Process for preparing salbutamol sulfate orally disintegrating tablets
A technology of albuterol sulfate and orally disintegrating tablets, which is applied in the field of medicine, can solve problems such as difficult industrial production, unqualified friability, and poor disintegration effect, and achieve low operating costs, low cost, and shortened disintegration time limit Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
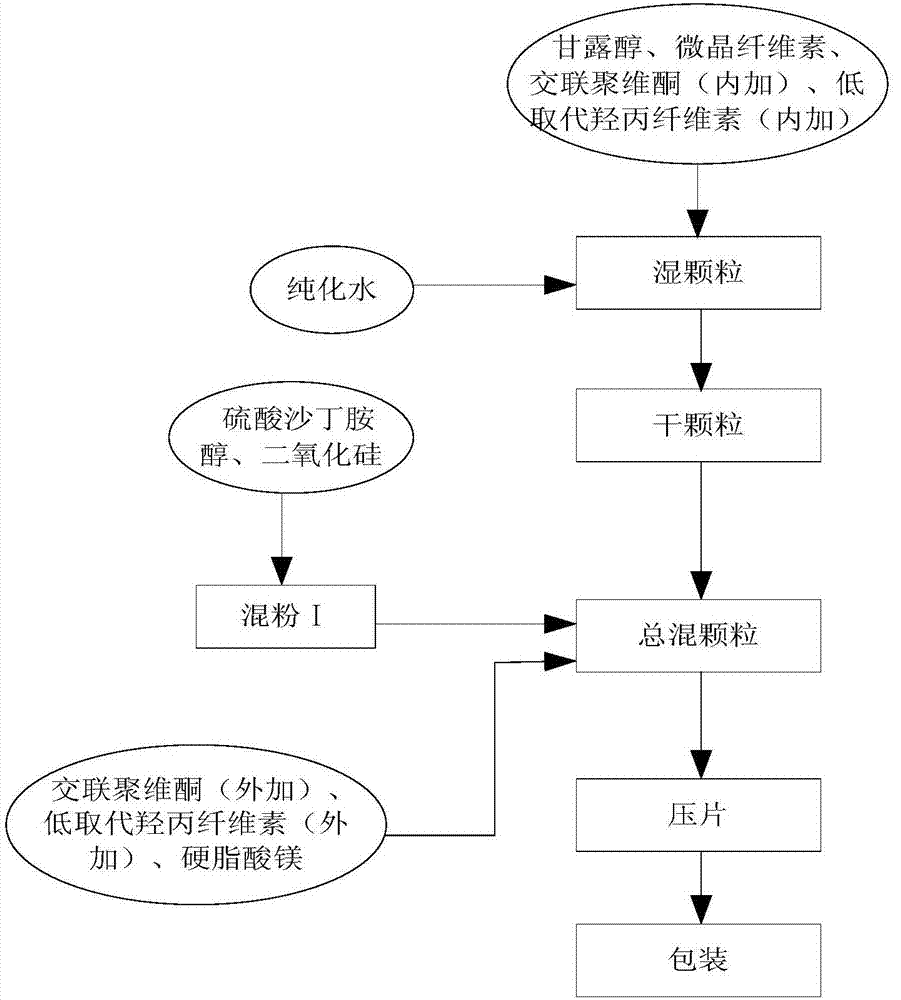
[0022] The preparation process of salbutamol sulfate orally disintegrating tablets is characterized in that it comprises the following steps:
[0023] S1. Weigh salbutamol sulfate, mannitol and microcrystalline cellulose, purified water, crospovidone and low-substituted hydroxypropyl cellulose, silicon dioxide and magnesium stearate according to the ratio; each component is calculated by weight percentage: Salbutamol sulfate 0.5 to 5%, silicon dioxide 0.5 to 5%, mannitol 10 to 65%, microcrystalline cellulose 10 to 65%, low-substituted hydroxypropyl cellulose 2 to 10%, crospovidone 5 to 20 %, 0.5-2% magnesium stearate;
[0024] S2. Mix salbutamol sulfate and silicon dioxide, pour out and pass through a 120-mesh sieve, which is mixed powder I;
[0025] S3. Mix mannitol, microcrystalline cellulose, cross-linked povidone and low-substituted hydroxypropyl cellulose with a total amount of 30-80%, add binder purified water and stir through 20-40 mesh sieve to make wet granules ;
[0026] S...
Embodiment 1
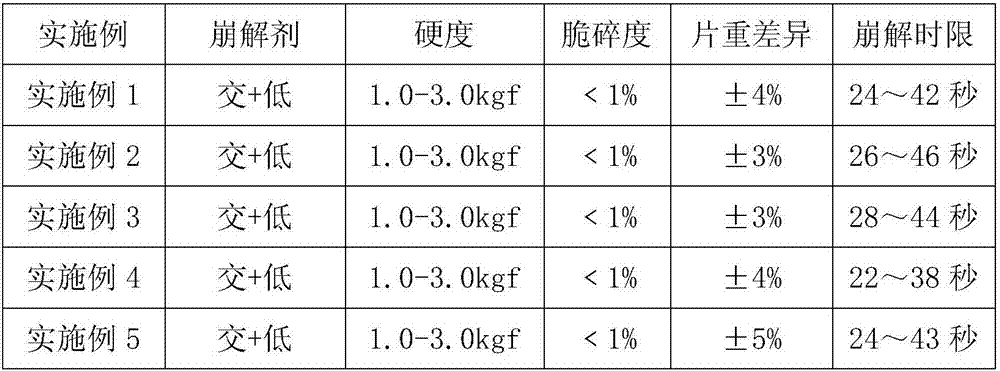
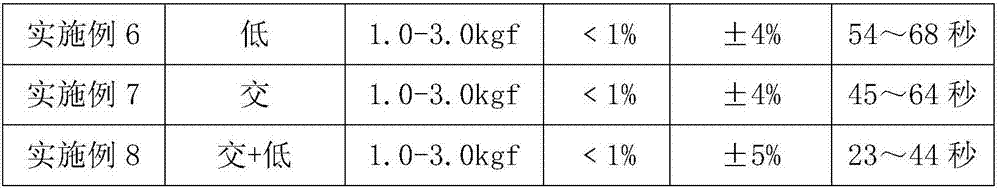
[0031] Example 1: Salbutamol sulfate orally disintegrating tablets, the disintegrant is crospovidone and low-substituted hydroxypropyl cellulose, wherein the distribution ratio of each component is calculated as weight percentage: salbutamol sulfate 0.9%, mannitol 62.5%, micro Crystal cellulose 15.6%, crospovidone 10.2%, low-substituted hydroxypropyl cellulose 7.8%, silicon dioxide 2%, magnesium stearate 1%.
[0032] A total of 1,000 tablets were prepared with the above formula with a specification of 64 mg. There was no stickiness during the compression process. Five volunteers complained of a sweet taste in a blind test, all with a smooth feeling but no grit feeling. The measured tablet hardness is 1.0-3.0kgf, the friability is less than 1%, the tablet weight difference is ±4%, and the disintegration time limit is 24 to 42 seconds.
Embodiment 2
[0033] Example 2: Salbutamol sulfate orally disintegrating tablets, the disintegrants are crospovidone and low-substituted hydroxypropyl cellulose, and the distribution ratio of each component is calculated as weight percentage: salbutamol sulfate 0.9%, mannitol 58.5%, micro Crystal cellulose 15.6%, crospovidone 20%, low-substituted hydroxypropyl cellulose 2%, silicon dioxide 2%, and magnesium stearate 1%.
[0034] A total of 1,000 tablets were prepared with the above formula with a specification of 64 mg. There was no stickiness during the compression process. Five volunteers complained of a sweet taste in a blind test, all with a smooth feeling but no grit feeling. The measured tablet hardness is 1.0-3.0kgf, the friability is less than 1%, the tablet weight difference is ±3%, and the disintegration time limit is 26 to 44 seconds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com