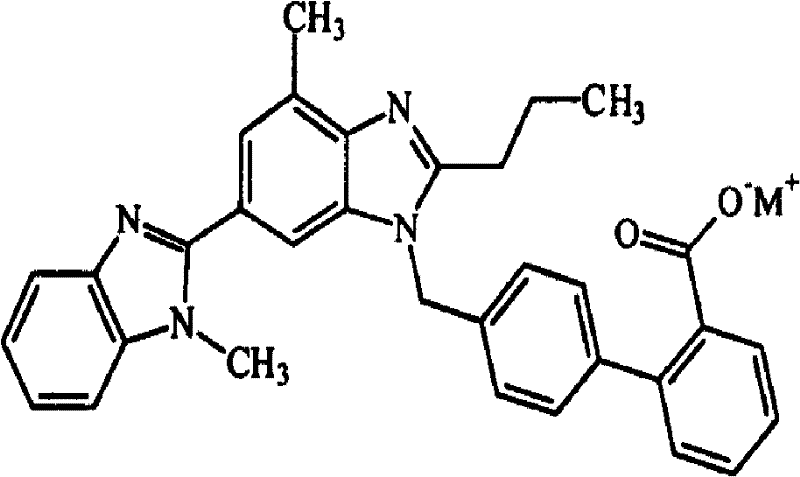
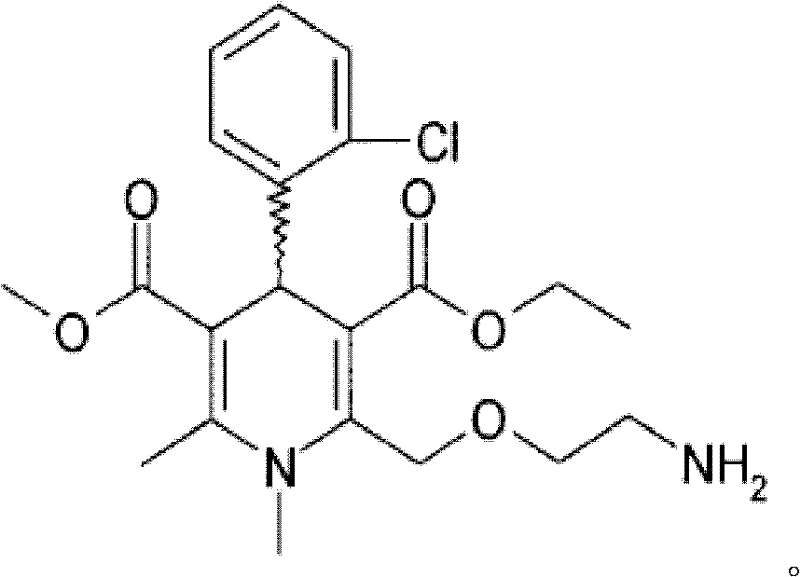
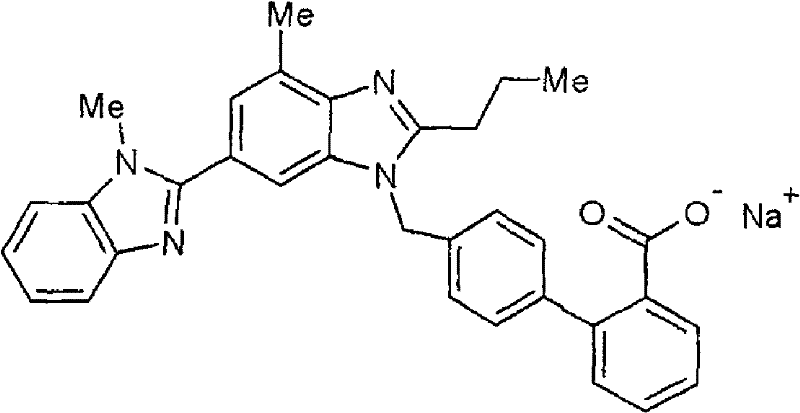
Pharmaceutical composition comprising telmisartan salt and calcium ion antagonist
The technology of a calcium ion antagonist, telmisartan, is applied in the directions of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., and can solve the problems of poor solubility, unsatisfactory results, poor bioavailability, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Tablets Comprising Telmisartan Sodium and Amlodipine Besylate
[0089] Name of raw material Dose 1 (mg) Dose 2 (mg) Dose 3 (mg) Dose 4(mg) Telmisartan Sodium 10.00 20.00 40.00 80.00 Amlodipine 6.94 6.94 6.94 6.94 microcrystalline cellulose 3.96 14.86 36.66 80.26 Povidone K30 2.50 5.00 10.00 20.00 Carboxymethyl Starch Sodium 0.82 1.65 3.30 6.60 Micropowder silica gel 0.20 0.40 0.80 1.60 Magnesium stearate 0.58 1.15 2.30 4.60 Sheet weight 25.00 50.00 100.00 200.00
[0090] Preparation:
[0091] (A) Telmisartan sodium and amlodipine besylate were dried at 80° C. for 6 hours respectively, crushed through an 80 mesh sieve, and then mixed uniformly in a mixer by an equal addition method;
[0092] (B) Microcrystalline cellulose, povidone K30, sodium carboxymethyl starch, micropowder silica gel and magnesium ste...
Embodiment 2
[0098] Example 2: Tablets Comprising Telmisartan Sodium and Amlodipine Besylate
[0099] Name of raw material Dose 1 (mg) Dose 2 (mg) Dose 3 (mg) Dose 4(mg) Telmisartan Sodium 20.00 40.00 80.00 160.00 Amlodipine 1.74 3.47 6.94 13.88 Optimized Microcrystalline Cellulose 8.48 16.96 33.91 67.82 Povidone K30 5.00 10.00 20.00 40.00 Carboxymethyl Starch Sodium 1.50 3.00 6.00 12.00 Micropowder silica gel 0.41 0.82 1.65 3.30 Magnesium stearate 0.38 0.75 1.50 3.00 Sheet weight 37.50 75.00 150.00 300.00
[0100] Preparation:
[0101] (A) Telmisartan sodium and amlodipine besylate were dried at 80° C. for 6 hours respectively, crushed through an 80 mesh sieve, and then mixed uniformly in a mixer by an equal addition method;
[0102] (B) Dry the optimized microcrystalline cellulose, povidone K30, sodium starch glycolate, micropowder ...
Embodiment 3
[0106] Example 3: Tablets Comprising Telmisartan Sodium and Amlodipine Besylate
[0107] Name of raw material Dose 1 (mg) Dose 2 (mg) Dose 3 (mg) Dose 4(mg) Telmisartan Sodium 10.00 20.00 40.00 80.00 Amlodipine 6.94 6.94 6.94 6.94 calcium carbonate 3.95 14.85 36.64 80.21 Povidone K30 2.50 5.00 10.00 20.00 sodium starch glycolate 0.55 1.10 2.20 4.40 sodium starch glycolate 0.28 0.55 1.10 2.20 Micropowder silica gel 0.20 0.41 0.82 1.65 Magnesium stearate 0.58 1.15 2.30 4.60 Sheet weight 25.00 50.00 100.00 200.00
[0108] Preparation:
[0109] (A) Telmisartan sodium and amlodipine besylate were dried at 80°C for 6 hours respectively, crushed through a 100-mesh sieve, and then mixed uniformly in a mixer by an equal addition method;
[0110](B) Calcium carbonate, povidone K30, sodium carboxymethyl starch added...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com