Salbutamol sulfate impurity and preparation method thereof
A salbutamol sulfate and impurity technology, applied in the field of drug synthesis, can solve the problems of preventing widespread use and high cost, and achieve the effects of simple operation, good reaction repeatability, and mild and controllable reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
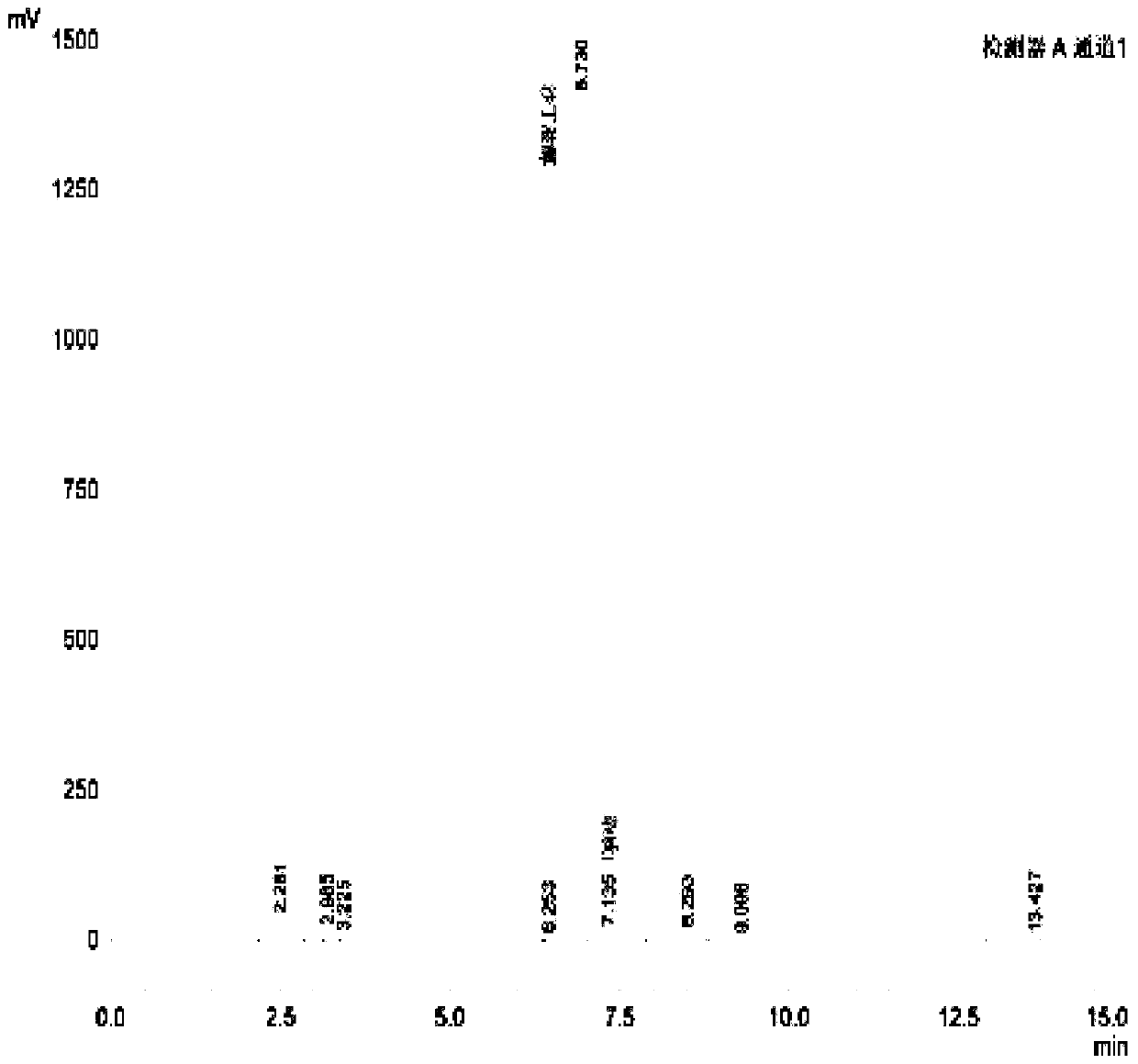
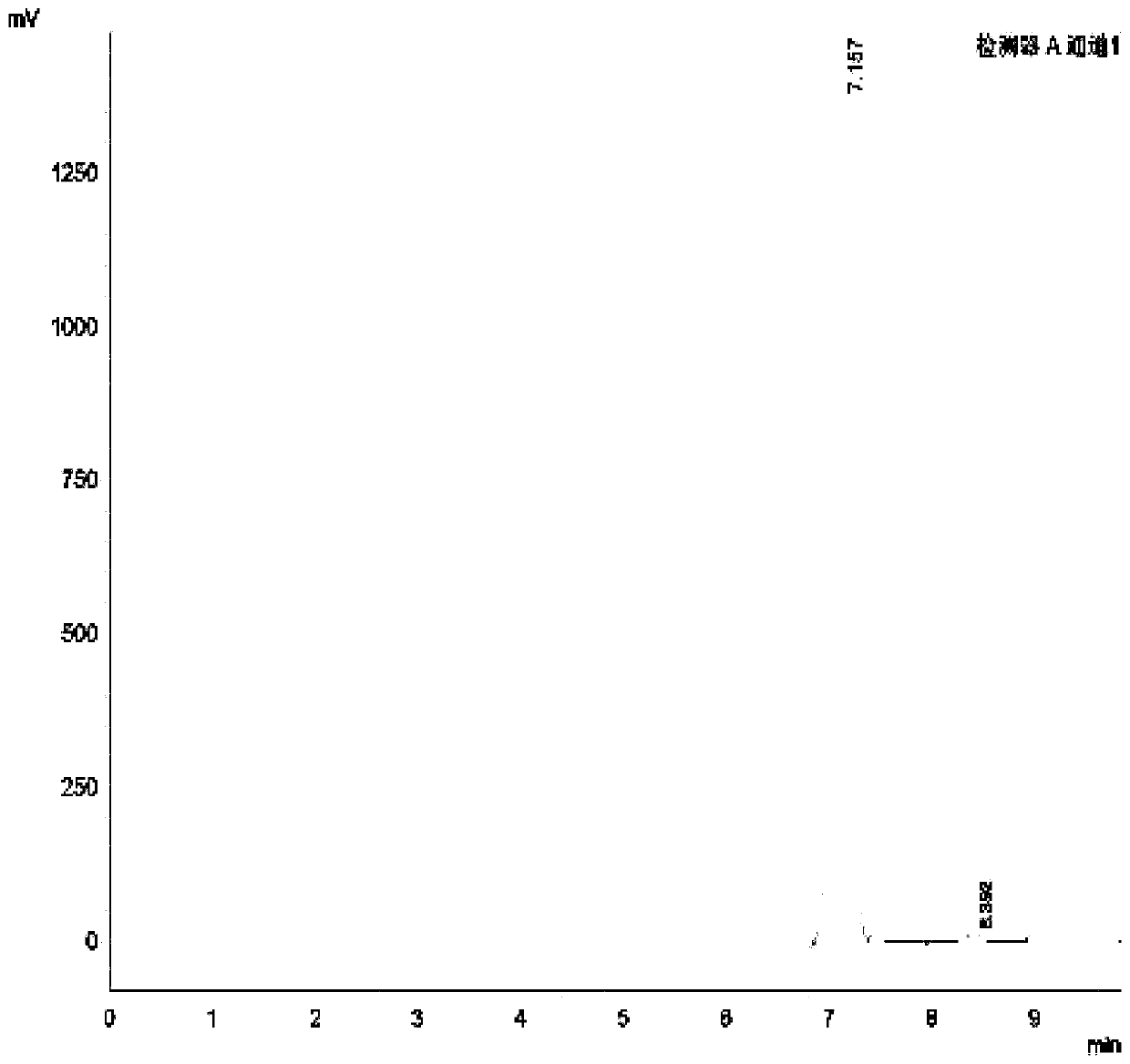
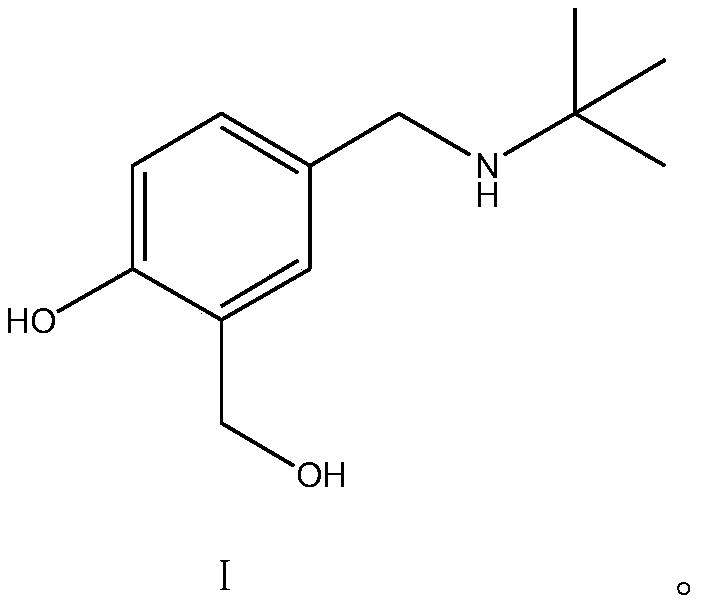
[0023] The preparation method of formula I compound (4-[(tert-butylamino) methyl]-2-(hydroxymethyl)phenol), comprises the steps:
[0024] 1. Dissolve 30.43g (0.2mol) of the compound of formula II in 120mL of ethanol, add an appropriate amount of 4A molecular sieve at a temperature below 10°C, stir evenly, add 21.94g (0.3mol) of tert-butylamine dropwise, and then heat up to 45°C, After reacting for 3 hours, the raw material (4-hydroxy-3-hydroxymethylbenzaldehyde) was monitored by TLC until the reaction was complete. Finally, the solvent in the reaction solution was concentrated to dryness without further purification to obtain 40.37 g (0.19 mol) of the compound of formula III.
[0025]
[0026] 2. Dissolve 20.72g (0.1mol) of the compound of formula III in 50mL of ethanol, add 9.08g (0.24mol) of sodium borohydride in batches at a controlled temperature of 0-20°C, slowly raise the temperature to 50°C for 4 hours, and then use 10 % dilute sulfuric acid to adjust the pH to 2-3, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com