Repaglinide troche and preparation method thereof
A tablet and formula technology, applied in the field of pharmaceutical preparations, can solve the problems of complicated operation steps, increased labor cost, uneven particle size distribution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
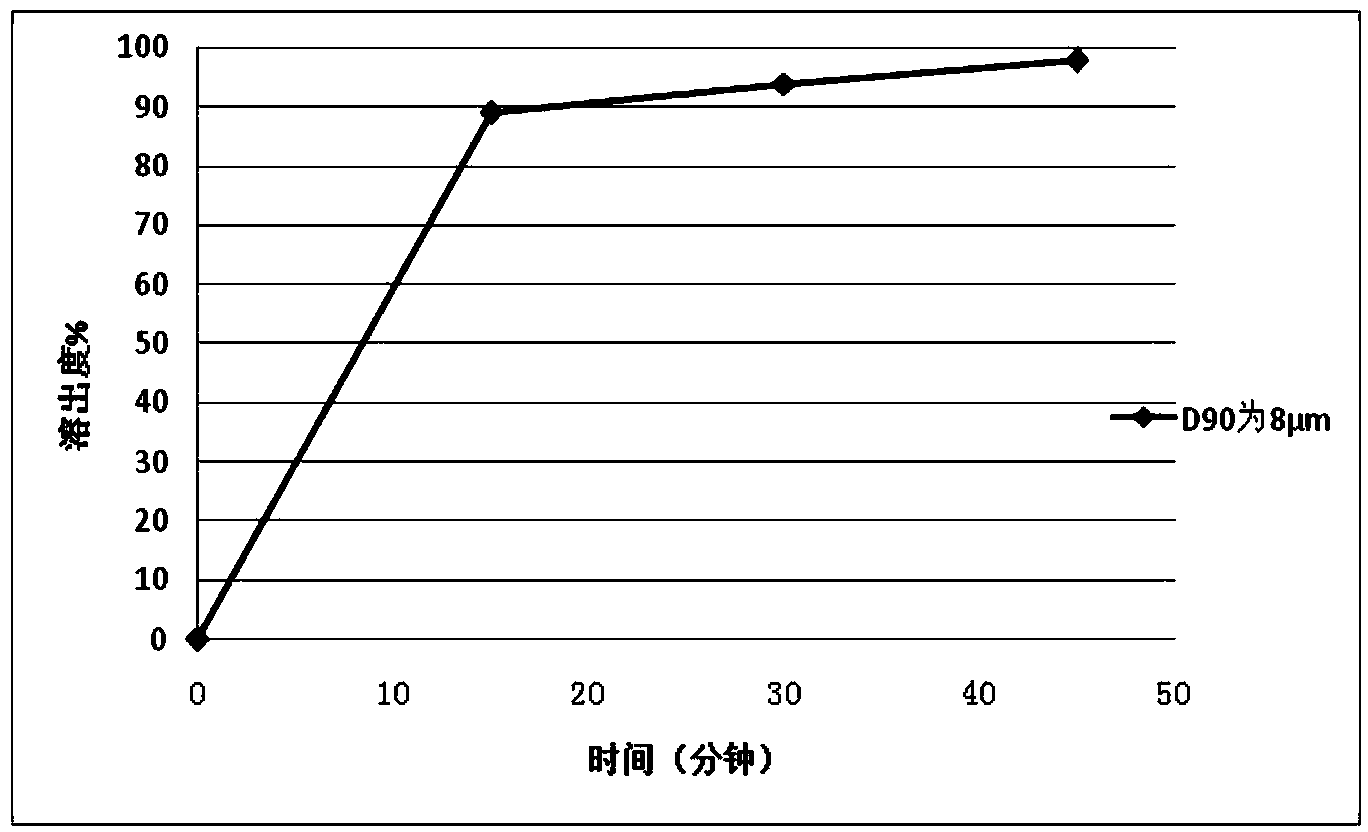
[0101] Example 1 Preparation of Repaglinide Tablets by Powder Direct Compression
[0102] Adopt commercially available repaglinide crude drug, pulverize with a jet mill, and detect the particle size after pulverization; repeat pulverization as required until the particle size is D 90 =8μm. The tablet prescription is shown in Table 1:
[0103] Table 1: Prescription for 1000 repaglinide tablets (2mg / tablet)
[0104] #
Material
1000 prescription quantity (g)
Percentage (%)
1
Repaglinide (8μm)
2
2
2
40
40
3
0.15
0.15
4
Poloxamer 188
2
2
5
Microcrystalline Cellulose PH102
54.15
54.15
6
Crospovidone
1
1
7
0.5
0.5
8
red iron oxide
0.2
0.2
[0105] Described tablet adopts direct compression method to prepare, and concrete steps are:
[0106] A. P...
Embodiment 2
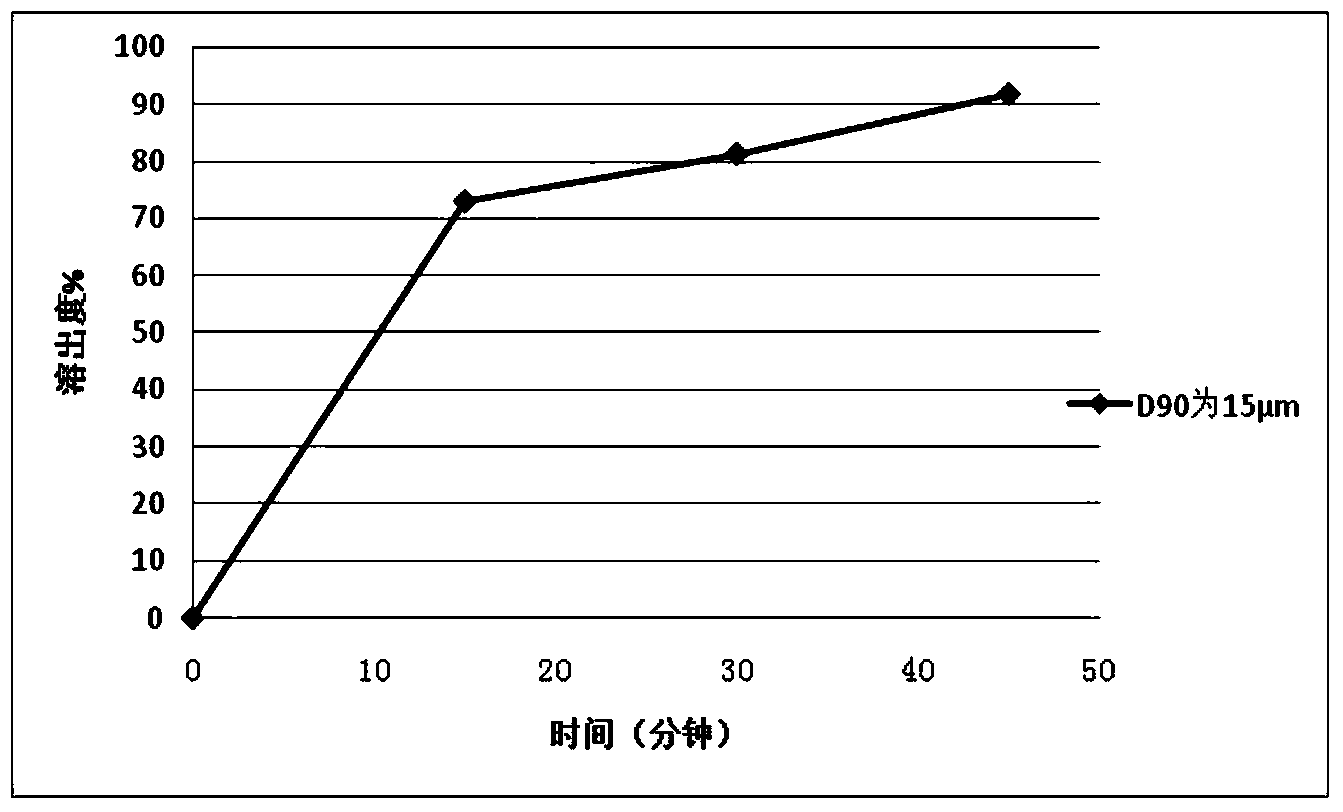
[0113] Example 2 Preparation of Repaglinide Tablets by Powder Direct Compression
[0114] Using the same prescription and process method in Example 1 to prepare 2mg / tablets, the difference is that the particle size D of repaglinide 90 is 15 μm.
Embodiment 3
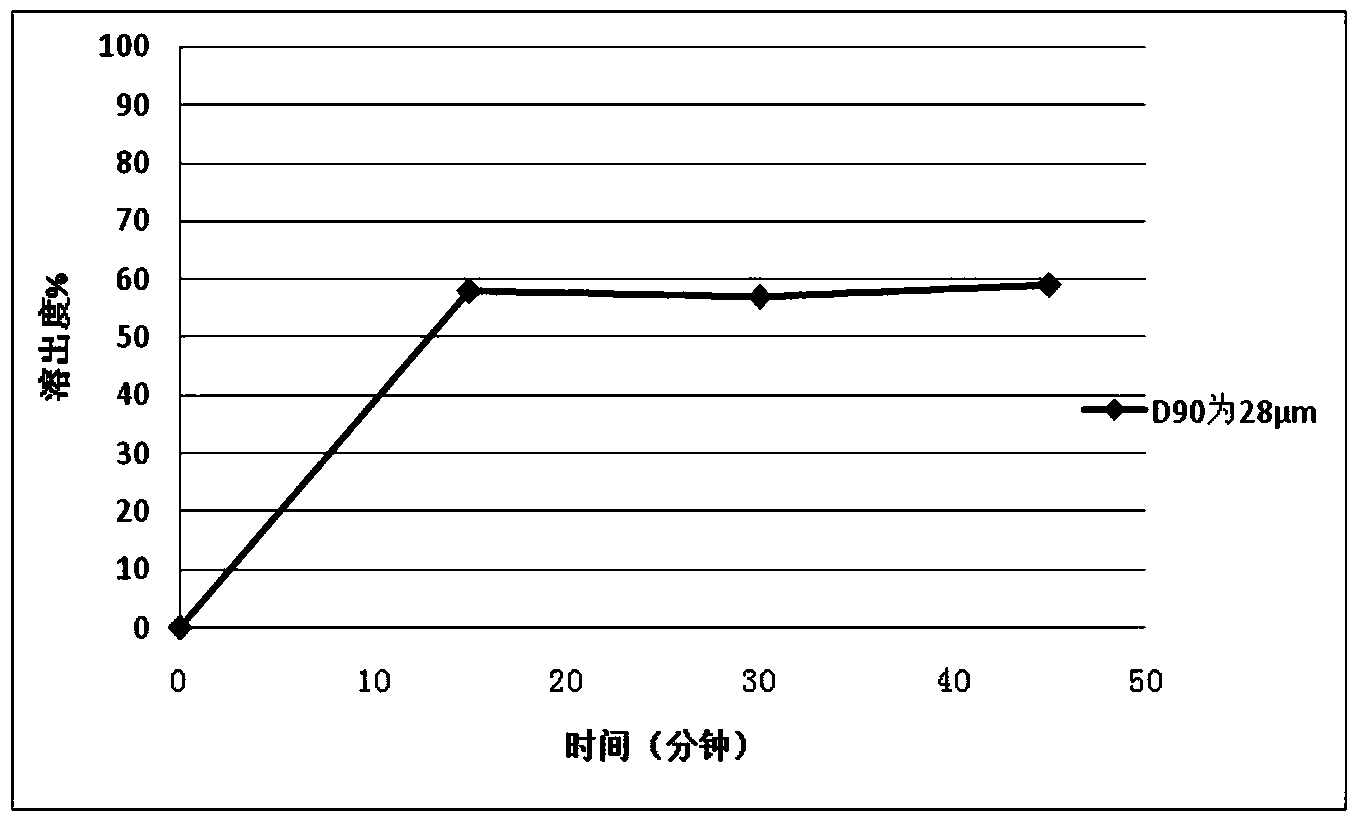
[0115] Example 3 Preparation of Repaglinide Tablets by Powder Direct Compression
[0116] Using the same prescription and process method in Example 1 to prepare 2mg / tablets, the difference is that the particle size D of repaglinide 90 is 28 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com