Patents
Literature
34 results about "Premature thelarche" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
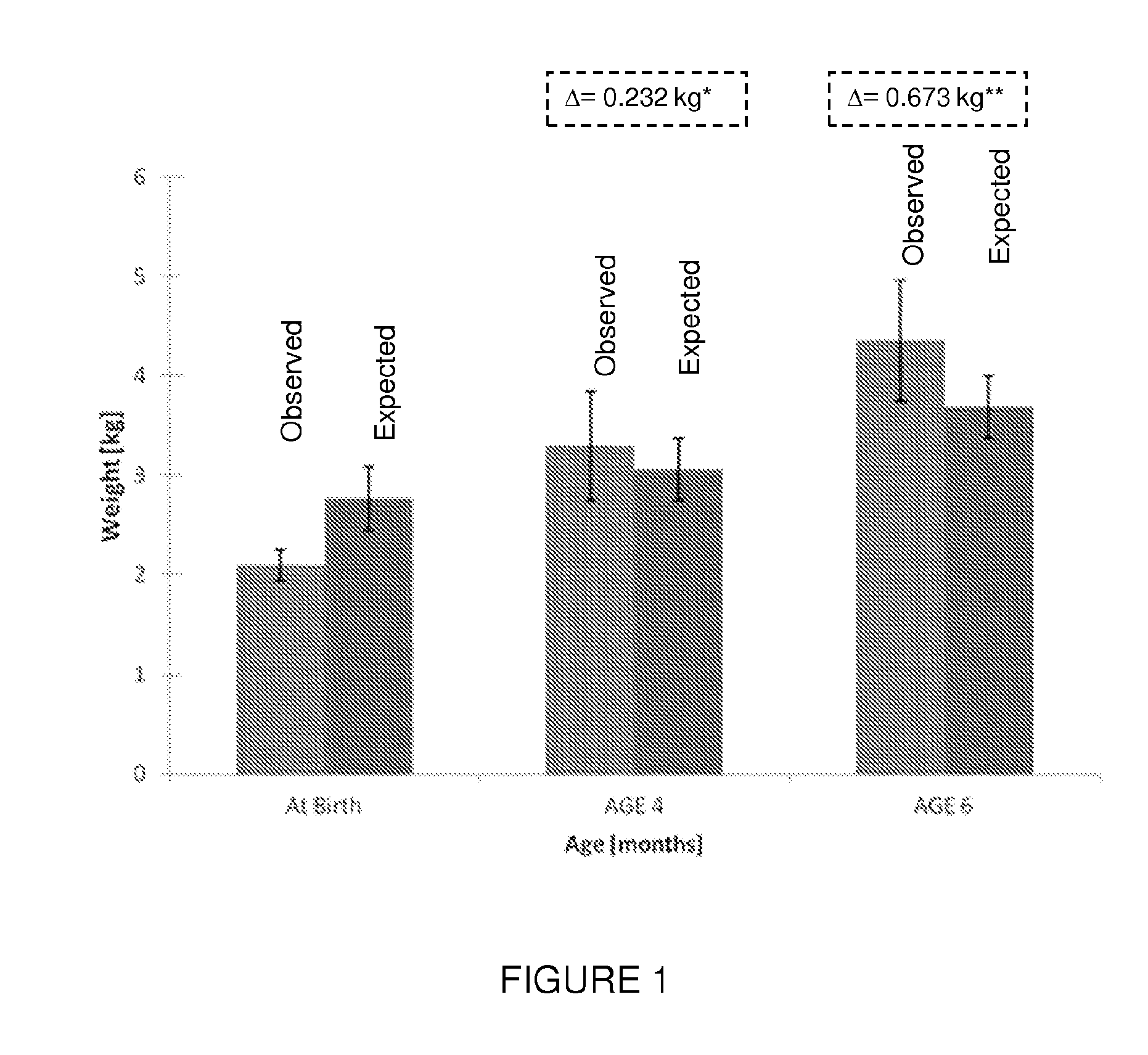
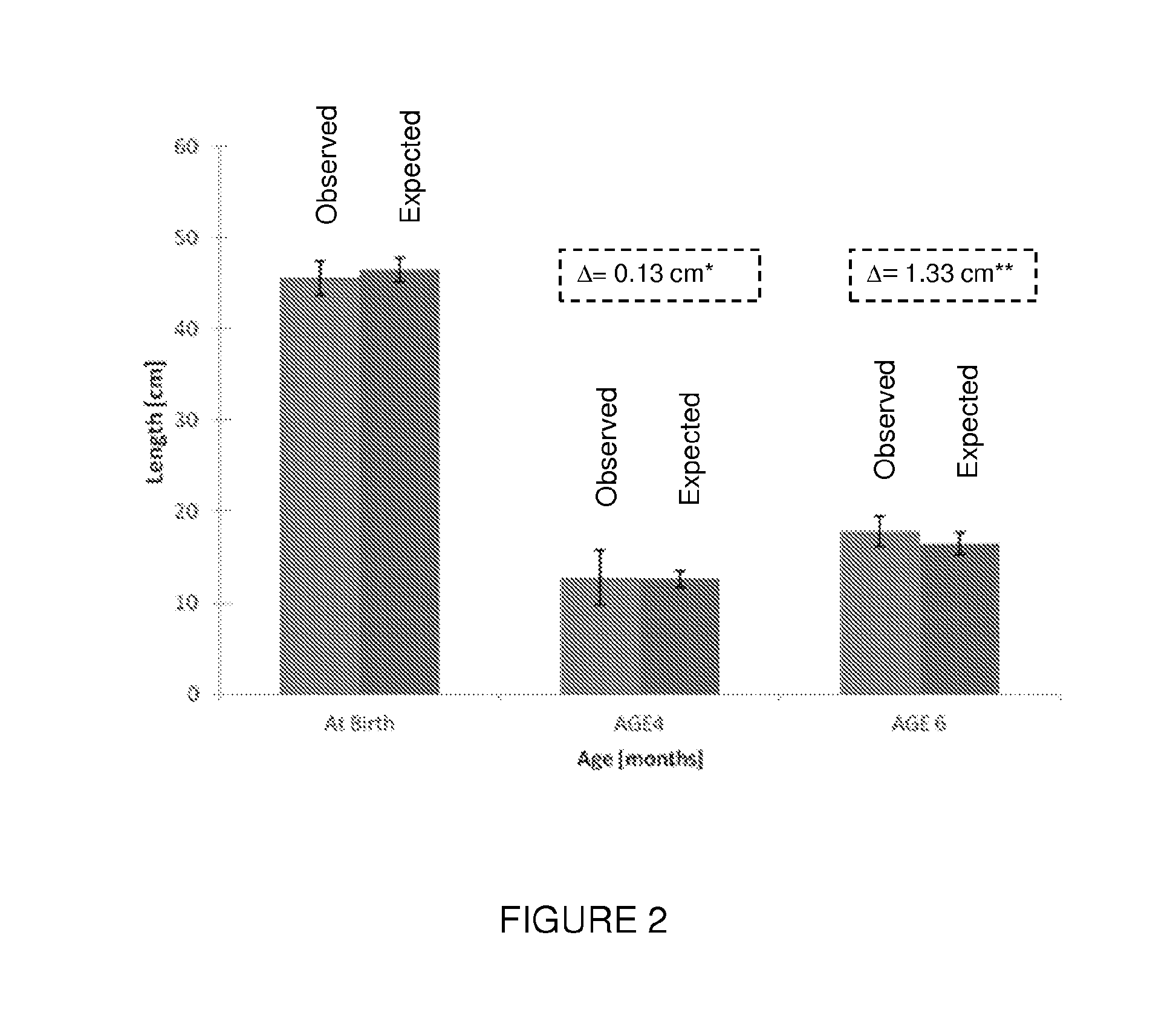
Premature thelarche (PT) is a medical condition, characterised by isolated breast development in female infants. It occurs in females younger than 8 years, with the highest occurrence before the age of 2. PT is rare, occurring in 2.2-4.7% of females aged 0 to 2 years old. The exact cause of the condition is still unknown, but it has been linked to a variety of genetic, dietary and physiological factors.
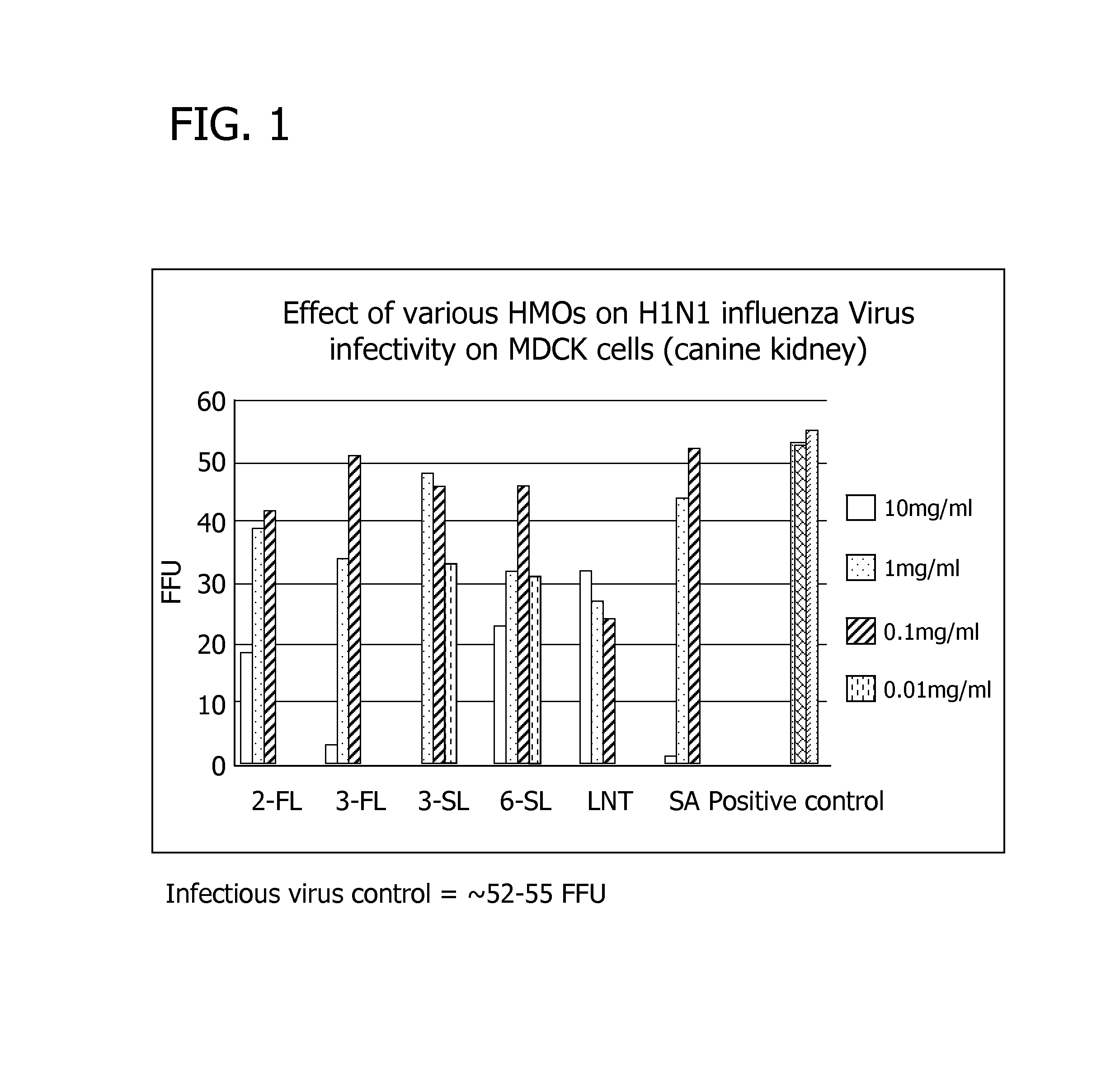
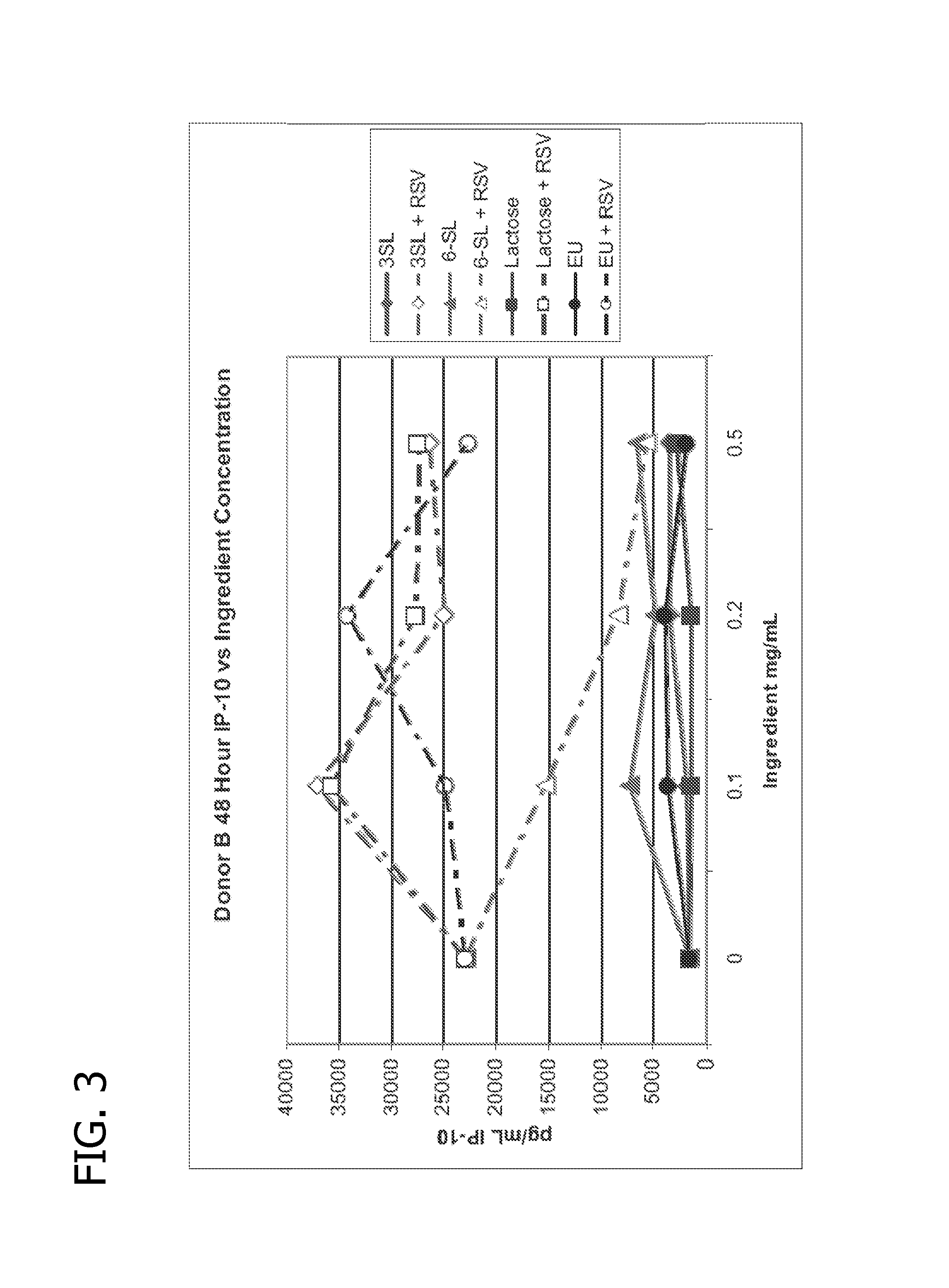
Human milk oligosaccharides to promote growth of beneficial bacteria
ActiveUS20120171165A1Improve immune system systemImprove system enteric nervous systemBiocideNervous disorderPremature thelarcheBacteroides
Disclosed are nutritional compositions including human milk oligosaccharides that can be administered to individuals including preterm infants, infants, toddlers, and children for improving gastrointestinal function and tolerance, as well as the growth of beneficial bacteria. Additional suitable methods of using the nutritional compositions including the human milk oligosaccharides are also disclosed.
Owner:ABBOTT LAB INC
Liquid nutritional product to supplement human milk
InactiveUS20060233915A1Improve solubilityImprove stabilityFood preparationPremature thelarcheNutrition supplementation
Owner:BRISTOL MYERS SQUIBB CO
Treatment of premature birth complications
InactiveUS20090136471A1Promote formationMany formatsAntibacterial agentsOrganic active ingredientsPremature thelarcheCord blood stem cell
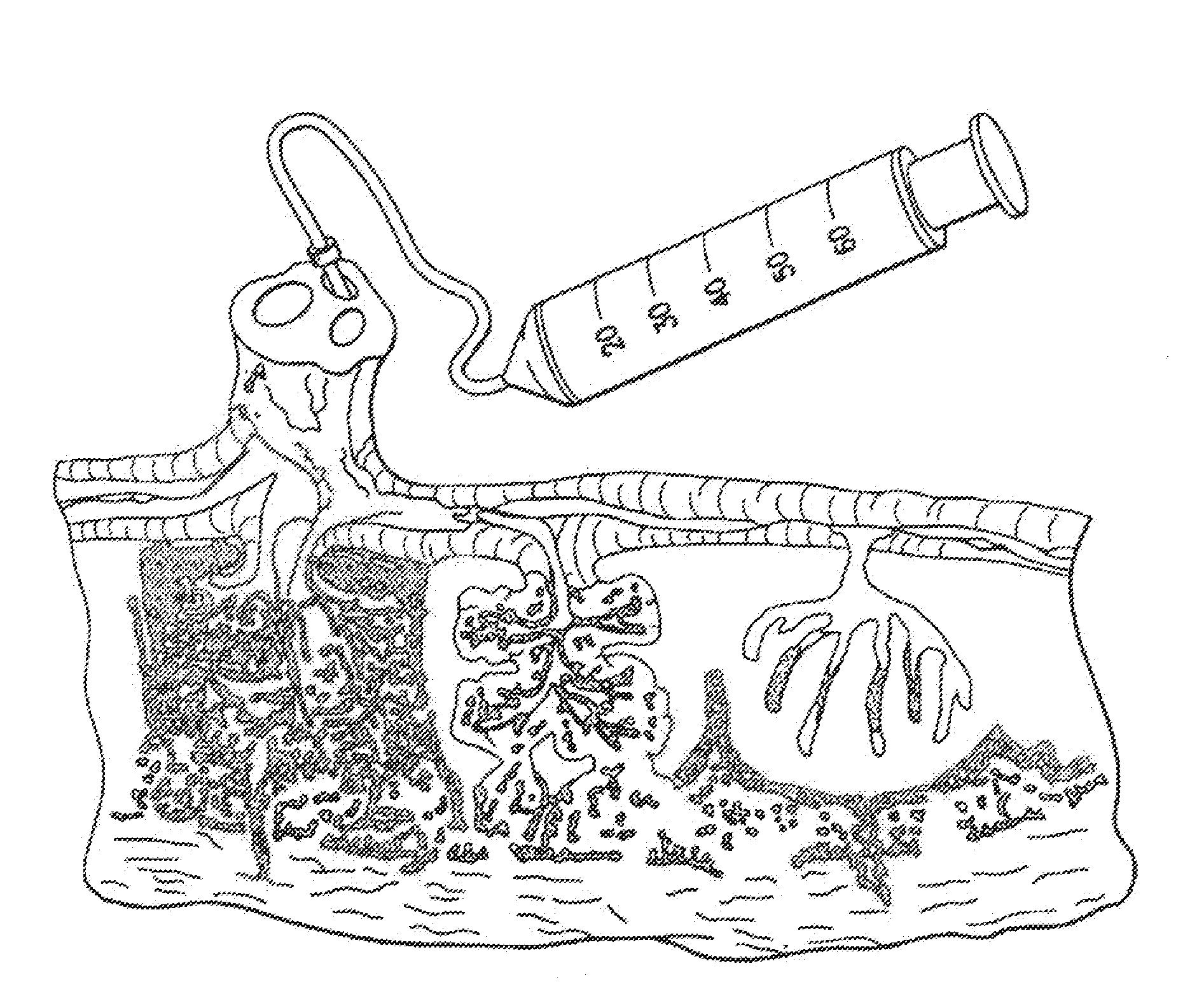
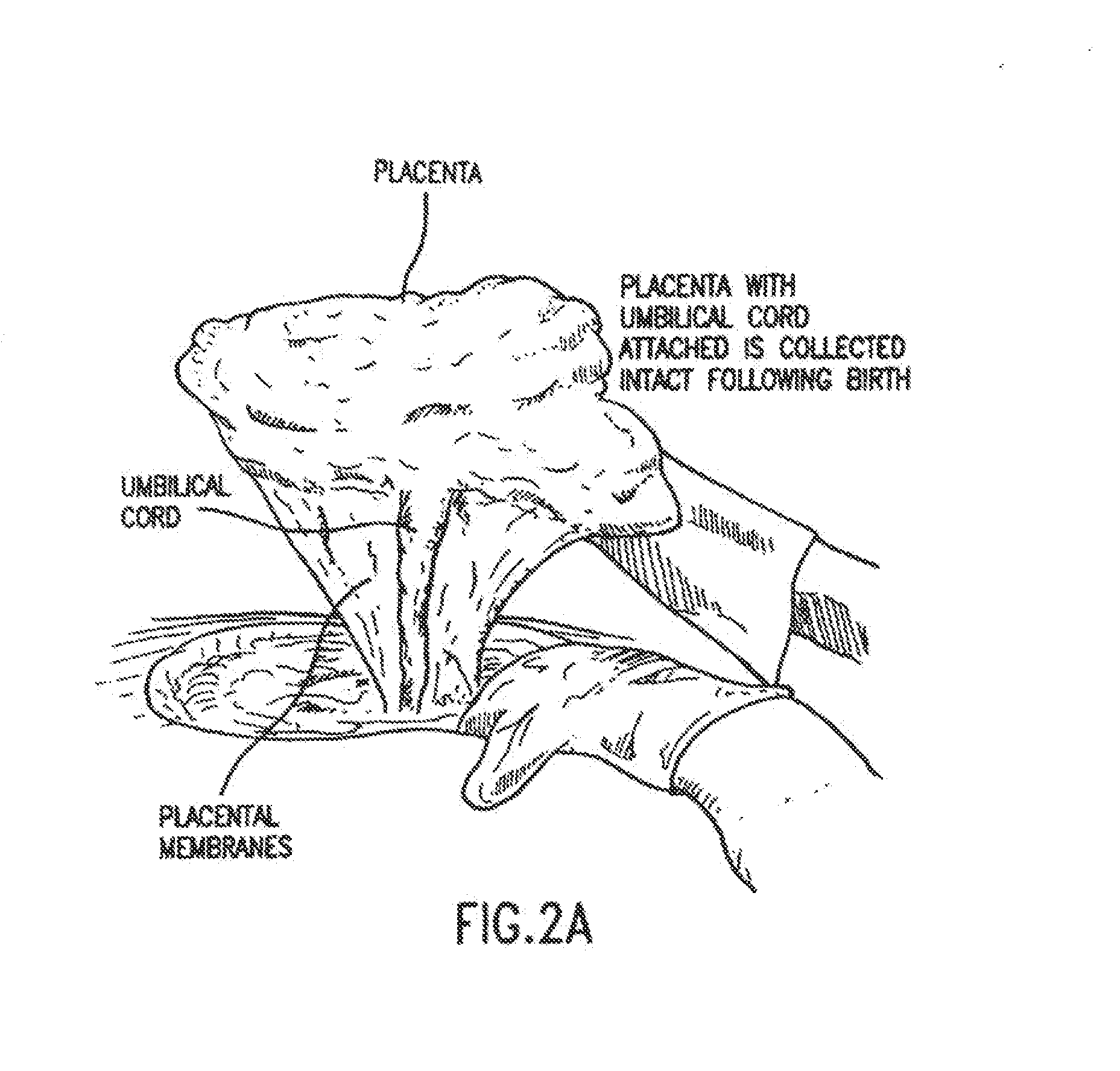
The present invention provides methods of treating one or more complications of premature birth suffered by premature infants, comprising administering to the premature infant umbilical cord blood stem cells and, optionally, placental stem cells. The present invention also provides methods of combining and administering, and compositions comprising, umbilical cord blood stem cells, particularly autologous cord blood cells, and placental stem cells for the treatment of premature infants.
Owner:CELULARITY INC
Methods of using human milk oligosaccharides for improving airway respiratory health
ActiveUS20120172331A1Reduce productionIncrease loadMilk preparationBiocidePremature thelarcheRespiratory health
Disclosed are nutritional compositions including human milk oligosaccharides that can be administered to preterm infants, term infants, toddlers, and children for improving airway defense mechanisms.
Owner:ABBOTT LAB INC
Human milk oligosaccharides to promote growth of beneficial bacteria
PendingUS20140249103A1Improving gut function and immunityAccelerated growth and maturationBiocideOrganic active ingredientsPremature thelarcheBacteroides
Disclosed are nutritional compositions including human milk oligosaccharides that can be administered to individuals including preterm infants, infants, toddlers, and children for improving gastrointestinal function and tolerance, as well as the growth of beneficial bacteria. Additional suitable methods of using the nutritional compositions including the human milk oligosaccharides are also disclosed.
Owner:ABBOTT LAB INC
Methods of using human milk oligosaccharides for improving airway respiratory health
ActiveUS8802650B2Reduce productionIncrease loadBiocideMilk preparationPremature thelarcheRespiratory health
Disclosed are nutritional compositions including human milk oligosaccharides that can be administered to preterm infants, term infants, toddlers, and children for improving airway defense mechanisms.
Owner:ABBOTT LAB INC
Human milk oligosaccharides for preventing injury and/or promoting healing of the gastrointestinal tract
ActiveUS20140335065A1High expressionImpaired ability to oxidize butyrateBiocideOrganic active ingredientsPremature thelarchePreventing injury
Owner:ABBOTT LAB INC
Methods for improving tolerance, digestion, and lipid soluble nutrient absorption in an infant, toddler, or child
ActiveUS20120172434A1Reduce the burden onImprove infant fat digestionBiocideHydroxy compound active ingredientsPremature thelarcheMonoglyceride
Disclosed are nutritional formulations including predigested fats that can be administered to preterm infants, infants, toddlers, and children for improving tolerance, digestion, and absorption of nutrients and for reducing the incidence of necrotizing enterocolitis, colic, and short bowel syndrome. The predigested fats include fatty acid-containing monoglycerides and / or a fatty acid component.
Owner:ABBOTT LAB INC
Method of reducing the risk of retinopathy of prematurity in preterm infants
InactiveUS20070166354A1Lessen risk of and severityReduce riskBiocideSenses disorderPremature thelarcheDocosahexaenoic acid
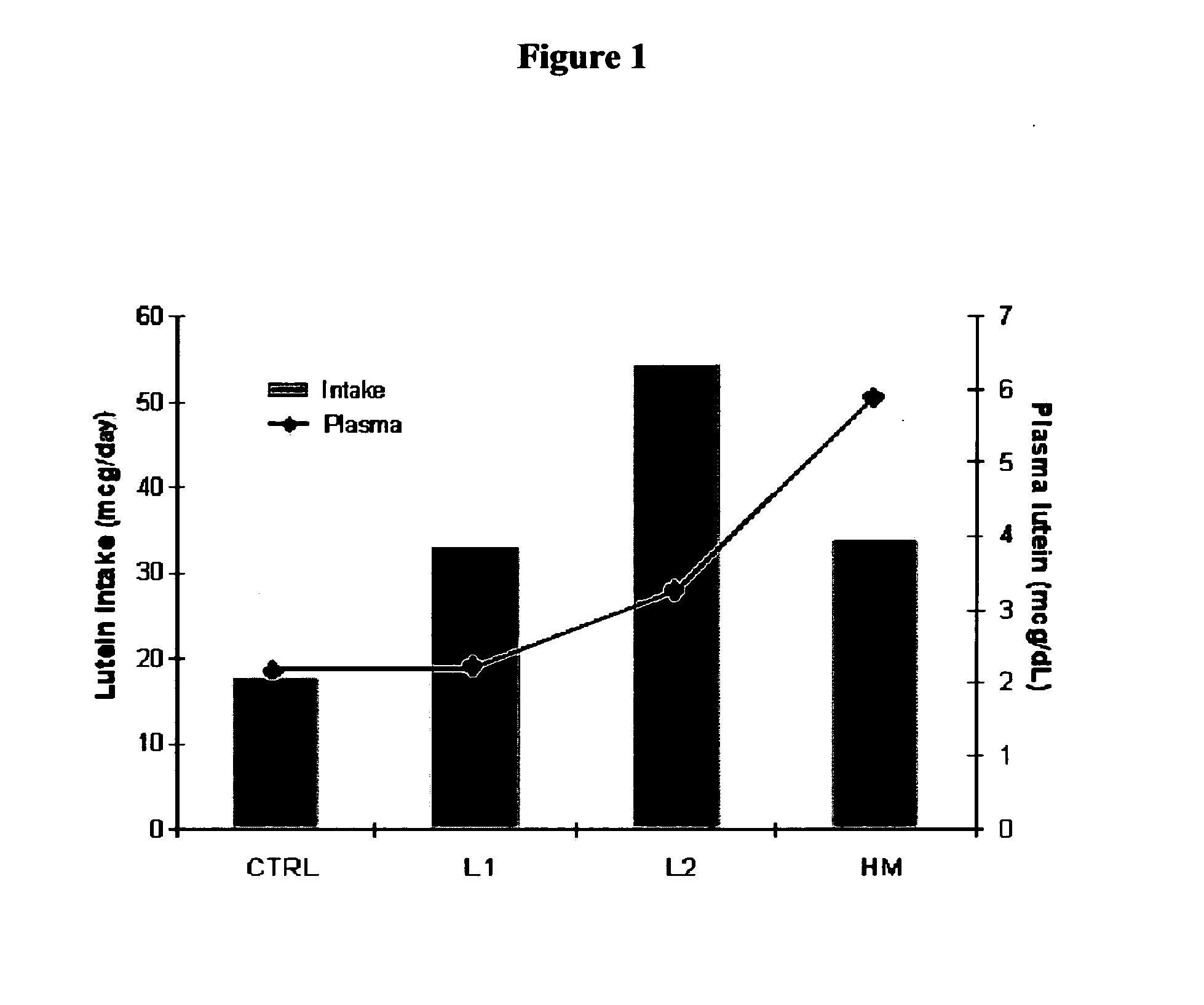
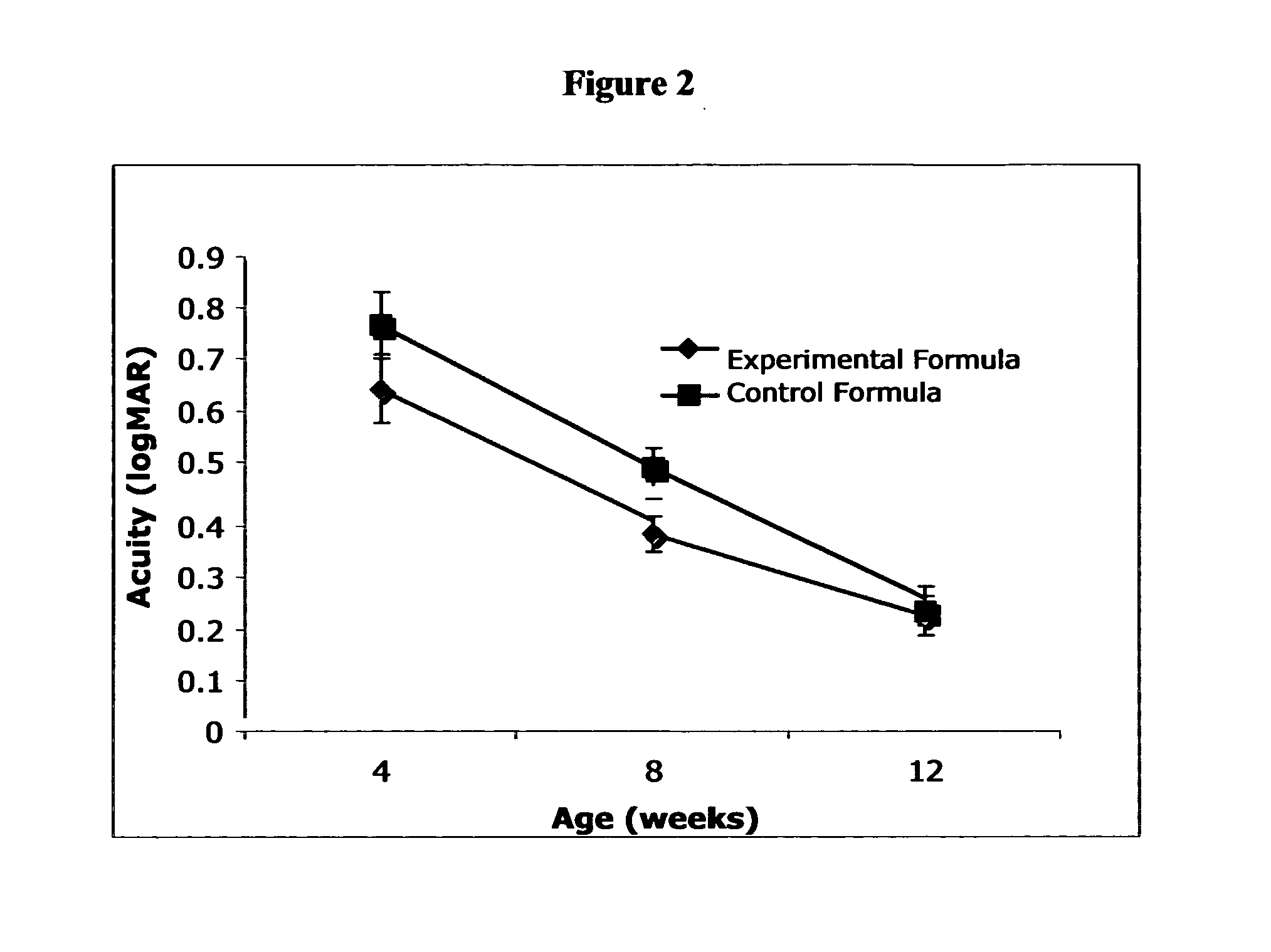
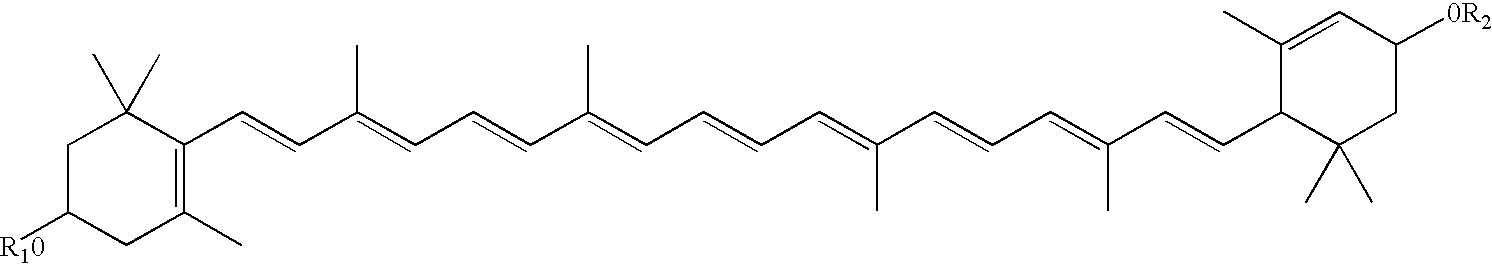
Disclosed is a method of reducing the risk or severity of retinopathy of prematurity in preterm infants. The method comprises (a) measuring skin carotenoid levels in preterm infants, preferably by Raman Spectroscopy, and then (b) administering supplemental carotenoids to those infants in need thereof, wherein the supplemental carotenoids comprise lutein, lycopene, beta-carotene, and zeaxanthin. The supplemental carotenoids may be provided by an infant formula comprising, on a ready-to-feed basis, from about 100 to about 2000 mcg / liter of total carotenoids, wherein the total carotenoids include at least about 50 mcg / liter of lutein. The formulas may further comprise docosahexaenoic acid.
Owner:ABBOTT LAB INC
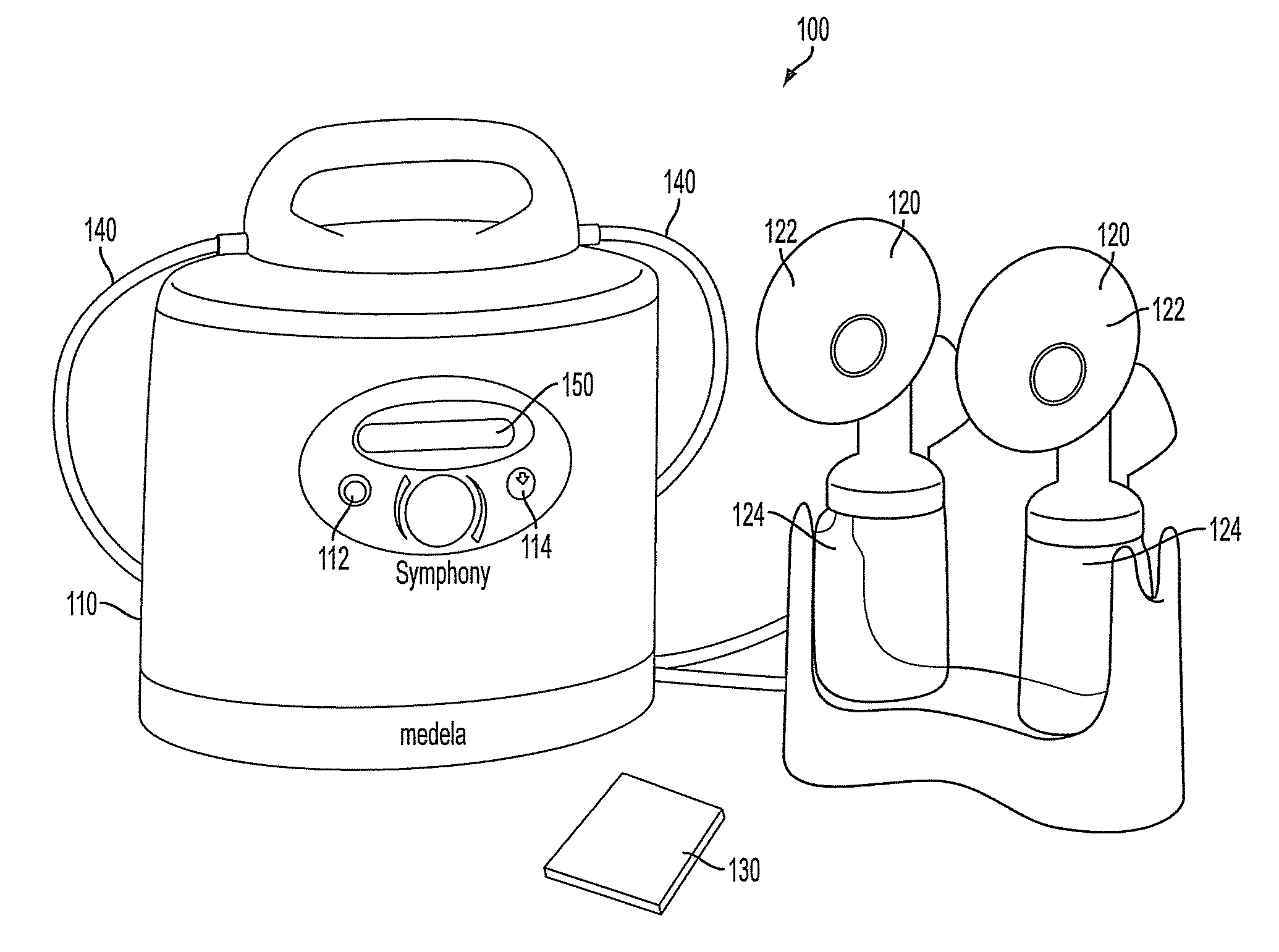
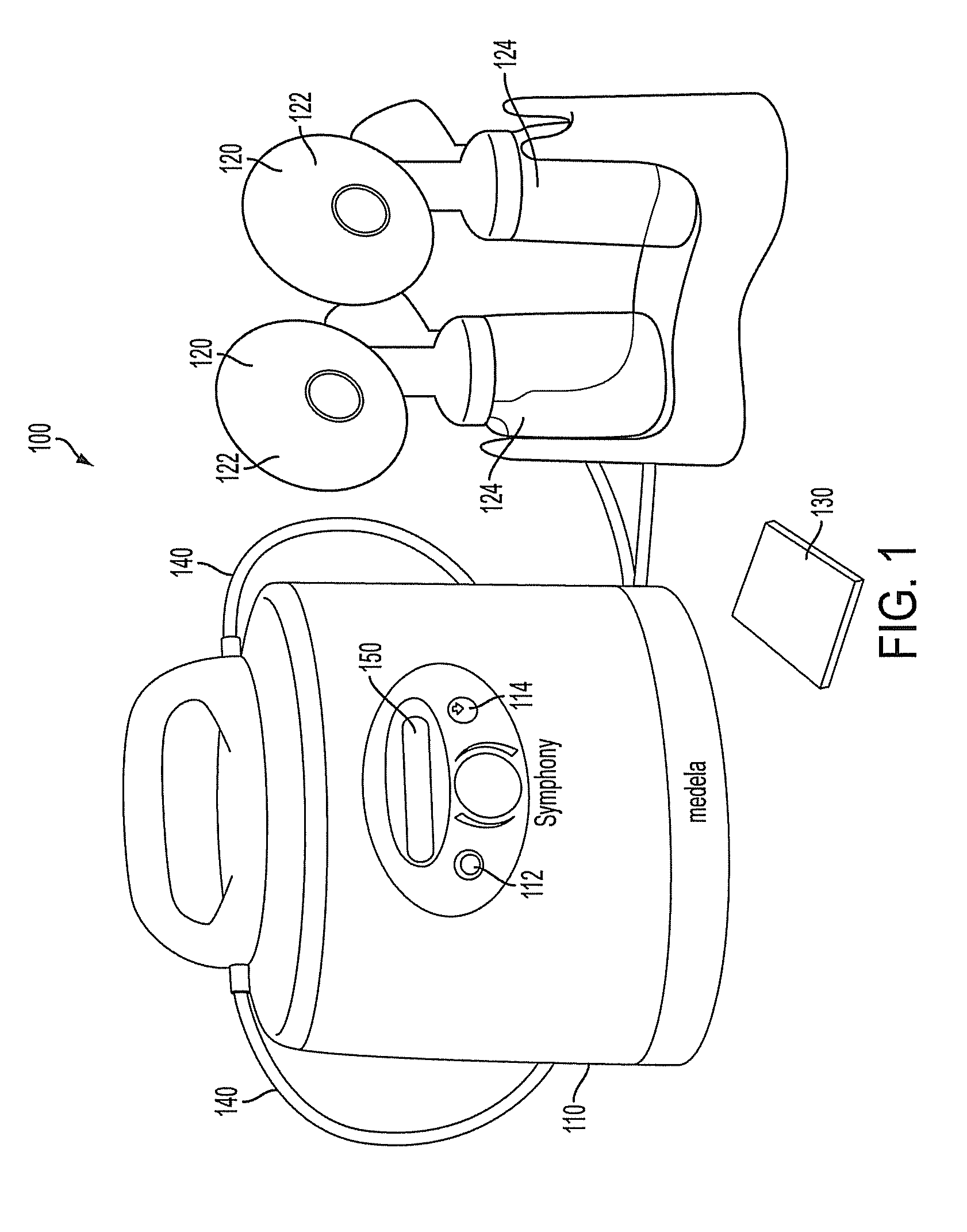
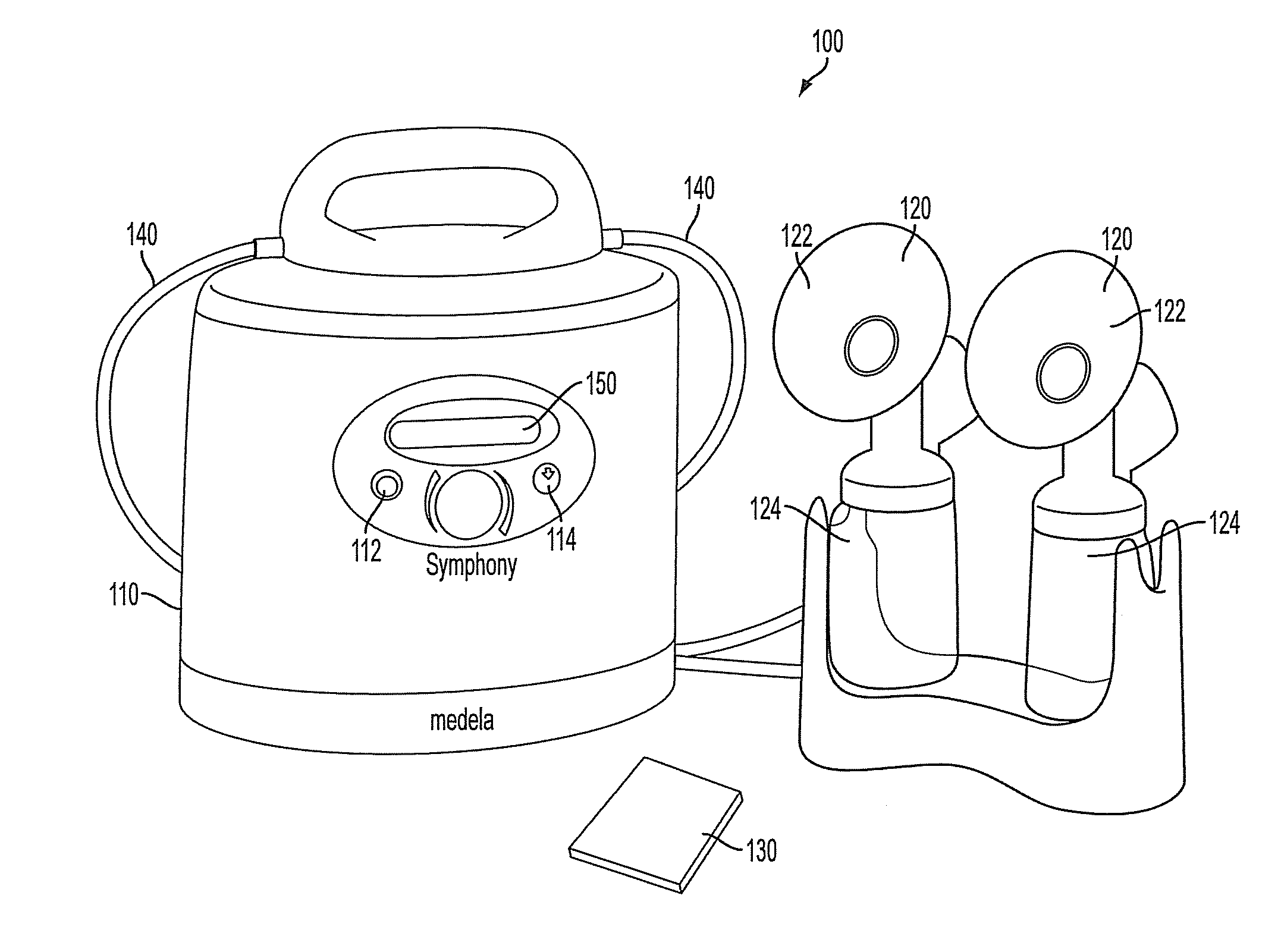
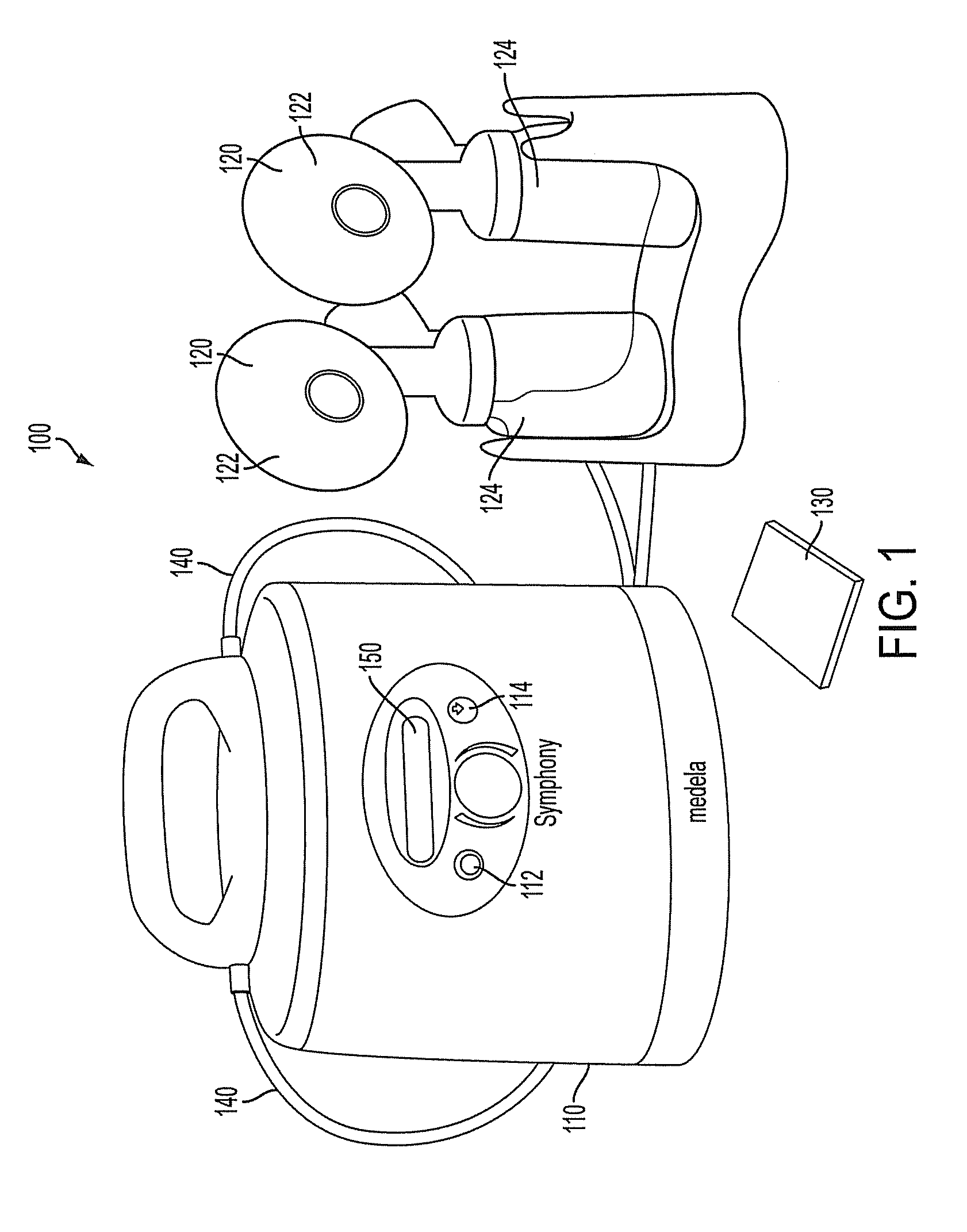
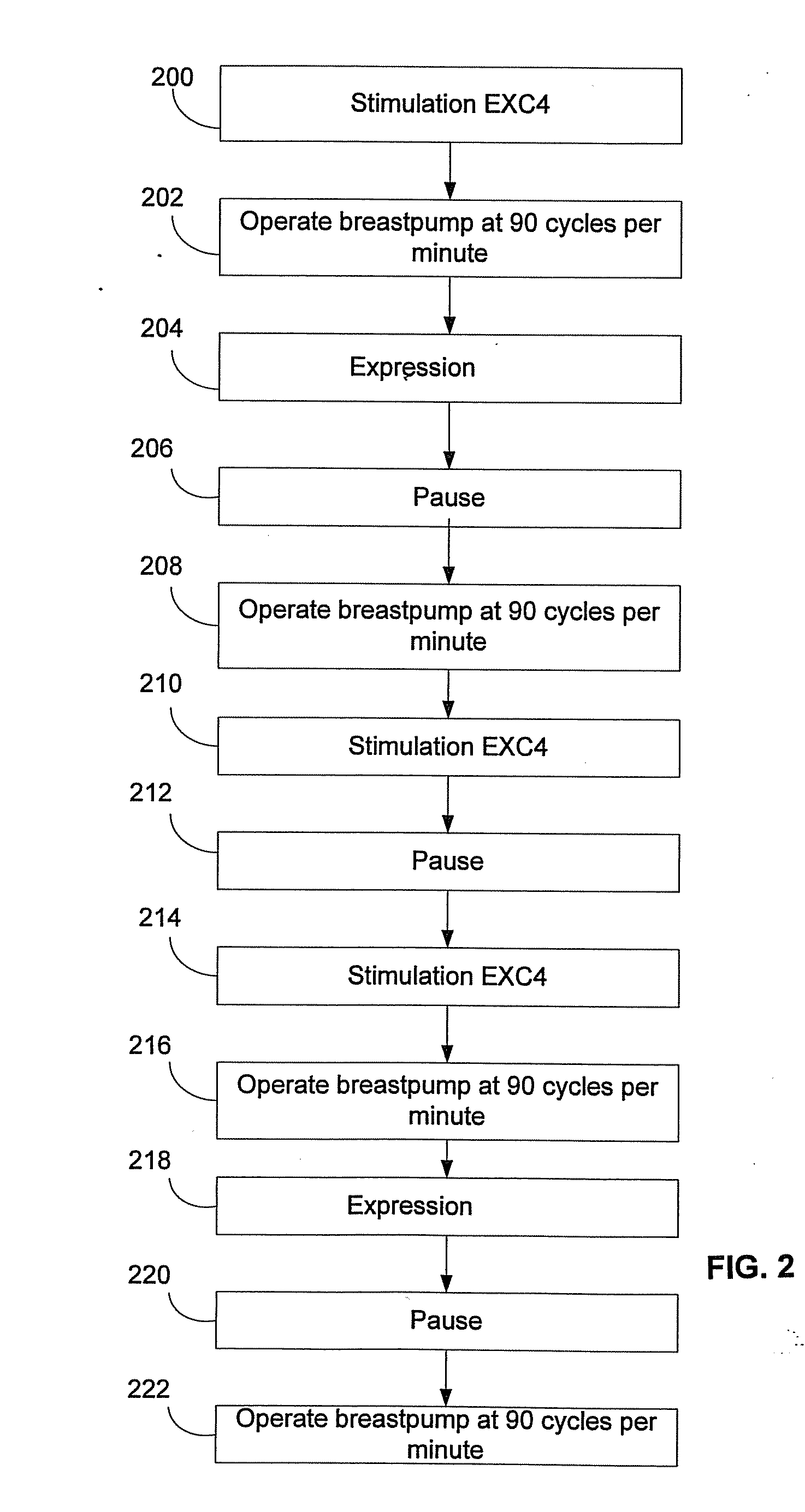
Process for use with breastpump to initiate milk in breastfeeding, particularly for premature infants
ActiveUS9050404B2Increase volumeIncrease productionMilking pumpMedical devicesPremature thelarcheBreastfeeding
Owner:MEDELA HLDG AG
Methods for improving tolerance, digestion, and lipid soluble nutrient absorption in an infant, toddler, or child
ActiveUS8754126B2Improved tolerance and digestion and absorptionReduce morbidityBiocideHydroxy compound active ingredientsPremature thelarcheMonoglyceride
Disclosed are nutritional formulations including predigested fats that can be administered to preterm infants, infants, toddlers, and children for improving tolerance, digestion, and absorption of nutrients and for reducing the incidence of necrotizing enterocolitis, colic, and short bowel syndrome. The predigested fats include fatty acid-containing monoglycerides and / or a fatty acid component.
Owner:ABBOTT LAB INC
Treatment of premature birth complications
InactiveUS20130259845A1Promote formationMany formatsAntibacterial agentsHeavy metal active ingredientsPremature thelarcheCord blood stem cell
The present invention provides methods of treating one or more complications of premature birth suffered by premature infants, comprising administering to the premature infant umbilical cord blood stem cells and, optionally, placental stem cells. The present invention also provides methods of combining and administering, and compositions comprising, umbilical cord blood stem cells, particularly autologous cord blood cells, and placental stem cells for the treatment of premature infants.
Owner:CELULARITY INC
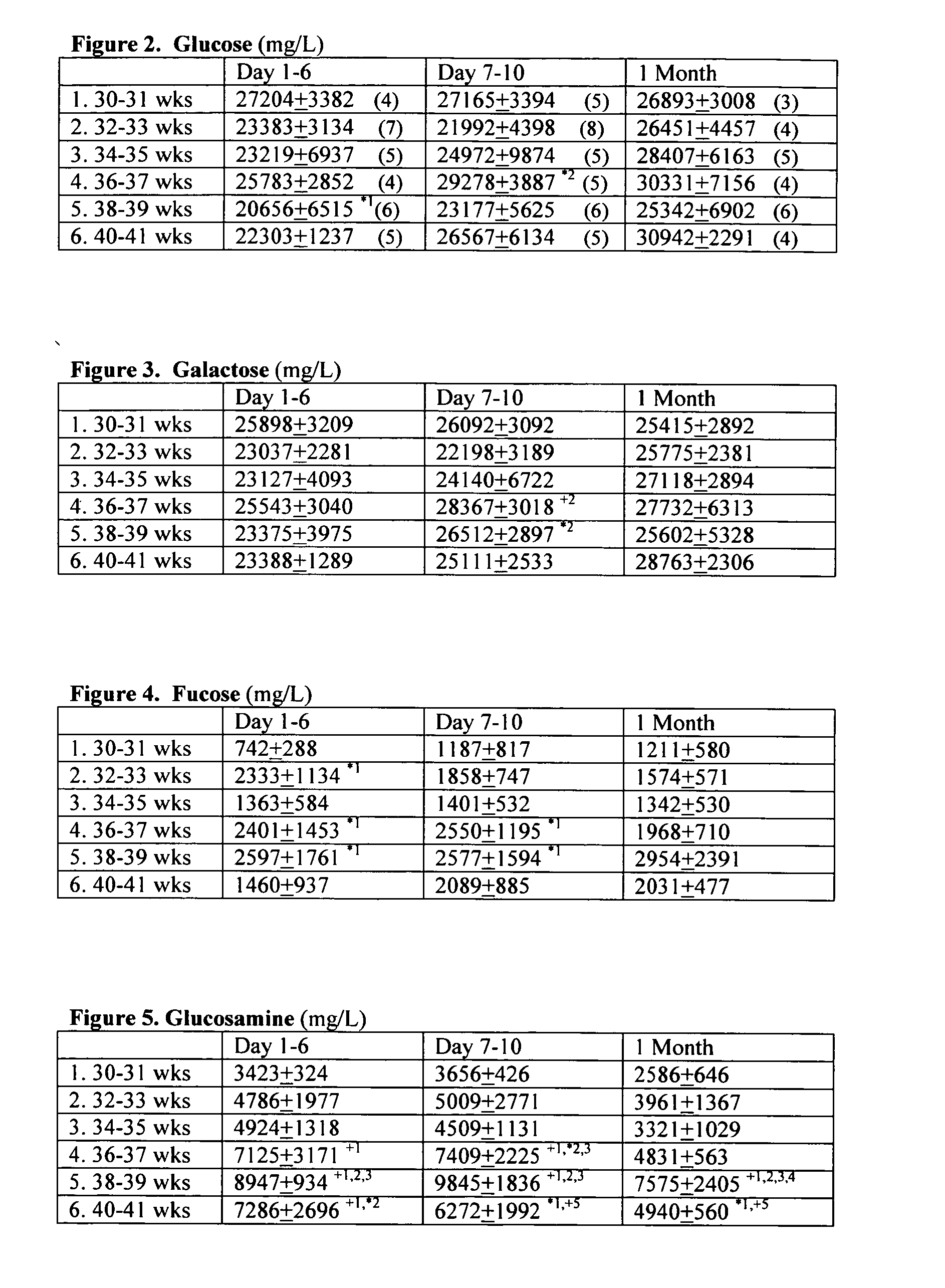
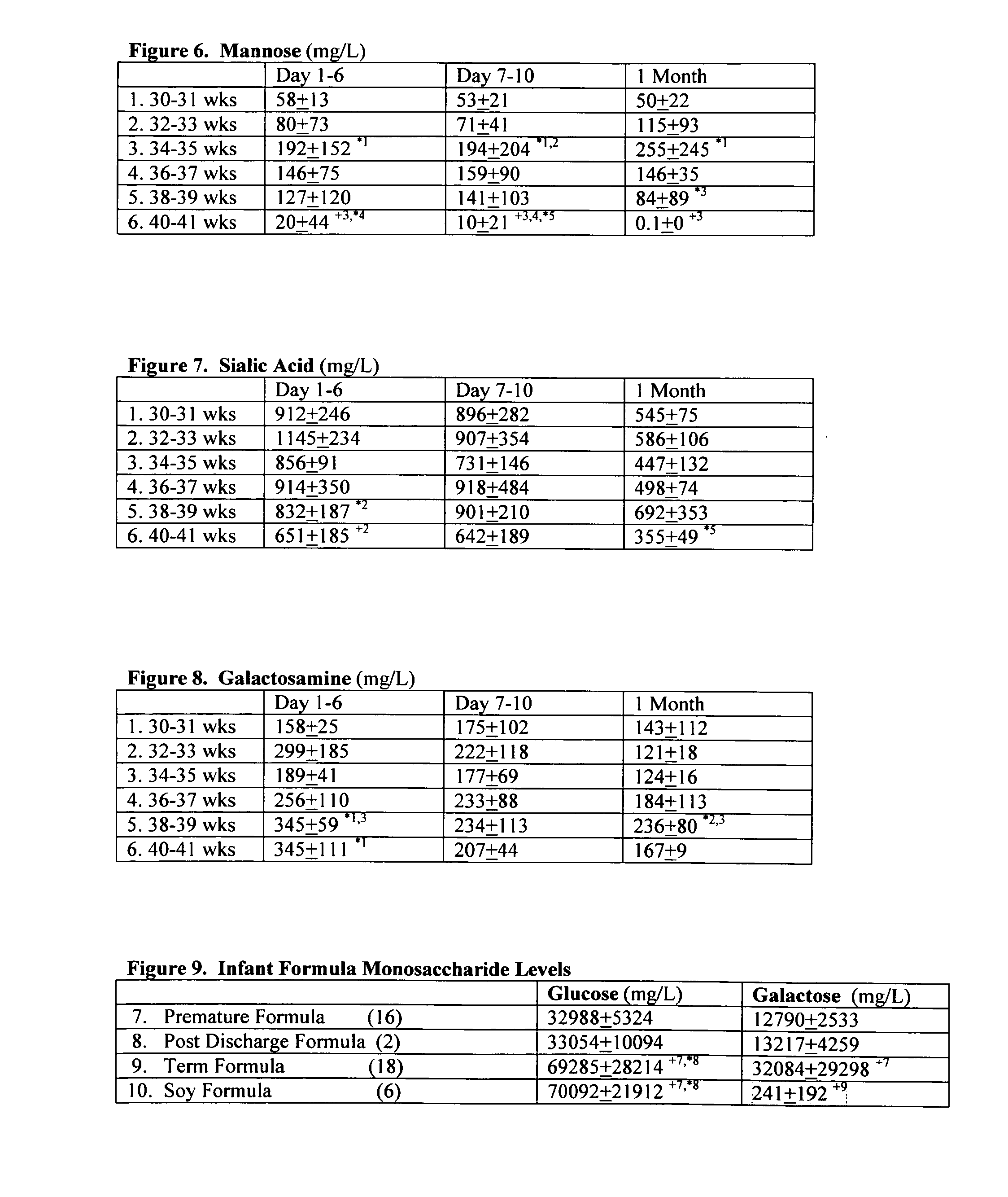
Prenatal and postnatal screening and treatment of critical monosaccharide deficiencies for neurologic and immunologic function
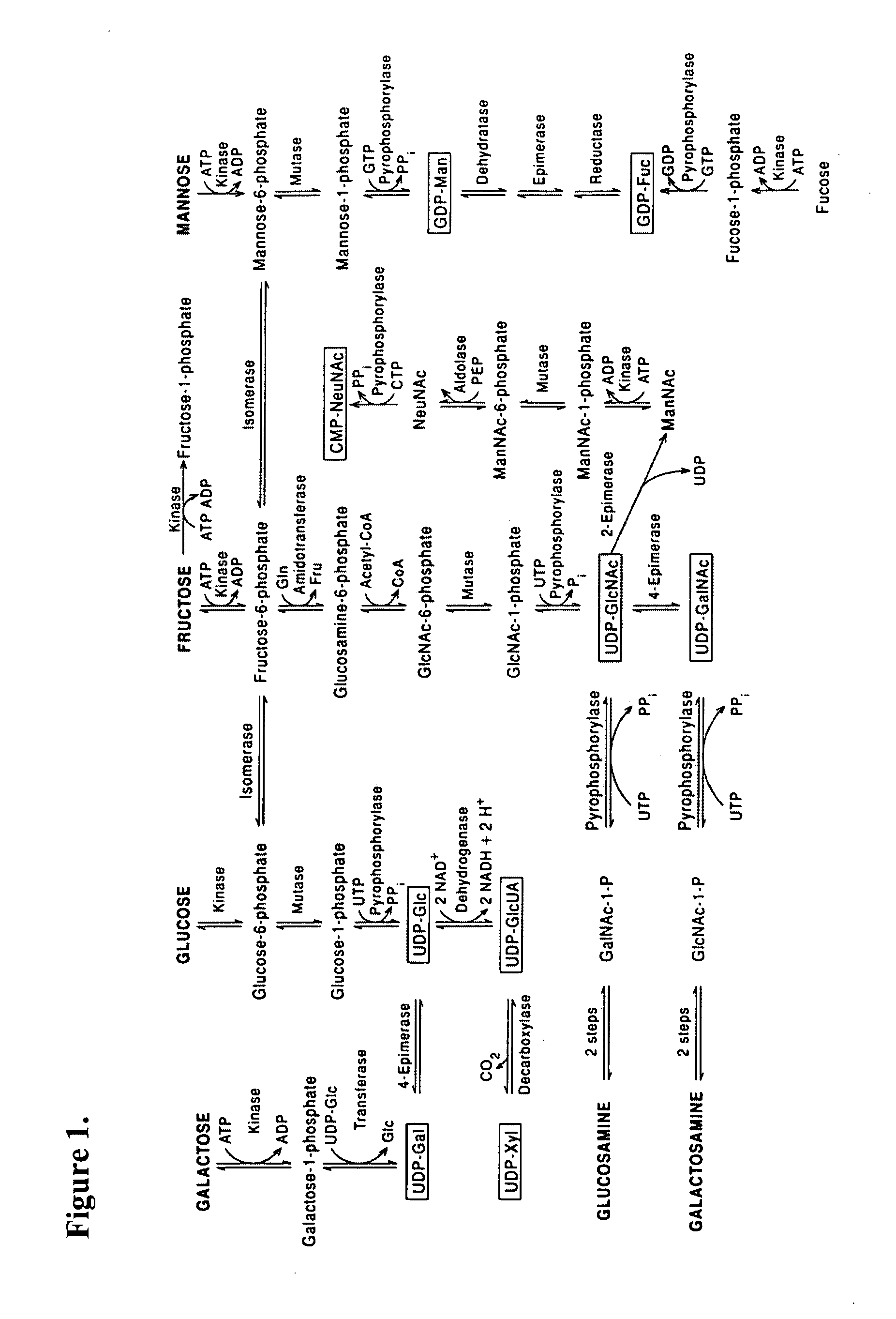
A method for determining the effect of critical glyconutrient dietary supplements in premature and term infants on growth, health, and brain function during early childhood development is described. The method comprises determining a critical target nutrient profile in typical samples of preterm and mother's milk, developing a supplement of the missing critical target nutrients to simulate mother's milk, performing an evaluation of early child development by correlating physiologic brain function with cognitive / behavioral brain function in low birth weight premature and term infants, assessing family influence and home environment on developmental outcome on infants under treatment, and comparing term siblings of low birth weight premature vs. term babies. A composition having the critical nutrients, an algorithm for screening and treatment, and a useful kit for employing the above are also described.
Owner:SZABO JOANNE
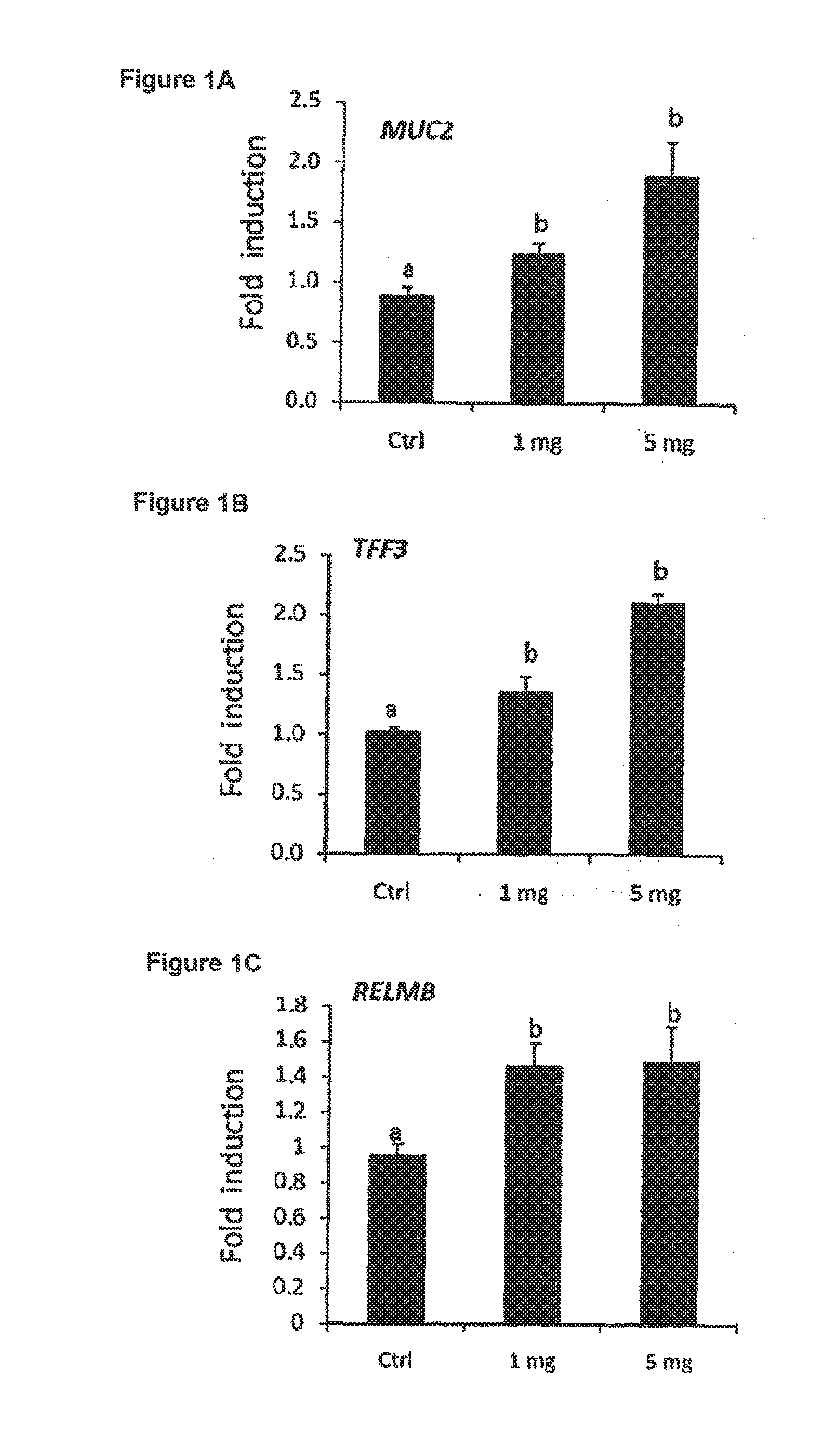
Galactooligosaccharides for preventing injury and/or promoting healing of the gastrointestinal tract
ActiveUS20140294789A1High expressionEnhanced barrier functionBiocideBacteria material medical ingredientsPremature thelarchePreventing injury
Disclosed are nutritional compositions including galactooligosaccharides that can be administered to individuals including preterm infants, infants, toddlers, children, and adults for preventing injury and / or improving the healing of the gastrointestinal tract. Additional suitable methods of using the nutritional compositions including the galactooligosaccharides are also disclosed.
Owner:ABBOTT LAB INC
Method for intrathecal administration of autologous stem cells in premature infants
ActiveUS20100226896A1Improve pathophysiologyReduce severityBiocideMammal material medical ingredientsPremature thelarcheCord blood stem cell
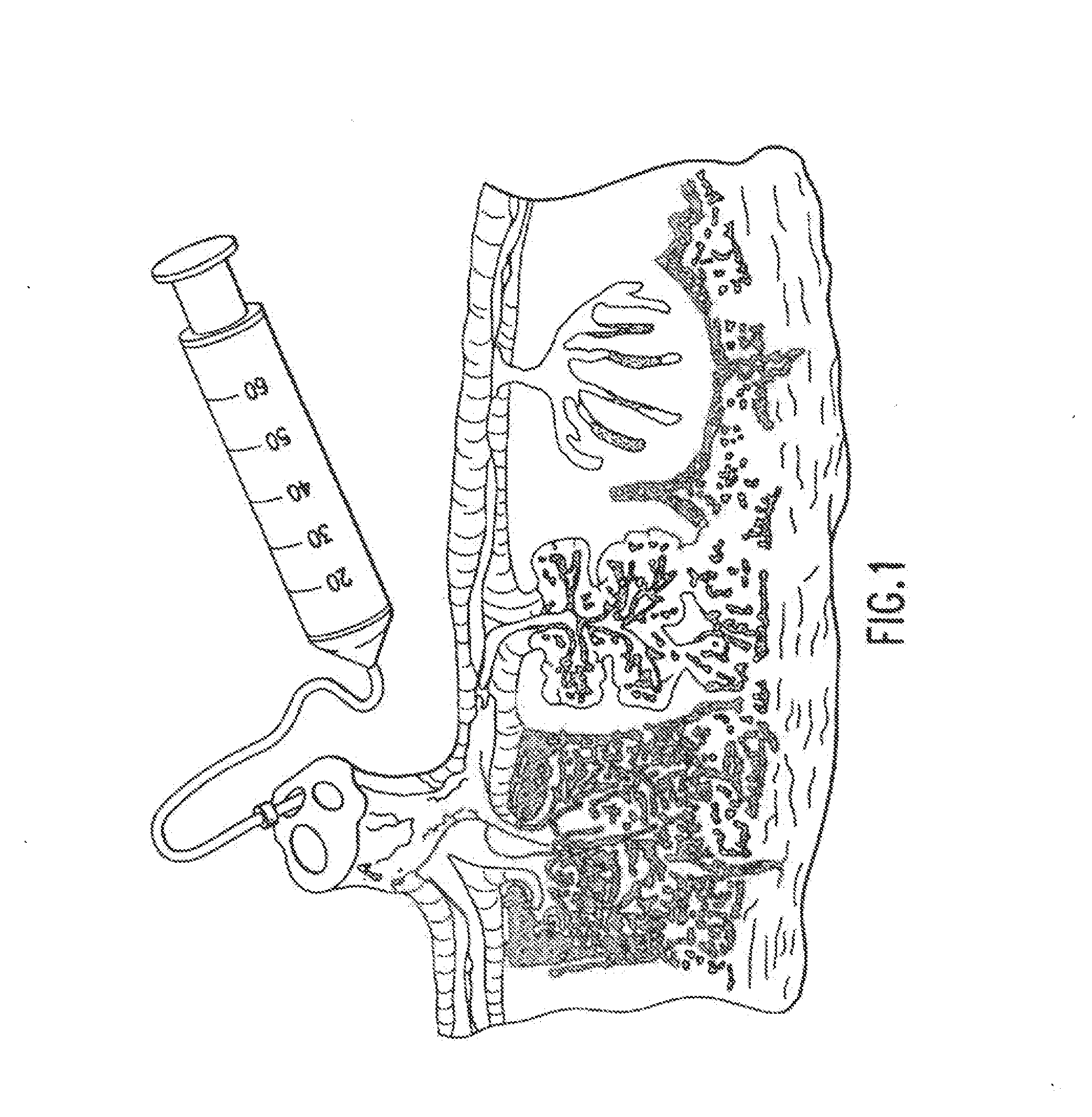
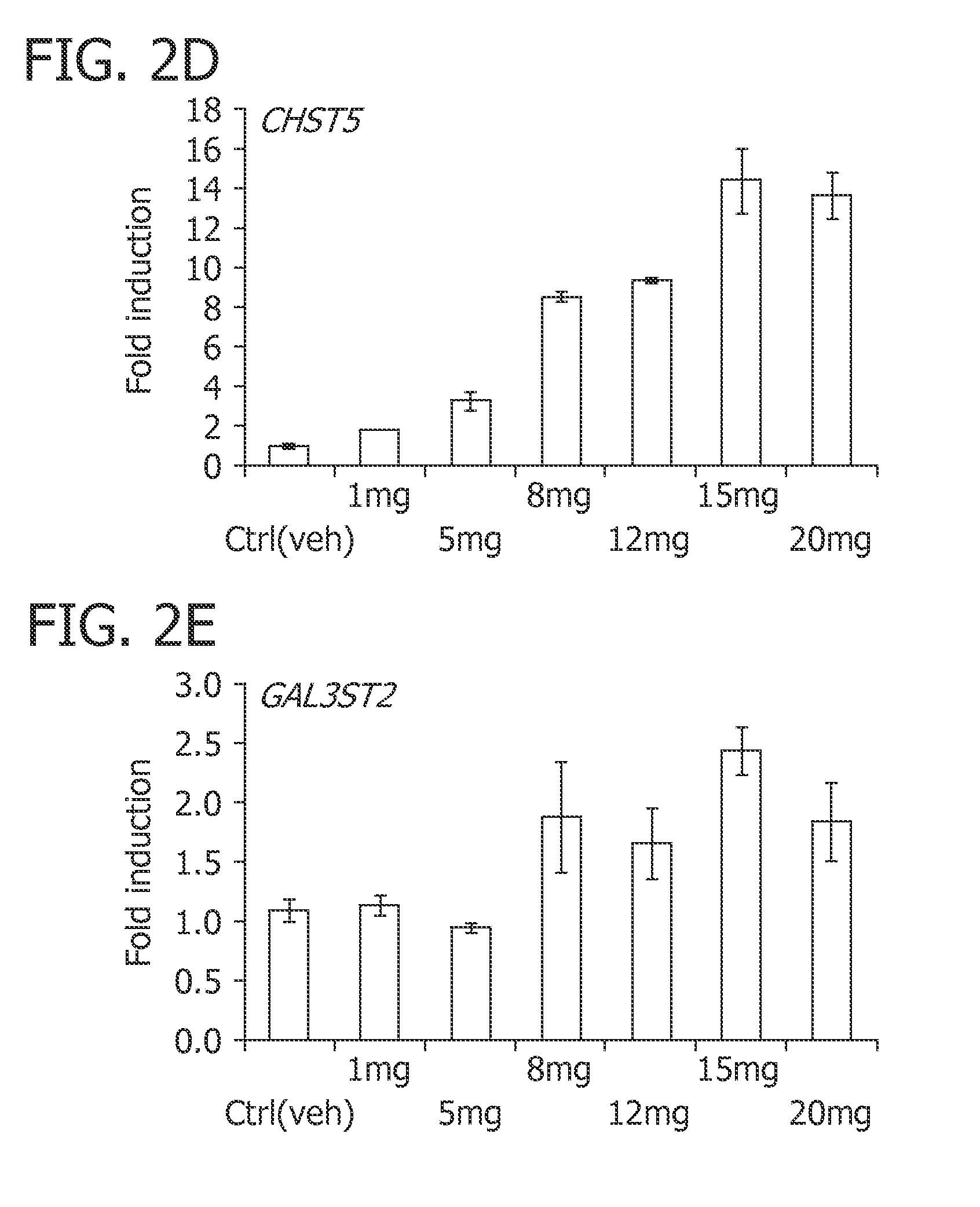
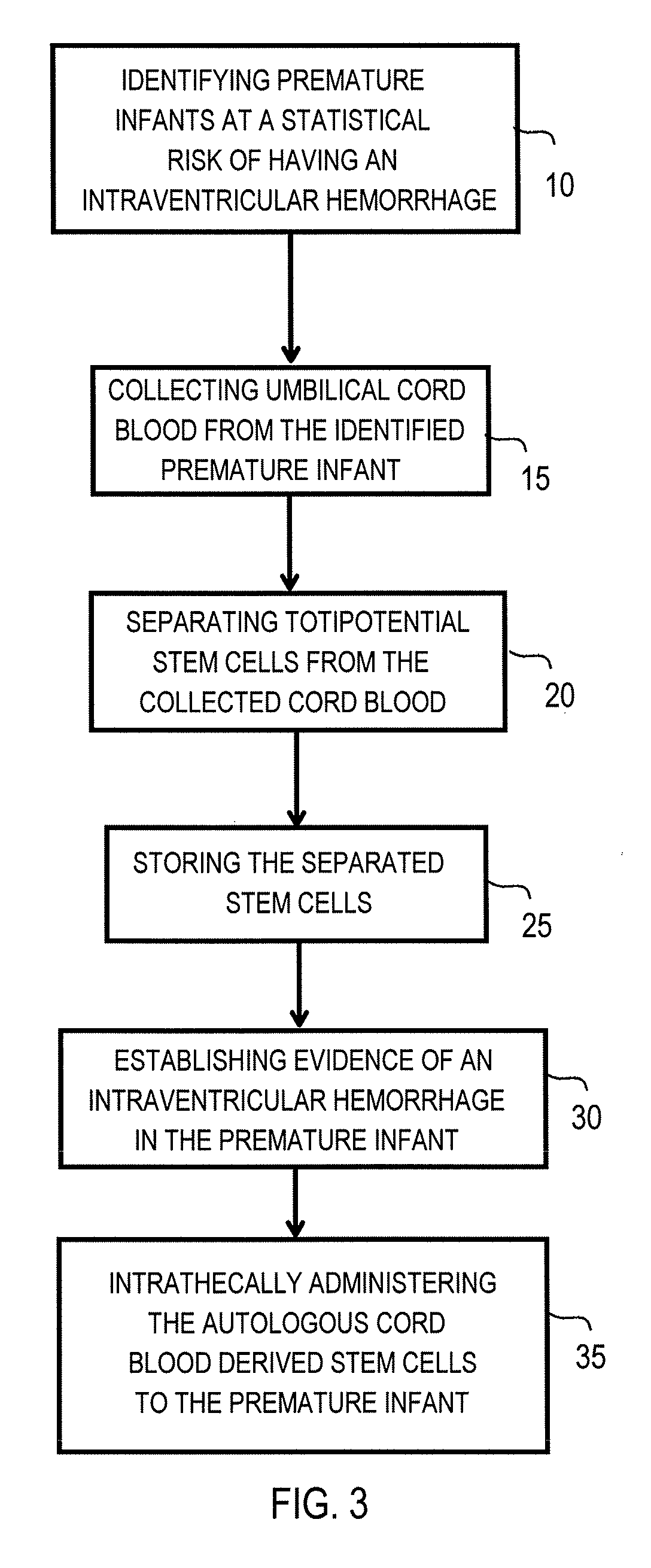
The invention provides a method of treating a premature infant at a statistical risk of sustaining an intraventricular hemorrhage. The method, or therapeutic protocol, can include at least one or more of the following steps: identifying premature infants at a statistical risk of having an intraventricular hemorrhage, collecting umbilical cord blood from the identified premature infant, separating totipotential stem cells (e.g., having the ability to proliferate and differentiate as neural stem cells) from the collected cord blood, storing the separated stem cells, establishing evidence of an intraventricular hemorrhage (preferably prior to Grade III / IV if possible) in the premature infant, and intrathecally administering the autologous cord blood derived stem cells to the premature infant.
Owner:DRACKER ROBERT A
Growth enhancement of infants
InactiveUS20150086622A1Enhance preterm gastrointestinal maturationIncrease ratingsPeptide/protein ingredientsMetabolism disorderPremature thelarcheSmall for gestational age
The present invention relates to compositions and methods for enhancing the growth of infants. Particularly, the present invention discloses the use of insulin for promoting the growth of low birth weight infants, including preterm infants and small for gestational age (SGA) infants over the expected rate.
Owner:NUTRINIA LTD
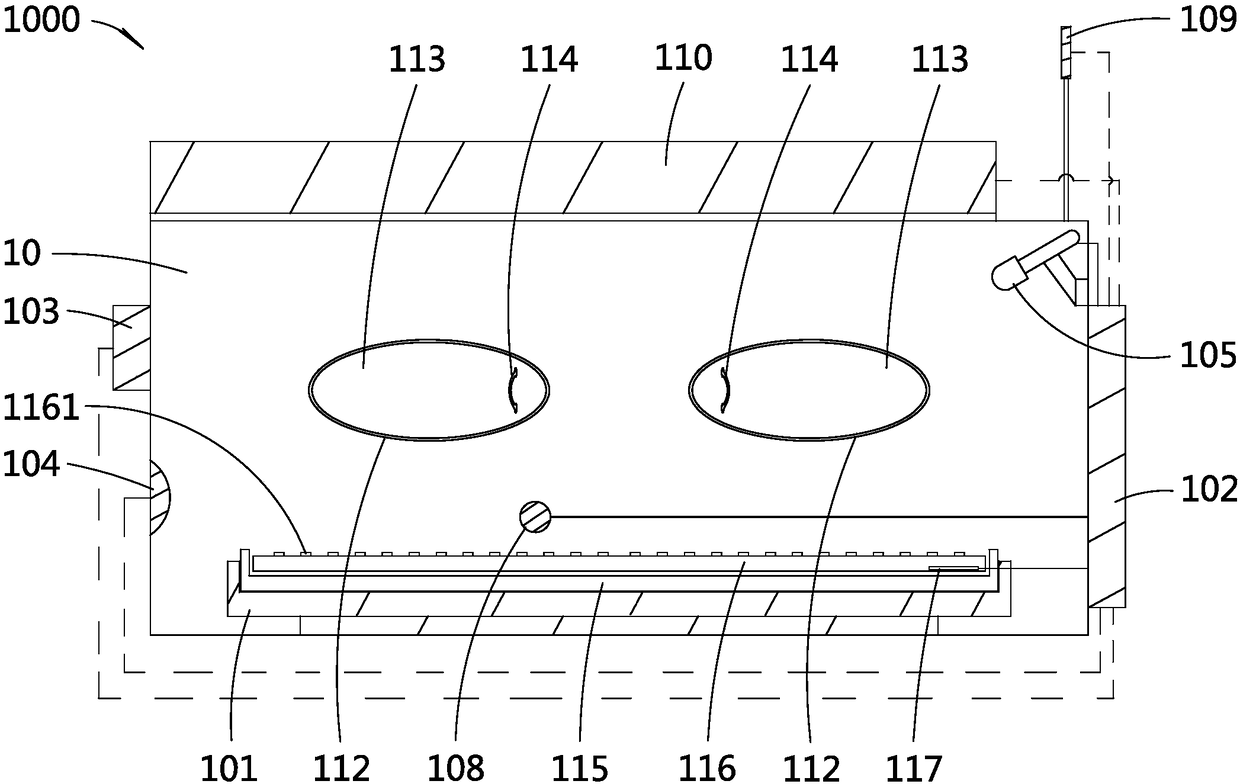
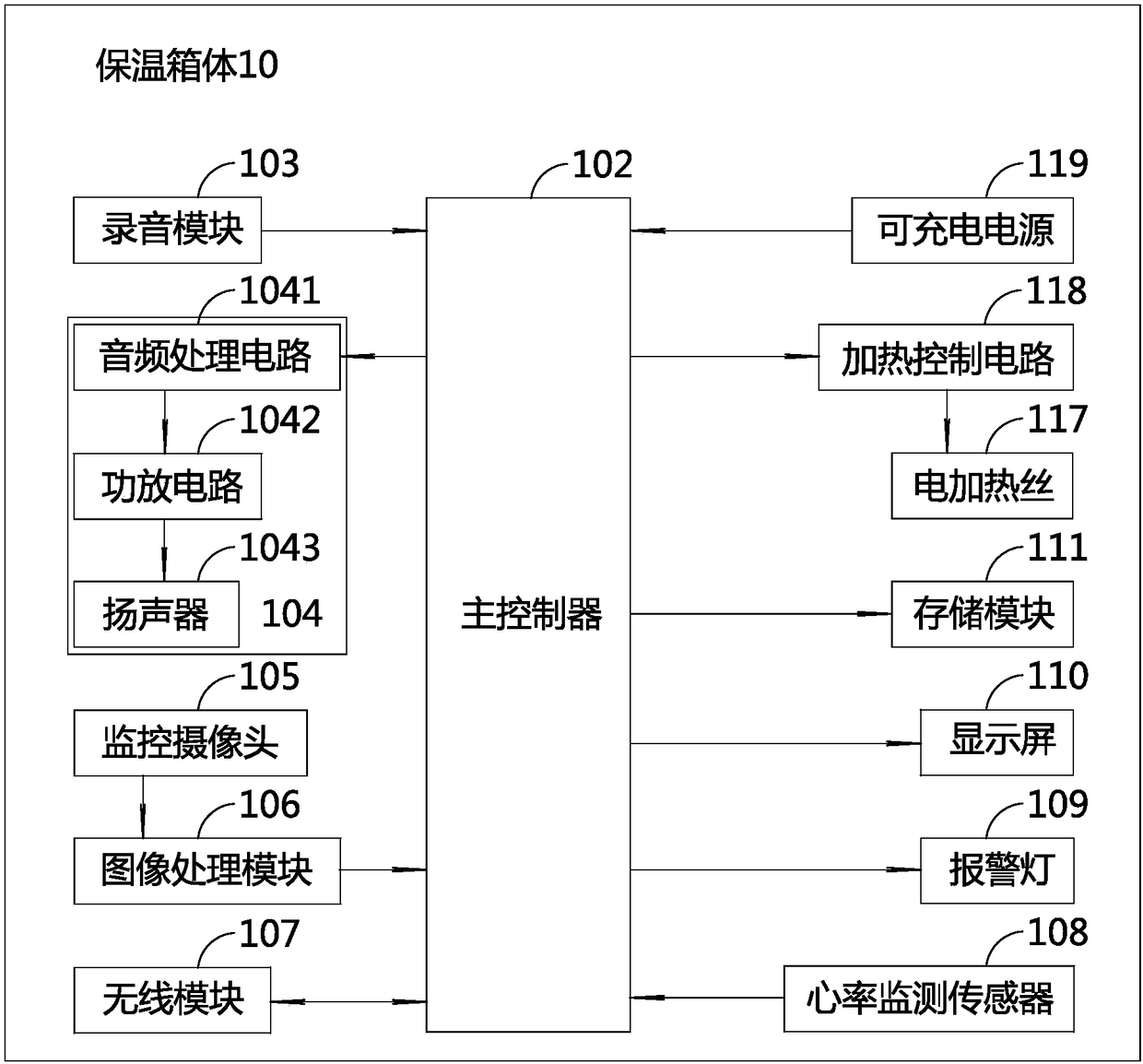
Incubator for premature infants and incubator monitoring system for premature infants
PendingCN108379007APlay an auxiliary role in soothingAppease effectiveBaby-incubatorsSensorsPremature thelarcheMonitoring system
The invention discloses an incubator for premature infants and an incubator monitoring system for premature infants. The incubator for premature infants comprises an incubator box body, a baby tray disposed in the incubator box body, a main controller, a recording module and a play module; the recording module and the play module are respectively connected with the main controller. The incubator for premature infants has the benefits that the recording module is convenient for recording sounds of mothers' of the premature infants, such as appeasing sounds, laugher voices, singing voices, amusing sounds and the like, the sounds can be played through the play module to realize an appeasing effect, and the psychological emotion of the premature infants is pacified effectively, so that the premature infants placed in the incubator box body lie meekly, relax physically and mentally, and grow joyfully and cheerfully. If any premature infant placed in the incubator is in an abnormal condition, a voice alarm will be output from the voice alarm, and the medical staff can know and handle in time, thereby saving the trouble for the medical staff to be on duty 24 hours a day to guard each premature infant in each incubator. Furthermore, the incubator for premature infants is good in use effect.
Owner:PEKING UNIV SHENZHEN HOSPITAL
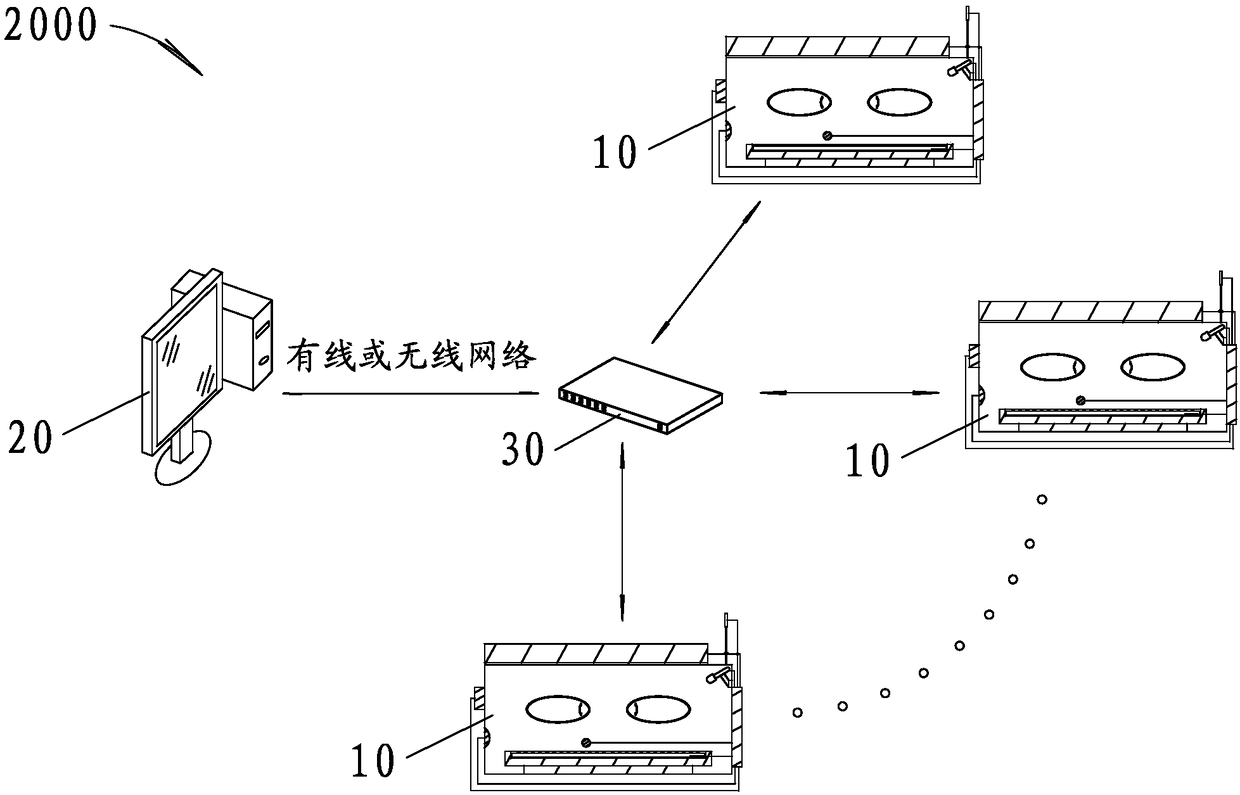
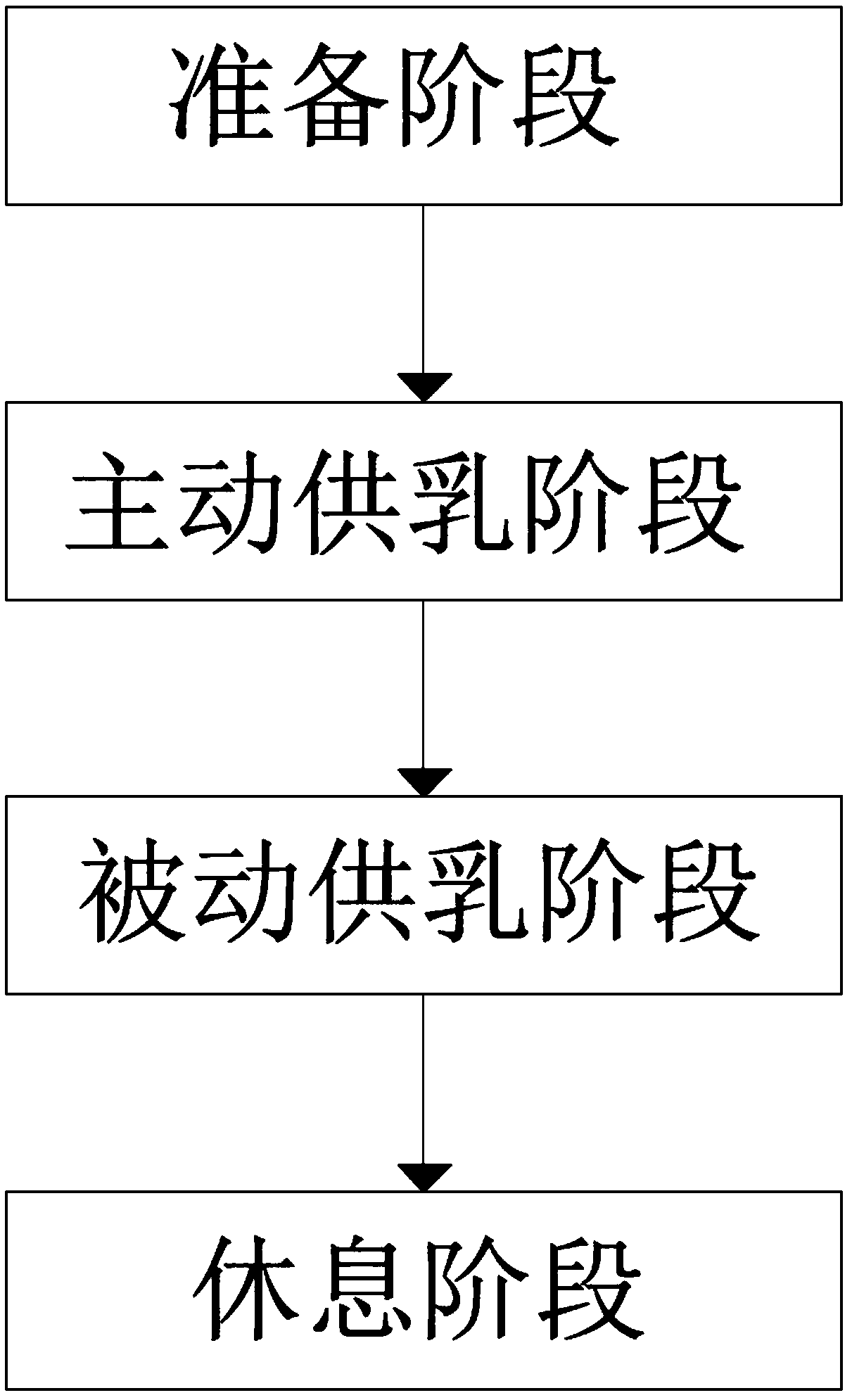
Automatic breastfeeding method and automatic breastfeeding machine for premature infant
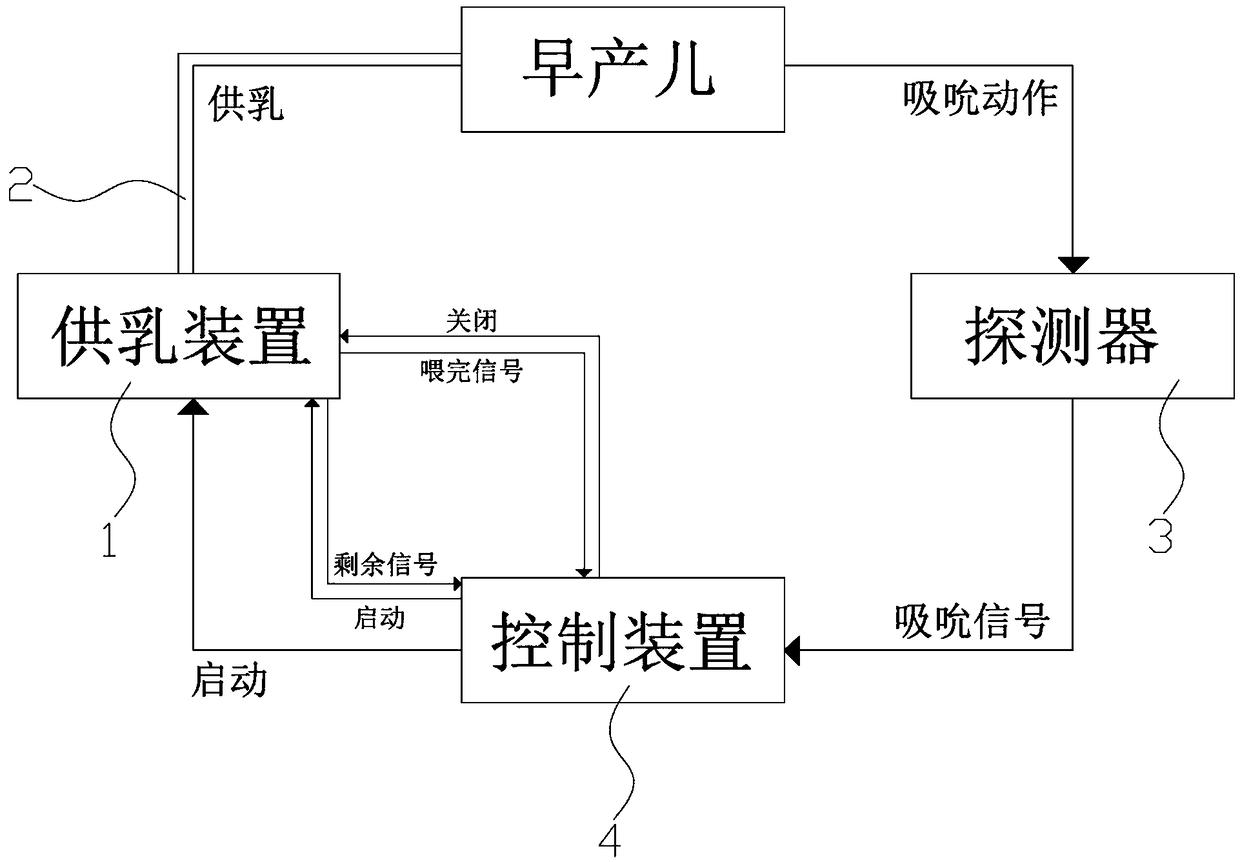
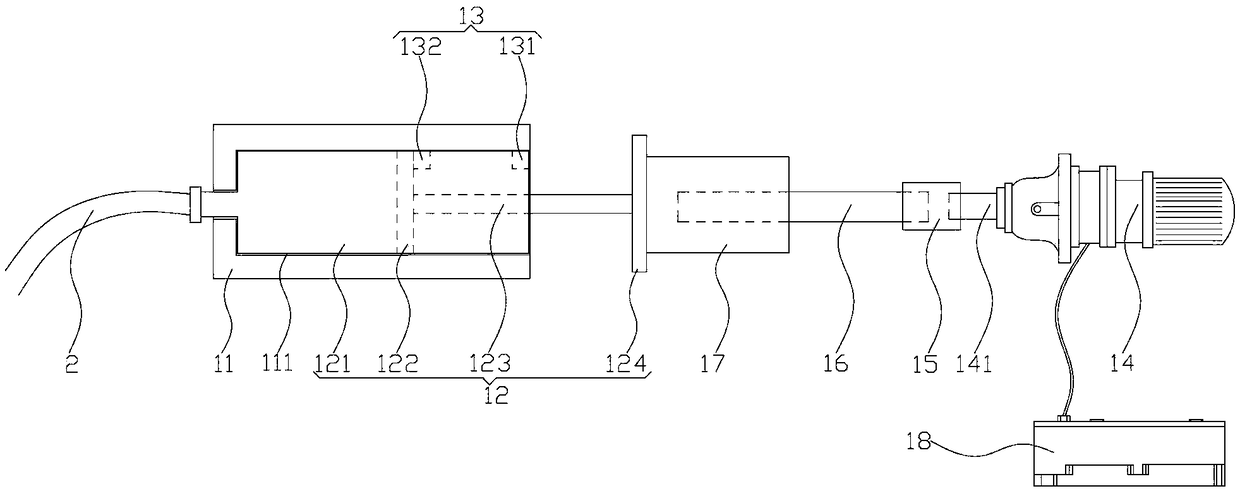
ActiveCN109106602ASelf-sucking ability trainingNo difference in dietDomestic articlesFeeding-tubesPremature thelarcheReflex
An automatic breastfeeding method of a premature infant comprises a preparation stage, an active lactation stage, a passive lactation stage and a rest stage; the invention also provides an automatic breastfeeding machine used for the automatic breastfeeding method of premature infant, comprising a stomach tube, a lactation supply device, a detector and a control device. One end of the stomach tubeis arranged in the stomach of the premature infant, and the other end is connected with the lactation supply device, the milk supply device is electrically connected with the control device, the control device is electrically connected with the detector, and the detector is arranged in the mouth of the premature infant. The self-sucking ability of the premature infant is trained, and the normal circulation of the mouth-pharyngeal-gastric-intestinal reflex of premature infants is realized, and the nutritional requirements of the premature infants are ensured taking into account the differencesof the premature infants, so that the premature infants have no difference in the care, diet, neurological development and growth and development with term infants in the future.
Owner:SHENZHEN CHILDRENS HOSPITAL
Glutamine enriched nutritional composition for preterm infants
InactiveUS20150024082A1Increase volumeOrganic active ingredientsDipeptide ingredientsBrain developmentPremature thelarche
The present invention relates to the use of nutritional compositions enriched in glutamineto improve structural brain development in preterm and / or low birth weight infants.
Owner:NV NUTRICIA
Process for Use with Breastpump to Initiate Milk in Breastfeeding, Particularly for Premature Infants
ActiveUS20100217182A1Increase volumeIncrease productionMilking pumpMedical devicesPremature thelarcheBreastfeeding
Owner:MEDELA HLDG AG
Soaking lotion for treatment of developmental retardation in premature infants and preparation method and application method thereof
InactiveCN110354165AImprove securityProlong the action timeAnthropod material medical ingredientsMetabolism disorderPremature thelarcheGrowth parameter
The invention provides a soaking lotion for treatment of developmental retardation in premature infants and a preparation method and an application method thereof, and belongs to the technical field of treatment of developmental retardation in premature infants. The soaking lotion for treatment of developmental retardation in premature infants includes an herbal medicinal composition and animal fat and other tissue combinations F. The animal fat and other tissue combinations include snake gall powder, red deer bone powder, hive powder, badger liver capsule oil and other animal fats. The herbalmedicinal composition includes the following raw materials (by weight): 1-20 parts of herba schizonepetae, 1-20 parts of divaricate saposhnikovia root, 1-20 parts of notopterygium root, 1-20 parts oflevisticum, 1-20 parts of Radix Peucedani, 1-20 parts of balloonflower root, 1-20 parts of light red tuckahoe, 1-20 parts of ligusticum chuanxiong hort, 1-20 parts of licorice, 1-20 parts of cicada slough, 1-20 parts of arctium fruit, and 1-20 parts of Artemisia capillaries. By the bubble bath mode for treating developmental retardation in premature infants and with the combination of nutritionalsupport, growth parameters of child patients can reach normal values in a short time.
Owner:贵州金宏基生物科技有限公司
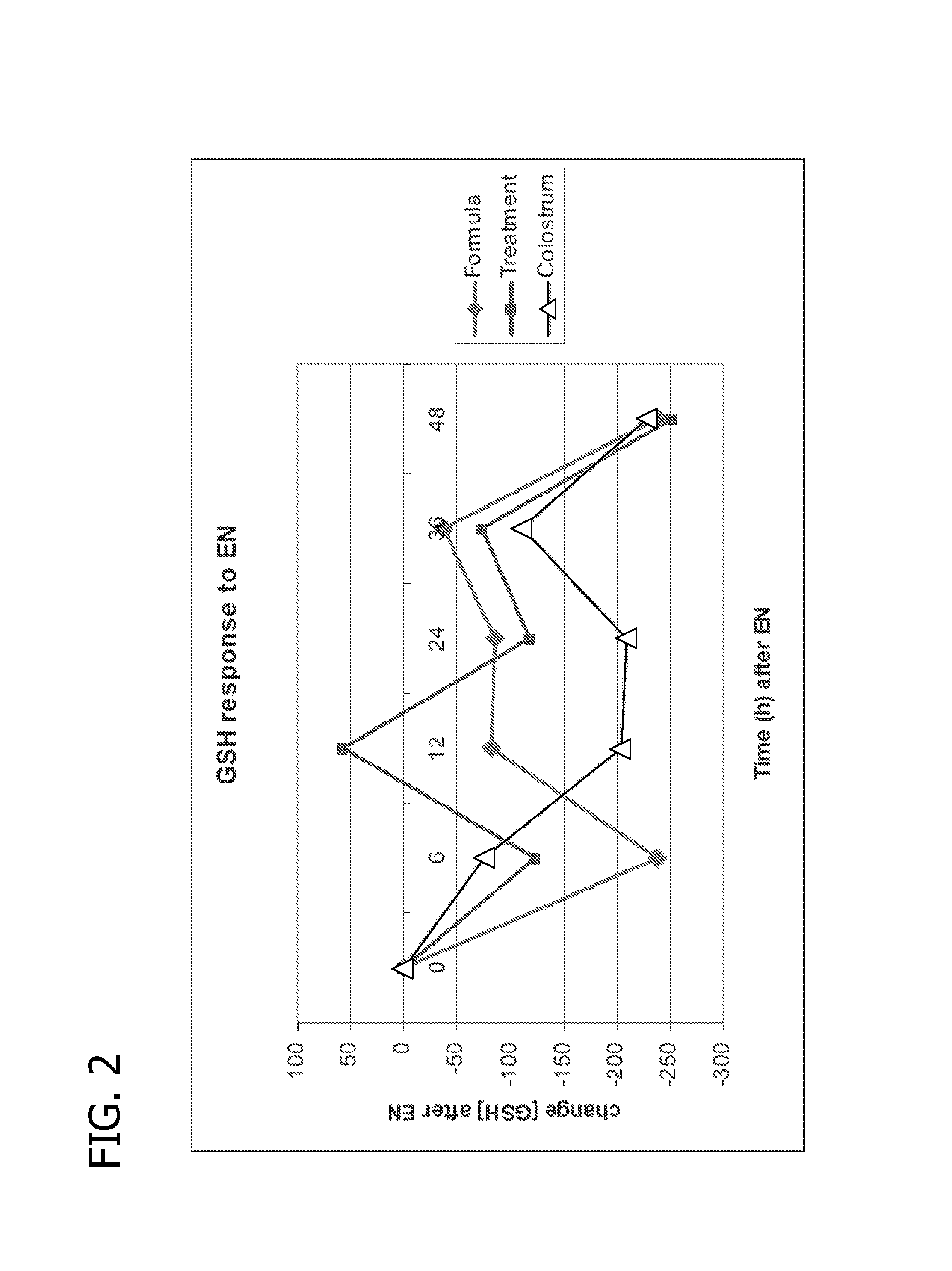
Free radical scavengers or promoters thereof as therapeutic adjuvants in preterm parturition
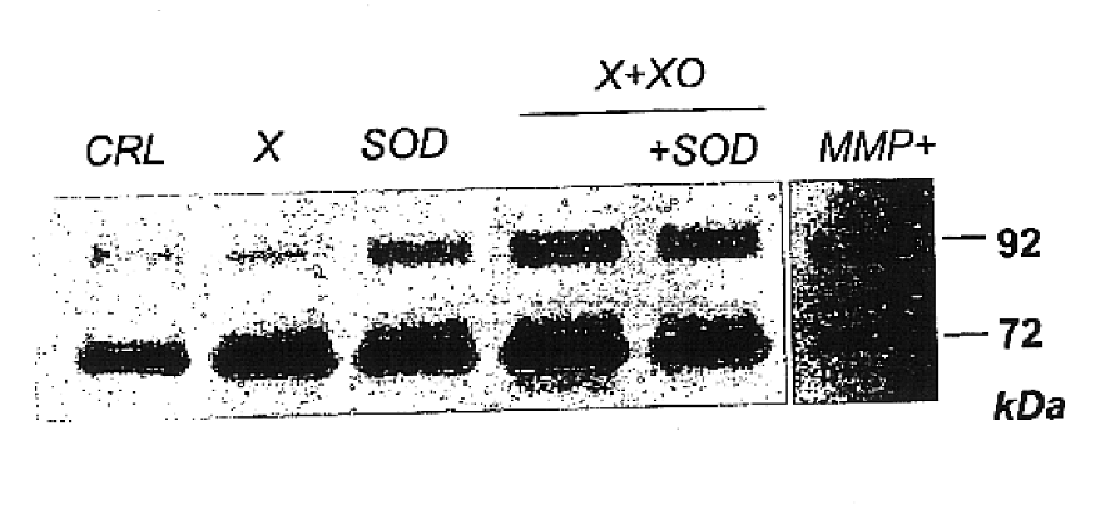
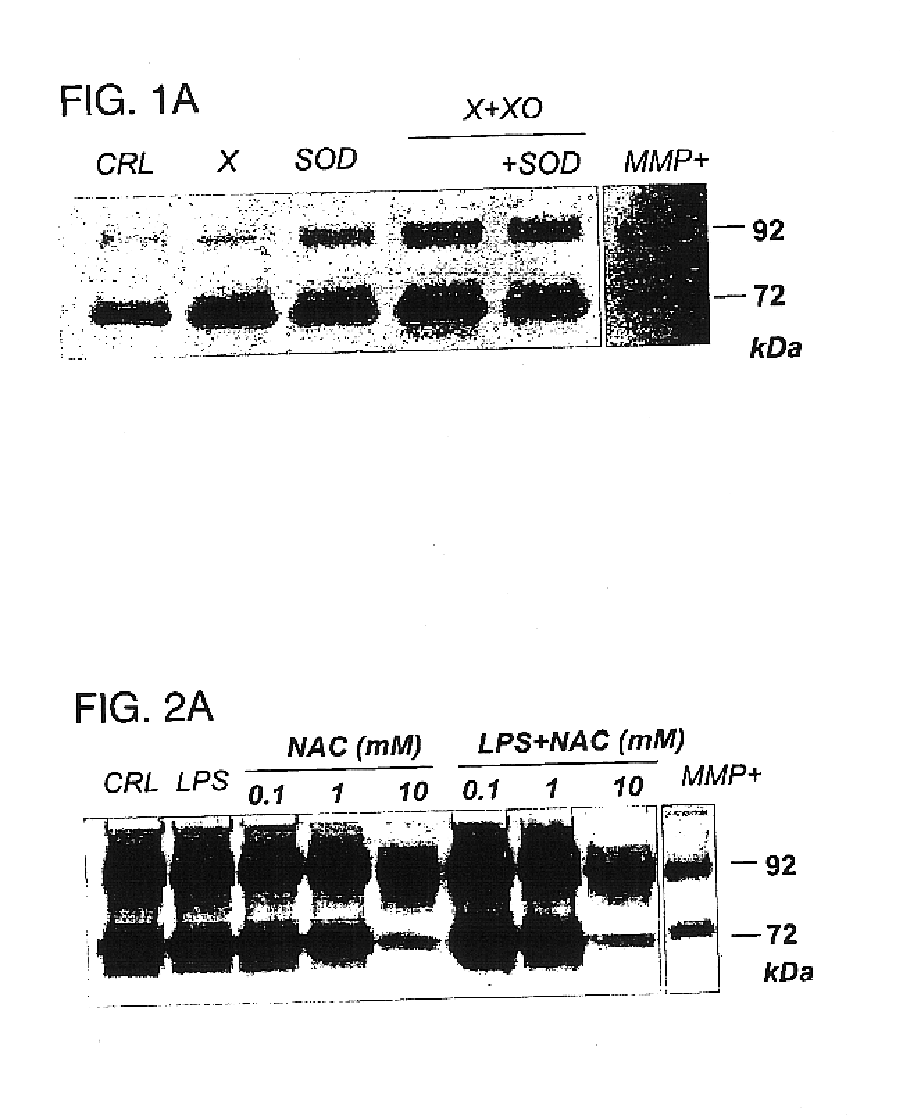
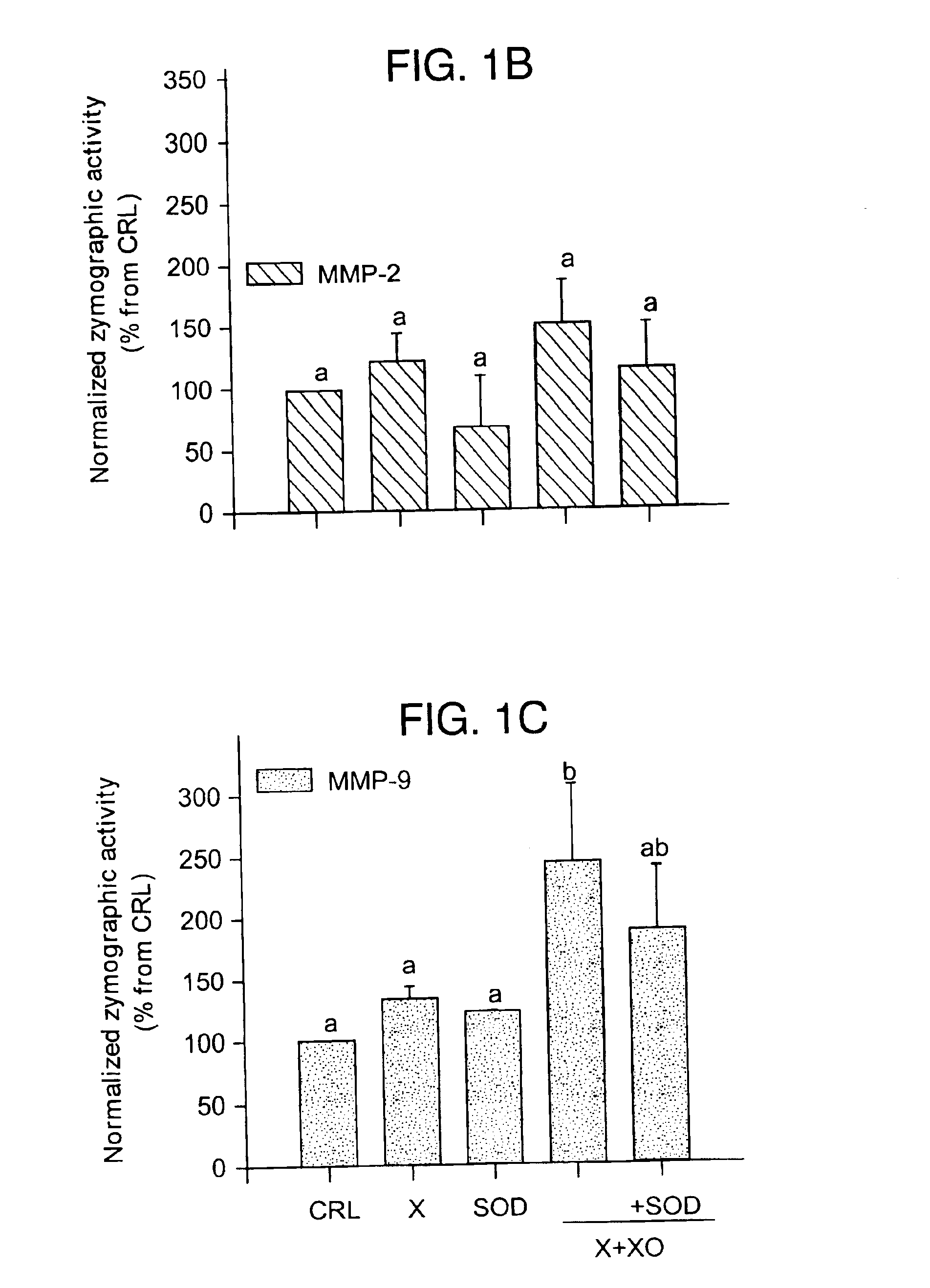
InactiveUS6852698B2Prevent ROS formationInhibition formationAntibacterial agentsBiocidePremature thelarchePreterm Births
The usage of compounds that improve fetal and neonatal outcome of preterm birth is described. These compounds are scavengers of ROS, their precursors, and agents that induce production of the scavengers. Examples of these compounds are glutathione, NAC, antioxidants, and spin trapping compounds. These compounds improve fetal outcome by inhibiting a fetal inflammatory process that may affect the fetus independently of prematurity. This fetal inflammatory response is characterized by increased cytokine and matrix metalloproteases (MMP) levels both in the mother and fetus and may be modulated by ROS at different levels. Targeting ROS formation with compounds such as specific antioxidants, glutathione or spin trapping compounds, their precursors, and / or agents which induce their production will suppress both the direct effects of ROS and its indirect effects through cytokines and MMPs already circulating in the system. This therapeutical intervention would limit the pathophysiologoical chain of events that ultimately leads to PPROM, preterm birth and / or adverse fetal and neonatal outcome.
Owner:PERINET INC
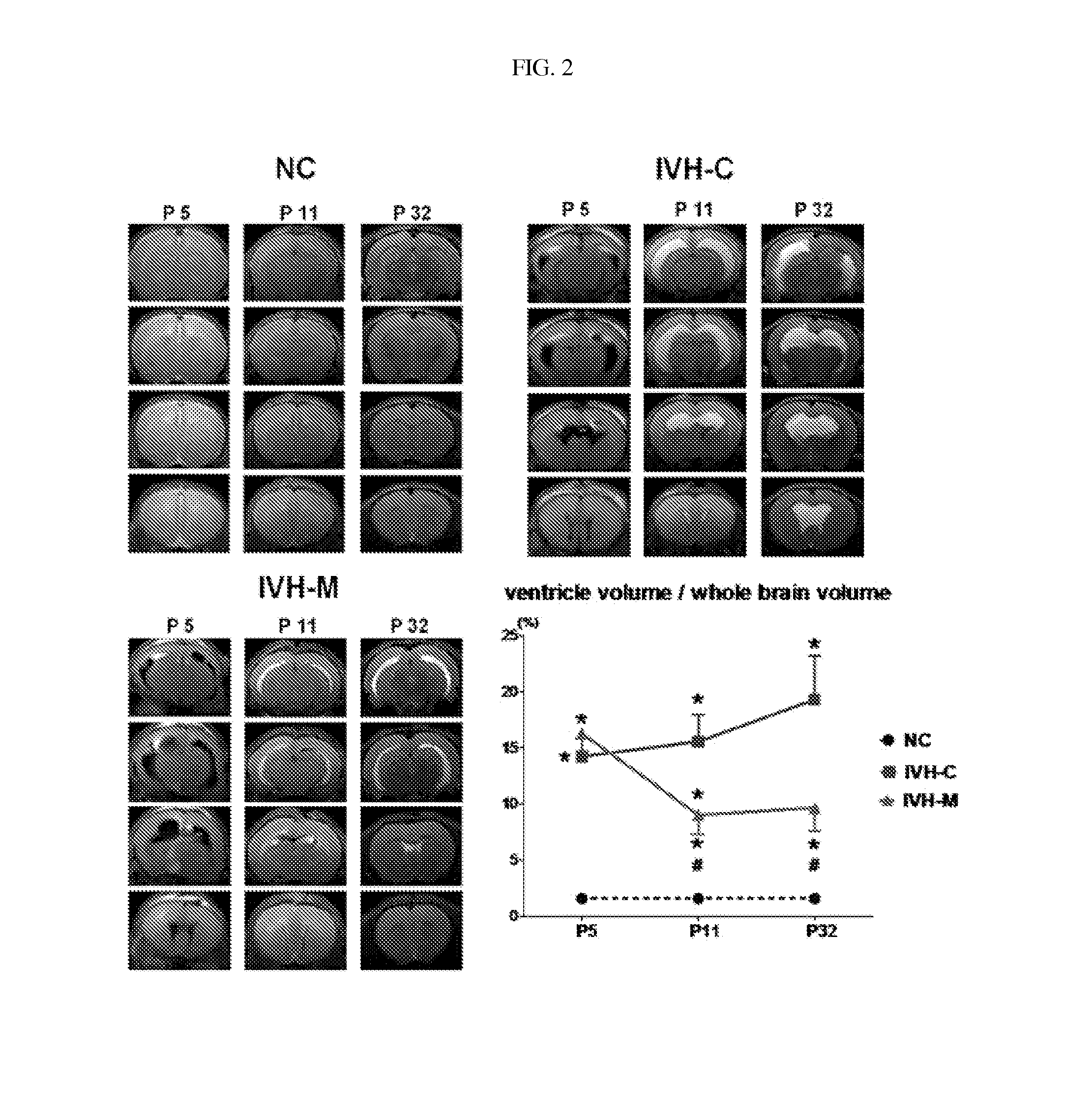
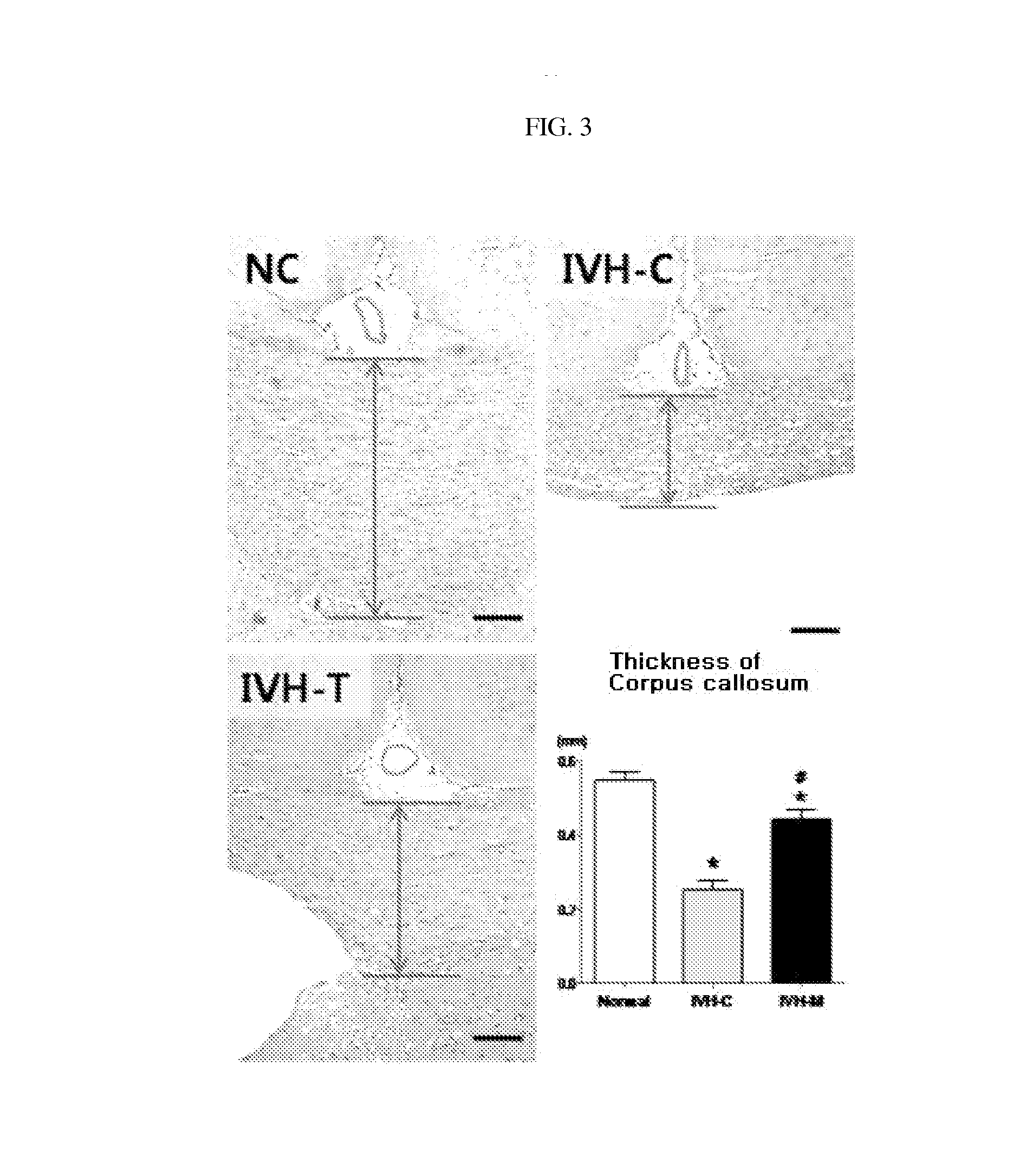
Composition for treating intraventricular hemorrhage in preterm infants comprising mesenchymal stem cells
ActiveUS20140072527A1Prevent ventricular dilatationLower Level RequirementsBiocideNervous disorderPremature thelarcheCerebral ventricular dilatation
Disclosed is a composition for the prophylaxis or therapy of intraventricular hemorrhage in preterm infants comprising mensenchymal stem cells. Functioning to prevent ventricular dilatation and reduce the level of inflammatory cytokines in cerebrospinal fluid, the composition comprising mesenchymal stem cells is advantageously useful for the prophylaxis or therapy of intraventricular hemorrhage in preterm infants. Accordingly, the composition is effectively preventive of hydrocephalus which occurs subsequent to intraventricular hemorrhage. In addition, the composition makes not only a histological and biochemical recuperation in the intraventricular hemorrhage-injured brain, but also significantly improves sensory motor functions. Mesenchymal stem cells can be used as an effective therapeutic agent because they are less prone to induce immune rejection responses and are highly likely to secrete proliferative, differentiative, and regulatory factors. Hence, mesenchymal stem cells are expected to play a critical role in the therapy of intraventricular hemorrhage in preterm infants.
Owner:MEDINNO INC
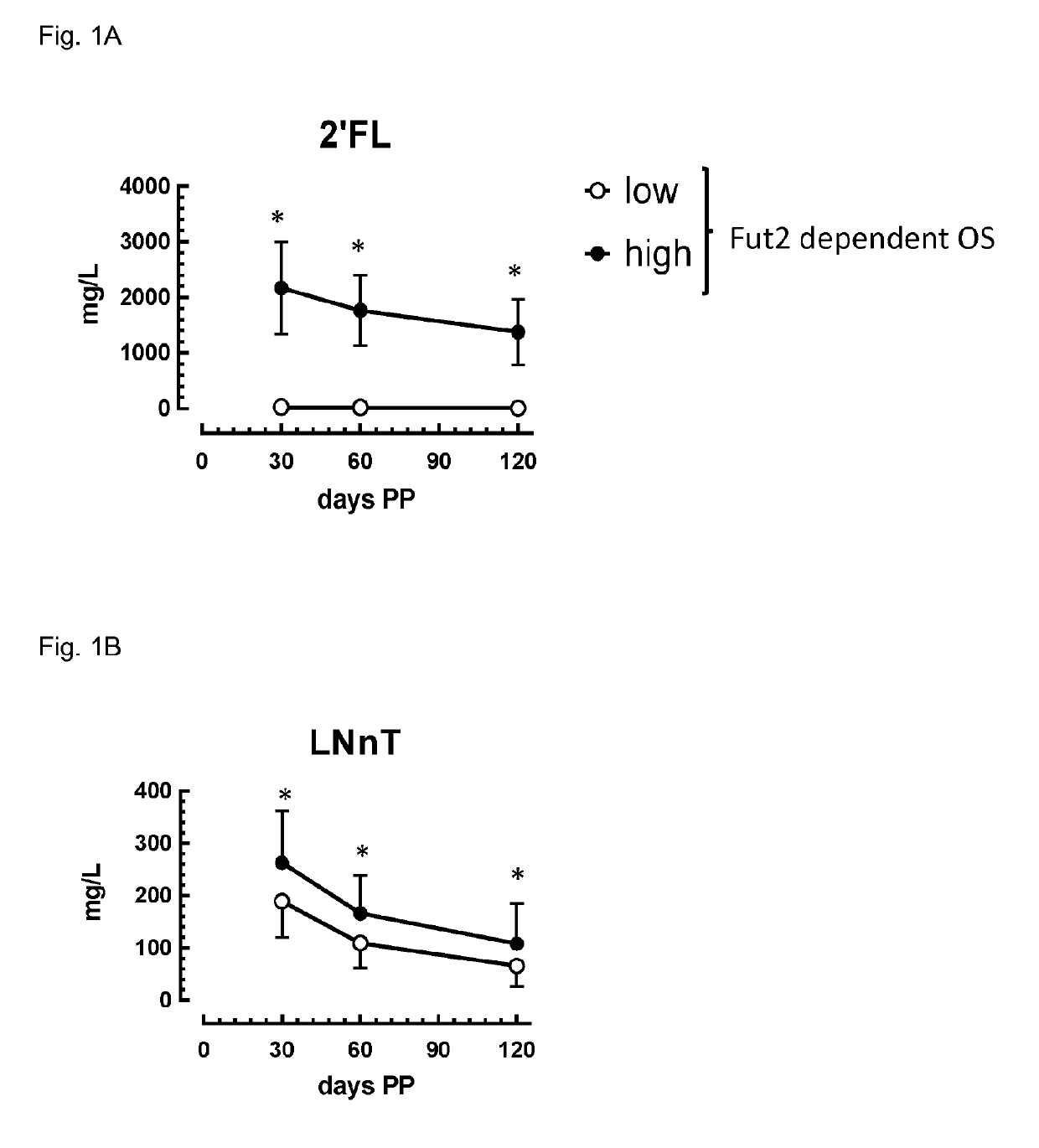
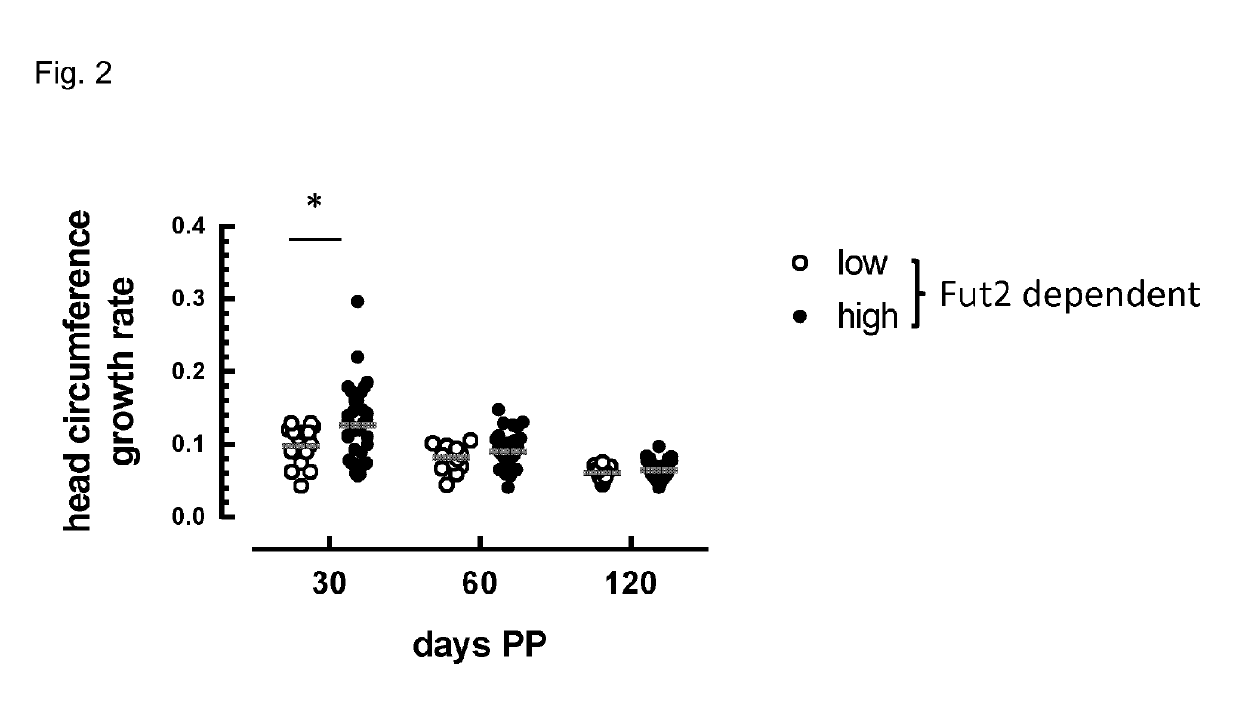
Composition comprising Fut2-dependent oligosaccharides and Lacto-N-neotetraose for use in promoting brain development and cognition
ActiveUS10328091B2Organic active ingredientsVitamin food ingredientsPremature thelarcheBrain development
This invention relates to the use of a nutritional composition comprising Fut2-dependent oligosaccharides for promoting brain growth and development in infants in the very early postnatal period. The nutritional composition comprises Fut2-dependent oligosaccharides and Lacto-N-neotetraose and is specifically for use in promoting brain catch-up growth in preterm infants or infants with low-birth weight. The invention further relates to a kit comprising at least two of these nutritional compositions.
Owner:SOC DES PROD NESTLE SA
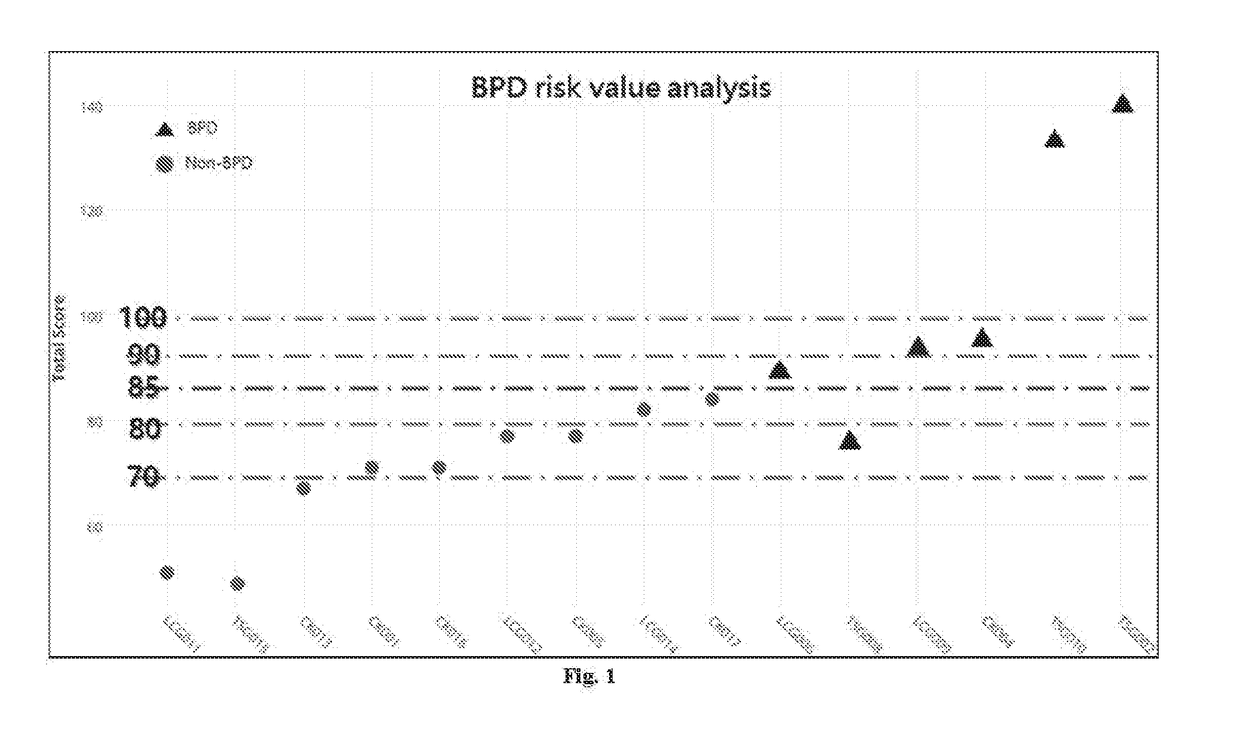
Method for identifying a greater risk for developing bronchopulmonary dysplasia
Disclosed is a method of identifying a greater risk for developing bronchopulmonary dysplasia (BPD) in a preterm infant. The method comprises obtaining a genomic DNA sample from the preterm infant's mother, identifying the nucleotide of rs2280789 SNP in the RANTES gene and the nucleotide of rs1800566 SNP in the NQO1 gene, and determining the preterm infant as being at risk of developing BPD when the genotype of rs2280789 SNP carries C nucleotide and the genotype of rs1800566 SNP carries T nucleotide.
Owner:MERIBANK BIOTECH CO LTD
Compositions comprising granulocyte-macrophage colony-stimulating factor for the treatment of inflammatory bowel disease
ActiveUS10105415B2Organic active ingredientsPeptide/protein ingredientsPremature thelarcheCrohn's disease
The present invention provides compositions comprising granulocyte-macrophage colony-stimulating factor and fosfomycin for the treatment, prevention or alleviation of an inflammatory bowel disease such as Crohn's disease, ulcerative colitis or necrotizing enterocolitis of newborn and premature infants by administration of the compositions into the intestinal lumen.
Owner:REPONEX PHARMA APS
Glutamine enriched nutritional composition for preterm infants
InactiveUS20140357571A1Long-term effectIncrease volumeBiocideOrganic active ingredientsPremature thelarcheBody weight
The present invention relates to the use of nutritional compositions enriched in glutamine to improve structural and functional brain development in preterm and / or low birth weight infants.
Owner:NUTRICIA
Method for monitoring fetus/preterm infant development, and for promoting the normal development of preterm infants
InactiveUS20190162731A1Prevent onset and progressionAvoid problemsPeptide/protein ingredientsDisease diagnosisPremature thelarcheDisease
A method for monitoring fetal and / or preterm infant development and a method to promote normal growth & development of preterm infants, especially in regards to each preterm infant's collective set of immature organs. Monitoring development is accomplished by using VEGF 121 as a biomarker in bodily fluid levels, to determine whether appropriate angiogenic activity is occurring to allow preterm infant or fetal development to proceed normally. The promotion of preterm infant normal development is accomplished by administering to a preterm infant human chorionic gonadotropin (hCG) and / or Luteinizing hormone (LH) and / or Luteinizing hormone releasing hormone (LHRH) in physiological amounts at appropriate intervals to raise and maintain the activation level of the patient's combined hCG / LH receptor activity, or VEGF 121 level, to a level that is normally present in fetuses of the equivalent developmental (gestational) age. By promoting normal development, we prevent onset or progression of disorders associated with premature organs rather than treat disorders associated with the premature organs after they occur.
Owner:ZIETCHICK RES INST
Method for using supplemental vascular endothelial growth factor (VEGF) or analog to prevent oxygen induced arrest of vessel growth and disease sequela of premature infant birth
InactiveUS20170224774A1Stimulates vessel growthPromotes normal vessel growthPeptide/protein ingredientsPharmaceutical delivery mechanismDiseasePremature thelarche
Disclosed herein is a method for administering systemic (e.g., by intravenous injection) supplemental vascular endothelial growth factor (VEGF) to premature infants to prevent the disease sequelae and complications including but not limited to retinopathy of prematurity (ROP) and / or paraventricular leukomalacia (PVL) and / or bronchopulmonary dysplasia (BPD) associated with premature births. The VEGF is administered to the premature infant to produce a physiologic serum VEGF level that is substantially similar to the VEGF level found in a normally developing fetus in the utero. By way of one example, the retinal blood vessel growth of the premature infant is monitored, and the administration of VEGF is terminated when the infant's retinal blood vessels are substantially fully grown to ROP Zone 3. By virtue of the method herein disclosed, normal vessel growth occurs, and lung disease, ROP with blindness, brain damage and other diseases associated with premature birth are substantially avoided.
Owner:WRIGHT KENNETH W
Intrathecal administration of autologous stem cells to treat intraventricular hemorrhage in premature infants
ActiveUS9168273B2Improve pathophysiologyReduce severityBiocideMammal material medical ingredientsPremature thelarcheCord blood stem cell
The invention provides a method of treating a premature infant at a statistical risk of sustaining an intraventricular hemorrhage. The method, or therapeutic protocol, can include at least one or more of the following steps: identifying premature infants at a statistical risk of having an intraventricular hemorrhage, collecting umbilical cord blood from the identified premature infant, separating totipotential stem cells (e.g., having the ability to proliferate and differentiate as neural stem cells) from the collected cord blood, storing the separated stem cells, establishing evidence of an intraventricular hemorrhage (preferably prior to Grade III / IV if possible) in the premature infant, and intrathecally administering the autologous cord blood derived stem cells to the premature infant.
Owner:DRACKER ROBERT A
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com