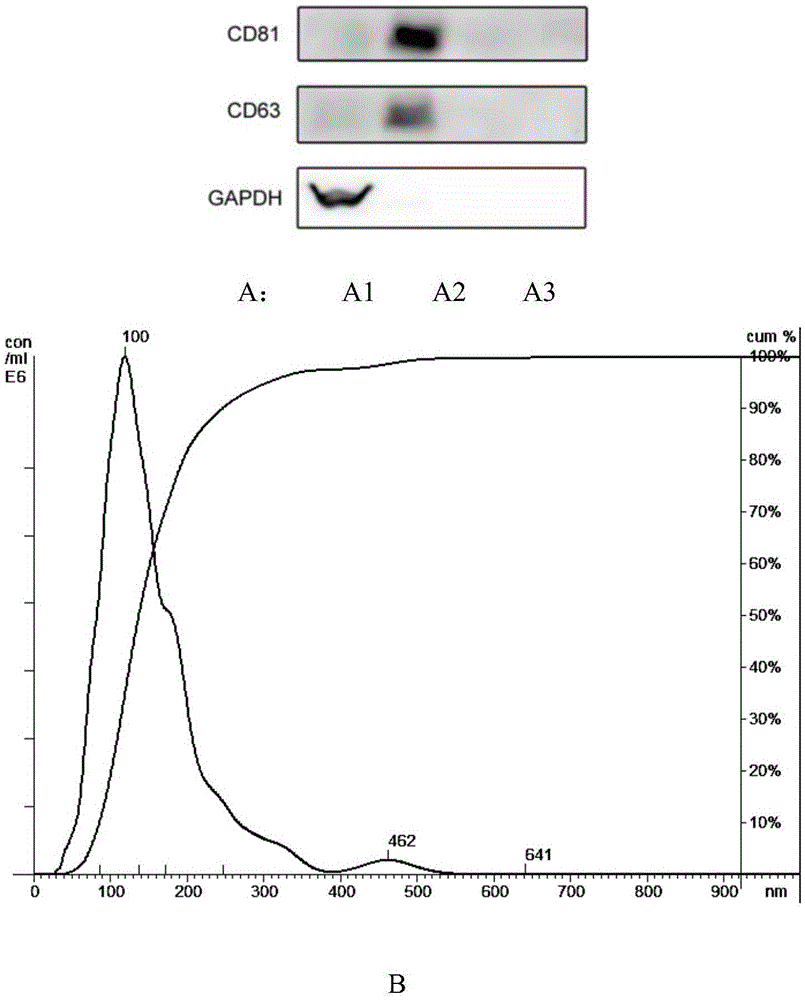
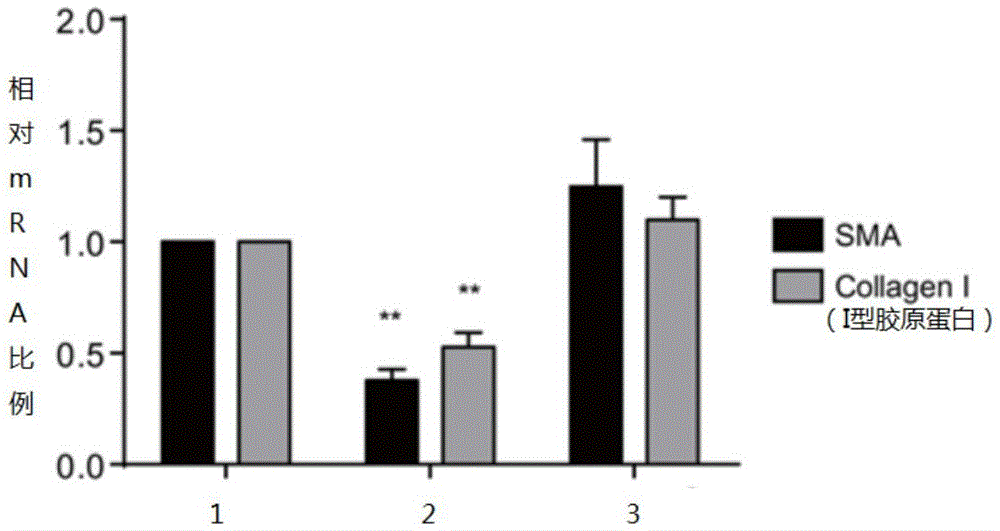
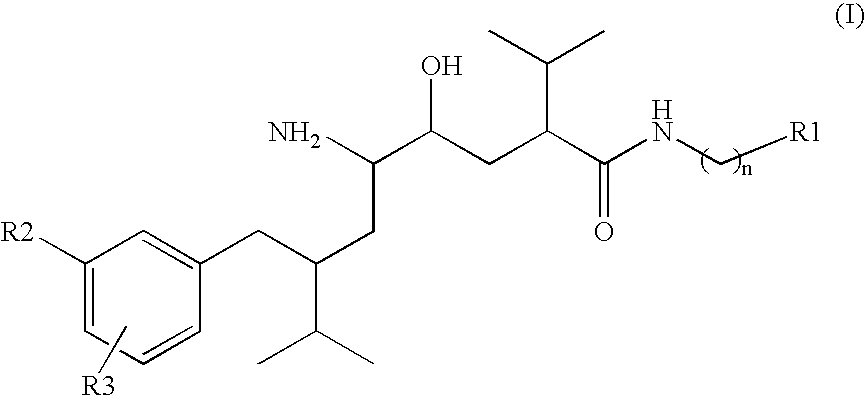
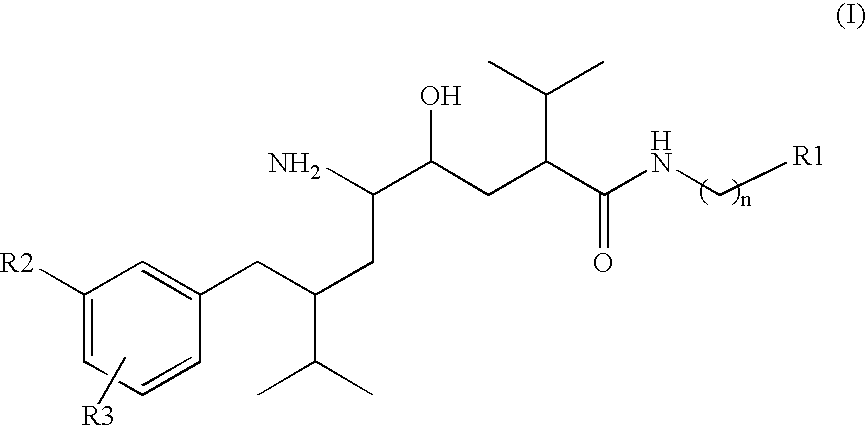
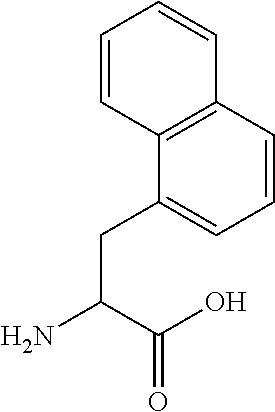
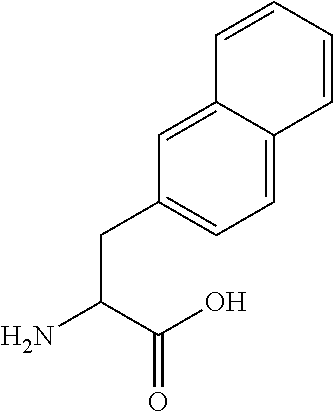
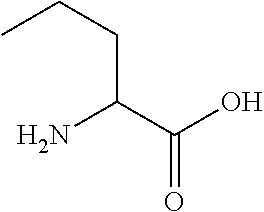
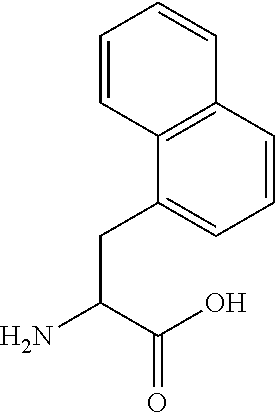
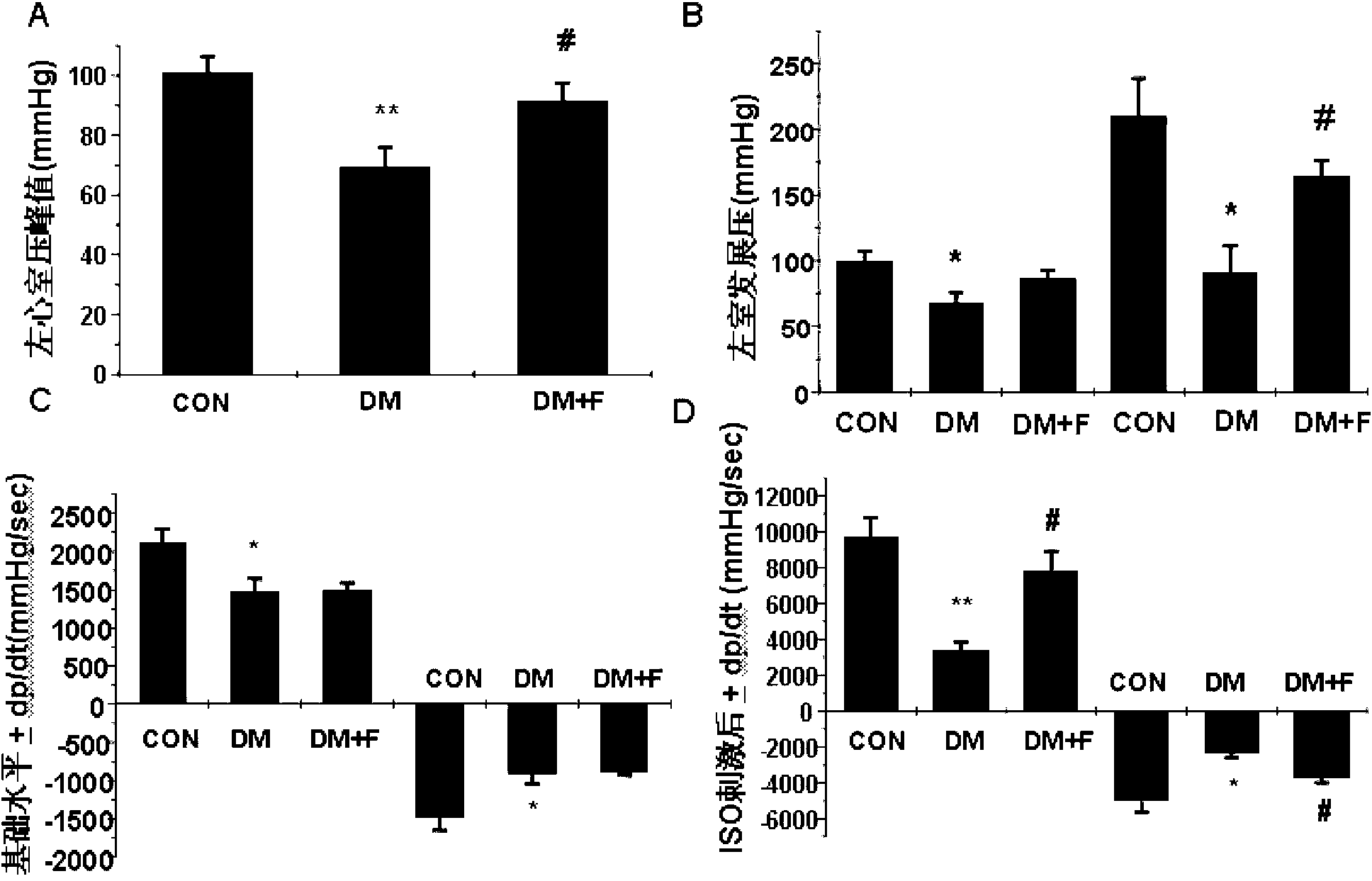
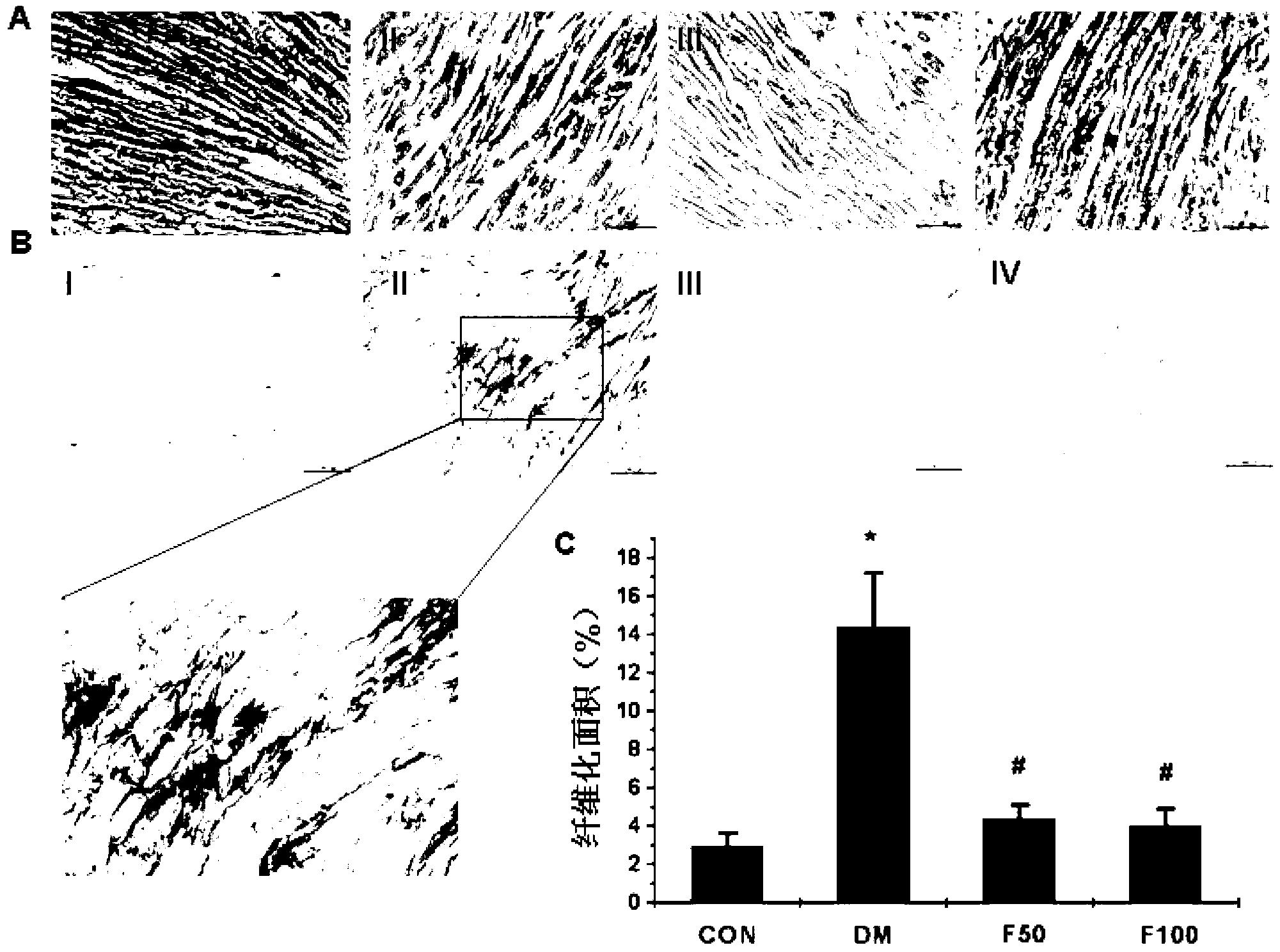
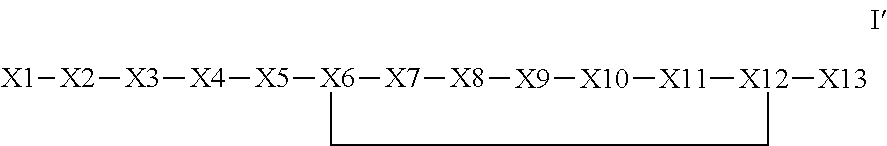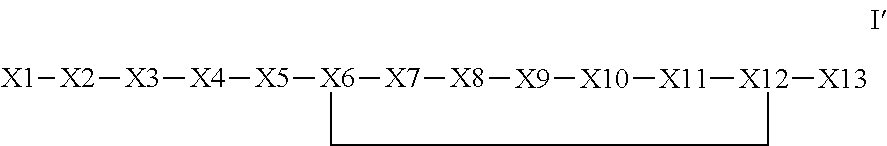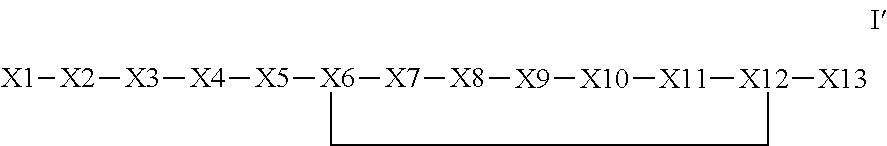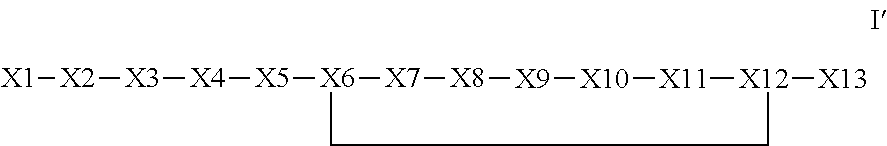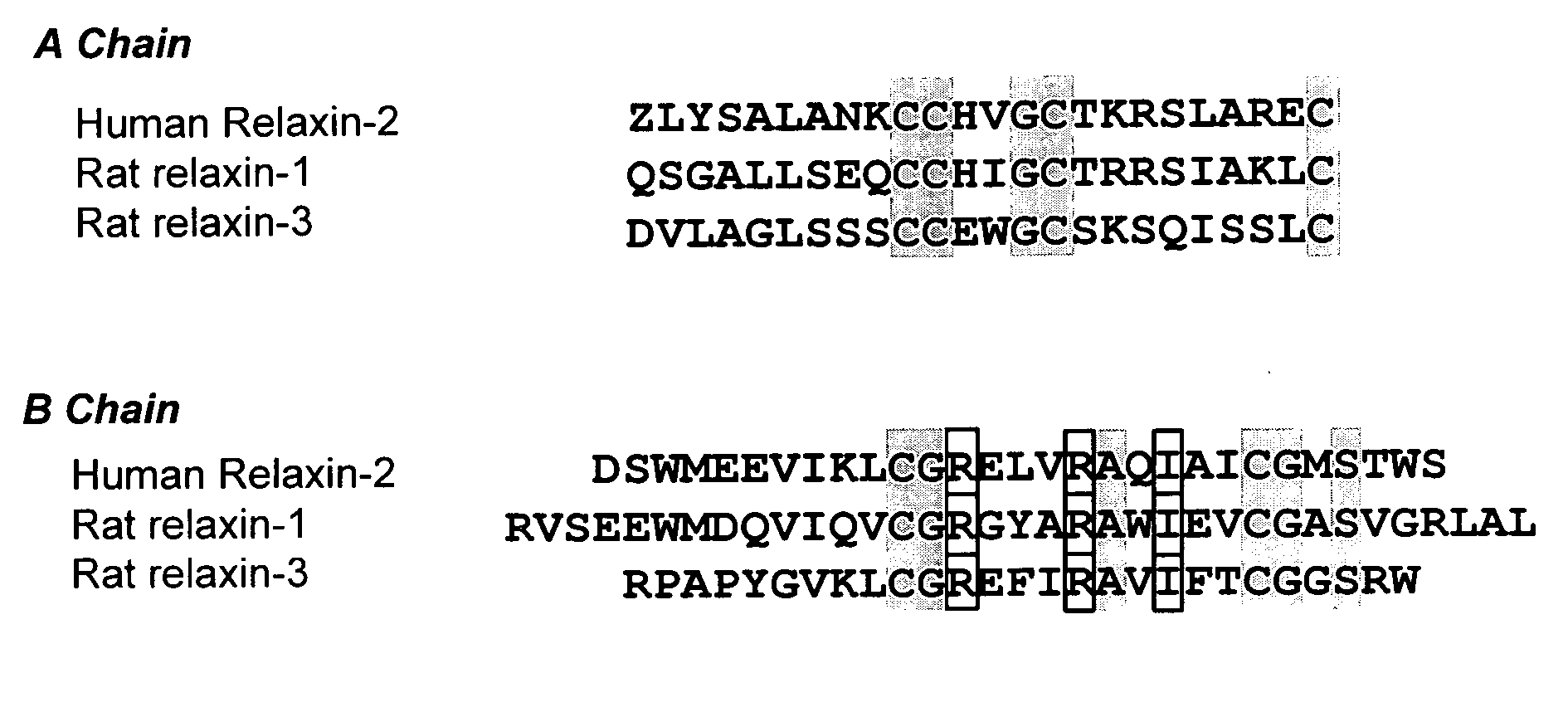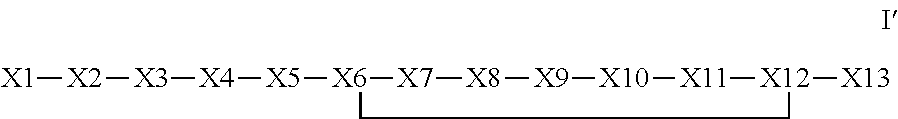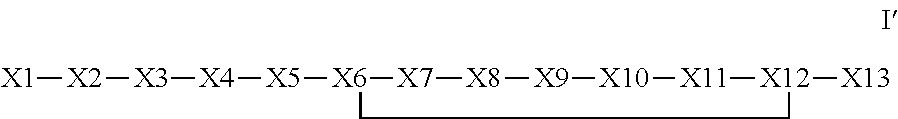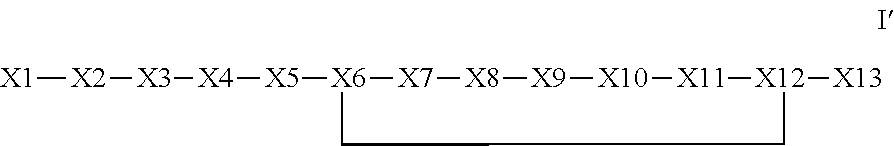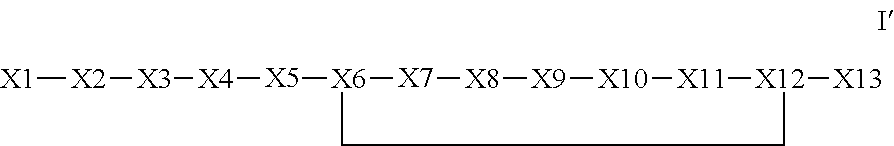Patents
Literature
163 results about "Cardiac fibrosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
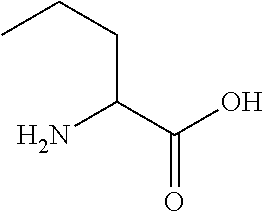
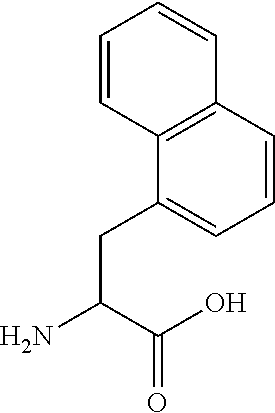
Cardiac fibrosis may refer to an abnormal thickening of the heart valves due to inappropriate proliferation of cardiac fibroblasts but more commonly refers to the excess deposition of extracellular matrix in the cardiac muscle. Fibrotic cardiac muscle is stiffer and less compliant and is seen in the progression to heart failure. The description below focuses on a specific mechanism of valvular pathology but there are other causes of valve pathology and fibrosis of the cardiac muscle.
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS8673848B2Extended half-lifeIncrease constraintsNervous disorderSkeletal disorderCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Application of exosome derived from human mesenchymal stem cells to resistance to tissue fibrosis and scar forming
InactiveCN105477016AAvoid cancer riskInhibition formationAerosol deliveryDigestive systemCardiac fibrosisTissue fibrosis
The invention belongs to the technical field of molecular biology and relates to application of an exosome derived from human mesenchymal stem cells to preparation of medicines for antagonizing scars or tissue and organ fibrosis. According to the application, the high-purity and high-bioactivity exosome is obtained by subjecting healthy human umbilical cords to primary in-vitro culture, collecting liquid supernatant after amplification, performing low-temperature ultracentrifugation, purifying and the like; the exosome is used individually or added into medicines, health care products and cosmetics according to a proportion to be developed into a product with a bioactivity function, thereby being capable of regulating organism cell signal transduction effectively, inhibiting fibrocyte differentiation and then antagonizing the tissue and organ fibrosis and scar forming. Through experimental verification, the exosome can play a remarkable role in repairing promotion in hypertrophic scar after skin injury, particularly pathological fibrosis proliferation such as scar contracture of the joint parts, hepatic fibrosis and cardiac fibrosis, and is high in safety, little in toxicity and convenient to storage and transport, thereby being wide in application prospect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Organic Compounds
InactiveUS20090076062A1Avoid actionReduce formationBiocideSenses disorderIntra ocular pressureCardiac fibrosis
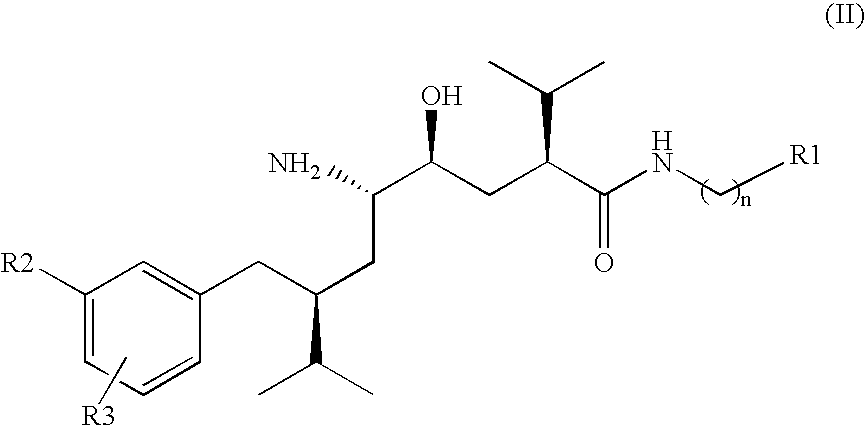
Disclosed are δ-amino-γ-hydroxy-ω-aryl-alkanoic acid amide compounds of formula (I)and the salts thereof, having renin-inhibiting properties. Also disclosed are pharmaceutical compositions comprising these compounds and methods of administering them for the treatment of hypertension, atherosclerosis, unstable coronary syndrome, congestive heart failure, cardiac hypertrophy, cardiac fibrosis, cardiomyopathy postinfarction, unstable coronary syndrome, diastolic dysfunction, chronic kidney disease, hepatic fibrosis, complications resulting from diabetes, such as nephropathy, vasculopathy and neuropathy, diseases of the coronary vessels, restenosis following angioplasty, raised intra-ocular pressure, glaucoma, abnormal vascular growth, hyperaldosteronism, cognitive impairment, alzheimers, dementia, anxiety states and cognitive disorders.
Owner:MAIBAUM JUERGEN KLAUS +2
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS20130196899A1Extended half-lifeIncrease constraintsNervous disorderSkeletal disorderCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
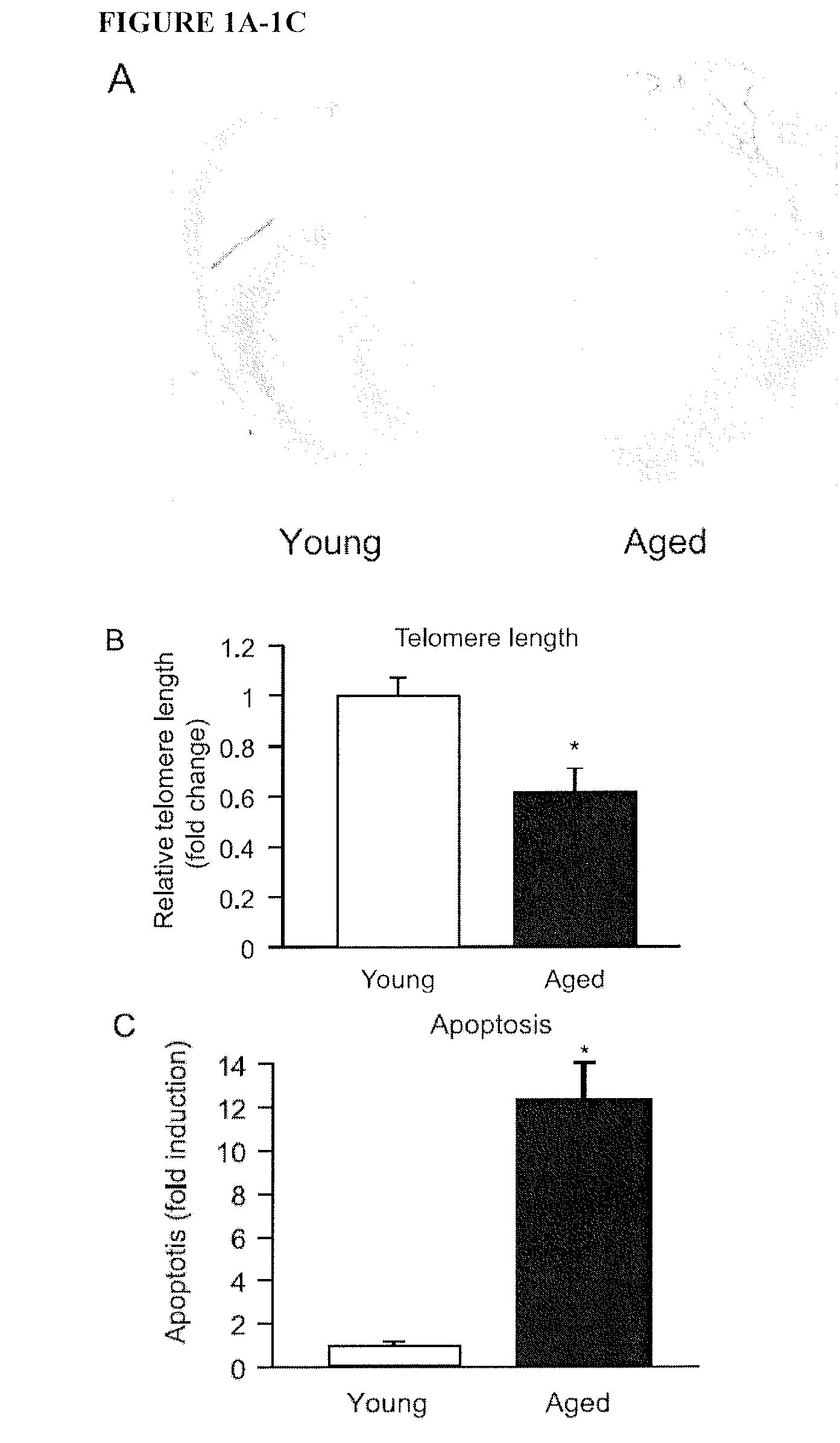
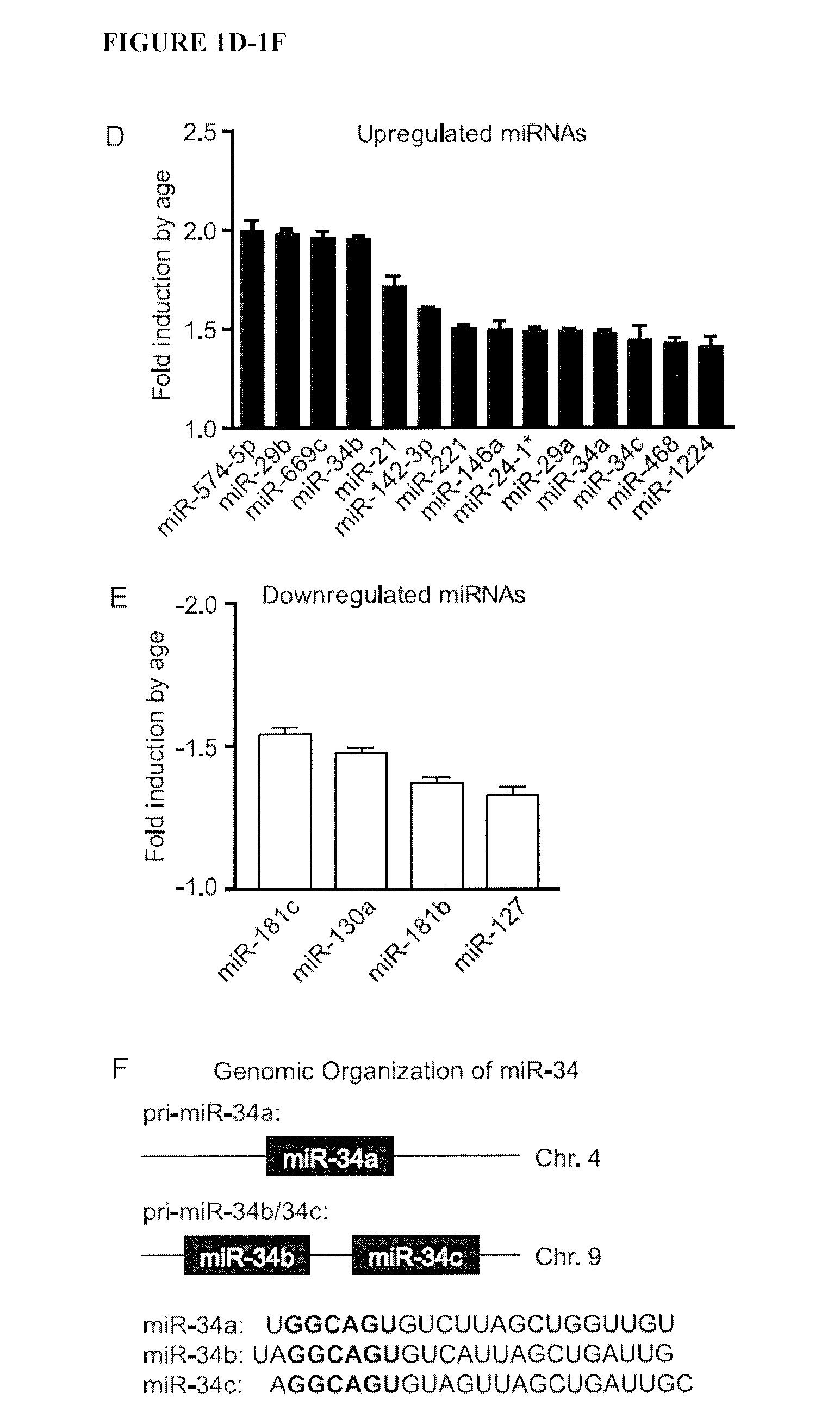
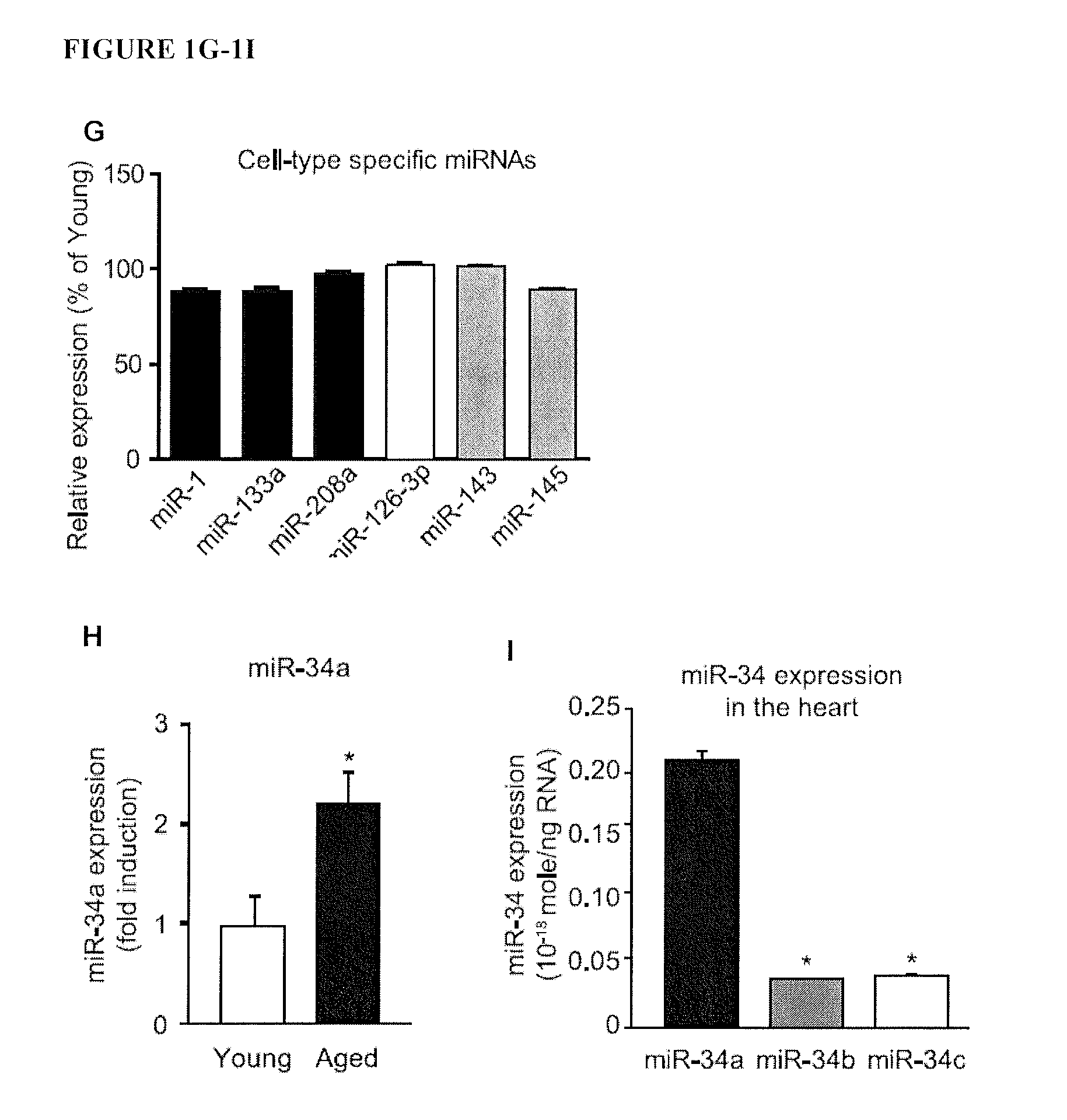
Micro-rna for the regulation of cardiac apoptosis and contractile function
InactiveUS20120238619A1Promote recoveryOrganic active ingredientsFermentationCardiac fibrosisApoptosis
The present invention relates to treating or preventing age-related cardiomyopathy by modulating the expression or activity of a miR-34 family member and / or PNUTS. Methods of treating or preventing age-related cardiomyopathy include administering an inhibitor of miR-34 expression or activity or an agonist of PNUTS expression or activity. Also provided herein are methods of treating or preventing cardiac fibrosis and myocardial infarction by administering an inhibitor of miR-34 expression or activity or an agonist of PNUTS expression or activity.
Owner:MIRAGEN THERAPEUTICS
Synthetic linear apelin mimetics for the treatment of heart failure
InactiveUS20140155315A1Extended half-lifeIncrease constraintsNervous disorderPeptide/protein ingredientsCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′ (SEQ ID NO: 1):X1-X2-X3-R—X5-X6-X7-X8-X9-X10-X11-X12-X13 Ior an amide, an ester or a salt thereof, wherein X1, X2, X3, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
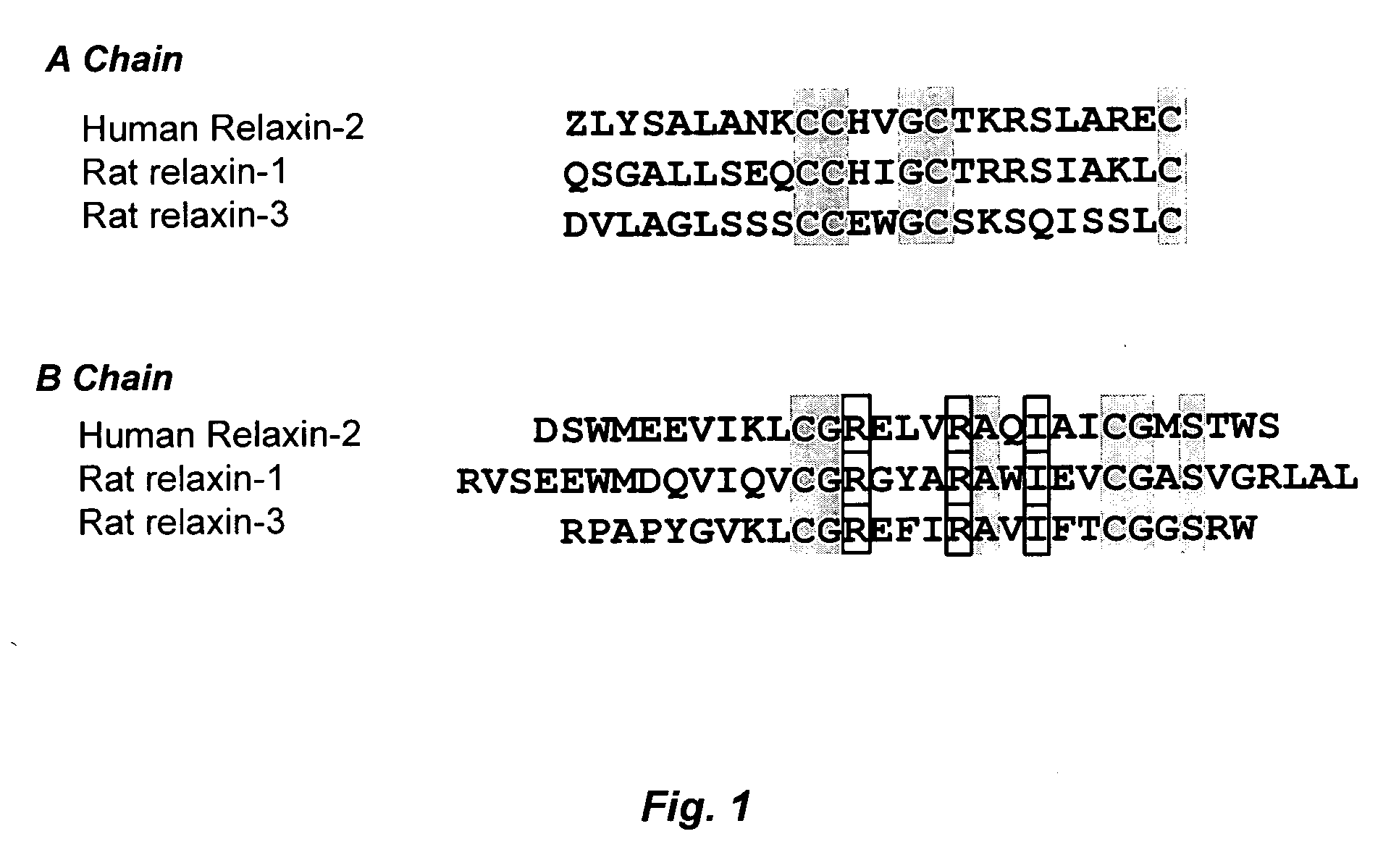
Prevention of fibrosis following cardiac injury
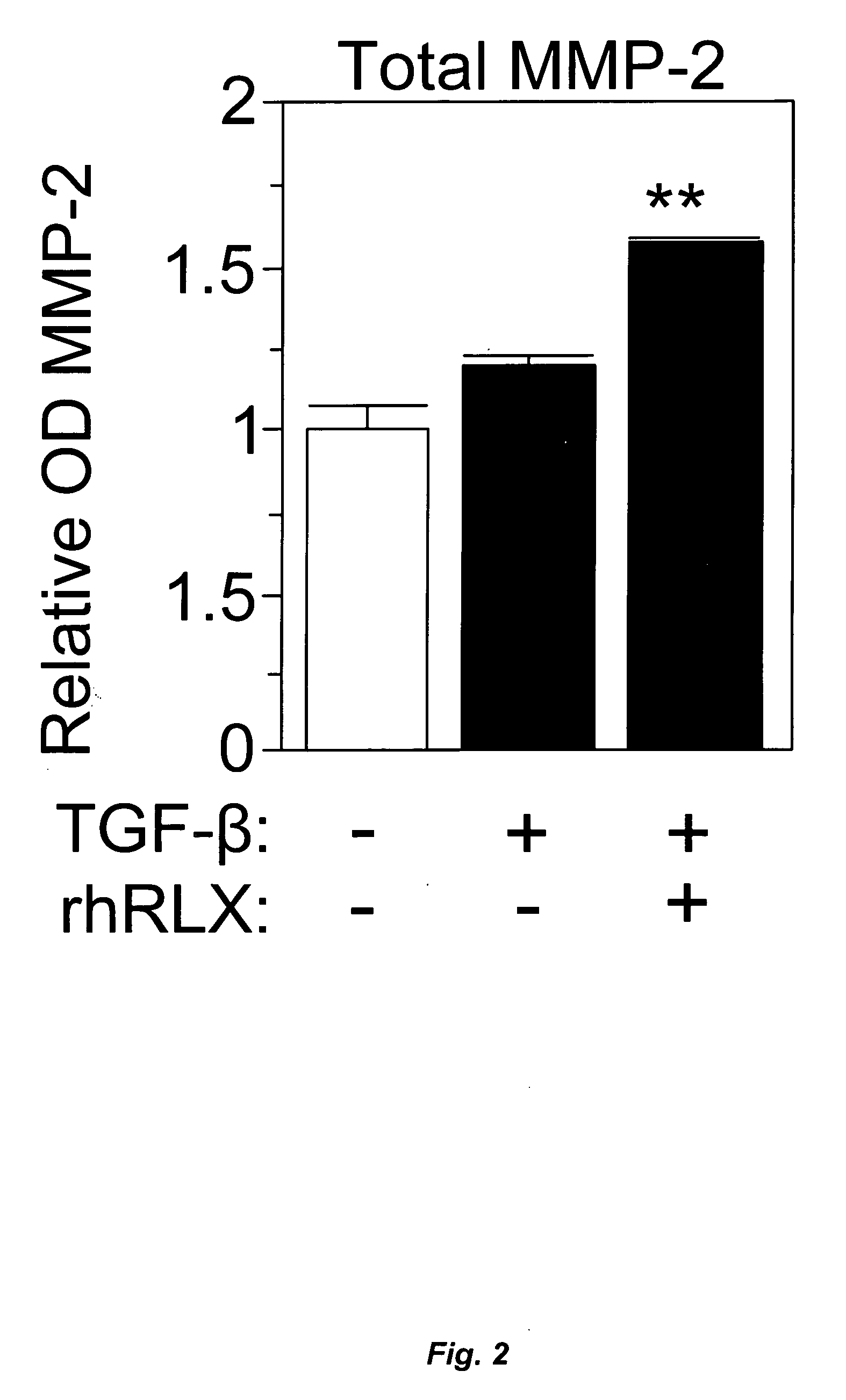
InactiveUS20060264367A1Reduce fibrosisReduce activationHormone peptidesPeptide/protein ingredientsCardiac fibrosisRelaxin
A method for treating cardiac fibrosis resulting from injury of a mammalian heart is described comprising the step of contacting a therapeutically effective amount of relaxin and / or an LGR7 activating agent with cardiac cells following the injury in an amount sufficient to reduce the fibrosis. Also described are methods for protecting the heart following an ischemic event, inhibiting the proliferation of activated fibroblasts and antagonising collagen secretion or deposition in a mammalian heart.
Owner:BAKER MEDICAL RES INST +1
Bioconjugates of synthetic apelin polypeptides
InactiveUS20150030594A1Extended half-lifeIncrease constraintsNervous disorderPeptide/protein ingredientsCardiac fibrosisVentricular tachycardia
The invention provides a bioconjugates comprising a synthetic polypeptide of Formula I′ (SEQ ID NO: 1):or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein and a half-life extending moiety wherein the peptide and the half-life extending moiety are covalently linked or fuse, optionally via a linker. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the bioconjugates of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Compositions and Methods of Treating Cardiac Fibrosis with Ifetroban
ActiveUS20150328190A1Preventing and treating and attenuating cardiac fibrosisReduce the impactBiocideOrganic chemistryCardiac fibrosisDefibrination syndrome
The present invention is directed to methods of treating, preventing, and / or ameliorating fibrosis syndrome, and in particular cardiac fibrosis, by administration of a therapeutically effective amount of ifetroban, or a pharmaceutically acceptable salt thereof.
Owner:CUMBERLAND PHARM INC +1
Identification of a micro-RNA that activates expression of β-myosin heavy chain
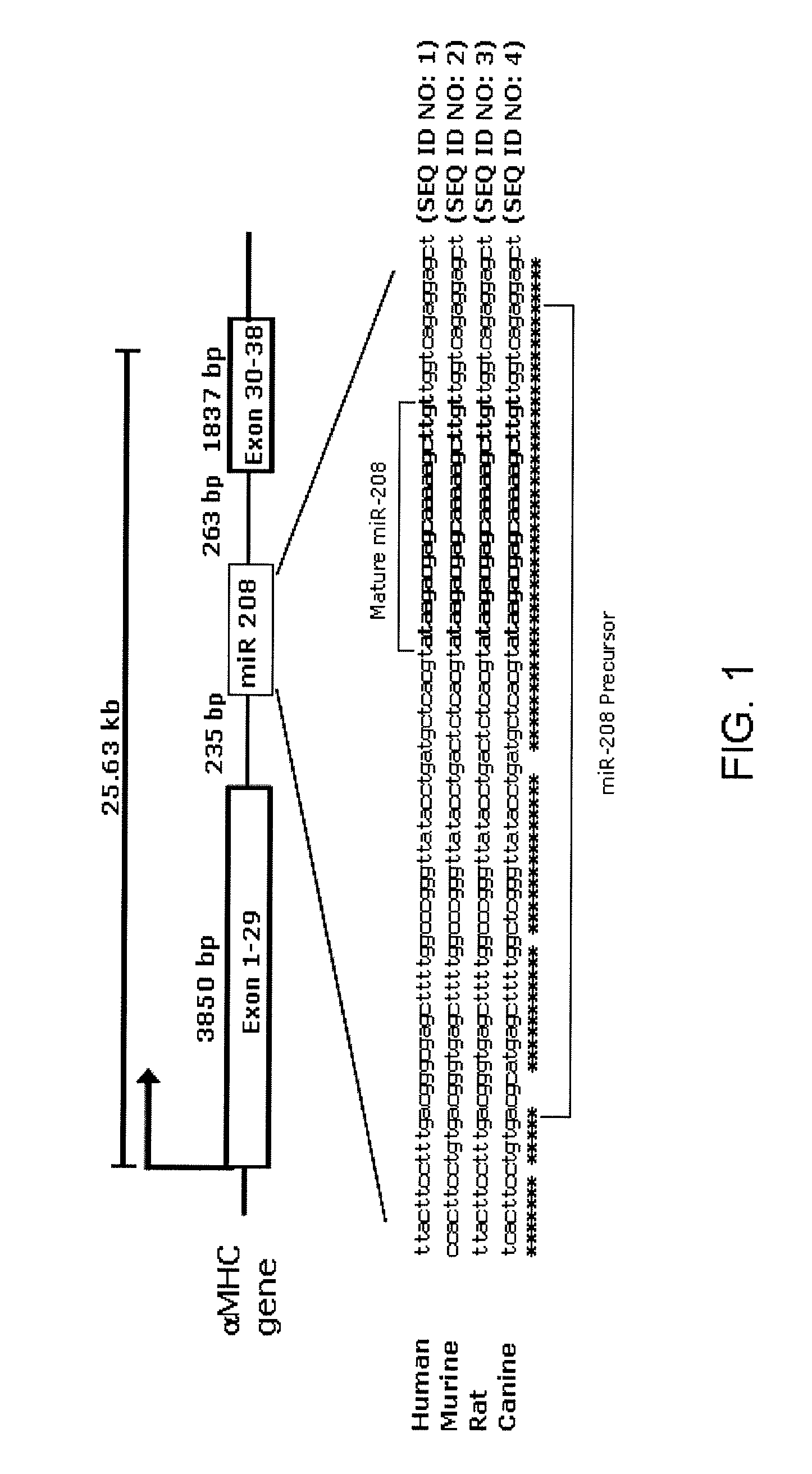
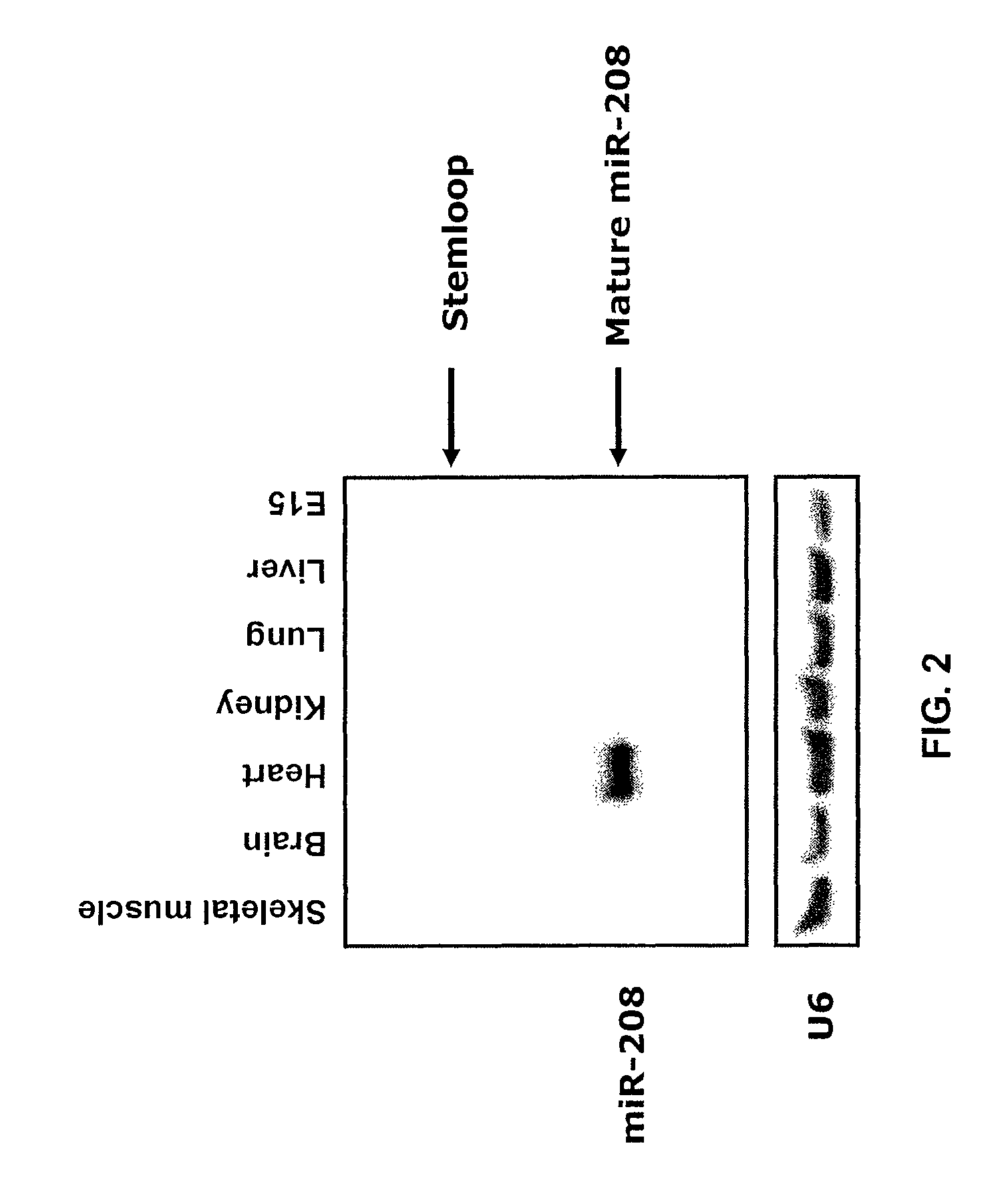
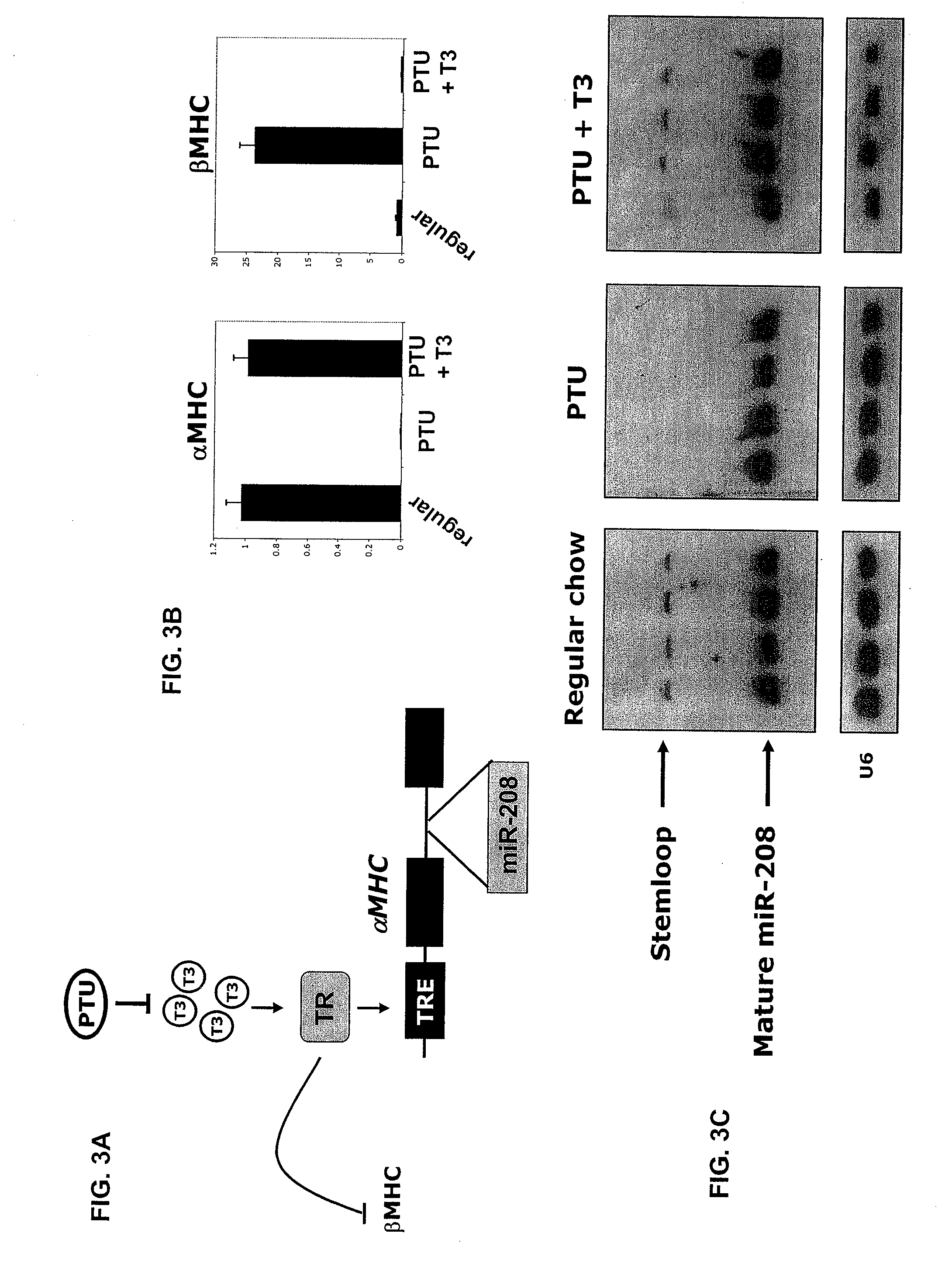
InactiveUS8304397B2Inhibit progressHigh expressionBiocideGenetic material ingredientsCardiac fibrosisFiber
The present invention relates to the identification of a microRNA, miR-208, that induces the expression of β-myosin heavy chain (β-MHC) and represses fast skeletal muscle contractile protein genes. Inhibition of this function is proposed as a treatment for cardiac fibrosis, hypertrophy and / or heart failure, and augmentation of this function can be used to repress slow fiber genes and activate fast fiber genes in the treatment of musculoskeletal disorders.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Na/K-ATpase Ligand
ActiveUS20090226513A1Prevent and reverse aging related lossSkin structure and functionOrganic active ingredientsCosmetic preparationsCardiac fibrosisATPase
This process relates to a pharmaceutical composition of an Na-K-ATPase ligand which will stimulate Na / K-ATPase signaling in a pharmaceutically acceptable vehicle. In one embodiment, the composition may be used to treat a skin disorder. In another embodiment, the composition may be used to inhibit cardiac fibrosis.
Owner:TOLEDO THE UNIV OF
Composition for repressing transformation growth factor beta
InactiveUS20060045905A1Improve securitySafe and effectiveBiocideSkeletal disorderCardiac fibrosisPercent Diameter Stenosis
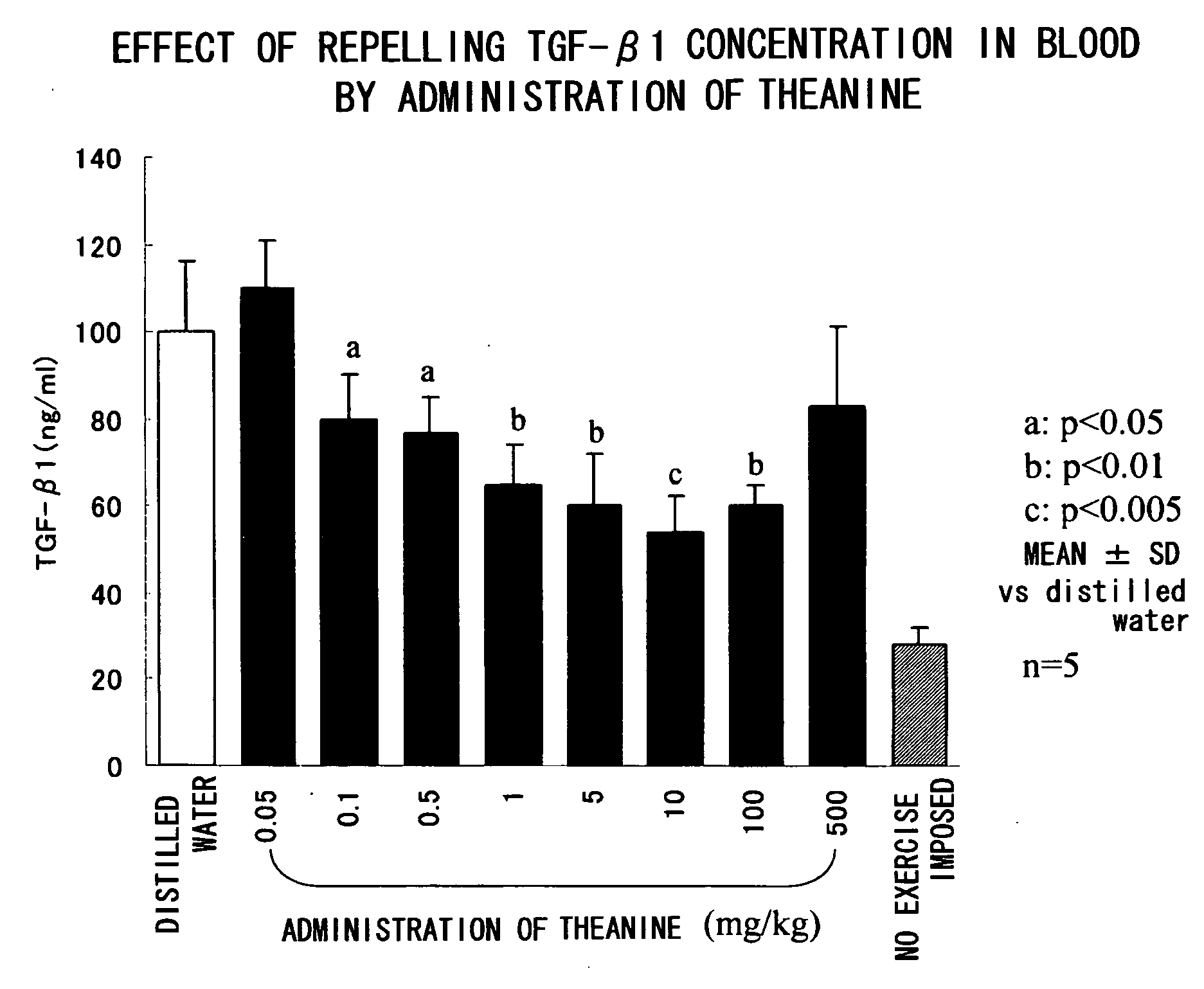
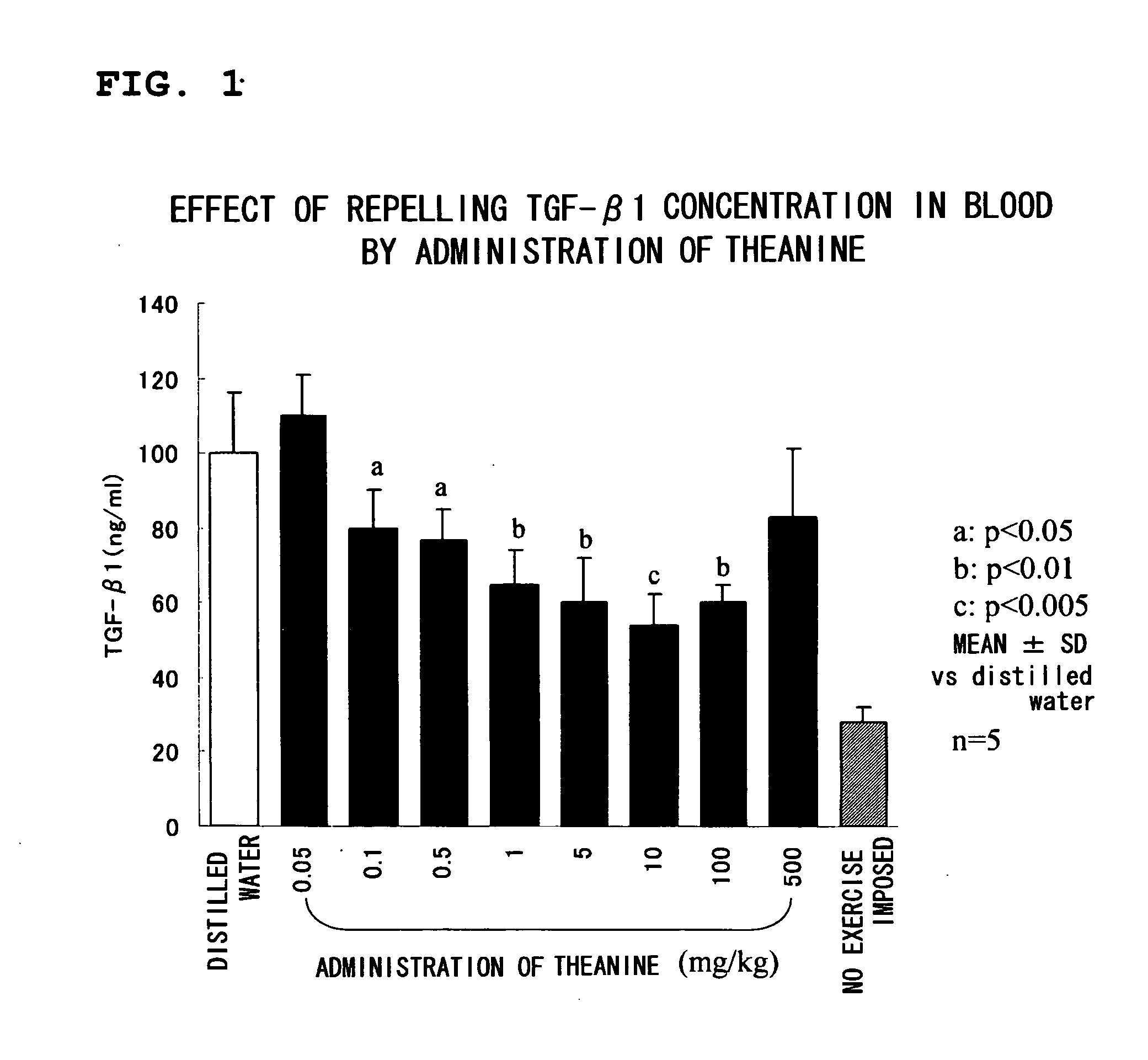
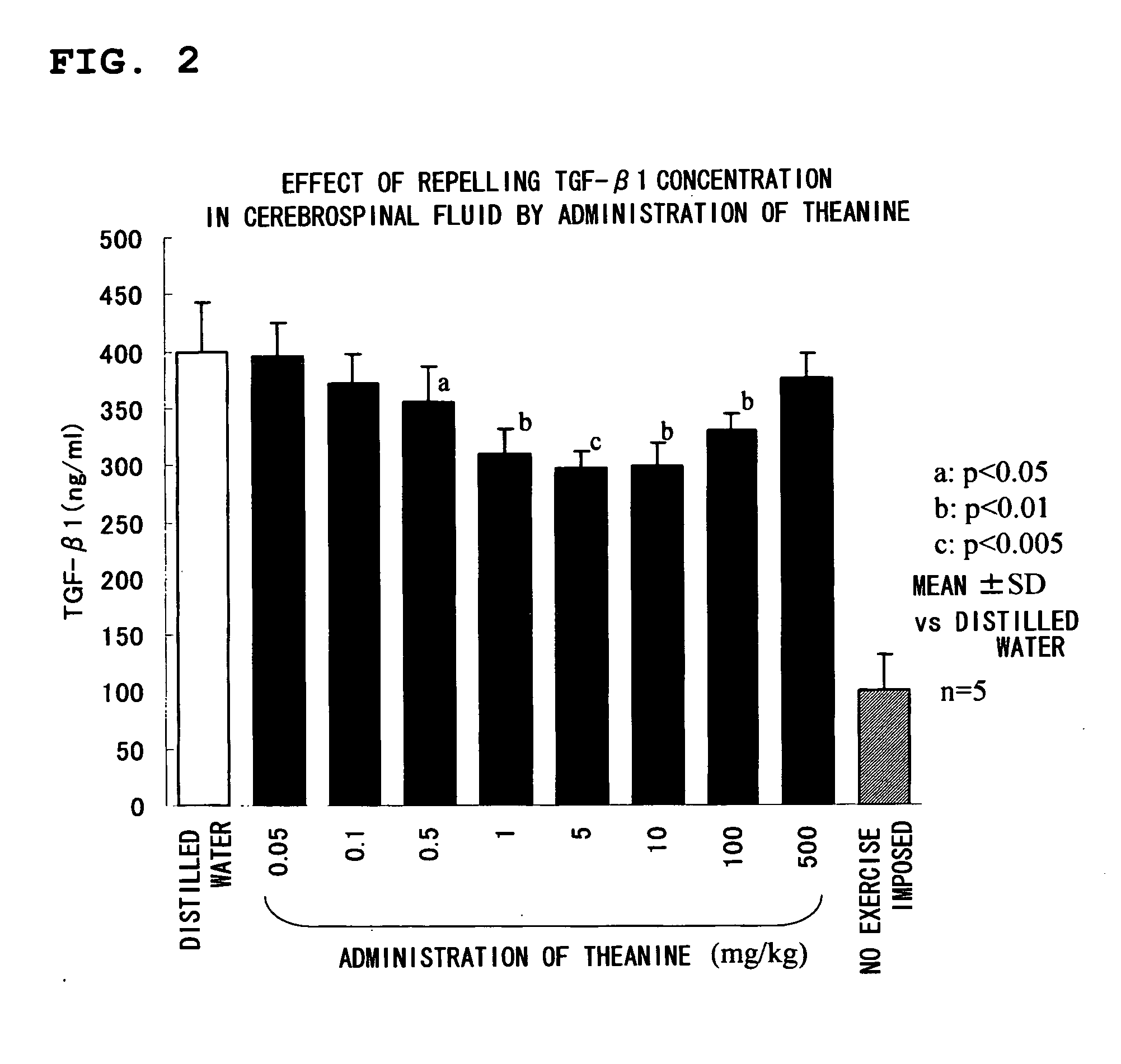
A composition for repressing transformation growth factor β contains theanine as an active substance. The composition is useful for preventing or treating chronic glomerulonephritis, renal interstitial fibrosis, hepatic fibrosis, hepatic cirrhosis, idiopathic interstitial pneumonia, keloid, hidebound disease, arterial sclerosis, myocardial infarction, cardiac fibrosis, restenosis, acute megakaryoblastic leukemia, adult T-cell leukemia, chronic fatigue syndrome or ordinary fatigue.
Owner:TAIYO KAGAKU CO LTD
Treatment of heart failure and related conditions
ActiveUS20140234319A1Easily damagedControlling signalOrganic active ingredientsPeptide/protein ingredientsCardiac fibrosisDisease
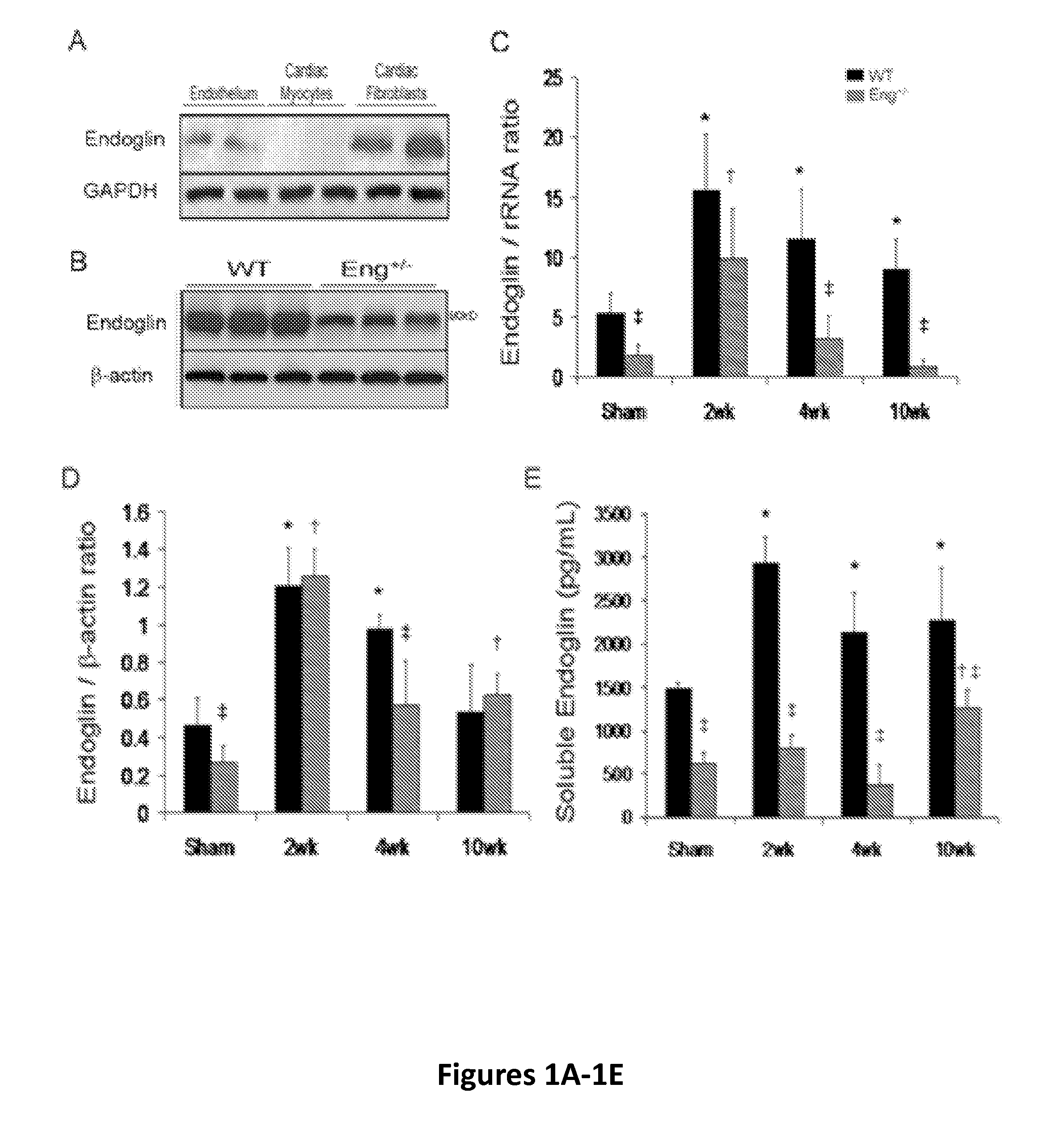
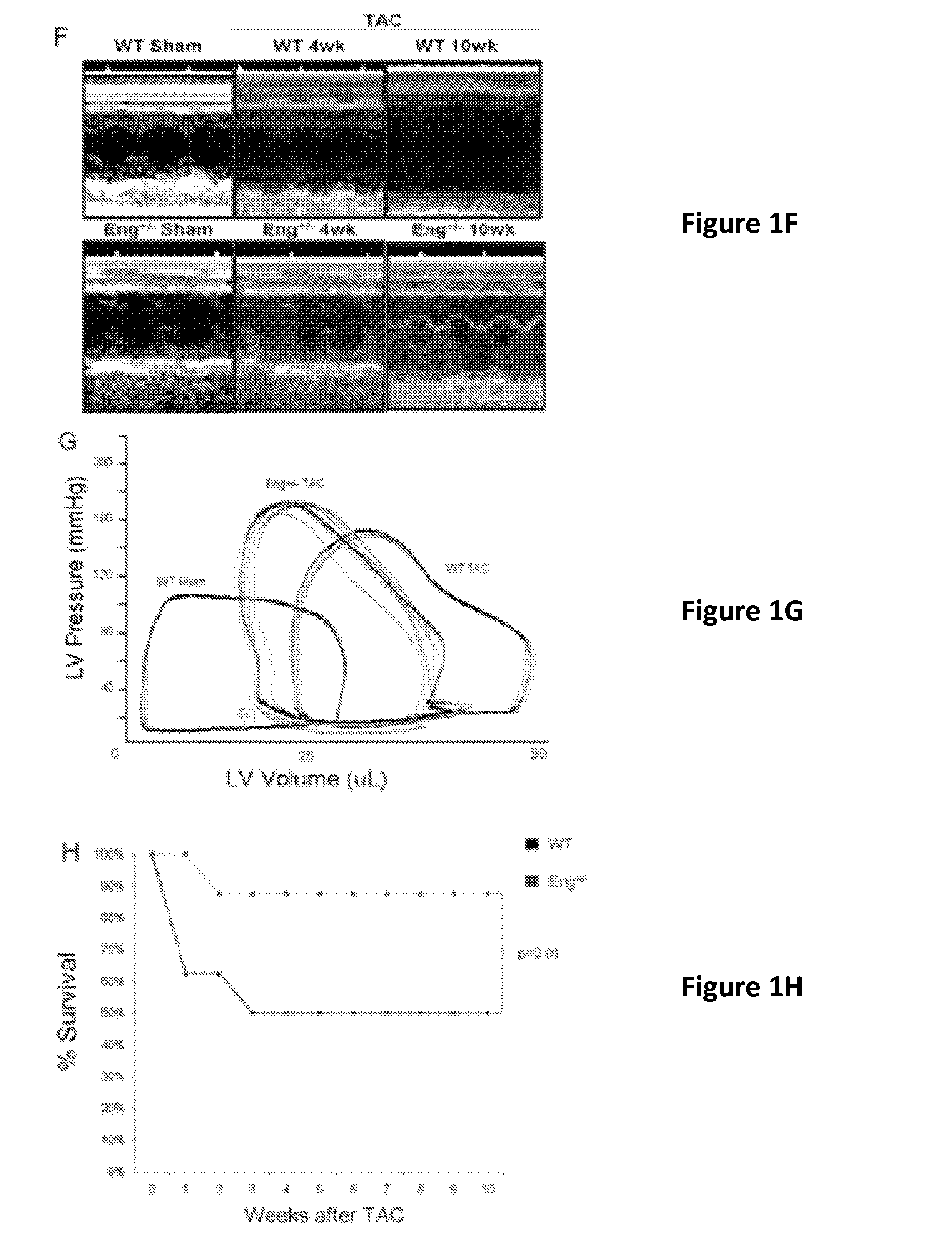
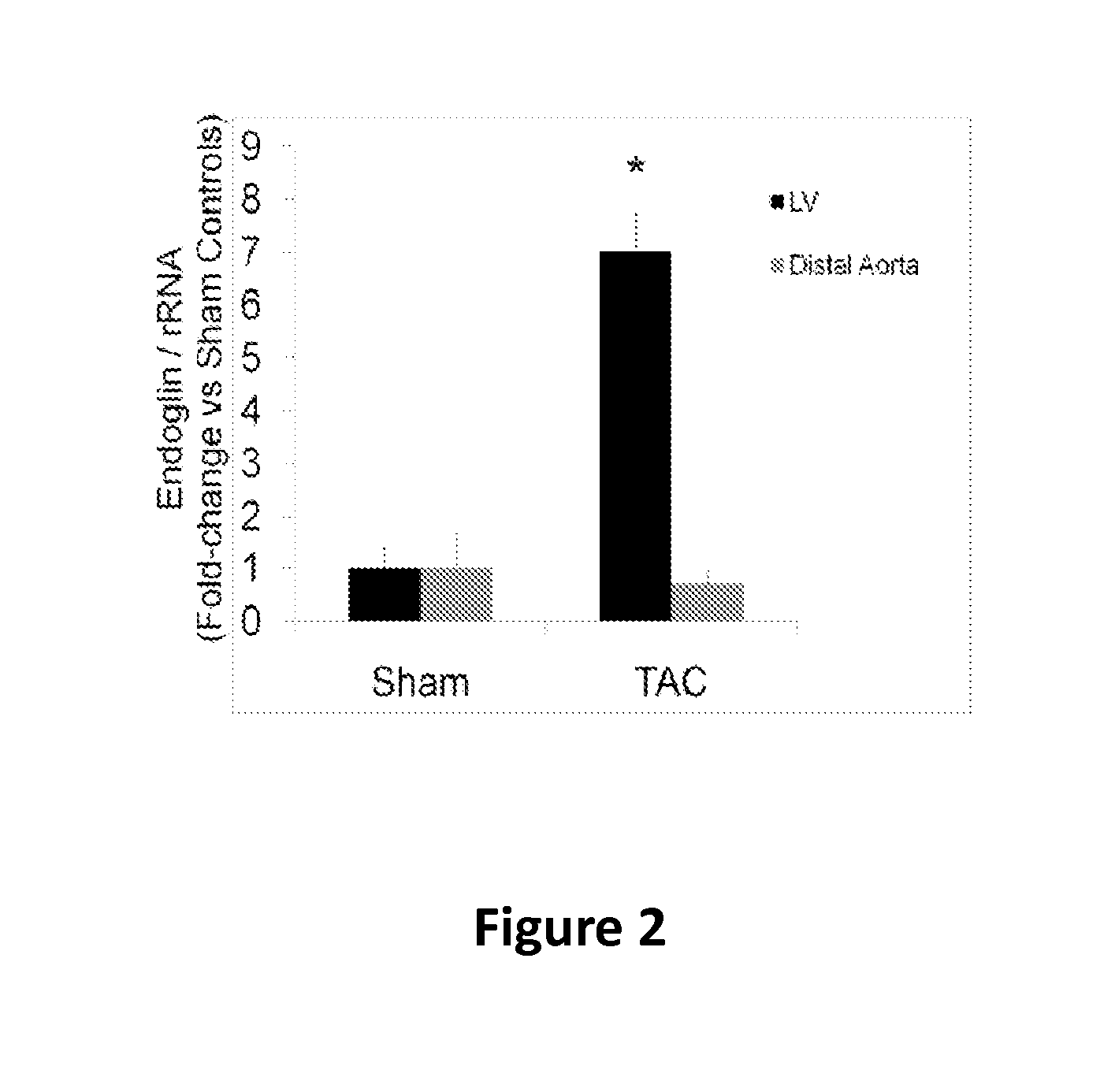
Endoglin has now been shown to be an important target of therapy to reduce disease symptoms associated with heart failure, particularly cardiac fibrosis. Soluble Endoglin is identified as an anatogonist to TGFβ1 activity, while membrane-bound Endoglin is identified as a necessary component to promote TGFβ1 activity in heart failure. The present invention therefore features methods and kits for treatment of subject having heart failure or a related disorder by administering a composition that decreases TGFβ1 signaling through either direct inhibition of membrane-bound Endoglin or promoting expression of soluble Endoglin.
Owner:TUFTS MEDICAL CENTER INC
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS20140142022A1Extended half-lifeIncrease constraintsNervous disorderPeptide/protein ingredientsCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Cyclic polypeptides for the treatment of heart failure
ActiveUS20150031604A1Extended half-lifeIncrease constraintsNervous disorderAntibody mimetics/scaffoldsCardiac fibrosisVentricular tachycardia
The invention provides a cyclic polypeptide of Formula I (SEQ ID NO: 1):X1-R-X3-X4-L-S-X7-X8-X9-X10-X11-X12-X13 Ior an amide, an ester or a salt thereof, or a bioconjugate thereof, wherein X1, X3, X4, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention or bioconjugates thereof, and their therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
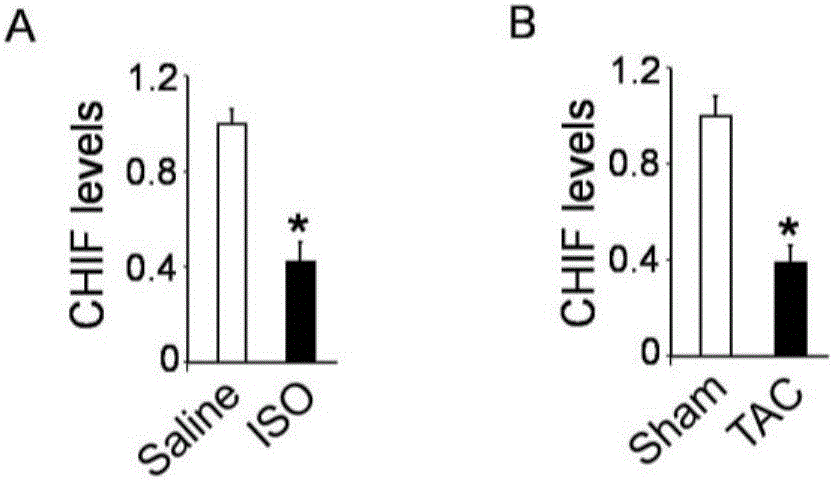
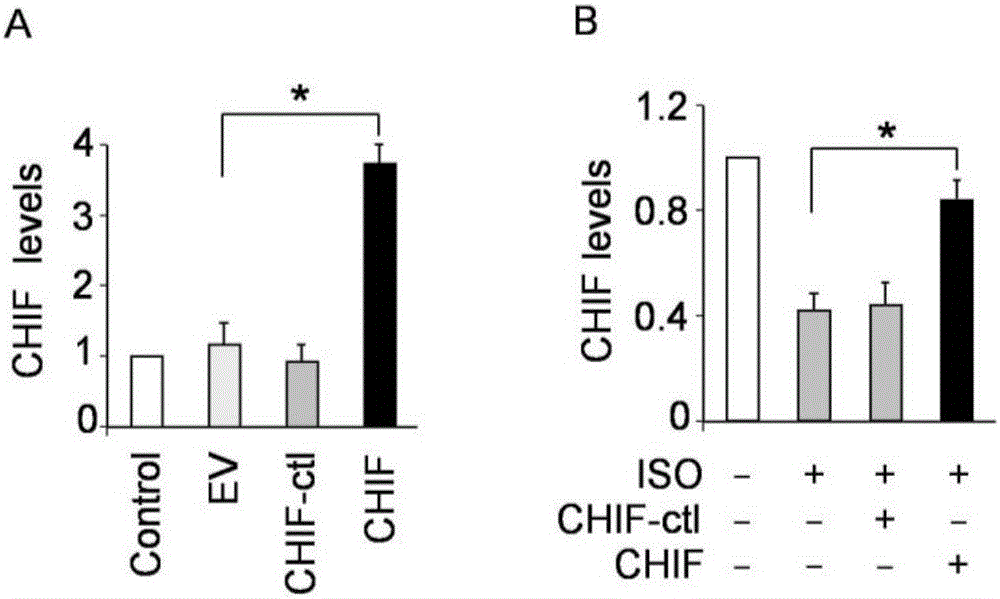
CircRNA CHIF nucleotide and pharmaceutical composition comprising such nucleotide and application thereof
ActiveCN106222174AReasonable formulaPharmacologically reliableOrganic active ingredientsCardiovascular disorderCardiac fibrosisCholesterol
The invention discloses circRNA CHIF nucleotide and a pharmaceutical composition comprising the circRNA CHIF nucleotide. The pharmaceutical composition comprises the circRNA CHIF nucleotide, an auxiliary, and a viral vector or an embedding vector, the embedding vector is cholesterol, nanoparticles or lipidosome, preferably lipidosome, the viral vector is one or more of adenovirus vector, lentiviral vector and retroviral vector, preferably adenovirus vector, and the auxiliary is one or more of mannitol, phosphate buffer solution and normal saline, preferably phosphate buffer solution, the circRNA MNCR viral vector is 1016 PFU in infectivity titer, and a mass ratio of the circRNA CHIF nucleotide to the lipidosome is 1:1.25; a mass ratio of the viral vector or embedding vector to the auxiliaries is 1:200. The pharmaceutical composition is used for preventing and treating cardiac hypertrophy, cardiac fibrosis, coronary heart disease and heart failure, has reasonable formula, is simple to manufacture, has significant therapeutic effect, is widely applicable and is friendly to service environments.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
RNA(Ribonucleic Acid)and application thereof in diseases of cardiovascular system
InactiveCN103114087APrevent proliferationPromote proliferationGenetic material ingredientsViruses/bacteriophagesDiseaseCardiac fibrosis
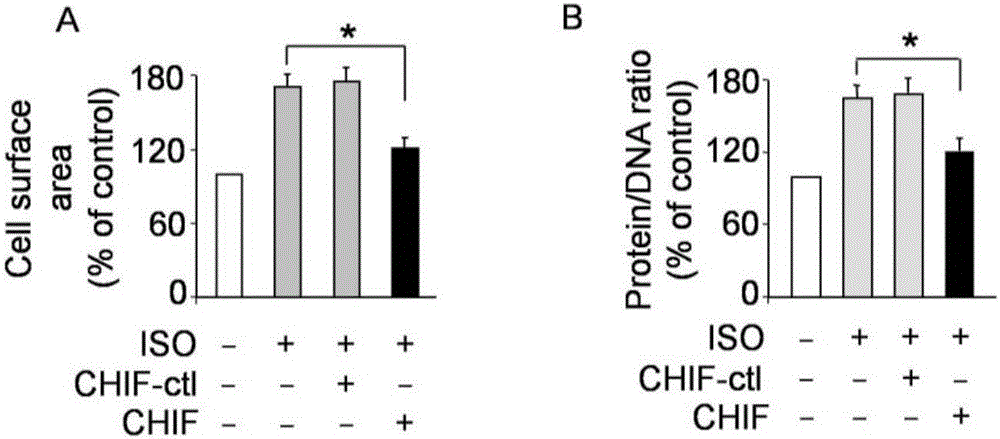
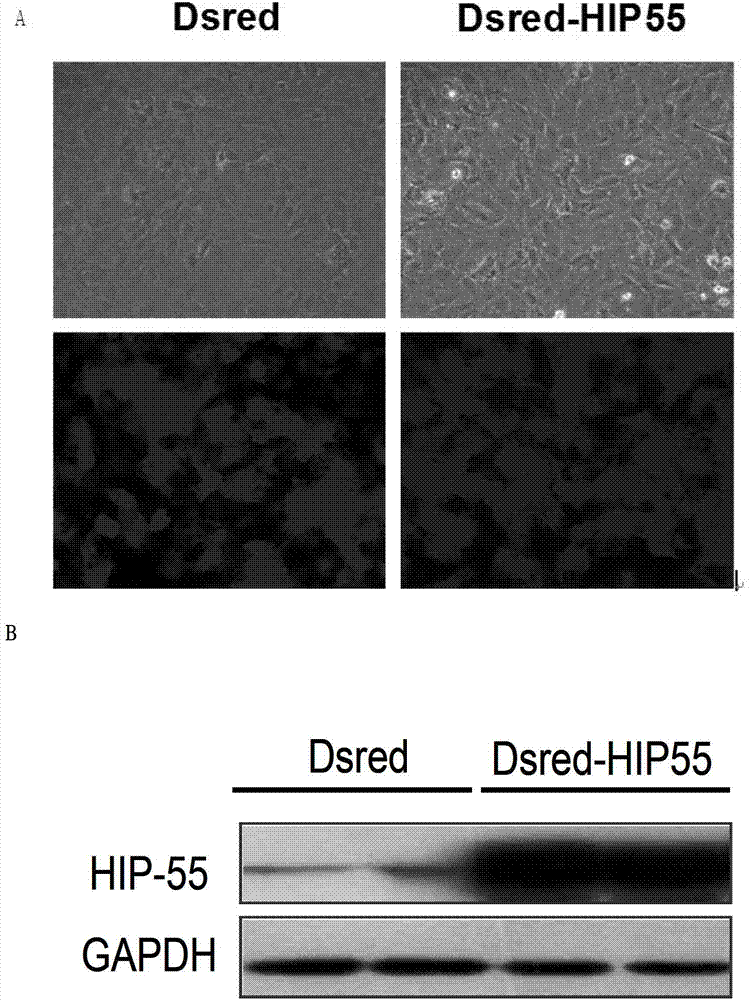
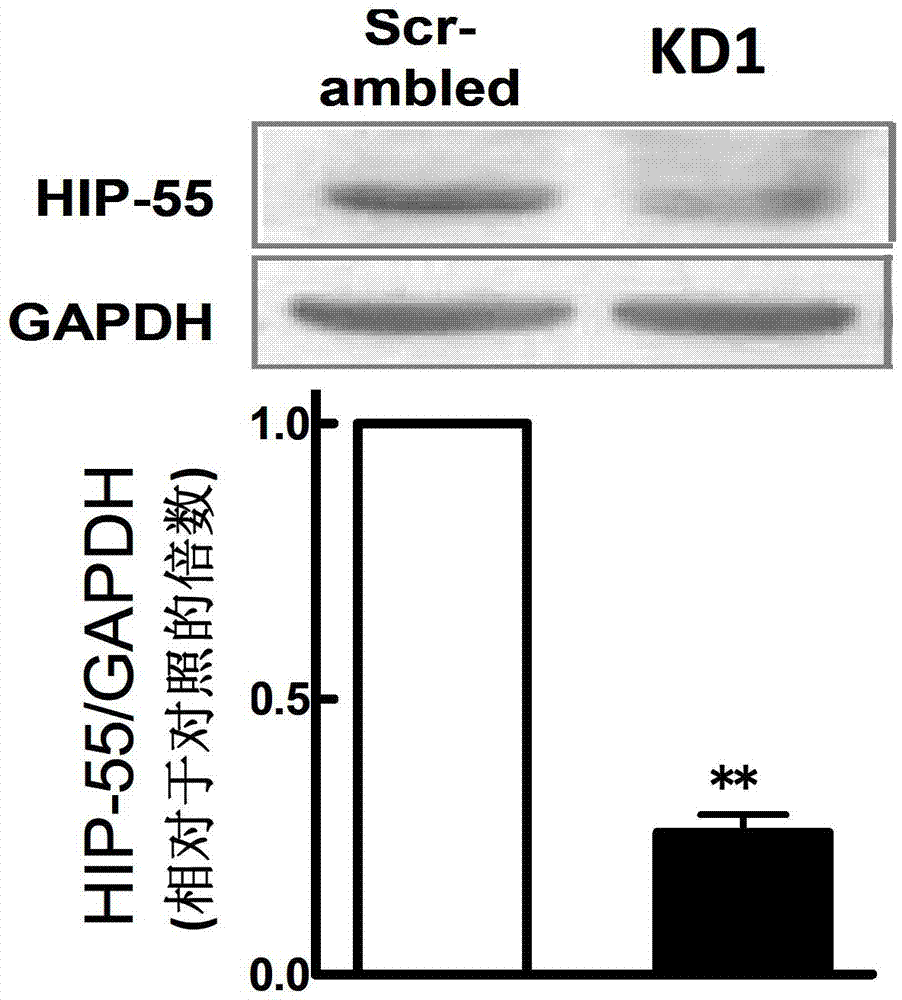
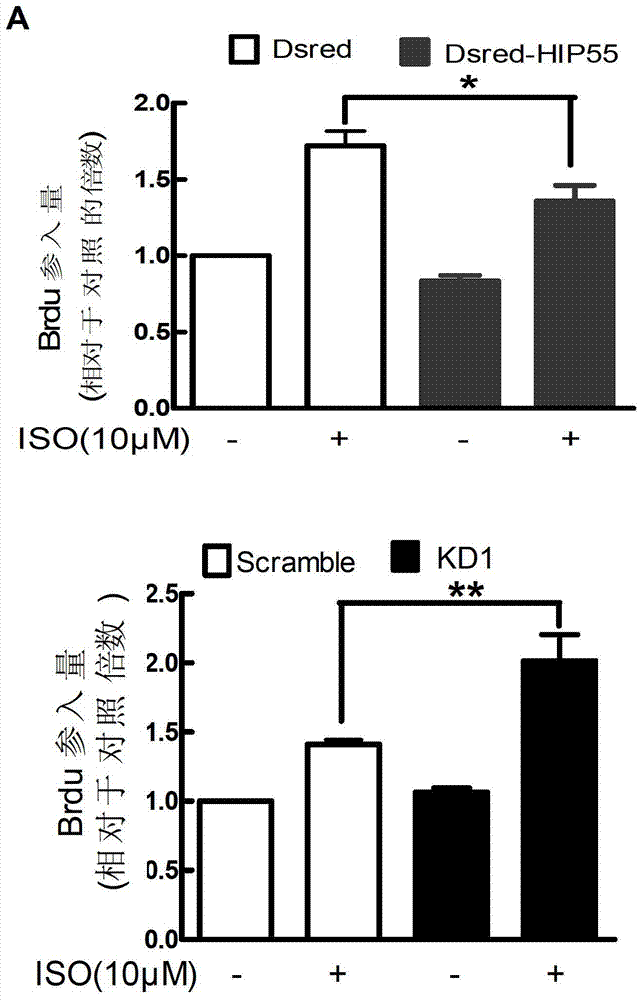
The invention discloses RNA and application of RNA in diseases of a cardiovascular system. According to RNA, the nucleotide sequence of RNA is a sequence 3 in a sequence table. The invention further provides a gene,encoding RNA. The nucleotide sequence of the gene of RNA is a sequence 4 in the sequence table. The experiment of the invention shows that RNA and the application of RNA in the diseases of the cardiovascular system find that by an induction of isoproterenol (ISO), RNAi method Knock down IHP-55 is used in neonatal rat cardiac fibroblasts to obviously promote proliferation of cardiac fibroblasts; ISO induced activation and kinase activity of P38MAPK can be enhanced in the Knock-down HIP-55 cardiac fibroblasts; and HIP-55 is a new protein which has a protective effect on cardiac fibrosis, and provides a new target for clinical diagnosis, treatment and new medicament development.
Owner:PEKING UNIV THIRD HOSPITAL
Cardiac wall tension relief device and method
Owner:DIAXAMED LLC
Method to treat cardiofibrosis with a combination therapy of an angiotensin II antagonist and epoxymexrenone
A therapeutic method is described for treating cardiofibrosis or cardiac hypertrophy using a combination therapy comprising a therapeutically-effective amount of an angiotensin II receptor antagonist and a therapeutically-effective amount of expoxymexrenone.
Owner:GD SEARLE & CO
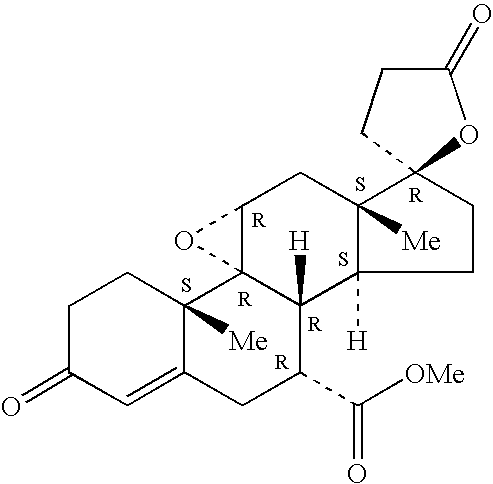
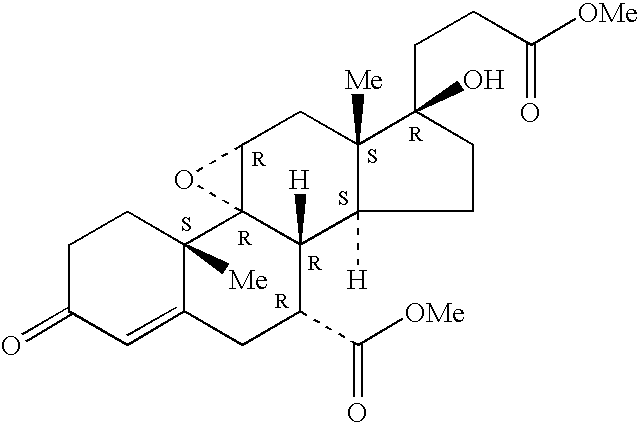
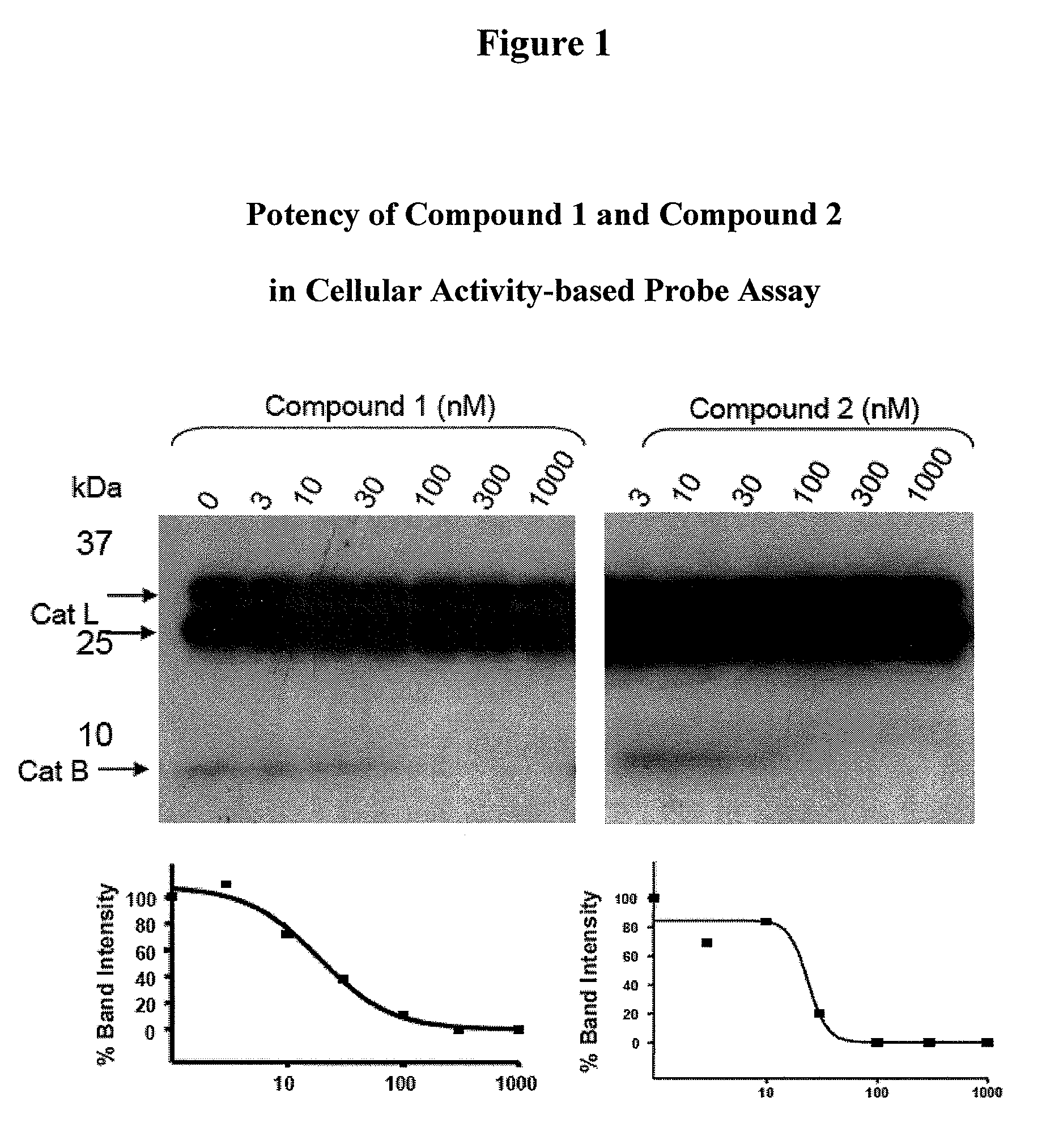
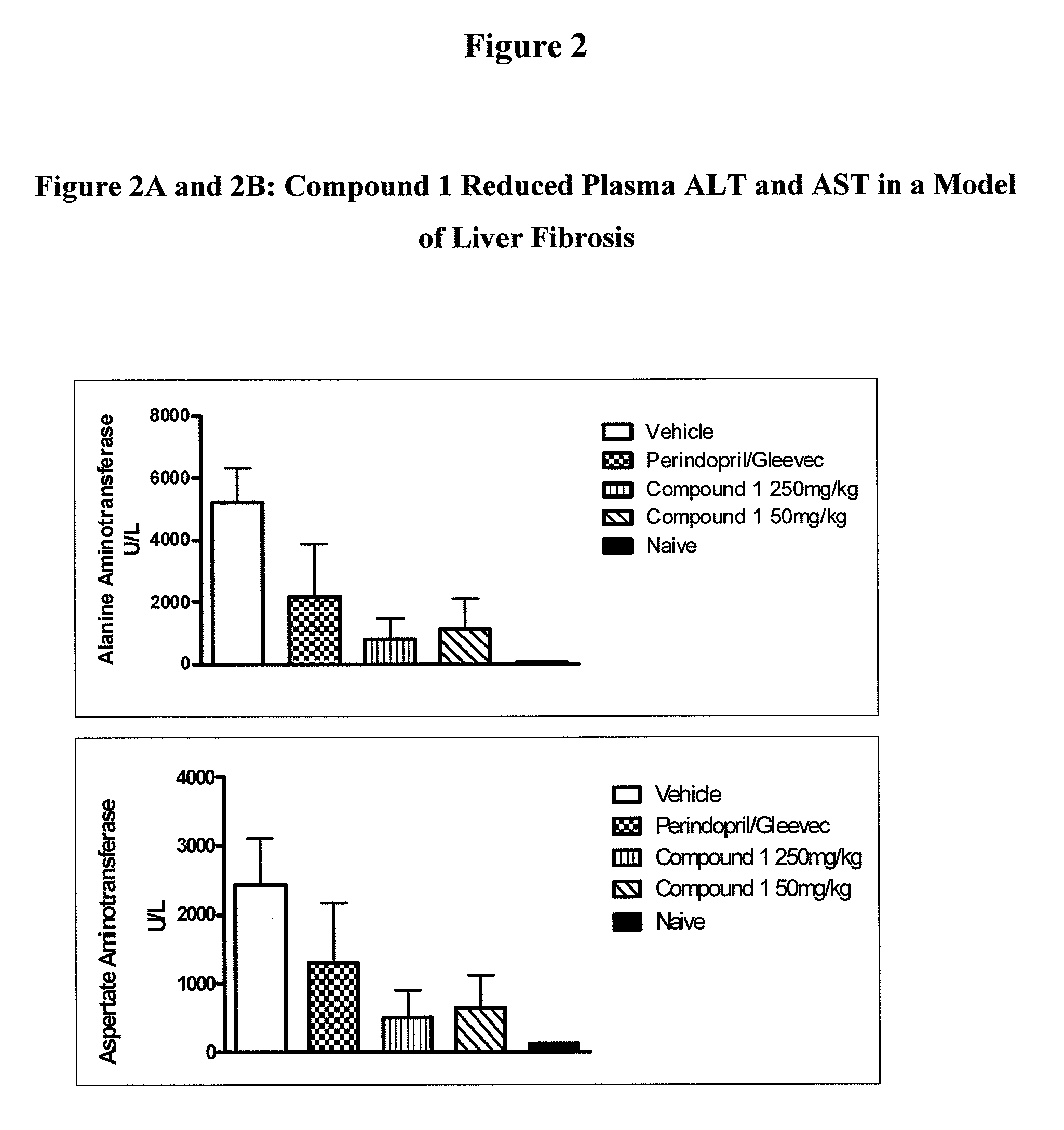
Inhibitors of cathepsin B
Owner:VIROBAY INC
Cyclic polypeptides for the treatment of heart failure
ActiveUS9266925B2Extended half-lifeIncrease constraintsAntibody mimetics/scaffoldsSerum albuminCardiac fibrosisVentricular tachycardia
The invention provides a cyclic polypeptide of Formula I (SEQ ID NO: 1):X1-R-X3-X4-L-S-X7-X8-X9-X10-X11-X12-X13 Ior an amide, an ester or a salt thereof, or a bioconjugate thereof, wherein X1, X3, X4, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention or bioconjugates thereof, and their therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Application of benzimidazole derivatives in preparation of medicines for treating congestive heart failure
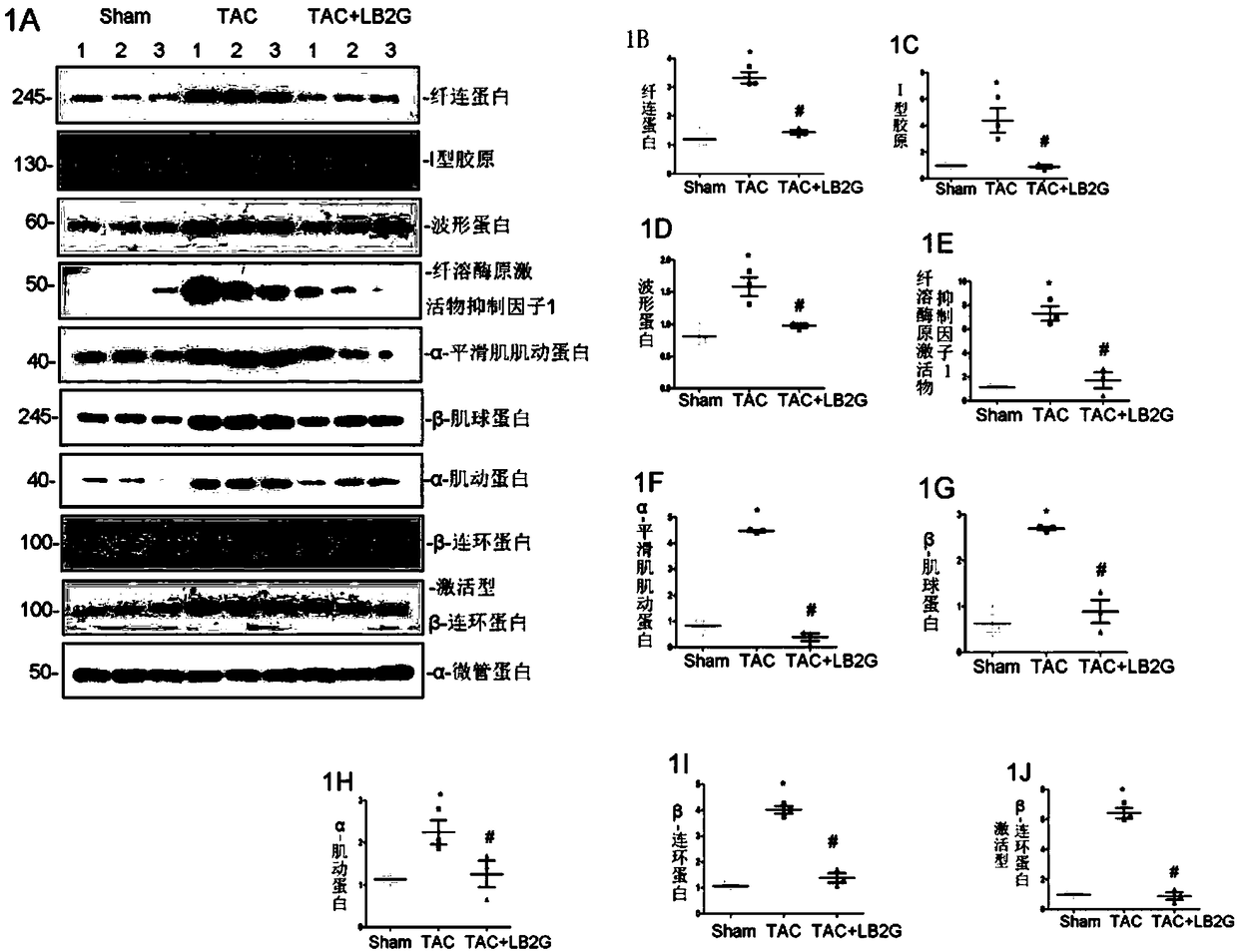
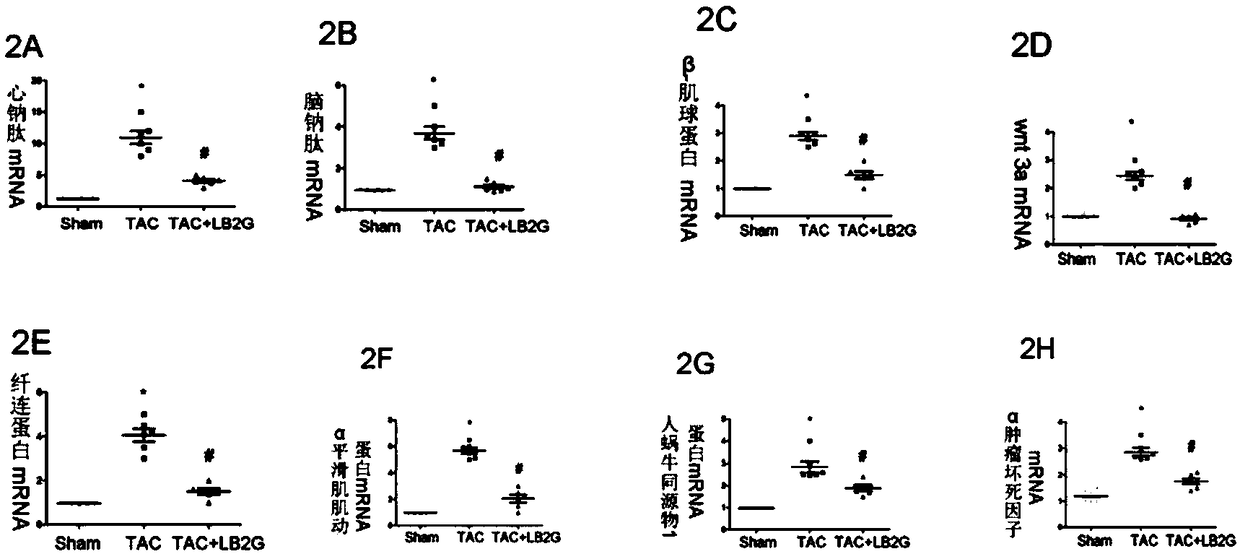
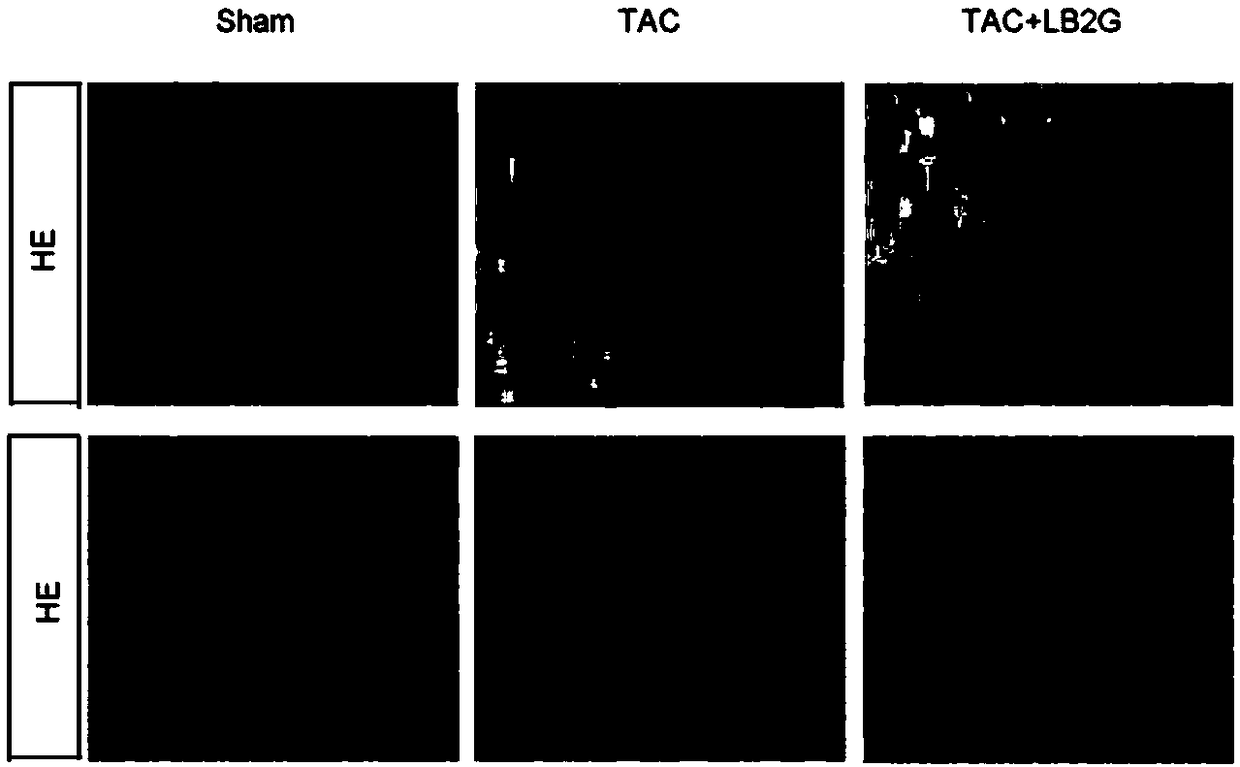
ActiveCN109331014ARetain activityGood water solubilityOrganic active ingredientsCardiovascular disorderCardiac fibrosisBenzimidazole derivative
The invention relates to LB2G serving as a novel and effective drug for blocking an in-vivo key signaling pathway for promoting cardiac hypertrophy and fibrosis so as to inhibit cardiac hypertrophy and cardiac fibrosis in the heart failure progression process and delay and / or reverse the disease course of the heart failure. The invention provides application of the LB2G in treatment of heart failure. The LB2G has obvious curative effects, and does not have any obvious toxic or side effect. Therefore, the LB2G disclosed by the invention can be prepared into a pharmaceutical preparation for treating the heart failure.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV +1
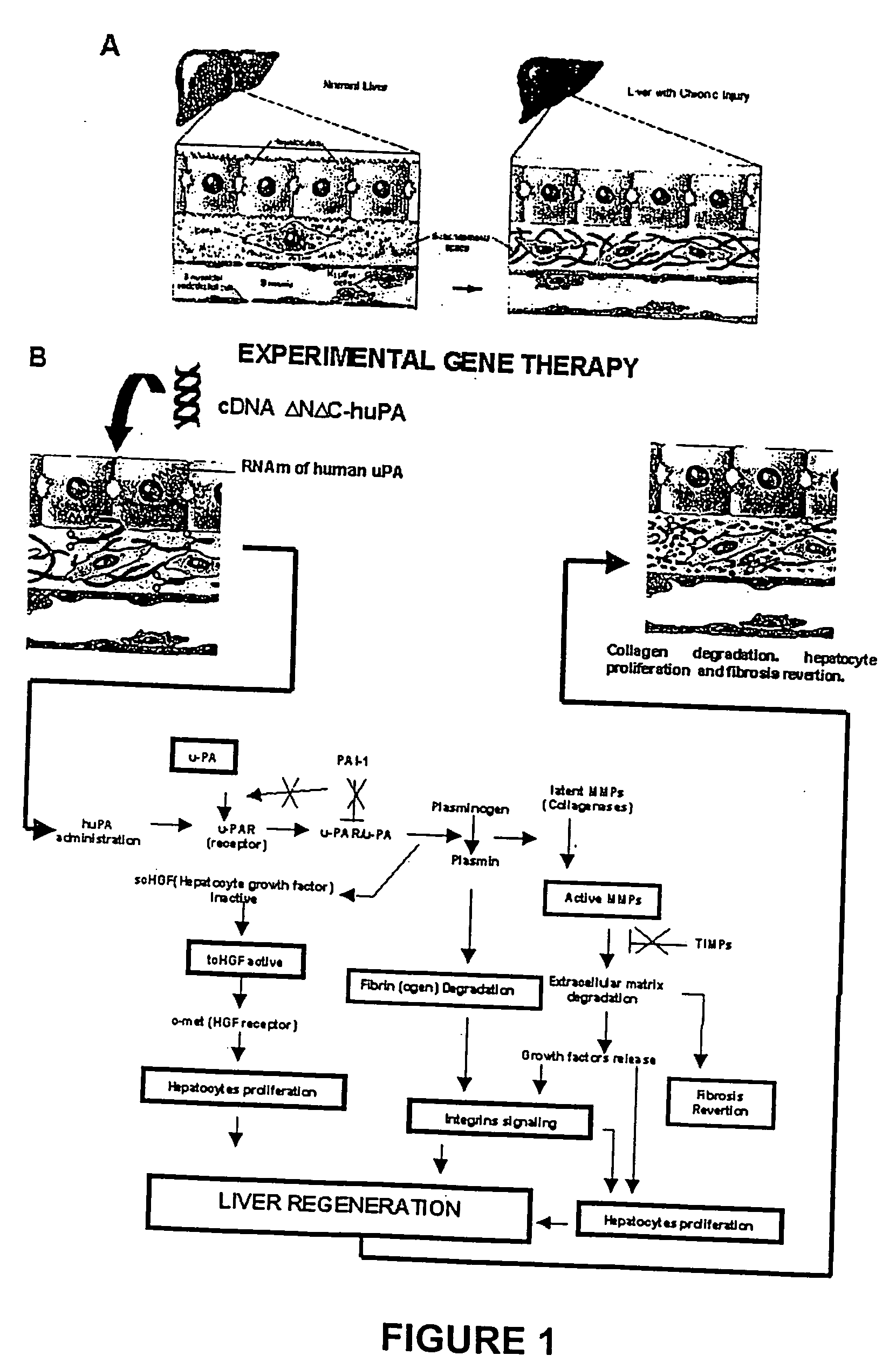
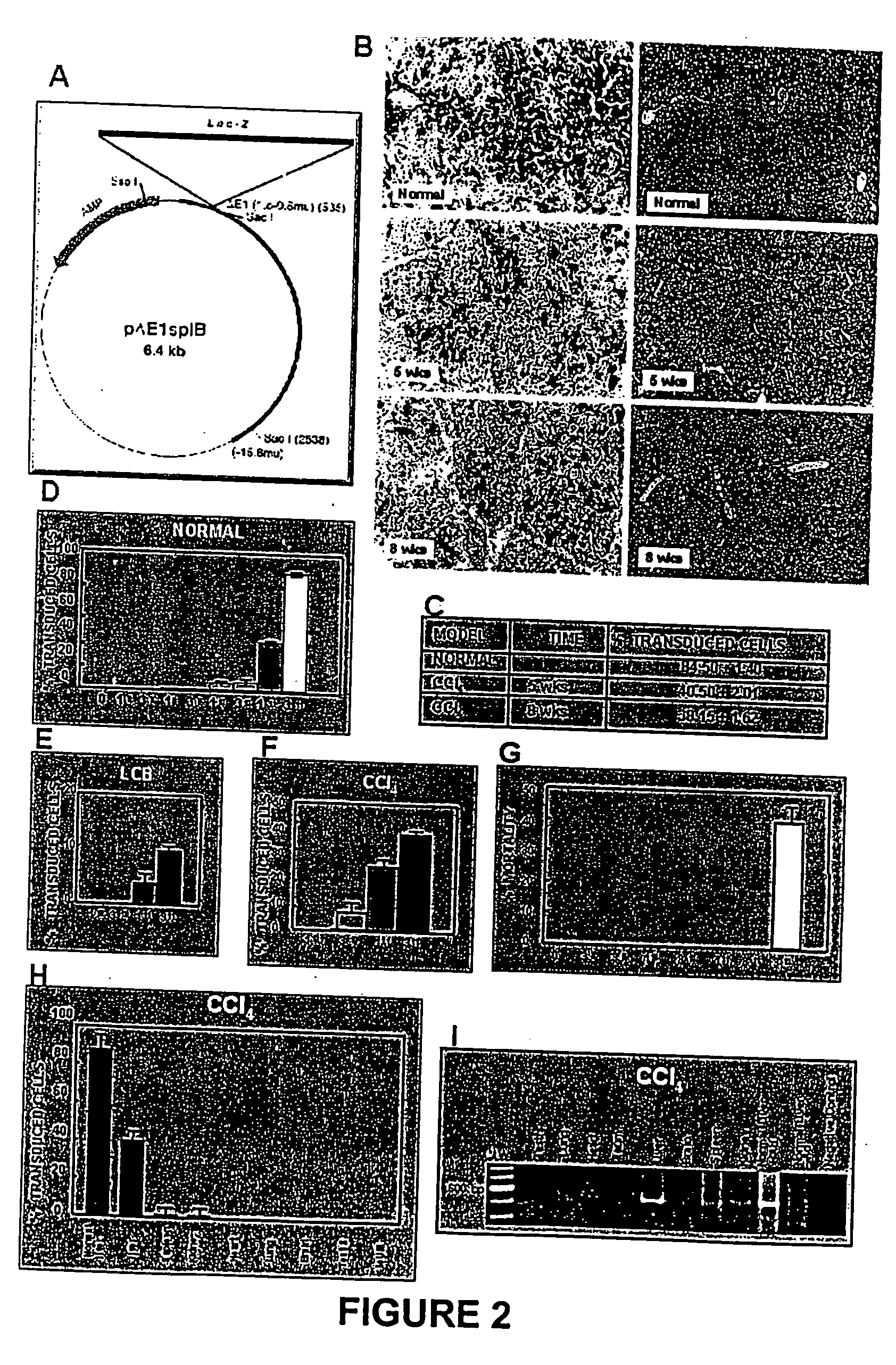
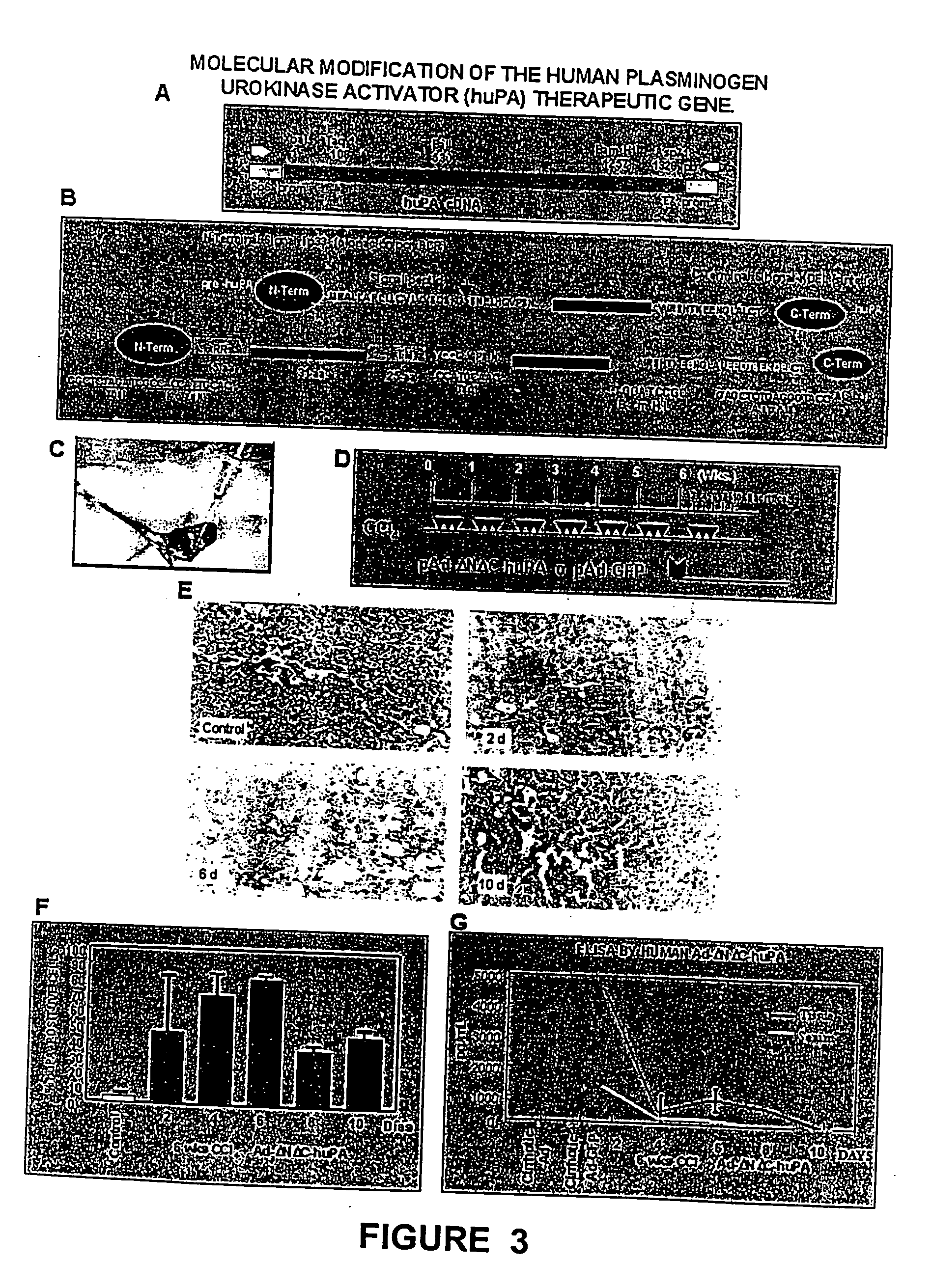
Recombinant viral and non-viral vectors containing the human urokinase plasminogen activator gene and its utilization in the treatment of various types of hepatic, pulmonary, pancreatic and cardiac fibrosis and hypertrophic scars
InactiveUS20040097455A1Reestablishment of liver functionFacilitated DiffusionPeptide/protein ingredientsGenetic material ingredientsCardiac fibrosisCause of death
Hepatic cirrhosis is considered a severe health problem in Mexico, since it is the third mortality cause in working-age people and there is no 100% effective treatment. Cirrhosis is characterized by an exacerbated increase of collagen in liver parenchyma, replacing the hepatocytes and thus provoking liver failure. This is one of the reasons why we have used a gene therapy through specific delivery to cirrhotic livers of the gene of human urokinase plasminogen activator (huPA), which activates mechanisms that induce the degradation of excess cellular matrix and stimulate hepatocyte proliferation, obtaining thus a fast re-establishment of the liver function. In the instant invention, the modified human uPA gene was inserted in the adenoviral vector (pAd-DeltahuPA), because it is not secreted and does not provoke hypercoagulation or spontaneous internal bleedings. Moreover, data from the bio-distribution essay with an adenoviral vector with reporter gene beta-gal have shown liver specificity as the target organ of the vector. Using ELISA, huPa protein was detected in liver homogenates (4500 pg / ml) in animals treated with pAd-DeltahuPA and was also intracellularly detected through immunochemistry in liver cuts (80% positive cells). huPa induced a dramatic fibrosis reduction (85%) on day 10 of vector administration, compared to control cirrhotic rats and 55% hepatocyte proliferation increase. Liver function tests (ALT, AST, alkaline phosphatase and bilirubin) dropped to nearly normal levels and hepatocyte proliferation was observed. Because of the two beneficial event cascades, gene therapy with modified huPA can be developed as a definite potential treatment for patients with liver cirrhosis.
Owner:TGT LAB DE C V
Inhibitors of cathepsin b
The present invention is directed to a method of using compounds of Formula (I) to inhibit Cathepsin B. Specifically the compounds of the present invention are useful as therapeutic agents for the treatment of tumor invasion, metastasis, Alzheimer's Disease, arthritis, inflammatory diseases such as chronic and acute pancreatitis, inflammatory airway disease, and bone and joint disorders, including osteoporosis, osteoarthritis, rheumatoid arthritis, psoriasis, and other autoimmune disorders, liver fibrosis, including liver fibrosis associated with HCV, all types of steatosis (including non-alcoholic steatohepatitis) and alcohol-associated steatohepatitis, non-alcoholic fatty liver disease, forms of pulmonary fibrosis including idiopathic pulmonary fibrosis, pathological diagnosis of interstitial pneumonia following lung biopsy, renal fibrosis, cardiac fibrosis, retinal angiogenesis and fibrosis / gliosis in the eye, schleroderma, and systemic sclerosis. The compounds of Formula (I) are also useful for treating subjects with both HCV and fibrosis in a mammal, particularly liver fibrosis, and subjects affirmatively diagnosed or at risk for both HCV and liver fibrosis.
Owner:VIROBAY INC
MiRNA (micro ribose nucleic acid)-874 and application of miRNA-874antisense nucleotide
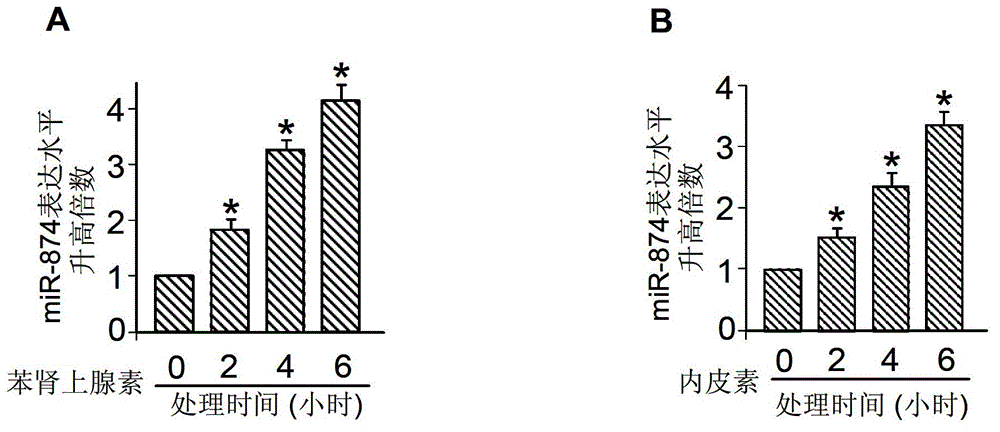
ActiveCN102973953AProtectiveOrganic active ingredientsGenetic material ingredientsCardiac fibrosisDisease
The invention discloses a (micro ribose nucleic acid)-874 and an application of a miRNA-874 antisense nucleotide, and in particular relates to the application of the miRNA-874 antisense nucleotide in preparing pharmaceutical compositions for treating and preventing serious heart diseases (myocardial hypertrophy and myocardial fibrosis) and the application of the miRNA-874 antisense nucleotide in preparing the pharmaceutical compositions for diagnosing or prognosing the heart diseases. The miRNA-874 antisense nucleotide can control cardiac hypertrophy and cardiomyocyte hypertrophy and also has the functions of improving a cardiac function and inhibiting cardiac structure remodeling and cardiac fibering. The miRNA-874 antisense nucleotide can be used as a novel target of drug action, has a function of controlling the myocardial hypertrophy and has an important meaning for clinically preventing and treating heart diseases of cardiac fibering, coronary heat disease, heart failure and the like which are caused by cardiomyocyte hypertrophy.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
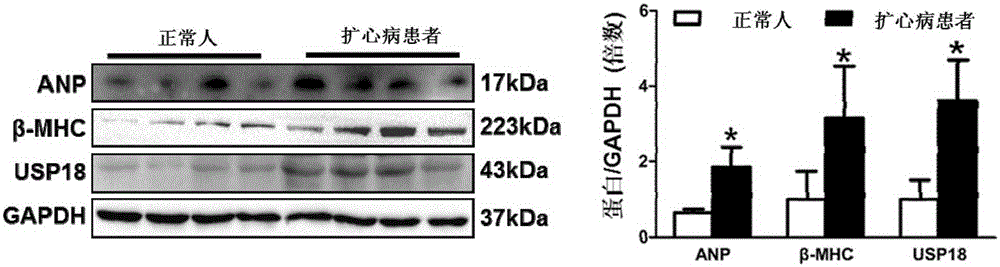
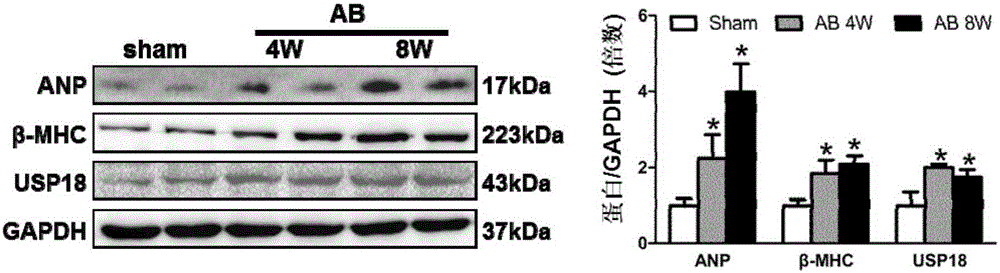
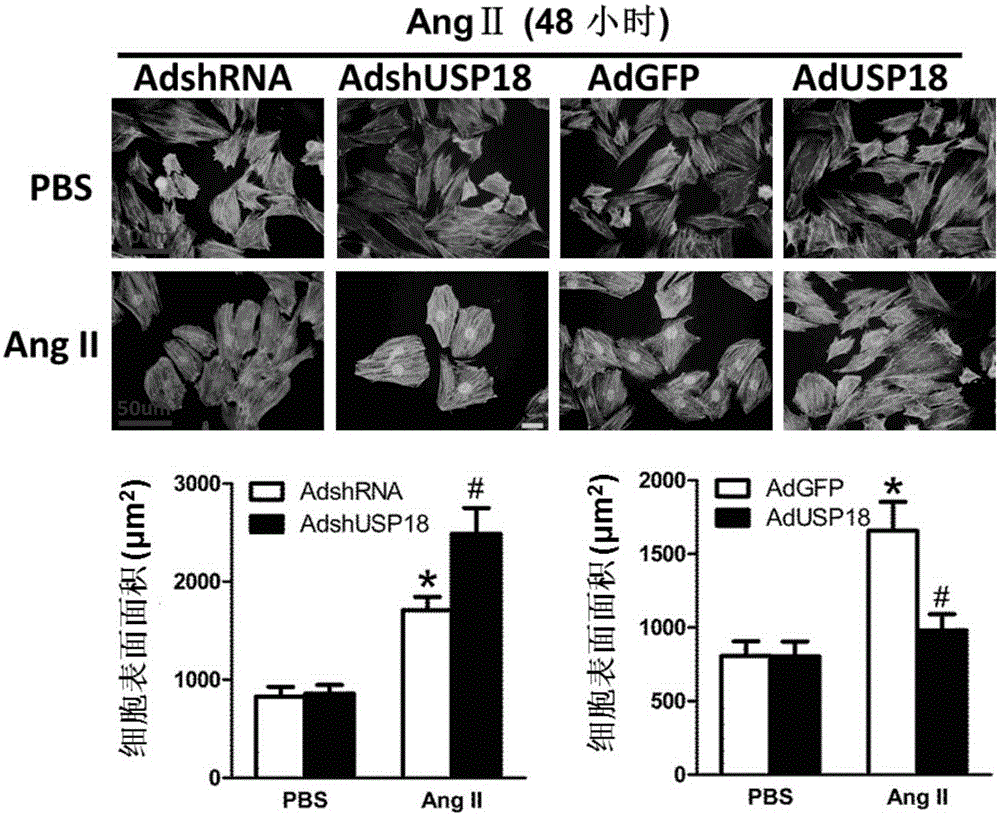
Function and application of ubiquitin-specific protease 18 (USP18) on treatment of cardiac hypertrophy
ActiveCN105194660AProtect heart functionProtects Against Cardiac FibrosisPeptide/protein ingredientsBiological testingCardiac fibrosisCardiac functioning
The invention discloses a function and application of ubiquitin-specific protease 18 (USP18) on treatment of cardiac hypertrophy, and belongs to the field of functions and application of genes. A mutual relation between USP18 expression and the cardiac hypertrophy is determined, and a research result shows that in a model suffering from the cardiac hypertrophy, USP18 expression is obviously lower than that of a normal group; and cardiac hypertrophy and fibrosis are promoted and a cardiac function is worsened by inhibiting USP18 expression, and cardiac hypertrophy and fibrosis are obviously inhibited and the cardiac function is protected by promoting USP18 overexpression. Therefore, the USP18 can serve as a target gene, is used for screening medicines for protecting the cardiac function, resisting cardiac fibrosis and / or preventing and relieving and / or treating cardiac hypertrophy, and is used for preparing medicines for protecting the cardiac function, resisting cardiac hypertrophy and / or preventing and relieving and / or treating cardiac hypertrophy, and a new effective path is provided for treatment of cardiac hypertrophy cardiac hypertrophy.
Owner:WUHAN UNIV
Synthetic linear apelin mimetics for the treatment of heart failure
InactiveUS8921307B2Extended half-lifeIncrease constraintsPeptide/protein ingredientsMetabolism disorderCardiac fibrosisVentricular tachycardia
Owner:NOVARTIS AG
Application of fucoidan polysaccharide sulfate in preparing drug for preventing and/or treating diabetic cardiomyopathy
ActiveCN103230411ALittle side effectsIncreased systolic functionOrganic active ingredientsMetabolism disorderCardiac fibrosisAntioxidative enzyme
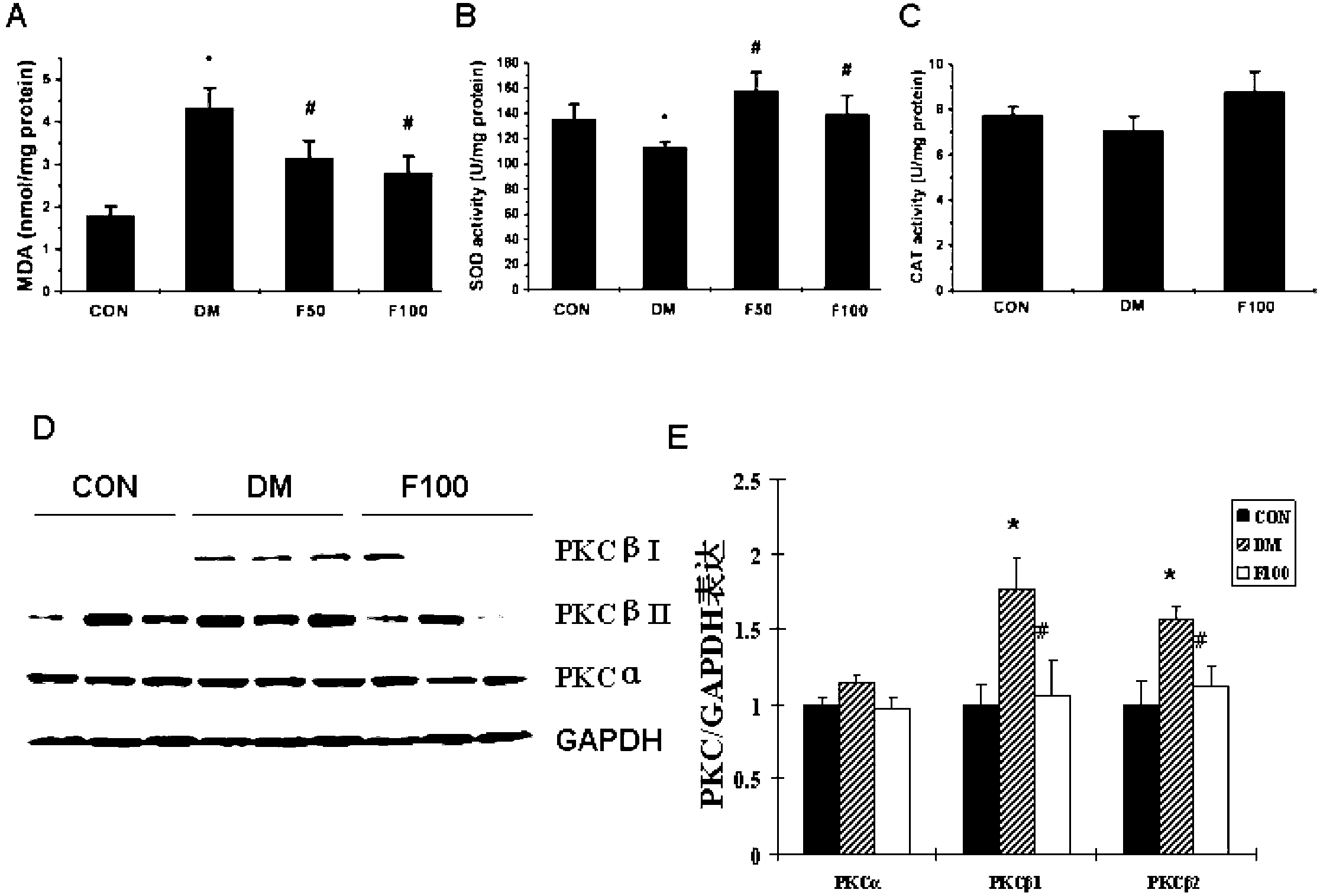
The invention discloses a drug novel use of fucoidan polysaccharide sulfate. The use is the application of the fucoidan polysaccharide sulfate in preparing the following products: (1), a product for preventing and / or treating diabetic cardiomyopathy; (2), a product for inhibiting diabetic myocardial oxidative stress; and (3), a product for inhibiting expression and activation of PKC (Protein Kinase C) beta in diabetic cardiomyopathy; and (4), a product for relieving cardiac interstitial fibrosis of a diabetic. The fucoidan polysaccharide sulfate is preferably LMWF (Fucoidan Polysaccharide Sulfate) with weight-average molecular weight of 3-30KD. According to the pharmacodynamic experiment, the LMWF can be used for generating beneficial interference to the development of pathogenetic condition of the diabetic cardiomyopathy by strengthening the myocardial systolic function and relieving cardiac fibrosis. More importantly, the LMWF can be used for effectively inhibiting oxidative stress by regulating the activity of antioxidase, and can be used for inhibiting the expression of protein kinase C beta (PKCbeta) subtype. The LMWF is from the natural existing kelp, so that the availability and safety of the kelp are beneficial to using the fucoidan polysaccharide sulfate as a potential clinical treatment drug and preventing the diabetic cardiomyopathy.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Methods and compositions for controlling cardiac fibrosis and remodeling
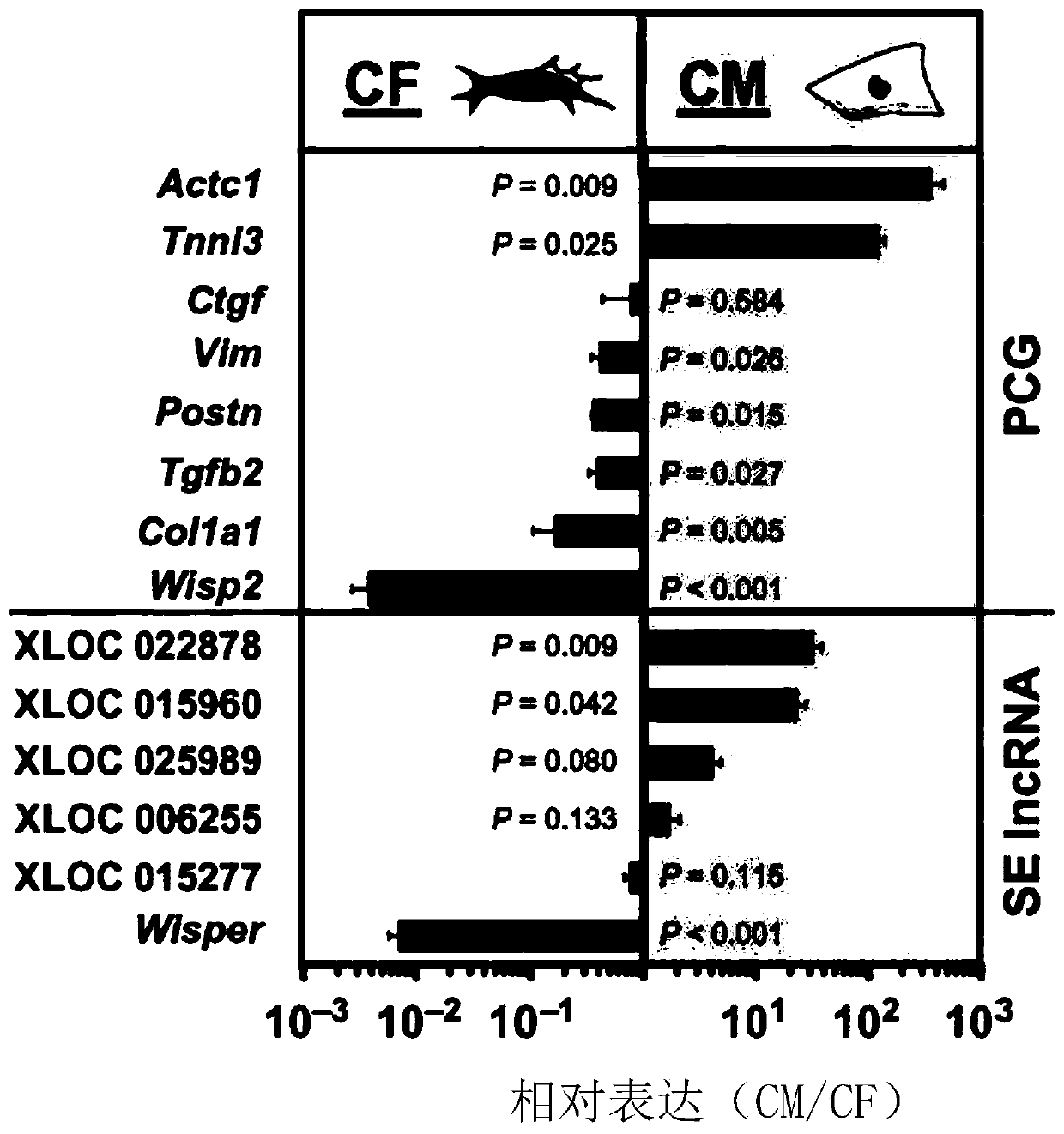
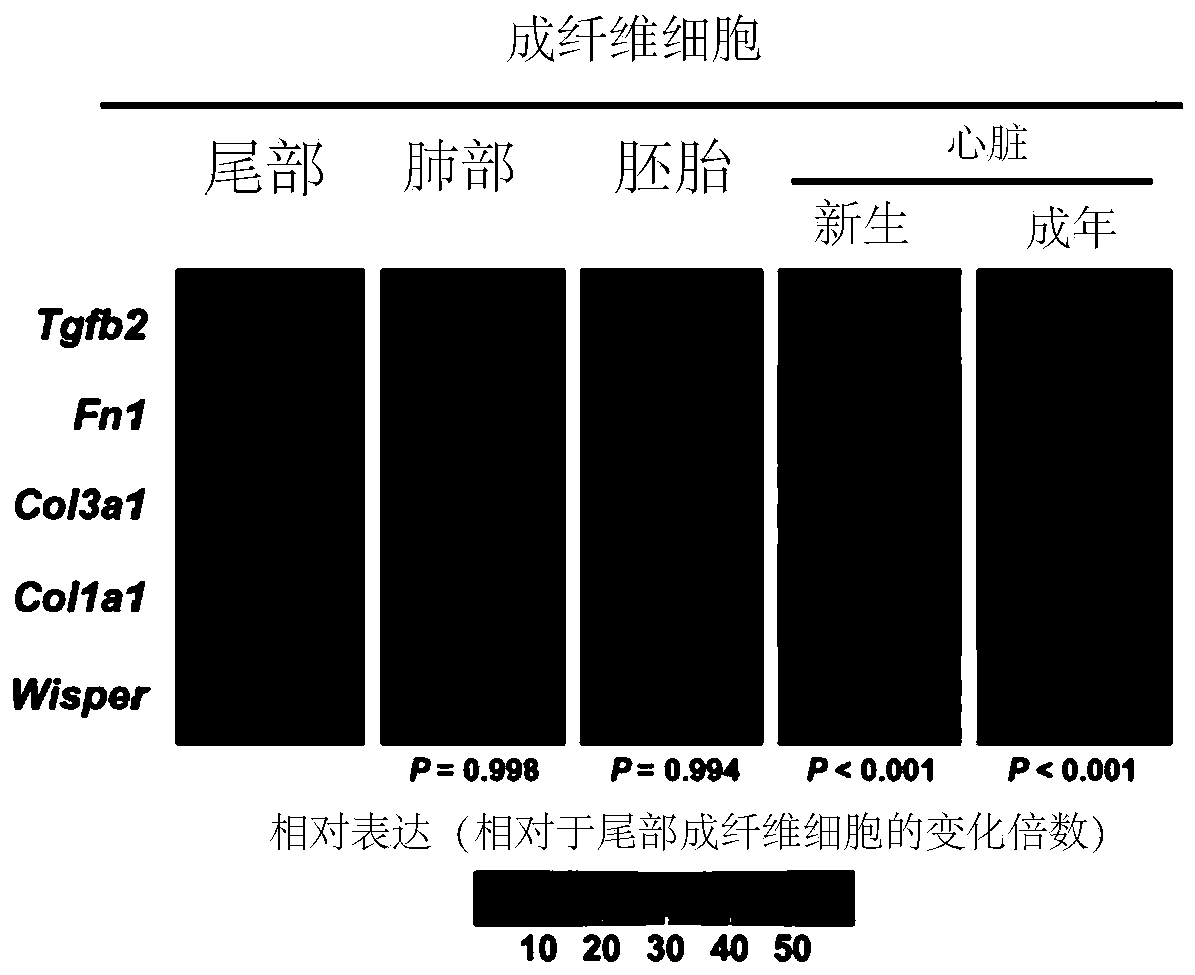
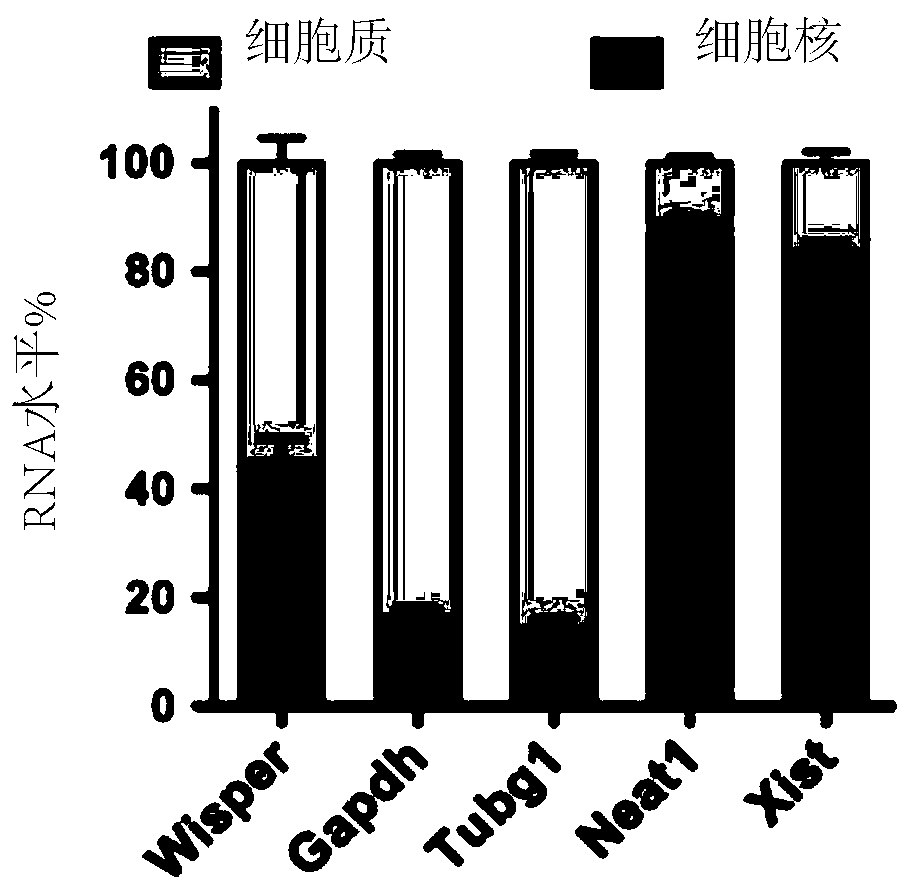
The present invention provides methods and compositions for controlling cardiac fibrosis and heart remodeling in a subject via modulating the expression of one or more lncRNA associated with cardiac-specific super-enhancers (SEs).
Owner:UNIVERSITY OF LAUSANNE
Compositions and methods of treating cardiac fibrosis with ifetroban
ActiveUS9693998B2Preventing and treating and attenuating cardiac fibrosisReduce impactOrganic active ingredientsOrganic chemistryCardiac fibrosisDefibrination syndrome
The present invention is directed to methods of treating, preventing, and / or ameliorating fibrosis syndrome, and in particular cardiac fibrosis, by administration of a therapeutically effective amount of ifetroban, or a pharmaceutically acceptable salt thereof.
Owner:CUMBERLAND PHARM INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com