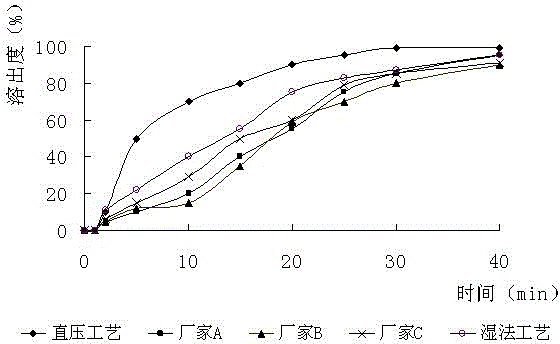
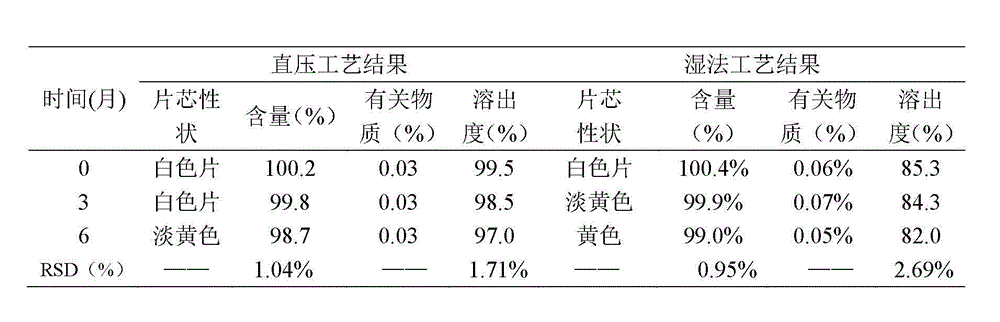
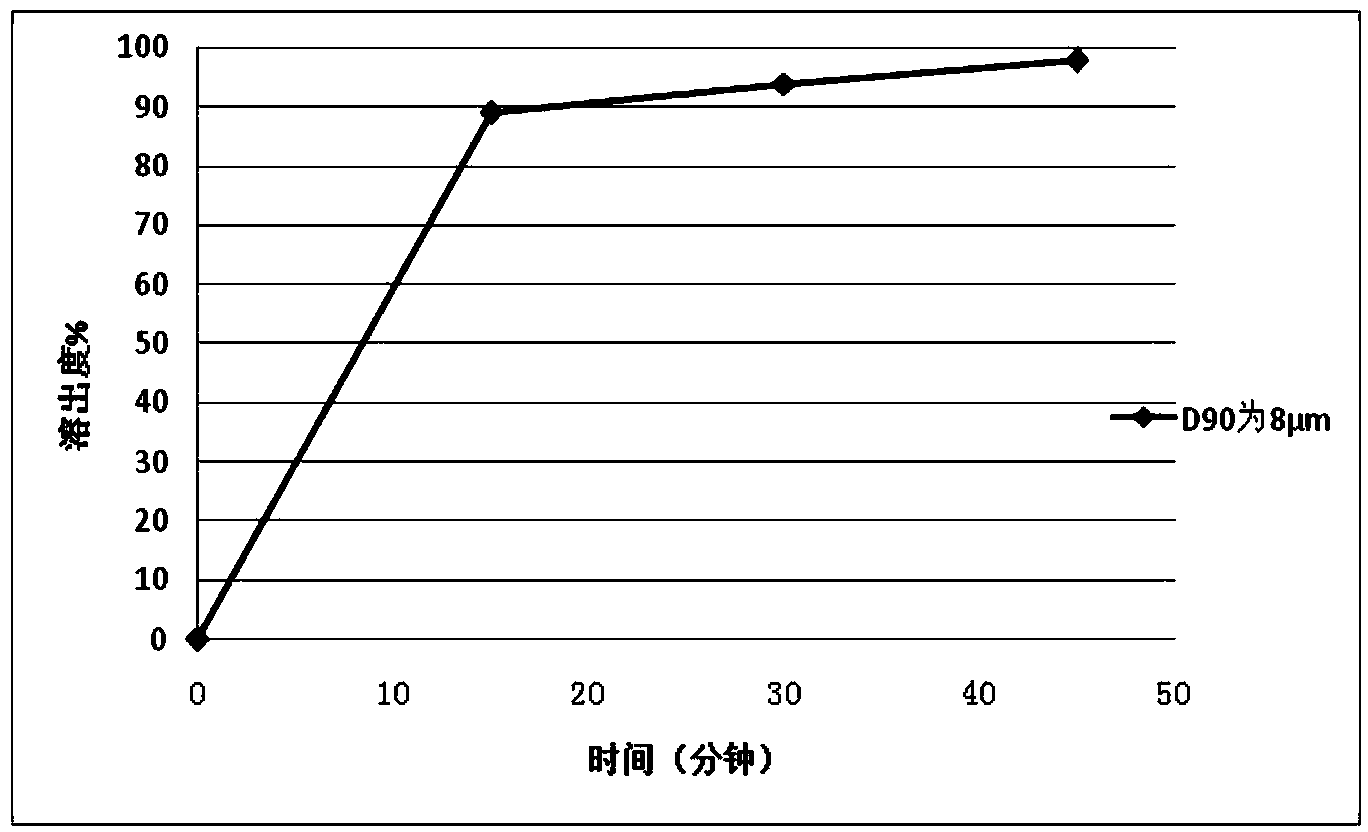
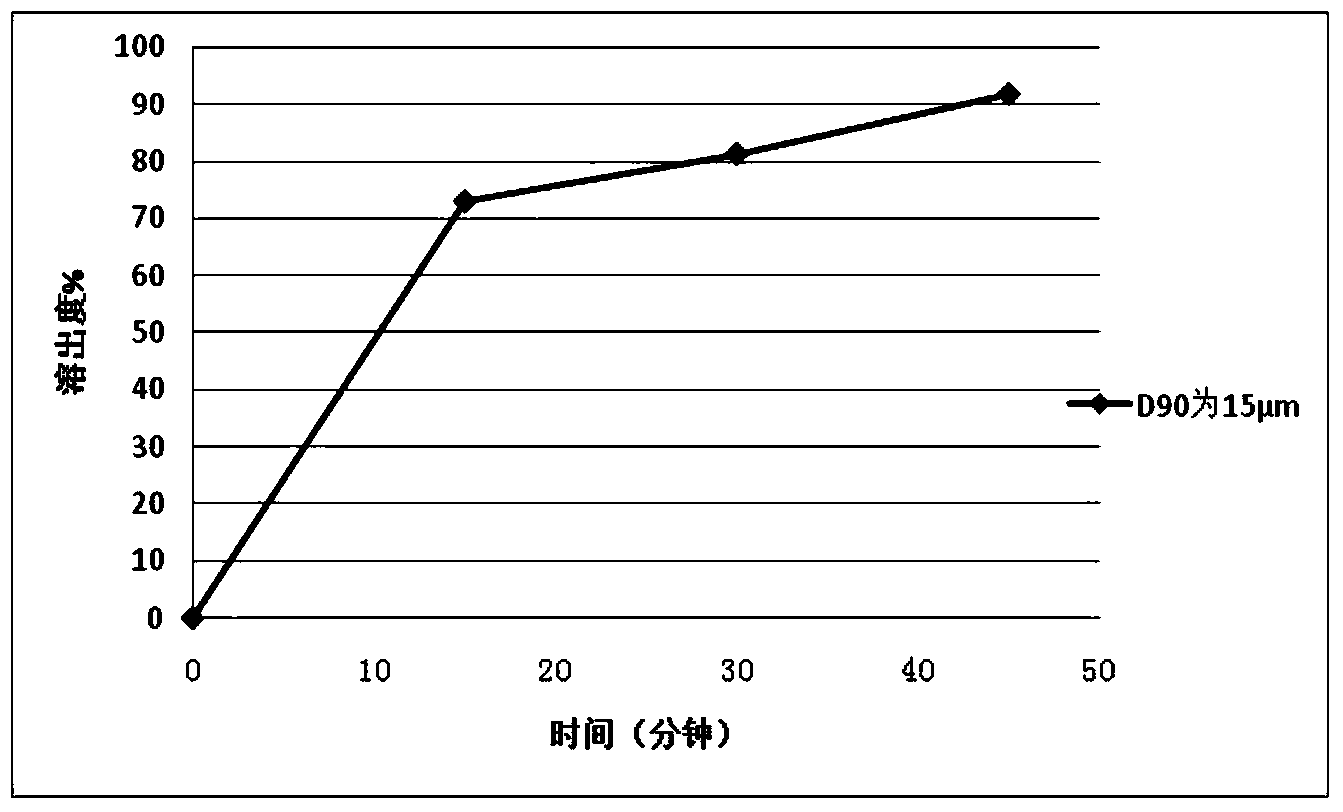
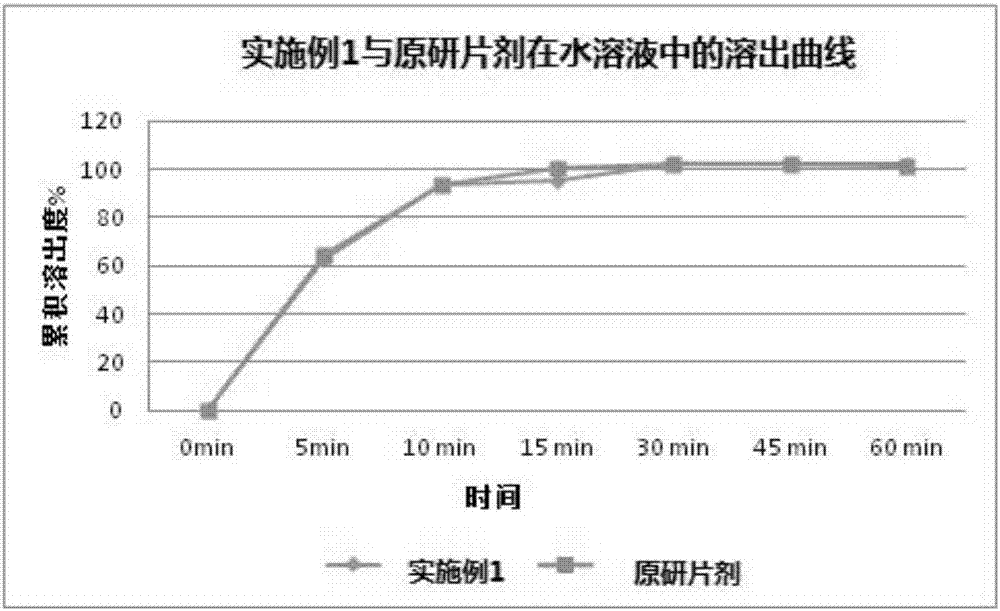
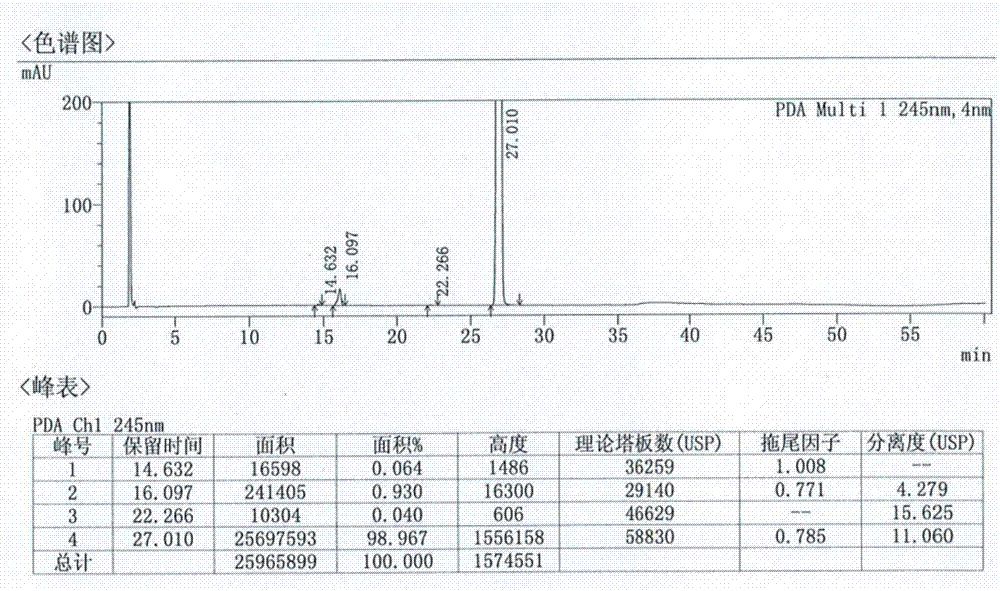
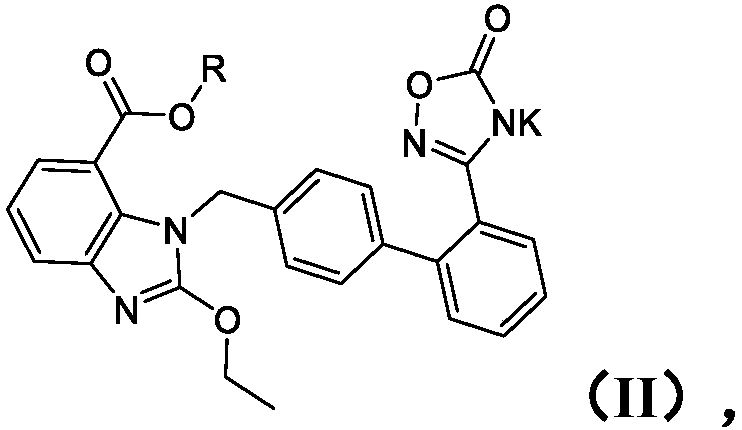
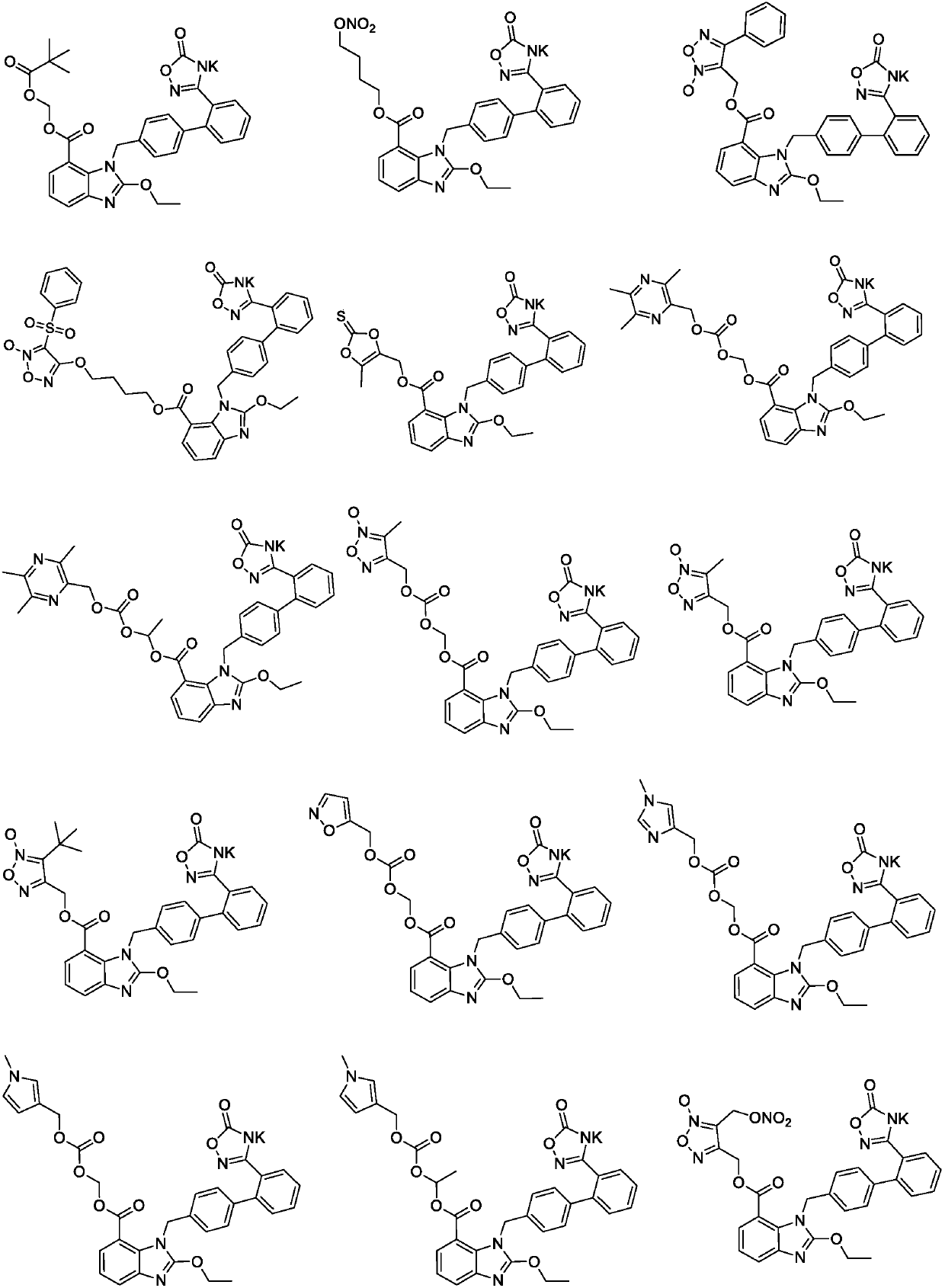
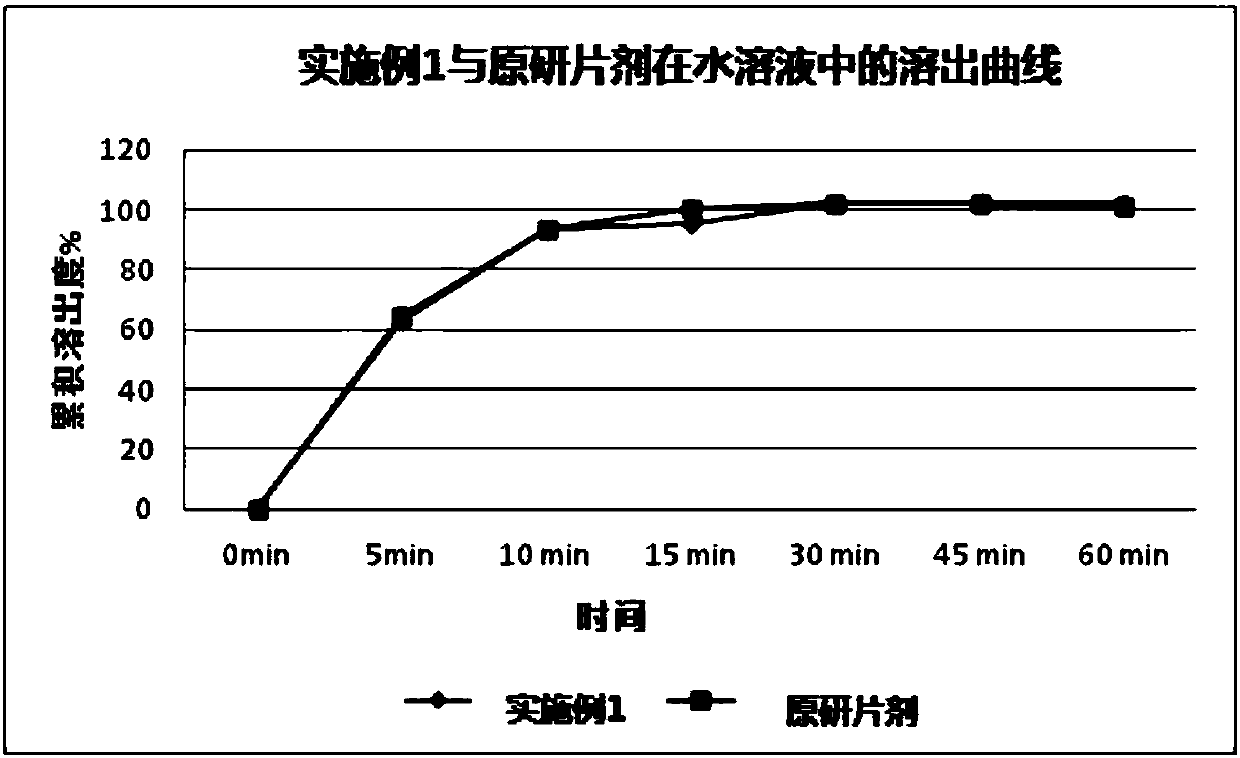
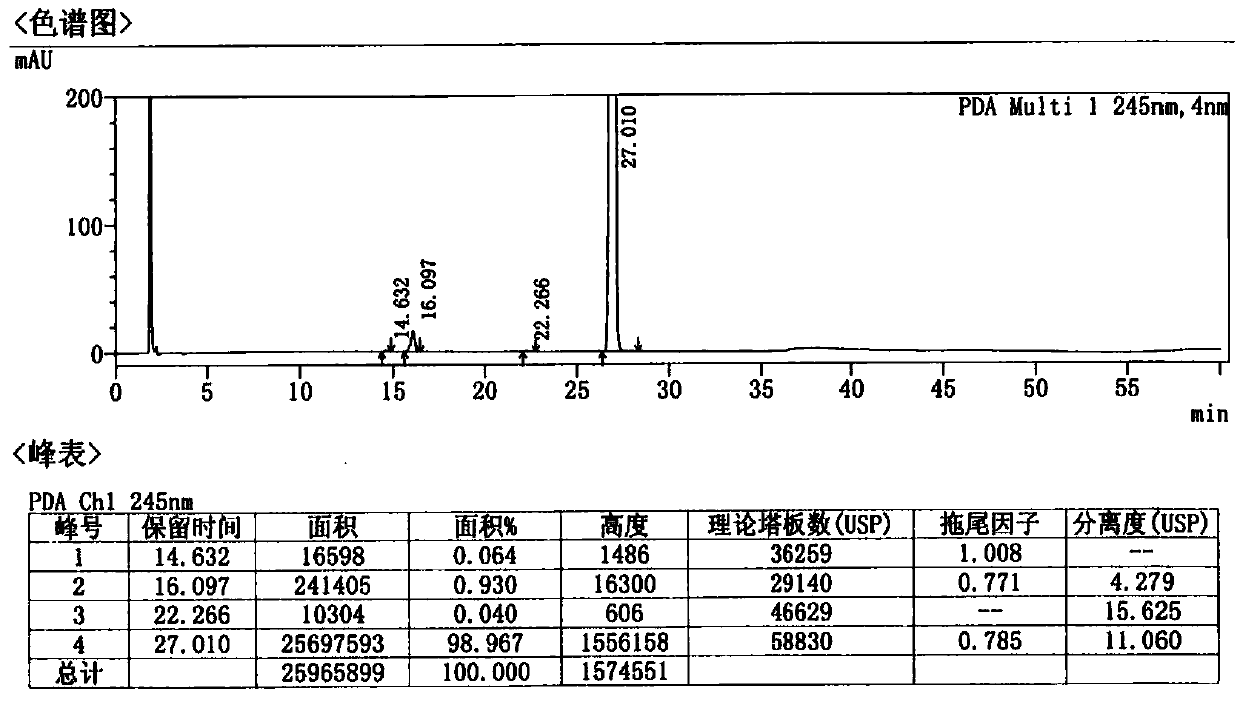
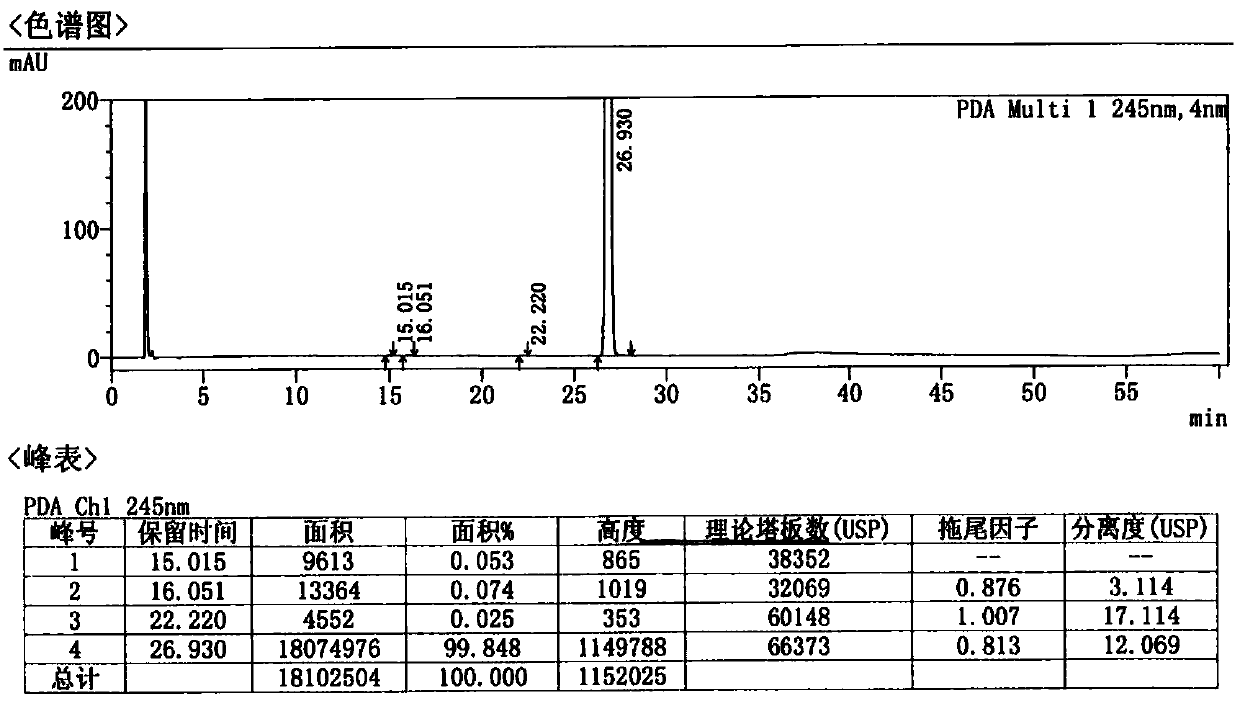
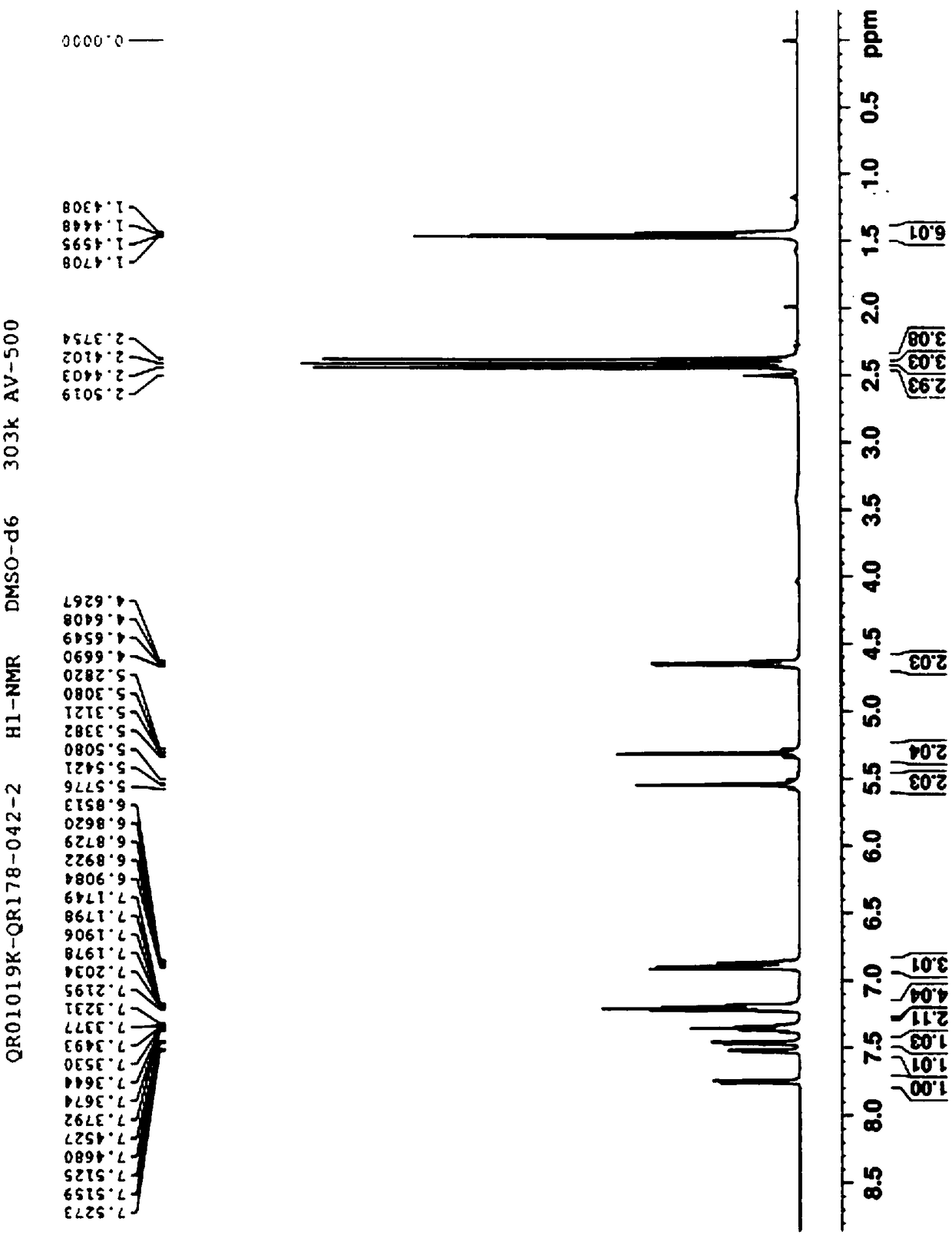
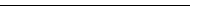Patents
Literature
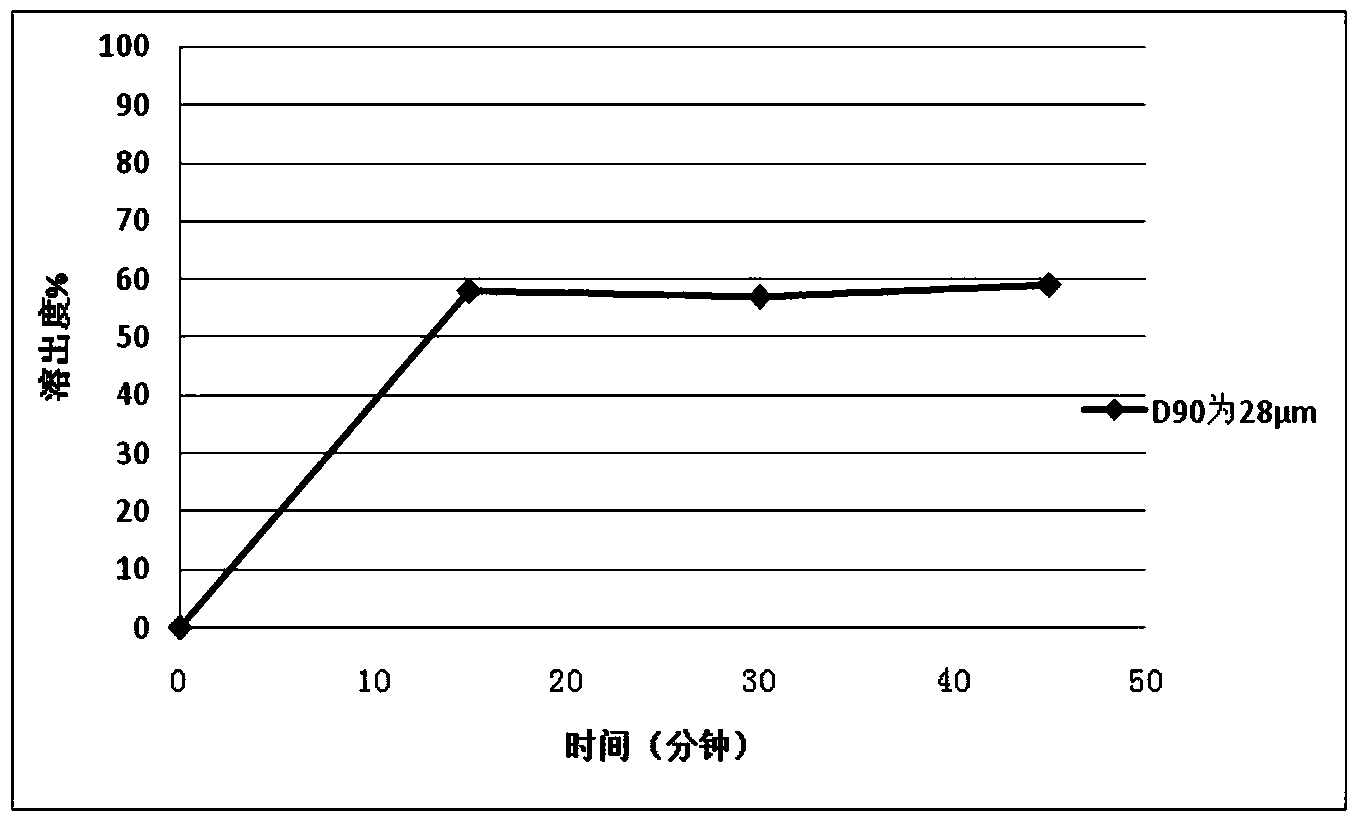
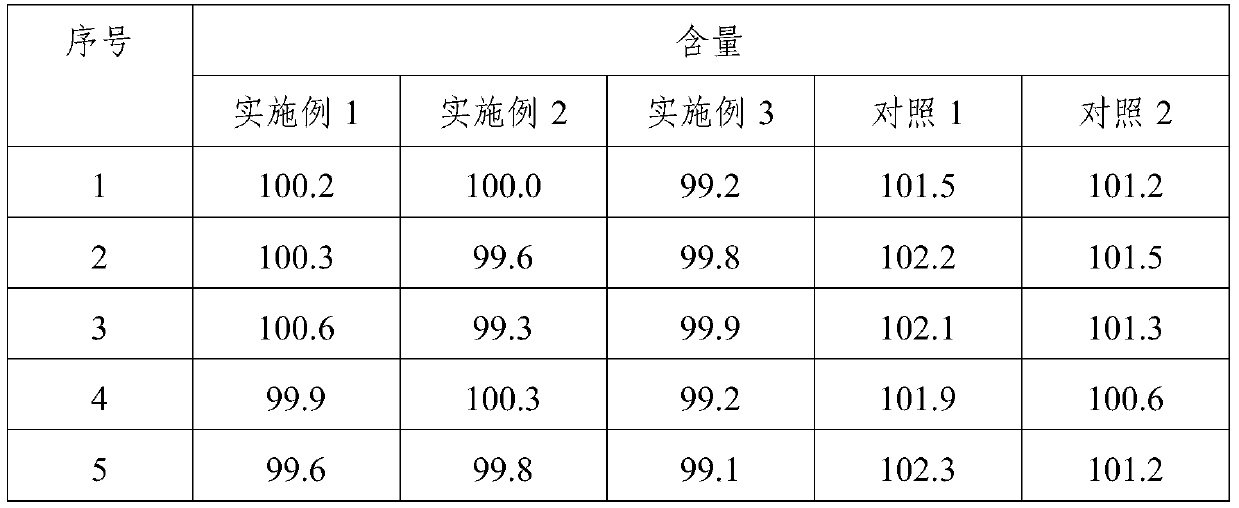
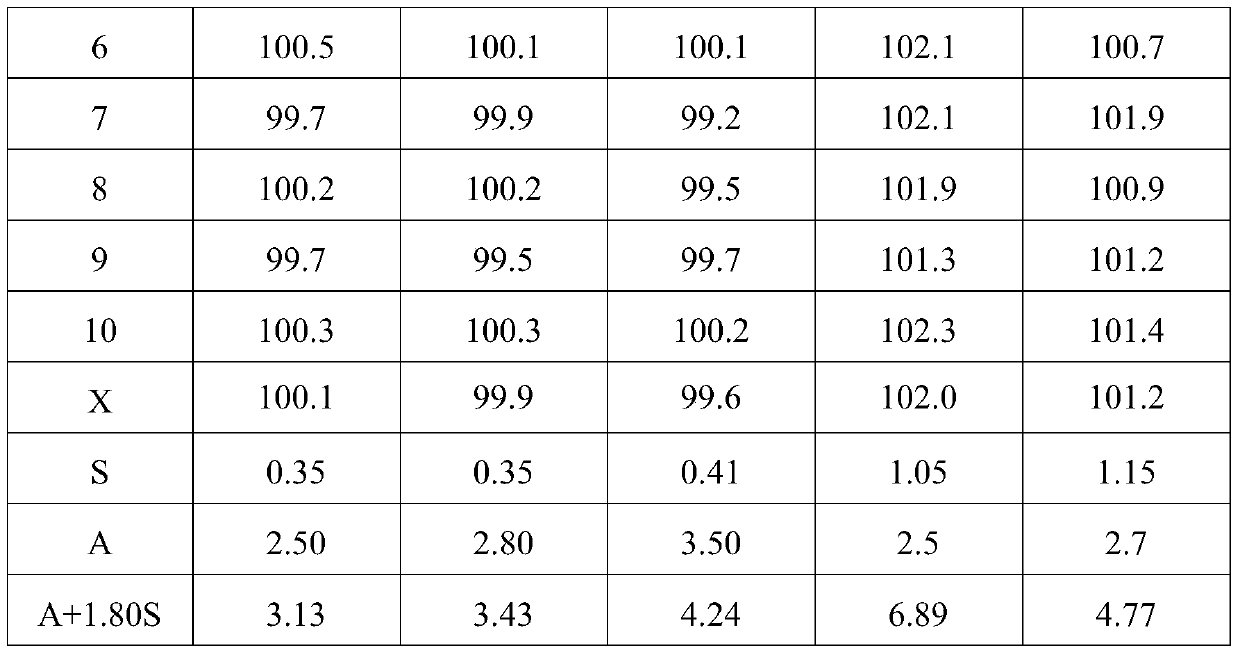
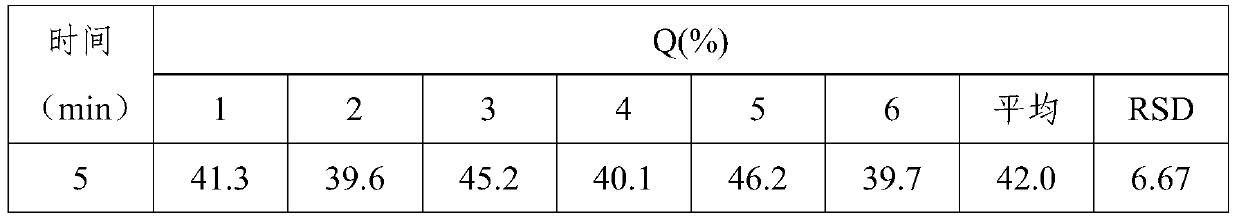
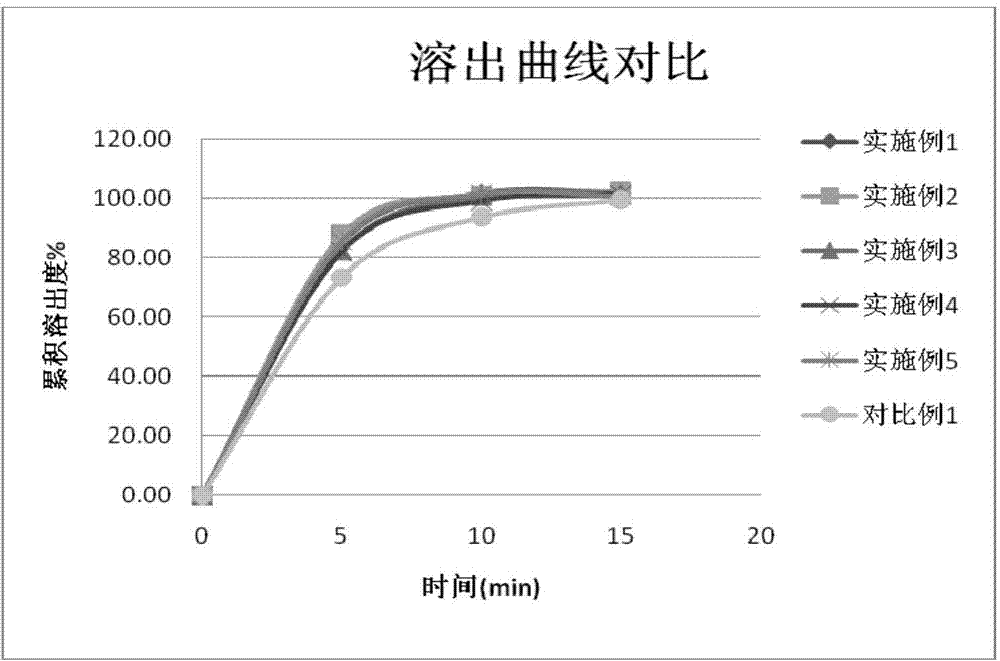
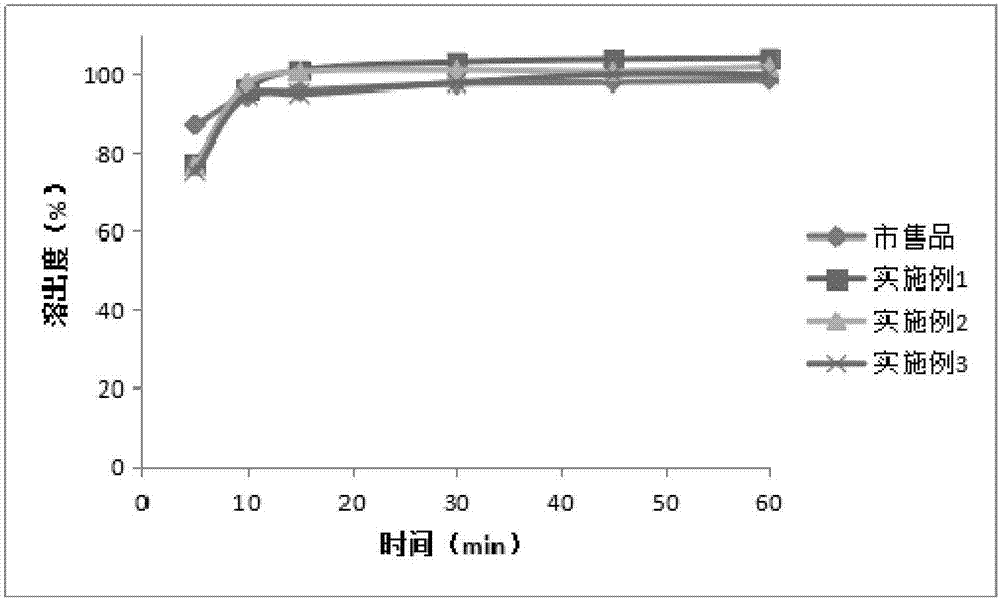
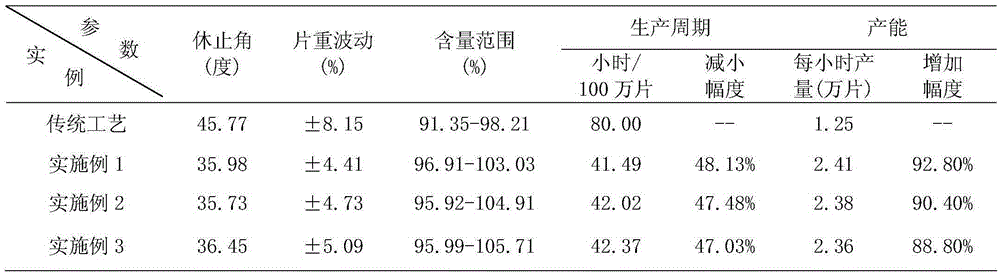
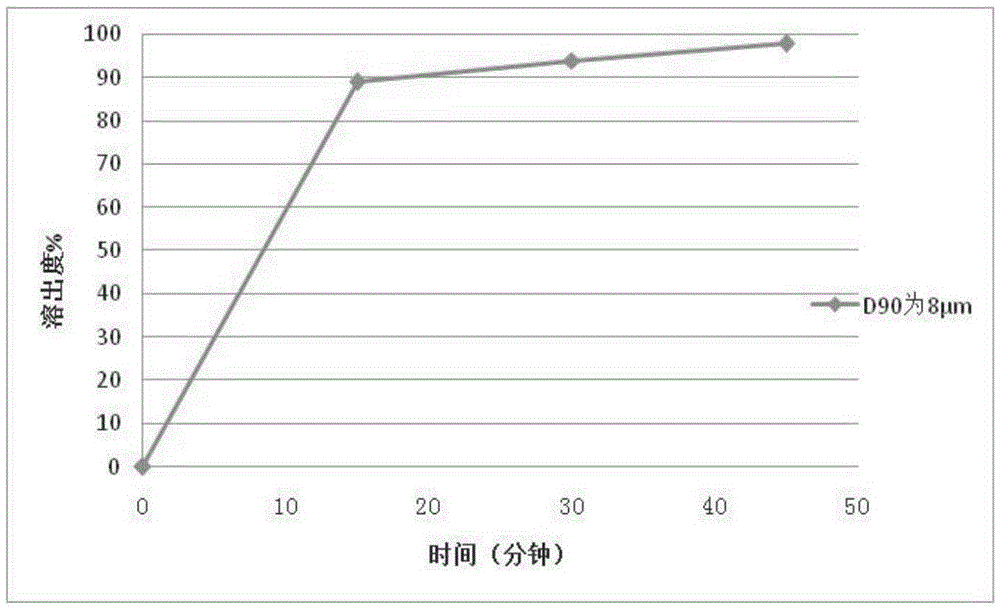
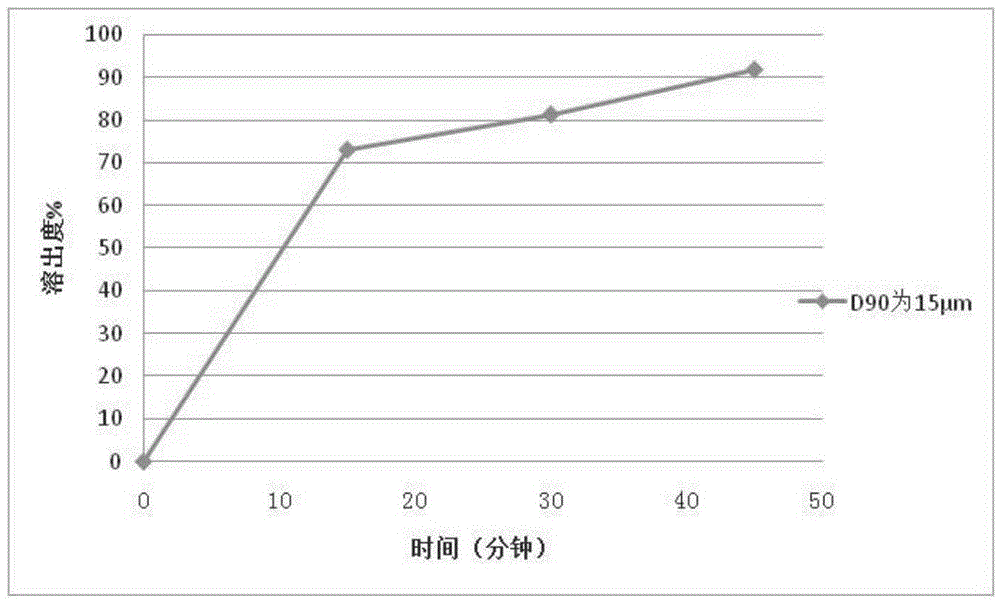
65results about How to "Little difference in tablet weight" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral disintegrant of compound paracetamol
InactiveCN1679525ASimple preparation processDisintegration has little adverse effectOrganic active ingredientsAntipyreticChlorphenamine maleateOrally disintegrating tablet
An oral disintegrating tablet of compound paracetamol is prepared from paracetamol, pseudoephedrine hydrochloride, dextromethorphan HBr, chlorphenamine maleate, filler, disintegrant, adhesive or moistening agent, lubricant, flavouring and pigment through direct tabletting.
Owner:FUDAN UNIV
Method for preparing norfloxacin tablets
ActiveCN102940612AImprove liquidityEasy to compressAntibacterial agentsOrganic active ingredientsGranularityHeight difference
The invention discloses a method for preparing norfloxacin tablets. The method comprises the following steps of: sieving norfloxacin with a sieve of 40 to 100 meshes, sieving a disintegrating agent with a sieve of 60 to 100 meshes, sieving a lubricating agent and a flow aid with a sieve of 80 to 120 meshes, mixing the norfloxacin with a large part of disintegrating agent uniformly, mixing less than 2 percent of disintegrating agent, the lubricating agent and the flow aid uniformly, adding into the mixed powder, mixing uniformly, tabletting, and thus obtaining the norfloxacin tablets. The norfloxacin tablets comprise the following raw materials in percentage by weight: 20 to 80 percent of norfloxacin, 5 to 79 percent of disintegrating agent, and 1 to 8 percent of lubricating agent and flow aid. 5 to 70 percent of filling agent can also be added. The mixed materials obtain high flowability, excellent compression property and adhesive property, high attachment and low sensitivity to the lubricating agent through a large quantity of tests, advantage complementation of auxiliary materials, proper formula proportioning, reasonable granularity distribution and accurate powder mixing sequence. The method overcomes the defects of common material non-layering, non-uniform content, high tablet height difference, tablet powder fall, slow disintegration and the like in the direct tabletting production process, and solves the problems of color change of tablet cores, low dissolubility and the like of the wet granulation process.
Owner:YUNNAN PHYTOPHARML
Repaglinide troche and preparation method thereof
ActiveCN103610677AAvoid stickingGood dispersionOrganic active ingredientsMetabolism disorderMedicineDissolution
The invention relates to an oral troche which contains repaglinide or pharmaceutically acceptable derivatives of repaglinide as well as a preparation method of the oral troche. According to the preparation method, powder of repaglinide or pharmaceutically acceptable derivatives of repaglinide is directly pressed into troche, so that the production cost is remarkably lowered, and the disintegration and the dissolution rate are greatly improved. The bioavailability and the stability of the medicine can be improved, and the problem of low content uniformity of existing small-dose medicines formed by the direct pressing method is overcome, so that the quality of the troche is better guaranteed.
Owner:华益泰康药业股份有限公司
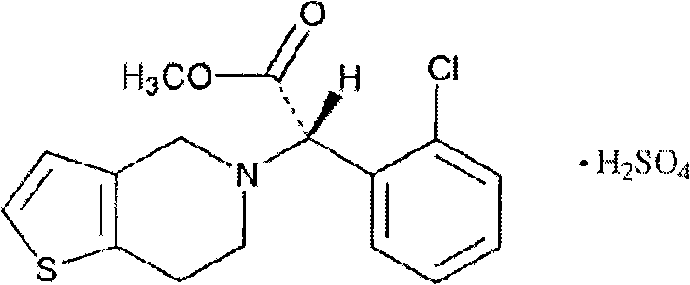
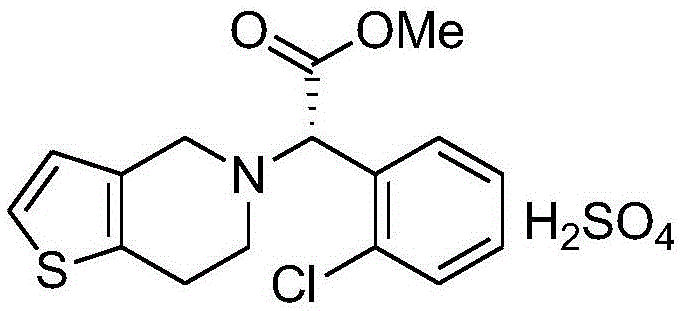
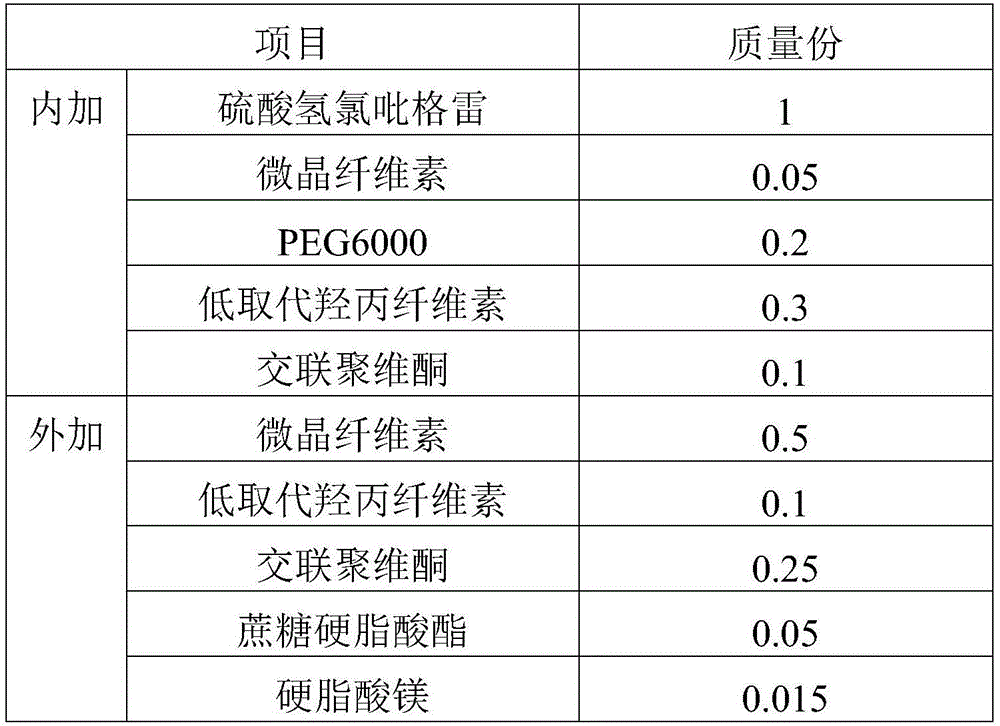
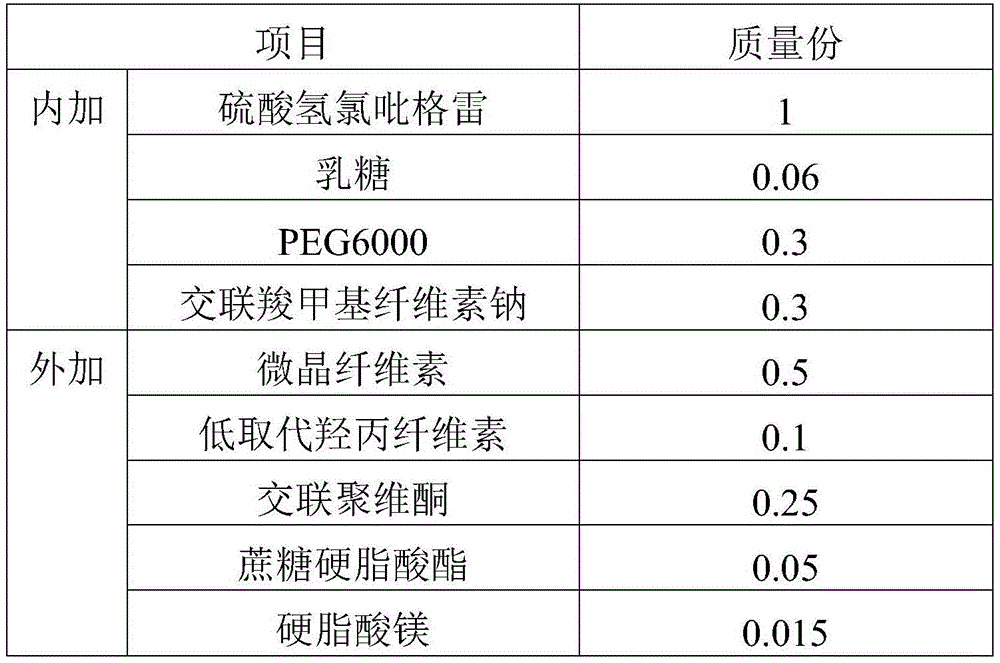
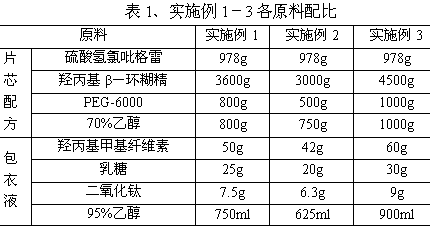
Clopidogrel hydrogen sulfate tablet and preparation method thereof
InactiveCN102247333AImprove liquiditySimple processOrganic active ingredientsBlood disorderAdhesiveMedicine
The invention relates to a preparation for preventing and treating adverse events of atherosclerosis and cardio-cerebrovascular embolism as well as the complications thereof, i.e. a clopidogrel hydrogen sulfate tablet and a preparation method thereof. The tablet comprises clopidogrel hydrogen sulfate, a filler, a disintegrating agent, a lubricating agent, an adhesive, and a film coating premixing agent. The preparation method is characterized by the steps of: weighing the filler, the disintegrating agent in a proportion specified in the prescription, mixing them well, making wet granules with the right amount of the adhesive, drying the wet granules at a temperature of 40-60DEG C, sieving the granules through a sieve of 24 meshes and finishing the granules, adding clopidogrel hydrogen sulfate and the lubricating agent and mixing well, then conducting tabletting, coating and packaging, thus obtaining the tablet. The tablet of the invention has the characteristics of simple prescription and process, good stability, high bioavailability, low cost, high production efficiency and the like.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Clopidogrel hydrogen sulfate solid preparation and preparation method thereof
InactiveCN105616407AImprove uniformityFlat surfaceOrganic active ingredientsPharmaceutical non-active ingredientsMedicineClopidogrel hydrogen sulfate
The invention provides a clopidogrel hydrogen sulfate solid preparation which is prepared by adopting a melt granulation technology, wherein the bulk drug content in obtained particles is high, the obtained preparation has the characteristics of good uniformity, smooth tablet surface and small tablet weight difference, the digestion performance meets the clinical medication requirement, and the effect is better when special crystal forms are used.
Owner:SHENZHEN SALUBRIS PHARMA +2
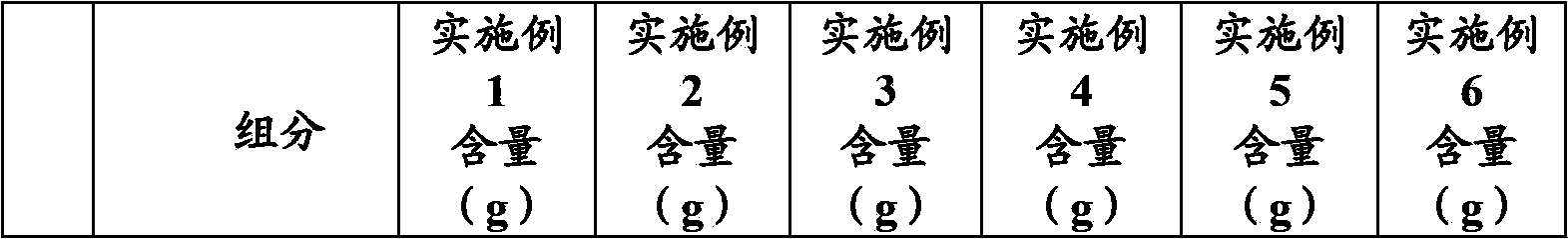
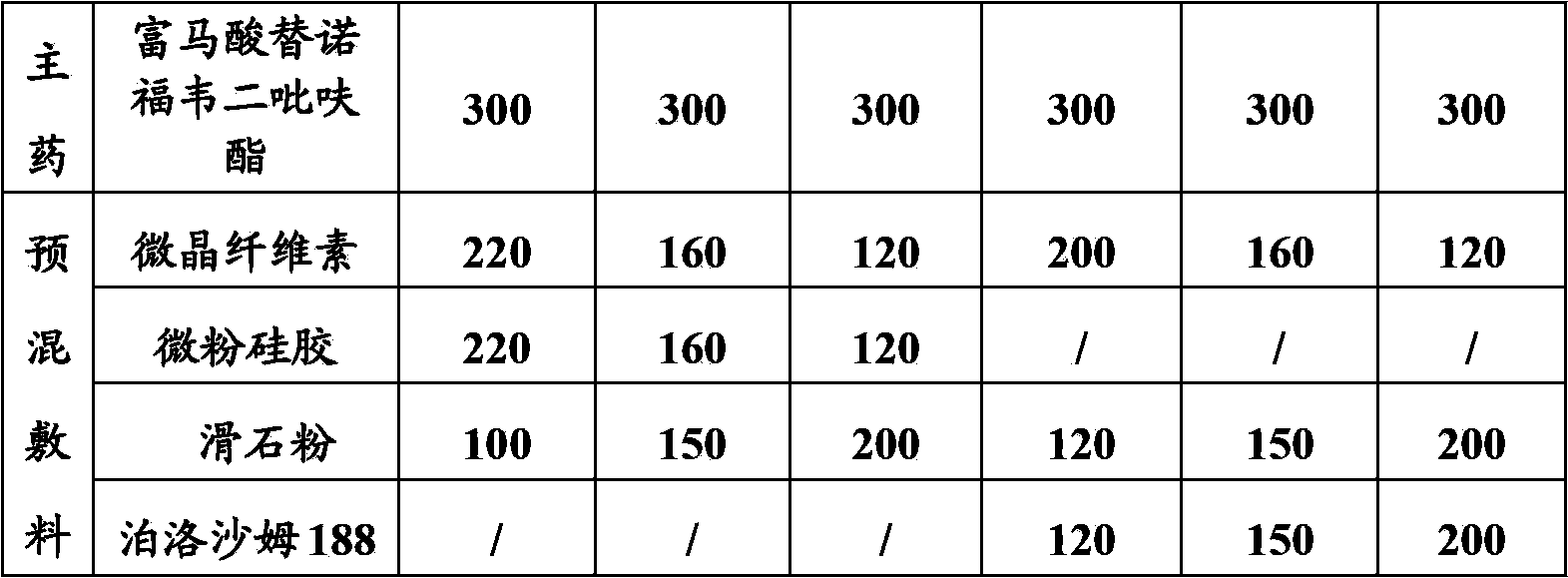
Tenofovir disoproxil fumarate tablets allowing direct powder compression and preparation method thereof
InactiveCN103830192ALow impurity contentLittle difference in tablet weightOrganic active ingredientsAntiviralsMedicineHardness
The invention relates to tenofovir disoproxil fumarate tablets and a preparation method thereof. The tablets mainly contain a main drug tenofovir disoproxil fumarate and a premixing accessory, wherein the premixing accessory is a mixture of uniformly mixed microcrystalline cellulose, talc powder and a component A, and the component A is aerosil or poloxamer 188. The preparation method provided by the invention effectively overcomes the defects of the prior art that the tenofovir disoproxil fumarate has relatively high viscosity and consequently the tablets are difficult to prepare by a direct powder compression method, thereby avoiding the degradation of tenofovir disoproxil fumarate by the moisture introduced in a preparation process of wet granulation in the prior art, and further improving the quality of the tablets. The prepared tablets have the advantages of little difference in tablet weight, moderate hardness, good dissolubility and low impurity content. Moreover, the production process of the tablets is simple and convenient to operate and suitable for industrial-scale production.
Owner:ANHUI BIOCHEM BIO PHARMA
Low dose diclofenac sodium lozenge and its preparation method
InactiveCN1413583AImprove liquidityLittle difference in tablet weightOrganic active ingredientsAntipyreticSolventChemistry
A low-dosage lozenge of diclofenac sodium contains diclofenac sodium, sugar powder, mannitol, maltose dextrin, menthol, polyvidone K30 solution and magnesium stearate, and is prepared by solvent dispersing method or particle coating method. It can be used for treating oral inflammation.
Owner:JIANGSU DURUI PHARMACEUTICAL CO LTD
Clopidogrel hydrogen sulfate tablets and production method thereof
InactiveCN102988321AFree from hydrolysisImprove stabilityOrganic active ingredientsPill deliverySolubilityMedicine
The invention discloses clopidogrel hydrogen sulfate tablets. Each tablet contains 25-75mg of clopidogrel hydrogen sulfate based on clopidogrel and also comprises a filling agent, an adhesive and a lubricant, wherein the filling agent is beta-cyclodextrin or hydroxypropyl beta-hydroxypropyl; the adhesive is ethanol with volume concentration of 70%; and the lubricant is PEG-6000, PEG-4000 or PEG-2000. Special accessory is adopted in the product; and through the protection effect of the holes of the filling agent on the main drug and the water solubility of the adhesive, the prepared tablet has the characteristics of high dissolution rate, uniform appearance, good stability and the like, and can meet the requirements of industrial production.
Owner:SHANGHAI MODERN HASEN SHANGQIU PHARMA
Preparation and prescription technology for berberine hydrochloride tablet preparation
InactiveCN108853035AIncrease contentAccelerate content dissolutionOrganic active ingredientsPill deliveryAdhesiveMagnesium stearate
The invention discloses preparation and a prescription technology for berberine hydrochloride tablet preparation. The berberine hydrochloride tablet preparation is prepared from the following raw materials in parts by weight: 1 to 15 parts of berberine hydrochloride, 0.36 to 36 parts of corn starch, 0.06 to 6 parts of carboxymethyl starch sodium, 0.25 to 25 parts of 70% ethyl alcohol, 0.20 to 20 parts of 8% starch slurry, 0.004 to 0.40 part of magnesium stearate and 0.004 to 0.40 part of silicon dioxide. By means of the preparation of the berberine hydrochloride tablet preparation, a berberinehydrochloride tablet can be prepared. According to the prepared berberine hydrochloride tablet, carboxymethyl starch sodium is utilized for improving internal disintegration of particles, so that content dissolution is quickened, and the efficiency is improved; the amount of utilizing a 70% ethanol solution as a wetting agent is reduced; the starch slurry is added to serve as an adhesive, so thatparticle formation and particle fluidity are facilitated; silicon dioxide is added, so that the sticking phenomenon in a particle tabletting process is improved, fluidity is improved, the phenomenonthat the berberine hydrochloride tablet particles are prone to sticking in a tabletting process is solved, tablet weight difference is reduced, a finished product yield is improved, and content dissolution is quick.
Owner:江苏长江药业有限公司
Calcium dobesilate dispersible tablet and preparation method thereof
InactiveCN103877038AFlat shapeBright and cleanSenses disorderPill deliveryCross-linkMoisture absorption
The present invention discloses a calcium dobesilate dispersible tablet and a preparation method thereof. The calcium dobesilate dispersible tablet contains, by weight, a main drug calcium dobesilate, and auxiliary materials such as 1-42% of cross-linked povidone, 0.5-42% of microcrystalline cellulose, 2-44% of lactose and 0.01-15% of magnesium stearate. According to the present invention, a direct powder tabletting method is adopted to prepare the calcium dobesilate dispersible tablet; the method has characteristics of simple operation, time saving, reduction of times of calcium dobesilate exposed to air and light, and reduction of moisture absorption and light instability; the prepared calcium dobesilate dispersible tablet has a smooth surface morphology, and can be completely disintegrated within 2 min and screened with a 2# sieve, and the dissolution rate of the prepared calcium dobesilate dispersible tablet in water and a dilute hydrochloric acid solution within 5 min can achieve more than 90%; and the preparation method has characteristics of simple preparation process and time saving, and the prepared calcium dobesilate dispersible tablet has characteristics of smooth surface, good dispersion uniformity and high dissolution rate, and is suitable for industrial production.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Preparation method of afatinib dimaleate tablets
ActiveCN107260698ASmooth appearanceHigh hardnessOrganic active ingredientsInorganic non-active ingredientsExternal calciumSulfate
The invention discloses a preparation method of afatinib dimaleate tablets. The preparation method comprises the following steps: 1) respectively weighing raw materials in parts by weight; 2) uniformly mixing afatinib dimaleate, a diluting agent, polyethylene glycol and internal calcium sulfate, heating to 60 to 70 DEG C, after the mixture reaches a predetermined temperature, keeping the temperature for 10 to 15min, cooling at room temperature, granulating, adding and uniformly mixing a disintegrating agent, external calcium sulfate, magnesium stearate and silicon dioxide, and tabletting; and 3) coating the tablets, thus obtaining the afatinib dimaleate tablet. The obtained tablets are stable in quality, uniform in content and good in in-vitro dissolution behavior.
Owner:GUANGDONG ANNOL PHARM CO LTD
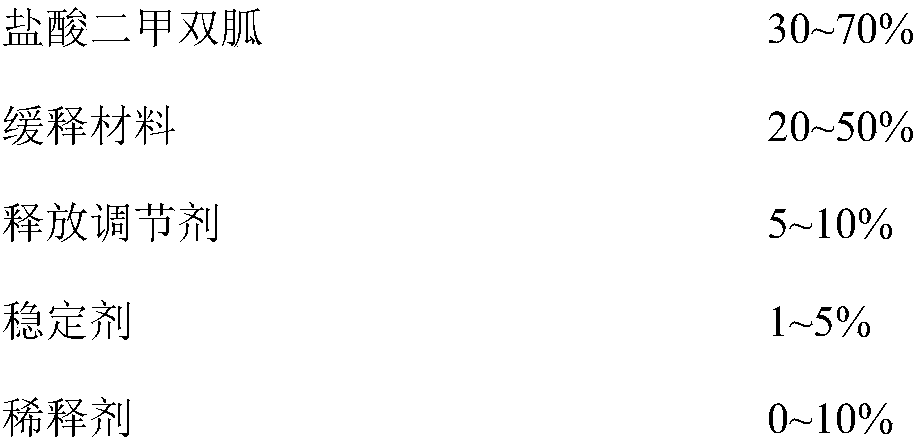
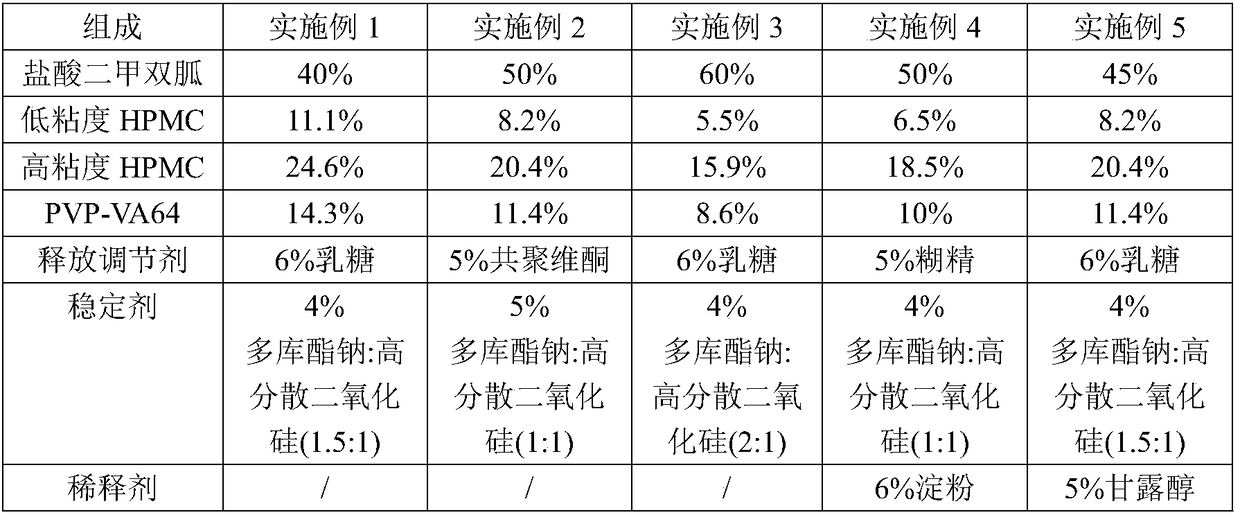
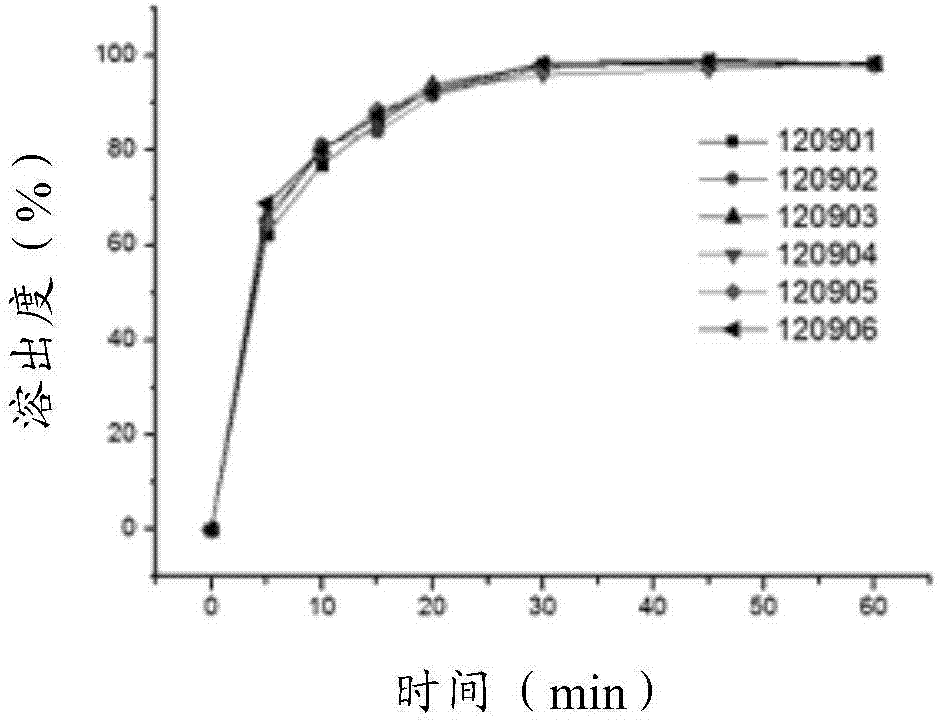
A kind of metformin hydrochloride sustained-release tablet and preparation method thereof
ActiveCN107184559BSlow release and dissolutionReduce hidden dangersOrganic active ingredientsMetabolism disorderMetformin HydrochlorideDrug release
The invention discloses a metformin hydrochloride sustained-release tablet and a preparation method thereof. The metformin hydrochloride sustained-release tablet is prepared by the following steps of using a hydroxypropyl methylcellulose and PVP-VA64 mixture as the sustained-release material, adding a certain amount of stabilizer and release adjuster, and preparing by a hot melting extrusion technique. The metformin hydrochloride sustained-release tablet has the advantages that the sustained-release material and the metformin hydrochloride are tightly bonded, the medicine is more smoothly and slowly released, the effect is obviously better than the in-vitro release conditions of the sustained-release tablet prepared by the conventional wet type granulating tabletting method and the market product (Glucophage SR), and the stability is better; under the condition of no coating, the adverse odor of the metformin hydrochloride can be covered, and the compliance of a patient is improved; the preparation technology is simple, the energy consumption is less, the solvent residue is avoided, the other impurities are not introduced in the whole process, and the continuous production can be easily realized.
Owner:广东赛康药业有限公司 +1
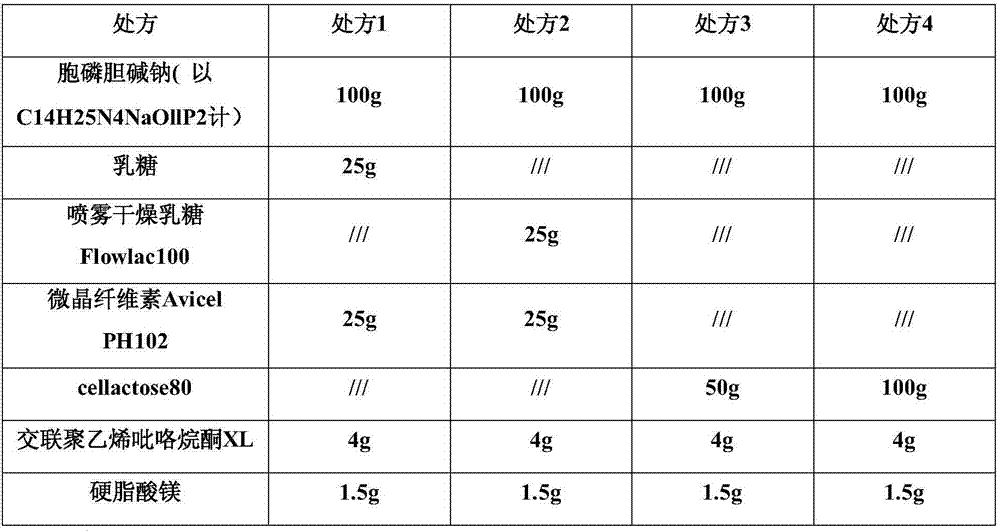
Citicoline sodium tablets and preparation method for directly compressing powder of citicoline sodium into tablets
ActiveCN107496369ASolve easy hydrolysisImprove securityOrganic active ingredientsNervous disorderCiticoline sodiumDissolution
The invention provides citicoline sodium tablets and a preparation method for directly compressing powder of citicoline sodium into tablets. The citicoline sodium tablets provided by the invention are prepared from citicoline sodium, cellactose 80, sodium carboxymethyl starch, magnesium stearate and micro-powder silica gel. The tablets provided by the invention are prepared by adopting a powder direct tablet compression method and the problem that citicoline sodium granulated by a traditional wet process is easy to hydrolyze is solved; meanwhile, the conditions that the tablet weight different is relatively great and the dissolution uniformly is relatively poor, which are easily caused by direct powder compression, are solved through screening prescriptions and setting technological parameters; the quality stability and the safety of products are greatly improved.
Owner:FUJIAN MINDONG REJUVENATION PHARMA
Clopidogrel hydrogen sulfate tablets and preparation method thereof
InactiveCN109771387AGuaranteed stabilityImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantLubricant
The invention relates to clopidogrel hydrogen sulfate tablets and a preparation method thereof. The tablets comprise the following components by weight percentage: clopidogrel hydrogen sulfate form II, a filler, a disintegrant, an antioxidant, a proper amount of a binder, and a proper amount of a lubricant. The tablets improve the stability of the obtained preparation, and the obtained preparationhas the characteristics of good uniformity, smooth surface of the tablets, small difference in tablet weight, and the like. The dissolution performance is in line with the clinical drug requirements,so that the effectiveness and safety of clinical drugs can be ensured. The preparation process also ensures that no sticking problem is generated during a tableting process.
Owner:HENAN TIANSHENG TAIFENG PHARM TECH CO LTD
Method for preparing isosorbide mononitrate orally disintegrating tablets
InactiveCN101342150ADelicate and cool tasteLittle difference in tablet weightPharmaceutical product form changePill deliveryOrally disintegrating tabletDissolution
The invention relates to a formula and a process of an isosorbide mononitrate orally disintegrating tablet produced with a direct powder tabletting method and a novel powder feed device for a tabletting machine. Compared with the conventional technology of the direct powder tabletting, the isosorbide mononitrate orally disintegrating tablet produced by the invention has cool and fine mouth feelings, small difference in tablet weight, favorable uniformity of content, fast disintegration and dissolution, high dissolution rate and dissolubility, strong rigidity and favorable compressibility.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
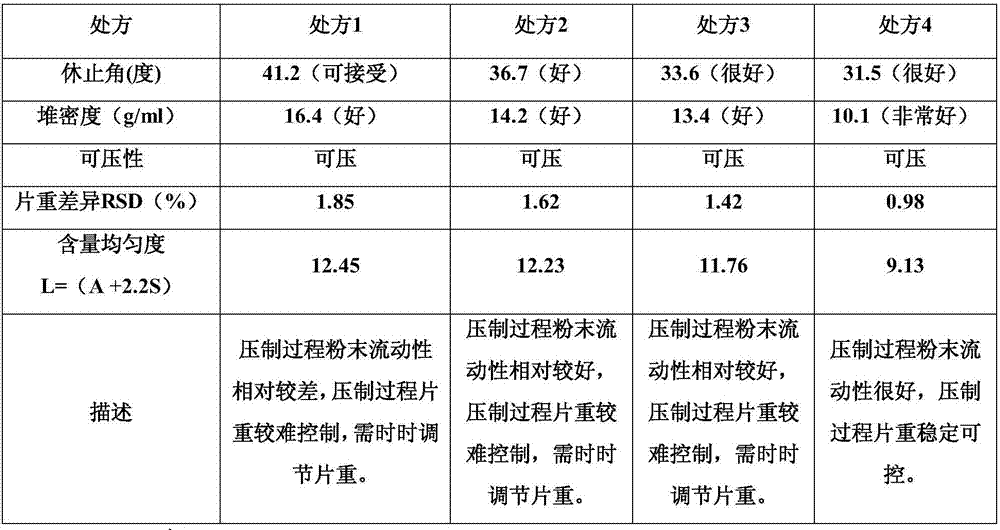
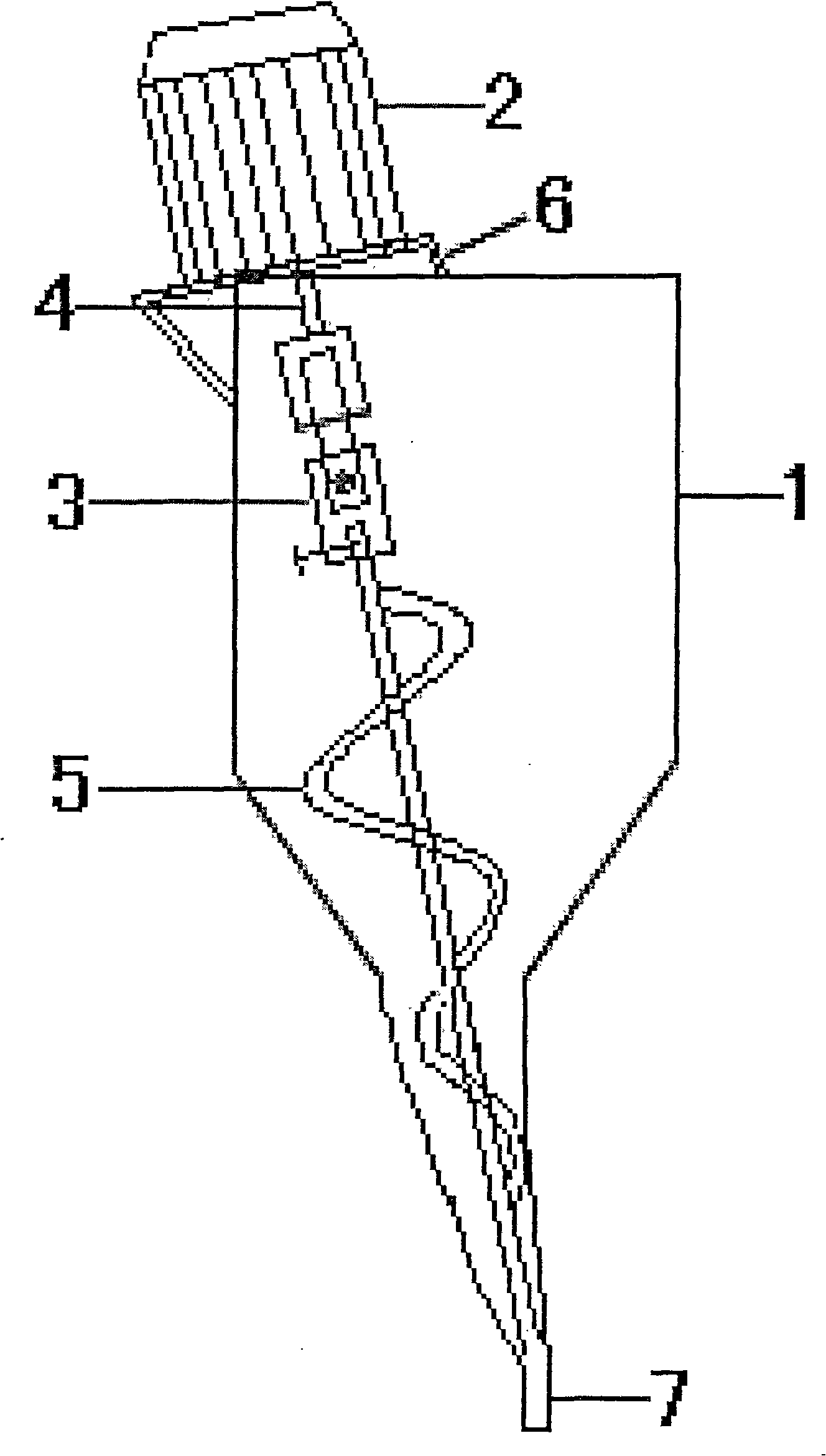
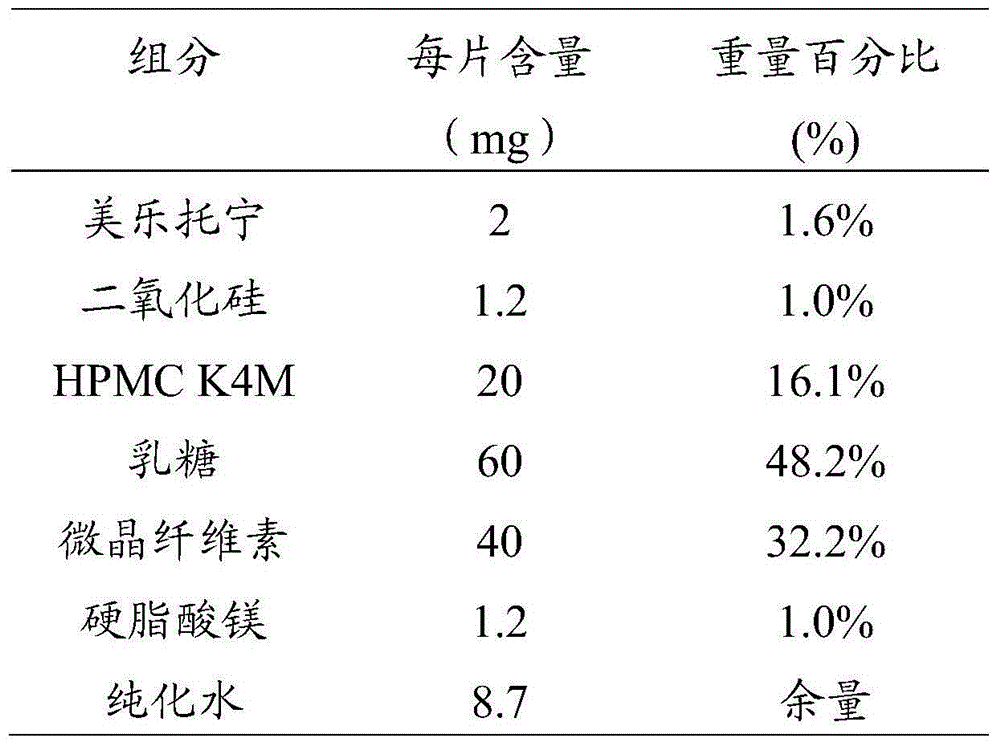
Preparation method of melatonin controlled-release composition and preparation method of melatonin controlled-release tablet
InactiveCN104546777AGood content uniformityImprove the phenomenon of easy adhesion on the inner wall of the metalOrganic active ingredientsInorganic non-active ingredientsControl releasePhysiology
The invention relates to the field of drug preparation and in particular relates to a preparation method of a melatonin controlled-release composition and a preparation method of a melatonin controlled-release tablet. The preparation method of the melatonin controlled-release composition comprises the following steps: mixing melatonin with an anti-sticking agent, and sieving, thereby obtaining a bulk drug premix; respectively mixing a filling agent, the bulk drug premix and a controlled-release material, adding a wetting agent, carrying out wet granulation, and then mixing with a lubricating agent, so that the melatonin controlled-release composition is obtained. By adopting the preparation method of the melatonin controlled-release composition, the content uniformity of the main drug in the melatonin controlled-release tablet is obviously improved.
Owner:HYBIO PHARMA
A kind of highly stable simvastatin tablet and preparation method thereof
ActiveCN105106198BImprove stabilityIncrease contentOrganic active ingredientsMetabolism disorderCooking & bakingCompressibility
The invention discloses a highly stable simvastatin tablet. The simvastatin tablet comprises the following components in parts by weight: 5-20 parts of simvastatin, 8-20 parts of pregelatinized starch, butylated hydroxyanisole 0.03‑0.06 part, 0.3‑1 part of povidone K30, 45‑55 part of lactose, 35‑45 part of microcrystalline cellulose, 3‑5 part of hypromellose, 0.5‑2 part of hypromellose, stearin Magnesium acid 0.2-1.2 parts, micro silica gel 0.6-2.4 parts. The preparation of the simvastatin tablet of the present invention adopts two-part granulation, the simvastatin and the pregelatinized starch adopt absolute ethanol, and are baked at a lower temperature to avoid degradation of the simvastatin; the other part is baked at a higher temperature. The granules prepared by the invention have good compressibility and fluidity. The disintegration time of the prepared tablet is suitable, the tablet content, content uniformity and dissolution rate all meet the requirements, has high stability, the production method is simple, and is suitable for industrialized production.
Owner:KAMP PHARMA
Tenofovir disoproxil fumarate tablet and preparation method thereof
InactiveCN104288118ASimple and fast operationAvoid destabilizing factorsOrganic active ingredientsPharmaceutical delivery mechanismCrospovidonesMedicine
The invention discloses a tenofovir disoproxil fumarate tablet and a preparation method thereof. The tenofovir disoproxil fumarate tablet is prepared from tenofovir disoproxil fumarate, anhydrous lactose, microcrystalline cellulose, crospovidone, sodium carboxymethyl starch, colloidal silica and talcum powder. Compared with dosage forms of tenofovir disoproxil fumarate in the prior art, the tenofovir disoproxil fumarate tablet has the advantages of good stability, simple preparation technology and controllable quality.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Azithromycin tablet and preparation method thereof
ActiveCN107281155AImprove reliabilitySolve for uniformityAntibacterial agentsOrganic active ingredientsActive agentSurface-active agents
The invention discloses an azithromycin tablet. The azithromycin tablet is prepared by taking azithromycin as an active ingredient and adding a filling agent, an adhesive, a disintegrating agent, a surfactant, a flow aid, a lubricating agent and a water-soluble polymer. The azithromycin tablet disclosed by the invention has the advantages of high quality, simple and convenient preparation technology and wide application prospect.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD
Preparation method of tablet for increasing bone density
The invention discloses a preparation method of a tablet for increasing the bone density. The table is mainly composed of red deer bone, calcium carbonate, hydrolyzed collagen, and sodium chondroitin sulfate. A boiling one-step granulation method is adopted. Three steps: mixing, granulation, and drying are carried out in a boiling enclosed chamber at a time. The prepared particles are uniform, the fluidity is good, the weight difference is small, and the dissolution rate is good. Compared with the conventional wet process granulation technology, the provided method has the advantages of fewer steps, less production equipment, higher safety, more output, and shorter production period, and the quality of obtained product is stable and controllable.
Owner:HARBIN PHARM GROUP SANJING PHARMACEUTICAL CO LTD
Preparation method of ethionamide tablet
InactiveCN103462912ALittle difference in tablet weightQuality controlAntibacterial agentsPill deliverySolventSieve
The invention discloses a preparation method of an ethionamide tablet, and the preparation method comprises the following steps: (1) weighing the following raw materials by weight: 250 parts of ethionamide; 40-55 parts of a filler; 15-25 parts of a disintegrating agent; 10-15 parts of a binder; 6.25 parts of a flow aid and 1 part of a lubricant; and (2) mixing the ethionamide and the flow aid, crushing, sieving by a 100 mesh standard sieve, adding the filler, the disintegrating agent and the binder for mixing, adding an auxiliary solvent with an amount being 6% of the total amount of the formula for wet granulation, baking at the temperature of 60DEG C for 20min, trimming granules by a 16-20 mesh standard sieve, adding the lubricant, and tabletting to obtain the ethionamide tablet. By using the preparation method of the invention, crushing and sieving of ethionamide raw material drug is convenient, the prepared tablet is small in tablet weight difference, and the quality of the drug is well controlled.
Owner:TIANJIN KUNJIAN BIOLOGICAL PHARMA
A kind of repaglinide tablet and preparation method thereof
ActiveCN103610677BAvoid stickingGood dispersionOrganic active ingredientsMetabolism disorderMedicineDissolution
The invention relates to an oral troche which contains repaglinide or pharmaceutically acceptable derivatives of repaglinide as well as a preparation method of the oral troche. According to the preparation method, powder of repaglinide or pharmaceutically acceptable derivatives of repaglinide is directly pressed into troche, so that the production cost is remarkably lowered, and the disintegration and the dissolution rate are greatly improved. The bioavailability and the stability of the medicine can be improved, and the problem of low content uniformity of existing small-dose medicines formed by the direct pressing method is overcome, so that the quality of the troche is better guaranteed.
Owner:华益泰康药业股份有限公司
Multivitamin chewable tablet and preparation method thereof
The invention discloses a multivitamin chewable tablet and a preparation method thereof. The multivitamin chewable tablet comprises multivitamin powder, xylitol granules, sorbitol and white granulatedsugar. According to the invention, xylitol granules are prepared through a specific process, the xylitol granules, sorbitol and white granulated sugar are preferably used as flavoring agents throughscreening of types and use amounts of auxiliary materials, and the use amounts are controlled as follows: the ratio of the multivitamin powder to the xylitol granules to the sorbitol to the white granulated sugar s in the range of (6-10) to (15-20) to (50-55) to (15-20), so that not only the problem of bitter and astringent taste is solved, but also the technical problems of poor compressibility,sticking, large tablet weight difference and the like are solved. The prepared multivitamin chewable tablet has no bitter taste, good taste and small tablet weight difference (-3.5% to +3.5%), and theproduct has good compressibility and can meet the requirement of continuous production of equipment.
Owner:BY HEALTH CO LTD
High-stability simvastatin tablet and preparation method thereof
ActiveCN105106198AImprove stabilityIncrease contentOrganic active ingredientsMetabolism disorderAlcoholCompressibility
The invention discloses a high-stability simvastatin tablet. The high-stability simvastatin tablet is prepared from, by weight, 5-20 parts of simvastatin, 8-20 parts of pre-gelatinized starch, 0.03-0.06 part of butyl hydroxy anisd, 0.3-1 part of povidone K30, 45-55 parts of lactose, 35-45 parts of microcrystalline cellulose, 3-5 parts of hydroxypropyl cellulose, 0.5-2 parts of hydroxypropyl methylcellulose, 0.2-1.2 parts of magnesium stearate and 0.6-2.4 parts of differential silica gel. Two-part pelleting is adopted in preparation of the simvastatin tablet, simvastatin and pre-gelatinized starch are baked through absolute ethyl alcohol at low temperature, and degradation of simvastatin is avoided; the other part is baked at high temperature. The prepared particles are good in compressibility and fluidity. The prepared tablet is proper in disintegration time, the content, content uniformity and dissolution rate of the tablet all meet requirements, high stability is achieved, a production method is simple, and the high-stability simvastatin tablet is suitable for industrial production.
Owner:KAMP PHARMA
Nisoldipine capsule and preparation method thereof
InactiveCN105168175AHigh dissolution rateLittle difference in tablet weightOrganic active ingredientsPharmaceutical non-active ingredientsAdhesiveDissolution
The invention belongs to the technical field of medicines, and concretely relates to a nisoldipine capsule and a preparation method thereof. The nisoldipine capsule is prepared from the following raw materials in parts by weight: 3.2-4.8 parts of nisoldipine, 44-132 parts of a filler, 0.25-0.75 parts of an adhesive, 6-18 parts of a solubilizer, and 0.4-1.2 parts of a lubricant. The technology is simple and easy to enforce, and the prepared nisoldipine capsule is steady for medicine release and high in medicine dissolution rate.
Owner:REYOUNG PHARMA
Preparation method of medical composition
ActiveCN110237072AGood disintegrationFacilitated releaseOrganic active ingredientsMetabolism disorderChemical compositionActive component
The invention belongs to the field of medicines, and particularly relates to a preparation method of a medical composition. The preparation technology can effectively solve the problem that the medical composition being good in properties cannot be prepared through a conventional technology, so that the problem that the preparation cannot be disintegrated is solved. A solid preparation can be promoted to disintegrate, and medicines are released. Through the adoption of the method, the flowing properties of materials are effectively improved, and the problem of decomposition of active components caused by high-temperature drying in the conventional technology can be solved. The preparation technology of the preparation is simple, and industrial mass production is easy to realize.
Owner:WUHAN LL SCI & TECH DEV
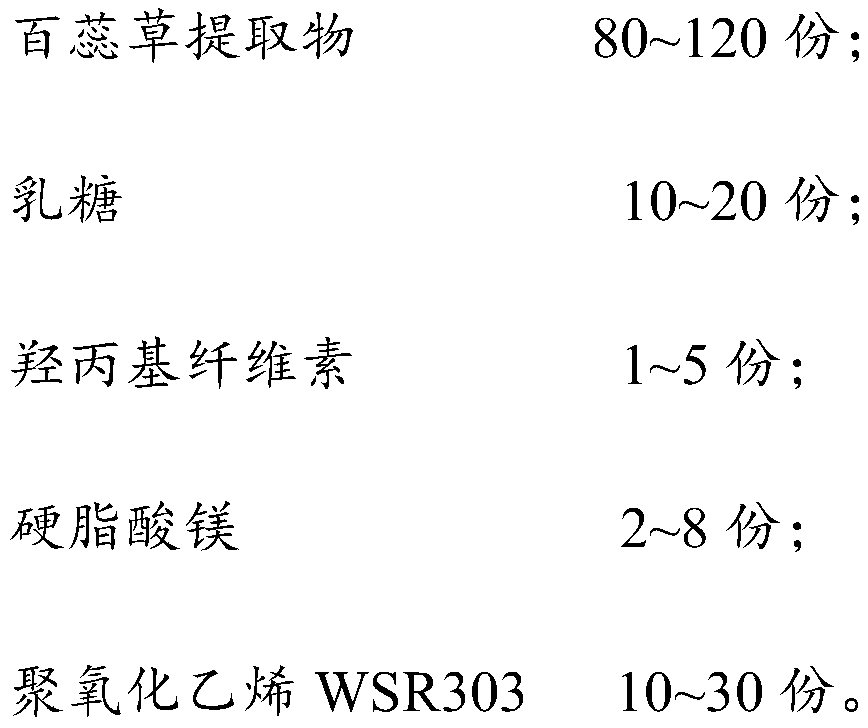
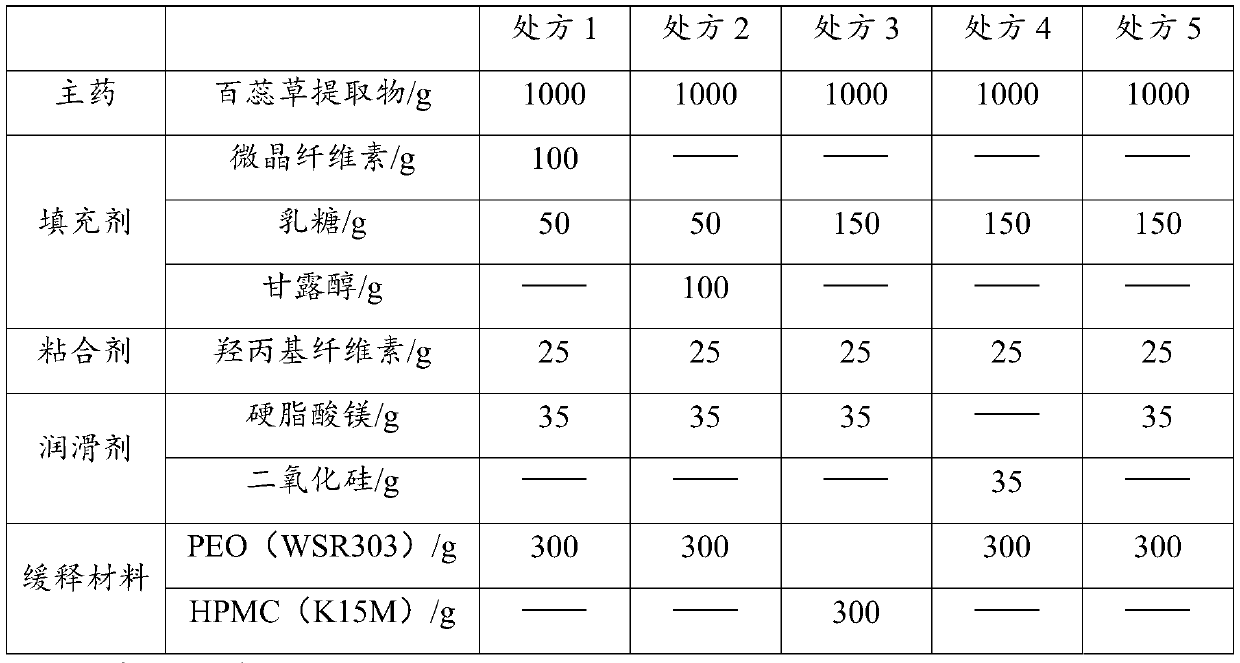
Thesium chinense tablet and preparation method and application thereof
ActiveCN110638774AGood sustained release effectImprove liquidityPharmaceutical non-active ingredientsPill deliveryCelluloseMagnesium stearate
The present invention relates to the field of thesium chinense preparations and particularly relates to a thesium chinense tablet and a preparation method and an application thereof. The thesium chinense comprises the following raw materials in parts by weight: 80-120 parts of a thesium chinense extract, 10-20 parts of lactose, 1-5 parts of hydroxypropyl cellulose, 2-8 parts of magnesium stearateand 10-30 parts of polyoxyethylene WSR 303. The prepared tablet is good in slow release effect, high in dissolution rate, good in stability, good in fluidity, smooth in surface and small in tablet weight difference.
Owner:HONG KONG JOWA & HUAYUAN GRP CHUZHOU PHARMA CO LTD
A kind of preparation method of Afatinib maleate tablet
ActiveCN107260698BSmooth appearanceHigh hardnessOrganic active ingredientsInorganic non-active ingredientsExternal calciumSulfate
The invention discloses a preparation method of afatinib dimaleate tablets. The preparation method comprises the following steps: 1) respectively weighing raw materials in parts by weight; 2) uniformly mixing afatinib dimaleate, a diluting agent, polyethylene glycol and internal calcium sulfate, heating to 60 to 70 DEG C, after the mixture reaches a predetermined temperature, keeping the temperature for 10 to 15min, cooling at room temperature, granulating, adding and uniformly mixing a disintegrating agent, external calcium sulfate, magnesium stearate and silicon dioxide, and tabletting; and 3) coating the tablets, thus obtaining the afatinib dimaleate tablet. The obtained tablets are stable in quality, uniform in content and good in in-vitro dissolution behavior.
Owner:GUANGDONG ANNOL PHARM CO LTD
Oral solid preparation for treating hypertension and related diseases
ActiveCN109316451AOvercome the insoluble problemGood disintegrationOrganic active ingredientsMetabolism disorderDiseaseAdhesive
The invention provides an oral solid preparation for treating hypertension and related diseases. The preparation is prepared from an active ingredient, a pH regulator, an excipient, a disintegrant, afiller, a lubricant, and an adhesive. The solid preparation has good disintegration performance and dissolution rate, and improves bioavailability, and can especially solve the problems that the active substances (active ingredients) with strong hygroscopicity become sticky after moisture absorption, which are not effectively disintegrated by using conventional prescriptions and processes, and have poor formulation fluidity leading difficulties in preparation. The invention also provides a preparation method and application of the oral solid preparation.
Owner:WUHAN LL SCI & TECH DEV
Avatrombopag maleate preparation composition as well as tablet or capsule prepared from composition and preparation method
PendingCN114010638AImprove uniformityUniform particle size distributionOrganic active ingredientsDigestive systemLactoseMedicinal chemistry
The invention discloses an avatrombopag maleate preparation composition. The composition comprises an active component avatrombopag maleate and a filler, the filler comprises lactose and microcrystalline cellulose, the mass ratio of the active component to the filler is 1:1 to 1:5, and the active component and the filler are co-ground to obtain the preparation composition. The composition prepared by the invention can be used for preparing the avatrombopag maleate tablets or capsules, the dissolution rate in a preparation batch is uniform, the shelf life and the dissolution rate are stable, and an effective and feasible production process can be provided for domestic popularization of the avatrombopag maleate preparation. The invention also discloses a tablet or a capsule prepared from the preparation composition. The invention also discloses a preparation method of a tablet or a capsule prepared from the avatrombopag maleate preparation composition.
Owner:南京唯创远医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com