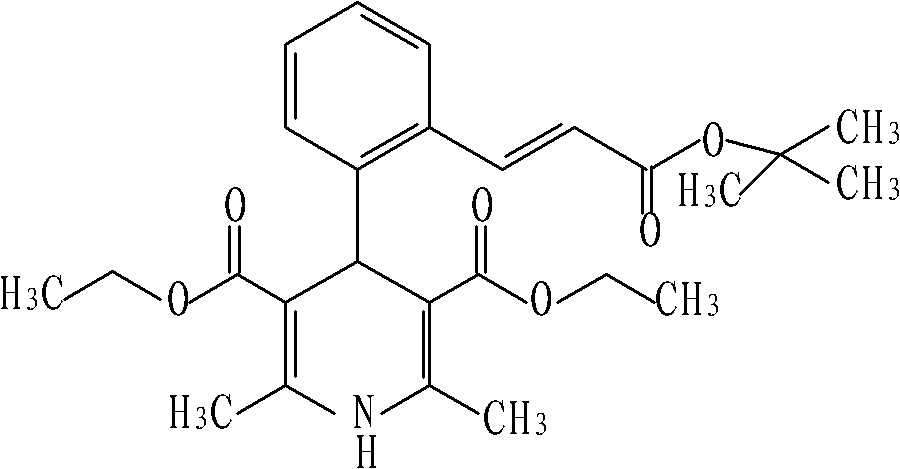
Lacidipine dispersible tablet and preparation method thereof
A technology of lacidipine and dispersible tablets, which is applied in the field of medicine, can solve problems such as easy agglomeration, enhanced hygroscopicity, and unfavorable storage, and achieve the effects of short disintegration time, enhanced compliance, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The present embodiment provides the prescription and preparation method thereof of preparing 2000 lacidipine dispersible tablets (specification is: lacidipine 4mg / sheet) as follows:
[0026] Plus prescription:
[0027]
[0028]
[0029] The preparation method is as follows:
[0030] 1) Pass the main ingredient and various auxiliary materials through a 100-mesh sieve for later use.
[0031] 2) Put sodium carboxymethyl starch, microcrystalline cellulose, and crospovidone into an oven and bake at 90°C for one hour.
[0032] 3) Weigh 8 g of lacidipine, 56 g of povidone K30, and 200 g of crospovidone respectively.
[0033] 4) Put lacidipine and povidone in a large beaker first, put them in a water bath at 80°C and stir evenly. Pour in 320ml of absolute ethanol, stir until it is completely dissolved, and stir for another 3 minutes if there is no solid matter.
[0034] 5) Pour all the crospovidone into the above-mentioned beaker at one time, and stir vigorously quic...
Embodiment 2
[0052] This embodiment provides a preferred process for establishing the preparation method of the present invention, including the following aspects:
[0053] (1) Differences in performance of products prepared before and after drying of auxiliary materials
[0054] Two parts (A and B) of the main drug and various auxiliary materials were respectively passed through a 100 mesh sieve for subsequent use, and two parts of the auxiliary materials sodium carboxymethyl starch, microcrystalline cellulose, and crospovidone were weighed respectively, and the A part was put into Bake in an oven at 90°C for one hour, and part B is not dried. Follow-up operations are performed with reference to Example 1 of the invention. The test results of the prepared dispersible tablets are as follows:
[0055]
A (drying accessories)
B (undried accessories)
dispersion
Compliance
did not qualified
4.8
5.3
disintegrate
2 minutes
5 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com