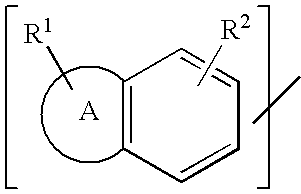
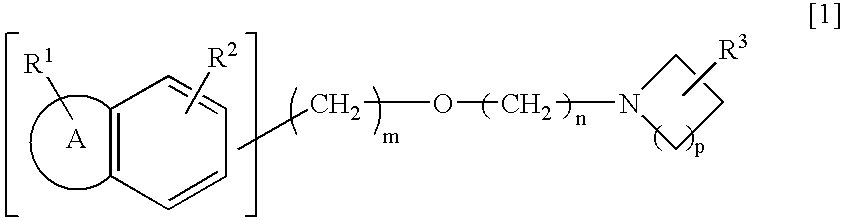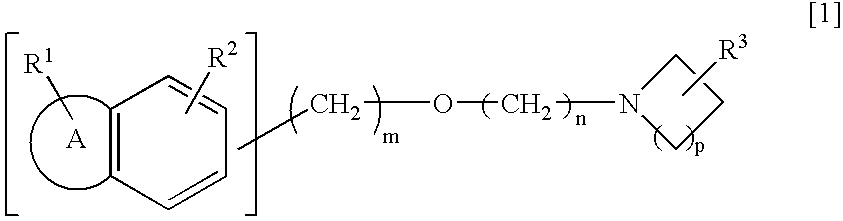Preventive/remedy for retinal nerve diseases containing alkyl ether derivatives or salts thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Production of 1-(2-(2-(1-benzothiophene-5-yl)ethoxy)ethyl)-3-azetidinol
[0143] (1) 1.20 g of 2-(2-(1-benzothiophene-5-yl)ethoxy)acetic acid was dissolved in 12 ml of methylene chloride. Thereafter, 2.3 ml of triethylamine and 0.38 g of imidazole were added to the obtained solution, and the mixture was then cooled to 5° C. Thereafter, 0.41 ml of thionyl chloride was added dropwise thereto, and the obtained mixture was stirred at the same above temperature for 1 hour. The reaction mixture was cooled to −60° C., and thereafter, 0.82 ml of triethylamine and 0.72 g of 3-azetidinol hydrochloride were added thereto. The mixture was stirred at the same above temperature for 1 hour and then at a room temperature for 1.5 hours. Thereafter, water was added to the reaction mixture, and the pH thereof was adjusted to pH 1.0 by addition of 6 mol / l hydrochloric acid. Thereafter, an organic layer was separated. The organic layer was washed with a saturated saline solution and then dried over anhydr...
production example 2
Production of 1-(2-(2-(1-benzothiophene-5-yl)ethoxy)ethyl)-3-azetidinol hydrochloride
[0147] 1.03 g of 1-(2-(2-(1-benzothiophene-5-yl)ethoxy)ethyl)-3-azetidinol was dissolved in 4.2 ml of ethyl acetate. Thereafter, 0.86 ml of an ethyl acetate solution containing 4.76 mol / l dry hydrogen chloride was added to the obtained solution, and the obtained mixture was stirred at a room temperature for 1 hour, and then at 5° C. for 1 hour. Thereafter, precipitated crystals were collected by filtration, washed with ethyl acetate, and then dried, so as to obtain 0.98 g of 1-(2-(2-(1-benzothiophene-5-yl)ethoxy)ethyl)-3-azetidinol hydrochloride.
[0148] Melting point: 101° C. to 102° C.
[0149] IR (KBr)cm−1: 3132, 2952, 1423, 1340, 1158, 814, 701
[0150] NMR (CDCl3)δppm: 2.97 (2H, t, J=7 Hz), 3.2-3.3 (2H, m), 3.69 (2H, t, J=7 Hz), 3.6-3.8 (2H, m), 3.9-4.1 (2H, m), 4.2-4.4 (2H, m), 4.6-4.8 (1H, m), 7.18 (1H, dd, J=1, 8 Hz), 7.29 (1H, d, J=5 Hz), 7.41 (1H, d, J=5 Hz), 7.65 (1H, d, J=1 Hz), 7.78 (1H, d,...
production example 3
Production of 1-(3-(2-(1-benzothiophene-6-yl)ethoxy)propyl)-3-azetidinol
[0151] 1.00 g of 6-(2-(3-chloropropoxy)ethyl)-1-benzothiophene was dissolved in 5 ml of dimethyl sulfoxide. Thereafter, 0.86 g of 3-azetidinol hydrochloride and 1.63 g of potassium carbonate were added to the obtained solution, and the obtained mixture was stirred at 75° C. for 2.5 hours, and then at 95° C. for 1.5 hours. Thereafter, the reaction solution was cooled, and thereafter, water and ethyl acetate were added to the reaction mixture. The pH of the obtained mixture was adjusted to pH 1 by addition of 6 mol / l hydrochloric acid, and a water layer was then separated. Ethyl acetate was added to the water layer, and the pH of the obtained mixture was adjusted to pH 10 by addition of a 2 mol / l aqueous sodium hydroxide solution, followed by separation of an organic layer. The organic layer was successively washed with water and a saturated saline solution, and then dried over anhydrous magnesium sulfate. Therea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com