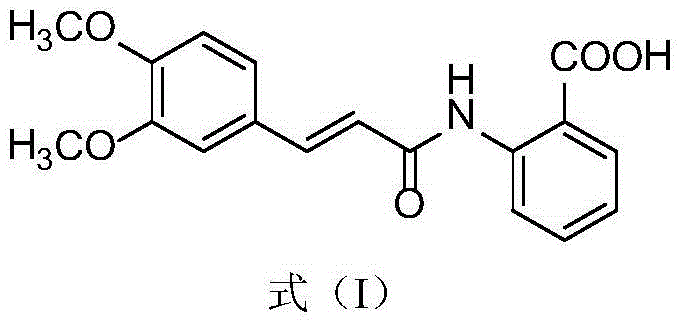
Synthesis method of tranilast
A synthetic method, the technology of special methyl ester, which is applied in the field of chemical synthesis and can solve the problems of high cost and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
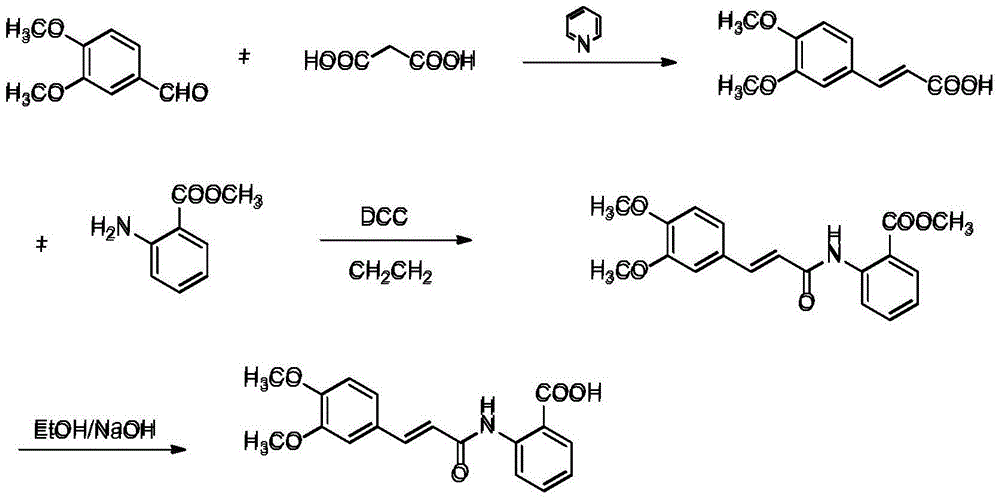
[0032] Tranilast production method of the present invention is as follows:
[0033] raw material:
[0034]
[0035] resolve resolution:
[0036] 1. Preparation of 3,4-dimethoxycinnamic acid: Weigh 117g of malonic acid and dissolve it in 200ml of dry pyridine. After stirring evenly, add 125g of 3,4-dimethoxybenzaldehyde and heat up to 90°C , react for 2 to 3 hours, after the reaction, cool slightly, pour the reaction liquid into 500ml of ice water, adjust the pH to 2 to 3 with concentrated hydrochloric acid, a large amount of solid precipitates, let stand, filter with suction, wash the filter cake with ice water until the filtrate The pH was 5-6 and dried to obtain 146 g of solid.
[0037] 2. Preparation of tranilast methyl ester: Weigh 146g of 3,4-dimethoxycinnamic acid and dissolve it in 700ml of dichloromethane, add 158g of DCC under stirring, cool the reaction solution to 0-5°C, and add in batches 105g of methyl anthranilate, stirred and reacted for 1h, reacted at roo...
Embodiment 2
[0040] raw material:
[0041]
[0042] Synthetic method is identical with example 1.
[0043] 1. Preparation of 3,4-dimethoxycinnamic acid: Weigh 234g of malonic acid and dissolve it in 470ml of dry pyridine. After stirring evenly, add 250g of 3,4-dimethoxybenzaldehyde and heat up to 90°C , react for 2 to 3 hours, after the reaction, cool slightly, pour the reaction solution into 800ml ice water, adjust the pH to 2 to 3 with concentrated hydrochloric acid, a large amount of solid precipitates, let stand, filter with suction, wash the filter cake with ice water until the filtrate The pH was 5-6 and dried to obtain 280 g of solid.
[0044] 2. Preparation of tranilast methyl ester: Weigh 280g of 3,4-dimethoxycinnamic acid and dissolve it in 1.4L of dichloromethane, add 305g of DCC under stirring, cool the reaction solution to 0-5°C, batch Add 205g of methyl anthranilate, stir and react for 1h, react at room temperature for 5-6h, filter to remove DCU, remove dichloromethane f...
Embodiment 3
[0047] raw material:
[0048]
[0049] In the synthetic method, the solvent in step 1 is changed to toluene, and the others are the same as in Example 1.
[0050] 1. Preparation of 3,4-dimethoxycinnamic acid: Weigh 150g of malonic acid and 780ml of pyridine and dissolve them in 800ml of dry toluene. After stirring evenly, add 160g of 3,4-dimethoxybenzaldehyde and heat up to 90°C, react for 7-8 hours, after the reaction, cool slightly, pour the reaction solution into 1.5L of ice water, adjust the pH to 2-3 with concentrated hydrochloric acid, a large amount of solid precipitates, let stand, filter with ice, and filter the cake with ice Wash with water until the pH of the filtrate is 5-6, and dry to obtain 150 g of solid.
[0051] 2. Preparation of tranilast methyl ester: Weigh 150g of 3,4-dimethoxycinnamic acid and dissolve in 720ml of dichloromethane, add 163g of DCC under stirring, cool the reaction solution to 0-5°C, and add in batches 109g of methyl anthranilate, stirr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com