Nisoldipine controlled-release patch and preparation method thereof
A nisoldipine and prescription technology, applied in the antihypertensive transdermal drug delivery system, the nisoldipine transdermal patch and the field of preparation thereof, can solve the problem of low bioavailability, large fluctuation of blood concentration, reduction of medication frequency, etc. problem, to achieve the effect of smooth percutaneous penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Composition of the drug-loaded polymer layer: 2 g of polyvinyl alcohol (PVA205), 0.8 g of polyvinylpyrrolidone (PVP), 0.375 g of azone, 0.375 g of propylene glycol, 0.6 g of glycerin, and 100 mg of nisoldipine.
[0026] Preparation method: add the prescribed amount of penetration enhancer and plasticizer to 65% ethanol and mix evenly, dissolve the prescribed amount of medicine, add polyvinyl alcohol and polyvinylpyrrolidone to swell overnight at 45°C, dissolve in a water bath at 60°C until clear, After magnetically stirring evenly, degas, coat on release paper, dry at 50°C, cover with aluminum foil and compound polyethylene, cut and divide the dose, and seal it for storage. Wherein the content of nisoldipine is 4mg / cm 2 .
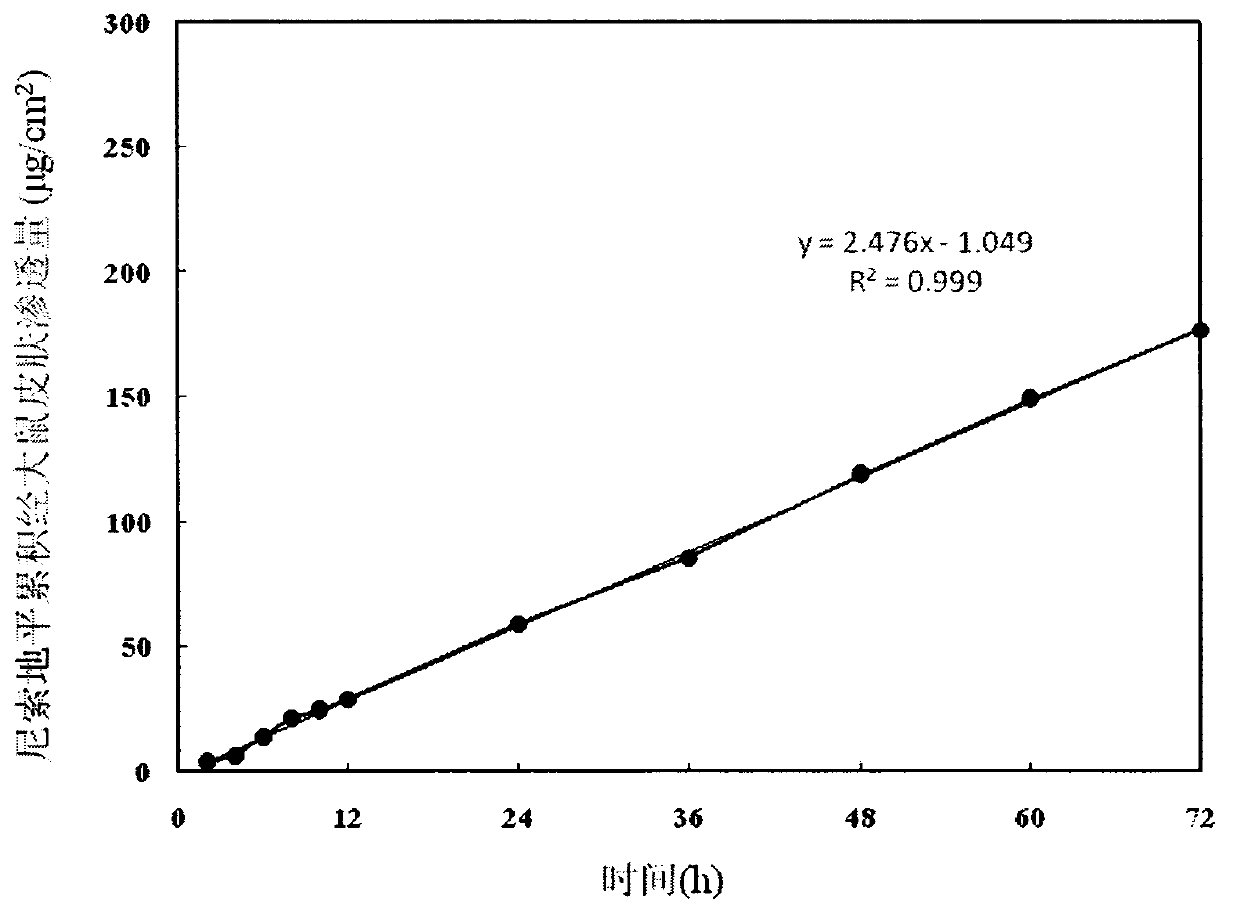
[0027] The in vitro permeation behavior of the drug-loaded patch through the shaved abdominal skin of rats was measured by a modified Franz diffusion cell, and the experiment lasted for 72 hours. The calculated permeation rate is 2.476μg / (cm 2 h),...
Embodiment 2
[0029] Composition of the drug-loaded polymer layer: 2.4 g of polyvinyl alcohol (PVA205), 0.4 g of polyvinylpyrrolidone (PVP), 0.75 g of propylene glycol, 0.6 g of glycerin, and 50 mg of nisoldipine.
[0030] With the preparation method of embodiment 1, it is 2mg / cm2 that nisoldipine content is obtained 2 patch.
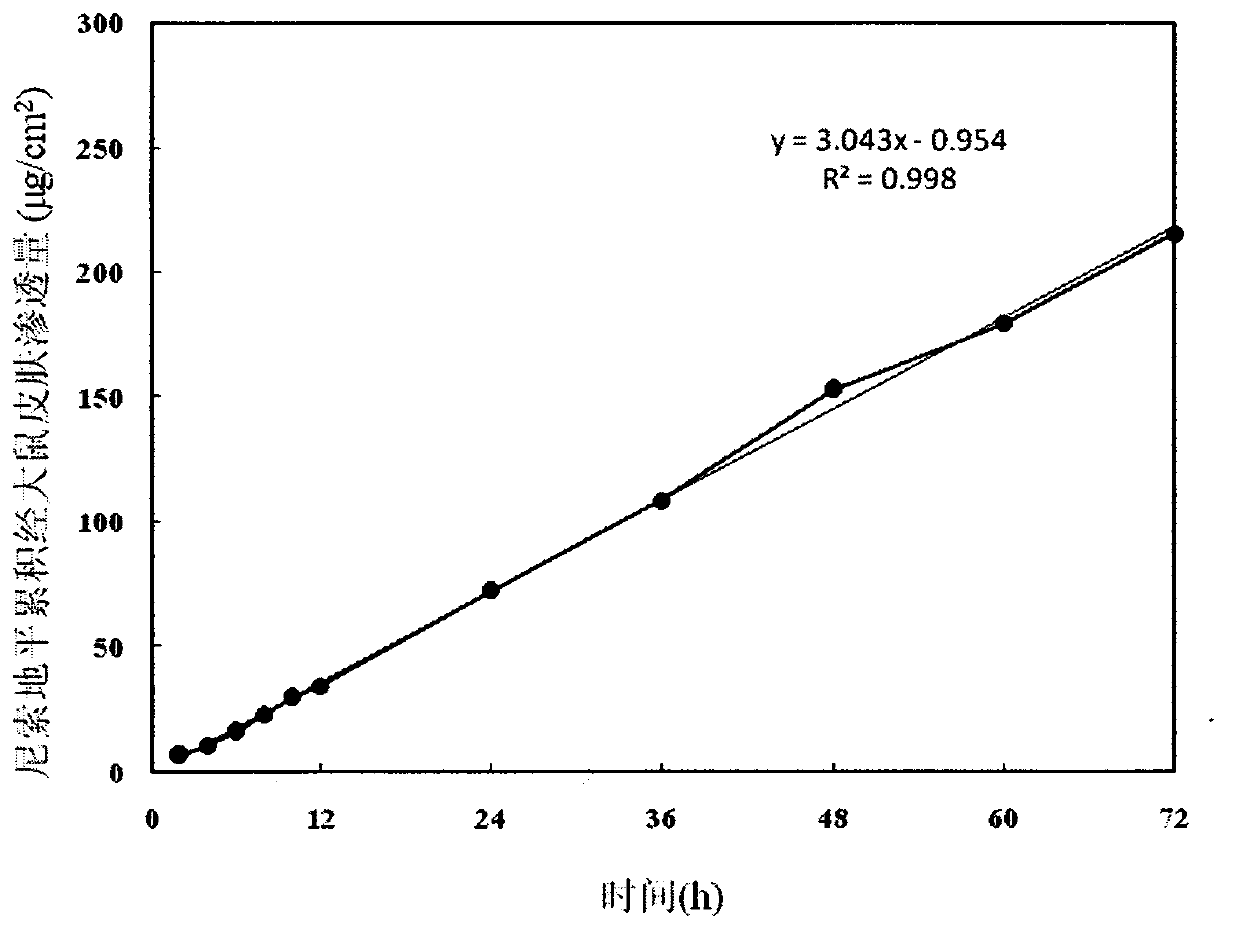
[0031] The same method as in Example 1 measured that the penetration rate through the skin of the abdomen of the rat was 3.043 μg / (cm) during the 72h period. 2 h), the cumulative percutaneous penetration is 215.34μg / cm 2 .
Embodiment 3
[0033] Composition of the drug-loaded polymer layer: 2.4 g of polyvinyl alcohol (PVA205), 0.4 g of hydroxymethylpropyl cellulose (HPMC), 0.9 g of oleic acid, 0.6 g of glycerin, and 25 mg of nisoldipine.
[0034] With the preparation method of embodiment 1, it is 1mg / cm to make nisoldipine content 2 patch.
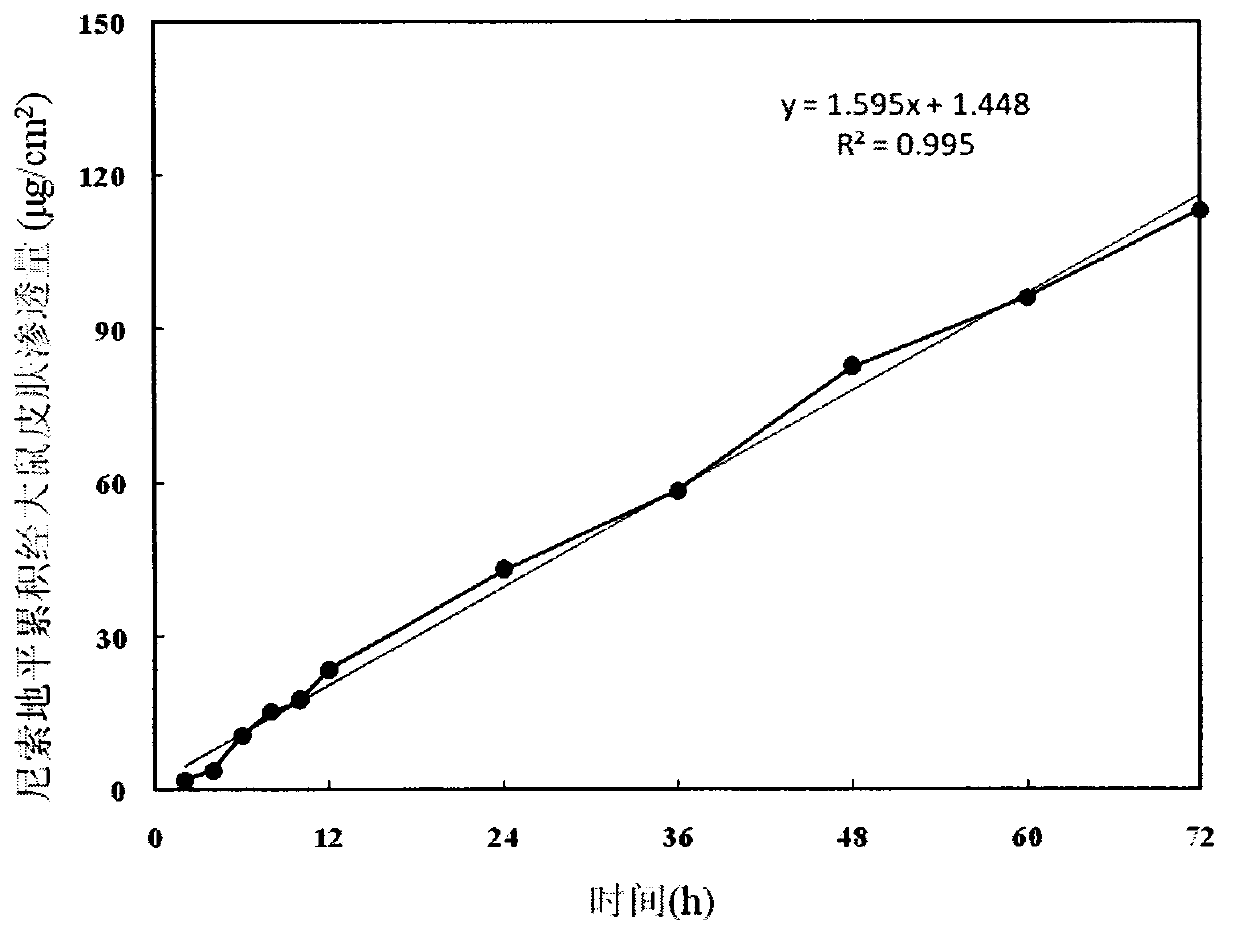
[0035] Measured with the method of Example 1 during 72 hours through the permeation rate of rat abdominal hair removal skin is 1.595 μ g / (cm 2 h), 72h cumulative transdermal penetration is 113.12μg / cm 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com