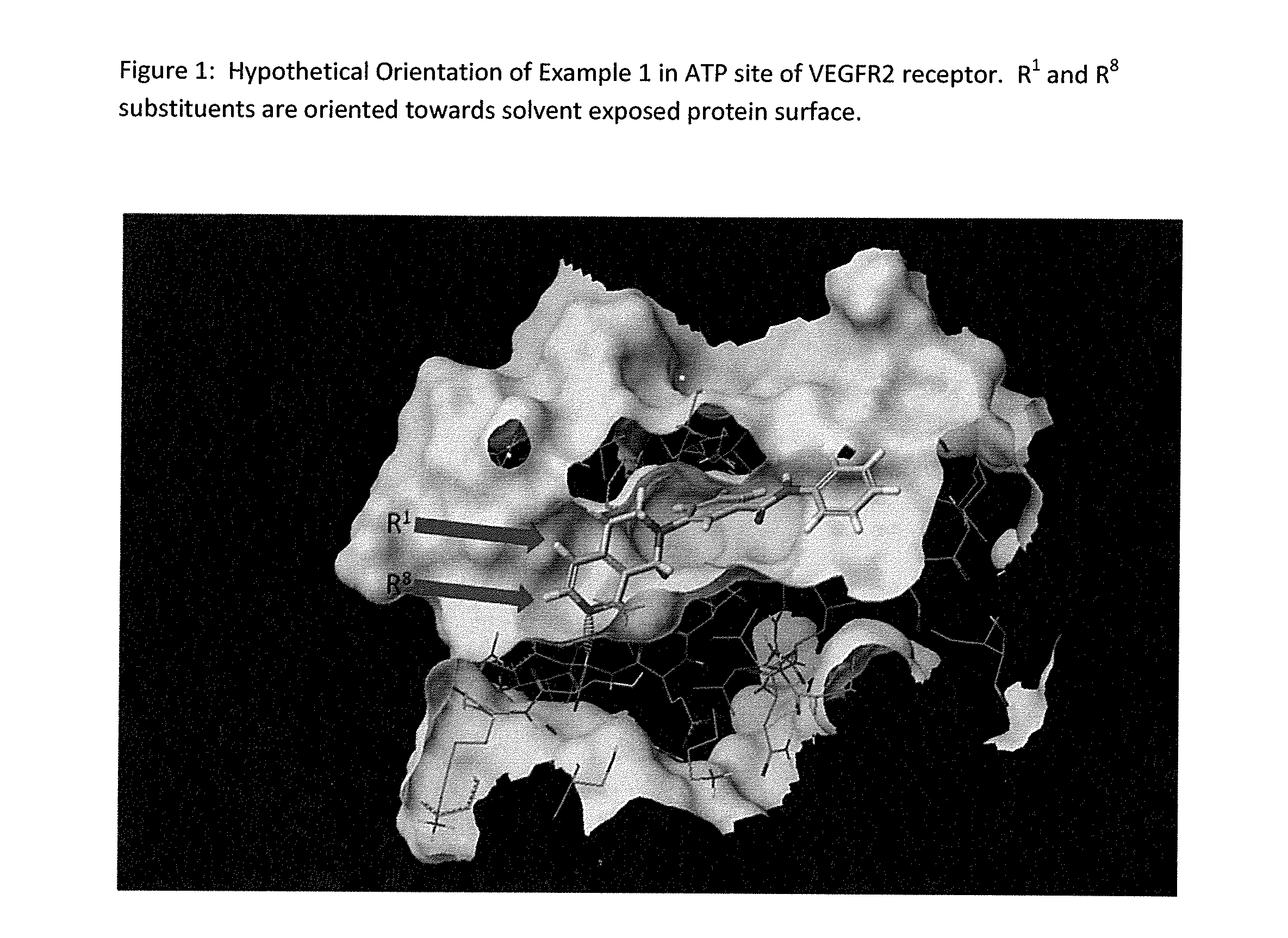
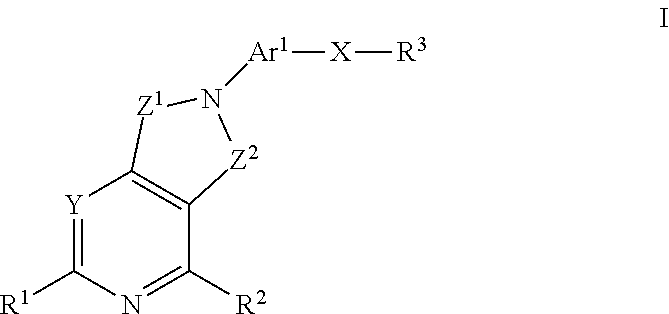
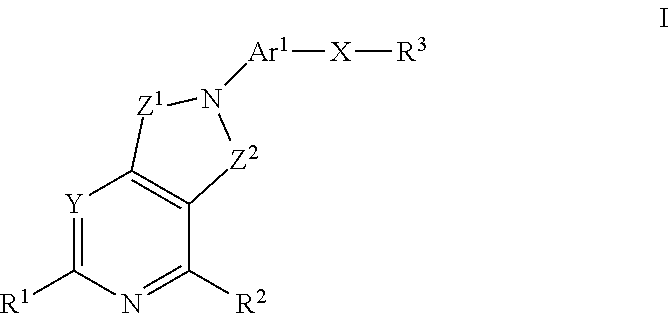
Method of treating conditiions with kinase inhibitors
a kinase inhibitor and conditiion technology, applied in the field of methods, can solve the problems of malignant tumor growth, abnormal blood vessel growth, malignant tumor growth, etc., and achieve the effect of increasing the hydrophilicity of the molecule and increasing the solubility of the otherwise hydrophobic compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation 1
[0105]
Methyl-3-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)-benzoate
[0106]Procedure adopted from Tetrahedron Lett., 2007, 48, 2519. A thick-walled glass reaction vessel was charged with palladium(II)acetate (235 mg, 1.05 mmol), X-Phos (500 mg, 1.05 mmol, 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl), cesium carbonate (6.82 g, 20.95 mmol) and 5:1 (v / v) toluene:t-BuOH (20 mL). The stirred contents were purged with nitrogen and a solution of 4-piperidone ethylene ketal (2.70 mL, 20.95 mmol) and methyl-3-bromobenzoate (4.95 g, 23.02 mmol) in 5:1 (v / v) toluene:t-BuOH (100 mL) was added. After stirring for 2 minutes, the vessel was sealed and the reaction mixture was heated at 120° C. for 16 hours. Upon cooling to room temperature, the reaction mixture was filtered through celite and the filtrate was concentrated.
[0107]Elution through a flash column (silica gel 60, 230-400 mesh, 7:3 hexanes:EtOAc) followed by concentration gave the title compound as an oil (5.64 g, 97%).
preparation 2
[0108]
Methyl-3-(4-oxopiperidin-1-yl)-benzoate
Method A:
[0109]A solution of methyl-3-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)-benzoate (3.85 g, 13.88 mmol) in 10% aqueous sulfuric acid (40 mL and THF (40 mL) was stirred at ambient temperature for 14 days. The reaction mixture was neutralized by cautious addition of NaHCO3 and simultaneously diluting with water. The aqueous mixture was extracted with EtOAc (2×75 mL) and the combined organic extracts were dried (MgSO4), filtered, and concentrated to give the title compound as an oil (2.75 g, 85%) which was used without further purification.
Method B:
[0110]A mixture of methyl-3-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)-benzoate (1.80 g, 6.49 mmol) and p-toluenesulfonic acid monohydrate (123 mg, 0.649 mmol) in water (30 mL) and acetone (15 mL) was heated at reflux for 17 hours. The organic solvent was removed in vacuo and the aqueous mixture was extracted with dichloromethane. The organic extract was washed with saturated NaHCO3(aq.), dried (MgSO4), ...
preparation 3
[0111]
Methyl-3-(3-dimethylaminomethylene-4-oxopiperidin-1-yl)-benzoate
[0112]A solution of methyl-3-(4-oxopiperidin-1-yl)-benzoate (2.72 g, 11.66 mmol) and DMF-dimethylacetal (6.24 mL, 46.64 mmol) in 1,4-dioxane (50 mL) was refluxed for 43 hours. The reaction mixture was concentrated and the residue was eluted through a flash column (silica gel 60, 230-400 mesh, 5% MeOH in EtOAc to 8% MeOH in EtOAc) to obtain the title compound as a red oil which slowly crystallized on standing (1.52 g, 45%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com