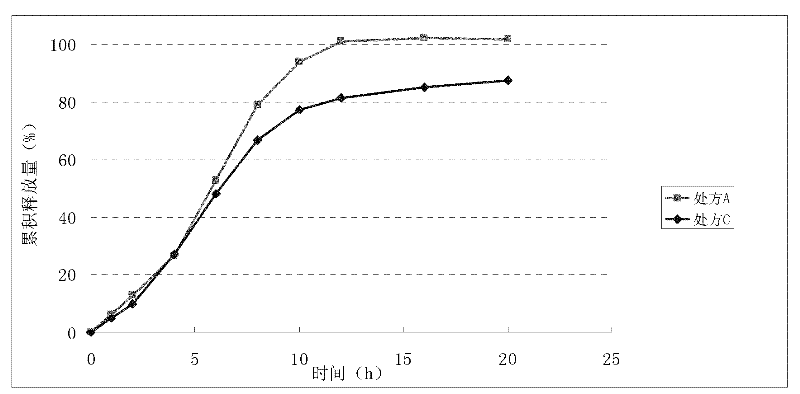
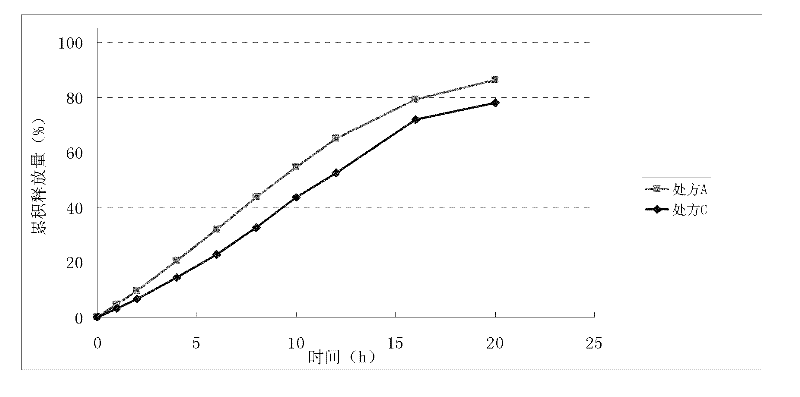
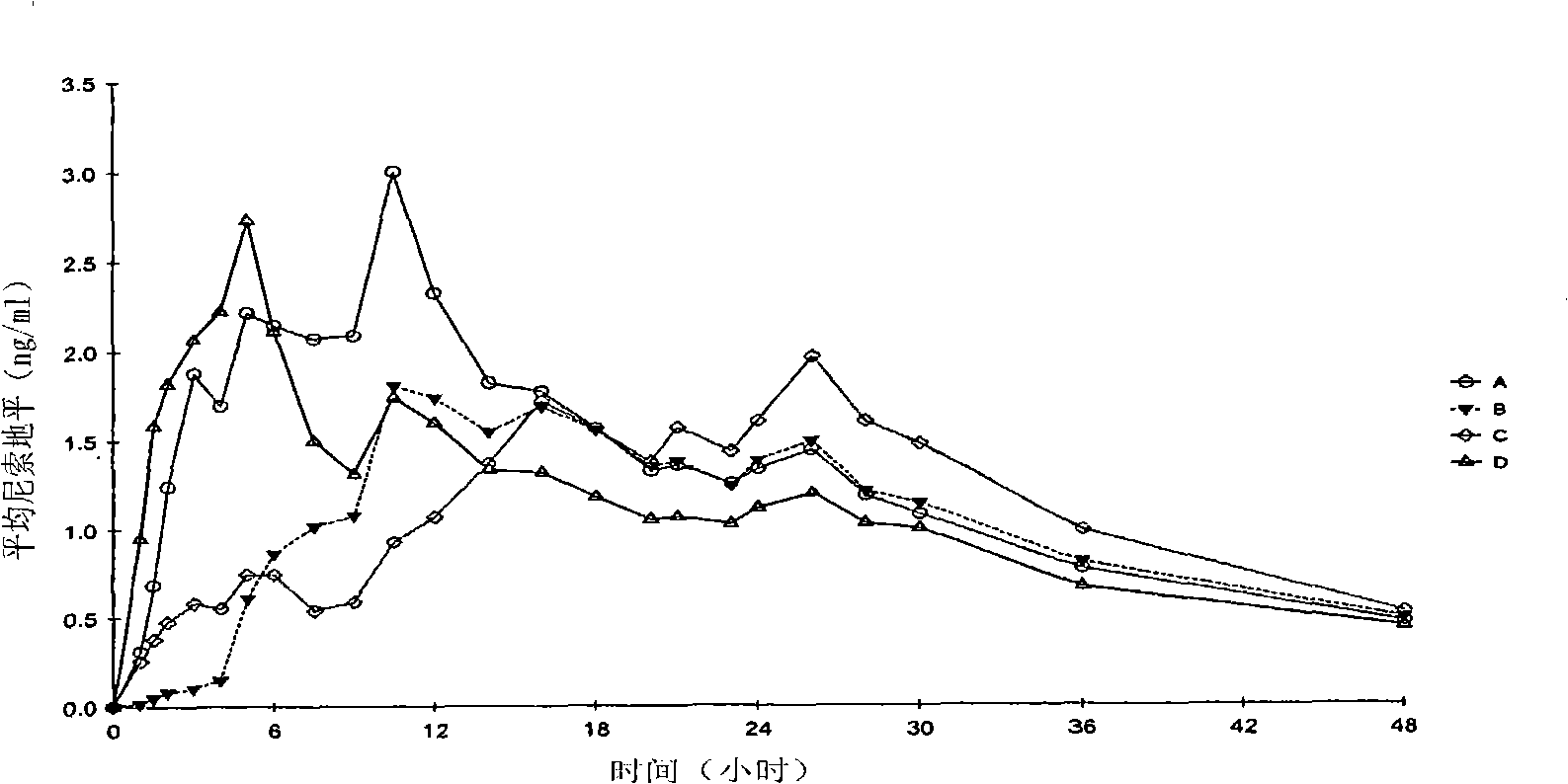
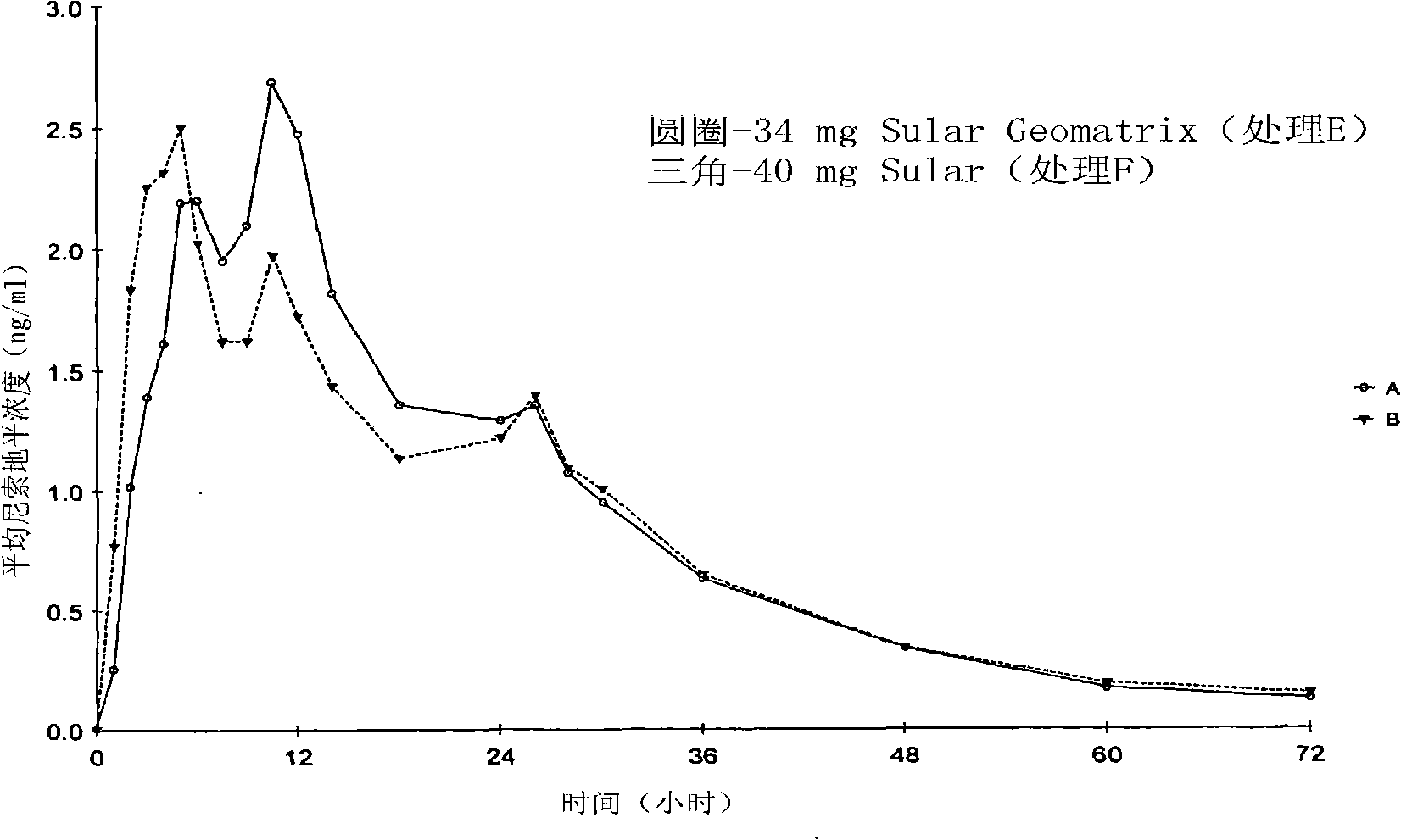
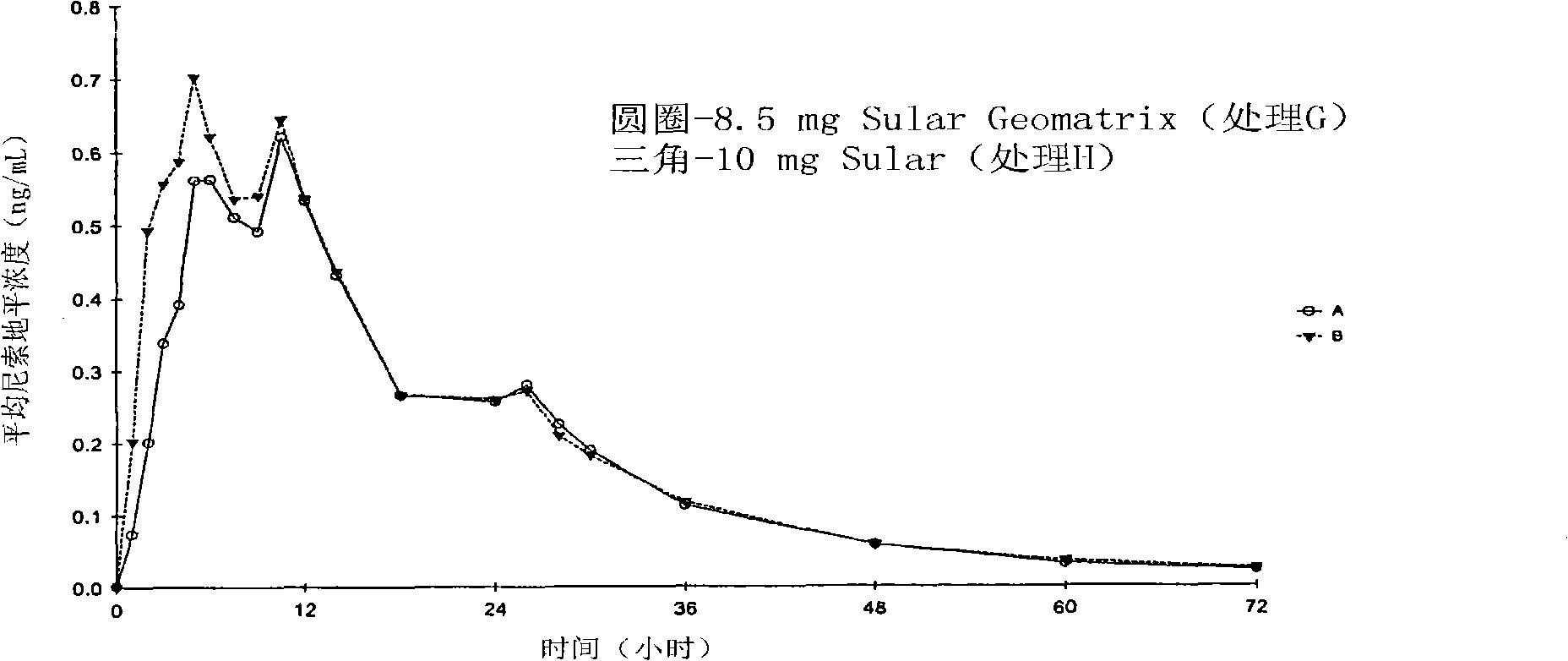
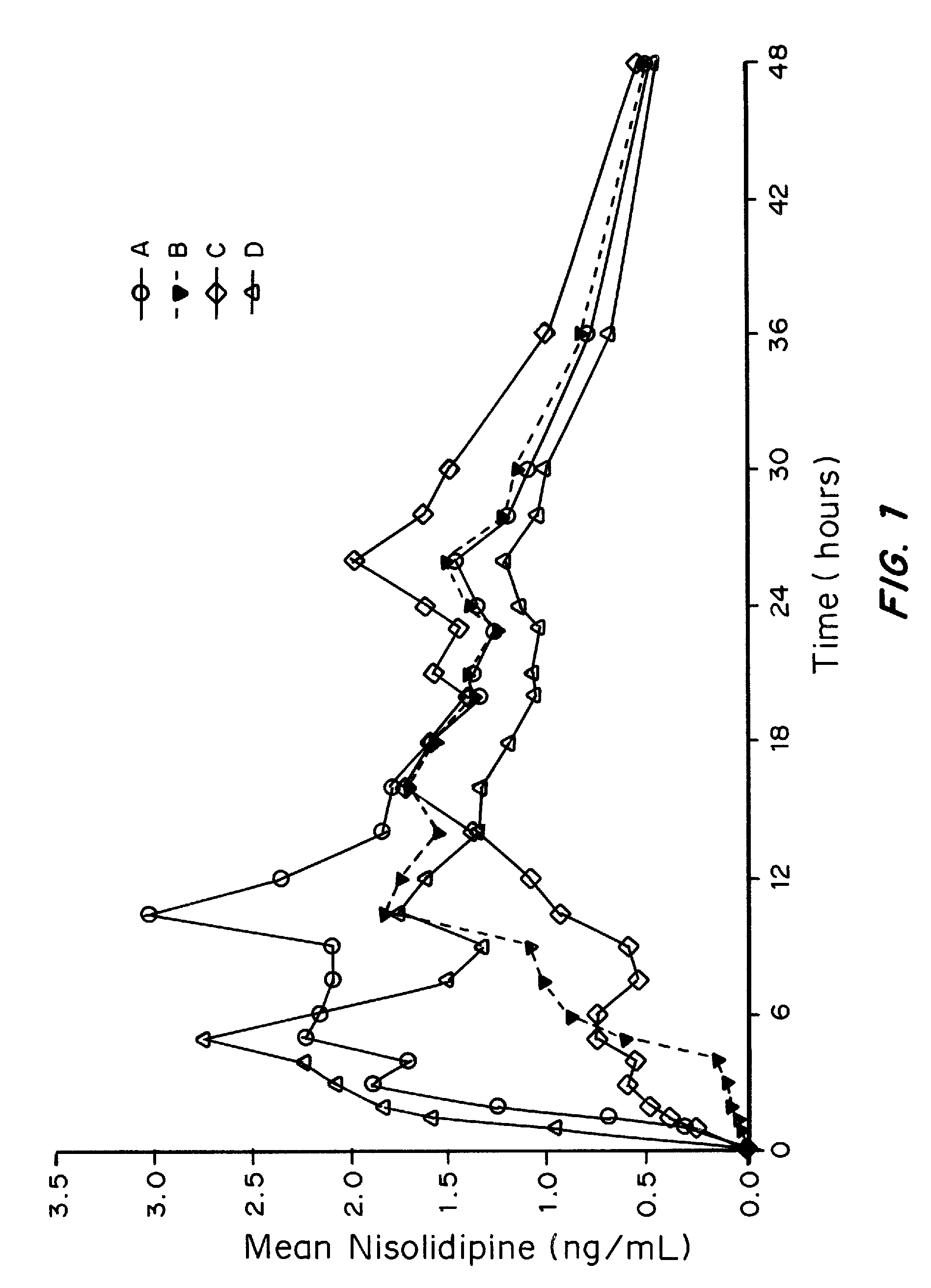
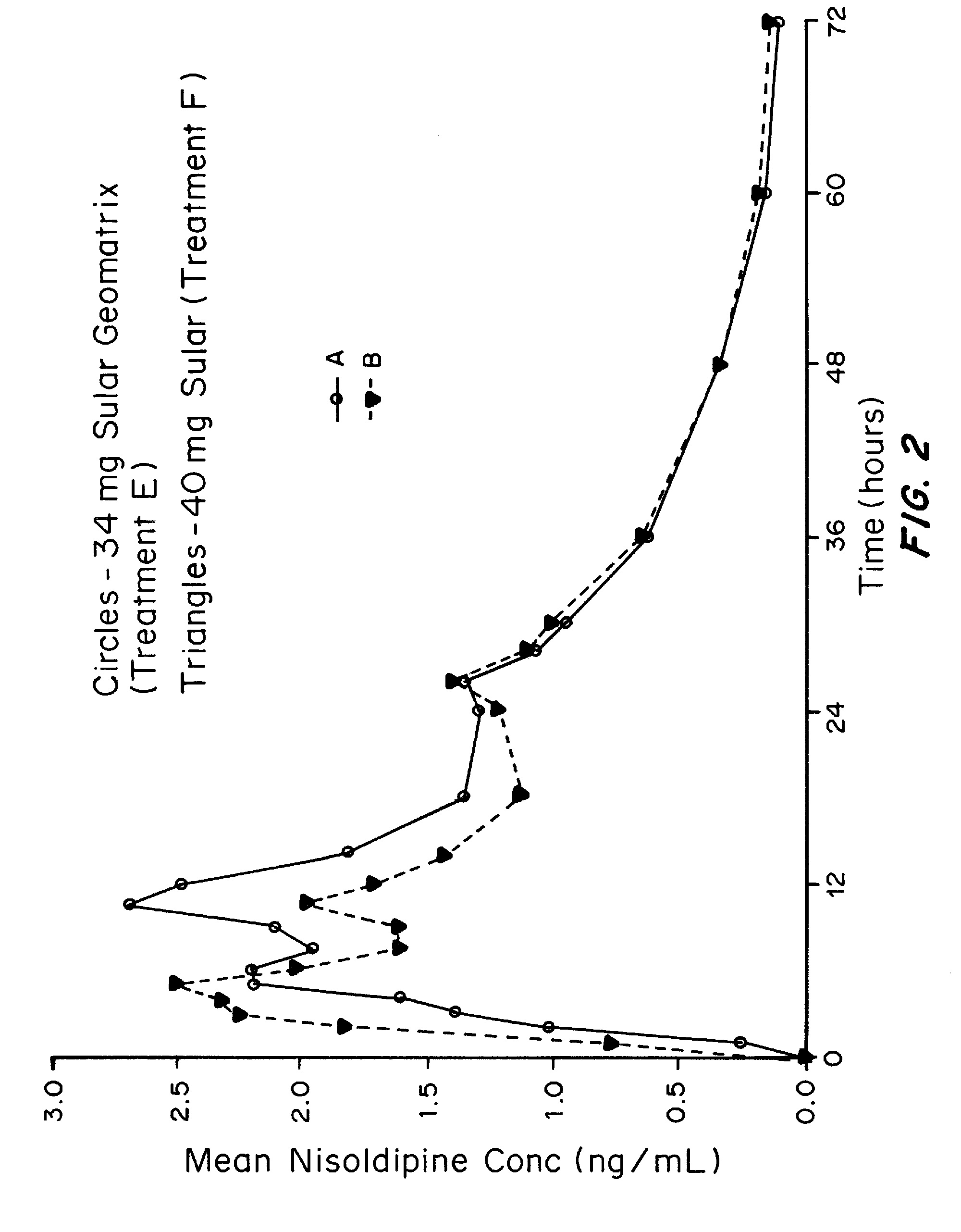
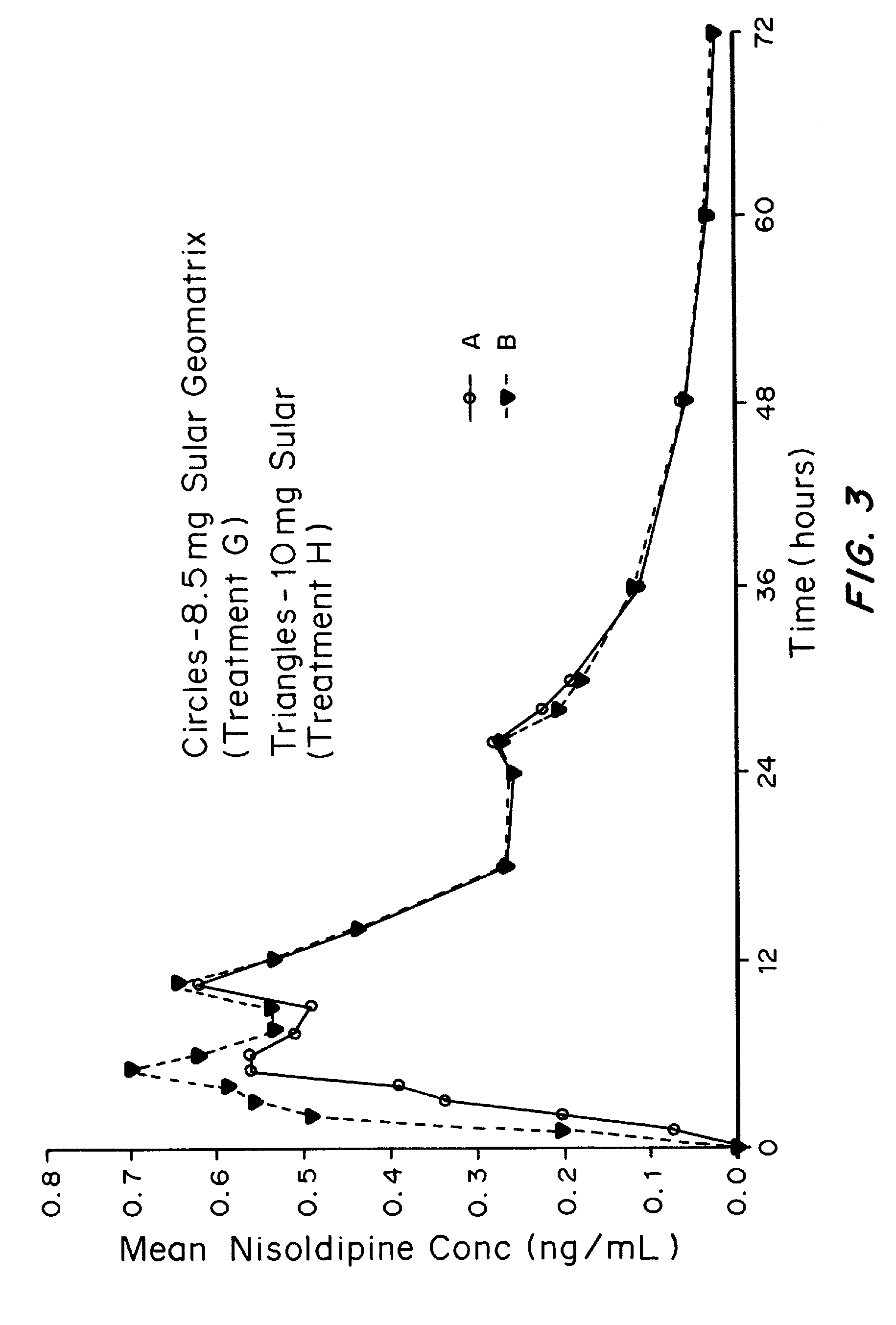
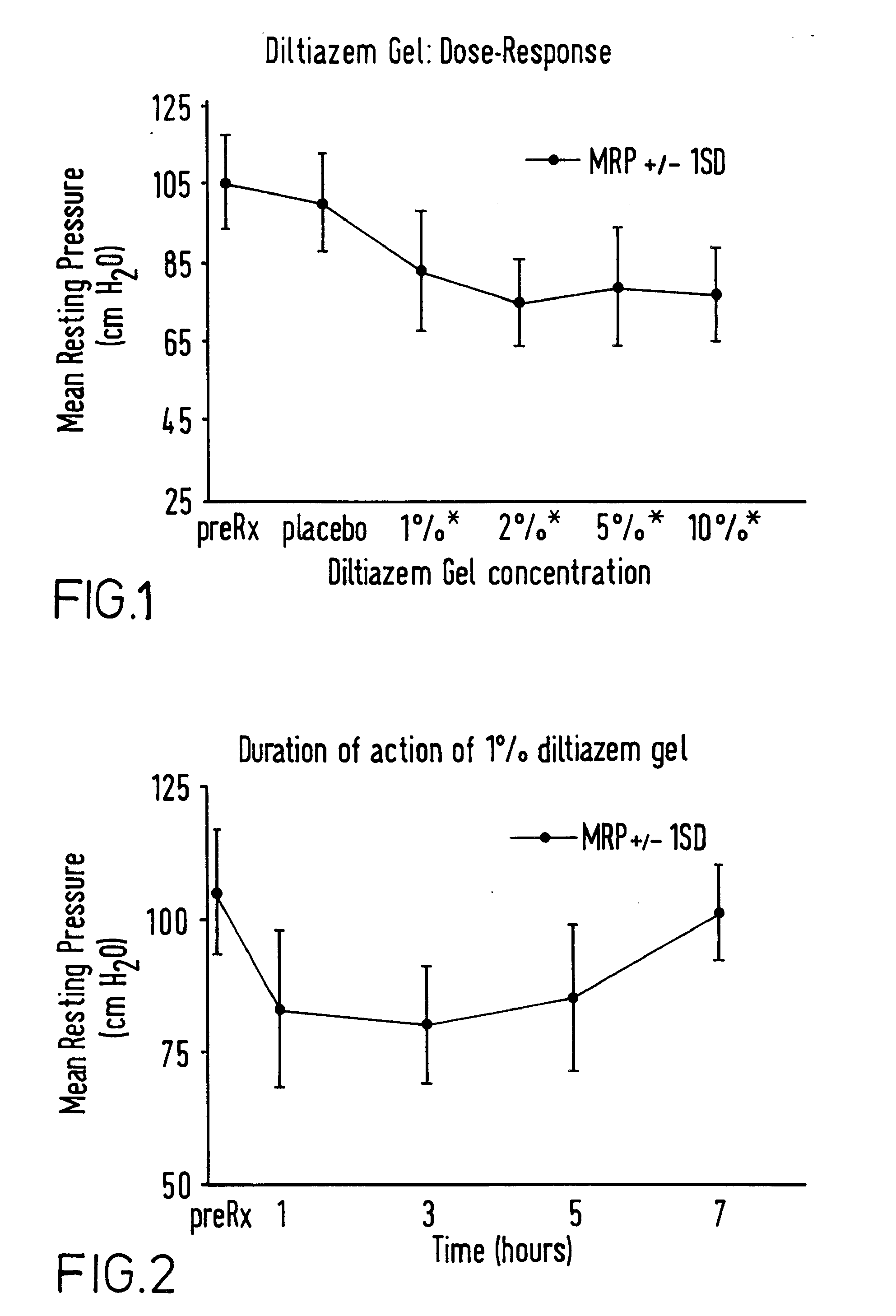
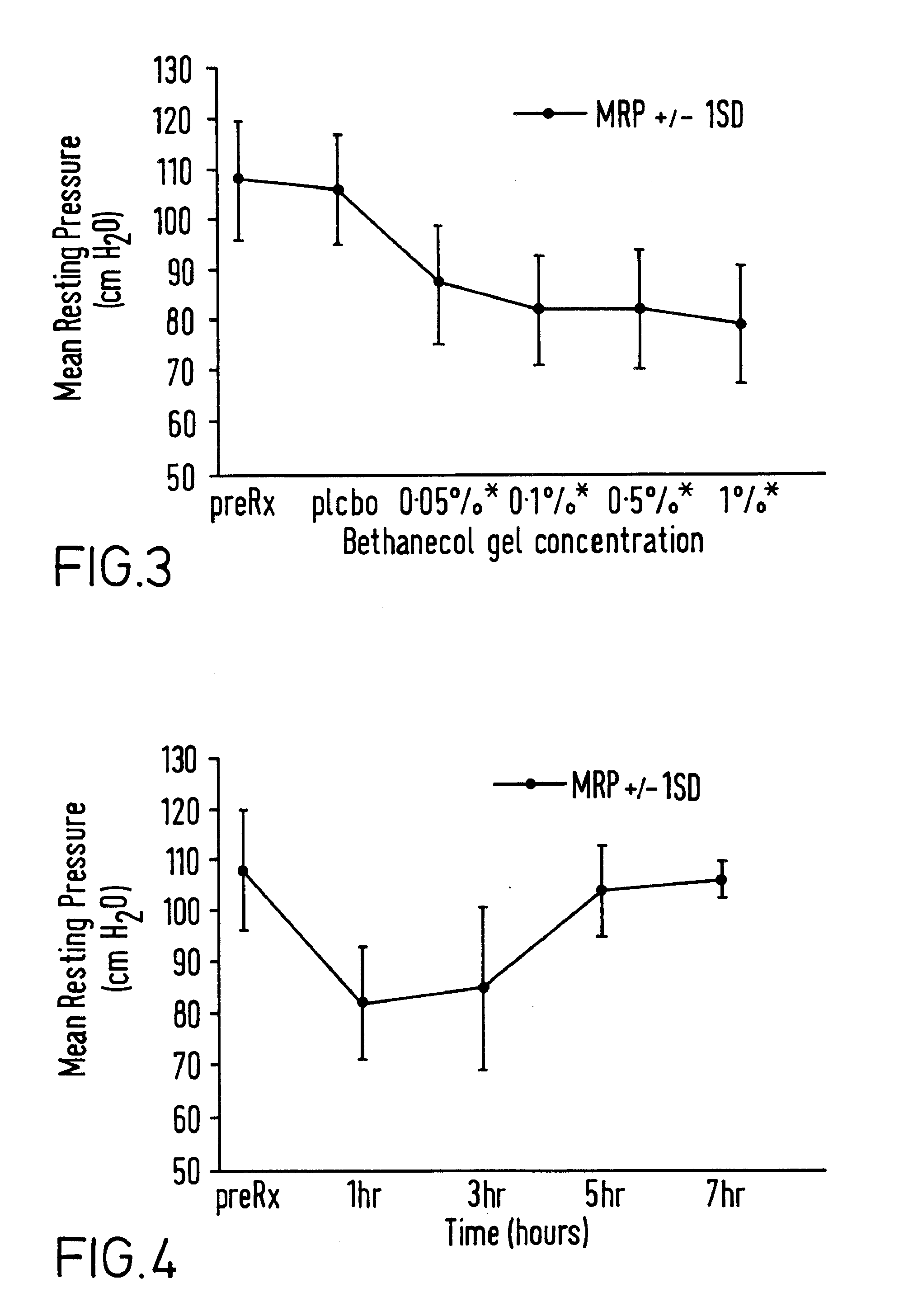
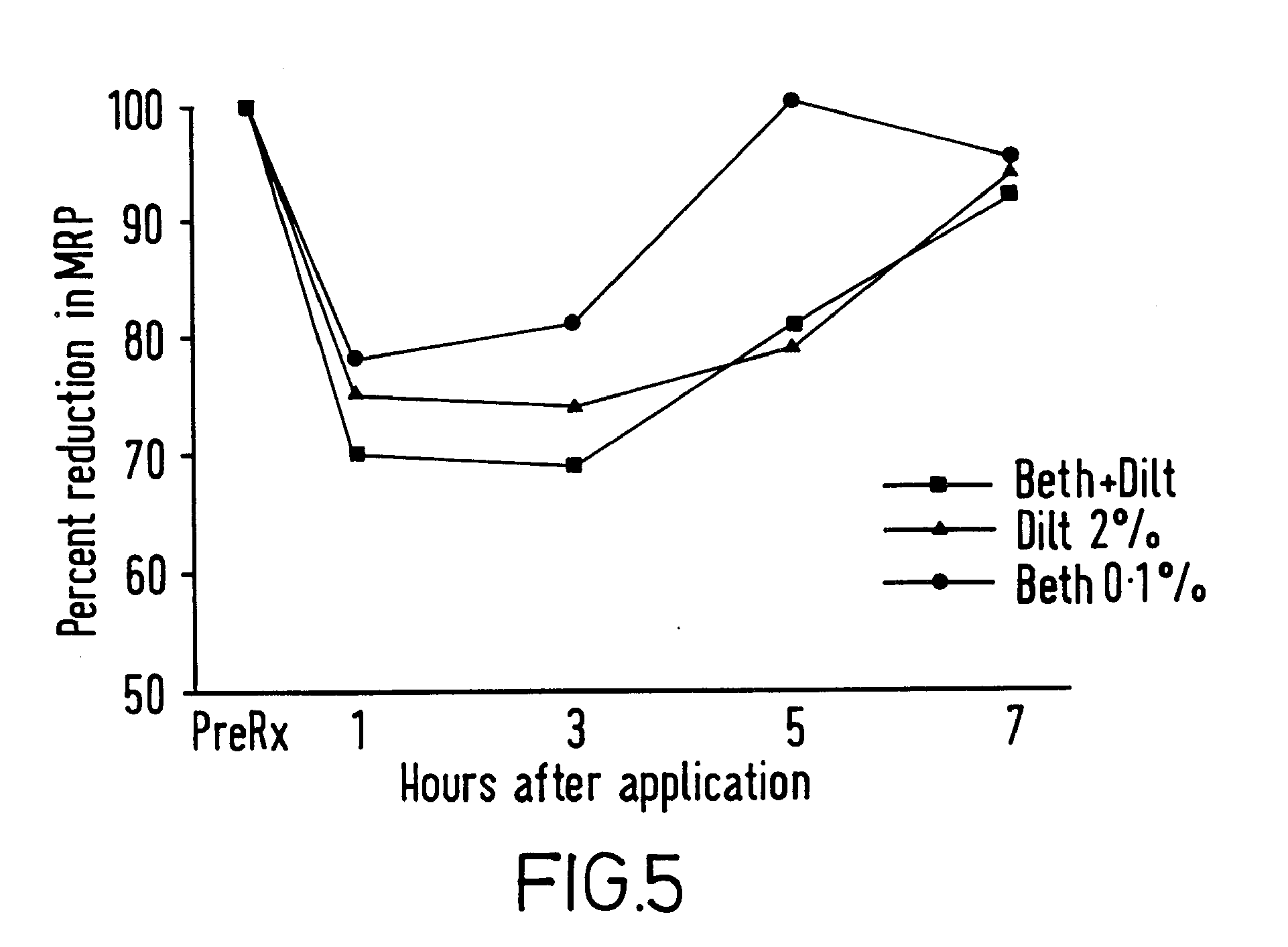
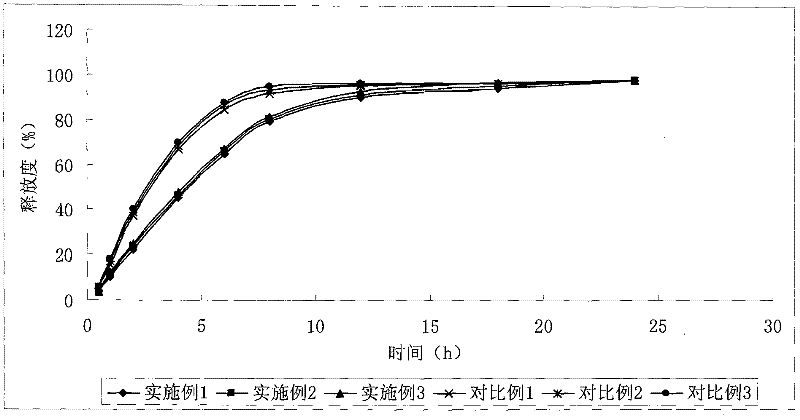
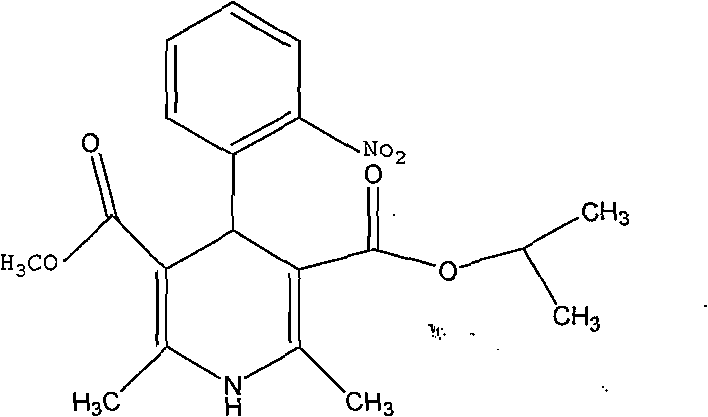
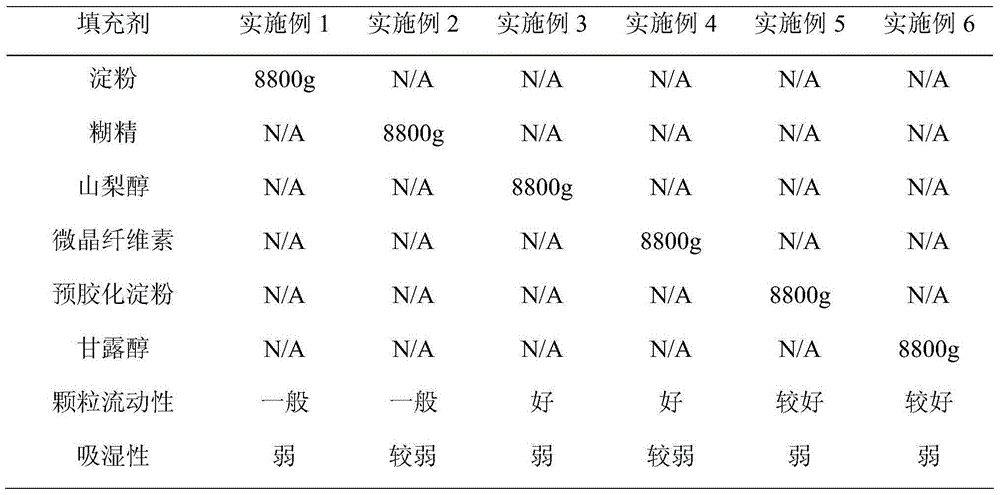
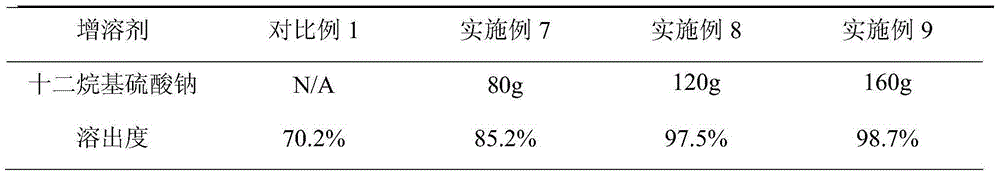
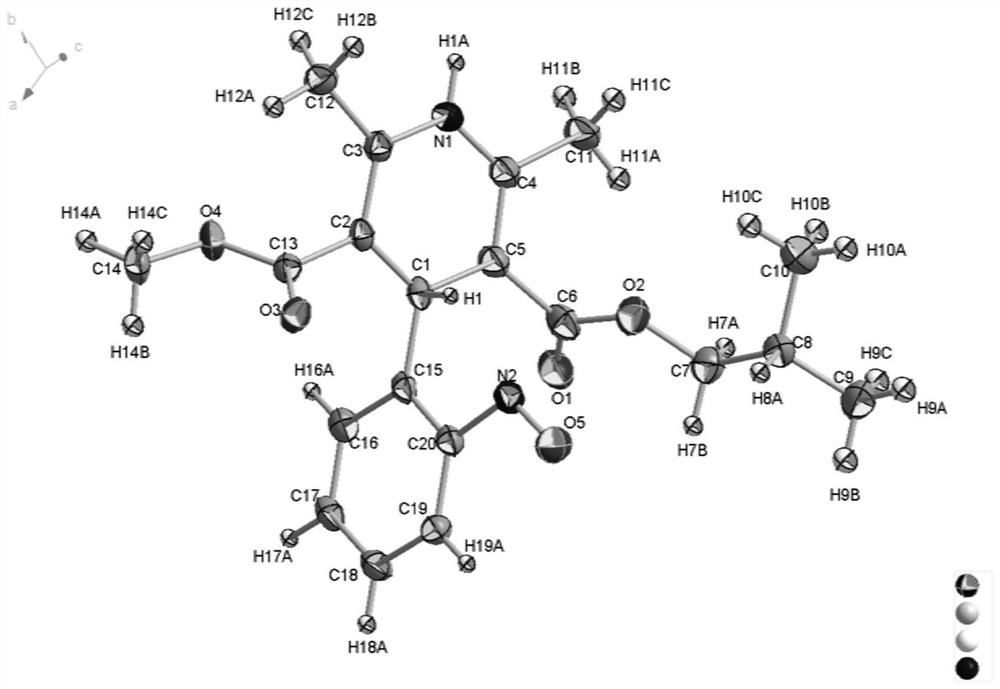
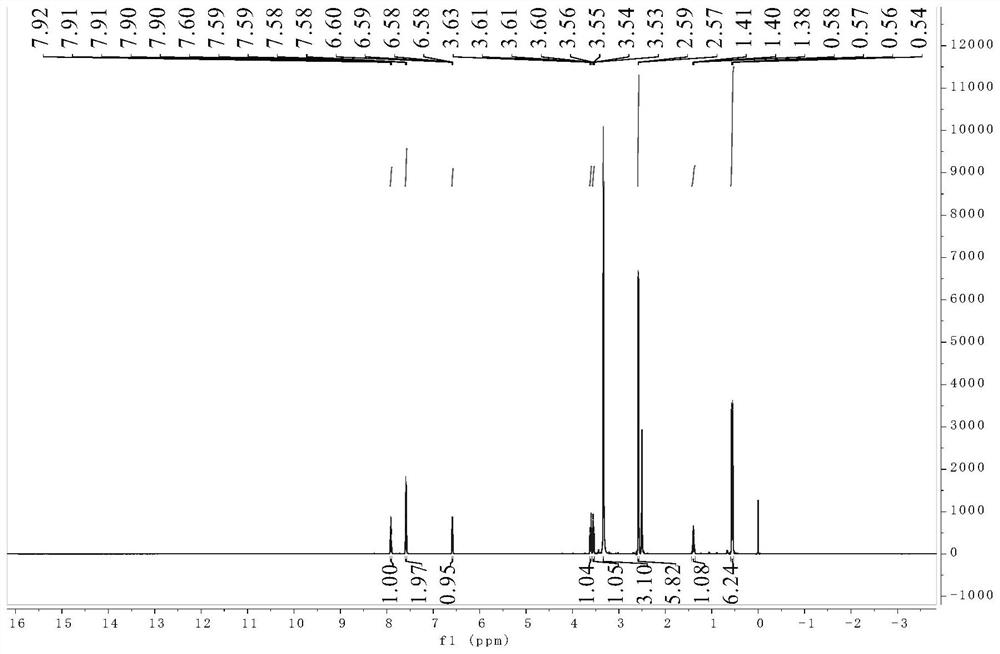
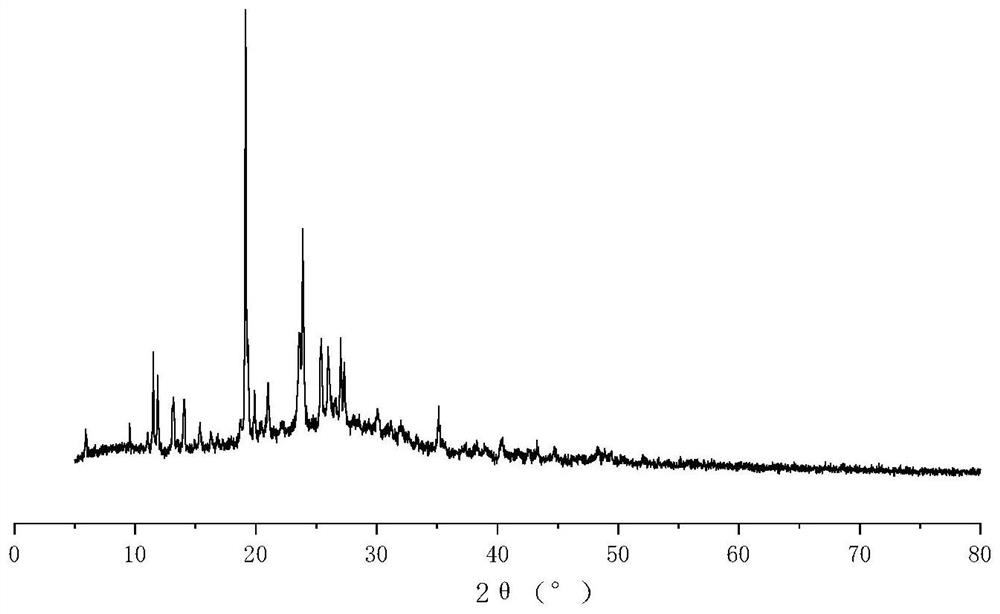
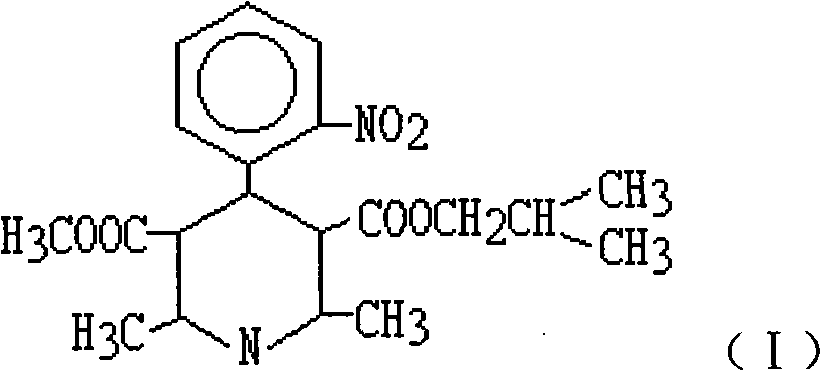Patents
Literature
32 results about "Nisoldipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nisoldipine is used with or without other medications to treat high blood pressure (hypertension).
Monolayer osmotic pump controlled releasing tablets of nimodipine
InactiveCN1552323ALasting effectGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismCellulose acetatePolyethylene glycol
A release controlled nisoldipine tablet with single-layer osmotic pump for treating hypertension, angina pectoris, heart failure, etc contains nisoldipine, the release-controlling auxidiary chosen from sodium chloride, potassium chloride, glyceride behenate, etc, and other auxiliaries. Its advantages are sure and durable curative effect and low toxic by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Composition for lowering blood pressure and application thereof
InactiveCN101890165AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderTasosartanValsartan
The invention provides a pharmaceutical composition which comprises calcium channel blockers of a medicinal dose, angiotensin II receptor antagonists of a medicinal dose, one or more of B vitamins of a medicinal dose and pharmaceutically acceptable carriers, wherein the calcium channel blockers are selected from amlodipine, felodipine, israbipine, nicardipine, nifedipine, nisoldipine, nitrendipine, lacidipine, diltiazem or verapamil; the angiotensin II receptor antagonists are selected from candesartan, telmisartan, losartan, valsartan, irbesartan, eprosartan, tasosartan or olmesartan; and the B vitamins are selected from one or more of vitamin B6, vitamin B12, folic acid and calcium leucovorin. The pharmaceutical composition of the invention can improve the curative effect of the hypotensor, enhance the target organ protecting action of the hypotensor, and reduce the morbidity of complications of angina, myocardial infarction and the like.
Owner:北京奥萨医药研究中心有限公司 +1
Nisoldipine double layer penetrated pump control releasing tablets
InactiveCN1439372AReduce the number of dosesEasy to takeOrganic active ingredientsPharmaceutical delivery mechanismCellulose acetateLeft ventricular size
A release controllable nisoldipine with dual-layer osmotic pump for treating cardiovascular diseases including hypertension, angine pectoris, left ventricular disfunction and congestive heart failure contains nisoldipine (5-20 wt.%), polyoxyethene and / or sodium chloride and / or hydroxypropyl methylcellulose as the release controlling agent of medicine layer, polyoxyethene and / or hydroxypropyl methylcellulose and / or ethylcellulose as the release controlling agent of boosting layer, and acetate cellulose and / or ethylcellulose and / or hydroxypropyl methylcellulose and / or polyethanediol as release-controlling membrane.
Owner:SHENYANG PHARMA UNIVERSITY
Controlled Release Nisoldipine Compositions
ActiveUS20080063711A1Improve bioavailabilityEffective amountBiocideAnimal repellantsActive agentAngina
Controlled release oral dosage formulations containing calcium channel blockers, and methods of use thereof, are provided for the once-a-day treatment of cardiovascular disorders, such as hypertension, angina, and cardiac arrhythmia. The active agent is preferably a dihydropyridine calcium channel blocker, such as nisoldipine. The formulation provides an increase in the bioavailability of the calcium channel blocker as compared to the bioavailability of the calcium channel blocker in other drug delivery formulations known in the art. In one embodiment, the formulation provides an increase in the bioavailability of the calcium channel blocker, nisoldipine, as compared to the same dose of nisoldipine in the coat-core version of the drug (SULAR®). The formulation can be in the form of a trilayer tablet containing a core or central layer and one or more barrier layers.
Owner:JAGOTEC AG
Nisoldipine controlled release tablet and preparation method thereof
ActiveCN102406623ASimple prescriptionEasy to prepareOrganic active ingredientsPharmaceutical non-active ingredientsWater insolubleControlled Release Tablet
The invention provides a nisoldipine controlled release tablet which comprises micronized nisoldipine and medicinal auxiliary materials, wherein the medicinal auxiliary materials include surfactants, pH-dependent auxiliary materials, water-soluble gel materials and / or water-insoluble gel materials, filling agents and lubricating agents. The nisoldipine controlled release tablet provided by the invention is a simple single-layer tablet of which the prescription and preparation method are simpler than those of the existing nisoldipine controlled release tablets such as core-spun tablets and three-layer tablets. The nisoldipine controlled release tablet can be prepared by using equipment for preparing common tablets without using special equipment, thereby improving the production efficiency and lowering the production cost. The release rate of the nisoldipine controlled release tablet provided by the invention in different pH environments can be controlled, and the tablet is stable.
Owner:SHANGHAI SUNTECH PHARMA +2
Controlled release solid oral dosage formulations comprising nisoldipine
InactiveCN101516352ASmall doseReduce manufacturing costOrganic active ingredientsPill deliveryActive agentAngina
Controlled release oral dosage formulations containing calcium channel blockers, and methods of use thereof, are provided for the once-a-day treatment of cardiovascular disorders, such as hypertension, angina, and cardiac arrhythmia. The active agent is preferably a dihydropyridine calcium channel blocker, such as nisoldipine. The formulation provides an increase in the bioavailability of the calcium channel blocker as compared to the bioavailability of the calcium channel blocker in other drug delivery formulations known in the art. In one embodiment, the formulation provides an increase in the bioavailability of the calcium channel blocker, nisoldipine, as compared to the same dose of nisoldipine in the coat-core version of the drug (SULAR TM ). The formulation can be in the form of a trilayer tablet containing a core or central layer and one or more barrier layers.
Owner:JAGOTEC AG
Controlled release nisoldipine compositions
InactiveUS20080221174A1Improve bioavailabilityEffective amountBiocideAnimal repellantsActive agentAngina
Controlled release oral dosage formulations containing calcium channel blockers, and methods of use thereof, are provided for the once-a-day treatment of cardiovascular disorders, such as hypertension, angina, and cardiac arrhythmia. The active agent is preferably a dihydropyridine calcium channel blocker, such as nisoldipine. The formulation provides an increase in the bioavailability of the calcium channel blocker as compared to the bioavailability of the calcium channel blocker in other drug delivery formulations known in the art. In one embodiment, the formulation provides an increase in the bioavailability of the calcium channel blocker, nisoldipine, as compared to the same dose of nisoldipine in the coat-core version of the drug (SULAR®). The formulation can be in the form of a trilayer tablet containing a core or central layer and one or more barrier layers.
Owner:JAGOTEC AG
Topical pharmaceutical composition comprising a cholinergic agent or a calcium channel blocker
A method and composition are provided for the treatment of an anorectal disorder and for controlling the pain associated therewith. The method comprises administering to a subject in need of such treatment therapeutically effective amounts of a calcium channel blocker either alone or together with a nitric oxide donor. Amlodipine, anipamil, barnidipine, benidipine, bepridil, darodipine, diltiazem, efonidipine, felodipine, isradipine, lacidipine, lercanidipine, lidoflazine, manidipine, mepirodipine, nicardipine, nifedipine, niludipine, nilvadipine, nimodipine, nisoldipine, nitrendipine, perhexiline, tiapamil, verapamil and pharmaceutically acceptable salts thereof, are suitable calcium channel blockers.
Owner:SLA PHARMA AG
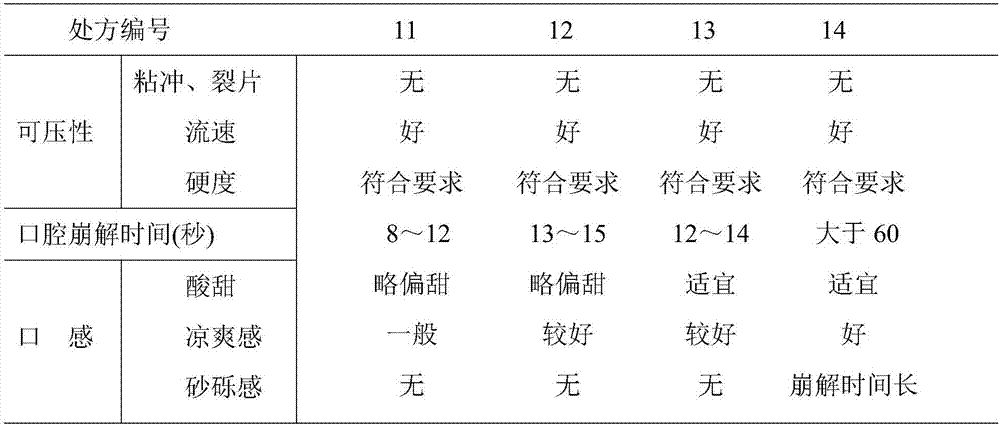
Nisoldipine oral disintegration tablet for treating hypertension and preparing method
InactiveCN1562013ANo grittinessMushy feeling noOrganic active ingredientsPill deliveryOrally disintegrating tabletCoronary heart disease
An oral disintegrating tablet for nisoldipine for treating coronary heart disease, hypertension, ischemic heart disease and congestive heart failure is prepared from nisoldipine, souring agnet, excipient and disintegrant.
Owner:JIANGXI HERBI SKY CO LTD
Nisoldipine compound and novel preparation method thereof
InactiveCN102491940AChange the status quo of low puritySolve puzzlesOrganic chemistryCardiovascular disorderActivated carbonSide effect
The invention discloses a high purity nisoldipine compound, which comprises the following steps: (1) dissolving a certain amount of rough nisoldipine products in an organic solvent, adding activated carbon into the organic solvent to absorb and filter, collecting filtrate, and reducing pressure and concentrating to obtain primary purified nisoldipine; (2) separating and purifying the primary purified nisoldipine by using a preparation type chromatographic column, and collecting eluent to obtain secondary purified nisoldipine; and (3) decompressing and concentrating the eluent, adding water while stirring, performing heating and backflow, performing cooling and crystallization, and centrifugally washing and drying precipitated crystals to obtain tertiary purified nisoldipine. By means of the method, the nisoldipine produced has high purity, the toxic and side effects of prepared pharmaceuticals for treating hemorrhagic cerebrovascular diseases, high blood pressure and the like are reduced, product quality of preparation is improved, and the high purity nisoldipine compound is suitable for large-scale industrialization production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Pharmaceutical composition comprising telmisartan salt and calcium ion antagonist
InactiveCN102266559AImprove solubilityHigh dissolution rateOrganic active ingredientsSenses disorderMagnesium saltDiltiazem
The present invention relates to a kind of pharmaceutical composition, it comprises telmisartan salt and calcium ion antagonist or its pharmaceutically acceptable salt and pharmaceutically acceptable carrier; Described telmisartan salt is selected from telmisartan Sodium salt, potassium salt, calcium salt, magnesium salt or amine salt of sartan, described calcium ion antagonist is selected from amlodipine, lacidipine, cilnidipine, lercanidipine, nisoldipine, nica Dipine, azedipine, barnidipine, manidipine, benidipine, verapamil, diltiazem, or a pharmaceutically acceptable salt thereof. The composition is used for preventing, delaying progress or treating patients with hypertension, angina pectoris, atherosclerosis, stroke, cardiac insufficiency, dyslipidemia, diabetes, renal function damage or hypertension accompanied by Alzheimer's disease, reducing Reduce the morbidity and / or mortality of cardiovascular and cerebrovascular diseases, reduce adverse drug reactions, and improve patients' compliance with medication.
Owner:王丽燕
Nisoldipine sustained-release dropping pill and preparation method thereof
InactiveCN101269046AIncrease oxygen supplyReduce afterloadOrganic active ingredientsPharmaceutical delivery mechanismSustained release drugAdditive ingredient
The invention belongs to the field of sustained-release drugs, particularly relates to a nisoldipine sustained-release dropping pill and the preparation method thereof, and aims to supplement the deficiency of the prior art and provide a sustained-release nisoldipine dropping pill formulation. The sustained-release nisoldipine dropping pill is prepared by adding stabilize Vitamin E and hydrophobic framework ingredients to guarantee no occurrence of an obvious change related to the substance content for the drug during the effective storage period and has the advantages of full release, controllable release time and high bioavailability simultaneously. The sustained-release nisoldipine dropping pill is suitable for clinical and family use.
Owner:北京博智绿洲医药科技有限公司
Topical pharmaceutical composition comprising a cholinergic agent or a calcium channel blocker
Owner:SLA PHARMA AG
Dispersion tablet of nisoldipine and preparation method
InactiveCN1695616AFast absorptionImprove bioavailabilityOrganic active ingredientsPill deliveryAdhesiveNisoldipine
A dispersing tablet of nisoldipine is proportionally prepared from nisoldipine, filler, adhesive and disintegrant. Its preparing process is also disclosed.
Owner:浙江南洋药业有限公司
Method for preparing standard of nisoldipine
This invention provides a novel method for preparing standard nisoldipine product. The method comprises: dissolving crude nisoldipine into C3-6 lower ketone and recrystallizing, or dissolving crude nisoldipine into C3-6 lower ketone, mixing with C3-6 lower alcohol, and recrystallizing. The method has such advantages as stable process, easy operation, high product purity (above 99.5%), and no pollution.
Owner:SHANDONG XINHUA PHARMA CO LTD
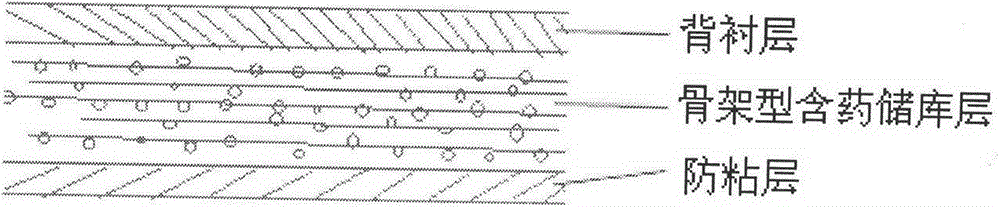
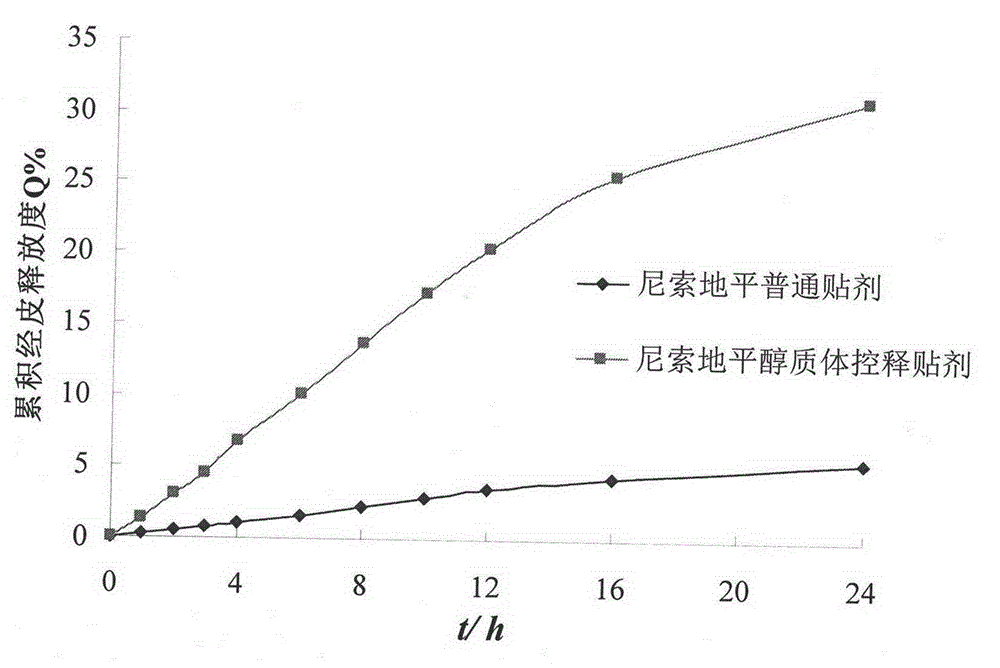
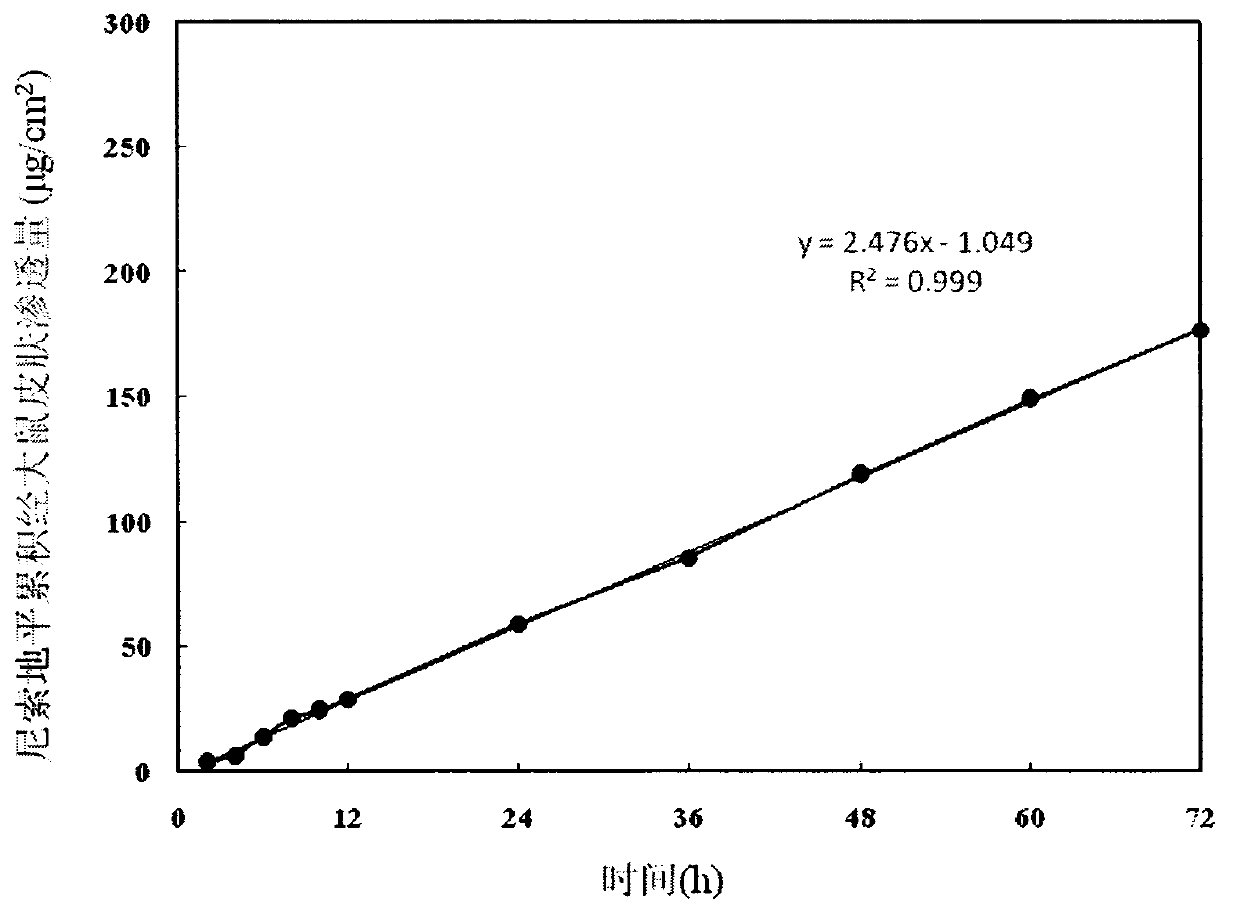
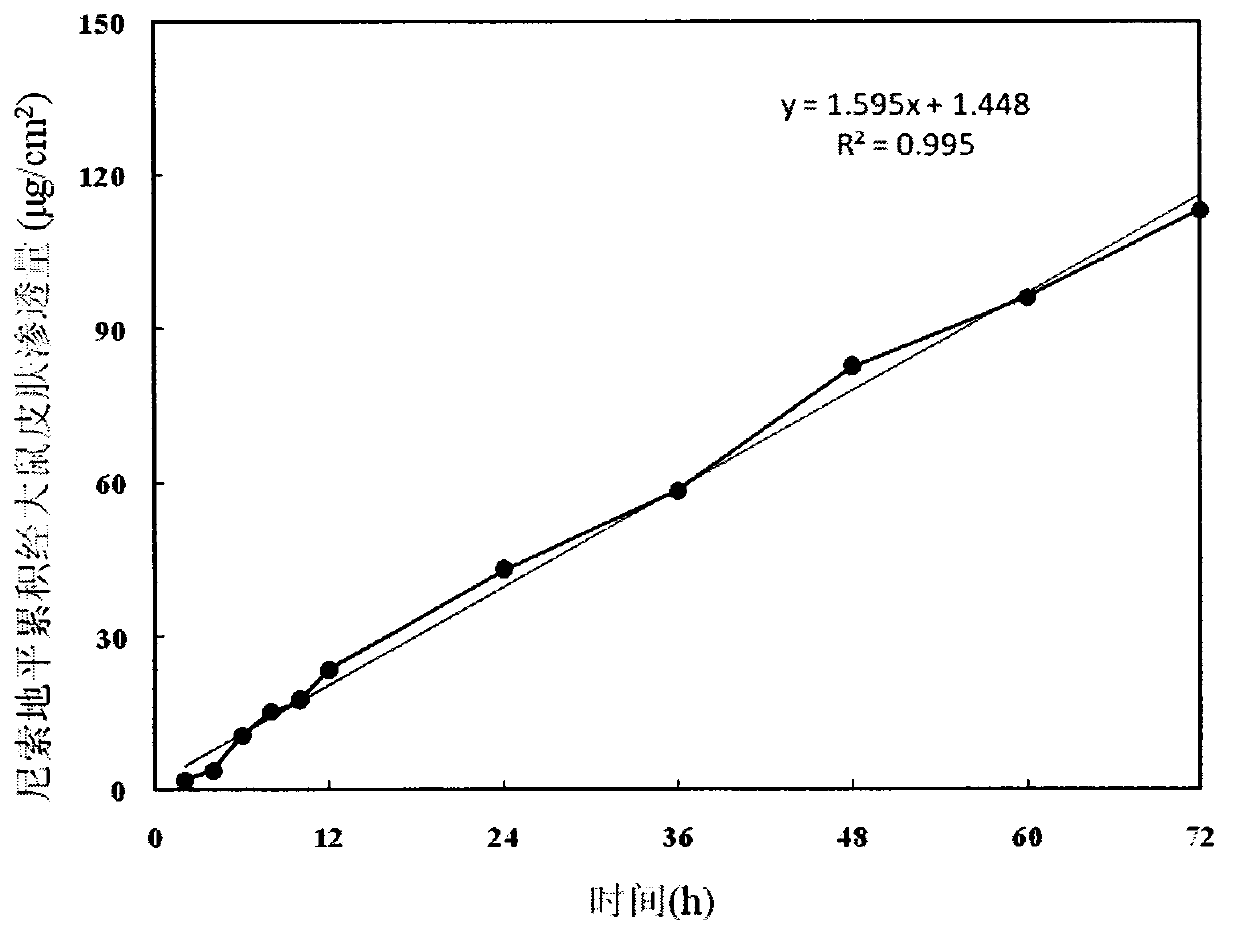
Nisoldipine ethosome controlled-released patch and preparation method thereof
InactiveCN105078928AImprove complianceSuitable for long-term prophylaxisOrganic active ingredientsPharmaceutical non-active ingredientsHepatic first pass effectPharmaceutical formulation
The invention relates to a nisoldipine ethosome controlled-released patch and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The nisoldipine ethosome controlled-released patch consists of a back lining, a frame type medicated storage cavern layer and an anti-adhesion layer, and the frame type medicated storage cavern layer contains nisoldipine ethosomes, pressure-sensitive adhesives, penetration enhancer, crosslinking agents, plasticizer and humectants. The ethosomes serve as transdermal drug delivery carriers, percutaneous permeation of the nisoldipine is improved, release of drug is controlled by the frame type pressure-sensitive adhesives, and the nisoldipine transdermal delivery patch is prepared. Compared with a nisoldipine oral preparation, the nisoldipine ethosome controlled-released patch can overcome shortcomings that a first-pass effect of oral delivery is obvious, bioavailability is low, and untoward effects of a digestive tract are obvious. Compared with a common nisoldipine patch, the nisoldipine ethosome controlled-released patch has the advantages that a controlled-released characteristic is good in a percutaneous penetration process, the percutaneous penetration rate of drug is increased for five times at least, stable high blood concentration can be maintained for a long time, relative bioavailability is improved for 4 times almost.
Owner:广东省中药研究所
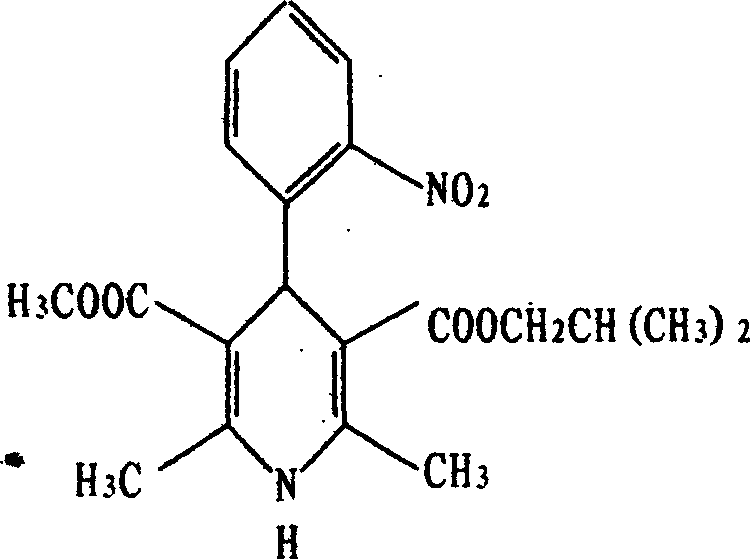
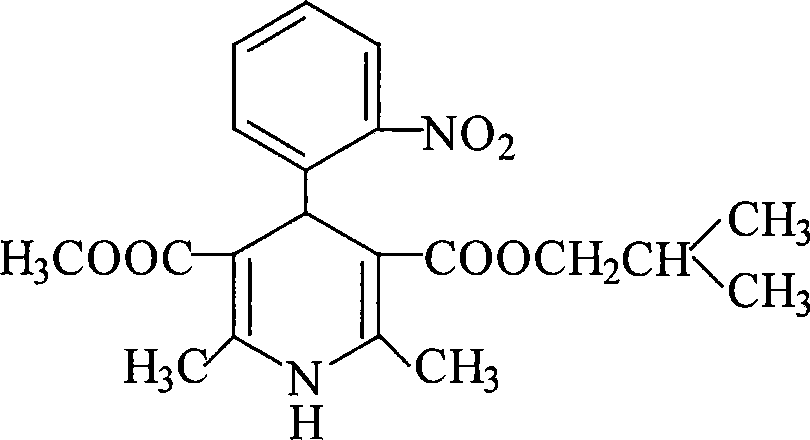
Industrial process for the synthesis of isobutyl methyl 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridine dicarboxylate (nisoldipine)
Synthetic process of isobutyl methyl 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)3,5-pyridine dicarboxylate (Nisoldipine) comprising on the reaction of isobutyl 2-(2-nitrobenzylidene)acetoacetate with methyl 3-aminocrotonate in an apolar solvent.
Owner:ERREGIERRE
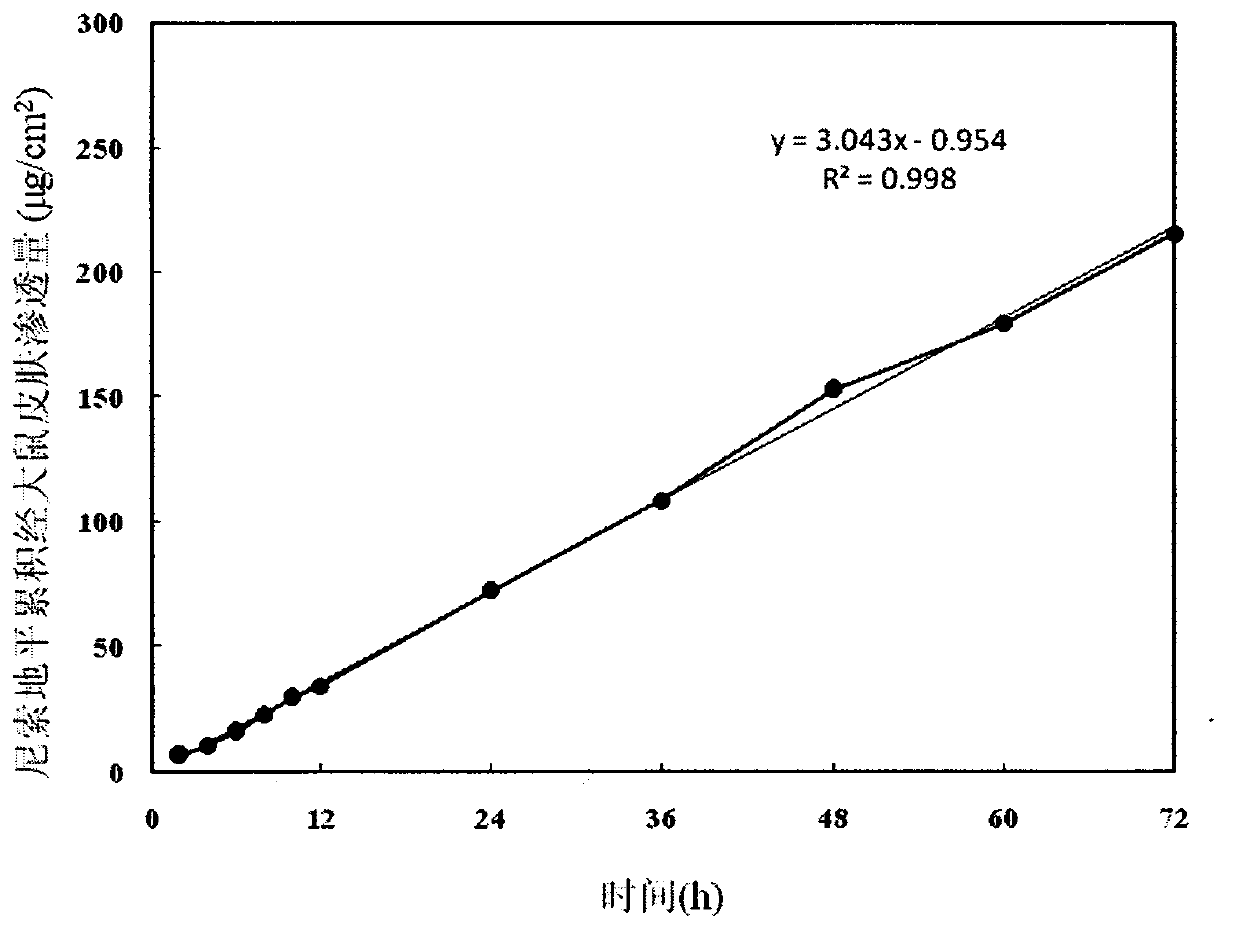
Nisoldipine controlled-release patch and preparation method thereof
InactiveCN103006620AImprove bioavailabilityGood affinity with skinOrganic active ingredientsSheet deliveryTransdermal patchControl release
The invention belongs to the field of pharmaceutic preparation, and particularly relates to a transdermal patch of antihypertensive drug nisoldipine, and a preparation method thereof. The nisoldipine controlled-release patch disclosed by the invention is composed of a backing layer, a drug-polymer layer and a release liner, wherein the drug-polymer layer is composed of drug nisoldipine, a drug-polymer, a penetration enhancer and a plasticizer. The nisoldipine transdermal patch prepared by the preparation method can lastingly release drug within three days at a constant speed.
Owner:CHINA PHARM UNIV
Nisoldipine liposome solid preparation
InactiveCN102406608AHigh encapsulation efficiencyImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention discloses a nisoldipine liposome solid preparation and a preparation method thereof. The liposome is prepared from an active component nisoldipine and specifically combined phosphatidyl ethanolamine, phosphatidylcholine distearate, acetyl cholesterol and tween 80, so that the stability, solubility and bioavailability of the medicament can be greatly improved, the action is smooth and durable, and the curative effect is remarkable. According to the preparation, the product quality of the preparation is increased, and toxic and side effects are reduced.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Nisoldipine capsule and preparation method thereof
InactiveCN105168175AHigh dissolution rateLittle difference in tablet weightOrganic active ingredientsPharmaceutical non-active ingredientsAdhesiveDissolution
The invention belongs to the technical field of medicines, and concretely relates to a nisoldipine capsule and a preparation method thereof. The nisoldipine capsule is prepared from the following raw materials in parts by weight: 3.2-4.8 parts of nisoldipine, 44-132 parts of a filler, 0.25-0.75 parts of an adhesive, 6-18 parts of a solubilizer, and 0.4-1.2 parts of a lubricant. The technology is simple and easy to enforce, and the prepared nisoldipine capsule is steady for medicine release and high in medicine dissolution rate.
Owner:REYOUNG PHARMA
Controlled release formulations of nisoldipine
InactiveUS9480681B2Simple processModulate the release of nisoldipineOrganic active ingredientsPowder deliveryActive agentPh independent
Controlled release oral dosage formulations containing calcium channel blocker and processes for preparation thereof, are provided for once a day treatment. The active agent is preferably a dihydropyridine calcium channel blocker, such as nisoldipine. In one embodiment, the formulation provides controlled release of micronized nisoldipine with one or more pH independent release controlling polymers. The controlled release matrix formulation is advantageous and can be prepared by a simple, economically viable process as compared to complex core-coat prior-art versions.
Owner:EMCURE PHARAMACEUTICALS LTD
Slow-release preparation of calcium antagonist and salt thereof and preparation method thereof
InactiveCN107126562AQuick effectStable blood concentrationPharmaceutical delivery mechanismPharmaceutical active ingredientsCilnidipineTreatment effect
The invention provides a slow-release preparation of calcium antagonist and salt thereof and a preparation method thereof. The slow-release preparation comprises a slow-release phase which contains active pharmaceutical ingredients, the active pharmaceutical ingredients include 5-40mg of amlodipine or salt thereof calculated by amlodipine, 4-32mg of lacidipine or salt thereof calculated by lacidipine, 5-40mg of nisoldipine or salt thereof calculated by nisoldipine, 5-40mg of cilnidipine or salt thereof calculated by cilnidipine, 2-32mg of benidipine or salt thereof calculated by benidipine, 10-80mg of lercanidipine or salt thereof calculated by lercanidipine and 5-80mg of manidipine or salt thereof calculated by manidipine; in release media meeting sink conditions, higher than 90% of weight of the active pharmaceutical ingredients are released within a release period of 6-14h. The slow-release preparation is convenient to use, good in treatment effect, high in taking compliance, little in adverse reaction, quick in action, capable of maintaining stable and effective blood concentration for a long time, ingenious in design, simple in structure, high in stability and suitable for large-scale popularization and application.
Owner:杨彦玲
Nisoldipine orally disintegrating tablet and preparation method thereof
InactiveCN107115303ASolve the phenomenon of sticking dieOrganic active ingredientsPill deliveryOrally disintegrating tabletCompressibility
The invention discloses a nisoldipine orally disintegrating tablet for treatment of high blood pressure and a preparation method thereof. The nisoldipine orally disintegrating tablet contains 2%-10% of nisoldipine, 45%-75% of filler, 10%-30% of a disintegrating agent, 0.1%-3% of a sour agent, 0.1%-10% of a flavoring agent, 0-5% of a flow aid, 0.1%-3% of a lubricant and 0.1-5% of a surfactant. The nisoldipine orally disintegrating tablet provided by the invention has the advantages of strong compressibility, fast disintegration, good drug dissolution and excellent taste.
Owner:JIANGXI HERBI SKY CO LTD
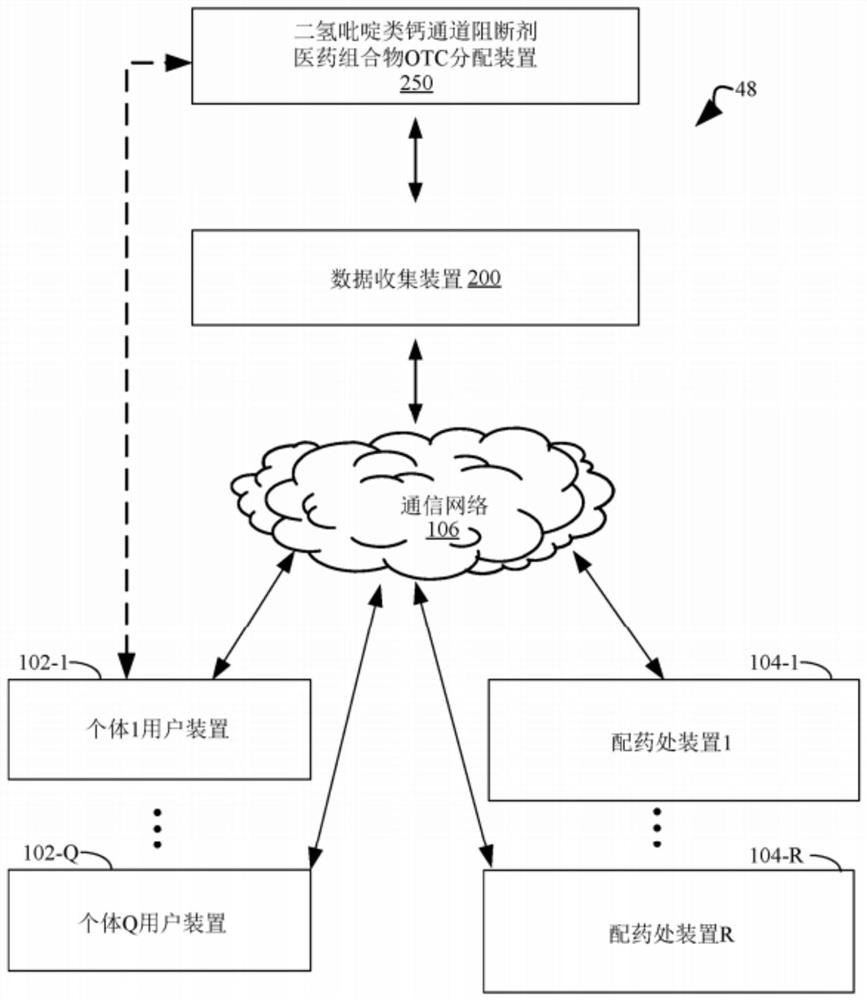
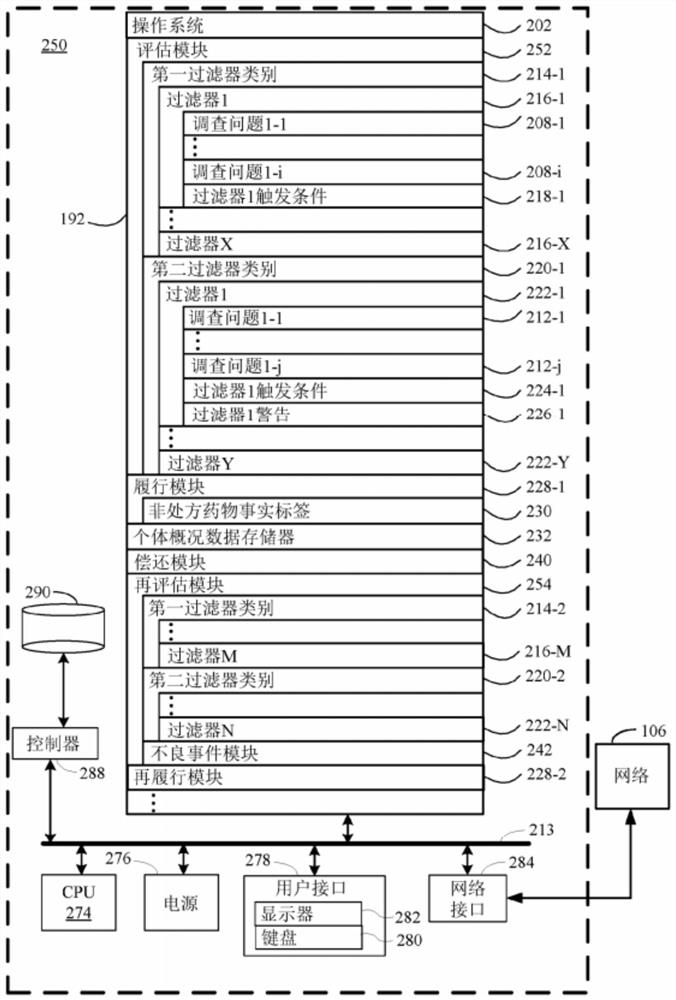
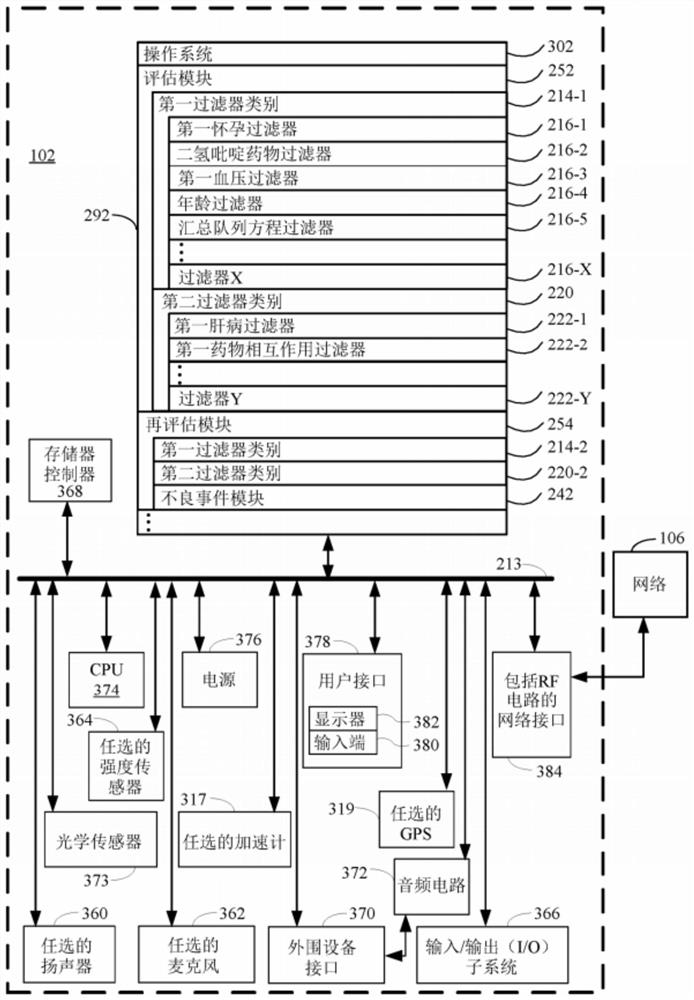
Methods for lowering blood pressure with a dihydropyridine-type calcium channel blocker pharmaceutical composition
PendingCN112955965AOrganic active ingredientsDrug and medicationsDihydropyridineCalcium channel blocker
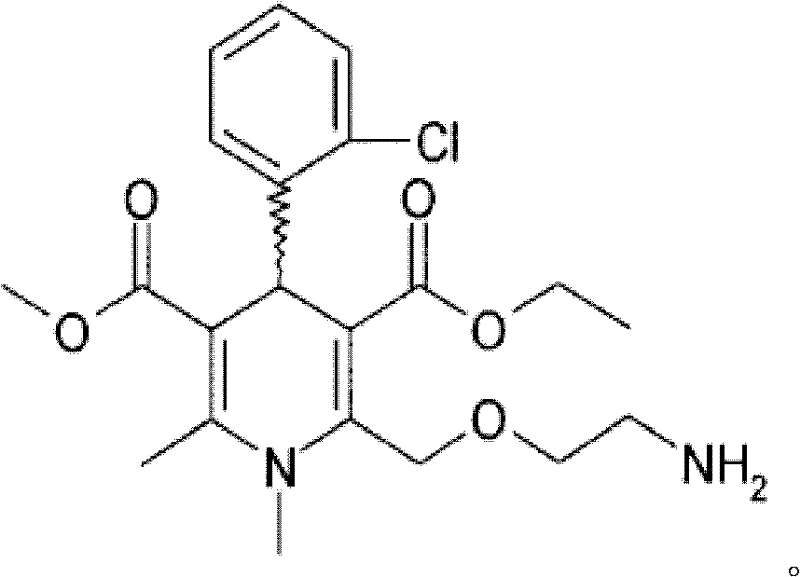
A method is provided for lowering blood pressure in a subject in need thereof by administering a dihydropyridine-type calcium channel blocker pharmaceutical composition to a subject qualified for over-the-counter access to the dihydropyridine-type calcium channel blocker pharmaceutical composition. In some embodiments, the dihydropyridine-type calcium channel blocker pharmaceutical composition includes isradipine, nifedipine, or nisoldipine. In some embodiments, the dihydropyridine-type calcium channel blocker pharmaceutical composition includes 3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2- chlorophenyl)-6-methyl-l,4-dihydropyridine-3,5-dicarboxylate or a pharmaceutically acceptable salt thereof.
Owner:ASTRAZENCA UK LTD
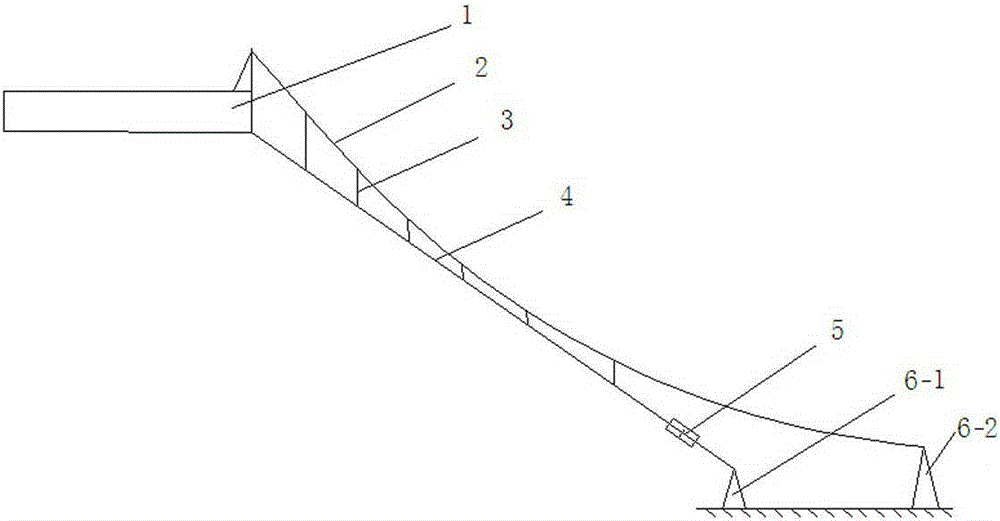
Double-cable composite damping cable
ActiveCN104404886BEliminate sagGreat longitudinal (axial) stiffnessBridge structural detailsBridge erection/assemblyEngineeringDouble barrier
The invention discloses a double-cable composited damping cable which comprises a main cable, an auxiliary cable, a first support, a second support and a viscous damper. The first support and the second support are installed on the ground, one end of the main cable is connected with a bridge main beam, the other end of the main cable is connected with one end of the viscous damper, the other end of the viscous damper is connected with the first support, the auxiliary cable is located above the main cable, two ends of the auxiliary cable are respectively connected to the bridge main beam and the second support, and the main cable and the auxiliary cable are connected through multiple vertical booms. The main cable and the auxiliary cable are combined, so that the sag of the main cable is basically eliminated, the longitudinal (axial) rigidity of the main cable is large due to small sag when larger-amplitude structural vibration is produced, and the cable force change is remarkable. Due to the fact the auxiliary cable is large in sag, the longitudinal rigidity of the auxiliary cable is small, the cable force change is very small, and conditions are provided for energy consumption on horizontally and remotely installed damper.
Owner:HUNAN UNIV OF SCI & TECH
Synthetic method of nisoldipine photolytic product reference substance
A synthesis method of a control for the photolytic product of nisoldipine relates to the field of drug production. The method comprises the following steps that: 1) an absolute ethanol saturated solution of nisoldipine is irradiated with light in a closed container at room temperature, until the color turns from yellow to aquamarine blue and then keeps stable; 2) the obtained solution is refluxed in water bath for 20 to 40 minutes at 70 to 80DEG C, followed by the elimination of insoluble substances under the heat; 3) the obtained is dried at 50 to 70 DEG C and; 4) the obtained remnant are re-crystallized for 2 or 3 times to obtain the refined product, which appears to be a powder in pale aquamarine blue. The invention has the advantages of easy operation, no requirement of toxic reagents, minor toxic effects on human body, and minor pollution on environment.
Owner:药大制药有限公司
Nisoldipine compound and novel preparation method thereof
Owner:HAINAN MEILAN SMITH KLINE PHARMA
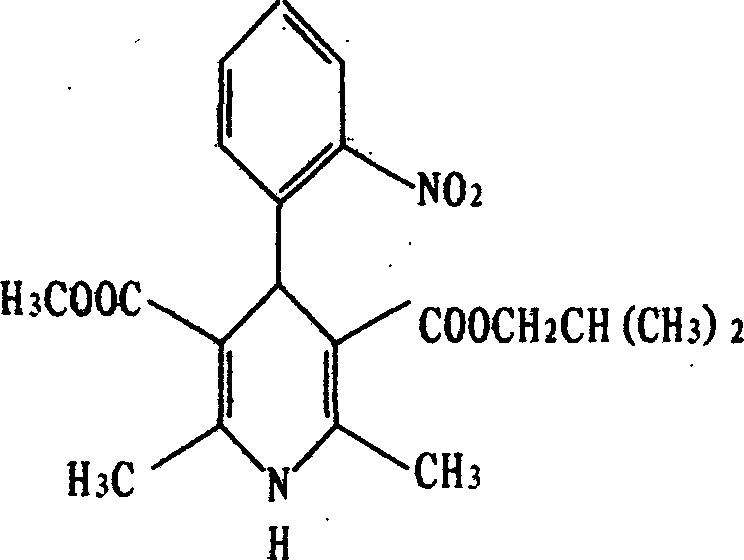
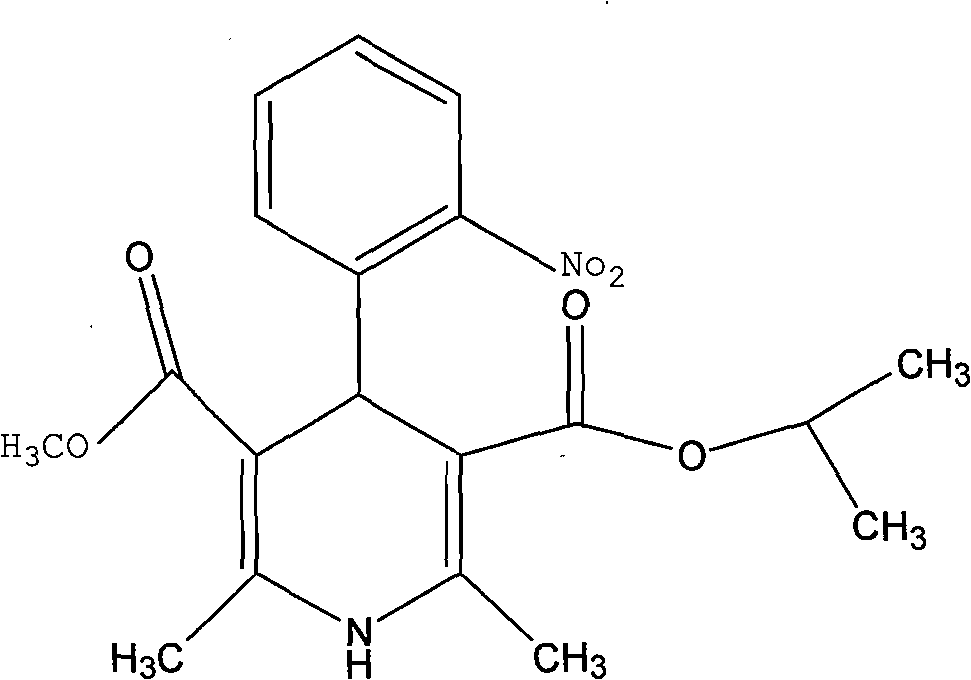
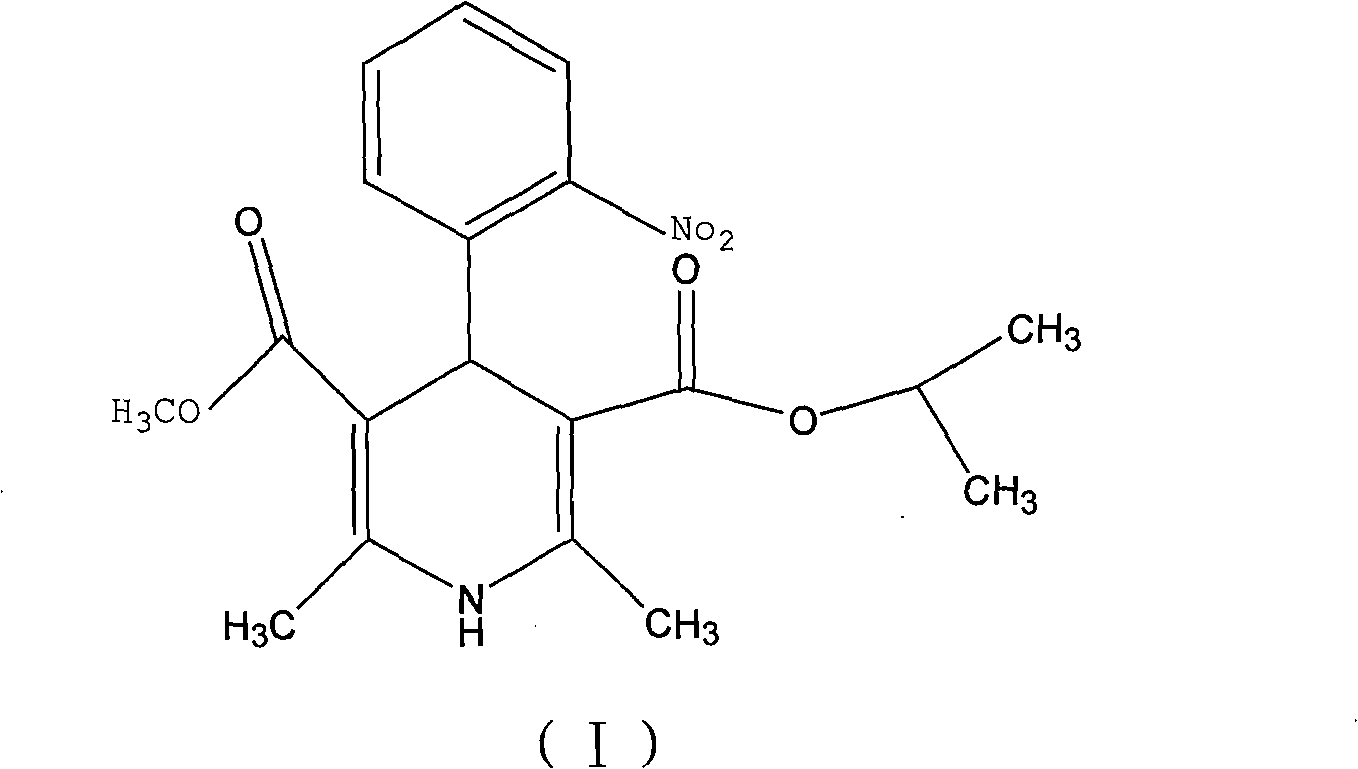
A kind of preparation method of nisoldipine impurity
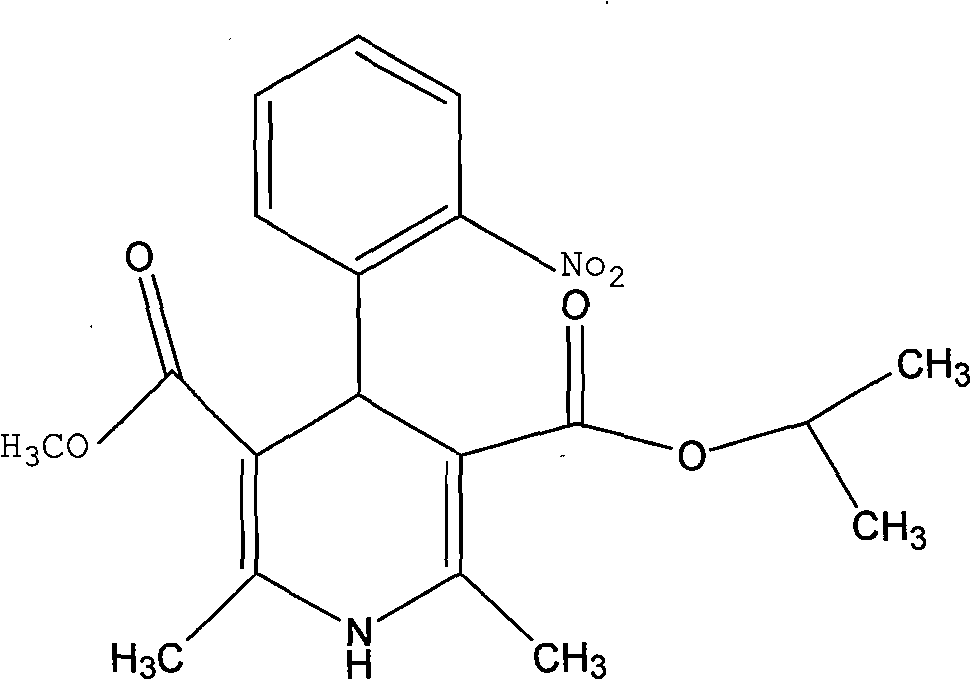
ActiveCN112979538BMild reaction conditionsEasy to prepareOrganic chemistry methodsNitrosoDihydropyridine
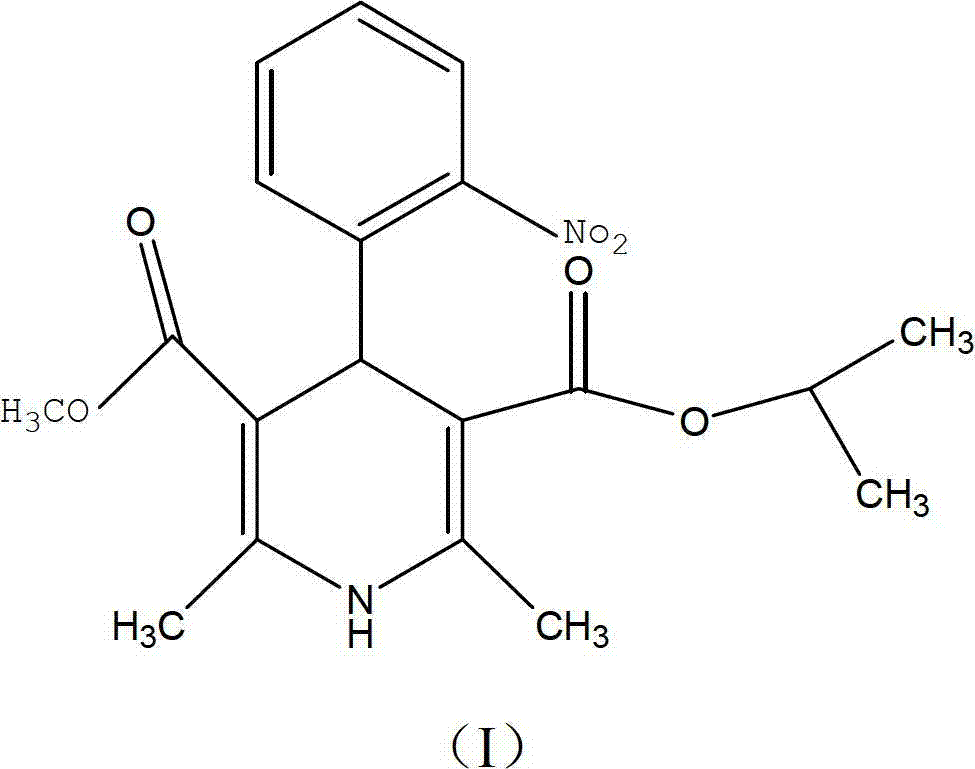
The invention discloses a preparation method of nisoldipine impurity. The nisoldipine impurity is 2,6-dimethyl-4-(2-nitrosophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate methyl isobutyl ester, the structure is as follows: Ⅰ) as shown. The preparation method of the nisoldipine impurity is prepared by taking nisoldipine as a raw material, adding a free radical initiator, and undergoing one-step photodegradation. The method adopted in the present invention is simple, easy to operate, mild in reaction conditions, and simple in post-treatment process, and provides a new way for further identifying, separating, preparing and characterizing the nisoldipine impurities, improving the quality control level and medication safety of nisoldipine, It is beneficial to the control of the quality of medicines in the production process.
Owner:SOUTHEAST UNIV
Industrial process for the synthesis of isobutyl methyl 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridine dicarboxylate (nisoldipine)
Synthetic process of isobutyl methyl 1,4,-dihydro-2,6-dimethyl-4-(2-nitrophenyl)3,5-pyridine dicarboxylate (Nisoldipine) comprising on the reaction of isobutyl 2-(2-nitrobenzylidene)acetoacetate with methyl 3-aminocrotonate in an apolar solvent.
Owner:ERREGIERRE
Preparation method of nisoldipine impurity
ActiveCN112979538AMild reaction conditionsEasy to prepareOrganic chemistry methodsNitrosoNitrobenzene
The invention discloses a preparation method of a nisoldipine impurity. The nisoldipine impurity is 2, 6-dimethyl-4-(2-nitrosophenyl)-1, 4-dihydro-3, 5-pyridine dicarboxylic acid methyl ester isobutyl ester, wherein the structure of the nisoldipine impurity is shown as a formula (I). According to the preparation method of the nisoldipine impurity, nisoldipine is taken as a raw material, a free radical initiator is added, and the nisoldipine impurity is prepared through one-step photodegradation; and the method is simple, simple and convenient to operate, mild in reaction condition and simple in post-treatment process, provides a new way for further identifying, separating, preparing and representing the nisoldipine impurities and improving the quality control level and medication safety of nisoldipine, and is beneficial to the control of the medicine quality in the production process.
Owner:SOUTHEAST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com