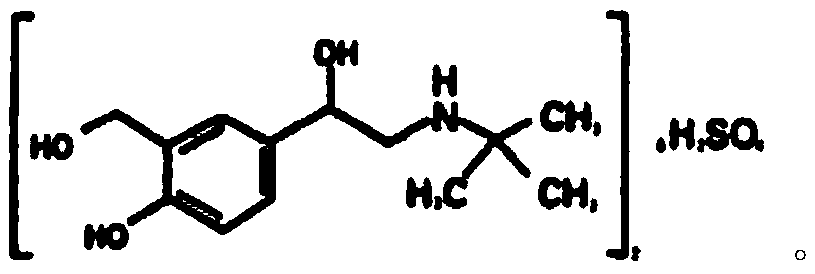
Salbutamol sulfate oral cavity disintegration tablets and preparation method thereof
A technology of albuterol sulfate and orally disintegrating tablets, which is applied in pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as hidden dangers of stability, increase of impurities in preparations, and reduction of product quality assurance. Improve particle fluidity and compressibility, facilitate commercial production, and avoid potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
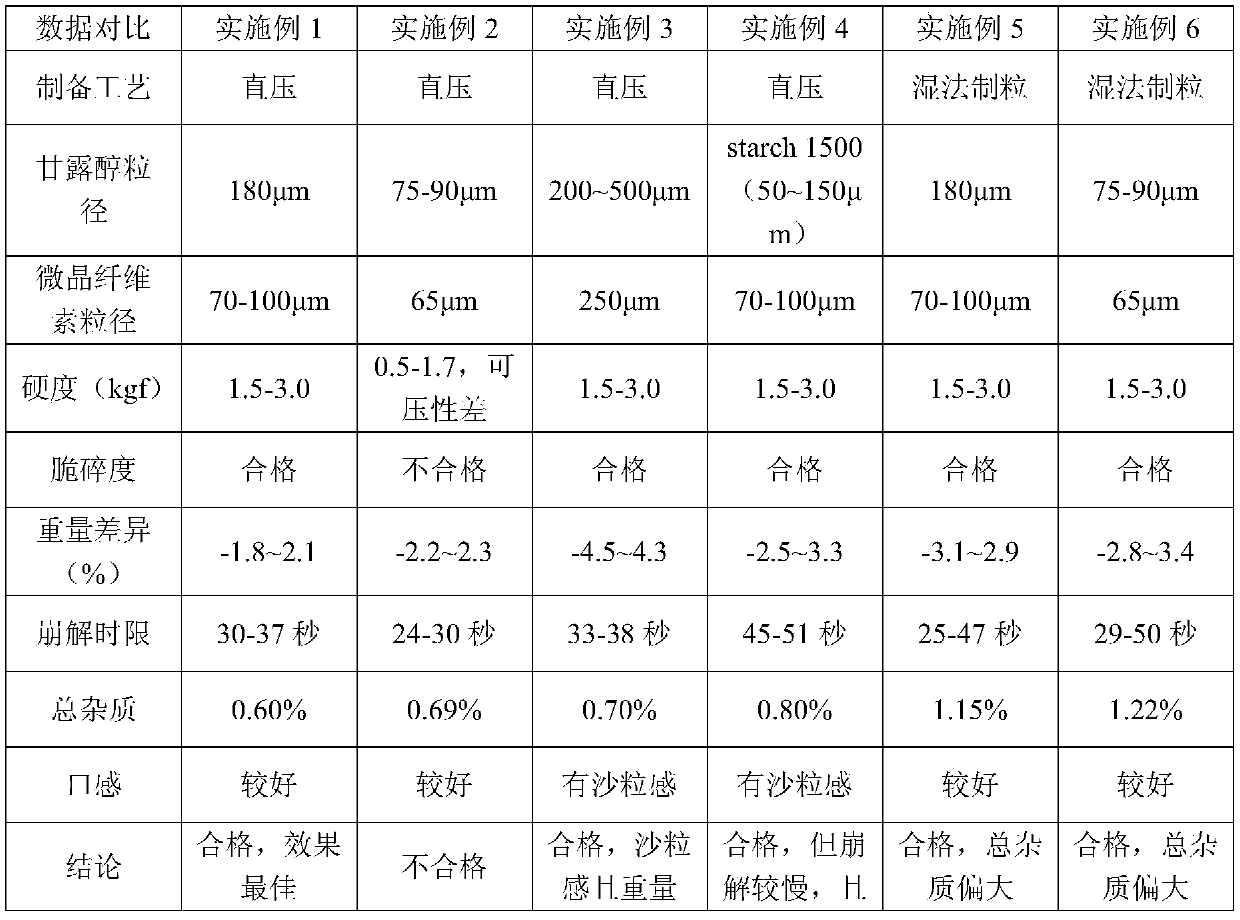
[0093] A salbutamol sulfate orally disintegrating tablet, wherein the distribution ratio of each component is calculated by weight percentage: salbutamol sulfate 1%, direct compression mannitol 50%, microcrystalline cellulose 35%, low-substituted hydroxypropyl cellulose 4%, cross-linked Povidone 8%, Magnesium Stearate 1%, Silicon Dioxide 1%.
[0094] Directly compressed mannitol was Pearlitol 200SD (average particle size 180 μm) produced by Roquette, and microcrystalline cellulose was PH112 (70-100 μm) from Luxi, Liaocheng, Shandong.
[0095] The preparation method of above-mentioned salbutamol sulfate orally disintegrating tablet, its step is as follows:
[0096] Mix salbutamol sulfate, direct pressure mannitol, microcrystalline cellulose, magnesium stearate, silicon dioxide, crospovidone and low-substituted hydroxypropyl cellulose in a three-dimensional mixer according to the above ratio; use DP30A Tablets are compressed by a single-punch tablet machine, the hardness is con...
Embodiment 2
[0099] A salbutamol sulfate orally disintegrating tablet, wherein the distribution ratio of each component is: salbutamol sulfate 1%, mannitol 50%, microcrystalline cellulose 35%, low-substituted hydroxypropyl cellulose 4%, crospovidone 8% , magnesium stearate 1%, silicon dioxide 1%.
[0100] Mannitol is common mannitol (75-90 μm) produced in Nanning, Guangxi, and microcrystalline cellulose is ZW-301 (average particle size: 65 μm) produced by Huzhou Zhanwang.
[0101] The preparation method of above-mentioned salbutamol sulfate orally disintegrating tablet, its step is as follows:
[0102] Mix albuterol sulfate, common mannitol, microcrystalline cellulose, magnesium stearate, silicon dioxide, crospovidone and low-substituted hydroxypropyl cellulose in a three-dimensional mixer according to the above proportion; press into tablets.
[0103] Above-mentioned formula prepares 1000 tablets altogether. The measured tablet hardness is 0.5-1.7kgf, the compressibility is poor when tabl...
Embodiment 3
[0105] A salbutamol sulfate orally disintegrating tablet, similar to Example 1, the difference is that the particle size of the filler is larger, wherein the distribution ratio of each component is calculated as: salbutamol sulfate 1%, mannitol 50%, microcrystalline fiber Vitamin 35%, low-substituted hydroxypropyl cellulose 4%, crospovidone 8%, magnesium stearate 1%, silicon dioxide 1%.
[0106] In this embodiment, mannitol is Pearlitol 300DC (particle size 200-500 μm) and microcrystalline cellulose (average particle size 250 μm) produced by Roquette.
[0107] The preparation method of above-mentioned salbutamol sulfate orally disintegrating tablet, its step is as follows:
[0108] Mix salbutamol sulfate, direct pressure mannitol, microcrystalline cellulose, magnesium stearate, silicon dioxide, crospovidone and low-substituted hydroxypropyl cellulose in a three-dimensional mixer according to the above ratio; Tablets are pressed by a punching machine, the hardness is controlle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com