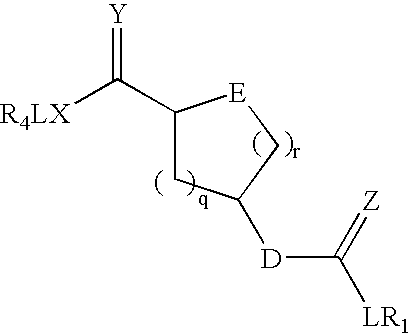
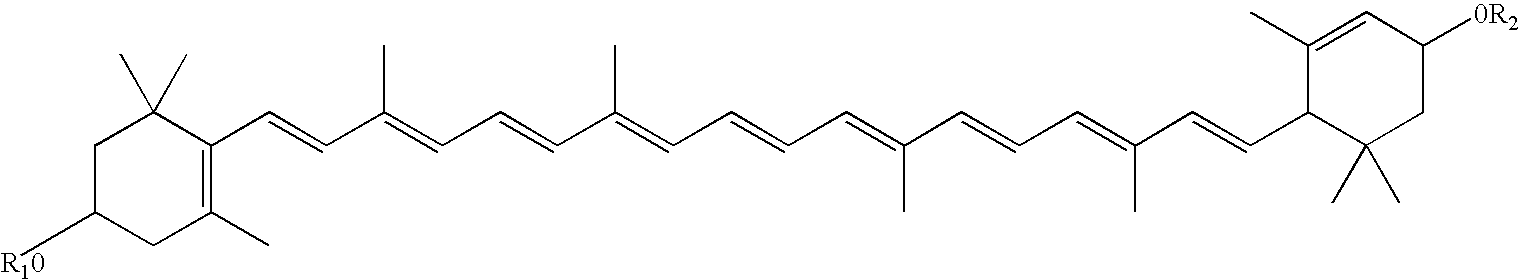
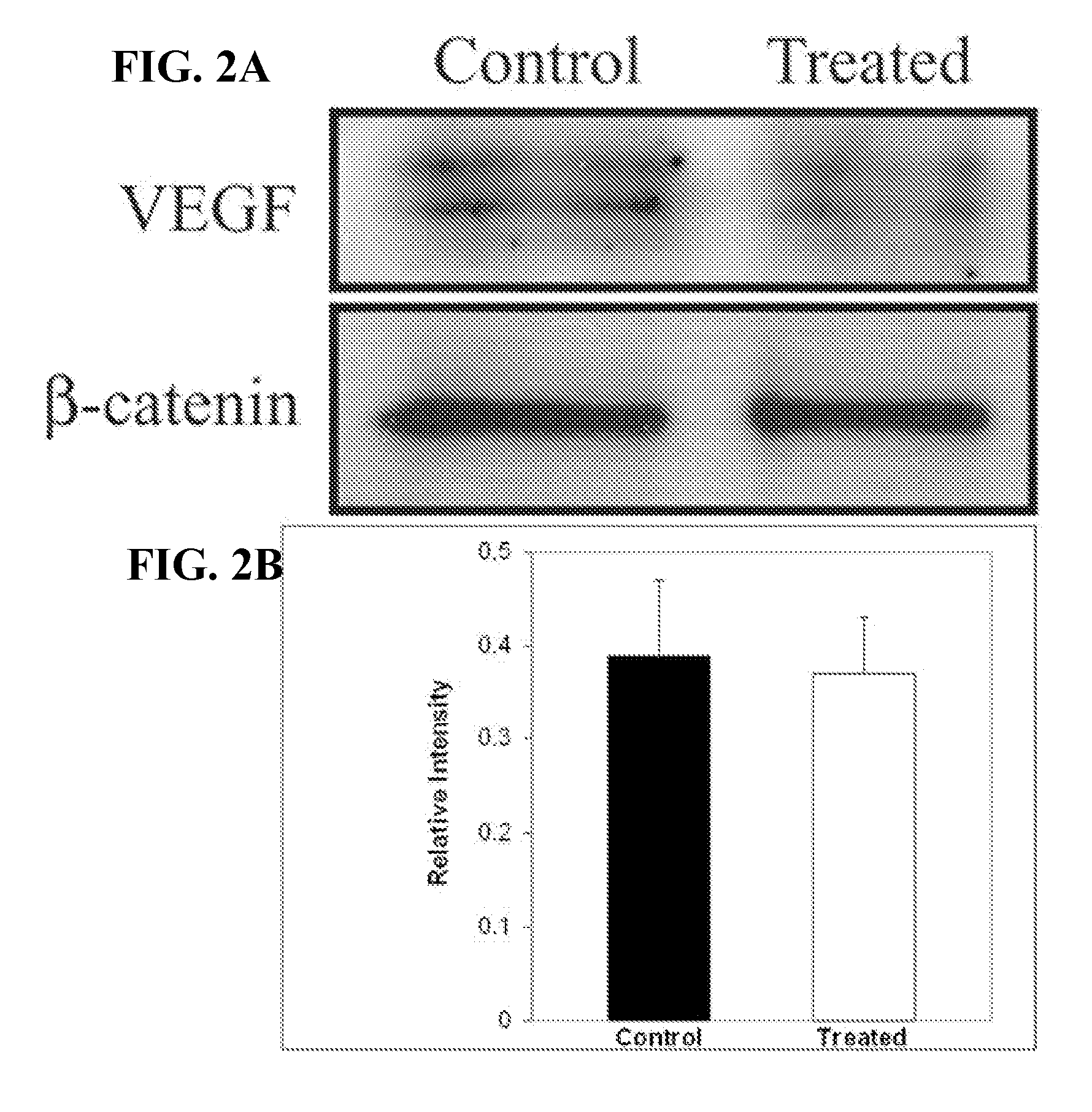
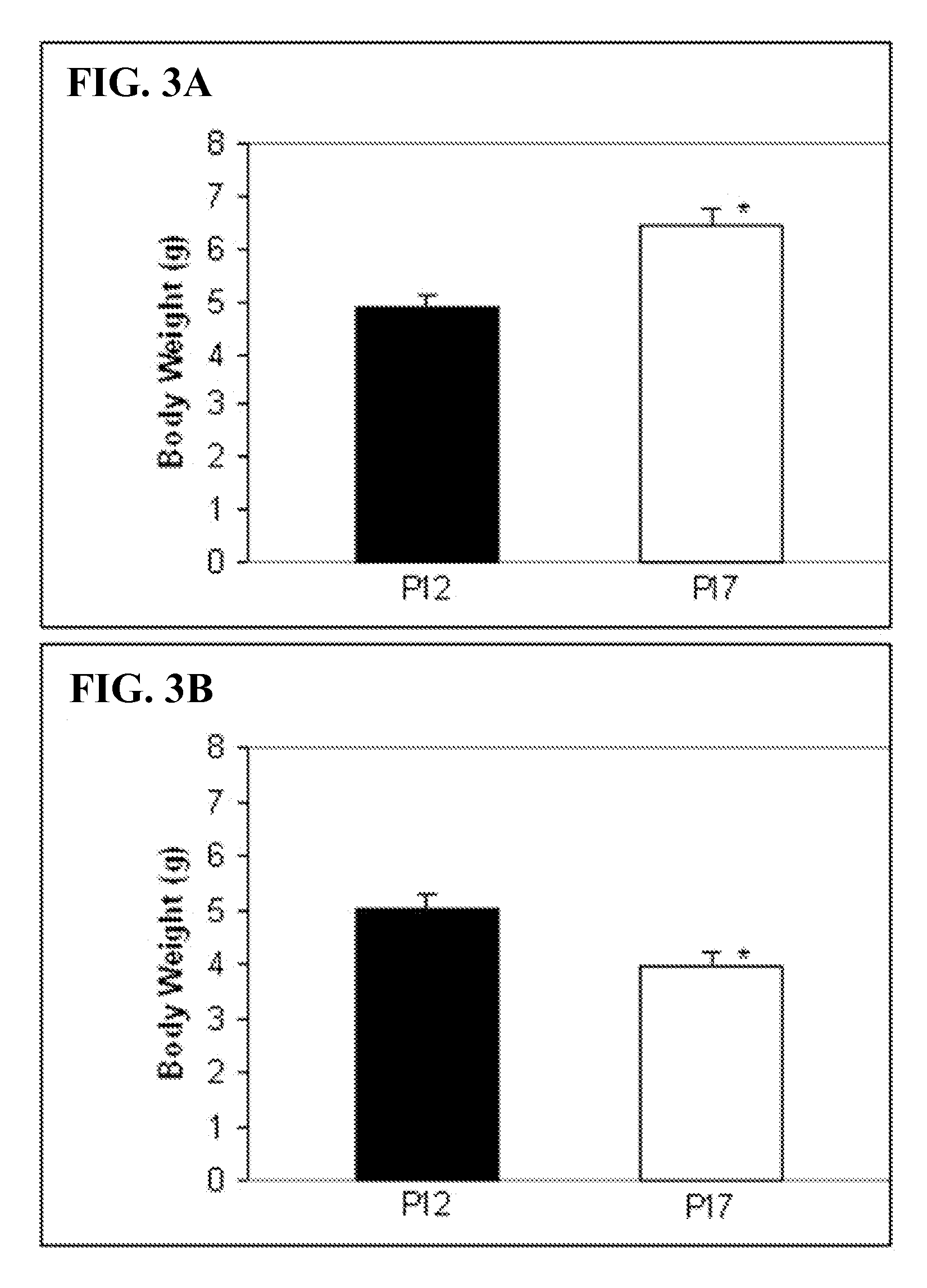
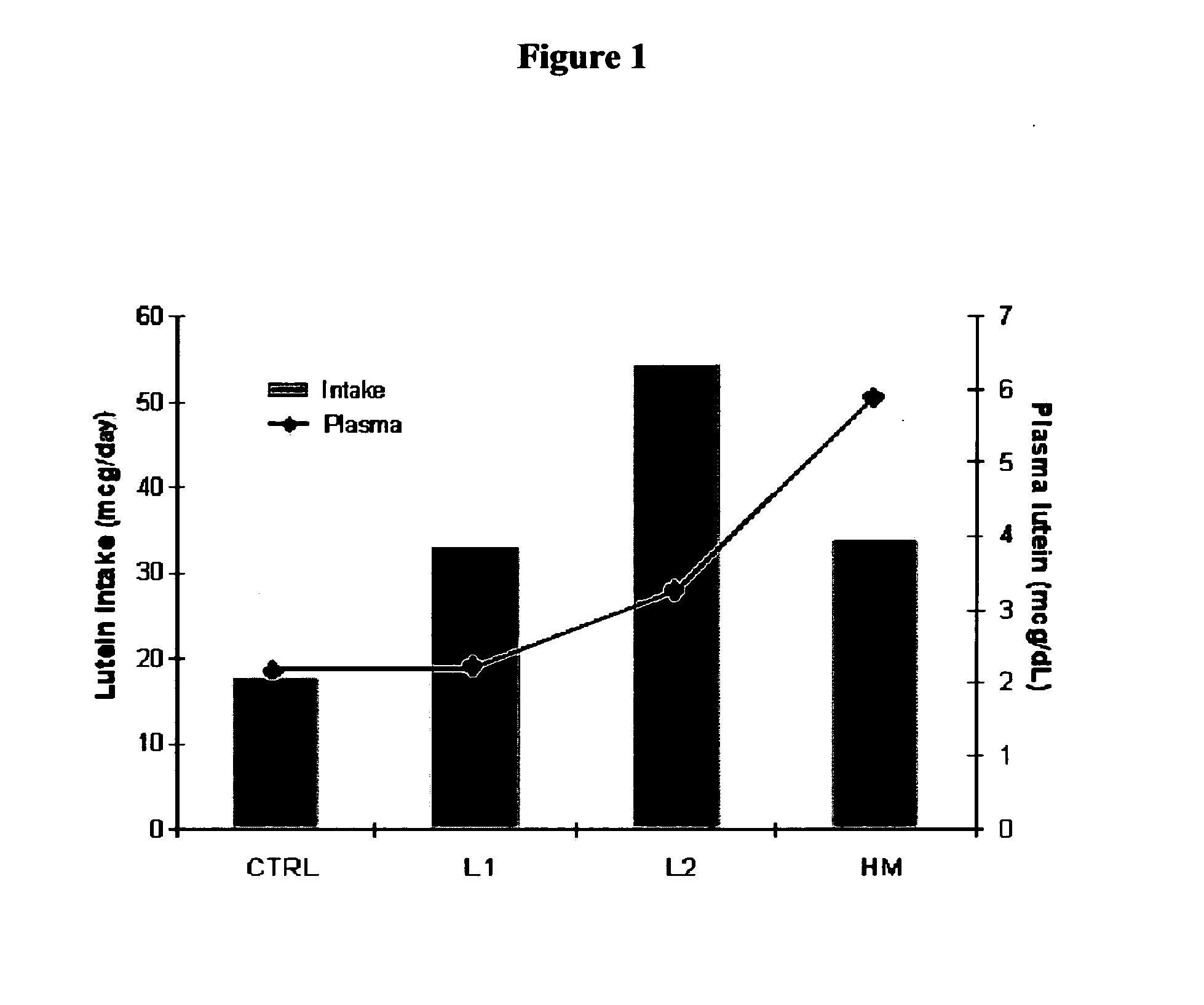
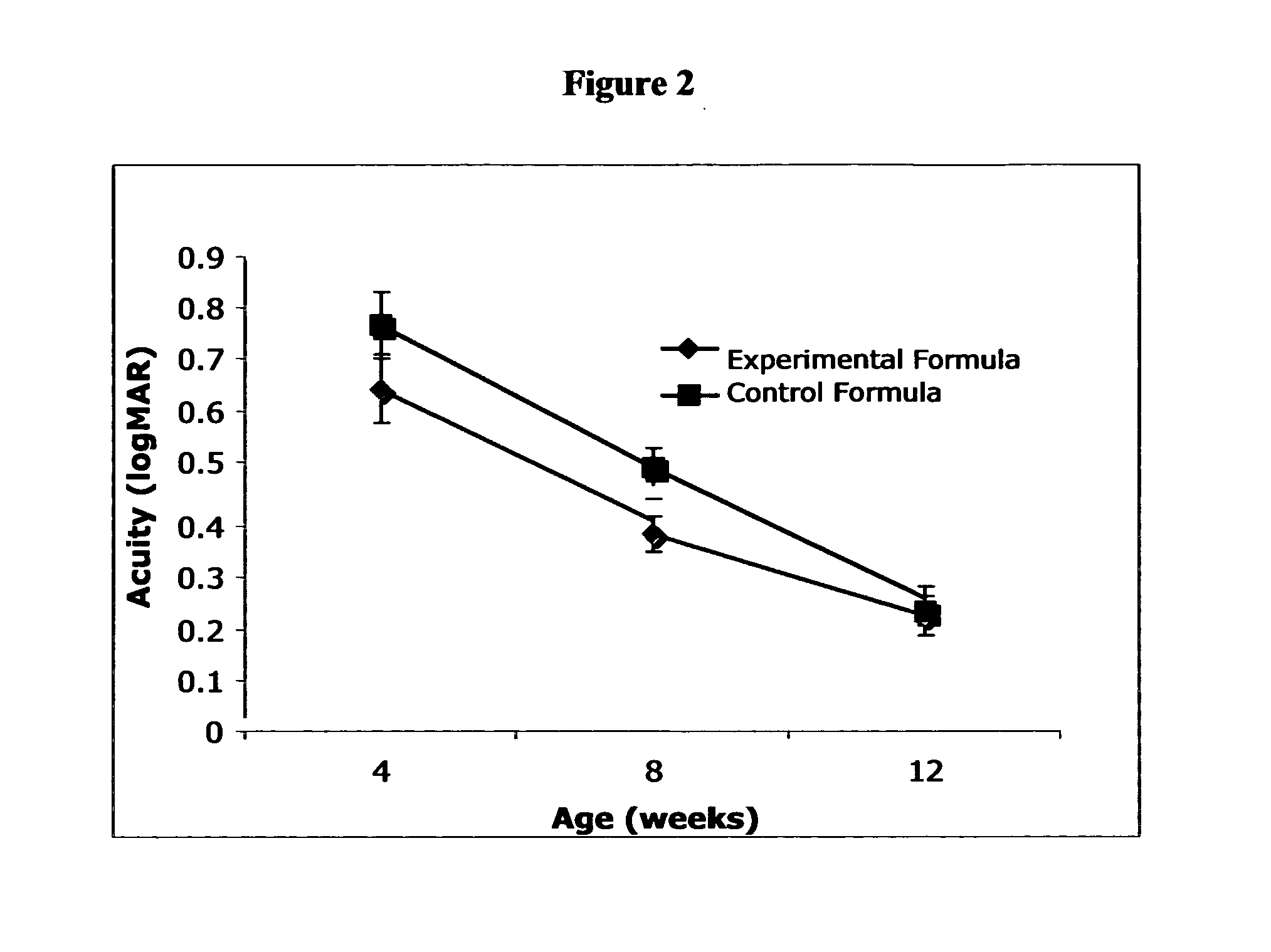
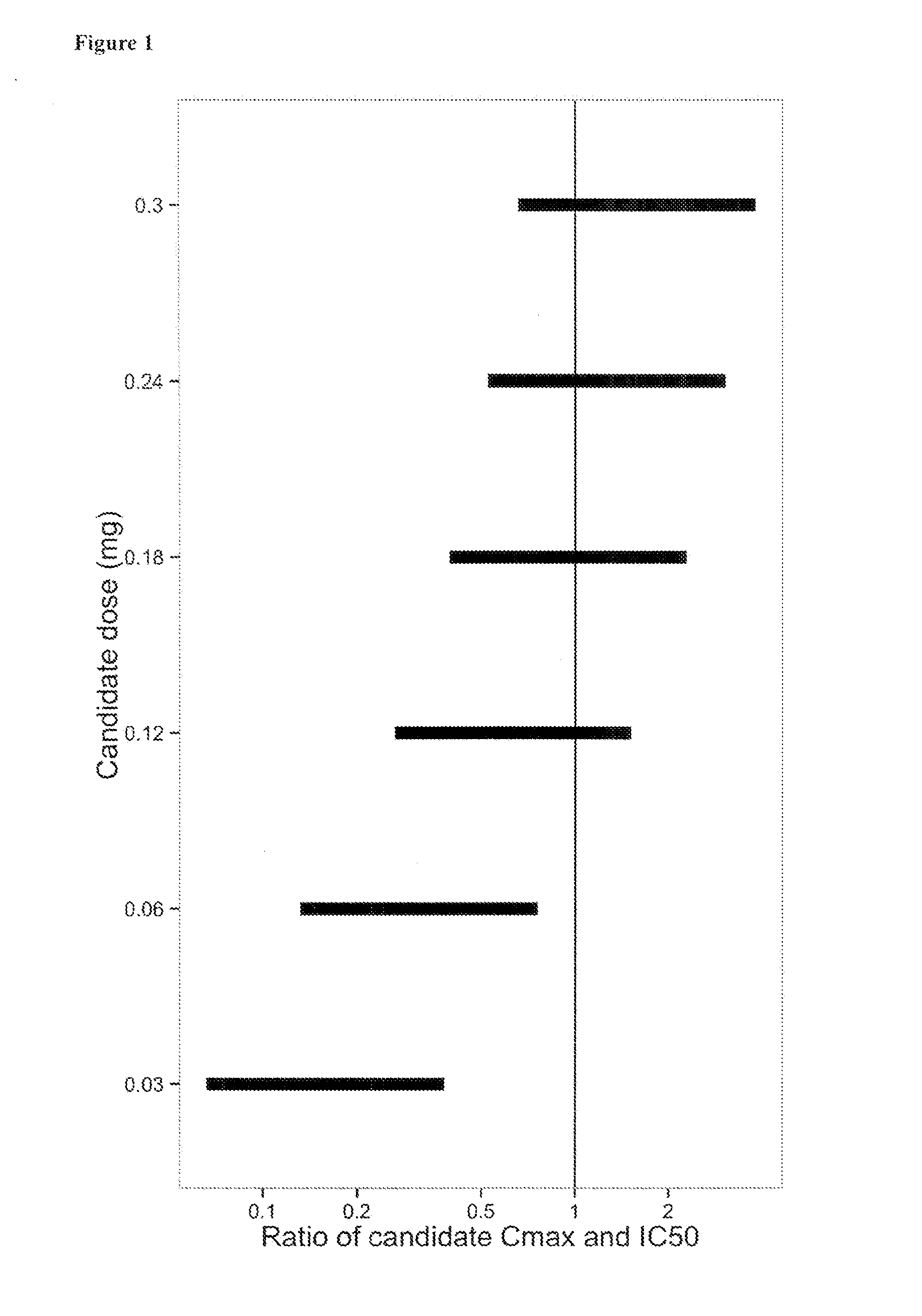
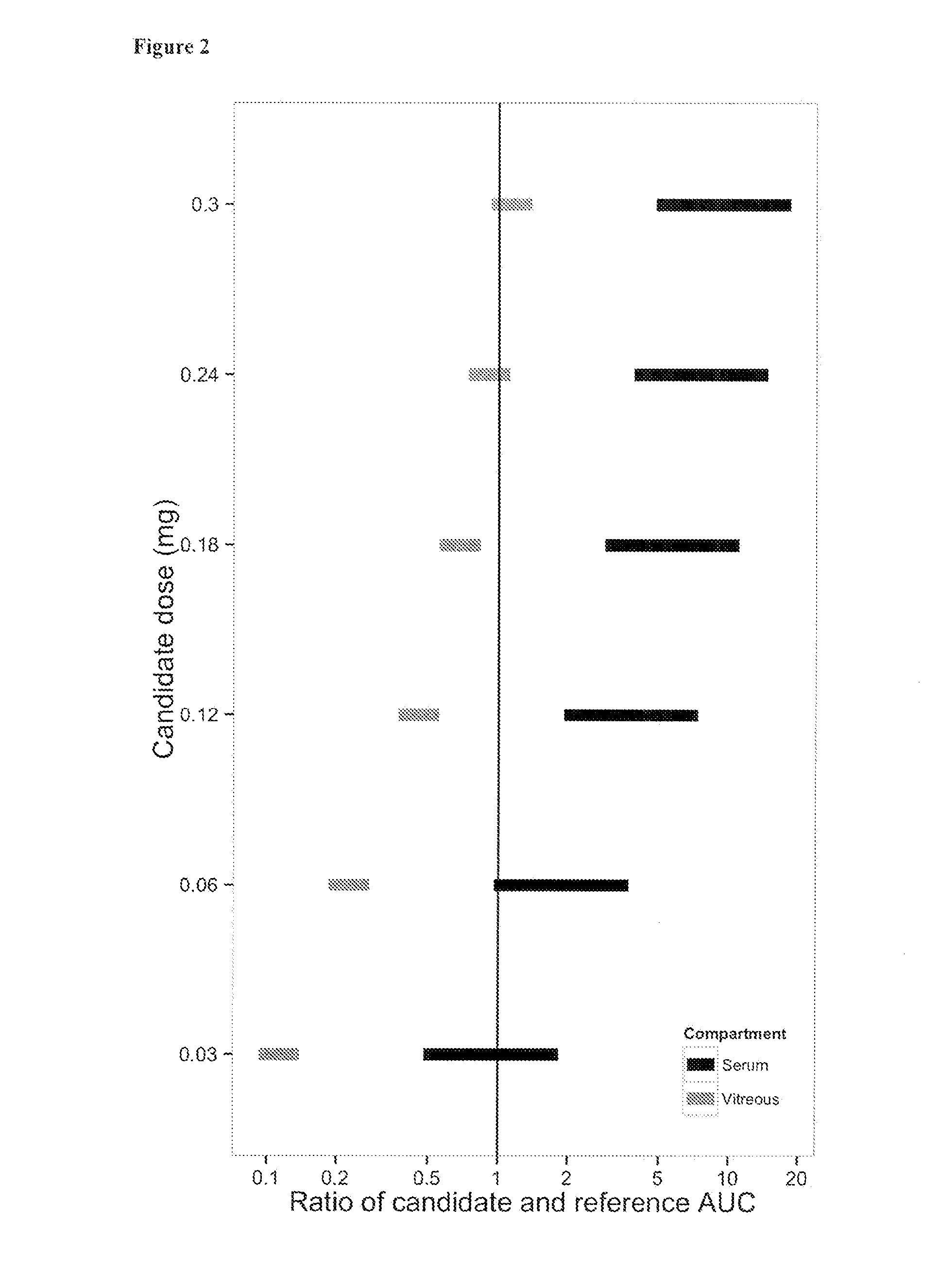
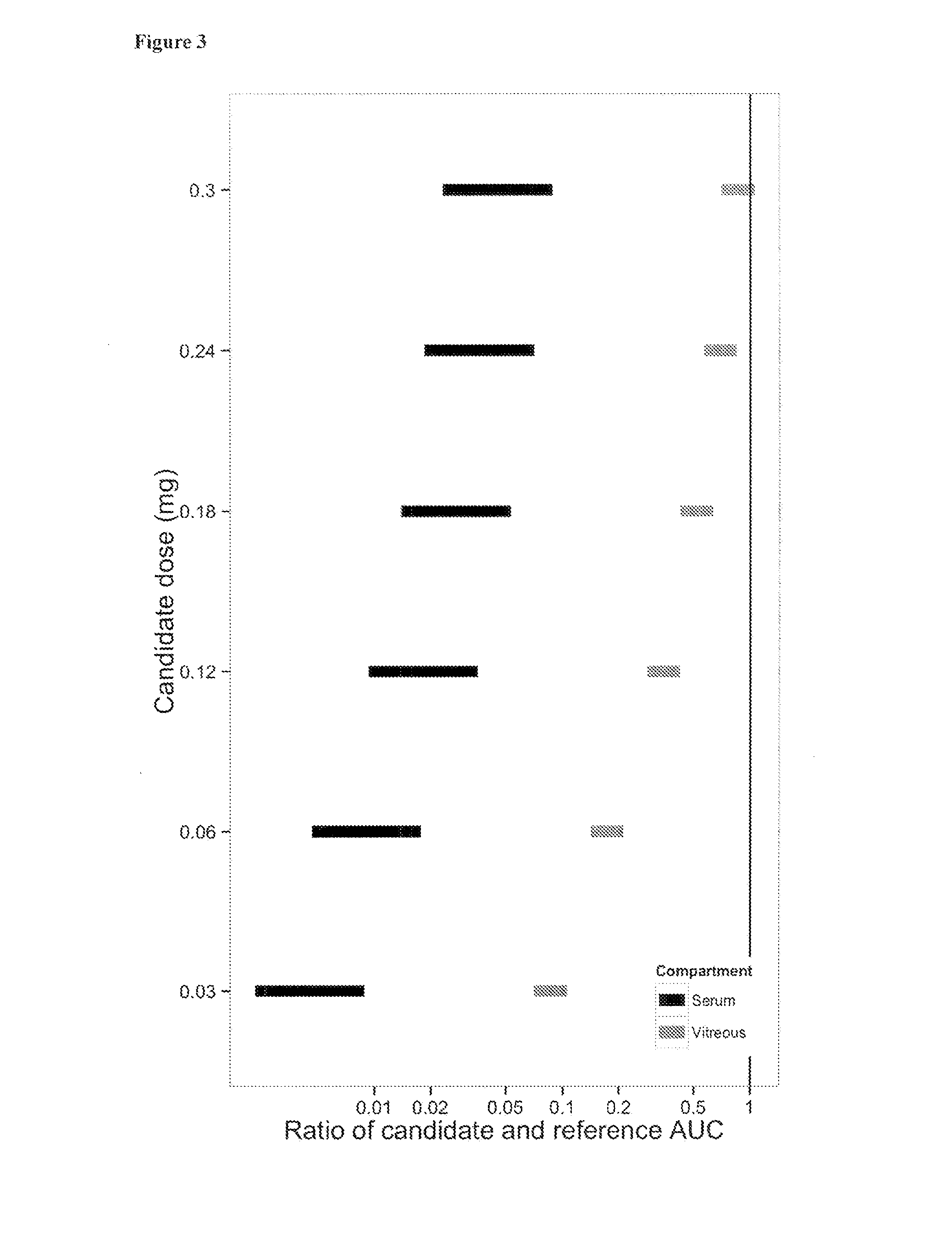
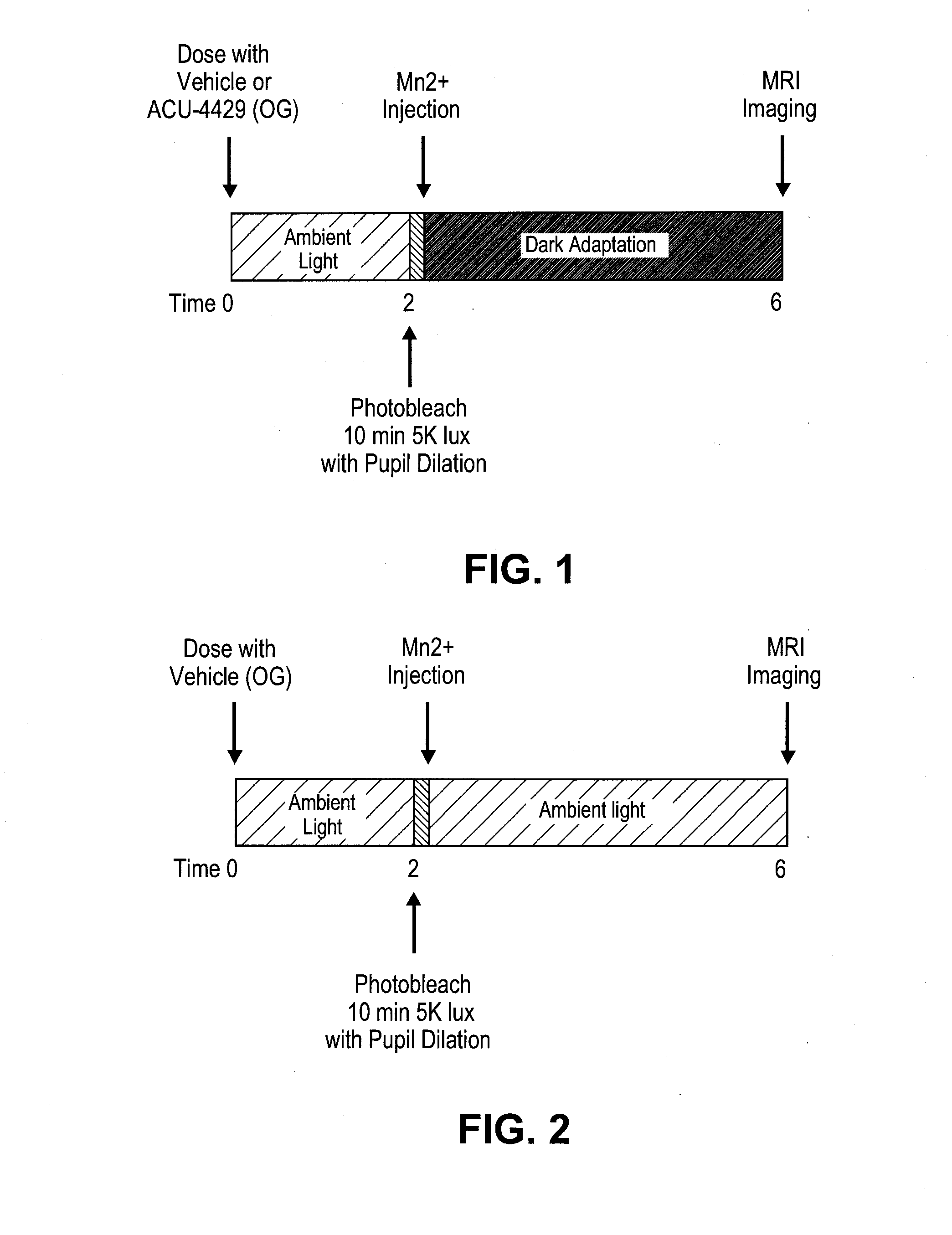
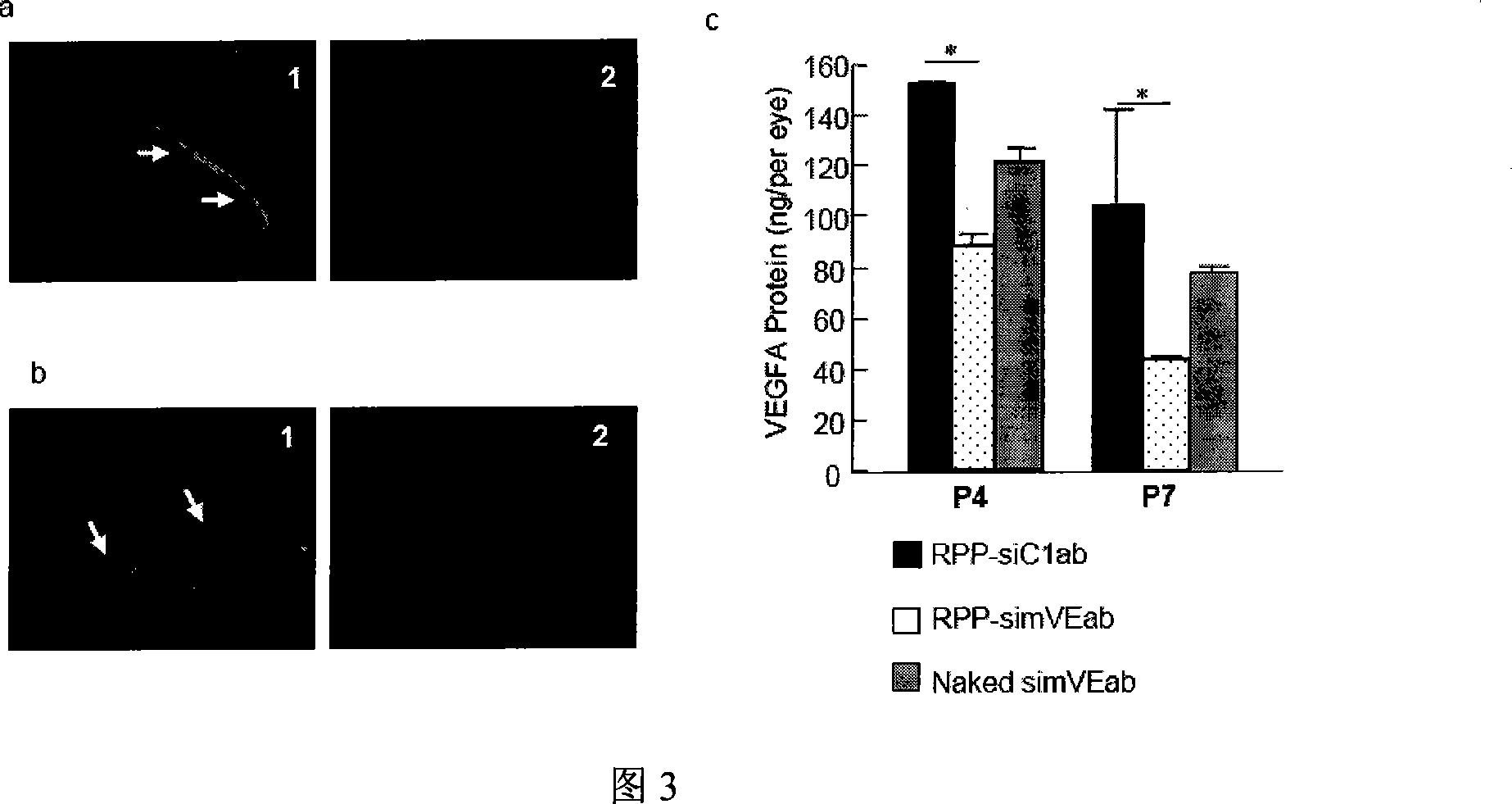
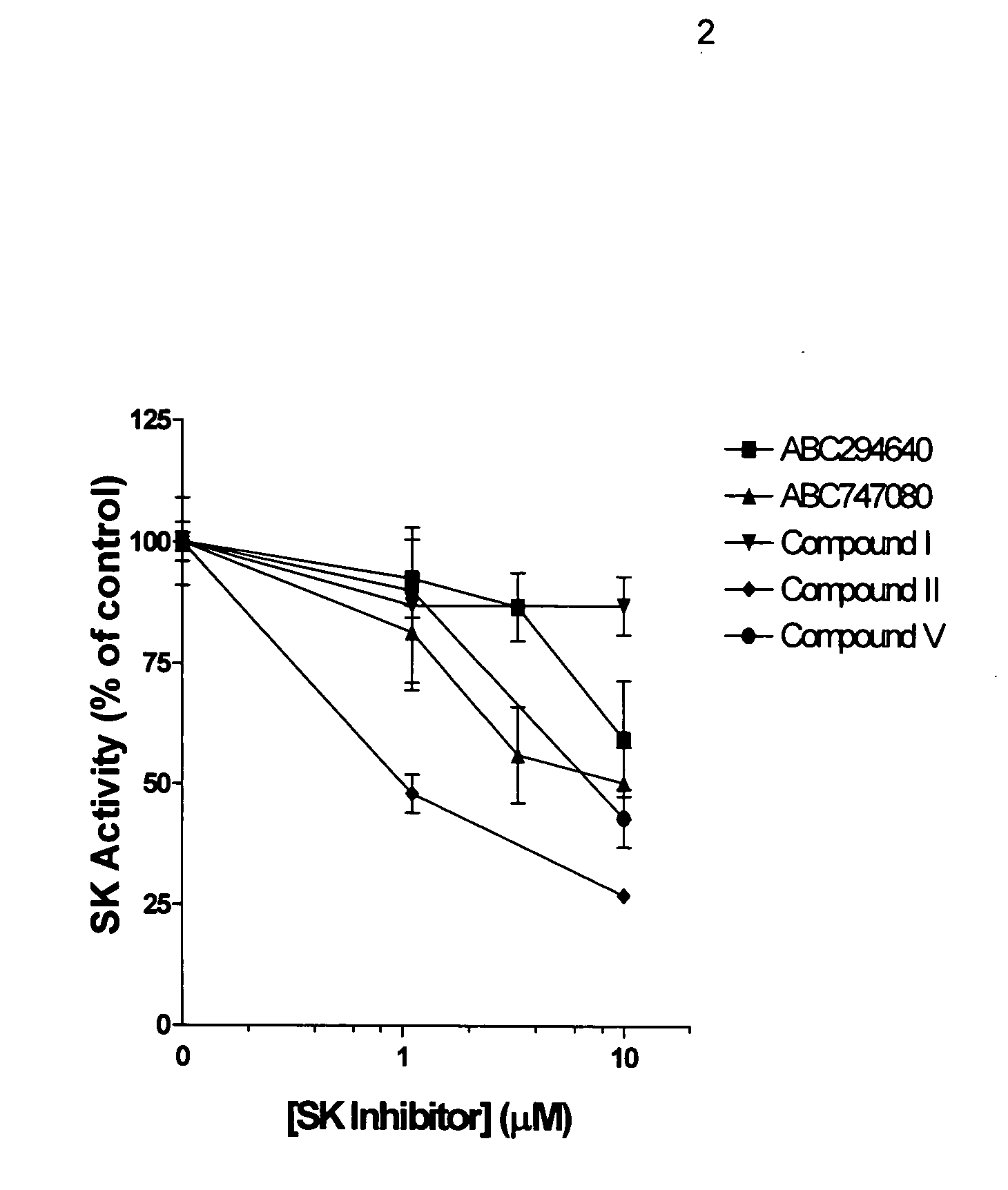
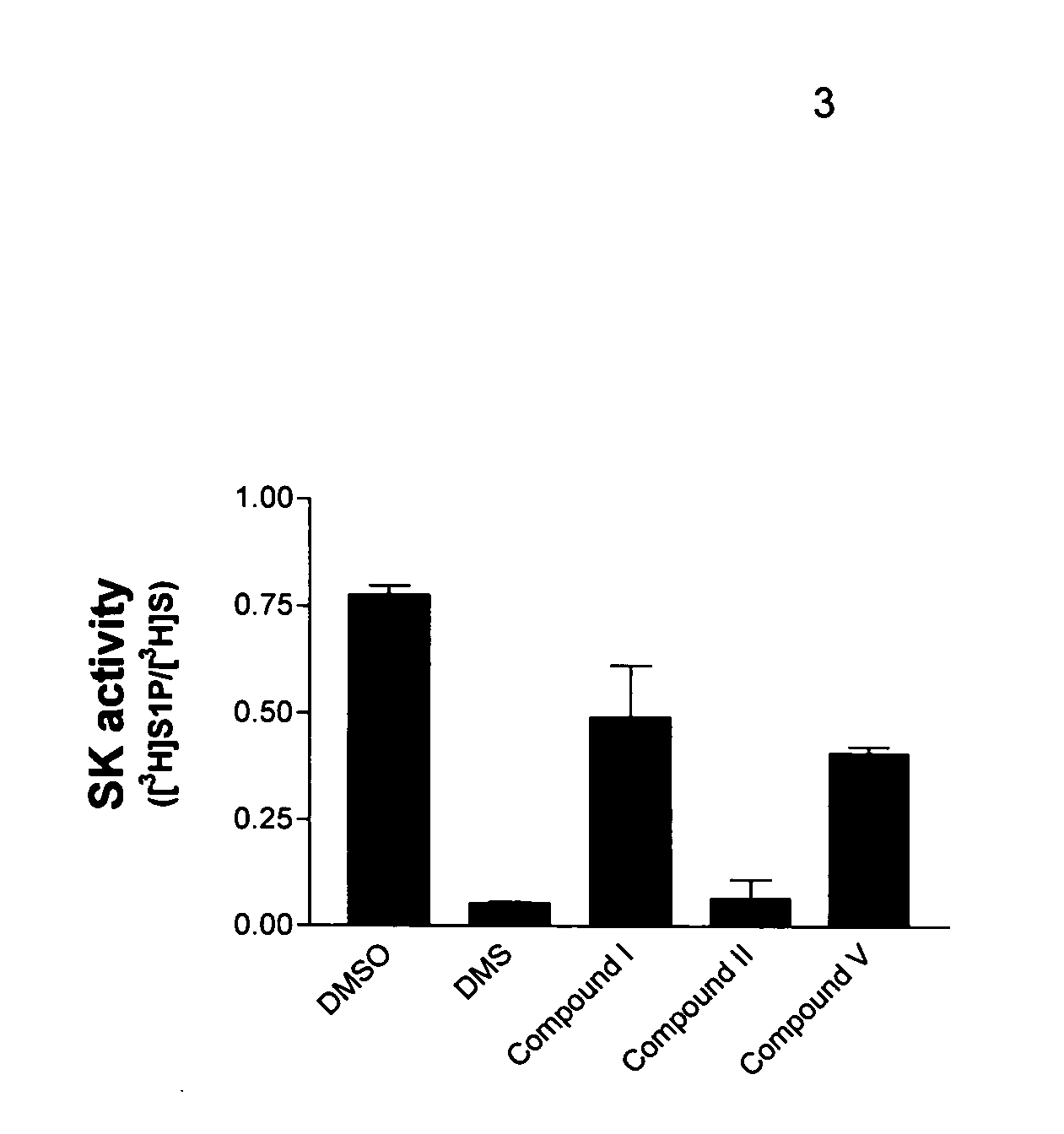
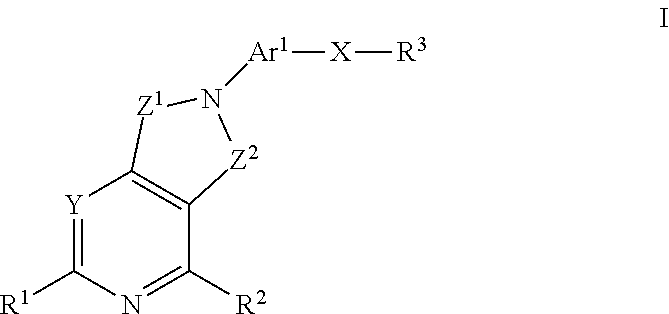
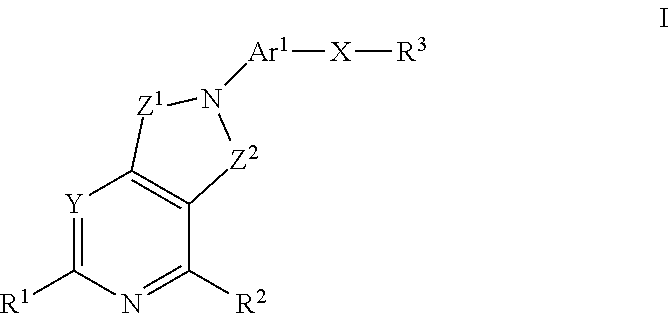
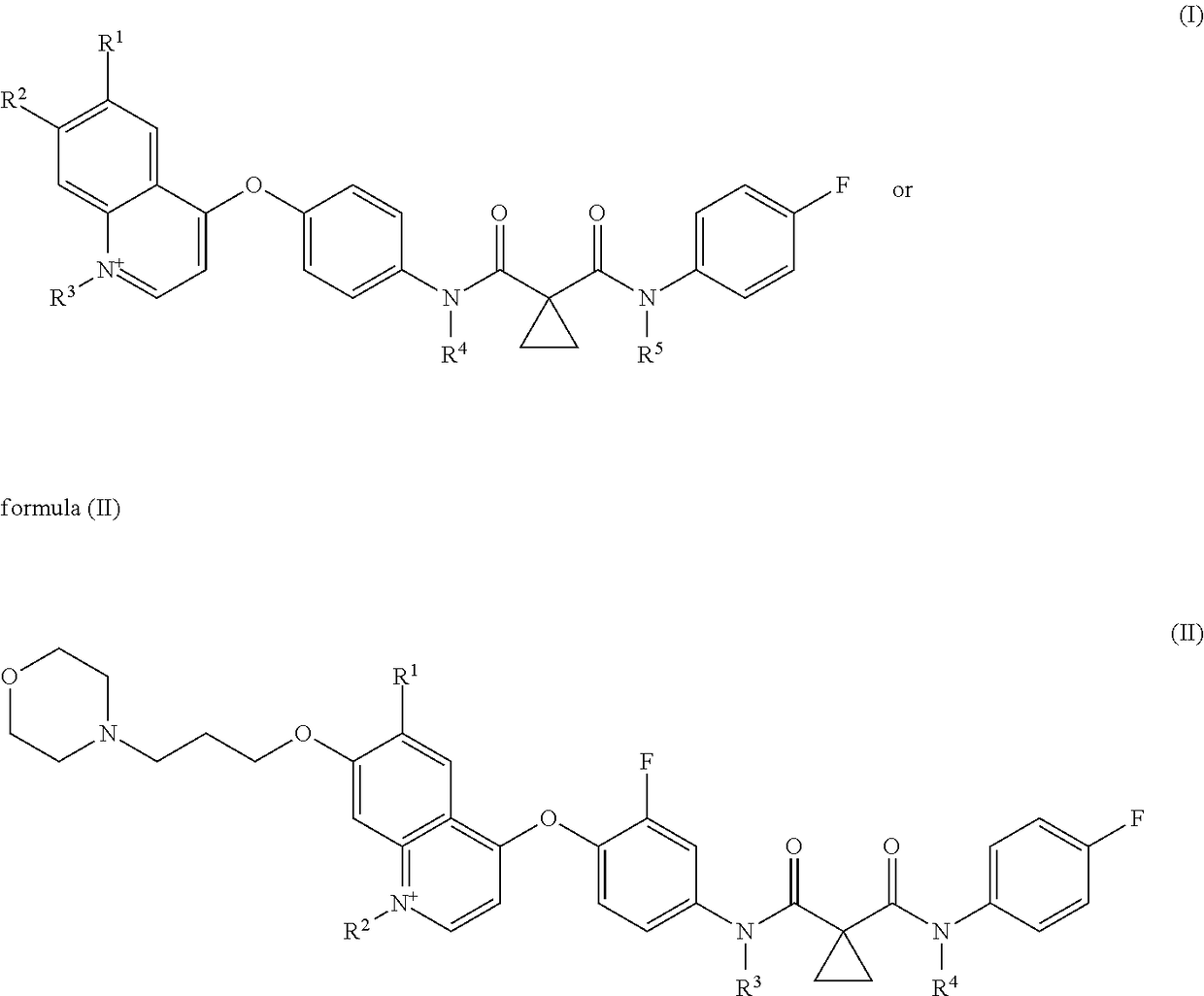
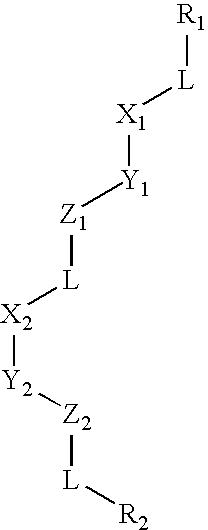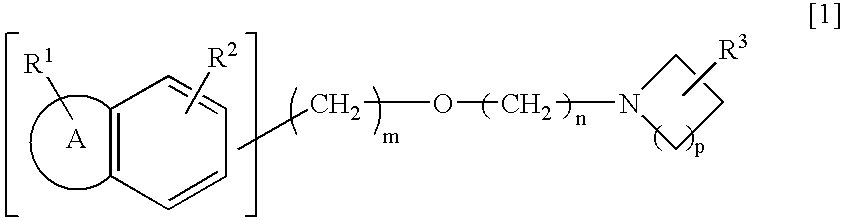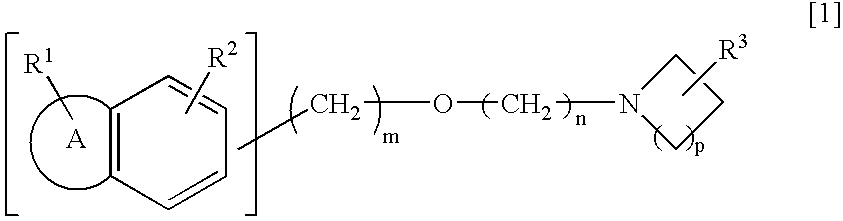Patents
Literature
86 results about "Retinopathy of prematurity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Retinopathy of prematurity (ROP), also called retrolental fibroplasia (RLF) and Terry syndrome, is a disease of the eye affecting prematurely born babies generally having received intensive neonatal care, in which oxygen therapy is used on them due to the premature development of their lungs. It is thought to be caused by disorganized growth of retinal blood vessels which may result in scarring and retinal detachment. ROP can be mild and may resolve spontaneously, but it may lead to blindness in serious cases. As such, all preterm babies are at risk for ROP, and very low birth-weight is an additional risk factor. Both oxygen toxicity and relative hypoxia can contribute to the development of ROP.
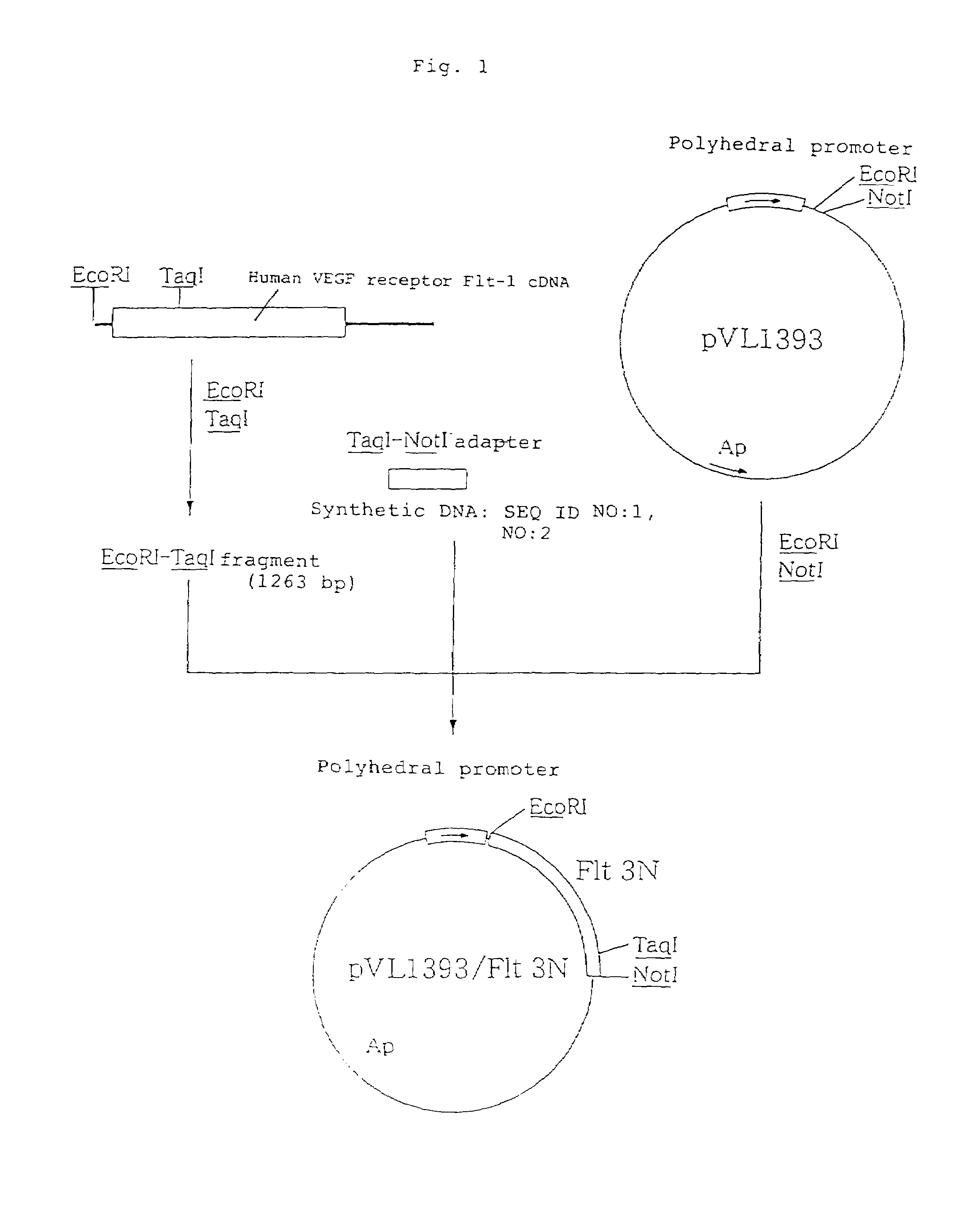
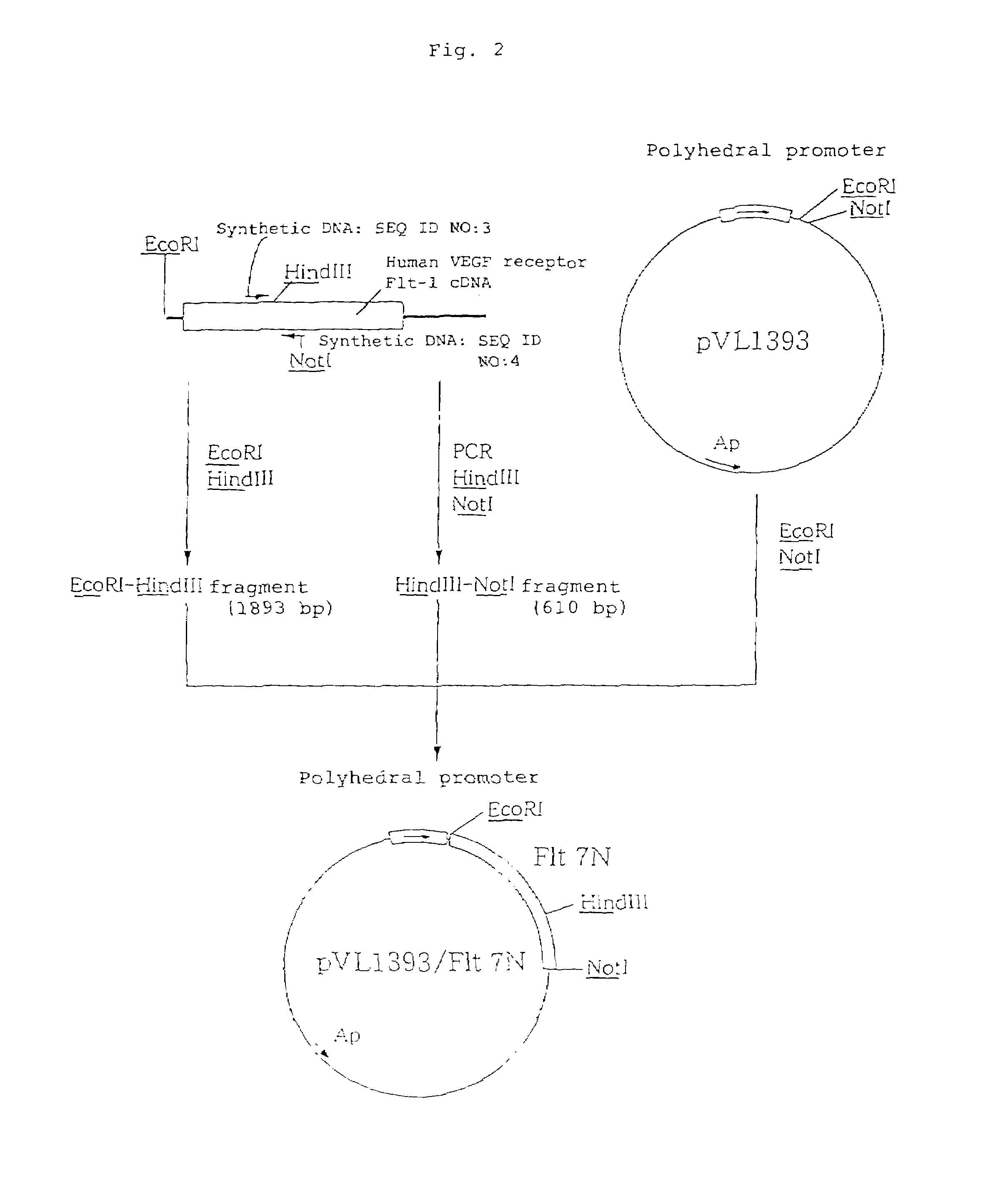
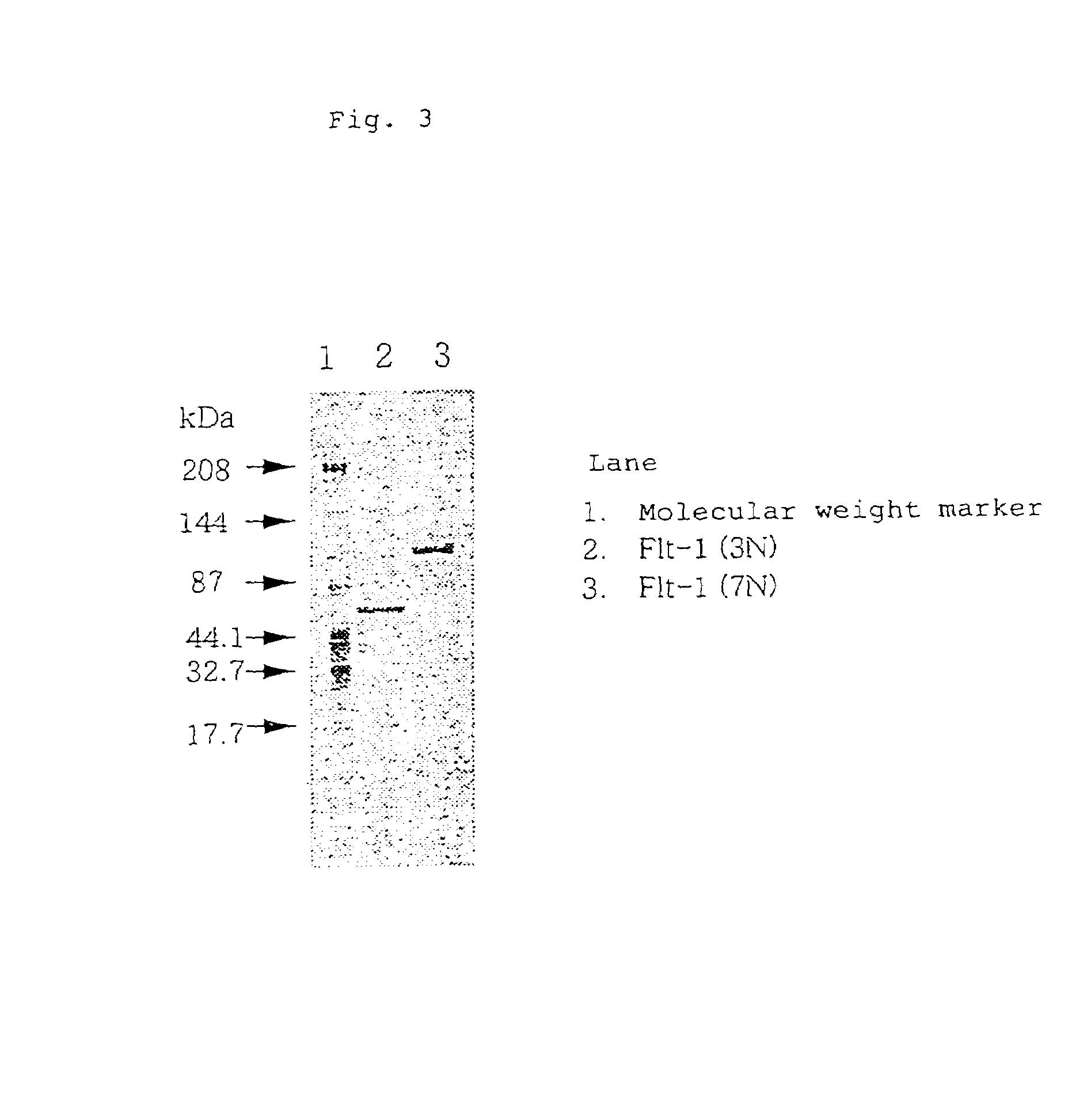
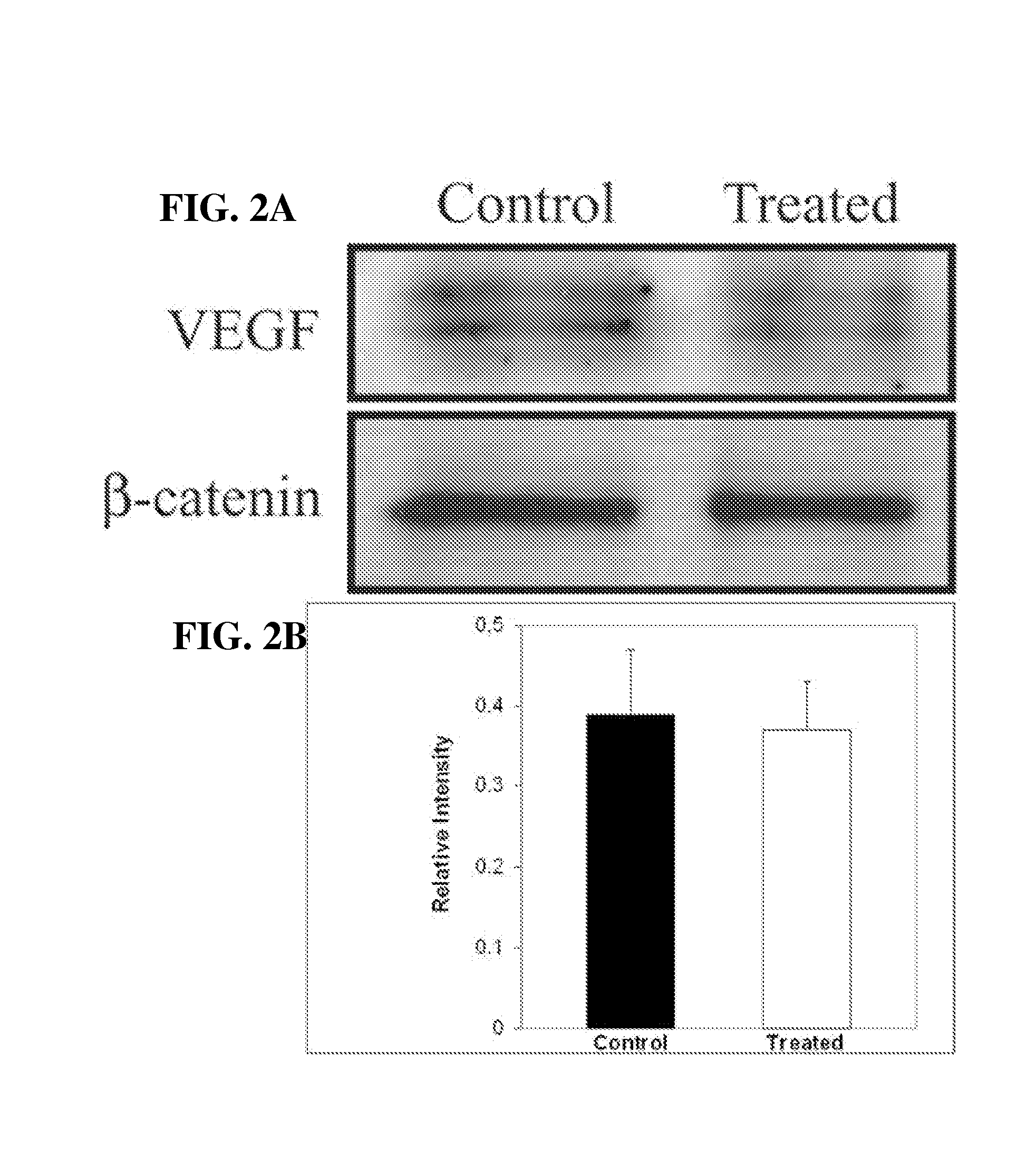
Anti-human VEGF receptor Flt-1 monoclonal antibody
The present invention provides an antibody or peptide which immunologically reacts with human VEGF receptor Flt-1 and cells in which human VEGF receptor Flt-1 is expressed on the cell surface and an antibody or peptide which inhibits binding of human VEGF to human VEGF receptor Flt-1. It also provides a means for the diagnosis or treatment of diseases in which their morbid states progress by abnormal angiogenesis, such as proliferation or metastasis of solid tumors, arthritis in rheumatoid arthritis, diabetic retinopathy, retinopathy of prematurity, psoriasis, and the like.
Owner:KYOWA HAKKO KIRIN CO LTD
Use of compounds that interfere with the hedgehog signaling pathway for the manufacture of a medicament for preventing, inhibiting, and/or reversing ocular diseases related with ocular neovascularization
InactiveUS7517870B2High unmet needControl rateBiocideOrganic active ingredientsDiabetic retinopathyHedgehog signaling pathway
The present invention concerns the use of compounds that interfere with the hedgehog signaling pathway for the manufacture of a medicament for preventing, inhibiting, and / or reversing ocular diseases related with ocular neovascularization. Particularly, the above-mentioned diseases are (wet) age-related macular degeneration, (proliferative) diabetic retinopathy, neovascular glaucoma, retinal vein occlusion, or retinopathy of prematurity (ROP).
Owner:FOND AZIONE TELETHON
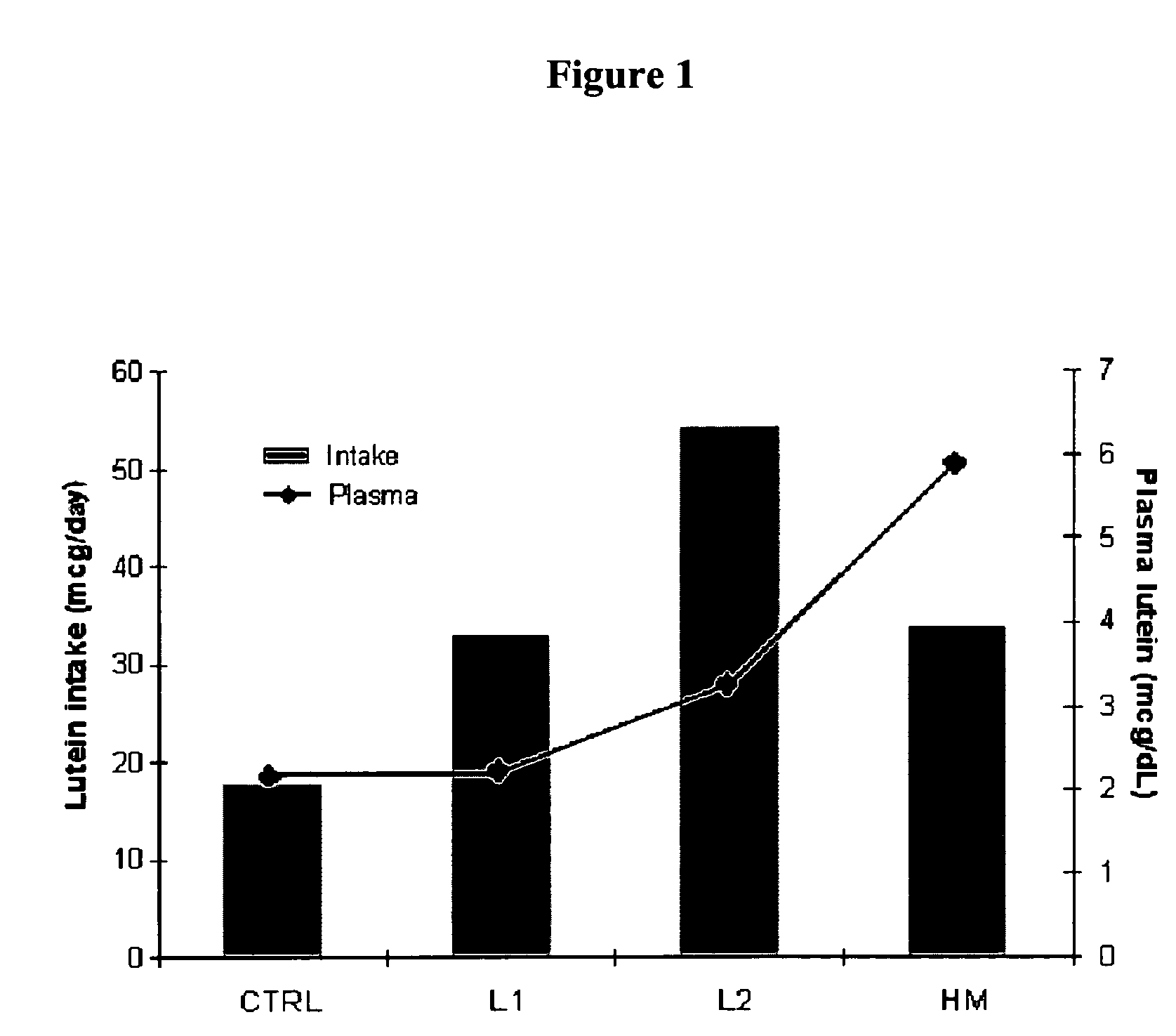
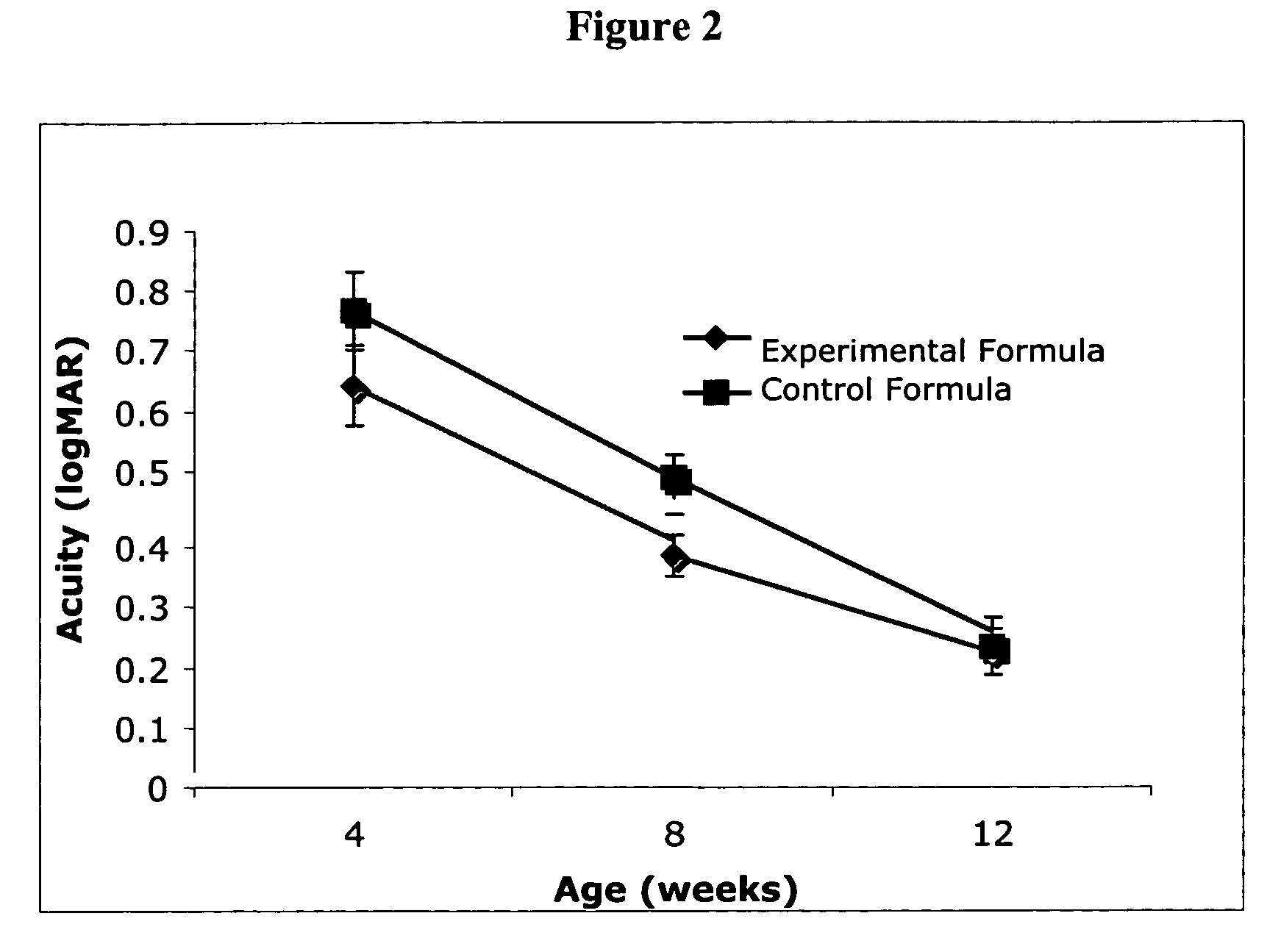
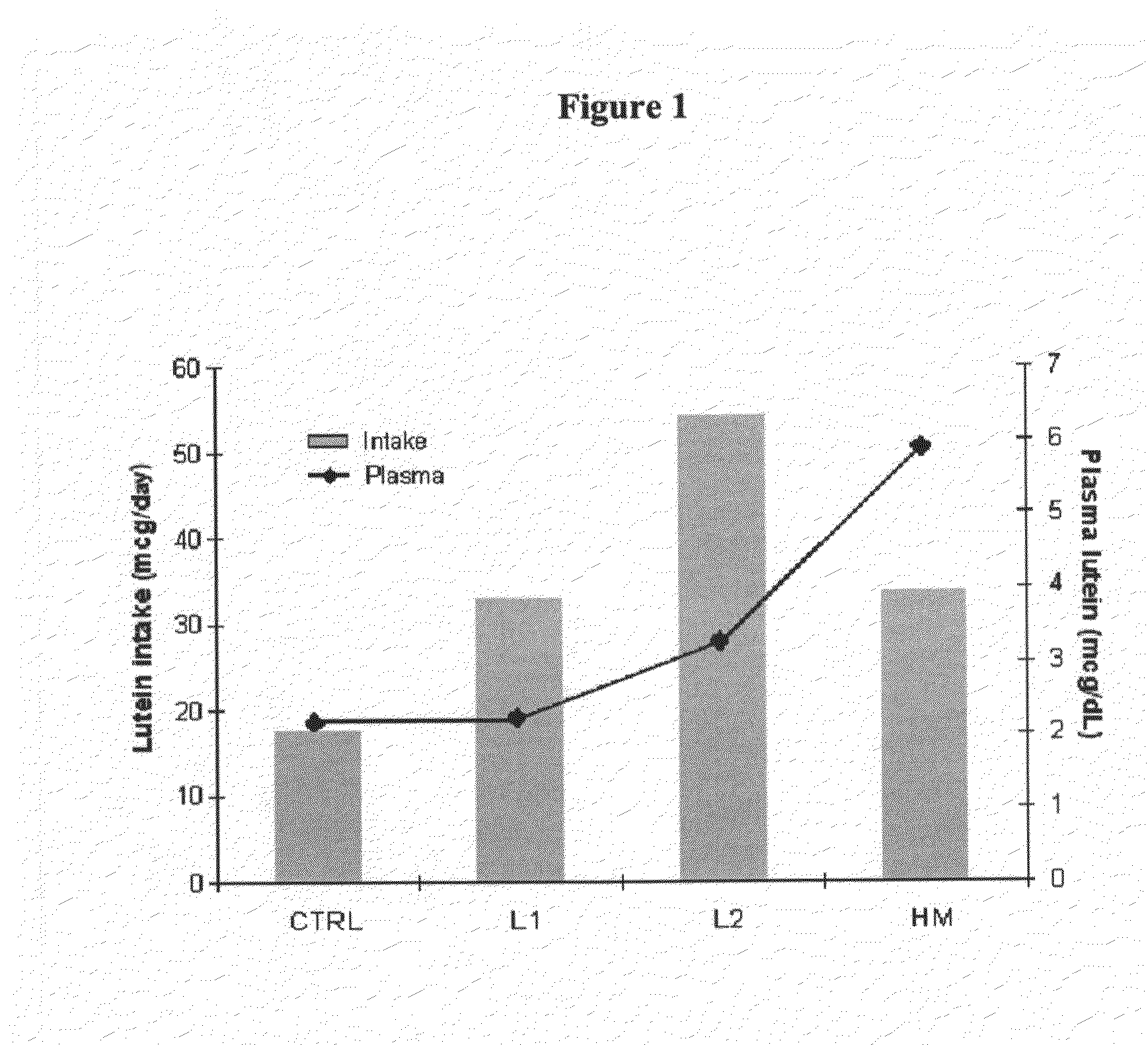
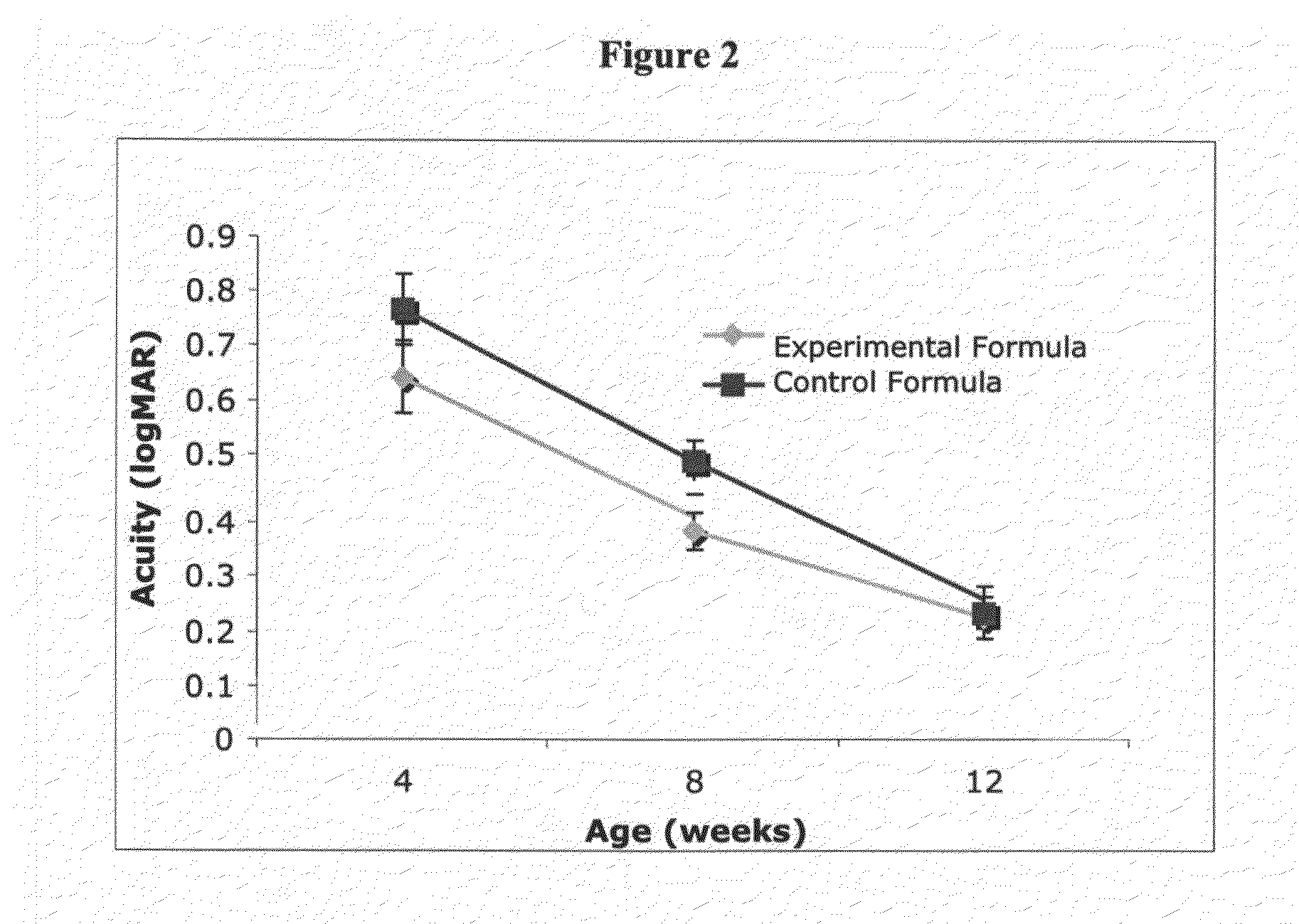
Infant formulas containing docosahexaenoic acid and lutein
ActiveUS20070098849A1Promote retinal healthPromote vision developmentBiocideHeavy metal active ingredientsLuteinDHA - Docosahexaenoic acid
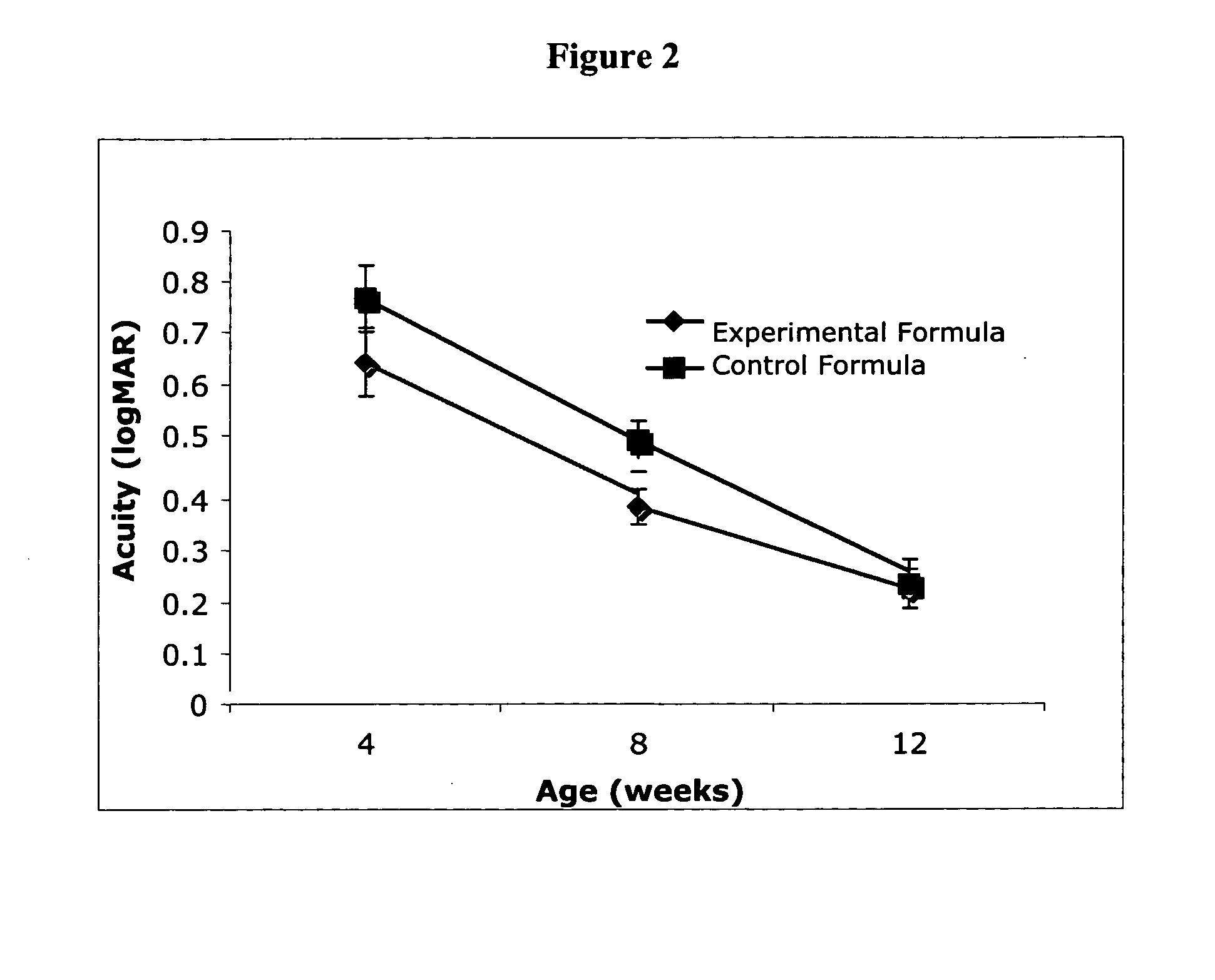
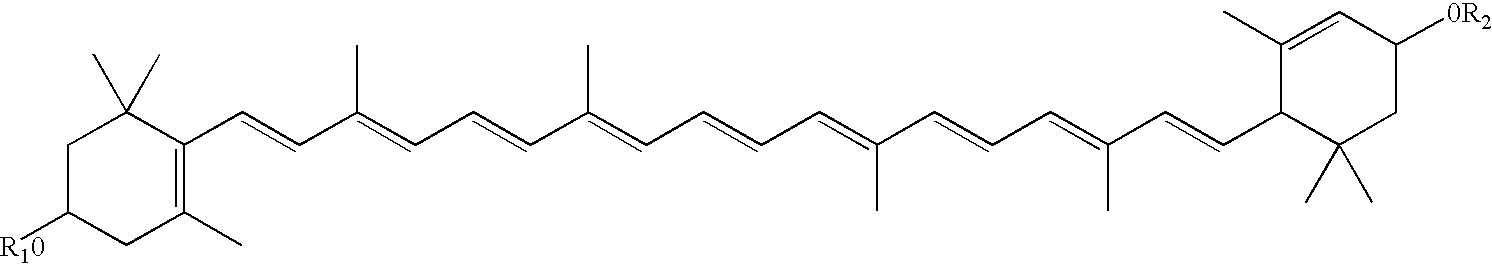
Disclosed are infant formulas and corresponding methods of using them to promote retinal health and vision development in infants. The formulas, which are free of egg phospholipids and comprise fat, protein, carbohydrate, vitamins, and minerals, including docosahexaenoic acid and, on a ready-to-feed basis, at least about 50 mcg / liter of lutein, wherein the weight ratio of lutein (mcg) to docosahexaenoic acid (mg) is from about 1:2 to about 10:1. The formulas are also believed to be especially useful in reducing the risk of retinopathy of prematurity in preterm infants.
Owner:ABBOTT LAB INC
Methods for treating acromegaly and giantism with growth hormone antagonists
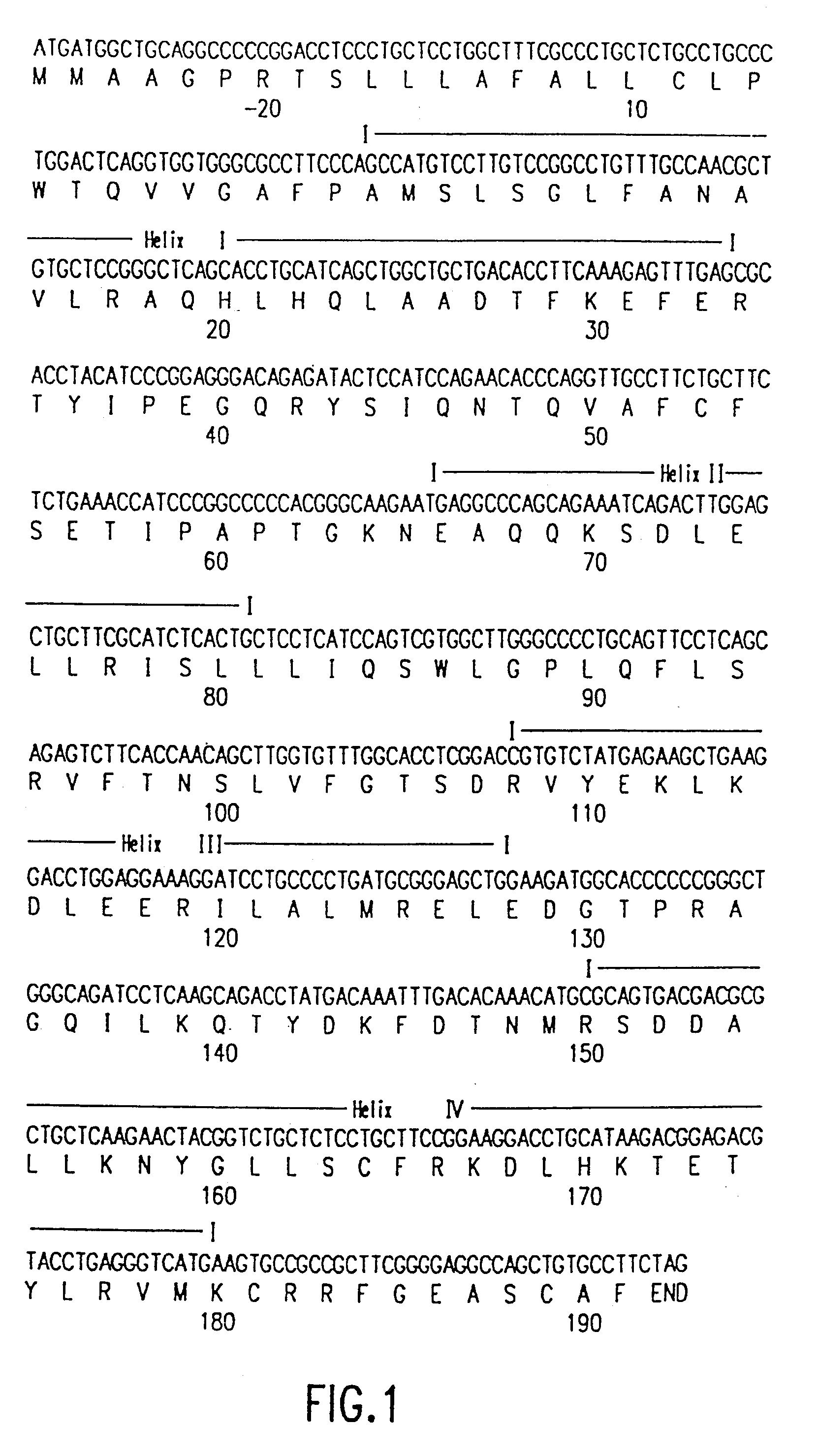
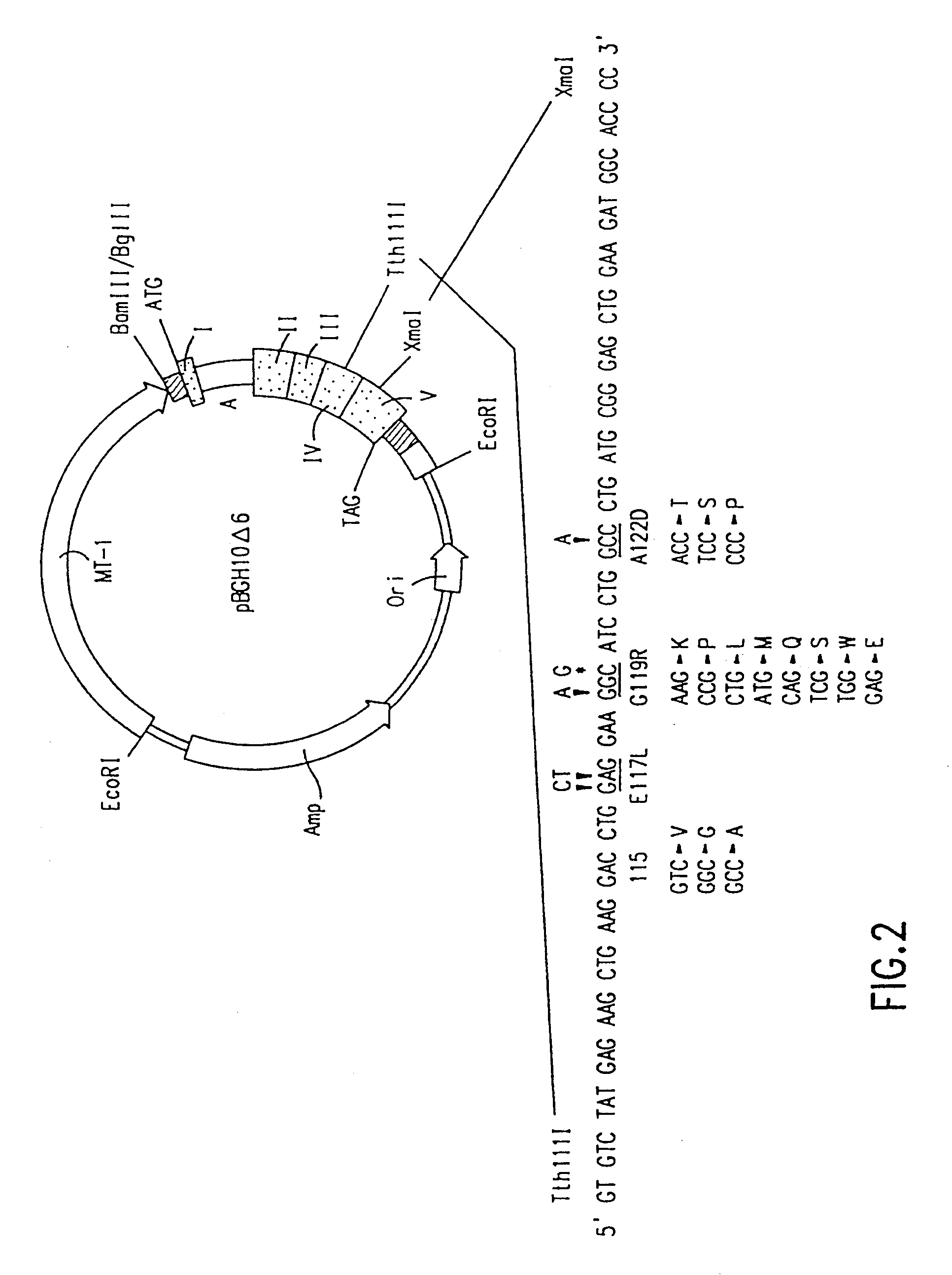
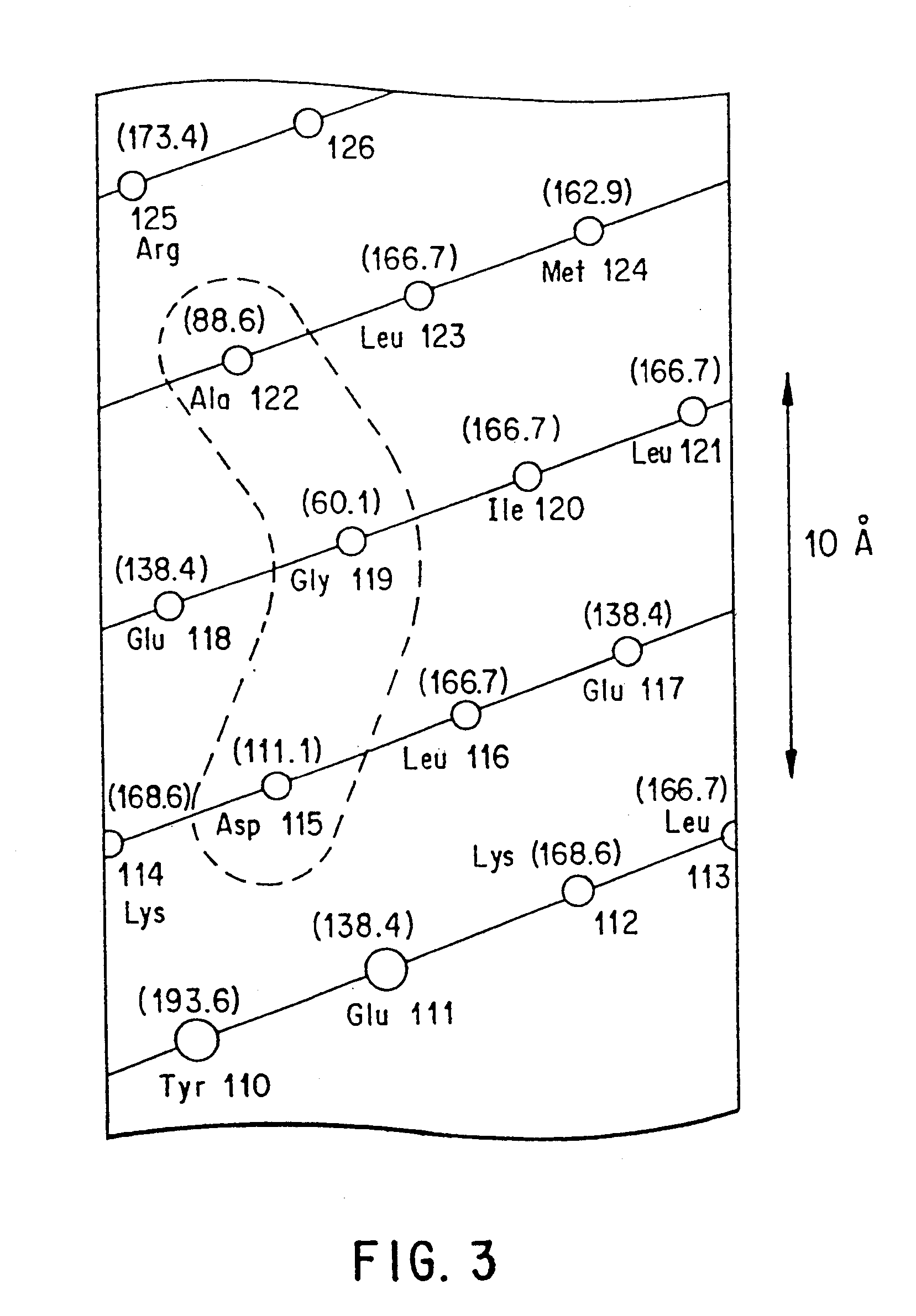
The present invention relates to antagonists of vertebrate growth hormones obtained by mutation of the third alpha helix of such proteins (especially bovine or human GHs). These mutants-have growth-inhibitory or other GH-antagonizing effects. These novel hormones may be administered exogenously to animals, or transgenic animals may be made that express the antagonist. Animals have been made which exhibited a reduced growth phenotype. The invention also describes methods of treating acromegaly, gigantism, cancer, diabetes, vascular eye diseases (diabetic retinopathy, retinopathy of prematurity, age-related macular degeneration, retinopathy of sickle-cell anemia, etc.) as well as nephropathy and other diseases, by administering an effective amount of a growth hormone antagonist. The invention also provides pharmaceutical formulations comprising one or more growth hormone antagonists.
Owner:OHIO UNIV EDISON ANIMAL BIOTECH INST
Use of cyclolignans for the treatment of type 2 diabetes and as contraceptives
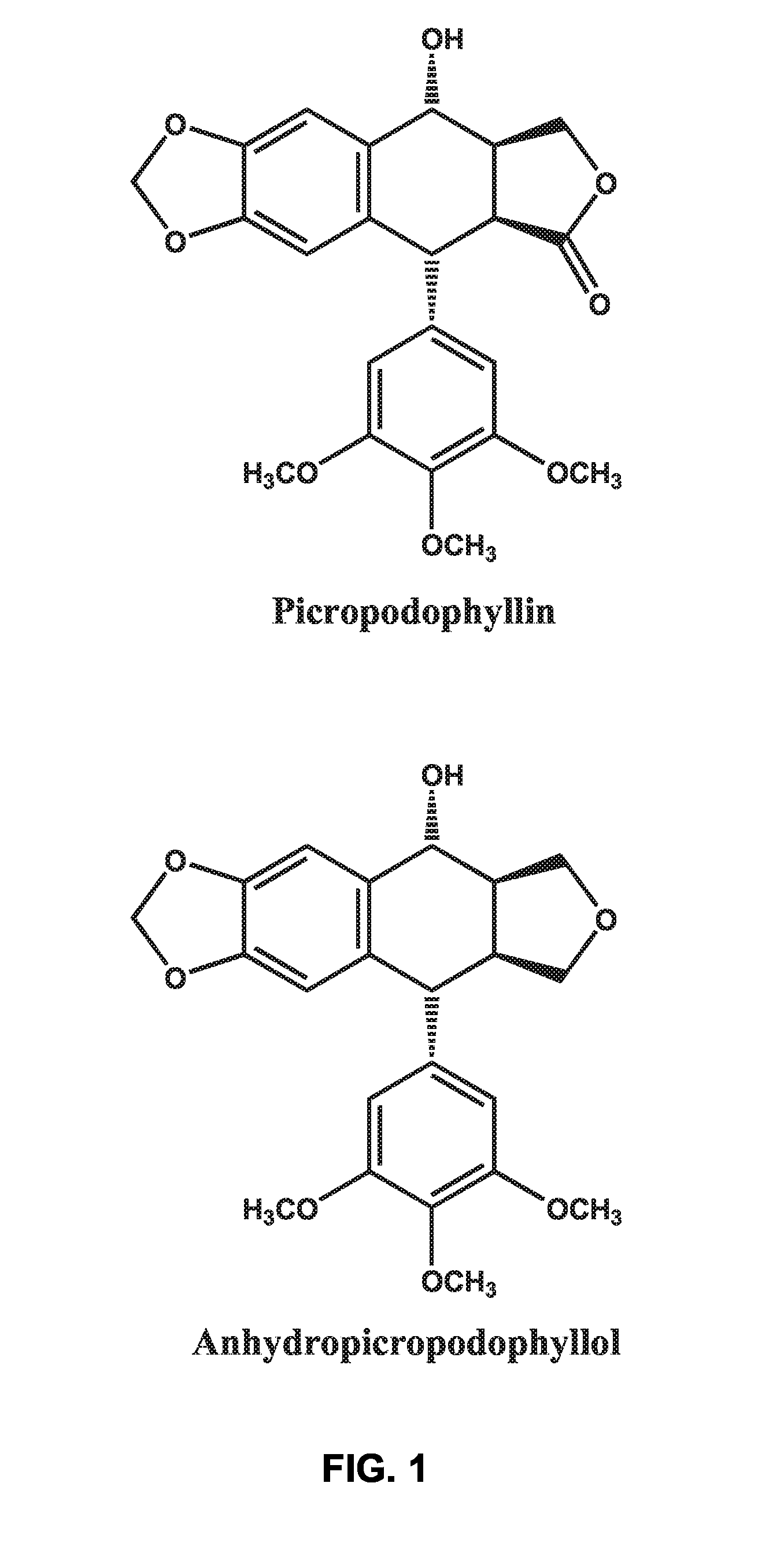
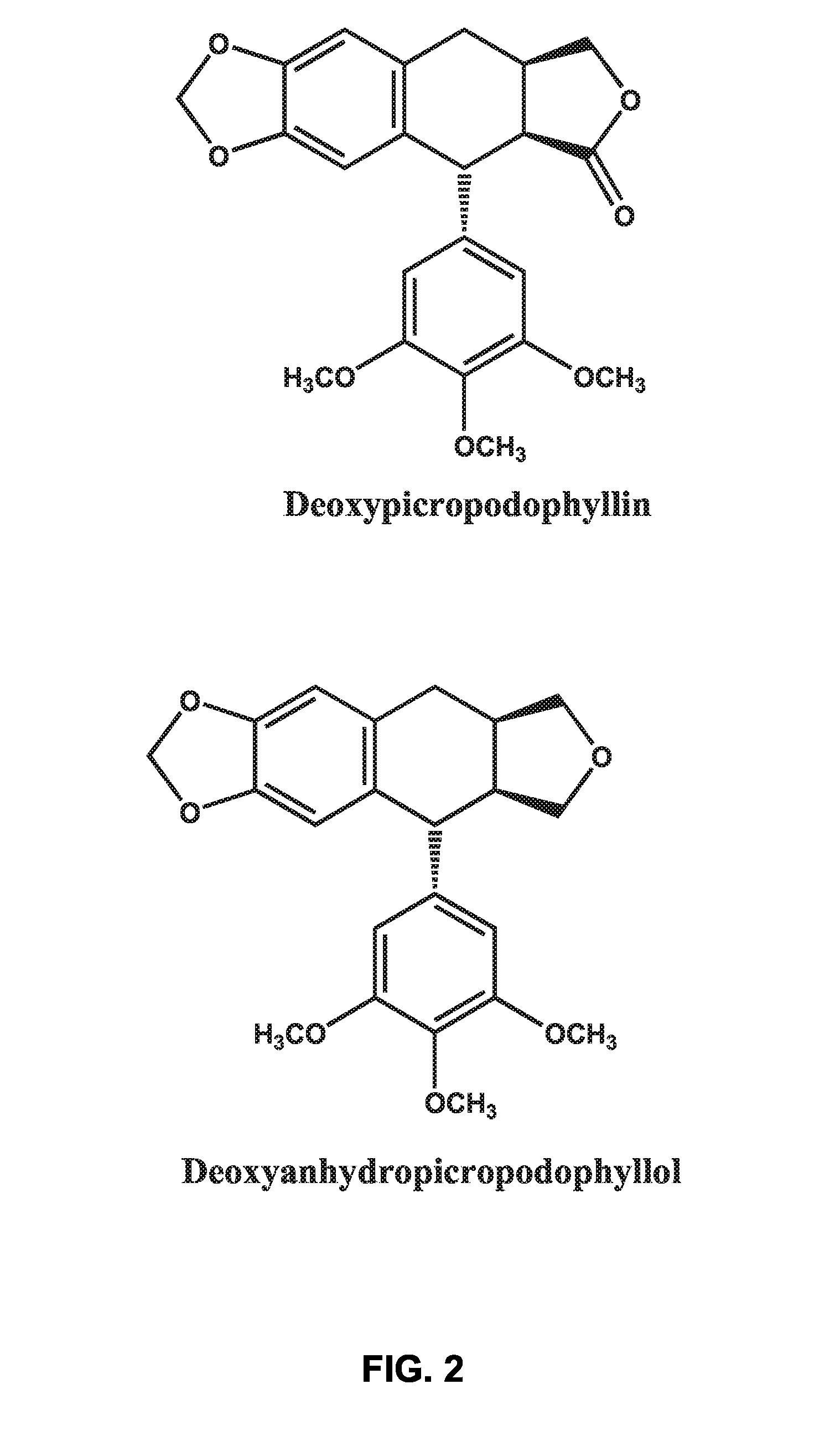
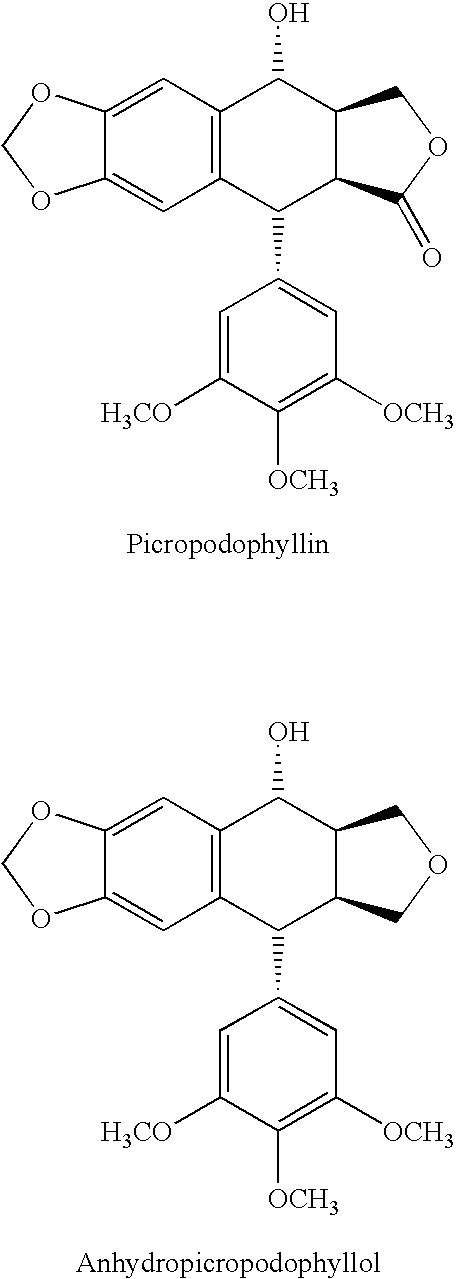
There is disclosed use of certain cyclolignans for prophylaxis or treatment of diabetes mellitus type 2, nephropathy, retinopathy, macular degeneration, retinopathy of prematurity, central retinal vein occlusion, branch retinal vein occlusion, rubeotic glaucoma, thyroid eye disease, corneal graft rejection and corneal chemical burns; and for contraception. Preferred compounds are picropodophyllin, deoxypicropodophyllin and anhydropicropodophyllol. There is also described a method of treatment of an eye disease.
Owner:AXELAR
Use of compounds that interfere with the Hedgehog signaling pathway for the manufacture of a medicament for preventing, inhibiting, and/or reversing ocular diseases related with ocular neovascularization
InactiveUS20060276391A1High unmet needControl rateBiocideOrganic active ingredientsDiabetic retinopathyHedgehog signaling pathway
The present invention concerns the use of compounds that interfere with the hedgehog signaling pathway for the manufacture of a medicament for preventing, inhibiting, and / or reversing ocular diseases related with ocular neovascularization. Partcularly, the above-mentioned diseases are (wet) age-related macular degeneration, (proliferative) diabetic retinopathy, neovascular glaucoma, retinal vein occlusion, or retinopathy of prematurity (ROP).
Owner:FOND AZIONE TELETHON
Infant formulas containing docosahexaenoic acid and lutein
ActiveUS7829126B2Promote retinal health and vision developmentReduce riskBiocideHeavy metal active ingredientsDocosahexaenoic acidLutein
Disclosed are infant formulas and corresponding methods of using them to promote retinal health and vision development in infants. The formulas, which are free of egg phospholipids and comprise fat, protein, carbohydrate, vitamins, and minerals, including docosahexaenoic acid and, on a ready-to-feed basis, at least about 50 mcg / liter of lutein, wherein the weight ratio of lutein (mcg) to docosahexaenoic acid (mg) is from about 1:2 to about 10:1. The formulas are also believed to be especially useful in reducing the risk of retinopathy of prematurity in preterm infants.
Owner:ABBOTT LAB INC
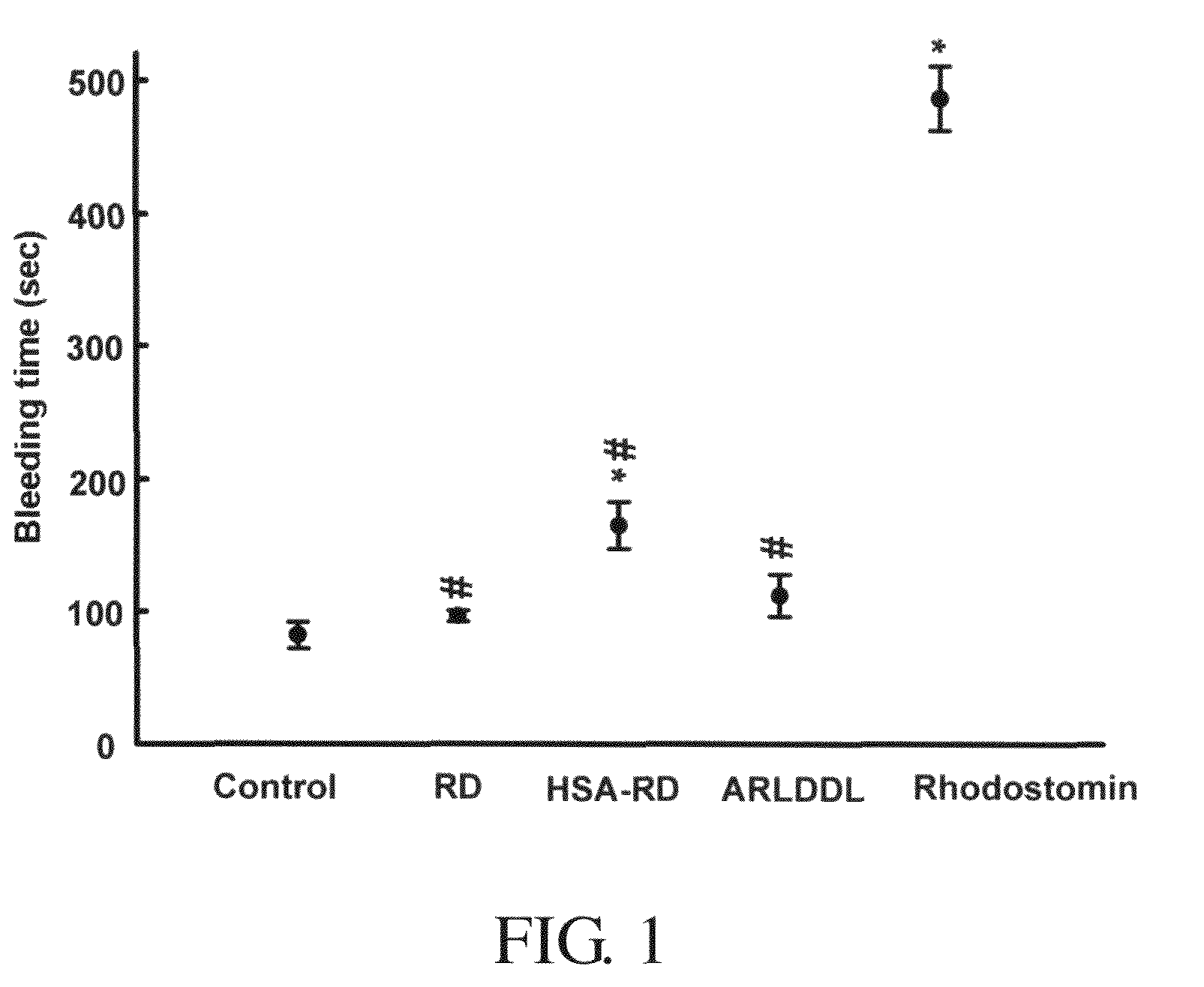
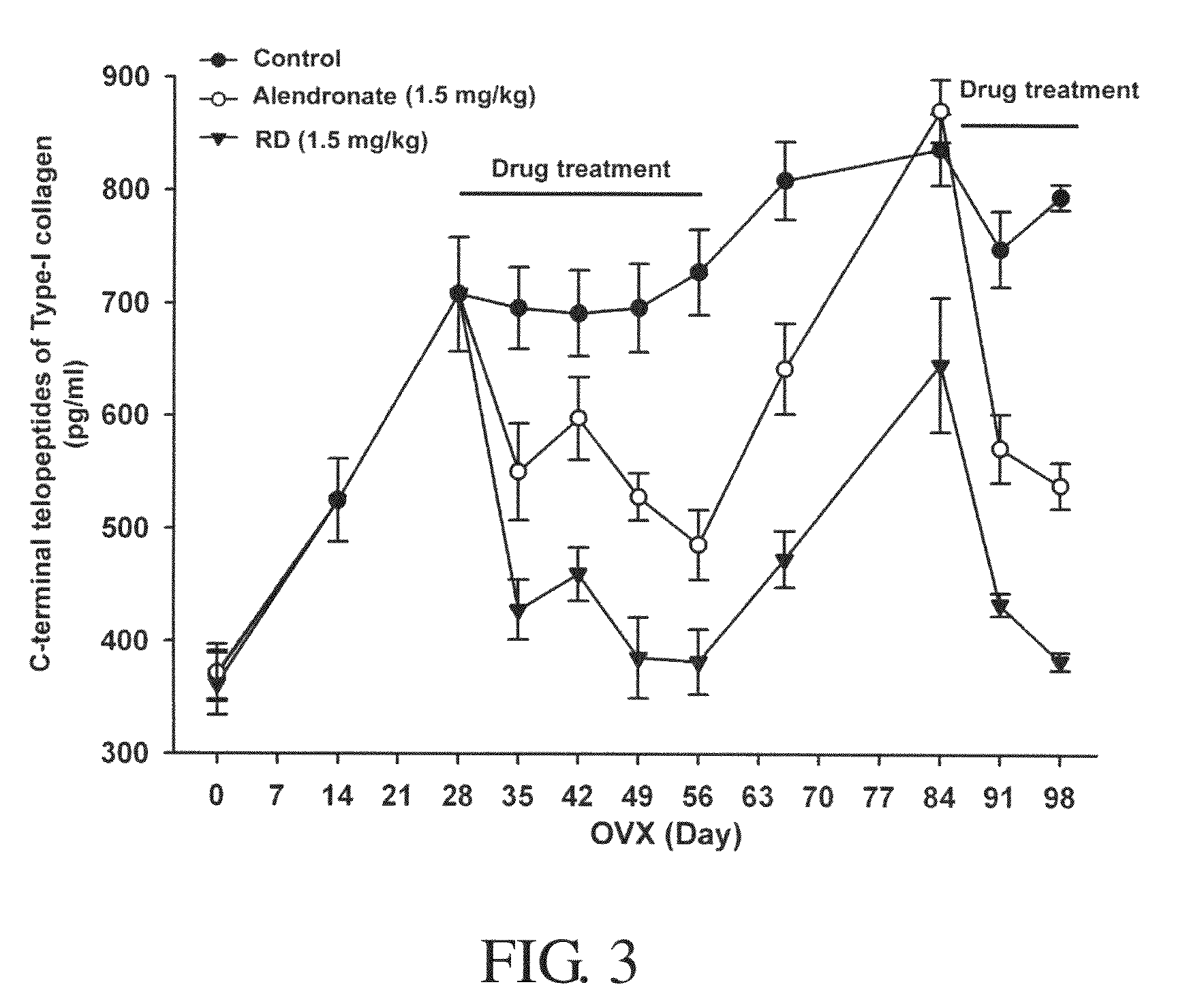
Disintegrin variants and pharmaceutical uses thereof
InactiveUS20080188413A1Treatment and prevention of osteoporosisIncreased formationSenses disorderAntipyreticDiseaseDiabetic retinopathy
Disintegrin variants and pharmaceutical uses thereof are disclosed. The disintegrin variant includes an isolated polypeptide that has integrin αvβ3 receptor-antagonist activity and substantially reduced integrin αllbβ3 and / or α5β1 receptor-blocking activity as compared to a wild-type disintegrin. The variant is encoded by a modified disintegrin nucleotide sequence that encodes a modified amino acid sequence, resulting in a polypeptide having substantially reduced affinity to integrin αllbβ3 and / or α5β1 as compared to a wild-type disintegrin. The variant is useful for treatment and / or prevention of αvβ3 integrin-associated diseases in a mammal, which include osteoporosis, bone tumor or cancer growth, angiogenesis-related tumor growth and metastasis, tumor metastasis in bone, malignancy-induced hypercalcemia, angiogenesis-related eye diseases, Paget's disease, rheumatic arthritis, and osteoarthritis. The angiogenesis-related eye diseases include age-related macular degeneration, diabetic retinopathy, corneal neovascularizing diseases, ischaemia-induced neovascularizing retinopathy, high myopia, and retinopathy of prematurity.
Owner:NAT CHENG KUNG UNIV +1
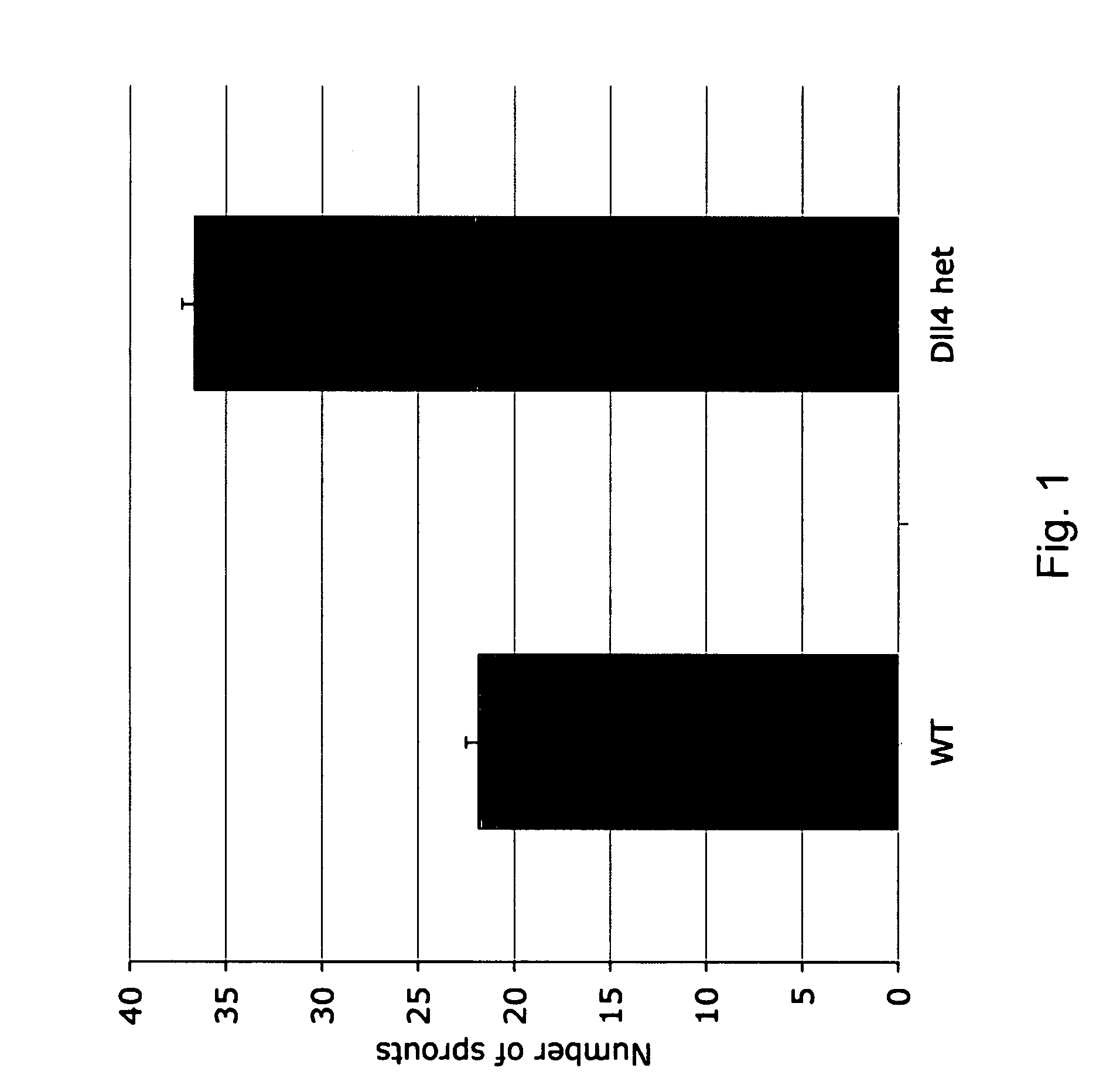
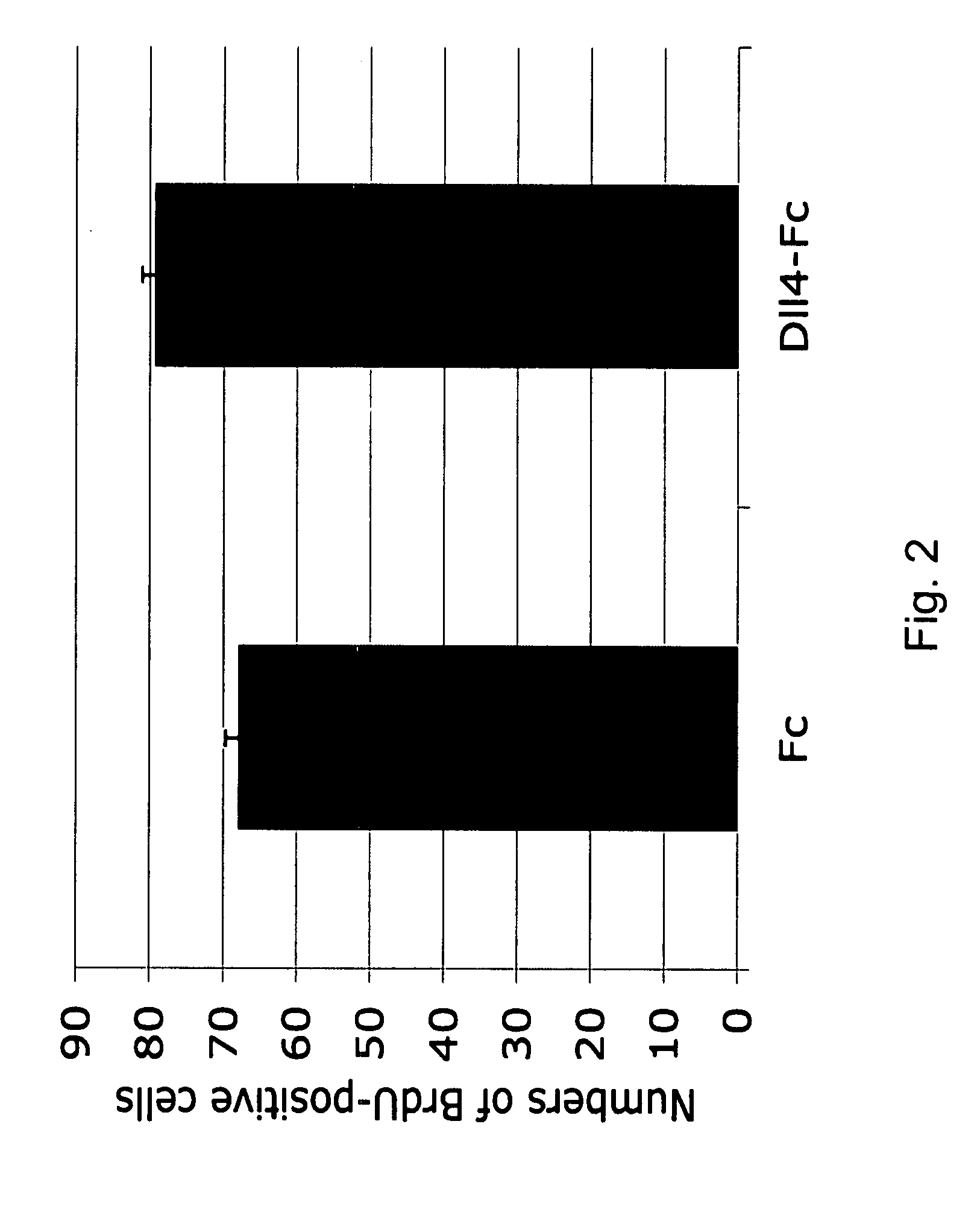
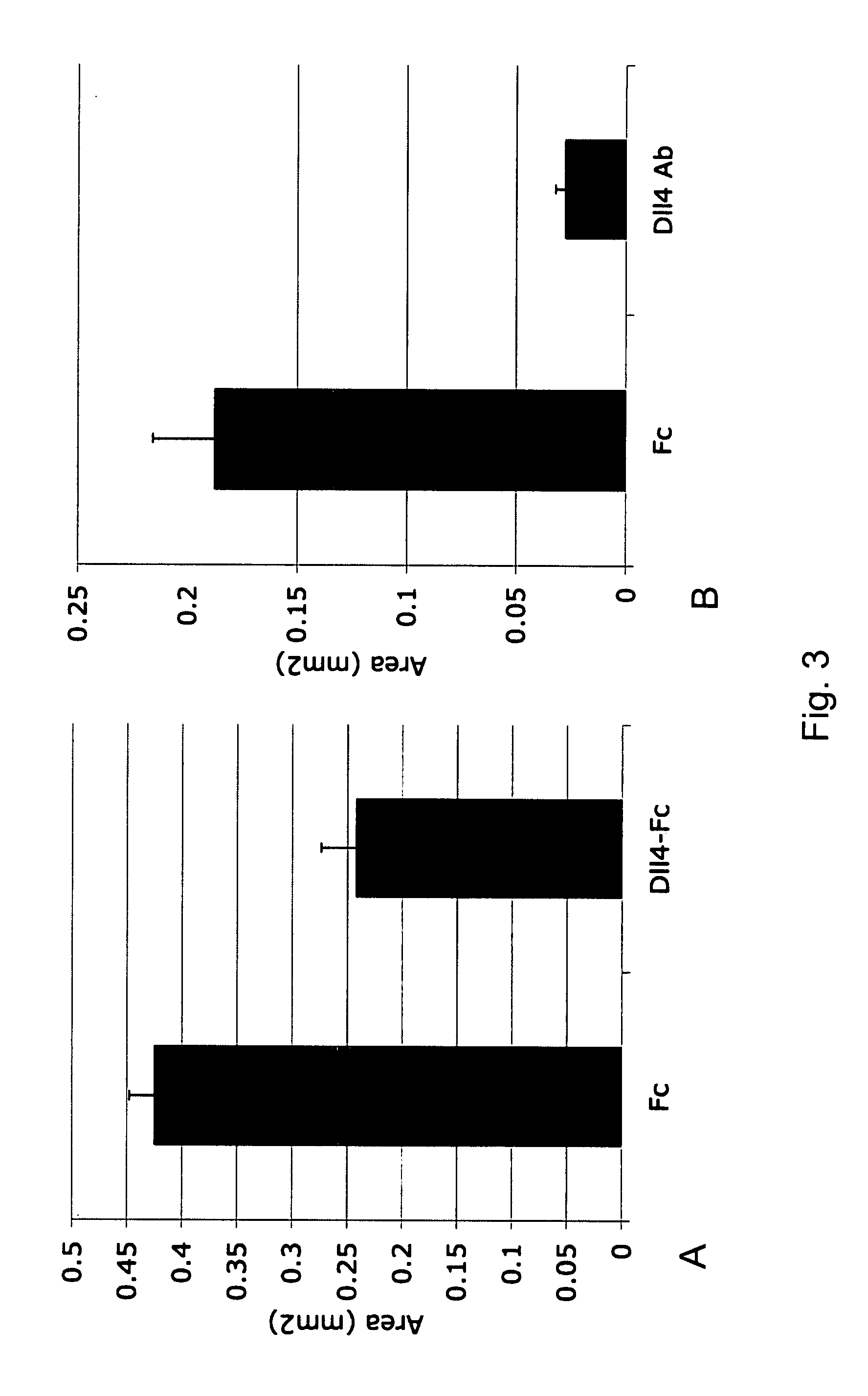
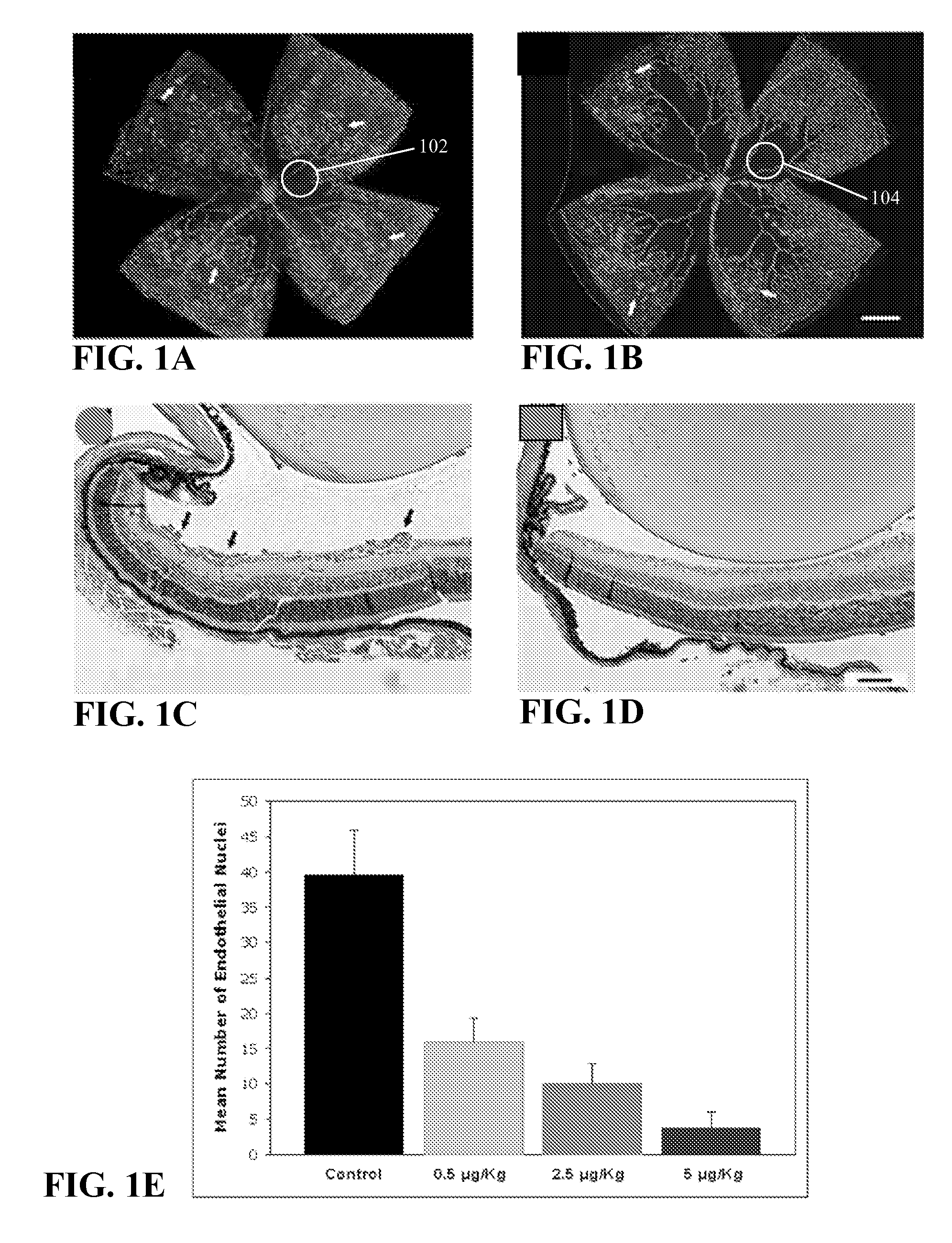
Therapeutic methods for treating vascular eye disorders with DII4 antagonists
InactiveUS20080181893A1Inhibition of developmentEnhanced regrowthSenses disorderPeptide/protein ingredientsDiabetic retinopathyIschemic retinopathy
A therapeutic method for treating ischemic or vascular disorders by administering an agent capable of inhibiting human delta-like ligand 4 (Dll4) activity to a subject in need thereof. In one embodiment, the agent is an anti-Dll4 antibody or antibody fragment capable of inhibiting the binding of Dll4 to a Notch receptor. The method of the invention is useful for treating eye disorders such as ischemic retinopathy, diabetic retinopathy, age related macular degeneration, corneal neovascularization, neovascular glaucoma, or retinopathy of prematurity. The method is also useful or treating ischemic or vascular disorders such as ischemic injury, cerebral ischemia, cardiac ischemia, ischemic conditions affecting the limbs and other organs or tissues, arteriovenous malformations, wound healing, organ or tissue transplantation, placental insufficiency, arterial narrowing and occlusion, atherosclerosis, and systemic or pulmonary hypertension.
Owner:REGENERON PHARM INC
Method of using calcitriol for treating intraocular diseases associated with angiogenesis
InactiveUS20070099879A1BiocideOrganic active ingredientsDiabetic retinopathyAngiogenesis growth factor
The present invention provides a method of treating pathologies resulting from neovascular growth in the eye such as those manifested as retinopathy of prematurity, diabetic retinopathy and macular degeneration. The invention comprises the administration of an effective amount of calcitriol that is administered at doses less than toxicity and results in a significant reduction in the formation of neo-vascular growth. The invention can be used to treat existing diseases or prophylactically to treat those at risk.
Owner:WISCONSIN ALUMNI RES FOUND
Method of reducing the risk of retinopathy of prematurity in preterm infants
InactiveUS20070166354A1Lessen risk of and severityReduce riskBiocideSenses disorderPremature thelarcheDocosahexaenoic acid
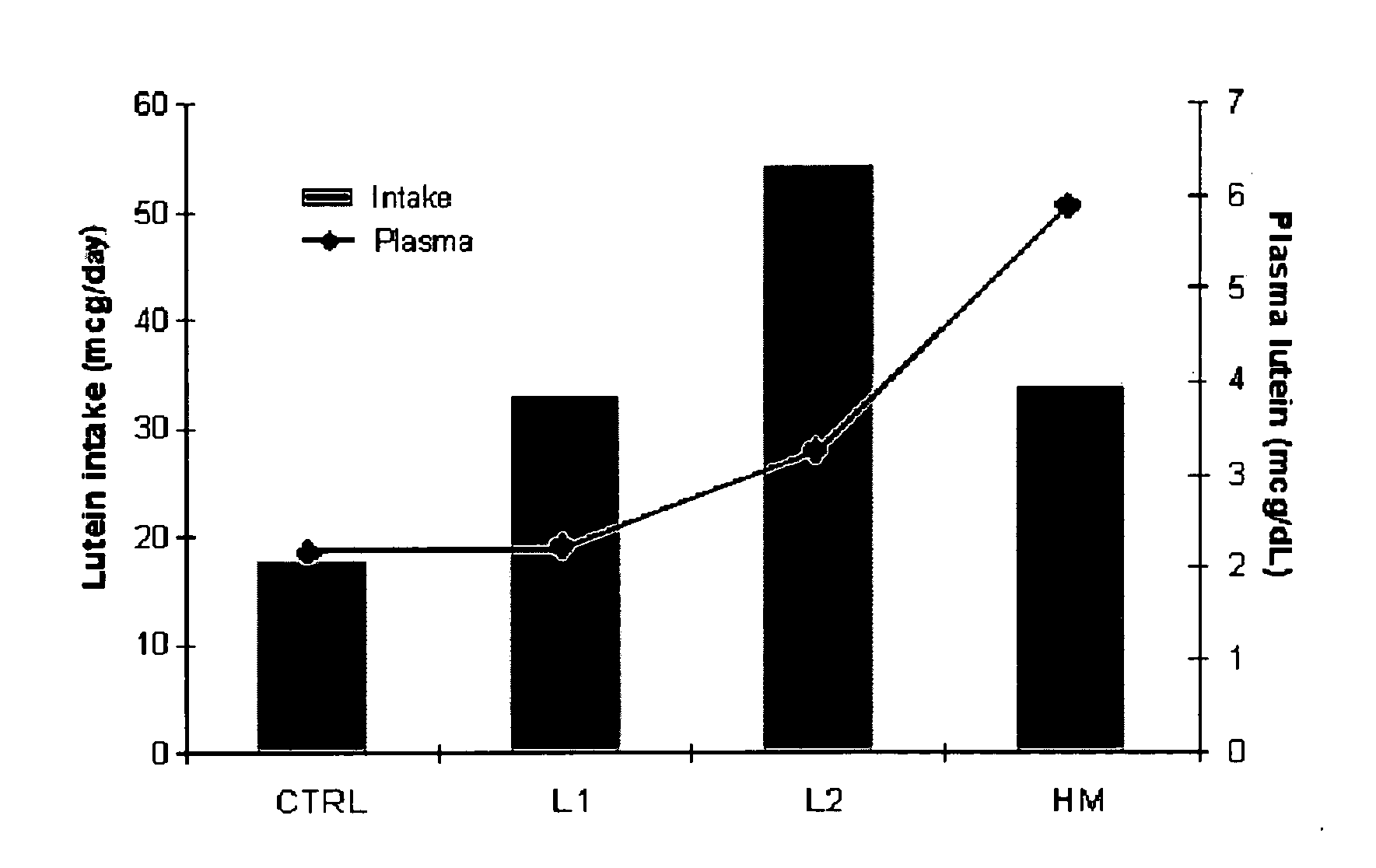
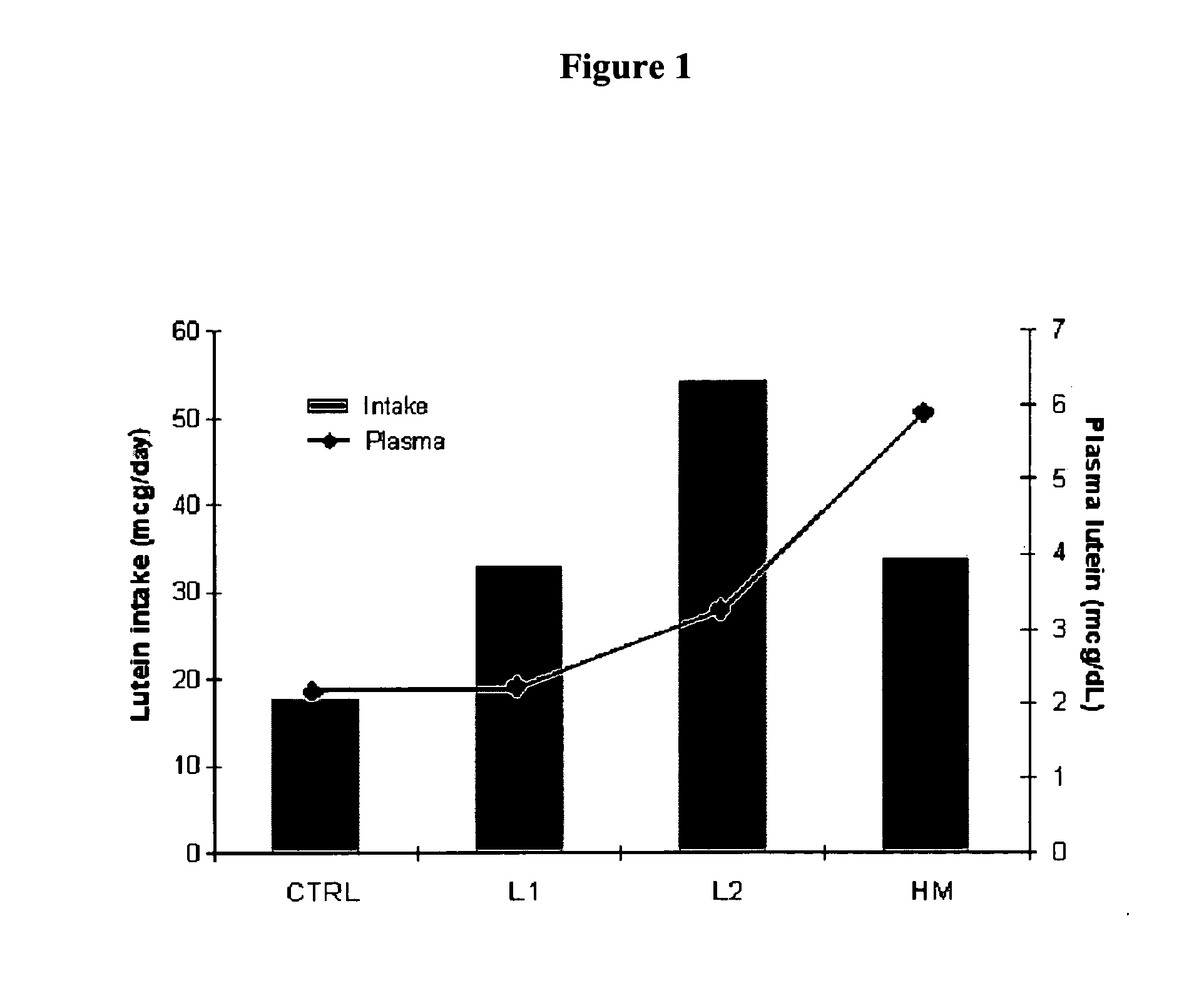
Disclosed is a method of reducing the risk or severity of retinopathy of prematurity in preterm infants. The method comprises (a) measuring skin carotenoid levels in preterm infants, preferably by Raman Spectroscopy, and then (b) administering supplemental carotenoids to those infants in need thereof, wherein the supplemental carotenoids comprise lutein, lycopene, beta-carotene, and zeaxanthin. The supplemental carotenoids may be provided by an infant formula comprising, on a ready-to-feed basis, from about 100 to about 2000 mcg / liter of total carotenoids, wherein the total carotenoids include at least about 50 mcg / liter of lutein. The formulas may further comprise docosahexaenoic acid.
Owner:ABBOTT LAB INC
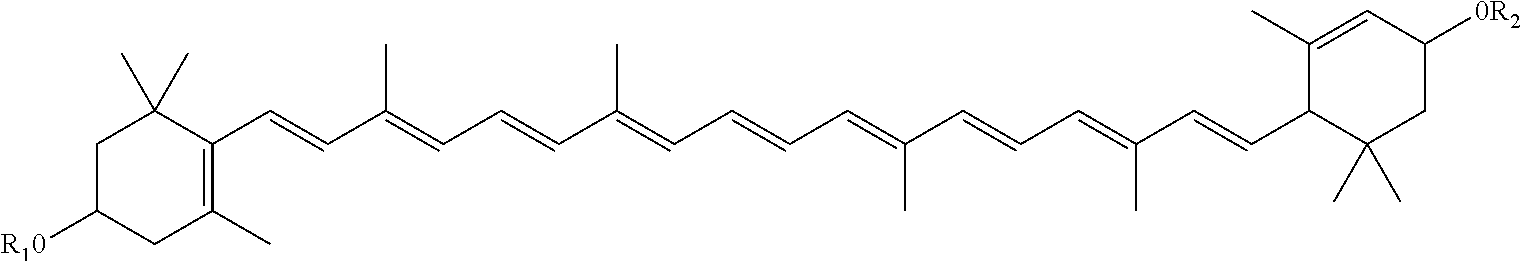
Preventive/remedy for retinal nerve diseases containing alkyl ether derivatives or salts thereof
An alkyl ether derivative represented by the following general formula [1]or its salt: wherein R1 and R2 represent each a substituent such as hydrogen, halogeno or alkyl; R3 represents alkylamino, amino or hydroxyl; the ring A represents a 5- or 6-membered aromatic heterocycle or a benzene ring; m and n are each an integer of from 1 to 6; and p is an integer of from 1 to 3; shows an effect of protecting retinal nerve cells and, therefore, is useful as a preventive and / or a remedy for retinal nerve diseases such as glaucoma, diabetic retinopathy, retinal artery obstruction, retinal venous obstruction, macular degeneration and retinopathy of prematurity.
Owner:TOYAMA CHEM CO LTD
Use of a VEGF Antagonist in Treating Retinopathy of Prematurity
InactiveUS20160159893A1Stopping abnormal blood vessel growthExtended half-lifeLaser surgerySenses disorderDiseaseRetina
The present invention relates to the use of a VEGF antagonist in the treatment of retinal neovascular disorders in infants. In particular, the invention provides a method for treating an infant having retinopathy of prematurity (ROP), wherein said method comprises administering to the eye of an infant a VEGF antagonist that either does not enter or is rapidly cleared from the systemic circulation. The term “infant” is typically used to refer to young children from birth up to the age of 12 months. The VEGF antagonist may be administered intravitreally, e.g. through injection, or topically, e.g. in form of eye drops.
Owner:NOVARTIS AG
Methods for the treatment of diabetic retinopathy and other ophthalmic diseases
ActiveUS20140039048A1Reduce rod energy demandImprove retinal functionBiocideSenses disorderDiseaseDiabetic retinopathy
Methods are provided herein for the treatment of ophthalmic diseases or conditions such as an ophthalmic disease or disorder associated with diabetes in a patient. Also provided herein are methods of treating retinopathy of prematurity in a patient. Further, provided herein are methods for treating wet age-related macular degeneration in a patient. The methods comprise administration of compounds disclosed herein to a patient in need thereof that inhibit or slow one or more signs or symptoms of such conditions.
Owner:ACUACELA INC
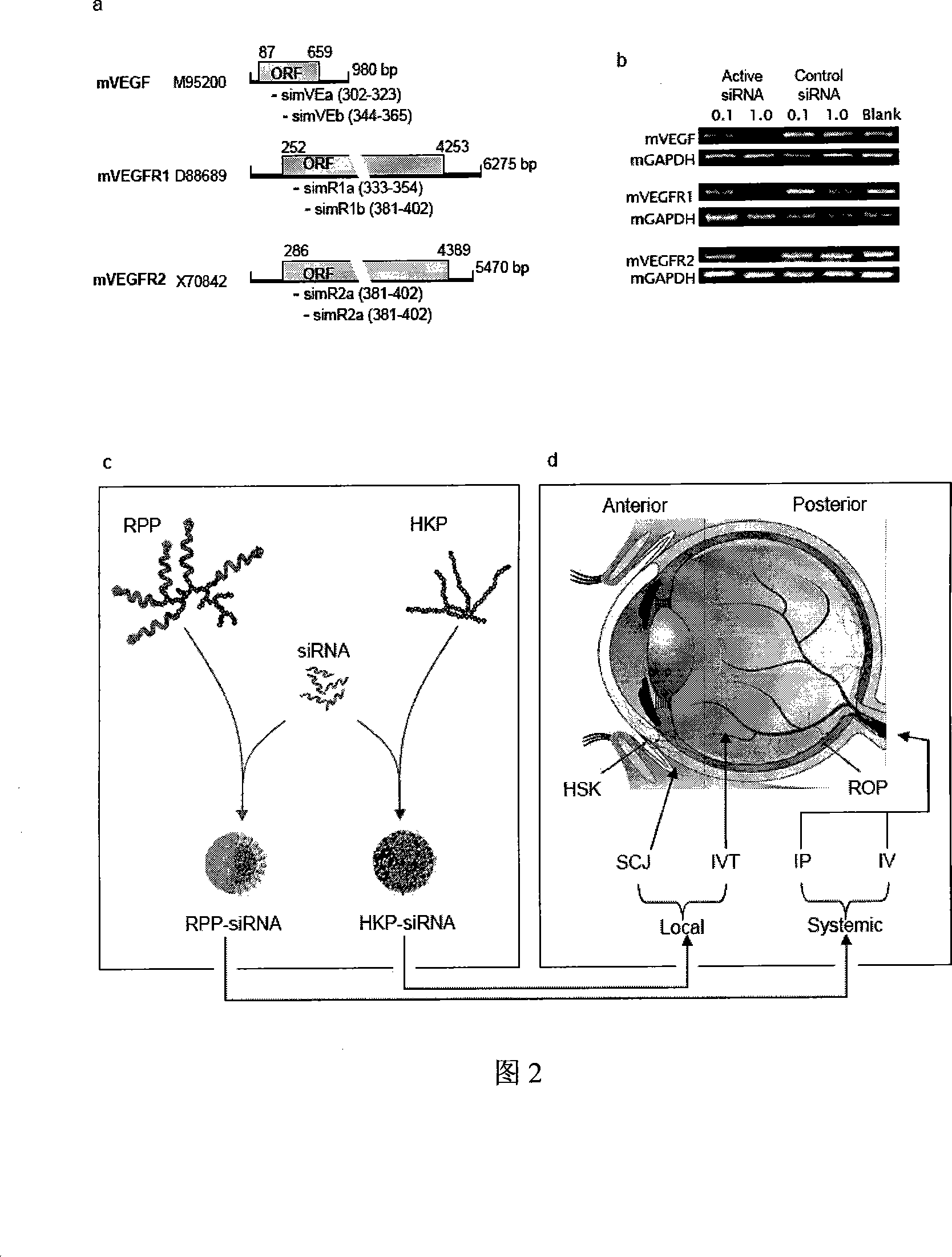
Multiple target point small interference RNA cocktail agent for treating ophthalmic disease and preparing method thereof
The invention discloses a multi-target small-interfering RNA cocktail preparation for treating ophthalmic diseases and the preparation method. The multi-target small-interfering-RNA cocktail preparation is composed of three or more than three small-interfering RNA aiming to three or more than three different genes and knocking down simultaneously a plurality of pathogenic genes; the multi-target small-interfering-RNA cocktail preparation is prepared by a plurality of small-interfering RNA in a certain proportion according to different diseases. The multi-target small-interfering-RNA cocktail preparation is a plurality of double-bond RNA molecules of different lengths from 19to 27nt, with blunt ends or overhanging ends; the RNA sequence in the multi-target small-interfering-RNA cocktail preparation has the homology to the gene targets of human, rat and other nonhuman primate; . The multi-target small-interfering-RNA cocktail preparation aiming to the following gene sequences: (1) virus-affection-related gene; (2) inflammation-arosing gene; (3) neovescular-related gene. The invention provides a novel treatment for a plurality of ophthalmic diseases, including retinopathy of prematurity, senile fundus macula lutea, retinopathy caused by senile diabetes, herpes simplex corneal stromal opacification and uveitis.
Owner:广州拓谱基因技术有限公司
Methods for the treatment and prevention of angiogenic diseases
InactiveUS20060270631A1Improve leakageLower Level RequirementsOrganic active ingredientsBiocideDiabetic retinopathyArthritis
The invention includes processes mainly for the treatment of angiogenic diseases, such as diabetic retinopathy, arthritis, cancer, psoriasis, Kaposi's sarcoma, hemangiomas, myocardial angiogenesis, atherosclerosis, and ocular angiogenic diseases such as choroidal neovascularization, retinopathy of prematurity (retrolental fibroplasias), macular degeneration, corneal graft rejection, rubeosis, neuroscular glacoma and Oster Webber syndrome. The processes involve treating a patient with a pharmaceutical composition containing an active ingredient that inhibits the activity of sphingosine kinase.
Owner:SMITH CHARLES D +2
Method for the Treatment of Retinopathy of Prematurity and Related Retinopathic Diseases
InactiveUS20080317721A1Easily damagedPromote recoveryOrganic active ingredientsBiocideProgenitorMammal
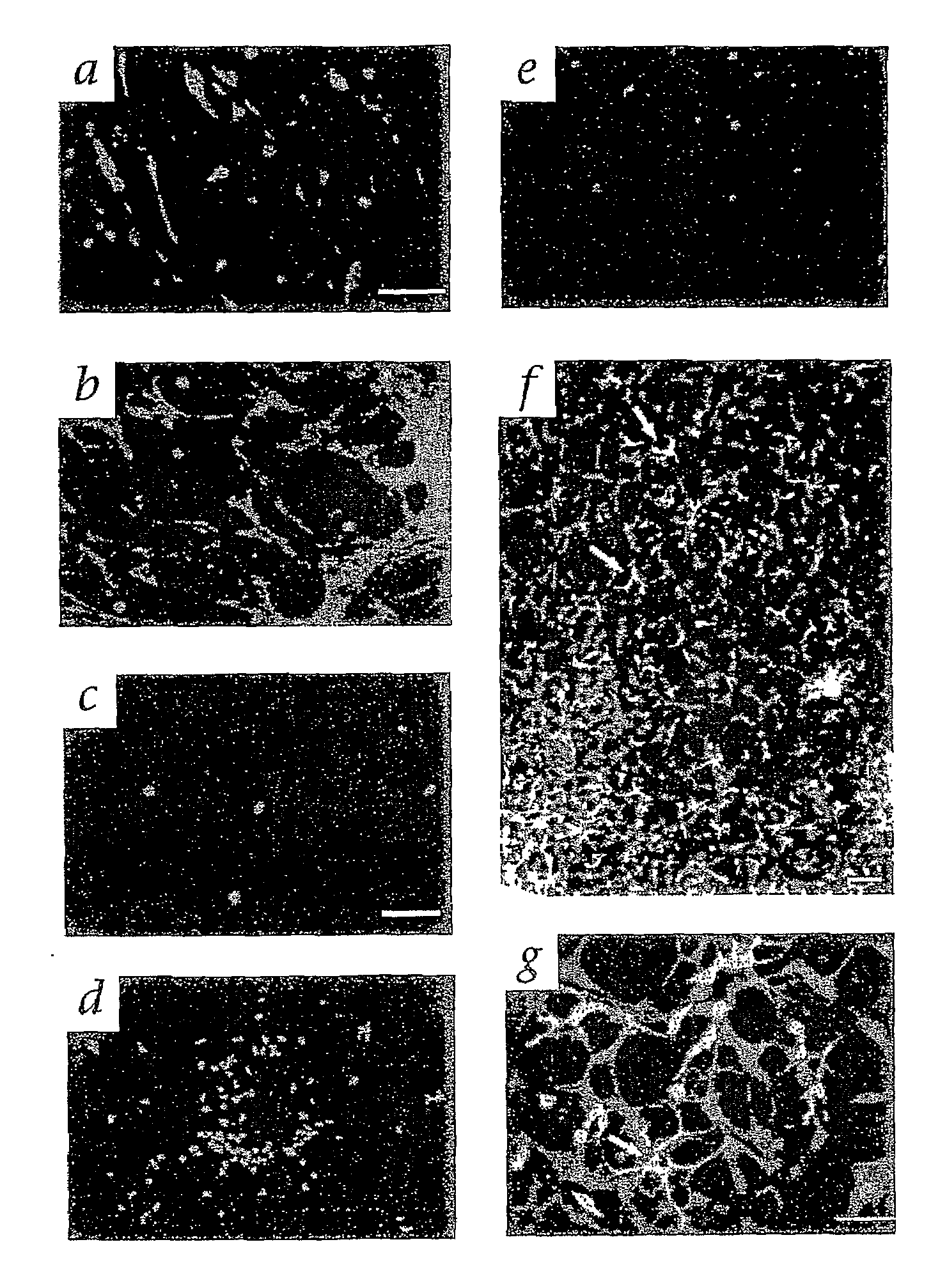
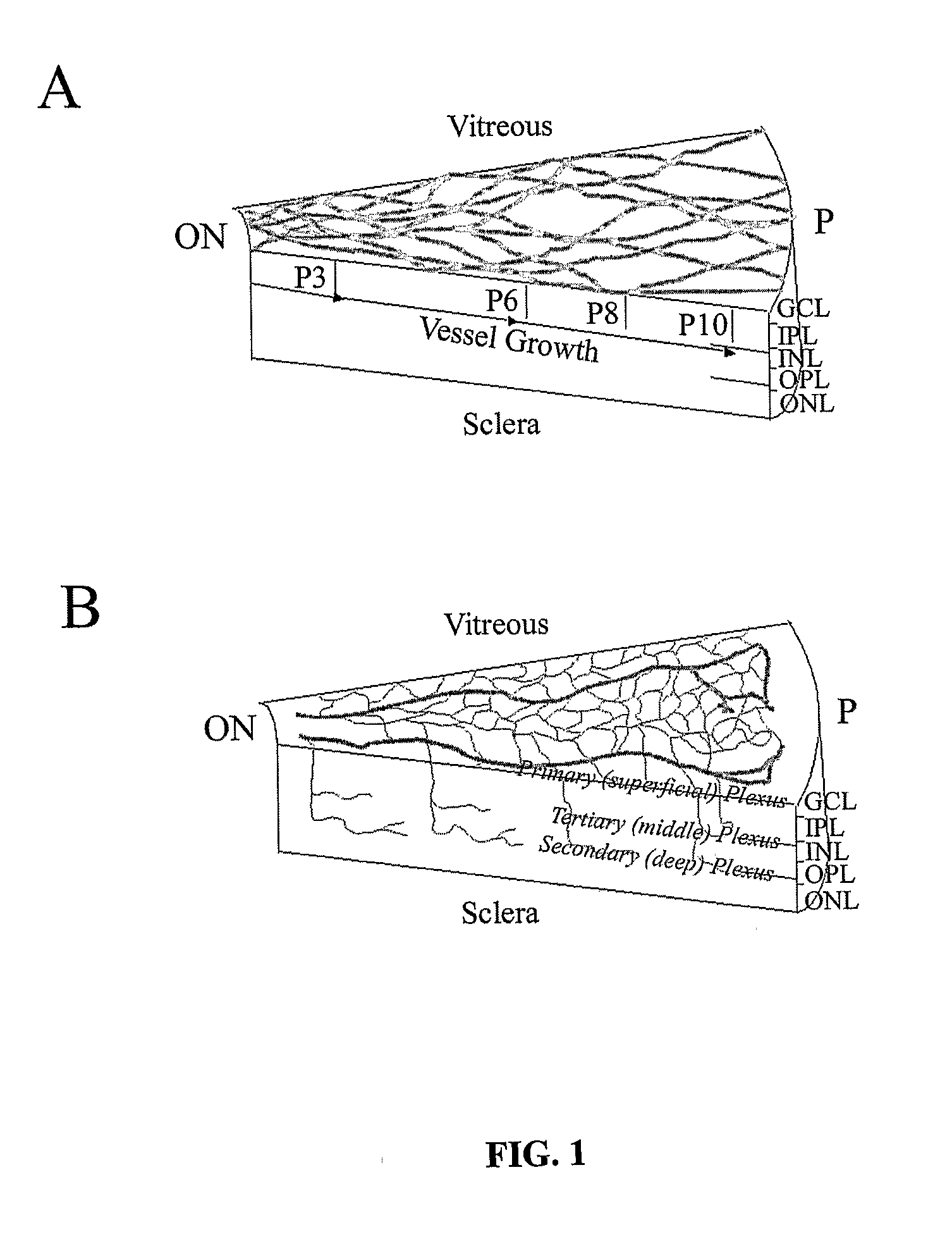
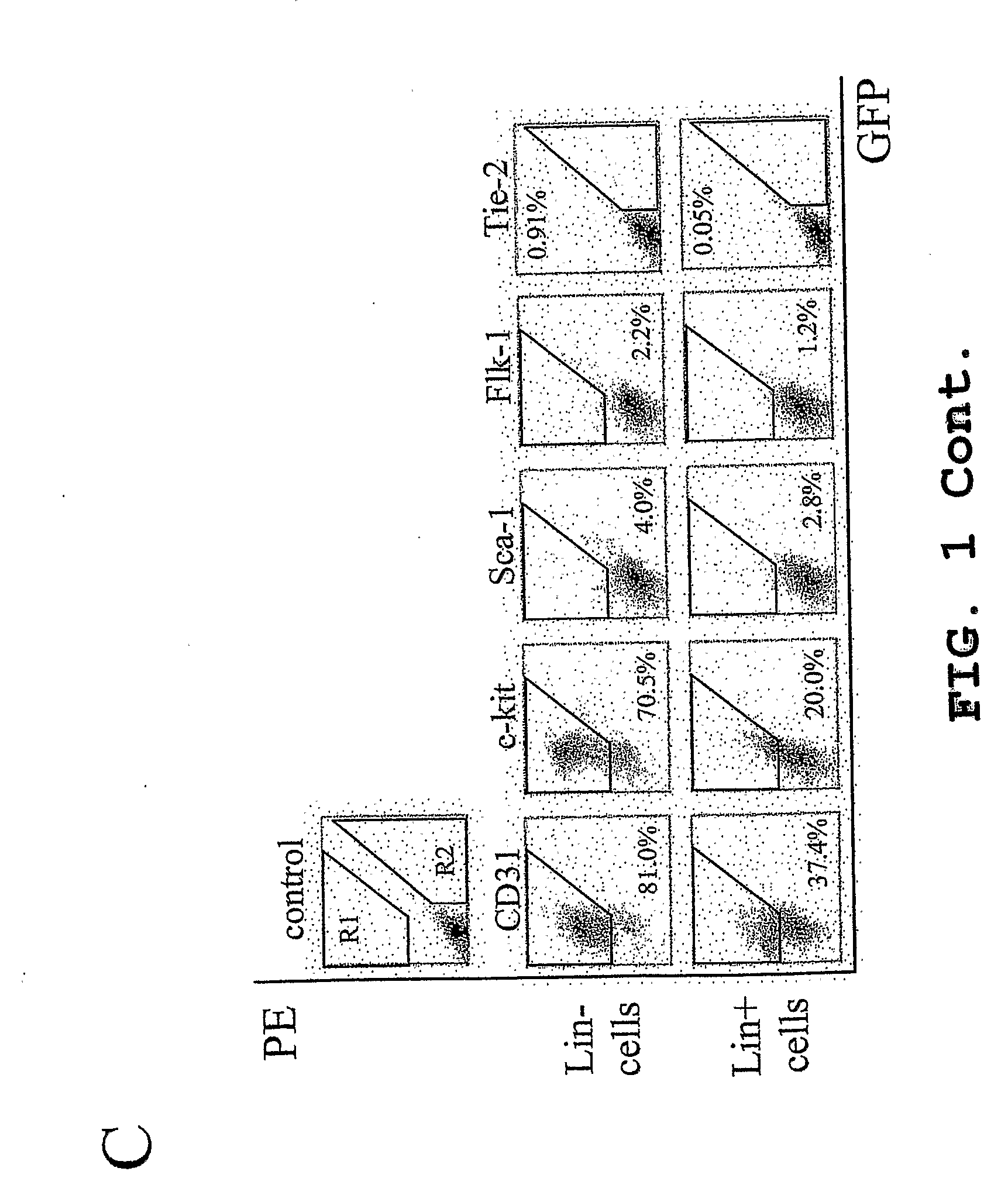
The present invention provides a method for treating retinopathy of prematurity (ROP) and related retinopathic diseases. The method comprises administering to the retina of a mammal suffering from, or at risk of developing, retinopathy of prematurity or a related retinopathic disease an amount of cells from a vasculotrophic lineage negative hematopoietic stem cell population, effective to promote beneficial physiological revascularization of damaged areas of the retina and to ameliorate damage to the retina caused by the disease. Preferably, the mammal is a human patient. In one preferred embodiment, the lineage negative hematopoietic stem cell population is a lineage negative hematopoietic stem cell population comprising hematopoietic stem cells and endothelial progenitor cells (i.e., Lin− HSC). In another preferred embodiment, the lineage negative hematopoietic stem cell population is an isolated myeloid-like bone marrow (MLBM) cell population in which the majority of the cells are lineage negative and express CD44 antigen and CD11b antigen. As an alternative, for treatment of newborn infants, a lineage negative hematopoietic stem cell population can be isolated from umbilical cord vein blood.
Owner:THE SCRIPPS RES INST
Inhibition of choroidal neovascularization
InactiveUS20120301439A1Promotes cholesterol effluxReduce activationBiocideSenses disorderDiabetic retinopathyWhole body
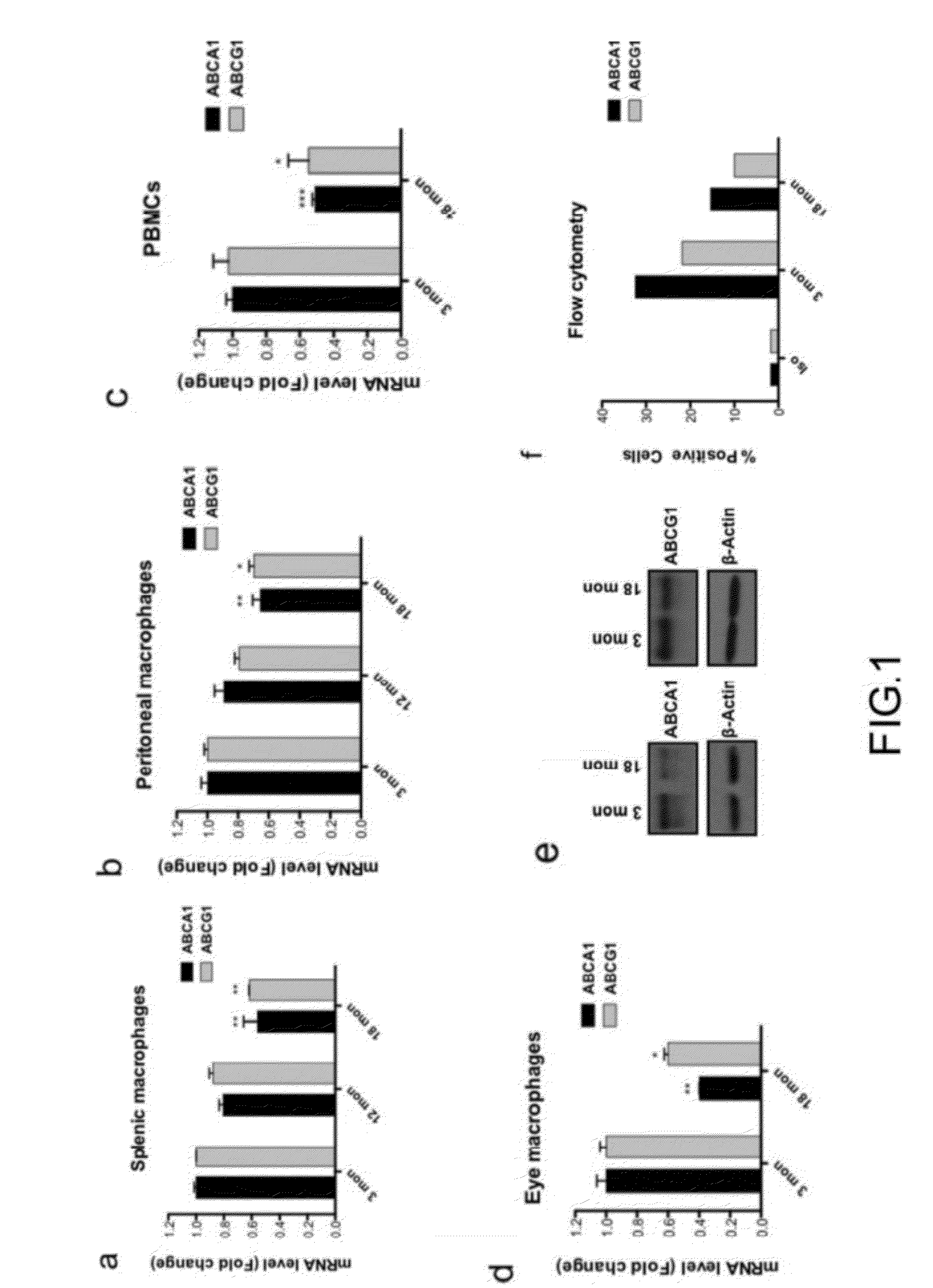
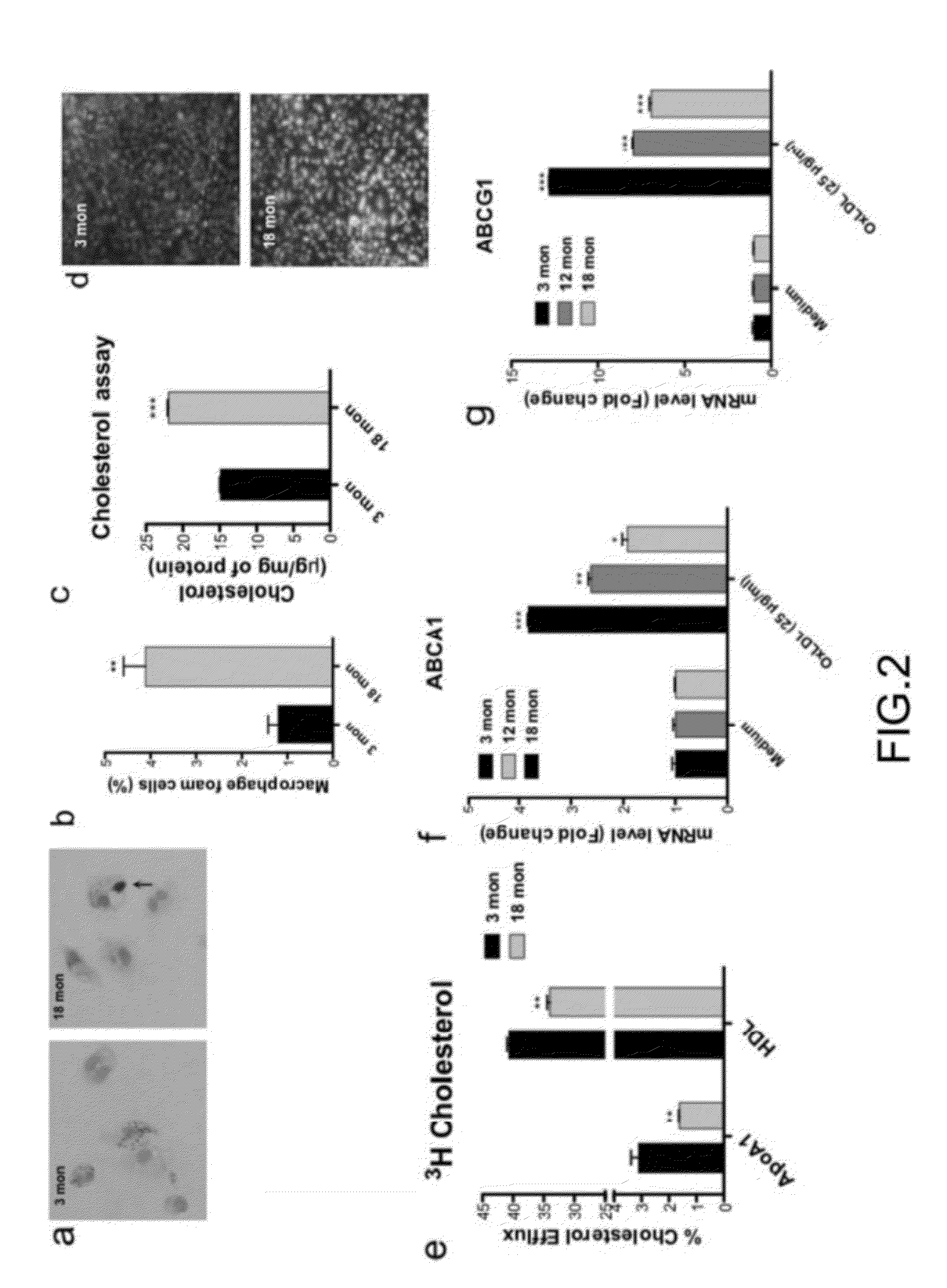
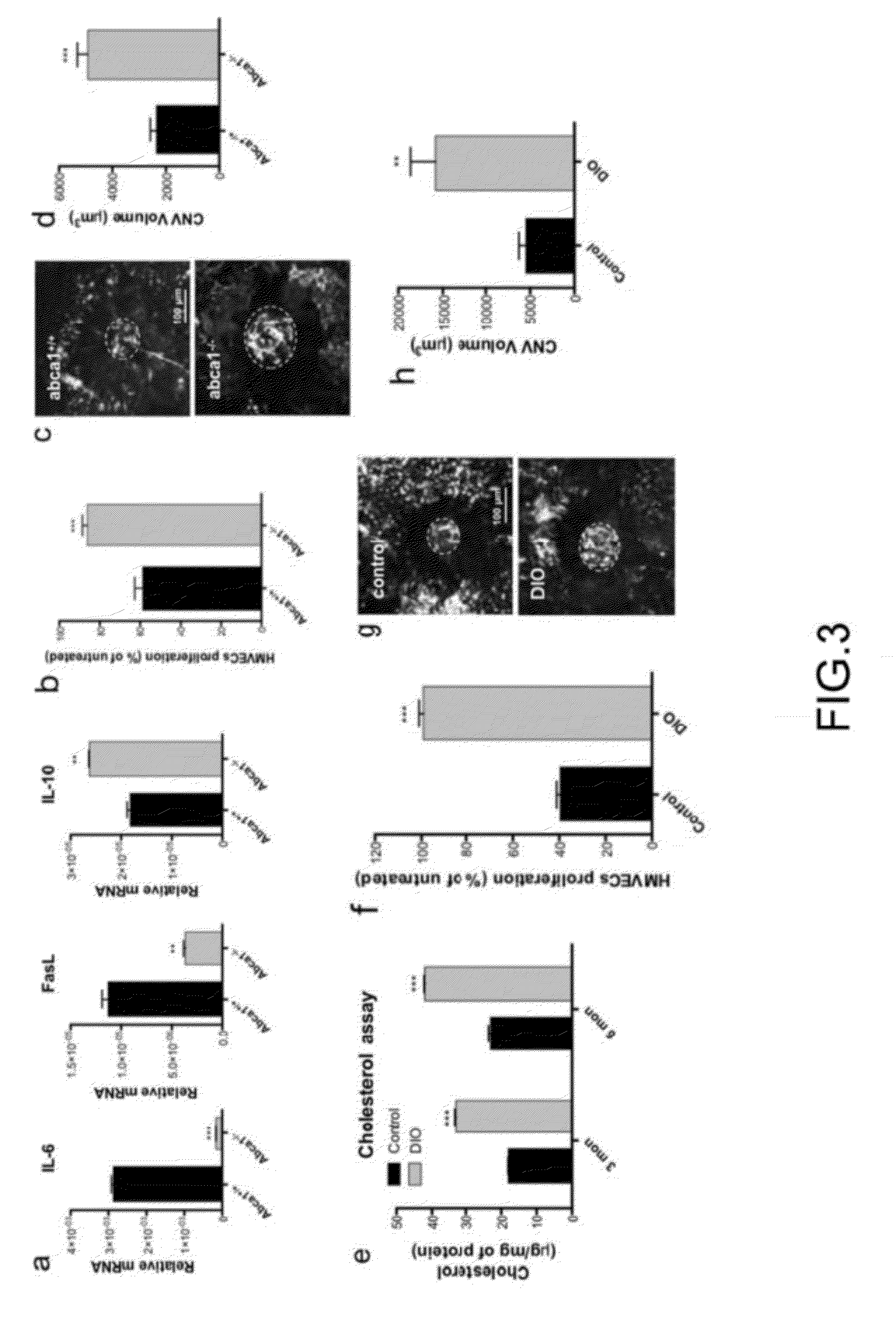
Methods of treatment of diseases that include or are characterized by inappropriate or pathological neovascularization are disclosed. These diseases include diseases of the eye, such as diabetic retinopathy, retinopathy of prematurity, and choroidal neovascularization which can occur in age-related macular degeneration (AMD). Disclosed methods include administering agents that cause directly or indirectly upregulation of the ABCA1 transporter protein in macrophages. These agents include, without limitation, LXR agonists. In some embodiments, inhibitors of CETP expression or activity can also be effective. Administration routes can include, without limitation, intraocular, periocular, or systemic administration.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Model newborn human eye and face manikin
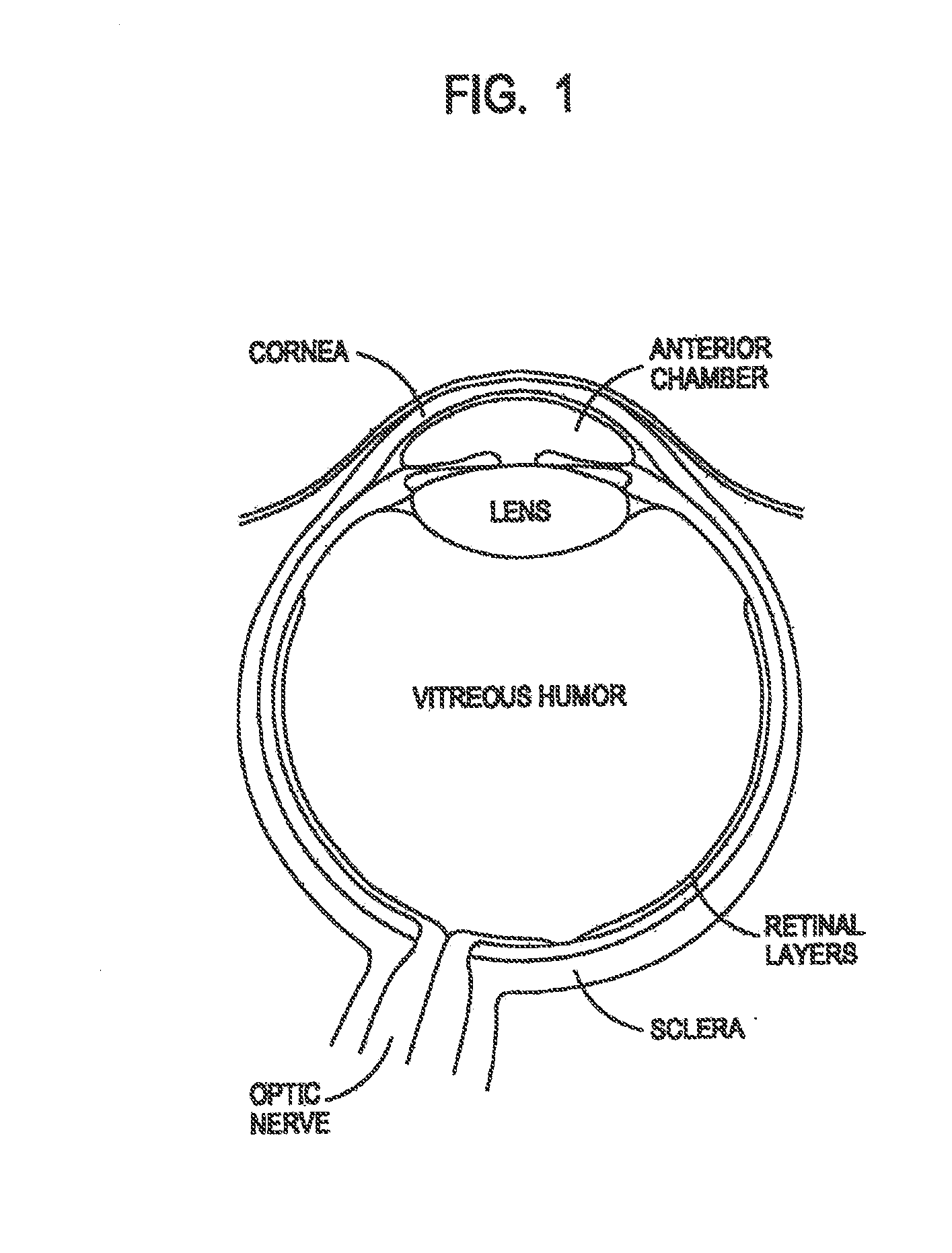
A model newborn human eye which includes a hemispherical-shaped, integrally molded top assembly comprising a visually transparent cornea portion surrounding a visually opaque sclera portion in combination with a hemispherical-shaped bottom assembly comprising a bowl-shaped substrate disposed therein. The model newborn human eye further comprises a retinal layer comprising a two dimensional image of retinal vasculature disposed on said substrate, where the model newborn human eye is dimensioned for diagnosing Retinopathy of Prematurity (“ROP”) in premature infants.
Owner:EYE CARE & CURE ASIA
Modulators of EphA2 and EphrinA1 for the treatment of fibrosis-related disease
InactiveUS20060122138A1Maintaining organizationInhibit and decrease angiogenesisSenses disorderAntipyreticPsoriatic arthropathyDiabetic retinopathy
The present invention relates to methods and compositions designed for the treatment, management, prevention and / or amelioration of non-neoplastic hyperproliferative epithelial and / or endothelial cell disorders, including but not limited to disorders associated with increased deposition of extracellular matrix components (e.g., collagen, proteoglycans, tenascin and fibronectin) and / or aberrant angiogenesis. Non-limiting examples of such disorders include cirrhosis, fibrosis (e.g., fibrosis of the liver, kidney, lungs, heart, retina and other viscera), asthma, ischemia, atherosclerosis, diabetic retinopathy, retinopathy of prematurity, vascular restenosis, macular degeneration, rheumatoid arthritis, osteoarthritis, infantile hemangioma, verruca vulgaris, Kaposi's sarcoma, neurofibromatosis, recessive dystrophic epidermolysis bullosa, ankylosing spondylitis, systemic lupus, Reiter's syndrome, Sjogren's syndrome, endometriosis, preeclampsia, atherosclerosis, coronary artery disease, psoriatic arthropathy and psoriasis. The methods of the invention comprise the administration of an effective amount of one or more agents that are modulators of EphA2 and / or its endogenous ligand, EphrinA1. The invention also provides pharmaceutical compositions comprising one or more EphA2 / EphrinA1 Modulators of the invention either alone or in combination with one or more other agents useful for therapy for such non-neoplastic hyperproliferative epithelial and / or endothelial disorders. Diagnostic methods and methods for screening for EphA2 / EphrinA1 Modulators are also provided.
Owner:MEDIMMUNE LLC
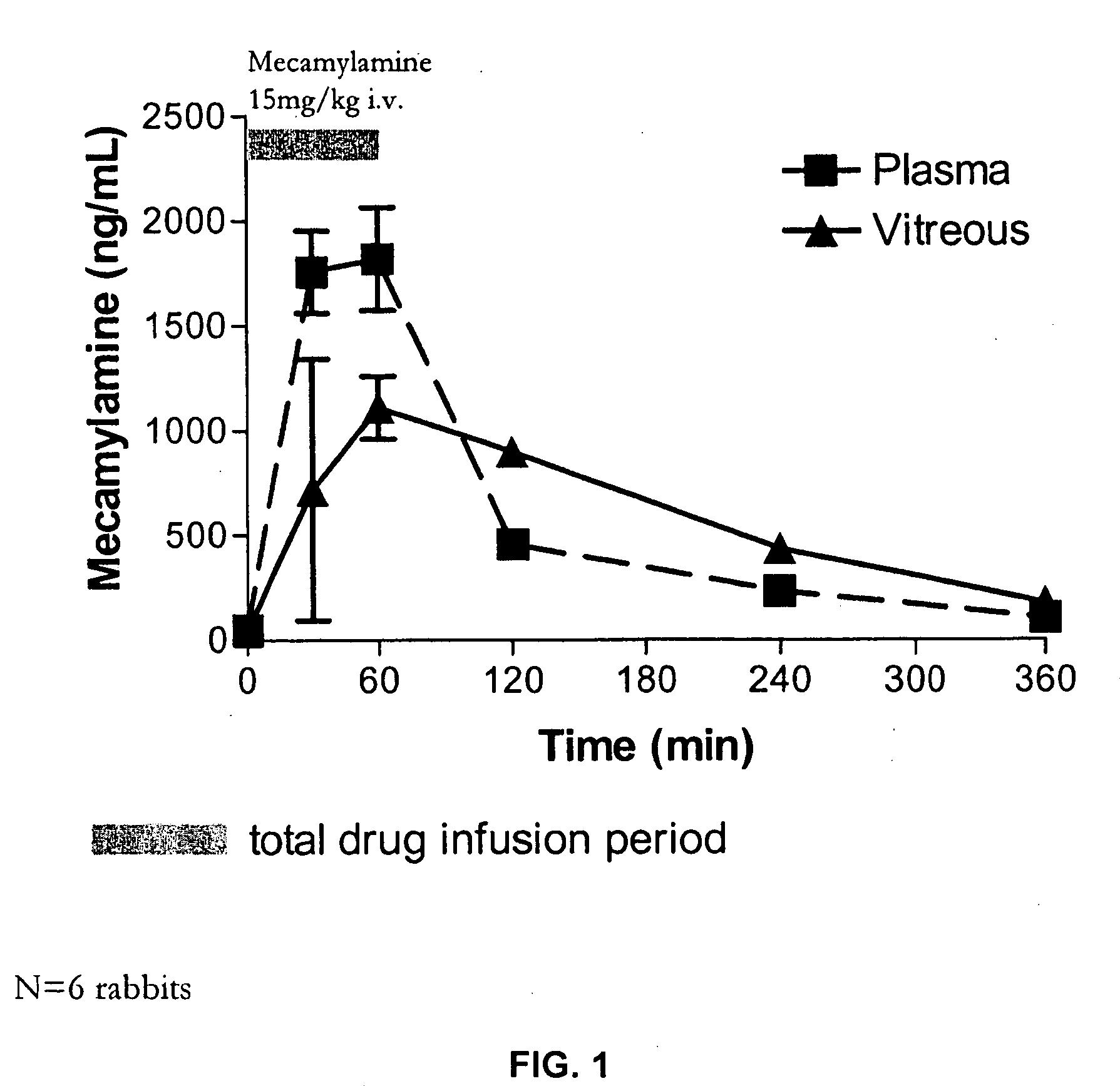
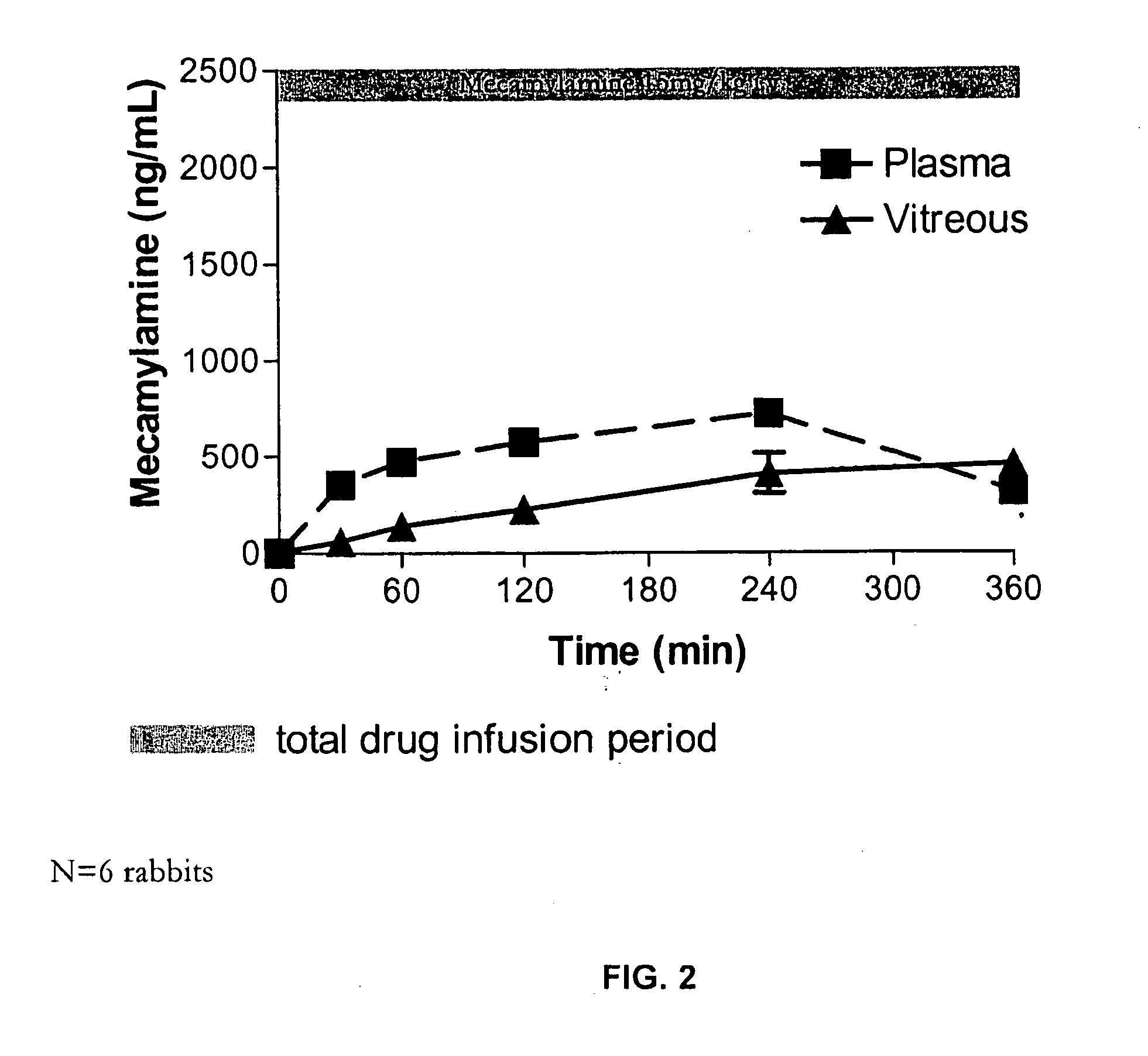
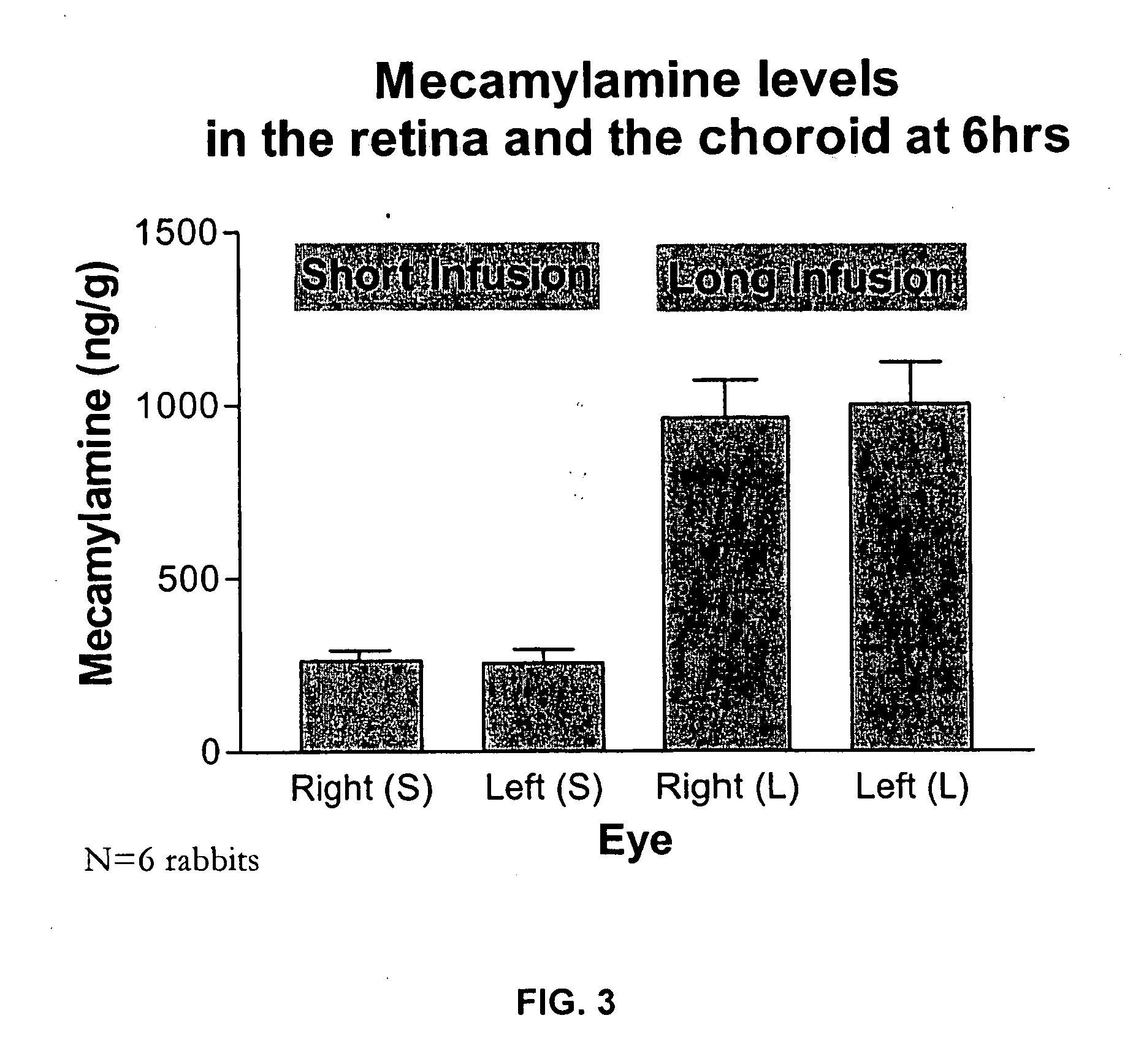
Topical mecamylamine formulations for ocular administration and uses thereof
Provided are methods, pharmaceutical formulations and kits thereof for the treatment and / or prevention of conditions mediated by neovascularization, abnormal angiogenesis, vascular permeability, or combinations thereof, of posterior and / or anterior tissues and fluids of the eye, including conditions associated with proliferative retinopathies, for example, diabetic retinopathy, age-related maculopathy, retinopathy of prematurity, retinopathy associated with macular edema, or retinopathy associated with sickle cell disease, using the topical administration of mecamylamine or a pharmaceutically acceptable salt thereof to the eye. Methods of preparing the pharmaceutical formulations are also provided.
Owner:COMENTIS
Disintegrin variants and their use in treating osteoporosis-induced bone loss and angiogenesis-related diseases
InactiveUS7943728B2Avoid problemsIncreased formationSenses disorderPeptide/protein ingredientsDiseaseDiabetic retinopathy
Disintegrin variants and pharmaceutical uses thereof are disclosed. The disintegrin variant includes an isolated polypeptide that has integrin αvβ3 receptor-antagonist activity and substantially reduced integrin αllbβ3 and / or α5β1 receptor-blocking activity as compared to a wild-type disintegrin. The variant is encoded by a modified disintegrin nucleotide sequence that encodes a modified amino acid sequence, resulting in a polypeptide having substantially reduced affinity to integrin αllbβ3 and / or α5β1 as compared to a wild-type disintegrin. The variant is useful for treatment and / or prevention of αvβ3 integrin-associated diseases in a mammal, which include osteoporosis, bone tumor or cancer growth, angiogenesis-related tumor growth and metastasis, tumor metastasis in bone, malignancy-induced hypercalcemia, angiogenesis-related eye diseases, Paget's disease, rheumatic arthritis, and osteoarthritis. The angiogenesis-related eye diseases include age-related macular degeneration, diabetic retinopathy, corneal neovascularizing diseases, ischaemia-induced neovascularizing retinopathy, high myopia, and retinopathy of prematurity.
Owner:NAT CHENG KUNG UNIV +1
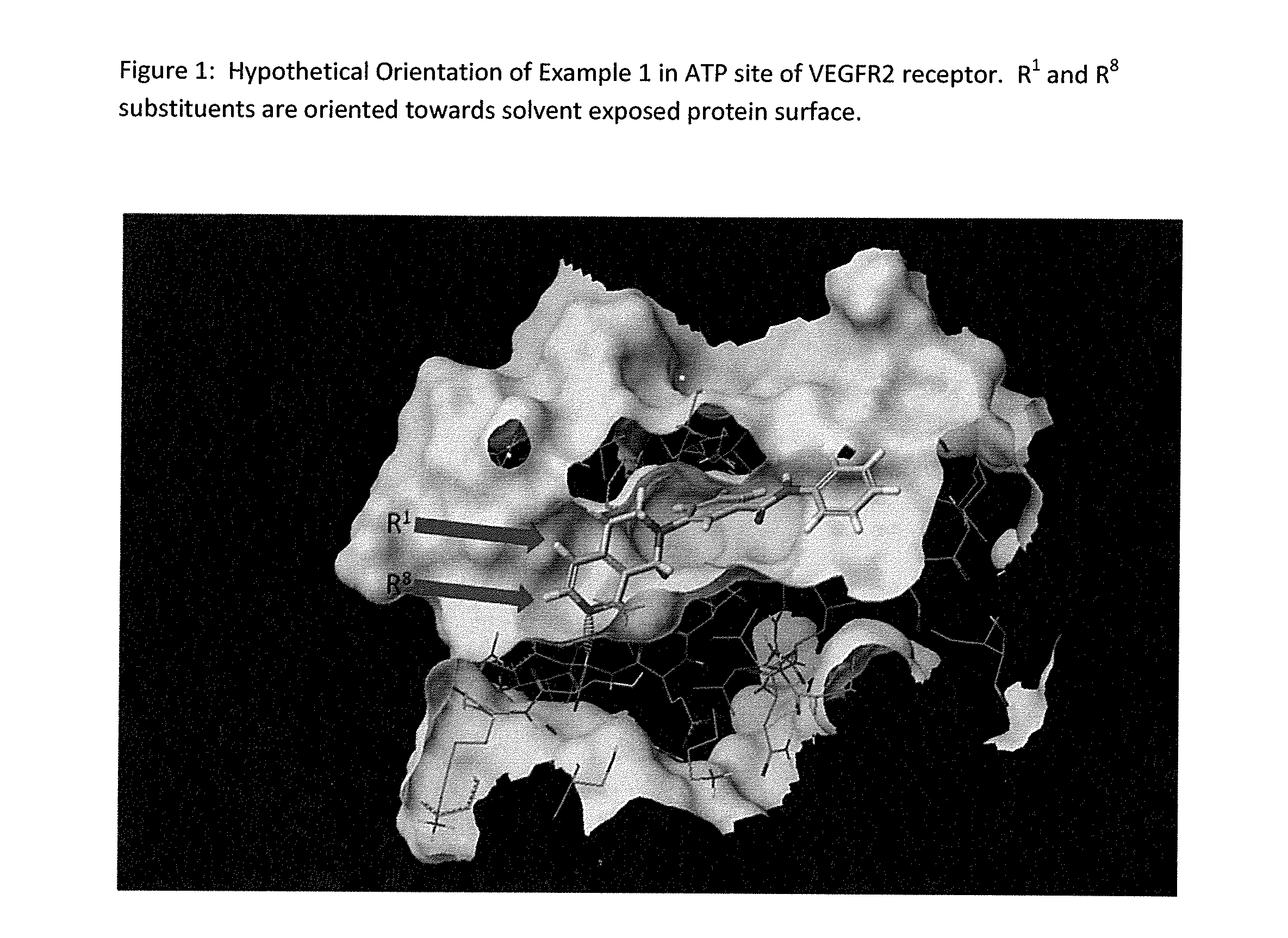
Method of treating conditiions with kinase inhibitors
ActiveUS20130237537A1Improve hydrophilicityImprove solubilityBiocideSenses disorderDiabetic retinopathyDisease
The present invention relates to a method of treating ophthalmic diseases and conditions, e.g. diabetic retinopathy, age-related macular degeneration, retinopathy of prematurity, etc., in a subject comprising administering to said subject a therapeutically effective amount of at least one compound of formula Ior a prodrug, pharmaceutically acceptable salt, racemic mixtures or enantiomers of said compound. The compounds of formula I are capable of modulating tyrosine kinase signal transduction in order to regulate, modulate and / or inhibit abnormal cell proliferation.
Owner:ALLERGAN INC
Multi-Tyrosine Kinase Inhibitors Derivatives and Methods of Use
InactiveUS20180009758A1Macular degeneration is suppressed and preventedWet macular degenerationOrganic chemistryPharmaceutical non-active ingredientsTyrosine-kinase inhibitorDrusen
The present invention is directed to multi-tyrosine kinase inhibitor compounds. The present invention is further directed to compositions comprising those compounds. Finally, the present invention is directed to methods of treating eye conditions including, but not limited to, diabetic background retinopathy, diabetic macular edema, diabetic proliferative retinopathy, diabetic macular edema with proliferative retinopathy, proliferative fibrovascular disease, diabetic macular edema with proliferative fibrovascular disease, retinopathy of prematurity, dry macular degeneration, dry macular degeneration with drusen and wet macular degeneration, using compounds and compositions of the invention.
Owner:ONTOGENESIS LLC
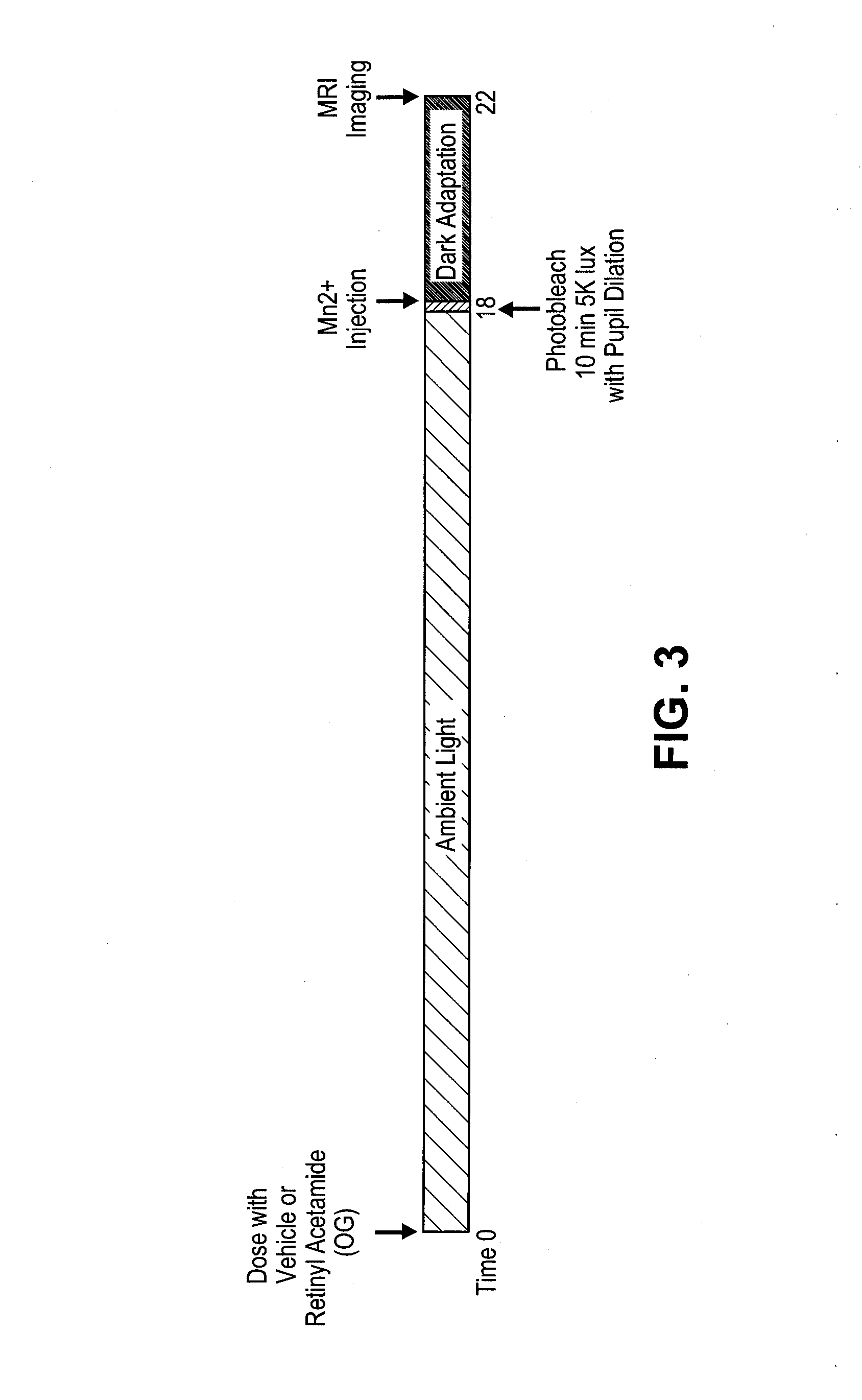
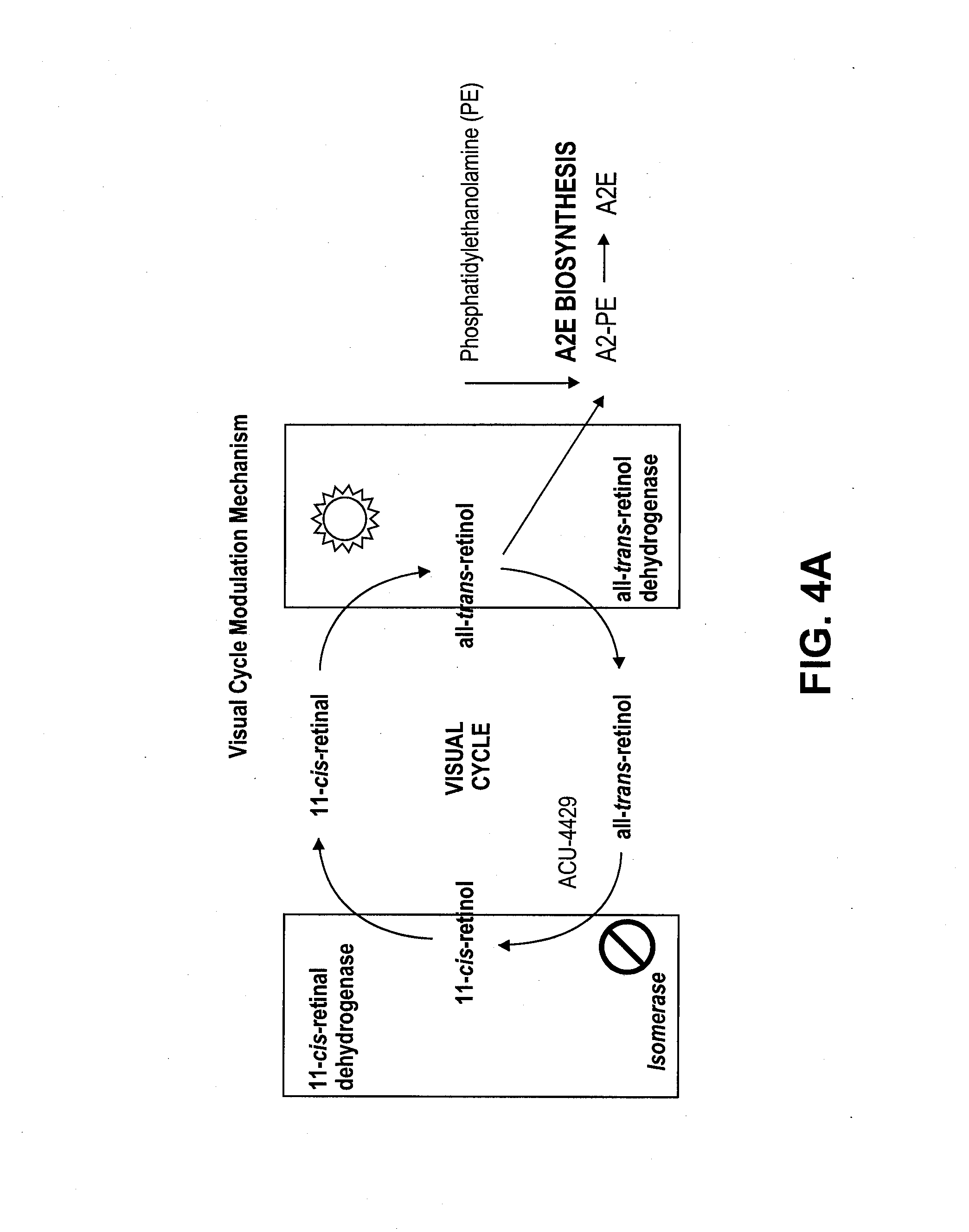
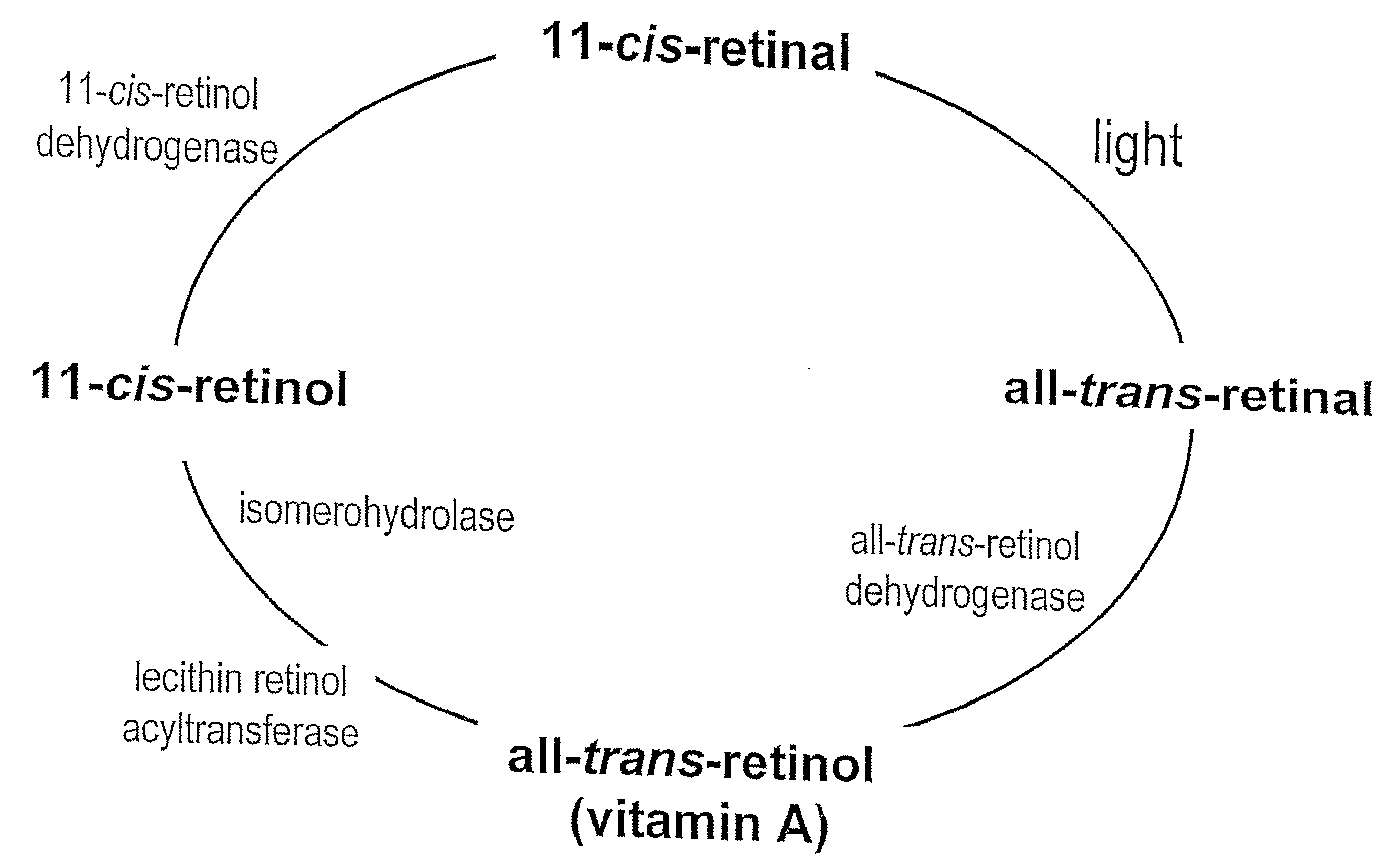
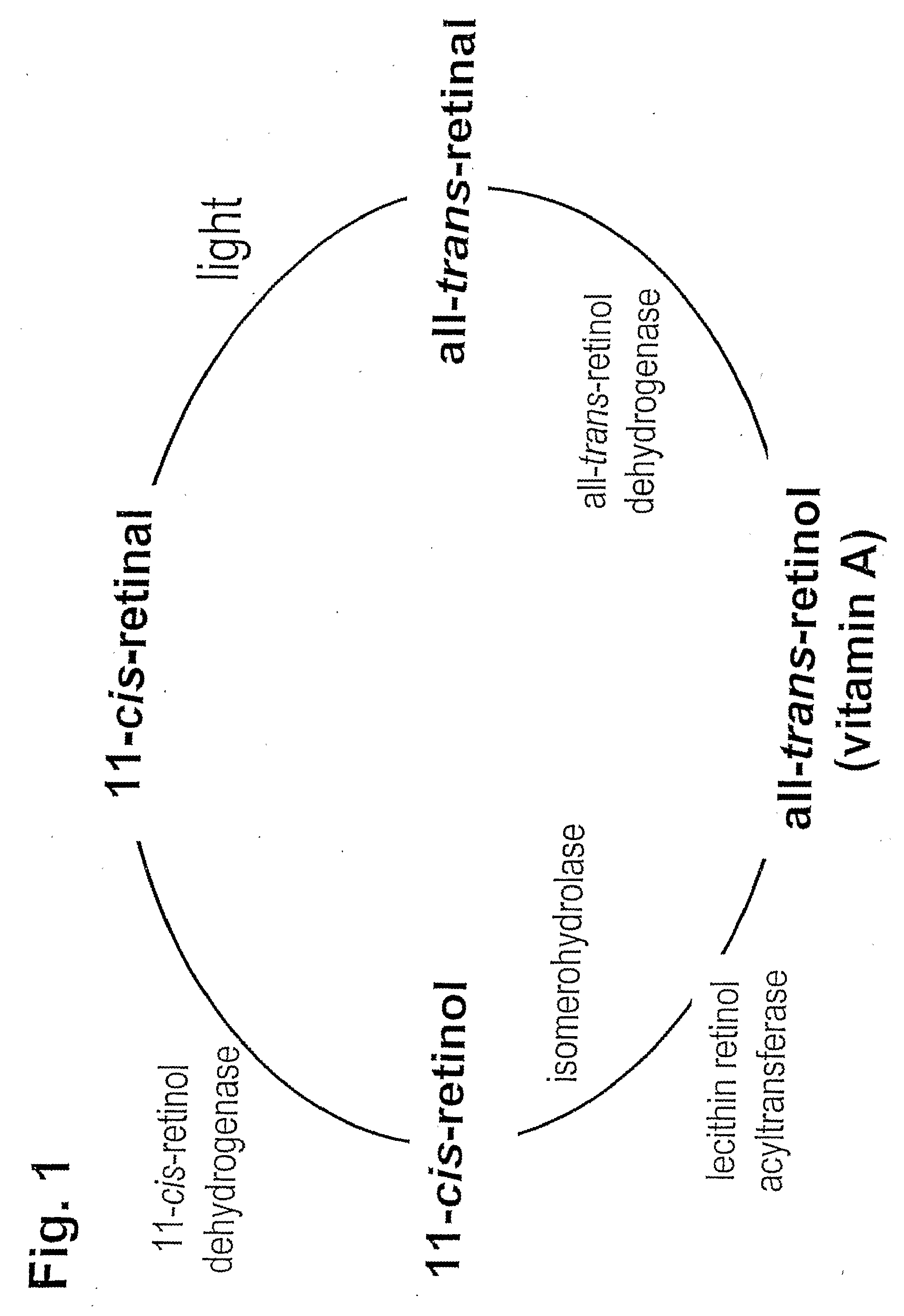
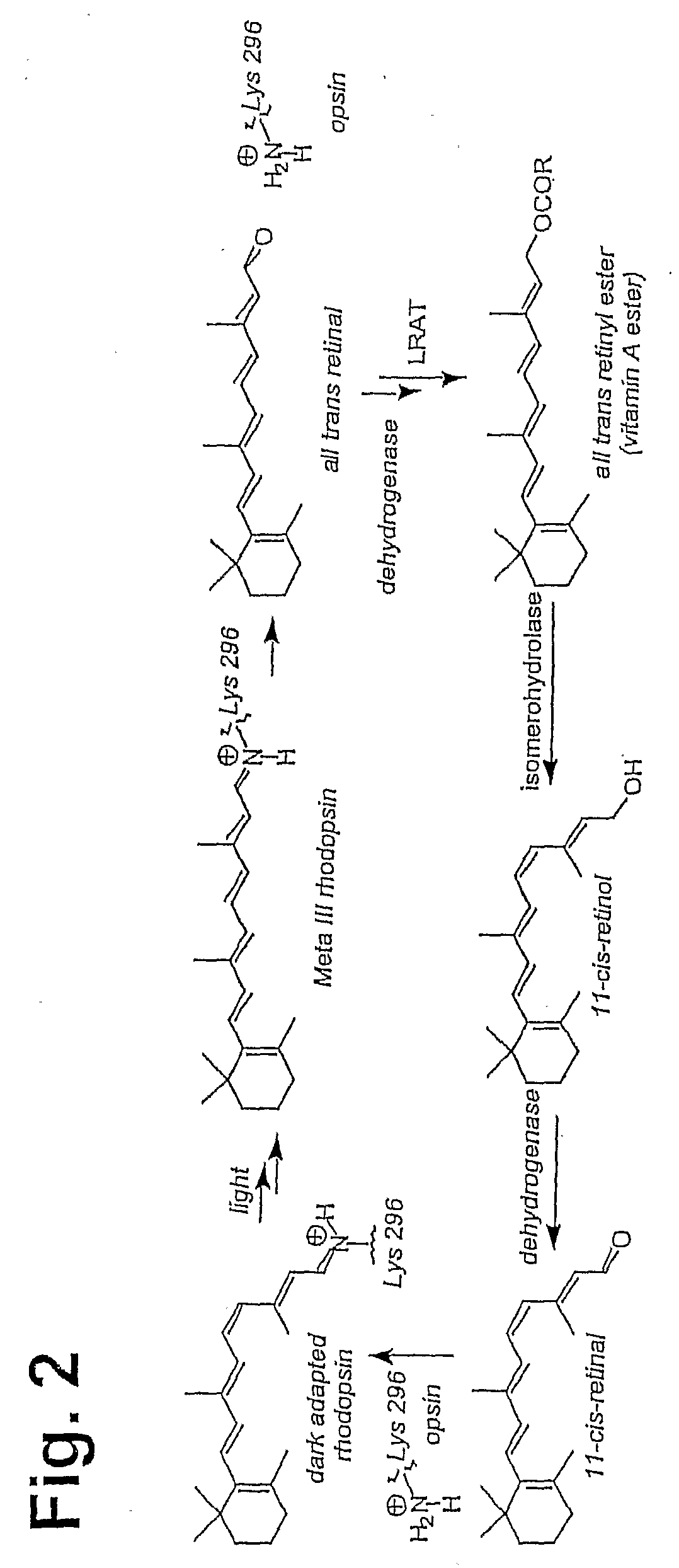
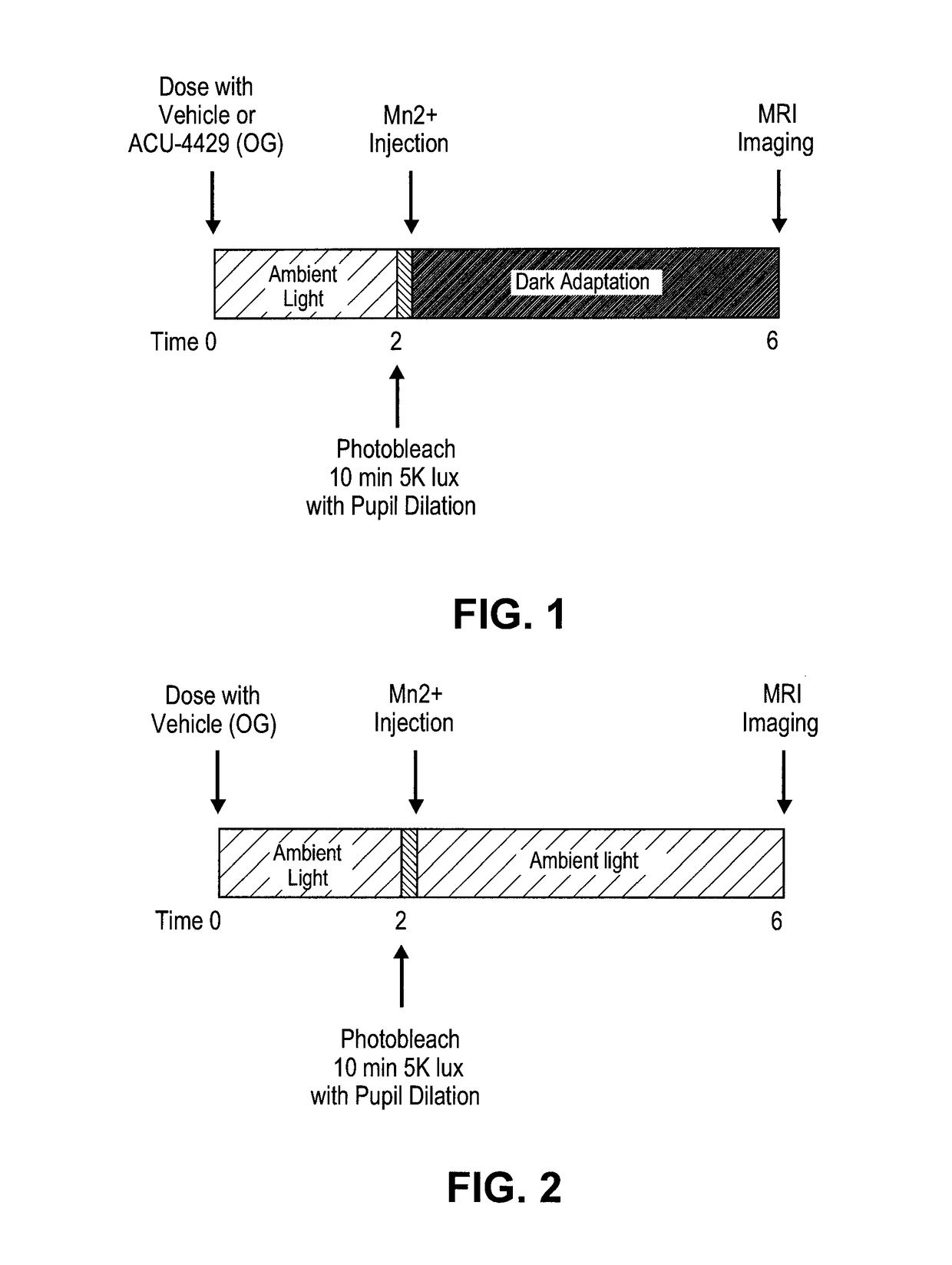
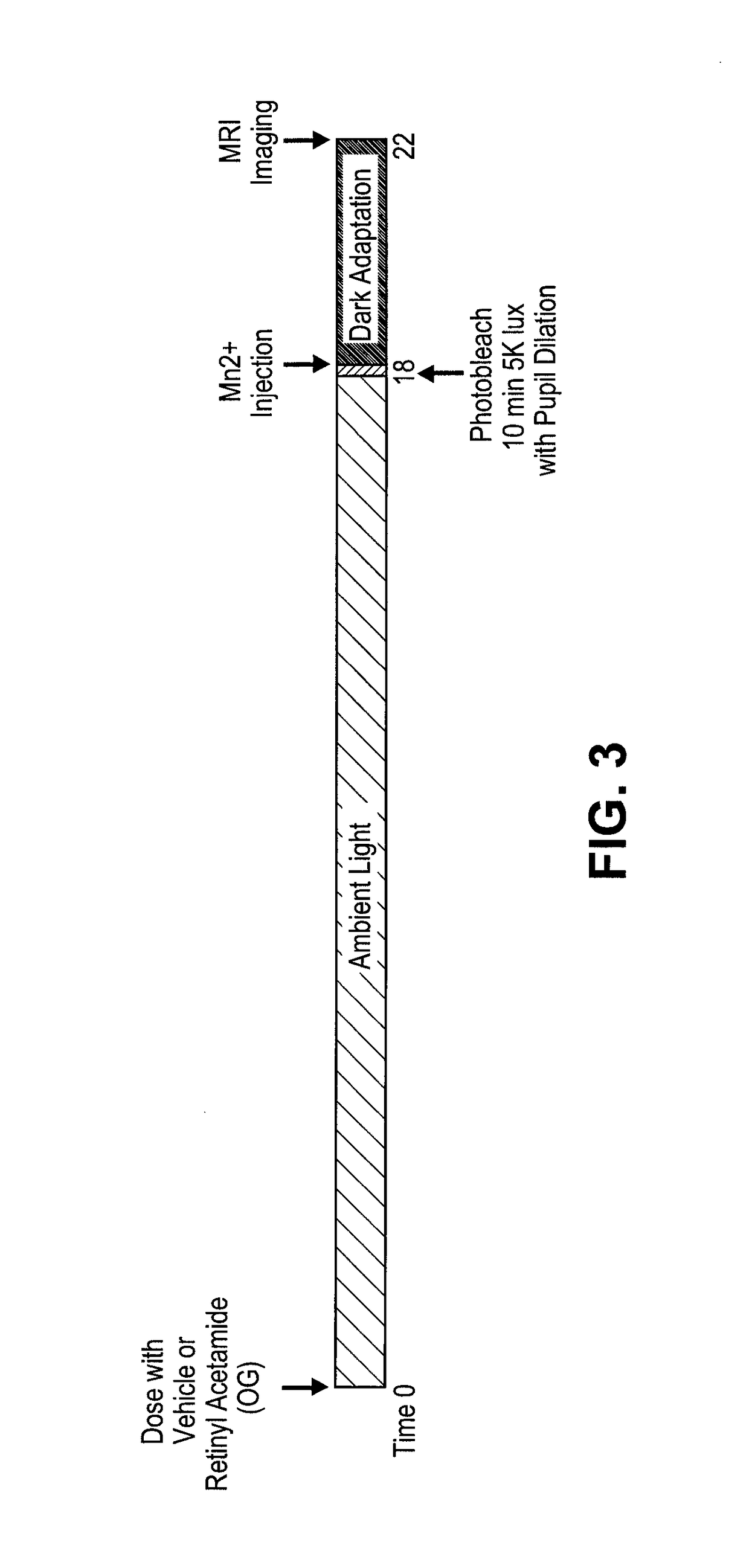
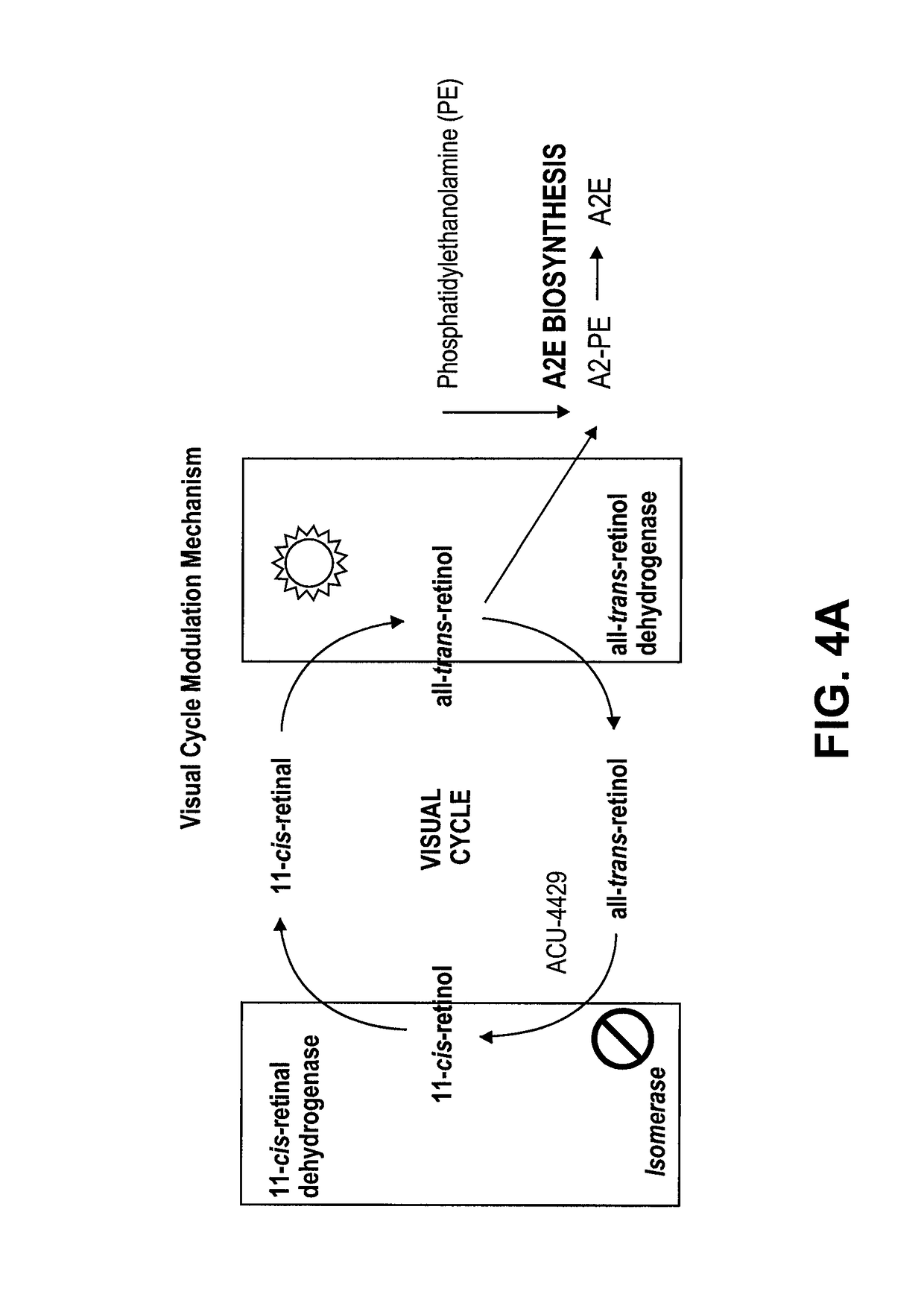
Prevention of Retinopathy by Inhibition of the Visual Cycle
The invention relates to compounds capable of inhibiting the visual cycle and / or dark adaptation and their use for treatment and prevention of non-degenerative retinal diseases with specific emphasis on the prevention and treatment of diabetic retinopathy, retinopathy of prematurity, branch retinal vein occlusion, central retinal vein occlusion, open-angle glaucoma, veovascular glaucoma, and other diseases of the retinal and / or optic nerve where an imbalance between metabolic demand and blood supply contribute to the development of tissue damage. The invention furthermore relates to pharmaceutical compositions comprising such compounds.
Owner:LARSEN
Infant formulas containing docosahexaenoic acid and lutein
ActiveUS20110213039A1Lessen risk of and severityReduce riskHeavy metal active ingredientsBiocideDocosahexaenoic acidBeta-Carotene
Disclosed is a method of reducing the risk or severity of retinopathy of prematurity in preterm infants. The method comprises (a) measuring skin carotenoid levels in preterm infants, preferably by Raman Spectroscopy, and then (b) administering supplemental carotenoids to those infants in need thereof, wherein the supplemental carotenoids comprise lutein, lycopene, beta-carotene, and zeaxanthin. The supplemental carotenoids may be provided by an infant formula comprising, on a ready-to-feed basis, from about 100 to about 2000 mcg / liter of total carotenoids, wherein the total carotenoids include at least about 50 mcg / liter of lutein. The formulas may further comprise docosahexaenoic acid.
Owner:ABBOTT LAB INC
Application of transthyretin in aspects of entering eye and preparing drop
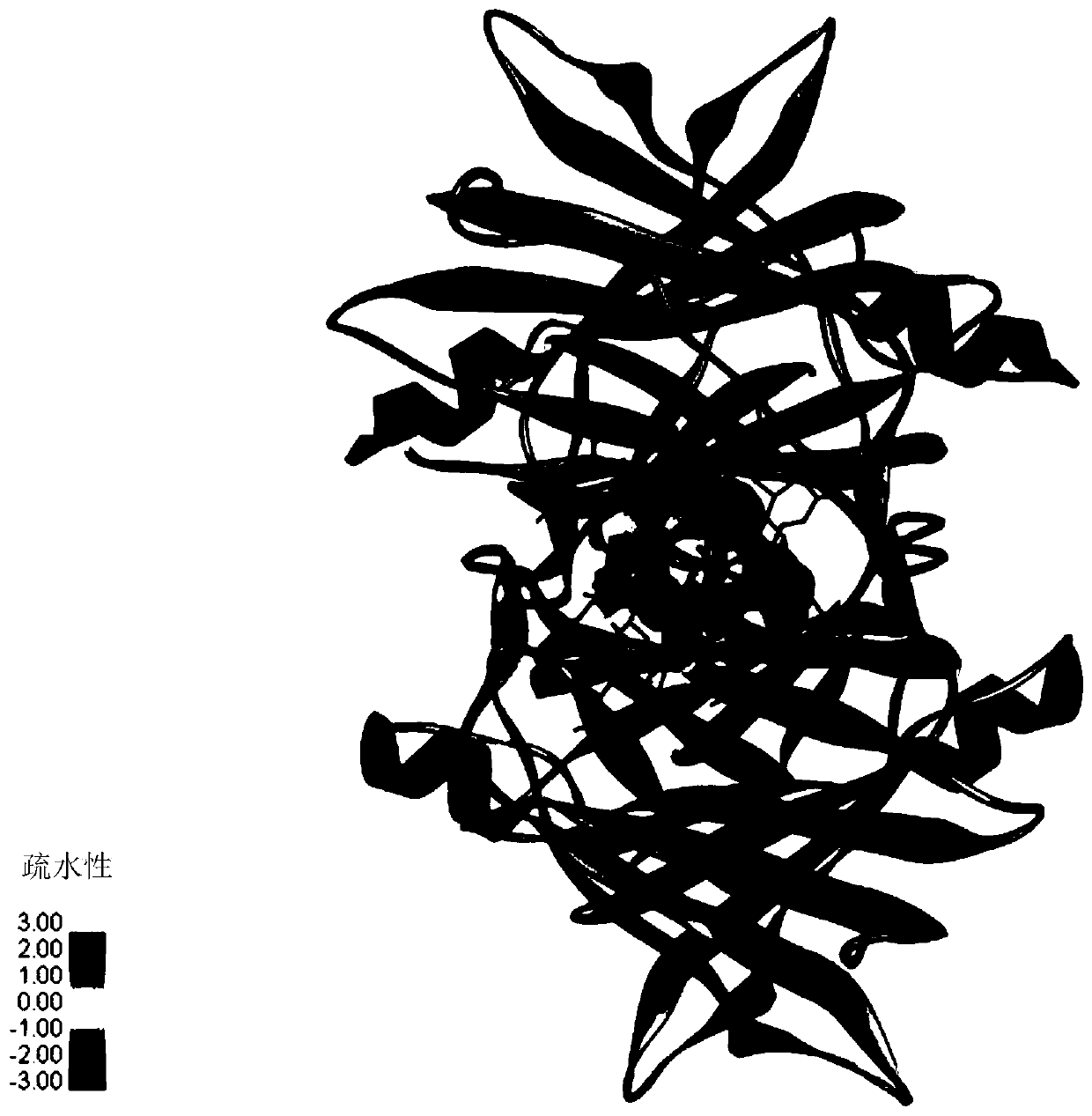
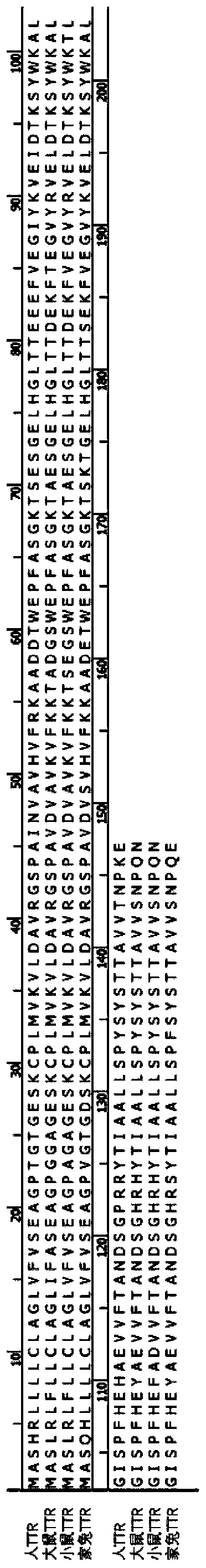
ActiveCN111437398AGood biocompatibilityHigh biosecuritySenses disorderAntibody mimetics/scaffoldsDiseaseAmino acid composition
The invention provides application of transthyretin in the aspect of serving as a carrier for a protein and / or polypeptide drug to enter an eye through an eye barrier. The transthyretin is a protein consisting of amino acid as shown in SEQ ID NO:1 or a mutation thereof or a modification thereof. The invention further provides application of the transthyretin and / or a fusion protein of the transthyretin and a drug to preparation of a drop and a drop. The drug is the protein and / or polypeptide drug. The transthyretin has good biocompatibility and safety in human bodies, can effectively convey aforeign protein and / or polypeptide into the eye and achieves an effect of treating eye diseases. In an eye dropping manner, when the eye diseases such as DR (Diabetic Retinopathy), AMD (Age-related macular degeneration), ROP (Retinopathy of Prematurity) and the like are treated, the transthyretin can enter a vitreous body and eye ground across a cornea barrier so as to obviously inhibit eyeball retina leakage, obviously reduce the number of retinal new blood vessels and effectively relieve the pathological phenomena of the eye diseases.
Owner:易舟(上海)生物医药有限公司
Methods and compositions for treating diseases associated with neovascualrization
InactiveUS20080076742A1Inhibit angiogenesisInhibiting neovascular growthOrganic active ingredientsBiocideDiabetic retinopathyAngiogenesis growth factor
Methods and compositions for treating pathologies resulting from neovascular growth in the eye such as those manifested as retinopathy of prematurity, diabetic retinopathy and macular degeneration. The invention comprises the administration of an effective amount of vitamin or a salt, prodrug or derivative thereof, administered at doses less than toxicity and results in a significant reduction in angiogenesis or the formation of neo-vascular growth. The invention can be used to treat existing diseases or prophylactically to treat those at risk.
Owner:WISCONSIN ALUMNI RES FOUND
Methods for the treatment of diabetic retinopathy and other ophthalmic diseases
ActiveUS9957224B2Reduce rod energy demandImprove retinal functionSenses disorderOrganic chemistryDiseaseDiabetic retinopathy
Owner:ACUACELA INC
Modulators of EphA2 and Ephrin-A1 for the treatment of fibrosis-related disease
InactiveUS20100260749A1Promote growthIncreased proliferationOrganic active ingredientsSenses disorderDiseasePsoriatic arthropathy
The present invention relates to methods and compositions designed for the treatment, management, prevention and / or amelioration of non-neoplastic hyperproliferative epithelial and / or endothelial cell disorders, including but not limited to disorders associated with increased deposition of extracellular matrix components (e.g., collagen, proteoglycans, tenascin and fibronectin) and / or aberrant angiogenesis. Non-limiting examples of such disorders include cirrhosis, fibrosis (e.g., fibrosis of the liver, kidney, lungs, heart, retina and other viscera), asthma, ischemia, atherosclerosis, diabetic retinopathy, retinopathy of prematurity, vascular restenosis, macular degeneration, rheumatoid arthritis, osteoarthritis, infantile hemangioma, verruca vulgaris, Kaposi's sarcoma, neurofibromatosis, recessive dystrophic epidermolysis bullosa, ankylosing spondylitis, systemic lupus, Reiter's syndrome, Sjogren's syndrome, endometriosis, preeclampsia, atherosclerosis, coronary artery disease, psoriatic arthropathy and psoriasis. The methods of the invention comprise the administration of an effective amount of one or more agents that are modulators of EphA2 and / or its endogenous ligand, EphrinA1. The invention also provides pharmaceutical compositions comprising one or more EphA2 / EphrinA1 Modulators of the invention either alone or in combination with one or more other agents useful for therapy for such non-neoplastic hyperproliferative epithelial and / or endothelial disorders. Diagnostic methods and methods for screening for EphA2 / EphrinA1 Modulators are also provided.
Owner:MEDIMMUNE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com