Detection method for related substances of salbutamol sulfate solution used for inhalation
A technology of salbutamol sulfate and detection method, applied in the detection field, can solve problems such as less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Solution preparation:
[0070] Diluent: mobile phase A.
[0071] Test solution: Take 1 stick of this product, accurately measure 1ml, put it in a 10ml measuring bottle, dilute to the mark with diluent, shake well, and you will get it.
[0072] System suitability solution: Accurately weigh an appropriate amount of 5-hydroxysalbutamol, impurity J, and salbutamol sulfate reference substance, dissolve and dilute with a diluent to make each 1ml contain 0.2 mg of salbutamol and 0.4 μg of impurities (including 5-hydroxysalbutamol) respectively mixed solution, as a system suitability solution.
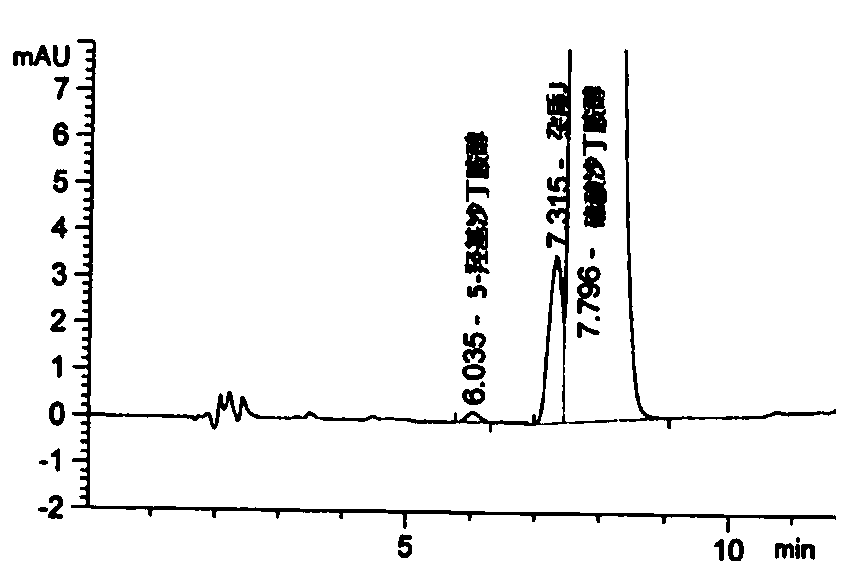
[0073] (1) Condition 1: Implement the chromatographic method of the import registration standard JX20160145
[0074] The method is confirmed, the chromatographic conditions are shown in Table 1, and the test results are shown in figure 1 .
[0075] Table 1 JX20160145 method confirmation chromatographic conditions
[0076]
[0077] The chromatographic method of JX20160145 was co...
Embodiment 2
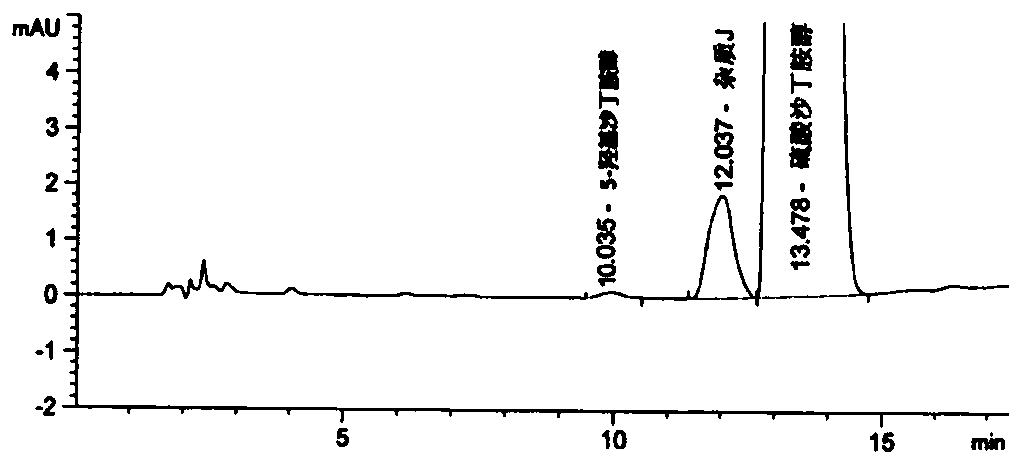
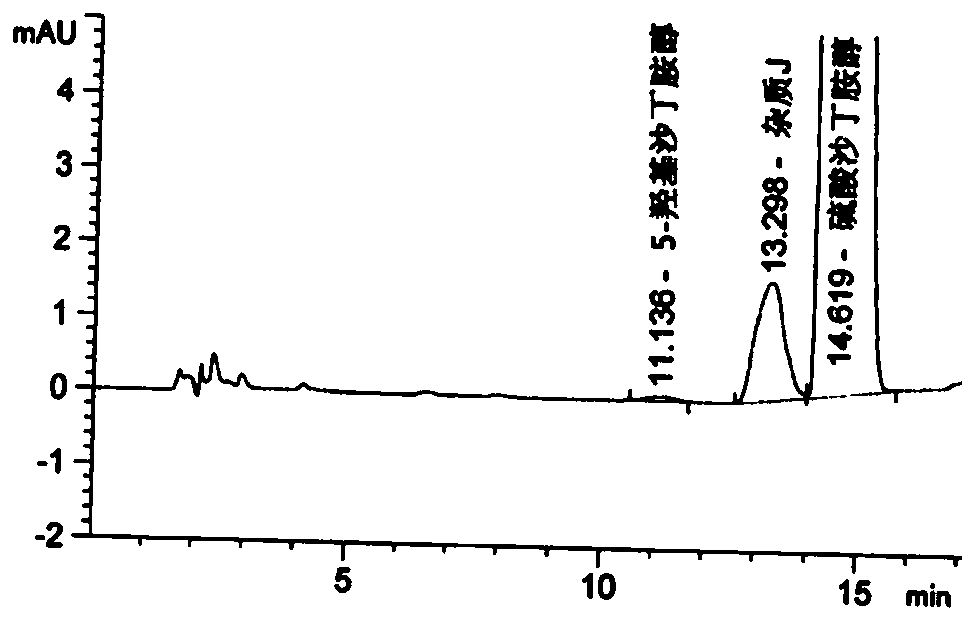
[0104] Embodiment 2 is to the detection of crude drug
[0105] Adopt the chromatographic conditions in table 6:
[0106] Table 6
[0107]
[0108] Image 6 It can be seen that the purity of the raw material drug is very high and hardly contains any impurities.
[0109] Methodology validation report:
[0110] Table 7 Summary of verification items and results
[0111]
[0112]
[0113] After the verification of the analytical method, the specificity of the method is good, the blank excipients do not interfere with the detection of impurities, the separation of each impurity and the main peak is good, the detection limit and quantification limit of each impurity meet the determination requirements and the linearity of each impurity is good; the method is proved by fine-tuning the chromatographic conditions Good durability.
[0114] In summary, the detection method of the present invention is better than the chromatographic conditions in the existing import registrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com