Method for preparing human immunoglobulin
A technology of human immunoglobulin and exchange chromatography, applied in the field of preparation of blood products, can solve the problems of high cost, difficult to realize large-scale industrial production, large volume of chromatography column, etc., and achieves low volume, good market application prospect, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
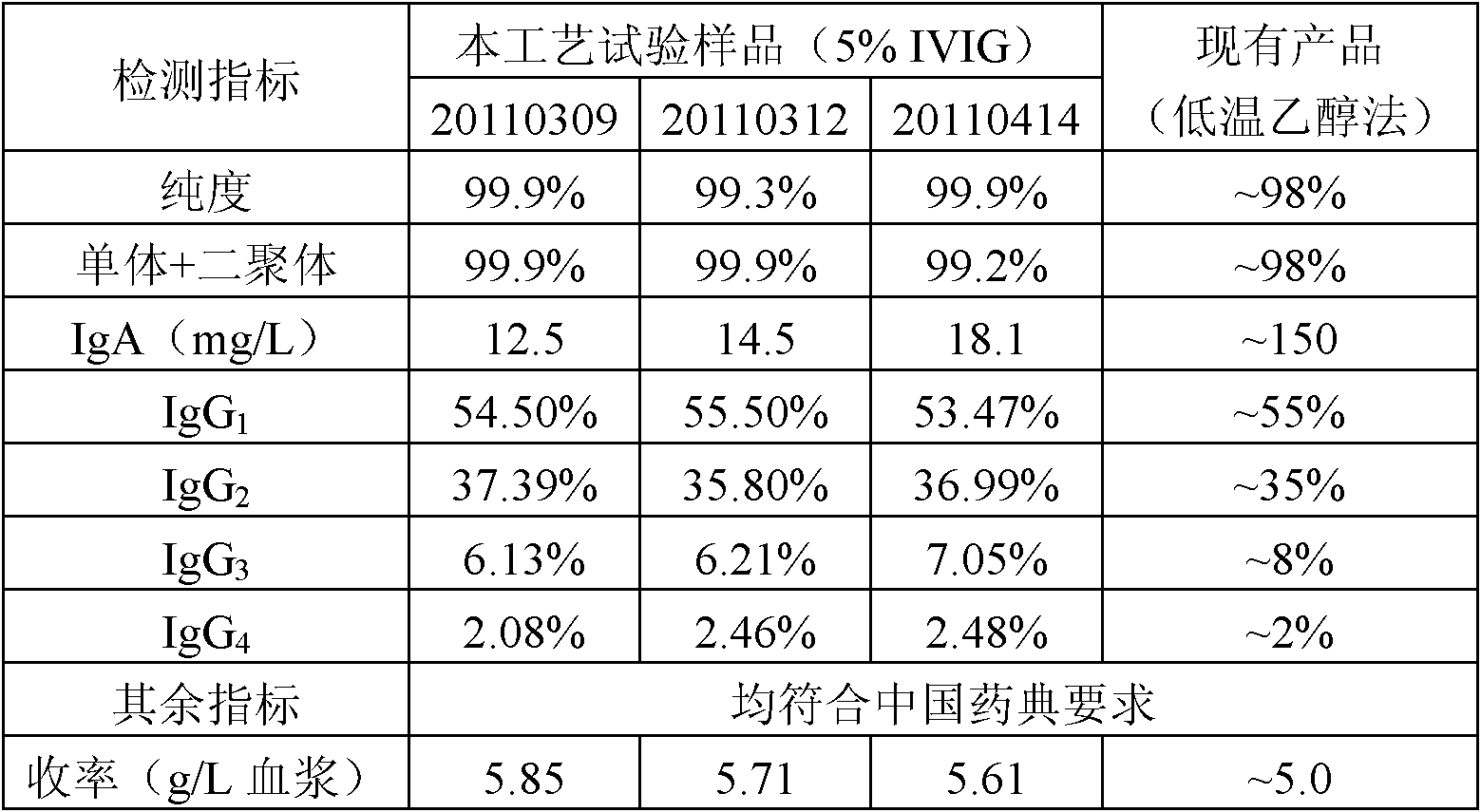
[0027] Embodiment 1 prepares human immunoglobulin by the method of the present invention
[0028] 1. Experimental materials
[0029] Cohn fraction I+II+III or fraction II+III: prepared according to the low-temperature ethanol precipitation method described in "Medical Biological Products" (People's Health Publishing House), second edition, page 1194.
[0030] Hydrochloric acid, caprylic acid, Capto Q filler, Gigacap Q filler, Unosphere Q filler, Macrocap Q, diatomaceous earth and Beckman IMMAGE IgA detection kit are all commercially available.
[0031] 2. Experimental method
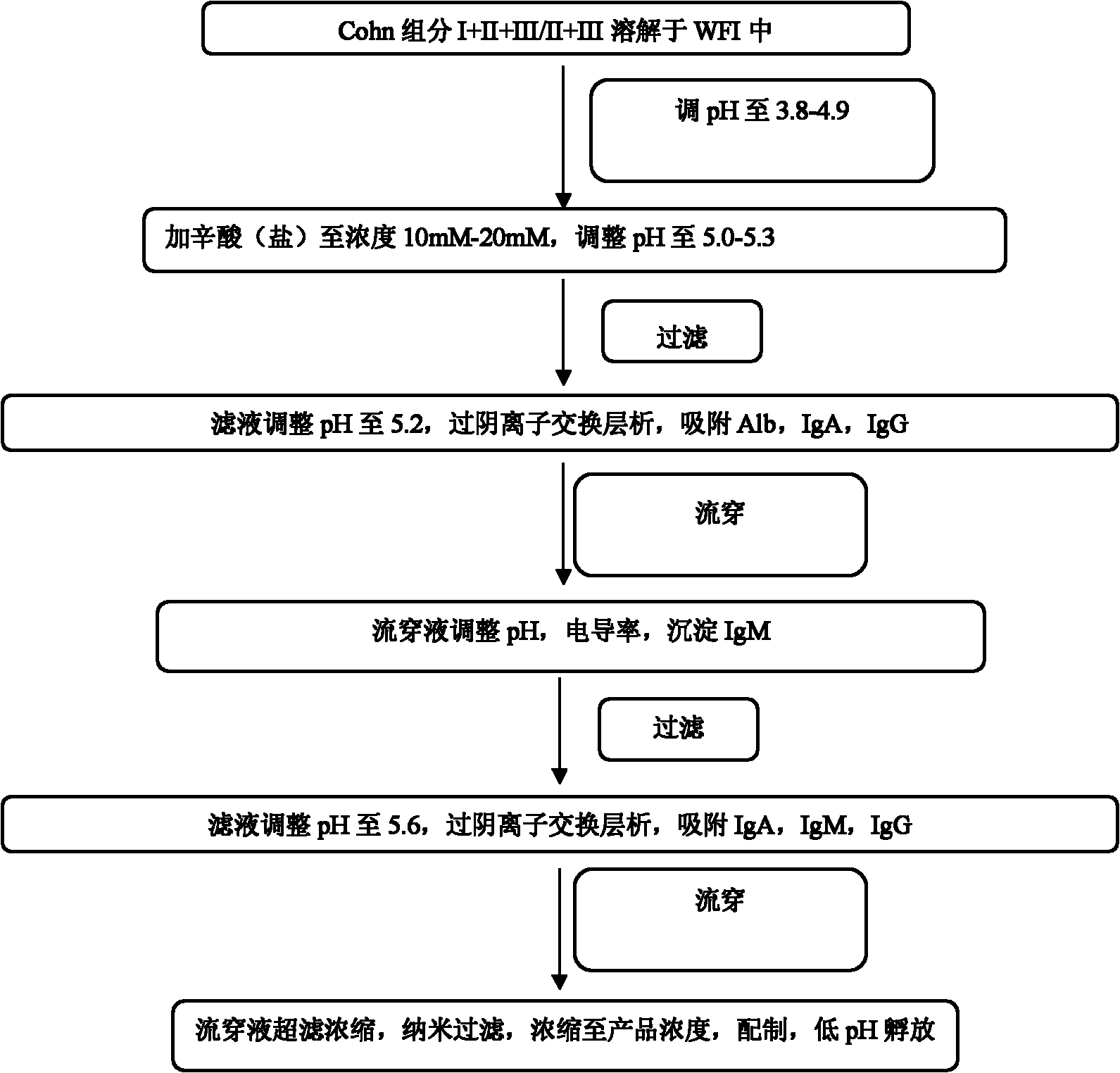
[0032] Such as figure 1 Prepare human immunoglobulins as indicated:
[0033] (1) Cohn Component I+II+III: Take 4kg Cohn Component I+II+III precipitate and dissolve it in 40L 5℃ WFI (Water for Injection), stir for 1h, use 0.5M hydrochloric acid to adjust the pH to 4.20, 25℃ water bath Heat and stir for 2h;
[0034] (2) Octanoic acid precipitation: directly add octanoic acid to a final concentration o...
Embodiment 2
[0049] Embodiment 2 parameter optimization experiment
[0050] Conductivity and pH value in the method step (4) of the present invention precipitate IgM and screen:
[0051] (1) Conductivity optimization experiment
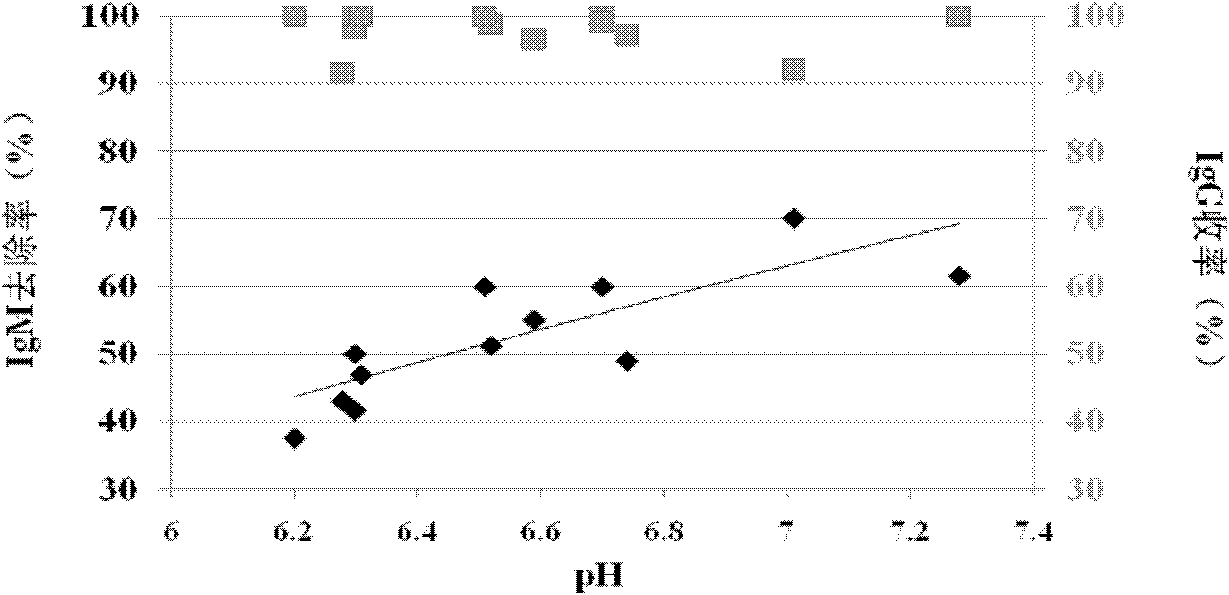
[0052] Experimental method: other conditions are constant, change the conductivity and pH in step (4) of Example 1, and detect the relationship between conductivity and IgM precipitation and IgG yield.
[0053] Experimental results: It has been observed through multiple experiments that when the conductivity of the chromatography flow-through solution is higher than 1ms / cm, IgM will not precipitate even if the pH of the solution is increased. When the conductivity of the chromatographic flow-through solution is between 500 and 1000 us / cm, adjust the pH to above 6.0, and IgM will be obviously precipitated.
[0054] (2) pH optimization experiment
[0055] Experimental method: other conditions are constant, change the pH in step (4) of Example 1, and detect the rela...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com