Preparing method of ambroxol salbutamol oral liquid
A technology of ambroxosalbutamol oral liquid and salbutamol sulfate, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as poor stability, unstable effect, and difficult quality control. problem, to achieve stable quality, remarkable effect, and the effect of treating respiratory diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
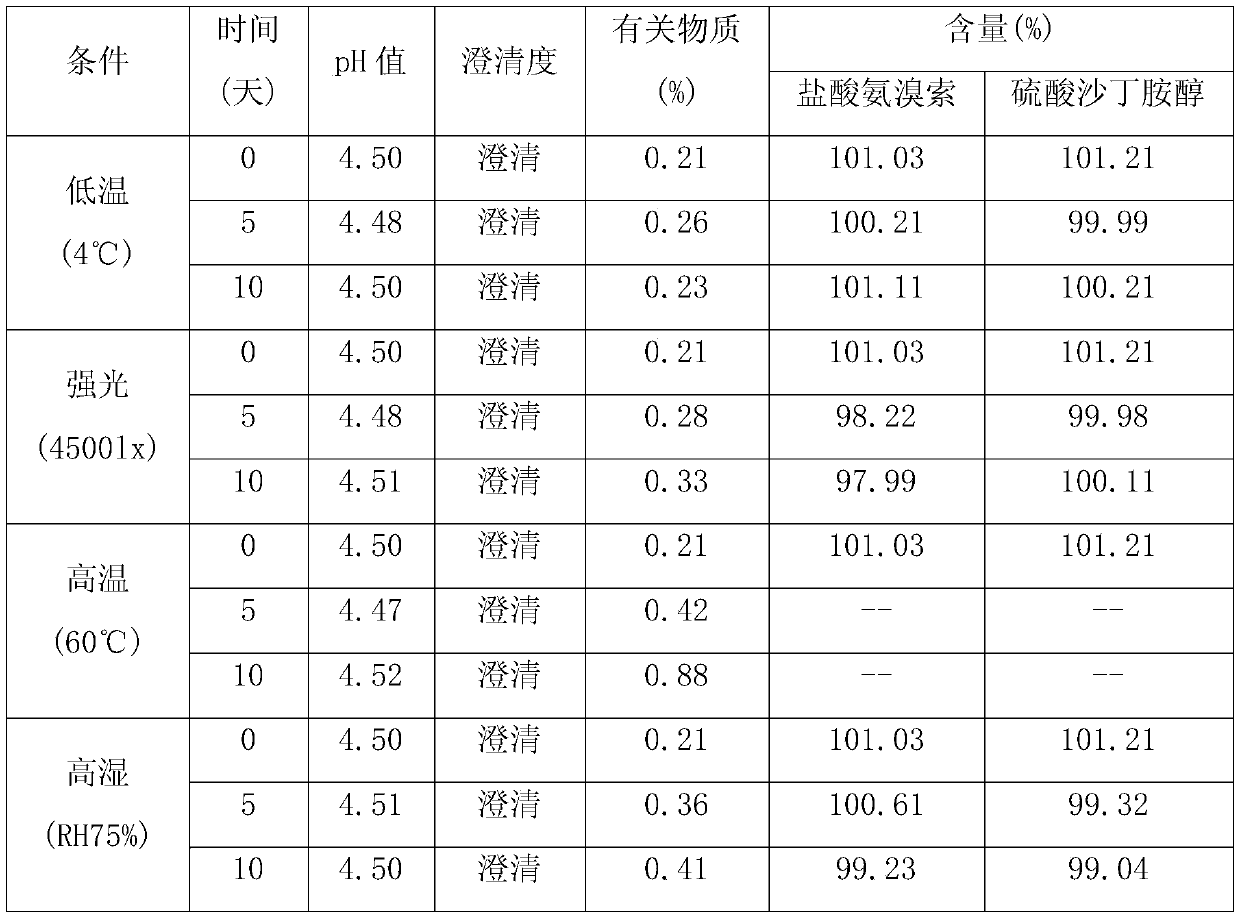
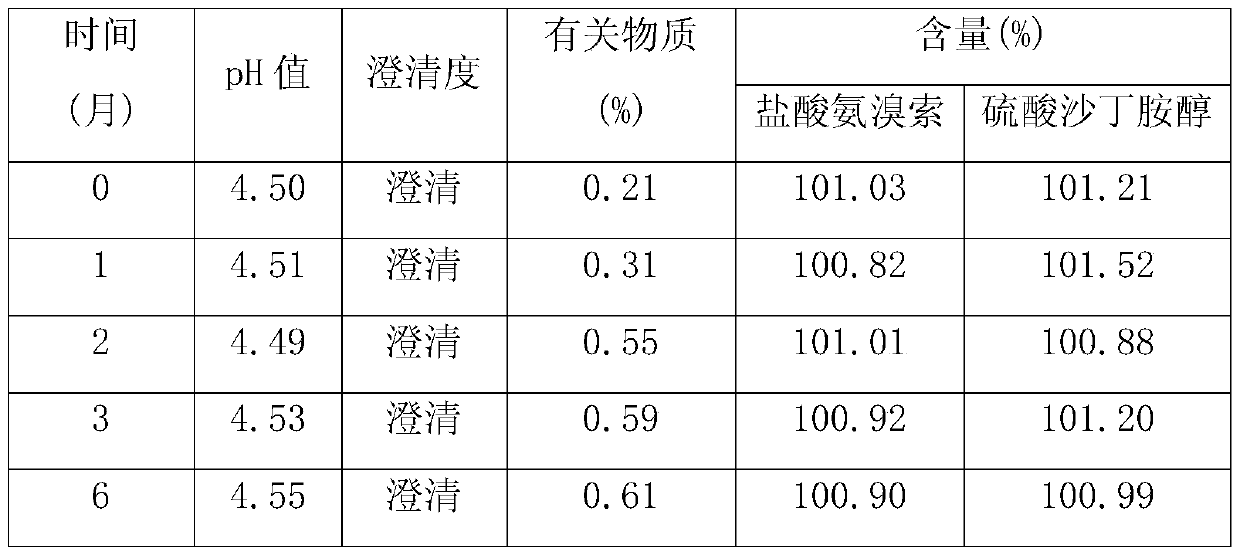
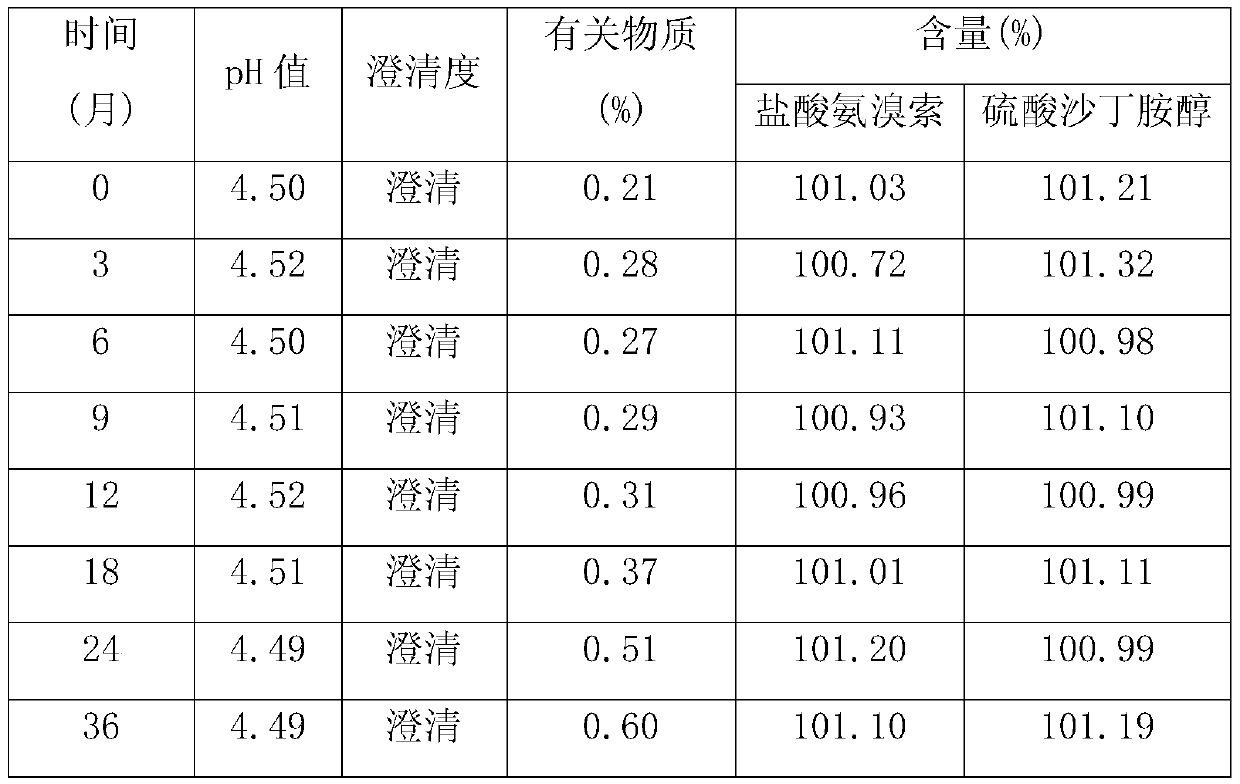
[0017] Weigh each component according to the following proportions: 2 g of ambroxol hydrochloride, 2 g of salbutamol sulfate, 3 g of propylene glycol, 0.5 g of glycerin, 0.2 g of methyl p-hydroxybenzoate, 0.3 g of sodium benzoate, 1 g of mannitol, 2 g of stevia, orange Essence 0.5g, appropriate amount of pH regulator, appropriate amount of purified water; take about 2 / 3 of purified water, add ambroxol hydrochloride, stir to dissolve completely, add salbutamol sulfate, propylene glycol, glycerin, methyl p-hydroxybenzoate, benzene Sodium formate, mannitol, stevioside and orange essence, stir evenly, adjust the pH value to about 4.0 with a pH regulator, add purified water to the full amount, filter with a 0.45μm microporous membrane, fill into an oral liquid bottle, seal, After passing the inspection, it is packaged and ready to be obtained.
Embodiment 2
[0019] Each component was weighed according to the following ratio: 5g of ambroxol hydrochloride, 3.6g of salbutamol sulfate, 2.5g of propylene glycol, 1g of glycerin, 0.3g of methyl p-hydroxybenzoate, 0.2g of sodium benzoate, 2g of mannitol, 3g of stevia, Orange flavor 0.2g, appropriate amount of pH regulator, appropriate amount of purified water; take about 2 / 3 of purified water, add ambroxol hydrochloride, stir to dissolve completely, add salbutamol sulfate, propylene glycol, glycerin, methyl p-hydroxybenzoate, Sodium benzoate, mannitol, stevia and orange flavor, stir well, adjust the pH value to about 4.5 with a pH regulator, add purified water to the full amount, filter with a 0.45μm microporous membrane, fill it into an oral liquid bottle, and seal it , passed the inspection, packaged, and obtained.
Embodiment 3
[0021] Weigh each component according to the following ratio: Ambroxol hydrochloride 7g, salbutamol sulfate 2g, propylene glycol 4g, glycerin 2g, methyl p-hydroxybenzoate 0.4g, sodium benzoate 0.1g, mannitol 3g, stevia 5g, orange essence 0.3g, appropriate amount of pH regulator, appropriate amount of purified water; take about 2 / 3 of purified water, add ambroxol hydrochloride, stir to dissolve completely, add salbutamol sulfate, propylene glycol, glycerin, methyl p-hydroxybenzoate, sodium benzoate , mannitol, stevia and orange essence, stir well, adjust the pH value to about 6 with a pH regulator, add purified water to the full amount, filter with a 0.45μm microporous membrane, fill it into an oral liquid bottle, seal it, and inspect Qualified, packed, ready to go.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com