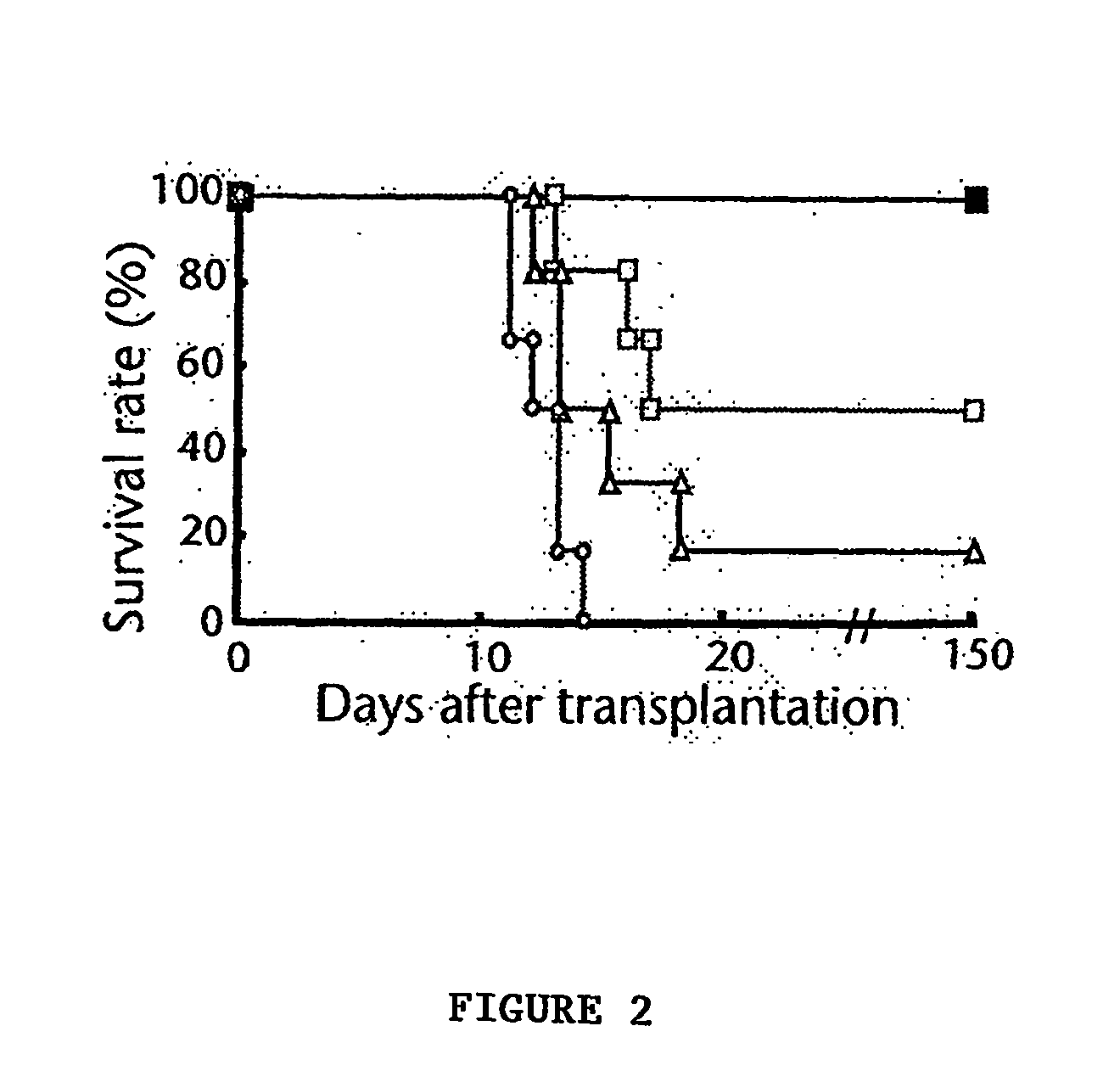
A stable liquid formulation includes a fusion protein having an Fc domain of a human immunoglobulin G (IgG), in particular, a protein in which an Fc domain of a human immunoglobulin G (IgG) and a soluble extracellular domain of a vascular endothelial growth factor (VEGF) receptor are fused (e.g., aflibercept)). A composition for stabilizing a protein and a method for stabilizing a protein in which an Fc domain of an IgG and a soluble extracellular domain of a VEGF receptor are fused are disclosed. The present invention improves therapeutic effects on various ophthalmic diseases (e.g., retinal vein occlusion, diabetic macular edema, choroidal neovascularization and wet age-related macular degeneration, etc.) caused by abnormal angiogenesis, while pursuing stabilization of bioactivity through a stable liquid formulation suitable for intravitreal injection of an anti-VEGF-Fc fusion protein including aflibercept.
Owner:ALTEOGEN
Isolation and mobilization of stem cells expressing vegfr-1
InactiveUS20050026220A1Improve isolationNervous disorderPeptide/protein ingredientsMyogenesisNeurogenesis
The present invention is directed to methods of isolating mammalian stem cells expressing the VEGF receptor VEGFR-1 and compositions thereof. The present invention is also directed to methods of using such isolated mammalian stem cells expressing VEGFR-1 to treat various conditions, which can involve inducing hematopoiesis, vasculogenesis and / or angiogenesis, myogenesis, and neurogenesis to treat the various condition. Finally, the present invention is directed to therapeutic methods using a molecule that binds and activates or stimulates VEGFR-1, for example, P1GF, to stimulate proliferation and / or differentiation and mobilization, i.e., motogenesis, of stem cells.
Owner:CORNELL RES FOUNDATION INC +1
Applications of VEGF receptor fusion protein in preparation of medicament for curing diseases about angiogenesis
The invention relates to an application of the fusion proteins FP1-FP8 of VEGF receptors on the treatment of the diseases relating to angiogenesis. The diseases relating to the angiogenesis includes autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, vasculitis, systemic sclerosis and other diseases caused by the angiogenesis such as bronchial asthma, obesity, nasal polyps, rhinitis, liver cirrhosis, endometriosis, uterine bleeding, warts, scars and ovarian cysts, etc.
Owner:CHENGDU KANGHONG BIOTECH
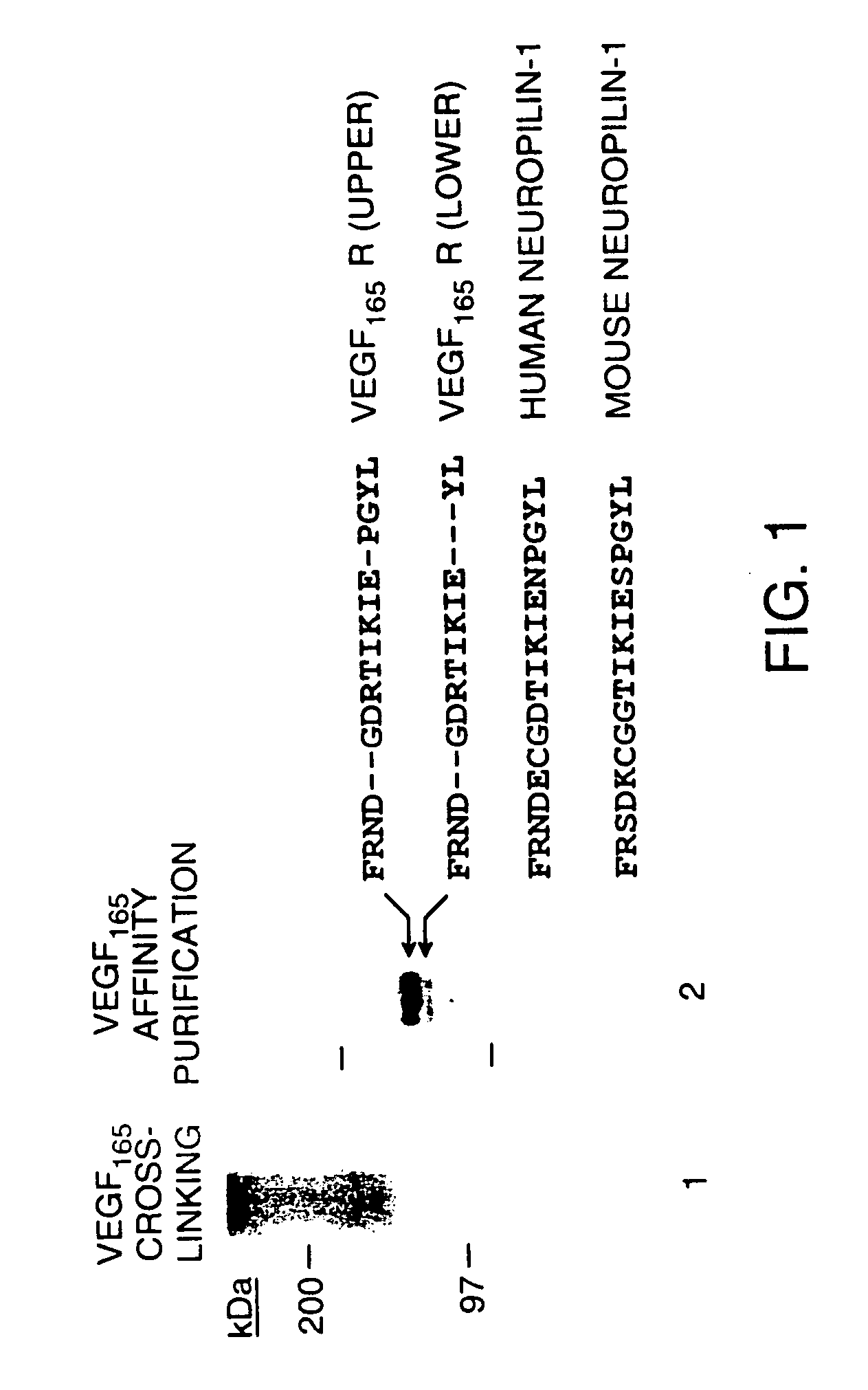
Use of vegf and homologues to treat neuron disorders
InactiveUS20030105018A1Impaired hypoxic upregulationDeterioration progressNervous disorderPeptide/protein ingredientsTruncal muscle weaknessSurvival of motor neuron
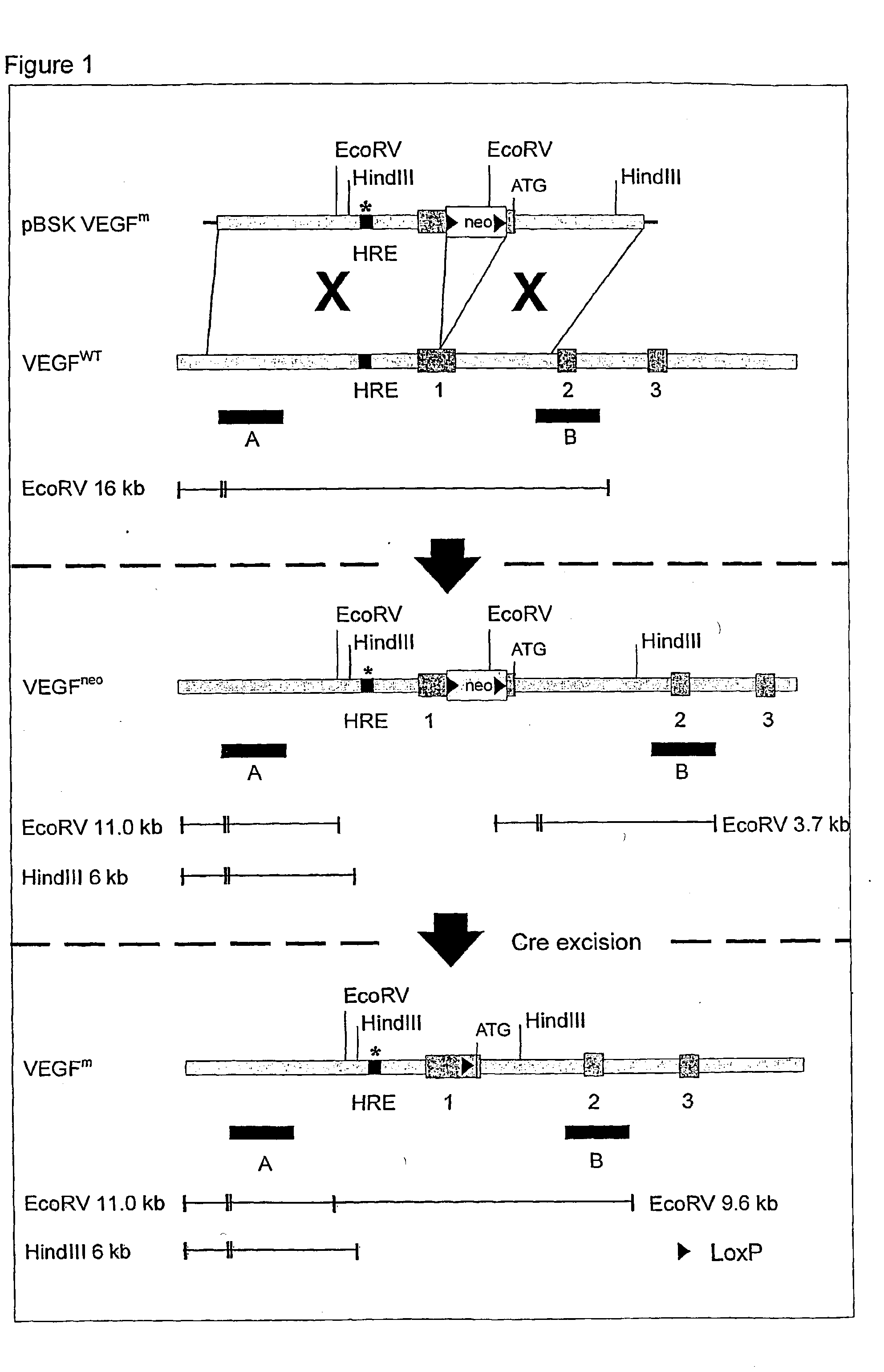
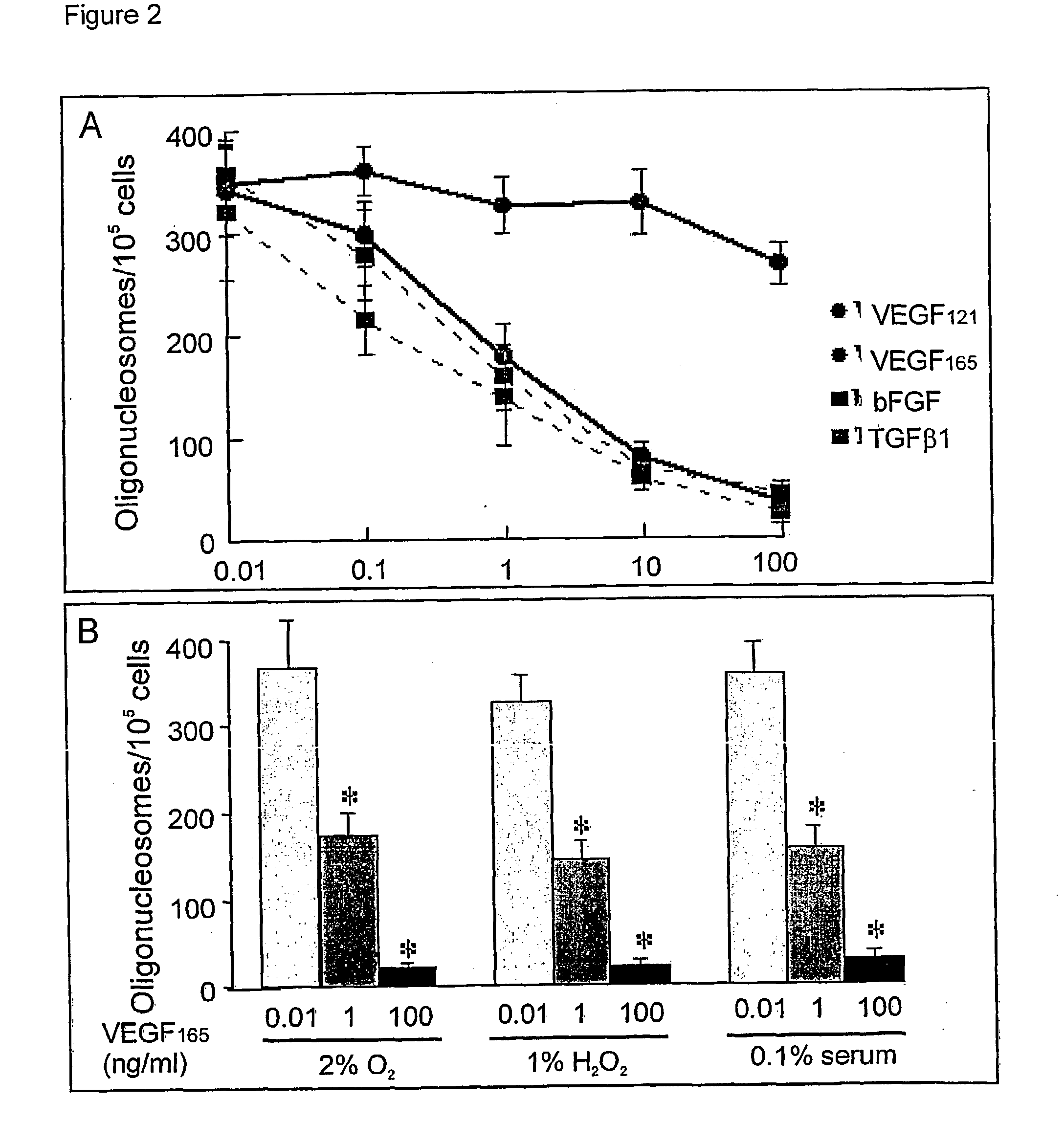
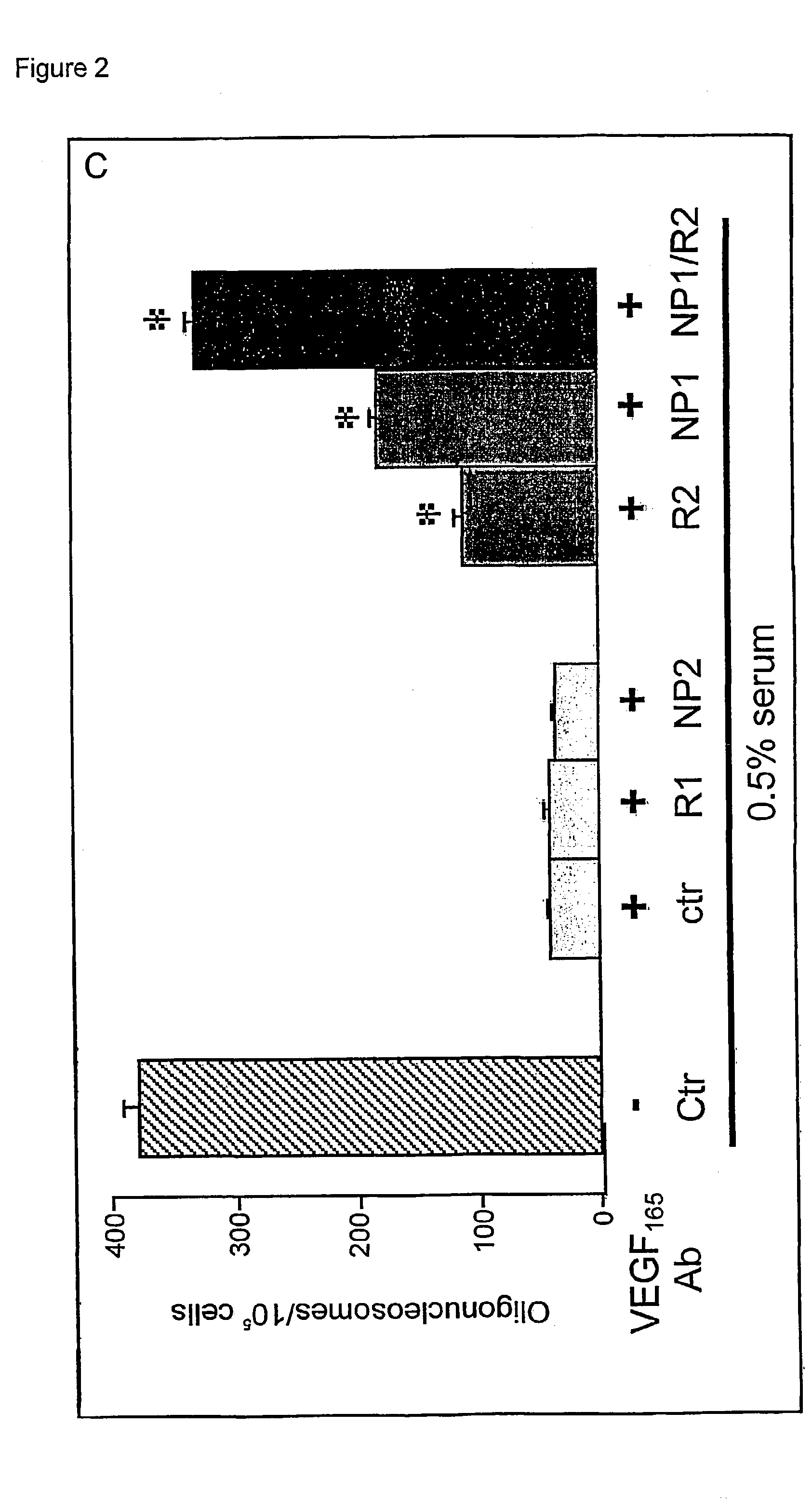
The present invention relates to neurological and physiological dysfunction associated with neuron disorders. In (particular, the invention relates to the involvement of vascular endothelial growth factor (VEGF) and homologues in the aetiology of motor neuron disorders. The invention further concerns a novel, mutant transgenic mouse (VEGFm / m) with a homozygous deletion in the hypoxia responsive element (HRE) of the VEGF promoter which alters the hypoxic upregulation of VEGF. These mice suffer severe adult onset muscle weakness due to progressive spinal motor neuron degeneration which is reminiscent of amyotrophic lateral sclerosis (ALS)-a fatal disorder with unknown aetiology. Furthermore, the neuropathy of these mice is not caused by vascular defects, but is due to defective VEGF-mediated survival signals to motor neurons. The present invention relates in particular to the isoform VEGF165 which stimulates survival of motor neurons via binding to neuropilin-1, a receptor known to bind semaphorin-3A which is implicated in axon retraction and neuronal death, and the VEGF Receptor-2. The present invention thus relates to the usage of VEGF, in particular VEGF165, for the treatment of neuron disorders and relates, in addition, to the usage of polymorphisms in the VEGF promotor for diagnosing the latter disorders.
Owner:LIFE SCI RES PARTNERS VZW +1
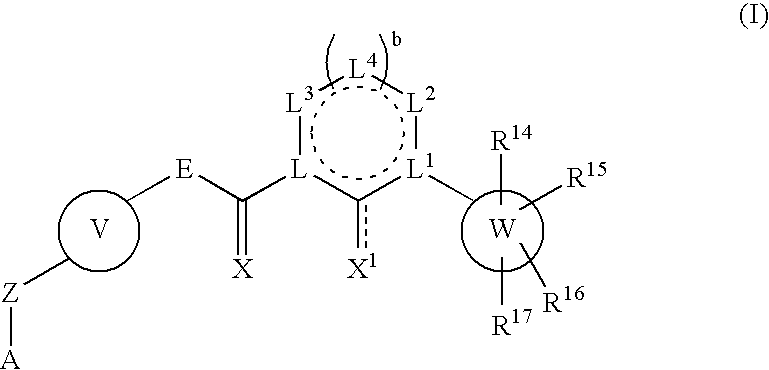
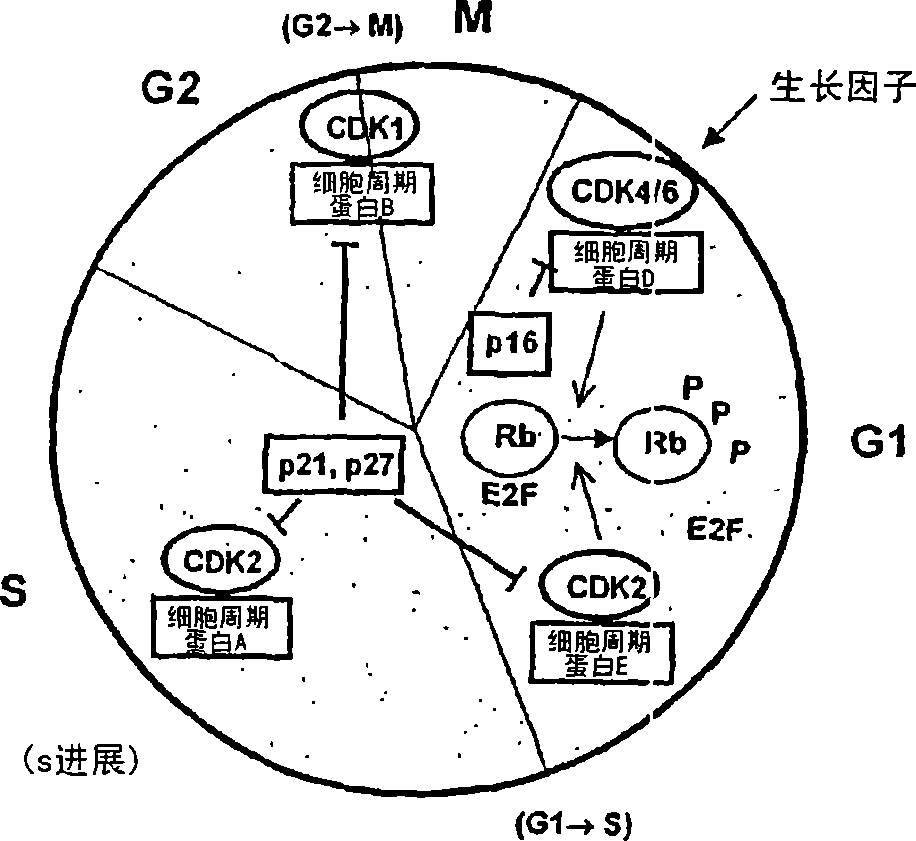
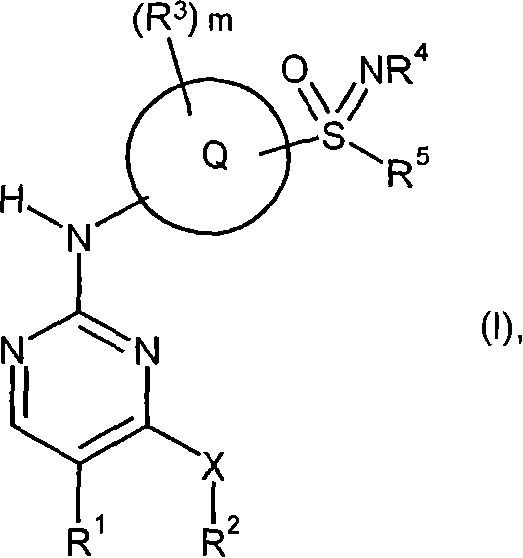
Sulfoximine-substituted pyrimidines as CDK-and/or VEGF inhibitors, their production and use as pharmaceutical agents
The present invention relates to pyrimidine derivatives of formula (I), which are useful as inhibitors of cyclin-dependent kinases and VEGF receptor tyrosine kinases. The present invention also relates to a method for preparing the derivative and its use as a medicine for treating various diseases.
Owner:BAYER IP GMBH
Liposome gel preparation and preparation method and application thereof
ActiveCN102697705AImprove poor water solubilityImprove stabilityAerosol deliveryOintment deliveryGel preparationLipid particle
The invention provides a liposome gel preparation for treating hypertrophic scars. The liposome gel preparation comprises liposome, gel material and glycerol, wherein the liposome is encapsulated with paeonol, and the surface of the liposome is bonded with vascular endothelial growth factor (VEGF) antibodies through polyethylene glycol. The invention provides a preparation method and application of the gel preparation at the same time. The gel preparation is encapsulated by using a material such as phospholipid with good physiological compatibility with the human body, so that the problems of poor water solubility and low stability of the paeonol are effectively solved. The VEGF antibodies are used for modifying the surface of the liposome, lipid particles enter the dermis through the epidermis by using the permeation promoting effect of liposome carriers and are directionally bonded with VEGF receptors of the pathological change skin part, and the encapsulated medicament is released at the same time, so that positioned retention of the medicament in the scar hyperplasia part is realized. Compared with other reported scar hyperplasia resistant medicament preparations or treatment schemes, the invention has the advantages that the liposome gel preparation is low in relapse rate, reduces the occurrence rate of the toxic or side effect and can be applied to any scar hyperplasia part of the body.
Owner:GUANGDONG PHARMA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com