Alendronate sodium powder inhalation used for respiratory drug delivery and preparation method and application thereof
A technology of alendronate sodium and powder spray, applied in the field of alendronate sodium powder spray, can solve the problems of low efficiency, increased risk of side effects such as kidney toxicity, etc., and achieves simple production and preparation process, prevention and safety. The effect of overcoming side effects and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196] Get 1000g of alendronate sodium crude drug, and load it into the QS100 pulverizer. The pulverization condition is 0.7MPa pulverization pressure, 3 hours, pulverization 4 times, obtain alendronate sodium fine powder.
[0197] Determination of alendronate sodium content, with 6mg / capsule sub-packed in hard capsules to get final product. Laser particle size measurement results: British Malvern Instruments Ltd. laser particle size analyzers measured the particle size of capsule content powders of different batches. As a result, the percentage of the above-mentioned samples with a particle size of less than 20 μm was greater than 90%, while the percentage of the portion with a particle size of less than 5 μm was less than 50%. Observe the capsule content powder with an electron microscope. Under a 10,000-fold SEM electron microscope, it can be seen that the powder is a single fine particle with no adhesion, no agglomerates, good dispersion, and an irregular three-dimensiona...
Embodiment 2
[0200] Get 50g of alendronate sodium crude drug, and load it into the AO pulverizer. The pulverization conditions were 0.7 MPa pulverization pressure, 2.5 hours, and pulverization twice. Obtain alendronate sodium micropowder. Determination of alendronate sodium content, with 2mg / capsule sub-package in hard capsules to get final product. Laser particle size measurement results: British Malvern Instruments Ltd. laser particle size analyzers measured the particle size of capsule content powders of different batches. As a result, the percentage of the above-mentioned samples with a particle size of less than 20 μm was greater than 90%, while the percentage of the portion with a particle size of less than 5 μm was less than 50%. Observe the capsule content powder with an electron microscope. Under a 10,000-fold SEM electron microscope, it can be seen that the powder is a single fine particle with no adhesion, no agglomerates, good dispersion, and an irregular three-dimensional st...
Embodiment 3
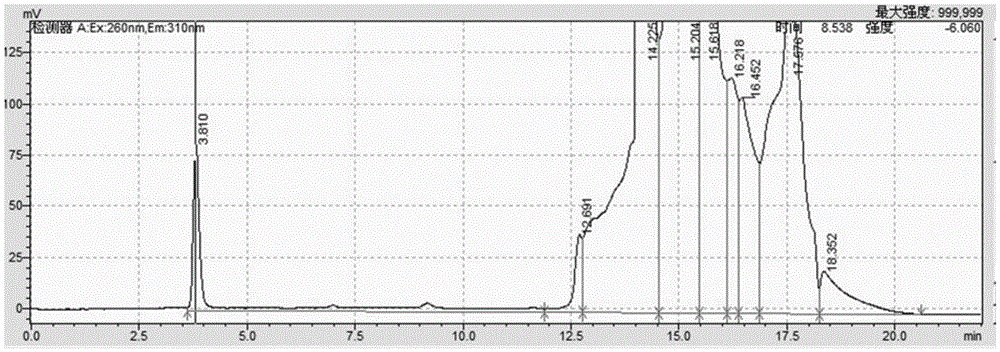
[0203] The alendronate sodium nebulizer prepared in Example 1 was used to measure the droplet distribution with an atomization absorption device, and 12.7% was deposited in the primary distribution bottle; 78.2% was deposited in the secondary distribution bottle. After the administration of the alendronate sodium powder spray in this example, the lungs of the rats were taken for HPLC analysis, and the results showed that the lung volume was 83.40% to 92.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com