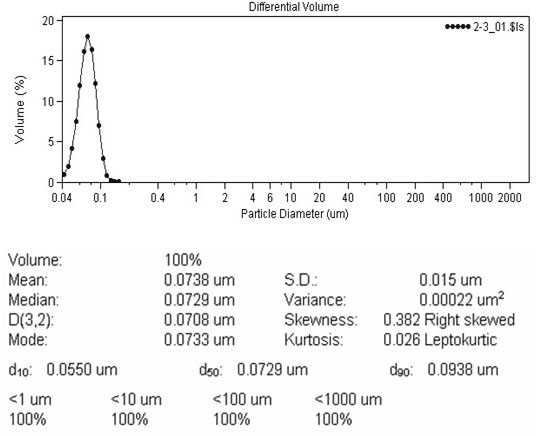
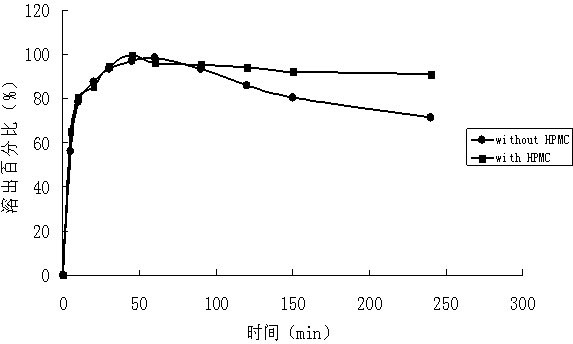
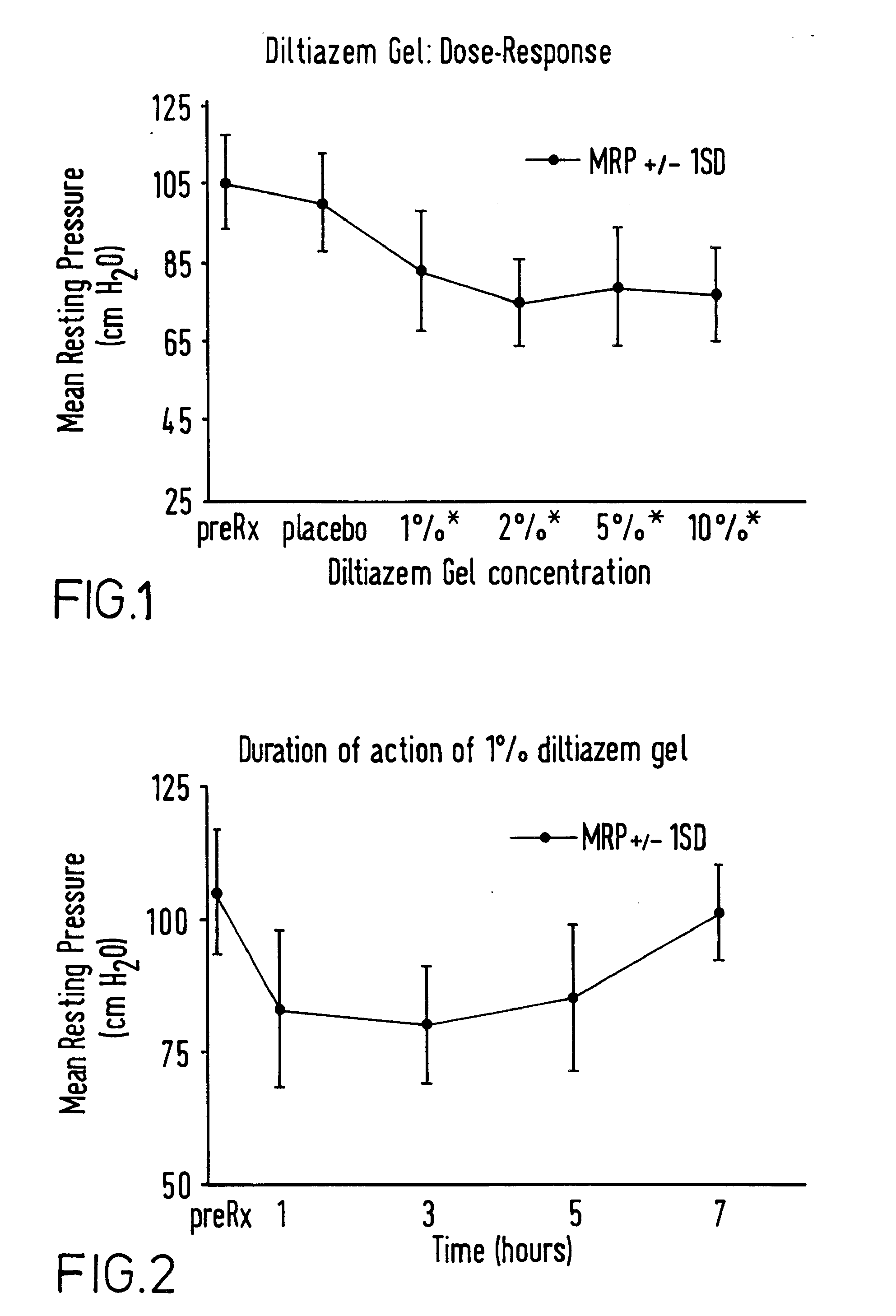
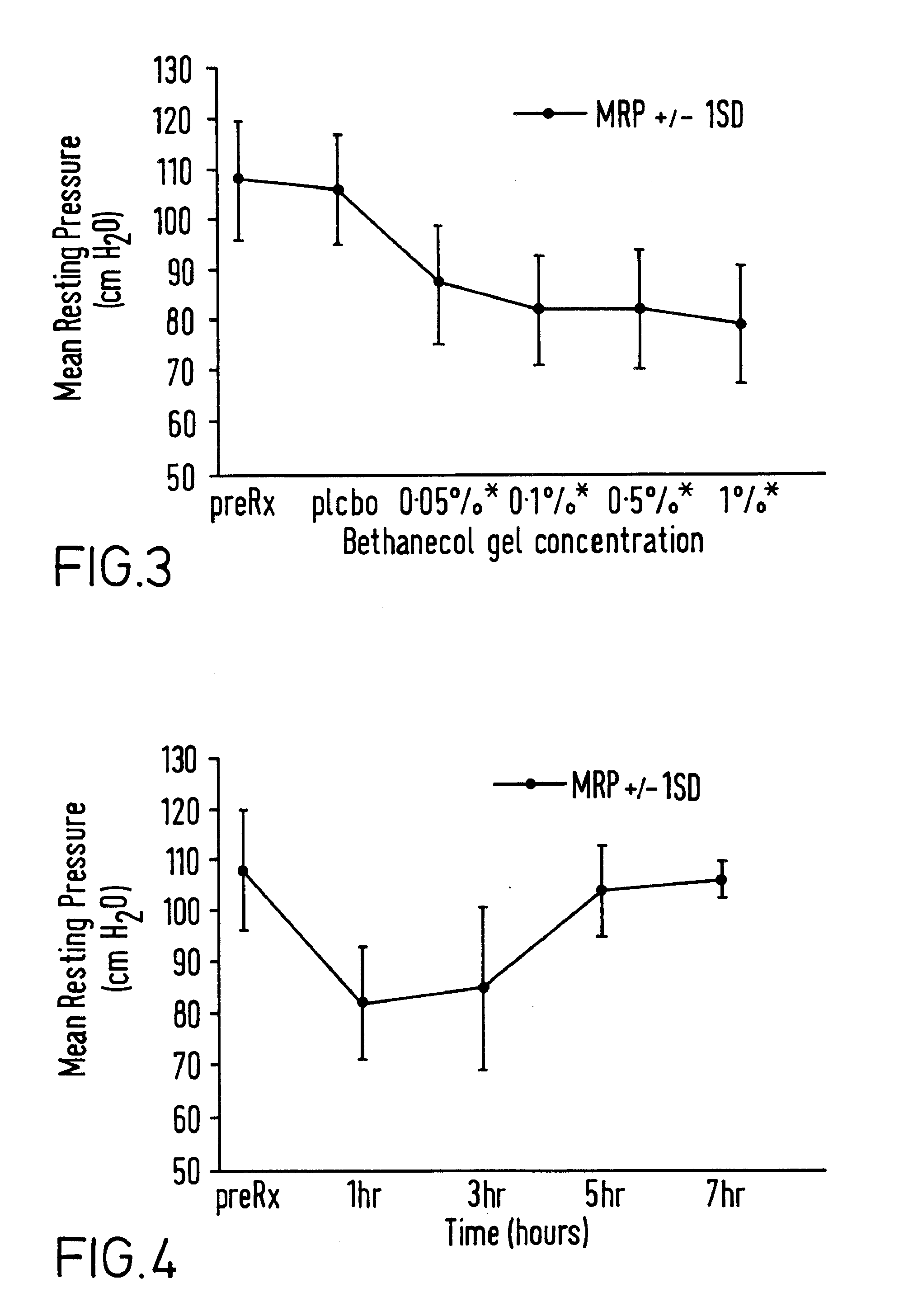
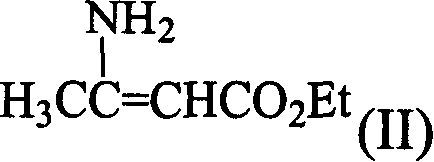Patents
Literature
44 results about "Lacidipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
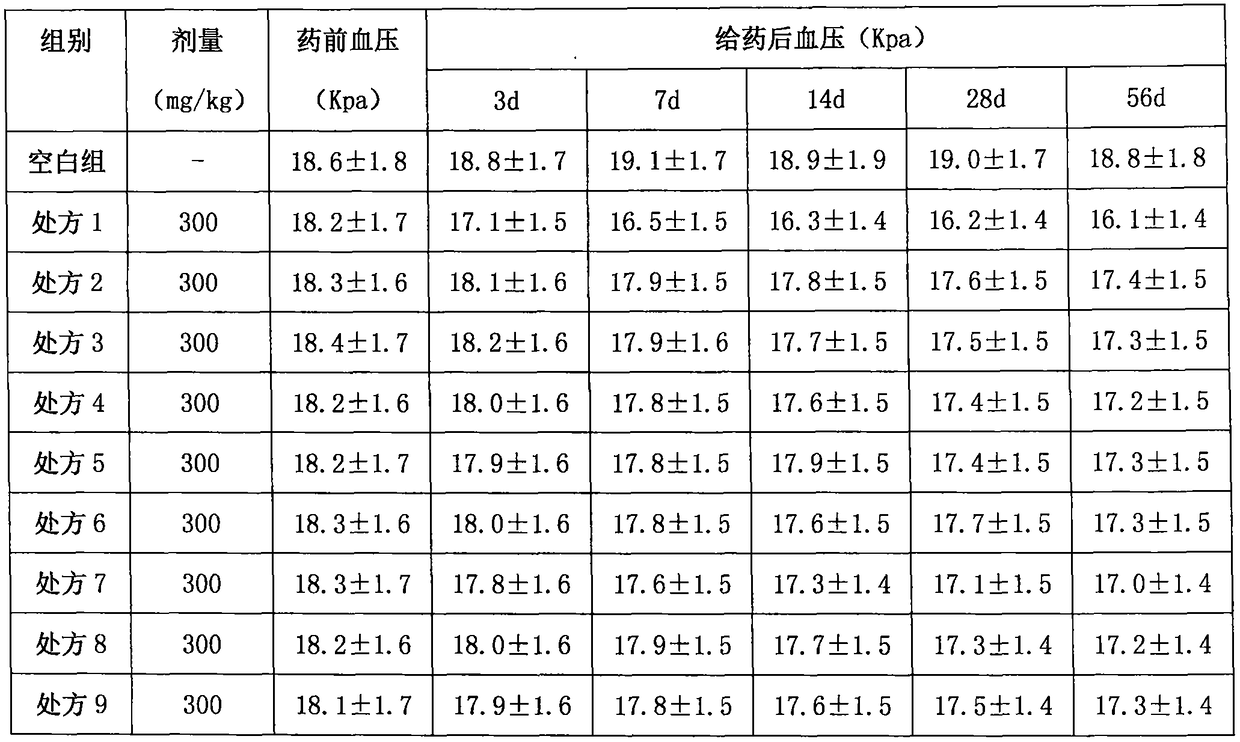
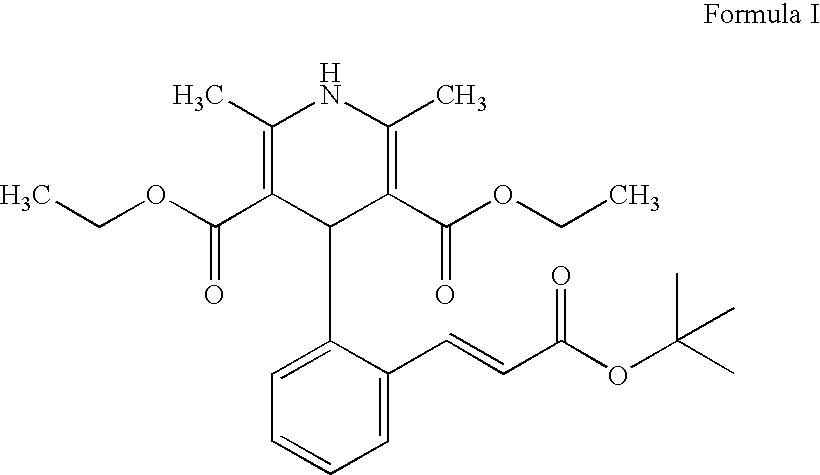
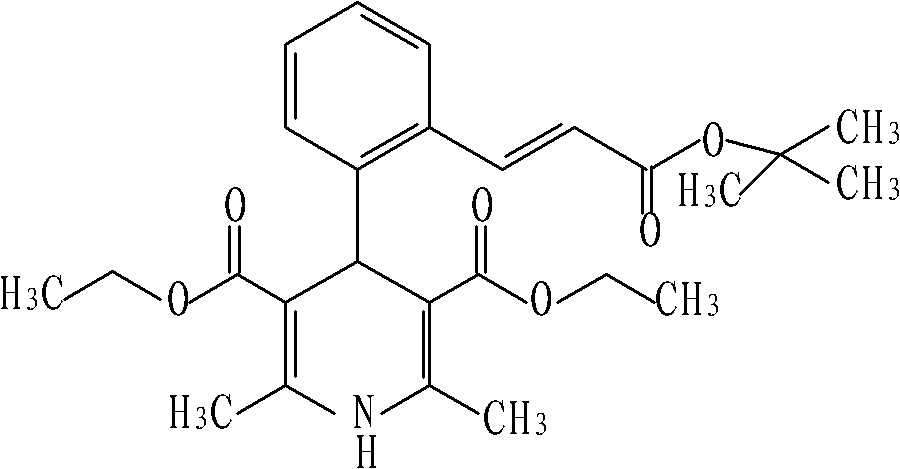
Lacidipine (tradenames Lacipil or Motens) is a calcium channel blocker. It is available as tablets containing 2 or 4 mg. It was patented in 1984 and approved for medical use in 1991.
Lacidipine tablets and preparation method thereof
InactiveCN101653423ASolve the phenomenon of content reduction in the crushing processKeep aliveOrganic active ingredientsPill deliveryLacidipineUltra fine
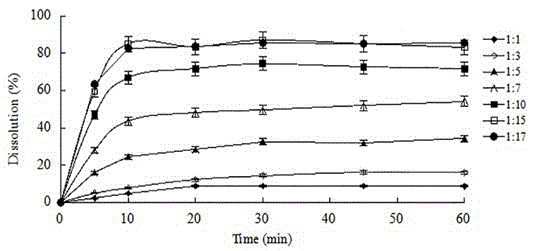
The invention provides lacidipine tablets and a preparation method thereof. The lacidipine tablets comprise 1-4% of lacidipine, 14-32% of hydroxypropyl cellulose or microcrystalline cellulose, 1-3% ofpoloxamer or sodium dodecyl sulfate or povidone or copovidone, 0.25-2% of magnesium stearate and the balance of lactose by weight percent, and the preparation method comprises the following steps ofevenly mixing raw material of the lacidipine with the lactose according to the weight ratio of 1:1 after ultra-fine smashing, further evenly mixing the mixture with the balance of the lactose and thehydroxypropyl cellulose or the microcrystalline cellulose, adding a water solution bonding agent prepared by the poloxamer or the odium dodecyl sulfate or the povidone or the copovidone, preparing appropriate soft materials, passing a 18-mesh sieve for granulation, drying at the temperature of 40-60 DEG C, passing a 16-mesh sieve for size stabilization, adding the magnesium stearate, evenly mixingand pressing tablets, thereby preparing products. The method can effectively improve the dissolution of the lacidipine tablets and lead the evenness index to achieve the more ideal value. The methodcan further improve the bioavailability of the lacidipine tablets and has the advantages of fast absorption, convenient administration and significant efficacy.
Owner:哈药集团人民同泰医药股份有限公司
Lacidipine pharmaceutical formulation preparing method
Owner:AVENTIS PHARMA HAINAN
Composition for lowering blood pressure and application thereof
InactiveCN101890165AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderTasosartanValsartan
The invention provides a pharmaceutical composition which comprises calcium channel blockers of a medicinal dose, angiotensin II receptor antagonists of a medicinal dose, one or more of B vitamins of a medicinal dose and pharmaceutically acceptable carriers, wherein the calcium channel blockers are selected from amlodipine, felodipine, israbipine, nicardipine, nifedipine, nisoldipine, nitrendipine, lacidipine, diltiazem or verapamil; the angiotensin II receptor antagonists are selected from candesartan, telmisartan, losartan, valsartan, irbesartan, eprosartan, tasosartan or olmesartan; and the B vitamins are selected from one or more of vitamin B6, vitamin B12, folic acid and calcium leucovorin. The pharmaceutical composition of the invention can improve the curative effect of the hypotensor, enhance the target organ protecting action of the hypotensor, and reduce the morbidity of complications of angina, myocardial infarction and the like.
Owner:北京奥萨医药研究中心有限公司 +1
Pharmaceutical composition containing calcium blocker, AII receptor blocker and statins
InactiveCN101618215AReduce morbidityImprove complianceSenses disorderMetabolism disorderCandesartanLacidipine
The invention relates to a pharmaceutical composition containing a calcium channel blocker (CCB) or the mixture thereof, an angiotonin 3II receptor blocker (ARB) or the mixture thereof, statins or the mixture thereof and a pharmaceutically acceptable carrier, wherein the CCB is selected from l-amlodipine, amlodipine, lacidipine, nitrendipine or the mixture thereof; the angiotonin II receptor blocker is selected from telmisartan, losartan, irbesartan, candesartan or the mixture thereof; and the statins are selected from atorvastatin, simvastatin, ruishufatadine, fluvastatin or the mixture thereof. The pharmaceutical composition is used for treating various high blood pressures and preventing or treating cardiovascular and cerebrovascular diseases relevant to the hypertension, reduces the disease rate and / or mortality rate of the cardiovascular and cerebrovascular diseases and also improves the adaptability for a sufferer taking medicine.
Owner:王丽燕
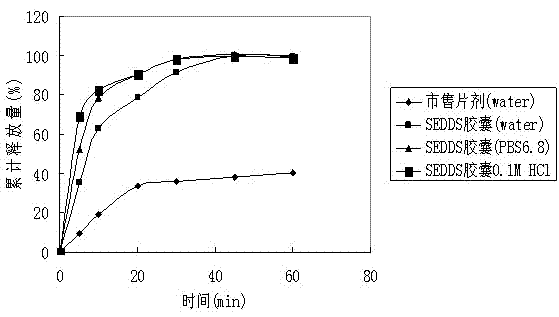
Lacidipine self-microemulsifying soft capsules and preparation method thereof
InactiveCN102008471AProtection stabilityImprove oral bioavailabilityOrganic active ingredientsCapsule deliveryLacidipineOil phase
The invention belongs to the technical field of medicines and relates to lacidipine self-microemulsifying soft capsules and a preparation method thereof. The lacidipine self-microemulsifying soft capsules is uniform transparent solution prepared from an oil phase, an emulsifier and an auxiliary emulsifier, wherein under the conditions of an ambient temperature and mild stirring, the lacidipine self-microemulsifying soft capsules spontaneously emulsify to form emulsion droplet smaller than 500 nanometers; the ratio of the lacidipine to the auxiliary emulsifier to the emulsifier to the oil phase is 1:1-30:1-60:0.5-10 or 1: 1-30:1-30:0.5-10; a water soluble macromoleclar polymer can be added for prolonging the maintenance time of the saturated state of the solution of the medicament; and an antioxygen can be added for improving the chemical stability of the preparation. In the invention, the biological utilization rate of oral lacidipine is improved; and the cost is low, and the process is simple.
Owner:SHENYANG PHARMA UNIVERSITY
Topical pharmaceutical composition comprising a cholinergic agent or a calcium channel blocker
A method and composition are provided for the treatment of an anorectal disorder and for controlling the pain associated therewith. The method comprises administering to a subject in need of such treatment therapeutically effective amounts of a calcium channel blocker either alone or together with a nitric oxide donor. Amlodipine, anipamil, barnidipine, benidipine, bepridil, darodipine, diltiazem, efonidipine, felodipine, isradipine, lacidipine, lercanidipine, lidoflazine, manidipine, mepirodipine, nicardipine, nifedipine, niludipine, nilvadipine, nimodipine, nisoldipine, nitrendipine, perhexiline, tiapamil, verapamil and pharmaceutically acceptable salts thereof, are suitable calcium channel blockers.
Owner:SLA PHARMA AG
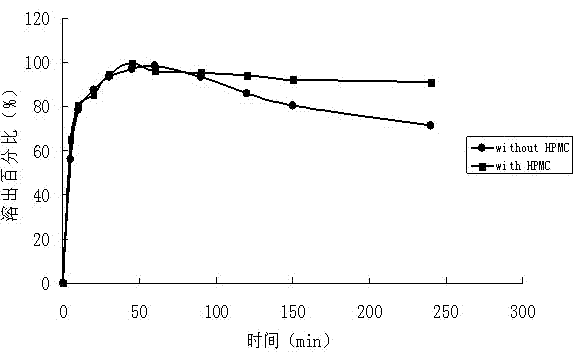
Lacidipine dispersible tablet and preparation method thereof
InactiveCN104000790AImprove solubilityImprove dissolution rateOrganic active ingredientsPill deliveryLacidipineSpray dried
The invention discloses a lacidipine dispersible tablet and a preparation method thereof. A solid dispersion is prepared by adopting lacidipine as a medicinal active component and polyvinylpyrrolidone as a carrier material through a spray drying technology, and the solid dispersion is processed to prepare the dispersible tablet. The preparation of the solid dispersion from lacidipine and a proper amount of polyvinylpyrrolidone increases the solubility and dissolve-out speed of a medicine, promotes the absorption of the medicine in bodies and improves the bioavailability of the medicine. The preparation of the dispersible tablet from the solid dispersion enhances the medicine administration compliance of a patient.
Owner:AVENTIS PHARMA HAINAN
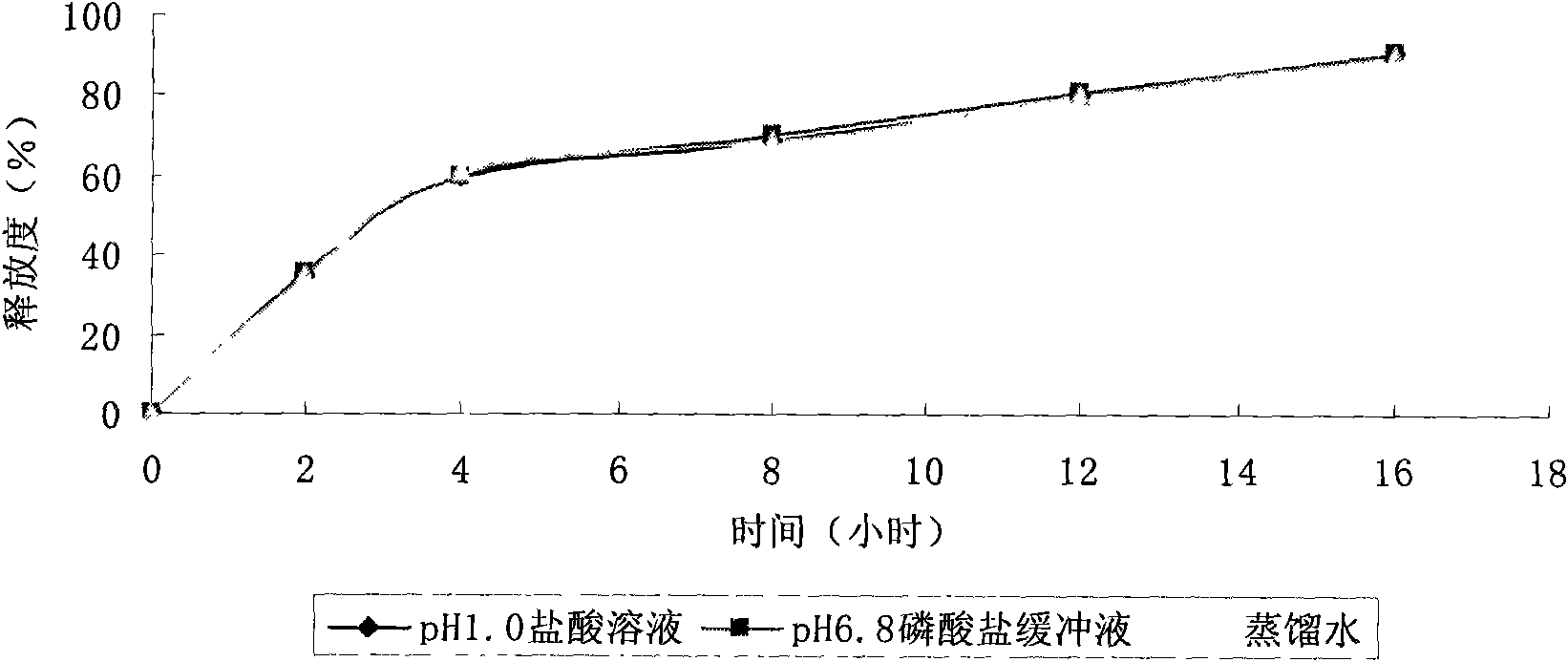
Lacidipine sustained-release preparation and preparation process thereof
ActiveCN101653410AOrganic active ingredientsPharmaceutical delivery mechanismBlood concentrationLacidipine
The invention discloses a lacidipine sustained-release preparation and a preparation process thereof. The lacidipine sustained-release preparation comprises an effective dose of lacidipine or a physiologically acceptable salt of the lacidipine and a pharmaceutically acceptable medicinal auxiliary material. The lacidipine sustained-release pharmaceutical preparation prepared by the preparation process can make medicaments released smoothly in vivo to ensure the smooth of the blood concentration in vivo, thereby ensuring that the effective blood concentration in vivo of a patient during medication is smooth, and fundamentally improving the safety and the effectiveness of the medicaments.
Owner:北京科信聚润医药科技有限公司
Compound blood pressure reducing prepn containing angiotonin converzyme inhibitor, calcium ion agonist and Estazolam
InactiveCN1526398AGood curative effectLittle side effectsOrganic active ingredientsPill deliveryCaptoprilSide effect
The present invention provides one new kind of compound blood pressure reducing preparation containing angiotonin converzyme inhibitor, calcium ion agonist, Estazolam and pharmaceutically acceptable carrier. The angiotonin converzyme inhibitor is selected from Enalapril, Ramipril, Benalapril, Lisinopril, Acertil, etc. as well as their mixture; and the calcium ion agonist is selected from Nitrendpine, Amlodipine Besylate, Nifedipine, Felodipine, etc. as well as their mixture. The present invention utilizes the synergistic effect between different medicines to raise the blood pressure lowering effect, reduce side effect and improve the compliance of patient.
Owner:杜晓锋
Pharmaceutical composition comprising telmisartan salt and calcium ion antagonist
InactiveCN102266559AImprove solubilityHigh dissolution rateOrganic active ingredientsSenses disorderMagnesium saltDiltiazem
The present invention relates to a kind of pharmaceutical composition, it comprises telmisartan salt and calcium ion antagonist or its pharmaceutically acceptable salt and pharmaceutically acceptable carrier; Described telmisartan salt is selected from telmisartan Sodium salt, potassium salt, calcium salt, magnesium salt or amine salt of sartan, described calcium ion antagonist is selected from amlodipine, lacidipine, cilnidipine, lercanidipine, nisoldipine, nica Dipine, azedipine, barnidipine, manidipine, benidipine, verapamil, diltiazem, or a pharmaceutically acceptable salt thereof. The composition is used for preventing, delaying progress or treating patients with hypertension, angina pectoris, atherosclerosis, stroke, cardiac insufficiency, dyslipidemia, diabetes, renal function damage or hypertension accompanied by Alzheimer's disease, reducing Reduce the morbidity and / or mortality of cardiovascular and cerebrovascular diseases, reduce adverse drug reactions, and improve patients' compliance with medication.
Owner:王丽燕
Lacidipine dispersible tablets and preparation method thereof
The invention provides Lacidipine dispersible tablets, which are medicines for treating hypertension, and a preparation method thereof. The Lacidipine dispersible tablets contain an effective amount of Lacidipine and pharmaceutical auxiliary materials. The auxiliary materials comprise polyethylene glycol, a diluent, a disintegrating agent, a lubricant and a flow aid. In the preparation process ofthe Lacidipine dispersible tablets, the polyethylene glycol is introduced as a medicine dispersion medium, the Lacidipine is formed into solid dispersion first and then mixed uniformly with other auxiliary materials, and the mixture is tabletted to form the disperse tablets. Compared with the common tablets, the Lacidipine dispersible tablets have the advantages of optimal dispersion state, shortdisintegrating time, quick medicine dissolution and the like. The Lacidipine dispersible tablets can be taken conveniently, the preparation process of the Lacidipine dispersible tablets is simple, special requirements on materials and equipment are avoided, and the industrial production can be realized easily.
Owner:ZHEJIANG BETTER PHARMA
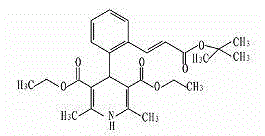
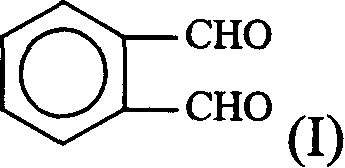
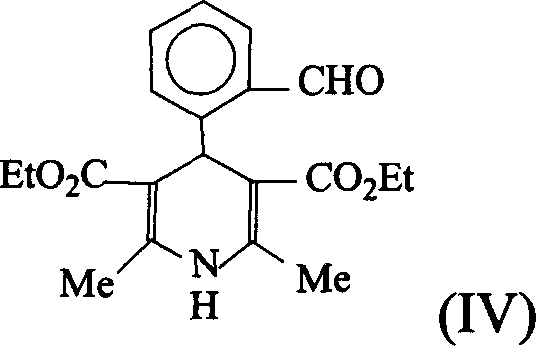
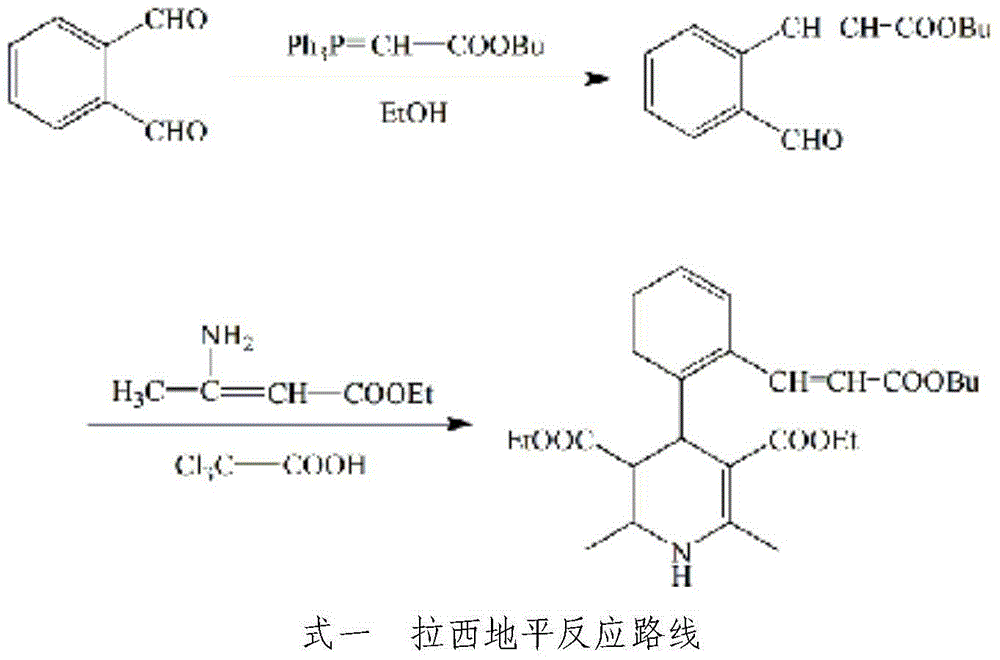
Process for synthesizing lacidipine of antihypertensive drugs
InactiveCN1594293AEasy to separateEasy to purifyGroup 5/15 element organic compoundsCardiovascular disorderPhosphoric Acid EstersOrganic acid
The invention discloses a novel process for synthesizing lacidipine of antihypertensive drugs which comprises the steps of, (a) subjecting ortho-xylenediol aldehyde (I) to reaction with beta-amido crotonic acid ethyl ester (II) in protonic or non-protonic solvent at the presence of organic acid catalyst, so as to obtain compound (IV), (2) reacting compound (IV) with phosphoric ester (III) in protonic or nonprotonic solvent at the presence of alkali.
Owner:PEKING UNIV
Subcutaneous implant line for long effect pressure lowering
InactiveCN108498508AOrganic active ingredientsPharmaceutical delivery mechanismCarboxymethyl starchLacidipine
The invention discloses a subcutaneous implant line for long effect pressure lowering. The implant line is prepared from raw medicine of lacidipine, aldactone and indapamide and auxiliary materials ofcrosslinked povidone, polyethylene glycol and sodium carboxymethyl starch. The implant line is a cylindrical implant line prepared by mixing biodegradable materials as carriers and hypotensive medicine. The pressure lowering speed is high; the blood pressure can be stable after implantating for 3 days; the goal of stabilizing the blood pressure for a long time can be achieved. In addition, the degradation is slow; the complete degradation can be completed after about 3 years; the line implant line use time is long; the frequent operation implantation is not needed; the operation pain of patients is reduced.
Owner:朱军星
Process for preparing lacidipine
Owner:DR REDDYS LAB LTD +1
Lacidipine solid dispersion and preparation method thereof
ActiveCN111467344AFacilitated releaseImprove oral bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsCrystallographyLacidipine
The invention belongs to the technical field of medicines, relates to a lacidipine solid dispersion and a preparation method thereof, and in particular relates to a lacidipine ternary solid dispersionand a preparation method thereof. The ternary solid dispersion comprises lacidipine, a crystal nucleus inhibitor and a dissolution promoter, wherein the crystal nucleus inhibitor is a water-soluble high polymer material, and the dissolution promoter is an anionic polymer. The crystal nucleus inhibitor can inhibit a drug from forming a crystal nucleus by effects of forming hydrogen bonds with thedrug and the like, so that the drug can maintain a supersaturated state for a long time. The dissolution promoter can stabilize crystal nuclei already appearing in a system and prevent the crystal nuclei from further growing. Meanwhile, the dissolution promoter has high water solubility, so that the release rate of lacidipine under a non-leaking condition can be further increased under combined action of the crystal nucleus inhibitor and the dissolution promoter. The system can significantly improve the dissolution rate and bioavailability of lacidipine.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of composition containing lacidipine
InactiveCN104693110AReduce processing operationsReduce stepsOrganic active ingredientsOrganic chemistryLacidipineOrganic chemistry
The invention provides a preparation method of a composition containing lacidipine, which is characterized in that lacidipine is synthesized by a one kettle way and a two-step reaction, and the lacidipine is taken as an effective component. The synthesis technology comprises the advantages of shortened steps, simple operation, and green and safety; the dissolution rate of the composition is obviously increased, stability is better under illumination, and the preparation method is benefit for industrialization and clinical application.
Owner:哈药集团人民同泰医药股份有限公司
Combination therapies of cicletanine and lacidipine
InactiveUS20060154971A1Treating and preventing nephropathyEliminate side effectsBiocideAnimal repellantsLacidipineCombination therapy
Preferred embodiments of the present invention are related to novel therapeutic drug combinations and methods for treating and / or preventing hypertension and complications in patients with diabetes and / or metabolic syndrome. More particularly, aspects of the present invention are related to using a combination of cicletanine and lacidipine (a preferred calcium antagonist) for treating and / or preventing hypertension and complications in patients with diabetes and / or metabolic syndrome.
Owner:NAVITAS PHARMA +1
Lacidipine self-microemulsifying soft capsules and preparation method thereof
InactiveCN102008471BHigh dissolution rateProtection stabilityOrganic active ingredientsCapsule deliveryLacidipineOil phase
The invention belongs to the technical field of medicines and relates to lacidipine self-microemulsifying soft capsules and a preparation method thereof. The lacidipine self-microemulsifying soft capsules is uniform transparent solution prepared from an oil phase, an emulsifier and an auxiliary emulsifier, wherein under the conditions of an ambient temperature and mild stirring, the lacidipine self-microemulsifying soft capsules spontaneously emulsify to form emulsion droplet smaller than 500 nanometers; the ratio of the lacidipine to the auxiliary emulsifier to the emulsifier to the oil phase is 1:1-30:1-60:0.5-10 or 1: 1-30:1-30:0.5-10; a water soluble macromoleclar polymer can be added for prolonging the maintenance time of the saturated state of the solution of the medicament; and an antioxygen can be added for improving the chemical stability of the preparation. In the invention, the biological utilization rate of oral lacidipine is improved; and the cost is low, and the process is simple.
Owner:SHENYANG PHARMA UNIVERSITY
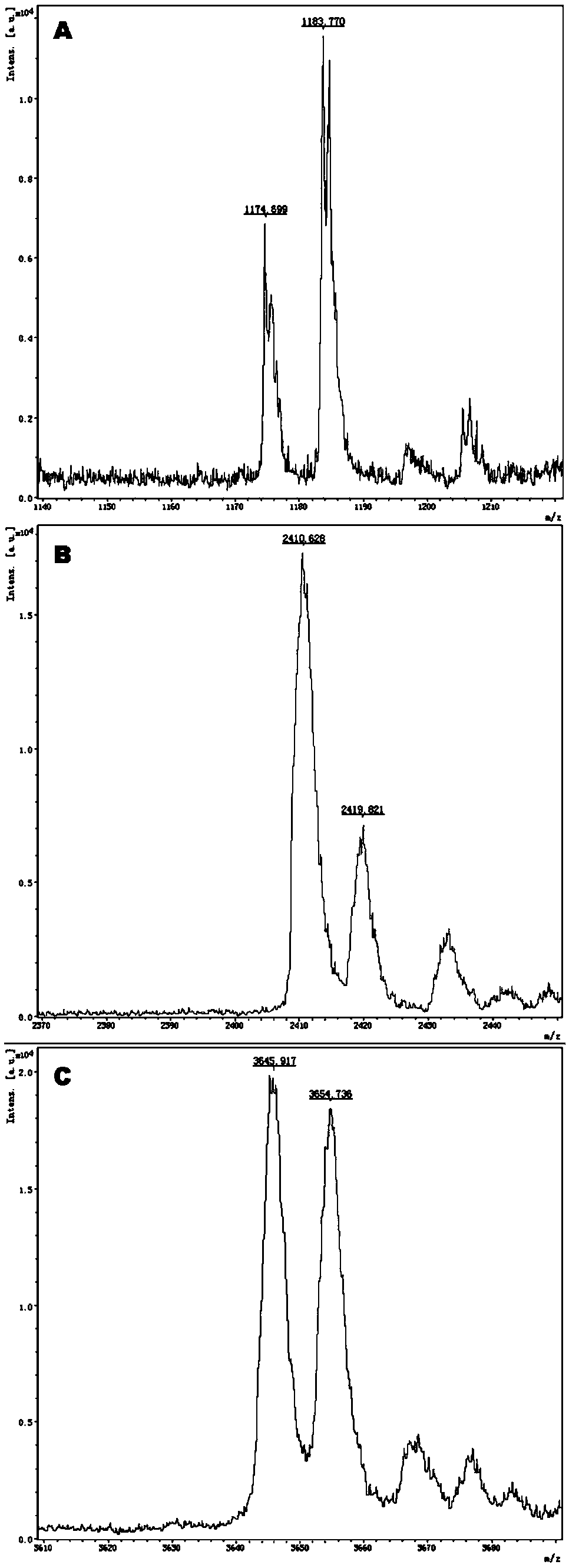
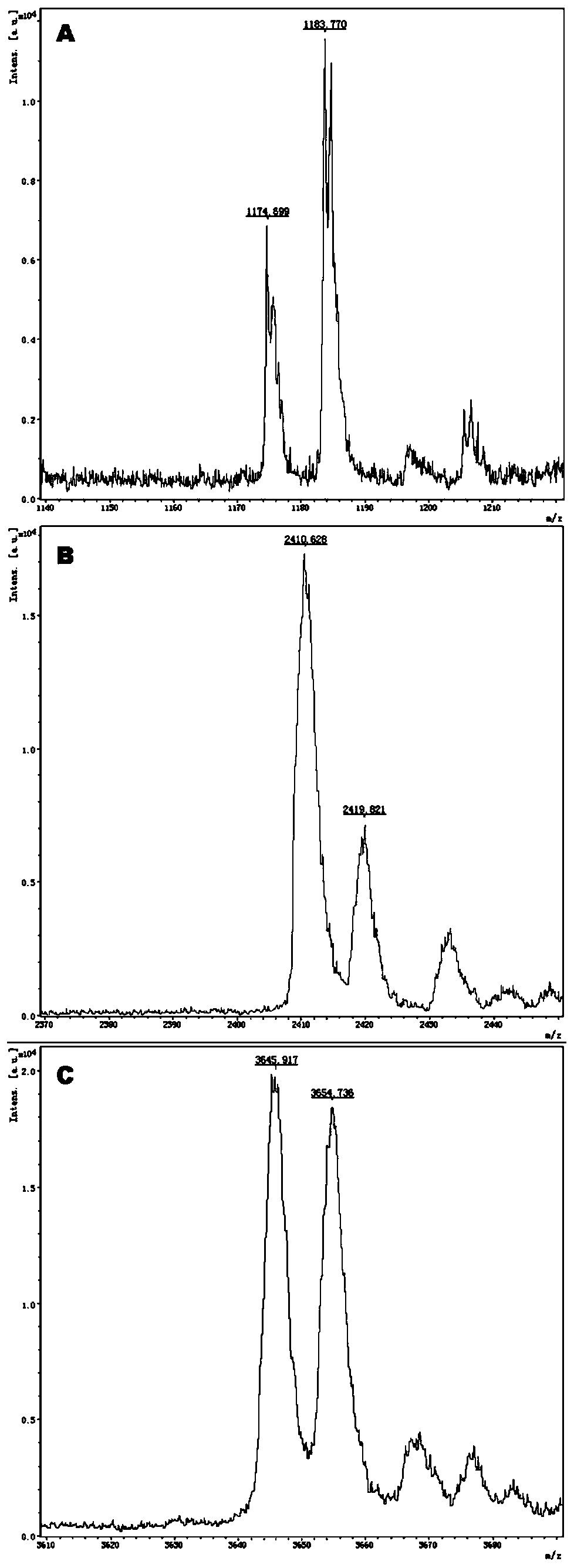
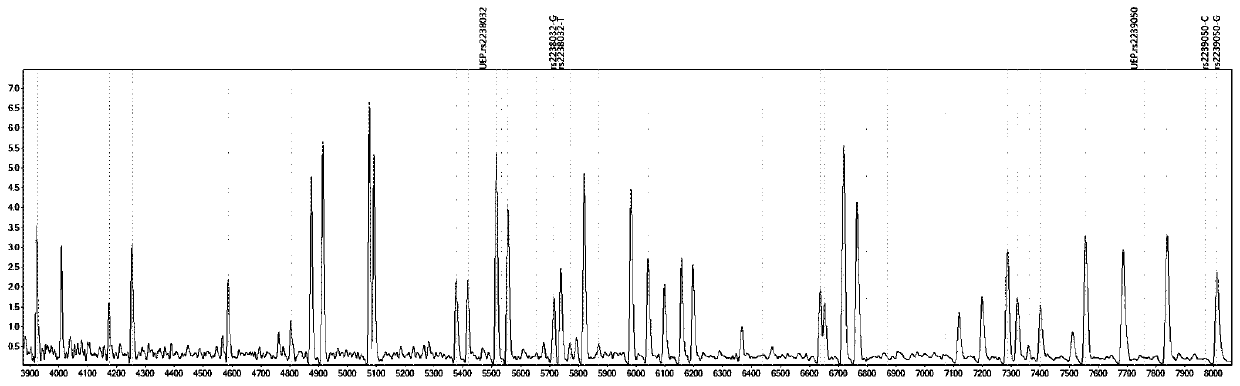
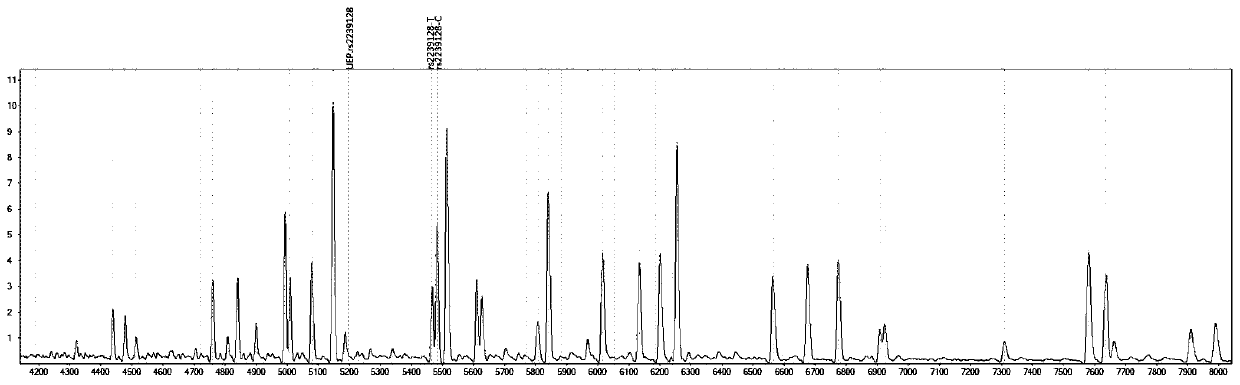
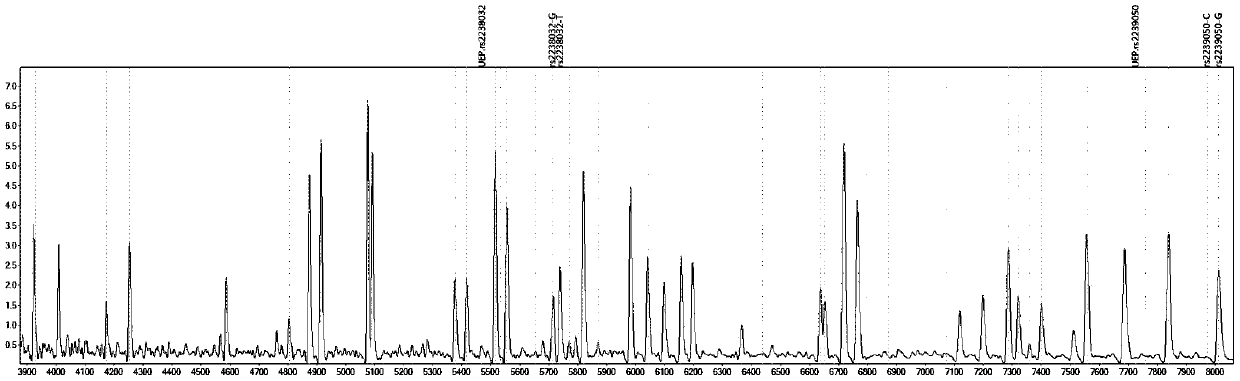
Gene detection kit for medication guidance of antihypertensive drug lacidipine
InactiveCN111235256AQuality Medical ServicesPromoting the process of rational drug useMicrobiological testing/measurementDNA/RNA fragmentationLacidipineNucleotide
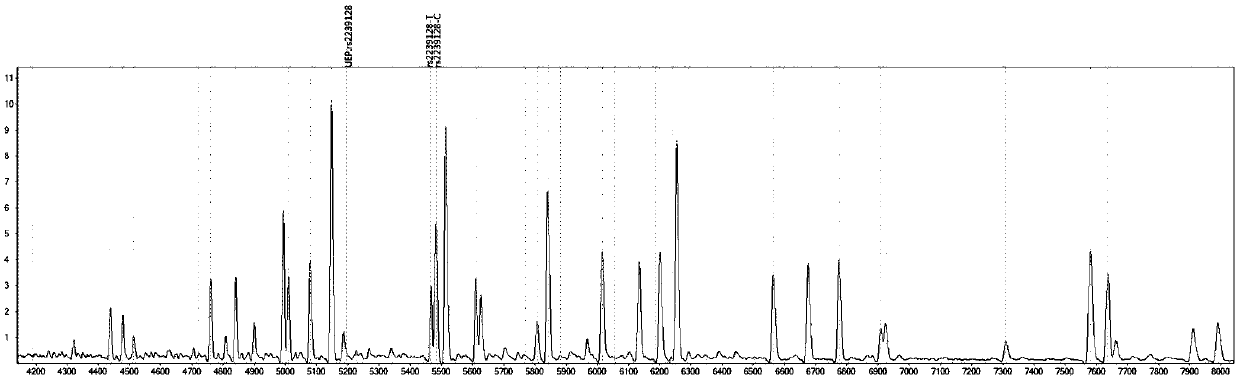
The invention provides a method utilizing extension primers with different molecular weights at different SNP (single nucleotide polymorphism) sites to detect multiple sites related to metabolism of an antihypertensive drug lacidipine by MALDI-TOF-MS (matrix-assisted laser desorption ionization time-of-flight mass spectrometry) and guide medication of lacidipine finally as well as a kit. The method comprises steps as follows: designing multiple amplification primers and extension primers respectively according to six target SNP sites to be detected; preparing a multi-amplification primer reaction system and an extension reaction system; performing amplification and single-base extension reactions on the six target SNP sites by multiple primers simultaneously respectively; performing time-of-flight mass spectrometry on products obtained after the single-base extension reaction, identifying genetypes of SNPs related to different drug metabolisms according to products, represented by massspectrum peaks, of extension primers with different molecular weights, and guiding the medication of the antihypertensive drug lacidipine. Meanwhile, the invention provides a detection kit utilizingthe method. The SNP sites related to metabolism of six antihypertensive drug lacidipine can be detected simultaneously, and the method has the advantages of low cost, no need of probe synthesis, low time consumption, simple and convenient result analysis and quite wide application field.
Owner:BIOYONG TECH
Mass spectrography method for differentiation of individualized medication of lacidipine through primer composition
InactiveCN111235258AQuality Medical ServicesPromoting the process of rational drug useMicrobiological testing/measurementDNA/RNA fragmentationHypertension medicationsMultiplex
The invention provides a mass spectrography method for differentiation of individualized medication of lacidipine through a primer composition. According to different single nucleotide polymorphism (SNP) sites having extension primers with different molecular weights, through matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS), a plurality of sites pertinentto metabolism of a hypertension reducing medicine namely lacidipine can be detected at the same time, and finally the mass spectrography method for differentiation of individualized medication of lacidipine through the primer composition is obtained. The method comprises the steps of: according to 6 target SNP sites to be detected, separately designing multiplex amplification primers and an extension primer; compounding a multiplex amplification primer reaction system and an extension primer reaction system; in the reaction systems, performing an amplification reaction and a single-base extension reaction on the 6 target SNP sites at the same time with multiple sets of primers; and performing time-of-flight mass spectrometric analysis on products after the single-base extension reaction,identifying the genotypes of different drug metabolism relevant SNPs according to extension primer products with different molecular weights and represented by mass spectrum peaks, and guiding the medication of the hypertension reducing medicine namely the lacidipine. The method disclosed by the invention can detect 6 metabolism pertinent SNP sites of the hypertension reducing medicine namely thelacidipine at the same time, and has the advantages of being low in cost, free from probe synthesis, short in time consumption, simple and convenient in result analysis, and extremely broad in application field.
Owner:BIOYONG TECH
Lacidipine containing pharmaceutical composition for treating hypertension and preparation method thereof
InactiveCN105687765AAvoid peripheral vasodilation responseGood treatment effectOrganic active ingredientsGranular deliveryLacidipineMaesa indica
The invention belongs to the technical field of medicines and particularly relates to a lacidipine containing pharmaceutical composition for treating hypertension and a preparation method thereof. The lacidipine containing pharmaceutical composition for treating the hypertension, provided by the invention, is prepared from the following raw pharmaceutical materials in parts by weight: 3-5 parts of lacidipine, 2,000-4,000 parts of Mesona chinensis, 2,000-4,000 parts of Maesa indica, 2,000-4,000 parts of Eurya japonica, 2,000-4,000 parts of acanthopanax brachypus and 2,000-4,000 parts of Bulbopyllum psychoon. According to the lacidipine containing pharmaceutical composition for treating the hypertension and the preparation method thereof, the traditional Chinese medicines and the lacidipine are combined, so that common adverse reactions of the lacidipine can be avoided, and the treatment effect on the hypertension is improved.
Owner:JINAN BANGWEN MEDICAL TECH
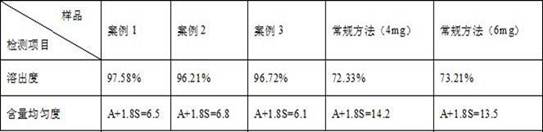
Preparation method of lacidipine composition
InactiveCN113230217AEvenly dispersedHigh dissolution rateOrganic active ingredientsPill deliveryLacidipinePyrrolidinones
The invention discloses a preparation method of a lacidipine composition. The preparation method is characterized by comprising the following steps: (1) dispersing lacidipine in molten poloxamer 188; (2) cooling and solidifying the product obtained in the step (1), and performing low-temperature crushing and sieving to obtain lacidipine solid dispersion powder; (3) mixing the mixture obtained in the step (2), polyvinylpyrrolidone (PVP) K30, cross-linked povidone and lactose, and carrying out dry granulation; and (4) uniformly mixing the mixture obtained in the step (3) with a proper amount of magnesium stearate and tabletting. According to the method, the content uniformity of the lacidipine preparation can be ensured, and the dissolution rate of the lacidipine preparation is improved, so that the bioavailability of the lacidipine is improved.
Owner:HAYAO GRP SANJING MINGSHUI PHARMA CO LTD
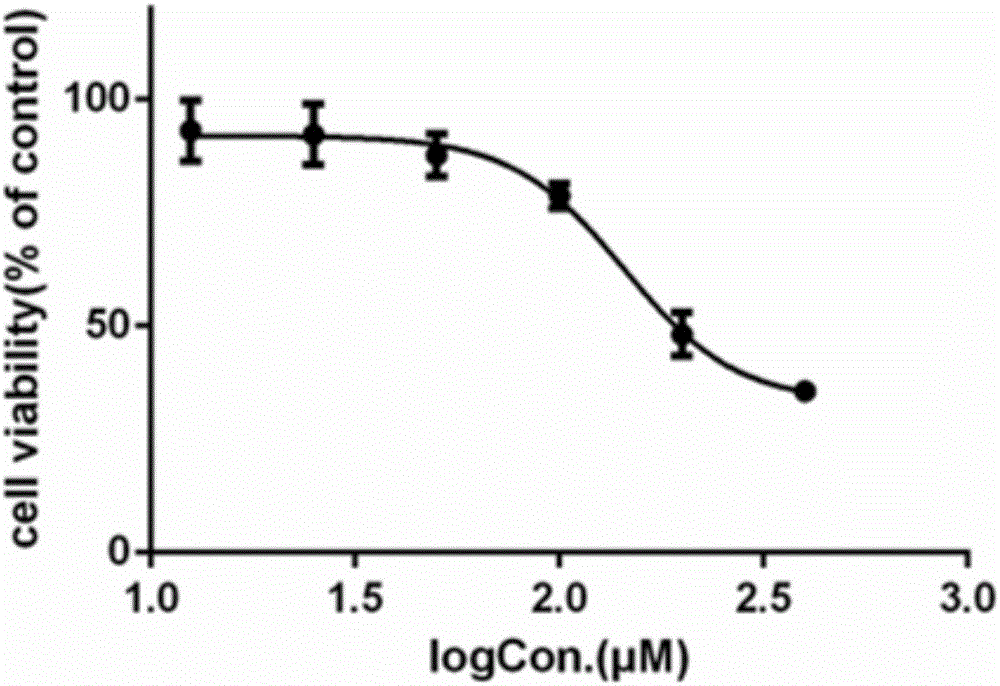
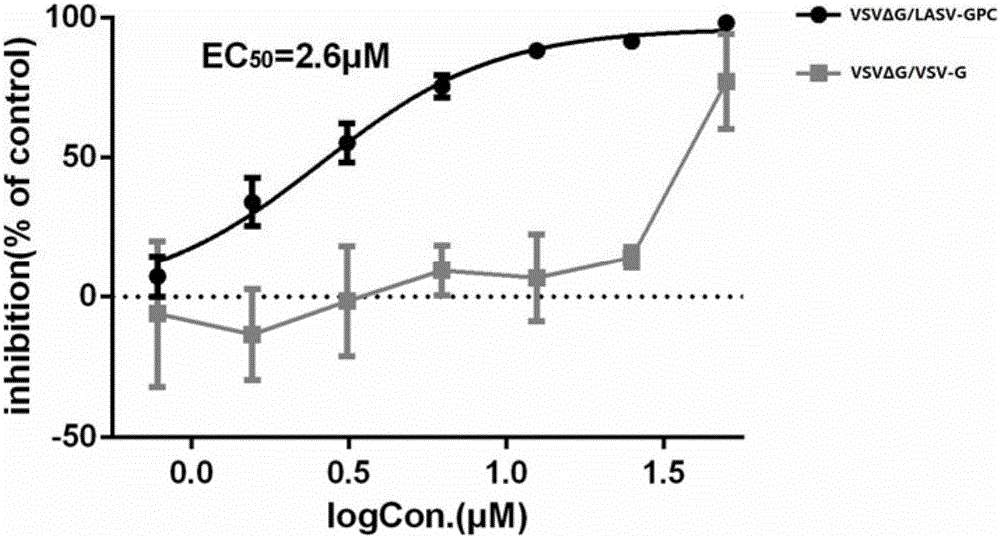
Application of lacidipine to preparing medicines for treating arenavirus infection
ActiveCN106511343AImprove securityAvoid infectionOrganic active ingredientsAntiviralsNon toxicityLacidipine
The invention provides application of lacidipine to preparing medicines for treating arenavirus infection, and belongs to the field of medicines. The application has the advantages that invasion of arenavirus into host cells can be effectively inhibited by the lacidipine in non-toxicity ranges, and accordingly virus infection due to the arenavirus can be effectively prevented and treated; the lacidipine is excellent in safety, further can be developed to obtain the medicines for treating or preventing the arenavirus infection and has a broad application prospect; the lacidipine further can be applied to preparing medicines for treating virus infection mediated by arenavirus envelope glycoprotein compounds and can act on the arenavirus envelope glycoprotein compounds, and accordingly the virus infection mediated by the arenavirus envelope glycoprotein compounds can be effectively inhibited; all different types of virus infection mediated by the arenavirus envelope glycoprotein compounds can be effectively treated and prevented by the lacidipine.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Method for determination of individualized medication of lacidipine through mass spectrography using detection products
InactiveCN111235257AQuality Medical ServicesPromoting the process of rational drug useMicrobiological testing/measurementDNA/RNA fragmentationHypertension medicationsMultiplex
The invention provides a method for determination of individualized medication of lacidipine through mass spectrography using detection products. Different single nucleotide polymorphism (SNP) sites have extension primers with different molecular weights, so that through matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS), a plurality of sites pertinent to metabolism of a hypertension reducing medicine namely lacidipine can be detected at the same time, and finally, the method for determination of individualized medication of lacidipine through mass spectrography using the detection products is obtained. The method comprises the steps of: according to 6 target SNP sites to be detected, separately designing multiplex amplification primers and an extension primer; compounding a multiplex amplification primer reaction system and an extension primer reaction system; in the reaction systems, performing amplification and a single-base extension reaction on the 6 target SNP sites at the same time with multiple sets of primers; and performing time-of-flight mass spectrometric analysis on products after the single-base extension reaction, identifyingthe genotypes of different SNPs relevant to drug metabolism according to extension primer products with different molecular weights represented by mass spectrum peaks, and guiding the medication of the hypertension reducing medicine namely the lacidipine. The method disclosed by the invention can detect 6 SNP sites pertinent to metabolism of the hypertension reducing medicine namely the lacidipineat the same time, and has the advantages of being low in cost, free from probe synthesis, short in time consumption, simple and convenient in result analysis, and extremely broad in application field.
Owner:BIOYONG TECH
Lacidipine dispersible tablet and preparation method thereof
InactiveCN102133203AReasonable compositionShort disintegration timeOrganic active ingredientsPharmaceutical non-active ingredientsAlcoholAdhesive
The invention relates to a lacidipine dispersible tablet and a preparation method thereof. The lacidipine dispersible tablet provided by the invention comprises the following components in parts by weight: 1 part of lacidipine, 5-12 parts of adhesives, 25-35 parts of disintegrating agents and 5-10 parts of diluents. The preparation method of the lacidipine dispersible tablet comprises the following steps of: (1) mixing the lacidipine and the adhesives, adding absolute ethyl alcohol and heating and stirring to complete dissolution; and (2) adding the disintegrating agents to the mixture obtained in the step (1), uniformly mixing, sieving and drying by blowing. The lacidipine dispersible tablet provided by the invention has the advantages of reasonable components, short disintegrating time, rapid medicine dissolution, rapid absorption and capability of improving medicine bioavailability, and the preparation process of the lacidipine dispersible tablet is simple and easy for enforcement and capable of realizing scale production.
Owner:佟兵 +1
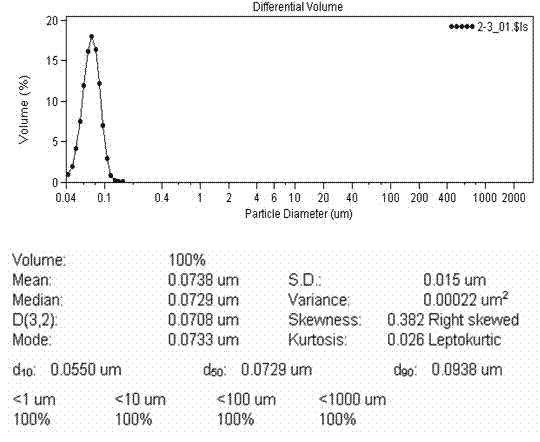
A kind of lacidipine silica gel adsorption solid dispersion and its preparation
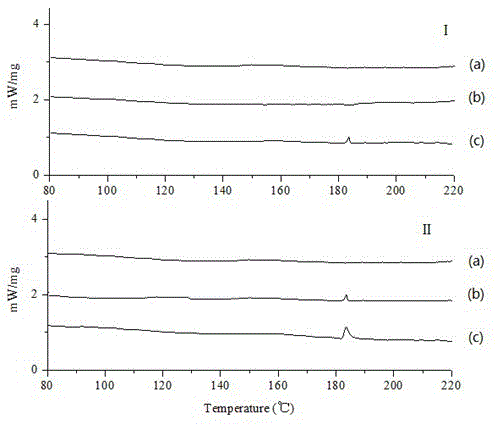
InactiveCN103784963BImprove in vitro dissolutionImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsOrganic solventGas phase
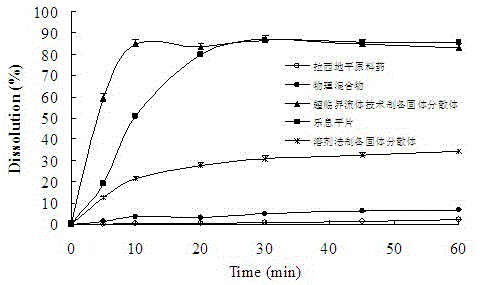
The invention discloses a lacidipine silica gel adsorption solid dispersion and a preparation method thereof. The method uses hydrophilic gas phase silica as a carrier and adopts supercritical fluid (SFC) technology to prepare the lacidipine silica gel adsorption solid dispersion. The method effectively increases the water dissolubility of lacidipine and improves the oral bioavailability of lacidipine. The preparation method does not use any organic solvent, overcomes the drug safety problem caused from organic solvent residues, is simple and easy to operate, and has low cost and good economical efficiency.
Owner:SHENYANG PHARMA UNIVERSITY
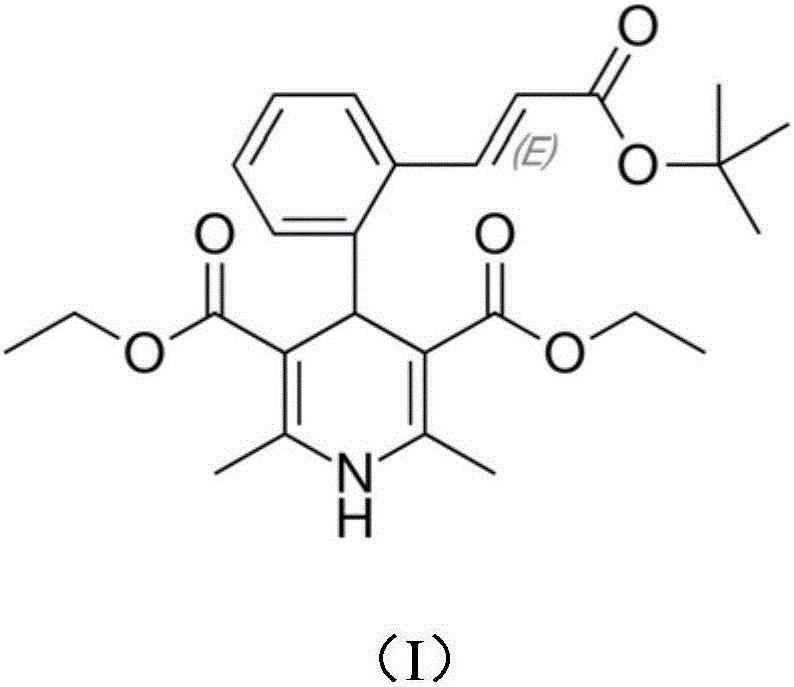
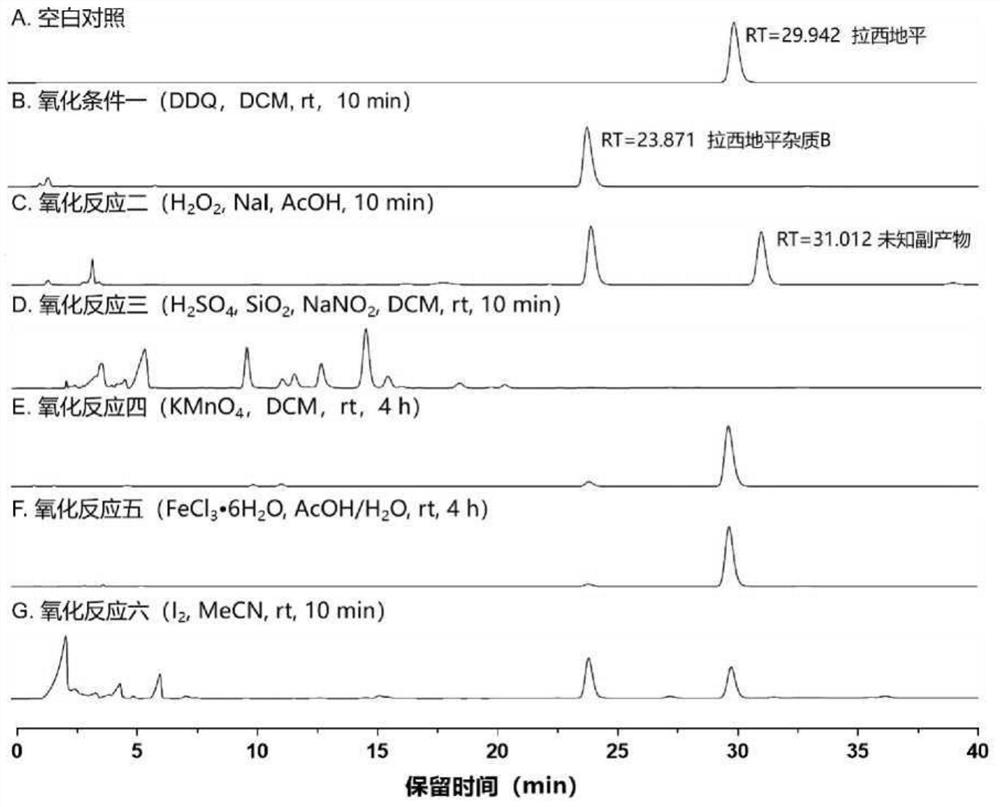
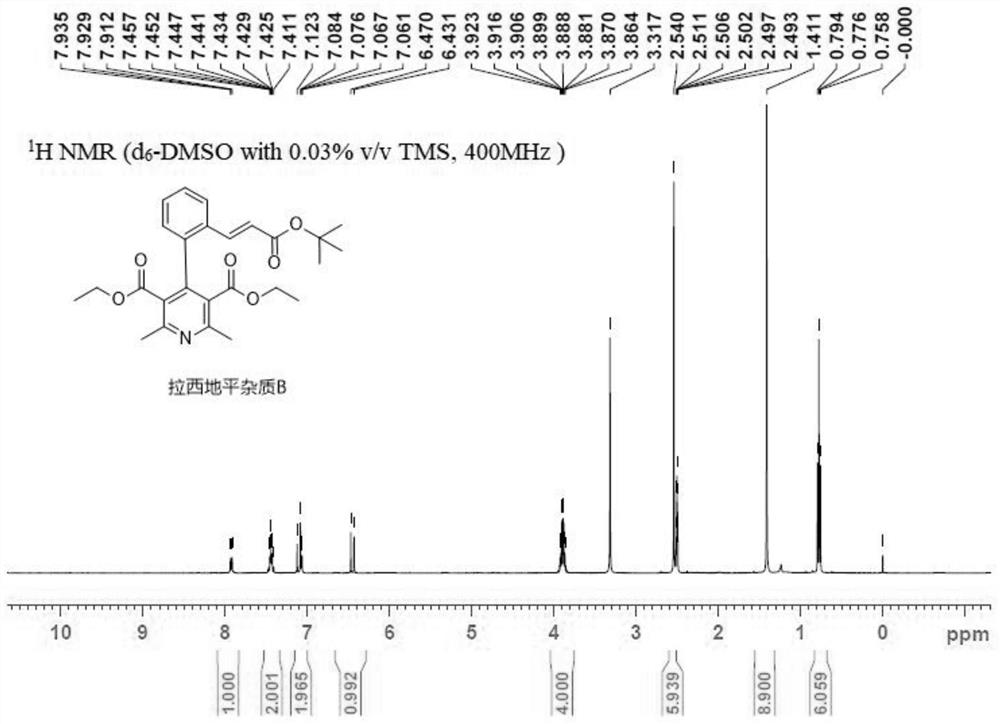
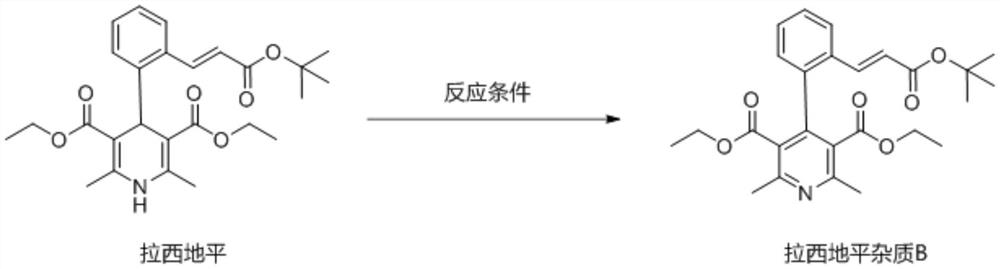
A kind of preparation method of lacidipine impurity b
The application relates to the field of pharmaceutical technology, and specifically discloses a preparation method of lacidipine impurity B. The method comprises the following steps: step 1), adding lacidipine into the solvent and stirring until dissolved to obtain a preparatory solution; step 2), slowly adding DDQ to the preparatory solution at a speed of 1.0-3.0 g / min, stirring at a speed of 250-350 rpm, Stir at 0~50°C for more than 10 minutes and then filter, the obtained organic phase is lacidipine impurity B solution, the molar ratio of lacidipine to DDQ is 1:1~3; step 3), lacidipine The impurity B solution was first washed with a weak alkaline aqueous solution, then washed with a saturated sodium chloride aqueous solution, and then spin-dried to obtain lacidipine impurity B in the form of a yellow solid. The application has the characteristics of short reaction time, simple preparation process, and can be applied to industrial production. The lacidipine impurity B prepared by the application has the advantages of high purity and good properties.
Owner:广州隽沐生物科技股份有限公司
Slow-release preparation of calcium antagonist and salt thereof and preparation method thereof
InactiveCN107126562AQuick effectStable blood concentrationPharmaceutical delivery mechanismPharmaceutical active ingredientsCilnidipineTreatment effect
The invention provides a slow-release preparation of calcium antagonist and salt thereof and a preparation method thereof. The slow-release preparation comprises a slow-release phase which contains active pharmaceutical ingredients, the active pharmaceutical ingredients include 5-40mg of amlodipine or salt thereof calculated by amlodipine, 4-32mg of lacidipine or salt thereof calculated by lacidipine, 5-40mg of nisoldipine or salt thereof calculated by nisoldipine, 5-40mg of cilnidipine or salt thereof calculated by cilnidipine, 2-32mg of benidipine or salt thereof calculated by benidipine, 10-80mg of lercanidipine or salt thereof calculated by lercanidipine and 5-80mg of manidipine or salt thereof calculated by manidipine; in release media meeting sink conditions, higher than 90% of weight of the active pharmaceutical ingredients are released within a release period of 6-14h. The slow-release preparation is convenient to use, good in treatment effect, high in taking compliance, little in adverse reaction, quick in action, capable of maintaining stable and effective blood concentration for a long time, ingenious in design, simple in structure, high in stability and suitable for large-scale popularization and application.
Owner:杨彦玲
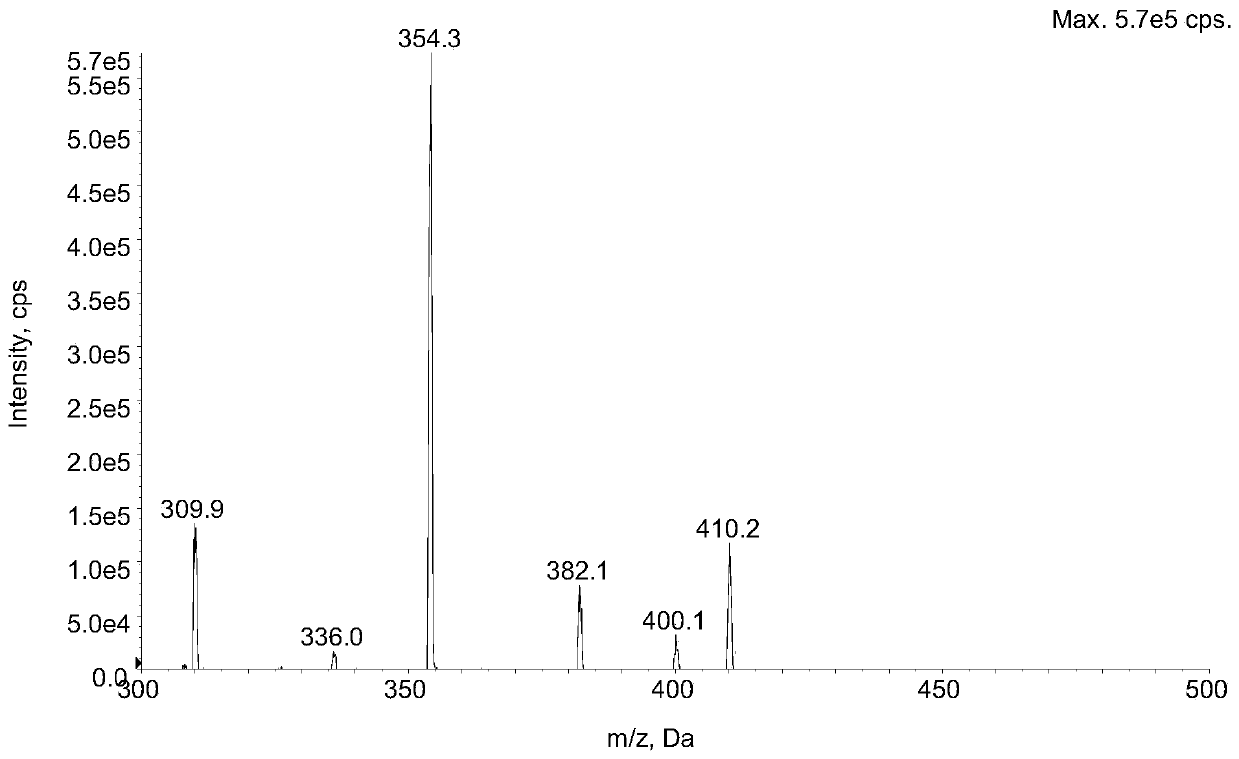
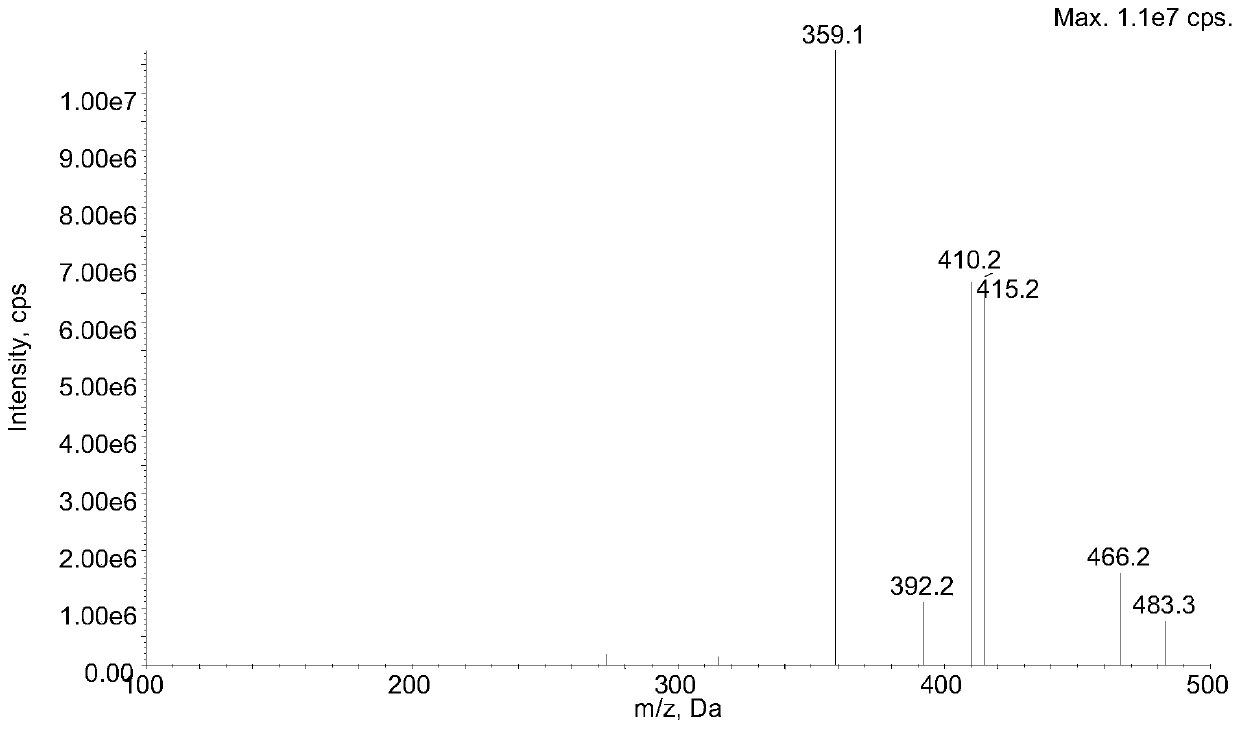
Method for determining concentration of lacidipine in blood plasma by adopting liquid chromatography-tandem mass spectrometry
InactiveCN111595977AHigh sensitivitySmall doseComponent separationChromatographic separationLacidipine
The invention discloses a method for determining concentration of lacidipine in blood plasma by adopting liquid chromatography-tandem mass spectrometry, and belongs to the technical field of pharmaceutical analysis. The method for determining the concentration of lacidipine in human plasma by adopting the liquid chromatography-tandem mass spectrometry is established in the invention, after a plasma sample is treated through using a protein precipitation method, a Luna PFP chromatographic column is adopted for rapid gradient elution chromatographic separation, and detection is conducted throughusing a tandem quadrupole mass spectrometer. The ion reactions monitored by mass spectrometry are as follows: m / z473.5 to 354.3(lacidipine) and m / z481.4 to 362.3(lacidipin-13C8). By adopting the method disclosed by the invention, the rapid and sensitive detection of lacidipine in the blood plasma can be realized, and the method can be applied to the bioequivalence research of lacidipine imitationmedicines.
Owner:苏州必宜生物科技有限公司
A kind of lacidipine solid dispersion and preparation method thereof
ActiveCN111467344BDoes not change or affect crystallization inhibitionStable release behaviorOrganic active ingredientsPharmaceutical non-active ingredientsCrystallographyLacidipine
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com