Nisoldipine ethosome controlled-released patch and preparation method thereof
A technology of nisoldipine and dipine alcohol, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of difficulty in reaching the therapeutic concentration of the drug, the limited amount of percutaneous penetration, and the impact of To avoid the hepatic first-pass effect, increase the rate of transdermal penetration, and enhance the effect of transdermal permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
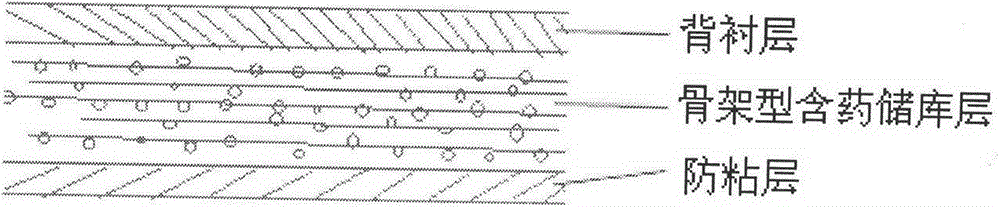
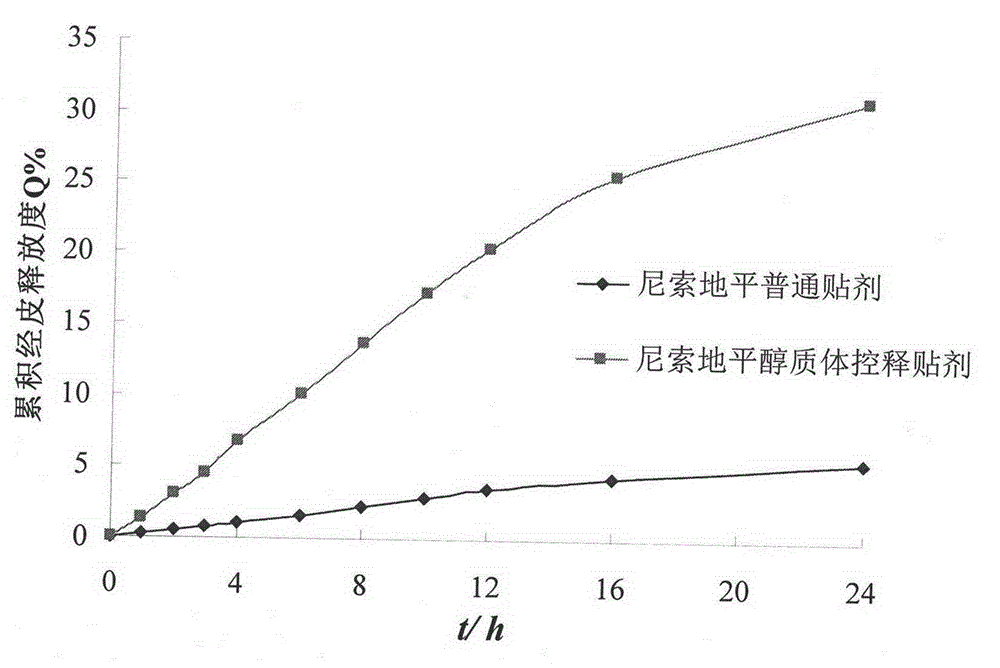
[0038] The nisoldipine elastosome controlled-release patch is composed of a backing layer, a skeleton drug-containing reservoir layer and an anti-adhesive layer, and its preparation method is as follows:
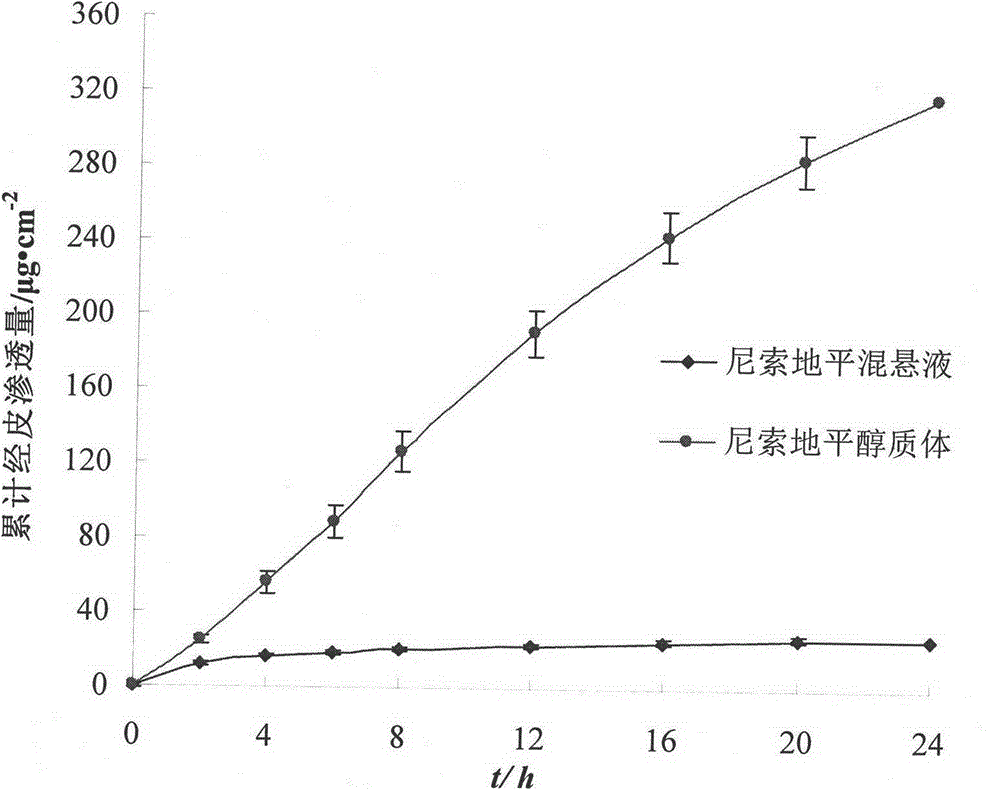
[0039] (1) Accurately weigh 0.4g of nisoldipine, 3.0g of phospholipids and 0.3g of cholesterol, add 45ml of absolute ethanol and stir until completely dissolved, under rapid stirring conditions, slowly drop into an aqueous solution containing 0.2% glacial acetic acid kept at 30°C In 55ml, the crude ethosome was obtained, ultrasonically dispersed for 30min, and then filtered through a 0.22 μm microporous membrane to obtain nisoldipine ethanol body fluid with uniform particle size.
[0040] (2) Accurately weigh 14.0g of EUDRAGITE100 (pressure-sensitive adhesive) and 0.7g of adipic acid, add 50ml of nisoldipine alcohol body fluid, stir until the pressure-sensitive adhesive is completely swollen, then add 2.0ml of azone, 1.0g of menthol, citrate Triethyl citrate 2.0ml and glycer...
Embodiment 2
[0042] The nisoldipine elastosome controlled-release patch is composed of a backing layer, a skeleton drug-containing reservoir layer and an anti-adhesive layer, and its preparation method is as follows:
[0043] (1) Accurately weigh 0.4g of nisoldipine, 2.0g of phospholipids and 0.4g of cholesterol, add 40ml of absolute ethanol and stir until they are completely dissolved. Under the condition of rapid stirring, slowly drip into the phosphate buffer (pH 5 .0) in 60ml, to obtain the alcoholic body crude product, which was homogeneously dispersed for 10 min, and then filtered through a 0.22 μm microporous membrane to obtain the nisoldipine alcoholic body fluid with uniform particle size.
[0044] (2) Precisely weigh 6.0g of EUDRAGITEPO (pressure-sensitive adhesive), 7.0g of EUDRAGITRLPO (pressure-sensitive adhesive) and 0.6g of adipic acid, add 50ml of nisoldipine ethanol body fluid, stir until the pressure-sensitive adhesive is completely swollen, and then add azone 1.0ml, euca...
Embodiment 3
[0046] The nisoldipine elastosome controlled-release patch is composed of a backing layer, a skeleton drug-containing reservoir layer and an anti-adhesive layer, and its preparation method is as follows:
[0047] (1) Accurately weigh 0.4g of nisoldipine, 4.0g of phospholipids and 0.45g of cholesterol, add 45ml of absolute ethanol and stir until completely dissolved, under rapid stirring conditions, slowly drop into an aqueous solution containing 0.2% glacial acetic acid kept at 30°C In 55ml, the crude ethosome was obtained, ultrasonically dispersed for 30min, and then filtered through a 0.22 μm microporous membrane to obtain nisoldipine ethanol body fluid with uniform particle size.
[0048] (2) Accurately weigh 7.0g of EUDRAGITS100 (pressure-sensitive adhesive), 6.0g of EUDRAGITL100 (pressure-sensitive adhesive) and 0.8g of succinic acid, add 50ml of nisoldipine alcohol body fluid, stir until the pressure-sensitive adhesive is completely swollen, and then add nitrogen Ketone ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com