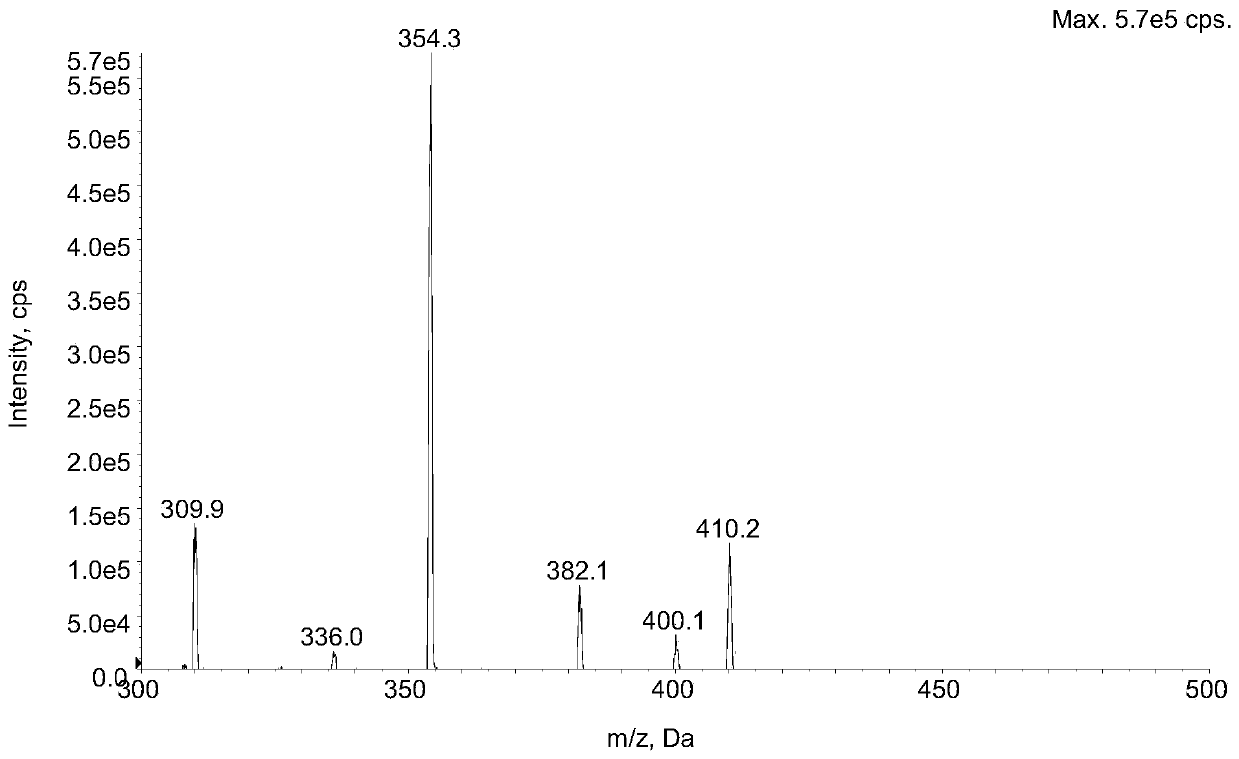
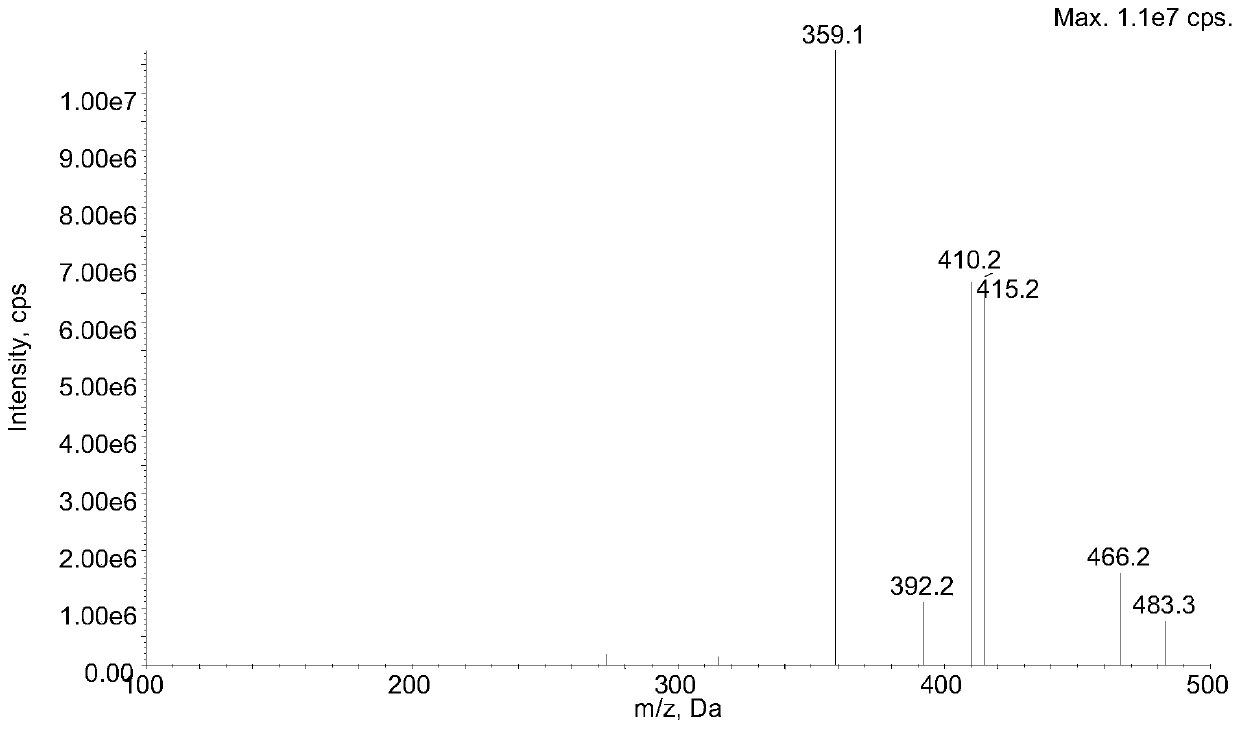
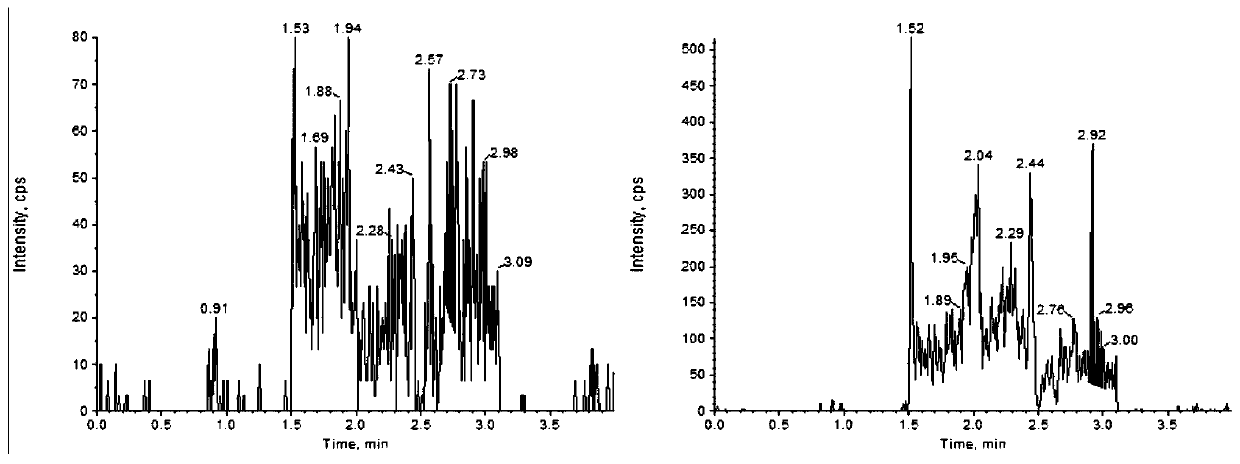
Method for determining concentration of lacidipine in blood plasma by adopting liquid chromatography-tandem mass spectrometry
A technology of tandem mass spectrometry and liquid chromatography, which is applied in the field of liquid chromatography-tandem mass spectrometry to determine the concentration of lacidipine in plasma, which can solve the problems of time-consuming concentration steps, poor sensitivity of detection methods, and long analysis time, so as to improve safety Effects of sex, dose reduction, and sensitivity enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Determination of lacidipine in plasma by liquid chromatography-tandem mass spectrometry
[0026] 1. Instrument:
[0027] Sciex's Triple Quad TM 6500 + Triple quadrupole tandem mass spectrometer and Analyst 1.6.3 data processing software; LC-30 high performance liquid chromatography system from Shimadzu Corporation, including DGU-20A5R degasser, LC-30AD infusion pump, SIL-30AC automatic sampling 器,CTO-20A column thermostat.
[0028] 2. Pretreatment of plasma samples:
[0029] Add 50 µL lacidipine to 50 µL plasma sample- 13 C 8 Internal standard solution (concentration of 50 ng / mL, solvent is a methanol: water mixture with a volume ratio of 50:50), then add 400 µL of acetonitrile, vortex for 10 minutes, and centrifuge at 3900 rpm for 10 minutes at 4°C. The supernatant after centrifugation was transferred to another 96-well plate. Take 10.0 µL of the supernatant for LC-MS / MS analysis.
[0030] 3. Preparation of standard series samples and quality control samples:
[00...
Embodiment 2
[0037] Example 2: Methodological verification
[0038] The methodological verification of the measurement method in Example 1 is as follows:
[0039] 1. Selectivity:
[0040] Analyze the selectivity of the evaluation method of blank plasma, hemolyzed plasma, hyperlipidemia plasma and the lower limit of quantification sample prepared by the blank plasma from 6 different sources.
[0041] The results show that endogenous substances do not interfere with lacidipine and lacidipine- 13 C 8 The determination.
[0042] 2. Standard curve:
[0043] Taking the theoretical concentration of Lassidipine as the abscissa (x), Lassidipine and Lassidipine- 13 C 8 The peak area ratio is the ordinate (y), the linear regression equation for regression analysis calculation, and the weighting factor W=1 / x 2 , Lacidipine plasma concentration range 0.025 ~ 10.0 ng . mL −1 The inner linear relationship is good. The typical standard curve regression equation is: y=0.392x+0.000911 (r=0.9968).
[0044] 3. Precisi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Collision gas | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com