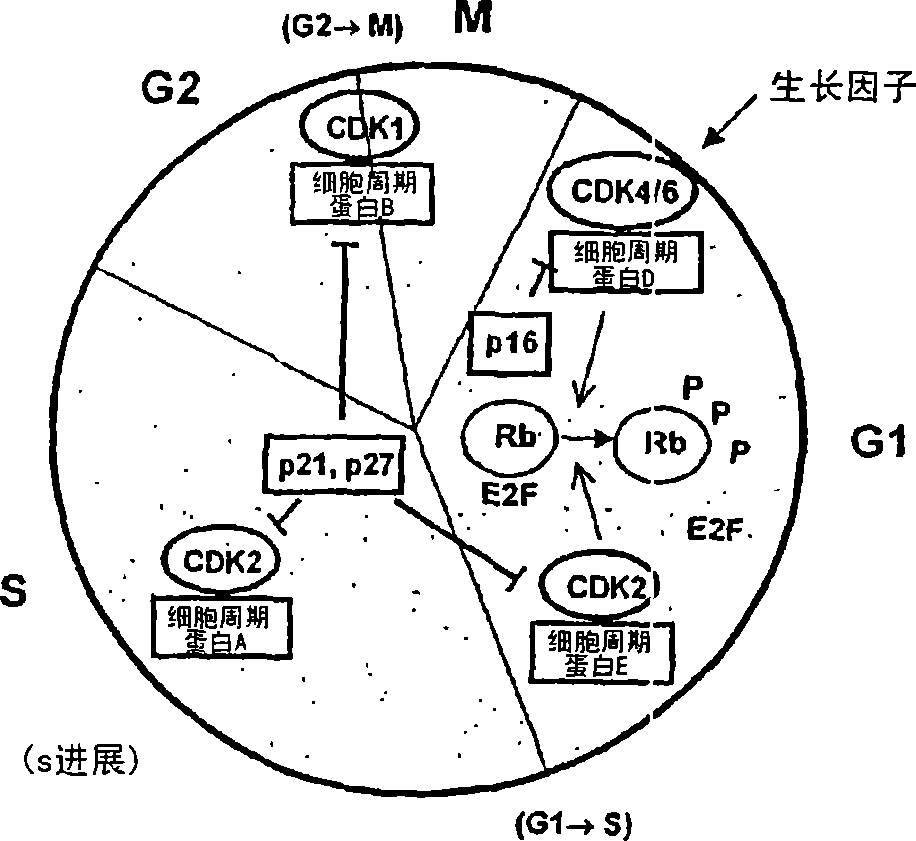
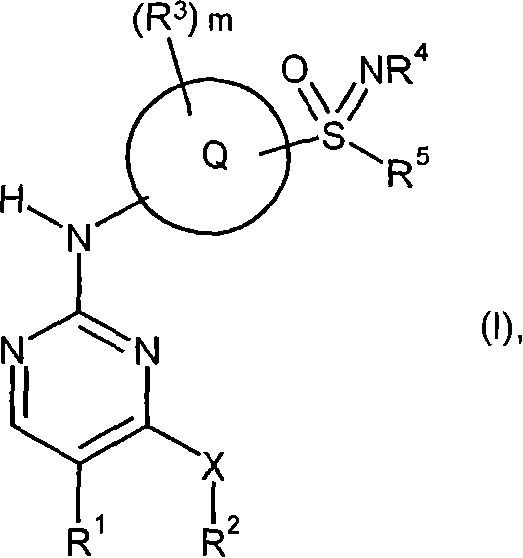
Sulfoximine-substituted pyrimidines as CDK-and/or VEGF inhibitors, their production and use as pharmaceutical agents
A compound and alkyl technology, which is applied in the field of drugs for the treatment of various diseases, can solve the problems of limited clinical application such as carbodehydratase inhibition and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175]
[0176] Substituent Q, R 1 , R 2 , R 3 , R 4 , R 5 and m are the same as defined in the general formula (I).
Embodiment 10
[0178] Preparation of (RS)-S-[4-({5-bromo-4-[(R)-(2-hydroxy-1-methylethyl)amino]pyrimidin-2-yl}amino)phenyl]-S -Methylaminosulfoxide
[0179]
[0180] Method A
[0181] 40 mg (0.23 mmol) of (RS)-S-(4-aminophenyl)-S-methylaminosulfoxide and 62 mg (0.23 mmol) of (R)-2-[(5-bromo -2-chloropyrimidin-4-yl)amino]propan-1-ol with 0.5 ml of 1-butyl-3-methyl-imidazolium tetrafluoroborate (review article on ionic liquids: a) T.Welton, Chem.Rev.1999,99,2071; b) H.Zhao, Aldrichimica Acta2002,35,75; c) M.J.Earle, K.R.Seddon, ACS Symposium Series 2002,819,10) mixed, and at room temperature Stir for 10 minutes. The reaction mixture was heated to 60°C and stirred at this temperature for a further 3 hours. Mix with 0.08 ml of 4M hydrochloric acid solution in dioxane and stir at 60° C. for 60 hours. After cooling, the reaction mixture was mixed with 10 ml of ethyl acetate and stirred for 10 minutes. The organic solvent was decanted off, and the residue was dissolved in 10 ml methanol. ...
Embodiment 11
[0196] Preparation of (RS)-S-[3-({5-bromo-4-[(R)-(2-hydroxy-1-methylethyl)amino]pyrimidin-2-yl}amino)phenyl]-S -Methyl-N-nitroaminosulfoxide
[0197]
[0198] A solution of 37mg (0.17mmol) of (RS)-S-(3-aminophenyl)-S-methyl-N-nitroaminosulfoxide in 3ml of acetonitrile was mixed with 91mg (0.34mmol) of (R)- 2-[(5-Bromo-2-chloropyrimidin-4-yl)amino]propan-1-ol and 0.06 ml of 4M hydrochloric acid solution in dioxane were mixed and stirred overnight at reflux. A further 0.05 ml of 4M hydrochloric acid solution in dioxane was added and refluxed for a further 6 hours. After TLC monitoring, it was mixed with 92 mg (0.34 mmol) of (R)-2-[(5-bromo-2-chloropyrimidin-4-yl)amino]propan-1-ol and refluxed overnight. After cooling, the reaction was made basic with saturated sodium bicarbonate solution, then extracted with ethyl acetate. The combined organic phases were dried (Na 2 SO 4 ), filtered and concentrated by evaporation. The residue obtained is purified by chromatography (DC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com