Method for determination of individualized medication of lacidipine through mass spectrography using detection products
A technology of lacidipine and mass spectrometry, applied in the field of oligonucleotide products, can solve problems such as unsuitability for multi-SNP detection, limitation of detection objects, and rising costs, and achieve high-quality medical services, simple and convenient result analysis, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: Primer Design and Synthesis
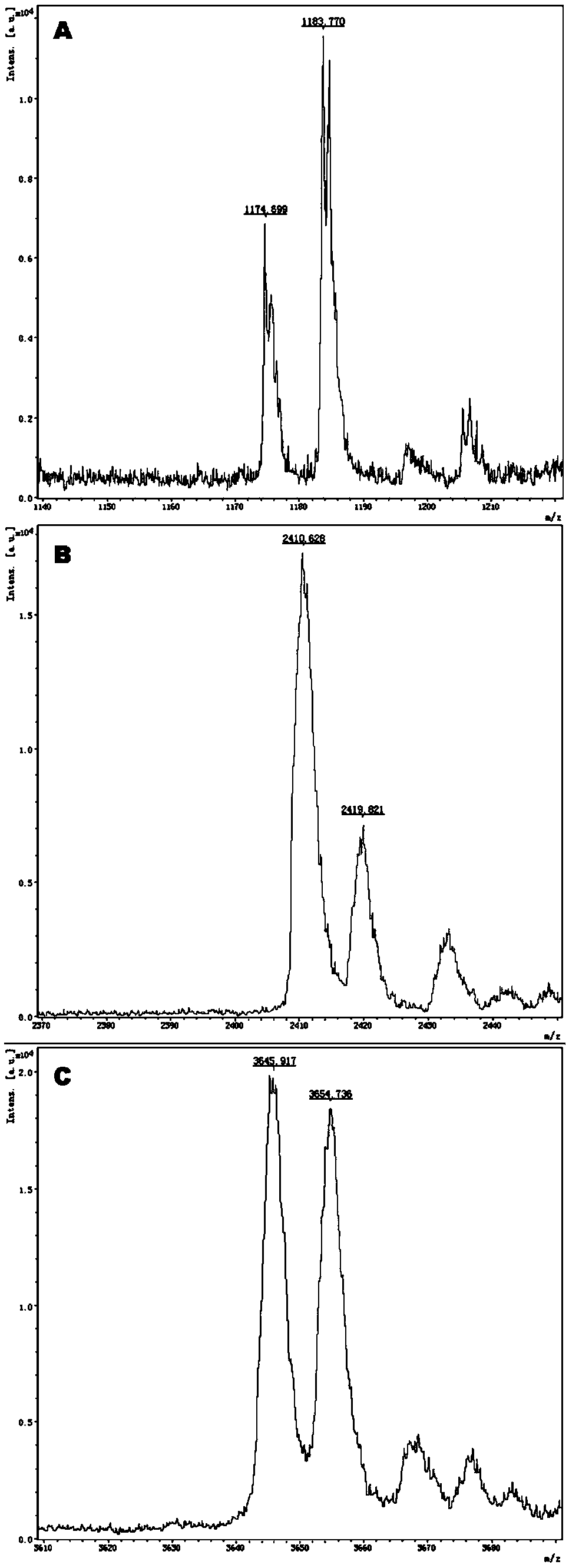
[0086] The surrounding sequences of the target SNP of this kit are queried in the db_SNP (build 132) and Hapmap (Rel 28, Phase II + III, Aug 10) databases, and these sequences are used to design multiplex PCR primers and single base extension primers.
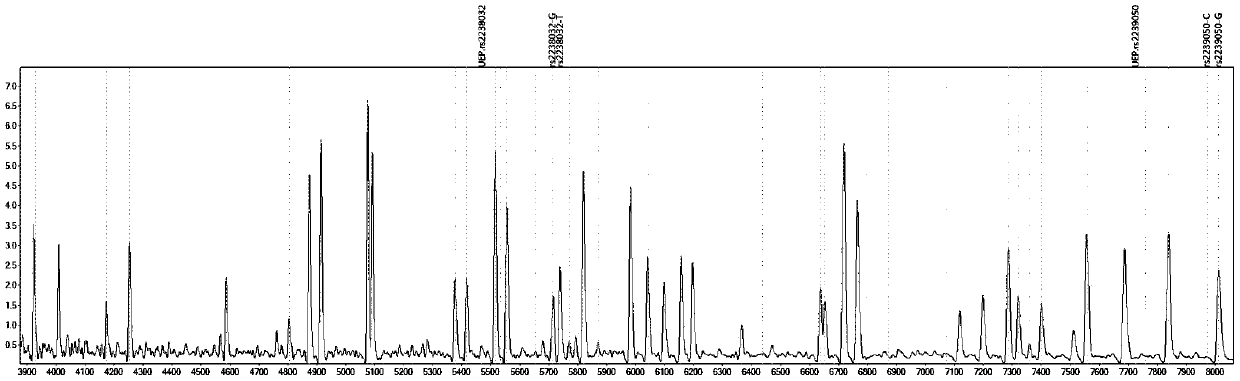
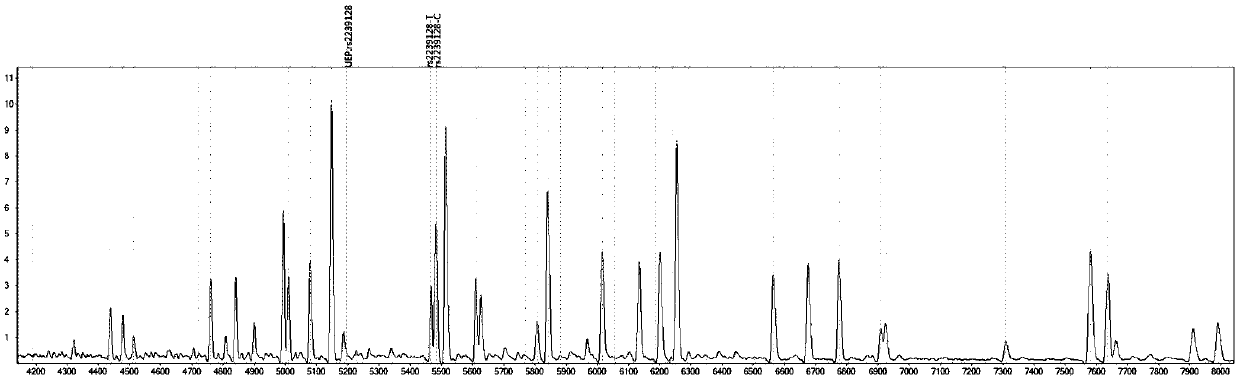
[0087] Design corresponding specific PCR primer core sequences (SEQ1a to SEQ6a) and specific extension primer core sequences (SEQ1b to SEQ6b) for 6 polymorphic sites related to discrimination of drug types, such as rs2238032, rs2239050, rs2239128, rs10898815, rs2429427, rs588076 . 6 pairs of PCR primers and 6 extension primers (SEQ1a / b to SEQ6a / b) constitute 2 independent reaction systems. In these two independent reaction systems, SEQ1a to SEQ3a participate in an independent multiplex PCR reaction, and SEQ1b to SEQ3b participate in a subsequent independent single base extension reaction; SEQ4a to SEQ6a participate in another independent multiplex PCR reaction, SEQ4b to SEQ6b to...
Embodiment 2
[0089] Embodiment 2: sample DNA extraction
[0090] A total of 10 DNA samples were collected from ordinary Chinese people, marked as A1-A10. Among them, sample collection, DNA extraction, etc. were collected in accordance with the requirements of the instructions, and human venous blood was collected with EDTA anticoagulant tubes. According to the instructions, the collected blood should not be stored at 2-8°C for more than one week, and at -20°C for no more than one month, and can be transported in a curling box with ice or a foam box with ice. It is recommended to use fresh blood as much as possible. Genomic DNA extraction. Since this kit does not provide human genomic DNA extraction reagents, a commercial nucleic acid extraction kit (such as DNeasy Blood and Tissuekit from QIAGEN Company) was used to extract human genomic DNA from 200 μl whole blood of each patient, and the DNA was extracted using NanoDrop 2000 ( Thermo Company) quantified and normalized to 30ng / μl (A1-A1...
Embodiment 3
[0091] Embodiment three: biological experiment
[0092] Using ABI9700 type PCR instrument, according to the instruction manual, check the 6 polymorphic sites that are used to identify the drug type.
[0093] The components used in the kit for PCR, PCR product purification and single base extension are:
[0094] serial number component name main ingredient Specification 1 PCR Primer Mix PCR primers 24μL / tube x1 tube 2 PCR reaction solution Taq enzyme, dNTP 72μL / tube x1 tube 3 Enzyme digestion reaction solution SAP enzyme 48μL / tube x1 tube 4 Extension Primer Mix extension primer 24μL / tube x1 tube 5 Extension reaction solution Single base elongase, ddNTP 24μL / tube x1 tube 6 positive control Human Genomic DNA (30ng / μL) 10μL / tube x1 tube
[0095] The concentration of each primer pair is 500nmol / L.
[0096] According to the manual, the specific operation method is as follows:
[0097] 1. PCR amplificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com