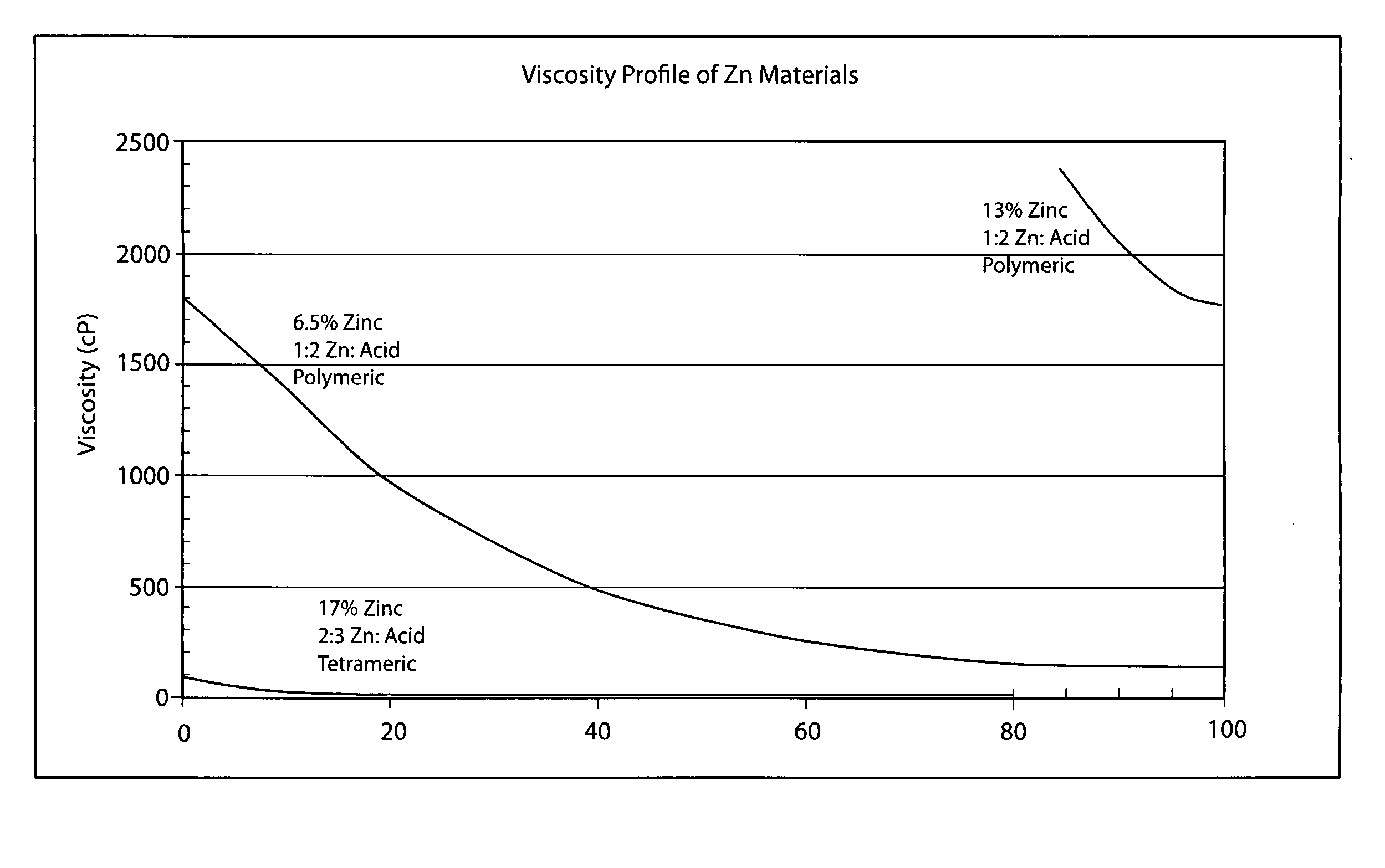
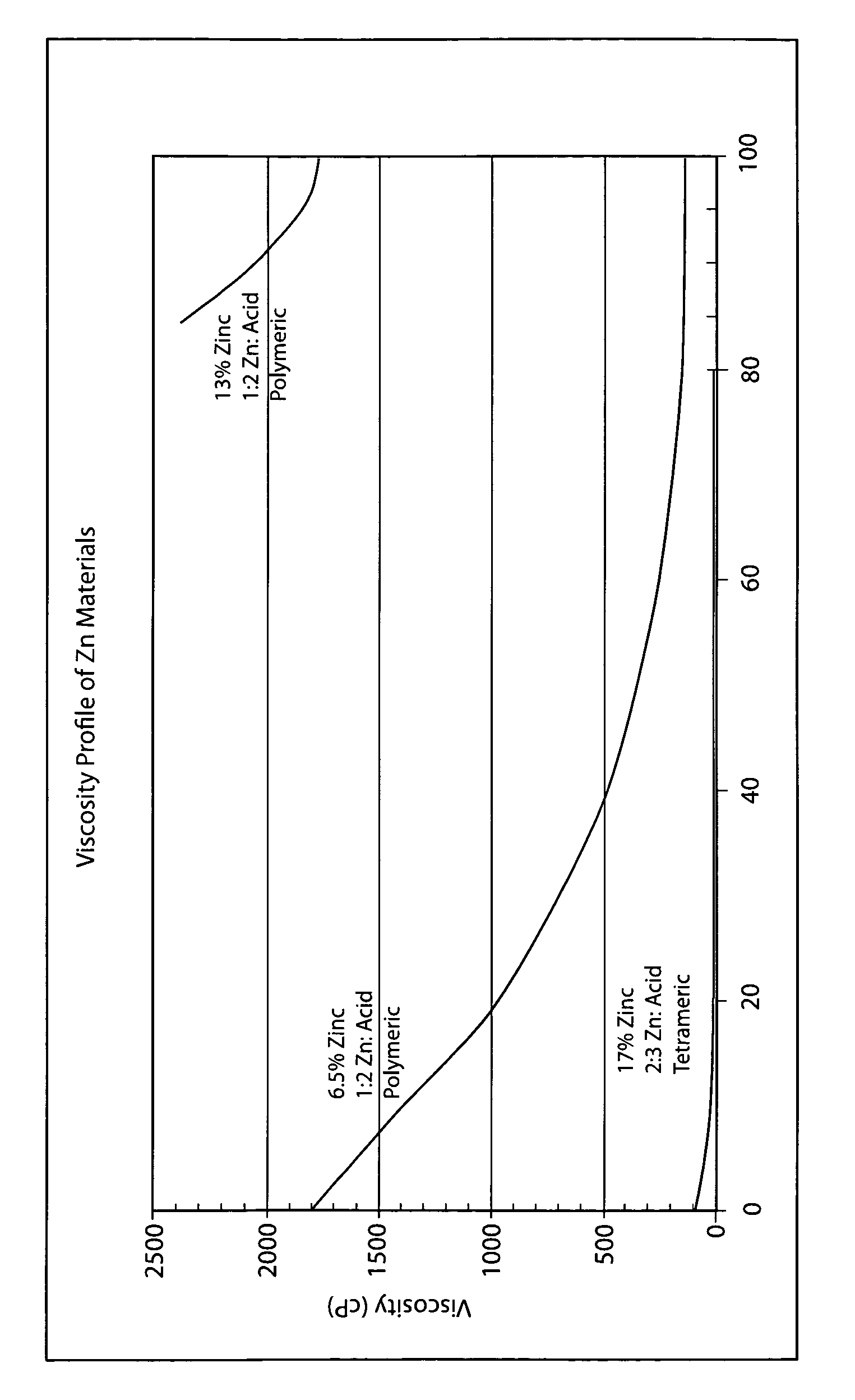
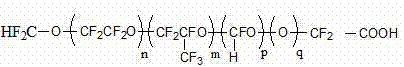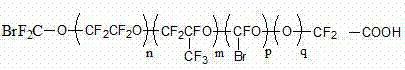Patents
Literature
687 results about "Octanoic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
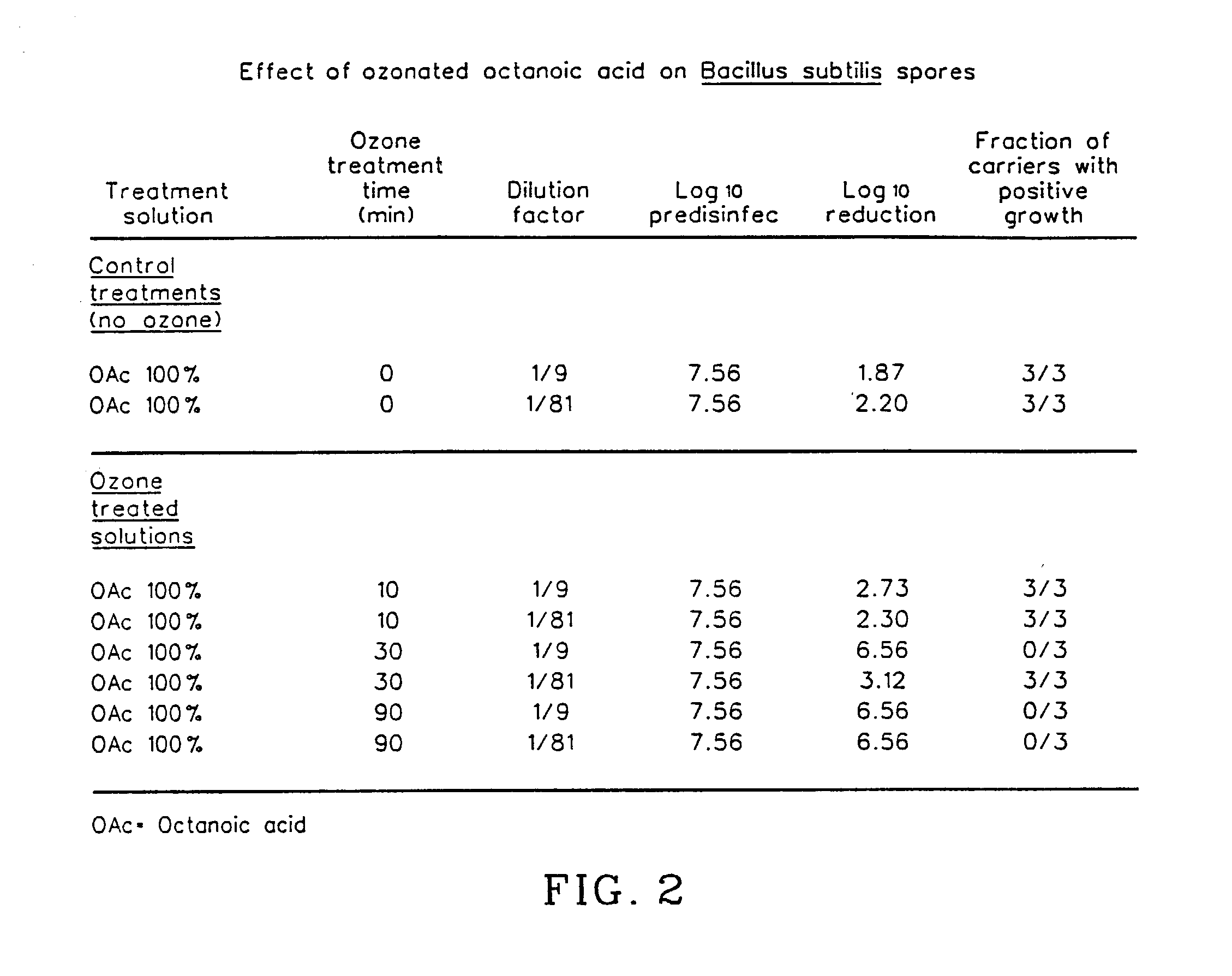
Methods of preparing antimicrobial compositions comprising ozone
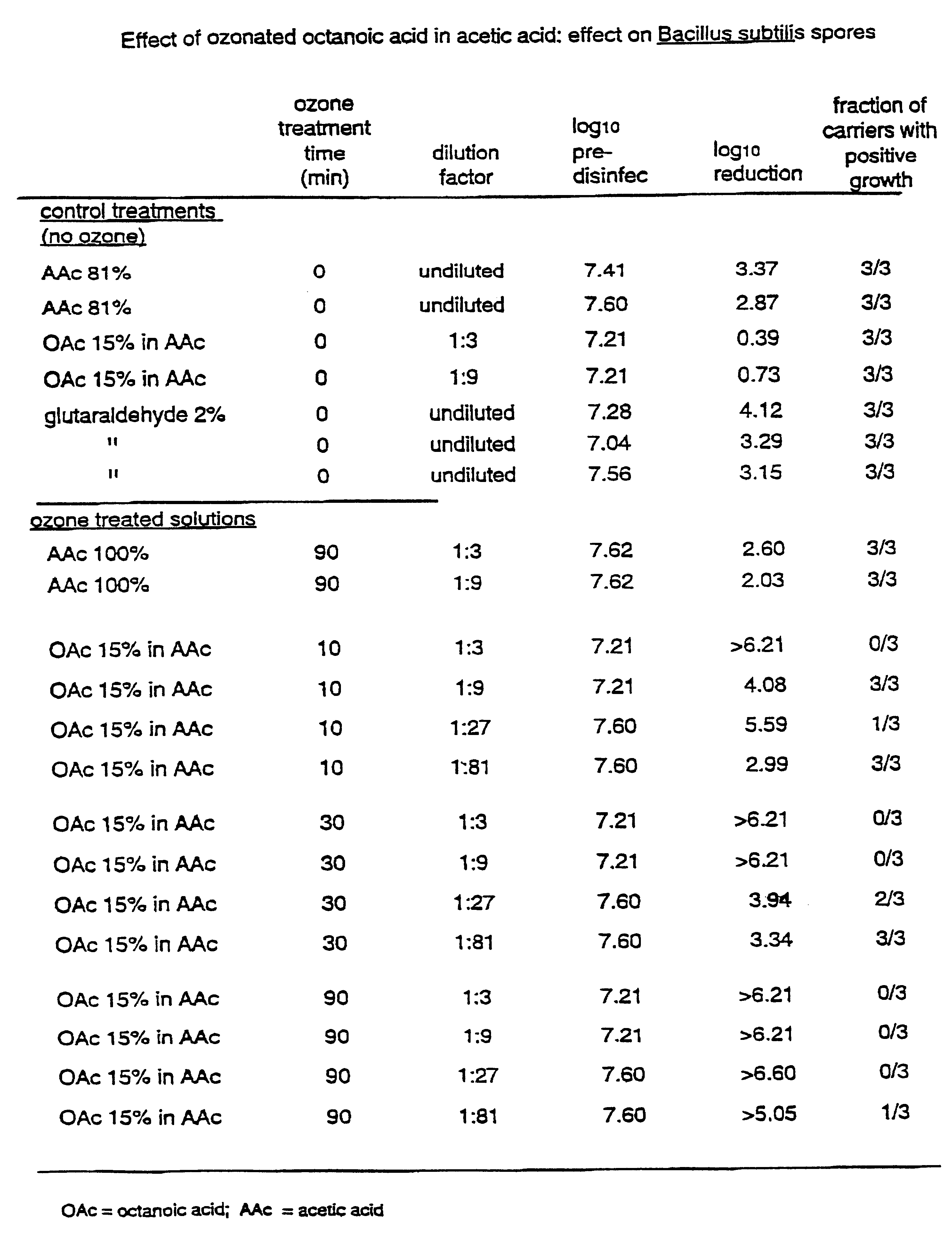
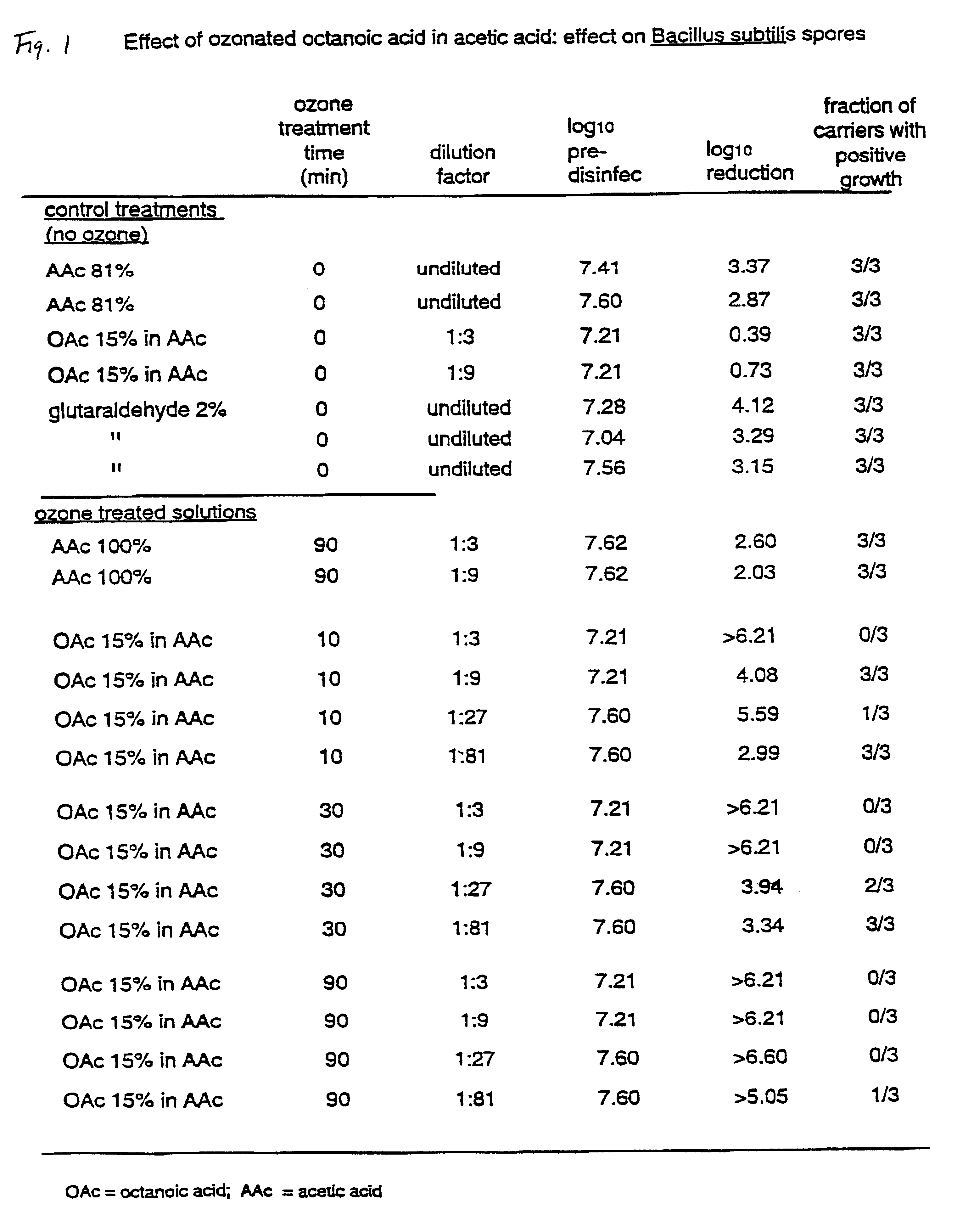
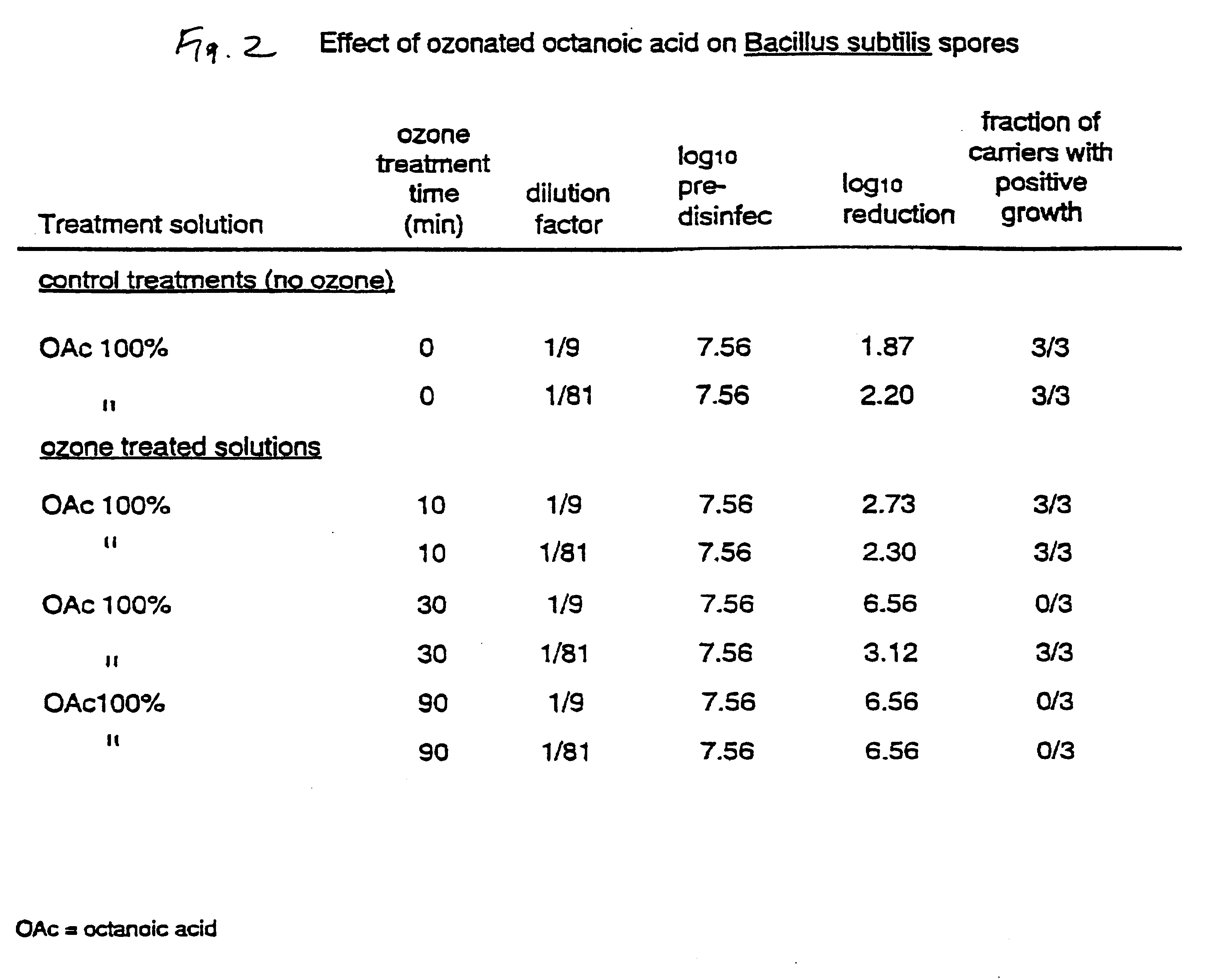
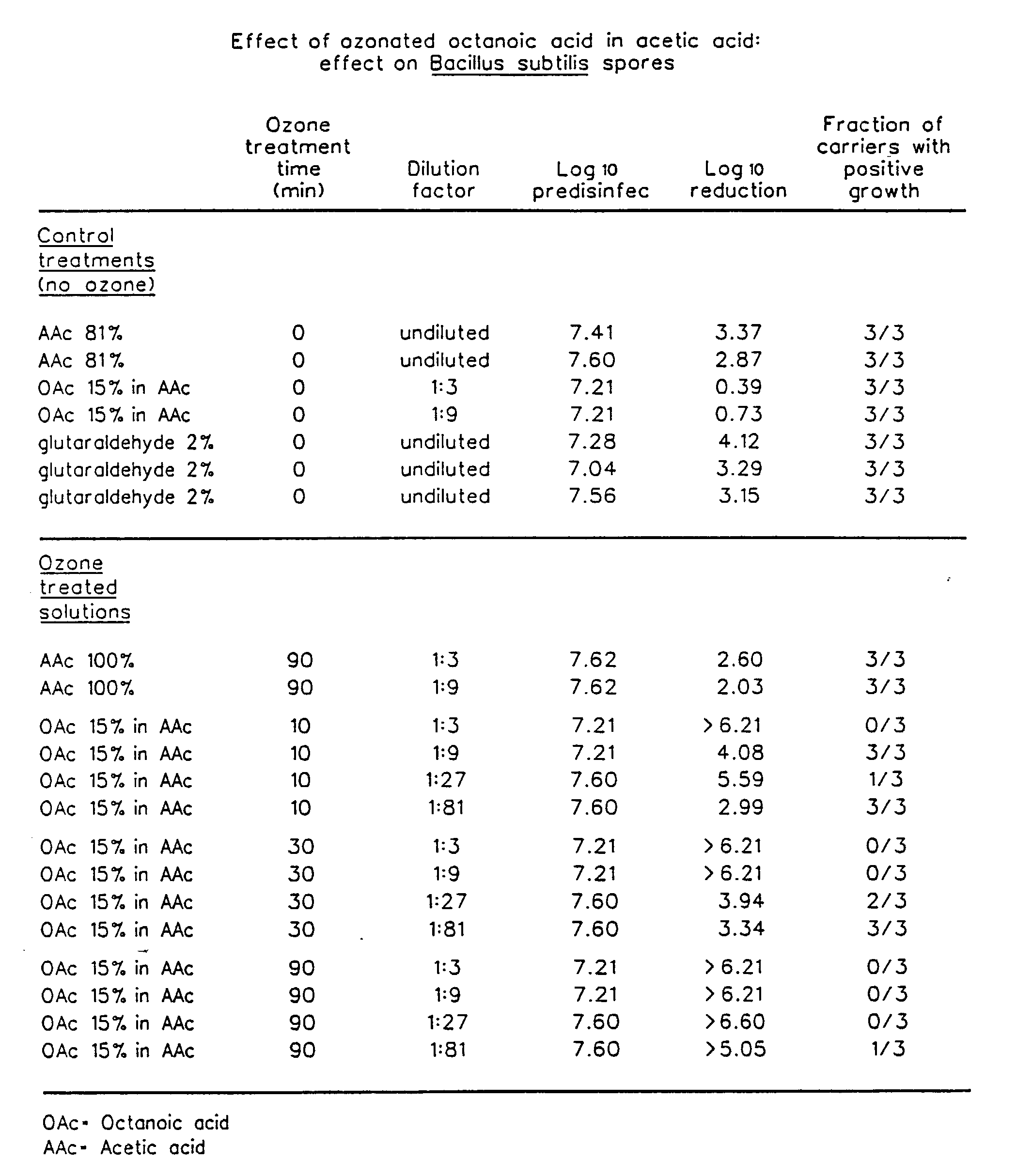
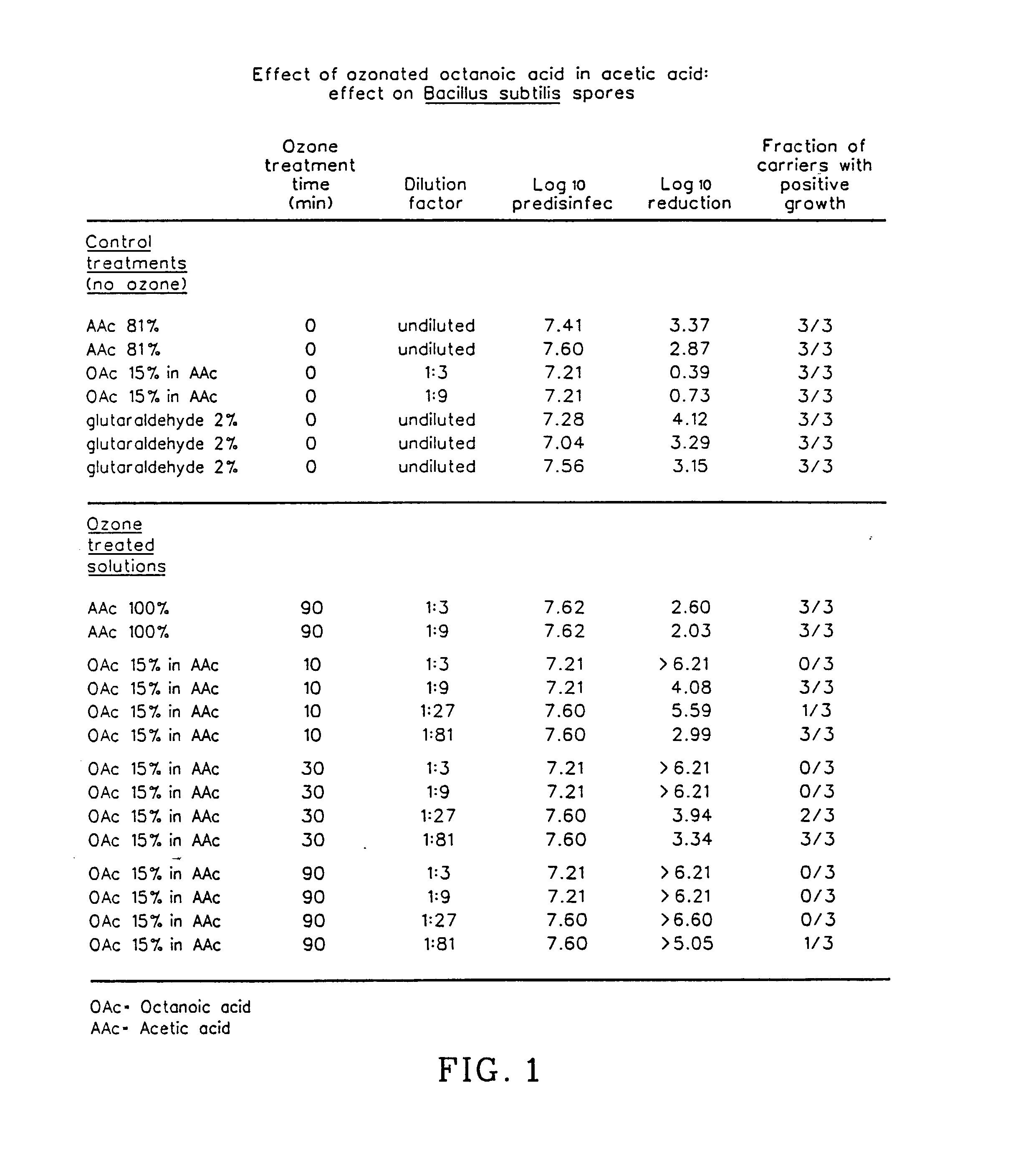
The invention relates to the formation of antimicrobial solutions formed by ozonating a liquid containing organic precursor molecules. The preferred organic precursor molecules include carboxylic acids, most particularly octanoic acid with or without acetic acid, and alcohols, most particularly greater than 80 weight percent ethanol. The ozonating step is preferably performed with minimal or no water present in the liquid containing the organic precursors. After ozonation is complete, the ozonated liquid may be diluted with water or other solvent to form a use solution for contacting and cleaning a microbially contaminated surface or other medium.
Owner:LYNNTECH
Electrically switchable polymer dispersed liquid crystal materials including transmissive holographic gratings
InactiveUS6667134B1Fast curingImprove clarityLiquid crystal compositionsPhotomechanical apparatusElectricityCrystallography
A new photopolymerizable material allows single-step, fast recording of volume holograms with properties that can be electrically controlled. Polymer-dispersed liquid crystals (PDLCs) in accordance with the invention preferably comprise a homogeneous mixture of a nematic liquid crystal and a multifunctional pentaacrylate monomer, in combination with photoinitiator, coinitiator and cross-linking agent. Optionally, a surfactant such as octanoic acid may also be added. The PDLC material is exposed to coherent light to produce an interference pattern inside, the material. Photopolymerization of the new PDLC material produces a hologram of clearly separated liquid crystal domains and cured polymer domains. Volume transmission gratings made with the new PDLC material can be electrically switched between nearly 100% diffraction efficiency and nearly 0% diffraction efficiency. By increasing the frequency of the switching voltage, switching voltages in the range of 50 Vrms can be achieved.
Owner:LEIDOS
Biocide formation via ozonation
Antimicrobial solutions formed by ozonating a liquid containing organic precursor molecules. The preferred organic precursor molecules include carboxylic acids, most particularly octanoic acid with or without acetic acid, and alcohols, most particularly at least about 70 weight percent ethanol. The ozonating step is preferably performed on the liquid containing the, organic precursors before diluting with water or other solvent to form a use solution for contacting and cleaning a microbially contaminated surface or other medium.
Owner:LYNNTECH
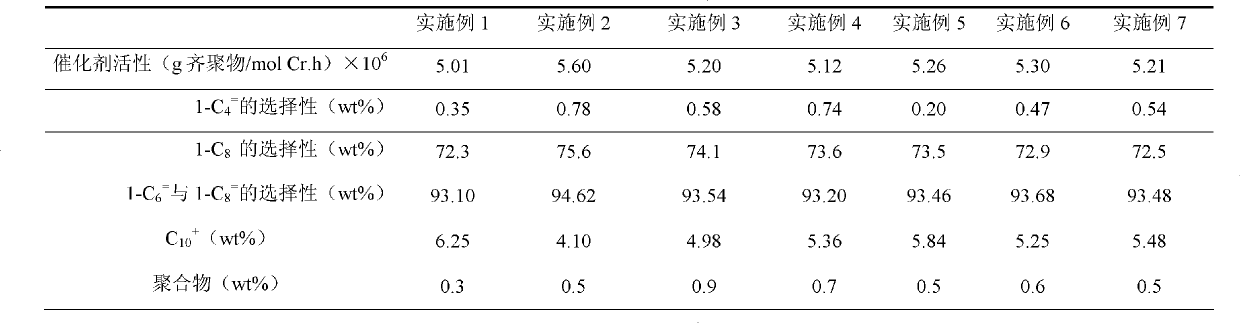
Ethylene oligomerization catalyst composition and application thereof
InactiveCN103285926AHigh activityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsArylAluminoxane
The invention relates to an ethylene oligomerization catalyst composition and an application thereof. The composition comprises the following components: a component a contains chromium acetylacetonate, tetrahydrofuran chromium chloride and / or chromium 2-ethylhexanoate; in the formula of a component b, R1, R2, R3 and R4 are phenyl, benzyl or naphthyl; and R5 is isopropyl, butyl, cyclopropyl, cyclopentyl, cyclohexyl or fluorenyl; a component c contains methyl aluminoxane, ethyl aluminoxane, propyl aluminoxane and / or butyl aluminoxane; a component d has a formula X1R6X2, wherein X1 and X2 are F, Cl, Br, I or alkoxy, and R6 is alkyl or aryl; in the formula of a component e, R is a linking group and selected from alkyl and derivatives thereof as well as aryl and derivatives thereof; and the molar ratio is 1: (0.5-10): (50-3000): (0.5-10): (0.5-10). The catalyst has the catalytic activity being more than 5.0*10<6>g mol<-1>Cr.h<-1>, and the mass of 1-hexene and 1-octylene is more than 93%.
Owner:PETROCHINA CO LTD
Liquid ester compositions and cosmetic compositions containing the same
ActiveUS20070110702A1Sustain feelingImprove gloss feelingCosmetic preparationsHair cosmeticsIsostearic acidOctanoic Acids
The present invention provides a liquid ester composition which is obtained by esterifying a branched isostearic acid such as 2-(1,3,3-trimethyl)butyl-5,7,7-trimethyl octanoic acid with dipentaerythritol, and said liquid ester composition having a viscosity at 25° C. of 100,000 to 2,000,000 mPa·s; hydroxyl value of 10 to 160; and cloud point of less than 5° C. This liquid ester composition has pigment dispersibility and hydrating ability that polybutene, which is one of the raw materials of oil compositions for cosmetic compositions, does not have, together with their abilities to sustain feeling of cosmetic film and improve gloss and moisture feeling of cosmetic compositions and shape retaining ability of lipsticks and the like equal to those containing polybutene.
Owner:THE NISSHIN OILLIO GRP LTD
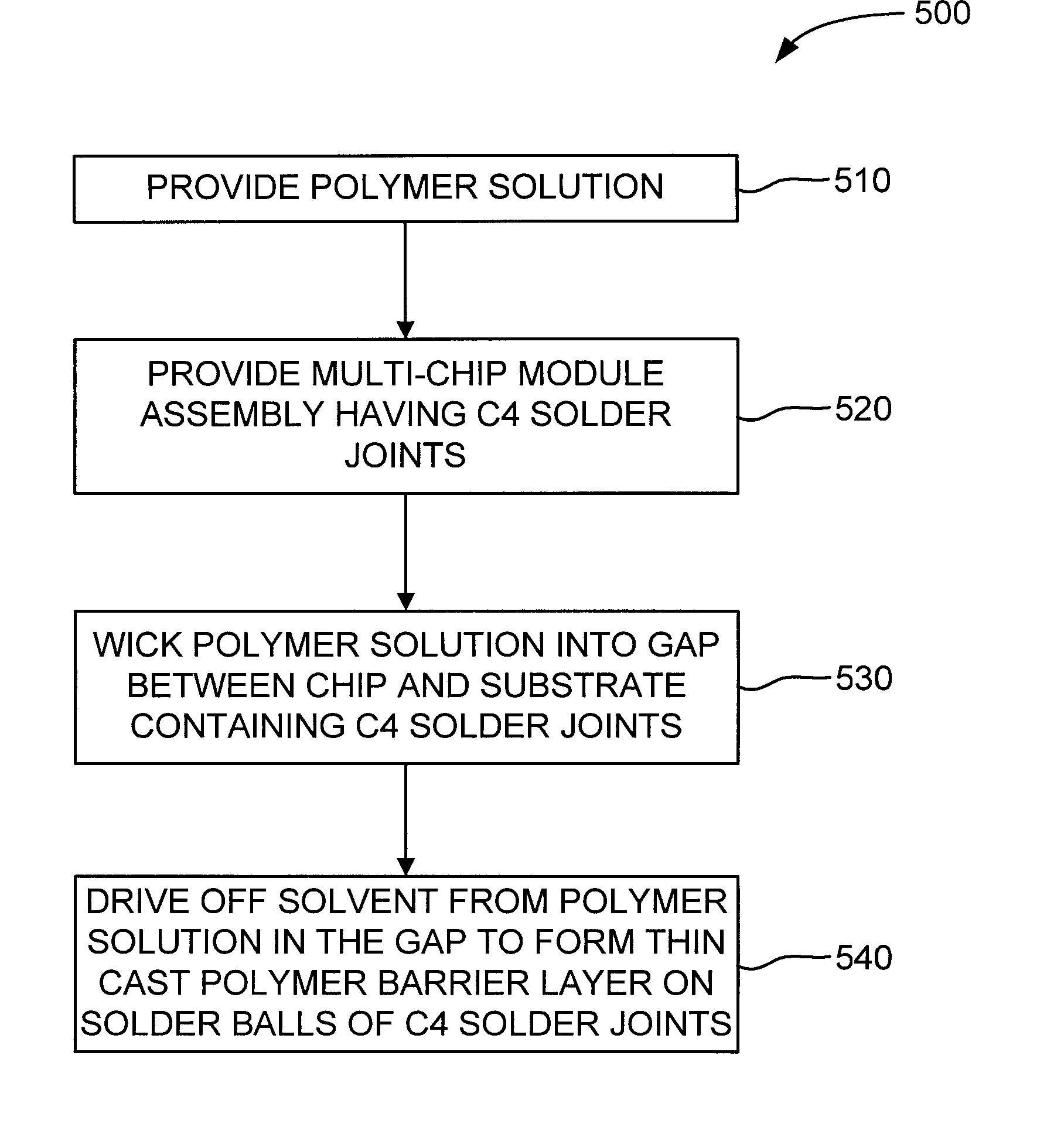
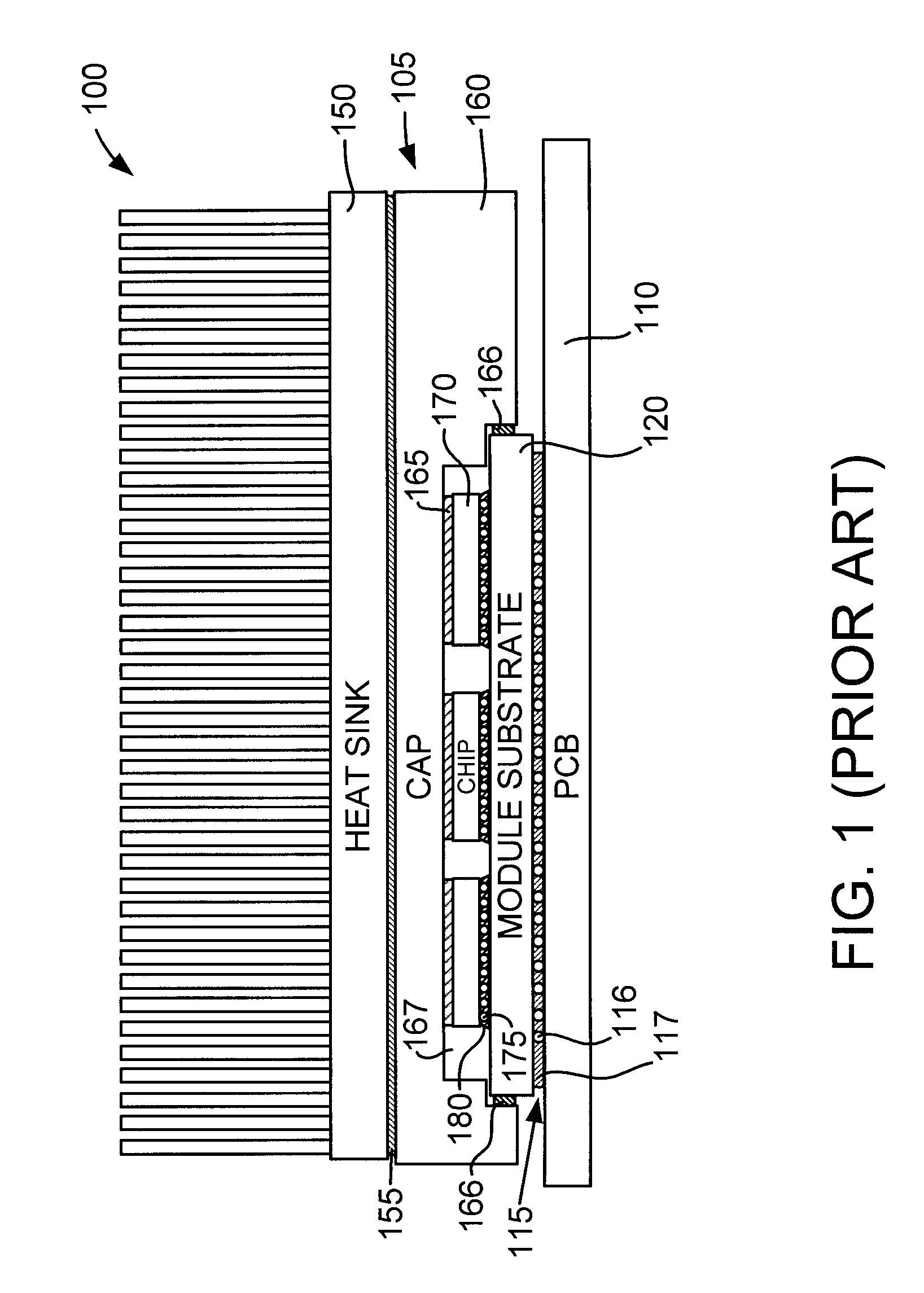
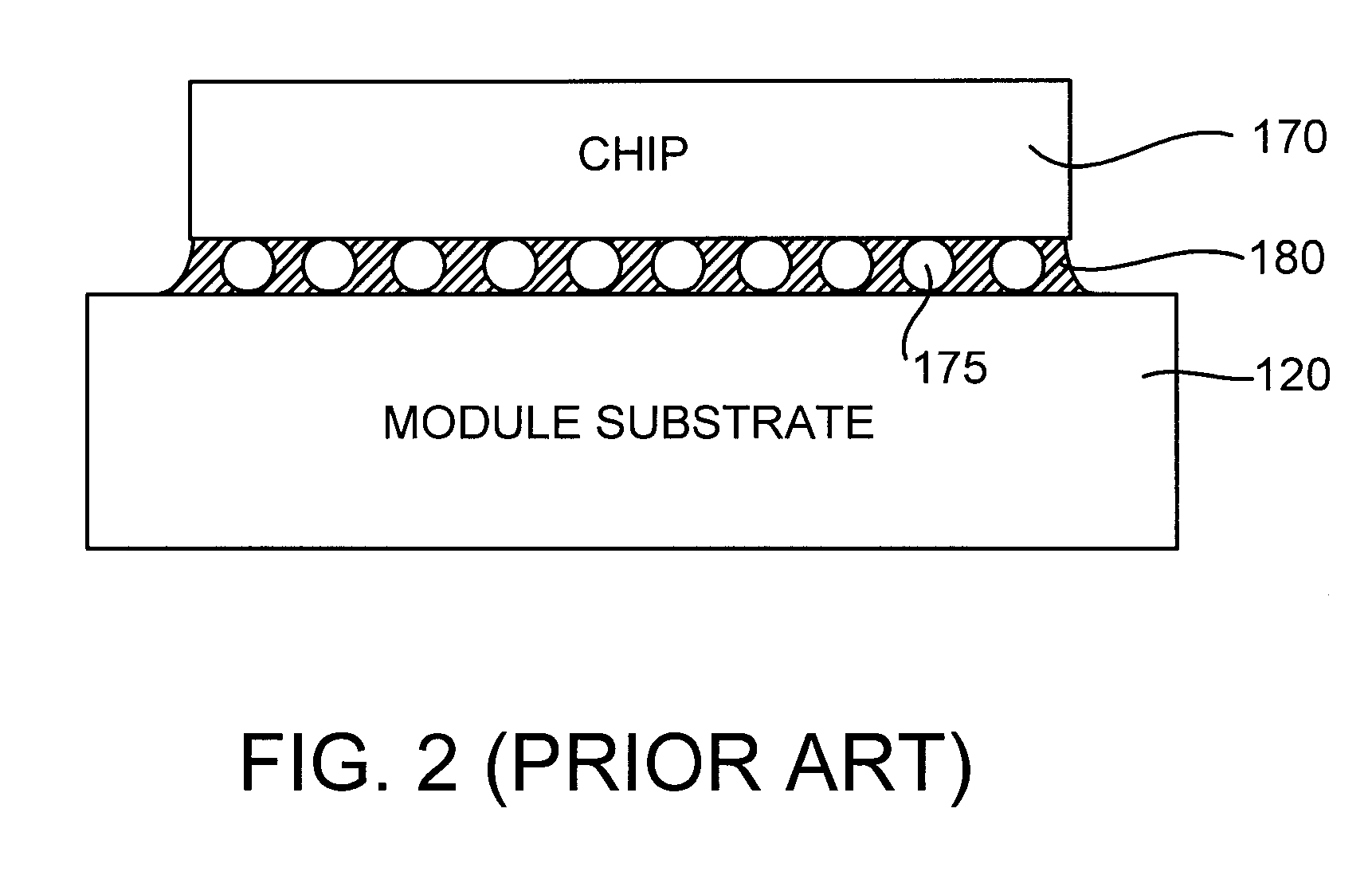
Chip Module Having Solder Balls Coated with a Thin Cast Polymer Barrier Layer for Corrosion Protection and Reworkability, and Method for Producing Same
InactiveUS20070257091A1Avoid corrosionSemiconductor/solid-state device detailsWelding/cutting auxillary devicesOctanoic AcidsDecomposition
A chip module apparatus includes one or more chips electronically connected to a substrate by controlled collapse chip connection (C4) solder joints. A thin cast polymer barrier layer is cast from solution and covers the C4 solder joints. The chips are enclosed within a cavity that includes a gaseous environment. The cast polymer barrier layer is exposed to, and protects the C4 solder joints from, the cavity's gaseous environment. Accordingly, the cast polymer barrier layer is able to protect the C4 solder joints from corrosion caused by corrosion inducing components (e.g., carbon dioxide, moisture, octanoic acid, etc.) present in the cavity's gaseous environment. To provide reworkability, the cast polymer barrier layer is thermally stable at least to the reflow temperature of the C4 solder joints and has a decomposition temperature below that of the substrate, and preferably has a melting point above the reflow temperature of the C4 solder joints.
Owner:IBM CORP
Anti-freezing fluid
ActiveCN101302424ATo achieve long-term goalsTo achieve the characteristics of green environmental protectionHeat-exchange elementsSodium molybdateAnti freezing
The invention discloses anti-freeze fluid. The compositions by weight percentage of the anti-freeze fluid are: 35 to 85 percent of glycol, 0.25 to 0.06 percent of sodium molybdate, 0.4 to 0.1 percent of sodium nitrate, 3.5 to 0.8 percent of potassium hydroxide, 1.2 to 0.2 percent of 85 percent phosphoric acid, 0.3 to 0.02 percent of metyl benzotriazole, 0.5 to 0.05 percent of benzotriazole, 3.5 to 0.8 percent of sebacic acid, 1.0 to 0.25 percent of undecanedioic acid, 1.0 to 0.2 percent of octanoic acid, 3.0 to 0.8 percent of sodium benzoate, 0.2 to 0.05 percent of hydrolytic-polymeric maleic anhydride, 0.003 to 0.01 percent of antifoaming agent, the balance being deionized water. The anti-freeze fluid is characterized by long effect and environmental protection, and can perform all-around multi-layer protection on an automobile cooling system.
Owner:张家港迪克汽车化学品有限公司
Non-aqueous coolant for engine
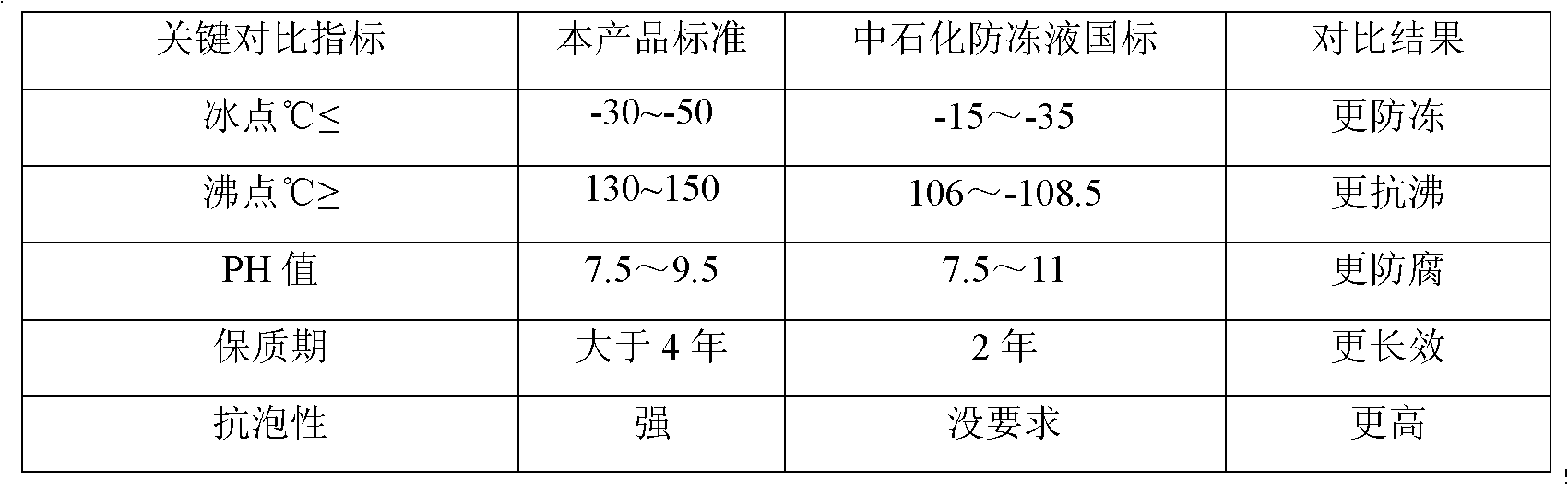
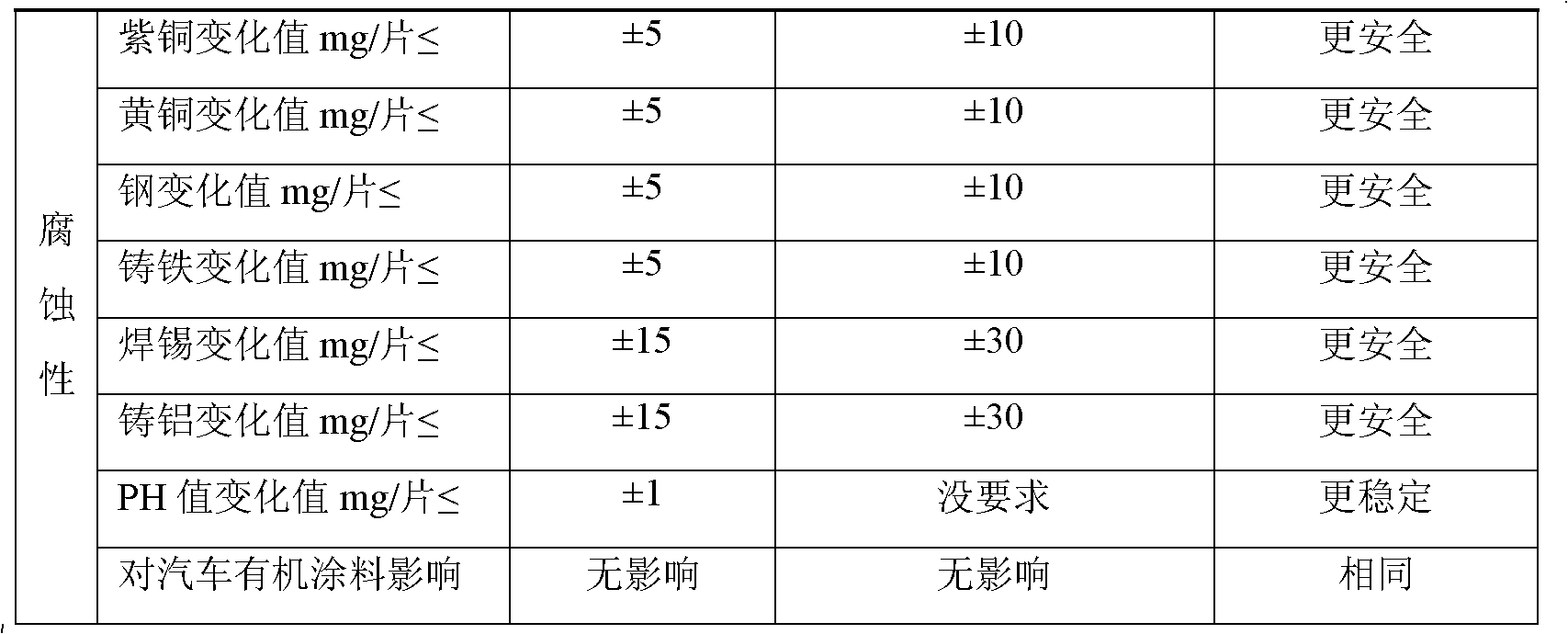
ActiveCN102002347AStable pHImprove antifreeze effectHeat-exchange elementsOctanoic AcidsPhosphoric acid
The invention relates to a non-aqueous coolant for an engine, which is characterized by comprising the following components in percent by weight: 10-80 percent of ethylene glycol, 10-80 percent of propylene glycol, 5-20 percent of polyol, 0.1-0.8 percent of phosphate-molybdate, 0.2-0.9 percent of silicate ester, 0.1-1 percent of 4-methyl 1H-benzotriazole, 0.2-0.8 percent of citric acid, 0.2-1 percent of sebacic acid, 0.1-1 percent of octanoic acid, 0.4-1.2 percent of citrate and 0.01-0.2 percent of cosolvent. The non-aqueous coolant has freezing point of -30--50 DEG C and boiling point of 130-150 DEG C, and ensures that the cold start of the engine is realized in cold weather; meanwhile, the working temperature of the engine is improved to 95-150 DEG C from the original 85-95 DEG C. The non-aqueous coolant belongs to a non-aqueous system, avoids electrochemical corrosion, and is a buffer system with pH of 7.5-9.5; and the added relevant auxiliaries have active corrosion resistance on aluminum and aluminum alloy and copper.
Owner:HEFEI UNIV OF TECH
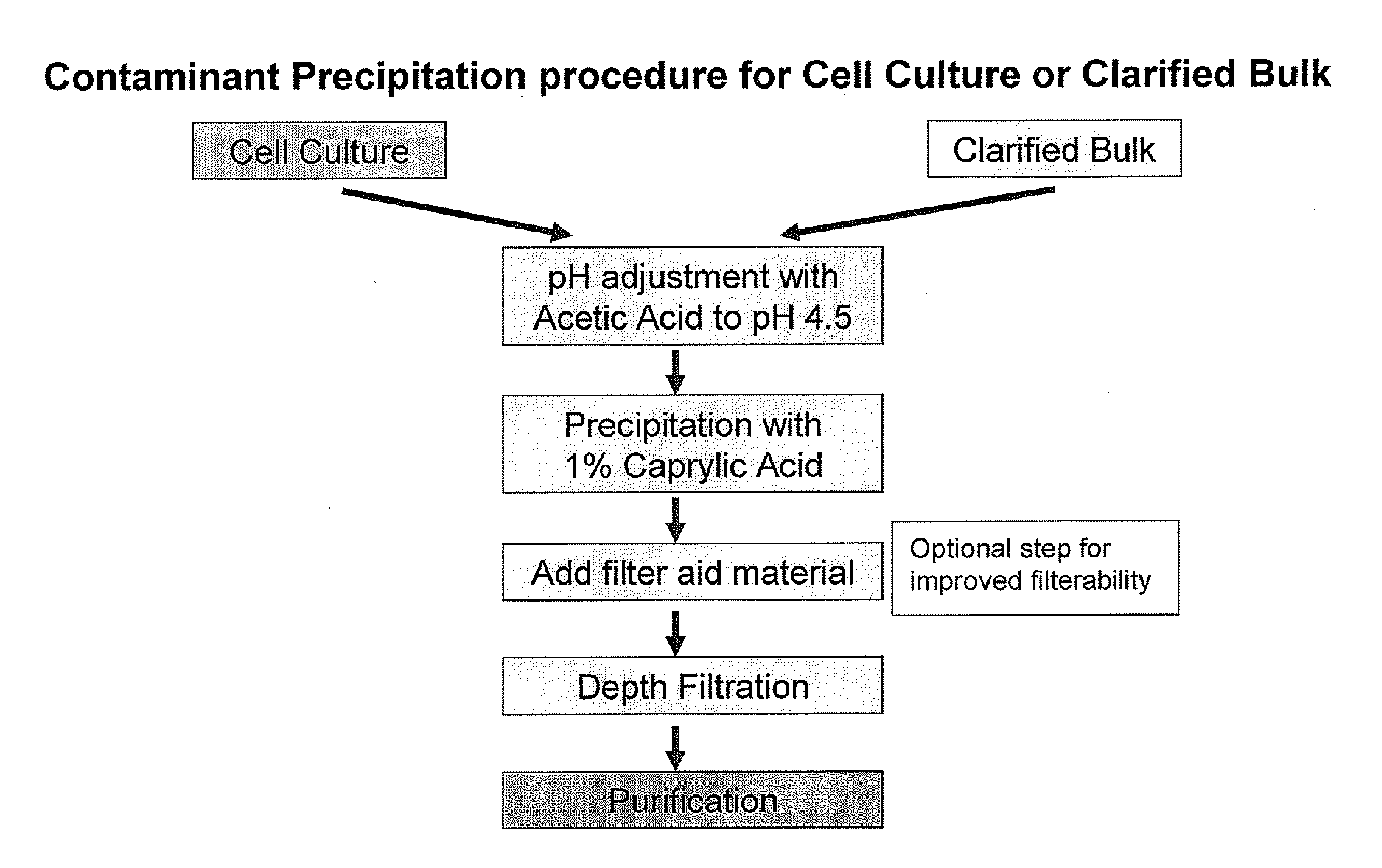
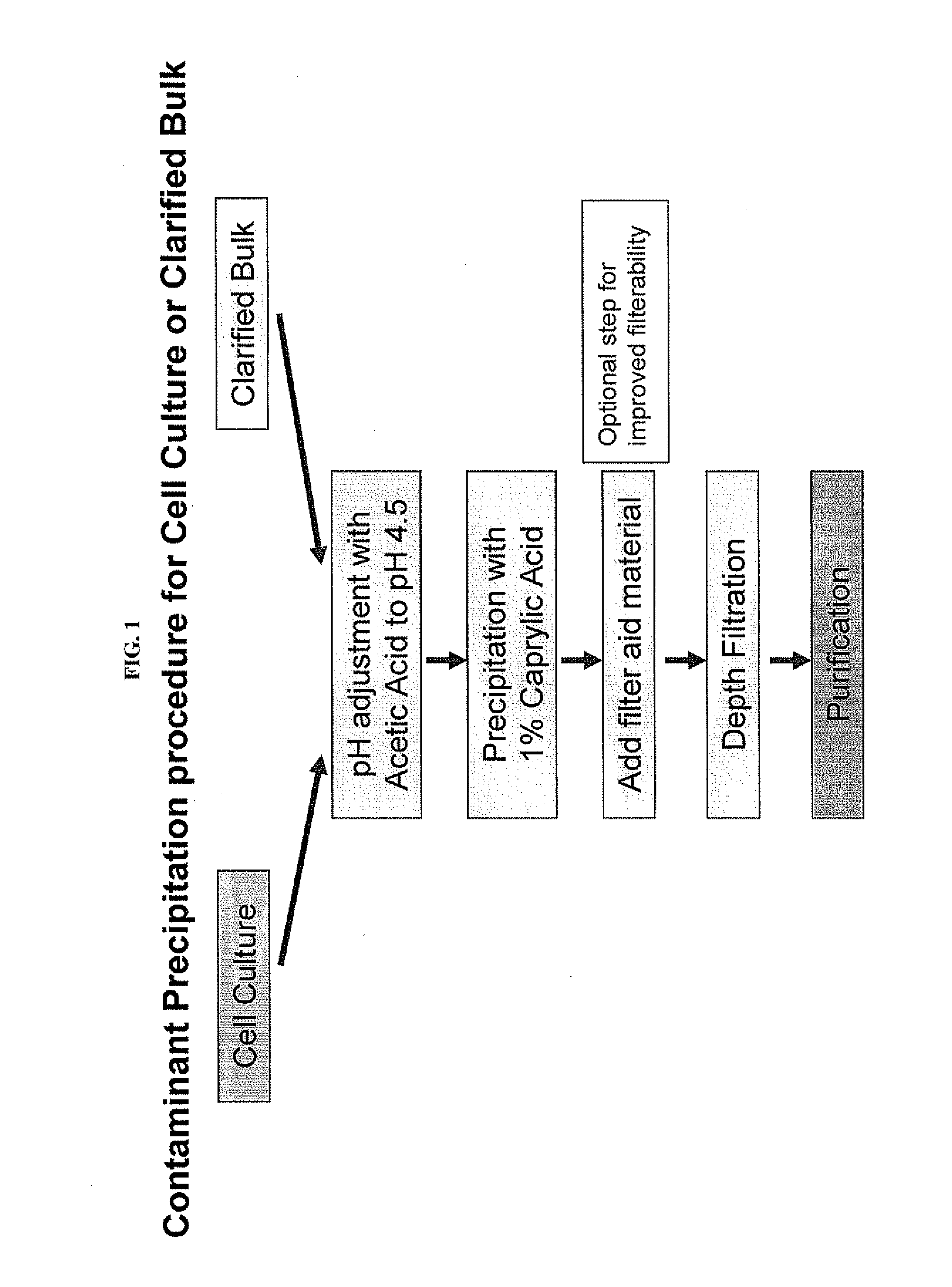
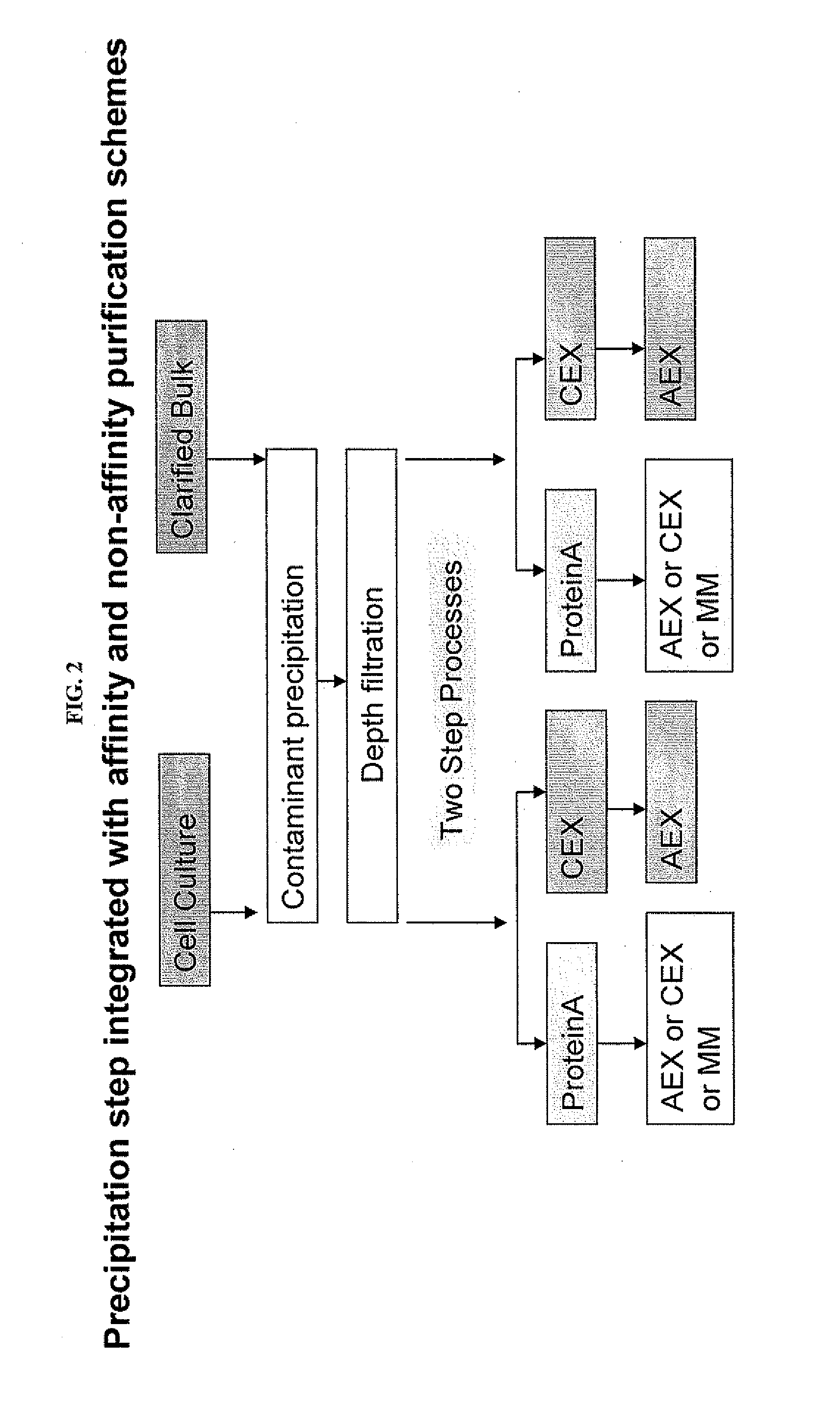
Protein purification by caprylic acid (octanoic acid) precipitation
InactiveUS20120101262A1Efficient purificationPeptide preparation methodsDepsipeptidesTherapeutic proteinMulti pollutant
The invention provides methods for a purifying protein of interest from a mixture comprising the protein of interest and one or more contaminants, including host cell DNA and proteins, by precipitation of the contaminants with caprylic acid. Such methods are particularly useful for purifying antibodies from cell cultures. Moreover, mixtures that have been depleted of contaminants using the methods of the invention can be used directly in downstream chromatography applications (e.g., ion exchange chromatography) without any further purification. These methods lead to manufacturing processes with a minimum number of unit operations and reduce the resource requirements, and thus positively influence the cost of goods for therapeutic protein production.
Owner:BRISTOL MYERS SQUIBB CO
Leadless radiation-proof organic glass and preparation method thereof
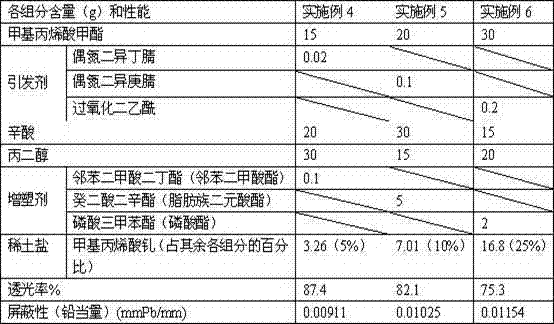
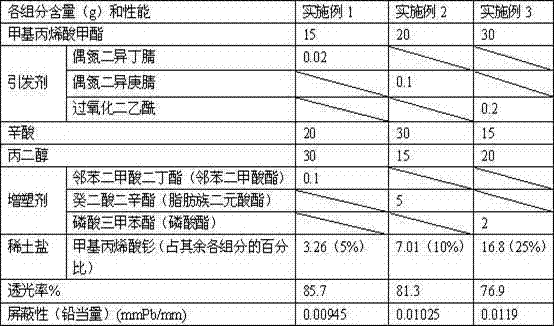
ActiveCN102558426AAvoid pollution and other hazardsImprove radiation protection effectShieldingOctanoic AcidsPlasticizer
The invention discloses leadless radiation-proof organic glass and a preparation method of the leadless radiation-proof organic glass, wherein the organic glass is obtained by the steps of dissolving organic rare-earth salt into octanoic acid and propylene glycol, and then performing copolymerization with methyl methacrylate and a plasticizer under the action of an initiator. The organic glass prepared by the method not only keeps good light transmission, but also effectively reduces radiation and avoids lead pollution.
Owner:ZHEJIANG GEELY AUTOMOBILE RES INST CO LTD +1
Method of removing hydrogen sulfide
ActiveUS8246813B2Reduce at least 50% of the sulfidesLow viscosityHydrocarbon purification/separationRefining with acid saltsOctanoic AcidsCarboxylic acid
Owner:ECOLAB USA INC
Duck plague yolk antibody freeze-dried powder and preparation method thereof
The invention relates to duck plague yolk antibody freeze-dried powder, which comprises the following components in percentage by weight: 87 to 90 percent of duck plague yolk antibody, 0.8 to 1.0 percent of octanoic acid, 0.2 to 0.3 percent of formaldehyde, 0.01 to 0.02 percent of thimerosal, 3 to 4 percent of glucose, 1 to 2 percent of sorbitol, 2 to 3 percent of glycine and 2 to 3 percent of mannitol. The freeze-dried powder has the advantages of long storage time, quick dissolution, high antibody valence and high bioactivity, and the purity of the antibody of the freeze-dried powder is higher than that of the conventional product in market. The invention also discloses a preparation method for the duck plague yolk antibody freeze-dried powder. The preparation method comprises the following steps of: separating strains to prepare vaccines, immunizing healthy ducks to obtain high-immunity eggs, performing sterilization, acidification, octanoic acid treatment, refining, extraction, purification, preparation, freeze drying and the like on the high-immunity eggs, and thus obtaining the duck plague high-immunity yolk antibody freeze-dried powder. According to the preparation method, yolk liquid is heated by using the temperature of pasteurization during acidification and water treatment, so that a sterilization effect is achieved, and the recovery rate of the antibody and the clarity of the solution during acidification can be improved at the same time; and the prepared yolk antibody has the advantages of high bioactivity, long storage time, retarded valence reducing speed and the like.
Owner:ZHENGZHOU HOUYI PHARMA
Esterification method of pentaerythritol
ActiveCN104086417AIncrease in positive chargePromote esterification reactionOrganic compound preparationCarboxylic acid esters preparationOctanoic AcidsAfter treatment
The invention provides an esterification method of pentaerythritol, which comprises the following step: under the action of a tin catalyst, carrying out esterification reaction on pentaerythritol and saturated fatty acid to obtain pentaerythritol ester, wherein the saturated fatty acid is one or more of butyric acid, valeric acid, hexanoic acid, heptylic acid, octanoic acid, pelargonic acid and capric acid. The esterification method provided by the invention has the advantages of higher product yield, higher product quality, simple esterification after-treatment and low pollution. The experimental result indicates that the conversion rate of the esterification reaction is greater than 99.5%, and the hydroxyl value in the reaction product is lower than 1mg KOH / g.
Owner:CHINA PETROLEUM & CHEM CORP
Preparation method for thermoset high temperature resistant methyl phenyl silicone resin
The invention discloses a preparation method for thermoset high temperature resistant methyl phenyl silicone resin, which relates to the technical field of resin preparation and comprises the steps of utilizing toluene or dimethylbenzene or mixed solution of the toluene and dimethylbenzene to serve as a solvent, utilizing methyl trichlorosilane, dichlorodimethylsilane and methylphenyldichloros as monomer raw materials, dropping mixed solution of acetone and water into the raw materials under the condition of ice-water bath, ensuring that the dropping is finished within 3-5 hours, obtaining a prepolymer of the methyl phenyl silicone resin, performing condensation and curing on the obtained prepolymer above 150 DEG C after pressure of the prepolymer is reduced and the prepolyme is concentrated, the adopted condensation catalyst is caprylate or naphthenate, particularly is stannous octoate, zinc isoocatanoate, cobalt naphthenate and dibutyltin dilaurate. The resin is semi-transparent hard or elastic solid, can not be dissolved or fused, and can be resistant to high temperature above 200 DEG C, and can be used as silicon rubber or a plastic filler.
Owner:JIANGSU UNIV
High-molecular mPEG-PLGA-mPEG accessory medicine for medicine use, prepu. method and application thereof
InactiveCN1537636AEvade captureAvoid the effect of adsorptionPharmaceutical non-active ingredientsLactidePolyethylene glycol
A high-molecular mPEG-PLGA-mPEG (PELGE) used as the axcessory of injection, oral-applied medicine, and the water-soluble medicine or the medicine difficult to dissolve in water is an amphipathic three-block copolymer, which is prepared from stannous octoate as catalyst, diisocyanate as coupling agent, polyethene glycol with single terminated end, glycollide, and lactide or polylactic acid-glycollic acid copolymer.
Owner:四川大学华西药学院 +1
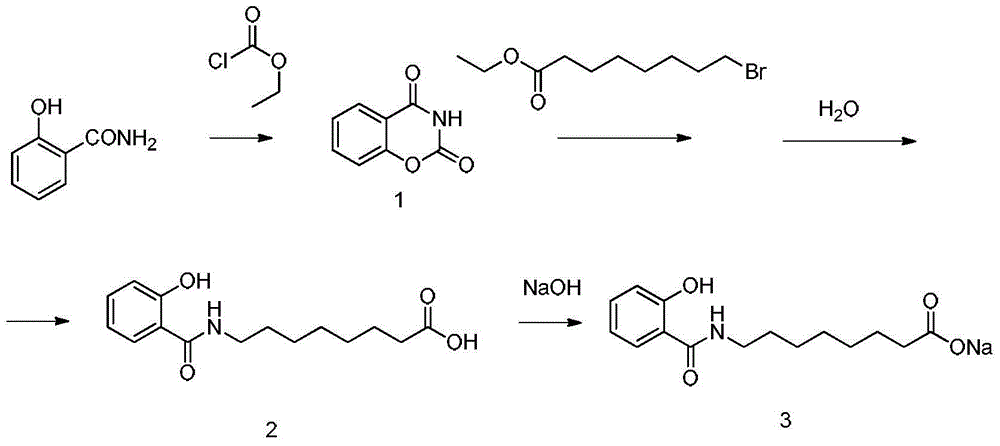
Method for preparing sodium, 8-(2-hydroxybenzamido)octanoate
InactiveCN104974060AGood choiceHigh yieldOrganic compound preparationCarboxylic acid amides preparationEthyl chloroformateOctanoic Acids
The invention discloses a method for preparing sodium, 8-(2-hydroxybenzamido)octanoate which is a drug intermediate. The method comprises the following steps of reacting salicylamide, which serves as a raw material, with ethyl chloroformate to produce an intermediate 2H-benzo[e][1,3]oxazine-2,4(3H)-dione, reacting the intermediate with 8-bromooctanoic acid ethyl ester, carrying out hydrolysis so as to obtain 8-(2-hydroxybenzamido)octanoic acid, and then, reacting 8-(2-hydroxybenzamido)octanoic acid with sodium hydroxide so as to obtain the final product, namely sodium, 8-(2-hydroxybenzamido)octanoate. According to the method, the raw material reacts with ethyl chloroformate to produce the intermediate 2H-benzo[e][1,3]oxazin-2,4(3H)-dione, so that the yield of the reaction is greatly increased, the selectivity of reaction is greatly improved, the production cost is reduced, the production of byproducts is lowered, industrial production is facilitated; and the method is environment-friendly.
Owner:CHEMSOON CO LTD
Duck virus hepatitis yolk antibody freeze-dried powder and preparation method thereof
The invention relates to a duck virus hepatitis yolk antibody freeze-dried powder which comprises the following components: 85-88% of duck virus hepatitis yolk antibody, 0.8-1.0% of octanoic acid, 0.01-0.02% of thimerosal, 0.2-0.3% of formaldehyde, 4-5% of glucose, 1-2% of sorbic alcohol, 2-3% of glycine and 2-3% of mannitol. The freeze-dried powder has the advantages of long preservation time, high dissolving speed, high antibody titer and excellent biological activity, and more importantly, the purity of the antibody of the product is higher than that of the existing product on the market. At the same time, the invention also discloses a preparation method of the duck virus hepatitis yolk antibody freeze-dried powder, comprising the following steps: voluntarily separating strains in a disease prevalence area to prepare a vaccine, immunizing healthy dusks to obtain hyper-immune eggs, ad performing disinfection, acidification, octanoic acid treatment, refining extraction and purification, preparation, freezing and the like on the hyper-immune eggs to obtain the duck virus hepatitis yolk antibody freeze-dried powder. According to the preparation method in the application, a yolk solution is heated at the temperature of pasteurization when the acidification water treatment is carried out, so that not only is the disinfection purpose fulfilled, but also the recovery rate of the antibody and the clarity of the solution in the acidification treatment are increased, the prepared yolk antibody has the advantages of high biological activity, long antibody preservation time, titer reduction, lowered speed and the like.
Owner:ZHENGZHOU HOUYI PHARMA
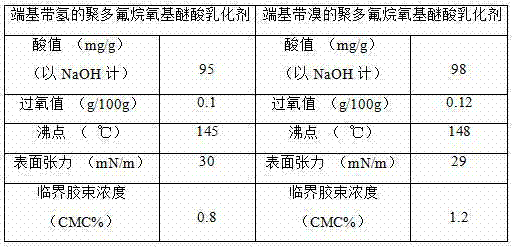
Fluorine-containing polymer and preparation method thereof
The invention discloses a fluorine-containing polymer and a preparation method thereof. One or more fluorinated monomers is / are subjected to liquid polymerization by using polyfluothane oxy ether acid as an emulsifier to obtain the fluorine-containing polymer free of perfluoro-octanoic acid or perfluoro-octylsulfonic acid, wherein the polyfluothane oxy ether acid is a halogen-terminated or hydrogen-terminated polyfluothane oxy ether acid emulsifier. The technical scheme mainly relates to the following three improvements: (1) the polyfluothane oxy ether acid or salt thereof selected instead of PFOA / PFOS as the fluorine-containing emulsifier can be effectively used in aqueous emulsion polymerization reaction of fluorinated monomers; (2) the prepared fluorine-containing polymer is free of perfluoro-octanoic acid or perfluoro-octylsulfonic acid, and thus, satisfies the environmental requirement; and (3) the fluorine-containing polymer conforms to the cost accounting, can greatly enhance the single-kettle yield of the equipment on the premise of not increasing the production equipment and personnel, and meanwhile, can enhance the polymerization time, polymerization yield and other production indexes.
Owner:成都晨光博达新材料股份有限公司
High temperature lubricant compositions
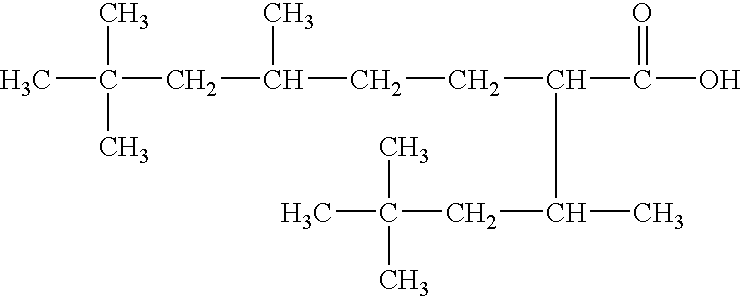
A lubricant composition useful for high temperature applications is provided comprising at least one polyol polyester derived from the reaction product of a neopentyl polyol with 5,7,7-trimethyl-2-(1,3,3-trimethylbutyl)-octanoic acid. The lubricants have low evaporation loss, high resistance to oxidation, and provide reduced deposits when utilized alone or in combination with other materials.
Owner:ZSCHIMMER & SCHWARZ INC
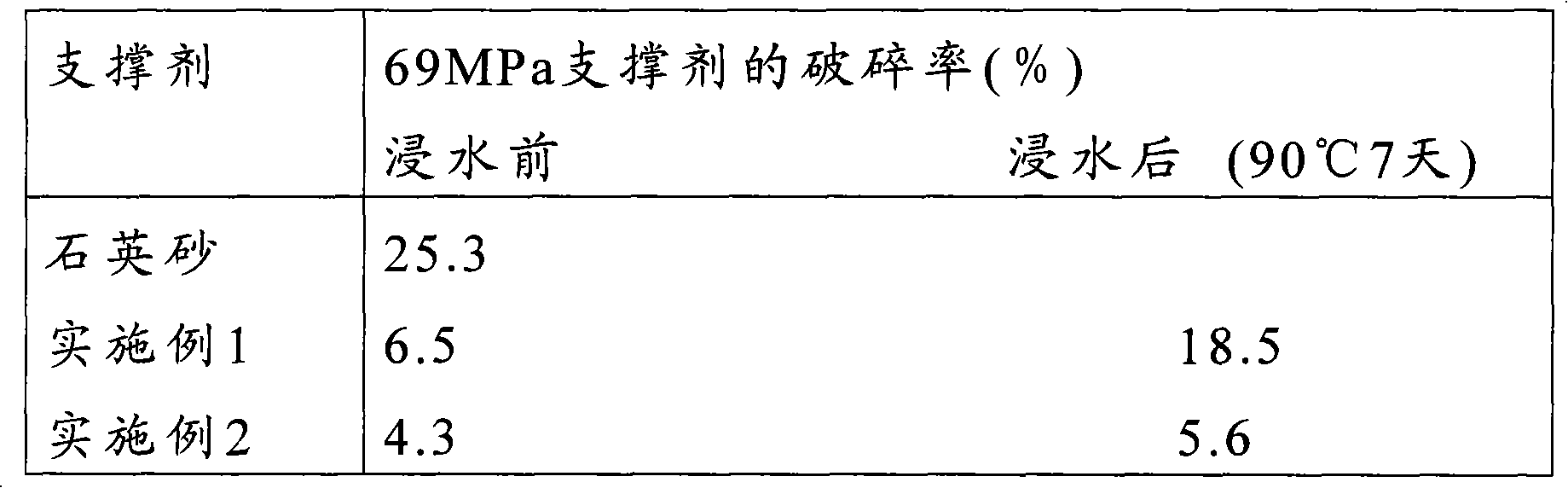
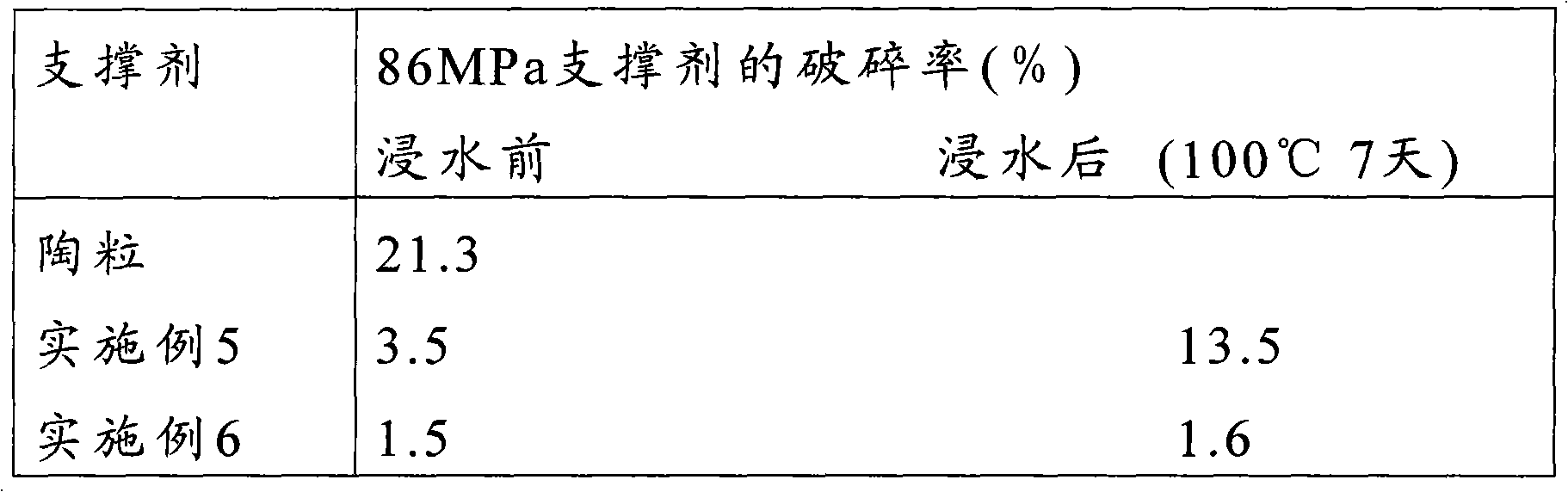
Precured resin coated propping agent and preparation method thereof
ActiveCN101580707ADoes not affect strengthReduce turbidityFluid removalDrilling compositionAntistatic agentOctanoic Acids
The invention relates to a precured resin coated propping agent and a preparation method thereof. The precured resin coated propping agent comprises an aggregate and a resin membrane which coats resin outside the aggregate; the resin membrane includes a resin membrane comprising an antistatic agent and is characterized in that the antistatic agent comprises acetylene black and one or variety selected from the group formed by isocaprylic acid, octanoic acid or naphthenic acid of the metals like Fe, Co, Cu, V, Cr and Sn with a plurality of valence states. The precured resin coated propping agent has excellent antistatic conduction performance and the water resistance thereof is greatly improved; the percentage of damage of the propping agent after being dipped into water is greatly reduced; the consumption of the resin is less and the intensity of the coated propping agent is high.
Owner:阳泉市长青石油压裂支撑剂有限公司 +2
Gas-phase anti-corrosion paint
InactiveCN101245218ADry fastHigh hardnessAnti-corrosive paintsPolyester coatingsBenzoic acidOctanoic Acids
The invention relates to a gas phase antirust coating, the components of the combination account according to parts by weight, and the scope is as follows: 8 to 25 parts of acrylate modified alkyd resin, 5 to 20 parts of 2402 phenolic resin, 0 to 15 parts of petroleum sulfonate barium, 0 to 25 parts of oxidized malthenes magnesium soap, 0 to 5 parts of dodecatylene base succinic acid imidazoline, 0 to 6 parts of sebacic acid morpholine, 0 to 6 parts of octanoic acid two cyclohexylamines, 0 to 6 parts of octanoic acid tributyl amine, 0 to 6 parts of capric acid tributyl amine, 0 to 15 parts of benzoic acid monoethanolamine, 0.1 to 1.0 part of drier, 0 to 5 parts of thixotropic agent, 1 to 5 parts of dibutyl phthalate, 0 to 5 parts of black paste, and the rest of xylene / 200 model solvent (1:1). The gas phase antirust coating of the invention has the advantages of fast table-drying speed, long antirust time, high rigidity, high brightness, good temperature resistance, etc., and can satisfy the requirements of the automatic pipeline production and long time open storage antirust need of oil steel pipes and other large pipes or profiles.
Owner:DALIAN YATAI SCI & TECH NEW MATERIAL
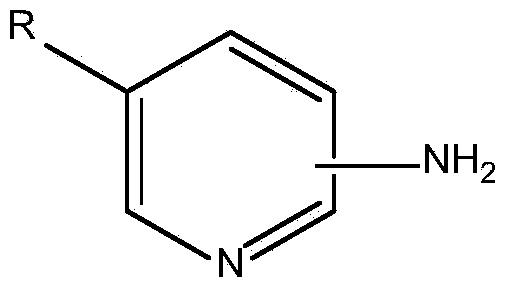
Method of aminopyridine modified resin for adsorbing rhodium octanoate dimer and metal ions
InactiveCN103586006AImprove adsorption capacitySimple methodIon-exchange process apparatusOther chemical processesOctanoic AcidsIndustrial waste water
The invention relates to a method of aminopyridine modified resin for adsorbing rhodium octanoate dimer and metal ions. The aminopyridine modified resin serves as an adsorbent, and adsorbs rhodium or the metal ions from a solution containing rhodium octanoate dimer or other metal ions on the surface, and metal is effectively recovered. The method comprises the steps of aminopyridine modified resin preparation and pretreatment. In an adsorbing step, the aminopyridine modified resin can be used independently or filled as an adsorbing column. The resin has good adsorbability for the metal ions, particularly rhodium octanoate dimer in industrial waste water, rhodium octanoate dimer can be directly recovered, and a rhodium octanoate dimer preparation process of condensing, burning and pickling rhodium octanoate dimer in the solution to prepare rhodium chloride to react with octanoic acid. The method is simple, and is very easy to implement in a laboratory or middle and small chemical plants.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Aqueous composition for plasticizing paint prior to strip
InactiveUS6153573AHigh speedImprove stripping efficiencyOther chemical processesTransportation and packagingPolymer scienceOctanoic Acids
PCT No. PCT / GB96 / 03219 Sec. 371 Date Sep. 11, 1998 Sec. 102(e) Date Sep. 11, 1998 PCT Filed Dec. 23, 1996 PCT Pub. No. WO97 / 24409 PCT Pub. Date Jul. 10, 1997A predominantly aqueous composition for plasticising or softening paint, varnish and similar coatings prior to stripping the coating from a surface includes preferably a combination of the active agents triethylphosphate and a co-agent selected from dimethyl adipate, 1,2,3-propanetriol triacetate, tri-n-butyl citrate, n-octyl acetate, methyl octanoate and 2-ethyl-l-butanol, as an emulsified hydrophobic phase in which the actives are partitioned between the hydrophobic and aqueous phases. The emulsion is preferably stabilized using a non-ionic water-soluble block copolymer surfactant, being a copolymer of more than one alkaline oxide. The composition may be thickened using a rheology control agent. Synthetic smectic clays are particularly suitable. The composition is non-toxic and consists predominantly of water, and yet exhibits effective paint, varnish and lacquer plasticising / softening characteristics.
Owner:ECO SOLUTIONS
Water-milk bilayer essence emulsion and preparation method thereof
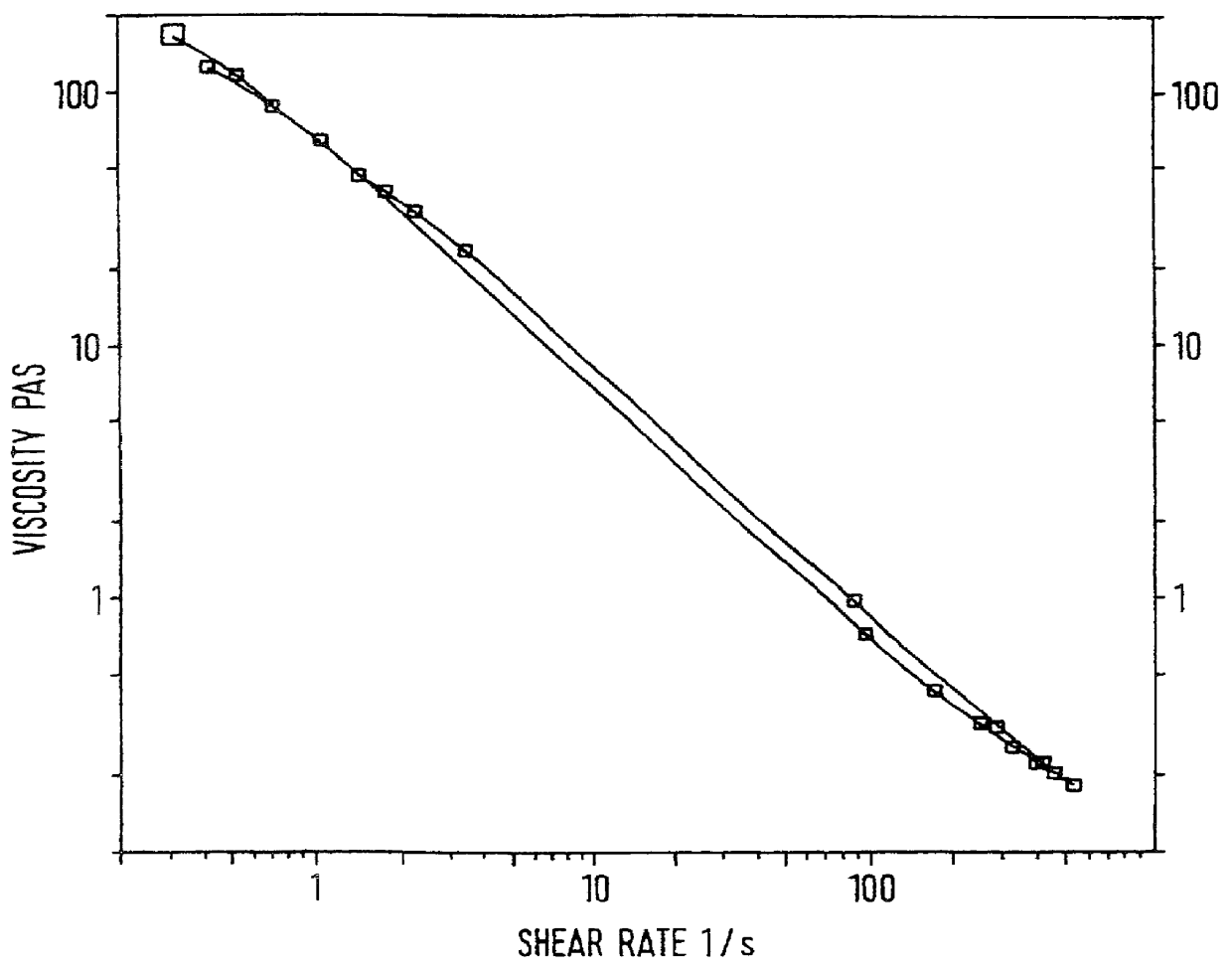
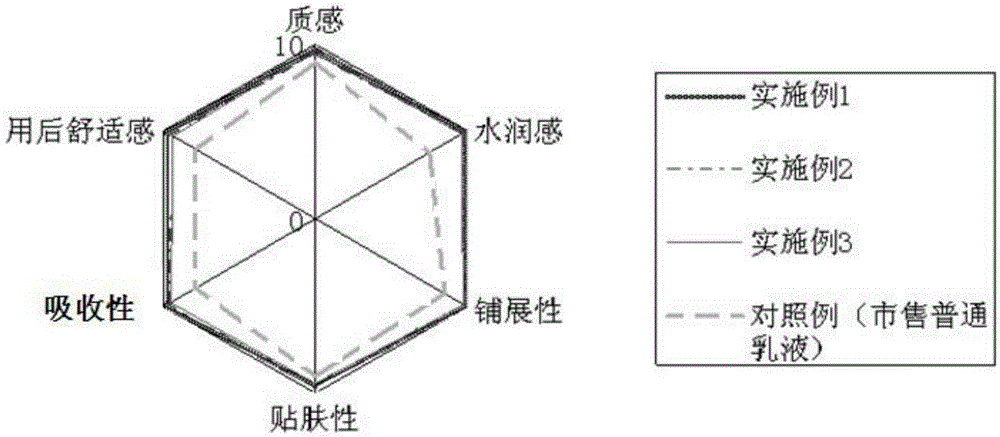
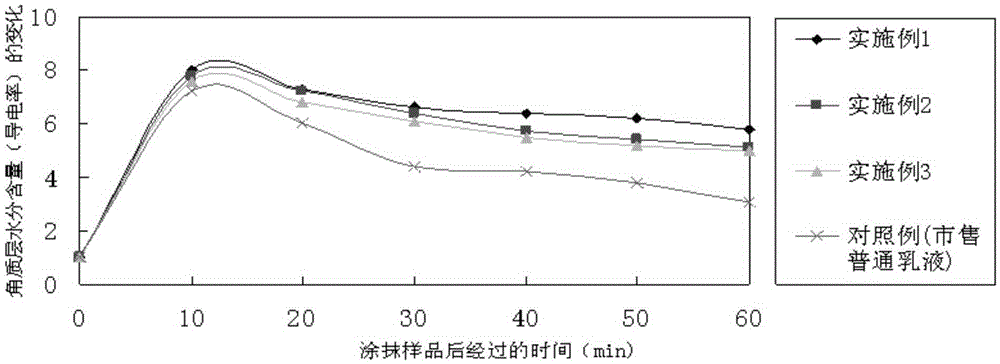
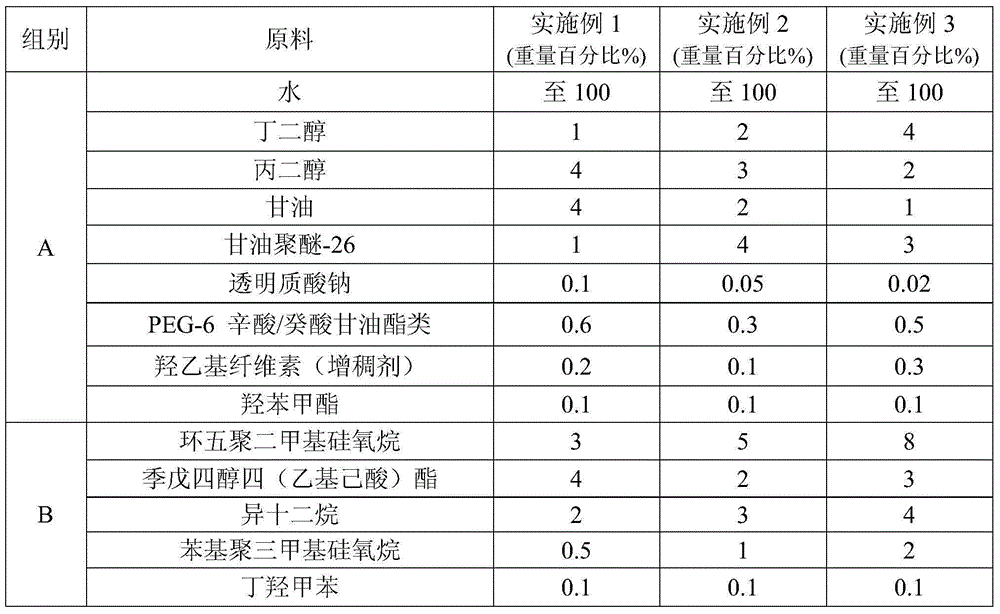
InactiveCN105147528AClear dividing lineNew and beautiful appearanceCosmetic preparationsToilet preparationsPEG-6 Caprylic/capric glyceridesCaprylyl Glycol
The invention provides a water-milk bilayer essence emulsion. The water-milk bilayer essence emulsion is composed of decamethyl cyclopentasiloxane, lubrajel, isododecane, pentaerythritol tetraester (ethylhexoic acid), butanediol, propanediol, glycerin polyether-26, glycerin, oat beta-glucosan, hamamalis water, phenyl trimethicone, phenoxyethanol, PEG-6 octanoic acid / capric acid glyceride, thickener, caprylyl glycol / ethylhexylglycerin, methylparaben, butylated hydroxytoluene, sodium hyaluronate, pigment and water. The water-milk bilayer essence emulsion is novel and beautiful in appearance, capable of realizing quick layering, stable in property and high in moisture retention performance.
Owner:GUANGZHOU SHEENCOLOR COSMETICS CO LTD
Method for preparing human immunoglobulin
ActiveCN102532307ASmall volumeReduce manufacturing costSerum immunoglobulinsPeptide preparation methodsOctanoic AcidsVirus inactivation
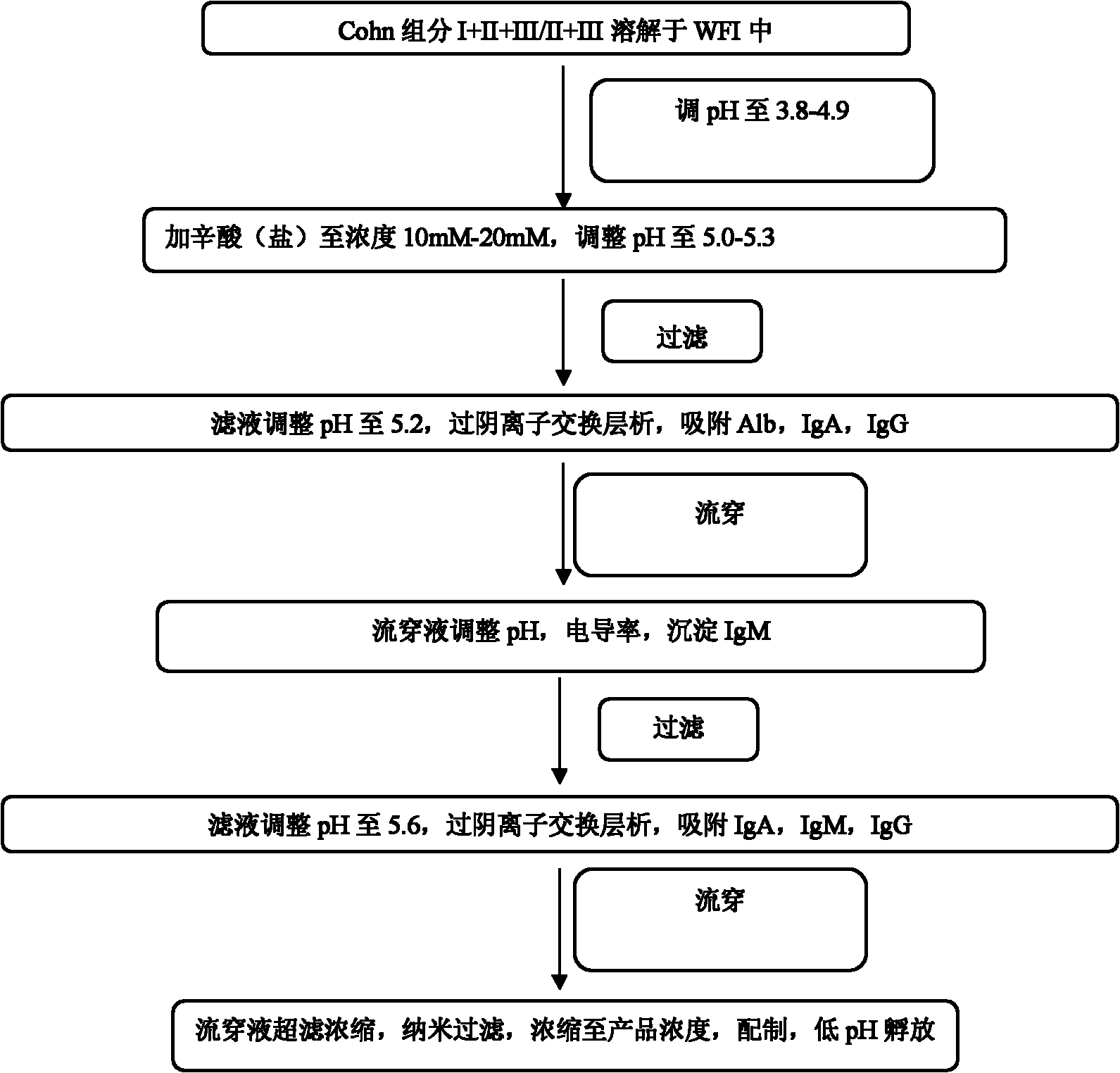
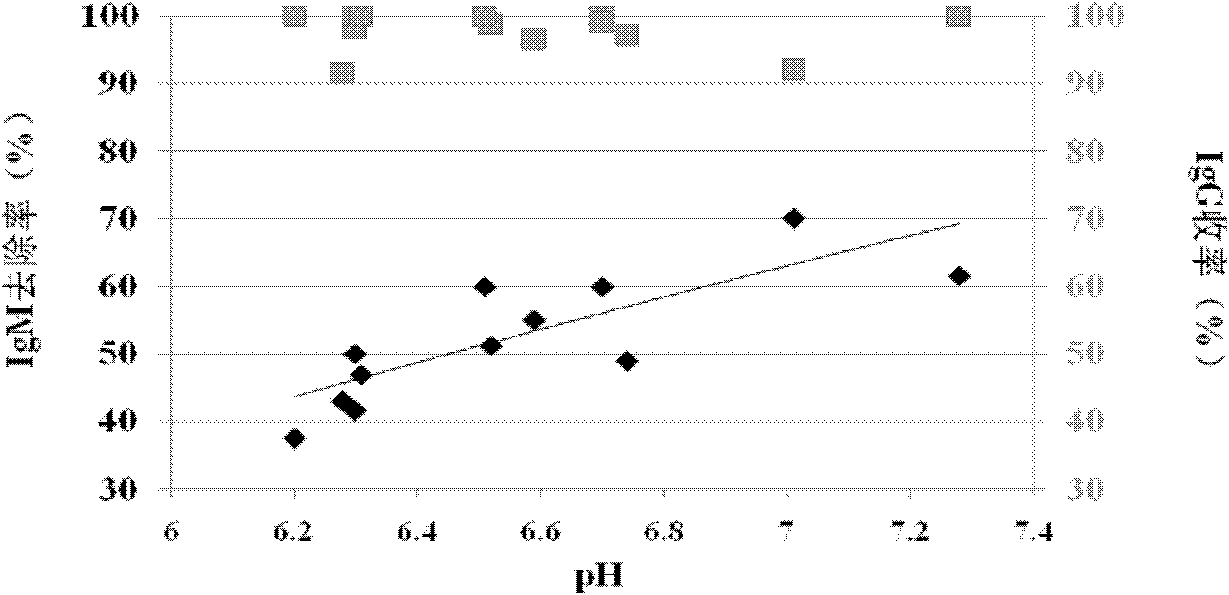
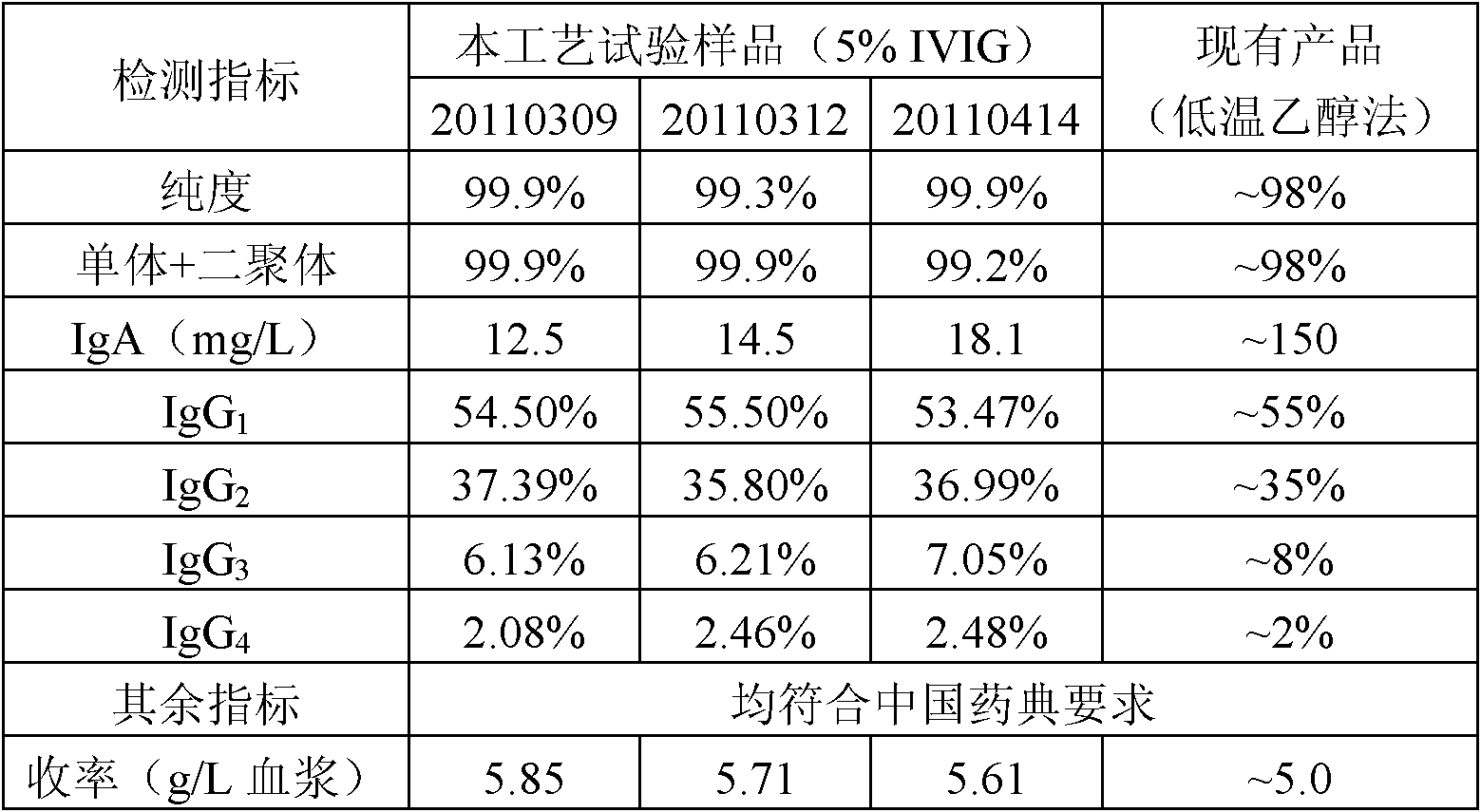
The invention provides a method for preparing human immunoglobulin. The method comprises the following steps of: dissolving Cohn components I, II and III and Cohn components II and III, precipitating by using octanoic acid, performing anion-exchange chromatography for the first time, precipitating IgM, performing anion-exchange chromatography for the second time, performing ultrafiltration or dialyzing, preparing, inactivating virus and the like to obtain the high-purity human immunoglobulin. The invention also provides the human immunoglobulin prepared by the method and a medicinal composition. The method for preparing the human immunoglobulin is simple, and low in cost and has good industrial application prospect.
Owner:CHENGDU RONGSHENG PHARMA
Preparation method of aminopyridine modified resin adsorbing material
InactiveCN103588912ALarge adsorption capacityQuick responseOther chemical processesIndustrial waste waterIon
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Acne cream capable of effectively removing acne and lightening acne mark and preparation method thereof
InactiveCN107753379AReduce the burden onAvoid stayingCosmetic preparationsToilet preparationsOctanoic AcidsMeadowfoam seed oil
The invention relates to acne cream capable of effectively removing acnes and lightening acne marks. The acne cream comprises water, glycerinum, butanediol, a hamamelis virginiana extract, an aloe vera extract, neopentyl glycol diheptanoate, octanoic acid / capric acid triglyceride, meadowfoam seed oil, squalane, macadimia nut oil, butyrospermum parkii kotschy grease, nicotinamide polydimethylsiloxane, cetostearyl alcohol, cetearyl glucoside, sorbitan olivate, cetearyl olivate, sodium hyaluronate, dipotassium glycyrrhizinate, oligopeptides-1, arbutin, L-ascorbyl dipalmitate, a calendula extract,a lavender extract, an acroloyl-dimethyl taurine amino / VP copolymer, a sodium acrylate / acroloyl-dimethyl taurine sodium copolymer, isohexadecane, polysorbate-80, fullerene, an angelica sinensis extract, p-hydroxyacetophenone, 1,2-hexanediol, sweet orange flower oil and EDTA (Ethylene Diamine Tetraacetic Acid). The acne cream is prepared through a series of procedures and has the characteristics of good acne treatment effect, no acne mark, no side effect, and the like.
Owner:达威控股有限公司
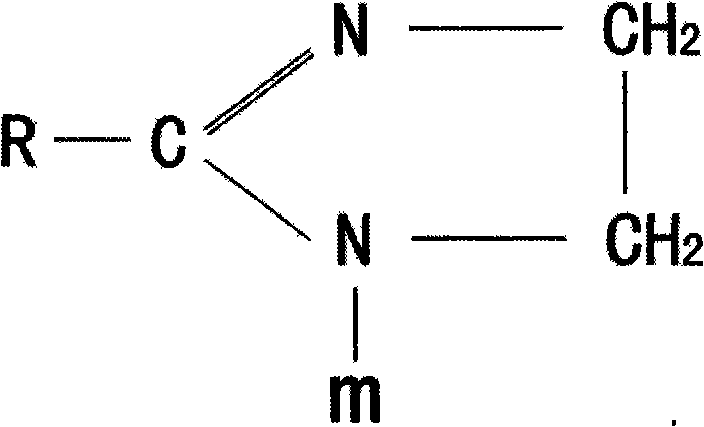
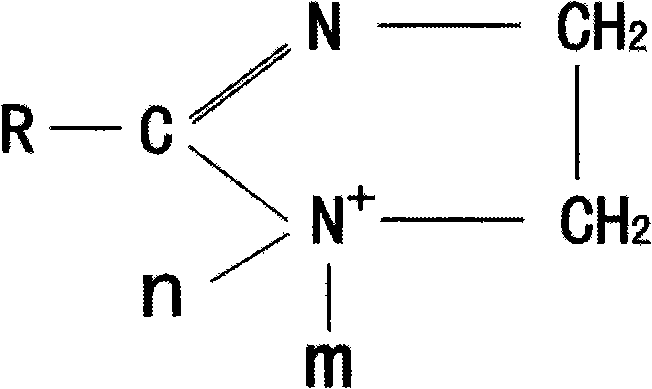
Method for synthesizing imidazoline intermediate and cationic derivative thereof
The invention relates to a method for synthesizing an imidazoline intermediate and a cationic derivative thereof. The method comprises the following process steps of: putting fatty acid, polyamine and water into a reaction kettle in a mole ratio of 1:1.1-2:1-5, heating to the temperature of between 100 and 180 DEG C to distill over the water added before and the water generated in the reaction, removing redundant polyamine and water under a pressure of between -0.04 and -0.1MPa and performing a reaction at a temperature of between 180 and 240 DEG C for 3 to 8 hours to synthesize the imidazoline intermediate; and reducing the temperature of the imidazoline intermediate to be below 80 DEG C, adding a solvent of which the mass is 30 to 100 percent of the imidazoline intermediate into the imidazoline intermediate, then adding 1 to 2mol of sulfate into the mixture and performing heat preservation reaction at the temperature of between 50 and 80 DEG C for 2 to 4 hours to synthesize cationic imidazoline. The fatty acid is oleic acid, linoleic acid, stearic acid, palmitic acid, lauric acid, coconut oil acid, capric acid and octanoic acid; the polyamine is ethylenediamine, diethylenetriamine, triethylene tetramine and tetraethylene pentamine; the solvent is isopropanol, absolute ethyl alcohol or 95 percent ethanol; and the sulfate is dithyl sulfate and dimethyl sulfate.
Owner:苗俊良
Antifreeze Concentrate and Coolant Compositions and Preparation Thereof
InactiveUS20090001313A1Improve thermal stabilityOther chemical processesHeat-exchange elementsBenzoic acidOctanoic Acids
A toxicological friendly antifreeze composition having improved thermal stability is provided. In one embodiment, the antifreeze composition comprises from 5 to 80 wt. % of an aqueous freezing point depressant selected from alkali metal salts of acetates, formates, proprionates, adipiates, and succinates, and mixtures thereof; 0.1 to 10 wt. % of at least one of a 2-ethylhexanoic acid, isononanoic acid and 3,5,5-trimethylhexanoic acid; and 0.1 to 10 wt. % of at least one of octanoic acid, nonanoic acid, decanoic acid, undecanoic acid, dodecanoic acid, neodecanoic acid, benzoic acid, 2-hydroxybenzoic acid, p-terbutylbenzoic acid, and mixtures thereof. In one embodiment, the composition is employed as a concentrate in admixture with 10 to 90 wt. % water.
Owner:CHEVROU USA INC
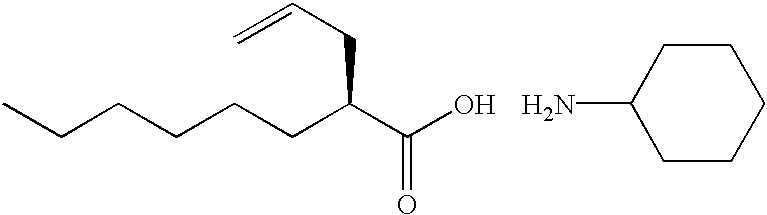
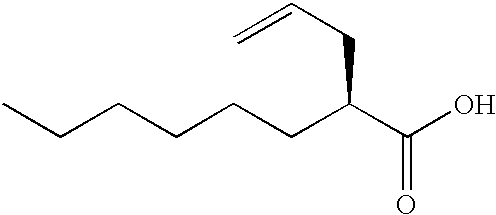
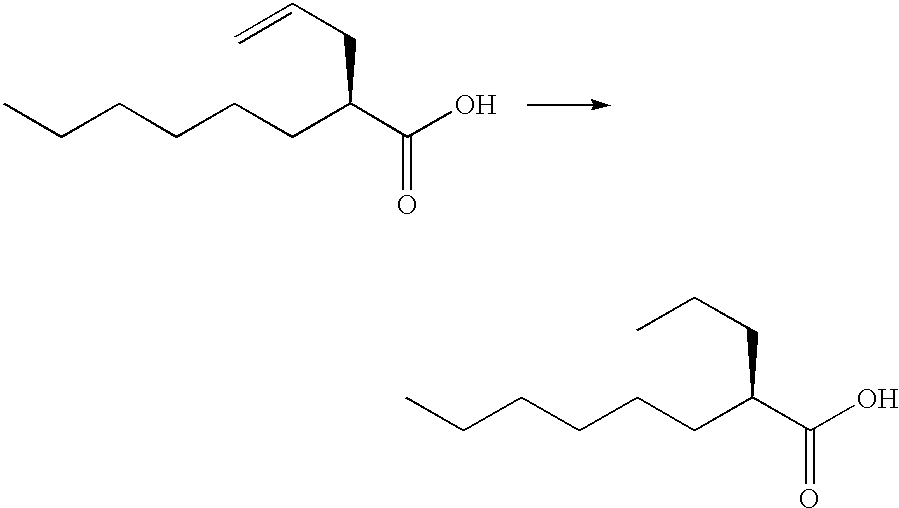
Process for the preparation of (2R)-2-propyloctanoic acid
InactiveUS6608221B1High optical purityNervous disorderOrganic compound preparationIsomerizationOctanoic Acids
A process for the preparation of (2R)-2-propyloctanoic acid which is characterized by subjecting (2S)-2-(2-propynyl)octanoic acid or (2S)-2-(2-propenyl)octanoic acid to reduction using platinum on carbon. This process gives (2R)-2-propyloctanoic acid having high optical purity without isomerization.
Owner:ONO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com